Abstract
Artificial light at night (ALAN) is predicted to have far-reaching consequences for natural ecosystems given its influence on organismal physiology and behaviour, species interactions and community composition. Movement and predation are fundamental ecological processes that are of critical importance to ecosystem functioning. The natural movements and foraging behaviours of nocturnal invertebrates may be particularly sensitive to the presence of ALAN. However, we still lack evidence of how these processes respond to ALAN within a community context. We assembled insect communities to quantify their movement activity and predation rates during simulated Moon cycles across a gradient of diffuse night-time illuminance including the full range of observed skyglow intensities. Using radio frequency identification, we tracked the movements of insects within a fragmented grassland Ecotron experiment. We additionally quantified predation rates using prey dummies. Our results reveal that even low-intensity skyglow causes a temporal shift in movement activity from day to night, and a spatial shift towards open habitats at night. Changes in movement activity are associated with indirect shifts in predation rates. Spatio-temporal shifts in movement and predation have important implications for ecological networks and ecosystem functioning, highlighting the disruptive potential of ALAN for global biodiversity and the provision of ecosystem services.
This article is part of the theme issue ‘Light pollution in complex ecological systems’.
Keywords: light pollution, ALAN, fragmented landscapes, activity pattern, foraging, crepuscular
1. Introduction
Artificial light at night (ALAN) is a rapidly increasing global phenomenon impacting the physiology and behaviour of organisms [1], their interactions [2,3] and space use [4,5], as well as the composition of species within and across communities [6–8]. It, therefore, has the potential to drastically alter natural ecosystems, and has been proposed as a major driver of insect decline [9,10].
To date, studies of the ecological impacts of ALAN have focused almost exclusively on the responses of animals near to individual bright sources of light (such as streetlights). However, ALAN also affects ecosystems much further from areas of human activity via the phenomenon known as ‘skyglow’, the diffuse and low-intensity artificial light that is reflected back to Earth by clouds and aerosols in the atmosphere [11–13]. The illuminance of skyglow is often far larger than starlight [14], and can approach the brightness of the full Moon [15]. Furthermore, in areas affected by skyglow, overcast nights are no longer dark [16], so the overall range of night-time illuminance experienced by the ecosystem is reduced by several orders of magnitude compared with natural conditions. For instance, it has been shown that even low levels of artificial light intensities have the capacity to modify foraging efficiency and the strength of interspecific interactions, which leads to corresponding changes in community structure [2], highlighting the need to study the community- and ecosystem-level effects of comparatively low-intensity skyglow [17,18].
To better address the effects of ALAN in general and skyglow in particular on biodiversity and ecosystem functioning, we require a holistic understanding of the underlying ecological processes that drive species' distributions and abundances, such as animal space use and biotic interactions. Movement is a key mediator of these processes as it enables animals to explore their environment for food, potential mates and suitable habitats. Unfortunately, the sensitivity of animal movement to the presence of diffuse night-time illuminance such as skyglow remains unclear.
Light pollution has been shown to have widespread effects on movement behaviours across several spatial scales, such as migration [19–21] and dispersal [22,23], as well as local movements within and between habitats [24–27]. These effects can be diverse [8]; for instance, at local scales there is evidence of reduced [28–31] as well as increased movement activity [32] in response to ALAN, which may be induced by the elevated risk or facilitation of predation, respectively. Moreover, animals may shift their activity temporally, with or without affecting their overall activity time budget [33–35]. Generally, the onset and duration of movement activity among visual predators depend on illumination levels that facilitate successful foraging activity [36]. This implies that ALAN acts on the temporal as well as spatial dimension of movement with diverse knock-on effects for encounter rates and interactions among hetero- and conspecifics.
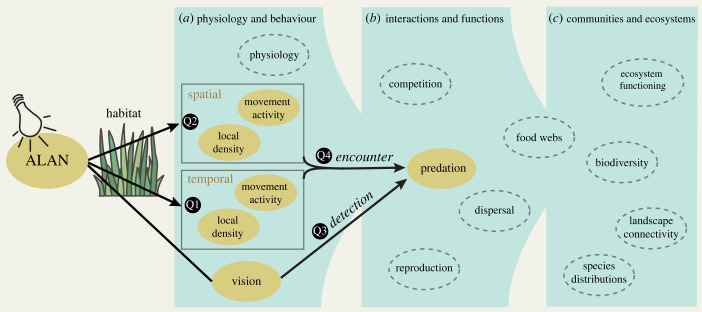
Predation is a key ecological interaction that determines the structure and functioning of ecosystems. ALAN can drive predation rates (i) by affecting spatio-temporal movement activity (i.e. where and when they move) and local densities, and thus encounters between predator and prey, or (ii) by affecting detection ability [37] (figure 1). Among animals that rely on visual cues to orient themselves or detect and capture their prey, visual acuity facilitates the processing of spatial information and increases the minimum distances at which potential prey becomes visible. Visual predators, particularly those that possess adaptations which increase visual acuity under the ambient light conditions of their temporal niche [38], are expected to be particularly sensitive to ALAN [2,39]. Habitat structure can modify the effects of ALAN on animal movement and foraging behaviour by altering the trade-off between foraging success and predation risk [25,34,40], for instance by impeding predators’ movements and their visual detection of prey or through the provision of prey refuges [41]. Moreover, ALAN can change species' preferences for certain habitats and thus influence space use, habitat connectivity [4,5,26] and (co-)occurrence, with profound effects on encounter probabilities. This demonstrates that ALAN has the potential to fundamentally disrupt trophic interactions, with implications for food webs, species distributions, biodiversity and ecosystem functioning (figure 1).
Figure 1.
Concept illustrating how ALAN may cascade from physiological and behavioural processes (a), to interactions and functions (b), and ultimately to community and ecosystem responses (c). Orange ovals and black arrows indicate our research questions: how does ALAN affect temporal (Q1) and spatial movement activity (Q2) in a patchy habitat? Does ALAN predominantly affect predation rates through detection probabilities (Q3), or are predation rates rather driven by the effects of spatio-temporal movement activity on encounter rates between predator and prey (Q4)? (Online version in colour.)
We designed an Ecotron experiment with simulated diel light and Moon cycles to elucidate the interactive effects of skyglow and habitat structure on movement and predation. We continuously tracked the movements of individual insects (792 individuals across seven beetle and one bug species) within experimental grassland-patch landscapes using radio frequency identification (RFID) tracking and measured predation rates on artificial caterpillar prey dummies. We quantified how temporal (Q1) and spatial movement activity (Q2) respond to night-time illuminance across a gradient from 0.001 lux (starlight) to 30 lux (under a streetlight). Furthermore, we measured the effect of light on predation rates and discuss their dependence on detection probabilities and encounter rates (Q3 and Q4) (figure 1).
