Abstract
Purpose
Pembrolizumab demonstrated antitumor activity in programmed death ligand 1 positive (combined positive score (CPS) ≥ 1) gastric/gastroesophageal junction cancer in KEYNOTE-059 (third line or beyond), KEYNOTE-061 (second line), and KEYNOTE-062 (first line). We characterized efficacy and safety of pembrolizumab monotherapy in Japanese patients across several lines of therapy in these studies.
Methods
This analysis was conducted in 34 patients from KEYNOTE-059 cohort 1 (all pembrolizumab), including 13 patients with CPS ≥ 1, 65 patients with CPS ≥ 1 from KEYNOTE-061 (pembrolizumab, n = 27; chemotherapy, n = 38), and 70 patients with CPS ≥ 1 from KEYNOTE-062 (pembrolizumab, n = 38; chemotherapy, n = 32). Overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and safety were evaluated.
Results
In KEYNOTE-059, ORR with pembrolizumab was 9%, median PFS was 2 months, and median OS was 10 months. In KEYNOTE-061, median OS was 12 months with pembrolizumab versus 10 months with chemotherapy (hazard ratio (HR), 0.67; 95% confidence interval (CI), 0.39–1.15). Median PFS (pembrolizumab vs. chemotherapy) was 2 months versus 4 months (HR, 1.21; 95% CI, 0.69–2.13); ORR was 7% versus 18%. In KEYNOTE-062, median OS was 20 months with pembrolizumab versus 18 months with chemotherapy (HR, 0.76; 95% CI, 0.43–1.33). Median PFS (pembrolizumab vs. chemotherapy) was 6 months versus 7 months (HR, 1.03; 95% CI, 0.61–1.74); ORR was 29% versus 34%.
Conclusions
The current analysis provides valuable information that anti–PD-1 therapies are worthy of further assessment for gastric cancer.
Trial Registration
ClinicalTrials.gov: NCT02335411 (KEYNOTE-059), NCT02370498 (KEYNOTE-061), and NCT02494583 (KEYNOTE-062).
Supplementary Information
The online version contains supplementary material available at 10.1007/s12029-023-00920-9.
Keywords: Biomarkers, Clinical trial, Gastrointestinal neoplasm, Pembrolizumab, Japan
Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide, and the geographic distribution of GC incidence and mortality is disproportionate, with the highest rates observed in east Asian countries, such as South Korea, Mongolia, and Japan [1, 2].
In Japan, the current standard of care for unresectable advanced, recurrent, or metastatic GC in the first-line setting is systemic chemotherapy with a fluoropyrimidine plus platinum-based agent with or without nivolumab for human epidermal growth factor receptor 2 (HER2)–negative tumors or a trastuzumab-containing regimen for HER2-positive tumors [3]. Recommendations for subsequent lines of therapy include paclitaxel plus ramucirumab in the second-line setting and irinotecan or the anti–programmed death 1 (PD-1) monoclonal antibody nivolumab in the third-line setting [3].
The PD-1 inhibitor pembrolizumab was approved by the US Food and Drug Administration—based on the KEYNOTE-059 study—for recurrent locally advanced or metastatic programmed death ligand 1 (PD-L1)–positive (combined positive score (CPS) ≥ 1) gastric/gastroesophageal junction (GEJ) adenocarcinoma with disease progression on or after ≥ 2 previous lines of therapy, including fluoropyrimidine- and platinum-containing chemotherapy, and, if appropriate, HER2/neu-targeted therapy [4]. In Japan, pembrolizumab is approved for the treatment of advanced/recurrent microsatellite instability-high (MSI-H) solid tumors, including GC [5], that progressed after chemotherapy [6].
In the single-arm, multicohort, phase 2 KEYNOTE-059 study, patients with advanced gastric/GEJ cancer in cohort 1 received pembrolizumab in the third-line or later setting [7]. Among 148 patients with PD-L1-positive tumors, durable antitumor activity was observed (objective response rate (ORR), 15.5%; median duration of response (DOR), 16.3 months) with a manageable safety profile [7].
KEYNOTE-061 was a randomized, phase 3 study of second-line therapy with pembrolizumab versus paclitaxel in advanced gastric/GEJ cancer [8]. In the primary analysis (395 patients with CPS ≥ 1), pembrolizumab did not significantly improve overall survival (OS) versus paclitaxel (hazard ratio (HR), 0.82; 95% confidence interval (CI), 0.66–1.03; one-sided P = 0.042) [8]; post hoc analysis of patients with CPS ≥ 10 revealed a greater survival benefit with pembrolizumab than paclitaxel (HR, 0.64; 95% CI, 0.41–1.02) [9]. Similar ORRs were reported for pembrolizumab and paclitaxel (15.8% and 13.6%, respectively) in patients with CPS ≥ 1, but median DOR was longer in the pembrolizumab group (18.0 vs. 5.2 months) [8]. Pembrolizumab did not prolong progression-free survival (PFS), but its safety profile when compared with that of paclitaxel was favorable [8].
