Abstract
Background:
Increased intra-abdominal pressure (IAP) in patients admitted to the intensive care unit leads to reduced abdominal perfusion pressure (APP), causing circulatory insufficiency and organ failure.
Aims:
To investigate the effect of maintaining a targeted APP on renal injury and the effect of increased IAP on the mortality rate in patients with septic shock.
Study Design:
Randomized, controlled, open-label study.
Methods:
A total of 72 patients were randomly divided into two groups (MAP65 or APP60). The MAP target for patients in the MAP65 group (n = 36) was 65 mmHg according to the Surviving Sepsis Guidelines. In the APP60 group (n = 36), the target APP was set to > 60 mmHg. The glomerular filtration rate (GFR), inotrope consumption, and IAP were recorded daily. The need for renal replacement therapy, decrease in GFR, and 30- and 90-day mortality rates were compared between the two groups.
Results:
In both the groups, the IAP was statistically similar (p = 0.458). The decreased in GFR was similar in both groups during the first 2 days. From day 3, there was a more statistically significant rapid decline in GFR in the MAP65 group than in the APP60 group. The GFR p-values on the 3rd, 4th, and 5th days were 0.040, 0.043, and 0.032, respectively. Eight patients (22.2%) in the MAP65 group and three patients (8.3%) in the APP group required renal replacement therapy (p = 0.101). The 30-day mortality rates in the MAP65 and APP60 groups were 61.1%, and 47.7%, respectively (p = 0.237). The 90-day mortality rates in the MAP65 and APP60 groups were 66.7% and 66.7%, respectively (p = 1).
Conclusion:
Setting an APP target limited the reduction in GFR. The mortality rates were similar in the two groups and there was no difference in the rate of end-stage renal failure between the groups.
INTRODUCTION
Septic shock, a subset of sepsis, substantially increases the morbidity and mortality rates. It is clinically diagnosed by the presence of persistent hypotension requiring vasoactive agents to maintain the mean arterial pressure (MAP) above 65 mmHg despite adequate fluid resuscitation and lactate levels > 2 mmol/l. Although it is recommended to maintain the MAP at > 65 mmHg for adequate circulation, it is not the only important factor. Increased intra-abdominal pressure (IAP), which is frequently seen in patients with septic shock, might lead to a decrease in the abdominal perfusion pressure (APP) despite adequate MAP levels. This, in turn, compromises the blood flow to the visceral organs, leading to organ failure. The IAP is < 8 mmHg in normal people and < 10 mmHg in critically ill patients. An IAP of > 12 mmHg is defined as intra-abdominal hypertension (IAH), and an IAP of > 20 mmHg signals abdominal compartment syndrome (ACS).1
APP is calculated by subtracting either the central venous pressure or IAP (whichever is high) from the MAP. The APP defines the blood flow to the target organ. The perfusion pressures of all the end-organs differ substantially; however, they are generally maintained above 60 mmHg for visceral organs. Levels < 60 mmHg can cause damage by reducing the blood flow to the end organ, thereby compromising the micro- and macro-circulation. Although the mechanism remains unelucidated, the increase in IAP and consequent decrease in APP possibly causes a decrease in the perfusion pressure in the abdominal organs, damaging the end organs, particularly the kidneys. A decrease in the perfusion pressure affects the kidneys both directly, by causing renal damage, and indirectly, by activating the renin-angiotensin-aldosterone system. Additionally, the increased IAP secondarily leads to deterioration of venous drainage, causing venous congestion in the gastrointestinal organs. This increased pressure on the cardiac and respiratory systems results in atelectasis, increased respiratory pressures, and possibly cardiac failure, which contribute significantly to the morbidity and mortality of patients admitted to the ICU.
This study aimed to investigate the effects of individualized APP secondary to increased IAP on renal injury. The primary endpoint of this study was whether APP optimization would reduce renal injury and the need for renal replacement therapy. The secondary endpoint was the effect of APP optimization on the patient’s 30- and 90-day mortality.
