Abstract
Background
People undergoing major vascular surgery have an increased risk of postoperative cardiac complications. Beta‐adrenergic blockers represent an important and established pharmacological intervention in the prevention of cardiac complications in people with coronary artery disease. It has been proposed that this class of drugs may reduce the risk of perioperative cardiac complications in people undergoing major non‐cardiac vascular surgery.
Objectives
To review the efficacy and safety of perioperative beta‐adrenergic blockade in reducing cardiac or all‐cause mortality, myocardial infarction, and other cardiovascular safety outcomes in people undergoing major non‐cardiac vascular surgery.
Search methods
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator searched the Specialised Register (January 2014) and the Cochrane Central Register of Controlled Trials (CENTRAL; 2013, Issue 12). We searched trials databases and checked reference lists of relevant articles.
Selection criteria
We included prospective, randomised controlled trials of perioperative beta‐adrenergic blockade of people over 18 years of age undergoing non‐cardiac vascular surgery.
Data collection and analysis
Two review authors independently performed study selection and data extraction. We resolved disagreements through discussion. We performed meta‐analysis using a fixed‐effect model with odds ratios (ORs) and 95% confidence intervals (CIs).
Main results
We included two studies in this review, both of which were double‐blind, randomised controlled trials comparing perioperative beta‐adrenergic blockade (metoprolol) with placebo, on cardiovascular outcomes in people undergoing major non‐cardiac vascular surgery. We included 599 participants receiving beta‐adrenergic blockers (301 participants) or placebo (298 participants). The overall quality of studies was good. However, one study did not report random sequence generation or allocation concealment techniques, indicating possible selection bias, and the other study did not report outcome assessor blinding and was possibly underpowered. It should be noted that several of the outcomes were only reported in a single study and neither of the studies reported on vascular patency/graft occlusion, which reduces the quality of evidence to moderate. There was no evidence that perioperative beta‐adrenergic blockade reduced all‐cause mortality (OR 0.62, 95% CI 0.03 to 15.02), cardiovascular mortality (OR 0.34, 95% CI 0.01 to 8.32), non‐fatal myocardial infarction (OR 0.83, 95% CI 0.46 to 1.49; P value = 0.53), arrhythmia (OR 0.70, 95% CI 0.26 to 1.88), heart failure (OR 1.71, 95% CI 0.40 to 7.23), stroke (OR 2.67, 95% CI 0.11 to 67.08), composite cardiovascular events (OR 0.87, 95% CI 0.55 to 1.39; P value = 0.57) or re‐hospitalisation at 30 days (OR 0.86, 95% CI 0.48 to 1.52). However, there was strong evidence that beta‐adrenergic blockers increased the odds of intra‐operative bradycardia (OR 4.97, 95% CI 3.22 to 7.65; P value < 0.00001) and intra‐operative hypotension (OR 1.84, 95% CI 1.31 to 2.59; P value = 0.0005).
Authors' conclusions
This meta‐analysis currently offers no clear evidence that perioperative beta‐adrenergic blockade reduces postoperative cardiac morbidity and mortality in people undergoing major non‐cardiac vascular surgery. There is evidence that intra‐operative bradycardia and hypotension are more likely in people taking perioperative beta‐adrenergic blockers, which should be weighed with any benefit.
Plain language summary
Beta‐blockers for cardiac risk reduction in people undergoing non‐cardiac vascular surgery
Background
As the population is ageing, more people will undergo major vascular surgery, which carries an increased risk of cardiac complications. The increased risk of cardiac complications is often the result of asymptomatic heart disease. Treating severe symptoms, such as critical limb ischaemia (severely narrowed arteries of the lower limbs resulting in rest pain, ulcers, or gangrene), in people with peripheral arterial disease is a common reason for undergoing vascular surgery, which carries an increased risk heart attack (myocardial infarction) ranging from 5% to 24% during and shortly after surgery. There is clear evidence for the use of beta‐blockers (a class of medications used to treat certain heart conditions as well as high‐blood pressure and other conditions) to reduce cardiac risk in people with known heart disease, and it has been suggested that beta‐blockers may reduce short‐term cardiac illness (morbidity) and death (mortality) in people undergoing major non‐cardiac vascular surgery.
Study characteristics
We identified two studies that evaluated beta‐blockers giving during surgery (perioperatively) in people undergoing major non‐cardiac vascular surgery, with follow‐up data on cardiovascular outcomes. A total of 599 participants were randomised to receive beta‐blockers (301 participants) or placebo (298 participants). Both studies were double‐blind (neither participants nor surgeon were aware of the treatment), randomised controlled trials evaluating the beta‐blocker, metoprolol.
Key results
The results of the analysis offered no clear evidence that perioperative beta‐blockers reduced death from any cause (all‐cause mortality), cardiovascular death, non‐fatal heart attack, irregular heartbeat (arrhythmia), heart failure, stroke, combined cardiovascular events or re‐hospitalisation at 30 days. There was evidence to support that beta‐blockers increased the risk of intra‐operative low heart rate (bradycardia) and low blood pressure (hypotension). These complications should be weighed with any benefit when considering the use of beta‐blockers in this population.
Quality of the evidence
Study quality was good for both trials. One trial did not adequately describe their randomisation techniques and the other trial did not report whether the outcome assessors were blinded to the treatment group, and was possibly underpowered. With only two studies included, several of the outcomes only had data from a single study, and neither of the studies reported on blockage or obstruction of blood vessels (vascular patency/graft occlusion), reducing the quality of evidence to moderate.
Background
Description of the condition
Non‐cardiac, vascular surgery includes a broad range of surgical types. These include, but are not limited to, bypass, angioplasty and stenting, aortic aneurysm repair, amputation, reconstruction of deep vein occlusion, dialysis access and treatment for thoracic outlet syndrome. Vascular surgery is associated with a high risk of perioperative and postoperative morbidity and mortality due to cardiovascular complications, largely because many people undergoing vascular surgery are at risk of coronary artery disease (ESC/ESA 2014). After aortic aneurysm repair, cardiac complications occur in 2.8% of people treated endovascularly and as high as 7.8% of people treated by open repair (Nowygrod 2006). Two studies found that perioperative myocardial infarction could range from 5% to as high as 24% (Landesberg 2003; Le Manach 2005). People with severe symptoms of peripheral arterial disease often require vascular surgery to manage their progressing disease. Using modelling methods, it is estimated that within European Union member states there are annually at least 167,000 cardiac complications due to non‐cardiac surgeries, with 19,000 being life‐threatening (ESC/ESA 2014). These numbers are expected to increase as the population ages and the number of cardiovascular risk factors also increases within this population. To try to manage this increase in cardiac risk the American College of Cardiology/American Heart Association has developed a task force to address risk evaluation of people receiving non‐cardiac surgery, in an attempt to reduce cardiac complications (Fleisher 2007), and the European Society of Cardiology and European Society of Anaesthesiology have put out joint guidelines for healthcare workers to offer best management strategies for this patient population (ESC/ESA 2014).
Description of the intervention
Beta‐adrenergic receptor blockers (beta‐blockers) are a class of medications taken orally or intravenously, that are used in the treatment of many forms of heart conditions, as well as suggested for migraines and muscle tremors. Beta‐adrenergic blockers have the ability to bind to adrenergic receptors meant for the catecholamines noradrenaline (norepinephrine) and adrenaline (epinephrine), which would normally act to increase the body's ability to withstand stress, by increasing the heart rate, heart muscle contraction, and blood pressure, and allowing more oxygen to reach the lungs (Frishman 2003). However, when a beta‐adrenergic blocker binds to the adrenergic receptor, the catecholamines cannot bind and there is a general slowing of the heart rate and reduced blood pressure. Adrenergic receptors are found in the heart, blood vessels, lungs and brain, and the various types of beta‐adrenergic blockers on the market will act comparatively on the heart, but with varying effects on the blood vessels and lungs (Frishman 2003).
