Abstract
The European Commission requested the EFSA Panel on Plant Health to prepare and deliver risk assessments for commodities listed in Commission Implementing Regulation (EU) 2018/2019 as ‘High‐risk plants, plant products and other objects’. This Scientific Opinion covers plant health risks posed by plants of Quercus robur imported from the UK as: (a) bundles of 1‐ to 2‐year‐old whips and seedlings, (b) 1‐ to 7‐year‐old bare root plants for planting and (c) less than 1‐ to 15‐year‐old plants in pots, taking into account the available scientific information, including the technical information provided by the UK. All pests associated with the commodity were evaluated against specific criteria for their relevance for this opinion. Two EU quarantine pests, Cronartium quercuum and Phytophthora ramorum (non‐EU isolates), two protected zone quarantine pests, Cryphonectria parasitica and Thaumetopoea processionea and four pests not regulated in the EU, Coniella castaneicola, Meloidogyne mali, Phytophthora kernoviae and Trinophylum cribratum, fulfilled all relevant criteria and were selected for further evaluation. For the selected pests, the risk mitigation measures included in the technical dossier from the UK were evaluated taking into account the possible limiting factors. For these pests an expert judgement is given on the likelihood of pest freedom taking into consideration the risk mitigation measures acting on the pest, including uncertainties associated with the assessment. In the assessment of risk, the age of the plants was considered, reasoning that older trees are more likely to be infested mainly due to longer exposure time and larger size. The degree of pest freedom varies among the pests evaluated, with C. castaneicola being the pest most frequently expected on the imported plants. The expert knowledge elicitation indicated with 95% certainty that between 9,711 and 10,000 per 10,000 less than 1‐ to 15‐year‐old plants in pots will be free from C. castaneicola.
Keywords: oak, European Union, commodity risk assessment, plant health, plant pest
1. Introduction
1.1. Background and Terms of Reference as provided by European Commission
1.1.1. Background
The Plant Health Regulation (EU) 2016/2031 1 , on the protective measures against pests of plants, has been applied from December 2019. Provisions within the above Regulation are in place for the listing of ‘high risk plants, plant products and other objects’ (Article 42) on the basis of a preliminary assessment, and to be followed by a commodity risk assessment. A list of ‘high risk plants, plant products and other objects’ has been published in Regulation (EU) 2018/2019 2 . Scientific opinions are therefore needed to support the European Commission and the Member States in the work connected to Article 42 of Regulation (EU) 2016/2031, as stipulated in the terms of reference.
1.1.2. Terms of Reference
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/2002 3 , the Commission asks EFSA to provide scientific opinions in the field of plant health.
In particular, EFSA is expected to prepare and deliver risk assessments for commodities listed in the relevant Implementing Act as ‘High risk plants, plant products and other objects’. Article 42, paragraphs 4 and 5, establishes that a risk assessment is needed as a follow‐up to evaluate whether the commodities will remain prohibited, removed from the list and additional measures will be applied or removed from the list without any additional measures. This task is expected to be on‐going, with a regular flow of dossiers being sent by the applicant required for the risk assessment.
Therefore, to facilitate the correct handling of the dossiers and the acquisition of the required data for the commodity risk assessment, a format for the submission of the required data for each dossier is needed.
Furthermore, a standard methodology for the performance of ‘commodity risk assessment’ based on the work already done by Member States and other international organizations needs to be set.
In view of the above and in accordance with Article 29 of Regulation (EC) No 178/2002, the Commission asks EFSA to provide scientific opinion in the field of plant health for Quercus robur from the United Kingdom (UK) taking into account the available scientific information, including the technical dossier provided by the UK.
1.2. Interpretation of the Terms of Reference
The EFSA Panel on Plant Health (hereafter referred to as ‘the Panel') was requested to conduct a commodity risk assessment of Quercus robur from the UK following the Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019) taking into account the available scientific information, including the technical information provided by the UK.
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Protocol on Ireland/Northern Ireland in conjunction with Annex 2 to that Protocol, for the purposes of this Opinion, references to the UK do not include Northern Ireland.
The EU quarantine pests that are regulated as a group in the Commission Implementing Regulation (EU) 2019/2072 4 were considered and evaluated separately at species level.
Annex II of Implementing Regulation (EU) 2019/2072 lists certain pests as non‐European populations or isolates or species. These pests are regulated quarantine pests. Consequently, the respective European populations, or isolates, or species are non‐regulated pests.
Annex VII of the same Regulation, in certain cases (e.g. point 32) makes reference to the following countries that are excluded from the obligation to comply with specific import requirements for those non‐European populations, or isolates, or species: Albania, Andorra, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Canary Islands, Faeroe Islands, Georgia, Iceland, Liechtenstein, Moldova, Monaco, Montenegro, North Macedonia, Norway, Russia (only the following parts: Central Federal District (Tsentralny federalny okrug), Northwestern Federal District (SeveroZapadny federalny okrug), Southern Federal District (Yuzhny federalny okrug), North Caucasian Federal District (Severo‐Kavkazsky federalny okrug) and Volga Federal District (Privolzhsky federalny okrug), San Marino, Serbia, Switzerland, Türkiye, Ukraine and the United Kingdom (except Northern Ireland 5 ).
Consequently, for those countries,
any pests identified, which are listed as non‐European species in Annex II of Implementing Regulation (EU) 2019/2072 should be investigated as any other non‐regulated pest.
any pest found in a European country that belongs to the same denomination as the pests listed as non‐European populations or isolates in Annex II of Implementing Regulation (EU) 2019/2072, should be considered as European populations or isolates and should not be considered in the assessment of those countries.
Pests listed as ‘Regulated Non‐Quarantine Pest’ (RNQP) in Annex IV of the Commission Implementing Regulation (EU) 2019/2072 and deregulated pests (i.e. pest which were listed as quarantine pests in the Council Directive 2000/29/EC and were deregulated by Commission Implementing Regulation (EU) 2019/2072) were not considered for further evaluation. In case a pest is at the same time regulated as a RNQP and as a Protected Zone Quarantine pest, in this Opinion it should be evaluated as Quarantine pest.
In its evaluation the Panel:
Checked whether the provided information in the technical dossier (hereafter referred to as ‘the Dossier’) provided by the applicant (UK, Department for Environment Food and Rural Affairs – hereafter referred to as ‘DEFRA’) was sufficient to conduct a commodity risk assessment. When necessary, additional information was requested to the applicant.
Selected the relevant Union quarantine pests and protected zone quarantine pests (as specified in Commission Implementing Regulation (EU) 2019/2072, hereafter referred to as ‘EU quarantine pests’) and other relevant pests present in the UK and associated with the commodity.
Did not assess the effectiveness of measures for Union quarantine pests for which specific measures are in place for the import of the commodity from the UK in Commission Implementing Regulation (EU) 2019/2072 and/or in the relevant legislative texts for emergency measures and if the specific country is in the scope of those emergency measures. The assessment was restricted to whether or not the applicant country implements those measures.
Assessed the effectiveness of the measures described in the Dossier for those Union quarantine pests for which no specific measures are in place for the importation of the commodity from the UK and other relevant pests present in the UK and associated with the commodity.
Risk management decisions are not within EFSA's remit. Therefore, the Panel provided a rating based on expert judgement regarding the likelihood of pest freedom for each relevant pest given the risk mitigation measures proposed by DEFRA of the UK.
2. Data and methodologies
2.1. Data provided by DEFRA of the UK
The Panel considered all the data and information (hereafter called ‘the Dossier’) provided by DEFRA of the UK in June 2022 including the additional information provided by DEFRA of the UK in January 2023, after EFSA's request. The Dossier is managed by EFSA.
The structure and overview of the Dossier is shown in Table 1. The number of the relevant section is indicated in the Opinion when referring to a specific part of the Dossier.
Table 1.
Structure and overview of the Dossier
| Dossier section | Overview of contents | Filename |
|---|---|---|
| 1.0 | Technical dossier | Quercus robur commodity information final |
| 2.0 | Pest list | Quercus_pest_list_final_checked |
| 3.0 | Additional information: answers | Quercus robur additional information 5 Jan 2023 |
| 4.0 | Additional information: distribution of Quercus robur plants | Quercus_robur_distribution (1) |
| 5.0 | Additional information: Pest details | Quercus_robur‐EFSA_pest_detail_request_Jan23 |
| 6.0 | Additional information: producers sample product list | Quercus_producers_sample_product_list |
The data and supporting information provided by DEFRA of the UK formed the basis of the commodity risk assessment. Table 2 shows the main data sources used by DEFRA of the UK to compile the Dossier (Dossier Sections 1.0 and 2.0).
Table 2.
Databases used in the literature searches by DEFRA of the UK
2.2. Literature searches performed by EFSA
Literature searches in different databases were undertaken by EFSA to complete a list of pests potentially associated with Q. robur. The following searches were combined: (i) a general search to identify pests reported on Q. robur in the databases and subsequently, (ii) a tailored search to identify whether the above pests are known to be present in the UK. The searches were run between September and November 2022. No language, date or document type restrictions were applied in the search strategy.
The Panel used the databases indicated in Table 3 to compile the list of pests associated with Q. robur. As for Web of Science, the literature search was performed using a specific, ad hoc established search string (see Appendix B). The string was run in ‘All Databases’ with no range limits for time or language filters. This is further explained in Section 2.3.2.
Table 3.
Databases used by EFSA for the compilation of the pest list associated with Quercus robur
Additional searches, limited to retrieve documents, were run when developing the Opinion. The available scientific information, including previous EFSA opinions on the relevant pests and diseases (see pest data sheets in Appendix A) and the relevant literature and legislation (e.g. Regulation (EU) 2016/2031; Commission Implementing Regulations (EU) 2018/2019; (EU) 2018/2018 and (EU) 2019/2072) were taken into account.
2.3. Methodology
When developing the Opinion, the Panel followed the EFSA Guidance on commodity risk assessment for the evaluation of high‐risk plant dossiers (EFSA PLH Panel, 2019).
In the first step, pests potentially associated with the commodity in the country of origin (EU‐quarantine pests and other pests) that may require risk mitigation measures are identified. The EU non‐quarantine pests not known to occur in the EU were selected based on evidence of their potential impact in the EU. After the first step, all the relevant pests that may need risk mitigation measures were identified.
In the second step, the implemented risk mitigation measures for each relevant pest were evaluated.
A conclusion on the pest freedom status of the commodity for each of the relevant pests was determined and uncertainties identified using expert judgements.
Pest freedom was assessed by estimating the number of infested/infected units out of 10,000 exported units. Further details on the methodology used to estimate the likelihood of pest freedom are provided in Section 2.3.4.
2.3.1. Commodity data
Based on the information provided by DEFRA of the UK the characteristics of the commodity were summarised.
2.3.2. Identification of pests potentially associated with the commodity
To evaluate the pest risk associated with the importation of the commodity from the UK, a pest list was compiled. The pest list is a compilation of all identified plant pests reported as associated with Q. robur and Quercus spp. based on information provided in the Dossier Sections 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0 and on searches performed by the Panel. The search strategy and search syntax were adapted to each of the databases listed in Table 3, according to the options and functionalities of the different databases and CABI keyword thesaurus.
The scientific names of the host plant (i.e. Quercus robur) were used when searching in the EPPO Global database and CABI Crop Protection Compendium. The same strategy was applied to the other databases excluding EUROPHYT and Web of Science.
EUROPHYT was investigated by searching for the interceptions associated with Q. robur imported from the whole world from 1995 to May 2020 and TRACES‐NT from May 2020 to 22 December 2022, respectively. For the pests selected for further evaluation, a search in the EUROPHYT and/or TRACES‐NT was performed for the years between 1995 and December 2022 for the interceptions from the whole world, at species level.
The search strategy used for Web of Science Databases was designed combining English common names for pests and diseases, terms describing symptoms of plant diseases and the scientific, and English common names of the commodity and excluding pests which were identified using searches in other databases. The established search strings are detailed in Appendix B and they were run on 21 September 2022.
The titles and abstracts of the scientific papers retrieved were screened and the pests associated with Q. robur were included in the pest list. The pest list was eventually further compiled with other relevant information (e.g. pest specific EPPO code, taxonomic information, categorisation, distribution) useful for the selection of the pests relevant for the purposes of this Opinion.
The compiled pest list (see Microsoft Excel® in Appendix F) includes all identified pests that use Q. robur as a host.
The evaluation of the compiled pest list was done in two steps: first, the relevance of the EU‐quarantine pests was evaluated (Section 4.1); second, the relevance of any other plant pest was evaluated (Section 4.2).
Pests for which limited information was available on one or more criteria used to identify them as relevant for this opinion, e.g. on potential impact, are listed in Appendix E (List of pests that can potentially cause an effect not further assessed).
2.3.3. Listing and evaluation of risk mitigation measures
All implemented risk mitigation measures were listed and evaluated. When evaluating the likelihood of pest freedom of the commodity, the following types of potential infection/infestation sources for Q. robur in export nursery were considered (see also Figure 1):
pest entry from surrounding areas,
pest entry with new plants/seeds,
pest spread within the nursery.
Figure 1.
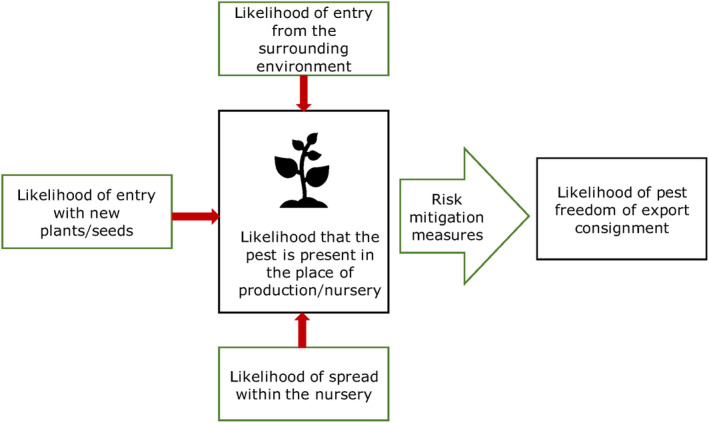
Conceptual framework to assess likelihood that plants are exported free from relevant pests (Source: EFSA PLH Panel, 2019)
The risk mitigation measures proposed by DEFRA of the UK were evaluated with expert knowledge elicitation (EKE) according to the Guidance on uncertainty analysis in scientific assessment (EFSA Scientific Committee, 2018).
Information on the biology, likelihood of entry of the pest to the export nursery, of its spread inside the nursery and the effect of measures on the specific pests were summarised in data sheets of pests selected for further evaluation (see Appendix A).
2.3.4. Expert knowledge elicitation
To estimate the pest freedom of the commodities an EKE was performed following EFSA guidance (Annex B.8 of EFSA Scientific Committee, 2018). The specific question for EKE was: ‘Taking into account (i) the risk mitigation measures in place in the nurseries and (ii) other relevant information, how many of 10,000 commodity units, either single plants or bundles of plants, will be infested with the relevant pest when arriving in the EU?’
The risk assessment considers a) bundles of 5, 10, 15, 25 or 50 plants for bare root whips and seedlings; b) 1‐ to 7‐year‐old bare root single plants, and c) less than 1‐ to 15‐year‐old single plants in pots.
The following reasoning is given for considering bundles of whips and seedlings:
There is no quantitative information available regarding clustering of plants during production.
Plants are grouped in bundles after sorting.
For the pests under consideration, a cross‐contamination during transport is possible.
The following reasoning is given for considering single plants (bare root or in pots):
The inspections before export are targeted on individual plants.
It is assumed that the product will be distributed in the EU as individual plants to the consumer.
The uncertainties associated with the EKE were taken into account and quantified in the probability distribution applying the semi‐formal method described in Section 3.5.2 of the EFSA‐PLH Guidance on quantitative pest risk assessment (EFSA PLH Panel, 2018). Finally, the results were reported in terms of the likelihood of pest freedom. The lower 5% percentile of the uncertainty distribution reflects the opinion that pest freedom is with 95% certainty above this limit.
3. Commodity data
3.1. Description of the commodity
The commodities of Q. robur (common name: common oak; family: Fagaceae) to be imported from the UK to the EU are whips, bare root plants and rooted plants in pots (Dossier Sections 1.0 and 3.0). According to the Dossier Section 3.0, none of the nurseries expected to export to the EU are using grafting in the production of Q. robur.
The commodities are as follows:
-
–
Whips and seedlings: the age of plants is between 1 and 2 years (Dossier Section 1.0). The diameter is between 4 and 10 mm. Whips are slender, unbranched trees. Whips can be bare root or containerised. Whips may have some leaves at the time of export, particularly when exported in November (Dossier Section 3.0). Seedlings are defined here as small plants which are grouped in larger bundles (see Section 3.3.6).
-
–
Bare root plants: the age of plants is between 1 and 7 years (Dossier Section 1.0). The diameter is between 30 and 40 mm for 7‐year‐old plants. Bare root plants may have some leaves at the time of export, particularly when exported in November (Dossier Section 3.0).
-
–
Rooted plants in pots: the age of plants ranges from less than 1 to 15 years (Dossier Section 1.0). The diameter is between less than 4 to 80 mm. The plants in pots may be exported with leaves, depending on the timing of the export (Dossier Section 3.0).
The growing media is virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) (Dossier Sections 1.0 and 3.0) complying with the requirements for growing media as specified in the Annex VII of the Commission Implementing Regulation 2019/2072.
According to ISPM 36 (FAO, 2019), the commodities can be classified as ‘bare root plants’ and ‘rooted plants in pots’.
According to the Dossier Section 1.0, the annual trade volume is up to 150,000 bare root plants and 50,000 rooted plants in pots. Trade of these plants will mainly be to Northern Ireland and the Republic of Ireland.
According to the Dossier Section 1.0, plants are supplied directly to professional operators and traders. Uses may include propagation, growing‐on, onward trading or direct sales to final consumers but will generally fall into the following categories:
-
–
Tree production and further growing‐on by professional operators;
-
–
Direct sales to final users as ornamentals;
-
–
Landscapers and garden centres, mainly for woodland and ornamental planting.
3.2. Description of the production areas
There are five known nurseries in the UK that are producing Q. robur plants for the export to the EU (Dossier Section 3.0). The nurseries are shown in a below Figure 2.
Figure 2.
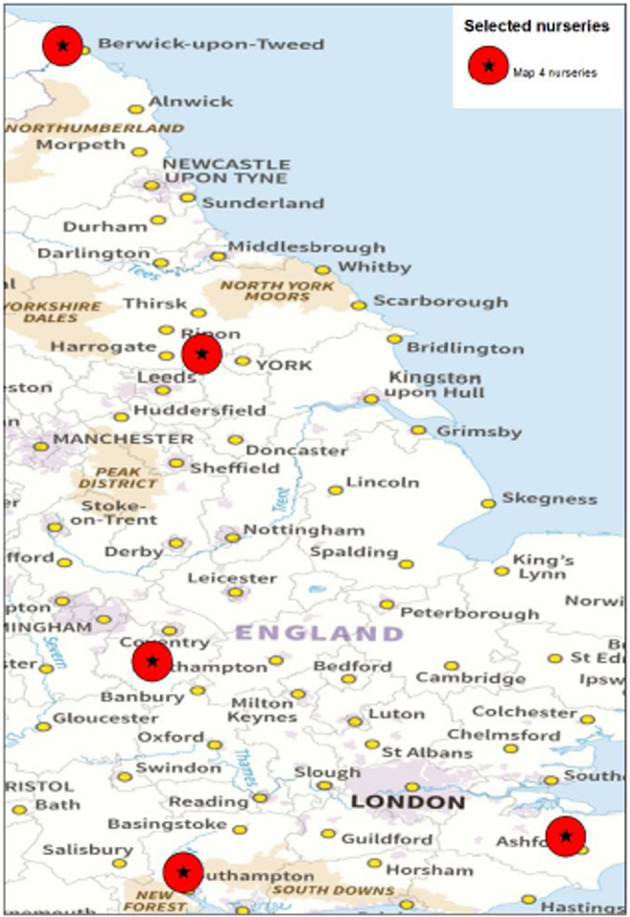
Nurseries in the UK of Quercus robur plants for the export to the EU (Source: Dossier Section 3.0)
Quercus species are grown in Great Britain in line with the Plant Health (Amendment etc.) (EU Exit) Regulations 2020 6 and the Plant Health (Phytosanitary Conditions) (Amendment) (EU Exit) Regulations 2020. 7 These regulations are broadly similar to EU phytosanitary regulation (Dossier Section 1.0). Producers do not set aside separate areas for export production. All plants within the UK nurseries are grown under the same phytosanitary measures, meeting the requirements of the UK Plant Passporting regime (Dossier Section 1.0).
The size of the nurseries is between 8 and 150 ha for container stock and up to 325 ha for field grown stock (Dossier Section 3.0).
The nurseries also grow other plant species as shown in the Appendix C. The minimum and maximum proportion of Q. robur compared to the other plant species grown in the nurseries is between 1% and 15%. The majority of the nurseries also produce plants for the local market, and there is no distancing between production areas for the export and the local market (Dossier Section 3.0).
The nurseries are kept clear of non‐cultivated herbaceous plants. In access areas, non‐cultivated herbaceous plants are kept to a minimum and only exist at nursery boundaries. Non‐cultivated herbaceous plants grow on less than 1% of the nursery area. The predominant species is rye grass (Lolium spp.). Other identified species include dandelions (Taraxacum officinale), hairy bittercress (Cardamine hirsuta), common daisy (Bellis perennis), creeping cinquefoil (Potentilla reptans) and bluebells (Hyacinthoides non‐scripta). These are all extremely low in number (Dossier Section 3.0).
There are hedges surrounding the export nurseries made up of a range of species including hazel (Corylus avellana), yew (Taxus baccata), holly (Ilex spp.), ivy (Hedera spp.), alder (Alnus glutinosa), laurel (Prunus laurocerasus), hawthorn (Crataegus spp.), blackthorn (Prunus spinosa) and leylandii (Cupressus × leylandii) (Dossier Section 3.0).
The closest Quercus plants grown in the surroundings are 5 m away from the nurseries (Dossier Section 3.0).
Nurseries are predominately situated in rural areas. The surrounding land would tend to be arable farmland with some pasture for animals and small areas of woodland. Hedges are often used to define field boundaries and grown along roadsides (Dossier Section 3.0).
Arable crops within a radius of 2 km from the nurseries are rotated in line with good farming practice and could include oilseed rape (Brassica napus), wheat (Triticum spp.), barley (Hordeum vulgare), turnips (Brassica rapa subsp. rapa), potatoes (Solanum tuberosum) and maize (Zea mays) (Dossier Section 3.0).
Pastures are present within a radius of 2 km from the nurseries and are predominantly ryegrass (Lolium spp.) (Dossier Section 3.0).
Woodland is present within a radius of 2 km from the nurseries. The nearest woodland in one of the nurseries borders the boundary fence. Woodlands tend to be a standard UK mixed woodland, with a range of the UK native trees such as oak (Q. robur), pine (Pinus spp.), poplar (Populus spp.), ash (Fraxinus spp.), sycamore (Acer pseudoplatanus), holly (Ilex spp.), Norway maple (Acer platanoides) and field maple (Acer campestre). The nearest woodland in one of the nurseries borders the boundary fence (Dossier Section 3.0).
It is not possible to identify what plant species which are growing within the gardens of private dwellings within a radius of 2 km from the nurseries (Dossier Section 3.0).
Other plants likely to be present in the surroundings of the nurseries (within 2 km radius) are: Abies spp., Acer spp., Adiantum spp., Aesculus spp., Annona spp., Arbutus spp., Arctostaphylos spp., Berberis spp., Camellia spp., Castanea spp., Cornus spp., Corylus spp., Cotoneaster spp., Crataegus spp., Fagus sylvatica, Fagus spp., Larix spp., Ligustrum vulgare, Liquidambar spp., Lithocarpus spp., Malus spp., Magnolia spp., Morus spp., Picea spp., Pieris spp., Pinus spp., Populus spp., Prunus spp., Pyracantha spp., Pyrus spp., Rhamnus spp., Rhododendron spp., Ribes spp., Rosa spp., Rubus spp., Sequoia spp., Sorbus spp., Syringa spp., Taxus spp., Ulmus spp., Vaccinium spp., Viburnum spp. and Vitis vinifera (Dossier Section 3.0).
Based on the global Köppen–Geiger climate zone classification (Kottek et al., 2006), the climate of the production areas of Q. robur in the UK is classified as Cfb, i.e. main climate (C): warm temperate; precipitation (f): fully humid; temperature (b): warm summer.
3.3. Production and handling processes
3.3.1. Source of planting material
The starting material of the commodities is a mix of seeds and seedlings depending on the nursery (Dossier Section 3.0).
Seeds purchased in the UK are certified under The Forest Reproductive Material (Great Britain) Regulations 2002. Seedlings sourced in the UK are certified with UK Plant Passports. Seedlings from the EU countries are certified with phytosanitary certificates. Some plants are obtained from EU (mostly the Netherlands) (Dossier Section 3.0).
None of the nurseries expected to export to the EU produce plants from grafting, they use only seed and seedlings, therefore there are no mother plants of Q. robur present in the nurseries (Dossier Section 3.0).
3.3.2. Production cycle
Plants are either grown in containers (cells, pots, tubes, etc.) or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown or field grown in containers (Dossier Section 1.0). Plants grown under protection are maintained in plastic polytunnels, or in glasshouses which typically consist of a metal or wood frame construction and glass panels. As the plants are intended for outdoor cultivation, normally, only certain growth stages are maintained under protection, such as young or seedling plants when there is an increased vulnerability to climatic conditions, including frost (Dossier Section 3.0). The Panel assumes that potted plants could be cultivated for the whole period in pots or grown in the field and then transplanted in pots at a later stage. In this last case it is assumed that the roots will be washed before potting and soil removed as required by the legislation for a commodity to be exported to the EU.
Bare root plants are planted from autumn until spring (October to April), Rooted plants in pots can be planted at any time of year, though winter is most common (Dossier Section 1.0).
According to the Dossier Section 1.0, bare root plants will be harvested from late autumn until early spring (October to April) to be able to lift plants from the field and because this is the best time to move dormant plants. Rooted plants in pots can be moved at any time in the year to fulfil consumer demand, but more usually September to May. These will likely be destined for amenity or garden centre trade rather than nurseries.
The growing media is virgin peat or peat‐free compost. This compost is heat‐treated by commercial suppliers during production to eliminate pests and diseases. It is supplied in sealed bulk bags or shrink‐wrapped bales and stored off the ground on pallets, these are free from contamination. Where delivered in bulk, compost is kept in a dedicated bunker, either indoors, or covered by tarpaulin outdoors, and with no risk of contamination with soil or other material (Dossier Section 1.0).
The irrigation would be done on the need basis and could be overhead, sub irrigation or drip irrigation. Water used for irrigation can be drawn from several sources, the mains water supply, bore holes or from rainwater collection/watercourses (Dossier Section 3.0). Additional information on water used for irrigation is provided in the Appendix D. Regardless of the source of the water used to irrigate, none of the nurseries have experienced the introduction of any pest/disease as a result of contamination of the water supply (Dossier Section 3.0).
Growers are required to assess water sources, irrigation and drainage systems used in the plant production for the potential to harbour and transmit plant pests. Water may be obtained from the mains water supply, bore holes, rivers or reservoirs/lagoons. Water is routinely sampled and sent for analysis (Dossier Section 1.0).
Growers must assess weeds and volunteer plants for the potential to host and transmit plant pests and have an appropriate programme of weed management in place on the nursery (Dossier Section 1.0).
General hygiene measures are undertaken as part of routine nursery production, including disinfection of tools and equipment between batches/lots and different plant species (Dossier Sections 1.0 and 3.0). The tools are dipped and wiped with a clean cloth between trees to reduce the risk of virus and bacterial transfer between subjects. There are various disinfectants available, with Virkon S being a common example (Dossier Section 3.0).
Growers keep records to allow traceability for all plant material handled. These records must allow a consignment or consignment in transit to be traced back to the original source, as well as forward to identify all trade customers to which those plants have been supplied (Dossier Section 1.0).
3.3.3. Pest monitoring during production
All producers are registered as professional operators with the UK Competent Authority via the Animal and Plant Health Agency (APHA) for England and Wales, or with SASA (Scotland), and are authorised to issue UK plant passports, verifying they meet the required national sanitary standards. The Competent Authority inspect crops at least once a year to check they meet the standards set out in the guides. Assessments are normally made based on visual examinations, but samples may be taken for laboratory analysis to get a definitive diagnosis (Dossier Section 1.0).
The Plant Health and Seeds Inspectorate (PHSI), part of the APHA, execute plant health policy, except forestry matters, in England and Wales under a Memorandum of Understanding with DEFRA and with the Welsh Government. In Scotland, this role is carried out by inspectors in the Rural Payments and Inspections Division and the Horticulture and Marketing Unit, in SASA. PHSI and Scottish inspectors carry out import, export, monitoring and survey inspections, issue phytosanitary certificates and oversee import controls, issuing of plant passports and eradication campaigns (Dossier Section 1.0).
The sanitary status of production areas is controlled by the producers as part of these schemes, as well as via official inspections by APHA PHSI or with SASA (Scotland) (Dossier Section 1.0).
All producers are subject to regular inspections by plant health inspectors as part of either Plant Passporting audits, or a programme of general surveillance of all registered producers (Dossier Section 1.0).
The UK plant health inspectors monitor for pests and diseases during crop certification and passporting inspections. In addition, the PHSI (in England and Wales) carry out a programme of Quarantine Surveillance in registered premises, inspecting plants grown and moving within the UK market. Similar arrangements operate in Scotland (Dossier Section 1.0).
According to the Dossier Section 1.0 the objective of the quarantine surveillance is to ensure that:
-
–
the plant passport regime is being operated effectively;
-
–
quarantine organisms are not spread on plants and plant produce which are not subject to plant passporting;
-
–
the UK plant health authorities have early warning of any new threat from a previously unknown pest or disease which has become established within the UK;
-
–
plant health authorities can take informed decisions on the scope and operation of the plant passport regime.
According to the Dossier Section 1.0 the quarantine surveillance programme centres on a risk‐based selection of premises to visit, based on size, types of plants grown, source of plants and the producer's track record of pest and disease issues. Guidance on visit frequency is given to inspectors to ensure that those sites which present the greatest risk are visited more frequently than those of lower risk. The risk category assigned to a premise determines the frequency of visit:
-
–
very high risk (multiple visits per year);
-
–
high risk (two/three visits per year);
-
–
medium risk (annual visit);
-
–
low risk (once every 3 years).
Inspections are targeted both at the plants or products which present the greatest risk, and also a wider range of plants and plant products which are monitored for more general risks, including those highly polyphagous pests whose range may be unknown or still increasing. The UK inspectors receive comprehensive training on the full range of symptoms caused by pests and diseases, to allow them to detect any new and emerging risks, and during a visit to a nursery they are free to inspect any plants on that nursery. Samples of pests and plants showing any suspicious symptoms are routinely sent to the laboratory for testing (Dossier Section 1.0).
In the last 3 years (2019–2022) there has been a substantial level of inspection of registered Quercus producers, both in support of the Plant Passporting scheme (checks are consistent with the EU legislation, with a minimum of one a year for authorised operators) and as part of the Quarantine Surveillance programme (Great Britain uses the same framework for its surveillance programme as the EU) (Dossier Section 1.0).
Plant material is regularly monitored for plant health issues. Pest monitoring is carried out by trained nursery staff via crop walking and records kept of this monitoring. Qualified agronomists also undertake crop walks to verify the producer's assessments. Curative or preventative actions are implemented together with an assessment of phytosanitary risk. Unless a pest can be immediately and definitively identified as non‐quarantine, growers are required to treat it as a suspect quarantine pest and notify the competent authority (Dossier Section 1.0).
The crops are inspected visually on a regular basis by competent nursery staff as part of the growing process. All plants are also carefully inspected by nurseries on arrival and dispatch for any plant health issues (Dossier Section 3.0).
It is a legal requirement under the UK Plant Health law for any person in charge of a premise to notify the Competent Authority of the presence, or suspected presence, of a plant pest. The requirement is not limited to those organisms listed in the UK legislation but is also required for any organism not normally present in the UK which is likely to be injurious to plants (Dossier Section 1.0).
The nurseries follow the Plant Health Management Standard issued by the Plant Healthy Certification Scheme of which DEFRA, Royal Horticultural Society and others contribute to via The Plant Health Alliance Steering Group (Dossier Section 3.0).
UK surveillance is based on visual inspection with samples taken from symptomatic material, and where appropriate, samples are also taken from asymptomatic material (e.g. plants, tubers, soil, watercourses). According to the Dossier Section 3.0, for sites with the likelihood of multiple pest and host combinations (e.g. ornamental and retail sites) standard methods are used for site selection and visit frequency, whereby clients are assessed taking into account business activity, size of business and source material, so for example a large propagator using third country material receives 10 visits per year whilst a small retailer selling locally sourced material is visited once every second year. Where pest specific guidelines are absent inspectors select sufficient plants to achieve a 95% probability of detecting symptoms randomly distributed on 1.5% of plants in a batch/consignment. For inspections of single hosts, possibly with multiple pests, survey site selection is often directed to specific locations identified by survey planners, for example 0.5% of ware production land is annually sampled for potato cyst nematode (PCN) with farms randomly selected and sampled at a rate of 50 cores per hectare (Dossier Section 3.0).
During production, in addition to the general health monitoring of the plants by the nurseries, official growing season inspections are undertaken by the UK Plant Health Service at an appropriate time, taking into consideration factors such as the likelihood of pest presence and growth stage of the crop. Where appropriate this could include sampling and laboratory analysis. Official sampling and analysis could also be undertaken nearer to the point of export depending on the type of analysis and the import requirements of the country being exported to. Samples are generally taken on a representative sample of plants, in some cases however where the consignment size is quite small all plants are sampled. Magnification equipment is provided to all inspectors as part of their standard equipment and is used during inspections when appropriate (Dossier Section 3.0).
Incoming plant material and other goods such as packaging material and growing media, that have the potential to be infected or harbour pests, are checked on arrival. Growers have procedures in place to quarantine any suspect plant material and to report findings to the authorities (Dossier Section 1.0).
3.3.4. Pest management during production
Crop protection is achieved using a combination of measures including approved plant protection products, biological control or physical measures. Plant protection products are only used when necessary and records of all plant protection treatments are kept (Dossier Section 1.0).
Pest and disease pressure varies from season to season. Product application takes place only when required and depends on situation (disease pressure, growth stage, etc. and environmental factors) at that time. Subject to this variation in pest pressure, in some seasons few, if any, pesticides are applied; in others it is sometimes necessary to apply preventative and/or control applications of fungicides, herbicides or insecticides. In many circumstances also, biological control is used to control outbreaks, rather than using chemical treatments (Dossier Section 3.0).
Examples of typical treatments used against mildew, grey mould, spider mites, aphids and thrips are detailed in the Dossier Section 3. These would be applied at the manufacturers recommended doses and intervals (Dossier Section 3.0).
There are no specific measures/treatments against the soil pests. However, containerised plants are grown in trays on top of protective plastic membranes to prevent contact with soil. Membranes are regularly refreshed when needed. Alternatively, plants may be grown on raised galvanised steel benches stood on gravel as a barrier between the soil and bench feet and/or concreted surfaces (Dossier Section 3.0).
Post‐harvest and through the autumn and winter, nursery management is centred on pest and disease prevention and maintaining good levels of nursery hygiene. Leaves, pruning and weeds are all removed from the nursery to reduce the number of overwintering sites for pests (insects, mites, pathogens, etc.) (Dossier Section 1.0).
3.3.5. Inspections before export
The UK NPPO carries out inspections and testing where required by the country of destination's plant health legislation, to ensure all requirements are fulfilled and a valid phytosanitary certificate with the correct additional declarations is issued (Dossier Section 1.0).
Separate to any official inspection, plant material is checked by growers for plant health issues prior to dispatch (Dossier Section 1.0).
A final pre‐export inspection is undertaken as part of the process of issuing a phytosanitary certificate. These inspections are generally undertaken as near to the time of export as possible, usually within 1–2 days, and not more than 2 weeks before export. Phytosanitary certificates are only issued if the commodity meets the required plant health standards after inspection and/or testing according to appropriate official procedures (Dossier Section 3.0).
The protocol for plants infested by pests during inspections before export is to treat the plants, if they are on site for a sufficient period of time, or to destroy any plants infested by pests otherwise. All other host plants in the nursery would be treated. The phytosanitary certificate for export will not be issued until the UK Plant Health inspectors confirm that the plants are free from pests (Dossier Section 3.0).
3.3.6. Export procedure
Bare root plants are lifted from late autumn until early spring (October to April) to be able to lift plants from the field and because this is the best time to move dormant plants (Dossier Section 1.0). Bare root plants are lifted, washed free from soil with a low‐pressure washer in the outdoors nursery area away from packing/cold store area (Dosser Section 3.0).
Rooted plants in pots can be moved at any point in the year to fulfil consumer demand, but more usually from September to May. These will likely be destined for amenity or garden centre trade rather than nurseries.
The maximum time from the harvesting of bare root plants to the export is up to 5 months. Plants are stored in cold store or heeled into soil (but before export they would be washed to ensure freedom from soil). Most plants for export would be kept in cold store (Dossier Section 3.0).
The preparation of the commodities for export is carried out inside the nurseries in a closed environment, e.g. packing shed (Dossier Section 3.0).
The commodities will be sent by lorry and can be exported either between November and April or any time of the year, depending on the type of the commodity. Bare root plants are exported from November and April, while rooted plants in pots are mainly exported between September and May, although these can be moved at any point in the year to fulfil consumer demand. Sensitive plants will occasionally be transported by temperature‐controlled lorry if weather conditions during transit are potentially harmful to plants (Dossier Section 1.0).
According to the Dossier Section 3.0, the commodities will be dispatched as single bare root trees and plants in pots or in bundles as follows:
-
–
25 or 50 for seedlings or transplants;
-
–
5, 10 or 15 for whips.
Bare root plants are placed in bundles, wrapped in polythene and packed and distributed on ISPM 15 certified wooden pallets or metal pallets. Alternatively, they may be placed in pallets which are then wrapped in polythene. Small volume orders may be packed in waxed cardboard cartons or polythene bags and dispatched via courier (Dossier Sections 1.0 and 3.0).
Rooted plants in pots are transported on Danish trolleys for smaller containers, or certified pallets, or individually in pots for larger containers (Dossier Section 1.0).
4. Identification of pests potentially associated with the commodity
The search for potential pests associated with the commodity rendered 1,707 species (see Microsoft Excel® file in Appendix F).
4.1. Selection of relevant EU‐quarantine pests associated with the commodity
The EU listing of union quarantine pests and protected zone quarantine pests (Commission Implementing Regulation (EU) 2019/2072) is based on assessments concluding that the pests can enter, establish, spread and have potential impact in the EU.
30 EU‐quarantine species that are reported to use commodity as a host plant were evaluated (Table 4) for their relevance of being included in this Opinion.
Table 4.
