Abstract
To study the substrate specificity of the oligopeptide transport system of Lactococcus lactis for its natural substrates, the growth of L. lactis MG1363 was studied in a chemically defined medium containing milk peptides or a tryptic digest of αs2-casein as the source of amino acids. Peptides were separated into acidic, neutral, and basic pools by solid-phase extraction or by cation-exchange liquid chromatography. Their ability to sustain growth and the time course of their utilization demonstrated the preferential use of hydrophobic basic peptides with molecular masses ranging between 600 and 1,100 Da by L. lactis MG1363 and the inability to use large, acidic peptides. These peptide utilization preferences reflect the substrate specificity of the oligopeptide transport system of the strain, since no significant cell lysis was inferred. Considering the free amino acid content of milk and these findings on peptide utilization, it was demonstrated that the cessation of growth of L. lactis MG1363 in milk was due to deprivation of leucine and methionine.
Lactococci have a limited capacity to synthesize amino acids (1). The amino acid requirements appear to be strain specific, but most Lactococcus lactis strains need at least Leu, Ile, Val, Met, and His for growth. Moreover, the addition of several other amino acids to a chemically defined medium (CDM) was found to be growth stimulatory (15, 22), indicating that the rate of their biosynthesis is too low to sustain a high growth rate. Consequently, optimal growth of L. lactis depends on the amino acid availability of the culture medium. In milk, three different sources of amino acids are used by lactococci, namely, free amino acids, peptides, and caseins.
Lactococci possess at least nine different amino acid transport systems, which theorically allow the strains to use all of the different amino acids in milk (19). However, the concentrations of several free amino acids, especially those of the essential amino acids Ile, Leu, and Met, are very low in milk. As a result, the use of the free amino acids in milk as the sole source of nitrogen allows lactococci to grow to low cell densities (about 3 × 107 CFU/ml) (8).
Utilization of milk peptides requires the concerted action of a peptide transport system(s) and peptidases. The intracellular localization of peptidases is now well accepted, which means it is necessary for peptide translocation to occur prior to intracellular hydrolysis (11). Three different peptide transport systems have been identified in lactococci (2, 23, 27). Inactivation of the two di- and tripeptide transport systems does not result in a significant growth defect in milk. In contrast, the oligopeptide transport (Opp) system has been shown to play a crucial role in milk peptide utilization (8).
Casein utilization by lactococci has been extensively studied in recent years, since it represents the main source of amino acids for growth in milk (for recent reviews, see references 10 and 21). However, casein utilization proceeds only during the last four generations, corresponding to a 10-fold increase in population density, compared to the growth of proteinase-negative (Prt−) strains (8, 17).
At the end of the growth of Prt− strains in milk, the amino acids and peptides in the milk are clearly not exhausted (8). However, the residual peptides are unable to sustain further growth (9). The reason why only some of the milk peptides are used during growth remains unknown. No clear data are available on the substrate specificity of the Opp system. Previous studies have been performed using model peptides, which do not necessarily mimic the composition of milk peptides. In fact, the size restriction of the Opp system was initially thought to be 8 residues (27), whereas evidence for translocation of β-casein-derived peptides containing up to at least 10 residues has been reported recently (10). To study further the size restriction of the Opp system and its natural substrate specificity, the assimilable peptides in milk have been analyzed. A study of the time course of their utilization during the growth of L. lactis MG1363 provides evidence for the preferential use of specific classes of peptides.
MATERIALS AND METHODS
Strain and culture conditions.
Proteinase-negative, plasmid-free L. lactis subsp. lactis MG1363 (3) was stored at −80°C in M17 broth (26) containing glucose (0.5% [wt/vol]) and glycerol (10% [wt/vol]). Cells were grown at 30°C in reconstituted skim milk (10% Nilac Low Heat milk powder; Netherlands Dairy Research Institute, Ede, The Netherlands) supplemented with glucose (1% [wt/vol]) or in CDM (20) containing peptides isolated from milk as the source of amino acids. The peptide concentration used in the CDM was about five times that of the peptides in milk, unless otherwise stated. Growth media were inoculated to approximately 106 CFU/ml with a preculture in the exponential stage of growth. The strain was precultured in M17 broth, washed twice in 50 mM KH2PO4-K2HPO4 (pH 6.8), and resuspended in CDM deprived of amino acids and peptides prior to inoculation.
Bacterial enumeration and statistical analysis.
All growth experiments were repeated four times, unless otherwise stated. Cell populations were estimated by spiral plating of appropriate dilutions on M17 agar. The accuracy and precision of this plating method have been assessed previously (5). Confidence limits (P = 0.95) of the means were calculated as described by Snedecor and Cochran (24).
Peptide isolation.
Milk proteins were precipitated by lowering the pH to 4.6 with HCl. After removal of the proteins by centrifugation (10,000 × g for 10 min at 4°C), the supernatant was ultrafiltered through a 10,000-Da cutoff membrane (YM10; Amicon Corp., Beverly, Mass.), unless otherwise stated. Peptides were then isolated from the ultrafiltrate by C18 solid-phase extraction using reverse-phase cartridges (Sep-Pak C18; Waters, Milford, Mass.). In some experiments, high-molecular-weight peptides were additionally discarded by performing a second ultrafiltration through a 1,000-Da cutoff membrane (YM1; Amicon). Ultimate fractionation of the peptides was achieved by solid-phase extraction on weak cation-exchanger and strong anion-exchanger cartridges (Sep-Pak Accell CM and Accell QMA, respectively; Waters) or by strong cation-exchange liquid chromatography (Waters Protein Pak FP8HR; Waters). Peptide concentration was estimated by the method of Lowry et al. (14), using bovine serum albumin as the standard, or by measuring the amino acid composition by ion-exchange chromatography with an LC 5000 amino acid analyzer (Biotronik, Eppendorf, Maintal, Germany) after acidic hydrolysis of the peptide mixture under vacuum in the presence of 5.7 N HCl for 24 h at 110°C. Acidic hydrolysis resulted in the conversion of Gln and Asn into Glu and Asp, respectively, whereas Trp was not detected. Nevertheless, both methods yielded the same results.
