Abstract
Hyphomonas strain MHS-3, a member of a genus of primary colonizers of surfaces immersed in marine water, synthesizes two structures that mediate adhesion to solid substrata, namely, capsular exopolysaccharide and fimbriae. Specific stains, gold-labelled lectins, and monoclonal antibodies, along with transmission electron microscopy of synchronized populations, revealed that both structures are polarly and temporally expressed. The timed synthesis and placement of the fimbriae and capsule correlated with the timing and locus of MHS-3 adhesion.
Members of the marine bacterial genus Hyphomonas have a biphasic life cycle; swarm cells are planktonic entities, and prosthecate cells are sessile, benthic forms (14). These organisms are primary colonizers of surfaces in the marine environment (1, 3, 19, 38, 39, 42, 54) and are important because they adhere and form microcolonies (8, 33) which modify and enrich the surfaces. Thus, they can render a surface more suitable for subsequent attachment of heterotrophic bacteria, microalgae, fungi, and protozoans (13, 17). Such an adherent community can have profound effects on the subsequent colonization of the surface by invertebrates and on its ultimate performance (5, 12, 56, 57).
In order to adhere, bacteria produce extracellular adhesive structures that bridge repulsive electrostatic forces on submerged substrata. Functionally, these structures reduce the effective radius of interaction between the surface and the cell, thereby lowering the energy barrier (32, 40). Several types of proteinaceous structures may mediate transitory (primary) attachment of bacteria to surfaces. Among these are polar flagella (40, 61) and fimbriae (27, 40). Permanent cementation to surfaces requires the synthesis of exopolysaccharides (EPS) (9, 16, 32). EPS form the hydrated matrix in which multiple layers of cells and other materials become embedded, forming a biofilm (7). It has also been suggested that bacterial EPS are involved in primary adhesion (12), and more than 80% of the marine bacteria associated with deep-sea aggregates have EPS capsules (10).
The positions of polar structures in bacteria often correlate with physiological function (31). A number of different species of bacteria produce polar adhesive structures (holdfasts), leading to cell asymmetry and cell attachment in a distinct orientation. In Thiothrix spp., Seliberia stellata, and members of the prosthecate genera Caulobacter and Asticcacaulis, cells attach to inanimate surfaces and to one another (forming rosette-like aggregates) by polar production of fimbriae and EPS holdfasts (22, 30, 42, 51, 59). Several members of the prosthecate genus Hyphomonas and the related genus Hyphomicrobium also attach polarly to surfaces and form rosettes (36, 55). It has been theorized, but not demonstrated, that a Hyphomicrobium cell adheres via a polar holdfast opposite the prosthecum (35).
Hyphomonas strain MHS-3 synthesizes copious amounts of an integral, capsule-like EPS, which is associated with flocculation in broth cultures and the formation of thick biofilms on both hydrophilic and hydrophobic surfaces. The MHS-3 EPS capsule has been observed only during the sessile stage (43), and its adhesive properties have been demonstrated (43, 47). The purified polymer has been partially characterized; one of its major components is galactosamine which appears to be acetylated (44). In this paper, we show that adhesive fimbriae and capsular EPS are expressed both polarly and temporally by MHS-3 and that the presence and location of these structures correlate with the previously reported timing and locus of cell adhesion (43).
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
Wild-type Hyphomonas strain MHS-3 was isolated from shallow-water sediments in Puget Sound in Washington by J. Smit and was kindly given to R.M.W. Reduced-adhesion (rad) phase variants were isolated on the basis of their different colony morphology on agar plates and were named for their low adhesion to surfaces and low biofilm production. These strains were cultured in marine broth 2216 (MB) (54) (37.4 g/liter; Difco Laboratories, Detroit, Mich.) at 25°C. Marine agar contained MB and 2% (wt/vol) agar.
Electron microscopy stains and supplies were purchased from Electron Microscopy Sciences (Fort Washington, Pa.) or Sigma Chemical Co. (St. Louis, Mo.). Other chemicals and supplies were obtained from VWR Scientific (Bridgeport, N.J.). Copper grids (200 or 400 mesh) were used for electron microscopy; all grids were coated with collodion and then coated with carbon by using a type MED 10 deposition system (Balzers Union, Fürstentum, Liechtenstein).
Negative staining.
Single drops of MHS-3 were placed on collodion-coated copper grids, cells were allowed to attach for 1 min, and the grids were blotted with filter paper. To negatively stain the cells, 5 drops of 1% uranyl acetate were placed on the grid (for <1 min) and then blotted. The stained cells were observed with a model JEM-100CX II transmission electron microscope (TEM) (JEOL Ltd., Tokyo, Japan).
Preparation of whole-cell antigen.
Cultures (100 ml) of MHS-3 in the early stationary phase were pelleted by centrifugation (10,000 × g), resuspended in 100 ml of a sterile 3% NaCl solution containing 0.337% formaldehyde, incubated at 25°C for 30 min, washed once with 100 ml of 3% NaCl and twice with sterile 0.1 M phosphate-buffered saline (PBS), and resuspended in 0.1 M PBS at a concentration of approximately 1.25 mg (dry weight)/ml.
Immunization of mice.
Six-week-old BALB/c mice were injected intraperitoneally (i.p.) with 0.2 ml of a 1:1 mixture of whole formalized cells (125 μg of dry wild-type cells) and complete Freund’s adjuvant. The mice were boosted i.p. with whole cells mixed as described above in incomplete Freund’s adjuvant five times at 2-week intervals. The serum antibody titer was monitored 3 days after each boost by performing an enzyme-linked immunosorbent assay (ELISA) (see below). If the antibody titer was sufficient (>10,000) after the sixth boost, the mice were sacrificed 3 days later.
Production of MAbs.
The weak immunogen method of Lane et al. (28) was used for spleenocyte fusions to Sp2/O myeloma cells. Hybridomas were grown, screened, cloned, and stored in liquid nitrogen as described elsewhere (18). The monoclonal antibody (MAb) isotype was determined by using a Screentype isotyping kit (Boehringer Mannheim Biochemicals, Indianapolis, Ind.). Ascites fluid was produced by using 6-week-old BALB/c mice injected i.p. with 0.3 ml of pristane (18). Antibodies were concentrated by ammonium sulfate precipitation (18). Immunoglobulin G (IgG) MAbs were purified by affinity chromatography with a MAC protein A capsule (Amicon, Beverly, Mass.) and were concentrated by using Centriprep concentrator centrifuge tubes (Amicon).
ELISA.
Serum titers and hybridomas were initially screened by an ELISA (15, 52), with each well of 96-well Falcon flexible microtiter plates coated overnight at 4°C with 100 μl of formalized MHS-3 cells (prepared as described above) in coating buffer (10.6 g of Na2CO3 per liter; pH 9.6). For serum testing, the titer was defined as the largest dilution showing a strong positive reaction, and only these antibodies were examined further.
Screening for anti-MHS-3 LPS MAbs.