How the intervention might work
Drugs that block beta‐adrenergic receptors have been established as a therapeutic intervention in prevention of cardiac complications in people with acute myocardial infarction, silent cardiac ischaemia, and heart failure. It has been proposed that beta‐adrenergic blockers reduce the risk of perioperative cardiac complications by slowing heart rate, decreasing blood pressure, and moderating haemodynamic stress responses (Frishman 2003). Doing this reduces the amount of oxygen consumed by the heart, which results in longer diastolic filling and decreased myocardial contractility (ESC/ESA 2014).
Why it is important to do this review
With an increase in the ageing population, the need for vascular surgery will increase, as will the risk of cardiac complications, as coronary artery disease is also on the rise. Treatments to reduce cardiac risk during vascular surgery are needed in order to treat people effectively and safely. One trial published in 1996, which evaluated perioperative beta‐adrenergic blockade in people considered at high risk of coronary artery disease and undergoing major surgical procedures, found reduced all‐cause mortality in the participants who received beta‐adrenergic blockade. This reduction was primarily due to a decrease in cardiovascular mortality in the group receiving beta‐adrenergic blockade in the first six to eight months after surgery (Mangano 1996). A more recent study also showed a decrease in composite 30‐day death, myocardial infarction and non‐fatal cardiac arrest, but the large decrease in non‐fatal myocardial infarction was offset by an increase in deaths in the beta‐adrenergic blocker group (POISE Trial 2006). Another study showed similar incidence of combined death, myocardial infarction, unstable angina, and heart failure at 30 days between the treatment groups, although it should be kept in mind that the risk factor profile of this trial was different (Juul 2006). These studies included participants undergoing all types of major surgery, and not specifically vascular surgery. It cannot currently be determined what results would be seen in people undergoing vascular surgery alone.
A large body of evidence evaluating perioperative beta‐adrenergic blockade in people undergoing vascular surgery came from a line of studies known as DECREASE 2010 (Dutch Echo‐cardiographic Cardiac Risk Evaluation Applying Stress Echocardiography). The primary investigator of the studies, Don Poldermans, has been removed from his position as the head of perioperative cardiac care at Erasmus Medical Centre in Rotterdam, due to scientific misconduct. After investigation, several of the DECREASE 2010 studies have been found to have untrustworthy findings due to breaches in protocol, and possible falsifications of data. The lack of integrity of the findings of these studies, which predominantly found beta‐adrenergic blockers to have positive effects on morbidity and mortality, has made understanding the role beta‐adrenergic blockers play in people undergoing vascular surgery confusing for both clinicians and researchers. Priebe 2014 highlighted this confusion in a commentary. Guidelines established by the American College of Cardiology Foundation/American Heart Association and the European Society of Cardiology are currently being re‐drafted in light of the concerns with the DECREASE 2010 trials as well as other recent findings (ESC 2013; ESC 2014). Erasmus Medical Center published a full copy of the report regarding the DECREASE 2010 studies (Erasmus MC 2012http://www.erasmusmc.nl/5663/135857/3675250/3706798/Integrity_report_2012‐10.pdf?lang=en). In consideration of these uncertainties, we plan to review the current literature and assess the overall efficacy and safety of perioperative beta‐adrenergic blockers in reducing cardiovascular morbidity and mortality in people undergoing major non‐cardiac vascular surgery.
Objectives
To review the efficacy and safety of perioperative beta‐adrenergic blockade in reducing cardiac or all‐cause mortality, myocardial infarction, and other cardiovascular safety outcomes in people undergoing major non‐cardiac vascular surgery.
Methods
Criteria for considering studies for this review
Types of studies
Prospective, randomised controlled trials of perioperative beta‐adrenergic blockade.
Types of participants
Adults over 18 years of age undergoing non‐cardiac vascular surgery.
Types of interventions
Intervention: perioperative beta‐adrenergic blockers of any dose, titration, duration and mode of administration.
Control: placebo or no treatment.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Cardiovascular mortality.
30‐day postoperative non‐fatal and fatal myocardial infarction.
Secondary outcomes
Arrhythmia (any variation from the normal rhythm of the heart beat) requiring treatment.
Heart failure.
Vascular patency/graft occlusion.
Stroke.
Composite 30‐day cardiovascular outcomes.
Intra‐operative bradycardia.
Intra‐operative hypotension.
Re‐hospitalisation at 30 days.
Search methods for identification of studies
We applied no language restrictions.
Electronic searches
The Cochrane Peripheral Vascular Diseases Group Trials Search Co‐ordinator (TSC) searched the Specialised Register (last searched January 2014) and the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 12, part of The Cochrane Library (www.thecochranelibrary.com). See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library (www.thecochranelibrary.com).
The TSC searched the following trial databases for details of ongoing and unpublished studies using the terms vascular surgery and beta:
World Health Organization (WHO) International Clinical Trials Registry (apps.who.int/trialsearch/);
ClinicalTrials.gov (clinicaltrials.gov/);
ISRCTN register (/www.controlled‐trials.com/isrctn/).
Searching other resources
We reviewed the reference lists of all included studies for further relevant studies.
Data collection and analysis
Selection of studies
Two review authors (KM and RB) independently used selection criteria to assess titles and abstracts of all studies identified in the search. We reviewed full references where necessary. We included studies meeting the inclusion criteria and documented reasons for exclusion of any article. We addressed disagreement of study selection through discussion.
Data extraction and management
Two review authors (KM and RB) independently processed articles that met the inclusion criteria for data extraction. We checked results for consistency using a prepared data extraction form. We extracted the following details from the included studies: number of participants in each arm, participant characteristics (age, gender, and cardiovascular risk factors), type of vascular surgery, specific beta‐adrenergic blocker used (including dose and duration), study outcomes, adverse effects reported, and length of trial follow‐up. We resolved any disagreements through discussion.
Assessment of risk of bias in included studies
Risk of bias was assessed by RB and MS according to The Cochrane Collaboration 'Risk of bias' tool (Higgins 2011). This tool consists of six domains of bias: selection bias, performance bias, detection bias, attrition bias, reporting bias and other potential sources of bias. We allocated scores of high risk and low risk of bias according to the guidelines described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If insufficient information was available to allocate high or low risk, we deemed the study to be of unclear risk of bias. We resolved any disagreements through discussion.
Measures of treatment effect
As all primary and secondary outcomes are dichotomous, we expressed data as odds ratio (ORs) with 95% confidence intervals (CIs) using a fixed‐effect model. However, if the test for heterogeneity yielded an I2 statistic greater than 50%, we used a random‐effects model.
Unit of analysis issues
Due to the nature of the condition and intervention, we considered only randomised participants for inclusion in analysis.
Dealing with missing data
We based quantitative analysis of outcomes on an intention‐to‐treat basis, using all participants randomised. If data on drop‐outs, withdrawals or other missing data were not reported, we attempted to contact study authors.
Assessment of heterogeneity
A test for heterogeneity examines the null hypothesis that all studies are evaluating the same effect. We obtained P values comparing the test statistic with a Chi2 distribution. To help readers assess the consistency of results of studies in a meta‐analysis Review Manager 5 software includes a method (I2 statistic) that describes the percentage of total variation across studies due to heterogeneity above what is expected by chance (RevMan 2012). A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity (Higgins 2003). We analysed data with more than 50% heterogeneity using a random‐effects model to account for issues of heterogeneity.
Assessment of reporting biases
To assess reporting bias, we planned to construct funnel plots for meta‐analyses with sufficient number of trials included (more than 10) (Higgins 2011). As only two studies were included, we did not evaluate reporting bias.