Peptide analysis.
Cells were removed from the CDM by centrifugation (10,000 × g for 10 min at 4°C), and the supernatant was filtered through a 0.22-μm-pore-size low-binding protein filter (Millipore Corp., Bedford, Mass.). The peptides were separated at 40°C by high-performance liquid chromatography (HPLC) on a reverse-phase C18 column (Nucleosil; 4.6 mm [inside diameter] by 250 mm; Shandon HPLC, Cheshire, United Kingdom). Solvents A and B were 0.11% (vol/vol) trifluoroacetic acid in MilliQ water and 0.1% (vol/vol) trifluoroacetic acid–60% (vol/vol) acetonitrile in MilliQ water, respectively. A 5-min isocratic phase in solvent A was followed by a linear gradient of 0 to 100% (vol/vol) solvent B in 40 min with a flow rate of 1 ml/min. Eluted peptides were detected simultaneously by using on-line A214 (prior to derivatization) and fluorescence after postcolumn derivatization of the eluted peptides with o-phthalaldehyde, as previously described (7). For fluorescence detection, the excitation and emission wavelengths were 340 and 425 nm, respectively.
Tryptic digestion of αs2-casein.
Whole casein was obtained by acidic precipitation of defatted milk obtained from a cow selected for production of homozygote caseins. αs2-Casein (A variant) was purified by DEAE-cellulose urea chromatography as previously described (16). The protein (5 mg in 3 ml of 100 mM phosphate buffer, pH 7.5) was digested by incubation for 5 h at 37°C with l-1-tosylamide-2-phenylethyl chloromethyl ketone-treated trypsin (Serva, Heidelberg, Germany) at a concentration of 5.1 U/mg of αs2-casein. Trypsin was inactivated by boiling the reaction mixture for 10 min. Peptides released from αs2-casein were separated by HPLC as described above and identified by mass spectrometry and sequence analysis.
Degradation of the αs2-casein tryptic digest by the pool of intracellular enzymes of L. lactis MG1363 was analyzed by HPLC as described above, following 3 h of incubation of the peptides with a cell extract at a concentration equivalent to 107 CFU/ml. Cell extract was obtained by mechanical disruption of growing cells with glass beads (Mini-Beadbeater-8 cell disrupter; Biospec Products, Bartlesville, Okla.) and centrifugation of the disrupted cells (10,000 × g for 10 min at 4°C).
Estimation of cell lysis.
Lysis of the cells during growth in CDM was estimated from the release of the intracellular aminopeptidase PepX into the growth medium (8). PepX activity was checked by using l-Ala-l-Pro-p-nitroanilide as the chromogenic substrate. After removal of the cells from the CDM by centrifugation (10,000 × g for 10 min at 4°C), the supernatant was tested for the presence of PepX by monitoring the liberation of p-nitroanilide from 0.25 mM substrate at 37°C by measuring the ΔA405. PepX activity was compared with that of a cell extract. The sensitivity threshold of this method was about 106 lysed cells per ml.
RESULTS
Growth of L. lactis in milk.
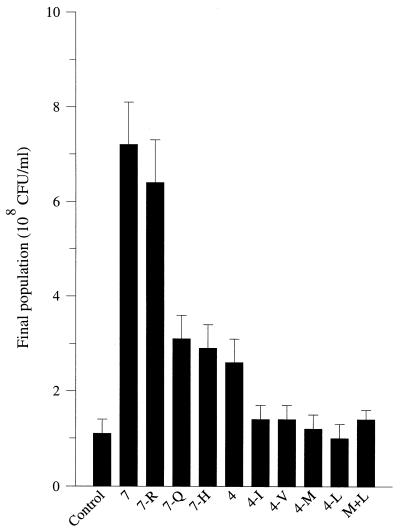
Previous studies (8) have demonstrated that the amino acids and peptides in milk are clearly not exhausted at the end of the growth of L. lactis Prt− strains. To determine which amino acid deficiency is responsible for the growth arrest, L. lactis MG1363 was grown in milk supplemented with different mixtures of free amino acids, the concentrations of which corresponded to those of CDM (20). The addition of a mixture containing all of the seven amino acids essential for L. lactis MG1363 (i.e., Arg, Met, Leu, Ile, Val, Gln, and His) resulted in a sevenfold increase in the maximal population density of the strain (Fig. 1). The extent of the stimulation was not really affected when Arg was removed from the added mixture, whereas removal of Gln or His reduced the extent of the stimulation, with a 2.7-fold increase in the maximal population density. The extent of the stimulation was in the same range (a 2.5-fold increase in population density) when only Met, Ile, Leu, and Val were added to the milk. Nevertheless, the presence of Met and Leu was required for stimulation. In fact, addition of these two amino acids to the milk resulted in significant (P = 0.95) stimulation, with a 30% increase in the final population density.
FIG. 1.
