Abstract
Background
The Infants and Toddlers Dermatology Quality of Life (InToDermQoL) is the dermatology-specific proxy health-related quality of life (HRQoL) instrument for children from birth to 4 years. The aim of the present study was to confirm the responsiveness and establish minimal clinically important difference (MCID) for the InToDermQoL.
Methods
Parents of children with skin diseases were asked to fill in the InToDermQoL at the initial visit (T1) and subsequent consultation (T2). We hypothesized that correlations between change scores of the InToDermQoL and change scores of global assessment of clinical severity by dermatologists and by patients’ parents should be above 0.3. The receiver operating characteristic (ROC) curves method was also used for confirmation of responsiveness and determination of MCIDs of the InToDermQoL. The area under the ROC curve (AUC) was used as an indicator of responsiveness.
Results
Results of 442 patients were included. Correlations between change scores of age-specific versions of the InToDermQoL and change scores of global assessment of clinical severity by dermatologists and by patients’ parents were above 0.3 (0.46-0.74). AUCs for age-specific versions of the InToDermQoL were acceptable (above 0.7) or excellent (above 0.8). Estimated MCIDs for the InToDermQoL were as follows: 3 points of total score change for 0–11 months, 5 for 1–2 years and 3 or 4 for 3–4 years version. Estimated MCIDs for the InToDermQoL version for 1–2-year-old children was higher than MCIDs for the 3–4-year-old version despite the higher number of items in the latter. Therefore a MCID of 5 was recommended for both these versions.
Conclusions
Responsiveness for all age-specific versions of the InToDermQoL questionnaire was confirmed. MCIDs for the InToDermQoL are proposed as follows: 3-point change of the total score for age version 0–11 months and 5-point for the age versions 1–2 years and 3–4 years.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-01022-x.
Keywords: Children, Dermatology, Infants and Toddlers Dermatology Quality of Life, Minimal clinically important difference, Quality of life, Responsiveness
Key Summary Points
| Why carry out this study? | |
| Responsiveness is an important validation characteristic of the health-related quality of life (HRQoL) instruments. | |
| Knowledge of what constitutes a minimal clinically important difference (MCID) allows clinicians and researchers to interpret the clinical meaning of a change in score of the HRQoL instrument. | |
| What did the study ask? | |
| The aim of the present study was to confirm the responsiveness and establish MCID for the Infants and Toddlers Dermatology Quality of Life (InToDermQoL) questionnaire. | |
| What was learned from the study? | |
| Responsiveness for all age-specific versions of the InToDermQoL questionnaire was confirmed. | |
| MCIDs for age-specific versions of the InToDermQoL were proposed. |
Introduction
The Infants and Toddlers Dermatology Quality of Life (InToDermQoL) questionnaire is the dermatology-specific proxy health-related quality of life (HRQoL) instrument for children from birth to 4 years [1]. Prior to the development of the InToDermQoL questionnaire, there were no dermatology-specific HRQoL instruments for this age group of patients. This resulted in attempts to use disease-specific questionnaires as dermatology-specific, to use dermatology-specific tools for children younger than the questionnaire minimal age limit, or to skip assessment of HRQoL in this age group [2, 3]. To avoid the problem of cross-cultural inequivalence, development and validation of the InToDermQoL were performed simultaneously in different national centers of the project [1, 4]. Results of the international field tests confirmed internal consistency, test–retest reliability, convergent and discriminant validity, and sensitivity to treatment of the InToDermQoL questionnaire [4, 5]. The first variant of score bands for the InToDermQoL questionnaire has been proposed [5]. The InToDermQoL was used to study QoL in children with seborrheic, allergic contact, and atopic dermatitis before and during the coronavirus disease 2019 (COVID-19) pandemic [6]. Furthermore, an epidermolysis-bullosa-specific module of the InToDermQoL was developed and underwent initial validation [7–9].
Responsiveness is one of the measurement properties that reflects the quality of outcome measurement. Responsiveness means that the instrument should detect change in the purported construct, but also that it should detect the right amount of change, that is, it should not under- or overestimate the real change in the construct that has occurred [10]. The European Academy of Dermatology and Venereology (EADV) Task Force on QoL and Patient-Oriented Outcomes consider responsiveness an important validation characteristic of the HRQoL instruments [11]. A clinically important difference represents a change that would be considered meaningful and worthwhile by the patient. The minimally clinically important difference (MCID) is a threshold value for such a change. The definition of a MCID would be particularly helpful in the evaluation of patient-reported outcomes [12]. The knowledge of what constitutes a MCID allows clinicians and researchers to interpret the clinical meaning of a change in score [13]. Some national and international guidelines contain detailed recommendations on treatment goals and changes of treatment approaches based on MCID [14].
The aim of the present study was to confirm the responsiveness and establish MCID for the InToDermQoL questionnaire.