Overview of the evaluation of the 30 EU‐quarantine pest species for which information was found in the Dossier, databases and literature searches that use Quercus robur or Quercus spp. as a host plant for their relevance for this opinion
| No. | Pest name according to EU legislation (a) | EPPO code | Group | Pest present in the UK | Quercus robur and Quercus spp. confirmed as a host (reference) | Pest can be associated with the commodity | Pest relevant for the Opinion |
|---|---|---|---|---|---|---|---|
| 1 | Anisandrus maiche as Scolytinae non‐European | ANIDMA | Insects | No | Quercus robur (EPPO, 2020) | Not assessed | No |
| 2 | Anoplophora chinensis | ANOLCN | Insects | No | Quercus petraea, Q. robur (CABI, online) | Not assessed | No |
| 3 | Anoplophora glabripennis | ANOLGL | Insects | No | Quercus (EPPO Bulletin, 2017) | Not assessed | No |
| 4 | Apriona germari | APRIGE | Insects | No | Quercus (EPPO, online) | Not assessed | No |
| 5 | Arrhenodes minutus | ARRHMI | Insects | No | Quercus (EPPO, online) | Not assessed | No |
| 6 | Bretziella fagacearum | CERAFA | Fungi | No | Quercus petraea (EPPO, online), Q. robur (CABI, online) | Not assessed | No |
| 7 | Bursaphelenchus xylophilus (1) | BURSXY | Nematodes | No | Quercus robur (Ferris, online) | Not assessed | No |
| 8 | Cronartium quercuum | CRONQU | Fungi | Yes | Quercus petraea, Q. robur (EPPO, online; Farr and Rossman, online) | Yes | Yes |
| 9 | Cryphonectria parasitica | ENDOPA | Fungi | Yes | Quercus petraea, Q. robur (Farr and Rossman, online) | Yes | Yes |
| 10 | Davidsoniella virescens | CERAVI | Fungi | No | Quercus robur (Farr and Rossman, online) | Not assessed | No |
| 11 | Diabrotica virgifera zeae | DIABVZ | Insects | No | Quercus (EPPO, online) | Not assessed | No |
| 12 | Euwallacea fornicatus sensu lato (including: Euwallacea fornicatus sensu stricto, Euwallacea fornicatior, Euwallacea kuroshio and Euwallacea perbrevis) |
XYLBFO EUWAWH EUWAFO EUWAKU EUWAPE |
Insects | No | Quercus, Quercus robur (EPPO, online) | Not assessed | No |
| 13 | Grapholita prunivora | LASPPR | Insects | No | Quercus (EPPO, online) | Not assessed | No |
| 14 | Homalodisca vitripennis | HOMLTR | Insects | No | Quercus (EPPO, online) | Not assessed | No |
| 15 | Massicus raddei | MALLRA | Insects | No | Quercus (EPPO, online) | Not assessed | No |
| 16 | Meloidogyne chitwoodi | MELGCH | Nematodes | No | Quercus (Dossier) | Not assessed | No |
| 17 | Neocosmospora euwallaceae | FUSAEW | Fungi | No | Quercus robur (EPPO, online) | Not assessed | No |
| 18 | Oemona hirta | OEMOHI | Insects | No | Quercus robur (EPPO, online) | Not assessed | No |
| 19 | Phytophthora ramorum (non‐EU isolates) | PHYTRA | Oomycetes | Yes | Quercus, Quercus robur (EPPO, online; CABI, online) | Yes | Yes |
| 20 | Popillia japonica | POPIJA | Insects | No | Quercus (EPPO Bulletin, 2017) | Not assessed | No |
| 21 | Pseudopityophthorus minutissimus | PSDPMI | Insects | No | Quercus (EPPO Bulletin, 2017) | Not assessed | No |
| 22 | Pseudopityophthorus pruinosus | PSDPPR | Insects | No | Quercus (EPPO Bulletin, 2017) | Not assessed | No |
| 23 | Scirtothrips citri | SCITCI | Insects | No | Quercus (EPPO, online) | Not assessed | No |
| 24 | Thaumatotibia leucotreta | ARGPLE | Insects | No | Quercus robur (EPPO, online) | Not assessed | No |
| 25 | Thaumetopoea processionea | THAUPR | Insects | Yes | Quercus petraea, Q. robur (CABI, online; EPPO, online) | Yes | Yes |
| 26 | Trirachys sartus | AELSSA | Insects | No | Quercus (EPPO, online) | Not assessed | No |
| 27 | Xiphinema americanum sensu stricto | XIPHAA | Nematodes | No | Quercus (Dossier) | Not assessed | No |
| 28 | Xiphinema rivesi (non‐EU populations) | XIPHRI | Nematodes | No | Quercus (Dossier) | Not assessed | No |
| 29 | Xiphinema tarjanense | XIPHTA | Nematodes | No | Quercus robur (Xu and Zhao, 2019) | Not assessed | No |
| 30 | Xylella fastidiosa | XYLEFA | Bacteria | No | Quercus (EPPO Bulletin, 2017) | Not assessed | No |
Commission Implementing Regulation (EU) 2019/2072.
The association with Q. robur is uncertain as it was found only as an experimental host.
The relevance of an EU‐quarantine pest for this opinion was based on evidence that:
the pest is present in the UK;
the commodity is host of the pest;
one or more life stages of the pest can be associated with the specified commodity.
Pests that fulfilled all criteria were selected for further evaluation.
Table 4 presents an overview of the evaluation of the 30 EU‐quarantine pest species that are reported as associated with the commodity.
Of these 30 EU‐quarantine pest species evaluated, 4 (Cronartium quercuum, Cryphonectria parasitica, Phytophthora ramorum (non‐EU isolates) and Thaumetopoea processionea) are present in the UK and can be associated with the commodity and hence were selected for further evaluation.
4.2. Selection of other relevant pests (non‐regulated in the EU) associated with the commodity
The information provided by the UK, integrated with the search performed by EFSA, was evaluated in order to assess whether there are other potentially relevant pests potentially associated with the commodity species present in the country of export. For these potential pests that are non‐regulated in the EU, pest risk assessment information on the probability of entry, establishment, spread and impact is usually lacking. Therefore, these pests were also evaluated to determine their relevance for this Opinion based on evidence that:
the pest is present in the UK;
the pest is (i) absent or (ii) has a limited distribution in the EU;
commodity is a host of the pest;
one or more life stages of the pest can be associated with the specified commodity;
the pest may have an impact in the EU.
For non‐regulated species with a limited distribution (i.e. present in one or a few EU MSs) and fulfilling the other criteria (i.e. c, d and e), either one of the following conditions should be additionally fulfilled for the pest to be further evaluated:
official phytosanitary measures have been adopted in at least one EU MS;
any other reason justified by the working group (e.g. recent evidence of presence).
Pests that fulfilled the above listed criteria were selected for further evaluation.
Based on the information collected, 1,673 potential pests known to be associated with the species commodity were evaluated for their relevance to this Opinion. Species were excluded from further evaluation when at least one of the conditions listed above (a–e) was not met. Details can be found in the Appendix F (Microsoft Excel® file). Of the evaluated EU non‐quarantine pests, four pests (Coniella castaneicola, Meloidogyne mali, Phytophthora kernoviae and Trinophylum cribratum) were selected for further evaluation because they met all of the selection criteria. More information on these four pests can be found in the pest datasheets (Appendix A).
4.3. Overview of interceptions
Data on the interception of harmful organisms on plants of Q. robur can provide information on some of the organisms that can be present on Q. robur despite the current measures taken. According to EUROPHYT, online (accessed on 22 December 2022) and TRACES‐NT, online (accessed on 22 December 2022), there were no interceptions of plants for planting of Q. robur from the UK destined to the EU Member States due to the presence of harmful organisms between the years 1995 and 22 December 2022.
There were 69 interceptions of plants for planting of Q. robur from Belgium and the Netherlands destined to other EU Member States due to the presence of harmful organism (Thaumetopoea processionea) between the years 1995 and 22 December 2022 (EUROPHYT, online).
4.4. List of potential pests not further assessed
From the list of pests not selected for further evaluation, the Panel highlighted 13 species (see Appendix E) for which currently available evidence provides no reason to select them for further evaluation in this Opinion. A specific justification of the inclusion in this list is provided for each species in Appendix E.
4.5. Summary of pests selected for further evaluation
The eight pests satisfying all the relevant criteria listed above in the Sections 4.1 and 4.2 are included in Table 5. The effectiveness of the risk mitigation measures applied to the commodity was evaluated for these selected pests.
Table 5.
List of relevant pests selected for further evaluation
| Number | Current scientific name | EPPO code | Name used in the EU legislation | Taxonomic information | Group | Regulatory status |
|---|---|---|---|---|---|---|
| 1 | Coniella castaneicola | – | – |
Diaporthales Schizoparmaceae |
Fungi | Not regulated in the EU |
| 2 | Cronartium quercuum | CRONQU | Cronartium spp. (non‐European) |
Pucciniales Cronartiaceae |
Fungi | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 3 | Cryphonectria parasitica | ENDOPA | Cryphonectria parasitica (Murrill) Barr |
Diaporthales Cryphonectriaceae |
Fungi | Protected Zone Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 4 | Meloidogyne mali | MELGMA | – |
Rhabditia Meloidogynidae |
Nematodes | Not regulated in the EU |
| 5 | Phytophthora kernoviae | PHYTKE | – |
Peronosporales Peronosporaceae |
Oomycetes | Not regulated in the EU |
| 6 | Phytophthora ramorum | PHYTRA | Phytophthora ramorum (non‐EU isolates) Werres, De Cock & Man in ‘t Veld |
Peronosporales Peronosporaceae |
Oomycetes | EU Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 7 | Thaumetopoea processionea | THAUPR | Thaumetopoea processionea L. |
Lepidoptera Notodontidae |
Insects | Protected Zone Quarantine Pest according to Commission Implementing Regulation (EU) 2019/2072 |
| 8 | Trinophylum cribratum | – | – |
Coleoptera Cerambycidae |
Insects | Not regulated in the EU |
5. Risk mitigation measures
For the selected pests (Table 5), the Panel evaluated the likelihood that it could be present in the Q. robur nurseries by evaluating the possibility that the commodity in the export nurseries is infested either by:
introduction of the pest from the environment surrounding the nursery;
introduction of the pest with new plants/seeds;
spread of the pest within the nursery.
The information used in the evaluation of the effectiveness of the risk mitigation measures is summarised in pest data sheets (see Appendix A).
5.1. Risk mitigation measures applied in the UK
With the information provided by the UK (Dossier Sections 1.0, 2.0, 3.0, 4.0, 5.0 and 6.0), the Panel summarised the risk mitigation measures (see Table 6) that are implemented in the production nursery.
Table 6.
Overview of implemented risk mitigation measures for Quercus robur plants designated for export to the EU from the UK
| Number | Risk mitigation measure | Implementation in the UK |
|---|---|---|
| 1 | Registration of production sites | All producers are registered as professional operators with the UK Competent Authority via the Animal and Plant Health Agency (APHA) for England and Wales, or with SASA (Scotland), and are authorised to issue UK plant passports, verifying they meet the required national sanitary standards (Dossier Section 1.0). |
| 2 | Physical separation | Producers do not set aside separate areas for export production. All plants within UK nurseries are grown under the same phytosanitary measures, meeting the requirements of the UK Plant Passporting regime (Dossier Section 1.0). |
| 3 | Certified plant material | Seeds purchased in the UK are certified under The Forest Reproductive Material (Great Britain) Regulations 2002. Seedlings sourced in the UK are certified with UK Plant Passports. Seedlings from the EU countries are certified with phytosanitary certificates. Some plants are obtained from EU (mostly the Netherlands) (Dossier Section 3.0). |
| 4 | Growing media | The growing media is virgin peat or peat‐free compost. This compost is heat‐treated by commercial suppliers during production to eliminate pests and diseases. It is supplied in sealed bulk bags or shrink‐wrapped bales and stored off the ground on pallets, these are free from contamination. Where delivered in bulk, compost is kept in a dedicated bunker, either indoors, or covered by tarpaulin outdoors, and with no risk of contamination with soil or other material (Dossier Section 1.0). |
| 5 | Surveillance, monitoring and sampling | For additional information see Section 3.3.3 Pest monitoring during production. |
| 6 | Hygiene measures |
Growers must assess weeds and volunteer plants for the potential to host and transmit plant pests and have an appropriate programme of weed management in place on the nursery (Dossier Section 1.0). General hygiene measures are undertaken as part of routine nursery production, including disinfection of tools and equipment between batches/lots (Dossier Section 1.0) and different plant species (Dossier Sections 1.0 and 3.0). The tools are dipped and wiped with a clean cloth between trees to reduce the risk of virus and bacterial transfer between subjects. There are various disinfectants available, with Virkon S being a common example (Dossier Section 3.0). |
| 7 | Removal of infested/infected plant material | Post‐harvest and through the autumn and winter, nursery management is centred on pest and disease prevention and maintaining good levels of nursery hygiene. Leaves, pruning and weeds are all removed from the nursery to reduce the number of over wintering sites for pests and diseases (Dossier Section 1.0). |
| 8 | Irrigation water | Water for irrigation is routinely sampled and sent for analysis (Dossier Section 1.0). |
| 9 | Application of pest control products |
Crop protection is achieved using a combination of measures including approved plant protection products, biological control or physical measures. Plant protection products are only used when necessary and records of all plant protection treatments are kept (Dossier Section 1.0). Examples of typical treatments used against mildew, grey mould, spider mites, aphids and thrips are detailed in the Dossier Section 3. These would be applied at the manufacturers recommended rate and intervals (Dossier Section 3.0). |
| 10 | Measures against soil pests | There are no specific measures/treatments against the soil pests. However, containerised plants are grown in trays on top of protective plastic membranes to prevent contact with soil. Membranes are regularly refreshed when needed. Alternatively, plants may be grown on raised galvanised steel benches stood on gravel as a barrier between the soil and bench feet and/or concreted surfaces (Dossier Section 3.0). |
| 11 | Inspections and management of plants before export |
The UK NPPO carries out inspections and testing where required by the country of destination's plant health legislation, to ensure all requirements are fulfilled and a valid phytosanitary certificate with the correct additional declarations is issued (Dossier Section 1.0). Separate to any official inspection, plant material is checked by growers for plant health issues prior to dispatch (Dossier Section 1.0). A final pre‐export inspection is undertaken as part of the process of issuing a phytosanitary certificate. These inspections are generally undertaken as near to the time of export as possible, usually within 1–2 days, and not more than 2 weeks before export. Phytosanitary certificates are only issued if the commodity meets the required plant health standards after inspection and/or testing according to appropriate official procedures (Dossier Section 3.0). The protocol for plants infested by pests during inspections before export is to treat the plants, if they are on site for a sufficient period of time, or to destroy any plants infested by pests otherwise. All other host plants in the nursery would be treated. The phytosanitary certificate for export will not be issued until the UK Plant Health inspectors confirm that the plants are free from pests (Dossier Section 3.0). |
| 12 | Separation during transport to the destination |
According to the Dossier Section 3.0 the commodities are dispatched as single bare root trees or in bundles as follows:
Bare root plants are placed in bundles, wrapped in polythene and packed and distributed on ISPM 15 certified wooden pallets or metal pallets. Alternatively, they may be placed in pallets which are then wrapped in polythene. Small volume orders may be packed in waxed cardboard cartons or polythene bags and dispatched via courier (Dossier Sections 1.0 and 3.0). Rooted plants in pots are transported on Danish trolleys for smaller containers, or certified pallets, or individually in pots for larger containers (Dossier Section 1.0). |
5.2. Evaluation of the current measures for the selected relevant pests including uncertainties
For each evaluated pest, the relevant risk mitigation measures acting on the pest were identified. Any limiting factors on the effectiveness of the measures were documented.
All the relevant information including the related uncertainties deriving from the limiting factors used in the evaluation are summarised in a pest data sheet provided in Appendix A. Based on this information, for each selected relevant pest, an expert judgement is given for the likelihood of pest freedom taking into consideration the risk mitigation measures and their combination acting on the pest.
An overview of the evaluation of each relevant pest is given in the sections below (Sections 5.2.1, 5.2.8–5.2.8). The outcome of the EKE regarding pest freedom after the evaluation of the currently proposed risk mitigation measures is summarised in Section 5.2.9.
5.2.1. Overview of the evaluation of Coniella castaneicola (Diaporthales; Schizoparmaceae)
| Overview of the evaluation of Coniella castaneicola for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,849 out of 10,000 bundles | 9,925 out of 10,000 bundles | 9,965 out of 10,000 bundles | 9,988 out of 10,000 bundles | 9,998 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 2 out of 10,000 bundles | 12 out of 10,000 bundles | 35 out of 10,000 bundles | 75 out of 10,000 bundles | 151 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Coniella castaneicola is present in the UK, although reports are still scattered. Quercus robur is reported as a host of this pathogen. Infection of leaves and twigs may occur by means of conidia through wounds. Infection courts represented by wounds and injuries of biotic and abiotic origin are expected to be present. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the treatment of the growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) the removal of infected plant material and (e) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. castaneicola between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of C. castaneicola for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,813 out of 10,000 plants | 9,906 out of 10,000 plants | 9,949 out of 10,000 plants | 9,976 out of 10,000 plants | 9,994 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 6 out of 10,000 plants | 24 out of 10,000 plants | 51 out of 10,000 plants | 94 out of 10,000 plants | 187 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Coniella castaneicola is present in the UK, although reports are still scattered. Quercus robur is reported as a host of the pathogen. Infection of leaves and twigs may occur by means of conidia through wounds. Infection courts represented by wounds and injuries of biotic and abiotic origin are expected to be present. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the treatment of the growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) the removal of infected plant material and (e) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. castaneicola between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of C. castaneicola for plants in pots up to 15 years old | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,711 out of 10,000 plants | 9,840 out of 10,000 plants | 9,905 out of 10,000 plants | 9,950 out of 10,000 plants | 9,985 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 15 out of 10,000 plants | 50 out of 10,000 plants | 95 out of 10,000 plants | 160 out of 10,000 plants | 289 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Coniella castaneicola is present in the UK, although reports are still scattered. Quercus robur is reported as a host of the pathogen. Infection of leaves and twigs may occur by means of conidia through wounds. Infection courts represented by wounds and injuries of biotic and abiotic origin are expected to be present. The hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) the treatment of the growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) the removal of infected plant material and (e) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. castaneicola between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Coniella castaneicola (Section A.1 in Appendix A).
5.2.2. Overview of the evaluation of Cronartium quercuum (Pucciniales; Cronartiaceae)
| Overview of the evaluation of Cronartium quercuum for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,881 out of 10,000 bundles | 9,944 out of 10,000 bundles | 9,979 out of 10,000 bundles | 9,995 out of 10,000 bundles | 9,999.85 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 0.15 out of 10,000 bundles | 5 out of 10,000 bundles | 21 out of 10,000 bundles | 56 out of 10,000 bundles | 119 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Cronartium quercuum has been reported as present in the UK, although uncertainties exist due to taxonomic issues. Quercus robur is reported to be telial host of the pathogen. Both telial and aecial hosts (Castanea spp., Castanopsis spp., Fagus japonica, Notholithocarpus densiflorus, Quercus spp., Rhus chinensis and Pinus spp., respectively) can be present at a suitable distance both in the nurseries and in the surroundings making it possible the infection of oak leaves by means of spores. Although bare root plants are mostly exported in a dormant phase, some leaves could still be attached to the plants at the time of export making the association with the commodity possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) the removal of infected plant material and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. quercuum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of C. quercuum for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,913 out of 10,000 plants | 9,953 out of 10,000 plants | 9,977 out of 10,000 plants | 9,992 out of 10,000 plants | 9,999.1 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 0.9 out of 10,000 plants | 8 out of 10,000 plants | 23 out of 10,000 plants | 47 out of 10,000 plants | 87 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Cronartium quercuum has been reported as present in the UK, although uncertainties exist due to taxonomic issues. Quercus robur is reported to be telial host of the pathogen. Both telial and aecial hosts (Castanea spp., Castanopsis spp., Fagus japonica, Notholithocarpus densiflorus, Quercus spp., Rhus chinensis and Pinus spp., respectively) can be present at a suitable distance both in the nurseries and in the surroundings making it possible the infection of oak leaves by means of spores. As some leaves could still be present on plants at the time of export, the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) the removal of infected plant material and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. quercuum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of C. quercuum for plants in pots up to 15 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,841 out of 10,000 plants | 9,922 out of 10,000 plants | 9,958 out of 10,000 plants | 9,981 out of 10,000 plants | 9,996 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 4 out of 10,000 plants | 19 out of 10,000 plants | 42 out of 10,000 plants | 78 out of 10,000 plants | 159 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Cronartium quercuum has been reported as present in the UK, although uncertainties exist due to taxonomic issues. Quercus robur is reported to be telial host of the pathogen. Both telial and aecial hosts (Castanea spp., Castanopsis spp., Fagus japonica, Notholithocarpus densiflorus, Quercus spp., Rhus chinensis and Pinus spp., respectively) can be present at a suitable distance both in the nurseries and in the surroundings making it possible the infection of oak leaves by means of spores. Plants in pots can be exported at any time depending on the demand, therefore leaves can be present on the plants at the time of export, making the association with the commodity possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) the removal of infected plant material and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. quercuum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Cronartium quercuum (Section A.2 in Appendix A).
5.2.3. Overview of the evaluation of Cryphonectria parasitica (Diaporthales; Cryphonectriaceae)
| Overview of the evaluation of Cryphonectria parasitica for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,927 out of 10,000 bundles | 9,961 out of 10,000 bundles | 9,979 out of 10,000 bundles | 9,991 out of 10,000 bundles | 9,998 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 2 out of 10,000 bundles | 9 out of 10,000 bundles | 21 out of 10,000 bundles | 39 out of 10,000 bundles | 73 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Cryphonectria parasitica is present in the UK, although not widely distributed, while its main host, i.e. Castanea spp., has scattered distribution in the UK. Quercus robur is reported as a host of the pathogen and infection courts (e.g. pruning wounds, accidental breaking of twigs before export) are expected to be present. The main hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) hygiene measures with particular reference to the disinfection of tools and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. parasitica between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of C. parasitica for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,880 out of 10,000 plants | 9,933 out of 10,000 plants | 9,966 out of 10,000 plants | 9,987 out of 10,000 plants | 9,998 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 2 out of 10,000 plants | 13 out of 10,000 plants | 34 out of 10,000 plants | 67 out of 10,000 plants | 120 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Cryphonectria parasitica is present in the UK, although not widely distributed, while its main host, i.e. Castanea spp., has scattered distribution in the UK. Quercus robur is reported as a host of the pathogen and infection courts (e.g. pruning wounds, accidental breaking of twigs before export) are expected to be present. The main hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) hygiene measures with particular reference to the disinfection of tools and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. parasitica between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of C. parasitica for plants in pots up to 15 years old | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,820 out of 10,000 plants | 9,896 out of 10,000 plants | 9,943 out of 10,000 plants | 9,976 out of 10,000 plants | 9,996 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 4 out of 10,000 plants | 24 out of 10,000 plants | 57 out of 10,000 plants | 104 out of 10,000 plants | 180 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Cryphonectria parasitica is present in the UK, although not widely distributed, while its main host, i.e. Castanea spp., has scattered distribution in the UK. Quercus robur is reported as a host of the pathogen and infection courts (e.g. pruning wounds, accidental breaking of twigs before export) are expected to be present. The main hosts can be present either inside or in the surroundings of the nurseries. Altogether, this suggests that the association with the commodity may be possible. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) hygiene measures with particular reference to the disinfection of tools and (d) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. parasitica between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Cryphonectria parasitica (Section A.3 in Appendix A).
5.2.4. Overview of the evaluation of Meloidogyne mali (Rhabditida; Meloidogynidae)
| Overview of the evaluation of Meloidogyne mali for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,901 out of 10,000 bundles | 9,940 out of 10,000 bundles | 9,960 out of 10,000 bundles | 9,975 out of 10,000 bundles | 9,989 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 11 out of 10,000 bundles | 25 out of 10,000 bundles | 40 out of 10,000 bundles | 60 out of 10,000 bundles | 99 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Meloidogyne mali is present in the UK with restricted distribution. Suitable hosts are present both in the nurseries and in the surroundings. Quercus robur is a host of M. mali. The pest can enter into the nurseries and spread within the nurseries with infected plant material and movement of soil attached to machinery and shoes and run‐off water. The plants could become infected during the growth in the soil in the fields. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the nematodes. These measures include (a) the use of certified plant material; (b) the use of heat‐treated growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) hygiene measures; and (e) separation of the pots from soil. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of M. mali between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures Low‐pressure water is used for washing roots before export. This washing may not be as effective as using high‐pressure water in removing the soil, thereby making symptoms less visible.
Main uncertainties
|
||||
| Overview of the evaluation of M. mali for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,792 out of 10,000 plants | 9,873 out of 10,000 plants | 9,927 out of 10,000 plants | 9,967 out of 10,000 plants | 9,994 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 6 out of 10,000 plants | 33 out of 10,000 plants | 73 out of 10,000 plants | 127 out of 10,000 plants | 208 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Meloidogyne mali is present in the UK with restricted distribution. Suitable hosts are present both in the nurseries and in the surroundings. Quercus robur is a host of M. mali. The pest can enter into the nurseries and spread within the nurseries with infected plant material and movement of soil attached to machinery and shoes. The plants could become infected during the growth in the soil in the fields. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the nematodes. These measures include (a) the use of certified plant material; (b) the use of heat‐treated growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) hygiene measures; and (e) separation of the pots from soil. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of M. mali between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures Low‐pressure water is used for washing roots before export. This washing may not be as effective as using high‐pressure water in removing the soil, thereby making symptoms less visible.
Main uncertainties
|
||||
| Overview of the evaluation of M. mali for plants in pots up to 15 years old | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,793 out of 10,000 plants | 9,866 out of 10,000 plants | 9,914 out of 10,000 plants | 9,953 out of 10,000 plants | 9,986 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 14 out of 10,000 plants | 47 out of 10,000 plants | 86 out of 10,000 plants | 134 out of 10,000 plants | 207 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Meloidogyne mali is present in the UK with restricted distribution. Suitable hosts are present both in the nurseries and in the surroundings. Quercus robur is a host of M. mali. The pest can enter into the nurseries and spread within the nurseries with infected plant material and movement of soil attached to machinery and shoes. The plants could become infected during the growth in the soil in the fields. Measures taken against the pest and their efficacy General measures taken by the nurseries are effective against the nematodes. These measures include (a) the use of certified plant material; (b) the use of heat‐treated growing media; (c) inspections, surveillance, monitoring, sampling and laboratory testing; (d) hygiene measures; and (e) separation of the pots from soil. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of M. mali between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures Low‐pressure water is used for washing roots before export. This washing may not be as effective as using high‐pressure water in removing the soil, thereby making symptoms less visible.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Meloidogyne mali (Section A.4 in Appendix A).
5.2.5. Overview of the evaluation of Phytophthora kernoviae (Peronosporales; Peronosporaceae)
| Overview of the evaluation of Phytophthora kernoviae for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,907 out of 10,000 bundles | 9,948 out of 10,000 bundles | 9,973 out of 10,000 bundles | 9,989 out of 10,000 bundles | 9,997 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 3 out of 10,000 bundles | 11 out of 10,000 bundles | 27 out of 10,000 bundles | 52 out of 10,000 bundles | 93 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora kernoviae is present in the UK with a restricted distribution. The pathogen has a wide host range including Quercus. The main hosts (e.g. Rhododendron spp.) can be present in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy Phytophthora kernoviae is a provisional quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of P. kernoviae between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of P. kernoviae for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,917 out of 10,000 plants | 9,957 out of 10,000 plants | 9,977 out of 10,000 plants | 9,990 out of 10,000 plants | 9,997.7 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 2.3 out of 10,000 plants | 10 out of 10,000 plants | 23 out of 10,000 plants | 43 out of 10,000 plants | 83 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora kernoviae is present in the UK with a restricted distribution. The pathogen has a wide host range including Quercus. The main hosts (e.g. Rhododendron spp.) can be present in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy P. kernoviae is a provisional quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of P. kernoviae between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of P. kernoviae for plants in pots up to 15 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,824 out of 10,000 plants | 9,905 out of 10,000 plants | 9,952 out of 10,000 plants | 9,981 out of 10,000 plants | 9,997 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 3 out of 10,000 plants | 19 out of 10,000 plants | 48 out of 10,000 plants | 95 out of 10,000 plants | 176 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora kernoviae is present in the UK with a restricted distribution. The pathogen has a wide host range including Quercus. The main host (e.g. Rhododendron spp.) can be present in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy P. kernoviae is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) application of pest control products and (d) removal of infected plant material. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of P. kernoviae between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Phytophthora kernoviae (Section A.5 in Appendix A).
5.2.6. Overview of the evaluation of Phytophthora ramorum (non‐EU isolates) (Peronosporales; Peronosporaceae)
| Overview of the evaluation of Phytophthora ramorum (non‐EU isolates) for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,872 out of 10,000 bundles | 9,922 out of 10,000 bundles | 9,957 out of 10,000 bundles | 9,981 out of 10,000 bundles | 9,995 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected bundles | 5 out of 10,000 bundles | 19 out of 10,000 bundles | 43 out of 10,000 bundles | 78 out of 10,000 bundles | 128 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including Quercus. The main hosts (e.g. Rhododendron spp., Larix spp., Viburnum spp. etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy P. ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of P. ramorum (non‐EU isolates) for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Pest free with some exceptional cases (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,886 out of 10,000 plants | 9,936 out of 10,000 plants | 9,964 out of 10,000 plants | 9,983 out of 10,000 plants | 9,995 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 5 out of 10,000 plants | 17 out of 10,000 plants | 36 out of 10,000 plants | 64 out of 10,000 plants | 114 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including Quercus. The main hosts (e.g. Rhododendron spp., Larix spp., Viburnum spp. etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy P. ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; and (c) application of pest control products. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of P. ramorum (non‐EU isolates) for plants in pots up to 15 years old | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,757 out of 10,000 plants | 9,860 out of 10,000 plants | 9,924 out of 10,000 plants | 9,968 out of 10,000 plants | 9,993 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infected plants | 7 out of 10,000 plants | 32 out of 10,000 plants | 76 out of 10,000 plants | 140 out of 10,000 plants | 243 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Phytophthora ramorum is present in the UK with a restricted distribution. The pathogen has a wide host range including Quercus. The main hosts (e.g. Rhododendron spp., Larix spp., Viburnum spp., etc.) can be present either inside or in the surroundings of the nurseries. Aerial inoculum could be produced on these host plants and cause bark and leaf infections on the commodity. Measures taken against the pest and their efficacy P. ramorum is a quarantine pest in the UK and under official control. General measures taken by the nurseries are effective against the pathogen. These measures include (a) the use of certified plant material and growing media; (b) inspections, surveillance, monitoring, sampling and laboratory testing; (c) application of pest control products and (d) removal of infected plant material. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Phytophthora ramorum (Section A.6 in Appendix A).
5.2.7. Overview of the evaluation of Thaumetopoea processionea (Lepidoptera; Notodontidae)
| Overview of the evaluation of Thaumetopoea processionea for bundles of whips and seedlings | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free bundles | 9,787 out of 10,000 bundles | 9,877 out of 10,000 bundles | 9,940 out of 10,000 bundles | 9,980 out of 10,000 bundles | 9,998 out of 10,000 bundles |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested bundles | 2 out of 10,000 bundles | 20 out of 10,000 bundles | 60 out of 10,000 bundles | 123 out of 10,000 bundles | 213 out of 10,000 bundles |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK in areas where some of the nurseries are located. Host species around the nurseries are widely distributed. Adults can fly and reach the nurseries. Plants produced in another nursery could carry the pest. Measures taken against the pest and their efficacy General measures taken in the nursery are effective against the pest, these measures include (a) the use of certified plant material; (b) monitoring and sampling; (c) removal of infected plant material; (d) application of pest control products and (e) inspections. However, the pest could go undetected during inspections. Interception records In the EUROPHYT/TRACES‐NT database there are 88 records of notification of Quercus plants for planting (Quercus cerris, Q. frainetto, Q. petraea, Q. robur, Q. × turneri) from the Netherlands, Germany and Belgium due to the presence of T. processionea between the years 1995 and December 2022, all for plants intended for planting, already planted (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of T. processionea for bare root plants/trees up to 7 years old with circumference below 80 mm at 1.2 m height | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,777 out of 10,000 plants | 9,855 out of 10,000 plants | 9,918 out of 10,000 plants | 9,967 out of 10,000 plants | 9,995 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 5 out of 10,000 plants | 33 out of 10,000 plants | 82 out of 10,000 plants | 145 out of 10,000 plants | 223 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK in areas where some of the nurseries are located. Host species around the nurseries are widely distributed. Adults can fly and reach the nurseries. Plants produced in another nursery could carry the pest. Measures taken against the pest and their efficacy General measures taken in the nursery are effective against the pest, these measures include (a) the use of certified plant material; (b) monitoring and sampling; (c) removal of infested plant material; (d) application of pest control products and (e) inspections. However, the pest could go undetected during inspections. Interception records In the EUROPHYT/TRACES‐NT database there are 88 records of notification of Quercus plants for planting (Quercus cerris, Q. frainetto, Q. petraea, Q. robur, Q. × turneri) from the Netherlands, Germany and Belgium due to the presence of T. processionea between the years 1995 and December 2022, all for plants intended for planting, already planted (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of T. processionea for plants in pots up to 15 years old with circumference below 80 mm at 1.2 m height | |||||
| Rating of the likelihood of pest freedom | Extremely frequently pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,733 out of 10,000 plants | 9,826 out of 10,000 plants | 9,902 out of 10,000 plants | 9,960 out of 10,000 plants | 9,994 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 6 out of 10,000 plants | 40 out of 10,000 plants | 98 out of 10,000 plants | 174 out of 10,000 plants | 267 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity The pest is present in the UK in areas where some of the nurseries are located. Host species around the nurseries are widely distributed. Adults can fly and reach the nurseries. Plants produced in another nursery could carry the pest. Measures taken against the pest and their efficacy General measures taken in the nursery are effective against the pest, these measures include (a) the use of certified plant material; (b) monitoring and sampling; (c) removal of infested plant material; (d) application of pest control products and (e) inspections. However, the pest could go undetected during inspections, especially in bigger trees. Interception records In the EUROPHYT/TRACES‐NT database there are 88 records of notification of Quercus plants for planting (Quercus cerris, Q. frainetto, Q. petraea, Q. robur, Q. × turneri) from the Netherlands, Germany and Belgium due to the presence of T. processionea between the years 1995 and December 2022, all for plants intended for planting, already planted (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Thaumetopoea processionea (Section A.7 in Appendix A).
5.2.8. Overview of the evaluation of Trinophylum cribratum (Coleoptera; Cerambycidae)
| Overview of the evaluation of Trinophylum cribratum for bundles of whips and seedlings | |
| Summary of the information used for the evaluation | The pest in not associated with this commodity because the diameter of plants at the base is not big enough to permit colonisation of the pest. |
| Overview of the evaluation of T. cribratum for bare root plants/trees up to 7 years old | |||||
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,996.5 out of 10,000 plants | 9,998.1 out of 10,000 plants | 9,998.9 out of 10,000 plants | 9,999.5 out of 10,000 plants | 9,999.91 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 0.09 out of 10,000 plants | 0.5 out of 10,000 plants | 1.1 out of 10,000 plants | 1.9 out of 10,000 plants | 3.5 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Trinophylum cribratum is present in the UK in central and southern England. It is a polyphagous pest that can colonise many tree species, included Quercus spp. Host species are present within 2 km from the nurseries. Moreover, the woodlands may be at the border of the nurseries, where the presence of declining or dead host trees suitable for the reproduction of the pest cannot be excluded. Adults can fly in search of suitable wood material to reproduce. Size of bigger plants are enough to permit colonisation by the pest. Measures taken against the pest and their efficacy Measures taken against the pest like registration of production sites, regular surveys carried out during the production or before export by visual inspection of the plants, or the removal of wilting branches, infested plants and pruning residues (either healthy or infested) will have a positive effect on the control of the pest. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of T. cribratum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
| Overview of the evaluation of T. cribratum for plants in pots up to 15 years old | |||||
| Rating of the likelihood of pest freedom | Almost always pest free (based on the Median). | ||||
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of pest‐free plants | 9,992.1 out of 10,000 plants | 9,995.1 out of 10,000 plants | 9,997.4 out of 10,000 plants | 9,999.04 out of 10,000 plants | 9,999.89 out of 10,000 plants |
| Percentile of the distribution | 5% | 25% | Median | 75% | 95% |
| Proportion of infested plants | 0.11 out of 10,000 plants | 0.96 out of 10,000 plants | 2.6 out of 10,000 plants | 4.9 out of 10,000 plants | 7.9 out of 10,000 plants |
| Summary of the information used for the evaluation |
Possibility that the pest could become associated with the commodity Trinophylum cribratum is present in the UK in central and southern England. It is a polyphagous pest that can colonise many tree species, included Quercus spp. Host species are present within 2 km from the nurseries. Moreover, the woodlands may be at the border of the nurseries, where the presence of declining or dead host trees suitable for the reproduction of the pest cannot be excluded. Adults can fly in search of suitable wood material to reproduce. Older pruned and potted trees that may be stressed or weakened may attract adults. Size of branches of bigger trees are enough to permit colonisation by the pest. Measures taken against the pest and their efficacy Measures taken against the pest like registration of production sites, regular surveys carried out during the production or before export by visual inspection of the plants, or the removal of wilting branches, infested plants and pruning residues (either healthy or infested) will have a positive effect on the control of the pest. Interception records In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of T. cribratum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online). Shortcomings of current measures/procedures None observed.
Main uncertainties
|
||||
For more details, see relevant pest data sheet on Trinophylum cribratum (Section A.8 in Appendix A).
5.2.9. Outcome of expert knowledge elicitation
Table 7 and Figures 3, 4 and 5 show the outcome of the EKE regarding pest freedom after the evaluation of the implemented risk mitigation measures for all the evaluated pests.
Table 7.
Assessment of the likelihood of pest freedom following evaluation of current risk mitigation measures against pests on Q. robur plants designated for export to the EU. In panel A, the median value for the assessed level of pest freedom for each pest is indicated by ‘M', the 5% percentile is indicated by ‘L' and the 95% percentile is indicated by ‘U'. The percentiles together span the 90% uncertainty range regarding pest freedom. The pest freedom categories are defined in panel B of the table
| Number | Group | Pest species/Commodity | Sometimes pest free | More often than not pest free | Frequently pest free | Very frequently pest free | Extremely frequently pest free | Pest free with some exceptional cases | Pest free with few exceptional cases | Almost always pest free |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Fungi | Coniella castaneicola/Bundles of whips and seedlings | L | M | U | |||||
| 2 | Fungi | Cronartium quercuum/Bundles of whips and seedlings | L | M | U | |||||
| 3 | Fungi | Cryphonectria parasitica/Bundles of whips and seedlings | L | M | U | |||||
| 4 | Nematodes | Meloidogyne mali/Bundles of whips and seedlings | L | MU | ||||||
| 5 | Oomycetes | Phytophthora kernoviae/Bundles of whips and seedlings | L | M | U | |||||
| 6 | Oomycetes | Phytophthora ramorum/Bundles of whips and seedlings | L | M | U | |||||
| 7 | Insects | Thaumetopoea processionea/Bundles of whips and seedlings | L | M | U | |||||
| 8 | Fungi | Coniella castaneicola/Bare root plants | L | M | U | |||||
| 9 | Fungi | Cronartium quercuum/Bare root plants | L | M | U | |||||
| 10 | Fungi | Cryphonectria parasitica/Bare root plants | L | M | U | |||||
| 11 | Nematodes | Meloidogyne mali/Bare root plants | L | M | U | |||||
| 12 | Oomycetes | Phytophthora kernoviae/Bare root plants | L | M | U | |||||
| 13 | Oomycetes | Phytophthora ramorum/Bare root plants | L | M | U | |||||
| 14 | Insects | Thaumetopoea processionea/Bare root plants | L | M | U | |||||
| 15 | Insects | Trinophylum cribratum/Bare root plants | LMU | |||||||
| 16 | Fungi | Coniella castaneicola/Plants in pots | L | M | U | |||||
| 17 | Fungi | Cronartium quercuum/Plants in pots | L | M | U | |||||
| 18 | Fungi | Cryphonectria parasitica/Plants in pots | L | M | U | |||||
| 19 | Nematodes | Meloidogyne mali/Plants in pots | L | M | U | |||||
| 20 | Oomycetes | Phytophthora kernoviae/Plants in pots | L | M | U | |||||
| 21 | Oomycetes | Phytophthora ramorum/Plants in pots | L | M | U | |||||
| 22 | Insects | Thaumetopoea processionea/Plants in pots | L | M | U | |||||
| 23 | Insects | Trinophylum cribratum/Plants in pots | L | MU |
Figure 3.
Elicited certainty (y‐axis) of the number of pest‐free bundles of Quercus robur whips and seedlings (x‐axis; log‐scaled) out of 10,000 bundles designated for export to the EU from the UK for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
Figure 4.
Elicited certainty (y‐axis) of the number of pest‐free up to 7y Quercus robur bare root plants (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU from the UK for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
Figure 5.