MATERIAL AND METHODS
This randomized, controlled, open-label study was approved by the hospital’s local ethics committee (no: 2003; date:11.11.2018) and conducted in the intensive care unit (ICU) of a tertiary teaching hospital between December 2, 2019 and September 21, 2022. The study was prospectively registered in the Protocol Registry System at Clinicaltrials.gov (no: NCT05358912) and was conducted in accordance with the principles of the Declaration of Helsinki.
In accordance with our primary objective of demonstrating whether there is an increased risk of renal damage due to the increased IAP, a preliminary study comparing the third-day glomerular filtration rate (GFR) values of 10 patients was conducted to determine the sample size. We determined that there should be 35 patients in each group to achieve 95% power with an alpha error of 5% and for an effect size “d” of 0.880 using the preliminary study data. Assuming a dropout rate of 15%, we planned to enroll a total of 85 patients. Of the 138 patients evaluated for eligibility, 85 patients were enrolled. Thirteen patients were excluded from the study during the follow-up for various reasons (the follow-up IAP measurements of five patients were > 25 mmHg, four patients died in the first 24 hours, and four patients required abdominal or urinary surgeries during treatment). Patients were randomized using the online randomization tool Research Randomizer (Randomizer.org), which uses a simple random allocation scheme without any blocks.
In the first group (MAP65; n = 36), the MAP was maintained at ³ 65 mmHg in accordance with the recommendations of the Surviving Sepsis Campaign guidelines. In the second group (APP60; n = 36), the minimum target APP was set to 60 mmHg, the minimum MAP target to 65 mmHg, and the maximum target MAP to 85 mmHg. Patients with initial IAPs > 25 mmHg were excluded from the study because they may have required additional treatment. Once allocated to a group, patients remained in the same group throughout the treatment course. Furthermore, there was no cross-over between the groups based on the later IAP measurements. The GFR values were calculated daily using the short formula, The Modification of Diet in Renal Disease. Renal replacement therapy was initiated in patients fulfilling one or more of the following criteria: severe pure metabolic acidosis (pH < 7.2), GFR < 10 ml/min/1.73 m2, hyperkalemia in the presence of electrocardiogram signs, presence of uremic symptoms (pericarditis, serositis, and encephalitis), and pulmonary edema that cannot be resolved by simple measures or diuretics.
Monitoring
Continuous invasive intra-arterial pressure measurements were obtained in all patients, preferably via the right radial artery. Measurements were obtained via the left radial artery and right femoral artery, when cannulations failed. The transducer used for obtaining pressure measurements was placed at the mid-axillary line, and a Philips mx550 (Philips Medical System DMC GmbH Boeblingen, Germany) was used for bedside monitoring.
Septic shock was diagnosed according to the Surviving Sepsis Campaign definition: sepsis which requires vasopressor support despite adequate fluid support and a lactate level of ³2 mmol/l. The patients were treated accordingly.
The IAP levels were measured indirectly via a urinary catheter. The patients were placed in the supine position, and the pressure transducer was zeroed in the mid-axillary line. The urinary catheters were clamped just proximal to the catheter-bag conjunction, and 25 ml of sterile saline was infused using a three-way tap. After waiting for the bladder to relax for 30-60 seconds, the end-expiratory bladder pressure was measured and recorded. The IAPs were measured twice at 12-hour intervals.