Methods
National centers of the InToDermQoL project were invited to participate in the study. Parents or other adult relatives of children with skin diseases from birth to 4 years old were asked to fill in the InToDermQoL questionnaire at the initial visit (T1) and subsequent consultation after 4–6 weeks (T2). Diagnoses of skin diseases were confirmed by dermatologists in all cases.
The data for the study were collected from September 2022 until May 2023.
The InToDermQoL (Table 1) questionnaire consists of three versions: 10 items for children under 1 year of age, 12 items for children 1–2 years of age, and 15 items for children 3–4 years of age. Responses of the InToDermQoL questionnaire are on a 4-point scale, from 0 to 3. The total score is calculated by summing the score of each question. Maximum total score for children under 1 year of age is 30. Maximum total score for children 1–2 years of age is 36, and maximum total score for children 3–4 years of age is 45 [1].
Table 1.
The Infants and Toddlers Dermatology Quality of Life Questionnaire
| Infants and Toddlers Dermatology Quality of Life (InToDermQoL) | |||
|---|---|---|---|
| The aim of this questionnaire is to measure how much your child’s skin problem has affected them over the last week | |||
| Child’s name: | Child’s age: | Child’s gender: | Date: |
| Diagnosis: | Disease severity: | Filled in by: mother/father/another person | |
| 1. Your child’s itching or scratching because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 2. Your child’s bleeding (from injured skin and/or mucosa) because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 3. Your child’s pain because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 4. Your child’s sleep problems because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 5. Your child’s mood changes because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 6. Your child’s bathing problems because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 7. Your child’s problems with dressing/undressing (irritation of lesions, pain) because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 8. Your child’s feeding problems because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 9. Your child’s problems during physical activity (infant’s movements or walking, running, crawling, etc.) | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 10. Your child’s problems with treatment (e.g., home treatment, bandaging, skin care, etc.) | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| If your child is over 1 year of age | |||
| 11. Your child’s tiredness because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 12. Restrictions and limitations (social, nutritional, physical activity, and sports, pets, etc.) your child had because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| If your child is over 3 years of age | |||
| 13. Do other peoples’ questions about your child’s skin disease affect your child? | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 14. Your child’s feeling of being different from peers because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
| 15. Rejection by other children because of their skin disease | Very much | □ | |
| Quite a lot | □ | ||
| Only a little | □ | ||
| Not at all | □ | ||
Supported by the EADV grant no. 2015–11
The anchor-based approach was used to study the responsiveness of the InToDermQoL questionnaire. There is no gold standard available, and therefore, the anchors used were the levels of improvement based on the global assessment of clinical severity by dermatologists and by patients’ parents. Hypotheses about the expected direction and magnitude of correlations between change scores on the instrument of interest and change scores of instruments that measure similar constructs (strong relationships, above 0.5) or instruments that measure unrelated constructs (weaker relationships, below 0.3) were used [10]. We consider disease severity and HRQoL as related but not similar constructs. Therefore, we hypothesized that correlations between change scores of the InToDermQoL and change scores of global assessment of clinical severity by dermatologists and by patients’ parents should be above 0.3. We also hypothesized that correlations between change scores of global assessment of clinical severity by dermatologists and change scores of global assessment of clinical severity by patients’ parents should be higher than correlations between change scores of the InToDermQoL, and that change scores of global assessment of clinical severity by dermatologists and by patients’ parents should be above 0.5 because they measure similar constructs. Pearson’s correlation coefficient was used to measure correlation between scores.
The receiver operating characteristic (ROC) curves method was also used for confirmation of responsiveness and determination of MCIDs of the age-specific versions of the InToDermQoL questionnaire. The area under the ROC curve (AUC) was used as an indicator of responsiveness. The AUC of an ROC curve represents the probability that scores will correctly discriminate between improved and non-improved patients. An area of 0.7–0.8 is considered acceptable and an area of 0.8–0.9 excellent [12]. MCIDs of the age-specific versions of the InToDermQoL questionnaire were estimated using the ROC method by comparing children with and without improvement assessed by dermatologists and by patients’ parents. The optimal cut-offs on the ROC curves were determined by using the optimal Youden’s index [15]. The nearest integers above the cut-off values were determined as MCIDs. The software StatPlus, AnalystSoft Inc., Version v7 was used in the analysis.
The EADV Task Force on Quality of Life and Patient Oriented Outcomes recommends using the word ‘‘quimp’’ (quality of life impairment) in routine clinical work and research [16, 17], and this word was used in this study.
This study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments. Ethical approval was obtained from the Commission on Bioethical Expertise and Ethics in Scientific Studies and other local ethical research committees where required. Informed consent from patients’ parents or guardians to participate and for publication was obtained in all cases.
Results
Parents or grandmothers of 442 children with skin diseases from Spain, Greece, Croatia, Romania, Malta, and Ukraine filled in national language versions of the InToDermQoL questionnaire at T1 and T2. Data from 20 parents were incomplete and 422 results were used for further analysis. Information on diagnoses of children with skin diseases is presented in Table 2. The questionnaires were filled in by mothers (87.79%), fathers (10.91%), and grandmothers (1.30%) of children with skin diseases.
