Abstract
Introduction
Near-complete skin clearance has become a rapidly achievable treatment goal for patients with psoriasis receiving systemic biologic therapies. However, real-world evidence for durability of near-complete skin clearance and risk factors associated with loss of near-complete skin clearance is limited.
Methods
This study described durability of near-complete skin clearance (≥ 90% improvement in Psoriasis Area and Severity Index from initiation; PASI90) and identified clinical factors or patient characteristics associated with loss of PASI90 among patients with psoriasis from the CorEvitas Psoriasis Registry (April 2015–August 2021). Included patients had PASI > 5 at biologic initiation and achieved PASI90 at approximately 6 months from initiation (index). A Kaplan-Meier estimate described time to loss of treatment response over 24 months follow-up from index. Proportional hazards regression was used to identify independent predictors of loss of treatment response.
Results
This study included 687 patient initiations (instances of patients initiating a biologic). Following achievement of PASI90, treatment response was maintained in more than half of patient initiations (54%). Treatment response was maintained at 6, 12, and 18 months from index in an estimated 73% (95% [confidence interval] CI 70–77%), 60% (95% CI 56–63%), and 50% (95% CI 47–54%) of patient initiations, respectively. Adjusted hazards regression suggested non-White race, full-time employment, greater body weight, concomitant psoriatic arthritis, prior use of biologics, and clinically meaningful skin symptoms were associated with loss of treatment response.
Conclusions
Among real-world patients with psoriasis who achieved PASI90 with biologic therapy, about one-quarter lost response at 6 months, and half lost response at 18 months. Prior use of a biologic therapy and clinically meaningful skin symptoms at index, including itch and skin pain, were associated with loss of treatment response. Therefore, dermatologists may consider focusing on patient-reported symptoms as part of any intervention designed to reduce the likelihood of loss of response to biologic therapies.
Trial Registration
ClinicalTrials.gov identifier, NCT02707341.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-01028-5.
Keywords: Biologic therapy, CorEvitas Psoriasis Registry, Interleukin, PASI, Psoriasis, Real-world evidence, Skin clearance
Plain Language Summary
Many people with psoriasis are treated with biologic medications that work to improve symptoms associated with psoriasis, including inflammation. These medications can lead to almost clear skin for many people. However, there is limited information available about how long almost clear skin can be maintained with biologic medications, and what predicts who is likely to lose it. To explore these questions, we examined a database of patients with psoriasis (the CorEvitas Psoriasis Registry) that records how clear patients’ skin is and the medications they take. Out of every 100 patients, 54 maintained almost clear skin and stayed on their original medication for 2 years after first having almost clear skin. Out of every 100 patients, 73, 60, and 50 maintained almost clear skin and remained on their original medication at 6, 12, and 18 months after they had achieved this response. The results indicated that patients who were not White, worked full time, previously used a biologic medication, or had itchy and/or painful skin after they had achieved almost-clear skin were more likely to change their medication and/or no longer have almost-clear skin. These results suggest that dermatologists may consider focusing on patient-reported characteristics when deciding how to treat their patients, to reduce the likelihood that they lose their response to the medication they are prescribed.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-01028-5.
Key Summary Points
| The objectives of this study were to describe the durability of response, following achievement of near-complete skin clearance (PASI90), and to identify patient characteristics or clinical factors associated with loss of near-complete skin clearance among patients with psoriasis treated with biologic therapies from the CorEvitas Psoriasis Registry. |
| Evidence for the durability of near-complete skin clearance for patients using biologic therapies has come primarily from randomized clinical trials, not necessarily reflecting the clinical experience of patients with psoriasis; few real-world data on the topic are available. |
| Among real-world patients with psoriasis in this study who achieved near-complete skin clearance (PASI90) with systemic biologic therapy, about one-quarter lost response at 6 months (27%), and half lost response at 18 months (50%) following achievement of PASI90. |
| Non-White race, full-time employment, greater body weight, concomitant psoriatic arthritis, prior use of biologics, and clinically meaningful skin symptoms, including itch and skin pain, were associated with a decreased likelihood of maintaining a treatment response that included near-complete skin clearance. |
| Dermatologists may consider focusing on patient-reported symptoms as a part of any intervention designed to reduce the likelihood of loss of response to biologic therapies. |
Introduction
Patients with psoriasis have indicated that treatment effectiveness is the main determinant of treatment satisfaction [1]. The Psoriasis Area and Severity Index (PASI) is the most commonly used quantitative measure of psoriasis disease severity in clinical research [2], and ≥ 75% improvement in PASI from therapy initiation (PASI75) is often used in clinical trials as a reasonable therapeutic target for systemic biologic treatment of psoriasis [3]. Patients with severe psoriasis who achieve this threshold of improvement in disease severity are considered responders by many regulatory agencies [3]. Near-complete skin clearance (≥ 90% improvement in PASI; PASI90) is a more stringent response measure that has been shown to be an achievable goal for treatment with biologic therapies [3].
While biologic therapies show rapid improvement for patients with moderate-to-severe psoriasis [4], given the chronic nature of this condition, it is important for patients to maintain response to treatment [5]. Therefore, understanding influences on the durability (maintenance of response over time) of near-complete skin clearance for patients treated with biologic therapies is critically important. Evidence for the durability of near-complete skin clearance for patients using biologic therapies has come primarily from randomized clinical trials [4, 6], which do not necessarily reflect the clinical experience of patients with psoriasis; few real-world data on the topic are available.
Furthermore, durability of response may be influenced by patient characteristics, yet few studies have identified which factors have the greatest impact [5, 7, 8]. Understanding durability of response with biologics and the contributing factors among real-world patients with psoriasis may inform approaches to patient care for dermatologists and determine whether maintaining near-complete skin clearance is an appropriate or realistic treatment target.
The objectives of this study were to describe the durability of response, following achievement of near-complete skin clearance (PASI90), and to identify patient characteristics or clinical factors associated with loss of near-complete skin clearance among patients with psoriasis treated with biologic therapies from the CorEvitas Psoriasis Registry.