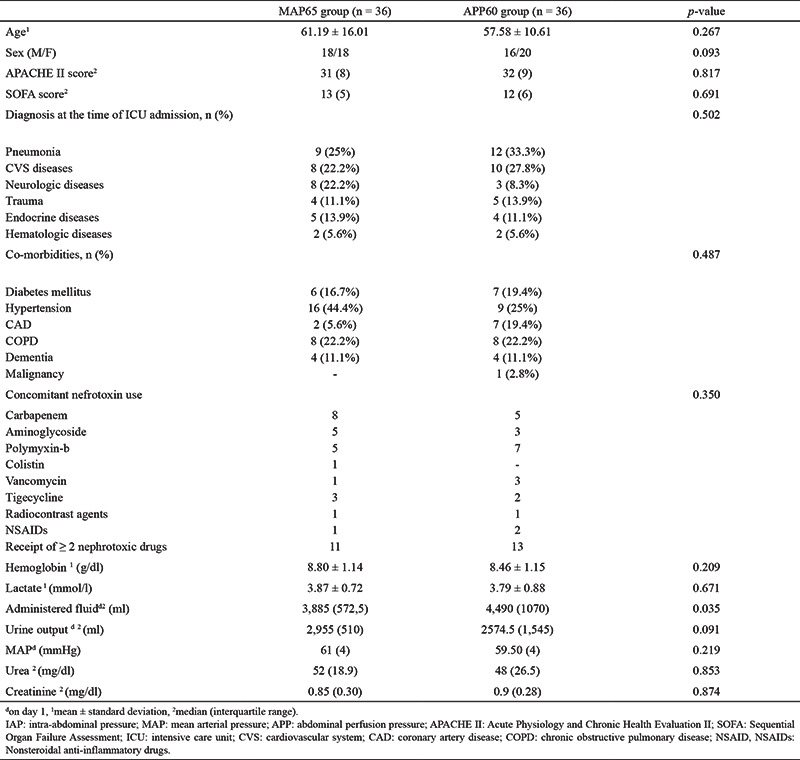
The following patient data were recorded: age, sex, diagnosis at admission, co-morbidities, first day APACHE-2 score, SOFA score, the serum urea, creatinine, aspartate transaminase, slanine transaminase, total bilirubin, and albumin levels, vasoactive agent doses, duration of ICU stay, mechanical ventilatory support requirement and its length of use in days when feasible, mortality status at the 30th and 90th day of ICU admission, renal replacement therapy requirement, and the presence of permanent kidney damage (as defined by KDIGO) (Table 1).
Table 1. Demographic Characteristics and initial Laboratory Findings of the Study Patients.
Treatment objectives
The MAP target was set to > 65 mm Hg for all patients. Patients were administered 30 ml/kg of crystalloid fluid upon establishing a diagnosis of septic shock (first 3 h). Broad-spectrum antibiotics were also administered, and the lactate levels were routinely monitored. Fluid responsiveness was monitored continuously using pulse pressure variation (PPV) where applicable. If hypotension (MAP < 65 mmHg) persisted despite fluid loading, norepinephrine was infused. While continuously monitoring the PPV using a bedside monitor (Philips Intellivue Mx 550; ), additional fluid (4 ml/kg) was administered in patients with a PPV £ 11%. After achieving the target PPV, fluid management was guided based on the patients’ daily fluid balance.
The patients were sedated using a propofol infusion (starting dose, 0.3 mg/kg/h; maximum dose < 4 mg/kg/h). The propofol dose was titrated to maintain the Richmond Agitation-Sedation Score between 0 and -3 for all patients. Additionally, tramadol (4 x 50 mg) was administered to all patients for analgesia.
Vasoactive agents were administered when necessary to maintain the MAP above the target level. Patients were randomized after calculating the IAP, and vasopressor doses were adjusted according to the treatment goals. Vasopressor agents were chosen in accordance with the Surviving Sepsis Guidelines recommendations. If the MAP target was not achieved with fluid resuscitation, norepinephrine was administered at an initial dose of 0.01 mg/kg/min, which could be increased up to a maximum dose of 1 mg/kg/min when necessary. Subsequently, adrenaline was infused.
Inclusion and exclusion criteria
Patients aged > 18 years who were diagnosed with septic shock were included in the study. Written informed consent was obtained from the patients or their legal guardians prior to enrollment. Patients aged < 18 years, pregnant women, patients with intra-abdominal diseases or diseases concerning the anterior abdominal wall, patients and patients with coexisting significant arrhythmias, uncontrolled hypertension, any renal or hepatic failure, retroperitoneal bleeding and abscess, bladder perforation, severe trauma, or burns were excluded. Patients in whom it was impossible or unsuitable to place a urinary catheter were also excluded from the study.