Lipopolysaccharide (LPS) samples were prepared with protease-treated whole-cell lysates and were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis by a modification of the method of Hitchcock and Brown (20). Lanes were loaded with the equivalent of 100 μg (dry weight) of whole cells. In the control gels, LPS was stained by the silver staining method of Tsai and Frasch (48), as modified by Hitchcock and Brown (20), in order to identify the MHS-3 LPS band pattern. Other gels were blotted onto nitrocellulose membrane filters (model 2117-250 Novablot electrophoretic transfer kit; Pharmacia LKB) by using continuous transfer buffer (39 mM glycine, 48 mM Tris, 0.375% [wt/vol] sodium dodecyl sulfate, 20% [vol/vol] methanol) and 140 mA.
To screen for anti-LPS MAbs, LPS-loaded nitrocellulose strips were blocked for 1 h with 5% skim milk (Difco) in PBS buffer and then individually incubated for 2 to 12 h with 50 ml of 5% skim milk (in PBS) containing 2 to 5 ml of supernatant from positive hybridoma cell lines. The blots were washed three times with PBS and incubated for 2 h with horseradish peroxidase-labelled goat anti-mouse IgG secondary antibody (GIBCO BRL, Grand Island, N.Y.) diluted 1:2,000 in milk-PBS. The strips were washed three times with PBS and incubated for 10 to 20 min in 50 ml of peroxidase substrate buffer (1 ml of 2 M Tris [pH 8.0], 20 mg of 4-chloro-1-naphthol, 10 ml of 30% hydrogen peroxide, and 49 ml of distilled H2O). Strips that developed band patterns identical to the pattern obtained with the LPS silver stain were considered positive, and the hybridoma cell lines were cloned and examined further. The hybridoma ultimately selected for this study produced an IgG2A kappa light chain.
Immunoelectron microscopy.
Cells were placed on collodion-coated copper grids (see above) and blocked for 10 min with 5% skim milk in PBS, and then the grids were inverted on 1 drop of a 1:750 anti-MHS-3 LPS MAb–skim milk solution for 10 min. The grids were rinsed by inverting them on 2 successive drops of skim milk, placed on 1 drop of 1:10 gold-conjugated protein A in skim milk (15-nm colloidal gold particles), and incubated for 10 to 30 min to allow the protein A to bind the IgG bound to the MHS-3 cell surface. Finally, the grids were rinsed by inverting them on 4 to 6 successive drops of distilled H2O (10 to 20 s each) and then observed with a model JEM-100CX II TEM (JEOL Ltd.).
Thin sectioning.
Cells were fixed by adding glutaraldehyde to mid-log-phase cultures of wild-type MHS-3 and rad phase variants (final concentration of fixative, 1%), incubated for 2 h at 25°C, and washed twice with 1× PBS. Cell pellets were resuspended in 1 ml of 1× PBS and mixed with an equal volume of molten agar (4%, 45°C), which was placed on Parafilm and allowed to harden. The agar was cut into 1- to 3-mm3 blocks, which were dehydrated by using a graded series of ethanol dilutions (30, 50, and 70% [twice], 30 min at each dilution), placed in microcentrifuge tubes containing LR White resin (LRW) and 70% ethanol (2:1) for 30 min, incubated with LRW for 1 h, transferred to tubes containing fresh LRW, incubated overnight at 25°C, transferred to tubes containing fresh LRW again, and incubated for 1 h. Finally, the blocks were placed in gelatin capsules with fresh LRW and incubated in a vacuum oven at 50°C for 72 h to allow the resin to polymerize.
Each gelatin capsule was removed from the polymerized resin, trimmed with a blade to expose the agar block region containing the embedded cells, and cut to make a 1-mm2 face. Thin sections (50 to 70 nm thick) were obtained with a model FC4E Ultracut E microtome (Reichert-Jung, Vienna, Austria) equipped with a diamond knife (Du Pont Co., Wilmington, Del.). For immunostaining, the sections were blocked with 5% skim milk and treated with anti-MHS-3 LPS MAb and protein A-gold as described above for whole cells.
Labelling of capsule with polycationic ferritin.
The procedure used to label capsule with polycationic ferritin was a modification of the protocol described by Jacques and Foiry (25). Briefly, mid-log-phase cultures of MHS-3 were fixed with glutaraldehyde as described above. The fixed bacteria were resuspended in cacodylate buffer (0.1 M sodium cacodylate, pH 7.0) and treated with polycationic ferritin (final concentration, 1.0 mg/ml) for 30 min at 25°C. The reaction was slowed by diluting the preparation 10-fold with buffer, and the cells were centrifuged and washed three times with cacodylate buffer. The bacteria were mixed with molten agar, embedded in LRW, and thin sectioned as described above.
Lectin-gold labelling of capsular EPS.
The procedure used for lectin-gold labelling of capsular EPS was carried out as reported previously (43). Gold-labelled Bauhinia purpurea lectin (BPA-Au) (10-nm gold particles; EY Laboratories, Inc., San Mateo, Calif.) was used to visualize MHS-3 capsular EPS. Briefly, 1 drop of a mid-log-phase culture was placed on a collodion-coated copper grid, incubated at room temperature for 1 min, blocked with a solution containing 5% bovine serum albumin in 0.1 M PBS for 5 to 10 min, incubated with a 1:10 dilution of BPA-Au in 5% bovine serum albumin for 10 to 15 min, and then rinsed five times with distilled water. The grids were observed with the TEM.
Synchronization of Hyphomonas strain MHS-3 cultures.
An effective protocol to isolate MHS-3 swarm cells is a modification of the differential size separation procedure used by Wali et al. (53) to synchronize cultures of Hyphomonas neptunium. Briefly, an early- to mid-log-phase broth culture of MHS-3 was chilled on ice and centrifuged at 2,500 × g for 3 to 5 min to pellet large flocs and cell aggregates. The pellet was discarded, and the chilled supernatant was passed through sterile 1.2-μm-pore-size membrane filters (Millipore Corp., Bedford, Mass.). Prosthecate cells were retained by the filters, and the filtrates, containing the swarm cells, were pooled and pelleted by centrifugation (16,000 × g, 30 min, 4°C). The pellet was resuspended in 10 ml of MB to start a synchronous culture at 25°C. These manipulations yielded a highly synchronous population; however, they also caused a lag period of approximately 90 min (14, 53).
Once the synchronous culture was initiated, 400-μl aliquots were removed at 15-min intervals, placed in microcentrifuge tubes with either glutaraldehyde (final concentration, 1%) or sodium azide (final concentration, 0.02%), and stored at 4°C. Glutaraldehyde was not used as a fixative for cells labelled with lectin-gold because it interfered with the binding of the conjugate to the MHS-3 capsule. Cells at different stages of development were negatively stained with uranyl acetate and labelled with lectin-gold as described above.
