Abstract
Papillary Thyroid Cancer (PTC) is the most common type of thyroid cancer. The membrane-associated glycoprotein cadherin-16 (CDH16) plays a significant role in the embryonal development of thyroid follicles and cell adhesion. Previous studies have indicated a substantial downregulation of CDH16 in PTC. However, its role in Middle Eastern PTC has not been elucidated. We analyzed a tissue microarray comprising 1606 PTC and 240 normal thyroid tissues using immunohistochemistry to assess CDH16 expression and determine its clinico-pathological associations. We also conducted BRAF and TERT mutations analyses through Sanger sequencing. Disease-free survival (DFS) was assessed using Kaplan–Meier curves. CDH16 immunostaining was seen in 100% of normal thyroid tissues but only in 9.4% of PTC tissues (p < 0.0001). The loss of CDH16 expression was associated with aggressive PTC characteristics including bilaterality, multifocality, extrathyroidal extension, tall cell variant, lymph node metastasis (LNM) and distant metastasis. Additionally a correlation between loss of CDH16 expression and BRAF and TERT mutations was identified. Intriguingly, upon conducting multivariate logistic regression analysis, CDH16 was determined to be an independent predictor for LNM (Odds ratio = 2.46; 95% confidence interval = 1.60–3.79; p < 0.0001). Furthermore, CDH16 loss was associated with a shorter DFS (p = 0.0015). However, when we further subdivided CDH16 negative patients based on the co-existence of TERT and/or BRAF mutations, we found that patients with both CDH16 negative expression and TERT mutation exhibited the shortest DFS (p < 0.0001). In conclusion, our results suggest that CDH16 protein expression could serve as a valuable diagnostic tool for PTC. Furthermore, these findings demonstrate that the loss of CDH16 expression is an independent predictor of LNM and may contribute to the aggressiveness of PTC. Therefore, downregulation of CDH16 in PTC might be a potential target for designing novel therapeutic strategies to treat PTC.
Subject terms: Biomarkers, Oncology
Introduction
Thyroid Cancer (TC) stands as the most prevalent endocrine malignancy1,2, with papillary thyroid cancer (PTC) comprising over 70% of all TC cases3,4. Over recent years, the global incidence of TC has seen a significant upsruge5–7. In Saudi Arabia, TC ranks as the second most prevalent cancer, following closely behind Breast Cancer8. While PTC is traditionally considered an indolent form of cancer, with a promising prognosis and favorable overall survival, it is worth noting that as many as 30% of TC patients face an unfavorable clinical course , marked by local recurrence, that necessitates additional medical and/or surgical interventions9–11. One of the paramount risk factors contributing to local recurrence is the presence of lymph node metastases (LNM)12,13. Therefore, identification of predictive markers for LNM holds immense importance in preventing recurrences and providing clinicians with valuable guidance when making therapeutic and follow-up decisions for PTC patients.
Cadherin-16 (CDH16) belongs to the Cadherin superfamily, which encompasses various calcium-dependent membrane-associated glycoproteins14. CDH16 serves a pivotal role in maintaining cell adhesion, embryonal development and cell growth14,15. Its crucial involvement in mediating cell–cell adhesion has led to several studies highlighting a strong association between abnormal CDH expression and tumor progression and metastasis16–18. A recent study has shed light on the prognostic significance of CDH16 in renal cell carcinoma, where a reduction in CDH16 expression was identified as a potent predictor of poor prognosis19. Additionally, CDH16 has been implicated in the development and differentiation of thyroid gland during embryogenesis20,21. Interestingly, our recent study, conducted in a smaller cohort encompassing multiple organ sites revealed a lower frequency of CDH16 expression in PTC compared to normal thyroid, follicular adenomas and follicular carcinomas22. RNA expression studies have also suggested a significant downregulation of CDH16 in PTC in comparison to thyroiditis23. Utilizing The Cancer Genome Atlas (TCGA) cohort and bioinformatics analysis, they showed an association between CDH16 expression and unfavorable clinico-pathological features in TC. Moreover, a recent investigation elucidated the mechanistic role of CDH16 in TC, where CDH16 overexpression inhibited cell proliferation and migration, while inducing apoptosis, affirming its role as tumor suppressor24. Furthermore, by analyzing a small cohort of 35 PTC patients, they demonstrated a significant correlation between low expression of CDH16 and tumor size, stage and LNM.
Despite these intriguing prior findings, research on the immunohistochemical expression of CDH16 has predominantly focused on renal cell carcinomas25–32, with only one recent study in PTC22. Additionally, publicly accessible RNA databases have indicated CDH16 expression in cervical, endometrial and ovarian cancers33–35. Nevertheless, comprehensive studies involving substantial cohorts of PTC patients remain scarce. As a result, this study was specifically designed to investigate the expression of CDH16 and its potential utility as a prognostic and diagnostic marker. We conducted our study using a cohort comprising 240 normal thyroid tissues and 1606 PTC samples by immunohistochemistry. Furthermore, we delved into the clinico-pathological and molecular correlations associated with CDH16 expression in Middle Eastern PTC cases.