Table 2.
Diagnoses of children with skin diseases whose parents filled in the Infants and Toddlers Dermatology Quality of Life questionnaire
| Diagnosis | 0–11 months (n = 169) |
1–2 years (n = 145) |
3–4 years (n = 128) |
|---|---|---|---|
| Atopic dermatitis | 79 | 67 | 36 |
| Seborrheic dermatitis | 6 | – | – |
| Pityriasis alba | 4 | 5 | – |
| Milia | 1 | – | – |
| Intertrigo | – | 1 | – |
| Pyoderma | 1 | 2 | 6 |
| Nevi | 7 | 3 | 4 |
| Giant nevus | 2 | – | – |
| Diaper dermatitis | 6 | 2 | – |
| Perioral dermatitis | 1 | 6 | 3 |
| Contact dermatitis | 3 | 3 | 2 |
| Impetigo | 2 | 2 | 2 |
| Urticaria | 2 | 2 | 3 |
| Papular urticaria | 8 | 2 | 3 |
| Urticaria pigmentosa | 3 | 2 | 1 |
| Hemangiomas | 8 | 3 | 2 |
| Xeroderma | 3 | – | – |
| Pediculosis | – | 1 | 2 |
| Ichtyosis | – | – | 1 |
| Prurigo | 2 | 3 | 2 |
| Eczema | 4 | 6 | 11 |
| Fungal infection | – | 5 | 10 |
| Hand eczema | 5 | 3 | 2 |
| Warts | 2 | 2 | 5 |
| Folliculitis | 2 | – | – |
| Molluscum contagiosum | 3 | 4 | 5 |
| Psoriasis | 1 | 1 | 1 |
| Keratosis pilaris | – | – | 1 |
| Scabies | 3 | 5 | 3 |
| Vitiligo | 1 | 1 | – |
| Pyogenic granuloma | 1 | – | – |
| Incontinentia pigmenti | 1 | – | – |
| Infantile cephalic pustulosis | 1 | – | – |
| Eczema herpeticum | 1 | – | – |
| Viral exanthem | 1 | 1 | 1 |
| Hypertrichosis | 1 | – | – |
| Epidermolysis bullosa | 1 | 1 | 2 |
| Capillary malformation | 1 | – | – |
| Aplasia cutis | 1 | – | – |
| Café au lait macule | 1 | 1 | – |
| Herpes | – | 2 | 2 |
| Nail dystrophy | – | 1 | – |
| Pityriasis rubra pilaris | – | 1 | – |
| Pilomatricoma | – | 1 | – |
| Alopecia areata | – | 1 | 9 |
| Gianotti–Crosti syndrome | – | 2 | – |
| Staphylococcal scaled skin syndrome | – | 1 | – |
| Cutaneous mosaicism | – | 1 | – |
| Bullous pemphigoid | – | 1 | – |
| Granuloma anulare | – | – | 3 |
| Pityriasis rosea | – | – | 3 |
| Dermatitis herpetiformis | – | – | 1 |
| Pigmented purpuric dermatitis | – | – | 1 |
| Xantogranuloma | – | – | 1 |
Correlations between change scores of age-specific versions of the InToDermQoL and change scores of global assessment of clinical severity by dermatologists and by patients’ parents, as well as between change scores of the InToDermQoL and change scores of global assessment of clinical severity by dermatologists and by patients’ parents, are presented in Table 3. Our hypotheses that correlations between change scores of the InToDermQoL and change scores of global assessment of clinical severity by dermatologists and by patients’ parents should be above 0.3 and that correlations between change scores of global assessment of clinical severity by dermatologists and change scores of global assessment of clinical severity by patients’ parents should be higher than correlations between change scores of the InToDermQoL and change scores of global assessment of clinical severity by dermatologists and by patients’ parents and should be above 0.5 were confirmed.
Table 3.
Correlations between change scores of age-specific versions of the Infants and Toddlers Dermatology Quality of Life (InToDermQoL) questionnaire and change scores of global assessment of clinical severity by dermatologists and by patients’ parents, and between change scores of the InToDermQoL and change scores of global assessment of clinical severity by dermatologists and by patients’ parents
| Age-specific versions of the InToDermQoL questionnaire | Correlation between change scores of the InToDermQoL and global disease severity assessed by dermatologists | Correlation between change scores of the InToDermQoL and global disease severity assessed by patients’ parents | Correlation between change scores of the global disease severity assessed by dermatologists and global disease severity assessed by patients’ parents |
|---|---|---|---|
| InToDermQoL version for 0–11 months (n = 164) | r = 0.57 | r = 0.53 | r = 0.76 |
| InToDermQoL version for 1–2 years (n = 137) | r = 0.46 | r = 0.59 | r = 0.63 |
| InToDermQoL version for 3–4 years (n = 121) | r = 0.68 | r = 0.74 | r = 0.85 |
The ROCs and AUCs for age-specific versions of the InToDermQoL developed on the basis of the global assessment of clinical severity by dermatologists and by patients’ parents are presented in Figs. 1, 2, 3, 4, 5, 6. AUCs for all age-specific versions of the InToDermQoL were acceptable (above 0.7) or excellent (above 0.8).
