Abstract
Several comprehensive and updated guidelines are available on the management of psoriasis with systemic treatments. However, there is a lack of updates in recommendations and guidelines on topical treatments, particularly regarding the latest evidence and developments in treatment formulations. Consequently, a comprehensive literature review on this topic, considering the continuous evolution of knowledge and evaluation of the relevance of the available literature evidence, represents a current need to improve the topical management of psoriasis. This study critically appraises the available literature on all topical treatments of psoriasis from the past 20 years to address some relevant issues, such as the vehicle associated with the highest effectiveness, the best vehicle for improving patient adherence, and the best strategy in terms of efficacy and safety for long-term treatment. The greater effectiveness of the foam formulation was demonstrated for calcipotriene/betamethasone dipropionate (Cal/BD) administration compared with the gel and ointment. Without a direct comparison, matching-adjusted indirect comparison analyses support the superiority of the foam versus the cream overall. In addition, the reduced treatment period required by the Cal/BD foam (4 weeks) may favor this formulation over cream (8 weeks). The literature evidence, supported by a broad clinical experience, reported high rates of acceptability and adherence for the foam vehicle. A growing consensus is shared among dermatologists sustaining the proactive approach as the best option for the long-term topical treatment of psoriasis in adults. The Cal/BD foam is the only treatment for which the approved label allows biweekly maintenance use (proactive management), thus representing the first option for long-term topical treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-01024-9.
Keywords: Psoriasis, Topical treatment, Betamethasone dipropionate, Calcipotriene, Vehicle, Adherence, Safety
Key Summary Points
| This study critically appraises the available literature on topical treatments of psoriasis to specifically address some relevant issues, namely the vehicle associated with the highest effectiveness, the best vehicle for improving adherence, and the best strategy in terms of efficacy and safety for long-term treatment. |
| The greater effectiveness of the foam formulation was demonstrated for calcipotriene/betamethasone dipropionate administration compared with the gel and ointment. |
| Literature evidence and clinical experience reported high rates of acceptability and adherence for the foam vehicle. |
| A growing consensus is shared among dermatologists sustaining the proactive approach as the best option for the long-term topical treatment of psoriasis in adults. |
Introduction
Psoriasis is a chronic inflammatory skin disease affecting up to 4% of the European population, characterized by well-demarcated erythematous plaques covered by silver scales, which can be painful and/or itchy [1, 2]. About 80% of patients with psoriasis suffer from localized, mild-to-moderate forms, characterized by plaques usually involving elbows, scalp, trunk, and/or knees, which can be effectively treated with topical treatments [3–6]. Among various topical options, guidelines and recommendations suggest the fixed-dose combination of corticosteroids (betamethasone dipropionate [BD]) and vitamin D analogs (calcipotriene [Cal]) as a first-line therapy in mild-to-moderate psoriasis because of its high effectiveness in reducing the number of daily applications [7–9].
However, while several guidelines are available on managing psoriasis with systemic treatments, there is a lack of updates in recommendations and guidelines on topical treatments, particularly regarding the most recent evidence and developments in treatment formulations [4]. Indeed, specific formulations of the same medication can play a major role in improving adherence, which has been highlighted to be very poor in psoriasis because of the inconvenience of use [10–15]. Consequently, a comprehensive and updated literature review on this topic, considering the continuous evolution of knowledge and the evaluation of the relevance of the available literature evidence, represents a current need for the improvement of the topical management of psoriasis.
Based on these needs, a group of dermatologists with proven experience in the management of psoriasis carried out a critical appraisal of the available literature regarding the topical treatment of psoriasis in adult patients, according to specific clinical questions, and assessed its relevance in the current clinical context. Within the project, studies were evaluated and selected according to an adapted form of the Downs and Black checklist created to assess the methodological quality of both randomized and non-randomized studies of healthcare interventions [16–18]. This study presents and critically discusses the results of this analysis.
Methods
Project Overview
A scientific board composed of five Italian experts in the management of psoriasis participated in this project. Four steps were applied by the participants to critically appraise the evidence regarding the topical management of psoriasis in adult patients: (1) definition of relevant research questions; (2) PubMed systematic literature search according to a pertinent keyword combination; (3) critical appraisal of literature according to an adapted form of the Downs and Black checklist (see the paragraph below for description) [16–18]; (4) a narrative review of each included study, according to the defined research questions.
Studies specifically addressing the management of difficult-to-treat areas were not considered.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Research Questions
The scientific board reviewed the literature regarding the topical treatment of psoriasis in adult patients to define the following research questions:
Which vehicle is associated with the highest effectiveness of the topical treatment of psoriasis in adults?
Which is the best vehicle for improving patient adherence to topical treatment of psoriasis in adults?
Which is the best strategy in terms of efficacy and safety for the long-term topical treatment of psoriasis in adults? (combination, sequential, reactive, pro-active, etc.). The long-term treatment has been defined as > 12 weeks [19].
The definition of the best topical treatment in terms of efficacy and safety was proposed as an additional clinical question and addressed through literature research. However, this question was not considered and not discussed in this literature critical appraisal because of the lack of well-grounded comparative studies between different actives.
Literature Search
Search Criteria
Studies that evaluated the topical treatment of psoriasis were searched on PubMed until 31 March 2022 according to the combination of pertinent keywords (psoriasis AND topical treatment; psoriasis AND therapy).
Type of Publications
Only human studies performed in adult patients in the last 20 years published in English were included. Original articles, clinical trials, randomized clinical trials (RCTs), and observational studies were considered. Other types of publications, including letters, editorials, reviews and systematic reviews, theses, and abstracts, were not evaluated. Studies evaluating non-pharmacological treatments, laser therapies, and pharmacological treatments not available on the market or not approved in Europe, or used off-label, were not included.
Study Eligibility Criteria
The eligibility criteria for included studies were designed according to the Population Intervention Comparators Outcomes Study (PICO) framework. The population included adult patients with psoriasis; the intervention of interest was the topical treatment. Any control treatment used in selected studies was included. Clinical success (defined as the complete resolution of signs and symptoms or their improvement at the end of treatment), safety and adherence were considered outcomes according to the clinical questions. Titles and abstracts from the literature research were screened independently by the authors. Eventual disagreement about paper inclusion was resolved by discussion. Full-text manuscripts were further reviewed for inclusion.
Critical Appraisal of Individual Studies
The Downs and Black checklist consisted of 27 items divided into five sub-scales (Supplementary Material) [16]: (1) reporting (10 items)—which assessed whether the information provided in the paper was sufficient to enable a reader to evaluate the findings of the study unbiasedly; (2) external validity (three items)—which addressed the extent to which the study findings could be generalized to the population from which the study subjects come; (3) bias (seven items)—which addressed biases in the measurement of the intervention and outcome; (4) confounding (six items)—which addressed bias in the selection of study subjects; (5) power (one item)—which attempted to assess whether the negative findings from a study could be due to chance. We used a modified version of the checklist, as reported in previous systematic reviews, to accommodate the characteristics of observational studies [17, 18]. According to the modified version, answers were scored 0 or 1, except for one item in the reporting subscale, which scored 0–2. The maximum score for quality was 28. Papers were classified as follows: excellent (26−28); good (20−25); fair (15−19); poor (≤ 14). Poor-quality studies were excluded from the review.