Methods
CorEvitas Psoriasis Registry Patient Population
This study utilized data from the CorEvitas Psoriasis Registry, an independent, prospective, multicenter, observational registry based in the US and Canada that was launched in April 2015 in collaboration with the National Psoriasis Foundation [9, 10]. Adult patients (≥ 18 years of age) are eligible to be enrolled in the registry if they have psoriasis diagnosed by a dermatologist, are willing to provide written informed consent for participation in the registry, and have initiated an eligible medication at enrollment or within the previous 12 months (Supplementary Material, Table S1). Patients participating in or planning to participate in an interventional clinical trial with a non-marketed or marketed investigational drug for psoriasis are ineligible.
Registry data are collected from participating dermatologists and enrolled patients via questionnaires during registry visits occurring at approximately 6-month intervals [11]. At each registry visit, providers update medication usage and document disease severity. Any change or planned change in therapy status occurring on the day of a registry visit is recorded at that registry visit with the reason for the change. Changes in biologic or non-biologic therapies that occur between registry visits are recorded along with the reason for the change at subsequent registry visits.
Providers record patient characteristics, clinical factors, and treatment changes at all registry visits. Patients also provide self-reported information on health-related quality of life (HRQoL; Dermatology Life Quality Index [DLQI]) and symptom burden (fatigue, itch, and skin pain 100-point visual analog scales [VAS]) at all registry visits.
This study was performed following the guidelines for Good Pharmacoepidemiology Practice [12] and in accordance with the current version of the applicable regulatory and International Council for Harmonisation Good Clinical Practice requirements [13], whose ethical principles have their origin in the Declaration of Helsinki, and the local laws of the countries involved. All participating investigators were required to obtain full board approval for conducting non-interventional research involving human patients with a limited dataset. Sponsor (CorEvitas, LLC) approval and continuing review were obtained through a central institutional review board (IRB; IntegReview, protocol number is Corrona-PSO-500). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained from the respective governing IRBs, and documentation of approval was submitted to CorEvitas, LLC, prior to the initiation of any study procedures. All registry patients were required to provide written informed consent prior to participating.
Study Design
This was a longitudinal cohort study which defined PASI response as a time-varying covariate (Supplementary Material, Fig. S1). All patients included in this study (April 2015–August 2021) had a baseline visit, at which the patient had plaque psoriasis with PASI > 5 and initiated a systemic biologic therapy within 42 days of the visit. These patient initiations were required to have an index visit at 5–9 months following initiation at which PASI90 was achieved, no systemic non-biologic therapies added from initiation, and no discontinuation of the initiated biologic therapy nor switch to another biologic therapy.
Patient initiations were followed from the index visit until (1) loss of treatment response, (2) discontinuation not related to treatment response, or (3) the last CorEvitas visit over the 24-month study follow-up period after the index visit. During the follow-up period, patient initiations were required to have at least one additional registry visit for inclusion in the study.
Study Outcome Measures
Loss of Treatment Response
Durability of treatment response is measured in this study as loss of treatment response, a composite measure of indicators based on clinical evaluation or change in therapy. Loss of treatment response, measured at registry visits following index, was defined as a loss of PASI90 or treatment failure (discontinuation of the initiated biologic therapy or addition of a systemic non-biologic therapy). Discontinuations for reasons other than poor response or adverse events were classified as non-failure discontinuations and were not considered a loss of treatment response; no discontinuation of the biologic prescribed at initiation or addition of a systemic non-biologic therapy was considered as persistence (Supplementary Material, Table S2).
The primary outcome of this study was an interval-censored, time-to-event measure, with time until loss of treatment response calculated from index. This measure had an upper and lower bound defining when loss of treatment response occurred. For patients that experienced loss of treatment response due to a loss of PASI90, the upper bound was defined as the time from index to the registry visit at which the loss of treatment response was observed and the lower bound was defined as the registry visit preceding the registry visit at which loss of treatment response due to PASI90 was observed. These bounds accounted for the possibility that loss of treatment response due to loss of PASI90 occurred between a visit at which PASI90 was observed and one at which it was lost.
For cases in which loss of treatment response was due to treatment failure observed at a registry visit (i.e., a treatment change was made at the visit), the time from index to the registry visit at which treatment failure was observed was used as both the lower and upper bound. In cases at which loss of treatment response due to treatment failure was not observed at a registry visit, the upper and lower bounds of the time-to-event measure were defined as the time from index to the registry visit at which treatment failure was recorded and the previous registry visit, respectively. Registry visits without an indication of a loss of treatment response (i.e., persistence or non-failure discontinuation) were considered right-censored, with the lower bound of the time-to-event measure being the time from index to the registry visit and the upper bound being undefined.
Due to the possibility of patients having multiple reasons for a loss of treatment response, this study considered the reason with the lesser lower bound as the instance of the loss of treatment response used to define the outcome. In cases at which the lower bound for the time-to-event measure was the same as the index visit, the lower bound was defined as 1 day. Data observed from all patient initiations were censored after the time-to-event measure was observed or at the last registry visit prior to the 24-month study follow-up period after the index visit, whichever occurred first (Supplementary Material, Fig. S2).
Other Response Variables at Index
The achievement of body surface area (BSA) ≤ 1%, DLQI = 0/1, or PASI ≤ 2 was described separately from achievement of PASI90 at the index visit. Patients whose data were analyzed for these secondary outcomes all achieved PASI90 at index, while patients with BSA ≤ 1% and DLQI ≤ 5 at baseline were excluded from each respective analysis.
Predictors
The following demographic and lifestyle characteristics were measured at baseline: age (years), gender (male, female), race (White, Black, Asian, other/unknown), ethnicity (Hispanic, non-Hispanic), health insurance (private, other), employment (full-time, part-time/unemployed), smoking status (never, former, current), body mass index (BMI; underweight/normal [< 25 kg/m2], overweight [25 to < 30 kg/m2], obese [≥ 30 kg/m2]), provider-reported history of anxiety/depression (yes, no), provider-reported histories of cardiovascular comorbidity (hyperlipidemia, diabetes, and hypertension) and non-cardiovascular comorbidity (chronic obstructive pulmonary disease, peptic ulcer disease, lymphoma, and solid tumor cancer [excluding non-melanoma of the skin]).
The following disease and treatment characteristics were measured at baseline: psoriasis duration (years), PASI > 12 (yes, no), history of psoriatic arthritis (PsA) as determined by a dermatologist report of PsA or any history of a Psoriasis Epidemiology Screening Tool score ≥ 3 (yes, no), prior biologic therapy use (bio-naïve/bio-experienced), prior systemic non-biologic therapy use, and concomitant therapy use at initiation (systemic non-biologic therapy, phototherapy, or topical therapy, separately).