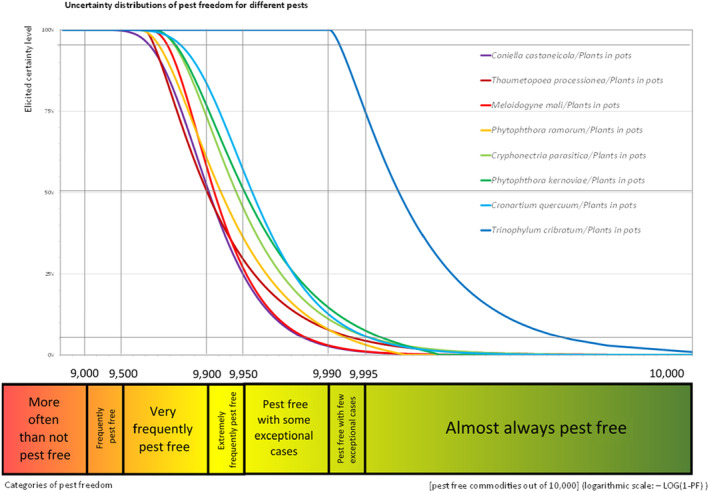
Elicited certainty (y‐axis) of the number of pest‐free Quercus robur plants in pots (x‐axis; log‐scaled) out of 10,000 plants designated for export to the EU from the UK for all evaluated pests visualised as descending distribution function. Horizontal lines indicate the percentiles (starting from the bottom 5%, 25%, 50%, 75%, 95%)
PANEL A
| Pest freedom category | Pest fee plants out of 10,000 | Legend of pest freedom categories | |||
|---|---|---|---|---|---|
| Sometimes pest free | ≤ 5,000 | L | Pest freedom category includes the elicited lower bound of the 90% uncertainty range | ||
| More often than not pest free | 5,000–≤ 9,000 | M | Pest freedom category includes the elicited median | ||
| Frequently pest free | 9,000–≤ 9,500 | U | Pest freedom category includes the elicited upper bound of the 90% uncertainty range | ||
| Very frequently pest free | 9,500–≤ 9,900 | ||||
| Extremely frequently pest free | 9,900–≤ 9,950 | ||||
| Pest free with some exceptional cases | 9,950–≤ 9,990 | ||||
| Pest free with few exceptional cases | 9,990–≤ 9,995 | ||||
| Almost always pest free | 9,995–≤ 10,000 | ||||
PANEL B
Figure 6 provides an explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the implemented risk mitigation measures for Q. robur plants in pots up to 15 years old designated for export to the EU for C. castaneicola.
Figure 6.
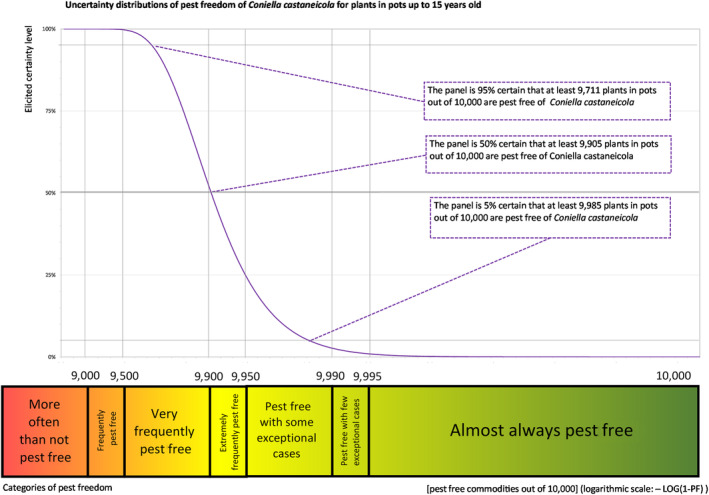
Explanation of the descending distribution function describing the likelihood of pest freedom after the evaluation of the implemented risk mitigation measures for plants designated for export to the EU based on based on the example of Coniella castaneicola on Quercus robur plants in pots up to 15 years old
6. Evaluation of the application of special requirements in the UK
Commission Implementing Regulation (EU) 2019/2072 specifies in point 22 of Annex X special requirements for plants for planting of Quercus L., other than Q. suber L., of a girth of at least 8 cm measured at 1,2 m height from the root collar, other than fruits and seeds for T. processionea.
| Special requirements as specified in Point 22 of Annex X of Commission Implementing Regulation (EU) 2019/2072 | Implementation of the special requirements in the UK according to information provided in the Dossier | Important remarks |
|---|---|---|
| ‘Official statement that: | ||
| a) the plants have been grown throughout their life in places of production in countries where T. processionea L. is not known to occur, OR | No, because the pest is present in the UK. | |
| b) the plants have been grown throughout their life in an area free from T. processionea L. established by the National Plant Protection Organisation in accordance with relevant International Standards for Phytosanitary Measures, OR | Yes, if the following conditions are fulfilled:
|
Some of the seedlings are originating from the EU infested areas (the Netherlands). One nursery indicated in the Dossier is in the buffer zone of 2022 shown in the map (Forestry Commission, online). |
| c) the plants have been grown throughout their life in a site with complete physical protection against the introduction of T. processionea L. and have been inspected at appropriate times and found to be free from T. processionea L.’ | No, because the physical protection of these larger trees is not foreseen. They are grown in the open fields. |
7. Conclusions
There are eight pests identified to be present in the UK and considered to be potentially associated with the commodities imported from the UK and relevant for the EU.
These pests are Coniella castaneicola, Cronartium quercuum, Cryphonectria parasitica, Meloidogyne mali, Phytophthora kernoviae, Phytophthora ramorum (non‐EU isolates), Thaumetopoea processionea and Trinophylum cribratum. The likelihood of the pest freedom after the evaluation of the implemented risk mitigation measures for the commodities designated for export to the EU was estimated. In the assessment of risk, the age of the plants was considered, reasoning that older trees are more likely to be infested mainly due to longer exposure time and larger size.
For Coniella castaneicola the likelihood of pest freedom for bundles of whips and seedlings following evaluation of current risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘very frequently pest free’ to ‘almost always pest free. The EKE indicated, with 95% certainty, that between 9,849 and 10,000 bundles of whips and seedlings per 10,000 will be free from C. coniella. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases'. The EKE indicated, with 95% certainty, that between 9,813 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from C. castaneicola. The likelihood of pest freedom for plants in pots up to 15 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases'. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,711 and 10,000 plants in pots up to 15 years old per 10,000 will be free from C. castaneicola.
For Cronartium quercuum the likelihood of pest freedom for bundles of whips and seedlings following evaluation of current risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘very frequently pest free’ to ‘almost always pest free. The EKE indicated, with 95% certainty, that between 9,881 and 10,000 bundles of whips and seedlings per 10,000 will be free from C. quercuum. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘pest free with some exceptional cases' with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,913 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from C. quercuum. The likelihood of pest freedom for plants in pots up to 15 years old was estimated as ‘pest free with some exceptional cases' with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,841 and 10,000 plants in pots up to 15 years old per 10,000 will be free from C. quercuum.
For Cryphonectria parasitica the likelihood of pest freedom for bundles of whips and seedlings following evaluation of current risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘extremely frequently pest free’ to ‘pest free with few exceptional cases’. The Expert Knowledge Elicitation indicated, with 95% certainty, that between 9,927 and 10,000 bundles of whips and seedlings per 10,000 will be free from C. parasitica. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,880 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from C. parasitica. The likelihood of pest freedom for plants in pots up to 15 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,820 and 10,000 plants in pots up to 15 years old per 10,000 will be free from C. parasitica.
For Meloidogyne mali the likelihood of pest freedom for bundles of whips and seedlings following evaluation of current risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘extremely frequently pest free’ to ‘pest free with some exceptional cases’. The EKE indicated, with 95% certainty, that between 9,901 and 10,000 bundles of whips and seedlings per 10,000 will be free from M. mali. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The EKE indicated, with 95% certainty, that between 9,792 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from M. mali. The likelihood of pest freedom for plants in pots up to 15 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with some exceptional cases’. The EKE indicated, with 95% certainty, that between 9,793 and 10,000 plants in pots up to 15 years old per 10,000 will be free from M. mali.
For Phytophthora kernoviae the likelihood of pest freedom for bundles of whips and seedlings following evaluation of current risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘extremely frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,907 and 10,000 bundles of whips and seedlings per 10,000 will be free from P. kernoviae. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘extremely frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,917 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from P. kernoviae. The likelihood of pest freedom for plants in pots up to 15 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,824 and 10,000 plants in pots up to 15 years old per 10,000 will be free from P. kernoviae.
For Phytophthora ramorum the likelihood of pest freedom for bundles of whips and seedlings following evaluation of current risk mitigation measures was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range reaching from ‘very frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,872 and 10,000 bundles of whips and seedlings per 10,000 will be free from P. ramorum. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘pest free with some exceptional cases’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,886 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from P. ramorum. The likelihood of pest freedom for plants in pots up to 15 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The EKE indicated, with 95% certainty, that between 9,757 and 10,000 plants in pots up to 15 years old per 10,000 will be free from P. ramorum.
For Thaumetopoea processionea the likelihood of pest freedom for bundles of whips and seedlings following evaluation of current risk mitigation measures was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range reaching from ‘very frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,787 and 10,000 bundles of whips and seedlings per 10,000 will be free from T. processionea. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,777 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from T. processionea. The likelihood of pest freedom for plants in pots up to 15 years old was estimated as ‘extremely frequently pest free’ with the 90% uncertainty range spanning from ‘very frequently pest free’ to ‘pest free with few exceptional cases’. The EKE indicated, with 95% certainty, that between 9,733 and 10,000 plants in pots up to 15 years old per 10,000 will be free from T. processionea.
The diameter at the base of whips and seedlings is not big enough to permit colonisation of Trinophylum cribratum and hence this commodity is considered free of T. cribratum. The likelihood of pest freedom for bare root plants/trees up to 7 years old was estimated as ‘almost always pest free’ with the 90% uncertainty range being in the category ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,996 and 10,000 bare root plants/trees up to 7 years old per 10,000 will be free from T. cribratum. The likelihood of pest freedom for plants in pots up to 15 years old was estimated as ‘almost always pest free’ with the 90% uncertainty range spanning from ‘pest free with some exceptional cases’ to ‘almost always pest free’. The EKE indicated, with 95% certainty, that between 9,992 and 10,000 plants in pots up to 15 years old per 10,000 will be free from T. cribratum.
Abbreviations
- APHA
Animal and Plant Health Agency
- CABI
Centre for Agriculture and Bioscience International
- DEFRA
Department for Environment Food and Rural Affairs
- EKE
Expert Knowledge Elicitation
- EPPO
European and Mediterranean Plant Protection Organization
- FAO
Food and Agriculture Organization
- ISPM
International Standards for Phytosanitary Measures
- NPPO
National Plant Protection Organisation
- PHSI
Plant Health and Seeds Inspectorate
- PLH
Plant Health
- PRA
Pest Risk Assessment
- RNQPs
Regulated Non‐Quarantine Pests
- SASA
Science and Advice for Scottish Agriculture
Glossary
- Control (of a pest)
Suppression, containment or eradication of a pest population (FAO, 1995, 2017).
- Entry (of a pest)
Movement of a pest into an area where it is not yet present, or present but not widely distributed and being officially controlled (FAO, 2017).
- Establishment (of a pest)
Perpetuation, for the foreseeable future, of a pest within an area after entry (FAO, 2017).
- Impact (of a pest)
The impact of the pest on the crop output and quality and on the environment in the occupied spatial units.
- Introduction (of a pest)
The entry of a pest resulting in its establishment (FAO, 2017).
- Measures
Control (of a pest) is defined in ISPM 5 (FAO, 2017) as ‘Suppression, containment or eradication of a pest population’ (FAO, 1995). Control measures are measures that have a direct effect on pest abundance. Supporting measures are organisational measures or procedures supporting the choice of appropriate risk mitigation measures that do not directly affect pest abundance.
- Pathway
Any means that allows the entry or spread of a pest (FAO, 2017).
- Phytosanitary measures
Any legislation, regulation or official procedure having the purpose to prevent the introduction or spread of quarantine pests or to limit the economic impact of regulated non‐quarantine pests (FAO, 2017).
- Protected zone
A Protected zone is an area recognised at EU level to be free from a harmful organism, which is established in one or more other parts of the Union.
- Quarantine pest
A pest of potential economic importance to the area endangered thereby and not yet present there, or present but not widely distributed and being officially controlled (FAO, 2017).
- Regulated non‐quarantine pest
A non‐quarantine pest whose presence in plants for planting affects the intended use of those plants with an economically unacceptable impact and which is therefore regulated within the territory of the importing contracting party (FAO, 2017).
- Risk mitigation measure
A measure acting on pest introduction and/or pest spread and/or the magnitude of the biological impact of the pest should the pest be present. A risk mitigation measure may become a phytosanitary measure, action or procedure according to the decision of the risk manager.
- Spread (of a pest)
Expansion of the geographical distribution of a pest within an area (FAO, 2017).
Appendix A – Data sheets of pests selected for further evaluation
A.1. Coniella castaneicola
A.1.1. Organism information
| Taxonomic information |
Current valid scientific name: Coniella castaneicola Synonyms: Anthasthoopa simba, Asteromella castaneicola, Coniella simba, Dothidella castaneicola, Embolidium eucalypti, Gloeosporium castaneicola, Phyllosticta castanicola, Pilidiella castaneicola (according to Index Fungorum) Name used in the EU legislation: – Order: Diaportales Family: Schizoparmaceae Common name: white rot, Coniella leaf blight Name used in the Dossier: Coniella castaneicola |
|
| Group | Fungi | |
| EPPO code | – | |
| Regulated status |
Coniella castaneicola is neither regulated in the EU, nor listed by EPPO. Coniella castaneicola is quarantine pathogen for New Zealand (MAF Biosecurity New Zealand, 2009), Western Australia (Australian Department of Agriculture, 2014) and Korea (Korea Government, 2013). |
|
| Pest status in the UK |
Coniella castaneicola is present in the UK, where it is found in the London area (Elmbridge, Wandsworth) and in south England (New Forest) (NBN Atlas, online; Dossier Section 5.0). The pathogen was recorded from England in 1991 (South Hampshire), 1997 (Surrey), 2001 (Surrey) and from Scotland in 2006 (Dawyck Botanic Garden) (NBN atlas, online). In 2015 it was found on cupules of Castanea sativa from Studland, Dorset, England (Dorset nature, online). |
|
| Pest status in the EU | Coniella castaneicola is reported in Germany on oak (Kehr and Wulf, 1993). In addition, it was found in Latvia on few strawberry plantations in Kurzeme, in 2007 and 2008 (Laugale et al., 2009). | |
| Host status on Quercus |
Coniella castaneicola was reported on Quercus robur in Germany (Kehr and Wulf, 1993). Coniella castaneicola is a pathogen of other Quercus species such as Q. acutissima, Q. mongolica var. grosseserrata, Q. rubra and Q. serrata (Kaneko, 1981). |
|
| PRA information | Available Pest Risk Assessment:
|
|
| Other relevant information for the assessment | ||
| Biology |
Coniella castaneicola is an ascomycete fungus causing rot of fruits and leaf spots on a number of hosts throughout the world, frequently found on living, decaying and dead leaves (Farr and Rossman, online). It is present in Africa (South Africa, Nigeria) (Van Niekerk et al., 2004; Australian department of Agriculture, 2014); Asia (China, Korea, India, Indonesia, Pakistan, Japan, Taiwan) (Farr and Rossman, online; Wang and Lin, 2004; Australian department of Agriculture, 2014); Australia (Australian department of Agriculture, 2014); North America and Caribbean (Canada, the US, Cuba) (Farr and Rossman, online); South America (Brazil) (Barreto et al., 2022). Coniella castaneicola is also present in Europe in Germany (Kehr and Wulf, 1993), Latvia (Laugale et al., 2009), Switzerland (Bissegger and Sieber, 1994), Russia (Melkumov, 2014) and the UK (GBIF, online; NBN Atlas, online). There is poor information on the biology and life cycle of C. castaneicola; however, its biology is considered very similar to that of Coniella diplodiella (Pilidiella diplodiella), so that the two species have been assessed together in Australia on grapevine (Australian Department of Agriculture, 2014). Coniella castaneicola is mostly known as a pathogen of grapevine, affecting peduncle, rachis, pedicel and berries; secondarily it is found on foliage of deciduous trees. Infections are frequently associated with hailstorms causing wounds on grapes and foliage. Heavy rain, sun scorch and wounding caused by insects can also facilitate infection to a lesser extent (Australian Department of Agriculture, 2014). The pathogen reproduces sexually and asexually, producing ascospores and conidia, respectively, both able to cause infection and dispersed by air or water. Conidia are also able to survive in the environment for long time. Infection rapidly develops at temperatures of 24–27°C, slowly at temperatures below 15°C and only slightly above 34°C. Incubation period varies from 3 to 8 days, depending on temperature, relative humidity, means of penetration and the tissue infected (Australian Department of Agriculture, 2014). Pycnidia and conidia of the pathogen overwinter on dead leaves and survive in the soil for long time (up to 15 years in case of C. diplodiella); conidia may germinate under favourable conditions and establish infection on suitable hosts. Conidia are dispersed over short distances by water splash from infected plant material as well as contaminated soil. On medium‐long distances, both ascospores and conidia may be dispersed by air currents. The movement of infected material or nursery stock and contaminated soil may also contribute to spreading of the pathogen (Australian Department of Agriculture, 2014). |
|
| Symptoms | Main type of symptoms |
Typical symptom on grapevine is white rot of peduncle, rachis, pedicel and berries. The infection begins as small, pale brown, elongated depressions, which may rapidly spread in favourable conditions, causing drying and falling of berries (Australian Department of Agriculture, 2014). According to Kaneko (1981), on Castanea and Quercus species in Japan the first symptom on leaves in summer is sparse small spots pale brown, becoming greyish white in colour. The spots increase in size and form irregular‐shaped lesions causing marked leaf blight. Pycnidia are produced in the lesions on both leaf surfaces as minute black points. Usually, the disease seems not causing premature defoliation. |
| Presence of asymptomatic plants | In Switzerland, C. castaneicola was isolated from young healthy in appearance shoots of Castanea sativa (Bissegger and Sieber, 1994). | |
| Confusion with other pests |
On grapevine, C. castaneicola and C. diplodiella cause very similar symptoms, hardly distinguishable. On deciduous trees, the symptoms of C. castaneicola may possibly be confused with those of foliage diseases caused by other ascomycete fungi, also depending on the host plant. Identification of the pathogen cannot be done on a symptomatic basis and requires examination of the mycelium and inoculum material by specialists. A good description of sexual morph of the pathogen on Castanea is provided by Jiang et al. (2021). |
|
| Host plant range |
Coniella castaneicola has a variety of hosts including Acer spp., Carya spp., Castanea sativa, C. crenata, C. mollissima, C. dentata, Castanea spp., Castanopsis sempervirens, Eucalyptus grandis, Eucalyptus spp., Fragaria spp., Liquidambar styraciflua, Mangifera indica, Quercus acutissima, Q. alba, Q. mongolica var. grosseserrata, Q. rubra, Q. serrata Quercus spp., Rhus copallina, Rhus spp., Rosa rugosa‐prostrata, Syzygium aromaticum, Vaccinium virgatum, Vitis cordifolia and V. vinifera (Kaneko, 1981; Crous and Van der Linde, 1993; Farr and Rossman, online). Other host plants recognised in Europe are Aesculus hippocastanum (Melkumov, 2014) and Quercus robur (Kehr and Wulf, 1993). |
|
| Reported evidence of impact |
Coniella castaneicola and C. diplodiella are mostly known as causing damage to grapevine berries, leading to crop losses and reduced marketability. In regions where hailstorms are frequent, white rot caused by C. castaneicola and C. diplodiella can lead to crop losses of 20–80% (Australian Department of Agriculture, 2014). Coniella castaneicola is also known to cause leaf and fruit diseases of strawberry in the US but no information on the economic significance was found (Australian Department of Agriculture, 2014). In Latvia the pathogen has only a little economic significance in strawberry plantations (Laugale et al., 2009). Coniella castaneicola is commonly found on leaves of Eucalyptus species, in plantations and nurseries in South Africa, Brazil and Australia, but is considered of minor importance as a pathogen causing leaf spot (Van Niekerk et al. 2004; Australian Department of Agriculture, 2014). In September 2020, C. castaneicola was observed on blueberries (Vaccinium virgatum) in Nanchang, China. The pathogen caused damage to the leaves (blight, curling, falling off), dieback and even shoot blight. Subsequently the pathogen lowered yield potential (floral buds' development was affected when the leaves fell off) (Lai et al., 2022). |
|
| Evidence that the commodity is a pathway | Although C. castaneicola has never been intercepted on plants for planting, the pathogen can move both via infected leaves on plants and contaminated soil in potted plants, therefore Quercus plants for planting may be a pathway. | |
| Surveillance information | Coniella castaneicola is not under official control (Dossier Section 5.0). | |
A.1.2. Possibility of pest presence in the nursery
A.1.2.1. Possibility of entry from the surrounding environment
Coniella castaneicola is present in the UK in the London area and southern England (South Hampshire, Surrey, Dorset) and Scotland (Dawyck Botanic Garden) (NBN atlas, online; Dorset nature, online; Dossier Section 5.0).
The pathogen can naturally spread with ascospores and conidia dispersed by air currents also over long distance, as well as with conidia transported with rain and water splash on short distances.
C. castaneicola can infect Acer spp., Castanea spp. (mostly C. sativa), Liquidambar spp., Quercus spp., Rosa spp., Vaccinium spp. and Vitis vinifera which are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
The presence of the pathogen on host plants in the surrounding area.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries from surrounding environment via conidia and ascospores transported by wind and air currents.
A.1.2.2. Possibility of entry with new plants/seed
The starting material of the commodities is a mix of seeds and seedlings. Seeds are certified and coming from the UK. Seedlings are obtained either from the UK or the EU (mostly the Netherlands) (Dossier Section 3.0).
In addition to Quercus plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are suitable hosts for the pathogen such as Acer spp., Aesculus hippocastanum, Castanea spp., Castanea sativa, Liquidambar spp., Rosa spp. etc. However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0).
Pycnidia and conidia of Coniella species can survive in the soil for long time (up to 15 years in case of C. diplodiella) (Australian Department of Agriculture, 2014), and therefore could potentially enter by this pathway. However, the growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
No information is available on the provenance of plants other than Quercus used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries via new seedlings of Quercus and plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.1.2.3. Possibility of spread within the nursery
Quercus plants are either grown in containers (cells, pots, tubes, etc.) outdoors, in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pathogen can infect other suitable plants, such as Acer spp., Aesculus hippocastanum, Castanea spp., Castanea sativa, Liquidambar spp. and Rosa spp. present within the nurseries (Dossier Sections 3.0 and 6.0).
C. castaneicola can naturally spread within the nurseries by rain, water splash, air currents and movement of soil.
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible by air currents, rain and water splash.
A.1.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. castaneicola between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online).
A.1.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on C. castaneicola is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
Although the pathogen is not regulated, the risk mitigation measure could have some effects in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
The risk mitigation measure could have some effects in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 4 | Growing media | Yes |
As the pathogen can survive in the soil for long time, this measure, in particular using heat‐treated growing media, could be effective in reducing the likelihood of introduction of the pathogen into the nurseries.
Uncertainties:
|
| 5 | Surveillance, monitoring and sampling | Yes |
Although the pathogen is not regulated, the risk mitigation measure could have some effects in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 6 | Hygiene measures | No | Not relevant. |
| 7 | Removal of infested/infected plant material | Yes |
This measure could have some effect.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Overhead irrigation could favour foliar infections and spread of the pathogen by water splash.
Uncertainties:
|
| 9 | Application of pest control products | Yes |
Some fungicides could reduce the likelihood of the infection by the pathogen.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
Although the pathogen is not regulated, the risk mitigation measure could have some effects in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. |
A.1.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.1.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Q. robur to be unsuitable/minor host for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections, and that infected leaves are removed from the ground thereby reducing the inoculum pressure.
A.1.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Q. robur to be a suitable host for the pathogen. The scenario also assumes that wounds (e.g. pruning wounds) representing infection courts may be present, that infected leaves are not completely removed from the ground, and that symptoms of the disease are not easily recognisable during inspections.
A.1.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection. Q. robur is considered minor host.
A.1.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.1.5.5. Elicitation outcomes of the assessment of the pest freedom for Coniella castaneicola on bundles of whips and seedlings
The following Tables show the elicited and fitted values for pest infection (Table A.1) and pest freedom (Table A.2).
Table A.1.
Elicited and fitted values of the uncertainty distribution of pest infection by Coniella castaneicola per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 15 | 30 | 80 | 200 | ||||||||||
| EKE | 1.01 | 1.30 | 1.99 | 3.86 | 7.17 | 12.3 | 18.7 | 35.3 | 59.2 | 75.5 | 97.0 | 122 | 151 | 175 | 200 |
The EKE results is the BetaGeneral (0.6983, 3.2249, 0.9, 280) distribution fitted with @Risk version 7.6.
Table A.2.
The uncertainty distribution of bundles free of Coniella castaneicola per 10,000 bundles calculated by Table A.1
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,800 | 9,920 | 9,970 | 9,985 | 9,999 | ||||||||||
| EKE results | 9,800 | 9,825 | 9,849 | 9,878 | 9,903 | 9,925 | 9,941 | 9,965 | 9,981 | 9,988 | 9,993 | 9,996 | 9,998.0 | 9,998.7 | 9,999.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.2.
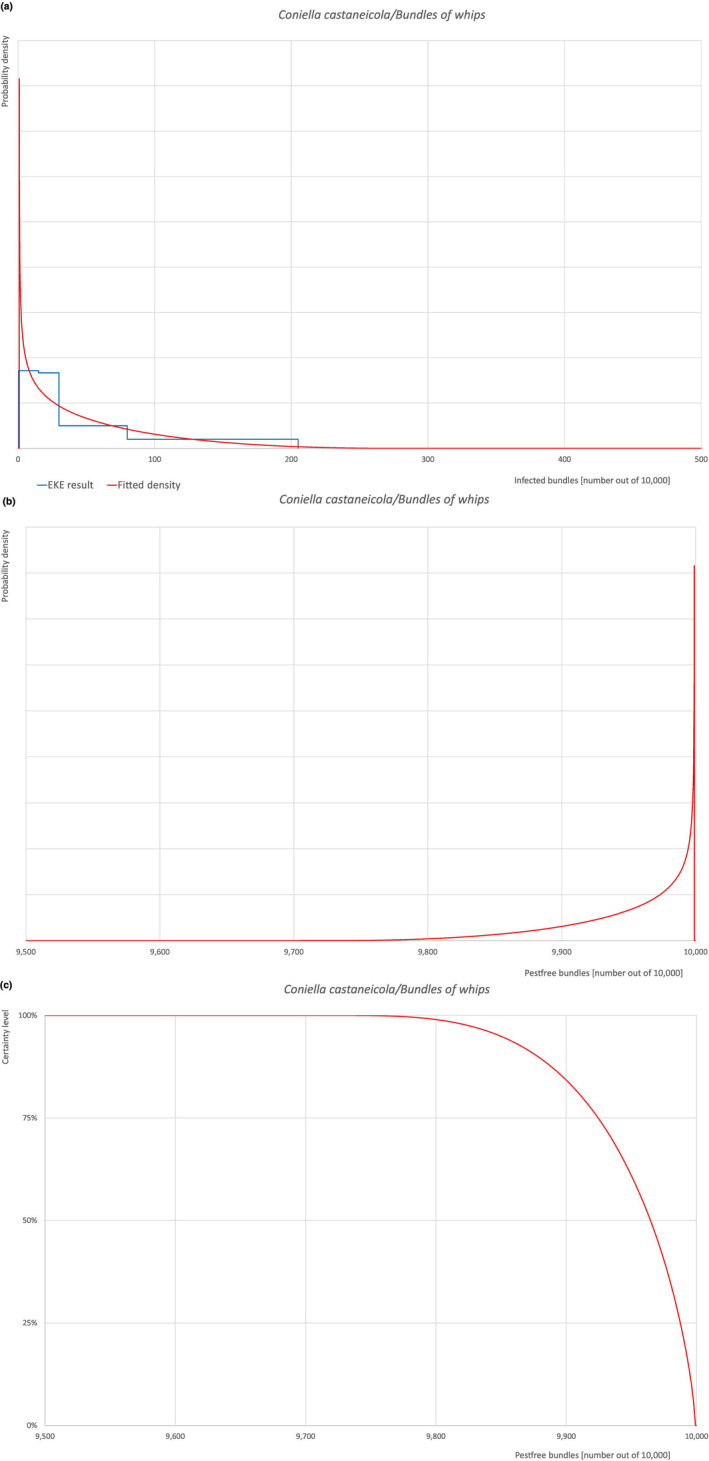
Figure A.1: (a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
A.1.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.1.6.1.Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Quercus robur to be minor host for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections, and that infected leaves are removed from the ground thereby reducing the inoculum pressure.
A.1.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Quercus robur to be a suitable host for the pathogen. The scenario also assumes that wounds (e.g. pruning wounds) representing infection courts may be present, that infected leaves are not completely removed from the ground, and that symptoms of the disease are not easily recognisable during inspections.
A.1.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection. Q. robur is considered minor host.
A.1.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.1.6.5. Elicitation outcomes of the assessment of the pest freedom for Coniella castaneicola on bare root plants/trees up to 7 years old
The following Tables show the elicited and fitted values for pest infection (Table A.3) and pest freedom (Table A.4).
Table A.3.
Elicited and fitted values of the uncertainty distribution of pest infection by Coniella castaneicola per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 25 | 50 | 95 | 300 | ||||||||||
| EKE | 1.60 | 3.34 | 5.90 | 10.6 | 16.7 | 24.3 | 32.4 | 51.3 | 76.5 | 93.8 | 118 | 147 | 187 | 226 | 276 |
The EKE results is the BetaGeneral (1.2653, 185.09, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.4.
The uncertainty distribution of plants free of Coniella castaneicola per 10,000 plants calculated by Table A.3
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,700 | 9,905 | 9,950 | 9,975 | 9,999 | ||||||||||
| EKE results | 9,724 | 9,774 | 9,813 | 9,853 | 9,882 | 9,906 | 9,923 | 9,949 | 9,968 | 9,976 | 9,983 | 9,989 | 9,994 | 9,997 | 9,998 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.4.
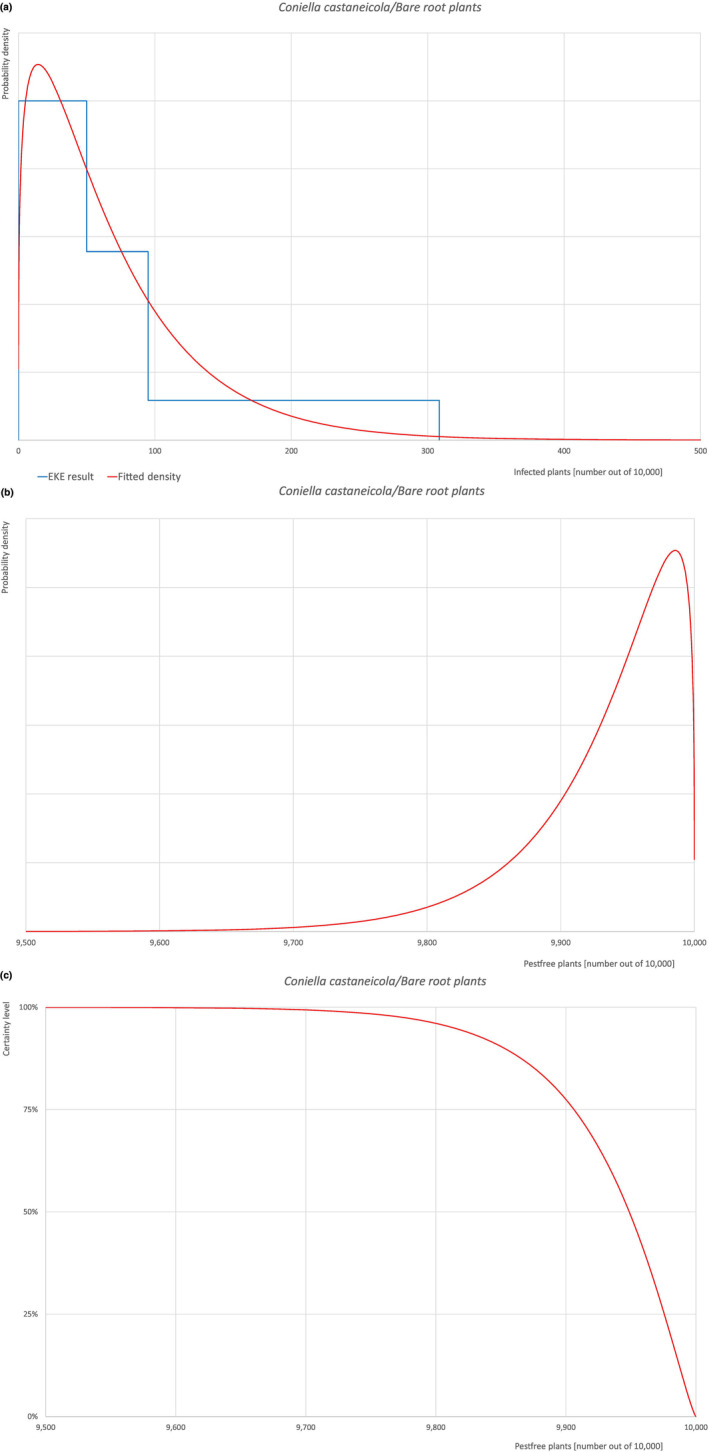
Figure A.2: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.1.7. Overall likelihood of pest freedom for plants in pots up to 15 years old
A.1.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots up to 15 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Q. robur to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections, and that infected leaves are removed from the ground thereby reducing the inoculum pressure during production and preventing the pathogen to be exported in plant material dropped on to the substrate present in pots.
A.1.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots up to 15 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Q. robur to be a suitable host for the pathogen. The scenario also assumes that several consignments are traded during the vegetation period (with leaves), that wounds representing infection courts are frequent, that infected leaves are not completely removed from the ground and that symptoms of the disease are not easily recognisable during inspections.
A.1.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots up to 15 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause infection. Q. robur is considered minor host.
A.1.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.1.7.5. Elicitation outcomes of the assessment of the pest freedom for Coniella castaneicola on plants in pots up to 15 years old
The following Tables show the elicited and fitted values for pest infection (Table A.5) and pest freedom (Table A.6).
Table A.5.
Elicited and fitted values of the uncertainty distribution of pest infection by Coniella castaneicola per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 5 | 50 | 95 | 160 | 400 | ||||||||||
| EKE | 5.11 | 9.41 | 15.1 | 24.7 | 36.2 | 50.0 | 64.0 | 95.0 | 134 | 160 | 195 | 236 | 289 | 339 | 402 |
The EKE results is the BetaGeneral (1.5486, 21.146, 0, 1,700) distribution fitted with @Risk version 7.6.
Table A.6.
The uncertainty distribution of plants free of Coniella castaneicola per 10,000 plants calculated by Table A.5
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,600 | 9,840 | 9,905 | 9,950 | 9,995 | ||||||||||
| EKE results | 9,598 | 9,661 | 9,711 | 9,764 | 9,805 | 9,840 | 9,866 | 9,905 | 9,936 | 9,950 | 9,964 | 9,975 | 9,985 | 9,991 | 9,995 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.6.
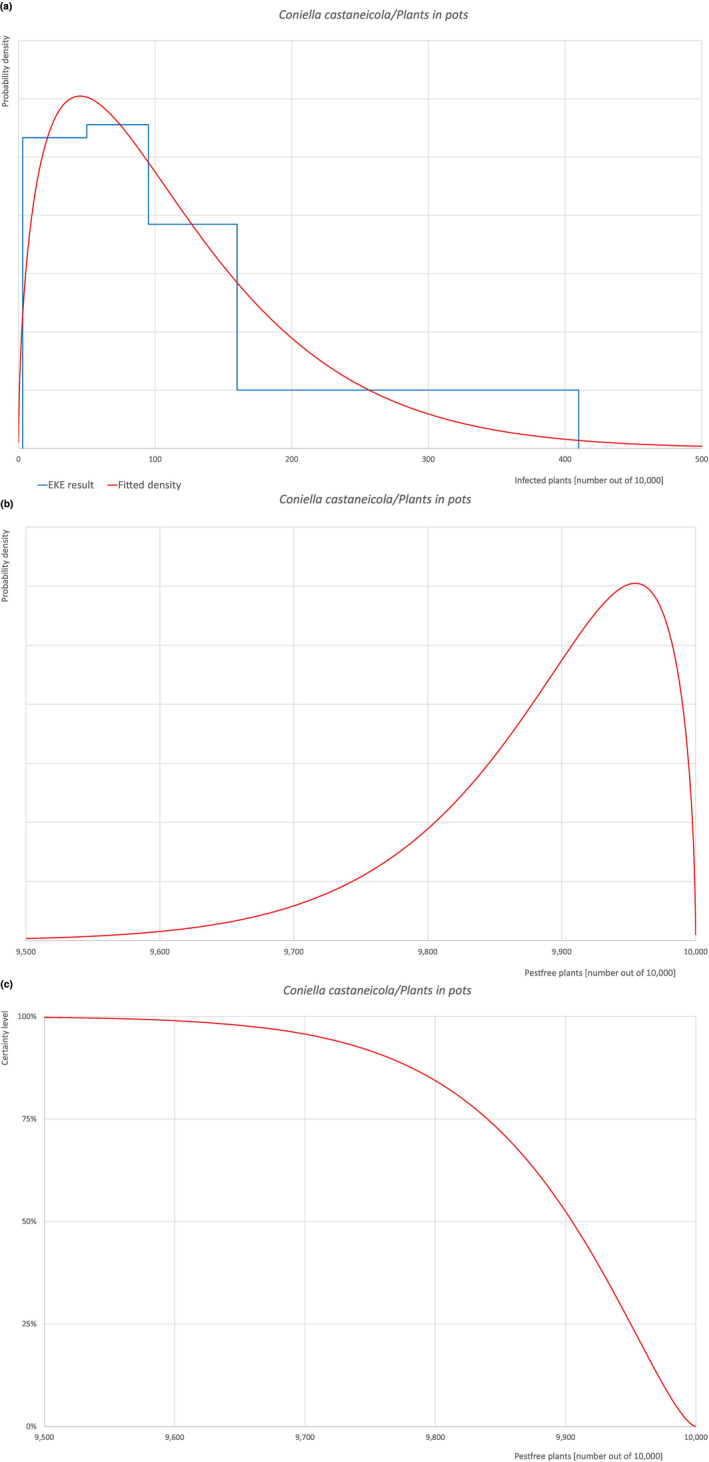
Figure A.3: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.1.8. Reference list
Australian Department of Agriculture, 2014. Draft report for the non‐regulated analysis of existing policy for table grapes from Japan. Department of Agriculture, 392 pp.
Barreto GG, Gusmão LFP and Dianese JC, 2022. Checklist of ascomycetes recorded on Eucalyptus in Brazil (1976–2022). Asian Journal of Mycology, 5, 107–129.
Bissegger M and Sieber TN, 1994. Assemblages of endophytic fungi in coppice shoots of Castanea sativa. Mycologia, 86, 648–655. https://doi.org/10.2307/3760535
Crous PW and Van der Linde EJ, 1993. New and interesting records of South African fungi. XI. Eucalyptus leaf fungi. South African Journal of Botany, 59, 300–304.
Dorset nature, online. Coniella castaneicola. Available online: http://www.dorsetnature.co.uk/pages-fungi/f-124.html [Accessed: 4 November 2022].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023a. Scientific Opinion on the commodity risk assessment of Acer campestre plants from the UK. EFSA Journal 2023;21(7):8071, 291 pp. https://doi.org/10.2903/j.efsa.2023.8071
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023b. Scientific Opinion on the commodity risk assessment of Acer palmatum plants from the UK. EFSA Journal 2023;21(7):8075, 228 pp. https://doi.org/10.2903/j.efsa.2023.8075
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023c. Scientific Opinion on the commodity risk assessment of Acer platanoides plants from the UK. EFSA Journal 2023;21(7):8073, 268 pp. https://doi.org/10.2903/j.efsa.2023.8073
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023d. Scientific Opinion on the commodity risk assessment of Acer pseudoplatanus plants from the UK. EFSA Journal 2023;21(7):8074, 271 pp. https://doi.org/10.2903/j.efsa.2023.8074
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en [Accessed: 22 December 2022].
Farr DF and Rossman AY. Fungal Databases, U.S. National Fungus Collections, ARS, USDA, online. Coniella castaneicola. Available online: https://data.nal.usda.gov/dataset/united-states-national-fungus-collections-fungus-host-dataset [Accessed: 18 February 2023].
GBIF (Global Biodiversity Information Facility) Secretariat, online. GBIF BackBone Taxonomy. Available online: https://www.gbif.org/ [Accessed: 18 February 2023].
Jiang N, Fan X and Tian C, 2021. Identification and characterization of leaf‐inhabiting fungi from Castanea plantations in China. Journal of Fungi, 7, 1–59. https://doi.org/10.3390/jof7010064
Kaneko S, 1981. Fungi inhabiting Fagaceous trees III Coniella leaf blight of Quercus and Castanea caused by Coniella castaneicola. Japanese Journal of Phytopathology, 47, 80–83.
Kehr RD and Wulf A, 1993. Fungi associated with above‐ground portions of declining oaks (Quercus robur) in Germany. European Journal Forest Pathology, 23, 18–27.
Korea Government, 2013. List of plant quarantine fungi in Korea newly revised in 2013. Research in Plant Disease, 19, 237–241.
Lai J, Xiong G, Liu B, Kuang W and Song S, 2022. First report of Coniella castaneicola causing leaf blight on blueberry (Vaccinium virgatum) in China. Plant Disease, 106, 1298.