2. Methods
(a) . General set-up and experimental design
We conducted our experiment at the iDiv Ecotron experimental facility, which is an indoor mesocosm facility consisting of independent, experimental chambers called ‘EcoUnits’ [42]. The Ecotron is located in Bad Lauchstädt, Saxony-Anhalt, Germany, at the Experimental Research Station of the Helmholtz Centre for Environmental Research (UFZ, 51.3917° N, 11.8762° E). Multiple environmental conditions in the EcoUnits can be fully controlled (e.g. nutrient supply and irrigation). Each EcoUnit has internal dimensions of 1.46 × 1.46 × 1.50 m (L × W × H, aboveground) and 1.24 × 1.24 × 0.80 m (L × W × H, belowground), with the soil surface area measuring 1.54 m2 [42]. We conducted the experiment in 12 EcoUnits from July to October 2020.
To assess the interactive effects of diffuse night-time illuminance and landscape structure on animal movement patterns, we established a patch-grassland system that consisted of four meadow patches within each of the corners of an EcoUnit, separated by an area of bare ground (figure 2a,b). The EcoUnits were filled with 1.23 m3 of unsterilized and homogenized soil from the vicinity of the iDiv Ecotron, and plant communities of 16 plant species were sown on 4 February 2020 (electronic supplementary material, table S1). We allowed a settlement phase of roughly five months before starting our measurements.
Figure 2.
Experimental design. (a) Interior view of the grassland habitat patches established in an EcoUnit. (b) Schematic of the patch design highlighting the distribution of radio frequency identification (RFID) sensors and prey dummies across the EcoUnit. (c) EcoUnits covered with black theatre curtains to prevent cross-contamination with light. (d) Pictures of a beetle with medium-sized RFID-tag (taken from an experiment using the same tracking approach and setting, [43]) and an artificial caterpillar prey dummy with bite marks. (Online version in colour.)
(b) . Light treatment
Across the 12 EcoUnits, we simulated diel light and Moon cycles and added a treatment of diffuse night-time illuminance including the full range of observed skyglow intensities [16,44,45].
(c) . Daylight
The daytime lighting (manufactured by Roschwege, Germany) within all EcoUnits was set to the same daylight settings. Photoperiods were adjusted every four weeks to approximate local sunrise and sunset times throughout the duration of the experimental period. Daylight was gradually (i.e. linearly) brightened or dimmed over the course of 2 h before sunrise and sunset, respectively. The maximum brightness of the daytime lighting was approximately 35 000 lux, which corresponds roughly to a sunny day in Germany, with a light spectrum that approximates sunlight.
(d) . Moonlight
At night, moonlight within each EcoUnit was simulated by a single Sunlike LED (SunLike3030 by Seoul Semiconductor Co., Korea) with a light spectrum that approximates sunlight (electronic supplementary material, figure S1). We simulated moonlight because complete darkness is not a meaningful control [46,47], and organisms have adapted to Moon cycles over the course of evolutionary history. Moonlight intensities for clear-sky conditions were modelled for the real time and location of the experiment using an astronomical model of solar and lunar illuminance. The illuminance model calculates direct and diffuse illuminance and was based on the model of Janiczek & DeYoung [48], with several enhancements to increase accuracy. Illuminance of the moonlight LED was adjusted automatically every minute using a Python script running on a Raspberry Pi, and could be adjusted to 57 illuminance levels spanning from 0 lux (off) to the maximal modelled moonlight brightness of approximately 0.274 lux.
(e) . Night-time illuminance
We established a skyglow treatment with a gradient of diffuse night-time illuminance that spanned from 0.0014 lux (slightly brighter than starlight) to 30 lux on a log10 scale. The very upper end of our gradient is brighter than the skyglow that is observed in nature today but might cover future scenarios of diffuse night-time illuminance. The levels of night-time illuminance at both ends of the gradient were replicated once. We used LED lights (type 2835 by HuiYuan Opto-Electronic Co., China) with a typical blue light peak within their spectrum (electronic supplementary material, figure S2). This resulted in illuminance of 0.0014 (no pollution control), 0.0087, 0.028, 0.081, 0.1, 0.3, 0.94, 3.03, 9.88 and 30.31 lux (in the absence of moonlight). Note that half of the gradient lies below the maximum brightness of the full Moon during the experimental phase (0.274 lux). We chose to cover these low light intensities for two major reasons: (i) they represent light levels organisms naturally experience and have adapted to for millions of years, and (ii) they reflect typical intensities of far-reaching skyglow [14]. To avoid stunning animals by sudden changes in brightness, the treatment lights were always switched on and off at sunrise and sunset, respectively (when daylight was at 50%). To avoid point sources of light and simulate diffuse night-time illuminance such as skyglow, the light was scattered using diffusion foil.
All units were covered with black theatre curtains to block light from outside (see figure 2c). The illuminance in the units was calibrated via a sky brightness measurement approach using a fisheye-lens camera [16].
(f) . Study animals
We collected the insects for the experimental communities in the area surrounding Leipzig, Saxony, Germany (51.3213° N, 12.3964° E and 51.2799° N, 12.4119° E) from June to August 2020 using pitfall traps. Our species selection (electronic supplementary material, table S2) depended on seasonal densities and occurrences, and consisted of seven species of carabid ground beetle (Coleoptera: Carabidae) which are primarily crepuscular or nocturnal (electronic supplementary material, table S2, [49]): Abax parallelus (Duftschmid), Calathus fuscipes (Goeze), Carabus granulatus (Linnaeus), Carabus nemoralis (Müller), Harpalus rufipes (De Geer), Nebria brevicollis (Fabricius) and Pterostichus melanarius (Illiger) (electronic supplementary material, table S2). All species were housed in separate containers that were bedded with moistened soil and foliage, and fed ad libitum with beetle jelly from a commercial supplier (The Pet Factory, Germany) prior to the experiment. In total, our Ecotron communities constituted a total of 792 RFID-tagged individuals from seven species across two orders, with body masses ranging from 47 mg (Calathus fuscipes) to 707 mg (Carabus nemoralis, electronic supplementary material, table S2) . We distributed them equally across the EcoUnits at densities that reflect a natural abundance–mass relationship (electronic supplementary material, table S2).
