Abstract
This experiment determined the effects of different HS models and pair-feeding (PF) on nutrient digestibility and markers of stress, inflammation, and metabolism in broilers. Birds (720 total) were allocated into 12 environmentally controlled chambers and reared under thermoneutral conditions until 20 d. Until 41 d birds were exposed to 4 treatments, including: thermoneutral at 24°C (TN-al), daily cyclic HS (12 h at 24 and 12 h at 35°C; cyHS), constant HS at 35°C (coHS), and PF birds maintained at 24°C and fed to equalize FI with coHS birds (TN-coPF). At d 41, ileal digesta were collected to determine nutrient apparent ileal digestibility (AID). Blood, liver, and breast tissues were collected from 8 birds per treatment to determine the mRNA expression of stress, inflammation, and metabolism markers. An additional 8 TN-al birds were sampled after acute HS exposure at 35°C for 4 h (aHS), and 8 cyHS birds were sampled either right before or 4 h after HS initiation. Data were analyzed by 1-way ANOVA and means were separated using Tukey's HSD test. Compared with TN-al birds, AID of nitrogen and ether extract were reduced in coHS birds, and both cyHS and coHS reduced (P < 0.05) AID of total essential amino acids. TNFα and SOD2 expression were increased (P < 0.05) under aHS, coHS, and TN-coPF conditions. IL6 and HSP70 were increased (P < 0.05) under coHS and aHS, respectively. Expression of lipogenic enzymes ACCα and FASN were reduced by coHS and TN-coPF, while coHS increased the lipolytic enzyme ATGL (P < 0.05). IGF1 was lowered in coHS birds, and p70S6K and MyoG were reduced under coHS and TN-coPF (P < 0.05). Interestingly, MuRF1 and MAFbx were increased (P < 0.05) under coHS only. Overall, these results indicate that coHS has a greater impact on nutrient digestibility and metabolism than aHS and cyHS. Interestingly, increased protein degradation during HS appears to be mostly driven by HS per se and not the reduced FI.
Key words: heat stress, digestibility, inflammation, gene expression, metabolism
INTRODUCTION
Average global temperatures are projected to warm by 1.5°C of over the next 20 yr, leading to an increased frequency of heat waves, longer warm seasons, and shorter cold seasons (Masson-Delmotte et al., 2021). Climate change has wide-ranging impacts on agriculture and is an important challenge for poultry production (Bhattacharyya et al., 2020), especially given that much of the global increase in poultry production is expected to occur in hot climate areas (Moekti, 2020). Poultry are particularly sensitive to high temperatures due to their feathering and the absence of sweat glands, making panting and wing movement (evaporative cooling) their primary means for heat dissipation. Furthermore, genetic selection for birds with the highest performance, and in turn, the highest metabolic rates and heat production, also contributes to the sensitivity of birds to hot environmental temperatures (Deeb and Cahaner, 1999; Rosa et al., 2007; Lara and Rostagno, 2013). Physiological heat stress (HS) occurs when the balance between heat production and dissipation is disturbed and body heat production surpasses heat loss capacities in the surrounding environment (Bernabucci et al., 2010; Akbarian et al., 2016). In an attempt to reduce heat production and its effects, birds generally decrease their feed intake (FI) and undergo several metabolic changes that reduce performance and can ultimately increase mortality. These HS-induced physiological changes cause extensive economic losses, making HS one of the most significant challenges for the poultry industry worldwide (St-Pierre et al., 2003; Zhang et al., 2017).
Several models of HS have been used to mimic the different types of high environmental temperature conditions that birds may encounter in the field. We previously presented the effects of chronic cyclic and constant HS on performance, carcass characteristics, and meat quality in broilers (Teyssier et al., 2022b). Cyclic and constant HS reduced FI by 16 and 45%, respectively, corresponding with 20 and 67% reductions in body weight gain (BWG). Observations of birds that were pair-fed the same amount of feed as birds subjected to constant HS showed that approximately 81% of the BWG reduction was caused by decreased FI, with the remaining 19% caused by physiological changes induced by HS per se. Similarly, 36% of the reduction in breast meat yield was attributed directly to HS, further reflecting the dramatic potential that HS can have on lean protein accretion in broilers (Teyssier et al., 2022b).
From a nutritional perspective, reduced nutrient digestibility and altered postabsorptive metabolism, including oxidative stress, can impair the performance of HS birds. Under thermoneutral (TN) conditions, the production of reactive oxygen species (ROS) and the antioxidant systems in broiler chickens are balanced and can adapt to overcome moderate challenges. Major enzymes, including glutathione peroxidase (GPX) and superoxide dismutase 1 (SOD1) and 2 (SOD2), detoxify ROS soon after their formation (Surai et al., 2019). However, acute and chronic HS have been shown to disturb this equilibrium due to an overproduction of ROS that surpasses the bird's antioxidant capacity, leading to oxidative stress (Lin et al., 2000, 2006; Azad et al., 2010a; Akbarian et al., 2016). Another cellular line of defense against HS is the increased synthesis of heat shock proteins (HSP), which function as chaperones proteins to inhibit the aggregation of non-native and misfolded proteins. They enhance cellular thermotolerance by modulating apoptotic and antiapoptotic signaling pathways and regulating cellular redox conditions (Shehata et al., 2020). In addition, systemic and local inflammation can occur with high temperatures (Song et al., 2017, 2018). Therefore, HS is an important stressor for poultry, particularly on the liver which is highly susceptible to external stressor due to its important role in avian metabolic activity and has high rates of substrate metabolism and energy expenditure in broilers (Sanchez-Valle et al., 2012; Emami et al., 2020). Indeed, the liver is the main site of de novo lipogenesis in avian species (Leveille, 1969), and increased fat deposition of broilers during HS (Geraert et al., 1996; Faria Filho et al., 2007; Zeferino et al., 2016; Lu et al., 2019b; Teyssier et al., 2022b) could be attributed to hepatic stress (Emami et al., 2020). In contrast to the increased energy deposition as lipid, HS results in a lower protein deposition (Temim et al., 2000; Faria Filho et al., 2007; Zhang et al., 2012; De Souza et al., 2016). Therefore, alterations in bird lipid and protein metabolism play an important role in the degradation of bird performance.
This study aimed to characterize the effects of 3 HS models: constant, cyclic, and acute, and 1 pair-feeding treatment on nutrient digestibility and markers of stress, inflammatory, and metabolism in broilers. We hypothesized that the expression of some heat shock proteins, cytokines, and HS markers will be differently impacted depending on the HS model. Our previous study revealed that a large proportion of the decrease in breast meat yield and increase in fat pad deposition was not explained by the decrease in FI, and thus, the present study addresses markers of protein and lipid metabolism. We also hypothesized that constant HS will have a greater impact on digestibility and cause more changes in protein and lipid synthesis/degradation pathways than reduced FI alone.
MATERIALS AND METHODS
All procedures involving live animals were approved by the Institutional Animal Care and Use Committee at the University of Arkansas. Descriptions of the facilities, diets, and animals, as well as the results for growth performance, carcass characteristics, and meat quality of broilers used in this experiment were previously reported by Teyssier et al. (2022). A brief summary and additional information on our experimental design as well as the methodology applied for the measurement of nutrient digestibility, stress, lipid, and protein metabolism markers are provided below.
Animals and Experimental Design
Seven hundred twenty male chicks from a Cobb 500 hybrid female breeder line were distributed into 12 environmentally controlled chambers with 60 chicks per chamber at 0 d posthatch. Each chamber was divided by wire paneling into 2 pens of 4.47 m2, with each equipped with 2 hanging pan feeders and nipple waters. Water was provided ad libitum throughout the experiment and a diet based on corn and soybean meal was formulated and fed in 3 phases: starter from d 0 to 13, grower from d 14 to 27, and finisher from d 28 to 42 (Supplementary Table 1). Birds were kept under standard conditions from d 0 to d 20, with the ambient temperatures of all the chambers gradually decreasing from 32 to 24°C on d 20. The experimental period was set from d 20 to d 41.
On d 20, an appropriate number of birds were removed from each pen to obtain groups of 25 birds/pen with similar weights across all treatment groups. From d 20 to 41, 3 different environmental conditions and 1 pair-fed (PF) treatment were applied. These included TN birds fed ad-libitum and kept at a constant 24°C temperature (TN-al); cyclic HS (cyHS) birds fed ad libitum in 4 cyclic HS chambers for which the temperature was maintained at 35°C for 12 h daily (from 7:30 to 19:30) and reduced to 24°C each night; constant HS (coHS) birds fed ad libitum in 4 chambers for which the temperature was set to and maintained at 35°C; and a group kept in TN chambers and PF to equalize feed intake to that of coHS bird (TN-coPF). Moreover, at sampling at 41 d, cyHS birds were sampled during both the early morning at the end of the cool phase (cyHScool) and 4 h after the beginning of the hot phase (cyHShot). Additionally, 2 birds from each TN-al pen were randomly selected and exposed at 35°C for 4 h to assess acute HS (aHS) in birds not previously exposed to HS. Birds were euthanized by CO2 inhalation and immediately dissected for whole blood and pectoralis major (P. major) collection.
Apparent Ileal Digestibility
On d 41, 4 birds per replicate from the TN-al, cyHS, coHS, and TN-coPF groups were randomly selected for sampling. Birds were euthanized by CO2 inhalation and immediately dissected for digesta collection. One-half of the ileum proximal to the ileocecal junction was flushed for collection of digesta samples, which were pooled per replicate, placed on ice, frozen, lyophilized, and ground using an electric coffee grinder. Dry matter (DM), ether extract (EE), nitrogen (N) concentration, and gross energy (GE) content of the diet and digesta were analyzed by a commercial laboratory (ATC scientific, Little Rock, AR). Amino acid (AA) concentrations were analyzed at the University of Arkansas with a high-performance liquid chromatography instrument (Waters Corporation, Milford, MA; methods 994.12; AOAC International, 2005).
Titanium dioxide concentration, used as an indigestible marker, was determined according to the procedures of Short et al. (1996). Apparent ileal digestibility (AID) of DM, EE, N, GE, and AA was calculated using the following formula:
where = ratio of nutrient or energy concentration (%) to TiO2 (%) in the diet or ileal digesta. Energy digestibility (%) values obtained from the equation above were multiplied by the gross energy content of the diet to calculate apparent ileal digestible energy (IDE) in units of kcal/kg.
Blood and Pectoralis Major Sampling
Blood was collected from the heart immediately postmortem, from 8 birds per group. One drop of whole blood was placed in TRIzol reagent (catalog #15596018, Life Technologies, Carlsbad, CA) for RNA extraction, snap-frozen in liquid N, and stored at -80°C for further analysis on the mRNA expression of HSP, cytokines, and oxidative stress markers. Moreover, approximately 3 mL of blood was stored on ice in heparin tubes before being centrifuged at 1,300 × g for 15 min at 4°C and plasma was harvested. Plasma samples were stored at -80°C for subsequent analysis of total protein (TP) and uric acid (UA) concentration. Additionally, a small sample (approximately 2 grams) of the left P. major muscle was collected, and frozen in liquid N and stored at -80°C for future mRNA expression analysis.
