Abstract
Resource exchanges in the form of invertebrate fluxes are a key component of aquatic-terrestrial habitat coupling, but this interface is susceptible to human activities, including the imposition of artificial light at night. To better understand the effects of spectral composition of light-emitting diodes (LEDs)—a technology that is rapidly supplanting other lighting types—on emergent aquatic insects and terrestrial insects, we experimentally added LED fixtures that emit different light spectra to the littoral zone and adjacent riparian habitat of a pond. We installed four replicate LED treatments of different wavelengths (410, 530 and 630 nm), neutral white (4000 k) and a dark control, and sampled invertebrates in both terrestrial and over-water littoral traps. Invertebrate communities differed among light treatments and between habitats, as did total insect biomass and mean individual insect size. Proportional allochthonous biomass was greater in the riparian habitat and among some light treatments, demonstrating an asymmetrical effect of differently coloured LEDs on aquatic-terrestrial resource exchanges. Overall, our findings demonstrate that variation in wavelength from LEDs may impact the flux of resources between systems, as well as the communities of insects that are attracted to particular spectra of LED lighting, with probable implications for consumers.
This article is part of the theme issue ‘Light pollution in complex ecological systems’.
Keywords: resource flux, resource subsidies, light pollution, invertebrate community composition, anthropogenic impacts, aquatic-terrestrial linkages
1. Introduction
Freshwaters and their adjacent riparian zones are closely linked by the reciprocal exchanges of materials—termed resource subsidies—that matter for the structure and functioning of each of these types of ecosystems [1–3]. Key resources that link the two habitats are fluxes of insects, with important consumers of these resources being fishes, bats, birds and spiders [1,4–7]. Despite the demonstrated importance of resource subsidies for both the donor and recipient ecosystem, human impacts to these subsidies are increasingly being reported [8,9] with poorly understood ecological consequences.
Artificial light at night (ALAN) is one of the most rapidly increasing anthropogenic changes to the environment, with a 6% global increase in exposure per year [10] and a 2.2% yearly average increase in brightness in continuously illuminated areas worldwide [11]. Approximately 83% of Earth's human population is exposed to light pollution [12] and 22% of the world's coastlines are exposed to ALAN [10]. As concern over ALAN grows, in recent years, researchers have documented its effects on numerous physiological and ecological processes including circadian rhythms [13,14], melatonin production [15,16], navigation [17], migration [18] and temporal niche partitioning [19] for organisms across aquatic and terrestrial realms.
Insects have the potential to be particularly affected by disruptions to natural light regimes, as 50% of taxa are adapted to nocturnal environments [10]. Nocturnal insects' compound eyes are adapted to function in light levels up to 11 orders of magnitude lower than daytime light [20]. Many nocturnal insects of aquatic origin are polarotaxic [21,22], being attracted to the polarized light that is reflected from the surface of water, a trait that enables the identification of sites for breeding and subsequent ovipositioning. This combination of traits and characteristics makes insects uniquely susceptible to the impacts of ALAN, and ALAN often creates patches of polarized light in the riparian zone, causing emergent aquatic insects to land in terrestrial environments and increased insect mortality [23].
Recent advances in lighting technology have resulted in a rapid shift away from widespread and long-used technologies such as high-pressure sodium, fluorescent and incandescent lighting, and towards the use of light-emitting diodes (LEDs). LED lighting often peaks in shorter blue wavelengths and can easily be tuned to emit specific coloured outputs [24] which potentially affects the attractiveness of LEDs to insects [25]. Despite a substantial body of literature on the ecological impacts of ALAN on invertebrate communities [26,27], including emergent-insect response [7,28–31], this tunability combined with the limited studies on the impacts of near-monochromatic light on invertebrates suggests this topic warrants further investigation.
Research has identified differences in the attractiveness to insects among lighting technologies [32–34], but separating the effects of commonly confounded factors such as illuminance and wavelength is needed to provide a more-mechanistic understanding of how the rapid global increase of ALAN is impacting insect communities and their role in the fluxes of resource subsidies. Entomologists have long known that insects are highly attracted to ultraviolet and short-wavelength light [35], but this work has primarily focused on mortality or numerical responses. LEDs are often tuned to very narrow bands of the electromagnetic spectrum (termed quasi-monochromatic light) to provide illumination of different colours. A small number of studies have identified impacts of light wavelength on the ecology of organisms including larval stages of emergent aquatic insects [36] and aphid-parasitoid food webs [37]. How this quasi-monochromatic light, and the common (but not universal) predominance of short wavelengths in LED white light, varies in its attractiveness to both aquatic and terrestrial insects have yet to be fully elucidated. Similarly, given the potential to impact aquatic and terrestrial insects differently, how this monochromatic lighting might alter the transfer of aquatic-terrestrial resource subsidies needs attention given the importance of these subsidies for both ecosystem types.
To help fill these knowledge gaps, we simultaneously performed a manipulative field experiment in adjacent terrestrial and aquatic ecosystems. The design allowed us to test hypotheses regarding the effect of ALAN of different wavelengths on insect community structure and aquatic-terrestrial resource exchanges. We hypothesized that different communities of insects would be attracted to different wavelengths of LED light, as more emergent aquatic-insect taxa would be drawn to shorter wavelength relative to longer wavelength light, and that we would observe a less pronounced effect of wavelength on terrestrial invertebrates. Additionally, we hypothesized that we would observe an effect of wavelength on insect biomass, with shorter wavelength treatments receiving greater biomass inputs owing to the heightened effect on emergent aquatic invertebrates. Lastly, we hypothesized this would create an asymmetrical effect on resource exchange, because the riparian habitat would receive a greater input of allochthonous insect biomass than would the littoral habitat. To our knowledge, this is the first time LED lamps of different wavelengths standardized for illuminance have been used to assess adult emergent aquatic invertebrate flux, and the first time the effect of coloured LED lighting on biomass and insect size has been assessed in a replicated field setting.
2. Methods
(a) . Study site
We conducted this study in the littoral zone of a small pond and its adjacent riparian zone (Waterfowl Pond, Seven Ponds Nature Center, Dryden, Michigan, USA. 42°55′51.2″ N, 83°11′23.9″ W). We chose this pond because of its location on a nature preserve in a rural setting with little skyglow that is minimally impacted by human activities and is not exposed to existing artificial light via direct glare. On the night of the experiment (3 June 2022) sunset occurred at 21.05 and sunrise the following morning occurred at 5.55. Air temperature overnight averaged 8.1°C and overnight water temperature averaged 19.5°C. There was 9% cloud cover and a waxing crescent moon, with winds westnorthwest at 3.2 kph.