Data synthesis
We planned to perform meta‐analyses for each primary and secondary outcome using fixed‐effect models. However, if the test for heterogeneity yielded an I2 statistic greater than 50%, we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
Where possible, we planned to analyse subgroups based on type of surgery, vascular risk of study population, duration of drug therapy, and intensity of drug therapy. However, neither of the two included studies provided outcome data based on these characteristics, therefore, we could not perform subgroup analysis.
Sensitivity analysis
If sufficient trials were available, we planned to conduct sensitivity analysis by excluding studies at high risk of bias. However, as we included only two studies, both of good quality, in the analysis, we could not perform sensitivity analysis.
Results
Description of studies
Results of the search
See Figure 1. Two studies (four records) met the review inclusion criteria (MaVS 2006; POBBLE 2005). We excluded 24 references from nine studies (DECREASE 2010; Duranay 2010; Juul 2006; Mangano 1996; POISE Trial 2006; Ralley 1988; Stone 1988; Suttner 2009; Zaugg 1999).
1.
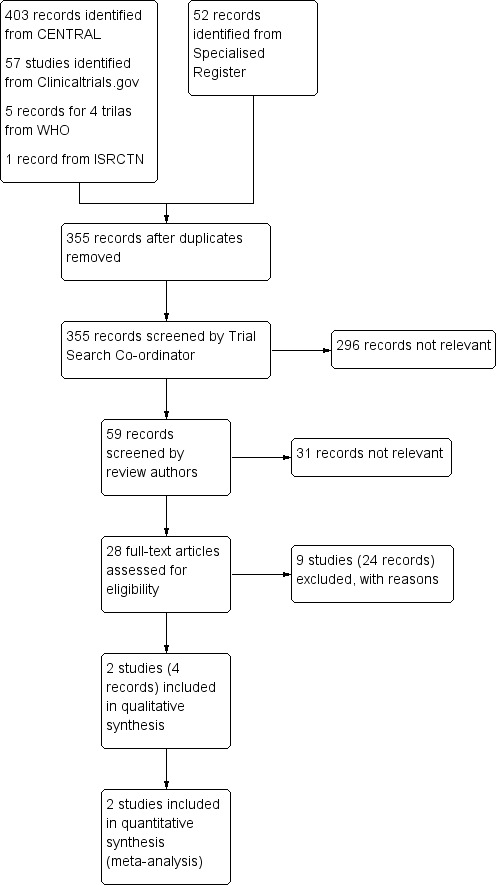
Study flow diagram.
Included studies
See Characteristics of included studies for more details.
We identified two double‐blind, randomised controlled trials that fulfilled our eligibility criteria (MaVS 2006; POBBLE 2005). A total of 599 participants were randomised to receive either perioperative beta‐adrenergic blockade or placebo; the MaVS 2006 study included 496 participants while the POBBLE 2005 study included 103. The POBBLE 2005 study excluded participants with the highest cardiovascular risk, such as people who had had a previous myocardial infarction. The MaVS 2006 study had more lenient criteria. The type of non‐cardiac vascular surgery varied between the two trials and included aortic aneurysm repair, aorto‐iliac grafts for stenosis, femo‐femoral cross‐over grafts, femoro‐popliteal bypass, femoro‐distal bypass, amputation, infrainguinal re‐vascularisation and axillofemoral re‐vascularisation.
The MaVS 2006 study followed a protocol with the beta‐adrenergic blocker metoprolol, using a weight‐based dosage: participants weighing 75 kg or greater received a dosage of 100 mg, participants weighing between 40 and 70 kg received 50 mg, and participants weighing 40 kg or less received 25 mg. The study medication was given orally (twice daily) or intravenously (every six hours) beginning two hours pre‐operatively until hospital discharge, or a maximum of five days postoperatively. The POBBLE 2005 study followed a different protocol, also with metoprolol, which was given in 2 to 4 mg intravenous injections beginning five to 10 minutes before intubation, followed by 50 mg twice daily (orally) for seven days after surgery. All participants in the POBBLE 2005 study underwent a test dosage of metoprolol prior to surgery to make sure the drug was well tolerated. Both studies were double‐blinded, and, therefore, used a placebo regimen that matched that of metoprolol.
The reported outcomes in the MaVS 2006 study were non‐fatal myocardial infarction, unstable angina, new congestive heart failure, new atrial or ventricular dysrhythmia requiring treatment, or cardiac death. The POBBLE 2005 study identified outcomes of interest as fatal and non‐fatal cardiovascular events within 30 days of surgery, myocardial ischaemia per 24 hours, length of postoperative hospital stay, and two‐year survival. Both studies included a composite 30‐day cardiovascular event outcome with the MaVS 2006 study combining cardiac death, non‐fatal myocardial infarction, congestive heart failure, unstable angina, and dysrhythmia requiring treatment, and the POBBLE 2005 study combining fatal and non‐fatal myocardial infarction, unstable angina, stroke, and ventricular tachycardia.
Excluded studies
See Characteristics of excluded studies for more details.
We excluded nine studies (DECREASE 2010; Duranay 2010; Juul 2006; Mangano 1996; POISE Trial 2006; Ralley 1988; Stone 1988; Suttner 2009; Zaugg 1999). We excluded the DECREASE 2010 studies due to concerns with integrity of the data and collection method. The Erasmus Medical Center produced a full report on the DECREASE 2010 studies (Erasmus MC 2012) and can be found at this link: http://www.erasmusmc.nl/5663/135857/3675250/3706798/Integrity_report_2012‐10.pdf?lang=en. We excluded five studies because the outcomes are not currently within the scope of this review (Duranay 2010; Ralley 1988; Stone 1988; Suttner 2009; Zaugg 1999). We excluded three studies because their study population included people undergoing other types of surgery, not just vascular, and subgroup data for people undergoing vascular surgery could not be extracted separately (Juul 2006; Mangano 1996; POISE Trial 2006). We contacted the authors of these three studies for outcome data specific to people undergoing vascular surgery.
Risk of bias in included studies
2.
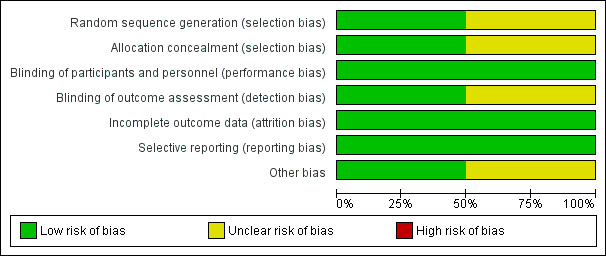
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Allocation bias for the MaVS 2006 study was unclear, as the randomisation sequence generation method was not fully clarified and allocation concealment was not addressed. The POBBLE 2005 study was at low risk of allocation bias as both randomisation sequence generation and allocation concealment were managed through a dedicated central website.
Blinding
Blinding of participants and personnel was adequate in both studies, with the use of identical placebos. In the POBBLE 2005 study, the anaesthesiologists were not blinded to the study drug, for safety reasons, but precautions were taken to ensure only the anaesthesiologists knew of the study medication, and no other carer or investigator. Blinding of assessors was adequate in the MaVS 2006 study, as a blinded adjudication committee was utilised;this was not adequately discussed in the POBBLE 2005 study, putting it an unclear risk of detection bias.
Incomplete outcome data
Attrition bias was low for both studies because they adequately reported participant drop‐out rates and reasons, with similar rates occurring between groups in both studies.
Selective reporting
Both the MaVS 2006 and POBBLE 2005 studies had low risk of reporting bias because all pre‐specified outcomes were reported on.
Other potential sources of bias
The MaVS 2006 study did not appear to have any other sources of bias, but the POBBLE 2005 study had unclear risk of other bias, as their power calculation determined a need of 300 participants in their study population, but they only recruited 103, leaving the study possibly underpowered.