Gene Expression in the Whole Blood and Pectoralis Major
Total RNA was extracted from the whole blood and P. major muscle using Trizol reagent (catalog #15596018, Life Technologies, Carlsbad, CA) according to the manufacturer's recommendations. For each sample, RNA concentrations and purity were determined by Take3 Micro-Volume Plate using a Synergy HT multimode microplate reader (BioTek, Winooski, VT). Ribonucleic acid samples were RQ1 DNase treated and reverse transcribed using qScript cDNA Synthesis SuperMix (catalog #95048-100, Quanta Biosciences, Gaithersburg, MD). The RT products (cDNA) were amplified by real-time quantitative PCR (Applied Biosystems 7500 Real-Time PCR System) with PowerUp SYBR green master mix (catalog #4312074, Life Technologies, Carlsbad, CA) as previously described (Rajaei-Sharifabadi et al., 2017; Greene et al., 2021a). Relative expressions of target genes were determined by the 2−∆∆Ct method and normalization was performed with the 18S rRNA as a housekeeping gene (Schmittgen and Livak, 2008). Oligonucleotide primer sequences specific for HSP27, HSP60, HSP70, HSP90, SOD1, SOD2, GPX1, IL6, IL10, IL18, tumor necrosis factor alpha (TNFα), C-reactive protein (CRP), adipose triglyceride lipase (ATGL), lipoprotein lipase (LPL), acetyl-CoA carboxylase (ACC) alpha, ATP-citrate lyase (ACLY), fatty acid synthase (FASN), insulin like growth factor 1 (IGF1), phosphoinositide 3-kinase (PI3K), extracellular signal-reduced protein kinase 1 (ERK1), AKT Serine/Threonine Kinase (AKT) 1, AMP-activated protein kinase (AMPK) alpha 1, mammalian target of rapamycin (mTOR), ribosomal protein S6 kinase (p70S6K), myogenic factor (MyoG), muscle RING-finger protein (MuRF) 1, and muscle atrophy F-box (MAFbx), also known as atrogenin-1 are presented in Table 1.
Table 1.
Oligonucleotide qPCR primers.
| Gene | Accession number1 | Primer sequence (5’→3’) | Orientation | Product size (bp) |
|---|---|---|---|---|
| HSP27 | XM_001231557 | TTGAAGGCTGGCTCCTGATC | For | 58 |
| AAGCCATGCTCATCCATCCT | Rev | |||
| HSP60 | NM_001012916 | CGCAGACATGCTCCGTTTG | For | 55 |
| TCTGGACACCGGCCTGAT | Rev | |||
| HSP70 | J02579 | GGGAGAGGGTTGGGCTAGAG | For | 55 |
| TTGCCTCCTGCCCAATCA | Rev | |||
| HSP90 | X07265 | TGACCTTGTCAACAATCTTGGTACTAT | For | 68 |
| CCTGCAGTGCTTCCATGAAA | Rev | |||
| SOD1 | NM_205064 | TGGCTTCCATGTGCATGAAT | For | 58 |
| AGCACCTGCGCTGGTACAC | Rev | |||
| SOD2 | NM_204211 | GCTGGAGCCCCACATCAGT | For | 61 |
| GGTGGCGTGGTGTTTGCT | Rev | |||
| GPX1 | NM_001277853 | TCCCCTGCAACCAATTCG | For | 57 |
| AGCGCAGGATCTCCTCGTT | Rev | |||
| TNFα | NM_204267 | CGTTTGGGAGTGGGCTTTAA | For | 61 |
| GCTGATGGCAGAGGCAGAA | Rev | |||
| CRP | NM_001039564 | AAGCTCAGGACAACGAGATCCT | For | 71 |
| TTTCCCCCCCACGTAGAAG | Rev | |||
| IL6 | NM_204628 | GCTTCGACGAGGAGAAATGC | For | 63 |
| GGTAGGTCTGAAAGGCGAACAG | Rev | |||
| IL10 | NM_001004414 | CGCTGTCACCGCTTCTTCA | For | 63 |
| CGTCTCCTTGATCTGCTTGATG | Rev | |||
| IL18 | GU119895 | TGCAGCTCCAAGGCTTTTAAG | For | 63 |
| CTCAAAGGCCAAGAACATTCCT | Rev | |||
| ATGL | EU240627 | GCCTCTGCGTAGGCCATGT | For | 60 |
| GCAGCCGGCGAAGGA | Rev | |||
| LPL | NM_205282 | GACAGCTTGGCACAGTGCAA | For | 62 |
| CACCCATGGATCACCACAAA | Rev | |||
| ACCα | NM_205505 | CAGGTATCGCATCACTATAGGTAACAA | For | 74 |
| GTGAGCGCAGAATAGAAGGATCA | Rev | |||
| ACLY | NM_001030540 | CTTTTAAGGGCATTGTTAGAGCAAT | For | 65 |
| CCTCACCTCGTGCTCTTTCAG | Rev | |||
| FASN | J03860 | ACTGTGGGCTCCAAATCTTCA | For | 70 |
| CAAGGAGCCATCGTGTAAAGC | Rev | |||
| IGF1 | NM_001004384 | GCTGCCGGCCCAGAA | For | 56 |
| ACGAACTGAAGAGCATCAACCA | Rev | |||
| PI3K | NM_001004410 | GCCATCTTACTCCAGGCGTATC | For | 70 |
| GAGGGACTTGGCTGTAGCTTCTC | Rev | |||
| ERK1 | NM_204150 | CGGACCATGATCACACAGGAT | For | 63 |
| CAGGAGCCCTGTACCAACGT | Rev | |||
| AKT1 | AF039943 | TTCAACGGTGATCTTTTGACTGA | For | 64 |
| CGGGAATGTCTCTTGGTGGAT | Rev | |||
| AMPKα1 | NM_001039603 | CCACCCCTGTACCGGAAATA | For | 68 |
| GGAAGCGAGTGCCAGAGTTC | Rev | |||
| mTOR | XM_417614.5 | CATGTCAGGCACTGTGTCTATTCTC | For | 77 |
| CTTTCGCCCTTGTTTCTTCACT | Rev | |||
| p70S6K | NM_001109771 | GTCAGACATCACTTGGGTAGAGAAAG | For | 60 |
| ACGCCCTCGCCCTTGT | Rev | |||
| MyoG | NM_204184 | GGAGAAGCGGAGGCTGAAG | For | 62 |
| GCAGAGTGCTGCGTTTCAGA | Rev | |||
| MuRF1 | XM_015297755 | TGGAGAAGATTGAGCAAGGCTAT | For | 64 |
| GCGAGGTGCTCAAGACTGACT | Rev | |||
| MAFbx | NM_001030956 | CCTTCCACCTGCTCACATCTC | For | 59 |
| CACAGGCAGGTCCACAAA | Rev | |||
| 18S | AF173612 | TCCCCTCCCGTTACTTGGAT | For | 60 |
| GCGCTCGTCGGCATGTA | Rev |
Accession number refer to GenBank (NCBI).
Total Protein and Uric Acid Concentrations in the Plasma
Uric acid and TP concentrations were measured in the plasma by a commercial laboratory (Veterinary Medical Diagnostic Laboratory, University of Missouri, Columbia, MO).
Statistical Analysis
To compare the effects of different environmental conditions, all data were subjected to a one-way ANOVA, with each environmental condition representing as a distinct factor level. Post-hoc comparisons of means were conducted using Tukey's HSD test to determine significant differences between the individual environmental conditions. Digestibility data were available for 4 treatments with 4 replicates per treatment, with each replicate corresponding to a pool of the 4 sampled birds from the same pen. Gene expression data analysis was performed on 6 treatments of 4 replicates, corresponding to the average value of the 2 sampled birds per replicate. Gene expression data were log-transformed to achieve a normal distribution for statistical analysis. Those data are presented as antilog of the geometric means and upper and lower 95% confidence interval of the log-transformed data (Olivier et al., 2008). It is worth noting that confidence intervals in the graphs may appear wider than the corresponding intervals from the log-transformed data used in our analysis. However, the log transformation reduced this apparent variability, playing a crucial role in obtaining valid statistical results. Model residuals were inspected for outliers using histograms and QQ-plots. Mean differences were considered significant when the p-value < 0.05, and analysis was performed using R (RStudio 2022.07.2).
RESULTS
The complete performance, carcass characteristics, and meat quality results from this experiment can be found in Teyssier et al. (2022b).
Apparent Ileal Digestibility
Apparent ileal digestibility values (%) of DM, EE, N, and energy, as well as ileal digestible energy values (kcal/kg) are presented in Table 2. The different environmental conditions did not affect the DM AID (P = 0.428), but the EE AID was decreased (P = 0.039) under coHS compared to TN-coPF conditions, and the N AID was decreased (P = 0.032) under coHS compared to TN-al conditions. No effects were observed on energy AID values expressed as percentage digestibility (P = 0.417) or IDE expressed in kcal/kg of diet (P = 0.415).
Table 2.
Ileal digestible energy and apparent ileal digestibility values of dry matter, ether extract, and nitrogen of broilers reared under different environmental conditions and feed regimens from 20 to 41 d with ileal content sampled at 41 d.
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Parameter3 | TN-al | cyHS | coHS | TN-coPF | SEM2 | P-values |
| Dry matter (%) | 74.1 | 70.8 | 69.9 | 70.9 | 3.70 | 0.428 |
| Ether extract (%) | 94.2ab | 93.8ab | 91.8b | 94.8a | 1.37 | 0.039 |
| Nitrogen (%) | 84.6a | 80.3ab | 79.3b | 82.3ab | 2.27 | 0.032 |
| Energy (%) | 78.3 | 75.1 | 73.6 | 75.9 | 3.89 | 0.417 |
| IDE4 (kcal/kg) | 3,167 | 3,036 | 2,975 | 3,069 | 157 | 0.415 |
TN-al: Birds reared under continuous 24°C and ad libitum feeding. cyHS: Birds reared under cyclic high temperature (8 h at 35˚C and 12 h at 24˚C) and ad libitum feeding. coHS: Birds reared under continuous 35°C and ad libitum feeding. TN-coPF: Birds reared under continuous 24°C and pair-fed to the coHS treatment.
SEM: pooled standard error of the mean.
Values are means of 4 replicates per treatment (pool of 4 birds per replicate).
IDE: Ileal digestible energy
Means within row without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's multiple comparison test.