First-line therapy with pembrolizumab with or without chemotherapy (cisplatin plus 5-fluorouracil or capecitabine) versus chemotherapy was assessed in the randomized, phase 3 KEYNOTE-062 study in patients with advanced/metastatic gastric/GEJ cancer [10]. In 506 patients with CPS ≥ 1, noninferiority of pembrolizumab monotherapy versus chemotherapy for OS was met, with an HR of 0.91 (99.2% CI, 0.69–1.18; prespecified noninferiority margin, 1.2); a lower ORR (15% vs. 37%) was also observed [10]. Predefined analysis of patients with CPS ≥ 10 revealed that OS was numerically prolonged with pembrolizumab monotherapy versus chemotherapy (HR, 0.69; 95% CI, 0.49–0.97), but this difference was not statistically tested. Pembrolizumab also demonstrated better tolerability than chemotherapy.
We retrospectively evaluated Japanese patients with gastric/GEJ cancer enrolled in KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 to characterize treatment response with pembrolizumab monotherapy across several lines of therapies.
Materials and Methods
Study Designs
Detailed descriptions of the study designs for KEYNOTE-059 cohort 1, KEYNOTE-061, and KEYNOTE-062 have been published [7, 8, 10]; additional details appear in Online Resource 1. The current analysis focused on the subgroup of patients enrolled at Japanese sites in each study.
The study protocols and all amendments were approved by the institutional review board or ethics committee at each institution. The studies were conducted in accordance with the protocol and its amendments, Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent.
Outcomes
OS, PFS, ORR, safety, and tolerability were evaluated in the pembrolizumab monotherapy and chemotherapy treatment groups.
Statistical Analyses
Efficacy data were reported for the subgroup of Japanese patients from KEYNOTE-059 cohort 1 and the subgroup of Japanese patients with CPS ≥ 1 and CPS ≥ 10 from both KEYNOTE-061 and KEYNOTE-062 who had received ≥ 1 dose of study drug. Safety data were reported for all Japanese patients from each study. Patients could have had more than 1 immune-mediated adverse events (imAEs). Results were analyzed for each of the trials separately (i.e., results were not pooled across trials). The primary efficacy analyses were performed in the CPS ≥ 1 populations of the KEYNOTE-059 (cohort 1) and in the CPS ≥ 1 and CPS ≥ 10 populations of the KEYNOTE-061 and KEYNOTE-062 studies.
The database cutoff dates for this analysis were August 8, 2018 (KEYNOTE-059; NCT02335411); October 26, 2017 (KEYNOTE-061; NCT02370498); and March 26, 2019 (KEYNOTE-062; NCT02494583).
Additional details can be found in Online Resource 1.
Results
Patients
This analysis of Japanese patients included 34 patients from KEYNOTE-059, 65 patients from KEYNOTE-061, and 70 patients from KEYNOTE-062 (Table 1). Patient demographics and baseline characteristics are summarized in Table 2.
Table 1.
Disposition of Japanese patients from the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 studies
| n (%) | KEYNOTE-059 | KEYNOTE-061 | KEYNOTE-062 | ||
|---|---|---|---|---|---|
| All patients | CPS ≥ 1 | CPS ≥ 1 | |||
| Pembrolizumab | Pembrolizumab | Chemotherapy | Pembrolizumab | Chemotherapy | |
| n = 34 | n = 27 | n = 38 | n = 38 | n = 32 | |
| Status for trial | |||||
| Discontinued | 31 (91) | 21 (78) | 35 (92) | 24 (63) | 25 (78) |
| Death | 31 (91) | 21 (78) | 34 (90) | 24 (63) | 25 (78) |
| Protocol violation | 0 | 0 | 1 (3) | 0 | 0 |
| Ongoing | 3 (9) | 6 (22) | 3 (8) | 14 (37) | 7 (22) |
| Status for study treatment | |||||
| Discontinued | 25 (93) | 36 (100) | 36 (95) | 31 (97) | |
| AE | — | 0 | 1 (3) | 3 (8) | 2 (6) |
| Clinical progression | — | 3 (11) | 3 (8) | 6 (16) | 1 (3) |
| Progressive disease | — | 22 (82) | 32 (89) | 25 (66) | 25 (78) |
| Noncompliance | — | 0 | 0 | 1 (3) | 1 (3) |
| Withdrawal by patient | — | 0 | 0 | 1 (3) | 2 (6) |
| Ongoing | — | 1 (4) | 0 | 1 (3) | 1 (3) |
AE adverse events, CPS combined positive score
Table 2.