Effects of interventions
Beta‐adrenergic blocker versus placebo or no treatment
All‐cause mortality
Both the MaVS 2006 and POBBLE 2005 studies reported all‐cause mortality for the comparison of beta‐adrenergic blockers and placebo. Due to heterogeneity, we used a random‐effects model (see Data synthesis), which found no clear difference between the two interventions (OR 0.62, 95% CI 0.03 to 15.02; P value = 0.77; I2 = 66%; n = 599). However, the CI is very wide, making any conclusion for the association difficult to make.
Cardiovascular mortality
Cardiovascular mortality was only evaluated in the MaVS 2006 study, and there was a single event in the placebo group, so there was no clear evidence that beta‐adrenergic blockers reduced cardiovascular mortality (OR 0.34, 95% CI 0.01 to 8.32; n = 496). No overall association could be determined from the model as only one study was included.
Non‐fatal myocardial infarction
Both the MaVS 2006 and POBBLE 2005 studies evaluated non‐fatal myocardial infarction, and the fixed‐effect model found no difference between the two treatment groups(OR 0.83, 95% 0.46 to 1.49; P value = 0.53; n = 599).
Arrhythmia
Only the MaVS 2006 recorded arrhythmia, so no overall association could be determined. There was no clear evidence that perioperative beta‐adrenergic blockers reduced arrhythmia (OR 0.70, 95% CI 0.26 to 1.88; n = 496).
Heart failure
Only the MaVS 2006 recorded heart failure, and there was no clear evidence to support beta‐adrenergic blockers reducing heart failure (OR 1.71, 95% CI 0.40 to 7.23; n = 496).
Vascular patency/graft occlusion
Neither of the included studies evaluated vascular patency/graft occlusion.
Stroke
The POBBLE 2005 study evaluated stroke, which found an OR of 2.67 (95% CI 0.11 to 67.08; n = 103), but only one event was recorded (in the beta‐adrenergic blocker group) so the CI is very wide and uninformative. No overall association could be derived from the single study.
Composite 30‐day cardiovascular outcomes
Th MaVS 2006 and POBBLE 2005 studies evaluated a composite of cardiovascular outcomes at 30 days. There was no statistically significant difference between beta‐adrenergic blockers and placebo (OR of 0.87, 95% CI 0.55 to 1.39; P value = 0.57; n = 599). It should be noted that although both studies were using a similar definition for a composite cardiovascular outcome, the two studies had slight differences in which events made up this outcome; this should be kept in mind when interpreting the findings.
Intra‐operative bradycardia
MaVS 2006 and POBBLE 2005 evaluated intra‐operative bradycardia, and the fixed‐effect model found a strong, statistically significant increase in the odds of intra‐operative bradycardia in the beta‐adrenergic blocker group compared with placebo(OR 4.97, 95% CI 3.22 to 7.65; P value < 0.00001; n = 599).
Intra‐operative hypotension
MaVS 2006 and POBBLE 2005 measured episodes of intra‐operative hypotension. The fixed‐effect model found a statistically significant increase in the odds of experiencing hypotension in the beta‐adrenergic blocker group compared with placebo (OR 1.84, 95% CI 1.31 to 2.59; P value = 0.0005; n = 599).
Re‐hospitalisation at 30 days
Only the MaVS 2006 study evaluated re‐hospitalisation at 30 days, and found no clear evidence supporting reduced re‐hospitalisation in the beta‐adrenergic blocker group (OR 0.86, 95% CI 0.48 to 1.52; n = 496).
Discussion
Summary of main results
We identified only two studies that met the eligibility criteria for inclusion in the review (MaVS 2006; POBBLE 2005). Both studies were double‐blind, randomised controlled trials comparing perioperative beta‐adrenergic blockade (metoprolol) with placebo on cardiovascular outcomes in people undergoing non‐cardiac vascular surgery. Combined, there were 599 participants included in this review; 301 received beta‐adrenergic blockers and 298 received placebo. The results give no clear evidence that perioperative beta‐adrenergic blockade reduces all‐cause mortality, cardiovascular mortality, or cardiovascular events. There was strong evidence that perioperative beta‐adrenergic blockers increase the odds of intra‐operative bradycardia and hypotension. For many of the outcomes, there were very few data points with data for some outcomes from only one study. This resulted in wide CIs for several outcomes, and unclear interpretation of the results. We conclude that, based on the current level of evidence, there is insufficient data to understand fully the effects of routine perioperative beta‐adrenergic blockade in people undergoing major non‐cardiac vascular surgery.
Overall completeness and applicability of evidence
The two studies included in this review offered relevant evidence to the review question. However, with only two studies, there was not enough data to address the objectives of the review sufficiently. The addition of relevant data was limited by study design, as several other studies addressing the same study question, but in a heterogeneous population of people undergoing major surgery, did not make outcome data available specific to people undergoing vascular surgery. There were differences between the two studies, with the POBBLE 2005 study population selected to have a lower cardiovascular risk, which was not done in the MaVS 2006 study. In addition, the composite cardiovascular events reported in the two studies were compiled of slightly different events. This review did not evaluate dosage or pre‐treatment of beta‐adrenergic blockers, and neither of the included studies titrated the beta‐adrenergic blocker to a target heart rate. In addition, as both included studies evaluated metoprolol, the current evidence is only specific to this medication.
Guidelines established in 2009 from the American College of Cardiology Foundation and American Heart Association (ACCF/AHA) recommend beta‐adrenergic blockers for people undergoing vascular surgery who are at a higher cardiac risk, but are uncertain to recommend beta‐adrenergic blockers in people undergoing vascular surgery with low or no cardiac risk (ACCF/AHA 2009). However, due to the uncertainty that has occurred after the controversies with the DECREASE 2010 trials became apparent (Erasmus MC 2012 : http://www.erasmusmc.nl/5663/135857/3675250/3706798/Integrity_report_2012‐10.pdf?lang=en), as well as other recent findings, the ACCF/AHA and the European Society of Cardiology (ESC) have published a joint statement that new guidelines are being constructed. In the interim, the recommendation is "initiation of beta blockers in patients who will undergo non‐cardiac surgery should not be considered routine, but should be considered carefully by each patient's treating physician on a case‐by‐case basis" (ESC 2013; ESC 2014).
Quality of the evidence
The quality of reporting in both studies was good. The MaVS 2006 study did not report how their randomisation sequence was generated or how they maintained allocation concealment, but was otherwise at low risk of bias. The quality of the POBBLE 2005 study was only limited by unclear reporting bias, as there were no two‐year data reported, as indicated by the study authors, and a possibly underpowered study population. However, with only two studies, totalling 599 randomised participants, differences in outcome reporting, and not all outcomes being reported on, the quality of the evidence was moderate, and not currently robust enough to draw any conclusions on whether perioperative beta‐adrenergic blockade reduces cardiovascular risk in people undergoing non‐cardiac vascular surgery.
Potential biases in the review process
Two review authors independently performed study selection, quality assessment and data extraction in order to reduce any bias. It was the choice of the review authors not to include the DECREASE 2010 studies due to the uncertainties of the integrity of the data. In addition, we could not use three excluded studies because they did not publish subgroup analysis of the outcomes of people who underwent vascular surgery. We attempted to contact study authors for this data, but received no response. While every attempt was made to identify any relevant studies, there is the possibility that we overlooked unpublished studies.
Agreements and disagreements with other studies or reviews
While there are several reviews that have evaluated cardiovascular outcomes comparing perioperative beta‐adrenergic blockade usage with a control in people undergoing major non‐cardiac surgery, this is the first review, to our knowledge, evaluating this comparison on people undergoing vascular surgery, using only prospective, randomised controlled trials.