The following patient-reported measures were recorded at the index visit: fatigue assessment on a 100-point VAS, DLQI, PASI change from baseline above PASI90 (PASI90 – PASI95, PASI95 – PASI100, PASI100), and presence of clinically meaningful skin symptoms (defined as having either ≥ 45 on the skin pain VAS or ≥ 30 on the itch VAS) [14, 15].
Statistical Analysis
Means (standard deviation [SD]), frequencies, and percentages were calculated for characteristics measured at baseline and index visits. Frequencies and percentages of persistence (remained on biologic prescribed at initiation), non-failure discontinuation, treatment failure, and loss of PASI90 over follow-up from index were calculated. The time from the index visit to loss of treatment response and time to discontinuation not due to loss of treatment response were summarized with medians and quartiles (first and third).
A non-parametric estimate of the survival function [16] describing the time until loss of treatment response was calculated over the 24-month follow-up period; the median time until loss of treatment response and percentage loss of treatment response at 6, 12, and 18 months after index were also calculated. Unadjusted proportional hazards regression models for interval-censored outcomes were fit separately for each potential predictor to calculate unadjusted hazard ratios (HRs) and 95% confidence intervals (CIs) estimating the relationships with loss of treatment response. Cut points on the hazard for this model were defined in 6-month intervals starting at zero months (index visit) until 24 months. To identify independent predictors of loss of response, an adjusted proportional hazards model was fit including age, gender, race, ethnicity, and other select characteristics (including body weight) having p ≤ 0.30 in the unadjusted models. The adjusted model excluded BMI because of its relationship with body weight.
All analyses were performed for the overall sample as well as separately for patients with and without prior biologic therapy use.
Results
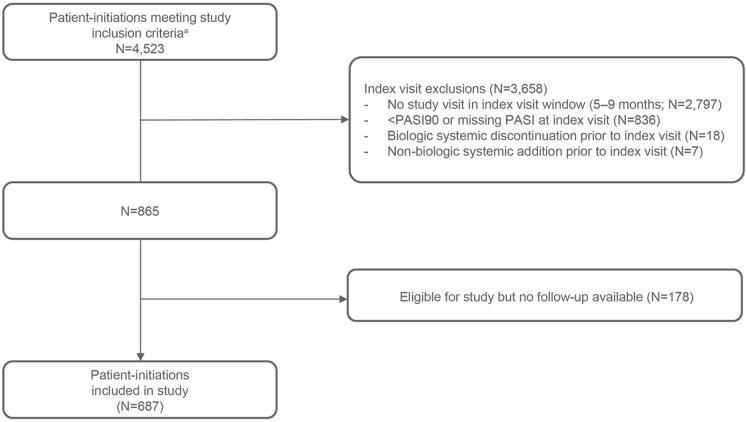
As of August 2021, there were 4523 instances of patients initiating a systemic biologic therapy (patient initiations) in the CorEvitas Psoriasis Registry meeting inclusion criteria at systemic biologic initiation, among which 687 patient initiations (from 679 patients; some patients had more than one initiation) were eligible for and included in this study on the basis of information collected at index and subsequent registry visits (Fig. 1).
Fig. 1.
Patient selection criteria and attrition. aIncluded adults (≥ 18 years of age) with plaque psoriasis at initiation, initiation of a biologic therapy at or up to 42 days following a registry visit, and PASI > 5 at initiation. N unique initiations, PASI Psoriasis Area and Severity Index
Patient Demographics and Disease Characteristics
Among the 687 patient initiations included in this study, mean patient age (SD) was 48.8 (15.1) years, 38% (n = 259) occurred among women, and 74% (n = 510) occurred among White patients. Most patient initiations occurred in full-time employees (62%; n = 425) and in those with private insurance (77%; n = 481). At baseline, 79% (n = 542) of patient initiations were recorded in overweight or obese patients, and 45% (n = 308) of patient initiations occurred in patients with a cardiovascular comorbidity (diabetes, hyperlipidemia, or hypertension; Table 1).
Table 1.
Characteristics of patients with psoriasis who achieved PASI90 at 6 months following biologic therapy initiation in the CorEvitas Psoriasis Registry
| Characteristic | Overall (N = 687) | Bio-naïve (N = 357) | Bio-experienced (N = 330) |
|---|---|---|---|
| Age (years), mean (SD) | 48.8 (15.1) | 47.2 (15.1) | 50.6 (14.9) |
| Gender (female), n (%) | 259 (37.7) | 132 (37.0) | 127 (38.5) |
| Race, n (%) | |||
| White | 510 (74.2) | 265 (74.2) | 245 (74.2) |
| Black | 21 (3.1) | 7 (2.0) | 14 (4.2) |
| Asian | 94 (13.7) | 52 (14.6) | 42 (12.7) |
| Other/unknown | 62 (9.0) | 33 (9.2) | 29 (8.8) |
| Ethnicity (Hispanic), n (%) | 66 (9.7) | 39 (11.0) | 27 (8.3) |
| Health insurance (private), n (%) | 481 (77.2) | 251 (78.4) | 230 (75.9) |
| Employment (full-time), n (%) | 425 (61.9) | 239 (66.9) | 186 (56.4) |
| Smoking history, n (%) | |||
| Never | 359 (52.9) | 192 (54.4) | 167 (51.4) |
| Former smoker | 210 (31.0) | 106 (30.0) | 104 (32.0) |
| Current smoker | 109 (16.1) | 55 (15.6) | 54 (16.6) |
| BMI, n (%) | |||
| Underweight/normal (< 25 kg/m2) | 137 (20.2) | 86 (24.4) | 51 (15.6) |
| Overweight (25 to < 30 kg/m2) | 211 (31.1) | 103 (29.2) | 108 (33.1) |
| Obese (≥ 30 kg/m2) | 331 (48.7) | 164 (46.5) | 167 (51.2) |
| Body weight (kg), mean (SD) | 91.7 (23.9) | 90.1 (24.2) | 93.4 (23.5) |
| Non-cardiovascular comorbidity, n (%)a | 51 (7.4) | 21 (5.9) | 30 (9.1) |
| Cardiovascular comorbidity, n (%)b | 308 (44.8) | 135 (37.8) | 173 (52.4) |
| History of diabetes | 112 (16.3) | 44 (12.3) | 68 (20.6) |