Statistical analysis
IBM SPSS (version 22.0; IBM Corp, Somers, NY, USA) was used for all statistical analyses. The categorical data are expressed as numbers (n) and percentages (%) of events, and they were compared using the Pearson chi-square test (sex, additional vasopressor requirement, mortality, renal replacement therapy requirements, and presence of arrhythmias) and linear-by-linear association (concomitant nefrotoxin use, diagnosis at the time of ICU admission, co-morbidities, and KDIGO). Normality was assessed using the Kolmogorov-Smirnov test. The continuous normally distributed data are expressed as mean ± standard deviation, and they were compared using the independent sample t-test (hemoglobin and lactate levels). The non-normally distributed data are expressed as median and interquartile range, and they were compared using the Mann-Whitney U test (fluid administered, urine output, MAP, serum urea and creatinine levels, SOFA and APACHE-2 scores, vasopressor-free days, IAP, GFR, days of mechanical ventilation use, LOS in ICU, and norepinephrine dose). The data were analyzed at a confidence level of 95%. A p-value of <0.05 was considered statistically significant.
RESULTS
A total of 85 patients were included in the study and randomly divided into two groups (MAP65 or APP60). During the follow-up period, 13 patients were excluded from the study for various reasons, leaving 36 patients in each group. Of the 72 patients, 34 were male and 38 were female. Pneumonia was the most common cause for admission. The patients’ admission APACHE-2 scores and first day laboratory findings are listed in Table 1.
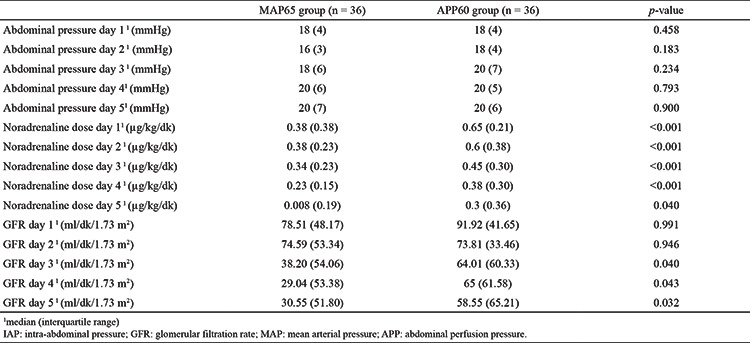
In both groups, the IAP was statistically similar (p = 0.458). The decrease in GFR was similar in both groups during the first 2 days. However, from day 3, there was a more rapid decline in the MAP65 group than in the APP60 group; this difference was statistically significant (Table 2). The GFR p-values on the 3rd, 4th, and 5th days were 0.040, 0.043, and 0.032, respectively. Eight patients (22.2%) in the MAP65 group and three patients (8.3%) in the APP60 group required renal replacement therapy (p = 0.101).
Table 2. The IAP, GFR, and Inotrope Dose used in the Study Participants During the 5-day Follow-up.
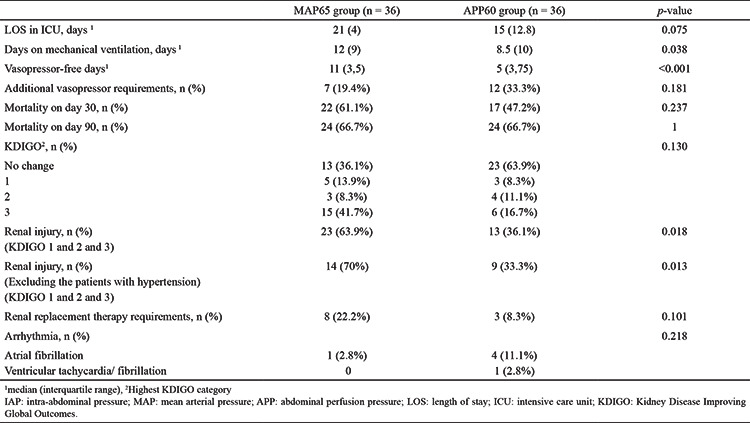
The 30-day mortality rates in the MAP65 and APP60 groups were 61.1% and 47.7%, respectively (p = 0.237). The 90-day mortality rates in the MAP65 and APP60 groups were 66.7% and 66.7%, respectively (p = 1).