Laugale V, Lepse L, Vilka L, and Rancāne R, 2009. Incidence of fruit rot on strawberries in Latvia, resistance of cultivars and impact of cultural systems. Sodininkystė ir daržininkystė, 28, 125–134.
MAF Biosecurity New Zealand, 2009. Import health standard commodity sub‐class: fresh fruit/vegetables mango, Mangifera indica from Australia. Ministry of Agriculture and Forestry, 19 pp.
Melkumov GM, 2014. Substrate specialization of causative agents of diseases of the tree component of the park areas of the city of Voronezh. Bulletin of the Voronezh State Agrarian University, (1–2), 57–62. (in Russian).
Nag Raj TR, 1993. Coelomycetous anamorphs with appendage‐bearing conidia. Mycologue Publications, Waterloo, Ontario, 1101 pp.
NBN Atlas (The National Biodiversity Network), online. Available online: https://nbnatlas.org/about-nbn-atlas/ [Accessed: 4 November 2022].
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Van Niekerk JA, Groenewald JZ, Verkley GJM, Fourie PH, Wingfield MJ and Crous PW, 2004. Systematic reappraisal of Coniella and Pilidiella, with specific reference to species occurring on Eucalyptus and Vitis in South Africa. Mycological Research, 108, 283–303. https://doi.org/10.1017/s0953756204009268
Wang CL and Lin CC, 2004. Five new records of ascomycetes in Taiwan. Fungal Science, 19, 21–29.
A.2. Cronartium quercuum
A.2.1. Organism information
| Taxonomic information |
Current valid scientific name: Cronartium quercuum Synonyms: Aecidium cerebrum, Aecidium giganteum, Cronartium asclepiadeum var. quercuum, Cronartium cerebrum, Cronartium fusiforme, Cronartium quercus, Dicaeoma quercus, Melampsora quercus, Peridermium cerebrum, Peridermium fusiforme, Peridermium giganteum, Peridermium mexicanum, Puccinia quercus, Uredo quercus, Uromyces quercus (according to Index Fungorum) Name used in the EU legislation: Cronartium spp. (non‐European) [1CRONG] Order: Pucciniales Family: Cronartiaceae Common name: eastern gall rust of pine Name used in the Dossier: Cronartium quercuum |
|
| Group | Fungi | |
| EPPO code | CRONQU | |
| Regulated status |
Cronartium quercuum is listed in Annex II/A of Commission Implementing Regulation (EU) 2019/2072 as Cronartium spp. (non‐European) [1CRONG], currently not present in the EU territories. Cronartium quercuum is listed in the Commission Implementing Regulation (EU) 2020/1217 as a pest of concern for Pinus parviflora. Cronartium quercuum is listed in the A1 EPPO list (EPPO, online_a). Cronartium quercuum is quarantine in Morocco, Norway and Tunisia. It is on A1 list of Georgia, Russia, Ukraine and EAEU (=Eurasian Economic Union – Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) (EPPO, online_b). |
|
| Pest status in the UK |
Cronartium quercuum is reported to be present in the UK (Dossier Section 2.0; GBIF, online; Farr and Rossman, online). The pathogen is known from England (East Sussex, North Devon, South Wiltshire, Suffolk and Surrey), Wales (Carmarthenshire and Pembrokeshire) and the Channel Islands (Guernsey) (Legon et al., online). According to the Dossier Section 5.0 the pathogen is present in the UK: not widely distributed and not under official control. |
|
| Pest status in the EU |
Cronartium quercuum is absent from the EU (EFSA PLH Panel, 2018; EPPO, online_b). However, in other databases C. quercuum is reported from Belgium, Italy, France, Germany, Spain and Portugal (Farr and Rossman, online; GBIF, online). EPPO (1997a) states that: ‘Cronartium quercuum is absent from the EU. The uredinial rust Uredo quercus is widely distributed but rather uncommon on Quercus throughout Europe and especially in Mediterranean countries. Viennot‐Bourgin (1956) mentions that the telial state has once been found in France, and identifies it as C. quercuum, but with little supporting detail. No corresponding aecial state has ever been found in Europe and, on this basis, ‘C. quercuum' would exist in Europe only as a short‐cycle uredinial rust (although it is not reported to behave in this way in North America).’ |
|
| Host status on Quercus |
Quercus petraea (from Japan) and Q. robur (from the UK) are reported hosts of Cronartium quercuum (Farr and Rossman, online; Legon et al., online). Cronartium quercuum is a pathogen of many other Quercus species such as Q. acutissima, Q. mongolica and Q. rubra (EPPO, online_c; Farr and Rossman, online). For a full list of Quercus species see Farr and Rossman (online). |
|
| PRA information | Pest Risk Assessment available:
|
|
| Other relevant information for the assessment | ||
| Biology |
Cronartium quercuum is present in Asia (China, India, Japan, North Korea, Philippines, South Korea, Taiwan), Central America (Belize, Costa Rica, Cuba, El Salvador, Honduras, Nicaragua, Panama), Europe (Russia), North America (Canada, Mexico, US) and South America (Guyana) (EPPO, online_b). It is also present in the UK (Dossier Sections 2.0 and 5.0). Cronartium quercuum is heteroecious rust that alternates its lifecycle between the aecial (Pinus species) and the telial hosts (EPPO, 1997a). Once the Pinus needles are infected, the pathogen takes one to several years to produce pycnidia and aecia. Pycnospores are not infectious and serve as spermatia. Aecia of C. quercuum appear 1 year after pycnia. Aecia then produce aeciospores, which are airborne and are able to travel long distances carried by wind (EPPO, 1997a). Once aeciospores reach a suitable telial host, infection occurs and uredinia will appear in about 1–3 weeks. Uredinia produce urediniospores which are airborne and able to re‐infect telial hosts during summer. Usually, 2 weeks after the appearance of uredinia, telia are produced in which basidiospores are formed. Basidiospores can be carried by wind up to 1.5 km distance and will infect Pinus trees via first year needles (EPPO, 1997a). There is no information available about overwintering of Cronartium quercuum. Similarly to Cronartium coleosporioides, the fungal mycelium may overwinter in bark and galls of Pinus species (EPPO, 1997b). There are four special forms of C. quercuum that have different host‐pathogen interactions with different species of pine. These are C. quercuum f.sp. banksianae (primarily pathogenic on Pinus banksiana), C. quercuum f.sp. echinatae (primarily pathogenic on Pinus echinata), C. quercuum f.sp. fusiforme (primarily pathogenic on Pinus taeda and Pinus elliottii var. elliottii) and C. quercuum f.sp. virginianae (primarily pathogenic on Pinus virginiana) (Burdsall and Snow, 1977). In North America, C. quercuum is causing damage mainly in nurseries and young plantations of Pinus species. It has been recorded to cause 25% losses on P. sylvestris (EPPO, 1997a). No damage information on Quercus is available. Possible pathways of entry for C. quercuum through aecial hosts are plants for planting, branches and non‐squared wood. Pathways of entry through telial hosts are plant for planting and branches with leaves. |
|
| Symptoms | Main type of symptoms |
On aecial hosts (Pinus spp.), Cronartium quercuum develops yellow/brown galls on stems, branches and on trunks, which can result in lesions. Infection of seedlings can cause severe stunting or rapid death (EPPO, 1997a). On telial hosts (including Quercus species), the infection is restricted only to the leaves (EPPO, 1997a). Cronartium spp. produce yellow spots (uredinia) on the lower side of leaves, yellow to necrotic leaf blotches and cause premature defoliation (Sinclair and Lyon, 2005). |
| Presence of asymptomatic plants |
No information on the presence of asymptomatic Quercus plants was found. Infected Pinus species will be asymptomatic for one or more years. |
|
| Confusion with other pests |
Early symptoms are generic and can be easily misidentified. Presence of yellow spots (uredinia) is usually visible and allows the identification of a rust fungus. The genus Cronartium can be identified by analysis of their spores. Cronartium quercuum can be distinguished from other Cronartium spp. by sequence analysis of the ITS region (Vogler and Bruns, 1998; Wijesinghe et al., 2019). |
|
| Host plant range |
Aecial host of C. quercuum is Pinus as a genus, including P. banksiana, P. densiflora, P. echinata, P. halepensis, P. mugo, P. nigra, P. parviflora, P. peuce, P. pinaster, P. sylvestris, P. thunbergii, P. virginiana and many more (EPPO, online_c; Farr and Rossman, online). |
|
|
Telial hosts are Castanea crenata, C. dentata, C. henryi, C. mollissima, C. pumila, C. sativa, Castanopsis cuspidata, C. sieboldii, Fagus japonica, Notholithocarpus densiflorus, Rhus chinensis and a large number of Quercus species including Q. petraea and Q. robur (EPPO, online_c; Farr and Rossman, online; Legon et al., online). For a full host list refer to EPPO (online_e) and Farr and Rossman (online). |
||
| Reported evidence of impact | Cronartium quercuum is EU quarantine pest. | |
| Evidence that the commodity is a pathway | Cronartium sp. was intercepted in 2000 in the UK on Mahonia plants for planting coming from China (EUROPHYT, online). Therefore, plants for planting are possible pathway of entry for C. quercuum. | |
| Surveillance information | According to the Dossier Section 5.0 the pathogen is not under official surveillance in the UK. | |
A.2.2. Possibility of pest presence in the nursery
A.2.2.1. Possibility of entry from the surrounding environment
Cronartium quercuum is present in the UK (Dossier Sections 2.0 and 5.0), it is known from England, Wales and the Channel Islands (Guernsey) (Legon et al., online).
The possible entry of C. quercuum from surrounding environment to the nurseries may occur through urediniospores or aeciospores carried by the wind from other telial or aecial hosts. Basidiospores can also enter from the surrounding environment and infect aecial hosts present in the nurseries.
Suitable telial host of C. quercuum like Castanea spp. and aecial host like Pinus spp. are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
The dispersal range of aeciospores and urediniospores of C. quercuum.
-
–
Presence of the pathogen in the surroundings and the distance between the nursery and the sources of pathogen in the surrounding environment.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nursery. The pathogen can be present in the surrounding areas on suitable hosts and enter the nursery through basidiospores, urediniospores or aeciospores carried by the wind.
A.2.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are obtained either from the UK or the EU (mostly the Netherlands) (Dossier Section 3.0). Seeds are not a pathway for the pathogen.
In addition to Quercus the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are suitable hosts for the pathogen such as Castanea spp. and Pinus spp. However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). Soil is not a pathway for the pathogen.
Uncertainties:
-
–
No information is available on the provenance of plants other than Q. robur used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries via new seedlings of Quercus and plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.2.2.3. Possibility of spread within the nursery
Quercus plants are either grown in containers (cells, pots, tubes, etc.) outdoors, in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
Spread within the nursery is possible if the pathogen fulfils its lifecycle within the nursery. For infection of telial host (Quercus spp.) to occur, telial and/or aecial hosts must be present within the nursery or in the vicinity of the nursery in a distance range of about 1.5 km. This requirement is fulfilled because the following hosts are present in the nursery: Castanea spp. and Pinus spp.
Uncertainties
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nursery is possible and can be enhanced by the presence and abundance of alternate telial and aecial hosts.
A.2.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. quercuum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online).
A.2.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on C. quercuum is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 2 | Physical separation | Yes |
Growing telial and aecial hosts at a distance of at least 1.5 km should reduce the likelihood of infection. However, there is no evidence that this requirement is met.
Uncertainties:
|
| 3 | Certified plant material | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 4 | Growing media | No | Not relevant. |
| 5 | Surveillance, monitoring and sampling | Yes |
This measure could have some effect. However, the pathogen is not under official surveillance in the UK.
Uncertainties:
|
| 6 | Hygiene measures | No | Not relevant. |
| 7 | Removal of infested/infected plant material | Yes |
This measure could have some effect although it would be impractical for a foliar disease.
Uncertainties:
|
| 8 | Irrigation water | No | Not relevant. |
| 9 | Application of pest control products | Yes |
Cronartium quercuum like other rusts could be controlled by using suitable fungicides.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
This measure could have some effect.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. |
A.2.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.2.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes absence or low presence of the pathogen in the nurseries and in the surroundings and that the distance between oaks intended for export and other telial or aecial hosts is relevant. The scenario also assumes that only a very few leaves are present on plants at the time of export and that signs of the disease (uredinia and telia) are promptly detected during inspections.
A.2.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present and that the distance between oaks intended for export and other telial or aecial hosts is limited. The scenario also assumes that infected leaves will remain on the plant and that symptoms and signs of the disease are not easily recognisable during inspections.
A.2.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the distance between oaks intended for export and other telial or aecial hosts is relevant. The scenario also assumes that a limited number of leaves are present on the plants at the time of export.
A.2.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The uncertainties and the limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.2.5.5. Elicitation outcomes of the assessment of the pest freedom for Cronartium quercuum on bundles of whips and seedlings
The following Tables show the elicited and fitted values for pest infection (Table A.7) and pest freedom (Table A.8).
Table A.7.
Elicited and fitted values of the uncertainty distribution of pest infection by Cronartium quercuum per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 7 | 15 | 60 | 150 | ||||||||||
| EKE | 0.0049 | 0.0341 | 0.148 | 0.643 | 1.91 | 4.53 | 8.43 | 20.8 | 41.1 | 55.6 | 74.7 | 95.9 | 119 | 135 | 150 |
The EKE results is the BetaGeneral (0.4724, 1.855, 0, 175) distribution fitted with @Risk version 7.6.
Table A.8.
The uncertainty distribution of bundles free of Cronartium quercuum per 10,000 bundles calculated by Table A.7
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,940 | 9,985 | 9,993 | 10,000 | ||||||||||
| EKE results | 9,850 | 9,865 | 9,881 | 9,904 | 9,925 | 9,944 | 9,959 | 9,979 | 9,992 | 9,995 | 9,998 | 9,999.36 | 9,999.85 | 9,999.97 | 10,000.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.8.
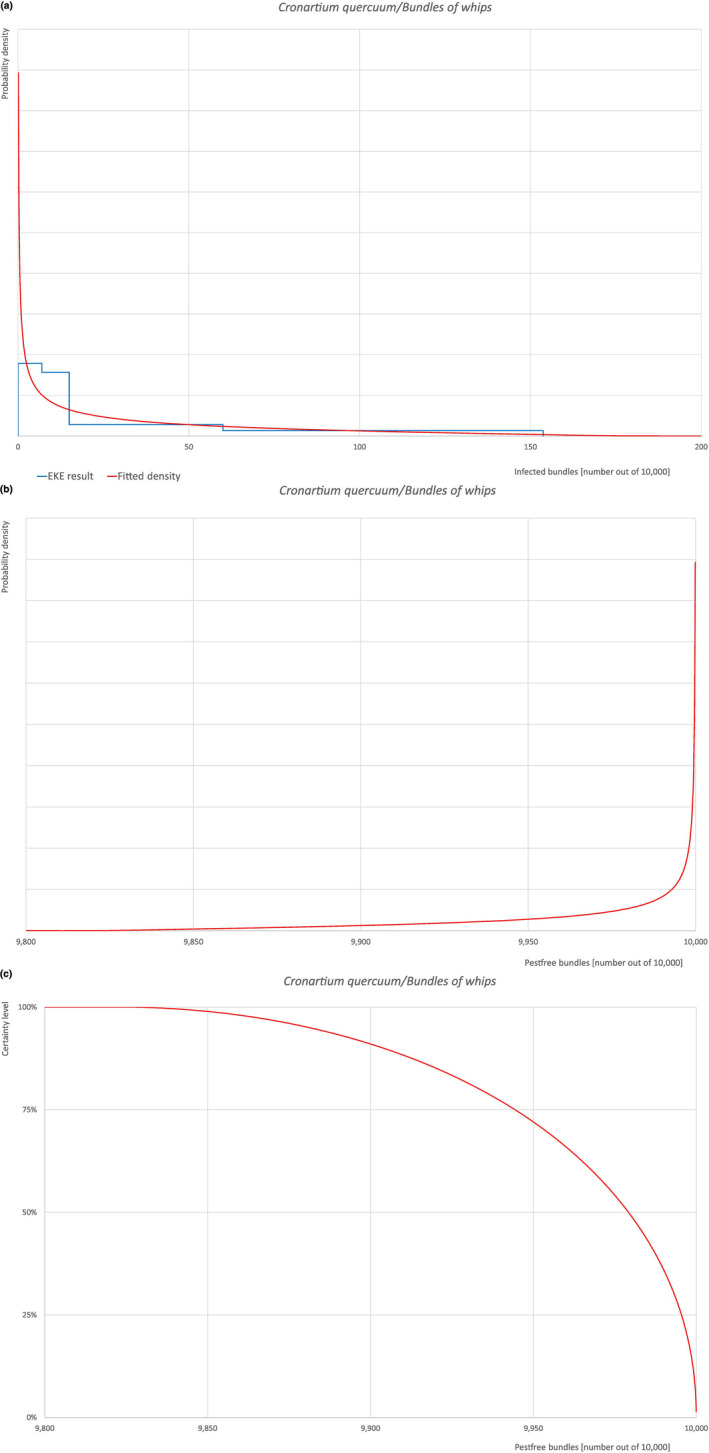
Figure A.4: (a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
A.2.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.2.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees up to 7 years old
The scenario assumes absence or low presence of the pathogen in the nurseries and in the surroundings and that the distance between oaks intended for export and other telial or aecial hosts is relevant. The scenario also assumes that only a very few leaves are present on plants at the time of export and that signs of the disease (uredinia and telia) are promptly detected during inspections.
A.2.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees up to 7 years old
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the distance between oaks intended for export and other telial or aecial hosts is limited. The scenario also assumes that a limited number of leaves are present on the plants at the time of export. In addition, the scenario assumes symptoms and signs are overlooked during inspections.
A.2.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the distance between oaks intended for export and other telial or aecial hosts is relevant. The scenario also assumes that a limited number of leaves are present on the plants at the time of export.
A.2.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The uncertainties and the limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.2.6.5. Elicitation outcomes of the assessment of the pest freedom for Cronartium quercuum on bare root plants/trees up to 7 years old
The following Tables show the elicited and fitted values for pest infection (Table A.9) and pest freedom (Table A.10).
Table A.9.
Elicited and fitted values of the uncertainty distribution of pest infection by Cronartium quercuum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 10 | 20 | 50 | 110 | ||||||||||
| EKE | 0.102 | 0.353 | 0.902 | 2.32 | 4.70 | 8.28 | 12.5 | 23.3 | 37.9 | 47.4 | 59.6 | 72.9 | 87.5 | 98.8 | 110 |
The EKE results is the BetaGeneral (0.7414, 2.4778, 0, 135) distribution fitted with @Risk version 7.6.
Table A.10.
The uncertainty distribution of plants free of Cronartium quercuum per 10,000 plants calculated by Table A.9
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,890 | 9,950 | 9,980 | 9,990 | 10,000 | ||||||||||
| EKE results | 9,890 | 9,901 | 9,913 | 9,927 | 9,940 | 9,953 | 9,962 | 9,977 | 9,987 | 9,992 | 9,995 | 9,998 | 9,999.1 | 9,999.6 | 9,999.9 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.10.
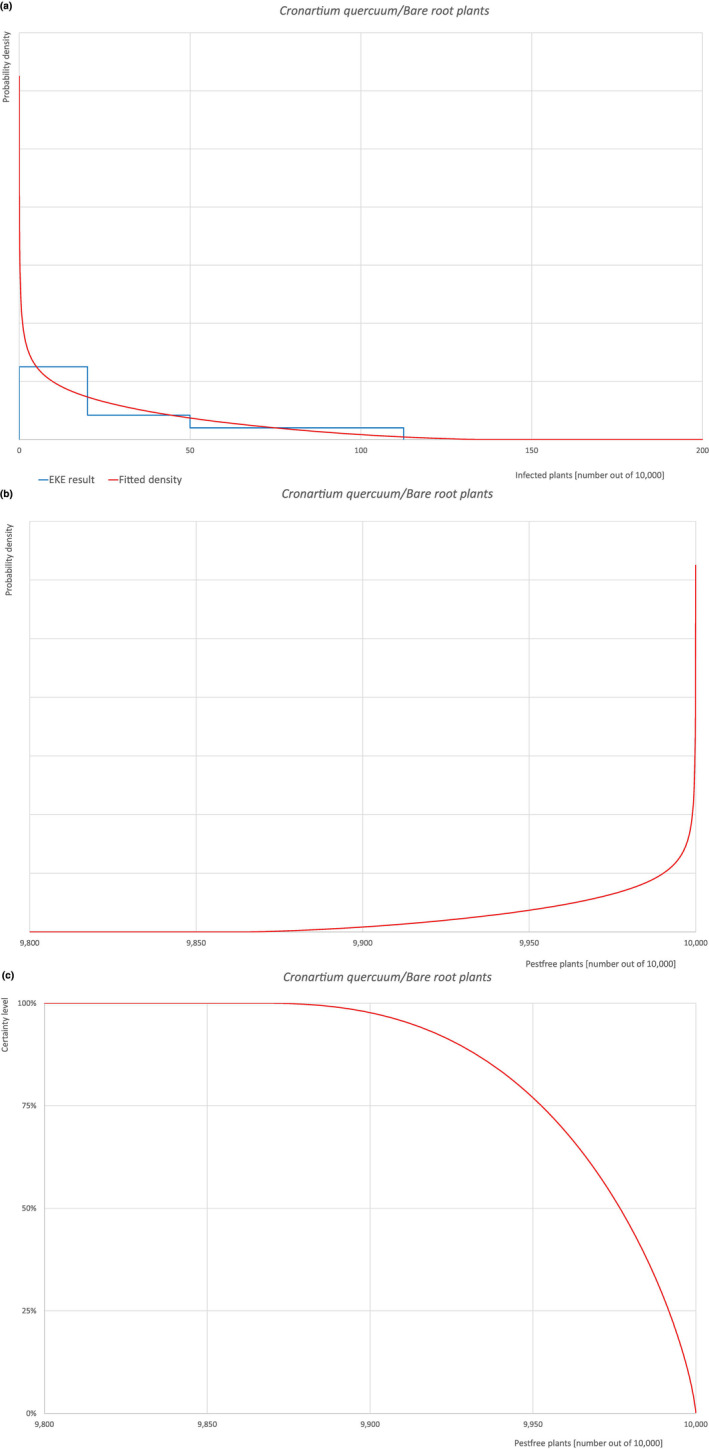
Figure A.5: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.2.7. Overall likelihood of pest freedom for plants in pots up to 15 years old
A.2.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots up to 15 years old
The scenario assumes absence or low presence of the pathogen in the nurseries and in the surroundings and that the distance between oaks intended for export and other telial or aecial hosts is relevant. The scenario also assumes that the majority of plants are young and exported in the dormant phase with a very few leaves attached and that signs of the disease (uredinia and telia) are promptly detected during inspections.
A.2.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots up to 15 years old
The scenario assumes high‐inoculum pressure of the pathogen in the nurseries and in the surroundings and that the distance between oaks intended for export and other telial or aecial hosts is limited. The scenario also assumes that the majority of plants are old and exported during the vegetative period, with leaves.
A.2.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots up to 15 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the distance between oaks intended for export and other telial or aecial hosts is relevant. The scenario also assumes that the majority of plants are young at the time of export, with limited presence of leaves.
A.2.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The uncertainties and the limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.2.7.5. Elicitation outcomes of the assessment of the pest freedom for Cronartium quercuum on plants in pots up to 15 years old
The following Tables show the elicited and fitted values for pest infection (Table A.11) and pest freedom (Table A.12).
Table A.11.
Elicited and fitted values of the uncertainty distribution of pest infection by Cronartium quercuum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 20 | 40 | 80 | 250 | ||||||||||
| EKE | 1.07 | 2.34 | 4.28 | 7.96 | 12.8 | 19.1 | 25.8 | 41.7 | 63.3 | 78.2 | 98.8 | 125 | 159 | 193 | 238 |
The EKE results is the BetaGeneral (1.1839, 208.43, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.12.
The uncertainty distribution of plants free of Cronartium quercuum per 10,000 plants calculated by Table A.11
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,920 | 9,960 | 9,980 | 10,000 | ||||||||||
| EKE results | 9,762 | 9,807 | 9,841 | 9,875 | 9,901 | 9,922 | 9,937 | 9,958 | 9,974 | 9,981 | 9,987 | 9,992 | 9,996 | 9,998 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.12.
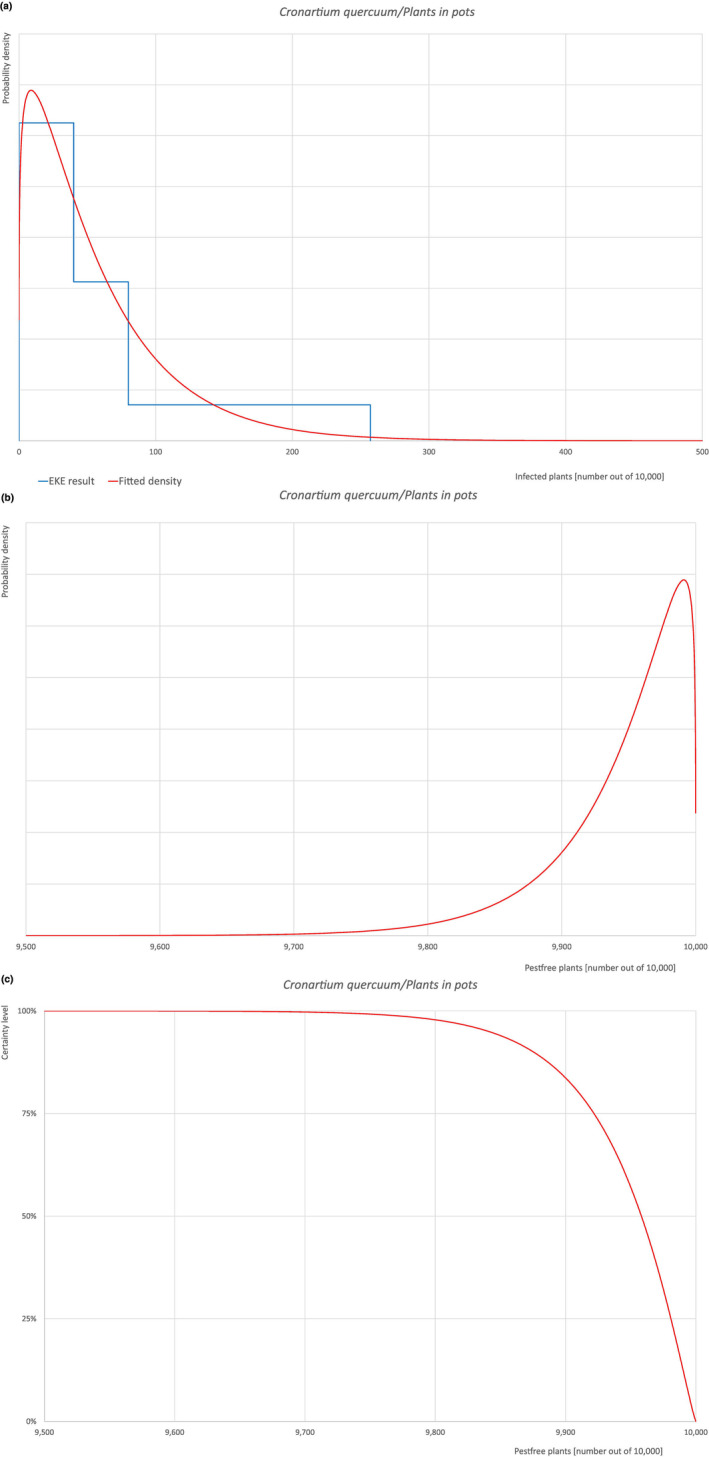
Figure A.6: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.2.8. Reference list
Burdsall Jr HH and Snow GA, 1977. Taxonomy of Cronartium quercuum and C. fusiforme. Mycologia, 69, 503–508. https://doi.org/10.2307/3758553
DEFRA (Department for Environment, Food & Rural Affairs), online. UK Risk Register Details for Cronartium quercuum. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=12320 [Accessed: 13 December 2022].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Boberg J, Jeger M, Pautasso M and Dehnen‐Schmutz K, 2018. Scientific Opinion on the pest categorisation of Cronartium spp. (non‐EU). EFSA Journal, 16(12), 5511, 30 pp. https://doi.org/10.2903/j.efsa.2018.5511
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Dehnen‐Schmutz K, Di Serio F, Gonthier P, Jacques M‐A, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Reignault PL, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Vettraino AM, Leuschner R, Mosbach‐Schulz O, Rosace MC and Potting R, 2019. Scientific Opinion on the commodity risk assessment of black pine (Pinus thunbergii Parl.) bonsai from Japan. EFSA Journal 2019;17(5):5667, 184 pp. https://doi.org/10.2903/j.efsa.2019.5667
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F and Gonthier P, 2022. Scientific Opinion on the commodity risk assessment of bonsai plants from China consisting of Pinus parviflora grafted on Pinus thunbergii. EFSA Journal 2022;20(2):7077, 301 pp. https://doi.org/10.2903/j.efsa.2022.7077
EPPO (European and Mediterranean Plant Protection Organization), 1997a. Data sheets on quarantine pests: Cronartium quercuum. In: Smith IM, McNamara DG, Scott PR and Holderness M (eds.). Quarantine Pests for Europe, 2nd Edition. CABI/EPPO, Wallingford. 5 pp.
EPPO (European and Mediterranean Plant Protection Organization), 1997b. Data sheets on quarantine pests: Cronartium coleosporioides. In: Smith IM, McNamara DG, Scott PR and Holderness M (Eds.), Quarantine Pests for Europe, 2nd Edition. CABI/EPPO, Wallingford. 5 pp.
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A1 List of pests recommended for regulation as quarantine pests, version 2022–09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A1_list [Accessed: 13 December 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Cronartium quercuum (CRONQU), Categorization. Available online: https://gd.eppo.int/taxon/CRONQU/categorization [Accessed: 13 December 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Cronartium quercuum (CRONQU), Distribution. Available online: https://gd.eppo.int/taxon/CRONQU/distribution [Accessed: 13 December 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Cronartium quercuum (CRONQU), Host commodities. Available online: https://gd.eppo.int/taxon/CRONQU/pathwayshosts [Accessed: 3 January 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_e. Cronartium quercuum (CRONQU), Host plants. Available online: https://gd.eppo.int/taxon/CRONQU/hosts [Accessed: 3 January 2023].
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en [Accessed: 22 December 2022].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://data.nal.usda.gov/dataset/united-states-national-fungus-collections-fungus-host-dataset [Accessed: 13 December 2022].
GBIF (Global Biodiversity Information Facility), online. Cronartium orientale S. Kaneko. in GBIF Secretariat (2021). GBIF Backbone Taxonomy. Available online: https://www.gbif.org/species/2517504 [Accessed: 13 December 2022].
Legon NW, Henrici A, Ainsworth AM, Roberts PJ, Spooner BM, Watling R, Cooper JA and Kirk PM, online. Checklist of the British and Irish Basidiomycota, online. Available online: http://www.basidiochecklist.info/DisplayResults.asp?intGBNum=32697 [Accessed: 30 November 2022].
Sinclair WA and Lyon HH, 2005. Diseases of Trees and Shrubs, 2nd Edition. Cornell University Press, Ithaca, NY. 660 pp.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Viennot‐Bourgin G, 1956. Mildious, oïdiums, caries, charbons, rouilles des plantes de France. Encyclopédie Mycologique, No. XXVI. Lechevalier, Paris, France.
Vogler DR and Bruns TD, 1998. Phylogenetic relationships among the pine stem rust fungi (Cronartium and Peridermium spp.). Mycologia, 90, 244–257. https://doi.org/10.2307/3761300
Wijesinghe S, McKenzie E, Wanasinghe DN, Boonmee S and Jayawardena RS, 2019. The genus Cronartium revisited. Plant Pathology and Quarantine, 9(1), 219–238. https://doi.org/10.5943/ppq/9/1/20
A.3. Cryphonectria parasitica
A.3.1. Organism information
| Taxonomic information |
Current valid scientific name: Cryphonectria parasitica Synonyms: Diaporthe parasitica, Endothia gyrosa var. parasitica, Endothia parasitica, Valsonectria parasitica (according to Index Fungorum) Name used in the EU legislation: Cryphonectria parasitica (Murrill) Barr [ENDOPA] Order: Diaporthales Family: Cryphonectriaceae Common name: chestnut blight, blight of chestnut, canker of chestnut, blight of oak Name used in the Dossier: Cryphonectria parasitica |
|
| Group | Fungi | |
| EPPO code | ENDOPA | |
| Regulated status |
The pathogen is listed in Annex III and in Annex VI of Commission Implementing Regulation (EU) 2019/2072 as Cryphonectria parasitica (Murrill) Barr. [ENDOPA]. It is EU protected zone quarantine pest of Czechia, Ireland, Sweden and the UK (Northern Ireland) and also RNQP (Regulated non‐quarantine pest) for plants for planting other than seeds of Castanea. Cryphonectria parasitica is a quarantine pest in Israel, Morocco, Norway and the US (EPPO, online_a). Cryphonectria parasitica is included in the EPPO A2 and in the A2 list of Jordan, Türkiye and COSAVE (Comité de Sanidad Vegetal del Cono Sur – Argentina, Brazil, Chile, Paraguay, Peru and Uruguay). It is also reported on A1 list of Argentina, Azerbaijan, Chile, the UK and IAPSC (Inter‐African Phytosanitary Council) (EPPO, online_a). |
|
| Pest status in the UK |
Cryphonectria parasitica is present in the UK (CABI, online; Farr and Rossman, online). The pathogen was apparently eradicated after the first findings in 2011, then newly recorded in 2016; it was suggested that C. parasitica has been introduced to the UK multiple times over at least two decades through international plant trade (Perez‐Sierra et al., 2019). According to EPPO (online_b) the pathogen is present in the UK with restricted distribution. During surveys held in 2017/18 and 2019/20 Cryphonectria parasitica was detected in Berkshire, Buckinghamshire, Cornwall, Derbyshire, Devon, Dorset, London, West Sussex, Jersey and Guernsey (Perez‐Sierra et al., 2019; Romon‐Ochoa et al., 2022; EPPO, online_c; Forestry Commission, online). According to the Dossier Section 5.0 C. parasitica is present, not widely distributed and under official control in Great Britain. It is present in central and southern England. In Northern Ireland the pathogen is not recorded. |
|
| Pest status in the EU |
Cryphonectria parasitica is present in the EU. It is widespread in Croatia, Italy and Portugal. It has restricted distribution in Austria, Belgium, Bulgaria, France, Germany, Greece, Hungary, Romania, Slovakia, Slovenia and Spain. The pathogen is present with few occurrences in Czechia and the Netherlands. In Poland, the pathogen was eradicated (EPPO online_b). Different areas in the EU have different strains of C. parasitica, the ability of new strains to spread in areas already infested by other strains seems to be very limited (EFSA PLH Panel, 2016). |
|
| Host status on Quercus |
Quercus robur is a reported host of Cryphonectria parasitica (Bissegger and Heiniger, 1991; Adamčíková et al., 2010; Rigling and Prospero, 2018; EPPO, online_d; Farr and Rossman, online). Cryphonectria parasitica is a pathogen of other Quercus species such as Quercus alba, Q. coccinea, Q. dentata, Q. frainetto, Q. ilex, Q. montana, Q. petraea, Q. prinus, Q. pubescens, Q. serrata, Q. stellata, Q. suber, Q. velutina and Q. virginiana (Rigling and Prospero, 2018; EPPO, online_d; Farr and Rossman, online). |
|
|
Both field observations and inoculation experiments have shown that European oak species are less susceptible to C. parasitica compared to Castanea sativa, the main host in Europe (Rigling and Prospero, 2018; Dennert et al., 2020). |
||
| PRA information | Available Pest Risk Assessment:
|
|
| Other relevant information for the assessment | ||
| Biology |
Cryphonectria parasitica is a pathogen in the family Cryphonectriaceae, native to East Asia (EPPO, online_b). It is present in Africa (Tunisia), Asia (China, India, Iran, Japan, North and South Korea, Taiwan), Europe, North America (Canada, the US) and Oceania (Australia) (EPPO, online_b). The biology section is based on the studies on chestnut, one of the major hosts. Cryphonectria parasitica is a bark pathogen that infects the tissue through wounds or growth cracks in the bark. The pathogen can also infect abandoned galls of the gall wasp Dryocosmus kuriphilus (Meyer et al., 2015). Hail wounds have been documented as important infection courts (Lione et al., 2020). The infection is caused by asexual and sexual spores. The infection develops in a lesion and a canker, which eventually kills the plant part distal to the infection. The pathogen can saprophytically colonise recently (1 year) dead stems or branches (Hepting, 1974; Prospero et al., 2006). Then stromata develop. Stromata can contain sexual fruiting bodies (perithecia), asexual ones (pycnidia) or both. Pycnidia produce conidia that are released in tendrils in moist condition and splash dispersed by rain in a few metres range. Conidia can also be dispersed by birds, insects and windborne dust over long distances (Wendt et al., 1983; Russin et al., 1984). Once in the ground conidia can survive for a long time (Heald and Studhalter, 1914). Perithecia produce ascospores that can be dispersed by wind over hundreds of metres and are relatively short‐lived. Ascospores are discharged from spring to autumn during warm rains (Heald and Gardner, 1914; Guérin et al., 2001). Sexual reproduction can be by both outcrossing and self‐fertilisation (Marra et al., 2004). In northern Italy, it has been reported that C. parasitica can release propagules all over the year, though with significant seasonal peaks in the spring and fall (Lione et al., 2022). Large propagule loads were significantly correlated with an increasing number of rainy days of the week (days providing 1–10 mm/day of water) (Lione et al., 2022). In newly established populations, asexual reproduction via conidia is often the predominant spreading mechanism (Rigling and Prospero, 2018). The canker growth can be as fast as 1 mm per day when the average daily temperature is 20°C, with a peak at 27°C and slowed down below 20°C (Bazzigher, 1981). The optimal germination temperature of conidia is 25–26°C, the ascospores' one is 21°C (Fulton, 1912). Humidity promotes spore release (Griffin, 1986). Drought stress can increase tree susceptibility and mortality caused by the pathogen (Roane et al., 1986; Waldboth and Oberhuber, 2009). The pathogen's ability to infect a new host is dependent on the age of the wound: on European chestnut C. parasitica cannot establish itself in wounds of 4 or more days (Bazzigher and Schmid, 1962). Cryphonectria parasitica can also show an endophytic behaviour, it has been found in symptomless stems 3 months after inoculation (Guérin and Robin, 2003) or developed its symptoms after 16 months of quarantine in Australia (Cunnington and Pascoe, 2003). On chestnut fruits, the fungus is associated with only the nutshell (Jaynes and Depalma, 1984). In newly colonised territories, the population usually consists of one or few genotypes, limiting sexual reproduction and long‐range dispersal via ascospores. In most populations in Europe, random mating has been ruled out and, even then, ascospores are not likely to be the primary inoculum (Milgroom and Cortesi, 1999). The main mycovirus acting as a biological control agent for C. parasitica, reducing its virulence, in Europe is Cryphonectria hypovirus 1 (CHV‐1), one of the four known species of the genus Hypovirus (Turina and Rostagno, 2007). CHV‐1 can spread via hyphal anastomosis from one individual to another or via conidia, but not via ascospores. Fungivorous mites can be important for the spread of CHV‐1 (Bouneb et al., 2016). Cryphonectria parasitica, like many fungi has a vegetative incompatibility (vic) mechanism. This mechanism usually hinders the transmission of mycoviruses including CHV1. Up to date, there are 64 genetically defined vic genotypes (Short et al., 2015). According to EFSA PLH Panel (2016), the main pathways of entry for C. parasitica are plants for planting (including seedlings, scions, rootstocks, ornamental plants), wood with bark (including chips, wood for tannin production, hoops for barrels), fruit (nuts), soil and growing media (including isolated chestnut bark), natural spread of airborne inoculum, biological agents able to mechanically transfer the fungus (e.g. birds, mammals, insects, mites, etc.) and machinery (construction, terracing, etc.) and pruning/cutting tools. According to EUROPHYT (online), Cryphonectria parasitica was intercepted 14 times on wood and bark of Castanea sp. or Castanea sativa. Once it was intercepted on Castanea sativa plants intended for planting (not yet planted). Cryphonectria parasitica is single‐handedly responsible for the removal from the forest dominant plane of Castanea dentata in North America. Impact of the pathogen is strongly dependent on host availability, host susceptibility and virulence of the C. parasitica strain. An in‐depth analysis of the impact of introduction of new strains of the pathogen in EU countries where C. parasitica is already established and in countries where it is absent is available in the EFSA Pest Risk Assessment for C. parasitica (EFSA PLH Panel, 2016). |
|
| Symptoms | Main type of symptoms |
Cryphonectria parasitica only attacks the above‐ground tree parts. Symptoms vary depending on the age of the host tree, its species and the virulence of the particular pathogen strain (Heiniger and Rigling, 1994; Prospero and Rigling, 2013). Virulent strains on susceptible trees produce in few months cankers that can kill branches or twigs (Diller, 1965). On susceptible Castanea species, one of the first symptoms is branch wilting with wilted leaves hanging on the branches. Cankers typically appear as sunken, reddish‐brown bark lesions. Below the cankers, trees can produce epicormic shoots. At the canker border and under the bark, the fungus develops pale brown mycelial fans. On more resistant tree species (Asian chestnut species, oaks), cankers typically have a swollen appearance and are superficial or callused. On oaks (Quercus petraea and Q. robur) in Slovakia the observed symptoms were branch dieback and cankers on stems and branches (Adamčíková et al., 2010). |
| Presence of asymptomatic plants | Cryphonectria parasitica can show an endophytic behaviour, imported chestnut plants have developed symptoms after 16 months of quarantine (Cunnington and Pascoe, 2003). | |
| Confusion with other pests |
Cryphonectria parasitica symptoms can be confused with other cankers in the first stages, but the presence of mycelial fans and appearance of the fruiting bodies makes the identification clear. Isolated on potato dextrose agar can identify also hypovirus‐infected fungi, and molecular methods have been developed for identification (EFSA PLH Panel, 2014). Some confusion can occur with cancers caused by Gnomonopsis castaneae (Lione et al., 2019). |
|
| Host plant range |
Main host of C. parasitica are Castanea dentata and C. sativa. Other hosts in the Castanea genus are C. crenata, C. henryi, C. mollissima, C. ozarkensis, C. pumila and C. seguinii. Among oaks the known hosts are Quercus alba, Q. coccinea, Q. dentata, Q. frainetto, Q. ilex, Q. montana, Q. petraea, Q. prinus, Q. pubescens, Q. robur, Q. serrata, Q. stellata, Q. suber, Q. velutina and Q. virginiana (Rigling and Prospero, 2018; EPPO, online_d; Farr and Rossman, online). Cryphonectria parasitica was also reported on Acer palmatum, Acer rubrum, Aesculus hippocastanum, Carya ovata, Carpinus betulus, Eucalyptus camaldulensis, E. haemastoma, E. microcorys, E. punctata, E. robusta, Rhus typhina and Fagus sylvatica (Anderson and Babcock, 1913; Shear et al., 1917; EPPO, online_d; Farr and Rossman, online). The reports for Fagus sylvatica are only taken from artificial inoculation (Dennert et al., 2020). |
|
| Reported evidence of impact | Cryphonectria parasitica is EU protected zone quarantine pest. | |
| Evidence that the commodity is a pathway | Host plants for planting, excluding seeds, but including dormant plants, have been identified as pathways by EFSA PLH Panel (2014), and have been historically pathways even after quarantine (Cunnington and Pascoe, 2003). | |
| Surveillance information | Cryphonectria parasitica is a GB regulated quarantine pest subject to eradication measures, unless in the wider environment where a containment policy may be taken dependent on the site. As part of an annual survey at ornamental retail and production sites (frequency of visits determined by a decision matrix) C. parasitica is inspected for on common hosts plants (Dossier Sections 3.0 and 5.0). | |
A.3.2. Possibility of pest presence in the nursery
A.3.2.1. Possibility of entry from the surrounding environment
Cryphonectria parasitica is present in the UK with restricted distribution mostly in central and southern England (Dossier Section 5.0; Forestry Commission, online).