Results
Patient and tumor characteristics
Median age of the study population was 38.0 years (range: 5.9–87.6 years), with a male to female ratio of 1:3. The majority of tumors were classical variant of PTC (63.3%; 1017/1606). 33.0% (530/1606) of tumors were bilateral and 50.4% (809/1606) were multifocal. 41.4% (664/1606) of tumors exhibited extrathyroidal extension and 26.8% (430/1606) showed lymphovascular invasion. LNM was noted in 48.7% (782/1606) and distant metastasis in 8.9% (143/1606) of PTCs. BRAF mutation was noted in 54.9% (882/1606) and TERT mutation in 11.9% (191/1606) of PTCs (Table 1). Of the 882 cases with BRAF mutation, the following variants were detected: BRAFV600E (n = 871), A1801G;K601E (n = 3), c.1799_1801delTGA:p.V600Efs (n = 2), c.1799_1801het_delTGA:p.V600fs (n = 2), A1844G;G615E (n = 1), 1799_1822_insAGGGGATTTTGGTCTGGCTACAGA (n = 1), c.1460_1473delTGACAGCACCTACAC:p.V487fs (n = 1) and c.1801A > G:p.K601E (n = 1). Among the cases with TERT mutation (n = 191), C228T was present in 165 cases, C250T in 24 cases, C228A in one case and combined C228T and C250T being present in one case.
Table 1.
Clinico-pathological associations of CDH16 expression in papillary thyroid cancer.
| Total | CDH16 negative | CDH16 positive | p value | ||||
|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | ||
| No. of patients | 1606 | 1455 | 90.6 | 151 | 9.4 | ||
| Age (years) | |||||||
| Median (range) | 38.0 (5.9–87.6) | 38.2 (5.9–87.6) | 37.2 (8.5–74.2) | 0.4884 | |||
| ≤ 55 | 1307 | 81.4 | 1181 | 81.8 | 126 | 83.4 | |
| > 55 | 299 | 18.6 | 174 | 18.2 | 25 | 16.6 | |
| Age group | |||||||
| Pediatric/adolescent (≤ 18 years) | 90 | 5.6 | 84 | 5.8 | 6 | 4.0 | 0.4581 |
| Adult (> 18 years) | 1516 | 94.4 | 1371 | 94.2 | 145 | 96.0 | |
| Sex | |||||||
| Female | 1219 | 75.9 | 1109 | 76.2 | 110 | 72.8 | 0.3622 |
| Male | 387 | 24.1 | 346 | 23.8 | 41 | 27.2 | |
| Histology type | |||||||
| Classical variant | 1017 | 63.3 | 940 | 64.6 | 77 | 51.0 | < 0.0001 |
| Follicular variant | 282 | 17.6 | 232 | 16.0 | 50 | 33.1 | |
| Tall-cell variant | 168 | 10.5 | 162 | 11.1 | 6 | 4.0 | |
| Other variants | 139 | 8.6 | 121 | 8.3 | 18 | 11.9 | |
| Tumor laterality | |||||||
| Unilateral | 1076 | 67.0 | 962 | 66.1 | 114 | 75.5 | 0.0169 |
| Bilateral | 530 | 33.0 | 493 | 33.9 | 37 | 24.5 | |
| Tumor focality | |||||||
| Unifocal | 797 | 49.6 | 709 | 48.7 | 88 | 58.3 | 0.0252 |
| Multifocal | 809 | 50.4 | 746 | 51.3 | 63 | 41.7 | |
| Extrathyroidal extension | |||||||
| Absent | 942 | 58.6 | 821 | 56.4 | 121 | 80.1 | < 0.0001 |
| Present | 664 | 41.4 | 634 | 43.6 | 30 | 19.9 | |
| Lymphovascular invasion | |||||||
| Absent | 1176 | 73.2 | 1062 | 73.0 | 114 | 75.5 | 0.5042 |
| Present | 430 | 26.8 | 393 | 27.0 | 37 | 24.5 | |
| pT | |||||||
| T1 | 640 | 39.8 | 580 | 40.0 | 60 | 39.7 | 0.4985 |
| T2 | 510 | 31.8 | 464 | 32.0 | 46 | 30.5 | |
| T3 | 332 | 20.7 | 295 | 20.3 | 37 | 24.5 | |
| T4 | 120 | 7.5 | 112 | 7.7 | 8 | 5.3 | |
| Unknown | 4 | 0.2 | |||||
| Lymph node metastasis | |||||||
| Absent | 679 | 42.3 | 584 | 44.1 | 95 | 69.8 | < 0.0001 |
| Present | 782 | 48.7 | 741 | 55.9 | 41 | 30.2 | |
| Unknown | 145 | 9.0 | |||||
| Distant metastasis | |||||||
| Absent | 1463 | 91.1 | 1318 | 90.6 | 145 | 96.0 | 0.0138 |
| Present | 143 | 8.9 | 137 | 9.4 | 6 | 4.0 | |
| Stage | |||||||
| I | 1354 | 84.3 | 1219 | 84.2 | 135 | 90.0 | 0.1860 |
| II | 168 | 10.5 | 157 | 10.8 | 11 | 7.4 | |