Final population densities of L. lactis MG 1363 grown in milk supplemented with free amino acids. Control; no addition; 7, addition of a mixture of seven amino acids, i.e., Arg (R; 0.7 mM), Gln (Q; 2.7 mM), His (H; 0.7 mM), Ile (I; 1.6 mM), Met (M; 0.8 mM), Leu (L; 3.6 mM), and Val (V; 2.8 mM); 7-R, 7-Q, and 7-H, mixture of six amino acids (same concentrations as before); 4, mixture of four amino acids, i.e., Met, Ile, Leu, and Val (same concentrations as before). The values are means of four determinations (the error bars show confidence limits; P = 0.95).
Utilization of milk peptides during growth.
Milk peptides were isolated from milk by isoelectric precipitation, ultrafiltration through a 10,000-Da cutoff membrane, and C18 solid-phase extraction and used as a source of amino acids in CDM. The nitrogenous material concentration after each isolation step is given in Table 1. The reduction in nitrogen concentration during the solid-phase extraction corresponded almost exactly to the reported free amino acid concentration present in the milk (84 ± 5 μg/ml) (8), suggesting that no significant loss of peptides occurred during this step.
TABLE 1.
Milk peptide isolation
| Isolation step | Nitrogenous materials | Nitrogenous material concn (μg/ml)a |
|---|---|---|
| Milk | Amino acids, peptides, proteins | 30 × 103 |
| Acidic precipitation | Amino acids, peptides, soluble proteins | (7.6 ± 1.3) × 103 |
| Ultrafiltrationb | Amino acids, peptides | 124 ± 29 |
| C18 solid-phase extraction | Peptides | 45 ± 9 |
Mean of four determinations ± confidence limits; P = 0.95.
Molecular size cutoff, 10,000 Da.
To evaluate the yield of the peptide isolation procedure, L. lactis MG1363 was grown in CDM containing milk peptides (45 μg/ml, i.e., the concentration measured in milk) and a mixture of amino acids whose composition and concentration mimicked those of the amino acids in milk. The maximal population density was 45% of that found in milk ([4.5 ± 2.9] × 107 CFU/ml compared with [1.0 ± 0.1] × 108 CFU/ml; mean of four determinations ± the confidence limits at P = 0.95). This suggested a loss of nitrogenous material during the isolation procedure. Additional experiments demonstrated that the loss of material occurred during the casein precipitation procedure, presumably as a consequence of coprecipitation of low-molecular-weight peptides together with milk proteins (data not shown).
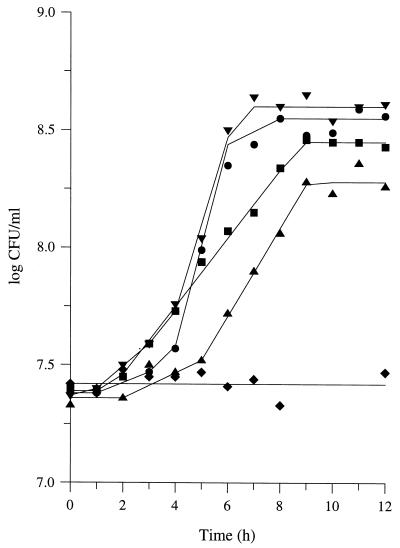
Milk peptides were added to the CDM as the sole source of amino acids at final concentrations about five times higher than those in milk. Surprisingly, L. lactis MG1363 was unable to grow. Significant growth was obtained when the medium was supplemented with Gln and His (Fig. 2). Nevertheless, acidic hydrolysis of the milk peptides revealed that they contained all of the amino acids (except, maybe, the nonessential amino acid Trp), including Glu/Gln and His. Similar results were obtained when a molecular mass cutoff of either 3 or 30 kDa, instead of 10 kDa, was used. These results, therefore, indicated that (i) milk peptides are unable to supply all of the amino acids required for the growth of L. lactis and (ii) not all milk peptides were used as a source of amino acids. In contrast, the use of only the lowest-molecular-weight peptides (isolated by a second ultrafiltration step through a 1,000-Da cutoff membrane) significantly decreased the final population density (Table 2). It therefore suggests that L. lactis MG1363 is able to translocate peptides which are retained by the 1,000-Da cutoff membrane.
FIG. 2.
Growth of L. lactis MG 1363 in CDM containing milk peptides as the main source of amino acids. Symbols: ⧫, milk peptides as the sole source of amino acids; ▴, ▪, •, and ▾, milk peptides supplemented with Gln and His; Gln, His, and Leu; Gln, His, and Met; and Gln, His, Leu, and Met; respectively. Peptides were isolated from milk by isoelectric precipitation, ultrafiltration through a 10,000-Da molecular size cutoff membrane, and C18 solid-phase extraction. The peptide concentration in the growth medium was five times that in milk (i.e., 225 μg/ml). Amino acid concentrations were the same as in Fig. 1.
TABLE 2.
Final population densities of L. lactis MG 1363 in CDM containing different pools of milk peptidesa
| Amino acid source in CDM | Final population size (CFU/ml)b with peptides having a molecular size of:
|
||
|---|---|---|---|
| 30 kDa | 10 kDa | 1 kDa | |
| Peptides alone | NGc | NG | NG |
| Peptides + Q, H, L, M | (1.9 ± 0.9) × 108 | (2.4 ± 0.5) × 108 | (3.7 ± 1.4) × 107 |
| Acidic peptides + Q, H, L, M | NDd | NG | NG |
| Neutral peptides + Q, H, L, M | ND | (6.3 ± 3.2) × 107 | (3.0 ± 2.2) × 107 |
| Basic peptides + Q, H, L, M | ND | (1.4 ± 0.3) × 107 | (5.9 ± 2.0) × 106 |
Peptides were isolated from milk by isoelectric precipitation, ultrafiltration, and C18 solid-phase extraction and added to CDM at final concentrations five times those of the peptides in milk, i.e., 500 μg/ml for the 30-kDa pool, 225 μg/ml for the 10-kDa pool, and 20 μg/ml for the 1-kDa pool. Gln (Q), His (H), Leu (L), and Met (M) were added at concentrations of 2.7, 0.7, 3.6, and 0.8 mM, respectively. Acidic, neutral, and basic peptides were obtained from the whole peptide pool by ion-exchange solid-phase extraction and used at final concentrations five times those of the peptides in milk. Respective ratios of the three peptides fractions were 1:1.5:1.5 in the 10-kDa pool and 0:24:1 in the 1-kDa fraction, respectively.
