Abstract
Introduction
The efficacy of conventional treatments for alopecia areata (AA) has been extremely variable and disappointing, with a high rate of relapse. Recent clinical trials and real-life studies have demonstrated efficacy and safety of baricitinib (an oral Janus kinase 1 and 2 inhibitor) in alopecia areata.
Methods
We retrospectively evaluated the effectiveness and tolerance of baricitinib in alopecia areata in a real-life Belgian monocentric adult cohort. The primary outcome was evaluated by the percentage of patients who achieved a Severity of Alopecia Tool (SALT) score of ≤ 20 at the end of the follow-up. All treatment-emergent adverse events were collected.
Results
In this 19-patient series, with a median ± interquartile range (IQR) follow-up duration of 13 ± 16.2 months, we demonstrated that: (i) hair regrowth was observed in nearly 90% of patients between 4 and 16 weeks after initiation of baricitinib; (ii) at the end of the follow-up, more than 70% and, in particular, 100% of patients with patchy AA, reached the primary outcome (SALT score ≤ 20); (iii) almost half of the patients, mostly with patchy AA, showed a complete hair regrowth (SALT score = 0), within a median ± IQR treatment time of 8.5 ± 10 months; (iv) baricitinib was discontinued in three patients with total hair regrowth, two of whom relapsed; and (v) no serious adverse events were reported.
Conclusion
Baricitinib is effective in treating patients with alopecia areata, particularly for the patchy phenotype, but with a risk of relapse after discontinuation. Safety data are reassuring, with lipid changes being the most frequent adverse event.
Keywords: Baricitinib, Alopecia areata, Real-life, JAK inhibitor, Effectiveness, Tolerance
Key Summary Points
| Why carry out this study? |
| Data on the real-world effectiveness and tolerance of baricitinib for treatment of alopecia areata are limited. |
| This retrospective observational study assessed the effectiveness and tolerance of baricitinib for the treatment of alopecia areata in a tertiary university hospital. |
| What was learned from this study? |
| This real-life study assessed the effectiveness and tolerance of baricitinib in alopecia areata in a series of 19 adults with a median follow-up of 13 months. |
| Results were better than in clinical trials; patients with patchy alopecia areata had a better hair regrowth prognosis than those with universalis. |
| Safety profile was reassuring; induced lipid changes were the most frequent adverse events. |
Introduction
Alopecia areata (AA) is an autoimmune disease, affecting approximately 2% of the general population [1, 2]. Although AA is not life-threatening, psychological comorbidities are common and result in major impacts on patients’ quality of life [3]. Response to conventional treatments is extremely variable and disappointing in most cases, with a high risk of relapse [1, 2, 4]. Recent clinical trials and real-life studies have demonstrated efficacy and safety of baricitinib (an oral Janus kinase 1 and 2 inhibitor) in AA, and it received European marketing authorization for the treatment of AA in June 2022 [5–9]. This study aimed to evaluate the effectiveness on hair regrowth and the tolerance of baricitinib in AA.
Methods
We retrospectively collected data from 1 January 2021 to 31 July 2023 from a monocentric cohort of adult patients with severe AA, with a Severity of Alopecia Tool (SALT) score of 50 or higher [range, 0 (no scalp hair loss) to 100 (complete scalp hair loss)], treated with baricitinib 4 mg orally daily. The primary outcome was evaluated by the percentage of patients who achieved a SALT score of ≤ 20 at the end of the follow-up. All adverse events were reported during the study. This study and data collection were conducted with the approval of the hospital and faculty institutional review board (Commission d’Ethique Biomédicale Hospitalo-Facultaire) of Université catholique de Louvain (UCLouvain), Belgium. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. The patients in this manuscript have given written informed consent to participate and for publication of their case details.