RESULTS
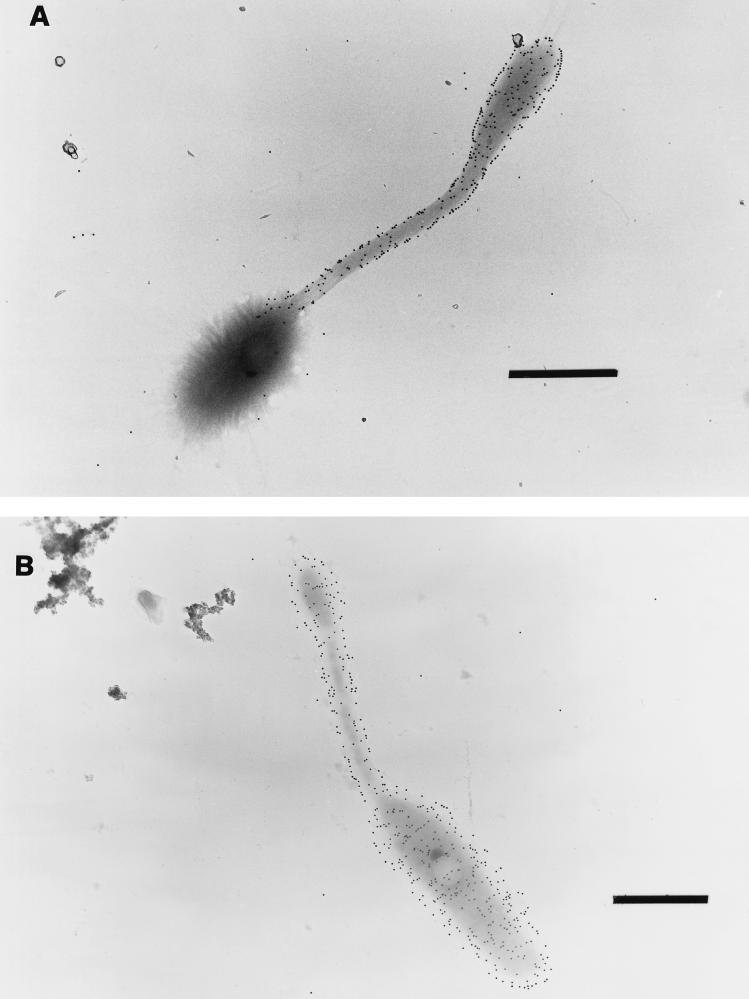
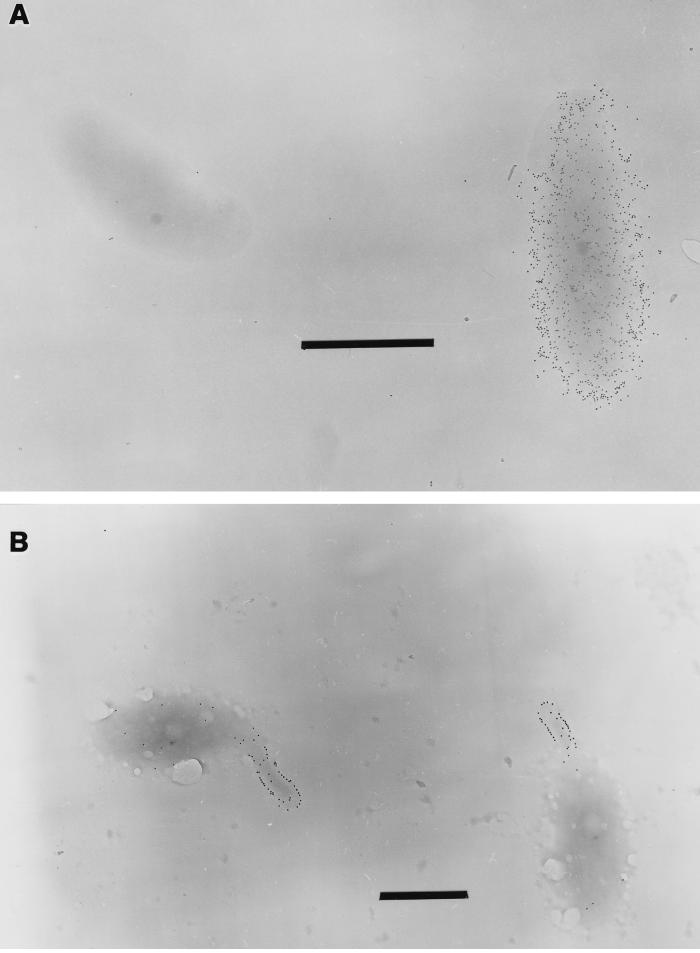
The location of the EPS capsule on Hyphomonas strain MHS-3 was determined by immunoprobing with EPS-specific stains and the TEM. An EPS-deficient mutant of MHS-3 (a rad strain [43]) was used as the negative control. The body, prosthecum, and bud were easily recognized in each prosthecate reproductive cell. In wild-type cells, MAb bound to swarm cells and to the prosthecum, but not the body, of a prosthecate reproductive cell (Fig. 1A). In a control rad strain cell, the MAb bound everywhere, including the body (Fig. 1B). Furthermore, when wild-type cells were sheared in a blender in the presence of EDTA to remove most of the capsular EPS, anti-LPS MAb also bound to the bodies of prosthecate reproductive cells (data not shown). We interpreted these results to mean that the EPS capsule blocked access of MAb to the outer membrane of the body of a wild-type cell. Thus, anti-LPS MAb served as a negative stain for the capsule, which was found to surround only the main body of each wild-type prosthecate cell.
FIG. 1.
Immunoelectron microscopy of Hyphomonas strain MHS-3 wild-type (A) and rad (B) whole cells. Anti-LPS MAb bound only the prosthecum and swarm cell in the wild-type strain (A); it labelled all the structures in the rad strain (B). Bars = 1 μm.