The pathogen can naturally spread with ascospores dispersed by air currents over hundreds of metres, as well as with conidia transported with rain splash over short distances. However, conidia can also be dispersed by birds, insects and wind over long distances (Wendt et al., 1983; Russin et al., 1984).
C. parasitica principally infects Castanea species mostly C. sativa, which is present within 2 km radius from the nurseries, together with other plants that the pathogen was reported on like Fagus spp. (Dossier Section 3.0).
Uncertainties:
-
–
The dispersal range of animals carrying C. parasitica inoculum (e.g. birds, insects and mites).
-
–
The role of animals in C. parasitica dispersal.
-
–
The distance of the nurseries to sources of pathogen and inoculum pressure in the surrounding environment.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for C. parasitica to enter the nurseries from surrounding environment via conidia and ascospores transported by air currents, birds and insects.
A.3.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are obtained either from the UK or the EU (mostly the Netherlands) (Dossier Section 3.0). Seeds are not a pathway for the pathogen.
In addition to Quercus the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are suitable hosts for the pathogen such as Castanea sativa and other plants that the pathogen was reported on like Acer spp., Aesculus hippocastanum, Carpinus betulus, Fagus sylvatica and Rhus spp. However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). Although soil and growing media are considered pathways of minor importance (EFSA, 2016), the conidia of C. parasitica can survive in the soil for long time (Heald and Studhalter, 1914) and therefore could potentially enter by this way. However, the growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
The susceptibility of plant species other than Castanea and Quercus in the nursery to the pathogen.
-
–
The provenance of plants other than Quercus used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries via new seedlings of Quercus and plants of other species used for plant production. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.3.2.3. Possibility of spread within the nursery
Quercus plants are either grown in containers (cells, pots, tubes, etc.) outdoors, in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pathogen can infect other plants, such as Acer spp., Aesculus spp., Castanea spp., Fagus spp., Rhus spp., etc. present within the nurseries (Dossier Sections 3.0 and 6.0).
If sporulating infections occur in the nurseries, C. parasitica can naturally spread within the nurseries by rain/water splash, air currents, transported by insects, mites and birds. Human assisted spread could be mostly via contaminated equipment, but tools used in the nurseries are disinfected before being used on different plants (Dossier Section 3.0).
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible by rain/water splash, air currents and transport of insects, mites and birds.
A.3.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of C. parasitica between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online).
A.3.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on C. parasitica is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
The risk mitigation measure is expected to be effective in reducing the likelihood of presence of the pathogen on the commodity.
Uncertainties:
|
| 4 | Growing media | No | Not relevant. |
| 5 | Surveillance, monitoring and sampling | Yes |
This measure could have some effect.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
The disinfection of tools with appropriate product can prevent the spread of the pathogen within the nurseries.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This measure could have some effect.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Overhead irrigation can increase the likelihood of spread of the pathogen by water splash.
Uncertainties:
|
| 9 | Application of pest control products | Yes |
Although C. parasitica is generally not a target of the pesticide treatments in the nurseries, some fungicides could reduce the likelihood of the infection by the pathogen.
Uncertainties:
|
| 10 | Measures against soil pests | No | Not relevant. |
| 11 | Inspections and management of plants before export | Yes |
This measure could have some effect.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. |
A.3.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.3.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. The plants are exposed to the pathogen for only short period of time. The scenario assumes Q. robur to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.3.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes Q. robur to be a suitable host for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.3.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings. Q. robur is considered minor host. The pathogen is a regulated quarantine pest in the UK and under official control.
A.3.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.3.5.5. Elicitation outcomes of the assessment of the pest freedom for Cryphonectria parasitica on bundles of whips and seedlings
The following Tables show the elicited and fitted values for pest infection (Table A.13) and pest freedom (Table A.14).
Table A.13.
Elicited and fitted values of the uncertainty distribution of pest infection by Cryphonectria parasitica per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 10 | 20 | 40 | 100 | ||||||||||
| EKE | 0.418 | 0.987 | 1.90 | 3.72 | 6.20 | 9.44 | 12.9 | 21.1 | 31.8 | 38.9 | 48.4 | 59.5 | 73.3 | 85.6 | 100 |
The EKE results is the BetaGeneral (1.0764, 6.8505, 0, 200) distribution fitted with @Risk version 7.6.
Table A.14.
The uncertainty distribution of bundles free of Cryphonectria parasitica per 10,000 bundles calculated by Table A.13
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,900 | 9,960 | 9,980 | 9,990 | 10,000 | ||||||||||
| EKE results | 9,900 | 9,914 | 9,927 | 9,940 | 9,952 | 9,961 | 9,968 | 9,979 | 9,987 | 9,991 | 9,994 | 9,996 | 9,998 | 9,999 | 10,000 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.14.
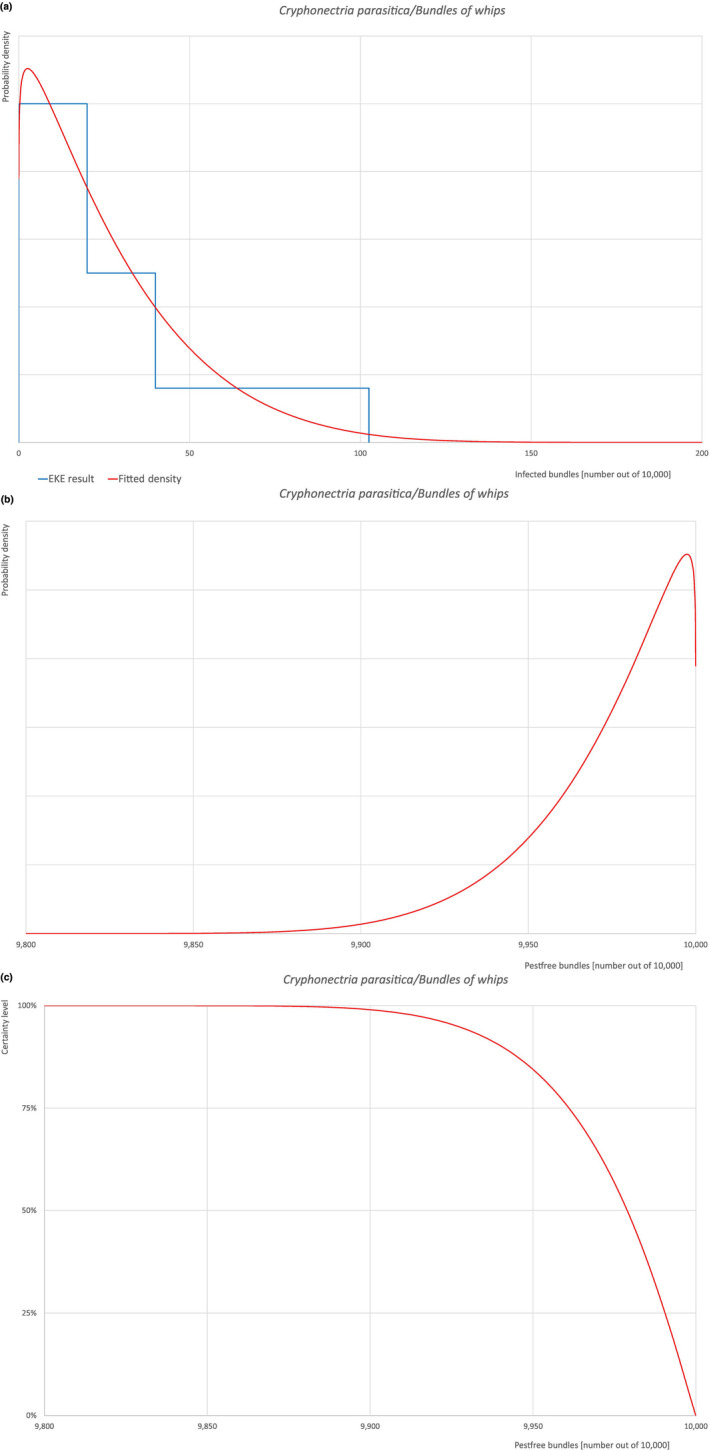
Figure A.7: (a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
A.3.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.3.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Q. robur to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.3.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Q. robur to be a suitable host for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.3.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause some infection. Q. robur is considered minor host. The pathogen is a regulated quarantine pest in the UK and under official control.
A.3.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.3.6.5. Elicitation outcomes of the assessment of the pest freedom for Cryphonectria parasitica on bare root plants/trees up to 7 years old
The following Tables show the elicited and fitted values for pest infection (Table A.15) and pest freedom (Table A.16).
Table A.15.
Elicited and fitted values of the uncertainty distribution of pest infection by Cryphonectria parasitica per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 15 | 30 | 70 | 150 | ||||||||||
| EKE | 0.215 | 0.680 | 1.63 | 3.92 | 7.57 | 12.9 | 19.0 | 34.0 | 53.9 | 66.8 | 83.0 | 101 | 120 | 135 | 150 |
The EKE results is the BetaGeneral (0.79863, 2.5561, 0, 185) distribution fitted with @Risk version 7.6.
Table A.16.
The uncertainty distribution of plants free of Cryphonectria parasitica per 10,000 plants calculated by Table A.15
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,930 | 9,970 | 9,985 | 10,000 | ||||||||||
| EKE results | 9,850 | 9,865 | 9,880 | 9,899 | 9,917 | 9,933 | 9,946 | 9,966 | 9,981 | 9,987 | 9,992 | 9,996 | 9,998 | 9,999 | 10,000 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.16.
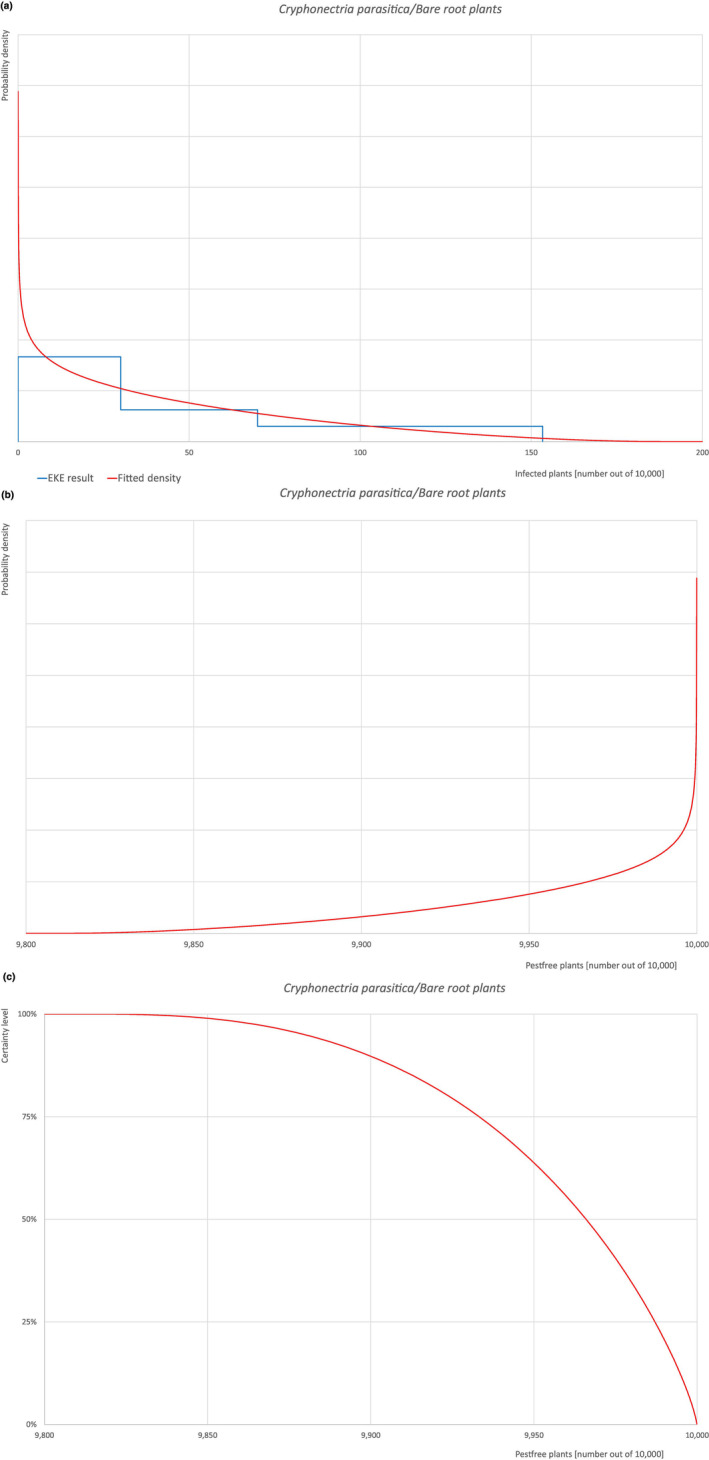
Figure A.8: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.3.7. Overall likelihood of pest freedom for plants in pots up to 15 years old
A.3.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots up to 15 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time. The scenario assumes Q. robur to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.3.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots up to 15 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. Older plants are exposed to the pathogen for longer period of time. The scenario assumes Q. robur to be a suitable host for the pathogen. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.3.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots up to 15 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings and that the plants are exposed to the pathogen for a sufficient period of time to cause some infection. Q. robur is considered minor host. The pathogen is a regulated quarantine pest in the UK and under official control.
A.3.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pathogen in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.3.7.5. Elicitation outcomes of the assessment of the pest freedom for Cryphonectria parasitica on plants in pots up to 15 years old
The following Tables show the elicited and fitted values for pest infection (Table A.17) and pest freedom (Table A.18).
Table A.17.
Elicited and fitted values of the uncertainty distribution of pest infection by Cryphonectria parasitica per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 25 | 55 | 105 | 225 | ||||||||||
| EKE | 0.764 | 2.00 | 4.18 | 8.78 | 15.4 | 24.3 | 33.9 | 56.5 | 85.3 | 104 | 127 | 152 | 180 | 203 | 225 |
The EKE results is the BetaGeneral (0.95432, 3.0154, 0, 290) distribution fitted with @Risk version 7.6.
Table A.18.
The uncertainty distribution of plants free of Cryphonectria parasitica per 10,000 plants calculated by Table A.17
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,775 | 9,895 | 9,945 | 9,975 | 10,000 | ||||||||||
| EKE results | 9,775 | 9,797 | 9,820 | 9,848 | 9,873 | 9,896 | 9,915 | 9,943 | 9,966 | 9,976 | 9,985 | 9,991 | 9,996 | 9,998 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.18.
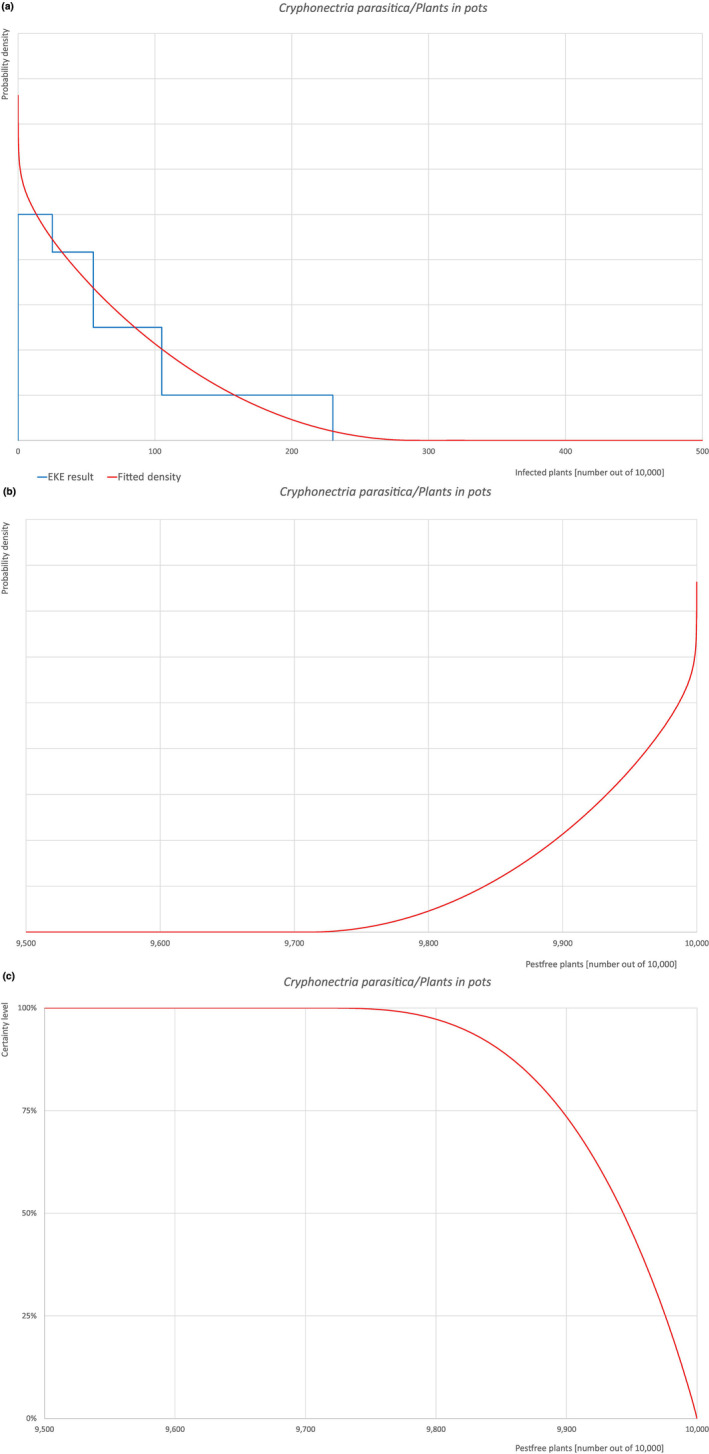
Figure A.9: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.3.8. Reference list
Adamčíková K, Kobza M and Juhásová G, 2010. Characteristics of the Cryphonectria parasitica isolated from Quercus in Slovakia. Forest Pathology, 40, 443–449. https://doi.org/10.1111/j.1439-0329.2009.00618.x
Anderson PJ and Babcock DC, 1913. Field studies on the dissemination and growth of the chestnut blight fungus. Pennsylvania Chestnut Tree Blight Commission, 3, 46.
Anderson A, Baker R, Parkinson N, Reed P and Woodward S, 2013. Rapid pest risk analysis for Cryphonectria parasitica. The Food and Environment Research Agency, 23 pp.
Baird RE, 1991. Growth and stromata production of hypovirulent and virulent strains of Cryphonectria parasitica on dead Quercus rubra and Acer rubrum. Mycologia, 83, 158–162. https://doi.org/10.2307/3759931
Bazzigher G, 1981. Selection of blight‐resistant chestnut trees in Switzerland. Forest Pathology, 11, 199–207. https://doi.org/10.1111/j.1439-0329.1981.tb00088.x
Bazzigher G and Schmid P, 1962. Methodik zur Prüfung der Endothia‐Resistenz bei Kastanien. Journal of Phytopathology, 45, 169–189.
Biosecurity Australia, 2006. Technical justification for Australia's requirement for wood packaging material to be bark free. Biosecurity Australia, Canberra, Australia. 123 pp.
Bissegger M and Heiniger U, 1991. Chestnut blight (Cryphonectria parasitica) north of the Swiss Alps. European Journal of Forest Pathology, 21, 250–252. https://doi.org/10.1111/j.1439-0329.1991.tb00976.x
Bouneb M, Turchetti T, Nannelli R, Roversi PF, Paoli F, Danti R and Simoni S, 2016. Occurrence and transmission of mycovirus Cryphonectria hypovirus 1 from dejecta of Thyreophagus corticalis (Acari, Acaridae). Fungal Biology, 120, 351–357. https://doi.org/10.1016/j.funbio.2015.11.004
CABI, online. Datasheet for Cryphonectria parasitica (blight of chestnut). Available online: https://www.cabi.org/isc/datasheet/21108 [Accessed: 28 November 2022].
Cunnington JH and Pascoe IG, 2003. Post entry quarantine interception of chestnut blight in Victoria. Australasian Plant Pathology, 32, 569. https://doi.org/10.1071/AP03067
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Cryphonectria parasitica. Available online: https://secure.fera.defra.gov.uk/phiw/riskRegister/viewPestRisks.cfm?cslref=11469 [Accessed: 28 November 2022].
Dennert F, Rigling D, Meyer JB, Schefer C, Augustiny E and Prospero S, 2020. Testing the pathogenic potential of Cryphonectria parasitica and related species on three common European Fagaceae. Frontiers in Forests and Global Change, 52, 8 pp. https://doi.org/10.3389/ffgc.2020.00052
Diller JD, 1965. Chestnut Blight. Forest Pest Leaflet 94. U.S. Department of Agriculture Forest Service, Washington, DC. 7 pp.
EFSA PLH Panel (EFSA Panel on Plant Health), 2014. Scientific Opinion on the pest categorisation of Cryphonectria parasitica (Murrill) Barr. EFSA Journal 2014;12(10):3859, 42 pp. https://doi.org/10.2903/j.efsa.2014.3859
EFSA PLH Panel (EFSA Panel on Plant Health), Jeger M, Bragard C, Chatzivassiliou E, Dehnen‐Schmutz K, Gilioli G, Jaques Miret JA, MacLeod A, Navajas Navarro M, Niere B, Parnell S, Potting R, Rafoss T, Urek G, Van Bruggen A, Van der Werf W, West J, Winter S, Maresi G, Prospero S, Vettraino AM, Vloutoglou I, Pautasso M and Rossi V, 2016. Risk assessment and reduction options for Cryphonectria parasitica in the EU. EFSA Journal 2016;14(12):4641, 54 pp. https://doi.org/10.2903/j.efsa.2016.4641
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F and Gonthier P, 2022. Scientific Opinion on the commodity risk assessment of Acer palmatum plants grafted on Acer davidii from China. EFSA Journal 2022;20(5):7298, 262 pp. https://doi.org/10.2903/j.efsa.2022.7298
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023a. Scientific Opinion on the commodity risk assessment of Acer campestre plants from the UK. EFSA Journal 2023;21(7):8071, 291 pp. https://doi.org/10.2903/j.efsa.2023.8071
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023b. Scientific Opinion on the commodity risk assessment of Acer palmatum plants from the UK. EFSA Journal 2023;21(7):8075, 228 pp. https://doi.org/10.2903/j.efsa.2023.8075
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023c. Scientific Opinion on the commodity risk assessment of Acer platanoides plants from the UK. EFSA Journal 2023;21(7):8073, 268 pp. https://doi.org/10.2903/j.efsa.2023.8073
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023d. Scientific Opinion on the commodity risk assessment of Acer pseudoplatanus plants from the UK. EFSA Journal 2023;21(7):8074, 271 pp. https://doi.org/10.2903/j.efsa.2023.8074
EPPO (European and Mediterranean Plant Protection Organization), online_a. Cryphonectria parasitica (ENDOPA), Categorization. Available online: https://gd.eppo.int/taxon/ENDOPA/categorization [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Cryphonectria parasitica (ENDOPA), Distribution. Available online: https://gd.eppo.int/taxon/ENDOPA/distribution [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Cryphonectria parasitica (ENDOPA), Distribution details in United Kingdom. Available online: https://gd.eppo.int/taxon/ENDOPA/distribution/GB [Accessed: 28 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Cryphonectria parasitica (ENDOPA), Hots plants. Available online: https://gd.eppo.int/taxon/ENDOPA/hosts [Accessed: 28 November 2022].
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en [Accessed: 22 December 2022].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://data.nal.usda.gov/dataset/united-states-national-fungus-collections-fungus-host-dataset [Accessed: 18 January 2023].
Fulton HR, 1912. Recent notes on the chestnut bark disease. Pennsylvania Chestnut Blight Conference Report, Harrisburg, PA, the US, 48–56.
Griffin GJ, 1986. Chestnut blight and its control. Horticultural Reviews, 8, 291–335.
Guérin L, Froidefond G and Xu X‐M, 2001. Seasonal patterns of dispersal of ascospores of Cryphonectria parasitica (chestnut blight): Dispersal of C. parasitica ascospores. Plant Pathology, 50, 717–724. https://doi.org/10.1046/j.1365-3059.2001.00600.x
Guérin L and Robin C, 2003. Seasonal effect on infection and development of lesions caused by Cryphonectria parasitica in Castanea sativa. Forest Pathology, 33, 223–235. https://doi.org/10.1046/j.1439‐0329.2003.00329.x
Heald FD and Gardner MW, 1914. Longevity of pycnospores of the chestnut blight fungus in soil. Journal of Agricultural Research, 2, 67–75.
Heald FD and Studhalter RA, 1914. Birds as carriers of the chestnut blight fungus. Journal of Agricultural Research, 2, 405–422.
Heiniger U and Rigling D, 1994. Biological control of Chestnut Blight in Europe. Annual Review of Phytopathology, 32, 581–599. https://doi.org/10.1146/annurev.py.32.090194.003053
Hepting GH, 1974. Death of the American Chestnut. Journal of Forest History, 18, 60–67. https://doi.org/10.2307/3983346
Jaynes RA and DePalma NK, 1984. Natural infection of nuts of Castanea dentata by Endothia parasitica. Phytopathology, 74, 296. https://doi.org/10.1094/Phyto-74-296
Lione G, Danti R, Fernandez‐Conradi P, Ferreira‐Cardoso JV, Lefort F, Marques G, Meyer JB, Prospero S, Radócz L, Robin C, Turchetti T, Vettraino AM and Gonthier P, 2019. The emerging pathogen of chestnut Gnomoniopsis castaneae: the challenge posed by a versatile fungus. European Journal of Plant Pathology, 153, 671–685. https://doi.org/10.1007/s10658-018-1597-2
Lione G, Giordano L, Turina M and Gonthier P, 2020. Hail‐induced infections of the chestnut blight pathogen Cryphonectria parasitica depend on wound size and may lead to severe diebacks. Phytopathology, 110, 1280–1293. https://doi.org/10.1094/PHYTO-01-20-0006-R
Lione G, Brescia F, Giordano L and Gonthier P, 2022. Effects of seasonality and climate on the propagule deposition patterns of the chestnut blight pathogen Cryphonectria parasitica in orchards of the Alpine district of NorthWestern Italy. Agriculture 12, 644. https://doi.org/10.3390/agriculture12050644
Marra RE, Cortesi P, Bissegger M and Milgroom MG, 2004. Mixed mating in natural populations of the chestnut blight fungus, Cryphonectria parasitica. Heredity, 93, 189–195. https://doi.org/10.1038/sj.hdy.6800492
Meyer JB, Gallien L and Prospero S, 2015. Interaction between two invasive organisms on the European chestnut: does the chestnut blight fungus benefit from the presence of the gall wasp? FEMS Microbiology Ecology, 91, fiv122. https://doi.org/10.1093/femsec/fiv122
Milgroom MG and Cortesi P, 1999. Analysis of population structure of the chestnut blight fungus based on vegetative incompatibility genotypes. Proceedings of the National Academy of Sciences, 96, 10518–10523. https://doi.org/10.1073/pnas.96.18.10518
Perez‐Sierra A, Romon‐Ochoa P, Gorton C, Lewis A, Rees H, Van Der Linde S and Webber J, 2019. High vegetative compatibility diversity of Cryphonectria parasitica infecting sweet chestnut (Castanea sativa) in Britain indicates multiple pathogen introductions. Plant Pathology, 68, 727–737. https://doi.org/10.1111/ppa.12981
Prospero S, Conedera M, Heiniger U and Rigling D, 2006. Saprophytic activity and sporulation of Cryphonectria parasitica on dead chestnut wood in forests with naturally established hypovirulence. Phytopathology, 96, 1337–1344. https://doi.org/10.1094/PHYTO-96-1337
Prospero S and Rigling D, 2013. Chestnut blight. Infectious Forest Diseases. In: Gonthier P, Nicolotti G. (Eds.), Infectious Forest Diseases. CAB International, Wallingford, UK, 318–339.
Rigling D and Prospero S, 2018. Cryphonectria parasitica, the causal agent of chestnut blight: invasion history, population biology and disease control: Cryphonectria parasitica. Molecular Plant Pathology, 19, 7–20. https://doi.org/10.1111/mpp.12542
Roane MK, Griffin GJ and Elkins JR, 1986. Chestnut blight, other Endothia diseases, and the genus Endothia. APS Press, American Phytopathological Society, St. Paul, MN, the US, vii + 53 pp.
Russin JS, Shain L and Nordin GL, 1984. Insects as carriers of virulent and cytoplasmic hypovirulent isolates of the chestnut blight fungus. Journal of Economic Entomology, 77, 838–846.
Shear CL, Stevens NE and Tiller RJ, 1917. Endothia parasitica and related species. Bulletin of the United States Department of Agriculture, 380, 1–82. https://doi.org/10.5962/bhl.title.64538
Short DPG, Double M, Nuss DL, Stauder CM, MacDonald W and Kasson MT, 2015. Multilocus PCR Assays Elucidate Vegetative Incompatibility Gene Profiles of Cryphonectria parasitica in the United States. Applied and Environmental Microbiology, 81, 5736–5742. https://doi.org/10.1128/AEM.00926-15
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Turina M and Rostagno L, 2007. Virus‐induced hypovirulence in Cryphonectria parasitica: still an unresolved conundrum. Journal of Plant Pathology, 14.
Waldboth M and Oberhuber W, 2009. Synergistic effect of drought and chestnut blight (Cryphonectria parasitica) on growth decline of European chestnut (Castanea sativa). Forest Pathology, 39, 43–55. https://doi.org/10.1111/j.1439-0329.2008.00562.x
Wendt R, 1983. Association of Endothia parasitica with mites isolated from cankers on American chestnut trees. Plant Disease, 67, 757. https://doi.org/10.1094/PD-67-757
A.4. Meloidogyne mali
A.4.1. Organism information
| Taxonomic information |
Current valid scientific name: Meloidogyne mali Synonyms: Meloidogyne ulmi Name used in the EU legislation: – Order: Rhabditia Family: Meloidogynidae Common name: apple root‐knot nematode Name used in the Dossier: Meloidogyne mali |
|
| Group | Nematodes | |
| EPPO code | MELGMA | |
| Regulated status |
Meloidogyne mali is included in the EPPO A2 list (EPPO, online_a) and was recently recommended for regulation as quarantine pest (EPPO, online_b). Meloidogyne mali is quarantine pest in the US and Morocco (EPPO, online_a) and listed as a ‘pest of quarantine interest’ in the Dominican Republic (EPPO, 2017); it is also regulated in Colombia, the Republic of Korea, Malaysia and Uruguay (EPPO, 2017). All Meloidogyne species are quarantine pests for Türkiye (EPPO, 2017). |
|
| Pest status in the UK |
Meloidogyne mali is present in the UK in Southern England ‐ two sites in Farnham and Surrey (Dossier Section 3.0) where it was found on elm trees in 2018, as consequence of introduction in the past of infected elms from the Netherlands (Prior et al., 2019). According to the Dossier Section 5.0 the nematode is present in the UK: not widely distributed and not under official control. |
|
| Pest status in the EU |
Meloidogyne mali is currently present in the EU in Austria (de Jong et al., online); it is also present in Belgium (Suwanngam and Wesemael, 2019), Italy (Palmisano and Ambrogioni, 2000) and the Netherlands (Ahmed et al., 2013), in all cases with few occurrences or restricted distribution (EPPO, online_c). M. mali was detected in France (Ile de France) in 2016, but it was eradicated in 2021 (EPPO, online_c). According to Ahmed et al. (2013) and EPPO (2017) M. mali may have a wider distribution in Europe, since elm plants growing in plots infested by the nematode in the Netherlands have been sent to other countries (Belgium, Denmark, France, Germany, Ireland, Italy, Spain, Slovakia, Romania, UK) to carry out resistance tests against the Dutch Elm Disease (DED). These programmes started from the 80's of the last century (Prior et al., 2019). |
|
| Host status on Quercus |
According to Ahmed et al. (2013) Q. robur is a host for Meloidogyne mali. There is no evidence that M. mali can infest other Quercus species. |
|
| PRA information | Available Pest Risk Assessments:
|
|
| Other relevant information for the assessment | ||
| Biology |
Meloidogyne mali is a root‐knot nematode inducing root galls on host plants; it is native to Asia (Japan), introduced decades ago to Europe and more recently also to the US (EPPO, 2017; Eisenback et al., 2017) and to the Republic of Korea (Kang et al., 2021). When found in Europe in 2000, the nematode was initially described as a new species, Meloidogyne ulmi (Palmisano and Ambrogioni, 2000) and elms remained long time the only known host plants. The synonymy with the well‐known species M. mali was found later, after comparison in the Netherlands with living material from Japan (Ahmed et al., 2013). Meloidogyne mali develops through three 6 stages: eggs, juveniles (four stages) and adults, all living in the root galls. Adult males, 2nd stage juveniles and eggs can live also free in the soil (EPPO, 2017). Information on M. mali biology mainly come from Malus sp. in Japan where the nematode and has one generation per year and the life cycle lasts 18–22 weeks. However, it is known that Meloidogyne species can frequently have more generations per year depending on the temperature and the feeding on perennial plants. Only few specific information on the life cycle of M. mali is available. Unlike similar species as M. chitwoodi and M. fallax which are parthenogenetic, M. mali reproduces sexually. Like all Meloidogyne root‐knot nematodes it deposits eggs in gelatinous sacs on the surface of galls or within them (EPPO, 2017; EFSA, 2019); in Japan the minimum hatching temperature range of M. mali eggs is 10–15°C (optimal 20–33°C) (EPPO, 2017). As usual in Meloidogyne species, the infective second‐stage juveniles move in the soil and attack the roots penetrating behind the root cap. They start to feed on cortical tissues inducing the formation of giant cells that cause swelling and finally root galls. After moulting, adults develop from the last juvenile stage; females remain into the roots where they lay eggs in a gelatinous matrix, while males leave the galls (EFSA, 2019). It is not clear in what extent the nematode can survive frost conditions during winter. Meloidogyne mali can probably overwinter in the roots of plants growing outdoors, possibly as young females, given that egg‐laying females have been observed in early March (EPPO, 2017). In the US the nematode seems able to survive at minimum winter temperature of −6°C (Pylypenko, 2016). Although Meloidogyne species are known not forming cysts to resist to the absence of host plants for long time, M. mali can survive for at least 2 years in root fragments in the soil after removal of infected trees; it is not known, however, if the nematode can also have a diapause period (EPPO, 2017). All Meloidogyne are strictly associated with the roots of plants and are known to be sedentary species, moving in the soil 1–2 m maximum per year and spread through the roots depending on their size, type of soil, water availability and other parameters (EFSA, 2019). As other species of root‐knot nematodes, the spread on medium‐long distance of Meloidogyne mali is by passive transport, and possible pathways are mainly plants for planting with infected roots, soil and growing media and also contaminated tools and machinery (EPPO, 2017). |
|
| Symptoms | Main type of symptoms |
Plants infected by M. mali show root‐knot galls on roots. The galls can be of different size also depending on the hosts and are always visible to the naked eye (0.5–2 cm in diameter) (EPPO, 2018). When a severe root infection occurs, as consequence of the developing of large number of galls the root system can be damaged, reducing uptake of water and minerals and causing symptoms on above‐ground part of plants. Common symptoms are little growth of primary shoots and increase of secondary shoots, leaf fall and general reduction of growth. No specific information about symptoms on Quercus robur was found. |
| Presence of asymptomatic plants | Plants infected by M. mali can remain asymptomatic. Damage on above‐ground part of plants goes often unnoticed in early infection stage or when underground attack on roots is light. 30‐year‐old elms gravely infected in the root system were uprooted by wind without any symptom on the crown or foliage (EPPO, 2017). | |
| Confusion with other pests |
Plants infected by M. mali appear similar to plants infected by other nematode species or root pathogens living in the soil. The identification of the nematode is not possible on the basis of sole galls. M. mali juveniles and adults are morphologically similar to other Meloidogyne nematodes. For identification to species level, laboratory tests on morphometric characters, electrophoresis or sequencing/DNA barcoding are needed (EPPO, 2018). |
|
| Host plant range |
Meloidogyne mali is a polyphagous nematode feeding on roots of several species of trees, shrubs and herbaceous plants. Some important woody hosts of M. mali are Acer × freemani, A. palmatum. A. pseudoplatanus, Castanea crenata, Euonymus kiautschovicus, E. fortunei, Fagus sylvatica, Lagerstroemia indica, Malus pumila, Morus alba, Prunus serrulata, Quercus robur, Sorbus aucuparia, Taxus baccata, Ulmus glabra, U. parvifolia, Vitis vinifera, Zelkova serrata (EPPO, 2017; DEFRA, online; Ferris, online). Common herbaceous hosts are: Dryopteris filix‐mas, D. carthusiana, Geranium robertianum, Geum coccineum, Impatiens parviflora, Rosa sp., Rubus fruticosus, Taraxacum officinale, Trifolium repens and Urtica dioica (EPPO, 2017; DEFRA, online). For a complete list of hosts see EPPO (2017) and DEFRA (online). |
|
| Reported evidence of impact |
Only poor information on economic impact caused by M. mali is available. In Japan, damage on Malus and Morus (15–43% growth reduction) was reported only following inoculation experiments. In Italy slowly declining elms were observed (Palmisano and Ambrogioni, 2000). In the UK, M. mali was only found in elms killed by DED (Prior et al., 2019). Roots damaged by M. mali may be also attacked by secondary pathogen agents. On elm trees in the Netherlands the infection by M. mali caused detriment of stability with uprooting by wind in urban areas (EPPO, 2017). No specific data about damage on Quercus robur was found. |
|
| Evidence that the commodity is a pathway | Meloidogyne mali can travel with plants for planting; although no specific evidence about Q. robur plants is found, they are certainly a possible pathway of entry for the nematode like other species as Acer, frequently intercepted mostly from Japan (EUROPHYT, online; TRACES‐NT,online). | |
| Surveillance information |
According to the Dossier Section 5.0, Meloidogyne mali is not under official surveillance, as does not meet criteria of quarantine pest for Great Britain. A survey was conducted to determine the extent of Meloidogyne mali presence in Surrey; all of the samples outside the two sites where the nematode was found in 2018 were negative, indicating that it has not spread off the sites (Dossier Section 3.0). A containment approach is being implemented in the two sites. No movement of soil from the sites is allowed. No movement of host plants from the sites is allowed. Staff and contractors coming into contact with host plants or soil on sites must remove soil from footwear and equipment before leaving the sites. Only non‐hosts should be planted at the sites (Dossier Section 3.0). |
|
A.4.2. Possibility of pest presence in the nursery
A.4.2.1. Possibility of entry from the surrounding environment
Meloidogyne mali is currently found in the UK territory only on Ulmus sp. in two sites in Southern England (Farnham, Surrey) (Prior, 2019; Dossier Sections 3.0 and 5.0). The pest is not regulated in the UK. No presence of the nematode outside the two known sites is reported and a containment approach has been implemented (Dossier Section 3.0).