(g) . Movement tracking via radio frequency identification tags
We used a radio frequency identification (RFID) tracking system consisting of passive RFID-tags, RFID-readers (transceivers) and a host system (controller) to track the movements of our study animals (see [43] for details). We distributed 36 RFID-sensors equally across patch and matrix areas in the EcoUnits (four sensors in each patch and 20 sensors in the matrix, figure 2b). Before adding the study animals to the EcoUnits, we weighed and tagged the individuals with a unique RFID-tag. We kept the insects at 4°C for 15 min before gluing the tag to the elytra of the beetles. We used medium-sized (size: 8.3 × 8.3 × 10.7 mm, reading range: 25 mm, mass: 35 mg, Murata LXMSAPHA17-176) and small RFID-tags (size: 3.2 × 3.2 × 0.75 mm, reading range: 12 mm, mass: 20 mg, Murata LXMS33HCNK-171), for large- (body mass > 200 mg) and small-bodied (body mass < 200 mg) species, respectively. We recorded the tag-ID together with the identity and body mass of the individual.
Movement tracking inside the EcoUnits was performed across two temporal experimental blocks, each corresponding to a period of approximately one lunar cycle (i.e. 28 days: experimental block I: 21 July 2020–18 August 2020, experimental block II: 15 September 2020–13 October 2020). Newly tagged individuals were added a few days prior to the start of the respective experimental block for acclimatization. During the tracking periods, individuals were identified with a unique timestamp when crossing a sensor and disturbances were minimized by only opening the EcoUnits once for the exchange of prey dummies. Together with the exact position of the RFID-sensor in the EcoUnit, this provides unique spatio-temporal information for each tagged individual. We defined detections as distinct and only counted them when (i) they occurred on different sensors or when (ii) at least 10 s had elapsed (without detection on the same sensor) between two consecutive detections on the same sensor. This prevented the repeated detection of resting or dead animals. We used the number of RFID detections as a measure of the movement activity of the community, which is the product of local densities and individual movement.
(h) . Predation rates
Predation rates were estimated across the skyglow gradient by recording bite marks on prey dummies [50–52]. We moulded artificial prey dummies from odourless, non-toxic green plasticine (Noris 8421 by Staedler, Germany) to resemble model caterpillars of a standardized appearance (figure 2d). We mounted 16 prey dummies on pins that were equally spaced within the four habitat patches of each EcoUnit (figure 2b) for two successive 14 day exposures within each four-week temporal experimental block. Two independent observers scored the prey dummies by identifying and counting the bite marks left by carabid predators. Although there are limits to the precision of identification [53], we were able to identify and group the parallel marks left by the mandibles of carabid beetles in order to identify the number of successful attacks on individual prey dummies, thereby quantifying predation rates during each 14 day exposure. This approach is likely only able to elucidate predation rates of visual predators, rather than predators that search via olfaction. Notably, visual hunters are the predators that are also likely to be affected by light.
(i) . Statistical analysis
We fitted generalized linear mixed effects models (GLMMs) using the ‘glmmTMB’ package [54] in R 4.2.2 [55] to investigate the effects of ALAN as diffuse night-time illuminance on the total movement activity, space use and predation rates in the insect communities. To test our first hypothesis, that ALAN alters animal movement activity (Q1), we modelled the interactive effects of diffuse night-time illuminance and diel light cycle (day versus night) on species' movement activity. Movement activity was estimated from the number of detections per day/night and analysed on both the community- and species-level. To assess the effects of ALAN on animal space use during each phase of the diel light cycle (Q2), we modelled the interactive effects of diffuse night-time illuminance and habitat (patch versus matrix) on species’ movement activity (sum of detections per day/night). We used a negative binomial distribution to account for overdispersion in the movement activity data and included the temporal experimental block as a random intercept to account for temporal replication of the four-week experimental tracking within the same EcoUnit. To test whether the effect of night-time illuminance on total movement activity of the community is driven more by individual movement or by local densities, we leveraged the individual-level information provided by the RFID-tags to correct the sum of detections for differences in local densities. Therefore, we included the number of unique RFID-tag detections, aggregated at the corresponding spatial and temporal scale (see electronic supplementary material, table S3 for detailed information), as an offset term to the GLMM models reported in our supplementary analyses. To evaluate if ALAN mediates the predation rates of visual predators by increasing the detection of prey dummies (Q3), we modelled the effect of diffuse night-time illuminance on predation rates (bite counts per 14 days). Predation rates during each of the two four-week experimental blocks were estimated by counting and summing the number of bite marks left by carabid beetles during two successive 14 day exposures. We modelled predation rates using a quasi-Poisson distribution to account for overdispersion, and included the temporal experimental block as a random intercept. To test our final hypothesis, that skyglow affects predation rates via an increased encounter rate with prey (Q4), we first aggregated the data on movement activity and predation rates to comparable spatial scales (figure 2b; electronic supplementary material, table S3): We modelled the effect of patch-level movement activity (sum of detections per 14 days within each patch, figure 2b) on patch-level predation rates (bite counts per 14 days within each patch) using a quasi-Poisson distribution. We included the number of prey dummies recovered from each patch as an offset term, with the temporal experimental block as a random intercept. The patch-level movement activity used in the analyses of predation rates excluded detections of species that are not expected to leave bite marks, i.e. species that are too small to reach mounted prey dummies (body size <200 mg). Figures were created using the R packages ‘ggplot2′ [56] and ‘ggeffects’ [57].
3. Results
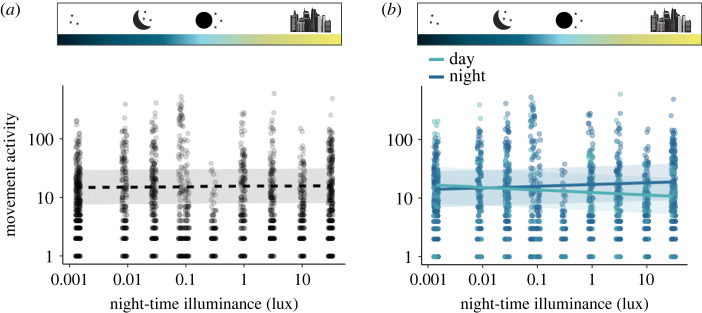
We recorded a total of 25 378 RFID-detections across all experimental insect communities. With regard to our first research question (Q1) on how movement activity (measured as the number of detections per day/night, see Methods) is altered by skyglow, we found no significant effect of night-time illuminance on overall movement activity (figure 3a, slope = 0.014, p = 0.530; electronic supplementary material, table S4a). However, with increased night-time illuminance and despite some variability across species (see electronic supplementary material, table S5), we found a significant decrease in movement activity at the community level during the day (figure 3b, slope = −0.099, p = 0.008; electronic supplementary material, table S6a) and a significant increase in movement activity during the night (figure 3b, slope = 0.069, p = 0.014; electronic supplementary material, table S6a). Together, these results imply a temporal shift in activity from day to night without effects on the overall activity time budget.
Figure 3.