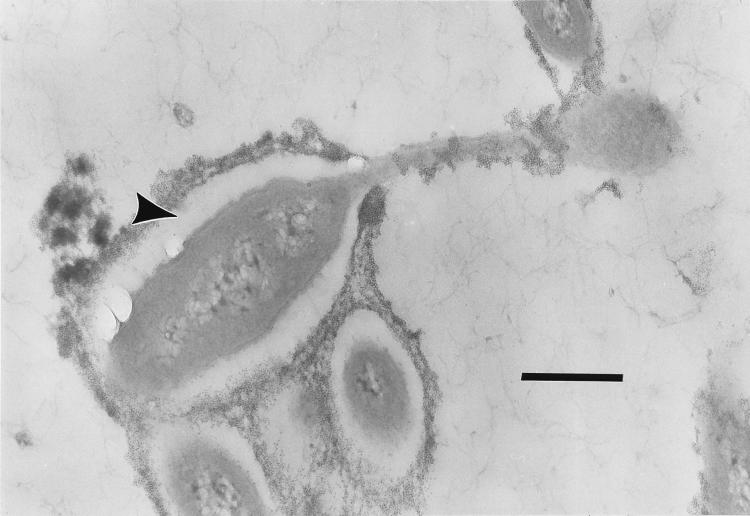
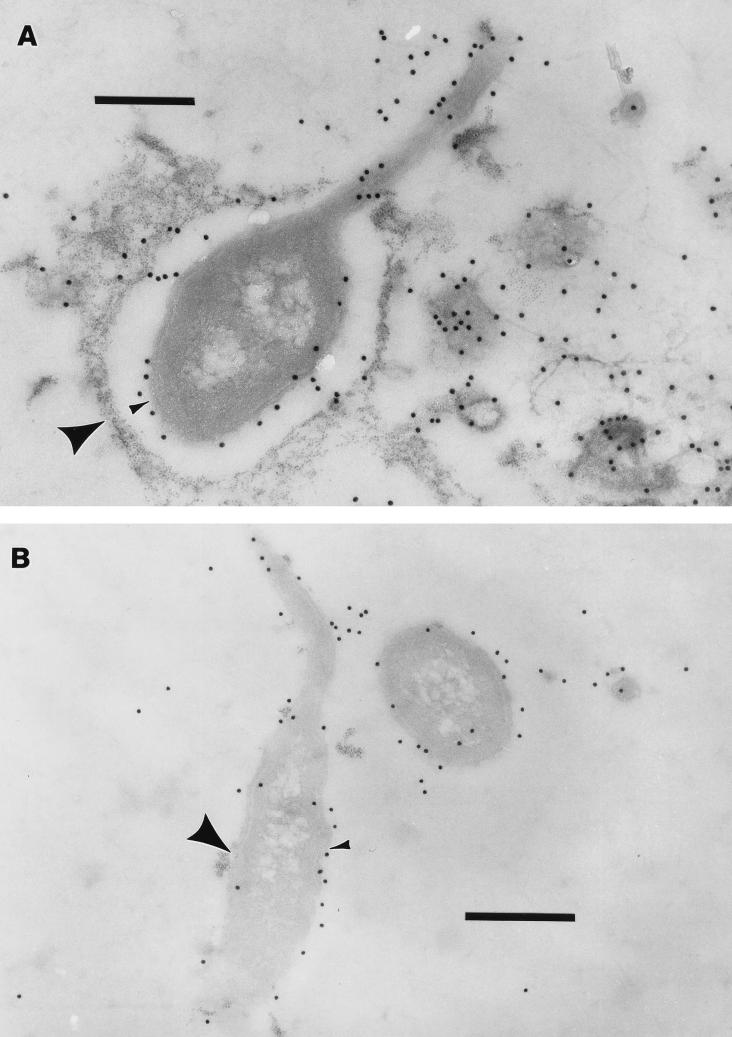
The same results were obtained when MHS-3 cells were treated with polycationic ferritin prior to fixing, embedding, and sectioning (Fig. 2). The polycationic ferritin penetrated the EPS capsule just enough to reveal its outer edge. When these cells were thin sectioned and then treated with anti-LPS MAb, the outer membrane was delineated, revealing that it was covered by the capsule (Fig. 3A), which was calculated to be 200 to 300 nm in diameter (by using the average diameter of the 15-nm gold particles as a reference). When the same experiment was repeated with the rad strain, the anti-LPS MAb and the polycationized ferritin each bound in the same location, the site of the outer membrane (Fig. 3B).
FIG. 2.
Thin section of polycationic ferritin-treated Hyphomonas wild-type strain MHS-3. Note the presence of a spatially defined capsule on the body of the prosthecate reproductive cell (arrowhead). Bar = 0.5 μm.
FIG. 3.
Immunoelectron microscopy of thin sections of Hyphomonas wild-type strain MHS-3 (A) and the rad strain (B) treated with polycationic ferritin by using anti-LPS MAb. Whole cells were stained with polycationic ferritin, fixed, embedded, and thin sectioned; the thin sections were exposed to the MAb. In wild-type strain MHS-3 the outer edge of the EPS capsule is delineated by polycationic ferritin binding (large arrowhead), while the outer membrane is clearly delineated by the binding of the MAb-protein A-gold complexes (small arrowhead). In the MHS-3 rad strain polycationic ferritin bound to the outer membrane of the cell (large arrowhead), as did anti-LPS MAb (small arrowhead), indicating that no capsule was present. Bars = 0.5 μm.
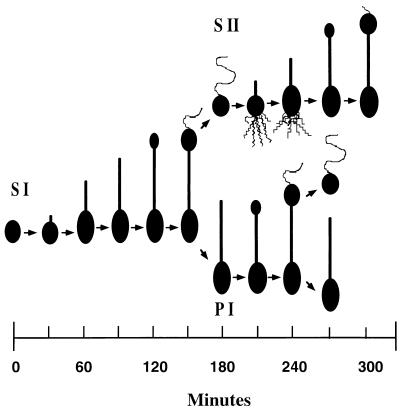
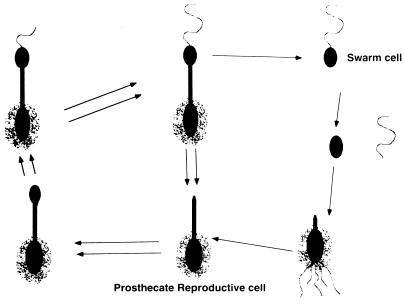
The temporality of capsule elaboration during the relatively complex life cycle of Hyphomonas strain MHS-3 was ascertained by using synchronous cultures. The postulated MHS-3 biphasic developmental cycle is diagrammed in Fig. 4. Generation I swarm (S I) cells required 30 min for maturation and initiation of prosthecal outgrowth and capsule deposition (swarm maturation period), which occurred concurrently (Fig. 4 and 5). The EPS appeared to be uniformly exported over the entire surface of the developing prosthecate reproductive cell. In Fig. 5A, which shows two adjacent young prosthecate reproductive cells, the onset of the timing of these processes is underscored. One cell was completely labelled with the MHS-3 capsular EPS-specific lectin, BPA-Au, while the other cell was not labelled (onset of capsule deposition). This difference was also revealed by using anti-LPS MAbs. Only the nascent prosthecae of cells at the same time point bound anti-LPS MAbs (Fig. 5B), leading to the conclusion that the onset of capsule deposition coincides with the initiation of prosthecal synthesis. S I prosthecate cells formed buds at 120 ± 15 min. After 150 min, S I cells were fully developed reproductive cells that budded at the distal ends of their prosthecae (bud maturation period, 30 min). The generation II swarm (S II) cells were released by the prosthecate reproductive cells at 165 ± 15 min during the synchronous cycle.
FIG. 4.
Timing of morphogenesis during synchronous growth of Hyphomonas strain MHS-3. S I cells, S II cells, and the first prosthecal generation (P I) are shown. The experimentally induced lag period was factored out.
FIG. 5.