Baseline characteristics of Japanese patients from the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 studies
| Characteristic | KEYNOTE-059 | KEYNOTE-061 | KEYNOTE-062 | ||
|---|---|---|---|---|---|
| All patients | CPS ≥ 1 | CPS ≥ 1 | |||
| Pembrolizumab | Pembrolizumab | Chemotherapy | Pembrolizumab | Chemotherapy | |
| n = 34 | n = 27 | n = 38 | n = 38 | n = 32 | |
| Median age, years (range) | 62 (39–83) | 68 (38–75) | 66 (27–77) | 67 (28–83) | 68 (44–85) |
| Male, n (%) | 24 (71) | 20 (74) | 25 (66) | 30 (79) | 24 (75) |
| ECOG performance status, n (%) | |||||
| 0 | 22 (65) | 21 (78) | 24 (63) | 30 (79) | 24 (75) |
| 1 | 12 (35) | 6 (22) | 14 (37) | 8 (21) | 8 (25) |
| Location of primary tumor, n (%) | |||||
| Gastric | — | 23 (85) | 34 (89) | 28 (74) | 29 (91) |
| GEJ | — | 4 (15) | 4 (11) | 10 (26) | 3 (9) |
| No. of metastases | |||||
| 0–2 | — | 27 (57) | 28 (53) | 24 (63) | 27 (84) |
| ≥ 3 | — | 20 (43) | 25 (47) | 12 (32) | 24 (13) |
| Missing | — | 0 | 0 | 2 (5) | 1 (3) |
| No. of previous therapies for metastatic disease, n (%) | |||||
| 2 | 11 (32) | — | — | — | — |
| 3 | 9 (27) | — | — | — | — |
| 4 | 11 (32) | — | — | — | — |
| ≥ 5 | 3 (9) | — | — | — | — |
| Previous gastrectomy, n (%) | 17 (50) | 6 (22) | 10 (26) | 11 (29) | 4 (13) |
| Histology, n (%) | |||||
| Tubular adenocarcinoma | 30 (88) | 5 (19) | 8 (21) | — | — |
| Signet-ring cell carcinoma | 2 (6) | 2 (7) | 1 (3) | — | — |
| Mixed carcinoma | 2 (6) | 0 | 0 | — | — |
| Adenocarcinoma | 0 | 16 (59) | 26 (68) | — | — |
| Poorly cohesive carcinoma | 0 | 4 (15) | 3 (8) | — | — |
| Histological subtype, n (%) | |||||
| Diffuse | — | 14 (52) | 16 (42) | 19 (50) | 14 (44) |
| Intestinal | — | 11 (41) | 20 (53) | 13 (34) | 14 (44) |
| Mixed | — | 1 (4) | 1 (3) | 3 (8) | 4 (13) |
| Unknown | — | 1 (4) | 1 (3) | 3 (8) | 0 |
| HER2-positive status, n (%) | 12 (35) | 4 (15) | 9 (24) | 0 | 0 |
| MSI-H status, n (%) | 1 (3) | 2 (7) | 2 (5) | 3 (8) | 1 (3) |
CPS combined positive score, ECOG Eastern Cooperative Oncology Group, GEJ gastroesophageal junction, HER2 human epidermal growth factor receptor 2, MSI-H microsatellite instability-high
Characteristics of patients with CPS ≥ 1 were generally similar between treatment groups in KEYNOTE-061 except for Eastern Cooperative Oncology Group performance status 1 (pembrolizumab, 22%; chemotherapy, 37%). Characteristics of patients with CPS ≥ 1 were generally similar between treatment groups in KEYNOTE-062 except for primary tumor location (GC—pembrolizumab, 74%; chemotherapy, 91%; GEJ cancer—pembrolizumab, 26%; chemotherapy, 9%) and previous gastrectomy (pembrolizumab, 29%; chemotherapy, 13%). In KEYNOTE-061, 82% of patients in the pembrolizumab group and 97% of patients in the chemotherapy group received subsequent therapy (Table S1). In KEYNOTE-062, 87% of patients in the pembrolizumab group and 84% of patients in the chemotherapy group received subsequent therapy (Table S2).
Overall Survival
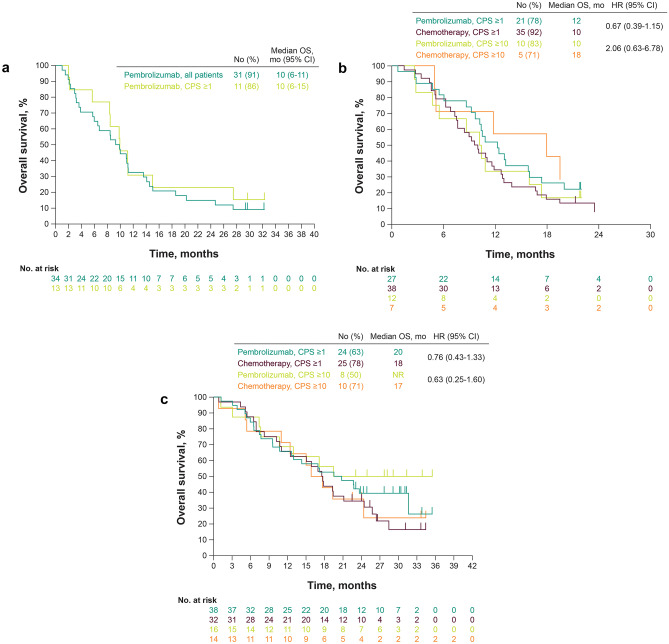
In KEYNOTE-059, median OS was 10 months in all patients and in the CPS ≥ 1 population (Fig. 1a).
Fig. 1.
Kaplan–Meier estimates of overall survival in Japanese patients from a KEYNOTE-059 cohort 1, b KEYNOTE-061, and c KEYNOTE-062. CPS combined positive score
In the CPS ≥ 1 population of KEYNOTE-061, median OS (pembrolizumab vs. chemotherapy) was 12 versus 10 months (HR, 0.67; 95% CI, 0.39–1.15); after adjusting for selected baseline characteristics in a multivariate analysis, the HR was 0.71 (95% CI, 0.40–1.25). In the CPS ≥ 10 population, median OS (pembrolizumab vs. chemotherapy) was 10 versus 18 months (HR, 2.06; 95% CI, 0.63–6.78) (Fig. 1b).
In the CPS ≥ 1 population of KEYNOTE-062, median OS (pembrolizumab vs. chemotherapy) was 20 versus 18 months (HR, 0.76; 95% CI, 0.43–1.33) (Fig. 1c); after adjusting for selected baseline characteristics in a multivariate analysis, the HR was 0.58 (95% CI, 0.30–1.10). In the CPS ≥ 10 population, median OS (pembrolizumab vs. chemotherapy) was not reached (NR) versus 17 months (HR, 0.63; 95% CI, 0.25–1.60) (Fig. 1c); after adjusting for selected baseline characteristics in a multivariate analysis, the HR was 0.49 (95% CI, 0.17–1.37).