| III | 24 | 1.5 | 22 | 1.5 | 2 | 1.3 | |
| IV | 53 | 3.3 | 51 | 3.5 | 2 | 1.3 | |
| Unknown | 7 | 0.4 | |||||
| BRAF mutation | |||||||
| Present | 882 | 54.9 | 853 | 59.9 | 29 | 19.2 | < 0.0001 |
| Absent | 692 | 43.1 | 570 | 40.1 | 122 | 80.8 | |
| Unknown | 32 | 2.0 | |||||
| TERT mutation | |||||||
| Present | 191 | 11.9 | 179 | 15.3 | 12 | 8.2 | 0.0211 |
| Absent | 1129 | 70.3 | 994 | 84.7 | 135 | 91.8 | |
| Unknown | 286 | 17.8 | |||||
| Disease-free survival time (months), median (range) | 38 (5–265) | 38 (5–267) | 40 (6–246) | ||||
Statistical analyses were performed only on available data and unknown cases were excluded.
CDH16 expression and its association with clinico-pathological characteristics
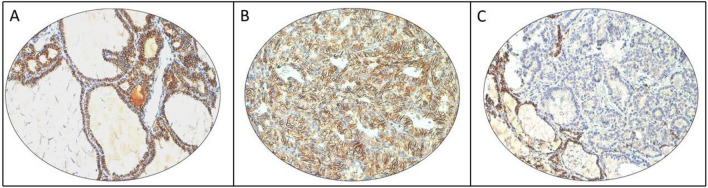
CDH16 immunostaining was predominantly membranous and was noted in 100% of normal thyroid tissues (Fig. 1A). In PTCs, CDH16 staining was noted in only 9.4% (151/1606) of cases (Fig. 1B,C). Loss of CDH16 immunostaining was significantly associated with adverse clinico-pathological characteristics such as tall-cell variant (p < 0.0001), bilateral tumors (p = 0.0169), multifocality (p = 0.0252), extrathyroidal extension (p < 0.0001), LNM (p < 0.0001) and distant metastasis (p = 0.0138) (Table 1). Interestingly, we also found a significant association between CDH16 loss and BRAF (p < 0.0001) as well as TERT (p = 0.0211) mutations (Table 1).
Figure 1.
Examples of CDH16 immunostaining in normal thyroid and papillary Thyroid cancer (PTC). (A) Representative section of normal thyroid tissue showing strong positive membrane expression of CDH16 in the follicular cells. (B) PTC tissue showing positive staining for CDH16 and (C) another PTC tissue showing negative staining for CDH16 with adjacent normal tissue showing positive expression. (20 X/0.70 objective on an Olympus BX 51 microscope (Olympus America Inc, Center Valley, PA, USA)).
We further analyzed the CDH16 expression in pediatric/adolescent patients (≤ 18 years) in our cohort. Patients aged ≤ 18 years constituted 5.6% (90/1606) of the total cases. CDH16 expression was noted in 6.7% (6/90) of pediatric/adolescent cases. However, the difference in CDH16 expression between pediatric/adolescent and adult PTC was not statistically significant (p = 0.4581) (Table 1).
Loss of CDH16 expression is an independent predictor of lymph node metastasis
Since LNM is an important prognostic marker in PTC, we sought to further analyze the association between CDH16 expression and LNM using logistic regression analyses. Univariate analysis revealed male sex (Odds ratio (OR) = 1.41; 95% confidence interval (CI) = 1.11–1.80; p = 0.0055), bilaterality (OR 2.11; 95% CI 1.68–2.64; p < 0.0001), multifocality (OR 1.71; 95% CI 1.39–2.10; p < 0.0001), extrathyroidal extension (OR 3.91; 95% CI 3.13–4.89; p < 0.0001), lymphovascular invasion (OR 1.86; 95% CI 1.46–2.39; p < 0.0001), T status (OR 1.62; 95% CI 1.27–2.06; p < 0.0001), distant metastasis (OR 2.44; 95% CI 1.45–4.13; p = 0.0008) and CDH16 loss (OR 2.94; 95% CI 2.01–4.31; p < 0.0001) were significant predictors of LNM. Multivariate analysis using these parameters indicated that only bilaterality (OR 1.74; 95% CI 1.23–2.45; p = 0.0016), extrathyroidal extension (OR 3.11; 95% CI 2.43–3.96; p < 0.0001) and CDH16 loss (OR 2.46; 95% CI 1.60–3.79; p < 0.0001) were significant independent predictors of LNM (Table 2).