In our study, AID values of total AA (essential plus nonessential AA) for broilers under coHS and cyHS were 4.4% units lower (P = 0.001; Table 3) than those of TN-al birds and intermediate for TN-coPF birds (-2.3% units). The same trend was observed for total essential AA digestibility (P < 0.001) and AID of Met (P = 0.005) and Lys (P = 0.017), but different responses were found among other individual essential AA. Specifically, leucine (P = 0.026) and histidine (P = 0.027) digestibility values were reduced under coHS compared to TN-al conditions and intermediate for cyHS and TN-coPF birds, and phenylalanine AID was reduced (P < 0.001) under cyHS and coHS compared to TN-al and TN-coPF conditions. Additionally, the AID of isoleucine (P = 0.049) was different among the treatments, but the Tukey HSD test did not result in separation of those means. Threonine (P = 0.189), valine (P = 0.074), and arginine (P = 0.243) AID were not affected by the different environmental treatments.
Table 3.
Apparent ileal digestibility coefficients of amino acids of broilers reared under different environmental conditions and feed regimens from 20 to 41 d and ileal content sampled at 41 d.
| Treatment1 | ||||||
|---|---|---|---|---|---|---|
| Parameter3 | TN-al | cyHS | coHS | TN-coPF | SEM2 | P-values |
| Essential amino acids | ||||||
| Methionine | 93.6a | 89.8b | 90.2b | 92.2ab | 0.94 | 0.005 |
| Lysine | 91.7a | 88.2b | 88.5b | 89.7ab | 1.29 | 0.017 |
| Threonine | 81.5 | 76.7 | 77.5 | 77.7 | 3.13 | 0.189 |
| Valine | 84.8 | 80.4 | 79.8 | 81.2 | 2.60 | 0.074 |
| Isoleucine | 86.0 | 81.4 | 81.4 | 82.9 | 2.30 | 0.049 |
| Leucine | 87.0a | 83.0ab | 81.9b | 84.7ab | 2.12 | 0.026 |
| Arginine | 92.6 | 90.4 | 90.4 | 92.1 | 1.78 | 0.243 |
| Phenylalanine | 88.3a | 83.4b | 84.3b | 87.7a | 1.26 | <0.001 |
| Histidine | 88.5a | 84.9ab | 83.9b | 87.1ab | 2.04 | 0.027 |
| Total essential amino acids | 88.2a | 83.9b | 84.2b | 86.1ab | 1.15 | <0.001 |
| Nonessential amino acids | ||||||
| Alanine | 86.8a | 83.0ab | 81.8b | 83.9ab | 2.22 | 0.043 |
| Aspartic acid | 85.2a | 80.4b | 83.2ab | 83.0ab | 1.50 | 0.006 |
| Cysteine | 80.5 | 73.6 | 71.3 | 79.0 | 4.36 | 0.089 |
| Glutamic acid | 90.0a | 86.3b | 87.3b | 88.2ab | 1.00 | 0.001 |
| Glycine | 82.7 | 77.6 | 77.2 | 79.0 | 2.86 | 0.068 |
| Proline | 85.0a | 80.0b | 80.7ab | 82.4ab | 2.22 | 0.031 |
| Serine | 86.5a | 82.5b | 82.6b | 83.3ab | 1.83 | 0.028 |
| Tyrosine | 90.4a | 87.0ab | 83.8b | 86.8ab | 2.61 | 0.030 |
| Total nonessential amino acids | 85.9a | 81.3b | 81.0b | 83.2ab | 1.68 | 0.005 |
| Total4 | 87.1a | 82.7b | 82.7b | 84.8ab | 1.34 | 0.001 |
TN-al: Birds reared under continuous 24°C and ad libitum feeding. cyHS: Birds reared under cyclic high temperature (8 h at 35˚C and 12 h at 24˚C) and ad libitum feeding. coHS: Birds reared under continuous 35°C and ad libitum feeding. TN-coPF: Birds reared under continuous 24°C and pair-fed to the coHS treatment.
SEM: pooled standard error of the mean.
Values are means of 4 replicates per treatment (pool of 4 birds per replicate).
Total = Sum of all reported essential and nonessential amino acids.
Means within row without a common superscript were determined to be significantly different (P < 0.05) by a Tukey's multiple comparison test.
Regarding the total nonessential AA, and as observed with the essential AA, a decrease (P = 0.005) in AID was observed under cyHS and coHS compared to TN-al conditions, with intermediate values for AID of nonessential AA for TN-coPF birds. Alanine (P = 0.043) and tyrosine (P = 0.030) AID was reduced under coHS compared to TN-al conditions, and values for cyHS and TN-coPF birds were intermediate. Additionally, the AID of glutamic acid (P = 0.001) and serine (P = 0.028) was reduced under both coHS and cyHS compared to TN-al conditions, with the TN-coPF treatment presenting intermediate values. Also, the AID of aspartic acid (P = 0.006) and proline (P = 0.031) was only reduced under cyHS compared to TN-al conditions, and the AID of those 2 AA was intermediate under cyHS and TN-coPF. Finally, no difference was observed among treatments for cysteine (P = 0.089) or glycine (P = 0.068) AID, despite large numerical differences in AID values between coHS and TN-al groups (-9.2% and -5.5% units, respectively).
Gene Expression of Stress and Inflammatory Markers in the Whole Blood
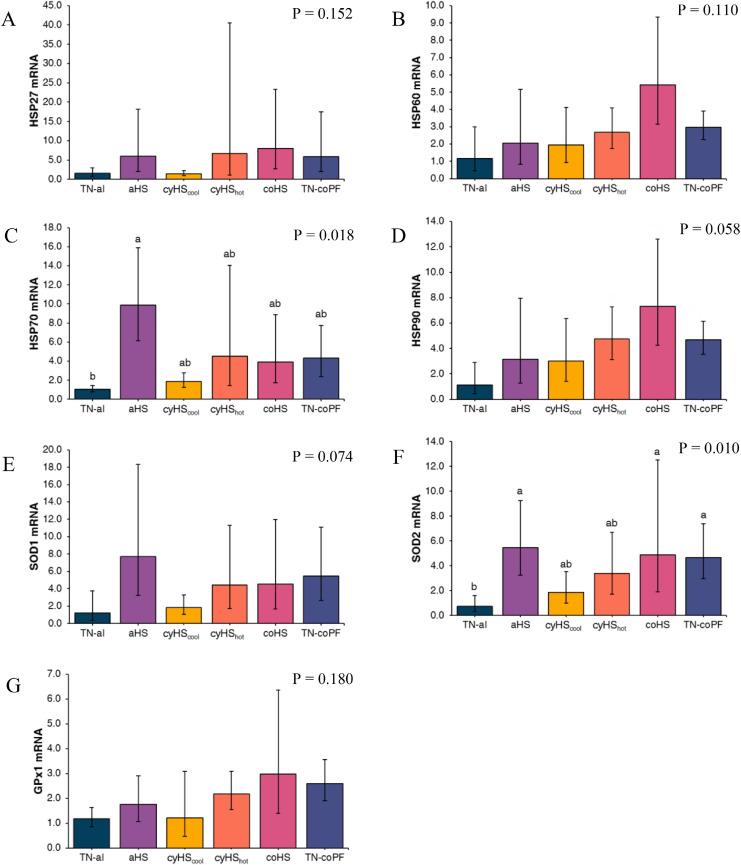
Gene expression data for HSP and oxidative stress (SOD1, SOD2, and GPX1) markers in whole blood, including the aHS group for which digestibility data were not determined, are presented in Figure 1. Compared to TN-al conditions, aHS increased blood gene expression of HSP70 (P = 0.018), and HSP90 tended to increase (P = 0.058) under coHS conditions. Both HSP27 (P = 0.152) and HSP60 (P = 0.110) were unaffected by the environmental conditions. For the oxidative stress markers, SOD2 gene expression was increased (P = 0.010) by aHS, coHS, and TN-coPF conditions, but no difference was observed for the gene expression of SOD1 (P = 0.074) and GPX1 (P = 0.180) among groups.
Figure 1.
Effect of different environmental conditions and feed regimen on blood mRNA expression of stress related transcription factors in broilers. Relative abundances of HSP27 (A), HSP60 (B), HSP70 (C), HSP90 (D), SOD1 (E), SOD2 (F), and GPX1 (G) mRNA was determined by real-time quantitative PCR and analyzed by the 2-ΔΔCt method using the TN-al group as calibrator. Data were log-transformed and are presented as antilog of the geometric means with error bars representing the 95% confidence interval: . a-b Different letters indicate significant difference at P < 0.05 by a Tukey's multiple comparison test. TN-al, birds reared under continuous 24°C and ad libitum feeding; aHS, birds reared under acute heat stress for 4 h on d 41; cyHScool, birds reared under cyclic high temperature (12 h at 35˚C and 12 h at 24˚C), ad libitum feeding, and sampled at the end of the cool phase of the night; cyHShot, same as cyHScool, but sampled after 4 h at 35°C; coHS, birds reared under continuous 35°C and ad libitum feeding; TN-coPF, birds reared under continuous 24°C and pair-fed to the coHS treatment
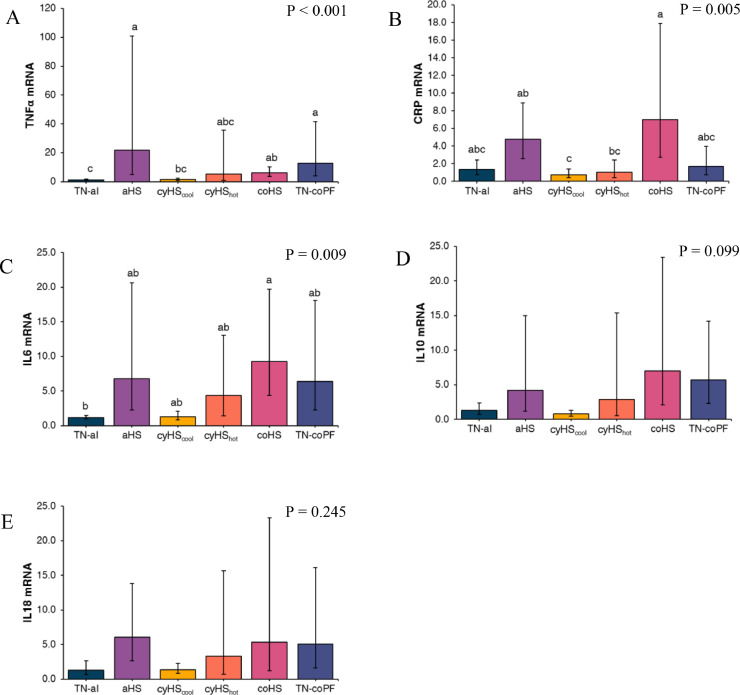
Concerning the inflammation markers presented in Figure 2, aHS, coHS, and TN-coPF conditions increased (P < 0.001) the gene expression of TNFα, and coHS birds had a higher (P = 0.005) CRP gene expression compared to cyHScool and cyHShot birds. Environmental condition did not affect IL10 (P = 0.099) or IL18 (P = 0.245) gene expression, but IL6 was upregulated (P = 0.009) under coHS compared to TN-al conditions.