One review conducted in 2010 evaluated perioperative beta‐adrenergic blockade in people undergoing vascular surgery, but the authors included retrospective and observational studies, in addition to randomised controlled trials, including the two trials included in our review (Brooke 2010). This review also included data from the DECREASE 2010 studies, as it was published before the controversy with its principal investigator. Meta‐analysis was not utilised as the outcomes of the included studies varied widely, and, therefore, the results were evaluated through narrative synthesis. The authors concluded that perioperative beta‐adrenergic blockade usage lowers the risk of myocardial infarction and cardiovascular death, but that the risk of adverse events, such as bradycardia, is increased. These conclusions were not drawn through quantitative evaluation, and it should be noted that the only two studies that met inclusion criteria for our own review showed no benefit of beta‐adrenergic blockers on cardiovascular events, whereas the remaining eight studies showed a benefit of beta‐adrenergic blockade, but for differing cardiac outcomes. The authors of the Brooke 2010 review also used studies evaluating people undergoing major surgery, not specific to vascular surgery, to draw their conclusions, making their findings not actually specific to people undergoing vascular surgery. The findings of our meta‐analyses do not currently agree with this review, as we found no clear evidence suggesting reduced cardiovascular risk from the use of perioperative beta‐adrenergic blockers in people undergoing vascular surgery.
Authors' conclusions
Implications for practice.
It is currently unclear whether perioperative beta‐adrenergic blockers reduce cardiac complications in people undergoing non‐cardiac vascular surgery. This review indicates that beta‐adrenergic blockers increase the risk of intra‐operative bradycardia and hypotension, which should be weighed against any possible benefit.
Implications for research.
There is a clear need for additional randomised controlled trials to evaluate the effects of perioperative beta‐adrenergic blockade in people undergoing non‐cardiac vascular surgery. Future research should aim to investigate pre‐treatment, dosage, and duration of beta‐adrenergic blockade, as these currently vary between studies. In addition, of great importance would be the evaluation of perioperative beta‐adrenergic blockade usage based on cardiovascular risk. There are already several large trials that evaluated the comparison of interest in people undergoing major, non‐cardiac surgery, that included vascular surgery, and if the study authors of these trials made the outcome data specific to people undergoing vascular surgery available, this would greatly increase relevant data available for this population.
History
Protocol first published: Issue 1, 2007 Review first published: Issue 1, 2015
| Date | Event | Description |
|---|---|---|
| 26 August 2009 | Amended | Converted to new review format. |
| 14 November 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors would like to thank Marlene Stewart for assistance with risk of bias and the Peripheral Vascular Diseases Review Group for the guidance during the review process.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Vascular Surgical Procedures] explode all trees | 12701 |
| #2 | MeSH descriptor: [Reconstructive Surgical Procedures] this term only | 623 |
| #3 | MeSH descriptor: [Arteries] explode all trees and with qualifier(s): [Surgery ‐ SU] | 1088 |
| #4 | MeSH descriptor: [Veins] explode all trees and with qualifier(s): [Surgery ‐ SU] | 714 |
| #5 | (vascular or prosthetic or arterial or aort* or (lower near/3 limb) or vein or venous) near/3 (graft or reconstruct* or surgery or bypass or revascular*) | 7485 |
| #6 | MeSH descriptor: [Peripheral Vascular Diseases] explode all trees and with qualifier(s): [Surgery ‐ SU] | 133 |
| #7 | MeSH descriptor: [Vascular Diseases] this term only and with qualifier(s): [Surgery ‐ SU] | 29 |
| #8 | MeSH descriptor: [Varicose Veins] explode all trees and with qualifier(s): [Surgery ‐ SU] | 250 |
| #9 | MeSH descriptor: [Telangiectasis] explode all trees and with qualifier(s): [Surgery ‐ SU] | 13 |
| #10 | MeSH descriptor: [Venous Insufficiency] explode all trees and with qualifier(s): [Surgery ‐ SU] | 76 |
| #11 | MeSH descriptor: [Arterial Occlusive Diseases] this term only and with qualifier(s): [Surgery ‐ SU] | 228 |
| #12 | MeSH descriptor: [Arteriolosclerosis] explode all trees and with qualifier(s): [Surgery ‐ SU] | 0 |
| #13 | MeSH descriptor: [Arteriosclerosis Obliterans] explode all trees and with qualifier(s): [Surgery ‐ SU] | 8 |
| #14 | MeSH descriptor: [Atherosclerosis] explode all trees and with qualifier(s): [Surgery ‐ SU] | 37 |
| #15 | MeSH descriptor: [Intermittent Claudication] explode all trees and with qualifier(s): [Surgery ‐ SU] | 61 |
| #16 | MeSH descriptor: [Aortic Diseases] explode all trees and with qualifier(s): [Surgery ‐ SU] | 655 |
| #17 | MeSH descriptor: [Aortic Aneurysm] explode all trees and with qualifier(s): [Surgery ‐ SU] | 590 |
| #18 | MeSH descriptor: [Iliac Aneurysm] explode all trees and with qualifier(s): [Surgery ‐ SU] | 10 |
| #19 | vascular near/2 surg* | 3086 |
| #20 | (non‐cardiac or noncardiac) near/2 surg* | 304 |
| #21 | ((abdominal near/3 aneury*) near/3 (graft or surg*)) | 573 |
| #22 | ((lower near/3 extrem*) near/4 (obstruct* or occlus* or steno* or block* or obliter*) and (surgery or graft or bypass or revascula* or reconstruct*)) | 94 |
| #23 | ((leg or limb) near/4 (obstruct* or occlus* or steno* or block* or obliter*) and (surgery or graft or bypass or revascula* or reconstruct*)) | 259 |
| #24 | (aort* or iliac or femoral or popliteal or femoropop* or fempop* or crural) near/3 (surg* or bypass or graft or reconstruct* or revascular*) | 3327 |
| #25 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #21 or #22 or #23 or #24 | 19560 |
| #26 | MeSH descriptor: [Adrenergic beta‐Antagonists] explode all trees | 4188 |
| #27 | (adrenergic near/3 (antagonist* or block*)) | 7418 |
| #28 | (betablocker* or beta‐blocker*) | 4910 |
| #29 | (beta* near/3 block*) | 8572 |
| #30 | acebutolol or atenolol or Tenormin or alprenolol | 3406 |
| #31 | betaxolol or bisoprolol or bupranolol | 1018 |
| #32 | carvedilol or Coreg or carteolol or celiprolol | 1227 |
| #33 | esmolol or labetalol or Normodyne or Trandate | 1004 |
| #34 | metoprolol or nadolol or nebivolol | 3075 |
| #35 | oxprenolol or penbutolol or pindolol | 1329 |
| #36 | Visken or practolol or propranolol or Inderal | 4564 |
| #37 | sotalol or timolol | 2269 |
| #38 | *lol | 15045 |
| #39 | #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 | 19860 |
| #40 | #25 and #39 in Trials | 403 |
Data and analyses
Comparison 1. Beta‐adrenergic blocker versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | 599 | Odds Ratio (M‐H, Random, 95% CI) | 0.62 [0.03, 15.02] |
| 2 Cardiovascular mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Non‐fatal myocardial infarction | 2 | 599 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.46, 1.49] |
| 4 Arrhythmia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Heart failure | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Stroke | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Composite cardiovascular events | 2 | 599 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.39] |
| 8 Intra‐operative bradycardia | 2 | 599 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.97 [3.22, 7.65] |
| 9 Intra‐operative hypotension | 2 | 599 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.31, 2.59] |
| 10 Re‐hospitalisation at 30 days | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
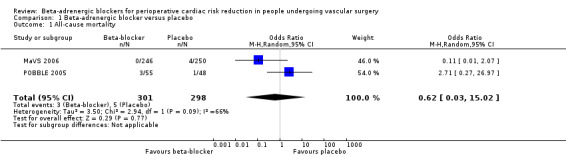
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 1 All‐cause mortality.
1.2. Analysis.
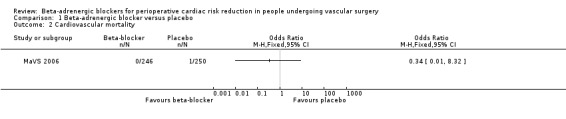
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 2 Cardiovascular mortality.