The nematode can only spread by passive transport with plants for planting with infected roots, infected soil and growing media, and possibly via contaminated tools and machinery. No other possibility of entry in the nurseries is known.
M. mali can infect Fagus sylvatica, Malus pumila, Morus alba, Taxus baccata, Q. robur, Rosa spp. and Ulmus spp. which are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
Pest pressure of the nematodes in the surrounding areas.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for M. mali to enter the nurseries from surrounding environment by infested machinery. In the surrounding area, suitable hosts are present but the nematode cannot enter by other way than human assisted spread.
A.4.2.2. Possibility of entry with new plants/seeds
The starting materials are only seeds and seedlings. Seeds are certified and coming from the UK. Seedlings are obtained either from the UK or the EU (mostly the Netherlands) (Dossier Section 3.0). Seeds are not a pathway for the nematode.
In addition to Quercus species, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are many suitable hosts for the nematode (such as Acer spp., Fagus sylvatica, Malus pumila, Rosa spp., Sorbus spp., Taxus baccata, Ulmus spp.). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the nematode could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). M. mali is able to survive both in the soil and in root fragments in the soil for 2 years (EPPO, 2007) and therefore could potentially enter with infested soil/growing media. However, the growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
No information is available on the provenance of plants other than Quercus used for plant production in the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the nematode to enter the nurseries via infected roots of new seedlings of Q. robur and plants of other species used for plant production in the area. The entry of the nematode with seeds and the growing media the Panel considers as not possible.
A.4.2.3. Possibility of spread within the nursery
Quercus plants are either grown in containers (cells, pots, tubes, etc.) outdoors in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The nematode can infect other suitable plants (such as Acer spp., Fagus sylvatica, Ulmus spp. etc.) present within the nurseries (Dossier Sections 3.0 and 6.0).
M. mali can spread within the nurseries by movement of soil, water, infested plant material and infected tools and machinery (EPPO, 2017). However, tools used in the nurseries are disinfected after operation on a stock and before being used on a different plant species (Dossier Section 3.0).
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the nematode within the nurseries is possible either by movement of infested soil, water and plant material.
A.4.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of M. mali between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online).
A.4.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on M. mali is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
As the plant passport is very similar to the EU one, the plants shall be free from quarantine and RNQP pests.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
Seedlings could be a pathway for the nematode. The certification could have an effect on preventing the nematode to enter into the nurseries.
Uncertainties:
|
| 4 | Growing media | Yes |
Heat treatment and protection of the treated growing media is effective against the nematode.
Uncertainties:
|
| 5 | Surveillance, monitoring and sampling | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Uncertainties:
|
| 9 | Application of pest control products | No | Not relevant, no nematicides are used in the nurseries. |
| 10 | Measures against soil pests | Yes |
Separation of the pots from soil is effective against the nematode.
Uncertainties:
|
| 11 | Inspections and management of plants before export | Yes |
This assessment can have some effect against the nematode.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. The nematode cannot spread between the roots of the plants when transported to the EU. |
A.4.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.4.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pest in the nurseries and in the surroundings. The plants are exposed to the nematode for only short period of time. The scenario also assumes that root galls are visible while inspecting plants before export and that the second juvenile stage are washed away during the root washing.
A.4.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pest in the nurseries and in the surroundings as many potential hosts are present. The scenario also assumes that root galls are not easily recognisable while inspecting plants before export and that the low‐pressure washing is not effective in removing the second juvenile stage before export.
A.4.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pest in the nurseries and the surroundings and that the plants are exposed to the nematode for only short period of time. The movement of soil from the surrounding into the nurseries is not expected to be significant.
A.4.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pests in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.4.5.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne mali on bundles of whips and seedlings
The following Tables show the elicited and fitted values for pest infection (Table A.19) and pest freedom (Table A.20).
Table A.19.
Elicited and fitted values of the uncertainty distribution of pest infection by Meloidogyne mali per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 3 | 25 | 40 | 60 | 150 | ||||||||||
| EKE | 5.51 | 8.12 | 11.0 | 15.3 | 19.9 | 25.0 | 29.8 | 40.0 | 52.2 | 60.1 | 70.5 | 83.0 | 99.1 | 114 | 134 |
The EKE results is the BetaGeneral (2.6372, 576.47, 0, 10,000) distribution fitted with @Risk version 7.6.
Table A.20.
The uncertainty distribution of bundles free of Meloidogyne mali per 10,000 bundles calculated by Table A.19
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,940 | 9,960 | 9,975 | 9,997 | ||||||||||
| EKE results | 9,866 | 9,886 | 9,901 | 9,917 | 9,929 | 9,940 | 9,948 | 9,960 | 9,970 | 9,975 | 9,980 | 9,985 | 9,989 | 9,992 | 9,994 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.20.
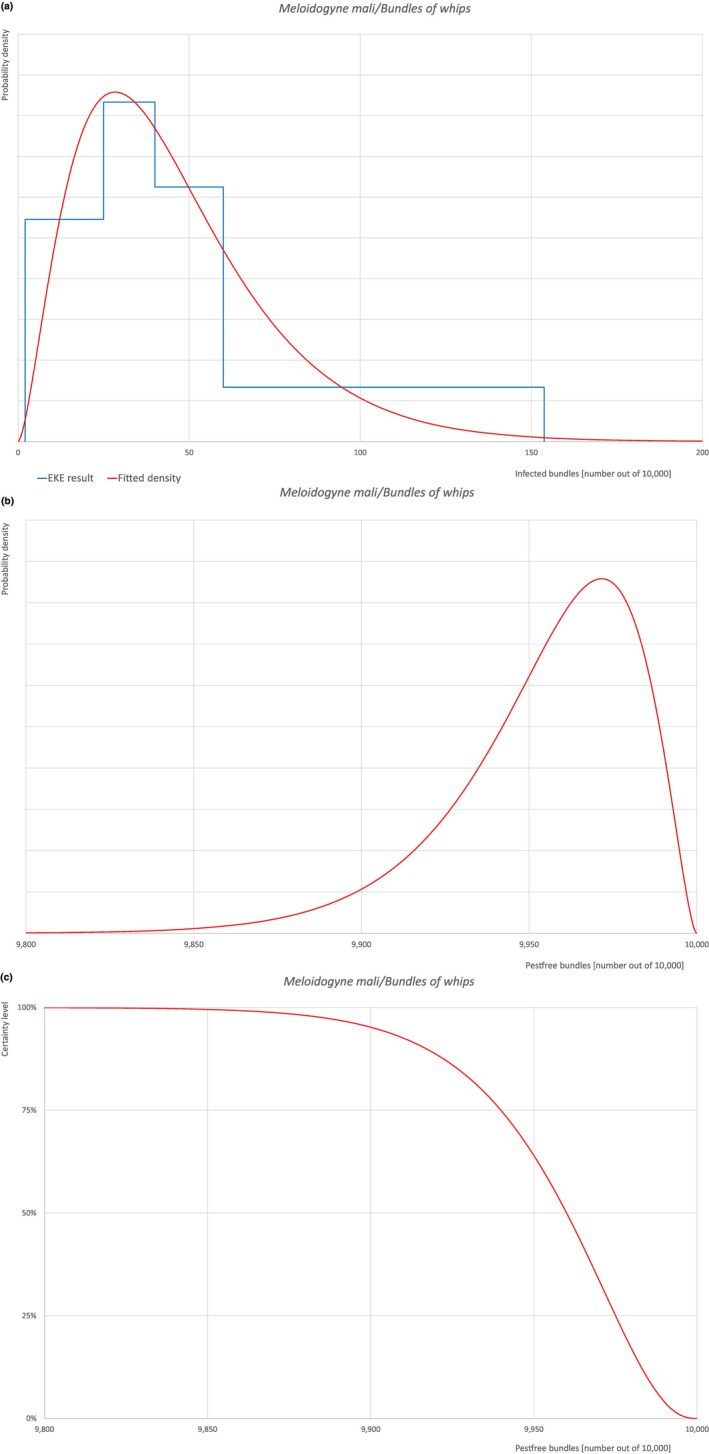
Figure A.10: (a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
A.4.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.4.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees up to 7 years old
The scenario assumes a low pressure of the pest in the nurseries and in the surroundings. Younger plants are exposed to the nematode for only short period of time. The scenario also assumes that root galls are visible while inspecting plants before export and that the second juvenile stage are washed away during the root washing of bare root plants.
A.4.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees up to 7 years old
The scenario assumes a high pressure of the pest in the nurseries and in the surroundings as many potential hosts are present. Older plants are exposed to the nematode for longer period of time. The scenario also assumes that root galls are not easily recognisable while inspecting plants before export and that the low‐pressure washing is not effective in removing the second juvenile stage before export.
A.4.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees up to 7 years old (Median)
The scenario assumes a limited presence of the pest in the nurseries and the surroundings and that the plants are exposed to the nematode for a sufficient period of time for infection to occur. The movement of soil from the surrounding into the nurseries is not expected to be significant.
A.4.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pests in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.4.6.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne mali on bare root plants/trees up to 7 years old
The following Tables show the elicited and fitted values for pest infection (Table A.21) and pest freedom (Table A.22).
Table A.21.
Elicited and fitted values of the uncertainty distribution of pest infection by Meloidogyne mali per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 35 | 70 | 130 | 250 | ||||||||||
| EKE | 1.31 | 3.22 | 6.41 | 12.8 | 21.7 | 33.2 | 45.5 | 73.1 | 107 | 127 | 153 | 179 | 208 | 229 | 250 |
The EKE results is the BetaGeneral (1.0205, 2.5146, 0, 297) distribution fitted with @Risk version 7.6.
Table A.22.
The uncertainty distribution of plants free of Meloidogyne mali per 10,000 plants calculated by Table A.21
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,870 | 9,930 | 9,965 | 9,999 | ||||||||||
| EKE results | 97,50 | 9,771 | 9,792 | 9,821 | 9,847 | 9,873 | 9,893 | 9,927 | 9,955 | 9,967 | 9,978 | 9,987.2 | 9,994 | 9,997 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.22.
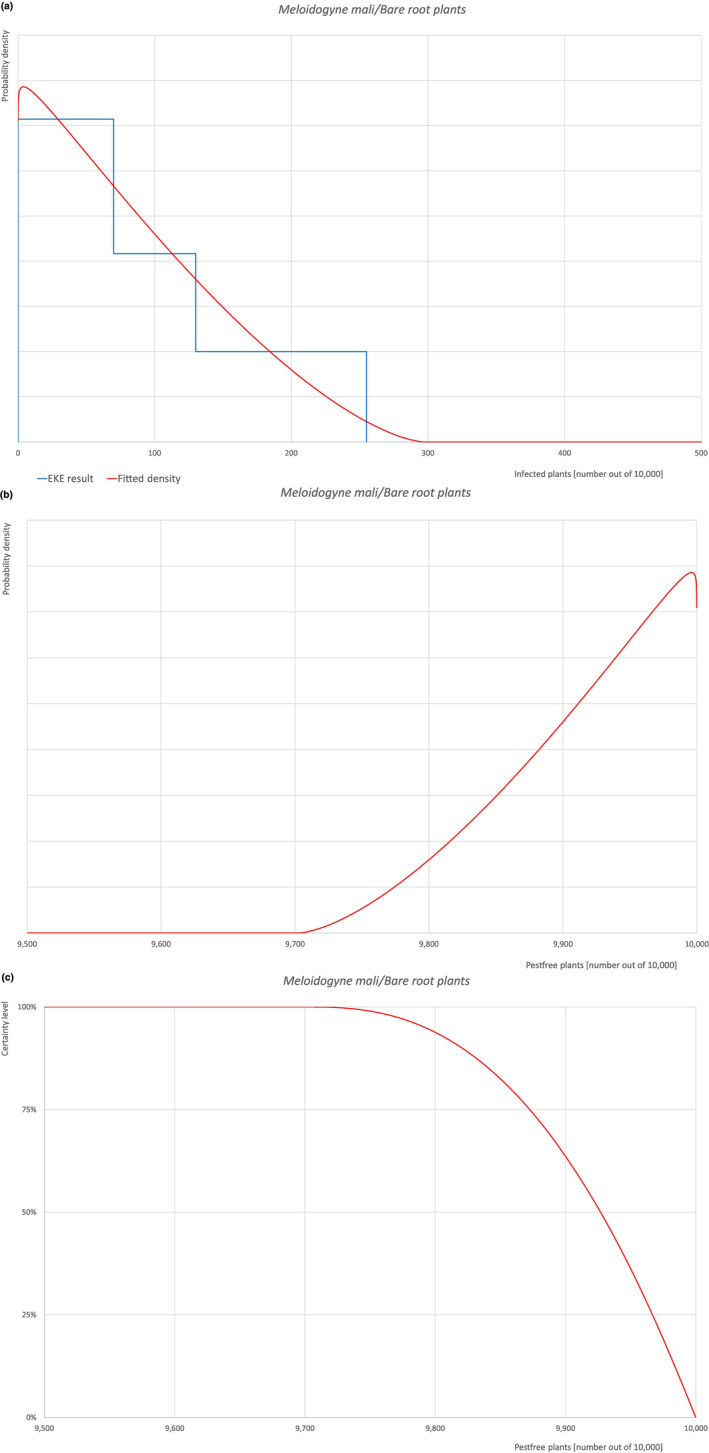
Figure A.11: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.4.7. Overall likelihood of pest freedom for plants in pots up to 15 years old
A.4.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots up to 15 years old
The scenario assumes a low pressure of the pest in the nurseries and in the surroundings. Younger plants are exposed to the nematode for only short period of time. The scenario also assumes that root galls are visible while inspecting plants before export and that the root systems of plants have undergone washing and inspection before being transplanted in pots.
A.4.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots up to 15 years old
The scenario assumes a high pressure of the pest in the nurseries and in the surroundings as many potential hosts are present. Older plants are exposed to the nematode for longer period of time. The scenario also assumes that root galls are not easily recognisable while inspecting plants before export and that the root systems of plants did not undergone washing and inspection before being transplanted in pots.
A.4.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots up to 15 years old (Median)
The scenario assumes a limited presence of the pest in the nurseries and the surroundings and that the plants are exposed to the nematode for a sufficient period of time for infection to occur. The movement of soil from the surrounding into the nurseries is not expected to be significant.
A.4.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on occurrence of the pests in the UK including the nurseries and the surroundings results in high level of uncertainties for infection rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.4.7.5. Elicitation outcomes of the assessment of the pest freedom for Meloidogyne mali on plants in pots up to 15 years old
The following Tables show the elicited and fitted values for pest infection (Table A.23) and pest freedom (Table A.24).
Table A.23.
Elicited and fitted values of the uncertainty distribution of pest infection by Meloidogyne mali per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1 | 45 | 90 | 130 | 250 | ||||||||||
| EKE | 4.68 | 8.79 | 14.3 | 23.5 | 34.3 | 47.0 | 59.5 | 85.7 | 116 | 134 | 157 | 181 | 207 | 228 | 250 |
The EKE results is the BetaGeneral (1.4846, 3.5229, 0, 320) distribution fitted with @Risk version 7.6.
Table A.24.
The uncertainty distribution of plants free of Meloidogyne mali per 10,000 plants calculated by Table A.23
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,870 | 9,910 | 9,955 | 9,999 | ||||||||||
| EKE results | 9,750 | 9,772 | 9,793 | 9,819 | 9,843 | 9,866 | 9,884 | 9,914 | 9,940 | 9,953 | 9,966 | 9,976.5 | 9,986 | 9,991 | 9,995 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.24.
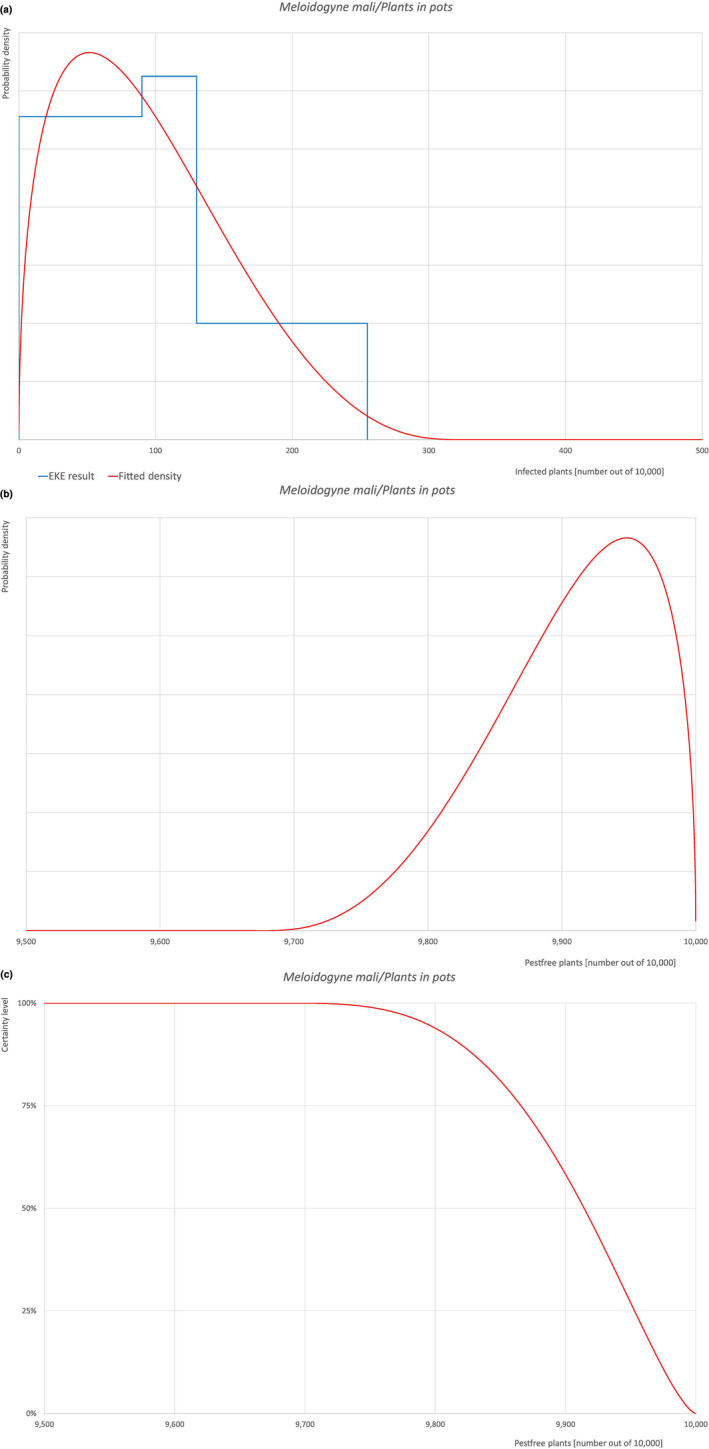
Figure A.12: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.4.8. Reference list
Ahmed M, van de Vossenberg BTLH, Cornelisse C and Karssen G, 2013. On the species status of the root‐knot nematode Meloidogyne ulmi Palmisano and Ambrogioni, 2000 (Nematoda, Meloidogynidae). ZooKeys, 362, 1–27. https://doi.org/10.3897/zookeys.362.6352
DEFRA (Department for Environment, Food and Rural Affairs), online. UK risk register details for Meloidogyne mali. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=16542 [Accessed: 30 November 2022].
de Jong Y, Verbeek M, Michelsen V, de Place Bjørn P, Los W, Steeman F, Bailly N, Basire C, Chylarecki P, Stloukal E, Hagedorn G, Wetzel FT, Glöckler F, Kroupa A, Korb G, Hoffmann A, Häuser C, Kohlbecker A, Müller A, Güntsch A, Stoev P and Penev L, online. Fauna Europaea ‐ all European animal species on the web. Biodiversity Data Journal. Available online: https://fauna-eu.org/ [Accessed: 3 November 2022].
EFSA (European Food Safety Authority), den Nijs L, Camilleri M, Diakaki M, Schenk M and Vos S, 2019. Pest survey card on Meloidogyne chitwoodi and Meloidogyne fallax. EFSA supporting publication 2019;EN‐1572, 20 pp. https://doi.org/10.2903/sp.efsa.2019.en-1572
EFSA PLH Panel (EFSA Panel on Plant Health), 2015. Scientific opinion on the risks to plant health posed by EU import of soil or growing media. EFSA Journal 2015;13(6):4132, 133 pp. https://doi.org/10.2903/j.efsa.2015.4132
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Gonthier P, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Zappalà L, Lucchi A, Gòmez P, Urek G, Bernardo U, Bubici G, Carluccio AV, Chiumenti M, Di Serio F, Fanelli E, Marzachì C, Kaczmarek A, Mosbach‐Schulz O and Yuen J, 2023a. Scientific Opinion on the commodity risk assessment of Malus domestica plants from United Kingdom. EFSA Journal 2023;21(5):8002, 146 pp. https://doi.org/10.2903/j.efsa.2023.8002
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Gonthier P, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H1‐H, Van der Werf W, Vicent Civera A, Zappalà L, Lucchi A, Gòmez P, Urek G, Bernardo U, Bubici G, Carluccio AV, Chiumenti M, Di Serio F, Fanelli E, Marzachì C, Kaczmarek A, Mosbach‐Schulz O and Yuen J, 2023b. Scientific Opinion on the commodity risk assessment of Malus sylvestris plants from United Kingdom. EFSA Journal 2023;21(6):8076, 122 pp. https://doi.org/10.2903/j.efsa.2023.8076
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023c. Scientific Opinion on the commodity risk assessment of Acer campestre plants from the UK. EFSA Journal 2023;21(7):8071, 291 pp. https://doi.org/10.2903/j.efsa.2023.8071
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023d. Scientific Opinion on the commodity risk assessment of Acer palmatum plants from the UK. EFSA Journal 2023;21(7):8075, 228 pp. https://doi.org/10.2903/j.efsa.2023.8075
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023e. Scientific Opinion on the commodity risk assessment of Acer platanoides plants from the UK. EFSA Journal 2023;21(7):8073, 268 pp. https://doi.org/10.2903/j.efsa.2023.8073
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023f. Scientific Opinion on the commodity risk assessment of Acer pseudoplatanus plants from the UK. EFSA Journal 2023;21(7):8074, 271 pp. https://doi.org/10.2903/j.efsa.2023.8074
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023f. Scientific Opinion on the commodity risk assessment of Fagus sylvatica plants from the UK. EFSA Journal 2023;21(7):8118, 151 pp. https://doi.org/10.2903/j.efsa.2023.8118
Eisenback JD, Graney LS and Vieira P, 2017. First report of the apple root‐knot nematode (Meloidogyne mali) in North America, found parasitizing Euonymus in New York. Plant Disease, 101, 510. https://doi.org/10.1094/pdis-06-16-0894-pdn
EPPO (European and Mediterranean Plant Protection Organization), 2017. Pest risk analysis for Meloidogyne mali, apple root‐knot nematode. EPPO, Paris, 38 pp.
EPPO (European and Mediterranean Plant Protection Organization), 2018. Diagnostics PM 7/136 (1) Meloidogyne mali. Bulletin OEPP/EPPO, 48, 438–445.
EPPO (European and Mediterranean Plant Protection Organization), online_a. Meloidogyne mali (MELGMA), Categorization. Available online: https://gd.eppo.int/taxon/MELGMA/categorization [Accessed: 30 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Meloidogyne mali (MELGMA), Documents. Available online: https://gd.eppo.int/taxon/MELGMA/documents [Accessed: 30 November 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Meloidogyne mali (MELGMA), Distribution. Available online: https://gd.eppo.int/taxon/MELGMA/distribution [Accessed: 30 November 2022].
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en [Accessed: 22 December 2022].
Ferris H, online. Nemaplex (The Nematode‐Plant Expert Information System). Available online: http://nemaplex.ucdavis.edu/ [Accessed: 4 December 2022].
GenBank (National Center for Biotechnology Information), online. Meloidogyne mali. Available online: https://www.ncbi.nlm.nih.gov/nuccore/?term=meloidogyne+mali [Accessed: 8 February 2023].
Kang H, Seo J, Ko HR, Park S, Park NS, Park BY and Choi I, 2021. First report of the apple root‐knot nematode, Meloidogyne mali, on maple trees in the Republic of Korea. Plant Disease. https://doi.org/10.1094/pdis-09-21-2121-pdn
Palmisano A and Ambrogioni L, 2000. Meloidogyne ulmi sp. n., a root‐knot nematode from elm. Nematologia Mediterranea, 28, 279–293.
Prior T, Tozer H, Yale R, Jones EP, Lawson R, Jutson L, Correia M, Stubbs J, Hockland S and Karssen G, 2019. First report of Meloidogyne mali causing root galling to elm trees in the UK. New Disease Reports, 39, 10. https://doi.org/10.5197/j.2044-0588.2019.039.010
Pylypenko LA, 2016. A quickscan pest risk analysis for the Meloidogyne mali. Interdepartmental Thematic Scientific Collection of Plant Protection and Quarantine, 62, 188–200. https://doi.org/10.36495/1606-9773.2016.62.188-200.
Suwanngam A and Wesemael WML, 2019. First report of the root‐knot nematode Meloidogyne mali infecting elm trees in Belgium. New Disease Reports, 40, 16. https://doi.org/10.5197/j.2044-0588.2019.040.016
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
A.5. Phytophthora kernoviae
A.5.1. Organism information
| Taxonomic information |
Current valid scientific name: Phytophthora kernoviae Synonyms: – Name used in the EU legislation: – Order: Peronosporales Family: Peronosporaceae Common name: – Name used in the Dossier: Phytophthora kernoviae |
|
| Group | Oomycetes | |
| EPPO code | PHYTKE | |
| Regulated status |
Phytophthora kernoviae is not regulated in the EU. The pathogen is included in the EPPO A2 list (EPPO, online_a). Phytophthora kernoviae is quarantine in Morocco. It is on A1 list of Chile, Egypt, Kazakhstan and EAEU (=Eurasian Economic Union: Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) and on A2 list of the UK (EPPO, online_b). |
|
| Pest status in the UK |
Phytophthora kernoviae is present in the UK: England, Scotland and Wales (Brasier et al., 2005; Webber, 2008; Elliot et al., 2013; EPPO, online_c; Farr and Rossman, online). From 2003 till January 2008 the pathogen was found mainly in the wild and only reported in three nurseries. In May 2008 it was found on imported plant material in a nursery in Kent (DEFRA, 2008). According to the Dossier Section 5.0 P. kernoviae is present not widely distributed, it is UK provisional quarantine pest and it is under official control in Great Britain. In Northern Ireland the pathogen is not recorded. |
|
| Pest status in the EU | Phytophthora kernoviae is present in Ireland (O'Hanlon et al., 2016; EPPO, online_c). It was first found on Rhododendron ponticum in woodlands in county Cork (South coast of Ireland) in 2008 (EPPO, online_d). | |
| Host status on Quercus |
Quercus robur is reported host of P. kernoviae in the UK (Brasier et al., 2005; EPPO, online_e; Farr and Rossman, online). Phytophthora kernoviae is a pathogen of other Quercus species such as Quercus ilex (Brasier et al., 2005; EPPO, online_e). |
|
| PRA information | Pest Risk Assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology |
Phytophthora kernoviae is present in Europe (Ireland, the UK), Oceania (New Zealand) and South America (Argentina, Chile) (EPPO, online_c; Farr and Rossman, online). The pathogen was first found on Fagus sylvatica and Rhododendron ponticum in Cornwall, south‐west England in 2003 during official surveillance activities for P. ramorum. Its origin is unclear (Brasier et al., 2005), but it is suggested to be native to New Zealand (Studholme et al., 2019). Phytophthora species generally reproduce through a) dormant (resting) spores which can be either sexual (oospores) or asexual (chlamydospores); and b) fruiting structures (sporangia) which contain zoospores (Erwin and Ribeiro, 1996). Phytophthora kernoviae belongs to clade 10c (Blair et al., 2008; Jung et al., 2022). The pathogen is self‐fertile (homothallic) and produces oogonia, oospores and highly caducous sporangia. Chlamydospores were not observed. The sporangia are either splash or wind dispersed over short distances (Brasier et al., 2005; DEFRA, 2008). Sporangia are only formed on hosts with susceptible foliage. Rhododendron is the most abundant sporulating host in Great Britain woodlands. Trunk cankers (e.g. on Fagus sylvatica) are not known to support sporulation and therefore do not transmit the pathogen. This appears to be a dead end for the pathogen (DEFRA, 2008). Optimum conditions for growth require temperatures between 18 and 26°C (Brasier et al., 2005) and moisture (DEFRA, 2008). |
|
|
Optimum temperature for infection on Rhododendron ponticum was observed to be between 15 and 20°C (Shelley et al., 2018). Oospore germination was optimal at 18 and 20°C. Germination was higher when oospores were exposed to continuous light compared to those in the dark, although not significantly for all isolates (Widmer, 2010). Phytophthora kernoviae infects leaves, shoots, stems, buds (DEFRA, 2008) and also roots (Fichtner et al., 2011). According to Brown and Brasier (2007), P. kernoviae commonly occupies xylem beneath phloem lesions and may spread within xylem and possibly recolonise the phloem from the xylem. Phytophthora kernoviae can remain viable within xylem for 2 or more years after the overlying phloem had been excised. Phytophthora kernoviae can be found in soil, leaf litter and water streams (DEFRA, 2008). According to Widmer (2011) oospores of P. kernoviae buried in a sand can survive for long periods of time at temperatures of 30°C and below. In the west of Scotland inoculum of P. kernoviae persisted in soil for at least 2 years after its hosts were removed (Elliot et al., 2013). In Chile, P. kernoviae was common to small forest streams (Jung et al., 2018). Phytophthora kernoviae can disperse by soil containing propagules on people's shoes, feet of animals and wheels of machinery (Brasier, 2008; DEFRA, 2008). Possible pathways of entry for P. kernoviae are plants for planting (excluding seed and fruit) of known susceptible hosts; plants for planting (excluding seed and fruit) of non‐host plant species accompanied by contaminated attached growing media; soil/growing medium (with organic matter) as a commodity; soil as a contaminant; foliage or cut branches; susceptible (isolated) bark and susceptible wood (EPPO, 2013). |
||
| Symptoms | Main type of symptoms |
According to DEFRA (2008) P. kernoviae causes three different types of disease:
Phytophthora kernoviae causes bark necrosis and bleeding stem lesions above‐ground level on Fagus sylvatica (Brasier et al., 2005). There is an uncertainty whether such symptoms develop on young plants and plants for planting. The pathogen was also observed to infect roots of F. sylvatica (Fichtner et al., 2012, citing others). On Rhododendron ponticum the pathogen causes shoot dieback, foliar necrosis, wilting, cankers, defoliation and death (Brasier et al., 2005; Beales et al., 2006). Symptoms on Drimys winteri in native forest of southern Chile showed necrosis around the midrib of leaves (Sanfuentes et al., 2016) and bleeding canker in the UK (EPPO, online_f). It was found to be infecting stems of Quercus robur and causing bleeding cankers in the UK (Brasier et al., 2005; DEFRA, 2008). |
| Presence of asymptomatic plants |
Phytophthora kernoviae was observed causing symptomless infections of leaves on Rhododendron ‘Cunninghams White’ and Quercus ilex (Denman et al., 2009) and symptomless infections of roots on R. ponticum (Fichtner et al., 2011). Application of some fungicides may reduce symptoms and therefore mask infection, making it more difficult to determine whether the plant is pathogen‐free (DEFRA, 2008). |
|
| Confusion with other pests | Phytophthora kernoviae can be easily distinguished from other Phytophthora species based on morphology (Brasier et al., 2005) and molecular tests (Beales et al., 2006; Hughes et al., 2011). | |
| Host plant range |
Phytophthora kernoviae has quite wide host range. Main host plants include Fagus sylvatica and Rhododendron ponticum (EPPO, online_e). Other hosts are Aesculus hippocastanum, Agathis australis, Annona cherimola, Berberis, Castanea sativa, Drimys winteri, Fagus grandiflora, Gevuina avellana, Hedera helix, Ilex aquifolium, Leucothoe fontanesiana, Liriodendron tulipifera, Lomatia myricoides, Magnolia amoena, M. cylindrica, M. delavayi, M. doltsopa, M. kobus, M. liliiflora, M. salicifolia, M. sargentiana, M. sprengeri, M. stellata, M. wilsonii, M. × brooklynensis, M. × soulangeana, Michelia doltsopa, Photinia sp., Pieris formosa, P. japonica, Pinus radiata, Podocarpus salignus, Prumnopitys ferruginea, Prunus laurocerasus, Quercus ilex, Q. robur, Sequoiadendron giganteum and Vaccinium myrtillus (Brasier et al., 2005; Dick et al., 2014; O'Hanlon et al., 2016; EPPO, online_e; Farr and Rosmann, online). Experimental hosts are Rhododendron macrophyllum, R. occidentale and Umbellularia californica (Fichtner et al., 2012; EPPO, online_e). Some of the hosts which have susceptible leaves and can produce infective sporangia are Drimys spp., Gevuina avellana, Ilex, Liriodendron tulipifera, Magnolia, Michelia, Prunus laurocerasus, Quercus ilex and Rhododendron ponticum (DEFRA, 2008). |
|
| Reported evidence of impact |
There is no data available on the actual impact that Phytophthora kernoviae has caused so far in the world. In the UK P. kernoviae appears to be a serious foliar pathogen on Rhododendron species (Webber, 2008). According to Beales et al. (2009) P. kernoviae has caused significant impact on ornamental plants and tree species since 2003 mainly in south‐west England. In New Zealand the pathogen together with Phytophthora pluvialis is connected to red needle cast disease (Dick et al., 2014) or needle blight of Pinus radiata (McDougal and Ganley, 2021). However, it has rarely been associated with plant disease (Scott and Williams, 2014). |
|
| Evidence that the commodity is a pathway | According to EPPO (2013), P. kernoviae can travel with plants for planting. Therefore, the commodity is a possible pathway of entry for P. kernoviae. | |
| Surveillance information |
This pathogen is UK provisional quarantine pest. It has been found in all three countries of Great Britain, with the highest concentration of confirmed cases in the counties of Devon and Cornwall in South‐West England. It has not been recorded in Northern Ireland (Dossier Section 5.0). As part of an annual survey at ornamental retail and production sites (frequency of visits determined by a decision matrix) P. kernoviae is inspected for on common hosts plants (Dossier Section 5.0). |
|
A.5.2. Possibility of pest presence in the nursery
A.5.2.1. Possibility of entry from the surrounding environment
P. kernoviae is present in the UK, it has been found in England, Scotland and Wales (Brasier et al., 2005; Webber, 2008; Elliot et al., 2013; EPPO, online_c; Farr and Rossman, online).
The possible entry of P. kernoviae from surrounding environment to the nurseries may occur through wind and rain (Brasier et al., 2005), water (Jung et al., 2018), people, animals and machinery entering the nursery with infested soil (Brasier, 2008).
P. kernoviae has wide host range and can infect number of different plants. Suitable hosts of P. kernoviae like Aesculus spp., Annona spp., Berberis spp., Castanea spp., Fagus sylvatica, Fagus spp., Magnolia spp., Pieris spp., Pinus spp., Prunus spp., Rhododendron spp. and Vaccinium spp. are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
Level of susceptibility to the pathogen of Quercus spp.
-
–
The dispersal range of P. kernoviae sporangia.
-
–
Possibility of the pathogen to enter nursery with irrigation water.
-
–
The presence/abundance of the pathogen in the area where the nurseries are located.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present and the pathogen can spread by wind, rain, water and infested soil propagules on machinery and feet of animals and humans entering the nurseries.
A.5.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are obtained either from the UK or the EU (mostly the Netherlands) (Dossier Section 3.0). Seeds are not a pathway for the pathogen.
In addition to Quercus plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are many suitable hosts for the pathogen (such as Aesculus spp., Berberis spp., Castanea spp., Fagus spp., Liriodendron tulipifera, Magnolia spp., Pinus spp., Prunus spp. etc.). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). P. kernoviae is able to survive in soil (Elliot et al., 2013) and therefore could potentially enter with infested soil/growing media. However, the growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
No information is available on the provenance of plants other than Quercus used for plant production in the area of the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries with new seedlings of Quercus and new plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.5.2.3. Possibility of spread within the nursery
Quercus plants are either grown in containers (cells, pots, tubes, etc.) or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pathogen can infect other suitable plants (such as Aesculus spp., Berberis spp., Castanea spp., Fagus spp., Liriodendron tulipifera, Magnolia spp., Pinus spp., Prunus spp. etc.) present within the nurseries and hedges surrounding the nurseries (Ilex spp. and Prunus spp.) (Dossier Sections 3.0 and 6.0).
P. kernoviae can spread within the nurseries by wind, rain, soil, water, movement of infested plant material, humans and animals (Davidson et al., 2002).
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible by wind, rain, soil, water, movement of infested plant material, humans and animals.
A.5.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of P. kernoviae between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online).
A.5.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on P. kernoviae is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
Phytophthora kernoviae is a UK provisional quarantine pest targeted by this measure.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
Phytophthora kernoviae is a UK provisional quarantine pest targeted by this measure.
Uncertainties:
|
| 4 | Growing media | Yes |
This measure should ensure pest‐free growing media and is expected to prevent the introduction of the pathogen into the nurseries with growing media.
Uncertainties:
|
| 5 | Surveillance, monitoring and sampling | Yes |
This measure has an effect as the pathogen would be detected on nursery‐grown plants, as well as on incoming plant material and growing media, and suspected plant material quarantined.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
General hygiene measures will reduce the likelihood of the pathogen being spread by tools and equipment, although this is not a major pathway for the pest.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This measure could have some effect by removing potentially infested plant material, thus reducing the spread of the pathogen within the nursery.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Testing of irrigation water would detect the pathogen, which can spread by water. Overhead irrigation could favour the spread of the pathogen by water splash.
Uncertainties:
|
| 9 | Application of pest control products | Yes |
Some fungicides could reduce the likelihood of infection by the pathogen. However, some fungicides may reduce symptoms and therefore mask infection, making it more difficult to determine whether the plant is pathogen‐free (DEFRA, 2008).
Uncertainties:
|
| 10 | Measures against soil pests | Yes |
This measure could have some effect by preventing root contact with soil where the pathogen may be present.
Uncertainties:
|
| 11 | Inspections and management of plants before export | Yes |
P. kernoviae is a UK provisional quarantine pest targeted by this measure.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. |
A.5.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.5.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. The plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Quercus to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.5.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects leaves, which may still be present on the plants at the time of export. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.5.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Quercus. The pathogen is a provisional quarantine pest in the UK and under official control.
A.5.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the susceptibility of Quercus and the occurrence of the pathogen in the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.5.5.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora kernoviae on bundles of whips and seedlings
The following Tables show the elicited and fitted values for pest infection (Table A.25) and pest freedom (Table A.26).
Table A.25.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora kernoviae per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1.5 | 13 | 24 | 55 | 115 | ||||||||||
| EKE | 1.51 | 1.87 | 2.59 | 4.34 | 7.13 | 11.2 | 15.9 | 27.3 | 42.6 | 52.4 | 64.8 | 78.3 | 93.1 | 105 | 116 |
The EKE results is the BetaGeneral (0.79767, 2.5374, 1.35, 142) distribution fitted with @Risk version 7.6.