Figure 2.
Effect of different environmental conditions and feed regimen on blood expression of inflammatory related transcription factors in broilers. Relative abundances of TNFα (A), CRP (B), IL6 (C), IL10 (D), and IL18 (E) mRNA was determined by real-time quantitative PCR and analyzed by the 2-ΔΔCt method using the TN-al group as calibrator. Data were log-transformed and are presented as antilog of the geometric means with error bars representing the 95% confidence interval: . a-b Different letters indicate significant difference at P < 0.05 by a Tukey's multiple comparison test. TN-al, birds reared under continuous 24°C and ad libitum feeding; aHS, birds reared under acute heat stress for 4 h on d 41; cyHScool, birds reared under cyclic high temperature (12 h at 35˚C and 12 h at 24˚C), ad libitum feeding, and sampled at the end of the cool phase of the night; cyHShot, same as cyHScool, but sampled after 4 h at 35°C; coHS, birds reared under continuous 35°C and ad libitum feeding; TN-coPF, birds reared under continuous 24°C and pair-fed to the coHS treatment.
Gene Expression of Lipid Metabolism Markers in the Liver
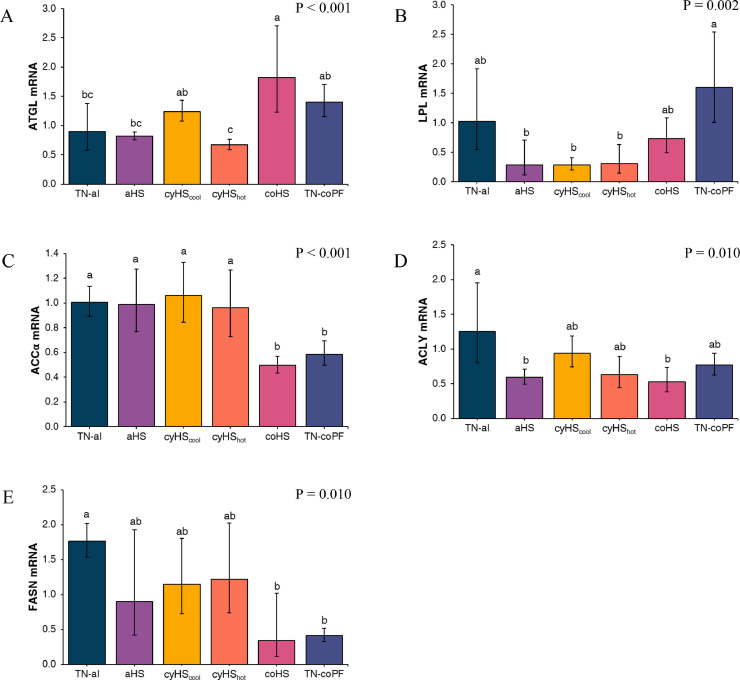
Gene expressions of lipid metabolism markers are presented in Figure 3. Constant HS increased (P < 0.001) ATGL expression and decreased ACCα (P < 0.001), ACLY (P = 0.010), and FASN (P = 0.010) expression compared to TN-al conditions, with ACCα and FASN also decreased for TN-coPF birds. LPL gene expression was increased (P = 0.002) under TN-coPF compared to aHS and cyHS conditions. Compared to TN-al birds, except for the lower (P = 0.010) gene expression of ACLY under aHS conditions, none of the lipid markers were affected by the aHS, cyHScool, and cyHShot treatments.
Figure 3.
Effect of different environmental conditions and feed regimen on liver expression of lipid metabolism related transcription factors in broilers. Relative abundances of ATGL (A), LPL (B), ACCα (C), ACLY (D), and FASN (E) mRNA was determined by real-time quantitative PCR and analyzed by the 2-ΔΔCt method using the TN-al group as calibrator. Data were log-transformed and are presented as antilog of the geometric means with error bars representing the 95% confidence interval: . a-b Different letters indicate significant difference at P < 0.05 by a Tukey's multiple comparison test. TN-al, birds reared under continuous 24°C and ad libitum feeding; aHS, birds reared under acute heat stress for 4 h on d 41; cyHScool, birds reared under cyclic high temperature (12 h at 35˚C and 12 h at 24˚C), ad libitum feeding, and sampled at the end of the cool phase of the night; cyHShot, same as cyHScool, but sampled after 4 h at 35°C; coHS, birds reared under continuous 35°C and ad libitum feeding; TN-coPF, birds reared under continuous 24°C and pair-fed to the coHS treatment.
Gene Expression of Protein Metabolism Markers in the Pectoralis Major Muscle
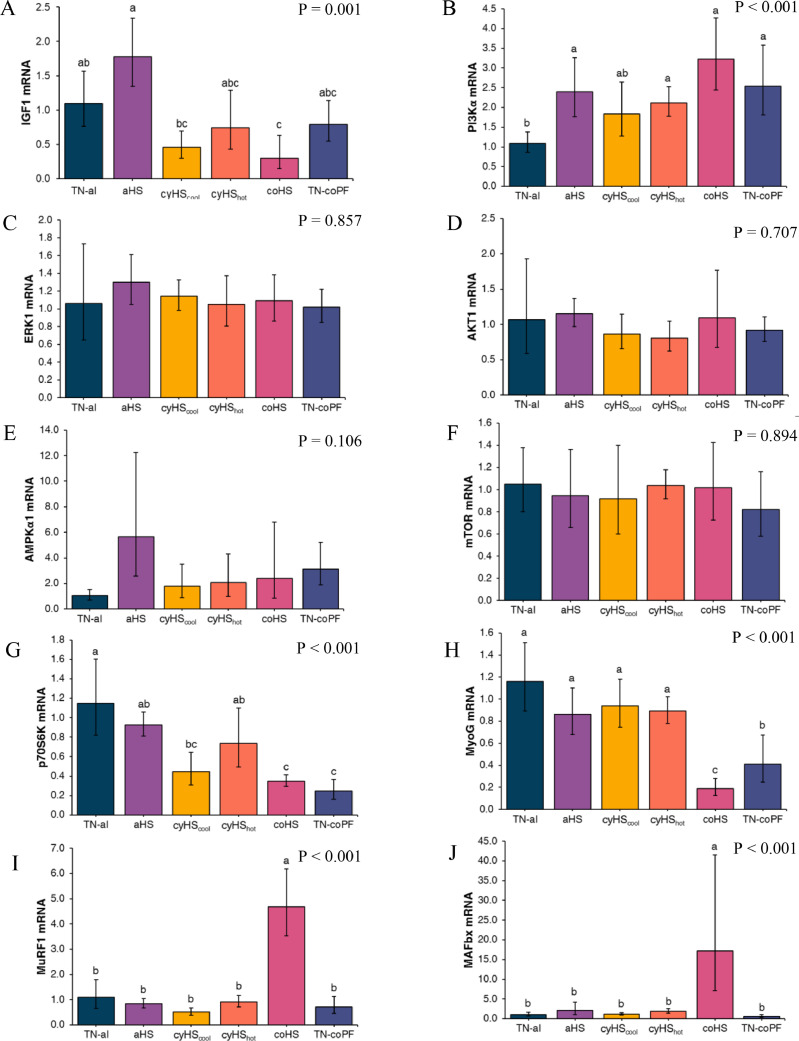
Gene expressions of protein metabolism markers are presented in Figure 4. Upstream of the mTOR pathway, IGF1 gene expression was decreased (P = 0.001) by coHS, and PI3Kα gene expression was increased (P < 0.001) by aHS, cyHShot, coHS, and TN-coPF compared to TN-al conditions. However, treatments did not affect the gene expression of ERK1 (P = 0.857), AKT1 (P = 0.707), AMPKα1 (P = 0.106), or mTOR (P = 0.894). Both coHS and TN-coPF conditions reduced the gene expression of p70S6K (P < 0.001) and MyoG (P < 0.001), as well as cyHScool for p70S6K. In addition, TN-coPF birds had a higher MyoG gene expression than coHS birds. Gene expression of the 2 protein catabolism markers MuRF1 and MAFbx was increased (P < 0.001) by coHS compared to TN-al conditions, but not affected by the other treatments.
Figure 4.
Effect of different environmental conditions and feed regimen on pectoralis major expression of protein metabolism related transcription factors in broilers. Relative abundances of IGF1(A), PI3Kα (B), ERK1 (C), AKT1 (D), AMPKα1 (E), mTOR (F), p70S6K (G), MyoG (H), MuRF1 (I), and MAFbx (J) mRNA was determined by real-time quantitative PCR and analyzed by the 2-ΔΔCt method using the TN-al group as calibrator. Data were log-transformed and are presented as antilog of the geometric means with error bars representing the 95% confidence interval: . a-b Different letters indicate significant difference at P < 0.05 by a Tukey's multiple comparison test. TN-al, birds reared under continuous 24°C and ad libitum feeding; aHS, birds reared under acute heat stress for 4 h on d 41; cyHScool, birds reared under cyclic high temperature (12 h at 35˚C and 12 h at 24˚C), ad libitum feeding, and sampled at the end of the cool phase of the night; cyHShot, same as cyHScool, but sampled after 4 h at 35°C; coHS, birds reared under continuous 35°C and ad libitum feeding; TN-coPF, birds reared under continuous 24°C and pair-fed to the coHS treatment.
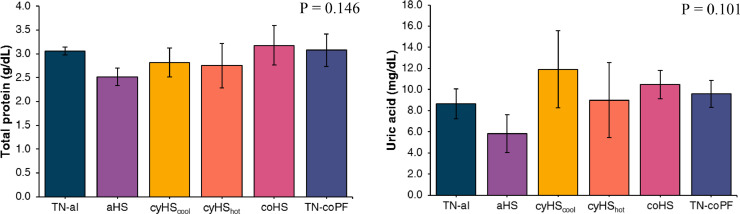
Total Protein and Uric Acid Concentrations in the Plasma
No differences in TP (P = 0.146) and UA (P = 0.101) concentrations in the plasma were observed among the different environmental conditions (Figure 5).
Figure 5.