The values shown are means of six experiments ± the confidence limits (P = 0.95).
NG, no growth (inoculation level of CDM was about 5 × 105 CFU/ml).
ND, not determined.
Fractionation of milk peptides.
To analyze further the nature of milk peptides used during the growth of L. lactis, the pool of milk peptides (molecular mass cutoff, 10 kDa) was separated into acidic, basic, and neutral fractions by ion-exchange solid-phase extraction, with a yield of about 80%. Basic and neutral fractions contained the same amount of peptides (13 mg/liter), whereas that of the acidic fraction was lower (8.5 mg/liter). Each peptide fraction still contained every amino acid (except, maybe, Trp), as indicated by amino acid composition analysis after acidic hydrolysis of the pool of peptides. As expected, none of the fractions was able to promote growth in the absence of free amino acids (Table 2). More surprising was the inability of the acidic fraction to sustain growth even in the presence of additional Gln, His, Leu, and Met. Significant growth was observed only when six of the seven essential amino acids (i.e., Gln, His, Leu, Ile, Val, and Met) were added to the acidic peptide fraction (data not shown). In contrast, basic and neutral fractions were able to sustain growth when supplemented with the essential amino acids Gln, His, Leu, and Met. However, the sum of the population densities reached with the different peptide fractions was consistently lower than that obtained when the unfractionated pool of peptides was used (7.7 × 107 and 2.4 × 108 CFU/ml, respectively).
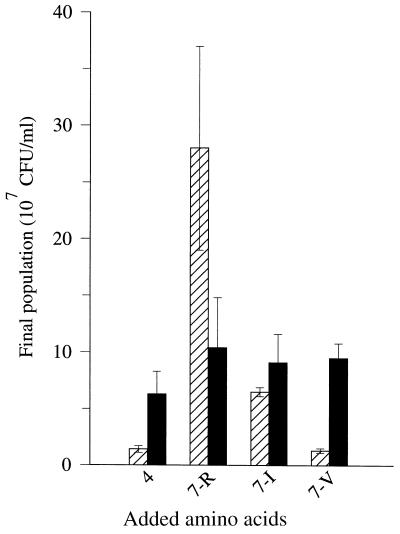
To explain this apparent discrepancy, different mixtures of amino acids were added to basic or neutral peptide fractions (Fig. 3). The addition of a mixture of essential amino acids deprived of valine to the basic fraction did not stimulate growth to a larger extent than did the addition of a mixture of Gln, His, Leu, and Met. This finding indicated that basic peptides represent a poor source of Val. In contrast, basic peptides provide a large amount of Arg, as addition of a mixture of amino acids deprived of Arg resulted in a 20-fold increase in population density. On the other hand, neutral peptides provide equal amounts of Arg, Val, and Ile, since addition of a mixture of essential amino acids deprived of Arg, Val, or Ile yielded the same level of growth stimulation. This suggests that the two peptide fractions have a synergistic effect on the growth of L. lactis MG1363, the basic peptide fraction representing the main source of Arg and the neutral peptide fraction containing most of the Val-containing peptides.
FIG. 3.
Final population densities of L. lactis MG1363 grown in CDM containing basic (▨) or neutral (▪) peptides isolated from milk and supplemented with different mixtures of amino acids. 4, mixture of four amino acids, i.e., Met, Ile, Leu, and Val. 7-R, 7-I, and 7-V, addition of a mixture of six amino acids among the following, i.e., Arg (R); Gln, His, and Ile (I); and Met, Leu, and Val (V). Amino acid concentrations were the same as in Fig. 1. The values are means of four determinations (the error bars show confidence limits; P = 0.95). Peptides were isolated from milk by isoelectric precipitation, ultrafiltration through a 10,000-Da molecular size cutoff membrane, C18 solid-phase extraction, and ion-exchange solid-phase extraction. The peptide concentration in the growth medium was five times that in milk, i.e., 65 μg/ml for each pool.
Low-molecular-weight milk peptide utilization.
Low-molecular-weight peptides were isolated from the acidic, basic, and neutral fractions by a second ultrafiltration procedure (molecular mass cutoff of 1,000 Da). Surprisingly, no significant amount of peptides could be detected in the acidic fraction, whereas 96 and 4% of the low-molecular-weight peptides were found in the neutral and basic fractions, respectively. Again, no growth was detected when basic or neutral peptides were used as the sole source of amino acids. Addition of a mixture of Gln, His, Leu, and Met to the culture medium enhanced growth (Table 2). About 15% of the growth measured on the whole low-molecular-weight fraction (i.e., without fractionation into acidic, basic, and neutral pools) was due to the use of the basic peptides, although this fraction represented only 4% of the pool of low-molecular-weight peptides.