Progression-Free Survival
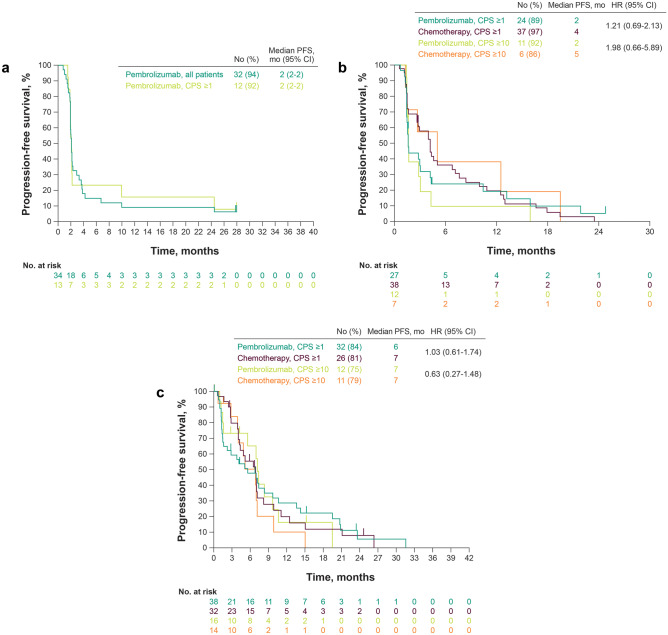
In KEYNOTE-059, median PFS based on independent central review was 2 months in all patients and in the CPS ≥ 1 population (Fig. 2a).
Fig. 2.
Kaplan–Meier estimates of progression-free survival in Japanese patients from a KEYNOTE-059 cohort 1, b KEYNOTE-061, and c KEYNOTE-062. CPS combined positive score
In the CPS ≥ 1 population of KEYNOTE-061, median PFS (pembrolizumab vs. chemotherapy) was 2 versus 4 months (HR, 1.21; 95% CI, 0.69–2.13) (Fig. 2b); after adjusting for selected baseline characteristics in a multivariate analysis, the HR was 1.22 (95% CI, 0.69–2.14). In the CPS ≥ 10 population, median PFS (pembrolizumab vs. chemotherapy) was 2 versus 5 months (HR, 1.98; 95% CI, 0.66–5.89).
In the CPS ≥ 1 population of KEYNOTE-062, median PFS (pembrolizumab vs. chemotherapy) was 6 versus 7 months (HR, 1.03; 95% CI, 0.61–1.74) (Fig. 2c); after adjusting for selected baseline characteristics in a multivariate analysis, the HR was 0.87 (95% CI, 0.49–1.55). In the CPS ≥ 10 population, median PFS (pembrolizumab vs. chemotherapy) was 7 months in each group (HR, 0.63; 95% CI, 0.27–1.48) (Fig. 2c); after adjusting for selected baseline characteristics in a multivariate analysis, the HR was 0.54 (95% CI, 0.21–1.41).
Response
In KEYNOTE-059, ORR was 9% (n = 3) (Table 3). In the CPS ≥ 1 population, ORR was 15% (n = 2). Among the three patients in the CPS ≥ 10 population, one patient achieved an objective response (partial response (PR)).
Table 3.
Summary of treatment response in Japanese patients from the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 studies
| n (%) | KEYNOTE-059 | KEYNOTE-059 | KEYNOTE-061 | KEYNOTE-061 | KEYNOTE-062 | KEYNOTE-062 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| All patients | CPS ≥ 1 | CPS ≥ 1 | CPS ≥ 10 | CPS ≥ 1 | CPS ≥ 10 | |||||
| Pembrolizumab | Pembrolizumab | Pembrolizumab | Chemotherapy | Pembrolizumab | Chemotherapy | Pembrolizumab | Chemotherapy | Pembrolizumab | Chemotherapy | |
| n = 34 | n = 13 | n = 27 | n = 38 | n = 12 | n = 7 | n = 38 | n = 32 | n = 16 | n = 14 | |
| ORR | 3 (9) | 2 (15) | 2 (7) | 7 (18) | 0 | 1 (14) | 11 (29) | 11 (34) | 5 (31) | 4 (29) |
| CR | 2 (6) | 1 (8) | 0 | 1 (3) | 0 | 0 | 3 (8) | 2 (6) | 2 (13) | 0 |
| PR | 1 (3) | 1 (8) | 2 (7) | 6 (16) | 0 | 1 (14) | 8 (21) | 9 (28) | 3 (19) | 4 (29) |
| SD | 6 (18) | 1 (8) | 10 (37) | 16 (42) | 5 (42) | 4 (57) | 9 (24) | 13 (41) | 5 (31) | 7 (50) |
| PD | 23 (68) | 10 (77) | 14 (52) | 12 (32) | 7 (58) | 2 (29) | 4 (11) | 2 (6) | 3 (19) | 1 (7) |
| Not availablea | 2 (6) | 0 | 1 (4) | 3 (8) | 0 | 0 | 5 (13) | 4 (13) | 3 (19) | 2 (14) |
CPS combined positive score, CR complete response, ORR objective response rate, PD progressive disease, PR partial response, SD stable disease
aNot available includes patients who were not evaluable and patients with no postbaseline assessment as of the data cutoff date
In the CPS ≥ 1 population of KEYNOTE-061, ORR (pembrolizumab vs. chemotherapy) was 7% versus 18% (Table 3). Among the four patients with MSI-H, one chemotherapy-treated patient achieved an objective response (PR); no pembrolizumab-treated patients achieved an objective response. In the CPS ≥ 10 population, one chemotherapy-treated patient achieved objective response (PR); no pembrolizumab-treated patients achieved objective response.