Fig. 1.
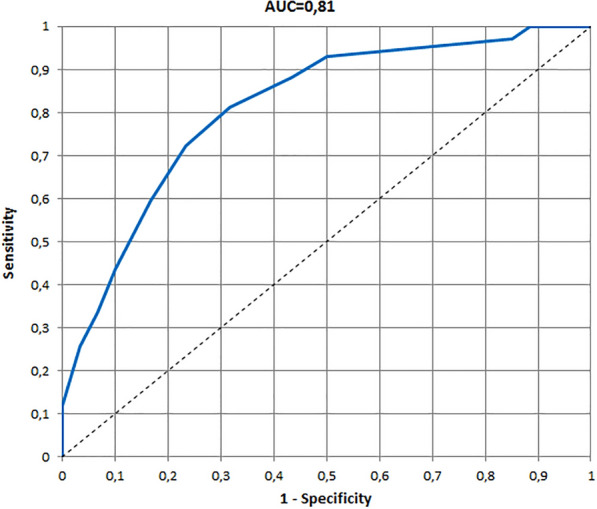
The receiver operating characteristic (ROC) and area under the ROC curve (AUC) for 0–11 months version of the Infants and Toddlers Dermatology Quality of Life questionnaire developed on the basis of the global assessment of clinical severity by dermatologists
Fig. 2.
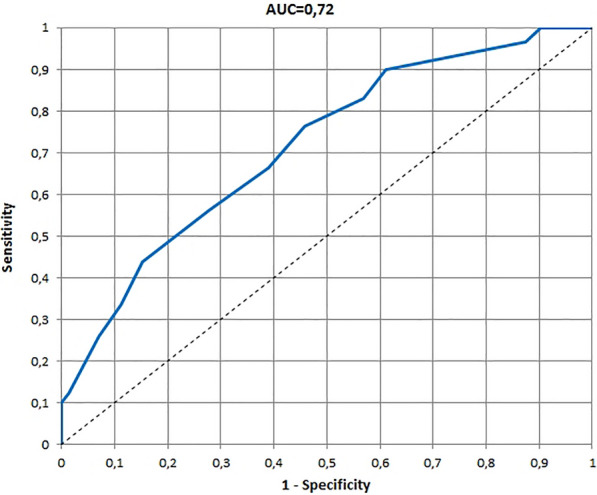
The receiver operating characteristic (ROC) and area under the ROC curve (AUC) for 0–11 months version of the Infants and Toddlers Dermatology Quality of Life questionnaire developed on the basis of the global assessment of clinical severity by patients’ parents
Fig. 3.
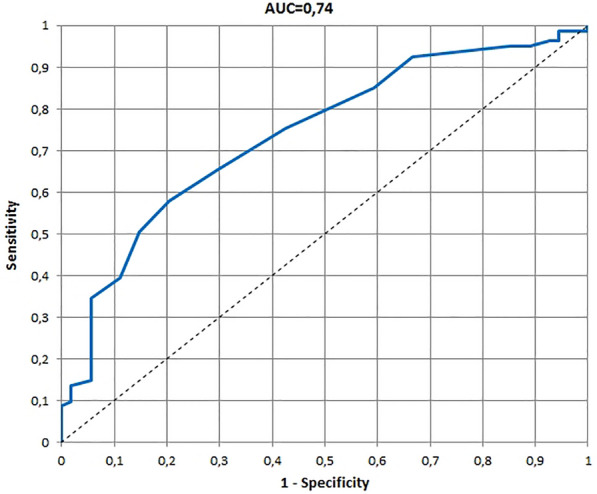
The receiver operating characteristic (ROC) and area under the ROC curve (AUC) for 1–2 years version of the Infants and Toddlers Dermatology Quality of Life questionnaire developed on the basis of the global assessment of clinical severity by dermatologists
Fig. 4.
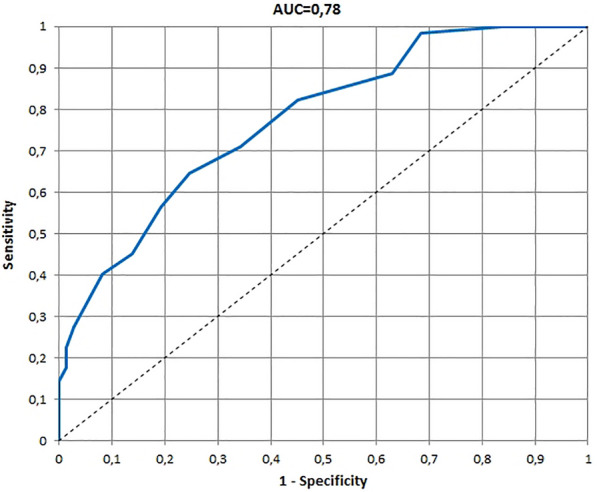
The receiver operating characteristic (ROC) and area under the ROC curve (AUC) for 1–2 years version of the Infants and Toddlers Dermatology Quality of Life questionnaire developed on the basis of the global assessment of clinical severity by patients’ parents
Fig. 5.