Results
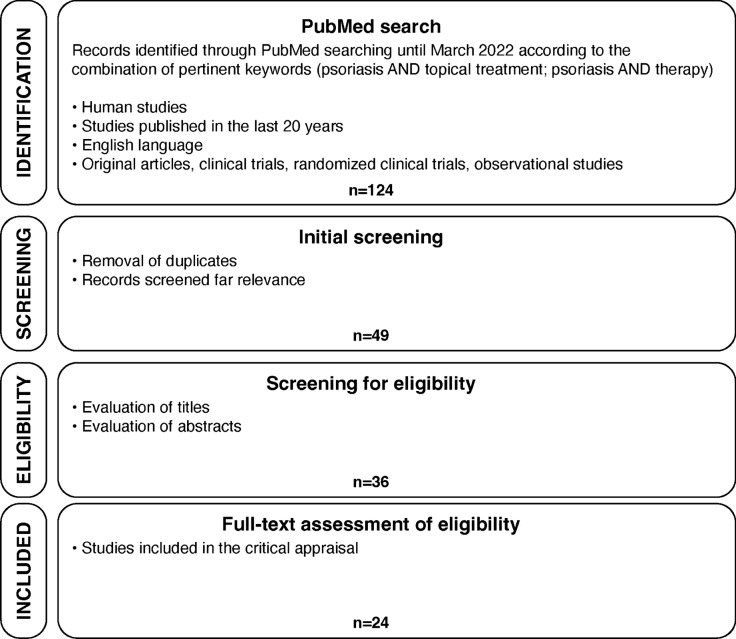
Overall, 124 potentially relevant articles evaluating the topical treatment of psoriasis were identified through the PubMed search. After screening for duplicates and assessing the full text for eligibility, 24 studies were considered since they answered the predefined clinical questions (Fig. 1). A list of included studies is provided in Table 1. Eleven out of 24 studies were RCTs.
Fig. 1.
Study selection flow diagram
Table 1.
Characteristics of included studies and rating according to the Downs and Black checklist
| Studies | Scoring | Classification | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Title | Author, year | Study design | Reporting | Ext. validity | Int. validity—bias | Int. validity—selection bias | Power | Total Score | Rating |
| A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed-dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis | Pinter (2022) [29] | Pooled analysis of two phase III clinical trials | 7 | 1 | 5 | 4 | 1 | 18 | Fair |
| Patient satisfaction with calcipotriol/betamethasone dipropionate cutaneous foam for the treatment of plaque psoriasis: The LION real-life multicenter prospective observational cohort study | Campanati (2021) [23] | Observational study | 10 | 3 | 6 | 2 | 1 | 22 | Good |
| Quality of life and patient-perceived symptoms in patients with psoriasis undergoing proactive or reactive management with the fixed-dose combination Cal/BD foam: A post-hoc analysis of PSO-LONG | Jalili (2022) [22] | Post-hoc analysis | 8 | 3 | 7 | 4 | 1 | 23 | Good |
| Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial) | Lebwohl (2021) [43] | RCT | 10 | 3 | 7 | 5 | 1 | 26 | Excellent |
| Calcipotriol/Betamethasone Dipropionate Aerosol Foam for Plaque Psoriasis: A Prospective, Observational, Non-Interventional, Single-Center Study of Patient Adherence and Satisfaction in Daily Use | Navarro-Triviño (2021) [38] | Observational study | 9 | 1 | 4 | 4 | 0 | 18 | Fair |
| A Phase 3, Randomized Trial Demonstrating the Improved Efficacy and Patient Acceptability of Fixed Dose Calcipotriene and Betamethasone Dipropionate Cream | Stein Gold (2021) [28] | RCT | 9 | 3 | 4 | 4 | 1 | 21 | Good |
| Patient and Physician Satisfaction with Calcipotriol and Betamethasone Dipropionate Aerosol Foam in the Treatment of Plaque Psoriasis on the Body | Velasco (2019) [42] | Observational study | 6 | 1 | 2 | 2 | 0 | 11 | Poor |
| Prospective, Observational, Non-Interventional, Multicentre Study on the Efficacy and Tolerability of a New Calcipotriol/Betamethasone Aerosol Foam (Enstilar®) in Patients with Plaque Psoriasis under Daily Practice Conditions | Gerdes (2017) [30] | Observational study | 7 | 3 | 5 | 2 | 0 | 17 | Fair |
| Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study | Hong (2017) [25] | RCT | 9 | 3 | 5 | 5 | 1 | 23 | Good |
| Calcipotriol Plus Betamethasone Dipropionate Aerosol Foam in Patients with Moderate-to-Severe Psoriasis: Sub-Group Analysis of the PSO-ABLE Study | Paul (2017) [26] | Subgroup analysis | 9 | 3 | 6 | 4 | 0 | 22 | Good |
| Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study | Paul (2017) [24] | RCT | 10 | 3 | 6 | 4 | 1 | 24 | Good |
| Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris–A randomized phase II study | Koo (2016) [27] | RCT | 10 | 3 | 6 | 5 | 0 | 24 | Good |
| Real-life effectiveness of once-daily calcipotriol and betamethasone dipropionate gel vs ointment formulations in psoriasis vulgaris: final analysis of the 52-week PRO-long study | Lambert (2015) [39] | Observational study | 6 | 1 | 4 | 0 | 0 | 11 | Poor |
| Practicability of combined treatment with calcipotriol/betamethasone gel (Daivobet® Gel) and improvement of quality of life in patients with psoriasis | Sticherling (2013) [31] | Observational study | 10 | 2 | 4 | 3 | 0 | 19 | Fair |
| Bioavailability, antipsoriatic efficacy and tolerability of a new light cream with mometasone furoate 0.1% | Korting (2012) [40] | RCT | 5 | 0 | 4 | 4 | 0 | 13 | Poor |
| The DESIRE study–psoriasis patients' satisfaction with topical treatment using a fixed combination of calcipotriol and betamethasone dipropionate in daily clinical practice | Claréus (2009) [32] | Observational study | 6 | 2 | 4 | 3 | 0 | 15 | Fair |
| Calcitriol ointment 3 microg/g is safe and effective over 52 weeks for the treatment of mild to moderate plaque psoriasis | Lebwohl (2009) [20] | Clinical Trial | 6 | 3 | 4 | 3 | 0 | 16 | Fair |
| A randomized, multicenter study of calcipotriene ointment and clobetasol propionate foam in the sequential treatment of localized plaque-type psoriasis: short- and long-term outcomes | Koo (2006) [33] | RCT | 6 | 0 | 6 | 4 | 0 | 16 | Fair |
| A 52-week randomized safety study of a calcipotriol/betamethasone dipropionate two-compound product (Dovobet/Daivobet/Taclonex) in the treatment of psoriasis vulgaris | Kragballe (2006) [21] | RCT | 10 | 3 | 6 | 6 | 1 | 26 | Excellent |
| Clobetasol propionate lotion, an efficient and safe alternative to clobetasol propionate emollient cream in subjects with moderate to severe plaque-type psoriasis | Lowe (2005) [34] | RCT | 7 | 0 | 4 | 4 | 1 | 16 | Fair |
| Clobetasol propionate lotion in the treatment of moderate to severe plaque-type psoriasis | Decroix (2004) [35] | RCT | 7 | 0 | 6 | 3 | 0 | 16 | Fair |
| Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis | Bergstrom (2003) [36] | RCT | 8 | 0 | 5 | 2 | 0 | 15 | Fair |
|
Calcipotriol versus coal tar: a prospective randomized study in stable plaque psoriasis |
Sharma (2003) [37] | RCT | 9 | 0 | 5 | 1 | 0 | 15 | Fair |
| Tacalcitol ointment for long-term control of chronic plaque psoriasis in dermatological practice | Lambert (2002) [41] | Clinical Trial | 6 | 1 | 3 | 1 | 0 | 11 | Poor |
RCT Randomized clinical trial, Cal/BD Calcipotriene/betamethasone dipropionate
Papers were listed in chronological order, starting with the most recent. Studies highlighted in gray were excluded from the analysis as their rating was classified as “poor”
Summary of Critical Appraisal
The rating of papers is reported in Table 1. Two studies were rated as “excellent” [20, 21], seven studies were rated as “good” [22–28], and 11 studies were rated as “fair” [20, 29–38]. Four studies were excluded as their rating was classified as “poor” [39–42]. Consequently, a total of 20 papers were considered to address the clinical questions (Table 1).