Because 96% of the low-molecular-weight peptides of milk were present in the neutral fraction when a weak cation-exchanger cartridge was used, a more discriminating procedure for peptide fractionation than solid-phase extraction was developed. Basic peptides were isolated from the pool of low-molecular-weight peptides by using a strong cation-exchanger HPLC column. About 55% of the low-molecular-weight peptides were retained by the column, indicating more selective retention of basic peptides. Retained peptides were used as a source of amino acids (together with free Gln, His, Leu, and Met) in CDM at a final concentration of 40 μg/ml (i.e., 18 times their concentration in milk). The final population of L. lactis MG1363 was (1.1 ± 0.6) × 108 CFU/ml (mean of four experiments ± the confidence limits; P = 0.95). On the other hand, 72 μg of the whole of the low-molecular-weight peptides per ml (i.e., prior to HPLC fractionation, the concentration being 18 times that in milk) together with the four free amino acids promoted the growth of L. lactis MG1363 to a final cell density of (1.4 ± 0.2) × 108 CFU/ml (mean of four experiments ± confidence limits; P = 0.95). Thus, basic peptides, which represented 55% of the low-molecular-weight peptides of milk, were responsible for about 80% of the growth.
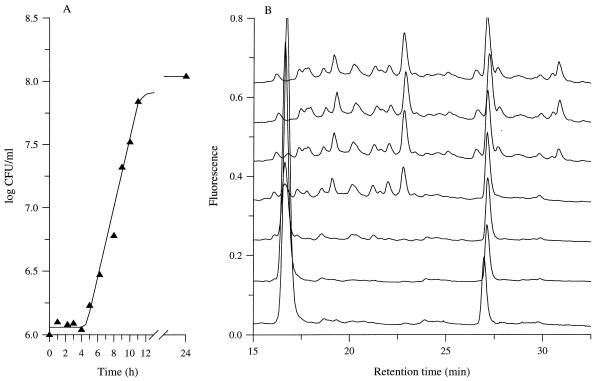
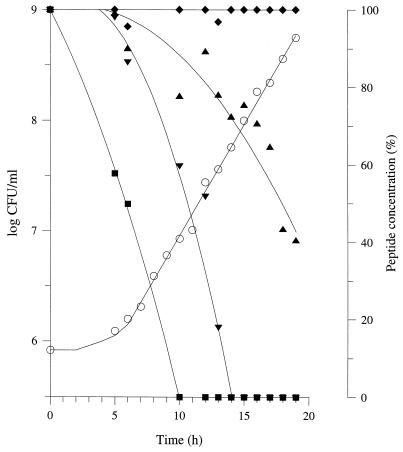
During the growth of L. lactis MG1363 in CDM containing the basic low-molecular-weight peptides (plus Gln, His, Leu, and Met) as nitrogen sources, the peptide content of the culture medium was analyzed by reverse-phase HPLC (Fig. 4). At the end of growth, the growth medium was clearly depleted of many peptides, although no release of PepX into the growth medium was detected during the exponential growth phase. After 8 h of incubation (i.e., corresponding to the mid-exponential growth phase), the culture medium was almost completely depleted of hydrophobic peptides (retention time longer than 23 min), whereas hydrophilic peptides (retention time less than 23 min) were not significantly utilized.
FIG. 4.
Growth of L. lactis MG1363 in CDM containing basic low-molecular-weight peptides isolated from milk and supplemented with Gln, His, Leu, and Met as the source of amino acids. A, Growth kinetics; B, peptide content of the growth medium (from the top to the bottom, after 0, 4, 6, 8, 10, 11, and 24 h of incubation). Peptides were isolated from milk with a five-step procedure (isoelectric precipitation, 30-kDa ultrafiltration, C18 solid-phase extraction, 1-kDa ultrafiltration, and cation-exchange liquid chromatography) and added to CDM at 40 μg/ml (i.e., 18 times the concentration in milk). Amino acid concentrations were the same as in Fig. 1. Peptide content of the growth medium was estimated by reverse-phase HPLC and fluorescence detection after postcolumn derivatization of the peptides with o-phthalaldehyde.
Utilization of a tryptic digest of αs2-casein as the peptide source.
To confirm the preferential use of hydrophobic basic peptides by L. lactis during growth, similar experiments were performed with a tryptic digest of αs2-casein supplemented with free Gln, His, Leu, and Met. The peptide content of the tryptic digest was identified by mass spectrometry analysis and sequencing (Table 3). On the basis of the time course of their consumption during growth (Fig. 5), three main classes of peptides can be distinguished: (i) the di- and tripeptides, which exhibited the highest rate of utilization; (ii) the oligopeptides, which were not used during growth; and (iii) the oligopeptides, which were consumed, the rates of consumption (expressed as the time at which 50% of the peptide was consumed) varying with the peptides (Table 3). Oligopeptides containing 11 residues were consumed at a higher rate than the tetrapeptides, suggesting that the size of the peptide was not the only criterion that determines the rate of utilization. All of the peptides which were not utilized at the end of growth were large, acidic phosphopeptides (i.e., containing phosphoserine residues), whose molecular masses ranged between 1,464 and 3,152 Da.
TABLE 3.