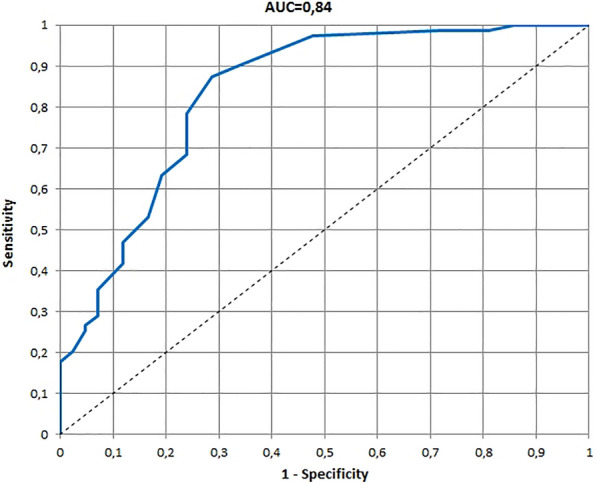
The receiver operating characteristic (ROC) and area under the ROC curve (AUC) for 3–4 years version of the Infants and Toddlers Dermatology Quality of Life questionnaire developed on the basis of the global assessment of clinical severity by dermatologists
Fig. 6.
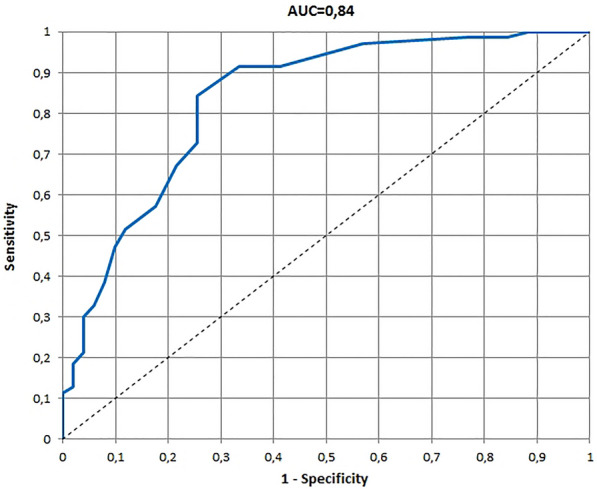
The receiver operating characteristic (ROC) and area under the ROC curve (AUC) for 3–4 years version of the Infants and Toddlers Dermatology Quality of Life questionnaire developed on the basis of the global assessment of clinical severity by patients’ parents
The 95% confidence intervals for AUCs, cut-off values, sensitivity, specificity, and MCIDs for age-specific versions of the InToDermQoL questionnaire are presented in Table 4. Estimated MCIDs for the InToDermQoL version for 3–4-year-old children appeared to be lower than the MCIDs for the InToDermQoL version for 1–2-year-old children despite a higher number of items in the version for 3–4-year-old children. Therefore, the highest MCID (five) was recommended for both these age-specific versions of the InToDermQoL questionnaire.
Table 4.
The 95% confidence intervals for areas under the receiver operating characteristic curves (AUCs), cut-off values, sensitivity, specificity, and minimal clinically important differences (MCIDs) for age-specific versions of the Infants and Toddlers Dermatology Quality of Life questionnaire
| AUC (95% CI) | Cut-off | Sensitivity (95% CI) | Specificity (95% CI) | MCID | |
|---|---|---|---|---|---|
| 0–11 months (n = 161) | |||||
| Dermatologists’ assessment | 0.81 (0.74; 0.88) | 2 | 0.81 (0.74; 0.89) | 0.68 (0.57; 0.80) | 3 |
| Parental assessment | 0.72 (0.64; 0.79) | 2 | 0.77 (0.68; 0.85) | 0.54 (0.43; 0.66) | 3 |
| 1–2 years (n = 135) | |||||
| Dermatologists’ assessment | 0.74 (0.66; 0.82) | 4 | 0.58 (0.47; 0.69) | 0.80 (0.69; 0.90) | 5 |
| Parental assessment | 0.76 (0.70; 0.85) | 4 | 0.65 (0.53; 0.76) | 0.75 (0.66; 0.85) | 5 |
| 3–4 years (n = 121) | |||||
| Dermatologists’ assessment | 0.84 (0.76; 0.92) | 2 | 0.87 (0.80; 0.95) | 0.71 (0.58; 0.85) | 3 |
| Parental assessment | 0.84 (0.76; 0.91) | 3 | 0.84 (0.76; 0.928) | 0.75 (0.63; 0.87) | 4 |
Discussion
The anchor-based approach used in our study confirmed the responsiveness of the InToDermQoL questionnaire. Our initial hypotheses were confirmed for all age-specific versions of the InToDermQoL questionnaire. AUCs based on either global assessment of clinical severity by dermatologists or on global assessment of clinical severity by patients’ parents were acceptable or excellent for all age-specific versions of the InToDermQoL. MCIDs based on cut-off values were proposed.