Research Question 1: Vehicle Associated with the Highest Effectiveness of the Opical Treatment of Psoriasis in Adults
Eight papers compared different vehicles for administering Cal/BD or clobetasol treatments, evaluating which vehicle had the highest effectiveness [24, 26–29, 34–36]. The main characteristics of the papers are summarized in Table 2. Half of the studies compared the foam vehicle with other vehicles [24, 26, 27, 36].
Table 2.
Main characteristics of considered studies evaluating the vehicle associated with the highest effectiveness of the topical treatment of psoriasis
| Author, year | Study design | Study population | Treatment and vehicle | Main conclusions on the vehicle associated with the highest effectiveness |
|---|---|---|---|---|
| Pinter (2022) [29] | Pooled analysis of two different phase III clinical trials (NCT03308799; NCT03802344) | 1286 patients | Cal/BD cream vs Cal/BD topical suspension vs cream vehicle |
PGA treatment success for the cream was greater than topical suspension (p < 0.0001) Mean percentage reduction in mPASI for the cream was greater than topical suspension (p < 0.0001) |
| Stein Gold (2021) [28] | Phase III, multicenter, randomized, active, and vehicle-controlled trial | 796 patients | Cal/BD cream vs Cal/BD topical suspension |
The proportion of patients achieving PGA treatment success after 8 weeks was greater for Cal/BD cream A similar statistically significant difference in favor of Cal/BD cream at week 8 was demonstrated for the percentage change in mPASI from baseline and the proportion of patients obtaining mPASI75 |
| Paul (2017) [24] | Phase III, prospective, multicenter, investigator-blinded study (PSO-ABLE) | 463 patients | Cal/BD foam vs Cal/BD gel | Cal/BD foam achieved higher treatment success rates and mPASI75 by week 4 than Cal/BD gel by week 8 |
| Paul (2017) [26] | Subgroup analysis of the phase III, prospective, multicenter, investigator-blinded PSO-ABLE study | 159 patients | Cal/BD foam vs Cal/BD gel |
Cal/BD foam showed to be more effective compared with Cal/BD gel A greater percentage of patients achieved DLQI of 0/1 at weeks 4, 8, and 12 |
| Koo (2016) [27] | Phase II, multicenter, investigator-blind, 4-week trial | 376 patients | Cal/BD foam vs Cal/BD ointment |
Treatment success rates and mean percentage decrease in mPASI at week 4 was higher for the Cal/BD foam group Percentage of patients achieving PASI75 or PASI50 was not statistically significant |
| Lowe (2005) [34] | Multicenter, randomized, active- and vehicle-controlled, parallel-group study | 192 patients | Clobetasol propionate lotion vs clobetasol propionate cream | Lotion and cream showed comparable efficacy profiles, which were superior to the vehicle, including a similar success rate at the end of the treatment period |
| Decroix (2004) [35] | Multicenter, randomized, active- and vehicle-controlled, parallel-group study | 222 patients | Clobetasol propionate lotion vs clobetasol propionate cream | Lotion and cream showed comparable efficacy profiles, which were superior to the vehicle, including a similar success rate at the end of the treatment period |
| Bergstrom (2003) [36] | Randomized, single-blind clinical trial | 32 patients | Clobetasol propionate foam vs a combined program of clobetasol cream and solution |
PASI score decrease was higher for the foam In the treatment of scalp psoriasis, the foam had greater improvement than the combined treatment |
Cal/BD: calcipotriene/betamethasone dipropionate; PGA: Physician Global Assessment; mPASI: modified Psoriasis Area Severity Index; DLQI: Dermatology Life Quality Index; PASI: Psoriasis Area Severity Index; PASI75: (improvement of) Psoriasis Area Severity Index of 75% and PASI50: (improvement of) Psoriasis Area Severity Index of 50%
The Cal/BD foam was compared with the Cal/BD gel in the phase III, randomized, multicenter, investigator-blinded PSO-ABLE study, which involved patients with mild-to-severe psoriasis [24]. The Cal/BD foam group achieved higher success rates (38% vs 22%, according to the physician’s global assessment of disease severity (PGA); p < 0.001) and mPASI75 (modified Psoriasis Area and Severity Index; 52% vs 35%; p < 0.001) after 4 weeks of treatment than the Cal/BD gel after 8 weeks of treatment [24]. In a subgroup analysis of the PSO-ABLE study, the once-daily treatment with the Cal/BD foam or gel or respective vehicles for up to 12 weeks was compared in 159 patients with moderate-to-severe psoriasis [26]. At week 12, reductions in mPASI and body surface area (BSA) were significantly higher with the foam compared with gel; the mPASI75 and mPASI90 achievement was 6% in the Cal/BD foam group and 12% in the Cal/BD gel group [26].
A phase II, multicenter, investigator-blind, 4-week trial compared the Cal/BD foam treatment versus ointment in 376 adult patients with mild-to-moderate psoriasis [27]. Treatment success at week 4 was significantly higher in the Cal/BD foam group (p = 0.025); the mean percentage decrease in mPASI was 74% and 63% at week 4 in the Cal/BD foam and in the Cal/BD ointment group, respectively [27].
The Cal/BD cream and the Cal/BD topical suspension were compared in terms of efficacy and acceptability in a phase III, vehicle-controlled trial enrolling 796 patients with moderate-to-severe psoriasis [28]. The proportion of patients achieving PGA treatment success after 8 weeks and mPASI75 was greater for the Cal/BD cream than for the Cal/BD topical suspension (p < 0.0001) [28]. In a subsequent study, data from this trial were pooled with data from another phase III, vehicle-controlled trial (not published as a single study) to compare the efficacy, safety, and quality of life (QoL) between the Cal/BD cream and topical suspension [29]. The pooled analysis confirmed the previous results [29].
Clobetasol propionate lotion and clobetasol propionate cream were compared in two multicenter, randomized, vehicle-controlled, parallel-group studies involving 192 and 222 subjects with moderate-to-severe psoriasis, respectively [34, 35]. In both studies, clobetasol lotion and cream showed comparable efficacy profiles, which were superior to the vehicle, including a similar success rate at the end of the 4-week treatment period [34, 35]. At the same time, the lotion showed a better remission profile after 4 weeks of treatment-free follow-up period [34]. A significantly higher cosmetic advantage was also attributed to the lotion [35].