(b) . Experimental design
(i) . Light treatments
We experimentally elevated ALAN levels by installing replicated light treatments in the littoral zone and adjacent riparian habitat (figure 1a). We replicated each treatment six times, with three replicates installed in the littoral zone and three in the riparian zone. We assigned light treatments randomly by rolling a die, and we installed five treatments of differing wavelength: violet (410 nm), green (530 nm), red (630 nm), neutral white (4000 k) and a dark control. Spectral information was obtained from the manufacturer for the 4000 k white light treatment which peaked at 453 nm (electronic supplementary material, figure S1). In each habitat, we separated each replicate by approximately 5 m and the habitat treatments by approximately 15 m (figure 1a). Light treatments consisted of battery-powered, 50.8 cm monochromatic (with the exception of the 4000 k white light treatment) LED strip lighting (5050 SMD LEDs, AA-20-Flex, LED supply, Randolph, VT, USA) mounted horizontally on the underside of an L-shaped brace positioned 0.60 m above the ground or water surface, with light projecting directly downwards (figure 1b). There was no overlap in light exposure between treatment sites and we measured illuminance between sites to ensure it fell below 1 lx, consistent with other non-illuminated areas and dark controls. We manually turned on the lights at sunset and manually turned them off at sunrise. We used a Digital Lux Meter (Dr. Meter, LX1330B) to measure illuminance directly below lights at the surface level of the pan traps (see details on pan traps below). To control for illuminance level we covered some diodes of red, green and white strips with electrical tape to standardize illuminance among these three colour treatments, and tested for differences in illuminance using analysis of variance (ANOVA, F2,15 = 0.90, p = 0.43). Violet was excluded from this standardization owing to the nature of near-ultraviolet wavelength becoming dangerous at high intensity, and it being dimmer than all other colours at an average of 13.7 lx. Average illuminance for the other four treatments was as follows: 43.1 lx for red, 40.8 lx for green, 42.5 lx for 4000 k white and 0.4 lx for dark controls. Illuminance was measured immediately after lights were turned on, and residual sunlight at dusk probably contributed to the higher than expected values in the dark controls. When illuminance was measured later in the season and well after sunset for a related experiment (with no change in experimental set-up) dark control illuminance averaged 0.03 lx. These illuminance levels fall within the bounds of previous ALAN research [26]. Irradiance in W m−2 was calculated for each treatment using the CIE photopic luminous efficiency function (V(λ)) and measured as follows: 16.5 W m−2 for violet, 0.069 W m−2 for green, 0.24 W m−2 for red and 0.14 W m−2 for 4000 k white. Like previous studies [26], ours did not standardize for irradiance because ALAN is typically designed for human vision.
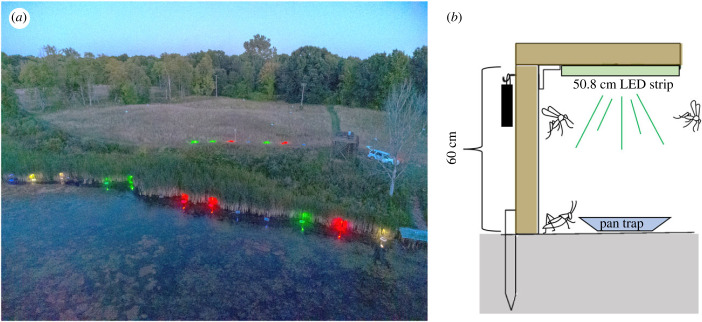
Figure 1.
(a) Oblique aerial view of the study site (Waterfowl Pond, Seven Ponds Nature Center in Dryden, Michigan, USA. 42°55′51.2″ N, 83°11′23.9″ W) showing partial experimental set-up. The photograph has been brightened for clarity. Light treatments were assigned at random. Photo credit: Sarah Griffith, Oakland University. (b) Diagram of an experimental unit, consisting of an L-brace, lighting treatment and pan trap. Pan traps were placed on either soil (riparian traps) or water (littoral traps). Littoral pan traps were secured to stakes with nylon rope to hold them in place. (Online version in colour.)
(ii) . Invertebrate collection
We collected invertebrates, (mostly insects, but also including spiders) using pan traps, each consisting of a rectangular plastic container (58.4 cm × 41.3 cm × 15.2 cm) that we filled with approximately 2 cm of a soap-water mixture. In littoral sites, we attached containers to L-braces using nylon rope and floated traps on the surface of the water; in the riparian sites, we placed the traps on the ground (figure 2b). In both the littoral and riparian treatments, we positioned traps directly underneath the lights, perpendicular to the L-brace. The morning following plot illumination we poured the contents of each pan trap through a 500 um sieve and then rinsed the sieve upside down over a collection tray with 70% ethanol to dislodge invertebrates. We then collected the invertebrates by hand and stored them in 70% ethanol before identifying them to family level [38].
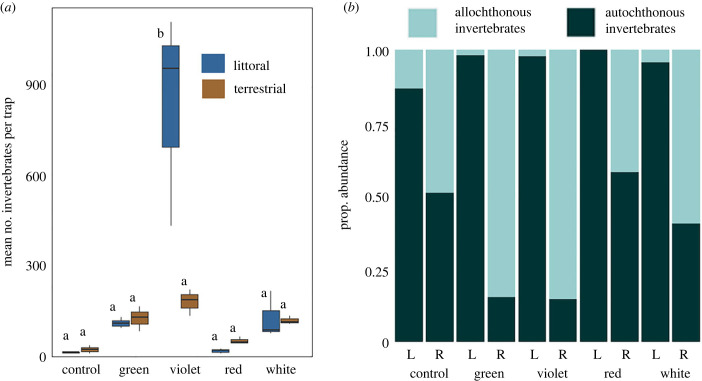
Figure 2.
Invertebrate abundance among colour treatments and between the habitats where invertebrates were collected. (a) Mean number of invertebrates per trap was greater in violet treatments than all other colours (p < 0.001) and greater in the littoral habitat than in the terrestrial habitat (p < 0.01). Littoral habitat is represented by blue bars; riparian habitat is represented by brown bars. Lines represent minimum and maximum values for each treatment. Traps sharing letters are statistically not different based on Tukey post hoc tests. (b) Proportional abundance of allochthonous and autochthonous invertebrates among colour treatments and between habitats, to distinguish the habitat where the organism acquired its biomass. Littoral traps of all colours had greater autochthonous proportional abundance than all white, green and violet riparian traps (p < 0.05). Allochthonous proportional abundance was greater in white, violet and green riparian traps compared to all other treatments (p < 0.05). Bars labelled ‘L’ represent littoral habitat and bars labelled ‘R’ represent riparian habitat. (Online version in colour.)