Peptide contents of the tryptic digest of αs2-casein
| Peptide sequence | Retention time (min) | Molecular mass (Da) | pI | T 50% (h)a |
|---|---|---|---|---|
| YQK | 15.3 | 437.3 | 10.1 | 11.89 |
| ISQR | 15.7 | 502.3 | 11.3 | 14.19 |
| HYQK | 15.7 | 574.3 | 10.3 | 14.19 |
| EVVR | 18.9 | 501.3 | 11.3 | 16.42 |
| LTEEEK | 19.4 | 747.4 | 4.1 | 16.42 |
| TVYQHQKb | 20.9 | 902.5 | 10.3 | 12.51 |
| EQLZTZEENSKKc | 20.9 | 1,538.6 | 4.7 | NDd |
| ITVDDK | 23.7 | 689.4 | 4 | 14.56 |
| YL | 26.7 | 294.2 | 6.1 | 5.70 |
| NMAINPSK | 27.5 | 873.5 | 10.4 | 12.29 |
| NANEEEYSIGZZZEEZAEVATEEVKb | 33.2 | 3,007.1 | 3.9 | NCe |
| AM*KPWIQPKc | 33.2 | 1,114 | 10.9 | ND |
| VIPYb | 33.8 | 490.3 | 6 | 15.14 |
| PWIQPKc | 33.8 | 767.5 | 10.4 | ND |
| KNTMEHVZZZEESIIZQETYKQEKb | 35.1 | 3,132.3 | 4.5 | NC |
| KNTMEHVZZZEESIIZQETYKb | 35.1 | 2,747.1 | 4.4 | NC |
| TVDMESTEVFTKKc | 35.1 | 1,593.4 | 4.5 | NC |
| TVDMEZTEVFTKKb | 35.8 | 1,465.6 | 3.9 | NC |
| LNFLKKb | 35.8 | 761.5 | 10.9 | 10.89 |
| RNAVPITPTLNRc | 35.8 | 1,350.8 | 12.6 | ND |
| NAVPITPTLNR | 36.3 | 1,194.7 | 11.3 | 13.57 |
| AMKPWIQPK | 36.9 | 1,097.6 | 10.9 | 12.19 |
| LNFLK | 38.6 | 633.4 | 10.4 | 10.43 |
| ALNEINQFYQK | 39.6 | 1,366.7 | 7.1 | 13.55 |
| NMAINPZKENLCSTFCK | 41.8 | 1,977.6 | 8.3 | 18.13 |
| FPQYLQY | 43.6 | 957.5 | 6 | 13.0 |
| FALPQYLK | 45.5 | 978.6 | 10 | 11.63 |
| LYQGPIVLNPWDQVK | 50.1 | 1,769 | 8.6 | 14.56 |
| FPQYLQYLYQGPIVLNPWDQVK | 59.7 | 2,708.4 | 8 | 14.95 |
Time at which 50% of the peptide was consumed by L. lactis MG1363 during growth in CDM containing a tryptic digest of αs2-casein as the nitrogen source.
Major coeluting peptide.
Minor coeluting peptide. M*, oxidized methionine. Z, phosphoserine.
ND, not detectable.
NC, no consumption.
FIG. 5.
Growth of L. lactis MG1363 in CDM containing a tryptic digest of αs2-casein and Gln, His, Leu, and Met as the sources of amino acids. Symbols: ○, growth kinetics; closed symbols, time course of peptide utilization; ▪, ▾, ▴, and ⧫, YL, LNFLK, NMAINPZKENLCSTFCK, and NANEEEYSIGZZZEEZA EVATEEVK, respectively (Z indicates phosphoserine). A tryptic digest of αs2-casein was added to CDM at 400 μg/ml. The amino acid concentration was the same as in Fig. 1. The time course of peptide utilization was estimated by reverse-phase HPLC analysis and fluorescence detection.
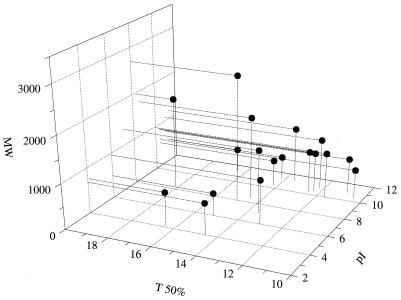
For a more precise classification of the consumed oligopeptides, the time at which 50% of the oligopeptide was consumed (T 50%) was plotted as a function of both the molecular weight and the isoelectric point of the peptide (Fig. 6). Estimation of the T 50% was not possible for four peptides which were present in small amounts together with coeluting peptides (i.e., EQLZTZEENSKK, AM*KPWIQPK [where M* indicates an oxidized methionine], PWIQPK, and RNAVPITPTLNR), although they were consumed during growth. An oligopeptide was considered basic or acidic when the pI was higher than 8.0 or lower than 6.0, respectively. The six oligopeptides which were utilized at a very high rate, i.e., a T 50% of less than 13 h (corresponding to the mid-exponential growth phase) were all basic peptides, with molecular masses ranging between 600 and 1,100 Da. In contrast, all of the oligopeptides which were consumed at a lower rate (T 50% longer than 13 h) either were basic peptides with molecular masses lower than 600 Da, basic peptides with molecular masses higher than 1,100 Da, or acidic peptides with molecular masses ranging between 600 and 1,100 Da. Among basic and neutral oligopeptides (i.e., pI higher than 6.0) whose molecular masses were lower than 1,100 Da (i.e., 11 peptides), the consumption rate during growth increased with the retention time of the peptide (Table 3), suggesting a preferential use of hydrophobic peptides. These results therefore confirm the above conclusions obtained by using peptides isolated from milk.
FIG. 6.
Consumption rate of oligopeptides released by tryptic digestion of αs2-casein by L. lactis during growth as a function of both the charge and the molecular weight (MW) of the peptides. Growth conditions were those described in the legend to Fig. 5. T 50% is the time at which 50% of the peptide was consumed.