In a randomized, single-blind study, the clobetasol propionate foam formulation was compared with a combined program consisting of applying clobetasol cream and solution in 32 subjects with mild-to-moderate psoriasis [36]. At the end of the 14-day treatment, the foam performed better than the combined program in several measures; the PASI score decrease was 41% (foam) versus 35% (cream/solution; p = 0.17) [36]. In treating scalp psoriasis, foam determined greater improvement than the solution in both absolute and percentage terms (p = 0.03 for both) [36]. When measuring global QOL, foam users had a significantly greater increase in EQ-5D than those using the combined program [36].
Research Question 2: Best Vehicle for Improving Patient Adherence to Topical Treatment of Psoriasis in Adults
Seven papers assessed the acceptability and/or adherence to the topical treatment according to different vehicles [23, 25, 28, 30–32, 38]. The main characteristics of the papers are summarized in Table 3. Four studies evaluated the Cal/BD foam vehicle [23, 25, 30, 38].
Table 3.
Main characteristics of considered studies evaluating the acceptability and/or adherence to the topical treatment according to different vehicles
| Author, year | Study design | Study population | Treatment and vehicle | Main conclusions on the acceptability and/or adherence |
|---|---|---|---|---|
| Campanati (2021) [23] | Real-life multicenter prospective observational cohort study | 256 patients | Cal/BD foam | More than 90% of patients evaluated the Cal/BD foam as more effective, easier to use, and better tolerated than previous topical treatments |
| Stein Gold (2021) [28] | Phase III, multicenter, randomized, active, and vehicle-controlled trial | 796 patients | Cal/BD cream vs Cal/BD topical suspension | Patient-reported treatment convenience for Cal/BD cream was rated superior to Cal/BD topical suspension |
| Navarro-Triviño (2021) [38] | Prospective, observational, single-center study | 65 patients | Cal/BD foam |
74% of patients showed high adherence at 12 weeks 71% of patients were completely satisfied |
| Gerdes (2018) [30] | Prospective, observational, non-interventional, multicenter study | 410 patients | Cal/BD foam | 82% of patients adhered to the Cal/BD foam therapy until the end of the 4-week observational period and were willing to continue the therapy thereafter |
| Hong (2017) [25] | Phase IIIb, prospective, multicenter (Canada/Germany), open-label, randomized, two-arm crossover study | 213 patients | Cal/BD foam and gel | Based on the Topical Product Usability Questionnaire (TPUQ), mean scores were high for both Cal/BD foam and gel overall. Cal/BD foam was generally preferred by younger patients (aged 18–39 years) |
| Sticherling (2013) [31] | Non-interventional, prospective trial | 579 patients | Cal/BD gel |
75% of patients were “very satisfied” with the Cal/BD gel vs 29% with the prior treatment Adherence was 95% 1 week after initiation of therapy and 81% after 4 weeks of treatment |
| Claréus (2017) [32] | Multicenter, single-group, international, real-world study | 1224 patients | Cal/BD gel | 80% of patients were highly satisfied with the treatment |
Cal/BD: calcipotriene/betamethasone dipropionate
The 4-week, observational LION study assessed patients’ satisfaction with the Cal/BD foam in a real-life setting involving 256 patients with mild (52%), moderate (43%), or severe (5%) psoriasis. The TSQM (Treatment Satisfaction Questionnaire for Medication)-9 median (25th–75th percentile) scores were 83.3 (66.7–88.9) for effectiveness, 77.8 (66.7–88.9) for convenience, and 78.6 (64.3–92.9) for global satisfaction (range 0–100) [23]. Specifically, patients were satisfied to extremely satisfied with the foam’s ability to manage (86%) and relief their symptoms (83%); > 80% of patients rated it from easy to extremely easy to use and plan when to use the Cal/BD foam [23]. Almost all (90%) patients previously treated for psoriasis evaluated the Cal/BD foam as more effective, easier to use, and better tolerated than previous topical treatments [23]. Full self-reported adherence to treatment was reported in 87% of patients; additional 10% of patients covered ≥ 80% of prescribed days [23].
Patient adherence and satisfaction with daily use of the Cal/BD foam were also evaluated in a prospective, observational, single-center study involving 65 patients with mild-to-moderate psoriasis [38]. Most patients (74%) showed high adherence at 12 weeks; 71% of patients were completely satisfied with the treatment, according to the TSQM-9 [38].
A high adherence rate (82%) to the Cal/BD foam treatment was reported in an observational, multicenter study involving 410 psoriasis patients under daily practice conditions [30]. After 4-week treatment, 58% of patients continued a once-daily regimen [30]. Overall, 71% of patients assessed the foam as very/quite cooling, 66% as very/quite skin satisfying, and 59% as very/quite itch relieving; the application was considered very/quite simple by 89% of patients [30].
In a phase III, multicenter, randomized, active, and vehicle-controlled trial enrolling 796 patients with moderate-to-severe psoriasis, the Cal/BD cream and Cal/BD topical suspension (once daily for 8 weeks) were compared in terms of efficacy and acceptability. At the end of therapy, treatment convenience with the Cal/BD cream was rated by patients as superior to the Cal/BD topical suspension, according to the Psoriasis Treatment Convenience Scale [28].
The phase IIIb, prospective, multicenter, open-label, randomized, PSO-INSIGHTFUL study evaluated the preference for the Cal/BD foam or Cal/BD gel in 213 patients with mild-to-moderate psoriasis [25]. Patients were randomly assigned to receive the Cal/BD foam once daily for 1 week, followed by the Cal/BD gel for 1 week, or vice versa [25]. The Topical Product Usability Questionnaire showed that both the Cal/BD foam and gel had high scores from the last topical treatment received [25].
A non-interventional, prospective trial was carried out to evaluate the practicability of the Cal/BD gel treatment in 579 patients with mild-to-moderate psoriasis [31]. Seventy-five percent of patients were “very satisfied” with the Cal/BD gel compared with 29% with the prior treatment (35% corticosteroids, 11% fixed combination corticosteroids and vitamin D3 analog in a different formulation, 10% salicylic acid, 9% vitamin D3 analog, 5% phototherapy, 6% other) [31]. Adherence was 81% after 4 weeks of treatment [31].
The multicenter, single-group, international DESIRE study assessed the satisfaction with the Cal/BD gel treatment for a minimum of 4 weeks in daily clinical practice in 1224 patients with different grades of psoriasis [32]. Twenty-three percent of patients had undergone repeated 4-week Cal/BD courses in the previous 6 months and 80% with high satisfaction [32]. Efficacy and ease of use were the main reasons for patients’ satisfaction assessment [32].
Research Question 3: Best Strategy in Terms of Efficacy and Safety for the Long-Term Topical Treatment of Psoriasis in Adults
Five papers compared different strategies for the long-term (> 12 weeks) topical treatment of psoriasis [20, 21, 33, 37, 43]. The main characteristics of the papers are summarized in Table 4. Among these studies, the PSO-LONG trial and the study by Kragballe and collaborators were rated excellent according to the Downs and Black checklist (Table 1) [21, 43].
Table 4.