(iii) . Insect biomass
We calculated biomass for 100 individual insects each from the five most abundant families—Leptoceridae, Caenidae, Hydroptillidae, Cicadellidae and Chironomidae—which represented 95.2% of the total of all individuals. In order to sub-sample these families for length measurements across all colour treatments, for all five families we assigned a numeric value (1–6) for replicates within each colour and used a random-number generator to determine which replicate to sub-sample. We repeated this process for each colour treatment in which that family was present. Each family was represented in each colour treatment with the exception of Leptoceridae, which was not present in white, green or red treatments. We took photographs of each individual invertebrate using an eFlex 75x/300x microscope against a micrometer-scale graticule, then measured body length digitally using ImageJ [39]. We entered length (in mm) into family-level allometric equations [40,41] to calculate individual biomass, and then calculated mean family biomass (in mg) per replicate by multiplying the mean individual biomass for each family by its abundance. We also used the 100 sampled individuals per family to calculate mean individual size (in mg) for each taxon.
(c) . Statistical analysis
(i) . Invertebrate community composition
We evaluated invertebrate community composition (insects and spiders) among colour treatments and between habitats using non-metric multidimensional scaling (NMDS) and adonis permutational multivariate analysis of variance using Bray–Curtis distances in package vegan [42] in program R [43]. We further evaluated community structure by comparing abundance and proportional allochthonous abundance among colour treatments and between habitats using two-way ANOVA in package car [44] in program R. We performed post hoc Tukey tests (electronic supplementary material, table S1) on all two-way ANOVA models; Levene's test indicated all data were normally distributed and homoscedastic for the parametric analyses performed. Colour treatment (which included dark controls) and habitat type were treated as fixed effects in all analyses.
(ii) . Biomass and size
We analysed total insect biomass and proportional allochthonous insect biomass among colour treatments and between habitat using two-way ANOVA and biomass per family among colour treatments and between habitat using two-way ANOVA. Biomass was log-transformed to achieve homoscedasticity. We also analysed mean individual insect size (mg) among colour treatments and between habitat using two-way ANOVA. We also used two-way ANOVA to analyse proportional biomass and abundance of the five most common taxa among treatments. Colour treatment (which includes dark controls) and habitat were treated as fixed effects in all analyses. We performed post hoc Tukey's tests (electronic supplementary material, table S2) on all ANOVA models; Levene's test indicated all data were normally distributed.
3. Results
In total, we collected 4699 invertebrates in pan traps, representing 34 families. Average invertebrate abundance per trap was 78% greater in the littoral habitat relative to the riparian habitat (ANOVA, F1,20 = 8.28, p < 0.001; electronic supplementary material, table S3) and was impacted by colour (ANOVA, F4,20 = 17.54, p < 0.001) (figure 2a). Traps in violet treatments had the greatest overall abundance of all colour treatments; abundance was 188% greater in violet traps than in dark controls. The effect of the light treatment depended on the habitat in which it was deployed, evidenced by a significant interaction between habitat and colour treatment (ANOVA, F4,20 = 9.68, p < 0.001). Post hoc tests revealed that abundance in littoral violet traps was consistently greater than all other colours in both littoral and terrestrial habitats. Allochthonous invertebrate abundance (originating from an outside habitat relative to the treatment) was proportionally more abundant in the riparian habitat (ANOVA, F1,20 = 116.49, p < 0.001) and was greater in white, violet and green riparian traps compared to all other treatments (ANOVA, F4,20 = 3.13, p < 0.05) (figure 2b).
(a) . Community structure across treatments
NMDS results (figure 3a–e) supported our hypothesis that different communities of insects would be attracted to different wavelengths (colours) of LED light, with a more pronounced effect of colour on emergent aquatic insects than terrestrial invertebrates. To facilitate interpretation of the overall NMDS plot (figure 3a) that included all colour treatments and both habitats, we separately present NMDS outputs by habitat (figure 3b) and colour treatment (figure 3c) as well as by individual colour treatment within each habitat (figure 3d,e).
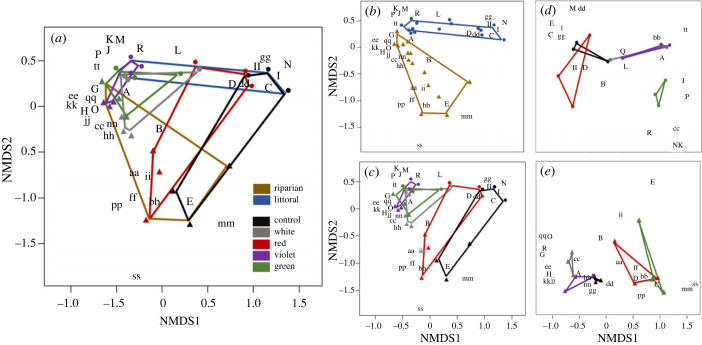
Figure 3.
NMDS ordination plot showing community composition across (a) all colour treatments and both habitat types, (b) between habitat type only, (c) among colour treatment only, (d) among colour treatments in the littoral habitat only, and (e) among colour treatments in the riparian habitat only. Capital letters represent aquatic-originating taxa and lowercase letters represent terrestrial-originating taxa. Triangles represent traps in the riparian habitat and circles represent traps in the littoral habitat. Invertebrate families are indicated by letter as follows: (A) Caenidae, (B) Hydroptillidae, (C) Tipulidae, (D) Chironomidae, (E) Coenagrionidae, (R) Leptoceridae, (G) Phryganidae, (H) Polycentropodidae, (I) Simuliidae, (J) Rhyacophilidae, (K) Corydalidae, (L) Culicidae, (M) Gerridae, (N) Notonecidae, (O) Psychomyiidae, (P) Helicopsyche, (Q), Hydropsychidae, (aa) Miturgidae, (bb), Cicadellidae, (cc) Noctuoidae, (dd) Dolichiopodidae, (ee) Muscidae, (ff) Tettigoniidae, (gg) Ephydridae, (hh) Formicidae, (ii) Hahnidae, (jj) Arctiinae, (kk) Erebidae, (ll) Cosmopterigidae, (mm) Rhopalosomatidae, (nn) Miridae, (pp) Diapridae, (qq) Geometridae, (ss) Scarabaetidae, (tt) Tetragnathidae. (Online version in colour.)
Community structure varied among colour treatments (adonis, R2 = 0.43, p = 0.001) across habitats (adonis, R2 = 0.23, p = 0.001) (figure 3a). Leptoceridae (R) was closely associated with violet treatments in the littoral habitat (figure 3a), and notably, of 686 individuals captured, only a single Leptoceridae individual was found in any non-violet treatment, despite being the second most overall abundant family. Leptoceridae proportional abundance was greater in violet littoral treatments than all other colours and dark controls (ANOVA, F4,20 = 7.74, p < 0.001). When comparing the differences between littoral and terrestrial communities (figure 3b), there is clear separation between the two, with Caenidae (A) and Hydroptillidae (B) (two of the five most abundant taxa) associated with both habitats. Caenidae (A) proportional abundance was impacted by colour (ANOVA, F4,20 = 26.78, p < 0.001), and within NMDS plots, situated within white, green and violet polygons (figure 3c), and distanced from red and dark controls. By contrast, Hydroptillidae (B) proportional abundance was not impacted by colour (ANOVA, F4,20 = 1.43, p = 0.26) and is centrally located within all colour treatments in NMDS plots (figure 3c).