It was previously reported that the variety of possible anchors and uncertainty in the anchor cut point that defines a minimal difference makes a single estimate of MCID problematic. It is recommended that the estimation of MCID for an instrument should be based primarily on relevant patient-based and clinical anchors. Multiple approaches to estimating the MCID will produce a range of different values, and decision guidance may often be needed to select a single value or narrow range of MCID values [18]. The only problem we encountered was that MCIDs for the version for 1–2 year olds appeared to be higher than MCIDs for the version for 3–4 year olds. On the basis of the higher number of items in the version for 3–4 year olds, the same MCID as for the version for 1–2 years old children was selected and approved.
There are a number of problems in defining a MCID, specifically those developed from patient-reported data. Problems may be associated with patients’ ability to understand the context of improvement. Retrospective judgments are subject to recall bias as the patients may fail to truly remember the intrinsic nature of their prior condition. Baseline severity of symptoms can also influence the outcome of the MCID. The MCID can vary depending on the variability of the health of the population ahead of time. Other forms of patient variation that can influence report of change include descriptive factors such as age, socioeconomic status, or education [19].
The choice of a subjective assessment as an external criterion is not ideal but is due to the lack of satisfying objective assessment, a situation that spurred the use of PRO in the first place. Global assessment scales have been shown to be very sensitive to change, both positive and negative. Anchor-based methods will produce different MCIDs depending on the criterion scale and the arbitrary selection or grouping of scale levels. Conceptually, a minimal difference is a difference between two adjacent levels on a scale, such as ‘‘unchanged’’ and ‘‘slightly better.’’ MCID would then depend on the number of levels on a scale: the larger the number of levels, the smaller the difference between two adjacent levels and the smaller the MCID [12].
We decided not to analyze answers of fathers and mothers separately because in almost 90% of cases, the person who fills in the InToDermQoL questionnaire was the mother and no significant difference between mothers’ and fathers’ assessment of disease-specific proxy questionnaire was previously reported [20, 21]. We used real-life data from dermatologic clinics and included patients with a wide spectrum of diagnoses. For some skin diseases, symptoms have the highest effects on quimp [7, 22]. Meanwhile, psychosocial problems have the main impact on quimp in other skin diseases [11, 23]. This may lead to a minimal or absence of quimp in children with a number of skin diseases because feelings of stigmatization are unlikely before the age of 3 years, and during the age period of 3–10 years of age, the majority of children are very optimistic and the memory of experiences of bullying might not persist [24]. However, such facts may be better reflected in proxy reports by parents. Here we should mention that skin disease in children often cause quimp in parents and other family members. Therefore, the EADV Task Force on QoL and Patient-Oriented Outcomes recommends that the measurement of the impact of a skin disease on family and caregivers should also be included in a thorough evaluation of the burden of disease [25].
It seems in any case irrelevant for clinical practice to provide different MCIDs for sexes, and difference of HRQoL instrument scores among the sexes should be studied in children matched by other factors [26, 27]. Parental assessment of HRQoL of their children with skin diseases may not be identical among different countries because of cultural, social, and climatic factors [28, 29]. External factors, as in the case of the COVID-19 pandemic, may have multidirectional effects on patient’s HRQoL [6, 30].
HRQoL instruments may vary by validation characteristics, scoring systems, included topics, and recall periods [31]. Use of validated international instruments with established score meaning bands and MCID makes comparison and interpretation of HRQoL assessment easy (as in case of the dermatology-specific HRQoL instrument for adults, the Dermatology Life Quality Index [32], and dermatology-specific HRQoL instrument for older children, the Children’s Dermatology Life Quality Index [33]). Such instruments may be included in guidelines, core outcome sets, and used in clinical trials and practice [14, 34]. There are many reasons to assess HRQoL in dermatologic clinical practice [35], and we hope that the InToDermQoL will be used internationally in pediatric dermatology for research and practical needs.
Conclusions
Acceptable or excellent responsiveness was confirmed for all age-specific versions of the InToDermQoL questionnaire. MCIDs for all three age-specific versions of the InToDermQoL were proposed as follows: 3 points of the InToDermQoL total score for age version 0–11 months and 5 points of the InToDermQoL total score for age versions 1–2 years and 3–4 years.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the participants of the study.