Movement activity (sum of detections per day/night) in response to night-time illuminance. EcoUnit-level daily movement activity (per 24 h) (a) and daytime (light blue) and night-time movement activity (dark blue) (b). Dashed lines represent non-significant relationships (p > 0.05). Shaded regions represent 95% confidence intervals. (Online version in colour.)
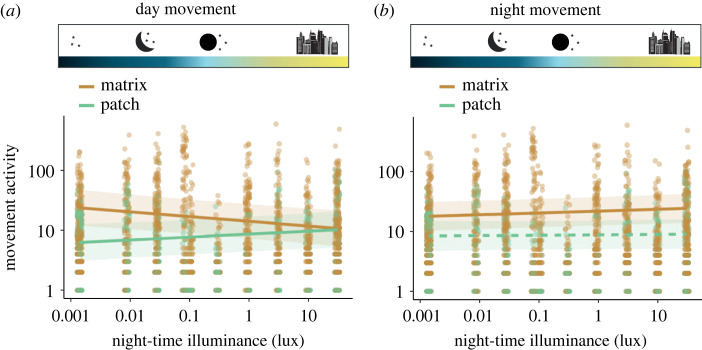
Furthermore, we observed a spatial shift in movement activity of the insect community in response to night-time illuminance (Q2). During daytime, the movement activity within the matrix decreased with night-time illuminance (figure 4a, slope = −0.183, p = 0.001; electronic supplementary material, table S7a), while the movement activity increased in the habitat patches (figure 4a, slope = 0.114, p = 0.048; electronic supplementary material, table S7a). This suggests a shift in space use towards denser habitats during daytime. By contrast, we found that night-time movement activity increased within the matrix in response to the effect of night-time illuminance (figure 4b, slope = 0.072, p = 0.028; electronic supplementary material, table S8a), while no significant effect was observed within habitat patches (figure 4b, slope = 0.018, p = 0.653; electronic supplementary material, table S7). This suggests that the increased nocturnal movement activity of the insect community as well as the corresponding decreased movement activity during day predominantly took place within the matrix.
Figure 4.
Movement activity (sum of detections per day/night) in habitat patches (green) and matrix (orange) in response to night-time illuminance during the day (a) and night (b). Dashed lines represent non-significant relationships (p > 0.05). Shaded regions represent 95% confidence intervals. (Online version in colour.)
To elucidate whether the effects of night-time illuminance on movement activity are driven by changes in individual movement or local densities, we performed all analyses (Q1 and Q2) with the local densities as an offset. All results remained virtually identical (see electronic supplementary material, figures S3 and S4 and tables S4b, S6b, S7b and S8b). Furthermore, the number of detected individuals per EcoUnit during the second half of each experimental block was not significantly affected by the diffuse night-time illumination (see electronic supplementary material, table S9), suggesting that abundances were unaffected by the light treatment. Together, this indicates that night-time illuminance drives the community-level movement activity mainly through changes in individual movement.
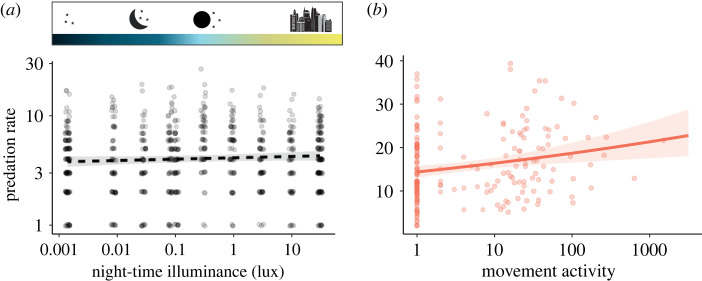
Contrary to our expectations, there was no significant effect of night-time illuminance on the overall predation rate (estimated by the number of bite marks on individual prey dummies) by the experimental insect community (figure 5a, slope = 0.029, p = 0.127; electronic supplementary material, table S10) (Q3). This reflects the neutral effect of night-time illuminance on the overall movement activity (figure 3a). In contrast to movement activity (figure 3b), bite marks could not be associated with night or day, as they were only collected every two weeks. However, we did find a strong correlation between patch-level predation rate and movement activity (figure 5b, slope = 0.131, p = 0.002; electronic supplementary material, table S11), i.e. we counted significantly more bite marks on prey dummies from patches that reported a higher movement activity. Together with our results showing no effect of night-time illuminance on local densities, this supports our expectation that higher movement activity, which enables more frequent encounters between predators and artificial caterpillar prey dummies, is the primary driver of predation rates (Q4).
Figure 5.
(a) EcoUnit-level predation rate (bite counts per 14 days) in response to night-time illuminance. (b) Patch-level predation rate (bite counts per 14 days per patch) in response to patch-level movement activity (sum of detections per 14 days per patch, figure 2). Dashed lines represent non-significant relationships (p > 0.05). Shaded regions represent 95% confidence intervals. (Online version in colour.)
4. Discussion
We experimentally exposed artificial grassland communities to a gradient of diffuse night-time illuminance, and demonstrated that night-time illuminance elicits spatio-temporal shifts in movement and predation of insects. We found shifts in community-level movement activity from daytime to night-time (Q1) as well as shifts in habitat use from vegetated habitat patches to open habitat at night (Q2). While we did not detect an overall response of predation rates to night-time illuminance, we deduce spatio-temporal shifts in predation rates via their strong correlation with patch-level movement activity (Q3 and Q4).
We tracked the movements of individual animals within experimental insect communities using an RFID-sensor array in order to investigate their response to diffuse night-time illuminance such as skyglow (Q1). The lightweight, passive RFID-tags [58] are well-suited to the tracking of small animals such as insects [59]. Moreover, this approach enabled us to track the movement of the insects in darkness as well as complex physical habitats, which is limited with other methods such as image-based tracking [60]. Despite finding no effect of night-time illuminance on the overall activity time budget (figure 3a), we did detect a temporal shift in movement activity from day to night (figure 3b). Our community is composed primarily of crepuscular and nocturnal species that are likely to be able to extend their temporal niche into the night when artificial light maintains their ability to see and thus forage. As nocturnal foraging probably evolved to reduce competition and predation pressure, crepuscular species may benefit from opportunities that reduce their interactions with diurnal species, which explains the simultaneous reduction in movement activity during the day. Furthermore, by keeping the total time budget constant, these species avoid an overall increase in their total energy expenditure.