Within the littoral habitat community structure was impacted by colour (adonis, R2 = 0.72, p < 0.001; figure 3d) and we observed relatively few terrestrial species (indicated by double lower case letters). Chironomidae (D), the third most abundant taxa, was proportionally more abundant in the littoral habitat than in the riparian habitat (ANOVA, F4,20 = 31.26, p < 0.001) and within the littoral habitat Chironomidae was proportionally more abundant in dark controls over green and violet treatments and red over violet treatments (ANOVA, F4,20 = 3.35, p < 0.05). Within the riparian habitat, colour also impacted community structure (adonis, R2 = 0.43, p < 0.001; figure 3e). In contrast with the littoral habitat that had few taxa of terrestrial origin, we observed a large number of emergent aquatic-insect taxa in the riparian habitat (figure 3e, uppercase letters), including Caenidae (A) which was the most abundant emergent aquatic family in riparian treatments. The most abundant family of terrestrial origin, Cicadellidae (bb), is closely associated in the riparian habitat with red treatments and dark controls and is proportionally more abundant in these treatments compared to violet and green treatments (ANOVA, F4,20 = 5.22, p < 0.05).
(b) . Biomass and insect size
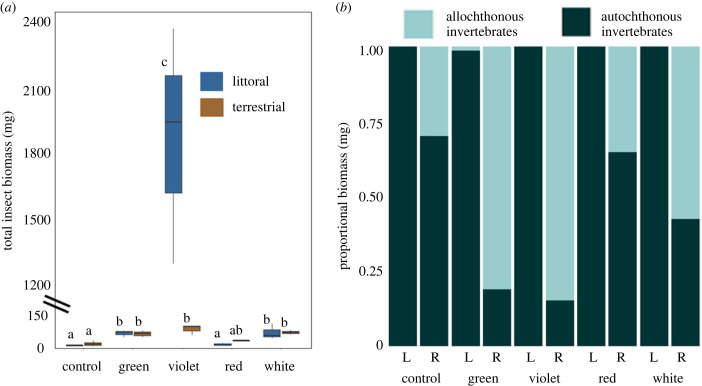
The five most abundant insect families, Caenidae (2860 individuals), Leptoceridae (686 individuals), Chironomidae (459 individuals), Cicadellidae (266 individuals) and Hydroptillidae (204 individuals) accounted for 95.2% of all invertebrates. Total biomass (mg) analysis of these families supports our proposed hypothesis that we would observe an effect of wavelength on insect biomass, with shorter wavelength treatments receiving greater biomass inputs. Total biomass was 184% greater in violet treatments than all other colours and was greater in white and green treatments than in red and dark controls (ANOVA, F4,20 = 44.85, p < 0.001). Total biomass was not impacted by habitat type, but the effect of colour was dependent on habitat (ANOVA, F4,20 = 13.74, p < 0.001), with violet littoral treatments having greater total biomass input than all other colour and habitat combinations. Littoral red treatments, littoral dark controls and riparian dark controls had lesser biomass than white, green and violet traps in both habitats (figure 4a).
Figure 4.
(a) Total insect biomass (mg) was greater in violet treatments than all other colours and was greater in white and green treatments than red and dark controls (p < 0.001). An interaction between colour and habitat was observed (p < 0.001) and treatments sharing a letter are statistically not different based on Tukey post hoc tests. Blue bars represent littoral treatments and brown bars represent riparian treatments. Lines represent minimum and maximum values for each treatment. (b) Proportional allochthonous biomass was greater in green and violet treatments than red and dark controls (p < 0.01) and greater in riparian habitat than littoral habitats (p < 0.001). Bars labelled ‘L’ represent littoral habitat and bars labelled ‘R’ represent riparian habitat. (Online version in colour.)
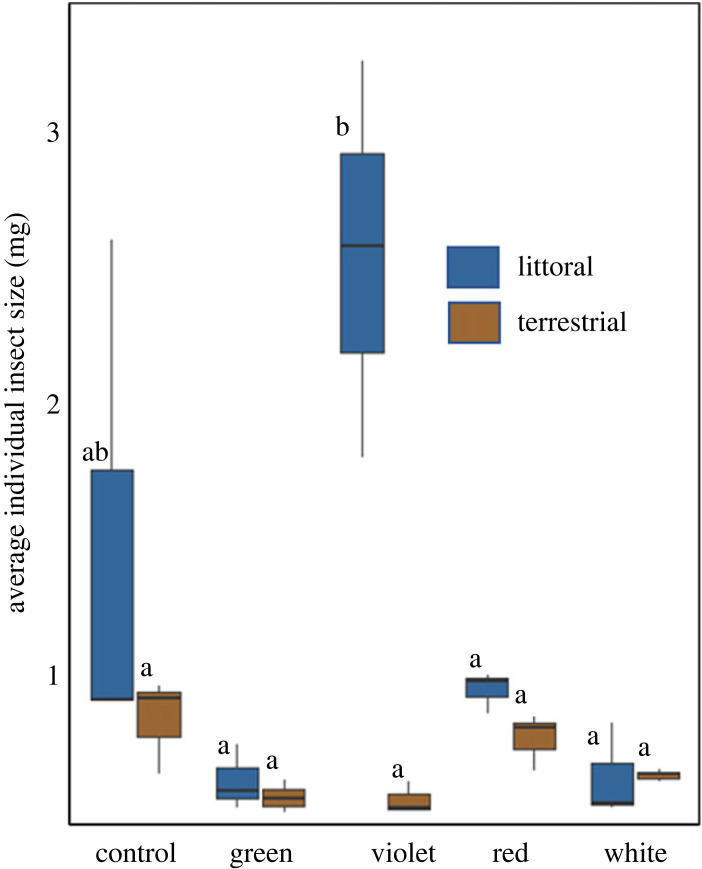
In addition to abundance, this biomass response is also a function of individual insect size (mg) among colour treatments and between habitats, with a large number of small-bodied insects potentially producing a similar biomass response as a small number of large-bodied insects. Mean insect body size was 61% larger in the littoral habitat than the riparian habitat (ANOVA, F1,20 = 15.50, p < 0.001) and was larger in violet treatments than white, green or red treatments (ANOVA, F4,20 = 6.07, p < 0.001) (figure 5). Insects in littoral violet treatments were larger on average than all other treatments in both habitats with the exception of littoral dark controls (ANOVA, F4,20 = 6.62, p < 0.01).
Figure 5.
Average individual insect biomass (mg) was greater in violet treatments than white, green or red treatments (p < 0.001) and was greater in the littoral habitat (p < 0.001). Littoral habitat is represented by blue bars and riparian habitat is represented by brown bars. Treatments sharing the same letter are statistically not different based on Tukey post hoc tests. (Online version in colour.)