The number of vasopressor-free days were significantly different between the groups. The MAP65 group had the most vasopressor-free days (n = 11; 4); the APP60 group had five (3.75) vasopressor-free days (p < 0.001). Furthermore, the vasopressor doses used were higher in the APP60 group than in the APP60 group (Table 2). Seven patients in the MAP65 group and 12 patients in the APP60 group required additional vasopressor therapy (p = 0.181).
The cumulative amount of fluid administered on the first day was highest [4,490 (702.85) cc] in the APP60 group (p = 0.035).
DISCUSSION
Our study findings suggest that renal injury can be alleviated by optimizing APP in addition to MAP. Although none of the patients progressed to ESRD at the 90-day follow-up, maintaining a certain level of APP reduced the estimated GFR from day 3 compared to the MAP65 group. There was no significant difference in 30- or 90-day mortality rates between the MAP65 and APP60 groups. In addition, the vasopressor dose used was significantly higher in the APP60 group than in the MAP65 group.
Renal injury in IAH usually develops secondary to a decrease in the renal blood flow.2,3 The increase in renal vascular resistance as well as the increased efferent arteriolar pressure reduce renal blood flow. Furthermore, the physical effect of the increased IAP on the kidneys contributes to the decrease in renal blood flow and GFR, resulting in oliguria at 15 mmHg and anuria at 30 mmHg.4 The decrease in GFR and deterioration of the tubular function decreases urine production. The fractional sodium excretion decreases,5 urinary sodium level decreases, and urinary potassium level increases.2 Thus, the renin-angiotensin-aldosterone system is activated, and the antidiuretic hormone activity increases significantly.6 Gül et al.7 explored the relationship between APP and the renal resistive index and determined that an APP threshold of 72 mmHg was related to a significant increase in the renal resistive index and impaired renal perfusion. Similarly, our study showed that individualizing the APP could reduce renal injury and that the GFR decrease was significantly less in the APP60 group than in the MAP65 group.
Approximately 40-70% of acute renal injuries are related to sepsis.8,9,10 There are several causes of sepsis-associated acute renal injury, including decreased global blood flow, tubular epithelial cell death, acute tubular necrosis, microcirculatory disorders, sepsis-associated microthrombi, oxygen radical-induced damage, shunts, and uncontrolled increase of inflammatory markers.11,12,13 The increased IAP and hypoperfusion caused by septic shock contribute to the acute renal injury. In such cases, even treatment may exacerbate the renal injury. Excessive amounts of fluid administration, which is often required in goal-oriented treatment plans, might contribute to the IAP elevation. McNelis et al.14 suggested that the positive fluid balance in patients with critical surgical conditions is the main determinant of increased IAP. In our study, we found that there was a positive fluid balance in both the groups, with the largest positive fluid balance occurring in the APP60 group.
IAP elevation not only affects renal function, but it also has deleterious effects on almost all organ systems and functions, such as respiratory failure,15 cardiac dysfunction,16 splanchnic hypoperfusion,17 and increased intracranial pressure.18 Increased IAP is transmitted to the mediastinum through the diaphragm, and therefore, may be related to increased central venous and pulmonary artery pressures.19,20 Disruption of the venous return may cause decreased cardiac output and increased tendency for peripheral venous thrombosis, which collectively result in pulmonary and peripheral edema.21,22,23 Studies suggest that an elevated IAP is closely related to mortality; furthermore, APP is a good predictor of visceral perfusion and a reliable indicator for resuscitation.16,24,25 Some studies have determined that the APP is superior to other commonly evaluated parameters (lactate levels, hourly urine output, and arterial pH) in predicting survival rates, which were found to be higher in patients with IAH and ACS, when the target APP was > 60 mmHg.24,25 In our study, there was no statistically significant difference in the mortality rates between the MAP65 and APP60 groups. However, our 30-day mortality rate was 66% and 47% in the MAP65 and APP60 groups, respectively.