| History of hyperlipidemia | 179 (26.1) | 78 (21.8) | 101 (30.6) |
| History of hypertension | 242 (35.2) | 110 (30.8) | 132 (40.0) |
| History of anxiety or depression, n (%) | 164 (23.9) | 80 (22.4) | 84 (25.5) |
| Psoriasis duration (years), mean (SD) | 15.9 (13.5) | 12.8 (12.5) | 19.2 (13.7) |
| History of PsA, n (%)c | 300 (43.7) | 116 (32.5) | 184 (55.8) |
| PASI > 12, n (%) | 290 (42.2) | 147 (41.2) | 143 (43.3) |
| PASI90–PASI95, n (%)d | 134 (19.5) | 65 (18.2) | 69 (20.9) |
| PASI95–PASI100, n (%)d | 100 (14.6) | 48 (13.5) | 52 (15.8) |
| PASI100, n (%)d | 453 (65.9) | 244 (68.4) | 209 (63.3) |
| Fatigue (VAS-100), mean (SD)d | 20.4 (25.1) | 18.1 (24.7) | 22.8 (25.4) |
| Skin pain (VAS-100), mean (SD)d | 5.6 (13.8) | 4.4 (12.4) | 6.9 (15.0) |
| Itch (VAS-100), mean (SD)d | 11.1 (20.5) | 11.6 (21.5) | 10.5 (19.5) |
| DLQI, mean (SD)d | 1.5 (2.7) | 1.2 (2.2) | 1.9 (3.1) |
| Clinically meaningful skin symptoms, n (%)d,e | 71 (12.6) | 35 (12.2) | 36 (12.9) |
| Concomitant therapy, n (%)f | |||
| Systemic non-biologic therapy | 34 (4.9) | 15 (4.2) | 19 (5.8) |
| Topical therapy | 348 (50.7) | 191 (53.7) | 157 (47.6) |
| Phototherapy | 25 (3.6) | 16 (4.5) | 9 (2.7) |
| Prior systemic non-biologic therapy use, n (%) | 357 (52.0) | 137 (38.4) | 220 (66.7) |
| Systemic biologic therapy class initiated, n (%) | |||
| TNFi | 81 (11.8) | 67 (18.8) | 14 (4.2) |
| IL-17Ai | 285 (41.5) | 110 (30.8) | 175 (53.0) |
| IL-12/23i or IL-23i | 321 (46.7) | 180 (50.4) | 141 (42.7) |
BMI body mass index, DLQI Dermatology Life Quality Index, i inhibitor, IL interleukin, PASI Psoriasis Area and Severity Index, PEST Psoriasis Epidemiology Screening Tool, PsA psoriatic arthritis, SD standard deviation, TNF tumor necrosis factor, VAS visual analog scale
aNon-cardiovascular comorbidities included chronic obstructive pulmonary disease, peptic ulcer disease, lymphoma, and solid tumor cancer (excluding non-melanoma of the skin)
bCardiovascular comorbidities included hyperlipidemia, diabetes, and hypertension
cDetermined by a dermatologist report of PsA or any history of a PEST score of 3 or greater
dCharacteristic was measured at index visit
eDefined as either having ≥ 45 on the skin pain VAS or ≥ 30 on the itch VAS
fOptions are not mutually exclusive, more than one category can be indicated
The mean (SD) psoriasis duration for patients included in patient initiations was 15.9 (13.5) years, and 44% (n = 300) of patient initiations occurred in patients with a history of PsA. Almost half (42%) of patient initiations occurred in patients with severe psoriasis as determined by PASI > 12. Among all patient initiations, 52% (n = 357) occurred in patients that had not previously received a biologic therapy (bio-naïve); the same proportion of patient initiations had previously received a systemic non-biologic therapy (Table 1).
At index, the mean (SD) DLQI was 1.5 (2.7), and the mean (SD) VAS-skin pain and VAS-itch scores were 5.6 (13.8) and 11.1 (20.5), respectively. Clinically meaningful skin symptoms were observed in 13% (n = 71) of patient initiations at index (Table 1).
When characteristics were compared between patient initiations that occurred in bio-naïve versus bio-experienced patients, the most notable variations were observed for cardiovascular comorbidities (38% versus 52%, respectively), history of diabetes (12% versus 21%), history of hyperlipidemia (22% versus 31%), history of hypertension (31% versus 40%), history of PsA (33% versus 56%), and prior systemic non-biologic therapy use (38% versus 67%). The systemic biologic therapy class initiated for patient initiations among those that were bio-naïve was more likely to be a tumor necrosis factor inhibitor than among those that were bio-experienced (19% versus 4%). Likewise, the biologic therapy class initiated by those who were bio-naïve was less likely to be an interleukin-17A inhibitor than among those who were bio-experienced (31% versus 53%). Across all patient initiations in the overall, bio-naïve, and bio-experienced groups, most patients who achieved PASI90 also achieved 100% improvement in PASI (PASI100; 66%, 68%, and 63%, respectively; Table 1).
Durability of Near-Complete Skin Clearance
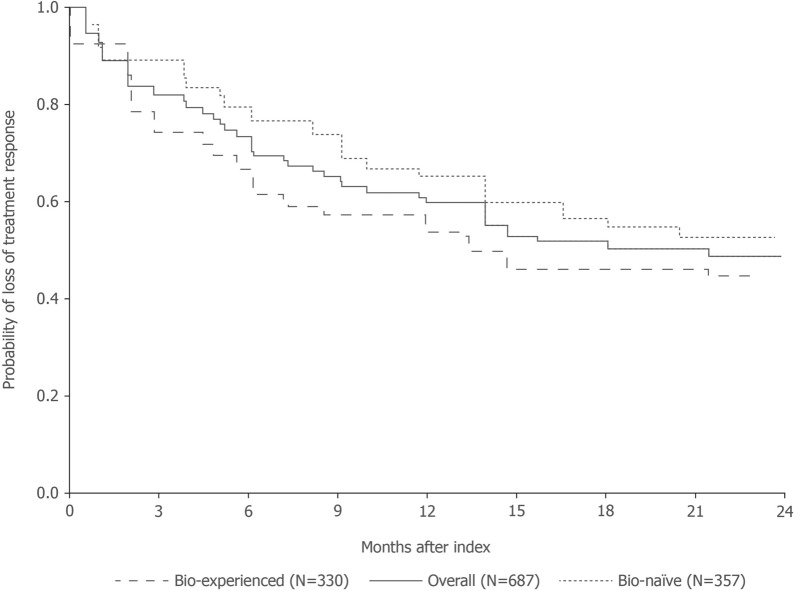
Over the 24 months of follow-up after achievement of PASI90, over half (54%; n = 373) of the patient initiations maintained treatment response through the last registry visit, with the majority persistent (51%; n = 351) on their initiated biologic at the end of follow-up (Supplementary Material, Tables S3 and S4). Of those patient initiations that ended in a loss of treatment response (46%; n = 314), 91% (n = 287/314) were due to a loss of PASI90 with the remainder (9%; n = 27/314) due to treatment failure (Supplementary Material, Table S4). Survival curve estimates indicated that 73% (95% CI 70, 77%), 60% (95% CI 56, 64%), and 50% (95% CI 47, 54%) of patient initiations maintained treatment response at 6, 12, and 18 months from index, respectively (Table 2 and Fig. 2).