1.3. Analysis.
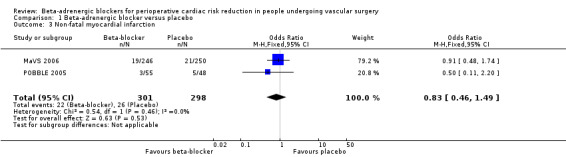
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 3 Non‐fatal myocardial infarction.
1.4. Analysis.
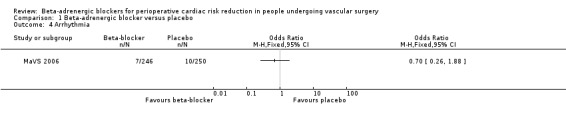
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 4 Arrhythmia.
1.5. Analysis.
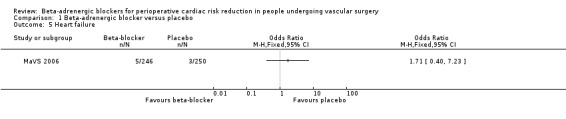
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 5 Heart failure.
1.6. Analysis.
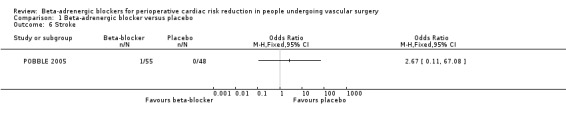
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 6 Stroke.
1.7. Analysis.
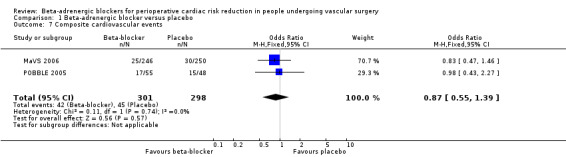
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 7 Composite cardiovascular events.
1.8. Analysis.
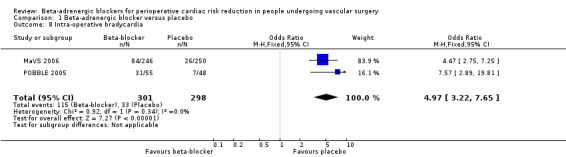
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 8 Intra‐operative bradycardia.
1.9. Analysis.
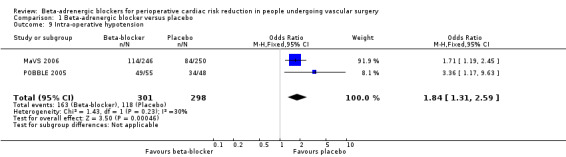
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 9 Intra‐operative hypotension.
1.10. Analysis.
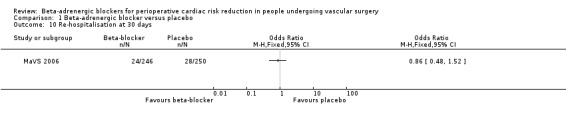
Comparison 1 Beta‐adrenergic blocker versus placebo, Outcome 10 Re‐hospitalisation at 30 days.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
MaVS 2006.
| Methods | Study type: double‐blind, randomised controlled trial Study aim: to investigate the effects of metoprolol and its effects on cardiac complications at 30 days and 6 months after vascular surgery Country: Canada Setting: multicentre; 3 tertiary care centres |
|
| Participants | Number randomised: total n = 496 (metoprolol n = 246; placebo n = 250) Age (mean (SD)): metoprolol = 66.4 (10.0) years; placebo = 65.9 (10.0) years Gender (M/F): metoprolol = 193/53; placebo = 184/66 Inclusion criteria: American Society of Anesthesiology class III or less; undergoing abdominal aortic surgery and infrainguinal or axillofemoral re‐vascularisation Exclusion criteria: current or recent beta‐adrenergic blocker use; current amiodarone use; airflow obstruction requiring treatment; history of CHF; history of atrioventricular block; previous adverse drug reactions to beta‐adrenergic blockers; previous participation in the MaVS study CVD risk factors: prior MI: metoprolol = 15.0%, placebo = 12.0%; angina: metoprolol = 7.3%, placebo = 10.0%; diabetes mellitus on treatment: metoprolol = 22.0%, placebo = 14.8%; permanent pacemaker: metoprolol = 0%, placebo = 0.4% Type of vascular surgery: abdominal aortic surgery, infrainguinal or axillofemoral re‐vascularisation |
|
| Interventions | Treatment: metoprolol; participants weighing ≥ 75 kg received 100 mg, between 40 and 75 kg received 50 mg and ≤ 40 kg received 25 mg, oral (twice daily) or intravenous route (every 6 hours) Control: placebo; participants weighing ≥ 75 kg received 100 mg, 40‐75 kg received 50 mg and ≤ 40 kg received 25 mg, oral or intravenous route Duration: 2 hours preoperatively until hospital discharge or maximum of 5 days postoperatively |
|
| Outcomes | Postoperative 30‐day composite incidence of non‐fatal MI, unstable angina, new CHF, new atrial or ventricular dysrhythmia requiring treatment or cardiac death The same composite outcome was evaluated over 6 months, but it should be noted that the outcome data between 1 and 6 months could not be used in the meta‐analysis as the data were presented graphically with no numerical data |
|
| Notes | It should be noted that Figure 1 (of Yang 2006), which is a flow diagram of patient screening indicates that 497 participants were randomised (metoprolol n = 247; placebo n = 250), but as the n = 496 was used twice later in the text, we assumed this is the correct number randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was constructed in blocks of 4 by the study statistician…" Comment: not enough information to determine adequate sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient description of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients, investigators, and all caretakers were blinded to the study randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent adjudication committee reviewed all primary composite outcomes", ...."in a blinded fashion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Discontinuation was similar between treatment groups, and reasons were also similar |
| Selective reporting (reporting bias) | Low risk | All outcomes indicated in methods appear to be reported on |
| Other bias | Low risk | No indication of other bias |
POBBLE 2005.