To make sure that the reported data were not the consequence of lysis of the cells, cell lysis was estimated during growth by measuring the release of PepX into the growth medium. Only very little PepX activity was detected at the end of incubation, corresponding to less than 1% lysis. To evaluate the potential effect of lysis, the pool of αs2-casein-derived peptides was incubated with a crude extract of L. lactis MG1363 mimicking the lysis of 107 cells per ml. Only the degradation of four peptides could be detected: EVVR, YL, FALPQYLK, and FPQYLQYLYQGPIVLNPWDQVK. The respective T 50% values of these peptides were 16.42, 5.70, 11.63, and 14.95 h. These results confirm that, except for, maybe, peptides EVVR and FPQYLQYLYQGPIVLNPWDQVK, the disappearance of the peptides from the medium during exponential growth was due to their translocation into the cell.
DISCUSSION
To be used as a source of amino acids by L. lactis, peptides have to be translocated into the cells (11). Therefore, the use of different mixtures of dairy peptides made it possible to define some preferences for their translocation and their utilization during the growth of L. lactis MG1363 in milk and related culture media. First, di- and tripeptides are consumed at a higher rate than oligopeptides, which is in perfect agreement with previous results (13). Translocation of oligopeptides is under the control of two main factors: the net charge of the peptide (i.e., its isoelectric point) and its molecular weight. Preferential use of basic peptides with molecular masses ranging between approximately 600 and 1,100 Da was observed. Among these, hydrophobic peptides were utilized prior to hydrophilic peptides. When the concentration of basic peptides with molecular masses ranging between 600 and 1,100 Da in the growth medium significantly decreased, L. lactis MG1363 used other peptides as the source of amino acids, i.e., either peptides in the same size range with a lower pI or basic peptides outside the optimal size range. Utilization of large peptides (containing up to 17 residues) was observed. Moreover, the consumption rate of these large oligopeptides was in the same range as that of tetrapeptides. These findings therefore indicate that the size of the oligopeptide is clearly not the only factor which determines translocation. Moreover, the complete lack of utilization of large, acidic peptides represents an additional factor in oligopeptide translocation by L. lactis MG1363.
Substrate specificities and binding affinities for oligopeptide transport by the Opp system are defined by the binding properties of oligopeptide-binding protein OppA (18). Therefore, the prevailing knowledge about peptide translocation in L. lactis MG1363 appear to conflict somewhat with the concept that OppA has a remarkably broad substrate specificity, binding peptides of two to five amino acid residues with high affinity but with little regard to sequence. It is worth noting that studies on the substrate specificity of OppA have been performed by using the gram-negative bacterium Escherichia coli or Salmonella typhimurium as the source of OppA (18, 25). Some crucial differences between OppA specificities from these microorganisms and L. lactis have already been reported: OppA from E. coli is able to bind di- and tripeptides and has a lower affinity for pentapeptides than for tetrapeptides (4), whereas OppA from L. lactis strains does not bind di- and tripeptides (13, 27). It should not be so surprising, then, that binding of peptides by OppA from these bacteria arises from different interactions. Strong hydrogen bonding and electrostatic interactions between the protein and the main chain of the ligand are responsible for peptide binding by OppA from S. typhimurium (25). According to our results, such bonds are unlikely to occur preferentially in L. lactis MG1363.
Elucidation of the specificity of milk peptide utilization by L. lactis made it possible to explain the cause of the cessation of growth of L. lactis MG1363 in milk. Transportable milk oligopeptides cannot provide Gln and His and represent a poor source of Leu and Met (Fig. 2). Glu/Gln and His were present in milk at relatively high concentrations (45 and 5 μg/ml, respectively), whereas Leu and Met were present at very low concentrations (<1 μg/ml) (8). Consequently, Leu and Met deprivation is expected to cause cessation of growth of L. lactis MG1363. On the other hand, basic milk peptides are deprived of Val and do not provide a high amount of Ile, whereas neutral milk peptides provide low but equal amounts of Ile and Val (Fig. 3). Again, the concentrations of these two amino acids in milk are low (0.6 and 2.1 μg/ml, respectively [8]). Consequently, growth cessation in milk supplemented with Leu and Met is expected to be due to depletion of Val and Ile. That is exactly what was observed in the present study (Fig. 1). These results suggest a synergism between free amino acids and milk peptides for the nitrogen supply of L. lactis.
In contrast, growth of a proteolytic L. lactis strain on β-casein as the source of amino acids is reported to be limited by His, Leu, Gln, Val, and Met (12), the corresponding peptides being released too slowly to support optimal growth (6). Thus, no synergistic effect is expected to occur between β-casein and milk peptides. This might reflect the origin of milk peptides. Complete identification of milk peptides should clarify this.