In addition to this temporal shift, we observed a change in the insect communities' space use in response to skyglow (Q2), marked by a concomitant increase in nocturnal- and decrease in diurnal movement activity within the bare-soil matrix. The dense vegetation within the habitat patches in our experimental landscapes (figure 2a) reduces light intrusion and visibility, in contrast to the open ground of the interstitial matrix area. This can have important implications for animal movement and foraging behaviour, for instance, by facilitating foraging or increasing predation risk [25,40,61]. The observed increase in movement activity in the matrix at night (figure 4b) fits our interpretation that crepuscular species shift their activity towards nocturnality owing to increased foraging and exploration opportunities. During daytime, not only does the overall movement activity decrease, but there is an additional shift in activity from the matrix to the habitat patches in response to increasing night-time illuminance (figure 4a). A shift towards nocturnal exploration activity could result in a preference for habitat patches that provide protection from potential predators during the day.
Predation rates can be driven by detection success as well as by the probability of encounters between predators and their prey. Higher detection ability facilitated by increased visibility under ALAN should generally lead to higher predation rates. However, as we did not find a significant effect of night-time illuminance on the total number of attacks (bite counts per 14 days) on prey dummies (Q3, figure 5a), we can deduce that there is likely also no significant effect on predation rates via detection probability within our experimental grassland communities. In addition to the predators’ movement activity, local densities and detection success, predation rates could also be influenced by the predators' decision to forage or the behaviour of the prey. In contrast to studies that employ immobile prey dummies, future studies that simultaneously track the movement of prey could elucidate the role of prey responses to ALAN in determining the outcomes of predator–prey interactions (e.g. [61]). Moreover, our use of prey dummies does not allow us to differentiate daytime and night-time predation rates; nevertheless, we found a strong spatio-temporal association between patch-level predation rate and movement activity (Q4). Therefore, based on the absence of an effect of night-time illuminance on movement activity (figure 3a), we expect a corresponding absence of an effect on predation rates when measured across day and night, which is supported by our analysis (figure 5a). This highlights that night-time illuminance drives predation through the rate of encounters between predators and their prey rather than via the predators’ visual detection of prey. Together with the observed temporal and spatial shift in movement activity (figures 3b and 4b, respectively), our results suggest that diffuse night-time illuminance leads to a congruent spatio-temporal shift in predation rates.
Organisms show diverse and context-dependent responses to ALAN [8,62], which was also reflected by some variability in how the species in our experimental community responded. This might translate to distinct community-level responses depending on, for example, the species composition of the community and the ecosystem it is integrated within (e.g. [63]). Here, we could show that in grasslands, diffuse night-time illuminance such as skyglow can influence movement and by extension prey encounters of seven predominantly crepuscular species. Our focus on diffuse night-time illuminance and the range of our experimental gradient covers most of the real-world light conditions from natural starlight to cities [14], suggesting that our results are relevant for light pollution experienced by invertebrates in open habitats such as grasslands and agricultural fields throughout the world. We were able to show that even low levels of night-time illuminance can cause substantial changes in animal movement and consequently predation rates. For instance, more than 50% of the observed change in movement activity (figure 3b) across the whole gradient of night-time illuminance occurred at illuminance levels that were below that of an average full Moon (approx. 0.3 lux). This strong response to low illuminance levels is to be expected among organisms that have adapted to respond to subtle changes in illuminance such as the Moon cycle [61,64] and to life under starlight [65].
Our evidence for skyglow affecting fundamental ecological processes such as the movement of invertebrates and predation suggests cascading and far-reaching repercussions for landscape connectivity, biodiversity and ecosystem functioning. For instance, the shift of predator activity to open habitats, as shown here, could increase the predation risk of dispersing nocturnal prey and diminish landscape connectivity. This is particularly relevant for animals that rely on behavioural shifts towards nocturnal activity to buffer against thermal and water stress [66–68]. Spatio-temporal shifts in predation rates can also have strong implications for species interactions, either by rewiring food webs or by modifying the strengths of interactions. This can fundamentally change the structure of food webs and their stability [69]: A temporal shift in the activity of crepuscular species into the night as found in our study could lead to new interactions with nocturnal species, and in turn, cause interactions with diurnal species to be lost or weakened. Furthermore, changes to encounter probabilities, and consequently interaction strengths can alter energy fluxes in food webs. For instance, an increase in predation rates at night could lead to higher energy fluxes, with knock-on effects on the stability of nocturnal as well as diurnal food webs [70–72]. Such spatio-temporal changes in movement activity have also been shown to affect other types of ecological networks such as plant–pollinator and host–parasitoid networks [2,7,73]. This suggests that skyglow, as a recent and intensifying anthropogenic disturbance [13], has far-reaching consequences and the potential to fundamentally disrupt natural communities and the services they provide.
Acknowledgements
We thank Jeff Conrad for creating the Windows executable program sunmoon, which was used to estimate the Moon illuminance. Thank you to Iris Veronika Heinrich for assisting us in the laboratory.
Ethics
This work did not require ethical approval from a human subject or animal welfare committee.
Data accessibility
The underlying data are available in an open access repository from the Zenodo repository: https://doi.org/10.5281/zenodo.8017438 [74].
Data are provided in the electronic supplementary material [75].
Declaration of AI use
We have not used AI-assisted technologies in creating this article.
Authors' contributions
A.D.: conceptualization, formal analysis, methodology, writing—original draft, writing—review and editing; R.R.: conceptualization, formal analysis, methodology, supervision, writing—original draft, writing—review and editing; U.B.: conceptualization, formal analysis, methodology, writing—review and editing; A.A.: methodology, writing—review and editing; N.B.: investigation, methodology; T.B.: conceptualization, methodology, software; S.F.B.: investigation, writing—review and editing; S.C.: investigation, writing—review and editing; N.E.: methodology, writing—review and editing; A.G.: methodology, software; J.H.: investigation, project administration, writing—review and editing; C.C.M.K.: methodology, writing—review and editing; M.H.M.M.: methodology, writing—review and editing; K.R.: methodology, software; T.S.: methodology, software; J.F.T.: investigation, methodology, writing—review and editing; M.R.H.: conceptualization, formal analysis, methodology, supervision, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
We gratefully acknowledge the support of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig funded by the German Research Foundation (DFG-FZT 118, 202548816). Funding for A.D. and U.B. was provided by the German Research Foundation (DFG) within the research unit DynaCom (DFG, FOR 2716). C.C.M.K. was supported by the Helmholtz Association Initiative and Networking Fund under grant no. ERC-RA-0031.