Table 2.
Logistic regression analysis for prediction of lymph node metastasis risk.
| Risk factor | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (> 55 years) | 0.90 (0.69–1.18) | 0.4439 | ||
| Male sex | 1.41 (1.11–1.80) | 0.0055 | 1.21 (0.92–1.59) | 0.1805 |
| Aggressive Histotypes | 1.12 (0.86–1.46) | 0.3982 | ||
| Bilateral tumors | 2.11 (1.68–2.64) | < 0.0001 | 1.74 (1.23–2.45) | 0.0016 |
| Multifocal tumors | 1.71 (1.39–2.10) | < 0.0001 | 0.99 (0.72–1.36) | 0.9406 |
| Extrathyroidal extension | 3.91 (3.13–4.89) | < 0.0001 | 3.11 (2.43–3.96) | < 0.0001 |
| Lymphovascular invasion | 1.86 (1.46–2.39) | < 0.0001 | 1.33 (0.99–1.78) | 0.0502 |
| T status (T3/4 vs T1/2) | 1.62 (1.27–2.06) | < 0.0001 | 1.14 (0.87–1.49) | 0.3338 |
| Distant metastasis | 2.44 (1.45–4.13) | 0.0008 | 1.39 (0.79–2.45) | 0.2542 |
| CDH16 negative | 2.94 (2.01–4.31) | < 0.0001 | 2.46 (1.60–3.79) | < 0.0001 |
OR odds ratio, CI confidence interval.
Disease-free survival
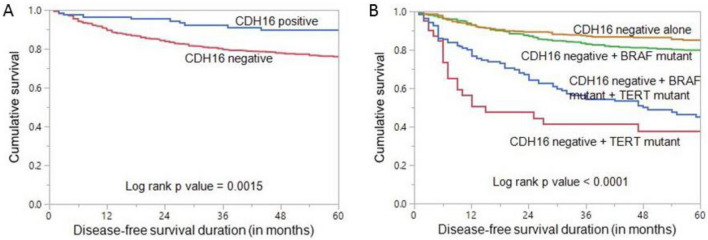
Loss of CDH16 expression was found to be associated with significantly shorter disease-free survival (DFS) (p = 0.0015, Fig. 2A). Given the significant association of BRAF and TERT mutations with CDH16, we further analyzed the effect of these mutations on CDH16 loss of expression in predicting DFS. We divided the patients with CDH16 loss into four categories: CDH16 negative alone, CDH16 negative + BRAF mutant, CDH16 negative + TERT mutant and CDH16 negative + BRAF mutant + TERT mutant. Interestingly, we found that CDH16 negative + TERT mutant patients had the worst DFS among the four groups (p < 0.0001, Fig. 2B).
Figure 2.
Disease-free survival (DFS). (A) Kaplan Meier survival plot showing statistically significant shorter DFS in CDH16 negative cases compared to CDH16 positive (p = 0.0015) (B) Kaplan Meier survival plot showing statistically significant shorter DFS in patients with co-existing CDH16 negative and TERT mutation (p < 0.0001), compared to other sub-groups.
Discussion
In this study, we observed a significant downregulation of CDH16 expression in PTC when compared to normal thyroid tissue. This loss of CDH16 was found to be associated with aggressive clinico-pathological characteristics and shorter DFS. The multivariate logistic regression analysis further confirmed that CDH16 stands as an independent predictor for LNM. Interestingly, when we sub-grouped CDH16 negative patients based on the presence of co-existing TERT and/or BRAF mutations, we discovered that patients with co-existing CDH16 negative expression and TERT mutation exhibited the shortest DFS.
In a recent study conducted by Lennartz et al.22, which analyzed over 10,000 tumors spanning more than 100 entities, CDH16 positivity was identified in 100% of normal thyroid tissues, 86% of 94 follicular adenomas, 60% of 67 follicular carcinomas and only 6.6% of 212 papillary thyroid carcinomas. In this study, a collection of tissue microarrays containing 1846 samples from 1606 PTC and 240 normal thyroid tissues revealed CDH16 membranous immunostaining in 100% of normal thyroid tissues, but only 9.4% of PTCs. Our finding regarding the low CDH16 positivity rate in PTC is in concordance with the results of Lennartz et al.22. However, it is worth noting that another study by Yang et al.24 reported CDH16 positivity in 51.4% of PTCs, albeit in a much smaller sample size of 35 cases. The presence of CHD16 expression in 100% of normal thyroid tissues is consistent with previous research, indicating that CDH16 expression is associated with the fully differentiated state of thyroid cells and plays a role in thyroid follicular polarity20,21,36. The striking absence of CDH16 expression in over 90% of PTC cases suggests a relevant clinical practical utility of using CDH16 immunostaining as a useful diagnostic tool for the identification of these tumors.