Table A.26.
The uncertainty distribution of bundles free of Phytophthora kernoviae per 10,000 bundles calculated by Table A.25
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,885 | 9,945 | 9,976 | 9,987 | 9,999 | ||||||||||
| EKE results | 9,884 | 9,895 | 9,907 | 9,922 | 9,935 | 9,948 | 9,957 | 9,973 | 9,984 | 9,989 | 9,993 | 9,996 | 9,997 | 9,998.1 | 9,998.5 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.26.
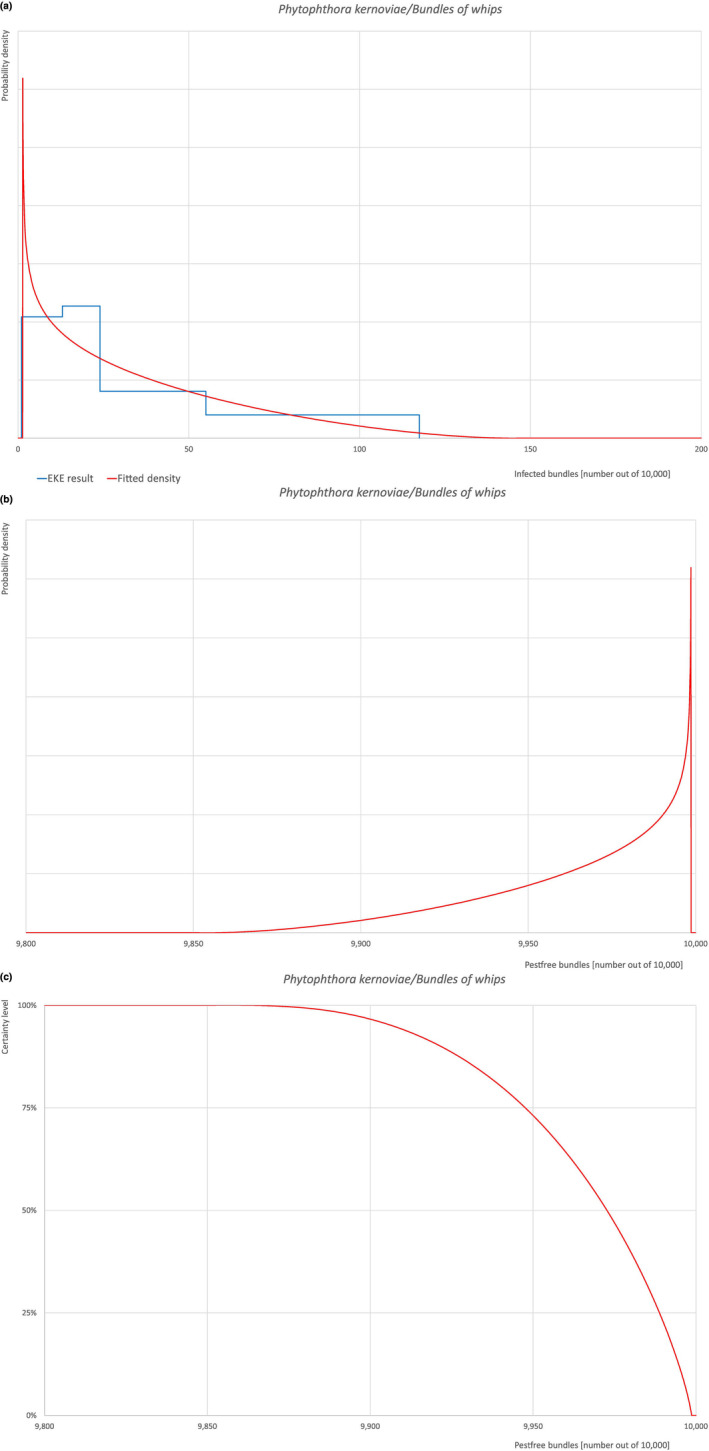
Figure A.13: (a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
A.5.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.5.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. The plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Quercus to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.5.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects leaves, which may still be present on the plants at the time of export. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.5.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Quercus. The pathogen is a provisional quarantine pest in the UK and under official control.
A.5.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the susceptibility of Quercus and the occurrence of the pathogen in the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.5.6.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora kernoviae on bare root plants/trees up to 7 years old
The following Tables show the elicited and fitted values for pest infection (Table A.27) and pest freedom (Table A.28).
Table A.27.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora kernoviae per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1.0 | 11 | 21 | 45 | 115 | ||||||||||
| EKE | 1.01 | 1.49 | 2.32 | 4.08 | 6.60 | 10.0 | 13.8 | 22.9 | 35.1 | 43.2 | 54.2 | 67.2 | 83.3 | 97.7 | 115 |
The EKE results is the BetaGeneral (0.97292, 6.4255, 0.7, 225) distribution fitted with @Risk version 7.6.
Table A.28.
The uncertainty distribution of plants free of Phytophthora kernoviae per 10,000 plants calculated by Table A.27
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,885 | 9,955 | 9,979 | 9,989 | 9,999 | ||||||||||
| EKE results | 9,885 | 9,902 | 9,917 | 9,933 | 9,946 | 9,957 | 9,965 | 9,977 | 9,986 | 9,990 | 9,993 | 9,996 | 9,997.7 | 9,998.5 | 9,999.0 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.28.
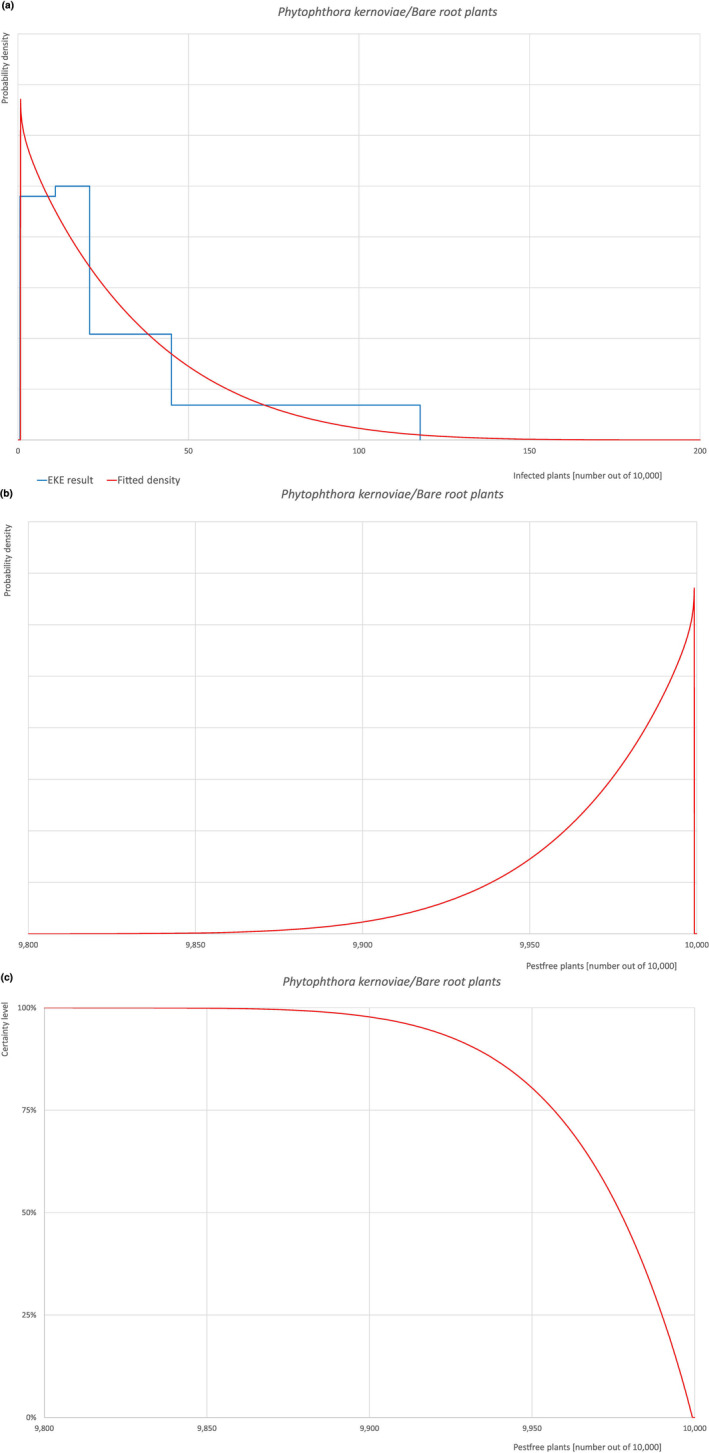
Figure A.14: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.5.7. Overall likelihood of pest freedom for plants in pots up to 15 years old
A.5.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots up to 15 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Quercus to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.5.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots up to 15 years old
The scenario assumes a high pressure of the pathogen in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects leaves, which may still be present on the plants at the time of export. Older trees are more likely to become infected due to longer exposure time and larger size. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.5.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots up to 15 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Quercus. The pathogen is a provisional quarantine pest in the UK and under official control.
A.5.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the susceptibility of Quercus and the occurrence of the pathogen in the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.5.7.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora kernoviae on plants in pots up to 15 years old
The following Tables show the elicited and fitted values for pest infection (Table A.29) and pest freedom (Table A.30).
Table A.29.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora kernoviae per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 1.5 | 22 | 42 | 100 | 225 | ||||||||||
| EKE | 1.49 | 2.11 | 3.39 | 6.49 | 11.5 | 18.7 | 27.2 | 48.1 | 76.4 | 95.0 | 119 | 146 | 176 | 200 | 225 |
The EKE results is BetaGeneral (0.79464, 2.9488, 1.2, 295) distribution fitted with @Risk version 7.6.
Table A.30.
The uncertainty distribution of plants free of Phytophthora kernoviae per 10,000 plants calculated by Table A.29
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,775 | 9,900 | 9,958 | 9,978 | 9,999 | ||||||||||
| EKE results | 9,775 | 9,800 | 9,824 | 9,854 | 9,881 | 9,905 | 9,924 | 9,952 | 9,973 | 9,981 | 9,989 | 9,994 | 9,997 | 9,998 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.30.
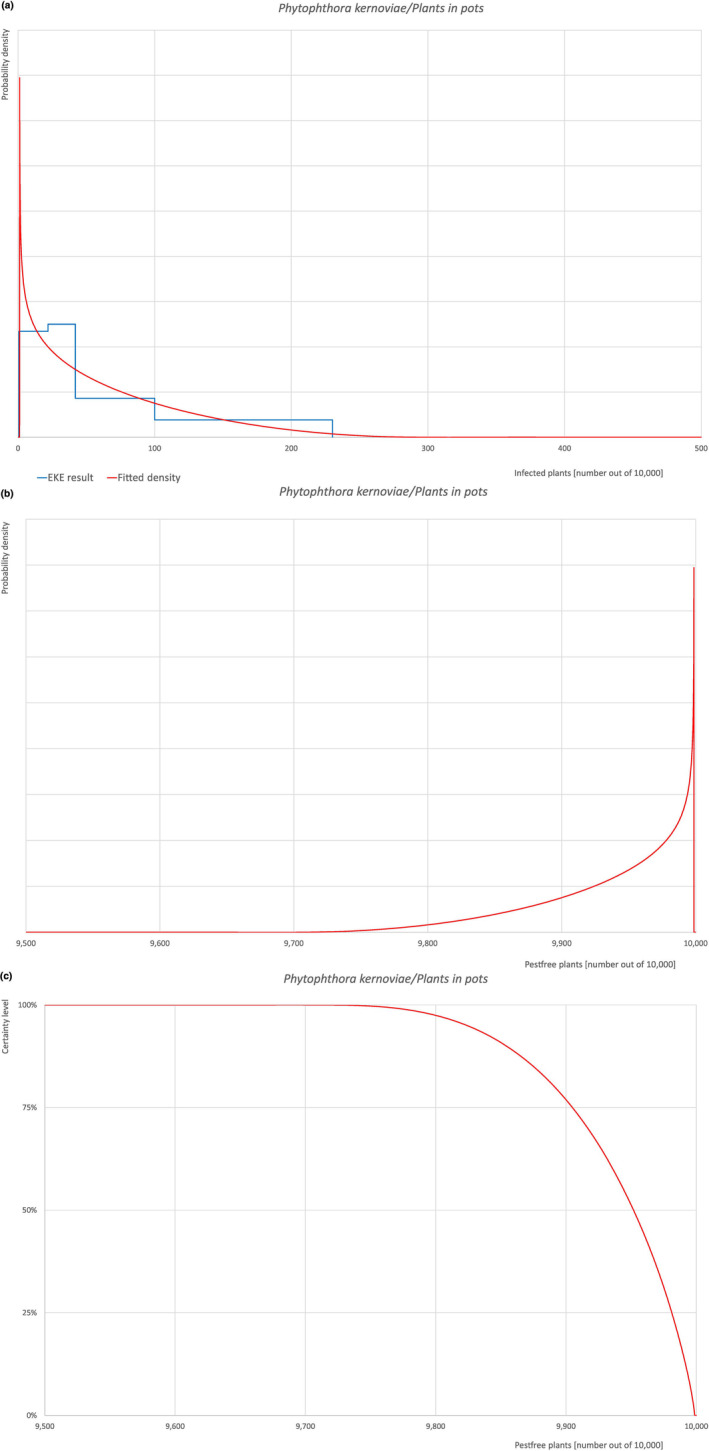
Figure A.15: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.5.8. Reference list
Beales PA, Lane CR, Barton VC and Giltrap PM, 2006. Phytophthora kernoviae on ornamentals in the UK. EPPO Bulletin, 36, 377–379. https://doi.org/10.1111/j.1365-2338.2006.01015.x
Beales PA, Giltrap PG, Payne A and Ingram N, 2009. A new threat to UK heathland from Phytophthora kernoviae on Vaccinium myrtillus in the wild. Plant Pathology, 58, 393. https://doi.org/10.1111/j.1365-3059.2008.01961.x
Blair JE, Coffey MD, Park SY, Geiser DM and Kang S, 2008. A multi‐locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology, 45, 266–277. https://doi.org/10.1016/j.fgb.2007.10.010
Brasier CM, Beales PA, Kirk SA, Denman S and Rose J, 2005. Phytophthora kernoviae sp. nov., an invasive pathogen causing bleeding stem lesions on forest trees and foliar necrosis of ornamentals in the UK. Mycological Research, 109, 853–859. https://doi.org/10.1017/s0953756205003357
Brasier C, 2008. Phytophthora ramorum + P. kernoviae = international biosecurity failure. In: Frankel SJ, Kliejunas JT, Palmieri KM (eds). Proceedings of the sudden oak death third science symposium. USDA Forest Service, Pacific Southwest Research Station, Albany, CA: US Department of Agriculture, 214, 133–139. https://doi.org/10.2737/psw-gtr-214
Brown AV and Brasier CM, 2007. Colonization of tree xylem by Phytophthora ramorum, P. kernoviae and other Phytophthora species. Plant Pathology, 56, 227–241. https://doi.org/10.1111/j.1365-3059.2006.01511.x
DEFRA (Department for Environment, Food and Rural Affairs), 2008. Consultation on future management of risks from Phytophthora ramorum and Phytophthora kernoviae. London, UK: Department for Environment, Food and Rural Affairs. 22 pp.
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Phytophthora kernoviae. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=25428 [Accessed: 18 January 2023].
Denman S, Kirk SA, Moralejo E and Webber JF, 2009. Phytophthora ramorum and Phytophthora kernoviae on naturally infected asymptomatic foliage. EPPO Bulletin, 39, 105–111. https://doi.org/10.1111/j.1365-2338.2009.02243.x
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023. Scientific Opinion on the commodity risk assessment of Fagus sylvatica plants from the UK. EFSA Journal 2023;21(7):8118, 151 pp. https://doi.org/10.2903/j.efsa.2023.8118
Elliot M, Meagher TR, Harris C, Searle K, Purse BV and Schlenzig A, 2013. The epidemiology of Phytophthora ramorum and P. kernoviae at two historic gardens in Scotland. In Frankel SJ, Kliejunas JT, Palmieri KM and Alexander JM (Eds.), Proceedings of the sudden oak death third science symposium. USDA Forest Service, Pacific Southwest Research Station, Albany, CA: US Department of Agriculture, 214, 23–32. https://doi.org/10.2737/psw-gtr-214
EPPO (European and Mediterranean Plant Protection Organization), 2013. Pest risk management for Phytophthora kernoviae and Phytophthora ramorum. EPPO, Paris. Available online: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A2 List of pests recommended for regulation as quarantine pests, version 2022–09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed: 18 January 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Phytophthora kernoviae (PHYTKE), Categorization. Available online: https://gd.eppo.int/taxon/PHYTKE/categorization [Accessed: 18 January 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Phytophthora kernoviae (PHYTKE), Distribution. Available online: https://gd.eppo.int/taxon/PHYTKE/distribution [Accessed: 18 January 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_d. First report of Phytophthora kernoviae in Ireland. Available online: https://gd.eppo.int/reporting/article-605 [Accessed: 18 January 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_e. Phytophthora kernoviae (PHYTKE), Host plants. Available online: https://gd.eppo.int/taxon/PHYTKE/hosts [Accessed: 18 January 2023].
EPPO (European and Mediterranean Plant Protection Organization), online_f. Phytophthora kernoviae (PHYTKE), Photos. Available online: https://gd.eppo.int/taxon/PHYTKE/photos [Accessed: 18 January 2023].
Erwin DC and Ribeiro OK, 1996. Phytophthora diseases worldwide. St. Paul, Minnesota: APS Press, American Phytopathological Society, 562 pp.
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en [Accessed: 22 December 2022].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://data.nal.usda.gov/dataset/united-states-national-fungus-collections-fungus-host-dataset [Accessed: 18 January 2023].
Fichtner EJ, Rizzo DM, Kirk SA and Webber JF, 2011. Root infections may challenge management of invasive Phytophthora spp. in UK woodlands. Plant Disease, 95, 13–18. https://doi.org/10.1094/pdis-03-10-0236
Fichtner EJ, Rizzo DM, Kirk SA and Webber JF, 2012. Infectivity and sporulation potential of Phytophthora kernoviae to select North American native plants. Plant Pathology, 61, 224–233. https://doi.org/10.1111/j.1365-3059.2011.02506.x
Hughes KJ, Tomlinson JA, Giltrap PM, Barton V, Hobden E, Boonham N and Lane CR, 2011. Development of a real‐time PCR assay for detection of Phytophthora kernoviae and comparison of this method with a conventional culturing technique. European Journal of Plant Pathology, 131, 695–703. https://doi.org/10.1007/s10658-011-9843-x
Jung T, Durán A, Sanfuentes von Stowasser E, Schena L, Mosca S, Fajardo S, González M, Navarro Ortega AD, Bakonyi J, Seress D, Tomšovský M, Cravador A, Maia C and Horta Jung M, 2018. Diversity of Phytophthora species in Valdivian rainforests and association with severe dieback symptoms. Forest Pathology, 48, 1–19. https://doi.org/10.1111/efp.12443
Jung T, Milenković I, Corcobado T, Májek T, Janoušek J, Kudláček T, Tomšovský M, Nagy ZÁ, Durán A, Tarigan M, Sanfuentes von Stowasser E, Singh R, Ferreira M, Webber JF, Scanu B, Chi NM, Thu PQ, Junaid M, Rosmana A, Baharuddin B, Kuswinanti T, Nasri N, Kageyama K, Hieno A, Masuya H, Uematsu S, Oliva J, Redondo M, Maia C, Matsiakh I, Kramarets V, O'Hanlon R, Tomić Ž, Brasie CM and Horta Jung M, 2022. Extensive morphological and behavioural diversity among fourteen new and seven described species in Phytophthora Clade 10 and its evolutionary implications. Persoonia‐Molecular Phylogeny and Evolution of Fungi, 49, 1–5. https://doi.org/10.3767/persoonia.2022.49.01
McDougal RL and Ganley RJ, 2021. Foliar Phytophthora in New Zealand plantation forests: historical presence of Phytophthora kernoviae and association with a previously undiagnosed disorder of Pinus radiata. Australasian Plant Pathology, 50, 747–759. https://doi.org/10.1007/s13313-021-00825-w
O'Hanlon R, Choiseul J, Corrigan M, Catarame T and Destefanis M, 2016. Diversity and detections of Phytophthora species from trade and non‐trade environments in Ireland. Bulletin OEPP/EPPO Bulletin, 46, 594–602. https://doi.org/10.1111/epp.12331
Sanfuentes E, Fajardo S, Sabag M, Hansen E and González M, 2016. Phytophthora kernoviae isolated from fallen leaves of Drymis winteri in native forest of southern Chile. Australasian Plant Disease Notes, 11, 1–3. https://doi.org/10.1007/s13314-016-0205-6
Scott P and Williams N, 2014. Phytophthora diseases in New Zealand forests. NZ Journal of Forestry, 59, 14–21.
Shelley BA, Luster DG, Garrett WM, McMahon MB and Widmer TL, 2018. Effects of temperature on germination of sporangia, infection and protein secretion by Phytophthora kernoviae. Plant Pathology, 67, 719–728. https://doi.org/10.1111/ppa.12782
Studholme DJ, Panda P, Sanfuentes Von Stowasser E, González M, Hill R, Sambles C, Grant M, Williams NM and McDougal RL, 2019. Genome sequencing of oomycete isolates from Chile supports the New Zealand origin of Phytophthora kernoviae and makes available the first Nothophytophthora sp. genome. Molecular Plant Pathology, 20, 423–431. https://doi.org/10.1111/mpp.12765
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Widmer TL, 2010. Phytophthora kernoviae oospore maturity, germination, and infection. Fungal Biology, 114, 661–668. https://doi.org/10.1016/j.funbio.2010.06.001
Widmer T, 2011. Effect of temperature on survival of Phytophthora kernoviae oospores, sporangia, and mycelium. New Zealand Journal of Forestry Science, 41, 15–23.
Webber JF, 2008. Status of Phytophthora ramorum and P. kernoviae in Europe. In Frankel SJ, Kliejunas JT and Palmieri KM (Eds.). Proceedings of the sudden oak death third science symposium. USDA Forest Service, Pacific Southwest Research Station, Albany, CA: US Department of Agriculture, 214, 19–26. https://doi.org/10.2737/psw-gtr-214
A.6. Phytophthora ramorum (non‐EU isolates)
A.6.1. Organism information
| Taxonomic information |
Current valid scientific name: Phytophthora ramorum Synonyms: – Name used in the EU legislation: Phytophthora ramorum (non‐EU isolates) Werres, De Cock & Man in ‘t Veld [PHYTRA] Order: Peronosporales Family: Peronosporaceae Common name: Sudden Oak Death (SOD), ramorum bleeding canker, ramorum blight, ramorum leaf blight, twig and leaf blight Name used in the Dossier: Phytophthora ramorum |
|
| Group | Oomycetes | |
| EPPO code | PHYTRA | |
| Regulated status |
The pathogen is listed in Annex II of Commission Implementing Regulation (EU) 2019/2072 as Phytophthora ramorum (non‐EU isolates) Werres, De Cock & Man in ‘t Veld [PHYTRA]. The EU isolates of P. ramorum are listed as regulated non‐quarantine pest (RNQP). The pathogen is included in the EPPO A2 list (EPPO, online_a). Phytophthora ramorum is quarantine in Canada, Israel, Mexico, Morocco and the UK. It is on A1 list of Brazil, Chile, Egypt, Kazakhstan, Türkiye and EAEU (=Eurasian Economic Union: Armenia, Belarus, Kazakhstan, Kyrgyzstan and Russia) (EPPO, online_b). |
|
| Pest status in the UK |
Phytophthora ramorum is present in the UK (Brown and Brasier, 2007; Dossier Sections 2.0 and 5.0; CABI, online; EPPO, online_c). According to the Dossier Section 5.0, European isolates of Phytophthora ramorum are present in the UK: not widely distributed and under official control. It has been found in most regions of the UK, but it is more often reported in wetter, western regions. |
|
| Pest status in the EU | Phytophthora ramorum is present in the EU and it is currently reported in the following EU Member States: Belgium, Croatia, Denmark, Finland, France, Germany, Ireland, the Netherlands, Poland, Portugal and Slovenia (EPPO, online_c). | |
| Host status on Quercus |
Phytophthora ramorum was reported on Quercus petraea and Q. robur (Farr and Rossman, online). They are listed as associated hosts (APHIS USDA, 2022) In inoculation experiments with P. ramorum, Q. petraea and Q. robur were found to have low to moderate susceptibility as foliar and bark hosts (Denman et al., 2005; Sansford et al., 2009). Phytophthora ramorum is a pathogen of other Quercus species such as Quercus agrifolia, Q. cerris, Q. chrysolepis, Q. falcata, Q. ilex, Q. kelloggii and Q. parvula var. shrevei, which are proven hosts (APHIS USDA, 2022). |
|
| PRA information | Pest Risk Assessments available:
|
|
| Other relevant information for the assessment | ||
| Biology |
Phytophthora ramorum is most probably native to East Asia (Poimala and Lilja, 2013; Jung et al., 2021). The pathogen is present in Asia (Japan, Vietnam), Europe (Belgium, Croatia, Denmark, Finland, France, Germany, Guernsey, Ireland, Luxembourg, the Netherlands, Norway, Poland, Portugal, Slovenia, the UK), North America (Canada, USA) and South America (Argentina) (EPPO, online_c). So far there are 12 known lineages of P. ramorum: NA1 and NA2 from North American, EU1 from Europe (including the UK) and North America (Grünwald et al., 2009), EU2 from Northern Ireland and western Scotland (Van Poucke et al., 2012), IC1 to IC5 from Vietnam and NP1 to NP3 from Japan (Jung et al., 2021). Phytophthora ramorum is heterothallic oomycete species belonging to clade 8c (Blair et al., 2008) with two mating types: A1 and A2 (Boutet et al., 2010). Phytophthora species generally reproduce through a) dormant (resting) spores which can be either sexual (oospores) or asexual (chlamydospores); and b) fruiting structures (sporangia) which contain zoospores (Erwin and Ribeiro, 1996). Phytophthora ramorum produces sporangia on the surfaces of infected leaves and twigs of host plants. These sporangia can be splash‐dispersed a short distance or carried by wind and rain over longer distances. The sporangia germinate to produce zoospores that penetrate and initiate an infection on new hosts. In infected plant material the chlamydospores are produced and can serve as resting structures (Davidson et al., 2005; Grünwald et al., 2008). Trunk cankers (e.g. on Quercus) are not known to support sporulation and therefore do not transmit the pathogen (DEFRA, 2008). The pathogen is also able to survive in soil (Shishkoff, 2007). In the west of Scotland, it persisted in soil for at least 2 years after its hosts were removed (Elliot et al., 2013). Oospores were only observed in pairing tests under controlled laboratory conditions (Brasier and Kirk, 2004). Optimal temperatures under laboratory conditions were 16–26°C for growth, 14–26°C for chlamydospore production and 16–22°C for sporangia production (Englander et al., 2006). Phytophthora ramorum is mainly a foliar pathogen, however it was also reported to infect shoots, stems and occasionally roots of various host plants (Parke and Lewis, 2007; Grünwald et al., 2008). According to Brown and Brasier (2007), P. ramorum commonly occupies xylem beneath phloem lesions and may spread within xylem and possibly recolonise the phloem from the xylem. Phytophthora ramorum can remain viable within xylem for two or more years after the overlying phloem had been excised. Phytophthora ramorum can disperse by aerial dissemination, water, movement of infested plant material and soil containing propagules on footwear, tires of trucks and mountain bikes or the feet of animals (Davidson et al., 2002; Brasier, 2008). Infected foliar hosts can be a major source of inoculum, which can lead to secondary infections on nearby host plants. Important foliar hosts in Europe are Rhododendron spp. and Larix kaempferi (Grünwald et al., 2008; Brasier and Webber, 2010). Possible pathways of entry for Phytophthora ramorum are plants for planting (excluding seed and fruit) of known susceptible hosts; plants for planting (excluding seed and fruit) of non‐host plant species accompanied by contaminated attached growing media; soil/growing medium (with organic matter) as a commodity; soil as a contaminant; foliage or cut branches; susceptible (isolated) bark and susceptible wood (EFSA PLH Panel, 2011). |
|
|
Phytophthora ramorum caused rapid decline of Lithocarpus densiflorus and Quercus agrifolia in forests of California and Oregon (Rizzo et al., 2005) and Larix kaempferi in plantations of south‐west England (Brasier and Webber, 2010). |
||
| Symptoms | Main type of symptoms |
Phytophthora ramorum causes different types of symptoms depending on the host species and the plant tissue infected. According to DEFRA (2008) P. ramorum causes three different types of disease:
Symptoms on Quercus species are cankers of red, brown or black colour on trunk, browning of the crown, gradual leaf loss and death of trees (Davidson et al., 2003). In inoculation experiments, P. ramorum induced bleeding stem lesions and leaf necrosis on Q. petraea and Q. robur (Sansford et al., 2009). Leaf lesions and shoot dieback can be observed on foliar hosts such as Rhododendron, Viburnum, Pieris and Camellia (Davidson et al., 2003; EPPO, online_e). On Larix kaempferi, P. ramorum causes foliage and bark infection that are visible as wilted shoot tips with blackened needles and stem lesions with resin bleeding (Braiser and Webber, 2010). Symptoms on Lithocarpus densiflorus are lesions on leaves, cankers on trunk, branches and twigs; shoot tip dieback, leaf flagging and formation of a Shepard's crook. The trees can die within 1 year (Davidson et al., 2003). |
| Presence of asymptomatic plants | If roots are infected by P. ramorum, the plants can be without above‐ground symptoms for months until developmental or environmental factors trigger disease expression (Roubtsova and Bostock, 2009; Thompson et al., 2021). | |
| Confusion with other pests |
Various symptoms caused by P. ramorum can be confused with other pathogens, such as: canker and foliar symptoms caused by other Phytophthora species (P. cinnamomi, P. citricola and P. cactorum); leaf lesions caused by rust in early stages; leafspots caused by sunburn; dieback of twigs and leaves caused by Botryosphaeria dothidea (Davidson et al., 2003). Phytophthora ramorum can be easily distinguished from other Phytophthora species based on morphology (Grünwald et al., 2008) and molecular tests. |
|
| Host plant range |
Phytophthora ramorum has a very wide host range, which is expanding. Main host plants include Camellia spp., Larix decidua, L. kaempferi, Pieris spp., Rhododendron spp., Syringa vulgaris, Viburnum spp. and the North American trees species, Lithocarpus densiflorus and Quercus agrifolia (EPPO online_d). Further proven hosts confirmed by Koch's postulates are Abies grandis, A. magnifica, Acer circinatum, A. macrophyllum, A. pseudoplatanus, Adiantum aleuticum, A. jordanii, Aesculus californica, A. hippocastanum, Arbutus menziesii, A. unedo, Arctostaphylos columbiana, A. glauca, A. hooveri, A. manzanita, A. montereyensis, A. morroensis, A. pilosula, A. pumila, A. silvicola, A. viridissima, Calluna vulgaris, Castanea sativa, Ceanothus thyrsiflorus, Chamaecyparis lawsoniana, Chrysolepis chrysophylla, Cinnamomum camphora, Corylus cornuta, Fagus sylvatica, Frangula californica, Frangula purshiana, Fraxinus excelsior, Gaultheria procumbens, G. shallon, Griselinia littoralis, Hamamelis virginiana, Heteromeles arbutifolia, Kalmia spp., Larix × eurolepis, Laurus nobilis, |
|
|
Lonicera hispidula, Lophostemon confertus, Loropetalum chinense, Magnolia × loebneri, M. oltsopa, M. stellata, Mahonia aquifolium, Maianthemum racemosum, Parrotia persica, Photinia fraseri, Phoradendron serotinum subsp. macrophyllum, Photinia × fraseri, Prunus laurocerasus, Pseudotsuga menziesii var. menziesii, Quercuscerris, Q. chrysolepis, Q. falcata, Q. ilex, Q. kelloggii, Q. parvula var. shrevei, Rosa gymnocarpa, Salix caprea, Sequoia sempervirens, Taxus baccata, Trientalis latifolia, Umbellularia californica, Vaccinium myrtillus, V. ovatum, V. parvifolium and Vinca minor (Cave et al., 2008; APHIS USDA, 2022). |
||
| Reported evidence of impact | Phytophthora ramorum is EU quarantine pest. | |
| Evidence that the commodity is a pathway | Phytophthora ramorum is continuously intercepted in the EU on different plant species intended for planting (EUROPHYT, online; TRACES‐NT, online) and according to EFSA PLH Panel (2011), P. ramorum can travel with plants for planting. Therefore, plants for planting are possible pathway of entry for P. ramorum. | |
| Surveillance information |
The UK has a containment policy in the wider environment with official action taken to remove infected trees (Dossier Section 3.0). Phytophthora ramorum at growing sites: infested plants are destroyed and potentially infested plants are ‘held’ (prohibited from moving). As part of an annual survey at ornamental retail and production sites (frequency of visits determined by a decision matrix) Phytophthora ramorum is inspected on common host plants. An additional inspection, during the growing period, is carried out at plant passport production sites. Inspections are carried out at a survey to 300 non‐woodland wider environment sites annually (Dossier Sections 3.0 and 5.0). |
|
A.6.2. Possibility of pest presence in the nursery
A.6.2.1. Possibility of entry from the surrounding environment
P. ramorum is present in the UK, it has been found in most regions of the UK, but it is more often reported in wetter, western regions (Dossier Section 5.0).
The possible entry of P. ramorum from surrounding environment to the nurseries may occur through aerial dissemination, water and animals (Davidson et al., 2002).
P. ramorum has wide host range and can infect a number of different plants. Suitable hosts of P. ramorum like Abies spp., Acer spp., Aesculus spp., Camellia spp., Castanea spp., Larix spp., Magnolia spp., Prunus spp., Rhododendron spp., Rosa spp., Salix spp., Syringa spp. and Viburnum spp. are present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
The dispersal range of P. ramorum sporangia.
-
–
No information available on the distance of the nurseries to sources of pathogen in the surrounding environment.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present and the pathogen can spread by wind, rain and infested soil propagules on feet of animals entering the nurseries.
A.6.2.2. Possibility of entry with new plants/seeds
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are obtained either from the UK or the EU (mostly the Netherlands) (Dossier Section 3.0). Seeds are not a pathway for the pathogen.
In addition to Quercus plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are many suitable hosts for the pathogen (such as Abies spp., Acer spp., Aesculus spp., Arbutus spp., Calluna spp., Castanea spp., Fagus spp., Larix spp., Viburnum spp., etc.). However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pathogen could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). P. ramorum is able to survive in soil (Shishkoff, 2007) and therefore could potentially enter with infested soil/growing media. However, the growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0).
Uncertainties:
-
–
No information is available on the provenance of plants other than Quercus used for plant production in the area of the nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pathogen to enter the nurseries with new seedlings of Quercus and new plants of other species used for plant production in the area. The entry of the pathogen with seeds and the growing media the Panel considers as not possible.
A.6.2.3. Possibility of spread within the nursery
Quercus plants are either grown in containers (cells, pots, tubes, etc.) or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pathogen can infect other suitable plants (such as Abies spp., Aesculus spp., Castanea spp., Larix spp., Fagus spp., etc.) present within the nurseries and hedges surrounding the nurseries (Prunus spp.) (Dossier Sections 3.0 and 6.0).
P. ramorum can spread within the nurseries by aerial dissemination, soil, water, movement of infested plant material and animals (Davidson et al., 2002).
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pathogen within the nurseries is possible either by aerial dissemination, animals, movement of infested plant material, soil and water.
A.6.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of P. ramorum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online).
A.6.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on P. ramorum is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
P. ramorum is a quarantine organism in the UK and targeted by this measure.
Uncertainties:
|
| 2 | Physical separation | No | Not relevant. |
| 3 | Certified plant material | Yes |
P. ramorum is a quarantine organism in the UK and targeted by this measure.
Uncertainties:
|
| 4 | Growing media | Yes |
This measure should ensure pest‐free growing media and is expected to prevent the introduction of the pathogen into the nurseries with growing media.
Uncertainties:
|
| 5 | Surveillance, monitoring and sampling | Yes |
This measure has an effect as the pathogen would be detected on nursery‐grown plants, as well as on incoming plant material and growing media, and suspected plant material quarantined.
Uncertainties:
|
| 6 | Hygiene measures | Yes |
General hygiene measures will reduce the likelihood of the pathogen being spread by tools and equipment, although this is not a major pathway for the pest.
Uncertainties:
|
| 7 | Removal of infested plant material | Yes |
This measure could have some effect by removing potentially infested plant material, thus reducing the spread of the pathogen within the nursery.
Uncertainties:
|
| 8 | Irrigation water | Yes |
Testing of irrigation water would detect the pathogen, which can spread by water. Overhead irrigation could favour foliar infections and spread of the pathogen by water splash.
Uncertainties:
|
| 9 | Application of pest control products | Yes |
Some fungicides could reduce the likelihood of foliar infection by the pathogen.
Uncertainties:
|
| 10 | Measures against soil pests | Yes |
This measure could have some effect by preventing root contact with soil where the pathogen may be present.
Uncertainties:
|
| 11 | Inspections and management of plants before export | Yes |
P. ramorum is a quarantine organism in the UK and the EU and this measure is expected to reduce the likelihood of infested plants being exported.
Uncertainties:
|
| 12 | Separation during transport to the destination | No | Not relevant. |
A.6.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.6.5.1. Reasoning for a scenario which would lead to a reasonably low number of infected bundles of whips and seedlings
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. The plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Quercus to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.6.5.2. Reasoning for a scenario which would lead to a reasonably high number of infected bundles of whips and seedlings
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects leaves, which may still be present on the plants at the time of export. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.6.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bundles of whips and seedlings (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Quercus. The pathogen is a regulated quarantine pest in the UK and under official control.
A.6.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the susceptibility of Quercus and the occurrence of the pathogen in the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.6.5.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on bundles of whips and seedlings
The following Tables show the elicited and fitted values for pest infection (Table A.31) and pest freedom (Table A.32).
Table A.31.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 3 | 20 | 40 | 80 | 150 | ||||||||||
| EKE | 3.00 | 3.62 | 4.85 | 7.74 | 12.2 | 18.6 | 25.7 | 42.8 | 64.4 | 77.7 | 93.9 | 111 | 128 | 140 | 151 |
The EKE results is the BetaGeneral (0.82439, 1.9948, 2.7, 170) distribution fitted with @Risk version 7.6.
Table A.32.
The uncertainty distribution of bundles free of Phytophthora ramorum per 10,000 bundles calculated by Table A.31
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,920 | 9,960 | 9,980 | 9,997 | ||||||||||
| EKE results | 9,849 | 9,860 | 9,872 | 9,889 | 9,906 | 9,922 | 9,936 | 9,957 | 9,974 | 9,981 | 9,988 | 9,992 | 9,995 | 9,996 | 9,997 |
The EKE results are the fitted values.
Based on the numbers of estimated infected bundles the pest freedom was calculated (i.e. = 10,000 – number of infected bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.32.