In the CPS ≥ 1 population of KEYNOTE-062, ORR (pembrolizumab vs. chemotherapy) was 29% versus 34% (Table 3). Among the four patients with MSI-H, one pembrolizumab-treated patient achieved an objective response (PR); no chemotherapy-treated patients achieved objective response. In the CPS ≥ 10 population, ORR (pembrolizumab vs. chemotherapy) was 31% versus 29% (Table 3).
Safety and Tolerability
Table 4 summarizes AEs reported in the Japanese subgroups of KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062. In KEYNOTE-059, 59% of patients had ≥ 1 treatment-related AE (TRAE); 18% experienced a grade 3 or 4 event, with no grade 5 events reported. The most common any-grade TRAE (≥ 10%) was rash (Table S3). Eight patients (24%) experienced imAEs. The most common imAE was hypothyroidism (n = 3; 9%), followed by infusion reactions (n = 2; 6%), colitis, encephalitis, hyperthyroidism, pneumonitis, and severe skin reactions (n = 1 each; 3%).
Table 4.
Summary of adverse events in Japanese patients from the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 studies
| n (%) | KEYNOTE-059 | KEYNOTE-061 | KEYNOTE-062 | ||
|---|---|---|---|---|---|
| All patients | All patients | All patients | |||
| Pembrolizumab | Pembrolizumab | Chemotherapy | Pembrolizumab | Chemotherapy | |
| n = 34 | n = 47 | n = 50 | n = 38 | n = 32 | |
| ≥ 1 AE | 30 (88) | 41 (87) | 48 (96) | 36 (95) | 32 (100) |
| TRAE | 20 (59) | 23 (49) | 46 (92) | 22 (58) | 30 (94) |
| Grade 3–5 AEs | 15 (44) | 14 (30) | 26 (52) | 14 (37) | 22 (69) |
| Grade 3–5 TRAE | 6 (18)a | 2 (4) | 22 (44) | 8 (21) | 19 (59) |
| Serious AE | 9 (27) | 7 (15) | 4 (8) | 8 (21) | 11 (34) |
| Serious TRAE | 4 (12) | 0 (0) | 1 (2) | 5 (13) | 7 (22) |
| Discontinuation due to AE | 2 (6) | 0 (0) | 3 (6) | 3 (8) | 9 (28) |
| Discontinuation due to TRAE | 2 (6) | 0 (0) | 3 (6) | 2 (5) | 9 (28) |
| Death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
AE adverse event, TRAE treatment-related adverse event
aNo grade 5 TRAEs occurred
In KEYNOTE-061, the incidence of any-grade TRAEs (pembrolizumab vs. chemotherapy) was 49% versus 92%; grade 3–5 events were reported in 4% versus 44% of patients (Table 4). The most common any-grade TRAEs with pembrolizumab (≥ 10%) were diarrhea, pruritus, and rash; the most common with chemotherapy (≥ 30%) were alopecia, decreased neutrophil count, and peripheral sensory neuropathy (Table S4). The incidence of imAEs (pembrolizumab vs. chemotherapy) was 19% (n = 9) versus 4% (n = 2). The most common imAE with pembrolizumab was hypothyroidism (n = 5, 11%), followed by infusion reactions (n = 2; 4%), hyperthyroidism, hypophysitis, and pneumonitis (n = 1 each; 2%).
In KEYNOTE-062, the incidence of any-grade TRAEs (pembrolizumab vs. chemotherapy) was 58% versus 94%; grade 3–5 events were reported in 21% versus 59% (Table 4). The most common TRAEs with pembrolizumab (≥ 10%) were pruritus, decreased appetite, diarrhea, and rash; the most common with chemotherapy (≥ 40%) were decreased appetite, nausea, decreased neutrophil count, and palmar-plantar erythrodysesthesia syndrome (Table S5). The incidence of imAEs (pembrolizumab vs. chemotherapy) was 16% (n = 6) versus 6% (n = 2). The most common imAE with pembrolizumab was colitis (n = 2; 5%), followed by adrenal insufficiency, hyperthyroidism, hypophysitis, hypothyroidism, myositis, and pneumonitis (n = 1 each; 3%).
Discussion
These subgroup analyses of Japanese patients with gastric/GEJ cancer demonstrated that pembrolizumab monotherapy, given as first-, second-, or third-line and later therapy, demonstrated a trend toward improvement in clinical outcomes, although the results were not statistically significant compared with placebo. Data from KEYNOTE-061 and KEYNOTE-062 also demonstrated numeric improvement in median OS with pembrolizumab monotherapy compared with chemotherapy in patients with PD-L1 CPS ≥ 1. Analyses from KEYNOTE-062 also suggested improvements in median OS and PFS with pembrolizumab in patients with CPS ≥ 10; however, these differences were not statistically tested. In all three studies, pembrolizumab was generally well tolerated, produced no unexpected toxicity, and had a better safety profile than chemotherapy.