Effect of different environmental conditions and feed regimen on plasma total protein and uric acid concentration in broilers. Data are presented as mean and error bars represent the 95% confidence interval: . TN-al, birds reared under continuous 24°C and ad libitum feeding; aHS, birds reared under acute heat stress for 4 h on d 41; cyHScool, birds reared under cyclic high temperature (12 h at 35˚C and 12 h at 24˚C), ad libitum feeding, and sampled at the end of the cool phase of the night; cyHShot, same as cyHScool, but sampled after 4 h at 35°C; coHS, birds reared under continuous 35°C and ad libitum feeding; TN-coPF, birds reared under continuous 24°C and pair-fed to the coHS treatment
DISCUSSION
Apparent Ileal Digestibility
Heat stress impairs nutrient digestibility by compromising intestinal barrier function, reducing upper gastrointestinal tract blood flow, and lowering expression and activity of digestive enzymes and several macronutrient transporters (Brugaletta et al., 2022; Teyssier et al., 2022a). However, in line with findings from other studies, no HS-induced losses in DM (Faria Filho et al., 2007; Seven and Seven, 2008; Attia et al., 2017) or energy (Bonnet et al., 1997; Faria Filho et al., 2007; De Souza et al., 2016) digestibility were observed in the current study. In contrast, a 5.3% reduction in ileal N digestibility under coHS was observed. This reduction aligns with results from other studies in broilers reared under hot temperatures, which have reported decreases in ileal or fecal N digestibility ranging between 1.5 and 10% (Zuprizal et al., 1993; Bonnet et al., 1997; Seven and Seven, 2008; Soleimani et al., 2010; Attia et al., 2017). Similar to De Souza et al. (2016), in this study there was a decrease in AID of N under constant HS that was not observed under cyclic HS, reflecting that the magnitude or duration of HS can influence its effects on N digestibility. Additionally, we observed reduced fat digestibility under constant HS compared to PF conditions, suggesting that, unlike proteins, fat digestibility may be more directly impacted by the heat per se rather than the reduced FI.
Our study also revealed the detrimental effect of HS on AA digestibility, with an average reduction of 5% in AID of total AA. Standardized and apparent digestibility values of several AA (i.e., Arg, His, Thr, Val, Lys, Ile, Leu, Phe, Cys, Gly, Ser, Ala, Pro, and Tyr) were also reduced by approximately 5.5% in a study by Soleimani et al. (2010). Likewise, Wallis and Balnave (1984) observed a slight decrease in the digestibility of Thr, Ala, Met, Ile, and Leu under constant HS at 31°C. The relatively greater decrease in AA digestibility compared to other macronutrients might be partly explain by a decrease in expression of several AA transporters (Habashy et al., 2017a), but several studies observed no influence of HS exposure on expression of AA transporters (Sun et al., 2015; Habashy et al., 2017b; Al-Zghoul et al., 2019).
In the current study, the reduction in FI appeared to be the main factor contributing to the performance degradation induced by HS, accounting for 81% of the BWG reduction in coHS birds. In contrast, reduced nutrient digestibility seemed to have only minimal impact on impaired feed efficiency under HS. Therefore, a more important role may be played by alterations in post absorptive nutrient utilization pathways, especially regulatory mechanisms of protein metabolism, as TBM yield were highly affected by both cyHS (-6.3%) and coHS (-16.3%) in the current experiment.
Gene Expression of Stress and Inflammatory Markers in the Whole Blood
Heat stress leads to excess ROS production, and both SOD and GPX are major antioxidant enzymes that detoxify and decompose free radicals and nonradical toxic products (Surai et al., 2019). Research in broiler chickens has shown that SOD enzyme activity was increased in plasma, heart, liver, and skeletal tissues under acute and chronic HS (Lin et al., 2000, 2006; Tan et al., 2010; Yang et al., 2010; Azad et al., 2010b; Ghazi Harsini et al., 2012; Huang et al., 2015; Habashy et al., 2019). However, the influence of HS on SOD activity is probably organ and time-dependent, as other studies did not observe increased SOD activity or mRNA expression under high temperatures (Lin et al., 2000; Willemsen et al., 2011; Xie et al., 2014; Rimoldi et al., 2015; Cramer et al., 2018; Emami et al., 2021; Chen et al., 2023). In the current study, we found increased blood mRNA expression of SOD2 under aHS, coHS, and TN-coPF conditions, suggesting SOD2 upregulation to cope with the increased ROS production.
Heat shock proteins are another defense mechanism to maintain the function and structural integrity of cells in response to HS. In our study, aHS increased HSP70 mRNA expression. In broilers, similar findings of increased HSP70 were observed in different tissues under both acute and chronic HS (Gabriel et al., 1996; Xie et al., 2014; Flees et al., 2017; Baxter et al., 2020; Emami et al., 2021). Interestingly, HSP 70 is widely expressed in chicken breeds naturally exposed to high temperatures and proven to be resistant to HS (Cedraz et al., 2017). Several studies also indicate that HSP70 plays an important role in preventing deleterious effects caused by oxidative stress (Guo et al., 2007; Hao et al., 2012).
Heat stress has also been shown to impact production of several cytokines. In the current study, proinflammatory cytokine IL6 was upregulated by coHS. Other studies have reported increased mRNA expression of some interleukins in various tissues after HS exposure (Ohtsu et al., 2015; Varasteh et al., 2015; He et al., 2019; Baxter et al., 2020). Consistent with findings from other studies (Alhenaky et al., 2017; He et al., 2019; Baxter et al., 2020; Alzarah et al., 2021; Emami et al., 2021; Greene et al., 2021b), TNFα exhibited a more consistent response to HS compared to other cytokines. Among the various inflammation markers measured in the current study, TNFα appeared to be the most sensitive cytokine marker to both acute and constant HS conditions. Interestingly, beyond its proinflammatory effect, TNFα may decrease muscle responsiveness to IGF1 (Frost and Lang, 2007) and interact with the antioxidant system by repressing SOD1 gene (Afonso et al., 2006).
Overall, compared to TN-al conditions, aHS and coHS caused a greater stress response than cyHS, as evidenced by increased HSP70, SOD2, and TNFα levels under aHS conditions, and higher expression of SOD2, TNFα, and IL6 under coHS. The absence of effects of cyHS in the current study seems to indicate that cyclic exposure to hot temperature may only have moderate effects on the expression of the measured oxidative stress, HSP, and inflammatory markers. Furthermore, similar mRNA expression profiles in aHS and cyHShot birds, both sampled 4h after HS exposure, suggest no physiological adaptation due to chronic HS exposure on the stress and inflammatory markers measured in the current study. Also, similar responses between coHS and TN-coPF birds indicate that these markers may be associated with reduced FI rather than HS per se. Interestingly, for all the stress and inflammation markers measured in the current study, the TN-al group exhibited the lowest variability, whereas the coHS or aHS groups displayed the highest variability. This may reflect varying capacities of individual birds to adapt and cope with HS through the pathways evaluated in this experiment.
Gene Expression of Lipid Metabolism Markers in the Liver
In birds, de novo lipogenesis in the liver plays an important role in lipid metabolism since this pathway is responsible for most of the endogenous body lipids (Goodridge and Ball, 1967; O'Hea and Leveille, 1968). The ACLY enzyme converts citrate into acetyl-CoA, which subsequently forms malonyl-CoA through ACC and is utilized by the FASN enzyme for fatty acid biosynthesis (Smink, 2012). In the current study, mRNA expression of these enzymes decreased under coHS, and ACLY was reduced under aHS. These findings are contradictory to the observed increase in abdominal fat at the carcass level (Teyssier et al., 2022b) and increased FASN and ACC mRNA levels in observed less intensive chronic HS conditions (Lu et al., 2017, 2019a,b,c; Kim et al., 2022). Interestingly, Flees et al. (2017) also measured reduced mRNA expression of these enzymes under constant and acute HS but did report an increase in protein levels for ACLY under constant HS, and ACLY and FASN under acute HS. Comparable changes were observed by Lu et al. (2019a,b,c) at the protein level and by Jastrebski et al. (2017) when using a nontargeted transcriptomic and metabolomic analysis. Therefore, the reduction in gene expression of these markers may not directly reflect protein levels, limiting interpretation of gene expression data. Heat stress might affect transcription and translation processes, and the pool of existing lipogenic mRNA could be quickly translated or degraded while the proteins accumulate due to their high half-life (Schwanhäusser et al., 2011; Flees et al., 2017). Furthermore, post-transcriptional modifications, such as phosphorylation, can significantly impact enzyme activity, making mRNA measurement less representative of their functional state. Additionally, similar levels of mRNA expression under coHS and TN-coPF treatments align with findings from Lu et al. (2019b) in birds after 14 d of HS exposure, indicating that mRNA expression regulation of these lipogenic enzymes is not solely due to the heat per se, but rather by the decreased FI. However, regulation at the protein level could be directly related to the increase in temperatures as suggested by research from Lu et al. (2019b), who obtained a lower protein expression of FASN and ACC under PF compared to HS conditions.
Concerning lipid degradation, the ATGL enzyme catalyzes the initial step in triglyceride hydrolysis, releasing a diglyceride and a nonesterified fatty acid (Zimmermann et al., 2004). Under coHS, ATGL mRNA expression was increased, consistent with the study of Flees et al. (2017) in HS birds maintained at 35°C for 3 wk. However, this contradicts with the increased fat accumulation observed in HS birds, and with the reduced ATGL mRNA expression observed by Huang et al. (2021) after exposure of preadipocyte cells at 41.5°C compared to 37°C for 2 and 8 d. Therefore, further research investigating lipolytic enzyme expression in liver and adipocyte tissues is still required to understand their role under HS conditions.
Ultimately, the mRNA expression results of the different markers associated with lipogenesis and lipolysis do not explain the results at the carcass level which indicate that chronic HS birds are energy-deficient due to the reduced FI, but are prone to accumulate fat (Teyssier et al., 2022b). The gene expression results diverge from protein-level findings in other studies, highlighting the need for additional investigations that address enzymes activation through phosphorylation and post-transcriptional regulatory mechanisms.
Gene Expression of Protein Metabolism Markers in the Pectoralis Major Muscle
Several markers of protein metabolism were measured to explain the decrease in total breast meat yield observed under chronic HS and PF conditions in the first part of our study (Teyssier et al., 2022b), as well as the reduced protein deposition and protein synthesis observed under HS conditions in other studies (Temim et al., 2000).
IGF1 plays an important role in muscle hypertrophy and hyperplasia during development and is consequently considered a marker of muscle growth and protein synthesis (McMurtry et al., 1997; Guernec et al., 2003; Duclos, 2005). It can also affect protein degradation by limiting the expression of the 2 ubiquitin ligase enzymes, MuRF1 and MAFbx (Sacheck et al., 2004; Stitt et al., 2004). In agreement with the reduced protein deposition caused by hot temperatures, coHS reduced IGF1 mRNA expression in chicken breast, as seen in other studies under constant and cyclic HS (Liu et al., 2014; Zuo et al., 2015; Ma et al., 2018; Roushdy et al., 2018). The binding of IGF1 to its receptor activates several intracellular signaling cascades including the PI3K/AKT and MAPK/ERK pathways (Weeks et al., 2017; Józefiak et al., 2021). Interestingly, the increase in IGF1 expression observed under coHS in the current study did not result in increased PI3K expression. Conversely, PI3K mRNA expression was increased under aHS, cyHShot, coHS, and TN-coPF birds compared with TN-al birds. Zuo et al. (2015) also observed an increase in PI3K mRNA expression in the thigh muscle under constant HS, while its expression in the breast was reduced. This upregulation of PI3K could be a negative feedback mechanism caused by reduced IGF1 expression or increased protein degradation markers to balance the loss of protein caused by HS and enhance survival (Zuo et al., 2015).