Initiation of capsular EPS synthesis by Hyphomonas wild-type strain MHS-3 in synchronous culture. The cell stages, labelled with BPA-Au (A) and anti-LPS MAb (B), correspond to 15 ± 15 min for S I cells and 195 ± 15 min for S II cells. EPS is deposited all over the body of the young prosthecate reproductive cells, as indicated by the binding pattern of BPA-Au (A) and the localized binding of the MAb (B), where only the nascent prosthecae are labelled. The timing of capsule deposition is readily apparent in panel A, in which one cell is heavily labelled with BPA-Au while the other cell, which is just beginning to excrete EPS, is lightly labelled. Bars = 1 μm.
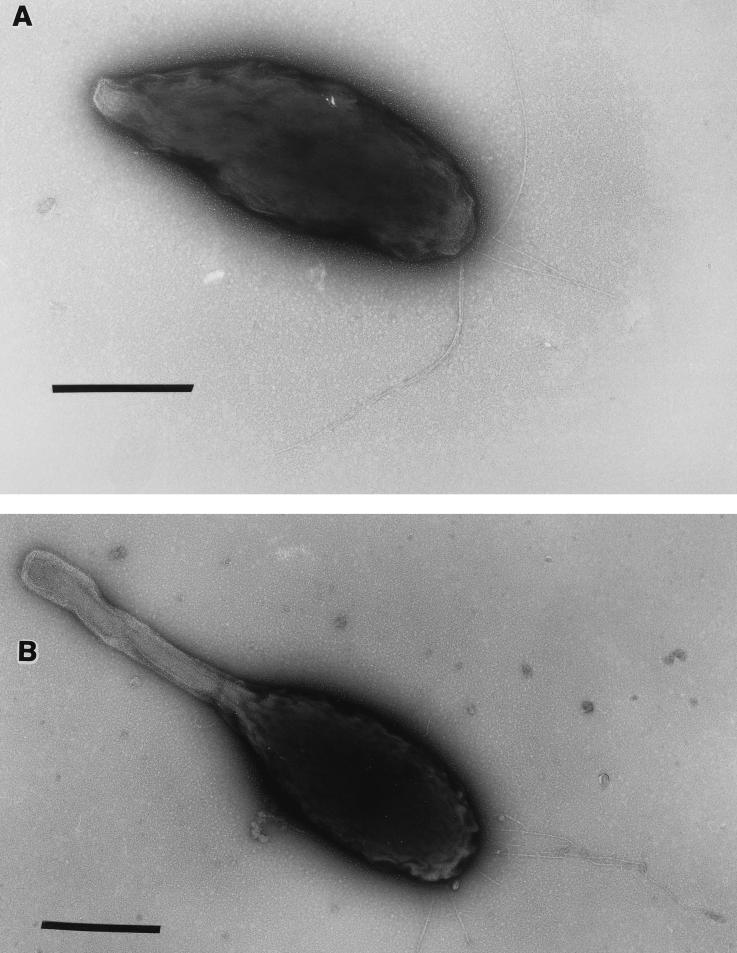
During the second swarm cell generation (S II) (Fig. 4) swarm cells matured and initiated prosthecal outgrowth and capsule deposition within 30 min after they were released, as they had during the first generation. New buds formed approximately 90 min into the S II phase. One unexpected generation II event, not observed during the first generation, occurred at 195 ± 15 min. Polar fimbriae were synthesized on the main body of each nascent reproductive cell (Fig. 6A). The fimbriae and capsule were produced simultaneously by the young prosthecate reproductive cells. Fimbriae were still evident at 225 ± 15 min (Fig. 6B), after which they were no longer observed on cells in subsequent S II stages. Thus, fimbrial elaboration in MHS-3 was confined to the swarm cell-to-prosthecate cell transition; fimbriae were present only during approximately 30 min of the developmental cycle (<20% of the time). In parallel experiments, synchronously cultured MHS-3 rad cells did not produce any capsular EPS at any stage of the life cycle. However, they did produce fimbriae during the same stages of the life cycle in which fimbriae were produced by the wild-type strain.
FIG. 6.
Uranyl acetate-stained Hyphomonas wild-type strain MHS-3 from a synchronous culture, showing polar fimbrial production by young prosthecate reproductive cells. The cell stages shown correspond to 195 ± 15 min (A) and 225 ± 15 min (B) for S II cells. These are the only stages in which fimbriae are found on MHS-3. Bars = 0.5 μm.
The prosthecal generation was also monitored (Fig. 4). Approximately 90 ± 15 min (180 to 270 min) was required for a prosthecate cell from the first generation to produce a second swarm cell (period of swarm maturation and separation).
DISCUSSION
This is the first report of temporal and spatial deposition of capsular EPS in the genus Hyphomonas. In strain MHS-3, it was found by using negative immunoelectron microscopy probing with anti-LPS MAb and EPS-specific stains that a capsule is synthesized only by prosthecate reproductive cells and only on the main body of a reproductive cell. These observations were verified in a synchronous culture, in which the temporality and polarity of fimbrial synthesis were also observed. These findings are summarized in Fig. 7.
FIG. 7.
Representation of the Hyphomonas strain MHS-3 developmental cycle, showing the temporality and polarity of capsule and fimbria expression.
It was confirmed by indirect immunostaining and direct ferritin staining that rad variants, which spontaneously arise at a low frequency (43), do not synthesize observable capsules, as hypothesized previously (43) on the basis of results obtained with direct lectin (BPA-Au) probes. The rad offspring may be less likely to be trapped in biofilms than the wild-type offspring, which would be advantageous in species dispersal.
Most EPS capsules are highly hydrated and require special treatment to stabilize their structure during fixation. During the graded specimen dehydration step required for embedding, most capsules usually collapse, and subsequent exposure to water fails to rehydrate the precipitated EPS (2). Several techniques involving antibody cross-linking, Lowcryl resin imbedding, or cationic ferritin binding have been developed to preserve the structure of capsules (2). In the case of MHS-3, although polycationic ferritin was used to label the outline of the capsule, it did not penetrate and bind the entire cross section of the capsule enough to stabilize it (Fig. 2). Furthermore, thin sections of wild-type cells that were not treated with polycationic ferritin revealed the faint outline of a well-preserved capsule after staining with uranyl acetate (data not shown). This suggests that standard glutaraldehyde fixation is sufficient to preserve the MHS-3 capsular structure. Moreover, the EPS capsules of marine bacteria are naturally stabilized by absorbing metals and are readily observed by TEM after glutaraldehyde fixation and embedding, with or without the use of heavy-metal stains (10).
Since the MHS-3 capsule blocks penetration of anti-LPS MAb (Fig. 1A) and polycationic ferritin (Fig. 2), it is probably an effective molecular sieve. The anti-LPS MAb used was a mouse IgG which has an approximate molecular weight of 150,000. The molecular weight of horse spleen ferritin (used to manufacture polycationic ferritin) is approximately 445,000. There is a long-held belief that EPS capsules of pathogenic bacteria interfere with host defense mechanisms, antibodies, and complement via masking mechanisms, rendering binding sites physically inaccessible (11). EPS capsules have been listed as virulence factors in pathogenic bacteria precisely because they prevent antibodies from binding to membrane epitopes (37). Such capsules are found on clinical isolates of Staphylococcus aureus and were identified by using EPS-specific MAbs to immunostain thin sections after standard fixation, dehydration, and embedding procedures (21), without any special capsule stabilization procedure. While MHS-3 is not a mammalian pathogen, its capsule also thwarts antibody binding to target sublayers (e.g., LPS).