References
- 1.Russart KLG, Nelson RJ. 2018. Artificial light at night alters behavior in laboratory and wild animals. J. Exp. Zool. Ecol. Integr. Physiol. 329, 401-408. ( 10.1002/jez.2173) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanders D, Kehoe R, Cruse D, van Veen FJF, Gaston KJ. 2018. Low levels of artificial light at night strengthen top-down control in insect food web. Curr. Biol. 28, 2474-2478. ( 10.1016/j.cub.2018.05.078) [DOI] [PubMed] [Google Scholar]
- 3.Grubisic M, van Grunsven RHA. 2021. Artificial light at night disrupts species interactions and changes insect communities. Curr. Opin. Insect Sci. 47, 136-141. ( 10.1016/j.cois.2021.06.007) [DOI] [PubMed] [Google Scholar]
- 4.Bliss-Ketchum LL, de Rivera CE, Turner BC, Weisbaum DM. 2016. The effect of artificial light on wildlife use of a passage structure. Biol. Conserv. 199, 25-28. ( 10.1016/j.biocon.2016.04.025) [DOI] [Google Scholar]
- 5.Laforge A, Pauwels J, Faure B, Bas Y, Kerbiriou C, Fonderflick J, Besnard A. 2019. Reducing light pollution improves connectivity for bats in urban landscapes. Landsc. Ecol. 34, 793-809. ( 10.1007/s10980-019-00803-0) [DOI] [Google Scholar]
- 6.Davies TW, Bennie J, Gaston KJ. 2012. Street lighting changes the composition of invertebrate communities. Biol. Lett. 8, 764-767. ( 10.1098/rsbl.2012.0216) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knop E, Zoller L, Ryser R, Gerpe C, Hörler M, Fontaine C. 2017. Artificial light at night as a new threat to pollination. Nature 548, 206-209. ( 10.1038/nature23288) [DOI] [PubMed] [Google Scholar]
- 8.Sanders D, Frago E, Kehoe R, Patterson C, Gaston KJ. 2020. A meta-analysis of biological impacts of artificial light at night. Nat. Ecol. Evol. 5, 74-81. ( 10.1038/s41559-020-01322-x) [DOI] [PubMed] [Google Scholar]
- 9.Owens ACS, Lewis SM. 2018. The impact of artificial light at night on nocturnal insects: a review and synthesis. Ecol. Evol. 8, 11 337-11 358. ( 10.1002/ece3.4557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Owens ACS, Cochard P, Durrant J, Farnworth B, Perkin EK, Seymoure B. 2020. Light pollution is a driver of insect declines. Biol. Conserv. 241, 108259. ( 10.1016/j.biocon.2019.108259) [DOI] [Google Scholar]
- 11.Aubé M. 2015. Physical behaviour of anthropogenic light propagation into the nocturnal environment. Phil. Trans. R. Soc. B 370, 20140117. ( 10.1098/rstb.2014.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kyba CCM, Hölker F. 2013. Do artificially illuminated skies affect biodiversity in nocturnal landscapes? Landsc. Ecol. 28, 1637-1640. ( 10.1007/s10980-013-9936-3) [DOI] [Google Scholar]
- 13.Kyba CCM, Altıntaş YÖ, Walker CE, Newhouse M. 2023. Citizen scientists report global rapid reductions in the visibility of stars from 2011 to 2022. Science 379, 265-268. ( 10.1126/science.abq7781) [DOI] [PubMed] [Google Scholar]
- 14.Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, Portnov BA, Rybnikova NA, Furgoni R. 2016. The new world atlas of artificial night sky brightness. Sci. Adv. 2, 1600377. ( 10.1126/sciadv.1600377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hänel A, et al. 2018. Measuring night sky brightness: methods and challenges. J. Quant. Spectrosc. Radiat. Transf. 205, 278-290. ( 10.1016/j.jqsrt.2017.09.008) [DOI] [Google Scholar]
- 16.Jechow A, Kyba C, Hölker F. 2019. Beyond all-sky: assessing ecological light pollution using multi-spectral full-sphere fisheye lens imaging. J. Imaging 5, 46. ( 10.3390/jimaging5040046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dominoni DM, Nelson RJ. 2018. Artificial light at night as an environmental pollutant: an integrative approach across taxa, biological functions, and scientific disciplines. J. Exp. Zool. Ecol. Integr. Physiol. 329, 387-393. ( 10.1002/jez.2241) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders D, Gaston KJ. 2018. How ecological communities respond to artificial light at night. J. Exp. Zool. Ecol. Integr. Physiol. 329, 394-400. ( 10.1002/jez.2157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Doren BM, Horton KG, Dokter AM, Klinck H, Elbin SB, Farnsworth A. 2017. High-intensity urban light installation dramatically alters nocturnal bird migration. Proc. Natl Acad. Sci. USA 114, 11 175-11 180. ( 10.1073/pnas.1708574114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cabrera-Cruz SA, Smolinsky JA, McCarthy KP, Buler JJ. 2019. Urban areas affect flight altitudes of nocturnally migrating birds. J. Anim. Ecol. 88, 1873-1887. ( 10.1111/1365-2656.13075) [DOI] [PubMed] [Google Scholar]
- 21.Rebke M, Dierschke V, Weiner CN, Aumüller R, Hill K, Hill R. 2019. Attraction of nocturnally migrating birds to artificial light: the influence of colour, intensity and blinking mode under different cloud cover conditions. Biol. Conserv. 233, 220-227. ( 10.1016/j.biocon.2019.02.029) [DOI] [Google Scholar]
- 22.Wilson P, Thums M, Pattiaratchi C, Meekan M, Pendoley K, Fisher R, Whiting S. 2018. Artificial light disrupts the nearshore dispersal of neonate flatback turtles Natator depressus. Mar. Ecol. Prog. Ser. 600, 179-192. ( 10.3354/meps12649) [DOI] [Google Scholar]
- 23.Foster JJ, Tocco C, Smolka J, Khaldy L, Baird E, Byrne MJ, Nilsson D-E, Dacke M. 2021. Light pollution forces a change in dung beetle orientation behavior. Curr. Biol. 31, 3935-3942. ( 10.1016/j.cub.2021.06.038) [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann J, Schirmer A, Eccard JA. 2019. Light pollution affects space use and interaction of two small mammal species irrespective of personality. BMC Ecol. 19, 1. ( 10.1186/s12898-019-0241-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voigt CC, Scholl JM, Bauer J, Teige T, Yovel Y, Kramer-Schadt S, Gras P. 2019. Movement responses of common noctule bats to the illuminated urban landscape. Landsc. Ecol. 35, 189-201. ( 10.1007/s10980-019-00942-4) [DOI] [Google Scholar]