In chickens, muscle fiber numbers are determined during embryonic development and cannot be increased, with subsequent muscle growth occurring via hypertrophy and protein deposition (Nawaz et al., 2021). Downstream of the mTOR pathway, p70S6K plays an important role in protein synthesis and muscle hypertrophy (Zanchi and Lancha, 2008). In the current study, mRNA expression of p70S6K was reduced in the breast muscle under cyHScool, coHS, and TN-coPF conditions, which is in line with other studies under constant HS (Zuo et al., 2015; Ma et al., 2018, 2021a; Lu et al., 2019a), but this reduced p70S6K expression observed under constant HS appears to be mainly caused by reduced FI. Furthermore, myogenin is a protein involved in the differentiation of myocytes into myoblasts and myotubes (Hernández-Hernández et al., 2017; Nawaz et al., 2021). Its direct involvement in muscle cell differentiation makes MyoG the most direct marker of protein synthesis among the pool of markers measured in the current study. Reduced MyoG mRNA expression under coHS aligns with the decrease in IGF1 mRNA expression, known to positively regulate myogenesis (Clemmons, 2009). Other researchers also reported decreased MyoG mRNA expression in embryos exposed to high temperatures (Gabriel et al., 2003), in the breast muscle of growing birds under constant HS (Ma et al., 2018), and in the liver of birds subjected to cyclic HS (Zaglool et al., 2019). Therefore, it seems that HS reduces muscle hypertrophy through downregulation of pathways downstream from mTOR, including p70S6K and MyoG (Ma et al., 2018). Also, although we observed an increase in MyoG mRNA expression under TN-coPF conditions compared to coHS conditions, the magnitude of the decrease in p70S6K and MyoG seems to indicate that most of the reduction in protein synthesis is explained by reduced FI.
Protein degradation in chickens is mainly induced by the ubiquitin–proteasome pathway, associated with increased expression of MAFbx and MuRF1, which catalyze the ubiquitination and degradation of key muscle proteins (Lecker et al., 1999; Sacheck et al., 2004). In the current study both MAFbx and MuRF1 were highly expressed under constant HS, which is in agreement with previous studies (Temim et al., 2000; Zuo et al., 2015; Lu et al., 2019a; Li et al., 2021; Ma et al., 2021a,b). During HS, protein degradation may serve to mobilize AA as substrates for gluconeogenesis in the liver (Ma et al., 2021a). Additionally, consistent with our results, several aforementioned studies (Zuo et al., 2015; Lu et al., 2019a; Li et al., 2021; Ma et al., 2021a) also reported elevated expression of MuRF1 and MAFbx mRNA in birds subjected to HS conditions, which was not observed in TN or PF conditions, except for Ma et al. (2021b) who observed intermediate expression in PF for MuRF1. This indicates that, contrary to protein synthesis, increased protein breakdown during HS could be directly associated with HS per se and not the reduced FI. Further analyses and research are required at the gene and protein level to elucidate the different interactions occurring in protein synthesis and degradation pathways.
CONCLUSIONS
In summary, HS markedly impairs broiler performance, mainly due to a reduction in FI in an attempt to minimize the endogenous heat production associated with nutrient digestion, absorption, and metabolism. Overall, constant HS caused greater aberrations in digestibility and markers of metabolism (i.e., mRNA expression levels) than cyclic HS. Part of this observation can be explained by the lower physiological stress response induced by cyclic exposure to hot temperatures, as suggested by the absence of difference in stress and inflammation markers between TN-al and cyHS birds. In addition, our study indicates that the reductions in digestibility of N, EE, and some AA induced by constant HS are independent of the reduced FI and driven by the heat per se. However, considering the magnitude of performance losses caused by constant HS, this reduced nutrient digestibility appears to have limited contribution in the impaired feed efficiency. Regulatory mechanisms of protein and lipid metabolism were also highly impacted by constant HS exposure. However, further research is required to explain the absence of correlation between the reduced lipogenic activity measured in our study under constant HS at the mRNA level and the increase in fat deposition observed at the carcass level. Interestingly, downregulation of protein synthesis mechanisms under constant HS also occurred with PF, whereas upregulation of protein degradation markers was directly associated with HS per se and not reduced FI. Furthermore, some of the stress, inflammation, and metabolism markers measured in this study were impacted by acute HS exposure when compared to TN-al conditions, but similar mRNA expression profiles between aHS and cyHShot birds, which were both sampled 4 h after the increase in temperature, do not support a physiological adaptation to chronic high-temperature exposure. Similarly, except for the ATGL mRNA expression, comparable mRNA expression profiles between cyHScool and cyHShot birds indicate an absence of acute regulatory mechanisms when birds are cyclically exposed to chronic high temperatures. In summary, our study emphasizes the importance of managing acute and chronic HS in broiler production by implementing strategies to address its deleterious effects on nutrient utilization, metabolic regulation, and physiological stress.
ACKNOWLEDGMENTS
This research was supported by a research grant from Adisseo France SAS and with funding from the University of Arkansas System Division of Agriculture.
DISCLOSURES
P. Cozannet is an employee of Adisseo France SAS. Otherwise, there are no conflicts of interest to declare.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.psj.2023.103048.
Appendix. Supplementary materials
REFERENCES
- Afonso V., Santos G., Collin P., Khatib A.-M., Mitrovic D.R., Lomri N., Leitman D.C., Lomri A. Tumor necrosis factor-α down-regulates human Cu/Zn superoxide dismutase 1 promoter via JNK/AP-1 signaling pathway. Free Radic. Biol. Med. 2006;41:709–721. doi: 10.1016/j.freeradbiomed.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:1–14. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhenaky A., Abdelqader A., Abuajamieh M., Al-Fataftah A.-R. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J. Therm. Biol. 2017;70:9–14. doi: 10.1016/j.jtherbio.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Alzarah M.I., Althobiati F., Abbas A.O., Mehaisen G.M.K., Kamel N.N. Citrullus colocynthis seeds: a potential natural immune modulator source for broiler reared under chronic heat stress. Animals. 2021;11:1951. doi: 10.3390/ani11071951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Alliftawi A.R.S., Saleh K.M.M., Jaradat Z.W. Expression of digestive enzyme and intestinal transporter genes during chronic heat stress in the thermally manipulated broiler chicken. Poult. Sci. 2019;98:4113–4122. doi: 10.3382/ps/pez249. [DOI] [PubMed] [Google Scholar]
- Attia Y.A., Al-Harthi M.A., El-Shafey A.S., Rehab Y.A., Kim W.K. Enhancing tolerance of broiler chickens to heat stress by supplementation with vitamin E, vitamin C and/or probiotics. Ann. Anim. Sci. 2017;17:1155–1169. [Google Scholar]
- Azad M.A.K., Kikusato M., Maekawa T., Shirakawa H., Toyomizu M. Metabolic characteristics and oxidative damage to skeletal muscle in broiler chickens exposed to chronic heat stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010;155:401–406. doi: 10.1016/j.cbpa.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Azad M.A.K., Kikusato M., Sudo S., Amo T., Toyomizu M. Time course of ROS production in skeletal muscle mitochondria from chronic heat-exposed broiler chicken. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010;157:266–271. doi: 10.1016/j.cbpa.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Baxter M.F.A., Greene E.S., Kidd M.T., Tellez-Isaias G., Orlowski S., Dridi S. Water amino acid-chelated trace mineral supplementation decreases circulating and intestinal HSP70 and proinflammatory cytokine gene expression in heat-stressed broiler chickens. J. Anim. Sci. 2020;98:skaa049. doi: 10.1093/jas/skaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabucci U., Lacetera N., Nardone A., Ronchi B., Ranieri M.S. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010;130:57–69. [Google Scholar]
- Bhattacharyya P., Pathak H., Pal S. In: Pages 17–32 in Climate Smart Agriculture: Concepts, Challenges, and Opportunities. Bhattacharyya P., Pathak H., Pal S., editors. Green Energy and Technology. Springer; Singapore, Singapore: 2020. Impact of climate change on agriculture: evidence and predictions. [Google Scholar]
- Bonnet S., Geraert P.A., Lessire M., Carré B., Guillaumin S. Effect of high ambient temperature on feed digestibility in broilers. Poult. Sci. 1997;76:857–863. doi: 10.1093/ps/76.6.857. [DOI] [PubMed] [Google Scholar]
- Brugaletta G., Teyssier J.-R., Rochell S.J., Dridi S., Sirri F. A review of heat stress in chickens. Part I: insights into physiology and gut health. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.934381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedraz H., Gromboni J.G.G., Garcia A.A.P., Farias Filho R.V., Souza T.M., de Oliveira E.R., de Oliveira E.B., do Nascimento C.S., Meneghetti C., Wenceslau A.A. Heat stress induces expression of HSP genes in genetically divergent chickens (F Gallyas, Ed.) PLoS One. 2017;12 doi: 10.1371/journal.pone.0186083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Liu H., Zhang J., Zhou B., He X., Wang T., Wang C. Dietary rutin improves breast meat quality in heat-stressed broilers and protects mitochondria from oxidative attack via the AMPK /PINK1–Parkin pathway. J. Sci. Food. Agric. 2023;103:12431. doi: 10.1002/jsfa.12431. [DOI] [PubMed] [Google Scholar]
- Clemmons D.R. Role of IGF-I in skeletal muscle mass maintenance. Trends Endocrinol. Metab. 2009;20:349–356. doi: 10.1016/j.tem.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Cramer T.A., Kim H.W., Chao Y., Wang W., Cheng H.W., Kim Y.H.B. Effects of probiotic (Bacillus subtilis) supplementation on meat quality characteristics of breast muscle from broilers exposed to chronic heat stress. Poult. Sci. 2018;97:3358–3368. doi: 10.3382/ps/pey176. [DOI] [PubMed] [Google Scholar]
- De Souza L.F.A., Espinha L.P., De Almeida E.A., Lunedo R., Furlan R.L., Macari M. How heat stress (continuous or cyclical) interferes with nutrient digestibility, energy and nitrogen balances and performance in broilers. Livest. Sci. 2016;192:39–43. [Google Scholar]