Findings from these Japanese subgroup analyses are comparable with outcomes observed in the intention-to-treat (ITT) populations of each respective study. In KEYNOTE-059, OS and PFS medians were similar between the Japanese subgroup and the ITT population [7]. Although the number of patients in the CPS ≥ 1 Japanese subgroup was small, response data were consistent with those of the ITT population with PD-L1-positive tumors [7].
Similar outcomes were also observed in the Japanese subgroup and the ITT population in KEYNOTE-061 patients with CPS ≥ 1 for OS and PFS medians, and no significant differences were observed in either population between pembrolizumab and chemotherapy [8].
In KEYNOTE-062, median OS in the Japanese subgroup with CPS ≥ 1 was higher in the pembrolizumab and chemotherapy groups (20 and 18 months, respectively) than in the ITT population (11 and 11 months), but no significant between-treatment differences were observed in either population; median PFS in the pembrolizumab group of the Japanese subgroup was also higher (6 months) than it was in the ITT population (2 months) [10]. It is notable that a greater proportion of the Japanese subgroup than the ITT population had 0 to 2 metastases (73% and 53%, respectively) and Eastern Cooperative Oncology Group performance status 0 (77% and 48%) [10].
When multiple factors influence prognosis, a combination of these factors can affect the OS HR between two treatment groups. To confirm whether the HRs were robust in our study, we performed multivariate analysis of OS after adjusting for selected baseline characteristics. We found that OS HRs ranged from 0.5 to 0.7 for an imbalanced combination of factors, supporting the robustness of our results. Furthermore, in KEYNOTE-062, 134 of 254 pembrolizumab-treated patients (53%) and 132 of 244 chemotherapy-treated patients (54%) received ≥ 1 subsequent anticancer therapy in the total population [10] compared with 33 of 38 patients (87%) and 27 of 32 (84%) patients, respectively, in the Japanese subgroup. As a result, the observed survival advantage in the Japanese subgroup may be the result of the higher proportion of patients receiving subsequent anticancer therapy. This suggests that switching to the next treatment at an appropriate time and to subsequent treatment may contribute to prolongation of survival. Further investigation of immunologic profile differences is also warranted.
PD-L1 expression can be used in clinical practice to help select patients for treatment with immunotherapy in tumor types with high PD-L1 expression. In GC, use of CPS is a robust and reproducible predictive marker to identify patients likely to respond to pembrolizumab [11]. Recent evidence suggests that enriching for PD-L1 status by increasing the minimum proportion of stained cells (e.g., from CPS ≥ 1 to CPS ≥ 10) is positively correlated with the OS benefit provided by PD-1/L1 inhibitor therapy in solid tumors [12]. In the current report, data from Japanese patients with CPS ≥ 10 from the KEYNOTE-062 study revealed an enhanced treatment effect with pembrolizumab monotherapy on survival outcomes, improving median OS (from 20 months (CPS ≥ 1) to NR (CPS ≥ 10)). Recent analyses of patients with CPS ≥ 10 from the ITT populations of KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 demonstrated improvement in OS with pembrolizumab monotherapy when a higher CPS cutoff was used [9], thus supporting the current findings in Japanese patients with CPS ≥ 10. Further enrichment of PD-L1 expression status in patients with gastric/GEJ cancer may serve as an important predictive biomarker for the selection of patients likely to benefit most from pembrolizumab monotherapy.
Pembrolizumab monotherapy was generally well tolerated in the Japanese subgroups of KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062, which were comparable with the ITT population [7, 8, 10]. Most TRAEs were mild or moderate in severity, discontinuation rates were low, and the incidence of imAEs was low. The consistency of these findings suggested that the use of pembrolizumab monotherapy is generally safe across first and subsequent lines of therapy in patients with advanced/metastatic gastric/GEJ cancer.
The results of the current report for the subgroup of patients enrolled at Japanese sites in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 studies demonstrated the efficacy and safety of pembrolizumab in previously untreated and treated Japanese patients and showed general consistency with data from globally conducted studies. These results not only build on existing data from global studies but add to the body of evidence evaluating pembrolizumab in various patient populations in the gastric cancer treatment setting. Analyses that differ in geographic region of enrollment enable regulatory agencies and health care providers to make decisions based on the benefit-to-risk ratio of pembrolizumab across various patient populations and ultimately facilitate swift access to needed treatments by patients, which is especially important in the gastric cancer disease setting.
Limitations of the current retrospective analysis include small sample sizes for the Japanese subgroups in each study (≤ 100 patients) as well subgroups for CPS ≥ 10 and MSI-H status; thus, results should be interpreted with caution. Additionally, the current analysis included the KEYNOTE-061 study, which did not meet its primary end points of OS and PFS in patients with a PD-L1 CPS ≥ 1.