- 26.Berger A, Lozano B, Barthel LMF, Schubert N. 2020. Moving in the dark—evidence for an influence of artificial light at night on the movement behaviour of European hedgehogs Erinaceus europaeus. Animals 10, 1306. ( 10.3390/ani10081306) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffmann J, Hölker F, Eccard JA. 2022. Welcome to the dark side: partial nighttime illumination affects night- and daytime foraging behavior of a small mammal. Front. Ecol. Evol. 9, 779825. ( 10.3389/fevo.2021.779825) [DOI] [Google Scholar]
- 28.Macgregor CJ, Evans DM, Fox R, Pocock MJO. 2016. The dark side of street lighting: impacts on moths and evidence for the disruption of nocturnal pollen transport. Glob. Change Biol. 23, 697-707. ( 10.1111/gcb.13371) [DOI] [PubMed] [Google Scholar]
- 29.Duarte C, et al. 2019. Artificial light pollution at night (ALAN) disrupts the distribution and circadian rhythm of a sandy beach isopod. Environ. Pollut. 248, 565-573. ( 10.1016/j.envpol.2019.02.037) [DOI] [PubMed] [Google Scholar]
- 30.Stone EL, Jones G, Harris S. 2009. Street lighting disturbs commuting bats. Curr. Biol. 19, 1123-1127. ( 10.1016/j.cub.2009.05.058) [DOI] [PubMed] [Google Scholar]
- 31.Allema AB, Rossing WAH, van der Werf W, Heusinkveld BG, Bukovinszky T, Steingröver E, van Lenteren JC. 2012. Effect of light quality on movement of Pterostichus melanarius (Coleoptera: Carabidae). J. Appl. Entomol. 136, 793-800. ( 10.1111/j.1439-0418.2012.01728.x) [DOI] [Google Scholar]
- 32.Polak T, Korine C, Yair S, Holderied MW. 2011. Differential effects of artificial lighting on flight and foraging behaviour of two sympatric bat species in a desert. J. Zool. 285, 21-27. ( 10.1111/j.1469-7998.2011.00808.x) [DOI] [Google Scholar]
- 33.Luarte T, Bonta CC, Silva-Rodriguez EA, Quijón PA, Miranda C, Farias AA, Duarte C. 2016. Light pollution reduces activity, food consumption and growth rates in a sandy beach invertebrate. Environ. Pollut. 218, 1147-1153. ( 10.1016/j.envpol.2016.08.068) [DOI] [PubMed] [Google Scholar]
- 34.Czarnecka M, Kakareko T, Jermacz L, Pawlak R, Kobak J. 2019. Combined effects of nocturnal exposure to artificial light and habitat complexity on fish foraging. Sci. Total Environ. 684, 14-22. ( 10.1016/j.scitotenv.2019.05.280) [DOI] [PubMed] [Google Scholar]
- 35.de Jong M, Jeninga L, Ouyang JQ, van Oers K, Spoelstra K, Visser ME. 2016. Dose-dependent responses of avian daily rhythms to artificial light at night. Physiol. Behav. 155, 172-179. ( 10.1016/j.physbeh.2015.12.012) [DOI] [PubMed] [Google Scholar]
- 36.Bauer T, Brauner U, Fischerleitner E. 1977. The relevance of the brightness to visual acuity, predation, and activity of visually hunting ground-beetles (Coleoptera, Carabidae). Oecologia 30, 63-73. ( 10.1007/bf00344892) [DOI] [PubMed] [Google Scholar]
- 37.Jolkkonen J, Gaston KJ, Troscianko J. 2023. Artificial lighting affects the landscape of fear in a widely distributed shorebird. Commun. Biol. 6, 131. ( 10.1038/s42003-023-04486-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer T, Kredler M. 1993. Morphology of the compound eyes as an indicator of life-style in carabid beetles. Can. J. Zool. 71, 799-810. ( 10.1139/z93-105) [DOI] [Google Scholar]
- 39.Becker A, Whitfield AK, Cowley PD, Järnegren J, Naesje TF. 2012. Potential effects of artificial light associated with anthropogenic infrastructure on the abundance and foraging behaviour of estuary-associated fishes. J. Appl. Ecol. 50, 43-50. ( 10.1111/1365-2664.12024) [DOI] [Google Scholar]
- 40.Straka TM, Wolf M, Gras P, Buchholz S, Voigt CC. 2019. Tree cover mediates the effect of artificial light on urban bats. Front. Ecol. Evol. 7, 91. ( 10.3389/fevo.2019.00091) [DOI] [Google Scholar]
- 41.Mocq J, Soukup PR, Näslund J, Boukal DS. 2021. Disentangling the nonlinear effects of habitat complexity on functional responses. J. Anim. Ecol. 90, 1525-1537. ( 10.1111/1365-2656.13473) [DOI] [PubMed] [Google Scholar]
- 42.Schmidt A, et al. 2021. The iDiv Ecotron—a flexible research platform for multitrophic biodiversity research. Ecol. Evol. 11, 15 174-15 190. ( 10.1002/ece3.8198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Terlau JF, et al. 2023. Microhabitat conditions remedy heat stress effects on insect activity. Glob. Change Biol. 29, 3747-3758. ( 10.1111/gcb.16712) [DOI] [PubMed] [Google Scholar]
- 44.Pun CSJ, So CW. 2011. Night-sky brightness monitoring in Hong Kong. Environ. Monit. Assess. 184, 2537-2557. ( 10.1007/s10661-011-2136-1) [DOI] [PubMed] [Google Scholar]
- 45.Kyba CCM, et al. 2015. Worldwide variations in artificial skyglow. Scient. Rep. 5, 8409. ( 10.1038/srep08409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aulsebrook AE, Jechow A, Krop-Benesch A, Kyba CCM, Longcore T, Perkin EK, van Grunsven RHA. 2022. Nocturnal lighting in animal research should be replicable and reflect relevant ecological conditions. Biol. Lett. 18, 20220035. ( 10.1098/rsbl.2022.0035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abelson E, Seymoure B, Jechow A, Perkin E, Moon H, Kyba C, Hoelker F, White J, Longcore T. 2023. Ecological aspects and measurement of anthropogenic light at night. SSRN. ( 10.2139/ssrn.4353905) [DOI] [Google Scholar]
- 48.Janiczek PM, DeYoung JA. 1987. Computer programs for Sun and Moon illuminance: with contingent tables and diagrams. Circular no. 171. Washington, DC: United States Naval Observatory. [Google Scholar]
- 49.Thiele H-U. 1977. Ecological aspects of activity patterns in carabids. In Carabid beetles in their environments, pp. 225-271. Berlin, Germany: Springer. [Google Scholar]
- 50.Meyer ST, Koch C, Weisser WW. 2015. Towards a standardized rapid ecosystem function assessment (REFA). Trends Ecol. Evol. 30, 390-397. ( 10.1016/j.tree.2015.04.006) [DOI] [PubMed] [Google Scholar]