(c) . Resource subsidy effects
We also hypothesized that we would observe an asymmetrical effect of wavelength on resource exchange, because the riparian habitat would receive a greater input of allochthonous insect biomass than the littoral habitat would receive from the riparian habitat. Our results support this hypothesis since proportional allochthonous biomass was greater in green and violet treatments than red treatments and dark controls (ANOVA, F4,20 = 5.49, p < 0.01) and greater in riparian habitat compared to littoral habitat (ANOVA, F4,20 = 165.75, p < 0.001) (figure 4b). Proportional allochthonous biomass was also greater in green and violet riparian treatments than all other habitat and colour combinations with the exception of white riparian treatments, which had greater proportional allochthonous biomass than all littoral colour treatments and littoral dark controls (ANOVA, F4,20 = 5.22, p < 0.01).
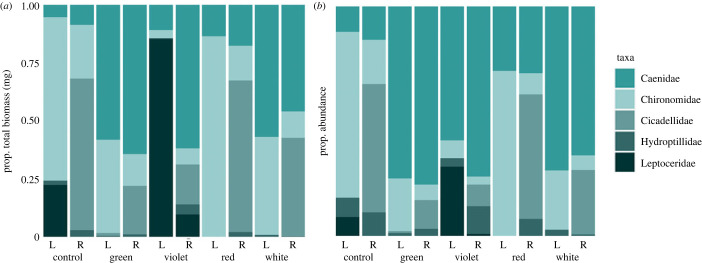
The asymmetrical effect of biomass that we observed was driven by the attraction of emergent aquatic insects to shorter wavelength LEDs in the riparian habitat (figure 6a). The aquatic family Caenidae (the most abundant family in our study) had proportionally greater biomass in short-wavelength violet treatments compared to dark controls, in short-wavelength green treatments compared to violet, red and dark controls, and in white treatments compared to red and dark controls (ANOVA, F4,20 = 1.47, p < 0.001). Chironomidae proportional biomass was also impacted by colour, but by longer wavelength treatments, as we observed a greater proportional biomass in red treatments and dark controls than violet treatments (ANOVA, F4,20 = 5.48, p < 0.01). Leptoceridae comprised a significant proportion of the biomass in violet treatments (ANOVA, F4,20 = 15.21, p < 0.001), with proportional biomass in littoral violet treatments greater than all other colours and dark controls in both habitats (ANOVA, F4,20 = 5.49, p < 0.001). Hydroptillidae proportional biomass was similar among all colour treatments. This asymmetrical effect of wavelength on resource exchange was also driven by the avoidance of short-wavelength colours in the only family of terrestrial origin abundant enough to be included in biomass analysis, Cicadellidae, which had greater proportional abundance in red treatments and dark controls than green and violet treatments (ANOVA, F4,20 = 5.22, p < 0.01).
Figure 6.
Proportional total biomass comprised the five most abundant taxa across all treatments (a) and proportional abundance comprised the five most abundant taxa across all treatments (b). Bars labelled ‘L’ represent littoral habitat and bars labelled ‘R’ represent riparian habitat. (Online version in colour.)
4. Discussion
As global exposure to ALAN continues to increase, the impact on aquatic and terrestrial ecosystems is likely to expand and intensify. With the increasingly widespread adoption of LED technology comes the ability to tune lighting to narrow spectral bands, presenting challenges and potential solutions to mitigating these impacts, particularly for insects. Improving our understanding of LED impacts on insects in aquatic and terrestrial realms is a vital part of filling knowledge gaps. Our results here provide novel insights into how emergent aquatic and terrestrial insect communities can respond to different LED spectra, and that these responses may impact the connections that exist in the form of fluxes of resources across ecosystem boundaries.
To address these and other gaps, we performed a manipulative field experiment and tested three hypotheses related to light spectra and the response of insect communities and resource fluxes. These hypotheses were each supported and were: that different communities of insects would be attracted to different wavelengths, that shorter wavelength treatments would have greater biomass inputs via emergent aquatic insects, and that resource exchange between habitats would be asymmetrically affected by colour treatments. Additionally, and interestingly, we found that mean individual body size of insects varied among colour treatments between habitats.
We found that community composition differed among colour treatments both across and within the littoral and riparian habitats. These community differences appear to be driven by taxa-specific responses—both attraction and avoidance—to particular colours of light. The most abundant terrestrial family, Cicadellidae, was most abundant in red treatments and dark controls, suggesting potential attraction to longer wavelengths or potential avoidance of shorter wavelengths. This is a stark contrast to abundant families of emergent aquatic insects such as Leptoceridae or Caenidae, which were much more abundant in short-wavelength light traps. Previous studies have seen similar variation between the response of terrestrial and aquatic emergent invertebrates to broad-spectrum ALAN [28,31,45]. These two invertebrate groups respond to light differently, with emergent invertebrates using light cues for ovipositing [31] and timing of emergence events [46]. Additionally, variations among taxa in photoreceptors and eye physiology [47] contribute to wavelength-specific responses. Better understanding of these taxa-specific responses to near-monochromatic LEDs is fertile ground for future research since it could lead to predictions of the impact of wavelength across ecosystems and communities.
In addition to being able to discern wavelength-specific community responses of invertebrate families in both littoral and riparian habitats, by categorizing invertebrate input as allochthonous and autochthonous, we observed an asymmetrical impact of ALAN on abundance and biomass fluxes of insects. Allochthonous proportional biomass was greater in violet and green riparian treatments, and allochthonous proportional abundance was greater in violet, green and white riparian treatments. This suggests short-wavelength LEDs have a stronger effect at pulling aquatic emergent invertebrates from the littoral habitat, but do not attract terrestrial invertebrates to the littoral zone in the same manner. With emergent aquatic invertebrates being more abundant than terrestrial invertebrates in both habitats, we suggest that these aquatic organisms are more susceptible to ALAN. While we have seen evidence of emergent aquatic-insect attraction to white, broad-spectrum lighting in multiple other studies [7,28–30,34] results from our study indicate that a narrower LED spectrum can alter this response.
Eisenbeis [23] proposed several mechanisms for the impact of ALAN on flying invertebrates and here we see evidence for both a barrier effect and vacuum effect. The barrier effect interrupts navigational clues, creating a barrier for dispersal and resulting in resource ‘recycling’ back into the environment of origin. The vacuum effect results in invertebrates being drawn from unilluminated areas to a light source. With autochthonous proportional abundance greater in the littoral zone and allochthonous proportional abundance greater in the riparian, there is evidence that short-wavelength LED lighting creates both dispersal barriers and an asymmetrical vacuum effect wherein aquatic emergent insects are more readily pulled from their habitat of origin than are terrestrial invertebrates. A recent study examining distance thresholds on adult aquatic insects [48] found that a majority of Trichoptera were captured much closer to the aquatic-riparian border than other taxa, such as Ephemeroptera, concurring with our findings that the capture versus vacuum effect varies among taxa. This taxa-specific response suggests a degree of predictability of communities and ecosystems to ALAN, and also highlights the context dependency of our work, performed in a single pond and on a single night.