Conclusions
These data in Japanese patients indicate that pembrolizumab monotherapy provides consistent survival benefit and an acceptable safety profile when used in the first-line (KEYNOTE-062), second-line (KEYNOTE-061), or third-line or later (KEYNOTE-059) settings in patients with advanced/metastatic gastric/GEJ cancers. The current analysis provides valuable insight and information that anti–PD-1 therapies are worthy of further assessment, particularly in patients with locally advanced/unresectable or metastatic gastric cancer. Furthermore, PD-L1 CPS ≥ 1 can be used as a predictive biomarker of response to pembrolizumab monotherapy; increasing this cutoff to CPS ≥ 10 has the potential to improve patient selection. Adequately powered, prospective clinical trials are needed to validate the optimal use of CPS as a predictive biomarker for patients with gastric/GEJ cancer.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients and their families and all investigators and site personnel. Medical writing and/or editorial assistance was provided by Holly C. Cappelli, PhD, CMPP, of ApotheCom (Yardley, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author Contribution
Conceptualization was done by Kei Muro, Kohei Shitara, Shinichi Shiratori, and Atsushi Ohtsu. Methodology was completed by Kei Muro, Kohei Shitara, Shinichi Shiratori, and Atsushi Ohtsu. Investigation was done by Kei Muro, Kohei Shitara, Takaki Yoshikawa, Hironaga Satake, Hiroki Hara, Naotoshi Sugimoto, Nozomu Machida, Masahiro Goto, Hisato Kawakami, Kenji Amagai, Yasushi Omuro, Taito Esaki, Tomohiro Nishima, Yoshito Komatsu, Hisahiro Matsubara, Shinichi Shiratori, Taroh Satoh, and Shuichi Hironaka. Formal analysis was done by Shinichi Shiratori and Taroh Satoh. Data interpretation was done by Kohei Shitara, Kensei Yamaguchi, Takaki Yoshikawa, Hiroki Hara, Hisato Kawakami, Tomohiro Nishima, Yoshito Komatsu, Shinichi Shiratori, and Atsushi Ohtsu. The original draft of the manuscript was written by Kohei Shitara. All authors contributed to manuscript review and editing. All authors had full access to the study data, were involved in the writing or reviewing and editing drafts of the manuscript, and approved the decision to submit for publication.
Funding
This study was sponsored by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Data Availability
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
Declarations
Ethics Approval and Consent to Participate
The studies were conducted in accordance with the protocol and its amendments, Good Clinical Practice guidelines, and the Declaration of Helsinki. The study protocols and all amendments were approved by the institutional review board or ethics committee at each institution. All patients provided written informed consent.
Competing Interests
KM has received grants from Sanofi, Astellas, Amgen, Solasia Pharma, Daiichi Sankyo, Parexel International, Taiho Pharmaceutical, MSD K. K., Merck Serono, Pfizer, and Ono; has received honoraria from Eli Lilly, Chugai Pharmaceutical, Takeda, Ono, Taiho Pharmaceutical, Sanofi, Bristol Myers Squibb, and Bayer; and has participated on data safety monitoring board or advisory board for AstraZeneca and Ono. KS has received grants from MSD K. K., Astellas, Lilly, Ono, Dainippon Sumitomo, Daiichi Sankyo, Taiho Pharmaceutical, Chugai Pharmaceutical, MSD K.K., Mediscience, and Eisai; consulting fees from Astellas, Lilly, Bristol Myers Squibb, Takeda, Pfizer, Ono, MSD K.K., Taiho Pharmaceutical, Novartis, AbbVie, GSK, Daiichi Sankyo, Amgen, and Boehringer Ingelheim; and honoraria from Novartis, AbbVie, and Yakult Honsha. KY reports no conflict of interest. TY has received consulting fees from MSD K. K. and honoraria for lectures from MSD K. K., Ono, and Bristol Myers Squibb. HS has received grants from Taiho Pharmaceutical, Chugai Pharmaceutical, and Yakult Honsha; consulting fees from Bristol Myers Squibb; and honoraria from Bristol Myers Squibb, Taiho Pharmaceutical, MSD K.K., Chugai Pharmaceutical, and Yakult Honsha. HH has received funding for medical writing assistance from MSD K.K.; grants from Astellas, AstraZeneca, Bayer, BeiGene, Boehringer Ingelheim, Chugai Pharmaceutical, Daiichi Sankyo, Dainippon Sumitomo, Eisai, Elevar Therapeutics, GSK, Incyte, Merck Biopharma, MSD K. K., Ono, Pfizer, and Taiho Pharmaceutical; consulting fees from Boehringer Ingelheim, Daiichi Sankyo, Dainippon Sumitomo, Lilly, MSD K. K., and Ono; and honoraria from Bayer, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Kyowa Hakko Kirin, Lilly, Merck Biopharma, MSD K. K., Ono, Sanofi, Taiho Pharmaceutical, Takeda, and Yakult Honsha. NS’s institution has received funds from MSD K. K., Astellas, Solasia Pharma, Daiichi Sankyo, and Ono. NM has received honoraria from Ono, Taiho Pharmaceutical, Lilly, Daiichi Sankyo, Takeda, Bristol Myers Squibb, and Yakult Honsha. MG reports no conflict of interest. K has received grants from Chugai Pharmaceutical, Taiho Pharmaceutical, and Eisai; honoraria from Bristol Myers Squibb, AstraZeneca, Bayer Yakuhin, Eli Lilly, MSD K. K., Ono, Chugai Pharmaceutical, Daiichi Sankyo, Takeda, and Taiho