- 51.Lövei GL, Ferrante M. 2017. A review of the sentinel prey method as a way of quantifying invertebrate predation under field conditions. Insect Sci. 24, 528-542. ( 10.1111/1744-7917.12405) [DOI] [PubMed] [Google Scholar]
- 52.Roslin T, et al. 2017. Higher predation risk for insect prey at low latitudes and elevations. Science 356, 742-744. ( 10.1126/science.aaj1631) [DOI] [PubMed] [Google Scholar]
- 53.Low PA, Sam K, McArthur C, Posa MRC, Hochuli DF. 2014. Determining predator identity from attack marks left in model caterpillars: guidelines for best practice. Entomol. Exp. Appl. 152, 120-126. ( 10.1111/eea.12207) [DOI] [Google Scholar]
- 54.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378-400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 55.R Core Team. 2022. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org. [Google Scholar]
- 56.Wickham H. 2016. ggplot2: Elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 57.Lüdecke D. 2018. ggeffects: Tidy data frames of marginal effects from regression models. J. Open Source Softw. 3, 772. ( 10.21105/joss.00772) [DOI] [Google Scholar]
- 58.Roberts CM. 2006. Radio frequency identification (RFID). Comput. Secur. 25, 18-26. ( 10.1016/j.cose.2005.12.003) [DOI] [Google Scholar]
- 59.Barlow SE, O'Neill MA. 2020. Technological advances in field studies of pollinator ecology and the future of e-ecology. Curr. Opin. Insect Sci. 38, 15-25. ( 10.1016/j.cois.2020.01.008) [DOI] [PubMed] [Google Scholar]
- 60.Dell AI, et al. 2014. Automated image-based tracking and its application in ecology. Trends Ecol. Evol. 29, 417-428. ( 10.1016/j.tree.2014.05.004) [DOI] [PubMed] [Google Scholar]
- 61.Blubaugh CK, Widick IV, Kaplan I. 2017. Does fear beget fear? Risk-mediated habitat selection triggers predator avoidance at lower trophic levels. Oecologia 185, 1-11. ( 10.1007/s00442-017-3909-1) [DOI] [PubMed] [Google Scholar]
- 62.Kehoe R, Sanders D, Cruse D, Silk M, Gaston KJ, Bridle JR, van Veen F. 2020. Longer photoperiods through range shifts and artificial light lead to a destabilizing increase in host–parasitoid interaction strength. J. Anim. Ecol. 89, 2508-2516. ( 10.1111/1365-2656.13328) [DOI] [PubMed] [Google Scholar]
- 63.Manfrin A, Singer G, Larsen S, Weiß N, van Grunsven RHA, Weiß N-S, Wohlfahrt S, Monaghan MT, Hölker F. 2017. Artificial light at night affects organism flux across ecosystem boundaries and drives community structure in the recipient ecosystem. Front. Environ. Sci. 5, 61. ( 10.3389/fenvs.2017.00061) [DOI] [Google Scholar]
- 64.Navara KJ, Nelson RJ. 2007. The dark side of light at night: physiological, epidemiological, and ecological consequences. J. Pineal Res. 43, 215-224. ( 10.1111/j.1600-079x.2007.00473.x) [DOI] [PubMed] [Google Scholar]
- 65.Somanathan H, Borges RM, Warrant EJ, Kelber A. 2008. Nocturnal bees learn landmark colours in starlight. Curr. Biol. 18, R996-R997. ( 10.1016/j.cub.2008.08.023) [DOI] [PubMed] [Google Scholar]
- 66.Fuller A, Mitchell D, Maloney SK, Hetem RS. 2016. Towards a mechanistic understanding of the responses of large terrestrial mammals to heat and aridity associated with climate change. Clim. Change Responses 3, 10. ( 10.1186/s40665-016-0024-1) [DOI] [Google Scholar]
- 67.Speights CJ, Harmon JP, Barton BT. 2017. Contrasting the potential effects of daytime versus nighttime warming on insects. Curr. Opin. Insect Sci. 23, 1-6. ( 10.1016/j.cois.2017.06.005) [DOI] [PubMed] [Google Scholar]
- 68.Gotcha N, Machekano H, Cuthbert RN, Nyamukondiwa C. 2020. Heat tolerance may determine activity time in coprophagic beetle species (Coleoptera: Scarabaeidae). Insect Sci. 28, 1076-1086. ( 10.1111/1744-7917.12844) [DOI] [PubMed] [Google Scholar]
- 69.Ryser R, Hirt MR, Häussler J, Gravel D, Brose U. 2021. Landscape heterogeneity buffers biodiversity of simulated meta-food-webs under global change through rescue and drainage effects. Nat. Commun. 12, 4716. ( 10.1038/s41467-021-24877-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Ruiter PC, Neutel A-M, Moore JC. 1995. Energetics, patterns of interaction strengths, and stability in real ecosystems. Science 269, 1257-1260. ( 10.1126/science.269.5228.1257) [DOI] [PubMed] [Google Scholar]
- 71.McCann K, Hastings A, Huxel GR. 1998. Weak trophic interactions and the balance of nature. Nature 395, 794-798. ( 10.1038/27427) [DOI] [Google Scholar]
- 72.Bascompte J, Melián CJ, Sala E. 2005. Interaction strength combinations and the overfishing of a marine food web. Proc. Natl Acad. Sci. USA 102, 5443-5447. ( 10.1073/pnas.0501562102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Borges RM, Somanathan H, Kelber A. 2016. Patterns and processes in nocturnal and crepuscular pollination services. Q. Rev. Biol. 91, 389-418. ( 10.1086/689481) [DOI] [PubMed] [Google Scholar]
- 74.Dyer A, et al. 2023. Data from: Insect communities under skyglow: diffuse night-time illuminance induces spatio-temporal shifts in movement and predation. Zenodo. ( 10.5281/zenodo.8017438) [DOI] [PMC free article] [PubMed]
- 75.Dyer A, et al. 2023. Insect communities under skyglow: diffuse night-time illuminance induces spatio-temporal shifts in movement and predation. Figshare. ( 10.6084/m9.figshare.c.6837645) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Dyer A, et al. 2023. Data from: Insect communities under skyglow: diffuse night-time illuminance induces spatio-temporal shifts in movement and predation. Zenodo. ( 10.5281/zenodo.8017438) [DOI] [PMC free article] [PubMed]
- Dyer A, et al. 2023. Insect communities under skyglow: diffuse night-time illuminance induces spatio-temporal shifts in movement and predation. Figshare. ( 10.6084/m9.figshare.c.6837645) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The underlying data are available in an open access repository from the Zenodo repository: https://doi.org/10.5281/zenodo.8017438 [74].
Data are provided in the electronic supplementary material [75].