| Methods | Study type: double‐blind, randomised placebo‐controlled trial Study aim: to assess whether a policy of perioperative beta‐adrenergic blockade with metoprolol reduced 30‐day CV morbidity and mortality and reduced the length of hospital stay in people undergoing infrarenal vascular surgery Country: UK Setting: multicentre, vascular surgical units in 4 hospitals |
|
| Participants | Number randomised: total n = 103 (metoprolol n = 55; placebo n = 48) Age (median (IQR)): metoprolol = 73 (61‐79) years; placebo = 74 (66‐76) years Gender (M/F): total = 80/23; metoprolol = 40/13*; placebo = 35/9*, *6 participants did not undergo the procedure Inclusion criteria: people undergoing major elective infrarenal vascular surgery under general anaesthesia Exclusion criteria: already taking beta‐adrenergic blockers; beta‐adrenergic blockers could be dangerous; receiving current treatment for asthma; had aortic stenosis; had bradycardia or hypotension; perioperative beta‐adrenergic blockade had already been shown to be beneficial; had unstable angina, or angina with positive dobutamine stress test; previous MI CVD risk factors: current/ex smoker: metoprolol = 43%/47%, placebo = 10%/30%; diabetes: metoprolol = 19%, placebo = 18% Type of vascular surgery: infrarenal; aortic aneurysm repair, aortoiliac grafts for stenosis, femofemoral cross‐over grafts, femoropopliteal bypasses, femorodistal bypasses, amputation |
|
| Interventions | Treatment: metoprolol; 2‐4 mg in a slow intravenous injection over 5‐10 minutes before intubation, then 50 mg twice daily, oral route Control: placebo; equivalent 2‐4 mg in a slow intravenous injection over 5‐10 minutes before intubation, then 50 mg twice daily, oral route Duration: 7 days after surgery |
|
| Outcomes | Fatal and non‐fatal CV events within 30 days of surgery, myocardial ischaemia per 24 hours while wearing the Holter monitor, length of postoperative hospital stay, 2‐year survival | |
| Notes | Study of average risk CV people undergoing vascular surgery, people with highest CV risk were excluded. All participants underwent a test dose of their allocated drug: metoprolol 50 mg or placebo equivalent for people weighing > 55 kg and 25 mg for people weighing ≤ 55 kg. People who did not tolerate the medication did not receive further beta‐adrenergic blockade | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization as performed centrally at TheSealedEnvelope.com web site. Treatment was allocated in a 1:1 ratio by using random permuted blocks of size 2, 4, and 6 within four stratification factors..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization as performed centrally at TheSealedEnvelope.com web site" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Trial drugs‐metoprolol and placebo‐of identical appearance were in gelatin‐coated capsules or ampoules..." Comment: study described as "double‐blind". For safety reasons the anaesthesiologists administering the study drug were unblinded, but precautions were taken to ensure remaining clinicians and trial co‐ordinators were blinded. There was no indication that blinding was broken for others involved in the trial, other than the anaesthesiologists |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study did not specify whether the outcome assessors were blinded or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate was even between treatment groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported on |
| Other bias | Unclear risk | Power calculations determined a study population of 300, but only 103 participants were recruited |
BP: blood pressure; HR: heart rate; IQR: interquartile range; CHF: congestive heart failure; CV: cardiovascular; CVD: cardiovascular disease; F: female; M: male; MI: myocardial infarction; n: number; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| DECREASE 2010 | The principal investigator, Don Poldermans, of the DECREASE studies was dismissed by Erasmus University in Rotterdam for alleged scientific misconduct that included failing to obtain written patient consent and negligent data collection. Data from these trials cannot be relied upon as valid. The Erasmus Medical Center published a full copy of the report (Erasmus MC 2012) which can be found here: http://www.erasmusmc.nl/5663/135857/3675250/3706798/Integrity_report_2012‐10.pdf?lang=en |
| Duranay 2010 | Outcomes of the study (estimated glomerular filtration rate, serum creatinine and nitrate levels) were not within the scope of this review |
| Juul 2006 | Outcome data for people undergoing vascular surgery, separately, were not available |
| Mangano 1996 | Outcome data for people undergoing vascular surgery, separately, were not available |
| POISE Trial 2006 | Outcome data for people undergoing vascular surgery, separately, were not available |
| Ralley 1988 | Outcomes of the study (haemodynamic outcomes) were not within the scope of this review |
| Stone 1988 | Outcomes of the study (myocardial ischaemia) were not within the scope of this review. If future data from the study is presented that includes outcome data relevant to our review, this study may be considered for inclusion |
| Suttner 2009 | Outcomes of the study (myocardial ischaemia) were not within the scope of this review. If future data from the study is presented that includes outcome data relevant to our review, this study may be considered for inclusion |
| Zaugg 1999 | Outcome data for people undergoing vascular surgery, separately, were not available |
Differences between protocol and review
According to updated guidelines from The Cochrane Collaboration, we assessed the methodological quality of the studies using the 'Risk of bias' tool described by Higgins 2011.
Contributions of authors
Katayoun Mostafaie reviewed articles for inclusion, assessed the quality of selected studies, extracted data and wrote the text of the review.
Rachel Bedenis reviewed articles for inclusion, assessed the quality of the selected studies, extracted data and assisted in drafting the review.
Darrell Harrington reviewed articles for inclusion, assessed the quality of selected studies, extracted data and contributed to the text of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, Scottish Government, UK.
The PVD Group editorial base is supported by the Chief Scientist Office.
Declarations of interest
KM: none known. RB: none known. DH: I have received consultancy fees from Jansenn Pharmaceutics, expert testimony fees from Massey and Associates Law Practice, Phoenix, AZ, payment for lectures from Jansenn Pharmaceuticals and American College of Physicians and travel and meeting expenses from BMS/Pfizer Pharmaceuticals and University of Massachusetts. My Institution has received grant funding from BMS/Pfizer Pharmaceuticals, National Institutes of Health and University of Massachusetts. The subject matter represented by my disclosures are limited to the field of venous thromboembolism and anticoagulation. I have no conflicts related to the current review topic.
New
References
References to studies included in this review
MaVS 2006 {published data only}
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. Metoprolol after vascular surgery. Canadian Journal of Anaesthesia 2004;51(Suppl 1):A7. [Google Scholar]
- Yang H. Raymer K. Butler R. Parlow J. Roberts R. The effects of perioperative beta‐blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. American Heart Journal 2006;152(5):983‐90. [DOI] [PubMed] [Google Scholar]
POBBLE 2005 {published data only}
- Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. POBBLE trial investigators. Perioperative beta‐blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double‐blind controlled trial. Journal of Vascular Surgery 2005;41(4):602‐9. [DOI] [PubMed] [Google Scholar]
- ISRCTN13072628. A prospective, randomised controlled trial of beta‐blockade in intermediate and high risk vascular surgery, 2002. www.controlled‐trials.com/ISRCTN13072628 (accessed 18 July 2014).
References to studies excluded from this review
DECREASE 2010 {published data only}
- Bakker EJ, Ravensbergen NJ, Voute MT, Hoeks SE, Chonchol M, Klimek M, et al. A randomised study of perioperative esmolol infusion for haemodynamic stability during major vascular surgery: rationale and design of DECREASE‐XIII. European Journal of Vascular and Endovascular Surgery 2011;42(3):317‐23. [DOI] [PubMed] [Google Scholar]
- Dunklegrun M, Boersma E, Koopman‐Van Gemert AW, Poorten F, Kalkman C, Schouten O, et al. Fluvastatin and bisoprolol for cardiac risk reduction in intermediate‐risk patients undergoing non‐cardiovascular surgery: a randomised controlled trial. European Heart Journal 2008;29(Suppl):602‐3. [Google Scholar]