Few other prokaryotes produce polar adhesive EPS. Previously published reports indicate that Caulobacter spp. (42), Asticcacaulis biprosthecum (51), Thiothrix spp. (30, 59), S. stellata (22), and Bradyrhizobium japonicum produce these molecules (31, 49, 50). The presence of an EPS holdfast has been inferred but not demonstrated in Hyphomicrobium spp. (36). The MHS-3 capsule is not just unusual; it is unique among the examples cited above because it is an extensive, integral EPS capsule, while the other structures are not.
S I cells required 165 ± 15 min to develop into mature prosthecate reproductive cells and release progeny. The only difference in the timing of S I and S II cells was in the prosthecal elongation period; S I cells required 90 min, which was 30 min longer than S II cells required. Swarm cell maturation and release required a little more than 30 min in each of the stable synchronous generations (Fig. 4). In Hyphomonas strain MHS-3, the hyphal growth period required a greater percentage of the cell cycle time than the percentage reported for Hyphomicrobium neptunium under similar growth conditions (53). For the first time, it was found by observing cells in synchronous cultures that the production of the capsule in the genus Hyphomonas is a temporally regulated event which is linked to major physiological and morphological changes during the transition from swarm cells to prosthecate cells. Thus, MHS-3 appears to be a member of a group of prosthecate bacteria in which the production of EPS is temporally governed by an obligate life cycle and occurs at specific stages of development. In contrast, in most other genera, the biosynthesis of EPS is regulated by nutritional (26, 62) and environmental (4) cues.
This is also the first report of fimbrial production in Hyphomonas, one of several genera that produce polar fimbriae. These fimbriae are expressed at a very specific stage during the transition from swarm cells to prosthecate cells (Fig. 6A). A close examination revealed that the fimbriae vary in diameter along their length, from about 5.0 to 8.0 nm, suggesting that they are flexible (58). Polar fimbriae of Pseudomonas aeruginosa have been implicated in adhesion to mammalian tissues during pathogenesis (60, 63) and to solid surfaces, such as stainless steel and polystyrene (23). Interestingly, Thiothrix spp., Caulobacter spp., and A. biprosthecum also produce polar tufts of fimbriae at the same locus as the holdfast (30, 42, 51, 59).
The primary role of fimbriae is to mediate bacterial adhesion to inanimate surfaces or to other cells. They help negatively charged bacteria bind to negatively charged substrata, bridging the electrostatic repulsive forces in accordance with the DLVO theory (bodies with very small radii experience less repulsion) (40). It has been proposed that fimbriae mediate primary (transitory) adhesion and that EPS promotes irreversible attachment to surfaces (16, 32). The experimental results obtained with MHS-3 are consistent with this hypothesis, except that it is possible that MHS-3 EPS mediates primary adhesion as well as permanent cementation, since both wild-type and rad strains produce fimbriae, but the wild-type (EPS-positive) cells adhere better (43). Similar results have been reported for A. biprosthecum, in which holdfast mutants failed to attach to surfaces (51). Thiothrix spp. attach to ciliated protozoa (34) and to one another (30) by means of EPS holdfasts. In Thiothrix nivea, the initial attachment step involves the fimbriated pole and is followed by the production of a holdfast (30). Another line of evidence which supports the importance of the capsule in primary adhesion is the transitory appearance of MHS-3 fimbriae. MHS-3 adheres at times when the fimbriae are not expressed.
In Caulobacter crescentus, fimbriae are functionally expressed during the swarm cell stage and disappear when a swarm cell differentiates into a prosthecate cell (29). Although the pilin monomer is present inside the cell throughout the entire Caulobacter life cycle (29), the fimbriae are assembled after separation of the swarm cell from the prosthecate cell (46). In MHS-3, as in C. crescentus, fimbriae are not shed into the culture medium, suggesting that they are probably also retracted into the cells (29, 46). Escherichia coli, Pseudomonas aeruginosa, and C. crescentus have been shown to have retractable fimbriae by using fimbria-specific bacteriophages to inhibit retraction (6, 25, 46). In the case of Pseudomonas syringae pv. phaseolicola, fimbria retraction pulls fimbria-associated bacteriophage f 6 through the EPS of the host and brings it into contact with the outer membrane, where membrane fusion can take place (45). Fimbria retraction may be a mechanism to bring cells closer to the surface once attachment has occurred (27). In contrast, A. biprosthecum and other bacteria shed their fimbriae (41).
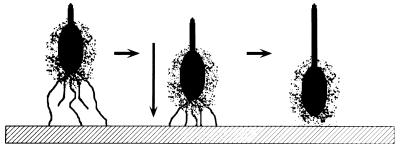
The temporal and spatial regulation of the adhesive structures of MHS-3 can be correlated with putative function (43). MHS-3 appears to synthesize capsular EPS and fimbriae simultaneously. The value of these events as an attachment strategy could be that two chemically distinct adhesive molecules are presented, which should allow interactions with a wide array of surfaces. MHS-3 fimbriae are 1,000 to 2,000 nm long, and the EPS capsule is 200 to 300 nm thick. Alternatively, fimbriae could mediate long-range, primary binding to surfaces, since they extend beyond the EPS capsule and can tether the cell to the surface and then retract and bring the EPS adhesive capsule in contact with the surface, as represented in Fig. 8.
FIG. 8.
Representation of fimbrial retraction during the process of adhesion of Hyphomonas strain MHS-3 to surfaces. Fimbriae putatively mediate long-range, primary binding to surfaces, retract, and bring the EPS adhesin involved in permanent attachment in contact with the surface.
Species that synthesize polar adhesive structures in lieu of surrounding capsules could conserve energy. Furthermore, adhesive structures (fimbriae, EPS) could foster polar attachment to surfaces, another potential survival advantage. This is exemplified by starved marine Vibrio strain DW1, which attaches perpendicularly to surfaces by an as-yet-unknown mechanism. During cell division, the original cells (analogous to the MHS-3 prosthecate reproductive cells) remain attached, while the progeny cells detach and become planktonic (32). This strategy is even more favorable for members of the genus Hyphomonas, since each swarm cell is released at the distal tip of the prosthecum, further elevating the cell. Therefore, prosthecate cells could release motile swarm cells closer to the water interface of the biofilm and into the water column to colonize new substrata.
ACKNOWLEDGMENTS
We thank G. Geesey for helpful discussions and M. Kessel for help and insights concerning fine structure.
This work was supported in part by grants from the Office of Naval Research and the Maryland Industrial Partnerships.