- Deeb N., Cahaner A. The effects of naked neck genotypes, ambient temperature, and feeding status and their interactions on body temperature and performance of broilers. Poult. Sci. 1999;78:1341–1346. doi: 10.1093/ps/78.10.1341. [DOI] [PubMed] [Google Scholar]
- Duclos M.J. Insulin-like growth factor-I (IGF-1) mRNA levels and chicken muscle growth. J. Physiol. Pharmacol. 2005;56(Suppl. 3):25–35. [PubMed] [Google Scholar]
- Emami N.K., Greene E.S., Kogut M.H., Dridi S. Heat stress and feed restriction distinctly affect performance, carcass and meat yield, intestinal integrity, and inflammatory (chemo) cytokines in broiler chickens. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.707757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Jung U., Voy B., Dridi S. Radical response: effects of heat stress-induced oxidative stress on lipid metabolism in the avian liver. Antioxidants. 2020;10:35. doi: 10.3390/antiox10010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria Filho D.E., Campos D.M.B., Torres K.A., Vieira B.S., Rosa P.S., Vaz A.M., Macari M., Furlan R.L. Protein levels for heat-exposed broilers: performance, nutrients digestibility, and energy and protein metabolism. Int. J. Poult. Sci. 2007;6:187–194. [Google Scholar]
- Flees J., Rajaei-Sharifabadi H., Greene E., Beer L., Hargis B.M., Ellestad L., Porter T., Donoghue A., Bottje W.G., Dridi S. Effect of Morinda citrifolia (noni)-enriched diet on hepatic heat shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front. Physiol. 2017;8:919. doi: 10.3389/fphys.2017.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R.A., Lang C.H. Protein kinase B/Akt: a nexus of growth factor and cytokine signaling in determining muscle mass. J. Appl. Physiol. 2007;103:378–387. doi: 10.1152/japplphysiol.00089.2007. [DOI] [PubMed] [Google Scholar]
- Gabriel J.E., Alvares L.E., Gobet M.C., de Paz C.C.P., Packer I.U., Macari M., Coutinho L.L. Expression of MyoD, myogenin, myostatin and Hsp70 transcripts in chicken embryos submitted to mild cold or heat. J. Therm. Biol. 2003;28:261–269. [Google Scholar]
- Gabriel J.E., Ferro J.A., Stefani R.M.P., Ferro M.I.T., Gomes S.L., Macari M. Effect of acute heat stress on heat shock protein 70 messenger RNA and on heat shock protein expression in the liver of broilers. Br. Poult. Sci. 1996;37:443–449. doi: 10.1080/00071669608417875. [DOI] [PubMed] [Google Scholar]
- Geraert P.A., Padilha J.C.F., Guillaumin S. Metabolic and endocrine changes induced by chronic heatexposure in broiler chickens: growth performance, body composition and energy retention. Br. J. Nutr. 1996;75:195–204. doi: 10.1079/bjn19960124. [DOI] [PubMed] [Google Scholar]
- Ghazi Harsini S., Habibiyan M., Moeini M.M., Abdolmohammadi A.R. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol. Trace Elem. Res. 2012;148:322–330. doi: 10.1007/s12011-012-9374-0. [DOI] [PubMed] [Google Scholar]
- Goodridge A., Ball E. Lipogenesis in the pigeon: in vivo studies. Am. J. Physiol. 1967;213:245–249. doi: 10.1152/ajplegacy.1967.213.1.245. [DOI] [PubMed] [Google Scholar]
- Greene E.S., Cauble R., Kadhim H., De Almeida Mallmann B., Gu I., Lee S.O., Orlowski S., Dridi S. Protective effects of the phytogenic feed additive “comfort” on growth performance via modulation of hypothalamic feeding- and drinking-related neuropeptides in cyclic heat-stressed broilers. Domest. Anim. Endocrinol. 2021;74 doi: 10.1016/j.domaniend.2020.106487. [DOI] [PubMed] [Google Scholar]
- Greene E.S., Emami N.K., Dridi S. Research Note: phytobiotics modulate the expression profile of circulating inflammasome and cyto(chemo)kine in whole blood of broilers exposed to cyclic heat stress. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guernec A., Berri C., Chevalier B., Wacrenier-Cere N., Bihan-Duval E.Le, Duclos M.J. Muscle development, insulin-like growth factor-I and myostatin mRNA levels in chickens selected for increased breast muscle yield. Growth Horm. IGF Res. 2003;13:8–18. doi: 10.1016/s1096-6374(02)00136-3. [DOI] [PubMed] [Google Scholar]
- Guo S., Wharton W., Moseley P., Shi H. Heat shock protein 70 regulates cellular redox status by modulating glutathione-related enzyme activities. Cell Stress Chaper. 2007;12:245. doi: 10.1379/CSC-265.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Adomako K., Attia Y.A., Rekaya R., Aggrey S.E. Effect of heat stress on amino acid digestibility and transporters in meat-type chickens. Poult. Sci. 2017;96:2312–2319. doi: 10.3382/ps/pex027. [DOI] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Fuller A.L., Attia Y.A., Rekaya R., Aggrey S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int. J. Biometeorol. 2017;61:2111–2118. doi: 10.1007/s00484-017-1414-1. [DOI] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Rekaya R., Aggrey S.E. Cellular antioxidant enzyme activity and biomarkers for oxidative stress are affected by heat stress. Int. J. Biometeorol. 2019;63:1569–1584. doi: 10.1007/s00484-019-01769-z. [DOI] [PubMed] [Google Scholar]
- Hao Y., Gu X.H., Wang X.L. Overexpression of heat shock protein 70 and its relationship to intestine under acute heat stress in broilers: 1. Intestinal structure and digestive function. Poult. Sci. 2012;91:781–789. doi: 10.3382/ps.2011-01627. [DOI] [PubMed] [Google Scholar]
- He S., Yu Q., He Y., Hu R., Xia S., He J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019;98:6378–6387. doi: 10.3382/ps/pez471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Hernández J.M., García-González E.G., Brun C.E., Rudnicki M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017;72:10–18. doi: 10.1016/j.semcdb.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Jiao H., Song Z., Zhao J., Wang X., Lin H. Heat stress impairs mitochondria functions and induces oxidative injury in broiler chickens1. J. Anim. Sci. 2015;93:2144–2153. doi: 10.2527/jas.2014-8739. [DOI] [PubMed] [Google Scholar]
- Huang Y., Xie H., Pan P., Qu Q., Xia Q., Gao X., Zhang S., Jiang Q. Heat stress promotes lipid accumulation by inhibiting the AMPK-PGC-1α signaling pathway in 3T3-L1 preadipocytes. Cell Stress Chaper. 2021;26:563–574. doi: 10.1007/s12192-021-01201-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastrebski S.F., Lamont S.J., Schmidt C.J. Chicken hepatic response to chronic heat stress using integrated transcriptome and metabolome analysis (MFW te Pas, Ed.) PLoS One. 2017;12 doi: 10.1371/journal.pone.0181900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Józefiak A., Larska M., Pomorska-Mól M., Ruszkowski J.J. The IGF-1 signaling pathway in viral infections. Viruses. 2021;13:1488. doi: 10.3390/v13081488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.Y., Lim B., Kim J.-M., Kil D.Y. Integrated transcriptome analysis for the hepatic and jejunal mucosa tissues of broiler chickens raised under heat stress conditions. J. Animal Sci. Biotechnol. 2022;13:79. doi: 10.1186/s40104-022-00734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara L.J., Rostagno M.H. Impact of heat stress on poultry production. Animals. 2013;3:356–369. doi: 10.3390/ani3020356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecker S.H., Solomon V., Mitch W.E., Goldberg A.L. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J. Nutr. 1999;129:227S–237S. doi: 10.1093/jn/129.1.227S. [DOI] [PubMed] [Google Scholar]
- Leveille G.A. In vitro hepatic lipogenesis in the hen and chick. Comp. Biochem. Physiol. 1969;28:431–435. doi: 10.1016/0010-406x(69)91357-7. [DOI] [PubMed] [Google Scholar]
- Li X., Zhang M., Feng J., Zhou Y. Myostatin and related factors are involved in skeletal muscle protein breakdown in growing broilers exposed to constant heat stress. Animals. 2021;11:1467. doi: 10.3390/ani11051467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Decuypere E., Buyse J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006;144:11–17. doi: 10.1016/j.cbpa.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Lin H., Du R., Zhang Z.Y. Peroxide status in tissues of heat-stressed broilers. Asian Australas. J. Anim. Sci. 2000;13:1373–1376. [Google Scholar]
- Liu L.L., He J.H., Xie H.B., Yang Y.S., Li J.C., Zou Y. Resveratrol induces antioxidant and heat shock protein mRNA expression in response to heat stress in black-boned chickens. Poult. Sci. 2014;93:54–62. doi: 10.3382/ps.2013-03423. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017;65:11251–11258. doi: 10.1021/acs.jafc.7b04428. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X.F., Ma B.B., Zhang L., Li J.L., Jiang Y., Zhou G.H., Gao F. The alleviative effects and related mechanisms of taurine supplementation on growth performance and carcass characteristics in broilers exposed to chronic heat stress. Poult. Sci. 2019;98:878–886. doi: 10.3382/ps/pey433. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X.F., Ma B.B., Zhang L., Li J.L., Jiang Y., Zhou G.H., Gao F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019;98:3695–3704. doi: 10.3382/ps/pez056. [DOI] [PubMed] [Google Scholar]
- Lu Z., He X., Ma B., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Dietary taurine supplementation decreases fat synthesis by suppressing the liver X receptor α pathway and alleviates lipid accumulation in the liver of chronic heat-stressed broilers. J. Sci. Food Agric. 2019;99:5631–5637. doi: 10.1002/jsfa.9817. [DOI] [PubMed] [Google Scholar]
- Ma B., He X., Lu Z., Zhang L., Li J., Jiang Y., Zhou G., Gao F. Chronic heat stress affects muscle hypertrophy, muscle protein synthesis and uptake of amino acid in broilers via insulin like growth factor-mammalian target of rapamycin signal pathway. Poult. Sci. 2018;97:4150–4158. doi: 10.3382/ps/pey291. [DOI] [PubMed] [Google Scholar]
- Ma B., Zhang L., Li J., Xing T., Jiang Y., Gao F. Heat stress alters muscle protein and amino acid metabolism and accelerates liver gluconeogenesis for energy supply in broilers. Poult. Sci. 2021;100:215–223. doi: 10.1016/j.psj.2020.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Zhang L., Li J., Xing T., Jiang Y., Gao F. Dietary taurine supplementation ameliorates muscle loss in chronic heat stressed broilers via suppressing the perk signaling and reversing endoplasmic reticulum-stress-induced apoptosis. J. Sci. Food Agric. 2021;101:2125–2134. doi: 10.1002/jsfa.10835. [DOI] [PubMed] [Google Scholar]
- Masson-Delmotte, V., P. Zhai, A. Priani, S. Connors, C. Péan, and S. Berger. 2021. IPCC, 2021: Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change.