Optimization of the APP may limit the injury to the visceral organs. We believe that our study results demonstrate this protective effect, at least for the short-term period. However, as none of the patients progressed to ESRD, we cannot conclude whether APP optimization could be beneficial in the long term.
Asfar et al.26 demonstrated that targeting a higher MAP (80-85 mmHg), rather than a lower MAP (65 mmHg), in septic shock reduces the renal replacement therapy requirements and blood creatinine doubling rate in patients with chronic hypertension. However, more patients in the MAP65 group experienced higher rates of atrial fibrillation due to increased vasopressor requirements than those in the APP60 group.26 Similarly, in our study, a larger vasopressor dose was required for a longer time in the APP60 group than in the MAP65 group. Although we found no statistical difference in the cardiac arrhythmia rates between the groups, more patients in the APP60 group experienced atrial fibrillation than in the MAP65 group. Furthermore, patients with chronic hypertension may require higher MAPs for renal autoregulation, and renal perfusion pressure may further decrease secondary to an increased IAP. Hypertension shifts the renal blood flow-renal perfusion pressure curve to the right, and renal autoregulation requires higher threshold pressures in patients with hypertension.27,28 Thus, we excluded patients with hypertension and compared the renal injury between two groups. However, we obtained similar results. This may be attributable to the insufficient number of patients included in the study and the exclusion of patients with uncontrolled hypertension. However, further studies are required to understand how hypertension affects renal damage.
In our study, we investigated the effect of intra-abdominal perfusion on renal injury. However, all of our patients had been diagnoses with septic shock. Septic shock can cause renal injury via various mechanisms. The antibiotics used to treat sepsis and the radiographic contrast agents used for imaging in these patients can exacerbate renal damage. In our study, although the majority of the patients had been exposed to nephrotoxic agents, there was no statistically significant difference among the nephrotoxic agents. Eleven patients in the MAP65 group and 13 patients in the APP60 group received two or more nephrotoxic agents.
In patients with sepsis, fluid management and vasopressor administration are crucial in terms of renal injury. The consensus report of the 28th Acute Disease Quality Initiative workgroup,29 after the Surviving Sepsis Guidelines, recommends fluid management based on the patient’s biochemical profile, selection of an appropriate fluid, and the use of diuretics, albumin, and sodium bicarbonate when necessary. Additionally, combining vasopressors with fluid administration can be beneficial. In patients with septic shock and increased IAP, the implementation of the four stages of fluid management (resuscitation, optimization, stabilization, and de-escalation) becomes extremely important.
Our study had some limitations. Blinding was not possible in this study because our outcomes included the measurement of data critical to patient management, such as MAP, which could have otherwise significantly jeopardized patient safety.
Our sample size was estimated to detect deteriorations in renal function rather than the rates of mortality, arrhythmia, or ESRD. Although we detected a clinical difference in the rates of arrhythmias and mortality between the groups, we were not able to demonstrate a statistically significant difference. Moreover, no patients in our study progressed to ESRD; hence, we could not demonstrate the effect of APP on the development of ESRD.
Our study established that personalizing the MAP target according to the IAP and targeting the APP levels reduced the acute renal injury in patients with septic shock. Personalizing IAP was not associated with an increased mortality, and there was no difference in the rate of ESRD between the groups. Furthermore, maintaining an APP target was associated with a higher inotropic agent requirement; However, no statistically significant side effect was observed.
Table 3. Comparison of the Mortality Rate and Other Relevant Data Between the Study Groups.
Footnotes
Ethics Committee Approval: This study was approved by the Ethics Committee of University of Health Sciences Türkiye, İzmir Bozyaka Training and Research Hospital.