Our analysis, encompassing a substantial cohort of over 1600 PTCs, enabled us to investigate in depth the correlation between the loss of CDH16 expression and various clinico-pathological and molecular parameters. Although the loss of CDH16 expression was unrelated to age, gender, tumor size and disease stage, it exhibited strong associations with several other aggressive features like tall cell variant, extrathyroidal extension, multifocality and bilateral tumors. Most notably, we observed a significant association between the absence of CDH16 and lymph node metastasis (LNM), as well as distant metastasis. Subsequent logistic regression analyses showed that downregulated expression of CDH16 was an independent predictor of LNM. Based on the identified role of CDH16 as cell adhesion molecule that preserves tissue integrity and inhibits cell migration and invasion24, the increased level of aggressiveness in PTC with the loss of CDH16 expression might be driven by lower degree of cells organization and higher tumor cell ability of motility and migration. Our findings are in concordance with results from previous studies that have described reduced CDH16 expression in association with aggressive PTC phenotype23,24. Both Li et al.23 (mRNA expression) and Yang et al.24 (protein expression) found a significant association between the downregulation of CDH16 and LNM, with Li et al. further demonstrating that reduced expression of CDH16 was an independent predictor of LNM, consistent with our study. These findings suggest that loss of CDH16 could be associated with PTC progression. We were particularly intrigued by the association of CDH16 expression loss and BRAF as well as TERT mutation. Previous studies have shown an interaction between CDH16 and other cancer genes23,37. In this cohort, lack of CDH16 expression was significantly associated with poor DFS in univariate analysis using the Kaplan–Meier curve. Since both BRAF and TERT mutations are known to be aggressive markers for PTC38–42 and both have been shown to be associated with poor DFS, we asked whether this survival effect of CDH16 loss of expression was affected by coexisting mutations in TERT and BRAF gene mutations. Specifically, we compared the DFS of PTC patients with coexisting TERT and BRAF mutations to those with a lack of CDH16 and found that PTC patients with coexisting lack of CDH16 and the presence of TERT mutation had the worst DFS. This is not surprising, given the clinical and functional role of TERT in PTC, which we and others have shown previously38,43,44. TERT is known to promote cancer progression, leading to the loss of epithelial cell adhesion molecule, E-cadherin and induction of several mesenchymal markers such as N-cadherin and vimentin38,45,46. The synergistic effect of TERT and CDH16 on cellular adhesion and cancer progression may explain the worse DFS when TERT mutation and CDH16 loss coexist.
Despite these interesting findings and the large cohort included in this study, this research still has some limitations. First, this is retrospective single institute cohort from Middle Eastern ethnicity where selection bias cannot be excluded. Second, the functional mechanism between CDH16 and other genes are mainly theoretical and should be further investigated and validated. Thirdly, the results of our findings cannot be generalized to the global population and hence, future large scale studies in other ethnicities is recommended.
In conclusion, our study has revealed that CDH16 protein expression is massively downregulated in Middle Eastern PTC when compared to normal tissue, indicating its potential as a valuable diagnostic tool for identification of these tumors. Furthermore, we have provided crucial clinical evidence that lack of CDH16 expression promotes PTC aggressiveness and serves as an independent predictor for LNM in PTC patients. Patients with both CDH16 expression loss and TERT mutation exhibited the worst DFS. This study underscores the potential of targeting CDH16 expression in PTC as a promising avenue for the development of therapeutic strategies to treat PTC.
Materials and methods
Patient selection and clinico-pathological data
One thousand six-hundred and six PTC patients diagnosed between 1988 and 2020 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) were included in the study. Cases were identified based on clinical history followed by fine needle aspiration cytology for confirmation. Baseline clinico-pathological data were collected from case records and has been summarized in Table 1. Staging of PTC was performed using the eighth edition of American Joint Committee on Cancer (AJCC) staging system47.
Ethics declarations
Institutional Review Board of King Faisal Specialist Hospital and Research Centre provided ethical approval for the current study. Research Advisory Council (RAC) granted waiver of informed consent for use of retrospective patient case data under project RAC# 2110 031 and 2211 168. All the methods were carried out in accordance with the Declaration of Helsinki.
BRAF and TERT mutation analysis
BRAF and TERT mutation status was assessed in our laboratory by utilizing Sanger sequencing technology and has been published by us previously38,48,49. BRAF mutation analysis was performed in 1574 cases and TERT mutation analysis was done in 1320 cases.