- Dunklegrun M, Boersma E, Schouten O, Koopman‐Van Gemert AW, Poorten F, Bax JJ, et al. Bisoprolol and fluvastatin for the reduction of perioperative cardiac mortality and myocardial infarction in intermediate‐risk patients undergoing non‐cardiovascular surgery: a randomized controlled trial (DECREASE‐IV). Annals of Surgery 2009;249(6):921‐6. [DOI] [PubMed] [Google Scholar]
- Lier F, Schouten O, Hoeks SE, Ven L, Stolker RJ, Bax JJ, et al. Impact of prophylactic beta‐blocker therapy to prevent stroke after noncardiac surgery. American Journal of Cardiology 2010;105(1):43‐7. [DOI] [PubMed] [Google Scholar]
- Poldermans D, Boersma E, Bax JJ, Thomson IR, Paelinck B, Ven LLM, et al. Bisoprolol reduces cardiac death and myocardial infarction in high‐risk patients as long as 2 years after successful major vascular surgery. European Heart Journal 2001;22(15):1353‐8. [DOI] [PubMed] [Google Scholar]
- Poldermans D, Boersma E, Bax JJ, Thomson IR, Ven LL, Blankensteijn JD, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high‐risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. New England Journal of Medicine 1999;341(24):1789‐94. [DOI] [PubMed] [Google Scholar]
- Schouten O, Poldermans D, Visser L, Kertai MD, Klein J, Urk H, et al. Fluvastatin and bisoprolol for the reduction of perioperative cardiac mortality and morbidity in high‐risk patients undergoing non‐cardiac surgery: rationale and design of the DECREASE‐IV study. American Heart Journal 2004;148(6):1047‐52. [DOI] [PubMed] [Google Scholar]
Duranay 2010 {published data only}
- Duranay M, Kanbay M, Akay H, Unverdi S, Surer H, Altay M, et al. Nebivolol improves renal function in patients who underwent angioplasty due to renal artery stenosis: a pilot study. Nephron. Clinical Practice 2010;114(3):c213‐7. [DOI] [PubMed] [Google Scholar]
Juul 2006 {published data only}
- Juul AB, Wetterslev J, Gluud C, Kofoed‐Enevoldsen A, Jensen G, Callesen T, et al. Effect of perioperative beta‐blockade in patients with diabetes undergoing major non‐cardiac surgery: randomised placebo controlled, blinded multicentre trial. BMJ 2006;332(7556):1482‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul AB, Wetterslev J, Kofoed‐Enevoldsen A, Callesen T, Jensen G, Gluud C, et al. The Diabetic Postoperative Mortality and Morbidity (DIPOM) trial: rationale and design of a multicenter, randomized, placebo‐controlled, clinical trial of metoprolol for patients with diabetes mellitus who are undergoing major noncardiac surgery. American Heart Journal 2004;147(4):677‐83. [DOI] [PubMed] [Google Scholar]
Mangano 1996 {published data only}
- Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. New England Journal of Medicine 1996;335(23):1713‐20. [DOI] [PubMed] [Google Scholar]
POISE Trial 2006 {published and unpublished data}
- Devereaux PJ. ACCEL: The Perioperative ISchemic Evaluation (POISE) trial: a randomized controlled trial of metoprolol versus placebo in patients undergoing noncardiac surgery. ACC Cardiosource Review Journal 2008;17(5):30‐2. [DOI] [PubMed] [Google Scholar]
- Devereaux PJ. The PeriOperative ISchemic Evaluation (POISE) trial: a randomized controlled trial of metoprolol versus placebo in patients undergoing noncardiac surgery. Clinical Research in Cardiology 2008; Vol. 97, issue 1:8. [DOI] [PubMed]
- Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Annals of Internal Medicine 2011;154:523‐8. [DOI] [PubMed] [Google Scholar]
- Leslie K, Myles P, Devereaux P, Williamson E, Rao‐Melancini P, Forbes A, et al. Neuraxial block, death and serious cardiovascular morbidity in the POISE trial. British Journal of Anaesthesia 2014;111(3):382‐90. [DOI] [PubMed] [Google Scholar]
- Leslie K, Myles P, Devereaux PJ, Forbes A, Rao‐Melancini P, Williamson E, et al. Nitrous oxide and serious morbidity and mortality in the POISE trial. Anesthesia and Analgesia 2013;116(5):1034‐40. [DOI] [PubMed] [Google Scholar]
- NCT00182039. POISE Trial: PeriOperative Ischemic Evaluation Study, 2005. clinicaltrials.gov/ct2/show/NCT00182039 (accessed 27 June 2014).
- POISE Trial Investigators, Devereaux PJ, Yang H, Guyatt GH, Leslie K, Villar JC, et al. Rationale, design, and organization of the PeriOperative ISchemic Evaluation (POISE) trial: a randomized controlled trial of metoprolol versus placebo in patients undergoing noncardiac surgery. American Heart Journal 2006;152(2):223‐30. [DOI] [PubMed] [Google Scholar]
- POISE study group. Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371(9627):1839‐47. [DOI] [PubMed] [Google Scholar]
Ralley 1988 {published data only}
- Ralley FE, Ramsay JR, Wynands JE, O'Connor JP, Bilodeau J. The efficacy of esmolol in the treatment of hypertension after aortic reconstructive surgery. Canadian Journal of Anesthesia 1988;35(1 Suppl):S73‐4. [Google Scholar]
Stone 1988 {published data only}
- Stone JG, Foex P, Sear JW, Johnson LL, Khambatta HJ, Triner L. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta‐adrenergic blocking agent. Anesthesiology 1988;68(4):495‐500. [PubMed] [Google Scholar]
Suttner 2009 {published data only}
- NCT00348101. Effects of beta‐blocker therapy and phosphodiesterase inhibition on cardiac neurohormonal activation, 2007. clinicaltrials.gov/ct2/show/NCT00348101 (accessed 18 July 2014).
- Suttner S, Boldt J, Mengistu A, Lang K, Mayer J. Influence of continuous perioperative beta‐blockade in combination with phosphodiesterase inhibition on haemodynamics and myocardial ischaemia in high‐risk vascular surgery patients. British Journal of Anaesthesia 2009;102(5):597‐607. [DOI] [PubMed] [Google Scholar]
Zaugg 1999 {published data only}
- Zaugg M, Tagliente T, Lucchinetti E, Jacobs E, Krol M, Boian C, et al. Beneficial effects from beta‐adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 1999;91(6):1674‐86. [DOI] [PubMed] [Google Scholar]
Additional references
ACCF/AHA 2009
- Fleischman KE, Beckman JA, Buller CE, Calkins H, Fleisher LA, Freeman WK, et al. 2009 ACCF/AHA focused update on perioperative beta blockade: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2009;120:2123‐51. [DOI] [PubMed] [Google Scholar]
Brooke 2010
- Brooke BS. Perioperative beta‐blockers for vascular surgery patients. Journal of Vascular Surgery 2010;51:515‐9. [DOI] [PubMed] [Google Scholar]
Erasmus MC 2012
- Erasmus MC Follow‐up Investigation Committee. Report on the 2012 follow‐up investigation of possible breaches of academic integrity, 2012. www.erasmusmc.nl/5663/135857/3675250/3706798/Integrity_report_2012‐10.pdf?lang=en. (accessed 5 January 2015).
ESC 2013
- ESC Press Office. Joint statement: issued by the American College of Cardiology, American Heart Association and the European Society of Cardiology, 2013. www.escardio.org/about/press/press‐releases/pr‐13/Pages/joint‐statement‐perioperative‐guidelines.aspx (accessed 5 January 2015).
ESC 2014
- ESC Press Office. Beta‐blockers and perioperative care: European Heart Journal editorial addresses controversy, 2014. www.escardio.org/about/press/press‐releases/pr‐14/Pages/Beta‐Blockers‐Perioperative‐Care‐EHJ‐Editorial‐addresses‐controversy.aspx?hit=dontmiss (accessed 5 January 2015).
ESC/ESA 2014
- The Joint Task Force on non‐cardiac surgery. 2014 ESC/ESA Guidelines on non‐cardiac surgery: cardiovascular assessment and management. European Heart Journal 2014;35:2383‐431. [DOI] [PubMed] [Google Scholar]
Fleisher 2007
- Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof EL, Fleischmann KE, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. Journal of the American College of Cardiology 2007;50(17):e159‐242. [DOI] [PubMed] [Google Scholar]
Frishman 2003
- Frishman WH. Beta‐adrenergic blockade. Circulation 2003;107:e117‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Landesberg 2003
- Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, et al. Association of cardiac troponin, CK‐MB, and postoperative myocardial ischemia with long‐term survival after major vascular surgery. Journal of the American College of Cardiology 2003;42(9):1547‐54. [DOI] [PubMed] [Google Scholar]
Le Manach 2005
- Manach YL, Perel A, Coriat P, Godet G, Bertrand M, Riou B. Early and delayed myocardial infarction after abdominal aortic surgery. Anesthesiology 2005;102:885‐91. [DOI] [PubMed] [Google Scholar]
Nowygrod 2006
- Nowygrod R, Egorova N, Greco G, Anderson P, Gelijns A, Moskowitz A, et al. Trends, complications and mortality in peripheral vascular surgery. Journal of Vascular Surgery 2006;43(2):205‐16. [DOI] [PubMed] [Google Scholar]
Priebe 2014
- Priebe HJ. The controversy of peri‐operative B‐blockade: what should I do?. European Journal of Vascular and Endovascular Surgery 2014;47(2):119‐23. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Yang 2006
- Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta‐blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. American Heart Journal 2006;152(5):983‐90. [DOI] [PubMed] [Google Scholar]