Informed Consent: Informed consent form was obtained from all patients for this study.
Data Sharing Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Authorship Contributions: Concept- H.Ö., Z.T.T., M.A., M.U.B., H.E.E., O.O., B.Ç.; Design- H.Ö., Z.T.T., M.A., M.U.B., H.E.E., O.O., B.Ç.; Data Collection or Processing- H.Ö., Z.T.T., M.A., M.U.B., H.E.E., O.O., B.Ç.; Analysis or Interpretation- H.Ö., Z.T.T., M.A., M.U.B., H.E.E., O.O., B.Ç.; Literature Search- H.Ö., Z.T.T., M.A., M.U.B., H.E.E., O.O., B.Ç.; Writing- H.Ö., Z.T.T., M.A., M.U.B., H.E.E., O.O., B.Ç.
Conflict of Interest: No conflict of interest was declared by the authors.
Funding: The authors declared that this study received no financial support.
References
- 1.Kirkpatrick AW, Roberts DJ, de Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenasky JH 2nd. The renal hemodynamic and functional effects of external counterpressure. Surg Gynecol Obstet. 1972;134:253–258. [PubMed] [Google Scholar]
- 3.Bradley SE, Bradley GP. The Effect of Increased Intra-Abdominal Pressure on Renal Function in Man. J Clin Invest. 1947;26:1010–1022. doi: 10.1172/JCI101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards WO, Scovill W, Shin B, Reed W. Acute renal failure associated with increased intra-abdominal pressure. Ann Surg. 1983;197:183–187. doi: 10.1097/00000658-198302000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Savino JA, Cerabona T, Agarwal N, Byrne D. Manipulation of ascitic fluid pressure in cirrhotics to optimize hemodynamic and renal function. Ann Surg. 1988;208:504–511. doi: 10.1097/00000658-198810000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bloomfield GL, Blocher CR, Fakhry IF, Sica DA, Sugerman HJ. Elevated intra-abdominal pressure increases plasma renin activity and aldosterone levels. J Trauma. 1997;42:997–1005. doi: 10.1097/00005373-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Gül F, Sayan İ, Kasapoğlu US, et al. Abdominal perfusion pressure is superior from intra-abdominal pressure to detect deterioration of renal perfusion in critically ill patients. Ulus Travma Acil Cerrahi Derg. 2019;25:561–566. doi: 10.14744/tjtes.2019.25263. [DOI] [PubMed] [Google Scholar]
- 8.Bagshaw SM, Laupland KB, Doig CJ, et al. Prognosis for long-term survival and renal recovery in critically ill patients with severe acute renal failure: a population-based study. Crit Care. 2005;9:700–709. doi: 10.1186/cc3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silvester W, Bellomo R, Cole L. Epidemiology, management, and outcome of severe acute renal failure of critical illness in Australia. Crit Care Med. 2001;29:1910–1915. doi: 10.1097/00003246-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Neveu H, Kleinknecht D, Brivet F, Loirat P, Landais P. Prognostic factors in acute renal failure due to sepsis. Results of a prospective multicentre study. The French Study Group on Acute Renal Failure. Nephrol Dial Transplant. 1996;11:293–299. doi: 10.1093/oxfordjournals.ndt.a027256. [DOI] [PubMed] [Google Scholar]
- 11.Seely KA, Holthoff JH, Burns ST, et al. Hemodynamic changes in the kidney in a pediatric rat model of sepsis-induced acute kidney injury. Am J Physiol Renal Physiol. 2011;301:209–217. doi: 10.1152/ajprenal.00687.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Backer D, Donadello K, Taccone FS, Ospina-Tascon G, Salgado D, Vincent JL. Microcirculatory alterations: potential mechanisms and implications for therapy. Ann Intensive Care. 2011;1:27. doi: 10.1186/2110-5820-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 14.McNelis J, Marini CP, Jurkiewicz A, et al. Predictive factors associated with the development of abdominal compartment syndrome in the surgical intensive care unit. Arch Surg. 2002;137:133–136. doi: 10.1001/archsurg.137.2.133. [DOI] [PubMed] [Google Scholar]