Results
Of the 19 patients included, 13 were female and the median age ± interquartile range (IQR) was 35 ± 12 years. The duration of the disease varied from 1 month to 63 years (median 13 years). Patients’ demographics, clinical characteristics, and treatment history are listed in Table 1. The median ± IQR follow-up duration of treatment with baricitinib was 13 ± 16.2 months (median follow-up duration was 23, 11.7, and 12 months for patchy, totalis, and universalis AA, respectively). Hair regrowth was observed in 17/19 (89.5%) patients between 4 and 16 weeks after initiation of treatment (median ± IQR of 6 weeks ± 2). At the end of the follow-up, 14/19 patients [73.7%–11/11 (100%) with patchy AA, 1/2 (50%) with totalis AA, 2/6 (33.3%) with universalis AA] reached the primary outcome (SALT score ≤ 20) (Fig. 1). Seven patients [36.8%–6/11 (54.5%) with patchy AA, 1/2 (50%) with totalis AA, 0/6 (0.0%) with universalis AA] showed complete hair regrowth (SALT score = 0). The median ± IQR treatment time required to obtain complete hair regrowth was 8.5 ± 10 months. Two patients with no signs of hair regrowth presented with long-lasting universalis AA. Baricitinib was discontinued in three patients of whom two relapsed (patients 5 and 13 relapsed 4 months and 1 month after discontinuation, respectively) and one (patient 6) maintained complete hair regrowth 12 months after discontinuation. Dosage was decreased to 2 mg daily in patient 11 after achieving complete hair regrowth; one small patch recurred 4 months after dose reduction (but SALT score remained ≤ 20).
Table 1.
Patients’ demographics and clinical characteristics
| Patient no. | Sex | Age (years) | AA types | Disease durationa | Previous treatments for AA | Time before first signs of regrowth with baricitinib | Regrowth description/SALT score at the end of follow-up | Time needed to reach complete regrowth | Follow-up duration | Adverse events |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 39 | Patchy (head, eyelashes, eyebrows) | 32 years | Topical CS, topical pimecrolimus, intralesional CS injections, minoxidil 2%, MTX, methylprednisolone infusions | 6 weeks |
Diffuse regrowth of hair, eyelashes, eyebrows Persistence of one patch with visible regrowth (SALT score ≤ 20) |
NA | 28 months | ALT 42 |
| 2 | F | 35 | Patchy (head) | 1.5 years | Topical CS, intralesional CS injections, minoxidil 2%/5%, diphencyprone | 6 weeks |
Complete regrowth (SALT score = 0) |
15 months | 31 months | None |
| 3 | M | 32 | Patchy (head, beard, eyelashes, eyebrows) | 13 months | Topical CS, minoxidil 5%, intralesional CS injections | 6 weeks |
Complete regrowth (SALT score = 0) |
20 months | 29 months | TG 203 |
| 4 | F | 27 | Patchy (head) | 15 years | Topical CS, intralesional CS injections, Methylprednisolone infusions | 6 weeks |
Persistence of three patches (SALT score ≤ 20) |
NA | 30 months | Acne |
| 5 | M | 30 | Patchy (head, beard) | 4 years | Topical CS, intralesional CS injections, topical pimecrolimus | 4 weeks |
Recurrence of one patch (SALT score ≤ 20) 4 months after achieving CR (SALT score = 0) and stopping treatment |
13.5 months | 24 months | Chol 233, LDL 147, TG 184 |
| 6 | F | 35 | Patchy (head) | 5 years | Topical CS, intralesional CS injections, minoxidil, cyclosporine | 6 weeks |
Complete regrowth (SALT score = 0), no recurrence 12 months after baricitinib discontinuation |
8.5 months | 17 months | None |
| 7 | F | 18 | Patchy (head) | 9 years | Topical CS, isoprinosine, minoxidil 2%/5% | 8 weeks |
Complete regrowth (SALT score = 0) |
13 months | 23 months | Chol 302, TG 213 |
| 8 | M | 22 | Totalis | 7 years | UV, intralesional CS injections, topical CS, minoxidil | 4 weeks |
Partial regrowth (SALT score ~ 50) |
NA | 16 months | None |
| 9 | M | 70 | Universalis | 63 years | Unknown | 12 weeks |
Partial regrowth in the beard and occipital area (SALT score ~ 80) |
NA | 17 months | None |