Main characteristics of considered studies evaluating the best strategy in terms of efficacy and safety for the long-term (> 8 weeks) topical treatment of psoriasis
| Author, year | Study design | Study population | Treatment and vehicle | Main conclusions on the strategy in terms of efficacy and safety for the long-term treatment |
|---|---|---|---|---|
| Jalili (2022) [22] | Post-hoc analysis of PSO-LONG | 545 patients | Cal/BD foam | Proactive management was associated with a significantly higher DLQI and a numerically lower EQ-5D-5L-PSO mean area under the curve score than proactive management |
| Lebwohl (2021) [43] | Phase III, multicenter trial (PSO-LONG) | 545 patients | Cal/BD foam |
Proactive management reduced the median time to first relapse and the number of relapses and increased the days in remission compared with reactive management The Cal/BD foam was well tolerated |
| Lebwohl (2009) [20] | Open-label, multicenter study | 324 patients | Calcitriol ointment |
A marked improvement in psoriasis symptoms from baseline was reported BSA was stable or improved in 97.7% of patients at 52 weeks Adverse events deemed related to study treatment were noted for 45 participants (14%), none was severe |
| Koo (2006) [33] | Phase II, multicenter, investigator-blind randomized study | 86 patients | Clobetasol foam + calcipotriene ointment (6 months) followed by 6 months of weekday calcipotriene ointment ± clobetasol foam weekend pulse therapy |
Combination therapy in the first 24 weeks decreased psoriasis scores compared with either agent The weekend pulse clobetasol foam was associated with a trend toward greater maintenance of remission than the vehicle |
| Kragballe (2006) [21] | Randomized clinical trial | 422 patients | Cal/BD gel applied for 52 weeks vs 52 weeks of alternating 4 weeks of Cal/BD gel and calcipotriol (alternating group, n = 213) or 4 weeks of the Cal/BD gel + 48 weeks of calcipotriol | Adverse drug reactions of concern associated with long-term topical corticosteroids were comparable among the three groups, suggesting that Cal/BD gel is safe and well tolerated in the long term whether used on its own or alternating every 4 weeks with calcipotriol treatment |
| Sharma (2003) [37] | Prospective, randomized study | 30 patients | Coal tar ointment on one side of the lesion and calcipotriol ointment on the other side |
Calcipotriol ointment produced a faster initial response, although after a long period of treatment, i.e., 12 weeks, coal tar ointment had comparable efficacy There was no statistically significant difference in the relapse rates between the two modalities |
Cal/BD: calcipotriene/betamethasone dipropionate; DLQI: Dermatology Life Quality Index; EQ-5D-5L-PSO: EuroQol five-dimensional 5L questionnaire for psoriasis; BSA: Body Surface Area
The PSO-LONG trial evaluated the long-term efficacy and safety of proactive management (biweekly Cal/BD foam for 52 weeks) [43]. A total of 545 patients who achieved treatment success in the open-label phase (Cal/BD foam once daily for 4 weeks) were randomized to proactive (n = 272) and reactive (treatment as needed; n = 273) management. Proactive management reduced the median time to first relapse (56 days versus 30 days in proactive and reactive groups, respectively) and number of relapses (3.1 in the proactive group and 4.8 in the reactive group) and increased remission days compared with reactive management (the proactive group had an additional 41 days in remission compared with the reactive group over 1 year, p < 0.001) [43]. A high tolerability profile was reported [44]. In a post hoc analysis, compared with reactive management, a significantly better Dermatology Life Quality Index (DLQI; 15% [p = 0.007]), Psoriasis Symptom Inventory (PSI; 15% [p = 0.013]) scores, and a numerically better EQ-5D-5L-PSO (EuroQol-5D for psoriasis) mean area under the curve score were reported for the proactive management (1% [p = 0.084]) [22].
Long-term use of calcitriol ointment was evaluated in an open-label, multicenter study involving 324 participants with mild-to-moderate psoriasis, treated twice daily as needed for up to 52 weeks [20]. A marked improvement in psoriasis symptoms from baseline was reported (64% of patients at week 52). Forty-three percent of patients achieved clear or minimal psoriasis at any given time point during the study; BSA was stable or improved in 98% of patients at 52 weeks [20]. Adverse events were noted for 45 participants (14%), and none were severe [20].
In a randomized multicenter study, the long-term outcomes of the sequential treatment with Cal ointment and clobetasol propionate foam were evaluated in 86 patients [33]. The sequential therapy consisted of 24 weeks of treatment with twice-daily clobetasol foam plus Cal ointment, followed by 6 months of treatment with weekday Cal ointment with or without weekend pulse clobetasol foam therapy. The combination therapy in the first 24 weeks reduced psoriasis scores compared with either agent (vs clobetasol foam group, p = 0.0017 for trunk and p = 0.0001 for extremity lesions; vs Cal group, p = 0.0001 for trunk and p = 0.0001 for extremity lesions) [33]. During the 6-month treatment, the weekend pulse clobetasol foam was associated with a trend toward greater maintenance of remission than the vehicle (92% improvement of trunk lesion vs 62%) [33].
A randomized study evaluated the safety of the Cal/BD gel applied for 52 weeks as needed (two-compound group, n = 212) compared with 52 weeks of alternating 4-week periods of the Cal/BD gel and calcipotriol (alternating group, n = 213) or 4 weeks of the Cal/BD gel followed by 48 weeks of calcipotriol (calcipotriol group, n = 209) [21]. Treatments were applied once daily [21]. Adverse drug reactions occurred in 5% of patients in the two-compound group, in 3% of patients in the alternating group, and in 3% of patients in the calcipotriol group. This suggests that the Cal/BD gel is safe and well tolerated in the long term, whether used as monotherapy or alternated treatment [21].
A prospective randomized study compared the application of a coal tar ointment overnight on one side of the lesion once daily and calcipotriol ointment on the other side of the same lesion twice daily in 30 patients [37]. Both sides were exposed to the sun for 2 h every day; data after a 12-week treatment period and an 8-week follow-up period were provided. Better cosmetic acceptability and faster initial response were related to calcipotriol ointment, although coal tar ointment had comparable efficacy after a long period of treatment, i.e., 12 weeks [reduction in Erythema, Scaling, and Induration (ESI) score: calcipotriol 71.9 ± 13% vs coal tar 69.4 ± 15% (p > 0.05)] [37]. No statistically significant difference in the relapse rates was found between the two treatments [37].
Discussion
A group of experts carried out a critical appraisal of the available literature regarding the topical treatment of psoriasis in adult patients to address three specific relevant questions, namely: (1) Which vehicle is associated with the highest effectiveness of the topical treatment? (2) Which is the best vehicle for improving patient adherence to topical treatment? (3) Which is the best strategy in terms of efficacy and safety for the long-term (> 12 weeks) topical treatment? A total of 24 papers was evaluated with a modified version of the Downs and Black checklist [16–18], and a selection of 20 papers was used.
The question about the best topical treatment in terms of efficacy and safety was not addressed because of the lack of well-grounded comparative studies between different actives.
Vehicle Associated with the Highest Effectiveness of the Topical Treatment of Psoriasis in Adults
Vehicles used to deliver topical therapy considerably impact efficacy as they directly affect percutaneous absorption and, consequently, allow different rates of the active drug through the stratum corneum, the skin’s major barrier [44, 45].
The formulation in lotion and cream showed comparable effectiveness profiles for the administration of clobetasol [34, 35]. Otherwise, the foam vehicle of clobetasol was associated with higher effectiveness compared with the cream [36].