- 15.Pelosi P, Quintel M, Malbrain ML. Effect of intra-abdominal pressure on respiratory mechanics. Acta Clin Belg. 2007;62(Suppl 1):78–88. doi: 10.1179/acb.2007.62.s1.011. [DOI] [PubMed] [Google Scholar]
- 16.Cheatham ML, Malbrain ML. Cardiovascular implications of abdominal compartment syndrome. Acta Clin Belg. 2007;62(Suppl 1):98–112. [PubMed] [Google Scholar]
- 17.Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intraabdominal pressure. J Surg Res. 1987;43:14–20. doi: 10.1016/0022-4804(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 18.De laet I, Citerio G, Malbrain ML. The influence of intraabdominal hypertension on the central nervous system: current insights and clinical recommendations, is it all in the head? Acta Clin Belg. 2007;62(Suppl 1):89–97. [PubMed] [Google Scholar]
- 19.Malbrain ML, Deeren D, de Potter TJ. Intra-abdominal hypertension in the critically ill: It is time to pay attention. Curr Opin Crit Care. 2005;11:156–171. doi: 10.1097/01.ccx.0000155355.86241.1b. [DOI] [PubMed] [Google Scholar]
- 20.ML M, ML C. Cardiovascular effects and optimal preload markers in intraabdominal hypertension. In: JL V, editor. Yearbook of Intensive Care and Emergency Medicine. Berlin. 2004:519–543. [Google Scholar]
- 21.Cullen DJ, Coyle JP, Teplick R, Long MC. Cardiovascular, pulmonary, and renal effects of massively increased intra-abdominal pressure in critically ill patients. Crit Care Med. 1989;17:118–121. doi: 10.1097/00003246-198902000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Barnes GE, Laine GA, Giam PY, Smith EE, Granger HJ. Cardiovascular responses to elevation of intra-abdominal hydrostatic pressure. Am J Physiol. 1985;17:208–213. doi: 10.1152/ajpregu.1985.248.2.R208. [DOI] [PubMed] [Google Scholar]
- 23.MacDonnell SP, Lalude OA, Davidson AC. The abdominal compartment syndrome: the physiological and clinical consequences of elevated intra-abdominal pressure. J Am Coll Surg. 1996;183:419–420. [PubMed] [Google Scholar]
- 24.Cheatham ML, White MW, Sagraves SG, Johnson JL, Block EF. Abdominal perfusion pressure: A superior parameter in the assessment of intra-abdominal hypertension. J Trauma. 2000;49:621–627. doi: 10.1097/00005373-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 25.M C, M M. Abdominal perfusion pressure [Internet]. In: Ivatury R, editor. Abdominal Compartment Syndrome. CRC Press; 2006. page 69–81.Available from: [Internet] https://www.taylorfrancis.com/books/9781498713214.
- 26.Asfar P, Meziani F, Hamel JF, et al. High versus Low Blood-Pressure Target in Patients with Septic Shock. N Engl J Med. 2014;370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 27.Wronski T, Seeliger E, Persson PB, et al. The step response: a method to characterize mechanisms of renal blood flow autoregulation. Am J Physiol Renal Physiol. 2003;285:758–764. doi: 10.1152/ajprenal.00420.2002. [DOI] [PubMed] [Google Scholar]
- 28.Semple SJ, De Wardener HE. Effect of Increased Renal Venous Pressure on Circulatory Autoregulation of Isolated Dog Kidneys. Circ Res. 1959;7:643–648. doi: 10.1161/01.res.7.4.643. [DOI] [PubMed] [Google Scholar]
- 29.Zarbock A, Nadim MK, Pickkers P, et al. Sepsis-associated acute kidney injury: consensus report of the 28th Acute Disease Quality Initiative workgroup. Nat Rev Nephrol. 2023;19:401–417. doi: 10.1038/s41581-023-00683-3. [DOI] [PubMed] [Google Scholar]