Author Contributions
Pavel V Chernyshov—concept and design, data analysis and drafting the manuscript; Servando E Marron, Dimitra Koumaki, Nives Pustišek, Liana Manolache, Carmen Salavastru, Alina Suru, Adelina Sendrea, Tetiana Svyatenko, Olga Statkevych, Michael J Boffa, Sara Borg Grech—data collection and critical review of the text; Sergii Zemskov, Pavlo Lishchynskyi, Volodymyr V Kuts—statistical analysis and critical review of the text; Andriy V Chernyshov and Lucia Tomas-Aragones—data analysis and critical review of the text. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of Interest
The authors have nothing to disclose.
Ethical Approval
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Ethical approval was obtained from the Commission on Bioethical Expertise and Ethics in Scientific Studies and other local ethical research committees where required. Informed consent from patients’ parents or guardians to participate and for publication was obtained in all cases.
References
- 1.Chernyshov PV, Boffa MJ, Corso R, et al. Creation and pilot test results of the dermatology-specific proxy instrument: the Infants and Toddlers Dermatology Quality of Life. J Eur Acad Dermatol Venereol. 2018;32:2288–2294. doi: 10.1111/jdv.15229. [DOI] [PubMed] [Google Scholar]
- 2.Chernyshov PV. Dermatological quality of life instruments in children. G Ital Dermatol Venereol. 2013;148:277–285. [PubMed] [Google Scholar]
- 3.Chernyshov P, de Korte J, Tomas-Aragones L, Lewis-Jones S, EADV Quality of Life Task Force EADV Taskforce’s recommendations on measurement of health-related quality of life in paediatric dermatology. J Eur Acad Dermatol Venereol. 2015;29:2306–16. doi: 10.1111/jdv.13154. [DOI] [PubMed] [Google Scholar]
- 4.Chernyshov PV, Sampogna F, Pustišek N, et al. Validation of the dermatology-specific proxy instrument the Infants and Toddlers Dermatology Quality of Life. J Eur Acad Dermatol Venereol. 2019;33:1405–1411. doi: 10.1111/jdv.15496. [DOI] [PubMed] [Google Scholar]
- 5.Chernyshov PV, Marron SE, Boffa MJ, et al. Sensitivity to treatment and score bands of the Infants and Toddlers Dermatology Quality of Life questionnaire. JAAD Int. 2022;10:61–67. doi: 10.1016/j.jdin.2022.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chernyshov PV, Vozianova SV, Chubar OV. Quality of life of infants, toddlers and preschoolers with seborrhoeic, allergic contact and atopic dermatitis before and during COVID-19 pandemic. Dermatol Ther (Heidelb) 2021;11:2017–2026. doi: 10.1007/s13555-021-00617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chernyshov PV, Suru A, Gedeon I, Derevyanko LA, Tiplica GS, Salavastru CM. Epidermolysis bullosa-specific module of the Infants and Toddlers Dermatology Quality of Life (InToDermQoL) questionnaire. J Eur Acad Dermatol Venereol. 2019;33:612–617. doi: 10.1111/jdv.15337. [DOI] [PubMed] [Google Scholar]
- 8.Chernyshov PV, Pustišek N, Gedeon I, et al. International pilot tests of the epidermolysis bullosa-specific module of the Infants and Toddlers Dermatology Quality of Life questionnaire. J Eur Acad Dermatol Venereol. 2020;34:e123–e124. doi: 10.1111/jdv.16051. [DOI] [PubMed] [Google Scholar]
- 9.Chernyshov PV, Marron SE, Tomas-Aragones L, et al. Initial validation of the epidermolysis bullosa-specific module of the Infants and Toddlers Dermatology Quality of Life questionnaire. Dermatol Ther. 2020;33:e14128. doi: 10.1111/dth.14128. [DOI] [PubMed] [Google Scholar]
- 10.Mokkink L, Terwee C, de Vet H. Key concepts in clinical epidemiology: responsiveness, the longitudinal aspect of validity. J Clin Epidemiol. 2021;140:159–162. doi: 10.1016/j.jclinepi.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Chernyshov PV, Tomas-Aragones L, Manolache L, et al. Quality of life measurement in vitiligo. Position statement of the European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient Oriented Outcomes with external experts. J Eur Acad Dermatol Venereol. 2023;37:21–31. doi: 10.1111/jdv.18593. [DOI] [PubMed] [Google Scholar]
- 12.Copay AG, Subach BR, Glassman SD, Polly DW, Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7:541–546. doi: 10.1016/j.spinee.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Finlay AY. Broader concepts of quality of life measurement, encompassing validation. J Eur Acad Dermatol Venereol. 2017;31:1254–1259. doi: 10.1111/jdv.14254. [DOI] [PubMed] [Google Scholar]
- 14.Chernyshov PV. The evolution of quality of life assessment and use in dermatology. Dermatology. 2019;235:167–174. doi: 10.1159/000496923. [DOI] [PubMed] [Google Scholar]
- 15.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Finlay AY. Quimp: a word meaning “quality of life impairment”. Acta Derm Venereol. 2017;97:546–547. doi: 10.2340/00015555-2650. [DOI] [PubMed] [Google Scholar]