| 10 | F | 33 | Universalis | 25 years | Intralesional CS injections, systemic CS, minoxidil, MTX | 6 weeks |
Almost complete regrowth, except for three small patches (SALT score ≤ 20) |
NA | 12 months | Chol 209, LDL 136, TG 224 |
| 11 | F | 53 | Universalis | 19 years | Topical CS, intralesional CS injections, minoxidil 5%, systemic CS, MTX | 4 weeks |
Complete regrowth (SALT score = 0) but recurrence of one small patch 4 months after dose reduction (SALT score remained ≤ 20) |
7 months | 12.5 months | None |
| 12 | M | 42 | Universalis | 15 years | Unknown | 8 weeks |
Partial regrowth (SALT score ~ 40) |
NA | 12 months | Mild headaches, Chol 211, LDL 218 |
| 13 | F | 38 | Patchy (head, eyebrows) | 26 years | Topical CS, intralesional CS injections, minoxidil 5%, systemic CS, MTX | 8 weeks |
Recurrence of four patches (SALT score remained ≤ 20) 1 month after reaching primary outcome and discontinuing baricitinib |
NA | 7.5 months | None |
| 14 | M | 31 | Patchy (head, beard, torso) | 2 years | Topical CS, minoxidil 5% | 16 weeks |
Persistence of one patch (SALT score ≤ 20) |
NA | 7 months | None |
| 15 | F | 42 | Universalis | 20 years | None | NA | No sign of regrowth | NA | 9.5 months | None |
| 16 | F | 31 | Patchy (head) | 22 years | Topical CS, intralesional CS injections, systemic CS, minoxidil 2%/5%, topical diphencyprone 0.1%, MTX | 8 weeks |
Complete regrowth (SALT score = 0) |
4.5 months | 9 months | None |
| 17 | F | 45 | Patchy (head) | 6 months | Topical CS, intralesional CS injections | 8 weeks |
Complete regrowth (SALT score = 0) |
4 months | 8 months | Mild headaches |
| 18 | F | 41 | Universalis | 6 years | Upadacitinib | NA | No sign of regrowth | NA | 6.5 months | None |
| 19 | F | 30 | Totalis | 1 month | Topical CS, systemic CS, minoxidil 5% | 6 weeks |
Complete regrowth (SALT score = 0) |
3.5 months | 7.5 months | Mild headaches, ALT 47, CPK 927 |
aAll patients were in the chronic phase of the disease at baseline, except for patient 17 and 19
AA alopecia areata, ALT alanine amino transferase (U/L), Chol cholesterol (mg/dL), CPK creatine phosphokinase (U/L), CR complete regrowth, CS corticosteroids, F female, LDL low-density lipoprotein (mg/dL), M male, MTX methotrexate, NA not applicable, SALT severity of alopecia tool, TG triglycerides (mg/dL), UV ultraviolet
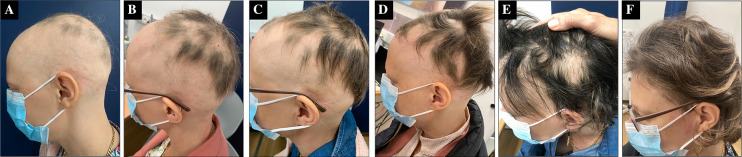
Fig. 1.
Clinical evolution of alopecia areata of patient 1 with baricitinib. At 1.5 months (A), at 3.5 months (B), at 5 months (C), at 7 months (D), at 15 months (E), and at 20 months (F)
Adverse events observed were mild and included acne [n = 1 (5.3%)], headaches [n = 3 (15.8%)], altered lipid status [n = 5 (26.3%)], transaminitis [n = 2 (10.5%)] and increased creatine phosphokinase [n = 1 (5.3%)]. No major adverse events were reported.
Discussion
Although the present real-life study is limited by the sample size and the retrospective design, the results were better than those reported from clinical trials [5, 6]. In two phase III trials (BRAVE-AA1 and BRAVE-AA2), 40.9% of patients achieved a SALT score of ≤ 20 at 52 weeks, compared with the 73.7% in the present real-life study [6]. Our observation that all patients with patchy AA reached a SALT score of 20 or less is encouraging for this patient subgroup. However, this study also confirmed a poorer response for universalis AA, as well as the risk of relapse after treatment discontinuation or dose reduction [4]. In this young population, high dose of baricitinib was well tolerated. The reassuring safety profile is consistent with the recent data about the safety of baricitinib in different indications, including AA [10].
A major problem of the accessibility of baricitinib for the treatment of AA (particularly in terms of reimbursement) remains in most European countries. Presently, because AA remains a largely underrecognized condition, particularly in terms of quality of life and psychological impact, it is still not considered as a priority by the health authorities.