Table 2.
Retention of treatment response at 6, 12, and 18 months after achievement of PASI90a
| 6 months | 12 months | 18 months | ||||
|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |
| Overall (N = 687) | 73.3 | (69.6, 76.7) | 59.8 | (55.9, 63.5) | 50.4 | (46.5, 54.2) |
| Bio-naïve (N = 357) | 79.5 | (74.6, 83.5) | 65.3 | (60.0, 70.2) | 54.7 | (49.2, 59.9) |
| Bio-experienced (N = 330) | 66.7 | (60.9, 71.8) | 53.8 | (48.1, 59.2) | 46.2 | (40.7, 51.6) |
CI confidence interval, PASI Psoriasis Area and Severity Index
aRetention of treatment response at 6, 12, and 18 months was estimated from the survival curve analysis using the Turnbull method [16]
Fig. 2.
Estimated survival plots for patients maintaining response after achievement of PASI90. PASI Psoriasis Area and Severity Index
In unadjusted analyses, body weight at baseline (10 kg increase; HR = 1.05; 95% CI 1.00, 1.09), non-cardiovascular comorbidity (HR = 1.60; 95% CI 1.11, 2.31), cardiovascular comorbidity (HR = 1.21; 95% CI 0.97, 1.51), history of PsA (HR = 1.43; 95% CI 1.15, 1.79), systemic non-biologic use (HR = 1.31; 95% CI 0.82, 2.08), fatigue as measured by VAS (HR = 1.03; 95% CI 1.01, 1.06), full-time work status (HR = 1.33; 95% CI 1.06, 1.66), presence of clinically meaningful skin symptoms at index (HR = 1.63; 95% CI 1.20, 2.21), and higher DLQI (HR = 1.72; 95% CI 1.46, 2.03) were associated with increased risk of loss of treatment response and met the criteria for inclusion in the subsequent multivariable analyses. Being bio-naïve at baseline was associated with a lower risk of loss of response (HR = 0.71; 95% CI 0.57, 0.89) and was also included in the multivariable analysis. A history of anxiety or depression was not associated with a loss of treatment response (HR = 0.93; 95% CI 0.72, 1.22; Table 3).
Table 3.
Association between patient characteristics and loss of treatment response following achievement of PASI90
| Characteristic | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Demographic factors | ||||||
| Age (10-year increase) | 1.03 | (0.96, 1.11) | 0.359 | 0.98 | (0.90, 1.07) | 0.612 |
| Gender (male–female) | 0.95 | (0.76, 1.19) | 0.660 | 0.99 | (0.77, 1.26) | 0.909 |
| Race (White–non-White) | 0.86 | (0.67, 1.11) | 0.251 | 0.80 | (0.61, 1.05) | 0.110 |
| Ethnicity (Hispanic–non-Hispanic) | 0.90 | (0.60, 1.34) | 0.598 | 0.93 | (0.61, 1.42) | 0.748 |
| Work status (full time–not full-time) | 1.33 | (1.06, 1.66) | 0.012 | 1.27 | (1.00, 1.62) | 0.052 |
| Health factors | ||||||
| BMI (underweight/normal–obese) | 0.83 | (0.62, 1.12) | 0.223 | – | – | – |
| BMI (overweight–obese) | 0.89 | (0.69, 1.15) | 0.374 | – | – | – |
| Body weight (10 kg increase) | 1.05 | (1.00, 1.09) | 0.052 | 1.05 | (1.00, 1.10) | 0.077 |
| Smoking status (never–current) | 0.95 | (0.69, 1.30) | 0.753 | – | – | – |
| Smoking status (former–current) | 1.05 | (0.75, 1.48) | 0.768 | – | – | – |
| Diabetes (yes–no) | 1.32 | (0.99, 1.76) | 0.060 | – | – | – |
| History of hyperlipidemia (yes–no) | 1.25 | (0.98, 1.60) | 0.075 | – | – | – |
| History of hypertension (yes–no) | 1.23 | (0.98, 1.55) | 0.078 | – | – | – |
| Cardiovascular comorbidity (yes–no) | 1.21 | (0.97, 1.51) | 0.097 | 1.00 | (0.77, 1.30) | 0.988 |
| Non-cardiovascular comorbidity (yes–no) | 1.60 | (1.11, 2.31) | 0.011 | 1.37 | (0.93, 2.03) | 0.112 |
| History of anxiety or depression (yes–no) | 0.93 | (0.72, 1.22) | 0.614 | – | – | – |
| Treatment and disease characteristics | ||||||
| Systemic non-biologic therapy use (yes–no) | 1.31 | (0.82, 2.08) | 0.262 | 1.26 | (0.79, 2.03) | 0.334 |
| Topical therapy use (yes–no) | 1.00 | (0.80, 1.25) | 0.988 | – | – | – |
| Phototherapy use (yes–no) | 0.83 | (0.43, 1.62) | 0.587 | – | – | – |
| Duration of psoriasis (5-year increase) | 1.01 | (0.97, 1.05) | 0.735 | – | – | – |
| Biologic experience (naïve–experienced) | 0.71 | (0.57, 0.89) | 0.002 | 0.78 | (0.62, 0.98) | 0.034 |
| History of PsA (likely–unlikely) | 1.43 | (1.15, 1.79) | 0.002 | 1.27 | (0.99, 1.62) | 0.059 |
| Quality of life factors | ||||||
| VAS-skin pain (5-unit increase)a | 1.05 | (1.02, 1.09) | 0.001 | – | – | – |
| VAS-fatigue (5-unit increase)a | 1.03 | (1.01, 1.06) | 0.001 | 1.02 | (0.99, 1.04) | 0.155 |
| VAS-itch (5-unit increase)a | 1.04 | (1.02, 1.06) | < 0.001 | – | – | – |
| DLQI (5-unit increase)a | 1.72 | (1.46, 2.03) | < 0.001 | – | – | – |
| Clinically meaningful skin symptoms (yes–no)a,b | 1.63 | (1.20, 2.21) | 0.002 | 1.47 | (1.06, 2.03) | 0.020 |
BMI body mass index, CI confidence interval, DLQI Dermatology Life Quality Index, HR hazard ratio, PASI Psoriasis Area and Severity Index, VAS visual analogue scale
aCharacteristic was measured at index visit
bDefined as either having ≥ 45 on the skin pain VAS or ≥ 30 on the itch VAS
In the adjusted multivariable regression model, bio-naïve status was associated with a lower risk of loss of treatment response (HR = 0.78; 95% CI 0.62, 0.98), while presence of clinically meaningful skin symptoms at index was associated with a higher risk of loss of treatment response (HR = 1.47; 95% CI 1.06, 2.03). HRs in the adjusted model also suggested non-White race, full-time work status, greater body weight, and concomitant PsA were associated with loss of treatment response, although CIs included 1.00 (Table 3).