Tissue microarray (TMA) construction and immunohistochemistry (IHC) analysis
Tissue microarray (TMA) format was utilized for immunohistochemical analysis of the PTC samples. TMA was constructed as previously described50. Briefly, modified semiautomatic robotic precision instrument (Beecher Instruments, Woodland, WI) was used to punch tissue cylinders with a diameter of 0.6 mm from representative tumor area of the donor tissue block and brought into the recipient paraffin block. Two 0.6-mm cores of PTC were arrayed from each case.
Tissue microarray slides were processed and stained manually as described previously51. Primary antibody against CDH16 (monoclonal Recombinant rabbit, MSVA-516R, MS Validated Antibodies, Hamburg, Germany) was used at a dilution of 1:200 (pH 9). The Dako Envision Plus System kit was used as the secondary detection system with 3, 30-diaminobenzidine as chromogen. All slides were counter stained with hematoxylin, dehydrated, cleared and mounted. Negative controls included omission of the primary antibody. Normal tissues of different organ system were also included in the TMA to serve as control. Only fresh cut slides were stained simultaneously to minimize the influence of slide aging and maximize reproducibility of the experiment.
Staining was scored as described previously19. Briefly, the percentage of CDH16 positive tumor cells was estimated and the staining intensity was semi-quantitatively assessed (0, 1+, 2+ and 3+). For statistical analyses, staining results were categorized into two groups: Negative—no staining at all; Positive—staining of any intensity.
Follow-up and study endpoint
Patients were regularly followed by both physical examinations and imaging studies to identify tumor persistence/recurrence. The median follow-up was 7.5 years (range 1.0–30.2 years). The study end-point was DFS. Patients were grouped according to disease status, with patients considered to be disease-free in the absence of clinical, biochemical (unstimulated serum thyroglobulin (Tg) levels of < 0.2 µg/L or stimulated Tg levels of < 1 µg/L in the absence of interfering thyroglobulin antibodies (TgAb)) or radiological evidence of disease persistence or recurrence. In contrast, active disease was defined by the presence of unstimulated serum Tg levels ≥ 0.2 µg/L or stimulated Tg levels ≥ 1 µg/L; a rising or denovo appearance of TgAb; or abnormal findings on radio-imaging.
Statistical analysis
The associations between clinico-pathological variables and CDH16 protein expression was performed using contingency table analysis and Chi square tests. Mantel-Cox log-rank test was used to evaluate DFS. Survival curves were generated using the Kaplan–Meier method. Logistic regression analysis was used for univariate and multivariate analysis. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. Data analyses was performed using the JMP14.0 (SAS Institute, Inc., Cary, NC) software package.
Acknowledgements
The authors would like to thank Felisa DeVera for her technical assistance. The authors did not receive funding from any organization for the submitted work.
Author contributions
A.K.S.: Study conception, Study design, Interpretation of data, Revised the article critically for important intellectual content; S.K.P.: Study design, Data analysis, Interpretation of data, Revised the article critically for important intellectual content; M.A.-R.: Performed experiments; P.A.: Performed experiments; N.S.: Performed experiments; M.L.: Study design, Interpretation of data, Revised the article critically for important intellectual content; S.S.A.: Acquisition of data, Interpretation of data; F.A.D.: Acquisition of data, Interpretation of data; G.S.: Study design, Interpretation of data, Revised the article critically for important intellectual content; K.S.A.: Study conception, Study design, Interpretation of data, Revised the article critically for important intellectual content; All authors were involved in writing the paper and had final approval of the submitted and published versions.
Data availability
All data generated or analyzed during this study are included in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Abdul K. Siraj and Sandeep Kumar Parvathareddy.
References
- 1.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd RV, Buehler D, Khanafshar E. Papillary thyroid carcinoma variants. Head Neck Pathol. 2011;5:51–56. doi: 10.1007/s12105-010-0236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coca-Pelaz A, et al. Papillary thyroid cancer—Aggressive variants and impact on management: A narrative review. Adv. Ther. 2020;37:3112–3128. doi: 10.1007/s12325-020-01391-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat. Rev. Endocrinol. 2020;16:17–29. doi: 10.1038/s41574-019-0263-x. [DOI] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 7.Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat. Rev. Endocrinol. 2016;12:646–653. doi: 10.1038/nrendo.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alrawaji et al. in Saudi Cancer Registry (ed Saudi Health Council) (Riyadh, 2018).