Pharmaceutical; and consulting fees from Bristol Myers Squibb, Eli Lilly, MSD K. K., Ono, Daiichi Sankyo, and Taiho Pharmaceutical. KA reports no conflict of interest. YO has received grants from MSD K. K., Ono, BeiGene, Astellas, and Daiichi Sankyo. TE has received grants (paid to the institution) from MSD K. K., Novartis, Dainippon Sumitomo, Ono, Daiichi Sankyo, Astellas, Astellas Amgen Biopharma, BeiGene, Pierre Fabre Medicament, Ignyta, Array BioPharma, and Merck Serono and honoraria from MSD K. K., Ono, Daiichi Sankyo, Merck Serono, Eli Lilly, Taiho Pharmaceutical, Chugai Pharmaceutical, Sanofi, Takeda, and Bayer. SH has received grants from Taiho Pharmaceutical, Chugai Pharmaceutical, and Yakult Honsha; consulting fees from Bristol Myers Squibb; and honoraria from Ono, Eli Lilly, Daiichi Sankyo, Bristol Myers Squibb, Taiho Pharmaceutical, Chugai Pharmaceutical, MSD K. K., and Yakult Honsha. TN has received personal fees from Ono, Taiho Pharmaceutical, Takeda, Chugai Pharmaceutical, and Daiichi Sankyo and grants from MSD K. K. YK has received grants from Taiho Pharmaceutical, Chugai Pharmaceutical, Takeda, Bayer Yakuhin, Sanofi-Aventis S. A., Ono, MSD K. K., Yakult Honsha, NanoCarrier, QuintilesIMS, SYSMEX Corp., Mediscience Planning Inc., Dainippon Sumitomo, Kyowa Kirin, Asahi Kasei Pharma, Nippon Zo Pharmaceutical, A2 Healthcare Corp., Daiichi Sankyo, Eisai, Parexel International Corp., Astellas, Incyte, Syneos Health, and IQVIA and personal fees from Taiho Pharmaceutical; Chugai Pharmaceutical; Takeda; Bayer Yakuhin; Sanofi-Aventis S. A.; Ono; Yakult Honsha; Daiichi Sankyo; EA Pharma Co., Ltd.; Eli Lilly Japan K. K.; Nipro Corp.; Bristol Myers Squibb; Moroo; Pfizer; Merck Biopharma; Medical Review Co., Ltd.; Sawai Pharmaceutical; Shiseido; Mitsubishi Tanabe; Nippon Kayaku; Shire Japan K. K.; and Novartis Pharma K. K. HM reports no conflict of interest. SS is an employee of MSD K.K., Tokyo, Japan and a stockholder in Merck & Co., Inc., Rahway, NJ, USA. SH reports no conflict of interest. TS has received grants from MSD K. K., Ono, Yakult Honsha, Chugai Pharmaceutical, Gilead, BeiGene, Eli Lilly, and Taiho Pharmaceutical; honoraria from Ono, Yakult Honsha, Chugai Pharmaceutical, Eli Lilly, and Taiho Pharmaceutical; and consulting fees from Takara-Bio. AO reports no conflict of interest.
Disclaimer
The study funder participated in study design, data interpretation, and the writing of this report. The sponsor maintained the study database.
Footnotes
Previous Presentation
Some data reported in this manuscript were presented at the 2020 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO-GI) (January 23-25, 2020; San Francisco, CA, USA).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Morgan E, Arnold M, Camargo MC, et al. The current and future incidence and mortality of gastric cancer in 185 countries, 2020–40: a population-based modelling study. EClinicalMedicine. 2022;47:101404. doi: 10.1016/j.eclinm.2022.101404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer. 2023;26(1):1–25. 10.1007/s10120-022-01331-8. Epub 2022 Nov 7. [DOI] [PMC free article] [PubMed]
- 4.KEYTRUDA® (pembrolizumab) injection, for intravenous use. 1/2023. Merck Sharp & Dohme LLC, Rahway, NJ, USA; 2023.
- 5.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merck Sharp & Dohme LLC, Rahway, NJ, USA. Merck’s KEYTRUDA® (pembrolizumab) receives five new approvals in Japan, including in advanced non-small cell lung cancer (NSCLC), as adjuvant therapy for melanoma, and in advanced microsatellite instability-high (MSI-H) tumors [press release]. Rahway, NJ, USA: Merck Sharp & Dohme LLC. 2019. https://www.merck.com/news/mercks-keytruda-pembrolizumab-receives-five-new-approvals-in-japan-including-in-advanced-non-small-cell-lung-cancer-nsclc-as-adjuvant-therapy-for-melanoma-and-in-advanced-microsa/. Accessed 3 Jan 2019
- 7.Fuchs CS, Doi T, Jang RW, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4:e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shitara K, Ozguroglu M, Bang YJ, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet. 2018;392:123–133. doi: 10.1016/S0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 9.Wainberg ZA, Fuchs CS, Tabernero J, et al. Efficacy of pembrolizumab (pembro) monotherapy versus chemotherapy for PD-L1–positive (CPS ≥10) advanced G/GEJ cancer in the phase II KEYNOTE-059 (cohort 1) and phase III KEYNOTE-061 and KEYNOTE-062 studies. J Clin Oncol. 2020;38:4275. doi: 10.1200/JCO.2020.38.4_suppl.427. [DOI] [Google Scholar]
- 10.Shitara K, Van Cutsem E, Bang Y-J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol. 2020;6:1571–1580. doi: 10.1001/jamaoncol.2020.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulangara K, Zhang N, Corigliano E, et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med. 2019;143:330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Guo C-Y, Tou F-F, et al. Association of PD-L1 expression status with the efficacy of PD-1/PD-L1 inhibitors and overall survival in solid tumours: a systematic review and meta-analysis. Int J Cancer. 2020;147:116–127. doi: 10.1002/ijc.32744. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.