- McMurtry J.P., Francis G.L., Upton Z. Insulin-like growth factors in poultry. Domest. Anim. Endocrinol. 1997;14:199–229. doi: 10.1016/s0739-7240(97)00019-2. [DOI] [PubMed] [Google Scholar]
- Moekti G.R. Industrial livestock production: a review on advantages and disadvantages. IOP Conf. Ser.: Earth Environ. Sci. 2020;492 [Google Scholar]
- Nawaz A.H., Amoah K., Leng Q.Y., Zheng J.H., Zhang W.L., Zhang L. Poultry response to heat stress: its physiological, metabolic, and genetic implications on meat production and quality including strategies to improve broiler production in a warming world. Front. Vet. Sci. 2021;8 doi: 10.3389/fvets.2021.699081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hea E.K., Leveille G.A. Lipogenesis in isolated adipose tissue of the domestic chick (Gallus domesticus) Comp. Biochem. Physiol. 1968;26:111–120. doi: 10.1016/0010-406x(68)90317-4. [DOI] [PubMed] [Google Scholar]
- Ohtsu H., Yamazaki M., Abe H., Murakami H., Toyomizu M. Heat stress modulates cytokine gene expression in the spleen of broiler chickens. J. Poult. Sci. 2015;52:282–287. [Google Scholar]
- Olivier J., Johnson W.D., Marshall G.D. The logarithmic transformation and the geometric mean in reporting experimental IgE results: what are they and when and why to use them? Ann. Allergy Asthma Immunol. 2008;100:333–337. doi: 10.1016/S1081-1206(10)60595-9. [DOI] [PubMed] [Google Scholar]
- Rajaei-Sharifabadi H., Ellestad L., Porter T., Donoghue A., Bottje W.G., Dridi S. Noni (Morinda citrifolia) modulates the hypothalamic expression of stress- and metabolic-related genes in broilers exposed to acute heat stress. Front. Genet. 2017;8:192. doi: 10.3389/fgene.2017.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimoldi S., Lasagna E., Sarti F.M., Marelli S.P., Cozzi M.C., Bernardini G., Terova G. Expression profile of six stress-related genes and productive performances of fast and slow growing broiler strains reared under heat stress conditions. Meta Gene. 2015;6:17–25. doi: 10.1016/j.mgene.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P., De Faria Filho D.E., Dahlke F., Vieira B.S., Macari M., Furlan R.L. Performance and carcass characteristics of broiler chickens with different growth potential and submitted to heat stress. Rev. Bras. Cienc. Avic. 2007;9:181–186. [Google Scholar]
- Roushdy E.M., Zaglool A.W., El-Tarabany M.S. Effects of chronic thermal stress on growth performance, carcass traits, antioxidant indices and the expression of HSP70, growth hormone and superoxide dismutase genes in two broiler strains. J. Therm. Biol. 2018;74:337–343. doi: 10.1016/j.jtherbio.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Sacheck J.M., Ohtsuka A., McLary S.C., Goldberg A.L. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 2004;287:E591–E601. doi: 10.1152/ajpendo.00073.2004. [DOI] [PubMed] [Google Scholar]
- Sanchez-Valle V., Chavez-Tapia N.C., Uribe M., Mendez-Sanchez N. Role of oxidative stress and molecular changes in liver fibrosis: a review. Curr. Med. Chem. 2012;19:4850–4860. doi: 10.2174/092986712803341520. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- Seven P.T., Seven İ. Effect of dietary turkish propolis as alternative to antibiotic on performance and digestibility in broilers exposed to heat stress. J. Appl. Anim. Res. 2008;34:193–196. [Google Scholar]
- Shehata A.M., Saadeldin I.M., Tukur H.A. Modulation of heat-shock proteins mediates chicken cell survival against thermal stress. Animals. 2020;10:2407. doi: 10.3390/ani10122407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short F.J., Gorton P., Wiseman J., Boorman K.N. Determination of titanium dioxide added as an inert marker in chicken digestibility studies. Anim. Feed Sci. Technol. 1996;59:215–221. [Google Scholar]
- Smink W. Wageningen University and Research; Wageningen, Netherland: 2012. Fatty Acid Digestion, Synthesis and Metabolism in Broiler Chickens and Pigs. [Google Scholar]
- Soleimani A.F., Meimandipour A., Azhar K., Ebrahimi M., Zulkifli I. Effects of heat exposure and sex on ileal digestibility of amino acids of soybean meal in broiler chickens. Arch. Geflügelk. 2010;74:249–255. [Google Scholar]
- Song Z., Cheng K., Zhang L., Wang T. Dietary supplementation of enzymatically treated Artemisia annua could alleviate the intestinal inflammatory response in heat-stressed broilers. J. Therm. Biol. 2017;69:184–190. doi: 10.1016/j.jtherbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Song Z.H., Cheng K., Zheng X.C., Ahmad H., Zhang L.L., Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018;97:430–437. doi: 10.3382/ps/pex312. [DOI] [PubMed] [Google Scholar]
- Stitt T.N., Drujan D., Clarke B.A., Panaro F., Timofeyva Y., Kline W.O., Gonzalez M., Yancopoulos G.D., Glass D.J. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol. Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- St-Pierre N.R., Cobanov B., Schnitkey G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 2003;86:E52–E77. [Google Scholar]
- Sun X., Zhang H., Sheikhahmadi A., Wang Y., Jiao H., Lin H., Song Z. Effects of heat stress on the gene expression of nutrient transporters in the jejunum of broiler chickens (Gallus gallus domesticus) Int. J. Biometeorol. 2015;59:127–135. doi: 10.1007/s00484-014-0829-1. [DOI] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Fisinin V.I., Kidd M.T. Antioxidant defence systems and oxidative stress in poultry biology: an update. Antioxidants. 2019;8:235. doi: 10.3390/antiox8070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.Y., Yang L., Fu Y.Q., Feng J.H., Zhang M.H. Effects of different acute high ambient temperatures on function of hepatic mitochondrial respiration, antioxidative enzymes, and oxidative injury in broiler chickens. Poult. Sci. 2010;89:115–122. doi: 10.3382/ps.2009-00318. [DOI] [PubMed] [Google Scholar]
- Temim S., Chagneau A.M., Peresson R., Tesseraud S. Chronic heat exposure alters protein turnover of three different skeletal muscles in finishing broiler chickens fed 20 or 25% protein diets. J. Nutr. 2000;130:813–819. doi: 10.1093/jn/130.4.813. [DOI] [PubMed] [Google Scholar]
- Teyssier J.R., Brugaletta G., Sirri F., Dridi S., Rochell S.J. A review of heat stress in chickens. Part II: insights into protein and energy utilization and feeding. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.943612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssier J.R., Preynat A., Cozannet P., Briens M., Mauromoustakos A., Greene E.S., Owens C.M., Dridi S., Rochell S.J. Constant and cyclic chronic heat stress models differentially influence growth performance, carcass traits and meat quality of broilers. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2022.101963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varasteh S., Braber S., Akbari P., Garssen J., Fink-Gremmels J. Differences in susceptibility to heat stress along the chicken intestine and the protective effects of galacto-oligosaccharides (A Bhunia, Ed.) PLoS One. 2015;10 doi: 10.1371/journal.pone.0138975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis I.R., Balnave D. The influence of environmental temperature, age and sex on the digestibility of amino acids in growing broiler chickens. Br. Poult. Sci. 1984;25:401–407. doi: 10.1080/00071668408454880. [DOI] [PubMed] [Google Scholar]
- Weeks K.L., Bernardo B.C., Ooi J.Y.Y., Patterson N.L., McMullen J.R. The IGF1-PI3K-Akt signaling pathway in mediating exercise-induced cardiac hypertrophy and protection. Adv. Exp. Med. Biol. 2017;1000:187–210. doi: 10.1007/978-981-10-4304-8_12. [DOI] [PubMed] [Google Scholar]
- Willemsen H., Swennen Q., Everaert N., Geraert P.-A., Mercier Y., Stinckens A., Decuypere E., Buyse J. Effects of dietary supplementation of methionine and its hydroxy analog dl-2-hydroxy-4-methylthiobutanoic acid on growth performance, plasma hormone levels, and the redox status of broiler chickens exposed to high temperatures. Poult. Sci. 2011;90:2311–2320. doi: 10.3382/ps.2011-01353. [DOI] [PubMed] [Google Scholar]
- Xie J., Tang L., Lu L., Zhang L., Xi L., Liu H.-C., Odle J., Luo X. Differential expression of heat shock transcription factors and heat shock proteins after acute and chronic heat stress in laying chickens (Gallus gallus) (S Cotterill, Ed.) PLoS One. 2014;9 doi: 10.1371/journal.pone.0102204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Tan G.-Y., Fu Y.-Q., Feng J.-H., Zhang M.-H. Effects of acute heat stress and subsequent stress removal on function of hepatic mitochondrial respiration, ROS production and lipid peroxidation in broiler chickens. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010;151:204–208. doi: 10.1016/j.cbpc.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Zaglool A.W., Roushdy E.M., El-Tarabany M.S. Impact of strain and duration of thermal stress on carcass yield, metabolic hormones, immunological indices and the expression of HSP90 and Myogenin genes in broilers. Res. Vet. Sci. 2019;122:193–199. doi: 10.1016/j.rvsc.2018.11.027. [DOI] [PubMed] [Google Scholar]
- Zanchi N.E., Lancha A.H. Mechanical stimuli of skeletal muscle: implications on mTOR/p70s6k and protein synthesis. Eur. J. Appl. Physiol. 2008;102:253–263. doi: 10.1007/s00421-007-0588-3. [DOI] [PubMed] [Google Scholar]
- Zeferino C.P., Komiyama C.M., Pelícia V.C., Fascina V.B., Aoyagi M.M., Coutinho L.L., Sartori J.R., Moura A.S.A.M.T. Carcass and meat quality traits of chickens fed diets concurrently supplemented with vitamins C and E under constant heat stress. Animal. 2016;10:163–171. doi: 10.1017/S1751731115001998. [DOI] [PubMed] [Google Scholar]
- Zhang Z.Y., Jia G.Q., Zuo J.J., Zhang Y., Lei J., Ren L., Feng D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012;91:2931–2937. doi: 10.3382/ps.2012-02255. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhao X.H., Yang L., Chen X.Y., Jiang R.S., Jin S.H., Geng Z.Y. Resveratrol alleviates heat stress-induced impairment of intestinal morphology, microflora, and barrier integrity in broilers. Poult. Sci. 2017;96:4325–4332. doi: 10.3382/ps/pex266. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Strauss J.G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- Zuo J., Xu M., Abdullahi Y.A., Ma L., Zhang Z., Feng D. Constant heat stress reduces skeletal muscle protein deposition in broilers. J. Sci. Food Agric. 2015;95:429–436. doi: 10.1002/jsfa.6749. [DOI] [PubMed] [Google Scholar]
- Zuprizal M.Larbier, Chagneau A.M., Geraert P.A. Influence of ambient temperature on true digestibility of protein and amino acids of rapeseed and soybean meals in broilers. Poult. Sci. 1993;72:289–295. doi: 10.3382/ps.0720289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.