Conclusion
Baricitinib demonstrates good effectiveness for the treatment of AA, particularly for the patchy phenotype, and could substantially improve the management of patients with AA. However, a poorer regrowth prognosis observed for patients with totalis or universalis AA, as well as the risk of relapse after treatment discontinuation, need to be considered.
Acknowledgements
We thank the participants of the study.
Medical Writing Assistance
We thank Dr. Mariana Andrade, M.D. (Andrade-Evrard SPRL), who provided editorial assistance.
Author Contributions
Axel De Greef: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft; Romane Thirion: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—original draft; Pierre-Dominique Ghislain: data curation, investigation, writing—review and editing; Marie Baeck: conceptualization, methodology, validation, formal analysis, investigation, resources, data curation, writing—review and editing, supervision.
Funding
No funding or sponsorship was received for this study, the editorial assistance or the publication of this article. The rapid service fee was funded by the authors.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Declarations
Conflict of interest
Pierre-Dominique Ghislain discloses his past participation as an investigator and as a scientific advisor for Lilly Eli. Pierre-Dominique Ghislain and Marie Baeck have previously participated as speakers in events sponsored by Lilly Eli. All of the authors declare that the present study was conducted in an independent manner.
Ethical Approval
This study and data collection were conducted with the approval of the hospital and faculty institutional review board (Commission d’Ethique Biomédicale Hospitalo-Facultaire) of Université catholique de Louvain (UCLouvain), Belgium. This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. The patients in this manuscript have given written informed consent to participate and for publication of their case details.
Footnotes
Axel De Greef and Romane Thirion have contributed equally to this work.
References
- 1.Zhou C, Li X, Wang C, Zhang J. Alopecia areata: an update on etiopathogenesis, diagnosis, and management. Clin Rev Allergy Immunol. 2021;61(3):403–423. doi: 10.1007/s12016-021-08883-0. [DOI] [PubMed] [Google Scholar]
- 2.Sterkens A, Lambert J, Bervoets A. Alopecia areata: a review on diagnosis, immunological etiopathogenesis and treatment options. Clin Exp Med. 2021;21(2):215–230. doi: 10.1007/s10238-020-00673-w. [DOI] [PubMed] [Google Scholar]
- 3.Toussi A, Barton VR, Le ST, Agbai ON, Kiuru M. Psychosocial and psychiatric comorbidities and health-related quality of life in alopecia areata: a systematic review. J Am Acad Dermatol. 2021;85(1):162–175. doi: 10.1016/j.jaad.2020.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burroway B, Griggs J, Tosti A. Alopecia totalis and universalis long-term outcomes: a review. J Eur Acad Dermatol Venereol. 2020;34(4):709–715. doi: 10.1111/jdv.15994. [DOI] [PubMed] [Google Scholar]
- 5.King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386(18):1687–1699. doi: 10.1056/NEJMoa2110343. [DOI] [PubMed] [Google Scholar]
- 6.Kwon O, Senna MM, Sinclair R, et al. Efficacy and safety of baricitinib in patients with severe alopecia areata over 52 weeks of continuous therapy in two phase III trials (BRAVE-AA1 and BRAVE-AA2) Am J Clin Dermatol. 2023;24(3):443–451. doi: 10.1007/s40257-023-00764-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan J, Cao J, Chen F, Jin Y, Huang C. Real-data on the use of baricitinib in adolescents with severe alopecia areata. J Eur Acad Dermatol Venereol. 2023 doi: 10.1111/jdv.19121. [DOI] [PubMed] [Google Scholar]
- 8.Moussa A, Eisman S, Sinclair RD, Bhoyrul B. Treatment of alopecia areata of the beard with baricitinib. J Am Acad Dermatol. 2023;88(4):948–950. doi: 10.1016/j.jaad.2022.11.028. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Liu T, Li S, et al. Efficacy and safety of baricitinib in patients with refractory alopecia areata. Dermatol Ther. 2022;35(12):e15845. doi: 10.1111/dth.15845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bieber T, Feist E, Irvine AD, et al. A review of safety outcomes from clinical trials of baricitinib in rheumatology, dermatology and COVID-19. Adv Ther. 2022;39(11):4910–4960. doi: 10.1007/s12325-022-02281-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.