- 17.Chernyshov PV, Linder MD, Pustisek N, et al. Quimp (quality of life impairment): an addition to the quality of life lexicon. J Eur Acad Dermatol Venereol. 2018;32:e181–e182. doi: 10.1111/jdv.14693. [DOI] [PubMed] [Google Scholar]
- 18.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61:102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Cook CE. Clinimetrics corner: the minimal clinically important change score (MCID): a necessary pretense. J Man Manip Ther. 2008;16:E82–E83. doi: 10.1179/jmt.2008.16.4.82E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holm EA, Esmann S, Jemec GB. Parent gender and assessment of infant life quality. J Eur Acad Dermatol Venereol. 2006;20:274–276. doi: 10.1111/j.1468-3083.2006.01421.x. [DOI] [PubMed] [Google Scholar]
- 21.Chernyshov PV. May the gender of a parent influence assessment of health-related quality of life, family impact and severity of atopic dermatitis in children? Pediatr Dermatol. 2009;26:99–100. doi: 10.1111/j.1525-1470.2008.00834.x. [DOI] [PubMed] [Google Scholar]
- 22.Misery L, Belloni Fortina A, El Hachem M, et al. A position paper on the management of itch and pain in atopic dermatitis from the International Society of Atopic Dermatitis (ISAD)/oriented patient-education network in dermatology (OPENED) task force. J Eur Acad Dermatol Venereol. 2021;35:787–796. doi: 10.1111/jdv.16916. [DOI] [PubMed] [Google Scholar]
- 23.Chernyshov PV, Tomas-Aragones L, Finlay AY, et al. Quality of life measurement in alopecia areata. Position statement of the European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient Oriented Outcomes. J Eur Acad Dermatol Venereol. 2021;35:1614–21. doi: 10.1111/jdv.17370. [DOI] [PubMed] [Google Scholar]
- 24.Chernyshov PV. Stigmatization and self-perception in children with atopic dermatitis. Clin Cosmet Investig Dermatol. 2016;9:159–166. doi: 10.2147/CCID.S91263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampogna F, Finlay AY, Salek SS, et al. Measuring the impact of dermatological conditions on family and caregivers: a review of dermatology-specific instruments. J Eur Acad Dermatol Venereol. 2017;31:1429–1439. doi: 10.1111/jdv.14288. [DOI] [PubMed] [Google Scholar]
- 26.Chernyshov PV. Gender differences in health-related and family quality of life in young children with atopic dermatitis. Int J Dermatol. 2012;51:290–294. doi: 10.1111/j.1365-4632.2011.04997.x. [DOI] [PubMed] [Google Scholar]
- 27.Chernyshov PV, Ho RC, Monti F, et al. Gender differences in self-assessed health-related quality of life in children with atopic dermatitis. J Clin Aesthet Dermatol. 2016;9:19–24. [PMC free article] [PubMed] [Google Scholar]
- 28.Chernyshov PV, Jirakova A, Ho RC, et al. An international multicenter study on quality of life and family quality of life in children with atopic dermatitis. Indian J Dermatol Venereol Leprol. 2013;79:52–58. doi: 10.4103/0378-6323.104669. [DOI] [PubMed] [Google Scholar]
- 29.Chernyshov PV, Jiráková A, Hercogová J. Comparative study of the quality of life of children with atopic dermatitis from Ukraine and the Czech Republic. J Eur Acad Dermatol Venereol. 2011;25:1483–1484. doi: 10.1111/j.1468-3083.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 30.Chernyshov PV, Tomas-Aragones L, Augustin M, et al. Position statement of the European Academy of Dermatology and Venereology Task Force on Quality of Life and Patient Oriented Outcomes on quality of life issues in dermatologic patients during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2020;34:1666–1671. doi: 10.1111/jdv.16720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marron SE, Chernyshov PV, Tomas-Aragones L. Quality-of-life research in acne vulgaris: current status and future directions. Am J Clin Dermatol. 2019;20:527–538. doi: 10.1007/s40257-019-00438-6. [DOI] [PubMed] [Google Scholar]
- 32.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)–a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 33.Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol. 1995;132:942–949. doi: 10.1111/j.1365-2133.1995.tb16953.x. [DOI] [PubMed] [Google Scholar]
- 34.Chernyshov PV, Evers AWM, Bewley A, et al. Quality of life assessment in core outcome sets: a position statement of the EADV task force on quality of life and patient oriented outcomes. J Eur Acad Dermatol Venereol. 2022;36:20–23. doi: 10.1111/jdv.17725. [DOI] [PubMed] [Google Scholar]
- 35.Finlay AY, Salek MS, Abeni D, et al. Why quality of life measurement is important in dermatology clinical practice: An expert-based opinion statement by the EADV Task Force on Quality of Life. J Eur Acad Dermatol Venereol. 2017;31:424–431. doi: 10.1111/jdv.13985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