Regarding Cal/BD topical administration, three out of five retrieved papers suggested that the foam vehicle was more effective than the gel and ointment [24, 26, 27]. Two studies compared the efficacy and safety associated with the cream formulation of Cal/BD with topical suspension/gel, suggesting the superiority of the cream versus gel [28, 29].
In the absence of head-to-head RCTs, two MAIC analyses were recently undertaken to evaluate the efficacy of the Cal/BD cream versus the Cal/BD foam when used for the recommended treatment duration (8 weeks for cream and 4 weeks for foam) [46, 47]. The analysis by Bewley and collaborators reported comparable effectiveness (i.e., no statistically significant difference) at the recommended treatment durations for cream and foam [46]. However, a MAIC analysis of five different trials by Papp et al. consistently showed that Cal/BD foam treatment for 4 weeks induced a significantly higher PGA success than 8 weeks of the Cal/BD cream, with an even greater difference seen between the two formulations after 4 weeks of each treatment [47]. Therefore, based on the licensed treatment periods for daily use of the two formulations, the Cal/BD foam appears to act faster than the cream; this may be associated with rapid improvement in skin symptoms and QoL, potentially increasing treatment adherence and effectiveness [45]. The foam, which does not crystallize, allows the rapid evaporation of the propellant, leaving a supersaturated layer of Cal/BD on the skin; this supersaturated layer has been associated with greater penetration even through thick lesions [48, 49]. Those characteristics are related to the associated rapid response of the treatment.
Best Vehicle for Improving Patient Adherence to Topical Treatment of Psoriasis in Adults
Topical products approved for the treatment of psoriasis are available in five topical semisolid formulations: ointment, gel, suspension, foam, and cream [44].
Therapy adherence and clinical efficacy of topical treatments for psoriasis have been related each other and in turn have been related to the vehicle characteristics, such as rheological and textural properties, both dependent on the excipients and the matrix structure [15]. According to clinical experience and literature data, patients prefer a topical formulation that dries quickly, and the greasiness of the topical therapy has been identified as the main reason for low adherence to treatment [50]. Vehicle choice is also associated with application time, number of applications per day, and duration of therapy, all of which can impact treatment adherence.
Acceptability and adherence to the foam formulation of the Cal/BD combination were assessed in most of the retrieved papers through treatment satisfaction questionnaires reported by patients in clinical practice. As the foam is formulated as a surfactant and alcohol-free foam with a non-skin-drying emollient vehicle, it has the potential for greater patient acceptability [51]. Results confirmed high rates of acceptability and adherence for the foam, supporting it as the best vehicle [23, 25, 30, 38].
Best Strategy in Terms of Efficacy and Safety for the Long-Term Topical Treatment of Psoriasis in Adults
There is growing consensus among dermatologists for a proactive approach (biweekly application on previously affected areas to prevent flares) to long-term management of psoriasis with topical therapies to maintain remission, increase adherence, and improve long-term outcomes [9, 14, 52–54]. Therefore, the inclusion in the guidelines of a proactive strategy among effective treatment options will be a fundamental step in the evolution of the therapeutic approach for treating mild-to-moderate psoriasis [14].
PSO-LONG trial is the only identified long-term study that compared the usual intermittent reactive treatment with regular biweekly maintenance therapy [43]. Results showed that the proactive management of psoriasis with the Cal/BD foam reduced the median time to first relapse and the number of relapses and increased remission days compared with the reactive management [43]. Given the results of the PSO-LONG trial, the Cal/BD foam is the only treatment for plaque psoriasis for which the approved label allows either reactive treatment of relapse or biweekly maintenance use (proactive management) [19].
Conclusions
This study critically appraises the available literature regarding the topical treatment of psoriasis to address three specific relevant issues, such as the vehicle associated with the highest effectiveness, the best vehicle for improving patient adherence, and the best strategy in terms of efficacy and safety for long-term treatment. Greater effectiveness of the foam formulation has been demonstrated for Cal/BD administration compared with the gel and ointment. Without a direct comparison, MAIC analyses support the superiority of the foam versus the cream overall. In addition, the reduced treatment period required by the Cal/BD foam reactive treatment (4 weeks) may favor this formulation over the cream (8 weeks). The literature evidence, supported by a broad clinical experience, has reported high rates of acceptability and adherence for the foam vehicle, which may be related to its surfactant- and alcohol-free formulation and the low residue on the skin as well as its demonstrated rapid effectiveness. Regarding clobetasol administration, even in this case, the foam formulation has been associated with greater effectiveness compared with the cream and lotion.
A growing consensus is shared among dermatologists sustaining the proactive approach as the best option for the long-term topical treatment of psoriasis in adults. The Cal/BD foam is the only treatment for which the approved label allows biweekly maintenance use (proactive management), thus representing the first option for long-term topical treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
Medical Writing/Editorial Assistance
Editorial and graphical assistance was provided by Simonetta Papa, PhD, Francesca Cappellini, PhD, Massimiliano Pianta, Valentina Attanasio and Aashni Shah (Polistudium SRL, Milan, Italy). This assistance was supported by LEO Pharma.
Author Contributions
Definition and contextualization of the paper’s contents: MCF, CS, PG, GP, PC-P; critical revision and editing of the first version of the manuscript: MCF, CS, PG, GP, PC-P; submission approval: MC F, CS, PG, GP, PCa-P.
Funding
This project was made possible thanks to the unconditional contribution from LEO Pharma, which includes the funding of the journal’s Rapid Service.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of Interest
Maria Concetta Fargnoli has served on advisory boards, received honoraria for lectures and research grants from AMGEN, Almirall, Abbvie, Leo Pharma, Janssen, Lilly, Novartis, Pfizer and UCB. Clara De Simone has served as a speaker, consultant or advisory board member and has received honoraria from Abbvie, Almirall,Amgen, Eli Lilly, Leo Pharma, Janssen, Novartis, and UCB Pharma. Giovanni Pellacani has no conflicts of interest to declare. Paolo Gisondi has served Amgen, Almirall, Abbvie, Leo Pharma, Eli Lilly, Pfizer, Janssen, Novartis, UCB for advisory boards and/or lectures. Piergiacomo Calzavara-Pinton has served as a speaker, consultant or advisory board member and has received honoraria from Galderma, Pierre-Fabre, Incyte, Almirall, Abbvie, Leo Pharma, UCB, Lilly, Pfizer, Janssen, Cantabria, Molteni and Novartis.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Maria Concetta Fargnoli, and Clara De Simone have contributed equally.
References
- 1.Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133(2):377–385. doi: 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong AW, Read C. Pathophysiology, Clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 3.Svendsen MT, Jeyabalan J, Andersen KE, Andersen F, Johannessen H. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatol Treat. 2017;28(5):374–383. doi: 10.1080/09546634.2016.1254331. [DOI] [PubMed] [Google Scholar]
- 4.Thaçi D, de la Cueva P, Pink AE, et al. General practice recommendations for the topical treatment of psoriasis: a modified-Delphi approach. BJGP Open. 2020;4(5):bjgpopen20X101108. doi: 10.3399/bjgpopen20X101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mihu C, Neag MA, Bocşan IC, et al. Novel concepts in psoriasis: histopathology and markers related to modern treatment approaches. Rom J Morphol Embryol. 2021;62(4):897–906. doi: 10.47162/RJME.62.4.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badri T, Kumar P, Oakley AM. Plaque Psoriasis. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. www.ncbi.nlm.nih.gov/books/NBK430879/. Accessed 11 Apr 2023.