The increased numerical response we observed in specific wavelengths did not always directly translate to an increased response in total biomass, a response owing to differences in mean individual insect size. A relatively smaller number of larger bodied individuals had a positive effect on biomass response. Taxa-specific response to wavelength, therefore, could have a positive impact for predators who rely on these particular resources, and a potentially negative impact on predators who do not. Previous studies on riparian spiders have indicated a numerical response to prey under artificially lit conditions [45,49,50] including an increase in body size under LED lamps [7]. The ‘rare large prey hypothesis' [51] suggests spiders obtain most of their energy from few large prey and a recent survey of orb-weaver species supports this conclusion [52]. In our study, we saw both a heightened numerical response, biomass response and body size response of emergent aquatic insects in shorter wavelength traps, suggesting these areas may draw a greater number of arachnid predators regardless of site selection mechanism. However, insects in our riparian control site were also larger on average than other sites, indicating light avoidance by larger bodied terrestrial insects (such as Cicadellidae here) could also influence this metric. Disentangling numeric response from size preference in predators such as spiders, which are responsible for a large portion of energy transfer from aquatic to terrestrial environments [53,54] is an important step in fully understanding the effects of light and spectral composition on invertebrate resource flux and an important next step in ALAN studies. These results also highlight that, like many anthropogenic activities, ALAN will benefit some taxa, and have adverse consequences for others.
The family-specific responses to light were a driving factor for all of our results. This taxa-specific response to different light treatments highlights uncertainty in generalizing about the effects of wavelength on other communities and the fluxes of resources across boundaries. Here we found that the family Caenidae drove the asymmetrical flux of resources between aquatic and terrestrial habitats. This order, Ephemeroptera, is well known for being intolerant to pollution, especially that related to water quality. Had we performed this study in a system with greater human impacts and with less abundant Ephemeroptera, a different result may have emerged, illustrating an as-of-yet untested mechanism of how human activities can impact resource subsidies. Similarly, our experiment was performed on a single evening. A key facet of insect emergence for some taxa is synchronous emergence over brief time periods. Had we performed this experiment later or earlier in the annual growing cycle, different taxa may have dominated emergence, and the impact on communities and biomass may have differed. Additionally, the sudden addition of light into a previously light-naive environment may have initial large impacts that dissipate over time in a continuously lit environment. Exploring how these patterns change over time is important to address the limitations of a single-night experimental design.
An additional essential next step to understanding the impact of ALAN on the riparian to terrestrial ecosystem pipeline is examining predator/prey interactions under varying wavelengths of LEDs. Recent studies observed wavelength-specific responses in a diurnal parasitoid predator–prey relationship [37], and an overall increase in predator abundance under ALAN [55], but to our knowledge a riparian predator response to prey under quasi-monochromatic lighting has yet to be observed. However, as better data emerges regarding taxa-specific responses to ALAN, so might more predictability for overall ALAN arthropod response. Our study, like much of the previous body of ALAN work, followed the convention of measuring illumination. Future studies into arthropod response to ALAN should consider other relevant metrics, such as irradiance and flicker rate in order to build a more complete picture of ALAN impact.
While LED lighting may offer practical advantages over other lighting technologies, such as being more energy efficient, its common peaks in blue/violet spectrum have resulted in documented impacts on animal and human health. Recent investigation into the effect of blue light on human behaviour has suggested a shift to violet emissions for LEDs [56] to achieve healthier lighting environments. Our findings suggest this be avoided, at least near aquatic systems, owing to potential impacts on non-human organisms. Our findings suggest wavelengths in the violet range could increase ALAN impacts on aquatic insects beyond what we have observed with older lighting technologies [28], which peaks in the orange/yellow spectrum and may not have the same attractive properties to aquatic emergent insects. While we did not test these peaks in broad-spectrum lighting directly, our research supports the idea that shorter wavelengths are more attractive to specific insect taxa. A logical next step would be comparing a variety of broad-spectrum LEDs to build on the results reported here.
Our findings suggest that lighting near shorelines could result in asymmetrical resource fluxes in terms of both insect abundance and biomass, driven by taxa-specific wavelength responses. Based on our findings of spectral sensitivity in adult emergent aquatic insects and recent findings of wavelength-specific responses of larval aquatic insects [36], the spectral composition of light sources should be considered when illuminating shoreline habitats if a goal is to minimize impacts to insect communities. Broad-spectrum, neutral white LEDs are quickly becoming the least expensive and most readily available light sources [12], and a benefit to this newer technology is the ability to tune it to particular narrow wavelength bands. Adjusting the wavelength of LED lighting in over-water and near shore situations could be a way to reduce the effects of ALAN on invertebrates. As LED becomes the norm for outdoor lighting, this adjustment could have ecosystem-wide consequences for how resources are transferred between connected habitats.
Acknowledgements
We would like to thank Daryl Bernard and Seven Ponds Nature Center for site use. We would also like to thank Jasmine Mancuso, Emily Bovee, Ryan Andrews Nick Parkinson, Zach Schwab and Chandler Schneider for help in the field, Katri Studtmann for field help and creation of L-braces, Sarah Griffith for photos, Cathy Starnes and Jan Bills for administrative support and LED Supply for providing spectral information for LEDs used in the study.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The data are provided in the electronic supplementary material [57].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
E.P.: conceptualization, data curation, formal analysis, investigation, writing—original draft, writing—review and editing; S.D.T.: conceptualization, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was funded by a National Science Foundation Graduate Research Fellowship to E.P.