Bio-Naïve and Bio-Experienced Cohorts
At baseline, 52% (357/687) of patient initiations occurred among bio-naïve patients and 48% (330/687) in bio-experienced patients. About half of patients in both groups (40% of bio-naïve, 52% of bio-experienced) experienced a loss of treatment response over follow-up (Supplementary Material, Table S3).
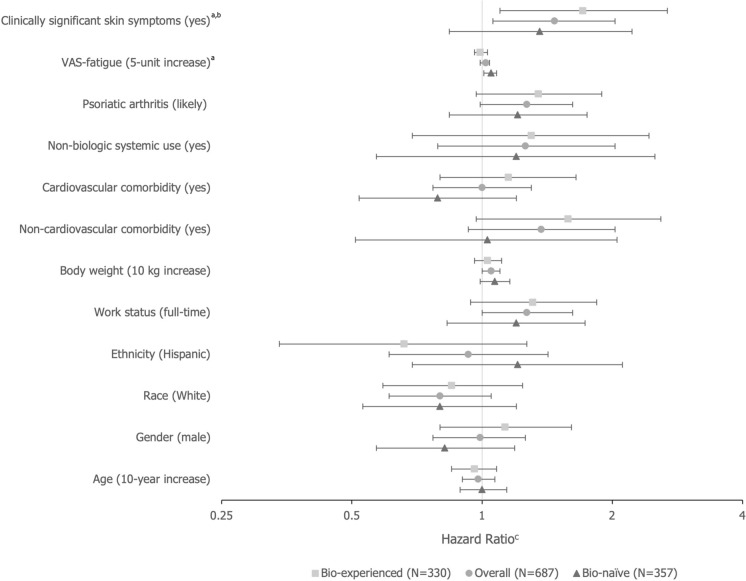
Associations between patient characteristics and loss of treatment response in the adjusted model for the bio-naïve and bio-experienced cohorts were similar to the overall population, with most CIs including 1.00. Exceptions were observed for fatigue (per 5-unit increase on 100-point VAS; HR = 1.05; 95% CI 1.01, 1.08) in the bio-naïve cohort and the presence of clinically meaningful skin symptoms at index (HR = 1.71; 95% CI 1.10, 2.68) in the bio-experienced cohort (Fig. 3).
Fig. 3.
Association between patient characteristics and loss of treatment response over 24 months of follow-up after achievement of PASI90 (adjusted model). aCharacteristic was measured at index visit; bDefined as either having ≥ 45 on the skin pain VAS or ≥ 30 on the itch VAS; cHazard ratios > 1 indicate factors that are more likely to be related to a loss of treatment response. PASI Psoriasis Area and Severity Index, VAS visual analog scale
Discussion
In this real-world study of patients with psoriasis who initiated a biologic therapy and achieved near-complete skin clearance at approximately 6 months follow-up from index, nearly half lost this level of response through 24 months of follow-up. Furthermore, the trend of loss of response from 6 to 18 months of follow-up was rapid, with maintenance of response dropping from nearly three-quarters of patient initiations to about half of patient initiations among those who achieved near-complete skin clearance. Additionally, patients who were bio-experienced or had clinically meaningful skin symptoms at the time of PASI90 achievement were more likely to lose treatment response over the follow-up period. Other patient characteristics and clinical factors that may predict loss of response were non-White race, full-time employment, greater body weight, and concomitant PsA.
Notably, a greater risk of loss of response was associated with such factors as increased DLQI (diminished HRQoL) and more burdensome disease symptoms (including fatigue), supporting the established connection between treatment effectiveness and improved patient satisfaction or QoL [1]. This has significant implications for dermatologists’ approaches to psoriasis treatment for their patients, as they would be more inclined to consider therapy comprehensively successful if resolution of physical skin symptoms is accompanied by improvements in QoL.
Near-complete skin clearance or better has become a reasonable target to measure effectiveness, as biologic therapies continue to demonstrate significant, rapid, and sustained improvements in skin outcomes [17, 18]. Randomized clinical trials have shown that patients with PASI90 response had a greater probability than those with PASI75 response of achieving meaningful improvements in patient-reported outcomes [19]. Near-complete skin clearance is increasingly being used as an endpoint in clinical trials for systemic biologic therapies and therefore the need to understand the maintenance of this response is important [3, 19].
Randomized clinical trials have explored the question of maintenance of near-complete skin clearance for specific biologic therapies up to 18 months post-treatment initiation, with some data showing associations between improved QoL-related patient-reported outcomes, such as DLQI, and other skin clearance measures [5, 7, 8]. As a result of different definitions for maintenance of response between these studies and the present study, comparisons would be inaccurate.