- 7.National Institute for Health and Excellence. NICE pathways. Topical therapy for psoriasis (2019). https://pathways.nice.org.uk/pathways/psoriasis. Accessed 11 Apr 2023.
- 8.Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35(2 Suppl 2):S36–44. doi: 10.12788/j.sder.2016.006. [DOI] [PubMed] [Google Scholar]
- 9.Fabbrocini G, De Simone C, Dapavo P, Malagoli P, Martella A, Calzavara-Pinton P. Long-term maintenance treatment of psoriasis: the role of calcipotriol/betamethasone dipropionate aerosol foam in clinical practice. J Dermatol Treat. 2022;4:1–8. doi: 10.1080/09546634.2021.1998310. [DOI] [PubMed] [Google Scholar]
- 10.Girolomoni G, Calzavara Pinton P, Cristaudo A, Cicchetti A. Back to the future: a new topical approach for mild-to-moderate psoriasis. G Ital Dermatol Venereol. 2018;153:375–382. doi: 10.23736/S0392-0488.16.05516-4. [DOI] [PubMed] [Google Scholar]
- 11.Alinia H, Moradi Tuchayi S, Smith JA, et al. Long-term adherence to topical psoriasis treatment can be abysmal: a 1-year randomized intervention study using objective electronic adherence monitoring. Br J Dermatol. 2017;176:759–764. doi: 10.1111/bjd.15085. [DOI] [PubMed] [Google Scholar]
- 12.Piaserico S, Manfredini S, Borghi A, et al. How to improve adherence to treatment in patients with mild-to-moderate psoriasis. G Ital Dermatol Venereol. 2018;153(5):692–697. doi: 10.23736/S0392-0488.17.05697-8. [DOI] [PubMed] [Google Scholar]
- 13.Gorelick J, Cantrell W, Kucera K, Veverka KA, Gooding K. Patient-reported satisfaction with the fixed combination calcipotriene/betamethasone dipropionate foam for plaque psoriasis. J Drugs Dermatol. 2018;17(8):880–884. [PubMed] [Google Scholar]
- 14.De Simone C, Dapavo P, Malagoli P, et al. Long-term proactive management of psoriasis with calcipotriol and betamethasone dipropionate foam: an Italian consensus through a combined nominal group technique and Delphi approach. Int J Dermatol. 2022;61(12):1543–1551. doi: 10.1111/ijd.16192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teixeira A, Teixeira M, Almeida V, et al. Does the vehicle matter? Real-world evidence on adherence to topical treatment in psoriasis. Pharmaceutics. 2021;13(10):1539. doi: 10.3390/pharmaceutics13101539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin B, Cabilan CJ, Ayoub B, et al. The effect of 20 minutes of cool running water first aid within three hours of thermal burn injury on patient outcomes: a systematic review and meta-analysis. Australas Emerg Care. 2022;25(4):367–376. doi: 10.1016/j.auec.2022.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Morton RW, Murphy KT, McKellar SR, et al. A systematic review, meta-analysis and meta-regression of the effect of protein supplementation on resistance training-induced gains in muscle mass and strength in healthy adults. Br J Sports Med. 2018;52(6):376–384. doi: 10.1136/bjsports-2017-097608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bark C, Brown C, Svangren P. Systematic literature review of long-term efficacy data for topical psoriasis treatments. J Dermatolog Treat. 2022;33(4):2118–2128. doi: 10.1080/09546634.2021.1925211. [DOI] [PubMed] [Google Scholar]
- 20.Lebwohl M, Ortonne JP, Andres P, Briantais P. Calcitriol ointment 3 microg/g is safe and effective over 52 weeks for the treatment of mild to moderate plaque psoriasis. Cutis. 2009;83(4):205–212. [PubMed] [Google Scholar]
- 21.Kragballe K, Austad J, Barnes L, et al. A 52-week randomized safety study of a calcipotriol/betamethasone dipropionate two-compound product (Dovobet/Daivobet/Taclonex) in the treatment of psoriasis vulgaris. Br J Dermatol. 2006;154(6):1155–1160. doi: 10.1111/j.1365-2133.2006.07236.x. [DOI] [PubMed] [Google Scholar]
- 22.Jalili A, Calzavara-Pinton P, Kircik L, et al. Quality of life and patient-perceived symptoms in patients with psoriasis undergoing proactive or reactive management with the fixed-dose combination Cal/BD foam: a post-hoc analysis of PSO-LONG. J Eur Acad Dermatol Venereol. 2022;36(1):60–67. doi: 10.1111/jdv.17673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campanati A, Atzori L, Potenza C, et al. Patient satisfaction with calcipotriol/betamethasone dipropionate cutaneous foam for the treatment of plaque psoriasis: The LION real-life multicenter prospective observational cohort study. Dermatol Ther. 2021;34(5):e15077. doi: 10.1111/dth.15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paul C, Stein Gold L, Cambazard F, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017;31(1):119–126. doi: 10.1111/jdv.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hong CH, Papp KA, Lophaven KW, Skallerup P, Philipp S. Patients with psoriasis have different preferences for topical therapy, highlighting the importance of individualized treatment approaches: randomized phase IIIb PSO-INSIGHTFUL study. J Eur Acad Dermatol Venereol. 2017;31(11):1876–1883. doi: 10.1111/jdv.14515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul C, Leonardi C, Menter A, et al. Calcipotriol plus betamethasone dipropionate aerosol foam in patients with moderate-to-severe psoriasis: sub-group analysis of the PSO-ABLE study. Am J Clin Dermatol. 2017;18(3):405–411. doi: 10.1007/s40257-017-0258-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koo J, Tyring S, Werschler WP, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris—a randomized phase II study. J Dermatolog Treat. 2016;27(2):120–127. doi: 10.3109/09546634.2015.1083935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein Gold L, Green LJ, Dhawan S, Vestbjerg B, Praestegaard M, Selmer J. A phase 3, randomized trial demonstrating the improved efficacy and patient acceptability of fixed dose calcipotriene and betamethasone dipropionate cream. J Drugs Dermatol. 2021;20(4):420–425. doi: 10.36849/JDD.2021.5653. [DOI] [PubMed] [Google Scholar]
- 29.Pinter A, Green LJ, Selmer J, et al. A pooled analysis of randomized, controlled, phase 3 trials investigating the efficacy and safety of a novel, fixed dose calcipotriene and betamethasone dipropionate cream for the topical treatment of plaque psoriasis. J Eur Acad Dermatol Venereol. 2022;36(2):228–236. doi: 10.1111/jdv.17734. [DOI] [PubMed] [Google Scholar]
- 30.Gerdes S, Krakor M, Anger T, Hutt HJ, Körber A. Prospective, observational, non-interventional, multicentre study on the efficacy and tolerability of a new calcipotriol/betamethasone aerosol foam (Enstilar®) in patients with plaque psoriasis under daily practice conditions. Dermatology. 2017;233(6):425–434. doi: 10.1159/000486700. [DOI] [PubMed] [Google Scholar]