References
- 1.Nakano S, Murakami M. 2001. Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proc. Natl Acad. Sci. USA 98, 166-170. ( 10.1073/pnas.98.1.166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baxter CV, Fausch KD, Carl Saunders W. 2005. Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw. Biol. 50, 201-220. ( 10.1111/j.1365-2427.2004.01328.x) [DOI] [Google Scholar]
- 3.Hampton SE, Fradkin SC, Leavitt PR, Rosenberger EE. 2011. Disproportionate importance of nearshore habitat for the food web of a deep oligotrophic lake. Mar. Freshw. Res. 62, 350. ( 10.1071/MF10229) [DOI] [Google Scholar]
- 4.Fukui D, Murakami M, Nakano S, Aoi T. 2006. Effect of emergent aquatic insects on bat foraging in a riparian forest. J. Anim. Ecol. 75, 1252-1258. ( 10.1111/j.1365-2656.2006.01146.x) [DOI] [PubMed] [Google Scholar]
- 5.Burdon FJ, Harding JS. 2008. The linkage between riparian predators and aquatic insects across a stream-resource spectrum. Freshw. Biol. 53, 330-346. ( 10.1111/j.1365-2427.2007.01897.x) [DOI] [Google Scholar]
- 6.Russo D, Cosentino F, Festa F, De Benedetta F, Pejic B, Cerretti P, Ancillotto L. 2019. Artificial illumination near rivers may alter bat-insect trophic interactions. Environ. Pollut. 252, 1671-1677. ( 10.1016/j.envpol.2019.06.105) [DOI] [PubMed] [Google Scholar]
- 7.Parkinson E, Lawson J, Tiegs SD. 2020. Artificial light at night at the terrestrial-aquatic interface: effects on predators and fluxes of insect prey. PLoS ONE 15, e0240138. ( 10.1371/journal.pone.0240138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stenroth K, Polvi LE, Faltstrom E, Johnsson M. 2015. Land-use effects on terrestrial consumers through changed size structure of aquatic insects. Freshw. Biol. 60, 136-149. ( 10.1111/fwb.12476) [DOI] [Google Scholar]
- 9.García–Girón J, Tolonen KT, Soininen J, Snåre H, Pajunen V, Heino J. 2022. Anthropogenic land–use impacts on the size structure of macroinvertebrate assemblages are jointly modulated by local conditions and spatial processes. Environ. Res. 204, 112055. ( 10.1016/j.envres.2021.112055) [DOI] [PubMed] [Google Scholar]
- 10.Hölker F, Wolter C, Perkin EK, Tockner K. 2010. Light pollution as a biodiversity threat. Trends Ecol. Evol. 25, 681-682. ( 10.1016/j.tree.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 11.Kyba CCM, et al. 2017. Artificially lit surface of Earth at night increasing in radiance and extent. Sci. Adv. 3, e1701528. ( 10.1126/sciadv.1701528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falchi F, Cinzano P, Duriscoe D, Kyba CC, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, Furgoni R. 2016. The new world atlas of artificial night sky brightness. Sci. Adv. 2, e1600377. ( 10.1126/sciadv.1600377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trotter AJ, Battaglene SC, Pankhurst PM. 2005. Buoyancy control and diel changes in swim-bladder volume in cultured striped trumpeter (Latris lineata) larvae. Mar. Freshw. Res. 56, 361. ( 10.1071/MF04209) [DOI] [Google Scholar]
- 14.Gaston KJ, Davies TW, Nedelec SL, Holt LA. 2017. Impacts of artificial light at night on biological timings. Annu. Rev. Ecol. Evol. Syst. 48, 49-68. ( 10.1146/annurev-ecolsys-110316-022745) [DOI] [Google Scholar]
- 15.Shuboni D, Yan L. 2010. Nighttime dim light exposure alters the responses of the circadian system. Neuroscience 170, 1172-1178. ( 10.1016/j.neuroscience.2010.08.009) [DOI] [PubMed] [Google Scholar]
- 16.Durrant J, Michaelides EB, Rupasinghe T, Tull D, Green MP, Jones TM. 2015. Constant illumination reduces circulating melatonin and impairs immune function in the cricket Teleogryllus commodus. PeerJ 3, e1075. ( 10.7717/peerj.1075) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dacke M, Baird E, Byrne M, Scholtz CH, Warrant EJ. 2013. Dung beetles use the milky way for orientation. Curr. Biol. 23, 298-300. ( 10.1016/j.cub.2012.12.034) [DOI] [PubMed] [Google Scholar]
- 18.Davies TW, Duffy JP, Bennie J, Gaston KJ. 2014. The nature, extent, and ecological implications of marine light pollution. Front. Ecol. Environ. 12, 347-355. ( 10.1890/130281) [DOI] [Google Scholar]
- 19.Bird BL, Branch LC, Miller DL. 2004. Effects of coastal lighting on foraging behavior of beach mice. Conserv. Biol. 18, 1435-1439. ( 10.1111/j.1523-1739.2004.00349.x) [DOI] [Google Scholar]
- 20.Warrant E, Dacke M. 2011. Vision and visual navigation in nocturnal insects. Annu. Rev. Entomol. 56, 239-254. ( 10.1146/annurev-ento-120709-144852) [DOI] [PubMed] [Google Scholar]
- 21.Horváth G, Mora A, Bernath B, Kriska G. 2011. Polarotaxis in non-biting midges: female chironomids are attracted to horizontally polarized light. Physiol. Behav. 104, 1010-1015. ( 10.1016/j.physbeh.2011.06.022) [DOI] [PubMed] [Google Scholar]
- 22.Kriska G, Horváth G, Andrikovics S. 1998. Why do mayflies lay their eggs on dry asphalt roads? Water imitating polarized light reflected from asphalt attracts Ephemeroptera. J. Exp. Biol. 201, 2273-2286. ( 10.1007/978-3-662-09387-0_22) [DOI] [PubMed] [Google Scholar]
- 23.Eisenbeis G. 2006. Artificial lighting and invertebrates. In Ecological consequences of artificial night lighting (eds Longcore T, Rich C), pp. 281-304. Washington, DC: Island Press. [Google Scholar]
- 24.Luginbuhl CB, Boley PA, Davis DR. 2014. The impact of light source spectral power distribution on sky glow. J. Quant. Spectrosc. Radiat. Transfer 139, 21-26. ( 10.1016/j.jqsrt.2013.12.004) [DOI] [Google Scholar]
- 25.Pawson SM, Bader MKF. 2014. LED lighting increases the ecological impact of light pollution irrespective of color temperature. Ecol. Appl. 24, 1561-1568. ( 10.1890/14-0468.1) [DOI] [PubMed] [Google Scholar]
- 26.Desouhant E, Gomes E, Mondy N, Amat I. 2019. Mechanistic, ecological, and evolutionary consequences of artificial light at night for insects: review and prospective. Entomol. Exp. Appl. 167, 37-58. ( 10.1111/eea.12754) [DOI] [Google Scholar]
- 27.Grubisic M, Van Grunsven RH. 2021. Artificial light at night disrupts species interactions and changes insect communities. Curr. Opin. Insect Sci. 47, 136-141. ( 10.1016/j.cois.2021.06.007) [DOI] [PubMed] [Google Scholar]
- 28.Perkin EK, Hölker F, Tockner K. 2014. The effects of artificial lighting on adult aquatic and terrestrial insects. Freshw. Biol. 59, 368-377. ( 10.1111/fwb.12270) [DOI] [Google Scholar]