REFERENCES
- 1.Baier R, Meyer A, DePalma V, King R, Fornalik M. Surface microfouling during the induction period. J Heat Transfer. 1983;105:618–624. [Google Scholar]
- 2.Bayer M E. Visualization of the bacterial polysaccharide capsule. Curr Top Microbiol Immunol. 1990;150:29–57. doi: 10.1007/978-3-642-74694-9_7. [DOI] [PubMed] [Google Scholar]
- 3.Beloni Z H, Avilov I A, Gromov B V. The peculiarities of soil strains of budding bacteria of the genus Hyphomicrobium. Biol Sci. 1984;6:71–75. [Google Scholar]
- 4.Berry A, DeVault J D, Chakrabarty A M. High osmolarity is a signal for enhanced algD transcription in mucoid and nonmucoid Pseudomonas aeruginosa strains. J Bacteriol. 1989;171:2312–2317. doi: 10.1128/jb.171.5.2312-2317.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bott T R. Introduction to the problem of biofouling in industrial equipment. NATO Adv Study Inst Ser Ser E Appl Sci. 1992;223:3–11. [Google Scholar]
- 6.Bradley D E. Pseudomonas aeruginosa pili. In: Bradley D E, Raizen E, Fives-Taylor P, Ou J, editors. Pili. Proceedings of the International Conference on Pili. Washington, D.C: American Society for Microbiology; 1978. pp. 319–338. [Google Scholar]
- 7.Christensen B E. The role of extracellular polysaccharides in biofilms. J Biotechnol. 1989;10:181–202. [Google Scholar]
- 8.Corpe W A. Microfouling: the role of primary film forming bacteria. In: Ackjer R F, Brown B F, DePalma J R, Verson W P, editors. Proceedings of the Third International Congress on Marine Corrosion Fouling. Evanston, Ill: Northwestern University Press; 1973. pp. 598–609. [Google Scholar]
- 9.Costerton J W. Mechanisms of microbial adhesion to surfaces. Direct ultrastructural examination of adherent bacterial populations in natural and pathogenic ecosystems. In: Klug M J, Reddy C A, editors. Current perspectives in microbial ecology. Proceedings of the 3rd International Symposium on Microbial Colonization. Washington, D.C: American Society for Microbiology; 1984. pp. 115–123. [Google Scholar]
- 10.Cowen J P. Morphological study of marine bacterial capsules: implications for marine aggregates. Mar Biol. 1992;114:85–95. [Google Scholar]
- 11.Cross A S. The biologic significance of bacterial encapsulation. Curr Top Microbiol Immunol. 1990;150:87–95. doi: 10.1007/978-3-642-74694-9_5. [DOI] [PubMed] [Google Scholar]
- 12.Decho A W. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr Mar Biol Annu Rev. 1990;28:73–153. [Google Scholar]
- 13.DiSalvo L H, Daniels G W. Observations on estuarine microfouling using the scanning electron microscope. Microb Ecol. 1975;2:234–240. doi: 10.1007/BF02010443. [DOI] [PubMed] [Google Scholar]
- 14.Emala M A, Weiner R M. Modulation of adenylate energy charge during the swarmer cycle of Hyphomicrobium neptunium. J Bacteriol. 1983;153:1558–1561. doi: 10.1128/jb.153.3.1558-1561.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70:419–438. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- 16.Fletcher M. Adherence of marine micro-organisms to smooth surfaces. In: Beachey E H, editor. Bacterial adherence. London, United Kingdom: Chapman & Hall; 1980. p. 345. [Google Scholar]
- 17.Gerchakov S M, Mardzalek D S, Roth F J, Udey L R. Proceedings of the 4th International Congress of Marine Corrosion Fouling. 1976. Succession of periphytic microorganisms on metal and glass surfaces in natural seawater. [Google Scholar]
- 18.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 19.Hirsch P. Budding bacteria. Annu Rev Microbiol. 1974;28:391–444. doi: 10.1146/annurev.mi.28.100174.002135. [DOI] [PubMed] [Google Scholar]
- 20.Hitchcock P, Brown T. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochkeppel H K, Braun D G, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan E L, Boutonnier A, Fournier J M. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol. 1987;25:526–530. doi: 10.1128/jcm.25.3.526-530.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hood M A, Schmidt J M. The examination of Seliberia stellata exopolymers using lectin assays. Microb Ecol. 1996;31:281–290. doi: 10.1007/BF00171572. [DOI] [PubMed] [Google Scholar]
- 23.Irvin R T. Hydrophobicity of proteins and bacterial fimbriae. In: Doyle R J, Rosenberg M, editors. Microbial cell surface hydrophobicity. Washington, D.C: American Society for Microbiology; 1990. pp. 137–177. [Google Scholar]
- 24.Jacobson A. Role of F pili in the penetration of bacteriophage f1. J Virol. 1972;10:835–843. doi: 10.1128/jvi.10.4.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacques M, Foiry B. Electron microscopic visualization of capsular material of Pasteurella multocida types A and D labeled with polycationic ferritin. J Bacteriol. 1987;169:3470–3472. doi: 10.1128/jb.169.8.3470-3472.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarman T R, Deavin L, Slocombe S, Righelato R C. Investigation of the effect of environmental conditions on the rate of exopolysaccharide synthesis in Azotobacter vinelandii. J Gen Microbiol. 1978;107:59–64. [Google Scholar]
- 27.Jones G W, Isaacson R E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10:229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- 28.Lane R D, Crissman R S, Lachman M F. Comparison of polyethylene glycols as fusogens for producing lymphocyte-myeloma hybrids. J Immunol Methods. 1984;72:71–76. doi: 10.1016/0022-1759(84)90434-4. [DOI] [PubMed] [Google Scholar]
- 29.Langenaur C, Agabian N. Caulobacter crescentus pili: structure and stage-specific expression. J Bacteriol. 1977;131:340–346. doi: 10.1128/jb.131.1.340-346.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larkin J M, Nelson R. Mechanism of attachment of swarm cells of Thiothrix nivea. J Bacteriol. 1987;169:5877–5879. doi: 10.1128/jb.169.12.5877-5879.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maddock J R, Alley M R K, Shapiro L. Polarized cells, polar actions. J Bacteriol. 1993;175:7125–7129. doi: 10.1128/jb.175.22.7125-7129.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall K C. Biofilms: an overview of bacterial adhesion, activity, and control at surfaces. ASM News. 1992;58:202–207. [Google Scholar]
- 33.Marshall K D, Stout R, Mitchell R. Selective sorption of bacteria from seawater. Can J Microbiol. 1971;17:1413–1416. doi: 10.1139/m71-225. [DOI] [PubMed] [Google Scholar]
- 34.Merkel G J. Observations of the attachment of Thiothrix to biological surfaces in activated sludge. Water Res. 1975;9:881–885. [Google Scholar]