One previous real-world evidence study assessed the maintenance of near-complete skin clearance over 48 weeks, a duration shorter than the maximum of 24 months in the present study, in patients receiving guselkumab. This study demonstrated that nearly three-quarters of patients maintained the response at this timepoint [20], a slightly higher rate than that observed in this study at 12 months, although this study includes biologics other than guselkumab. Furthermore, a previous study reporting data from the DERMBIO registry investigated durability of response over 6 months in biologic-naïve patients treated with adalimumab, etanercept, infliximab, secukinumab, or ustekinumab. It was identified that greater body weight and smoking were associated with reduced treatment response [21]. However, to our knowledge, this is the first study to identify predictive risk factors for the durability of response over 24 months among patients with psoriasis.
Previous registry studies have also identified similar risk factors for treatment discontinuation. Notably, data from the British Association of Dermatologists Biologic Interventions Register (BADBIR) have identified a high baseline DLQI as a predictor of treatment discontinuation, in addition to female gender and current smoking status [22]. Furthermore, a high PASI at the time of switching to a second-line biologic therapy has been reported as a predictor of discontinuation [23], supporting findings in the current study in which patients with clinically meaningful skin symptoms were more likely to lose treatment response over follow-up. Similarly, the PSOriasis Longitudinal Assessment and Registry (PSOLAR) has reported lack of treatment effectiveness as the most common risk factor for biologic discontinuation [24]. Together, these real-world data may help to inform dermatologists in the treatment and management of patients with psoriasis.
Strengths and Limitations
A particular strength of the present study involves its focus on specific risk factors for loss of PASI90, which allows for easier identification of patients who may have difficulty maintaining near-complete skin clearance. Such information would be valuable to practicing dermatologists, who would be empowered to use patient-specific risk factors to individualize management of psoriasis disease severity in routine clinical practice.
However, generalizability of these findings may be limited as this is a US and Canadian registry and participation of dermatologists and patients is voluntary. Furthermore, only patients who did not experience biologic therapy interruptions for more than 180 days were included. Nevertheless, the CorEvitas Psoriasis Registry collects data from patients seen during routine dermatology visits at both academic and private practices, thus these patients are more likely to reflect the typical real-world patient population than those participating in clinical trials.
Registry data are collected prospectively and include extensive clinical data that are not available in other real-world data sources, such as disease activity and reasons for starting and stopping systemic therapies. This facilitated a more comprehensive definition of loss of response. However, some treatment failures or non-failures may have been misclassified if the reasons for therapy discontinuation were recorded as “other.”
Although this study was designed to identify predictive risk factors for the durability of response to biologic therapy, the study was not designed to be comparative and therefore specific conclusions relating to different classes and mechanisms of action of biologics cannot be drawn. The design of the registry (6-month intervals between registry visits) also results in some uncertainty in the precise timing of a loss of treatment response for any given patient. Finally, CIs for HRs of several risk factors were large, thus limiting the ability to identify some important predictors of durability of response.
Conclusion
Among real-world patients with psoriasis who achieved near-complete skin clearance (PASI90) with systemic biologic therapy, about one-quarter lost response at 6 months, and half lost response at 18 months. Prior use of a biologic therapy and clinically meaningful skin symptoms at index, including itch and skin pain, were associated with a decreased likelihood of maintaining a treatment response that included near-complete skin clearance. Therefore, dermatologists may consider focusing on patient-reported symptoms as a part of any intervention designed to reduce the likelihood of loss of response to biologic therapies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all of the investigators, their clinical staff and the patients who participate in the CorEvitas Psoriasis Registry.
Medical Writing/Editorial Assistance.
The authors also acknowledge Patrick Reilly, BS, and Aaron Keeling, BA, Costello Medical, Boston, Massachusetts, USA, for medical writing and editorial assistance based on the authors’ input and direction.
Author Contributions
Access to study data was limited to CorEvitas, LLC and CorEvitas, LLC statisticians completed all analyses; all authors contributed to the interpretation of the results. Substantial contributions to study conception and design: Robert R. McLean, Adam P. Sima, Eric A. Jones, Thomas Eckmann, Rebecca L. Spitzer; substantial contributions to analysis and interpretation of the data: Robert R. McLean, Adam P. Sima, Silky Beaty, Eric Jones, Thomas Eckmann, Robert Low, Laura McClung, Rebecca L. Spitzer, Jeffrey Stark, April Armstrong; drafting the article or revising it critically for important intellectual content: Robert R. McLean, Adam P. Sima, Silky Beaty, Eric Jones, Thomas Eckmann, Robert Low, Laura McClung, Rebecca L. Spitzer, Jeffrey Stark, April Armstrong; final approval of the version of the article to be published: Robert R. McLean, Adam P. Sima, Silky Beaty, Eric Jones, Thomas Eckmann, Robert Low, Laura McClung, Rebecca L. Spitzer, Jeffrey Stark, April Armstrong.
Funding
This study was sponsored by CorEvitas, LLC, and the analysis was funded by UCB Pharma, 1950 Lake Park Drive, Smyrna, GA, USA 30080. Medical writing and editorial assistance, and publication fees (including rapid service and open access) were funded by UCB Pharma.
Data Availability
Data are CorEvitas, LLC through a commercial subscription agreement and are not publicly available. No additional data are the authors.
Declarations
Conflict of Interest
Robert R. McLean, Adam P. Sima, Eric A. Jones, Thomas Eckmann, Rebecca L. Spitzer: Employees of CorEvitas; Silky Beaty, Robert Low, Laura McClung, Jeffrey Stark: Employees and shareholders of UCB Pharma; April Armstrong: Has served as a research investigator and/or scientific advisor to AbbVie, Almirall, Arcutis, ASLAN, Boehringer Ingelheim, BMS, Dermavant, Dermira, Eli Lilly, EPI, Incyte, Janssen, LEO Pharma, Nimbus, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sun Pharma, Sanofi, and UCB Pharma; CorEvitas, LLC, has been supported through contracted subscriptions in the last two years by AbbVie, Amgen, Arena, Boehringer Ingelheim, BMS, Celgene, Chugai, Eli Lilly, Genentech, Gilead, GSK, Janssen, LEO Pharma, Novartis, Ortho Dermatologics, Pfizer, Regeneron, Sanofi, Sun Pharma, and UCB Pharma. The CorEvitas Psoriasis Registry was developed in collaboration with the National Psoriasis Foundation.