- 31.Sticherling M, Eicke C, Anger T. Practicability of combined treatment with calcipotriol/betamethasone gel (Daivobet® Gel) and improvement of quality of life in patients with psoriasis. J Dtsch Dermatol Ges. 2013;11(5):420–427. doi: 10.1111/ddg.12029. [DOI] [PubMed] [Google Scholar]
- 32.Claréus BW, Houwing R, Sindrup JH, Wigchert S. The DESIRE study–psoriasis patients' satisfaction with topical treatment using a fixed combination of calcipotriol and betamethasone dipropionate in daily clinical practice. Eur J Dermatol. 2009;19(6):581–585. doi: 10.1684/ejd.2009.0767. [DOI] [PubMed] [Google Scholar]
- 33.Koo J, Blum RR, Lebwohl M. A randomized, multicenter study of calcipotriene ointment and clobetasol propionate foam in the sequential treatment of localized plaque-type psoriasis: short- and long-term outcomes. J Am Acad Dermatol. 2006;55(4):637–641. doi: 10.1016/j.jaad.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 34.Lowe N, Feldman SR, Sherer D, et al. Clobetasol propionate lotion, an efficient and safe alternative to clobetasol propionate emollient cream in subjects with moderate to severe plaque-type psoriasis. J Dermatolog Treat. 2005;16(3):158–164. doi: 10.1080/09546630510041060. [DOI] [PubMed] [Google Scholar]
- 35.Decroix J, Pres H, Tsankov N, Poncet M, Arsonnaud S. Clobetasol propionate lotion in the treatment of moderate to severe plaque-type psoriasis. Cutis. 2004;74(3):201–206. [PubMed] [Google Scholar]
- 36.Bergstrom KG, Arambula K, Kimball AB. Medication formulation affects quality of life: a randomized single-blind study of clobetasol propionate foam 0.05% compared with a combined program of clobetasol cream 0.05% and solution 0.05% for the treatment of psoriasis. Cutis. 2003;72(5):407–411. [PubMed] [Google Scholar]
- 37.Sharma V, Kaur I, Kumar B. Calcipotriol versus coal tar: a prospective randomized study in stable plaque psoriasis. Int J Dermatol. 2003;42(10):834–838. doi: 10.1046/j.1365-4362.2003.01974.x. [DOI] [PubMed] [Google Scholar]
- 38.Navarro-Triviño FJ, Lozano-Lozano M, Ruiz-Villaverde R. Calcipotriol/betamethasone dipropionate aerosol foam for plaque psoriasis: a prospective, observational, non-interventional, single-center study of patient adherence and satisfaction in daily use. Dermatol Pract Concept. 2021;11(3):e2021056. doi: 10.5826/dpc.1103a56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambert J, Hol CW, Vink J. Real-life effectiveness of once-daily calcipotriol and betamethasone dipropionate gel vs. ointment formulations in psoriasis vulgaris: final analysis of the 52-week PRO-long study. J Eur Acad Dermatol Venereol. 2015;29(12):2349–2355. doi: 10.1111/jdv.13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Korting HC, Schöllmann C, Willers C, Wigger-Alberti W. Bioavailability, antipsoriatic efficacy and tolerability of a new light cream with mometasone furoate 0.1% Skin Pharmacol Physiol. 2012;25(3):133–141. doi: 10.1159/000335656. [DOI] [PubMed] [Google Scholar]
- 41.Lambert J, Trompke C. Tacalcitol ointment for long-term control of chronic plaque psoriasis in dermatological practice. Dermatology. 2002;204(4):321–324. doi: 10.1159/000063376. [DOI] [PubMed] [Google Scholar]
- 42.Velasco M, González-Fernández D, Rodriguez-Martín M, Sánchez-Regaña M, Pérez-Barrio S. Patient and physician satisfaction with calcipotriol and betamethasone dipropionate aerosol foam in the treatment of plaque psoriasis on the body. Actas Dermosifiliogr (Engl Ed). 2019;110(9):752–758. doi: 10.1016/j.ad.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Lebwohl M, Kircik L, Lacour JP, et al. Twice-weekly topical calcipotriene/betamethasone dipropionate foam as proactive management of plaque psoriasis increases time in remission and is well tolerated over 52 weeks (PSO-LONG trial) J Am Acad Dermatol. 2021;84(5):1269–1277. doi: 10.1016/j.jaad.2020.09.037. [DOI] [PubMed] [Google Scholar]
- 44.Selmin F, Franzè S, Casiraghi A, Cilurzo F. Spotlight on calcipotriol/betamethasone fixed-dose combination in topical formulations: is there still room for innovation? Pharmaceutics. 2022;14(10):2085. doi: 10.3390/pharmaceutics14102085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman SR, Housman TS. Patients' vehicle preference for corticosteroid treatments of scalp psoriasis. Am J Clin Dermatol. 2003;4(4):221–224. doi: 10.2165/00128071-200304040-00001. [DOI] [PubMed] [Google Scholar]
- 46.Bewley A, Barker E, Baker H, et al. An anchored matching-adjusted indirect comparison of fixed-dose combination calcipotriol and betamethasone dipropionate (Cal/BDP) cream versus Cal/BDP foam for the treatment of psoriasis. J Dermatolog Treat. 2022;33(8):3191–3198. doi: 10.1080/09546634.2022.2116924. [DOI] [PubMed] [Google Scholar]
- 47.Papp KA, Thoning H, Gerdes S, et al. Matching-adjusted indirect comparison of efficacy outcomes in trials of calcipotriol plus betamethasone dipropionate foam and cream formulations for the treatment of plaque psoriasis. J Dermatolog Treat. 2022;33(7):3005–3013. doi: 10.1080/09546634.2022.2095330. [DOI] [PubMed] [Google Scholar]
- 48.Lind M, Nielsen KT, Schefe LH, et al. Supersaturation of calcipotriene and betamethasone dipropionate in a novel aerosol foam formulation for topical treatment of psoriasis provides enhanced bioavailability of the active ingredients. Dermatol Ther (Heidelb) 2016;6(3):413–425. doi: 10.1007/s13555-016-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rudnicka L, Olszewska M, Goldust M, et al. Efficacy and safety of different formulations of calcipotriol/betamethasone dipropionate in psoriasis: gel, foam, and ointment. J Clin Med. 2021;10(23):5589. doi: 10.3390/jcm10235589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eastman WJ, Malahias S, Delconte J, DiBenedetti D. Assessing attributes of topical vehicles for the treatment of acne, atopic dermatitis, and plaque psoriasis. Cutis. 2014;94(1):46–53. [PubMed] [Google Scholar]
- 51.Kim ES, Frampton JE. Calcipotriol/betamethasone dipropionate foam: a review in plaque psoriasis. Drugs. 2016;76(15):1485–1492. doi: 10.1007/s40265-016-0643-7. [DOI] [PubMed] [Google Scholar]
- 52.Segaert S, Calzavara-Pinton P, de la Cueva P, et al. Long-term topical management of psoriasis: the road ahead. J Dermatolog Treat. 2022;33(1):111–120. doi: 10.1080/09546634.2020.1729335. [DOI] [PubMed] [Google Scholar]
- 53.Carrascosa JM, Theng C, Thaçi D. Spotlight on topical long-term management of plaque psoriasis. Clin Cosmet Investig Dermatol. 2020;13:495–498. doi: 10.2147/CCID.S254114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mrowietz U. Implementing treatment goals for successful long-term management of psoriasis. J Eur Acad Dermatol Venereol. 2012;26(Suppl 2):12–20. doi: 10.1111/j.1468-3083.2011.04411.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.