- 9.Ritter A, et al. Detecting recurrence following lobectomy for thyroid cancer: Role of thyroglobulin and thyroglobulin antibodies. J. Clin. Endocrinol. Metab. 2020;105:dgaa152. doi: 10.1210/clinem/dgaa152. [DOI] [PubMed] [Google Scholar]
- 10.Xing M, et al. Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J. Clin. Oncol. 2015;33:42. doi: 10.1200/JCO.2014.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nixon IJ, et al. The results of selective use of radioactive iodine on survival and on recurrence in the management of papillary thyroid cancer, based on memorial Sloan-Kettering cancer center risk group stratification. Thyroid. 2013;23:683–694. doi: 10.1089/thy.2012.0307. [DOI] [PubMed] [Google Scholar]
- 12.Medas F, et al. Predictive factors of recurrence in patients with differentiated thyroid carcinoma: A retrospective analysis on 579 patients. Cancers. 2019;11:1230. doi: 10.3390/cancers11091230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Carvalho AY, Kohler HF, Gomes CC, Vartanian JG, Kowalski LP. Predictive factors for recurrence of papillary thyroid carcinoma: Analysis of 4085 patients. Acta Otorhinolaryngol. Ital. 2021;41:236. doi: 10.14639/0392-100X-N1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulpiau P, Van Roy F. Molecular evolution of the cadherin superfamily. Int. J. Biochem. Cell Biol. 2009;41:349–369. doi: 10.1016/j.biocel.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Wendeler M, et al. Ksp-cadherin is a functional cell–cell adhesion molecule related to LI-cadherin. Exp. Cell Res. 2004;294:345–355. doi: 10.1016/j.yexcr.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Casal JI, Bartolomé RA. Beyond N-cadherin, relevance of cadherins 5, 6 and 17 in cancer progression and metastasis. Int. J. Mol. Sci. 2019;20:3373. doi: 10.3390/ijms20133373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaszak I, et al. Role of cadherins in cancer: A review. Int. J. Mol. Sci. 2020;21:7624. doi: 10.3390/ijms21207624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu W, Yang L, Li T, Zhang Y. Cadherin signaling in cancer: Its functions and role as a therapeutic target. Front. Oncol. 2019;9:989. doi: 10.3389/fonc.2019.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lennartz M, et al. Urologic Oncology: Seminars and Original Investigations. Elsevier; 2021. [Google Scholar]
- 20.de Cristofaro T, et al. An essential role for Pax8 in the transcriptional regulation of cadherin-16 in thyroid cells. Mol. Endocrinol. 2012;26:67–78. doi: 10.1210/me.2011-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koumarianou P, Goméz-López G, Santisteban P. Pax8 controls thyroid follicular polarity through cadherin-16. J. Cell Sci. 2017;130:219–231. doi: 10.1242/jcs.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lennartz M, et al. Cadherin-16 (CDH16) immunohistochemistry: A useful diagnostic tool for renal cell carcinoma and papillary carcinomas of the thyroid. Sci. Rep. 2023;13:12917. doi: 10.1038/s41598-023-39945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li P, et al. Downregulation of CDH16 in papillary thyroid cancer and its potential molecular mechanism analysed by qRT-PCR, TCGA and in silico analysis. Cancer Manag. Res. 2019;11:10719. doi: 10.2147/CMAR.S229631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang X, Li Y, Liu G, Zha W, Liu Y. Cadherin-16 inhibits thyroid carcinoma cell proliferation and invasion. Oncol. Lett. 2022;23:1–12. doi: 10.3892/ol.2022.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han G, et al. Oncocytic papillary renal cell carcinoma: A clinicopathological and genetic analysis and indolent clinical course in 14 cases. Pathol. Res. Pract. 2017;213:1–6. doi: 10.1016/j.prp.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Iribe Y, et al. Immunohistochemical characterization of renal tumors in patients with Birt–Hogg–Dubé syndrome. Pathol. Int. 2015;65:126–132. doi: 10.1111/pin.12254. [DOI] [PubMed] [Google Scholar]
- 27.Gaut JP, Crimmins DL, Lockwood CM, McQuillan JJ, Ladenson JH. Expression of the Na+/K+-transporting ATPase gamma subunit FXYD2 in renal tumors. Mod. Pathol. 2013;26:716–724. doi: 10.1038/modpathol.2012.202. [DOI] [PubMed] [Google Scholar]
- 28.Yasir S, et al. CD10 (+) and CK7/RON (−) immunophenotype distinguishes renal cell carcinoma, conventional type with eosinophilic morphology from its mimickers. Appl. Immunohistochem. Mol. Morphol. 2012;20:454–461. doi: 10.1097/PAI.0b013e31823fecd3. [DOI] [PubMed] [Google Scholar]
- 29.Kuehn A, et al. Expression analysis of kidney-specific cadherin in a wide spectrum of traditional and newly recognized renal epithelial neoplasms: Diagnostic and histogenetic implications. Am. J. Surg. Pathol. 2007;31:1528–1533. doi: 10.1097/PAS.0b013e318058818c. [DOI] [PubMed] [Google Scholar]