Ethical Approval
This study was performed following the guidelines for Good Pharmacoepidemiology Practice [12] and in accordance with the current version of the applicable regulatory and International Council for Harmonisation Good Clinical Practice requirements [13], the ethical principles that have their origin in the principles of the Declaration of Helsinki, and the local laws of the countries involved. All patients provided written informed consent for participation in the registry. All participating investigators were required to obtain full board approval for conducting non-interventional research involving human subjects with a limited dataset. Sponsor (CorEvitas, LLC) approval and continuing review was obtained through a central IRB (IntegReview, protocol number is Corrona-PSO-500). For academic investigative sites that did not receive a waiver to use the central IRB, full board approval was obtained from the respective governing IRBs, and documentation of approval was submitted to CorEvitas, LLC, prior to the initiation of any study procedures.
References
- 1.Van Cranenburgh O, De Korte J, Sprangers M, De Rie M, Smets E. Satisfaction with treatment among patients with psoriasis: a web-based survey study. Br J Dermatol. 2013;169(2):398–405. doi: 10.1111/bjd.12372. [DOI] [PubMed] [Google Scholar]
- 2.Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4(1):1–7. doi: 10.1186/1477-7525-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puig L. PASI 90 response: the new standard in therapeutic efficacy for psoriasis. J Eur Acad Dermatol Venereol. 2015;29(4):645–648. doi: 10.1111/jdv.12817. [DOI] [PubMed] [Google Scholar]
- 4.Reich K, Gordon K, Strober B, et al. Five-year maintenance of clinical response and health-related quality of life improvements in patients with moderate-to-severe psoriasis treated with guselkumab: results from VOYAGE 1 and VOYAGE 2. Br J Dermatol. 2021;185(6):1146–1159. doi: 10.1111/bjd.20568. [DOI] [PubMed] [Google Scholar]
- 5.Reich K, Griffiths CE, Gordon KB, et al. Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: results from the VOYAGE 1 and VOYAGE 2 trials. J Am Acad Dermatol. 2020;82(4):936–945. doi: 10.1016/j.jaad.2019.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Kotb I. Evidence for the long-term efficacy and safety of guselkumab. Br J Dermatol. 2021;185(6):1087–1088. doi: 10.1111/bjd.20716. [DOI] [PubMed] [Google Scholar]
- 7.Gordon KB, Armstrong AW, Foley P, et al. Guselkumab efficacy after withdrawal is associated with suppression of serum IL-23-regulated IL-17 and IL-22 in psoriasis: VOYAGE 2 study. J Invest Dermatol. 2019;139(12):2437–2446.e1. doi: 10.1016/j.jid.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Menter A, Feldman SR, Weinstein GD, et al. A randomized comparison of continuous vs intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31e1–e15. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Strober B, Karki C, Mason M, et al. Characterization of disease burden, comorbidities, and treatment use in a large, US-based cohort: results from the Corrona Psoriasis Registry. J Am Acad Dermatol. 2018;78(2):323–332. doi: 10.1016/j.jaad.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 10.National Psoriasis Foundation. CorEvitas (formerly Corrona) Psoriasis Patient Registry; 2015 [updated 2022]. https://www.psoriasis.org/corevitas-psoriasis-patient-registry/. Accessed 29 June 2022.
- 11.Clinicaltrials.gov. The Corrona Psoriasis (PSO) Registry: National Library of Medicine; 2016 [updated 2022]. https://clinicaltrials.gov/ct2/show/NCT02707341. Accessed 29 June 2022.
- 12.Public Policy Committee International Society of Pharmacoepidemiology Guidelines for good pharmacoepidemiology practice (GPP) Pharmacoepidemiol Drug Saf. 2016;25(1):2–10. doi: 10.1002/pds.3891. [DOI] [PubMed] [Google Scholar]
- 13.International Council for Harmonisation Integrated addendum to ICH E6 (R1): guideline for good clinical practice E6 (R2) Curr Step. 2015;2:1–60. [Google Scholar]
- 14.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4(7):407–414. doi: 10.1016/S1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 15.Reich A, Chatzigeorkidis E, Zeidler C, et al. Tailoring the cut-off values of the visual analogue scale and numeric rating scale in itch assessment. Acta Derm Venereol. 2017;97(6–7):759–760. doi: 10.2340/00015555-2642. [DOI] [PubMed] [Google Scholar]
- 16.Turnbull BW. Nonparametric estimation of a survivorship function with doubly censored data. J Am Stat Assoc. 1974;69(345):169–173. doi: 10.1080/01621459.1974.10480146. [DOI] [Google Scholar]
- 17.Schmitt-Egenolf M. Complete skin clearance and beyond. Br J Dermatol. 2021;184(1):3. doi: 10.1111/bjd.19544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strober B, Papp KA, Lebwohl M, et al. Clinical meaningfulness of complete skin clearance in psoriasis. J Am Acad Dermatol. 2016;75(1):77–82.e7. doi: 10.1016/j.jaad.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 19.Manalo IF, Gilbert KE, Wu JJ. Time to raise the bar to psoriasis area severity index 90 and 100. J Drugs Dermatol. 2015;14(10):1086–1088. [PubMed] [Google Scholar]
- 20.Mastorino L, Siliquini N, Avallone G, et al. Guselkumab shows high efficacy and maintenance in the improvement of response until week 48, a real-life study. Dermatol Ther. 2022;35:e15670. doi: 10.1111/dth.15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwarz CW, Loft N, Rasmussen MK, et al. Predictors of response to biologics in patients with moderate-to-severe psoriasis: a Danish Nationwide Cohort Study. Acta Derm Venereol. 2021;101(10):adv00579. doi: 10.2340/actadv.v101.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren RB, Smith CH, Yiu ZZN, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Invest Dermatol. 2015;135(11):2632–2640. doi: 10.1038/jid.2015.208. [DOI] [PubMed] [Google Scholar]
- 23.Iskandar IYK, Warren RB, Lunt M, et al. Differential drug survival of second-line biologic therapies in patients with psoriasis: observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Invest Dermatol. 2018;138(4):775–784. doi: 10.1016/j.jid.2017.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menter A, Papp KA, Gooderham M, et al. Drug survival of biologic therapy in a large, disease-based registry of patients with psoriasis: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) J Eur Acad Dermatol Venereol. 2016;30(7):1148–1158. doi: 10.1111/jdv.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are CorEvitas, LLC through a commercial subscription agreement and are not publicly available. No additional data are the authors.