REFERENCES
- 1.Chopin A. Organization and regulation of genes for amino acid biosynthesis in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:21–38. doi: 10.1111/j.1574-6976.1993.tb00011.x. [DOI] [PubMed] [Google Scholar]
- 2.Foucaud C, Kunji E R S, Hagting A, Richard J, Konings W N, Desmazeaud M, Poolman B. Specificity of peptide transport systems in Lactococcus lactis: evidence for a third system which transports hydrophobic di- and tripeptides. J Bacteriol. 1995;177:4652–4657. doi: 10.1128/jb.177.16.4652-4657.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guyer C A, Morgan D G, Staros J V. Binding specificity of the periplasmic oligopeptide-binding protein from Escherichia coli. J Bacteriol. 1986;168:775–779. doi: 10.1128/jb.168.2.775-779.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassan A I, Deschamps N, Richard J. Précision des mesures de vitesse de croissance des streptocoques lactiques dans le lait basées sur la méthode de dénombrement microbien par formation de colonies. Etude de référence avec Lactococcus lactis. Lait. 1989;69:433–447. [Google Scholar]
- 6.Helinck S, Richard J, Juillard V. The effects of adding lactococcal proteinase on the growth rate of Lactococcus lactis in milk depend on the type of the enzyme. Appl Environ Microbiol. 1997;63:2124–2130. doi: 10.1128/aem.63.6.2124-2130.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herraiz T, Casal V, Polo M C. Reversed-phase HPLC analysis of peptides in standard and dairy samples using on-line absorbance and post-column OPA-fluorescence detection. Z Lebensm Unters Forsch. 1994;199:265–269. doi: 10.1007/BF01193309. [DOI] [PubMed] [Google Scholar]
- 8.Juillard V, Le Bars D, Kunji E R S, Konings W N, Gripon J-C, Richard J. Oligopeptides are the main source of nitrogen for Lactococcus lactis during growth in milk. Appl Environ Microbiol. 1995;61:3024–3030. doi: 10.1128/aem.61.8.3024-3030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juillard V, Richard J. Indirect interaction in milk between proteolytic and isogenic non-proteolytic strains of Lactococcus lactis. I. Effect of pre-culturing by a nonproteolytic variant. Lait. 1990;70:425–438. [Google Scholar]
- 10.Kunji E R S, Mierau I, Hagting A, Poolman B, Konings W N. The proteolytic systems of lactic acid bacteria. Antonie van Leeuwenhoek. 1996;70:187–221. doi: 10.1007/BF00395933. [DOI] [PubMed] [Google Scholar]
- 11.Kunji E R S, Mierau I, Poolman B, Konings W N, Venema G, Kok J. Fate of peptides in peptidase mutants of Lactococcus lactis. Mol Microbiol. 1996;21:123–131. doi: 10.1046/j.1365-2958.1996.6231339.x. [DOI] [PubMed] [Google Scholar]
- 12.Kunji E R S, Hagting A, de Vries C J, Juillard V, Haandrikman A J, Poolman B, Konings W N. Transport of β-casein-derived peptides by the oligopeptide transport system is a crucial step in the proteolytic pathway of Lactococcus lactis. J Biol Chem. 1995;270:1569–1574. doi: 10.1074/jbc.270.4.1569. [DOI] [PubMed] [Google Scholar]
- 13.Kunji E R S, Smid E J, Plapp R, Poolman B, Konings W N. Di-tripeptides and oligopeptides are taken up via distinct transport mechanisms in Lactococcus lactis. J Bacteriol. 1993;175:2052–2059. doi: 10.1128/jb.175.7.2052-2059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 15.Marshall V M E, Law B A. The physiology and growth of dairy lactic acid bacteria. In: Davies F L, Law B A, editors. Advances in the microbiology and biochemistry of cheese and fermented milk. Amsterdam, The Netherlands: Elsevier; 1983. pp. 67–98. [Google Scholar]
- 16.Mercier J C, Maubois J L, Poznanski S, Ribadeau-Dumas B. Fractionnement préparatif des caséines de vache et de brebis par chromatographie sur DEAE cellulose, en milieu urée et 2-mercaptoethanol. Bull Soc Chim Biol. 1968;50:521–530. [PubMed] [Google Scholar]
- 17.Mills O E, Thomas T D. Nitrogen sources for growth of lactic streptococci in milk. N Z J Dairy Sci Technol. 1981;16:43–55. [Google Scholar]
- 18.Payne J W, Smith M W. Peptide transport by micro-organisms. Adv Microbial Physiol. 1994;36:1–80. doi: 10.1016/s0065-2911(08)60176-9. [DOI] [PubMed] [Google Scholar]
- 19.Poolman B. Energy transduction in lactic acid bacteria. FEMS Microbiol Rev. 1993;12:125–148. doi: 10.1111/j.1574-6976.1993.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 20.Poolman B, Konings W N. Growth of Streptococcus lactis and Streptococcus cremoris in relation to amino acid transport. J Bacteriol. 1988;170:700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poolman B, Kunji E R S, Hagting A, Juillard V, Konings W N. The proteolytic pathway of Lactococcus lactis. J Appl Bacteriol Symp Suppl. 1995;79:65–75. [PubMed] [Google Scholar]
- 22.Reiter R, Oram J D. Nutritional studies on cheese starters. I. Vitamin and amino acid requirements of single strain starter. J Dairy Res. 1962;29:63–77. [Google Scholar]
- 23.Smid E J, Driessen A J M, Konings W N. Mechanism and energetics of dipeptide transport in membrane vesicles of Lactococcus lactis. J Bacteriol. 1989;171:292–298. doi: 10.1128/jb.171.1.292-298.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snedecor G W, Cochran W G. Statistical methods. Ames: Iowa State University Press; 1957. [Google Scholar]
- 25.Tame J R H, Dodson E J, Murshudov G, Higgins C F, Wilkinson A J. The crystal structures of the oligopeptide-binding protein OppA complexed with tripeptide and tetrapeptide ligands. Structure. 1995;3:1395–1406. doi: 10.1016/s0969-2126(01)00276-3. [DOI] [PubMed] [Google Scholar]
- 26.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tynkkynen S, Buist G, Kunji E, Kok J, Poolman B, Venema G, Haandrikman A. Genetic and biochemical characterization of the oligopeptide transport system of Lactococcus lactis. J Bacteriol. 1993;175:7523–7532. doi: 10.1128/jb.175.23.7523-7532.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]