- 30.Adley BP, et al. Expression of kidney-specific cadherin in chromophobe renal cell carcinoma and renal oncocytoma. Am. J. Clin. Pathol. 2006;126:79–85. doi: 10.1309/JFE2B57YQFPWPL10. [DOI] [PubMed] [Google Scholar]
- 31.Shen SS, Krishna B, Chirala R, Amato RJ, Truong LD. Kidney-specific cadherin, a specific marker for the distal portion of the nephron and related renal neoplasms. Mod. Pathol. 2005;18:933–940. doi: 10.1038/modpathol.3800373. [DOI] [PubMed] [Google Scholar]
- 32.Mazal PR, et al. Expression of kidney-specific cadherin distinguishes chromophobe renal cell carcinoma from renal oncocytoma. Hum. Pathol. 2005;36:22–28. doi: 10.1016/j.humpath.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Lonsdale J, et al. The genotype-tissue expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lizio M, et al. Update of the FANTOM web resource: expansion to provide additional transcriptome atlases. Nucleic Acids Res. 2019;47:D752–D758. doi: 10.1093/nar/gky1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bloomstein JD, et al. Validated limited gene predictor for cervical cancer lymph node metastases. Oncotarget. 2020;11:2302. doi: 10.18632/oncotarget.27632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calì G, et al. CDH16/Ksp-cadherin is expressed in the developing thyroid gland and is strongly down-regulated in thyroid carcinomas. Endocrinology. 2012;153:522–534. doi: 10.1210/en.2011-1572. [DOI] [PubMed] [Google Scholar]
- 37.di Martino E, Kelly G, Roulson J-A, Knowles MA. Alteration of cell-cell and cell-matrix adhesion in urothelial cells: An oncogenic mechanism for mutant FGFR3Mutant FGFR3 alters adhesive properties of urothelial cells. Mol. Cancer Res. 2015;13:138–148. doi: 10.1158/1541-7786.MCR-14-0022. [DOI] [PubMed] [Google Scholar]
- 38.Bu R, et al. Telomerase reverse transcriptase mutations are independent predictor of disease-free survival in Middle Eastern papillary thyroid cancer. Int. J. Cancer. 2018;142:2028–2039. doi: 10.1002/ijc.31225. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, et al. BRAFV600E is correlated with recurrence of papillary thyroid microcarcinoma: A systematic review, multi-institutional primary data analysis, and meta-analysis. Thyroid. 2016;26:248–255. doi: 10.1089/thy.2015.0391. [DOI] [PubMed] [Google Scholar]
- 40.Enumah S, et al. BRAFV600E mutation is associated with an increased risk of papillary thyroid cancer recurrence. World J. Surg. 2020;44:2685–2691. doi: 10.1007/s00268-020-05521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer. 2013;20:603–610. doi: 10.1530/ERC-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melo M, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014;99:E754–E765. doi: 10.1210/jc.2013-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panebianco F, Nikitski AV, Nikiforova MN, Nikiforov YE. Spectrum of TERT promoter mutations and mechanisms of activation in thyroid cancer. Cancer Med. 2019;8:5831–5839. doi: 10.1002/cam4.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Hu N. Telomerase reverse transcriptase induced thyroid carcinoma cell proliferation through PTEN/AKT signaling pathway. Mol. Med. Rep. 2018;18:1345–1352. doi: 10.1007/s00109-018-1696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pestana A, Vinagre J, Sobrinho-Simões M, Soares P. TERT biology and function in cancer: Beyond immortalisation. J. Mol. Endocrinol. 2017;58:R129–R146. doi: 10.1530/JME-16-0195. [DOI] [PubMed] [Google Scholar]
- 46.Liu Z, et al. Telomerase reverse transcriptase promotes epithelial–mesenchymal transition and stem cell-like traits in cancer cells. Oncogene. 2013;32:4203–4213. doi: 10.1038/onc.2012.441. [DOI] [PubMed] [Google Scholar]
- 47.Amin MB, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 48.Siraj AK, et al. PD-L1 Is an independent prognostic marker in Middle Eastern PTC and its expression is upregulated by BRAFV600E mutation. Cancers. 2021;13:555. doi: 10.3390/cancers13030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parvathareddy SK, et al. TERT promoter mutations are an independent predictor of distant metastasis in Middle Eastern papillary thyroid microcarcinoma. Front. Endocrinol. 2022;13:808298. doi: 10.3389/fendo.2022.808298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siraj A, et al. Genome-wide expression analysis of Middle Eastern papillary thyroid cancer reveals c-MET as a novel target for cancer therapy. J. Pathol. 2007;213:190–199. doi: 10.1002/path.2215. [DOI] [PubMed] [Google Scholar]
- 51.Bavi P, et al. Prevalence of fragile histidine triad expression in tumors from Saudi Arabia: A tissue microarray analysis. Cancer Epidemiol. Prev. Biomark. 2006;15:1708–1718. doi: 10.1158/1055-9965.EPI-05-0972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.