- 35.Moore R L. The biology of Hyphomicrobium and other prosthecate, budding bacteria. Annu Rev Microbiol. 1981;35:567–594. doi: 10.1146/annurev.mi.35.100181.003031. [DOI] [PubMed] [Google Scholar]
- 36.Moore R L, Marshall K C. Attachment and rosette formation by hyphomicrobia. Appl Environ Microbiol. 1981;42:751–757. doi: 10.1128/aem.42.5.751-757.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moxon E R, Kroll J S. The role of bacterial polysaccharide capsules as virulence factors. Curr Top Microbiol Immunol. 1990;150:65–85. doi: 10.1007/978-3-642-74694-9_4. [DOI] [PubMed] [Google Scholar]
- 38.Nikitin D I, Nikitina E S. Environmental processes of self-clarifying and bacterial parasites (genus Bdellovibrio) Moscow, USSR: Nauka; 1978. p. 26. [Google Scholar]
- 39.Nikitin D I, Vishnewetskaya O Y, Chumakov K M, Zlatkin I V. Evolutionary relationships between some stalked and budding bacteria (genera Caulobacter, “Hyphobacter,” Hyphomonas and Hyphomicrobium) as studied by the new integral taxonomical method. Arch Microbiol. 1990;153:123–128. doi: 10.1007/BF00247808. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira D R. Physico-chemical aspects of adhesion. NATO Adv Study Inst Ser Ser E Appl Sci. 1992;223:45–58. [Google Scholar]
- 41.Pate J L, Petzold S J, Umbreit T H. Two flagellotropic phages and one pilus-specific phage active against Asticcacaulis biprosthecum. Virology. 1979;94:24–37. doi: 10.1016/0042-6822(79)90435-5. [DOI] [PubMed] [Google Scholar]
- 42.Poindexter J S. The caulobacters: ubiquitous unusual bacteria. Microbiol Rev. 1981;45:123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quintero E J, Weiner R M. Evidence for the adhesive function of the exopolysaccharide of Hyphomonas strain MHS-3 in its attachment to surfaces. Appl Environ Microbiol. 1995;61:1897–1903. doi: 10.1128/aem.61.5.1897-1903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quintero E J, Weiner R M. Physical and chemical characterization of the polysaccharide capsule of the marine bacterium, Hyphomonas strain MHS-3. J Ind Microbiol. 1995;15:347–351. [Google Scholar]
- 45.Romantschuk M, Bamford D H. Function of pili in bacteriophage f 6 penetration. J Gen Virol. 1985;66:2461–2469. doi: 10.1099/0022-1317-66-11-2461. [DOI] [PubMed] [Google Scholar]
- 46.Sommer J M, Newild A. Sequential regulation of developmental events during polar morphogenesis in Caulobacter crescentus: assembly of pili on swarmer cells requires cell separation. J Bacteriol. 1988;170:409–415. doi: 10.1128/jb.170.1.409-415.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suci P A, Frølund B, Quintero E J, Weiner R M, Geesey G G. Adhesive extracellular polymers of Hyphomonas MHS-3: interaction of polysaccharides and proteins. Biofouling. 1995;9:95–114. [Google Scholar]
- 48.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 49.Tsien H C, Schmidt E L. Polarity in the exponential-phase Rhizobium japonicum cell. Can J Microbiol. 1977;23:1274–1284. doi: 10.1139/m77-191. [DOI] [PubMed] [Google Scholar]
- 50.Tsien H C, Schmidt E L. Localization and partial characterization of soybean lectin-binding polysaccharide of Rhizobium japonicum. J Bacteriol. 1981;145:1063–1074. doi: 10.1128/jb.145.2.1063-1074.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Umbreit T H, Pate J L. Characterization of the holdfast region of wild-type cells and holdfast mutants of Asticcacaulis biprosthecum. Arch Microbiol. 1978;118:157–168. [Google Scholar]
- 52.Voller A, Bidwell D E, Bartlett A. The enzyme linked immunosorbent assay (ELISA). A guide with abstracts of microplate applications. Guernsey, Great Britain: Dynatech Europe, Burough House; 1979. [Google Scholar]
- 53.Wali T M, Hudson G R, Danald D A, Weiner R M. Timing of swarmer cell cycle morphogenesis and macromolecular synthesis by Hyphomicrobium neptunium in synchronous culture. J Bacteriol. 1980;144:406–412. doi: 10.1128/jb.144.1.406-412.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weidner S, Arnold W, Pühler A. Diversity of uncultured microorganisms associated with the seagrass Halophila stipulacea estimated by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA. Appl Environ Microbiol. 1996;62:766–771. doi: 10.1128/aem.62.3.766-771.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiner R M, Devine R A, Powell D M, Dagasan L, Moore R L. Hyphomonas oceanitis sp. nov., Hyphomonas hirschiana sp. nov., and Hyphomonas jannaschiana sp. nov. Int J Syst Bacteriol. 1985;35:237–243. [Google Scholar]
- 56.Weiner R M, Walch M, Labare M P, Bonar D B, Colwell R R. Effect of biofilms of the marine bacterium Alteromonas colwelliana (LST) on set of the oysters Crassostrea gigas (Thunberg, 1793) and C. virginica (Gmelin, 1791) J Shellfish Res. 1989;8:117–123. [Google Scholar]
- 57.Weiner R M, Sledjeski D, Quintero E, Coon S, Walch M. Abstracts of the 6th International Symposium on Microbial Ecology, Barcelona, Spain. Barcelona, Spain: Spanish Society for Microbiology; 1992. Periphytic bacteria cue oyster larvae to set on fertile benthic biofilms, abstr. S1-5-2. [Google Scholar]
- 58.Weiss R L. The structure and occurrence of pili (fimbriae) on Pseudomonas aeruginosa. J Gen Microbiol. 1971;67:135–143. doi: 10.1099/00221287-67-2-135. [DOI] [PubMed] [Google Scholar]
- 59.Williams T M, Unz R F, Doman J T. Ultrastructure of Thiothrix spp. and “type 021N” bacteria. Appl Environ Microbiol. 1987;53:1560–1570. doi: 10.1128/aem.53.7.1560-1570.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woods D E, Strauss D C, Johanson W G, Berry V K, Bass J A. The role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980;29:1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu H S, Ji W S, Xu B. Abstracts of the First International Marine Biotechnology Conference, Baltimore. Arlington, Va: Society for Industrial Microbiology; 1989. A study on the attachment of marine bacteria to different surfaces; p. 80. [Google Scholar]
- 62.Zhan H, Lee C C, Leigh J A. Induction of the second exo- polysaccharide (EPSb) in Rhizobium meliloti SU47 by low phosphate concentrations. J Bacteriol. 1991;173:7391–7394. doi: 10.1128/jb.173.22.7391-7394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zoutman D E, Hulbert W C, Pasloske B L, Joffe A M, Volpel K, Trebilcock M K, Prancych W. The role of polar pili in the adherence of Pseudomonas aeruginosa to injured canine tracheal cells: a semiquantitative morphologic study. Scanning Microsc. 1991;5:109–126. [PubMed] [Google Scholar]