- 29.Manfrin A, Singer G, Larsen S, Weiß N, Van Grunsven RH, Weiß NS, Wohlfahrt S, Monaghan MT, Hölker F. 2017. Artificial light at night affects organism flux across ecosystem boundaries and drives community structure in the recipient ecosystem. Front. Environ. Sci. 5, 61. ( 10.3389/fenvs.2017.00061) [DOI] [Google Scholar]
- 30.Larsson M, Göthberg A, Milberg P. 2020. Night, light and flight: light attraction in Trichoptera. Insect Conserv. Divers. 13, 296-302. ( 10.1111/icad.12379) [DOI] [Google Scholar]
- 31.Lockett MT, Jones TM, Elgar MA, Gaston KJ, Visser ME, Hopkins GR. 2021. Urban street lighting differentially affects community attributes of airborne and ground-dwelling invertebrate assemblages. J. Appl. Ecol. 58, 2329-2339. ( 10.1111/1365-2664.13969) [DOI] [Google Scholar]
- 32.Longcore T, Aldern HL, Eggers JF, Flores S, Franco L, Hirshfield-Yamanishi E, Petrinec LN, Yan WA, Barroso AM. 2015. Tuning the white light spectrum of light emitting diode lamps to reduce attraction of nocturnal arthropods. Phil. Trans. R. Soc. B 370, 20140125. ( 10.1098/rstb.2014.0125) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakefield A, Broyles M, Stone EL, Jones G, Harris S. 2016. Experimentally comparing the attractiveness of domestic lights to insects: do LEDs attract fewer insects than conventional light types? Ecol. Evol. 6, 8028-8036. ( 10.1002/ece3.2527) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakefield A, Broyles M, Stone EL, Harris S, Jones G. 2018. Quantifying the attractiveness of broad-spectrum street lights to aerial nocturnal insects. J. Appl. Ecol. 55, 714-722. ( 10.1111/1365-2664.13004) [DOI] [Google Scholar]
- 35.Hollingsworth JP, Hartstack AW, Lindquist DA. 1968. Influence of near-ultraviolet output of attractant lamps on catches of insects by light traps. J. Econ. Entomol. 61, 515-521. ( 10.1093/jee/61.2.515) [DOI] [Google Scholar]
- 36.Kuhne JL, Van Grunsven RHA, Jechow A, Holker F. 2021. Impact of different wavelengths of artificial light at night on phototaxis in aquatic insects. Integr. Comp. Biol. 61, 1182-1190. ( 10.1093/icb/icab149) [DOI] [PubMed] [Google Scholar]
- 37.Sanders D, Baker DJ, Cruse D, Bell F, Van Veen FJF, Gaston KJ. 2022. Spectrum of artificial light at night drives impact of a diurnal species in insect food web. Sci. Total Environ. 831, 154893. ( 10.1016/j.scitotenv.2022.154893) [DOI] [PubMed] [Google Scholar]
- 38.Merritt RW, Cummins KW, Berg MB. 2019. An introduction to the aquatic insects of North America, 5th edn. Dubuque, IA: Kendall Hunt. [Google Scholar]
- 39.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671-675. ( 10.1038/nmeth.2089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sample BE, Cooper RJ, Greer RD, Whitmore RC. 1993. Estimation of insect biomass by length and width. Am. Midland Naturalist 129, 234. ( 10.2307/2426503) [DOI] [Google Scholar]
- 41.Sabo JL, Bastow JL, Power ME. 2002. Length–mass relationships for adult aquatic and terrestrial invertebrates in a California watershed. J. North Am. Benthol. Soc. 21, 336-343. ( 10.2307/1468420) [DOI] [Google Scholar]
- 42.Oakensen J, et al. 2013. Vegan: community ecology package. R package version 2.0-.10. See http://CRAN.R-project.org/package=vegan. [Google Scholar]
- 43.R Core Team. 2022. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 44.Fox J, Weisberg S. 2011. An {R} companion to applied regression, 2nd edn. Thousand Oaks, CA: Sage. See http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. [Google Scholar]
- 45.Sullivan SMP, Hossler K, Meyer LA. 2019. Artificial lighting at night alters aquatic-riparian invertebrate food webs. Ecol. Appl. 29, e01821. ( 10.1002/eap.1821) [DOI] [PubMed] [Google Scholar]
- 46.Corbet PS. 1964. Temporal patterns of emergence in aquatic insects. Can. Entomol. 96, 264. ( 10.4039/Ent96264-1) [DOI] [Google Scholar]
- 47.Van Der Kooi CJ, Stavenga DG, Arikawa K, Belusic G, Kelber A. 2021. Evolution of insect color vision: from spectral sensitivity to visual ecology. Annu. Rev. Entomol. 66, 435-461. ( 10.1146/annurev-ento-061720-071644) [DOI] [PubMed] [Google Scholar]
- 48.Carannante D, Blumenstein CS, Hale JD, Arlettaz R. 2021. LED lighting threatens adult aquatic insects: impact magnitude and distance thresholds. Ecol. Solut. Evidence 2, e12053. ( 10.1002/2688-8319.12053) [DOI] [Google Scholar]
- 49.Manfrin A, Lehmann D, van Grunsven RH, Larsen S, Syväranta J, Wharton G, Voigt CC, Monaghan MT, Hölker F. 2018. Dietary changes in predators and scavengers in a nocturnally illuminated riparian ecosystem. Oikos 127, 960-969. ( 10.1111/oik.04696) [DOI] [Google Scholar]
- 50.Davies TW, Bennie J, Gaston KJ. 2012. Street lighting changes the composition of invertebrate communities. Biol. Lett. 8, 764-767. ( 10.1098/rsbl.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venner S, Casas J. 2005. Spider webs designed for rare but life-saving catches. Proc. R. Soc. B 272, 1587-1592. ( 10.1098/rspb.2005.3114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blackledge TA. 2011. Prey capture in orb weaving spiders: are we using the best metric? J. Arachnol. 39, 205-210. ( 10.1636/Chi10-52.1) [DOI] [Google Scholar]
- 53.Akamatsu F, Toda H, Okino T. 2004. Food source of riparian spiders analyzed by using stable isotope ratios. Ecol. Res. 19, 655-662. [Google Scholar]
- 54.Iwata T. 2007. Linking stream habitats and spider distribution: spatial variations in trophic transfer across a forest–stream boundary. Ecol. Res. 22, 619-628. ( 10.1007/s11284-006-0060-6) [DOI] [Google Scholar]
- 55.Mcmunn MS, et al. 2019. Artificial light increases local predator abundance, predation rates, and herbivory. Environ. Entomol. 48, 1331-1339. ( 10.1093/ee/nvz103) [DOI] [PubMed] [Google Scholar]
- 56.Moore-Ede M, Heitmann A, Guttkuhn R. 2020. Circadian potency spectrum with extended exposure to polychromatic white LED light under workplace conditions. J. Biol. Rhythms 35, 405-415. ( 10.1177/0748730420923164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parkinson E, Tiegs SD. 2023. Spectral composition of light-emitting diodes (LED) impacts aquatic and terrestrial invertebrate communities with potential implications for cross-ecosystem subsidies. Figshare. ( 10.6084/m9.figshare.c.6856475) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Parkinson E, Tiegs SD. 2023. Spectral composition of light-emitting diodes (LED) impacts aquatic and terrestrial invertebrate communities with potential implications for cross-ecosystem subsidies. Figshare. ( 10.6084/m9.figshare.c.6856475) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in the electronic supplementary material [57].