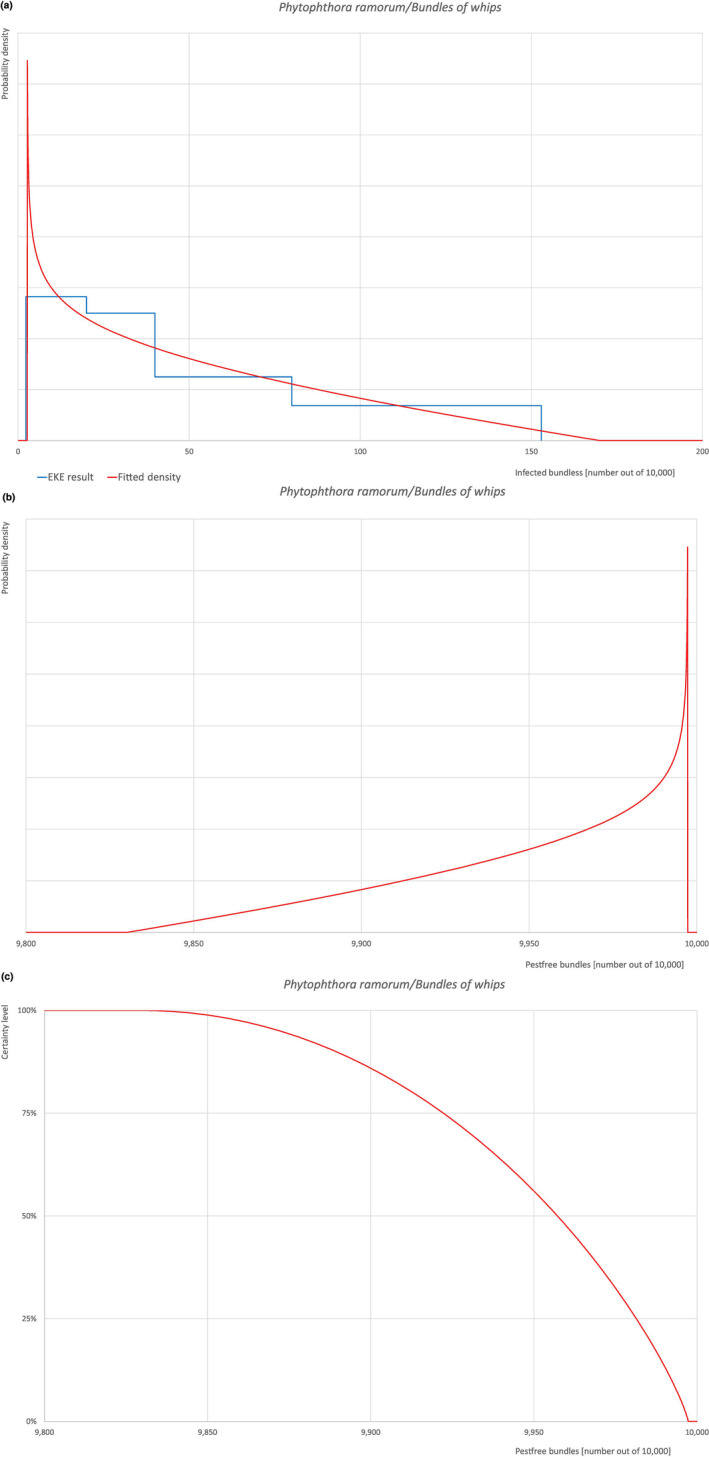
Figure A.16: (a) Elicited uncertainty of pest infection per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 bundles
A.6.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.6.6.1. Reasoning for a scenario which would lead to a reasonably low number of infected bare root plants/trees up to 7 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Quercus to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.6.6.2. Reasoning for a scenario which would lead to a reasonably high number of infected bare root plants/trees up to 7 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects leaves, which may still be present on the plants at the time of export. Older trees are more likely to become infected due to longer exposure time and larger size. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.6.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected bare root plants/trees up to 7 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Quercus. The pathogen is a regulated quarantine pest in the UK and under official control.
A.6.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the susceptibility of Quercus and the occurrence of the pathogen in the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.6.6.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on bare root plants/trees up to 7 years old
The following Tables show the elicited and fitted values for pest infection (Table A.33) and pest freedom (Table A.34).
Table A.33.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 2 | 18 | 35 | 65 | 150 | ||||||||||
| EKE | 2.01 | 3.04 | 4.67 | 7.81 | 12.0 | 17.4 | 23.1 | 36.2 | 53.0 | 63.9 | 78.0 | 94.4 | 114 | 131 | 150 |
The EKE results is the BetaGeneral (1.1205, 5.2894, 1.2, 250) distribution fitted with @Risk version 7.6.
Table A.34.
The uncertainty distribution of plants free of Phytophthora ramorum per 10,000 plants calculated by Table A.33
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,850 | 9,935 | 9,965 | 9,982 | 9,998 | ||||||||||
| EKE results | 9,850 | 9,869 | 9,886 | 9,906 | 9,922 | 9,936 | 9,947 | 9,964 | 9,977 | 9,983 | 9,988 | 9,992 | 9,995 | 9,997 | 9,998 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.34.
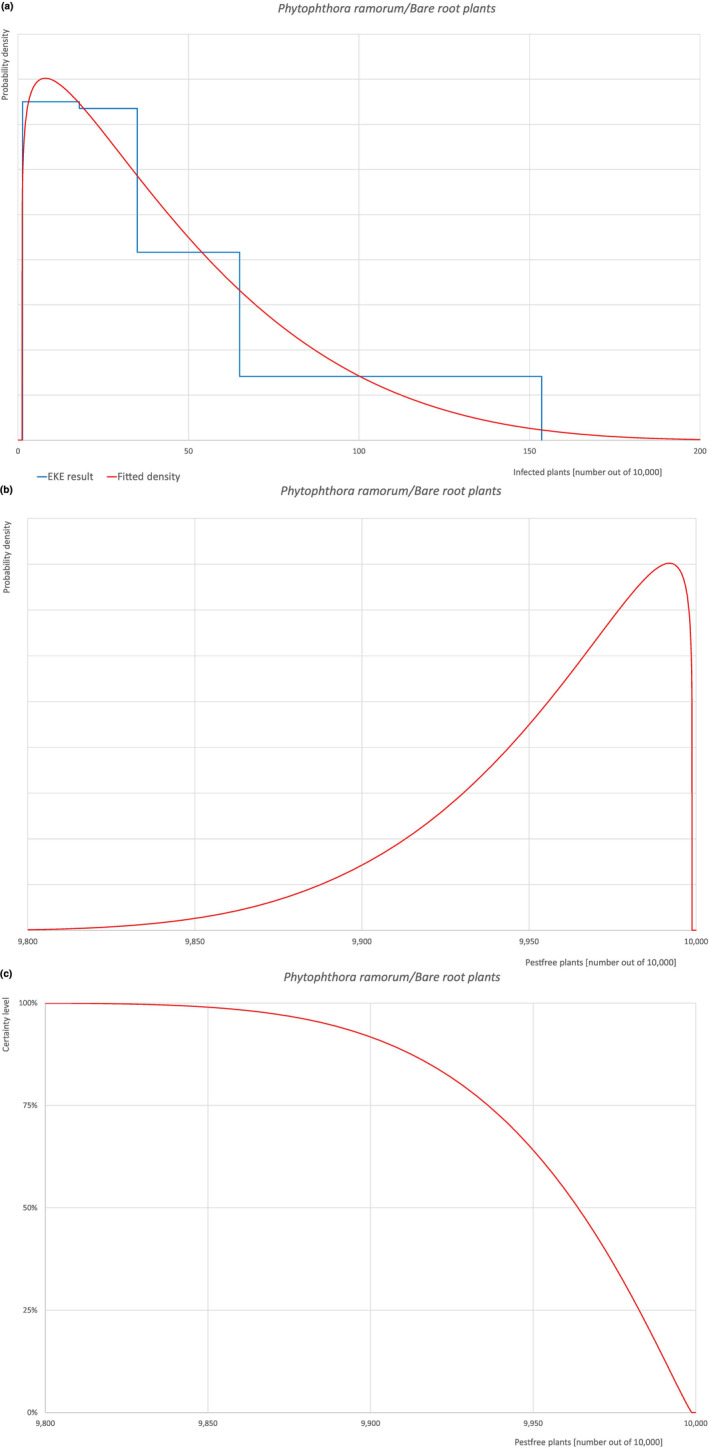
Figure A.17: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.6.7. Overall likelihood of pest freedom for plants in pots up to 15 years old
A.6.7.1. Reasoning for a scenario which would lead to a reasonably low number of infected plants in pots up to 15 years old
The scenario assumes a low pressure of the pathogen in the nurseries and in the surroundings. Younger plants are exposed to the pathogen for only short period of time and are exported without leaves. The scenario assumes Quercus to be minor hosts for the pathogen. The scenario also assumes that symptoms of the disease are visible and promptly detected during inspections.
A.6.7.2. Reasoning for a scenario which would lead to a reasonably high number of infected plants in pots up to 15 years old
The scenario assumes a high pressure of the pathogen in the nurseries and in the surroundings as suitable hosts are present. The scenario assumes that the pathogen infects leaves, which may still be present on the plants at the time of export. Older trees are more likely to become infected due to longer exposure time and larger size. The scenario also assumes that symptoms of the disease are not easily recognisable during inspections.
A.6.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infected plants in pots up to 15 years old (Median)
The scenario assumes a limited presence of the pathogen in the nurseries and the surroundings, and a limited susceptibility of Quercus. The pathogen is a regulated quarantine pest in the UK and under official control.
A.6.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The limited information on the susceptibility of Quercus and the occurrence of the pathogen in the nurseries and the surroundings results in high level of uncertainties for infestation rates below the median. Otherwise, the pest pressure from the surroundings is expected to be low giving less uncertainties for rates above the median.
A.6.7.5. Elicitation outcomes of the assessment of the pest freedom for Phytophthora ramorum on plants in pots up to 15 years old
The following Tables show the elicited and fitted values for pest infection (Table A.35) and pest freedom (Table A.36).
Table A.35.
Elicited and fitted values of the uncertainty distribution of pest infection by Phytophthora ramorum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 3 | 35 | 70 | 145 | 300 | ||||||||||
| EKE | 3.00 | 4.30 | 6.75 | 12.3 | 20.5 | 32.1 | 44.9 | 75.6 | 115 | 140 | 172 | 206 | 243 | 272 | 301 |
The EKE results is the BetaGeneral (0.8746, 2.6336, 2.3, 370) distribution fitted with @Risk version 7.6.
Table A.36.
The uncertainty distribution of plants free of Phytophthora ramorum per 10,000 plants calculated by Table A.35
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,700 | 9,855 | 9,930 | 9,965 | 9,997 | ||||||||||
| EKE results | 9,699 | 9,728 | 9,757 | 9,794 | 9,828 | 9,860 | 9,885 | 9,924 | 9,955 | 9,968 | 9,979 | 9,988 | 9,993 | 9,996 | 9,997 |
The EKE results are the fitted values.
Based on the numbers of estimated infected plants the pest freedom was calculated (i.e. = 10,000 – number of infected plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.36.
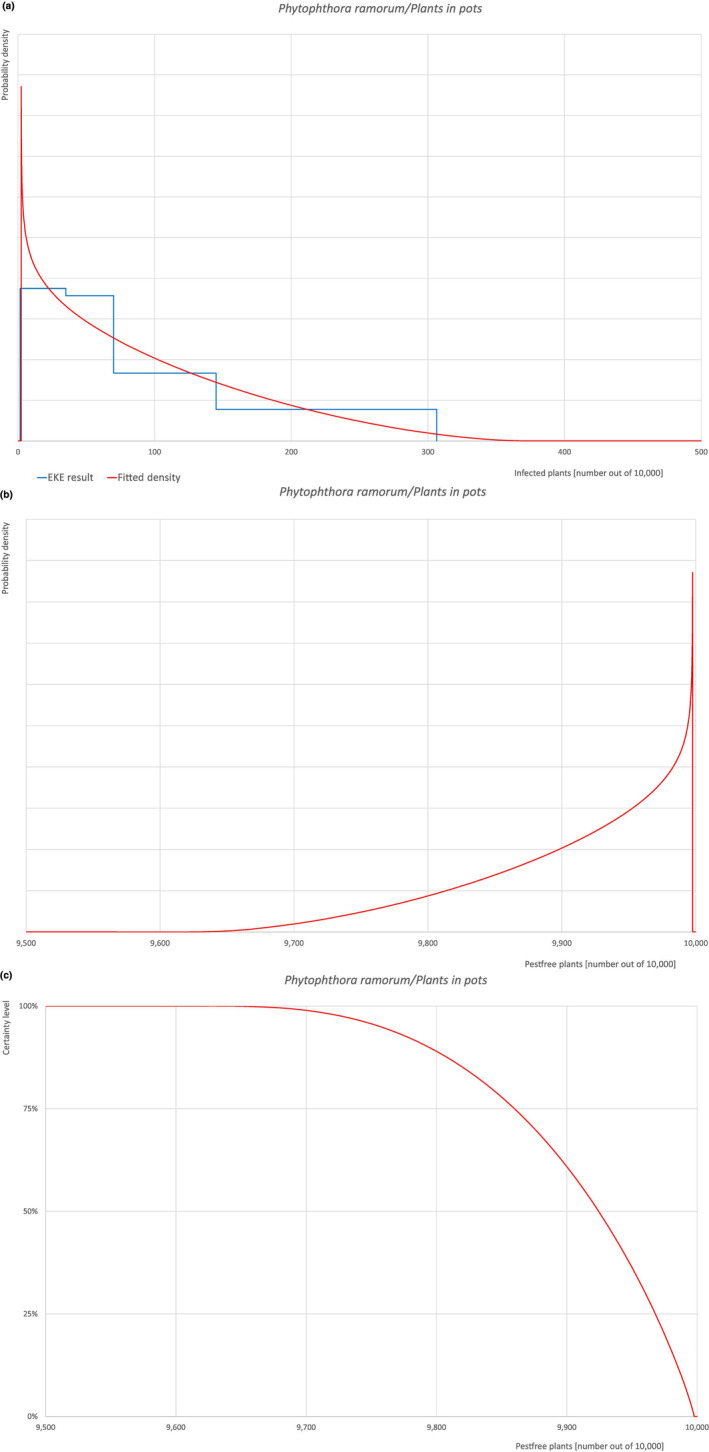
Figure A.18: (a) Elicited uncertainty of pest infection per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infection proportion expressed as percentage); (c) descending uncertainty distribution function of pest infection per 10,000 plants
A.6.8. Reference list
APHIS USDA (Animal and Plant Health Inspection Service U.S. Department of Agriculture), 2022. APHIS lists of proven hosts of and plants associated with Phytophthora ramorum. September 2022. 12 pp. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/pram/downloads/pdf_files/usdaprlist.pdf
Blair JE, Coffey MD, Park SY, Geiser DM and Kang S, 2008. A multi‐locus phylogeny for Phytophthora utilizing markers derived from complete genome sequences. Fungal Genetics and Biology, 45, 266–277. https://doi.org/10.1016/j.fgb.2007.10.010
Boutet X, Vercauteren A, Heungens C and Kurt A, 2010. Mating of Phytophthora ramorum: functionality and consequences. In: Frankel SJ, Kliejunas JT; Palmieri KM (eds.). Proceedings of the Sudden Oak Death Fourth Science Symposium. Albany, CA: US Department of Agriculture, Forest Service, Pacific Southwest Research Station, 229, 97–100.
Brasier C, 2008. Phytophthora ramorum + P. kernoviae = international biosecurity failure. In: Frankel SJ, Kliejunas JT, Palmieri KM (eds). Proceedings of the sudden oak death third science symposium. USDA Forest Service, Pacific Southwest Research Station, Albany, CA: US Department of Agriculture, 214, 133–139.
Brasier C and Kirk S, 2004. Production of gametangia by Phytophthora ramorum in vitro. Mycological research, 108, 823–827. https://doi.org/10.1017/s0953756204000565
Brasier C and Webber J, 2010. Sudden larch death. Nature 466, 824–825. https://doi.org/10.1038/466824a
Brown AV and Brasier CM, 2007. Colonization of tree xylem by Phytophthora ramorum, P. kernoviae and other Phytophthora species. Plant Pathology, 56, 227–241. https://doi.org/10.1111/j.1365-3059.2006.01511.x
CABI (Centre for Agriculture and Bioscience International), online. Phytophthora ramorum (Sudden Oak Death (SOD)). Available online: https://www.cabi.org/cpc/datasheet/40991 [Accessed: 27 September 2022].
Cave GL, Randall‐Schadel B and Redlin SC, 2008. Risk analysis for Phytophthora ramorum Werres, de Cock & Man in't Veld, causal agent of sudden oak death, ramorum leaf blight, and ramorum dieback. US Department of Agriculture, Animal and Plant Health Inspection Service, Raleigh, NC. 88 pp.
Davidson JM, Rizzo DM, Garbelotto M, Tjosvold S and Slaughter GW, 2002. Phytophthora ramorum and sudden oak death in California: II. Transmission and survival. In: Standiford RB, McCreary D and Purcell KL (Eds.), Proceedings of the fifth symposium on oak woodlands: Oaks in California's challenging landscape. San Diego, California, US Department of Agriculture, Forest Service, Pacific Southwest Research Station: 184, 741–749.
Davidson JM, Werres S, Garbelotto M, Hansen EM and Rizzo DM, 2003. Sudden oak death and associated diseases caused by Phytophthora ramorum. Plant Health Progress, 4, 12. https://doi.org/10.1094/php-2003-0707-01-dg
Davidson JM, Wickland AC, Patterson HA, Falk KR and Rizzo DM, 2005. Transmission of Phytophthora ramorum in mixed‐evergreen forest in California. Phytopathology, 95, 587–596. https://doi.org/10.1094/phyto-95-0587
DEFRA (Department for Environment, Food and Rural Affairs), 2008. Consultation on future management of risks from Phytophthora ramorum and Phytophthora kernoviae. London, UK: Department for Environment, Food and Rural Affairs. 22 pp.
DEFRA (Department for Environment, Food and Rural Affairs), online. UK Risk Register Details for Phytophthora ramorum. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=23022 [Accessed: 12 December 2022].
Denman S, Kirk SA, Brasier CM and Webber JF, 2005. In vitro leaf inoculation studies as an indication of tree foliage susceptibility to Phytophthora ramorum in the UK. Plant Pathology, 54, 512–521. https://doi.org/10.1111/j.1365-3059.2005.01243.x
EFSA PLH Panel (EFSA Panel on Plant Health), 2011. Scientific Opinion on the Pest Risk Analysis on Phytophthora ramorum prepared by the FP6 project RAPRA. EFSA Journal 2011;9(6):2186, 108 pp. https://doi.org/10.2903/j.efsa.2011.2186
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023a. Scientific Opinion on the commodity risk assessment of Acer campestre plants from the UK. EFSA Journal 2023;21(7):8071, 291 pp. https://doi.org/10.2903/j.efsa.2023.8071
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023b. Scientific Opinion on the commodity risk assessment of Acer palmatum plants from the UK. EFSA Journal 2023;21(7):8075, 228 pp. https://doi.org/10.2903/j.efsa.2023.8075
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023c. Scientific Opinion on the commodity risk assessment of Acer platanoides plants from the UK. EFSA Journal 2023;21(7):8073, 268 pp. https://doi.org/10.2903/j.efsa.2023.8073
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023d. Scientific Opinion on the commodity risk assessment of Acer pseudoplatanus plants from the UK. EFSA Journal 2023;21(7):8074, 271 pp. https://doi.org/10.2903/j.efsa.2023.8074
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023e. Scientific Opinion on the commodity risk assessment of Fagus sylvatica plants from the UK. EFSA Journal 2023;21(7):8118, 151 pp. https://doi.org/10.2903/j.efsa.2023.8118
Elliot M, Meagher TR, Harris C, Searle K, Purse BV and Schlenzig A, 2013. The epidemiology of Phytophthora ramorum and P. kernoviae at two historic gardens in Scotland. In Frankel SJ, Kliejunas JT, Palmieri KM and Alexander JM (Eds.), Sudden oak death fifth science symposium. Albany, CA, the US: US Department of Agriculture, Forest Service, Pacific Southwest Research Station, 23–32.
Englander L, Browning M and Tooley PW, 2006. Growth and sporulation of Phytophthora ramorum in vitro in response to temperature and light. Mycologia, 98, 365–373. https://doi.org/10.3852/mycologia.98.3.365
EPPO (European and Mediterranean Plant Protection Organization), 2013. Pest risk management for Phytophthora kernoviae and Phytophthora ramorum. EPPO, Paris. Available online: http://www.eppo.int/QUARANTINE/Pest_Risk_Analysis/PRA_intro.htm
EPPO (European and Mediterranean Plant Protection Organization), online_a. EPPO A2 List of pests recommended for regulation as quarantine pests, version 2021–09. Available online: https://www.eppo.int/ACTIVITIES/plant_quarantine/A2_list [Accessed: 27 September 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Phytophthora ramorum (PHYTRA), Categorization. Available online: https://gd.eppo.int/taxon/PHYTRA/categorization [Accessed: 27 September 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. Phytophthora ramorum (PHYTRA), Distribution. Available online: https://gd.eppo.int/taxon/PHYTRA/distribution [Accessed: 27 September 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Phytophthora ramorum (PHYTRA), Host plants. Available online: https://gd.eppo.int/taxon/PHYTRA/hosts [Accessed: 27 September 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_e. Phytophthora ramorum (PHYTRA), Photos. Available online: https://gd.eppo.int/taxon/PHYTRA/photos [Accessed: 27 September 2022].
Erwin DC and Ribeiro OK, 1996. Phytophthora diseases worldwide. St. Paul, Minnesota: APS Press, American Phytopathological Society, 562 pp.
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en [Accessed: 22 December 2022].
Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://data.nal.usda.gov/dataset/united-states-national-fungus-collections-fungus-host-dataset [Accessed: 13 December 2022].
Grünwald NJ, Goss EM and Press CM, 2008. Phytophthora ramorum: a pathogen with a remarkably wide host range causing sudden oak death on oaks and ramorum blight on woody ornamentals. Molecular Plant Pathology, 9, 729–740. https://doi.org/10.1111/j.1364-3703.2008.00500.x
Grünwald NJ, Goss EM, Ivors K, Garbelotto M, Martin FN, Prospero S, Hansen E, Bonants PJM, Hamelin RC, Chastagner G, Werres S, Rizzo DM, Abad G, Beales P, Bilodeau GJ, Blomquist CL, Brasier C, Brière SC, Chandelier A, Davidson JM, Denman S, Elliott M, Frankel SJ, Goheen EM, de Gruyter H, Heungens K, James D, Kanaskie A, McWilliams MG, Man in ‘t Veld W, Moralejo E, Osterbauer NK, Palm ME, Parke JL, Perez Sierra AM, Shamoun SF, Shishkoff N, Tooley PW, Vettraino AM, Webber J and Widmer TL, 2009. Standardizing the nomenclature for clonal lineages of the sudden oak death pathogen, Phytophthora ramorum. Phytopathology, 99, 792–795.
Jung T, Jung MH, Webber JF, Kageyama K, Hieno A, Masuya H, Uematsu S, Pérez‐Sierra A, Harris AR, Forster J, Rees H, Scanu B, Patra S, Kudláček T, Janoušek J, Corcobado T, Milenković I, Nagy Z, Csorba I, Bakonyi J and Brasier CM, 2021. The destructive tree pathogen Phytophthora ramorum originates from the laurosilva forests of East Asia. Journal of Fungi, 7, 226, 32 pp. https://doi.org/10.3390/jof7030226
Parke JL and Lewis C, 2007. Root and stem infection of Rhododendron from potting medium infested with Phytophthora ramorum. Plant Disease, 91, 1265–1270. https://doi.org/10.1094/pdis-91-10-1265
Poimala A and Lilja A, 2013. NOBANIS – Invasive Alien Species Fact Sheet – Phytophthora ramorum. From: Online Database of the European Network on Invasive Alien Species. 14 pp. Available online: https://www.nobanis.org/globalassets/speciesinfo/p/phytophthora-ramorum/phytophthora_ramorum.pdf [Accessed: 12 December 2022].
Rizzo DM, Garbelotto M and Hansen EM, 2005. Phytophthora ramorum: integrative research and management of an emerging pathogen in California and Oregon forests. Annual Review of Phytopathology, 43, 13.1–13.27. https://doi.org/10.1146/annurev.phyto.42.040803.140418
Roubtsova TV and Bostock RM, 2009. Episodic abiotic stress as a potential contributing factor to onset and severity of disease caused by Phytophthora ramorum in Rhododendron and Viburnum. Plant Disease, 93, 912–918. https://doi.org/10.1094/pdis-93-9-0912
Sansford CE, Inman AJ, Baker R, Brasier C, Frankel S, de Gruyter J, Husson C, Kehlenbeck H, Kessel G, Moralejo E, Steeghs M, Webber J and Werres S, 2009. Report on the risk of entry, establishment, spread and socio‐economic loss and environmental impact and the appropriate level of management for Phytophthora ramorum for the EU. Deliverable Report 28. EU Sixth Framework Project RAPRA. 310 pp.
Shishkoff N, 2007. Persistence of Phytophthora ramorum in soil mix and roots of nursery ornamentals. Plant Disease, 91, 1245–1249. https://doi.org/10.1094/pdis-91-10-1245
Thompson CH, McCartney MM, Roubtsova TV, Kasuga T, Ebeler SE, Davis CE and Bostock RM, 2021. Analysis of volatile profiles for tracking asymptomatic infections of Phytophthora ramorum and other pathogens in Rhododendron. Phytopathology, 111, 1818–1827. https://doi.org/10.1094/phyto-10-20-0472-r
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Van Poucke K, Franceschini S, Webber J, Vercauteren A, Turner JA, Mccracken AR, Heungens K and Brasier C, 2012. Discovery of a fourth evolutionary lineage of Phytophthora ramorum: EU2. Fungal Biology, 116, 1178–1191. https://doi.org/10.1016/j.funbio.2012.09.003
A.7. Thaumetopoea processionea
A.7.1. Organism information
| Taxonomic information |
Current valid scientific name: Thaumetopoea processionea Synonyms: Cnethocampa processionea, Traumatocampa processionea Name used in the EU legislation: Thaumetopoea processionea L. Order: Lepidoptera Family: Notodontidae Common name: oak processionary moth (OPM), oak processionary caterpillar Name used in the Dossier: Thaumetopoea processionea |
|
| Group | Insects | |
| EPPO code | THAUPR | |
| Regulated status |
Thaumetopoea processionea is listed in the Annex III of Commission Implementing Regulation (EU) 2019/2072 as protected zone quarantine pest for Ireland. It is protected zone quarantine pest in the UK, and included in A1 lists for Argentina and Türkiye (EPPO, online_a). The Panel noted that the species is native to Türkiye (Groenen and Meurisse, 2012). |
|
| Pest status in the UK |
Thaumetopoea processionea is established in the UK since 2006. It is a species under official control, currently found in the London area and in the Southeast of England (EPPO, online_b). According to the Dossier Section 5.0 T. processionea is present in Great Britain, except in specified pest‐free areas. In Northern Ireland the pest is absent: the entire country is pest free. In 2022, the T. processionea was found in Jersey (Channel Islands) where it is currently under eradication (EPPO, online_c), same as in the pest‐free area in Hampshire (Dossier Section 5.0). According to Suprunenko et al. (2022) the eradication of T. processionea from the UK territory is ‘no longer considered a feasible option’. |
|
| Pest status in the EU |
Thaumetopoea processionea is a native European species reported to be present in 21 EU member states. It is absent only from Estonia, Finland, Ireland (introduced in 2020, eradicated in 2021), Latvia, Lithuania and Malta (EPPO, online_d; GBIF, online; de Jong et al., online). According to Groenen and Meurisse (2012) the discontinuous occurrence of T. processionea in central‐northern Europe in the last two centuries, and its recent massive reappearance in north‐western Europe, are due to long‐term population fluctuations rather than range expansion. |
|
| Host status on Quercus sp. | Quercus sp., Q. robur is a host of T. processionea (Baker et al., 2009; CABI, online; DEFRA, online; EPPO, online_f). | |
| PRA information | Available Pest Risk Assessment:
|
|
| Other relevant information for the assessment | ||
| Biology |
Thaumetopoea processionea is native to southern and central Europe, where it is more abundant and widespread in warm and sunny sites; in central and western Europe its presence is mainly dependent on population fluctuations which can be determined by aridity and climate change (Groenen and Meurisse, 2012; Csoka et al., 2018). The moth is also present in Türkiye and in the Middle East (Syria, Lebanon, Jordan, Israel) (Groenen and Meurisse, 2012; Battisti et al., 2015; Basso et al., 2017; CABI, online). |
|
|
T. processionea has four life stages: egg, larva (six instars), pupa and adult; it is a univoltine species, overwintering as 1st instar larva inside the egg (Zielonka, 2020; CABI, online; Forestry Commission, online). Adults, 25–35 mm wingspan, fly from July to September and can survive 4–10 days. Females lay 30–200 eggs, occasionally up to 300 (CABI, online), which are 2 mm long. The eggs are laid in batches on small branches of oaks (3.5–10 mm diameter). In autumn 1st instar larvae are found within the eggs; eggs and larvae are known to withstand up to −30°C, and a 90% rate of survival of overwintering eggs is observed after severe winters (Baker et al., 2009; Battisti et al., 2015). Egg hatching in April–May is usually well synchronised with oak bud flushing. The larval stage can last 60–70 days. Larvae feed on foliage gregariously from April to July and build a silky nest for each of the instars (CABI, online); however, a large bag‐shaped nest weaved with silk is built only at 5th–6th larval stage in the medium‐lower part of the trunk. The 35–40 mm mature caterpillars rest in the nest during the day and move in head‐to‐tail processions during the night in search of food. Larvae from 3rd instar onwards develop urticating hairs on the dorsal part of abdomen (Zielonka, 2020; CABI, online; EPPO, online_e). In the UK, the mature larvae pupate inside the nests from June to early September and adult flight can be normally observed from end July to late September (Forestry Commission, online). Natural dispersal of T. processionea is through adult flight. Larvae move in processions only to very short distances from one tree to another only when there is no food left (Stigter et al., 1997). Adults are good flyers (up to 50–100 km for males and up to 5–20 km for females); windborne spread of adults is also possible (Baker et al., 2009; EPPO, online_c). Males are known to be able to fly over the Channel from France to southern England; this is considered unlikely for females, which are heavier (Evans, 2007; Battisti et al., 2015; EPPO, online_e). In the UK, T. processionea has recently increased its expansion rate, passing from 1.66 km/year in 2006–2014 to 6.17 km/year in 2015–2019 (Suprunenko et al., 2022). The spread of T. processionea can also be human supported, mostly via trading of plants for planting carrying eggs, larvae and pupae. Cut branches and round wood with bark are considered pathways of lesser importance (Evans, 2008; Baker et al., 2009; EPPO, online_e). According to Stigter et al. (1997), larvae were found in oak nurseries in Northern Brabant. The presence of the pest in nurseries is confirmed by Baker et al. (2009) based on reports of the Dutch PPO. |
||
| Symptoms | Main type of symptoms |
Main symptoms caused by larvae of T. processionea on oaks are skeletonisation of leaves and defoliation; presence of silken nests mainly on the lower branches and the lower part of the trunk; processions of caterpillars on the branches and trunks; egg batches in rows covered by scales, mostly on 1–2 years old twigs. Symptoms on humans and animals due to urticating hairs are skin rash, eye irritation, sore throat and breathing difficulty. |
| Presence of asymptomatic plants | No information on the presence of asymptomatic plants was found. | |
| Confusion with other pests | Thaumetopoea processionea is one of 15 species belonging to the genus Thaumetopoea worldwide, recently revised by Basso et al. (2017). The species is easily identified by both morphological features of adults, and features and host plants of larvae (it is the sole Thaumetopoea feeding on Quercus sp.) so that no confusion with other similar species is possible. | |
| Host plant range |
Thaumetopoea processionea is a specialist herbivore feeding on oaks in Europe (Damestoy, 2019). Quercus species known to be hosts of T. processionea are Quercus boissieri, Q. calliprinos, Q. cerris, Q. frainetto, Q. infectoria, Q. ilex, Q. palustris, Q. petraea, Q. pubescens, Q. pyrenaica, Q. robur and Q. × turneri (Baker et al., 2009; DEFRA, online; EPPO, online_f; EUROPHYT, online). Secondary, occasional hosts, only attacked during outbreaks are Acacia, Betula, Carpinus, Castanea, Corylus, Crataegus, Juglans, Fagus, Pistacia, Pinus, Robinia and Sorbus. However, beside Quercus, the development of larvae to adults is known only for Fagus (Stigter et al., 1997; EPPO online_e, f). |
|
| Reported evidence of impact |
Thaumetopoea processionea is both an important defoliating insect for oak species and a threat to human and domestic animal health. Marzano et al. (2020) provide a useful summary of how the multi‐face OPM problem is currently felt by people and managers in the UK. The impact of T. processionea on forest health is variable: it is considered a minor pest for oak forests in Ukraine, Romania, Hungary, Slovenia; severe damage was instead reported from Germany, Italy, France, Belgium and Spain (Baker et al., 2009). In western Europe (Belgium, the Netherlands) and in the UK, the pest is mainly harmful to urban and road trees, as well as to amenity oak trees in parks, forest edges and countryside hedgerows (Battisti et al., 2015). Both in canopied stands and open forests, oaks weakened after severe defoliation by the T. processionea become more susceptible to secondary pests as buprestid beetles, bark and ambrosia beetles or root rot fungi. T. processionea may be hence considered a contributing factor in the oak decline, also resulting in loss of biodiversity (Baker et al., 2009; CABI, online). Impact on human health may be relevant mostly in urban areas, due to the severe pseudo‐allergenic reactions caused by the contact of urticating hairs released by the larvae with skin, eyes and respiratory system. A good synthesis on health effects of T. processionea is provided by Rhalenbeck and Utikal (2015). Urticating hairs released by larvae spread by air currents also from nests, exuviae, pupal cases and may remain active in the soil or in the litter for several years lengthening the social impact of the species (Baker et al., 2009). |
|
| Evidence that the commodity is a pathway | Thaumetopoea processionea was very frequently intercepted on Quercus plants for planting from EU countries to the UK and Ireland, on plant of very similar size to those produced in the UK nurseries (EUROPHYT, online; TRACES‐NT, online). In all probability, T. processionea has been introduced in the London area in 2005 via plants for planting of fastigiated oaks (Baker et al., 2009). Depending on the season, eggs, larvae and pupae may be present on host plants in nurseries everywhere the pest is present in exporting countries. | |
| Surveillance information |
Thaumetopoea processionea is a quarantine pest under official control in the UK. As part of an annual survey at ornamental retail and production sites (frequency of visits determined by a decision matrix), T. processionea is inspected for on Quercus. An additional inspection, during the growing period, is carried out at plant passport production sites. Nursery staff is aware of T. processionea and check all Quercus products for signs, even where the pest is not present in the area. Movement restrictions for growing sites are enforced in the infested area and buffer zone. There is an eradication policy for the buffer zone and pest‐free area (Dossier Section 3.0). The Panel noted that the movement within the UK territory is only restricted to larger trees of Quercus. According to GOV.UK (online): ‘Movement of oak trees in Great Britain: Restrictions on moving large oak trees (Quercus L.), with a girth (circumference) at 1.2 m above the root collar of 8 cm (2.55 cm diameter approx.) in GB vary dependent on what OPM management zone the trees are in.’ |
|
A.7.2. Possibility of pest presence in the nursery
A.7.2.1. Possibility of entry from the surrounding environment
Thaumetopoea processionea is present in the UK territory with distribution restricted to a boundary including 86 local authorities in the London area and South East of England; recently (2022) the pest has also extended its presence to the previous pest‐free area of Hampshire (Dossier Section 5.0).
Adult moths have considerable spreading capacities (up to 50–100 km for males and up to 5–20 km for females); in the UK, the pest has strongly increased its expansion rate, passing from 1.66 km/year in 2006–2014 to 6.17 km/year in 2015–2019 (Suprunenko et al., 2022).
T. processionea breeds on Quercus species. On Fagus the mature larvae can complete the development according to Stigter et al. (1997) but oviposition and young larvae were never observed. Other secondary hosts are Betula, Carpinus, Castanea, Corylus, Crataegus, Juglans, Pinus, Robinia and Sorbus. All these species, mostly Quercus and Fagus, are widely present within 2 km from the nurseries (Dossier Section 3.0).
Uncertainties:
-
–
The pest pressure from the surrounding area of nurseries.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for T. processionea to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present and flying adult moths can easily reach the nurseries.
A.7.2.2. Possibility of entry with new plants/seed
The starting materials are only seeds and seedlings. Seeds are certified and coming from the UK. Seedlings are obtained either from the UK or the EU (mostly the Netherlands) (Dossier Section 3.0). Seeds are not a pathway for the pest.
In addition to Quercus plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, only Fagus sp. are hosts on which the pest can complete the life cycle. However, there is no information on how and where the plants are produced. Therefore, if the plants are first produced in another nursery, the pest could possibly travel with them.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). The growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0). Soil and growing media are not pathways for T. processionea.
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nurseries via new seedlings of Quercus and Fagus plants used for plant production in the area. The entry of the pest with seeds and the growing media the Panel considers as not possible.
A.7.2.3. Possibility of spread within the nursery
Quercus plants are either grown in containers (cells, pots, tubes, etc.) outdoors/ in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
The pest can infest other suitable plants mainly Quercus present within the nurseries (Dossier Sections 3.0 and 6.0).
T. processionea can spread within the nurseries by movement of larvae, adult flight and infested plant material.
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of the pest within the nurseries is possible both by movement of infested plant material and larvae, and flight of adult moths.
A.7.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are 88 records of notification of Quercus plants for planting (Quercus cerris, Q. frainetto, Q. petraea, Q. robur, Q. × turneri) from the Netherlands, Germany and Belgium due to the presence of T. processionea between the years 1995 and December 2022, all for plants intended for planting, already planted (EUROPHYT, online; TRACES‐NT, online).
A.7.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on T. processionea is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
The registration and the release of UK plant passport should be enough to warrant pest‐free plant material for a quarantine pest in the UK.
Uncertainties:
|
| 2 | Physical separation | No |
As the production is not carried out in separate areas, the possibility that the pest can move from the outside to the nurseries and from one tree species to another within the nurseries is concrete. |
| 3 | Certified plant material | Yes |
The use of certified material should be enough to warrant pest‐free status.
Uncertainties:
|
| 4 | Growing media | No |
The pest is not affected by the growing medium as in the nurseries all the stages develop above ground. |
| 5 | Surveillance, monitoring and sampling | Yes |
Regular surveys are carried out during the production by visual inspection of the plants. Any report of quarantine pest is provided.
Uncertainties:
|
| 6 | Hygiene measures | No |
Weeding and disinfection are not relevant for this pest. |
| 7 | Removal of infested plant material | Yes |
The removal of infested plants at the larval stage will have a positive effect although it would be difficult with the egg stage as egg masses are detectable only through a careful inspection of all the twigs.
Uncertainties:
|
| 8 | Irrigation water | No |
Water is not relevant for this pest. |
| 9 | Application of pest control products | Yes |
The pest is easy to control at the larval stage and being a quarantine pest, its presence must be reported and measures taken. However, the egg masses are not susceptible to any crop protection method and there are no treatments available against the moths.
Uncertainties:
|
| 10 | Measures against soil pests | No |
Soil is not relevant for this pest. |
| 11 | Inspections and management of plants before export | Yes |
Inspections carried out before export will be visual and would be enough to warrant that commodities are free of larvae. However, the detection of egg masses is difficult and it should require the individual checking of every twig in each plant.
Uncertainties:
|
| 12 | Separation during transport to the destination | Yes |
The separation of the plants during the transport would reduce the possibility that larvae are moving among plants if the transport happens when green leaves are occurring between April and August. Separation is not affecting the egg stage as they are not mobile.
Uncertainties:
|
A.7.5. Overall likelihood of pest freedom for bundles of whips and seedlings
A.7.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bundles of whips and seedlings
The scenario assumes that the nurseries are located in a pest‐free area for the whole period of plant development and the plant material taken to the nurseries originate only from pest‐free areas within the UK.
A.7.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bundles of whips and seedlings
The scenario assumes that the nurseries are not in a pest‐free area and plant material taken to the nursery could originate from infested areas in the EU and in the UK. This scenario also assumes a high difficulty in eradicating the pest. It also assumes that there is no restriction of trade in smaller plants in infested areas and buffer zones. The scenario assumes that although they are smaller plants, a bundle effect is expected. Finally, this scenario assumes that interceptions have occurred mostly on smaller plants.
A.7.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bundles of whips and seedlings (Median)
The median is skewed to the left (lower values) because the pest is of concern in the UK and measures are taken against this pest. Furthermore, the plants are young and there is only 1 year time for oviposition of the pest.
A.7.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The uncertainty is almost equally distributed around the median (the third quartile shows slightly less uncertainty) because measures are taken again the pest in the UK, and because the plants are 1–2 years old and therefore there is less time for oviposition (and infection) in such young plants.
A.7.5.5. Elicitation outcomes of the assessment of the pest freedom for Thaumetopoea processionea on bundles of whips and seedlings
The following Tables show the elicited and fitted values for pest infestation (Table A.37) and pest freedom (Table A.38).
Table A.37.
Elicited and fitted values of the uncertainty distribution of pest infestation by Thaumetopoea processionea per 10,000 bundles
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.0 | 25.0 | 50.0 | 130.0 | 250.0 | ||||||||||
| EKE | 0.152 | 0.605 | 1.72 | 4.89 | 10.7 | 19.8 | 31.0 | 59.9 | 98.7 | 123 | 153 | 183 | 213 | 234 | 251 |
The EKE results is the BetaGeneral (0.66538, 1.6786, 0, 275) distribution fitted with @Risk version 7.6.
Table A.38.
The uncertainty distribution of bundles free of Thaumetopoea processionea per 10,000 bundles calculated by Table A.37
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,870 | 9,950 | 9,975 | 10,000 | ||||||||||
| EKE results | 9,749 | 9,766 | 9,787 | 9,817 | 9,847 | 9,877 | 9,901 | 9,940 | 9,969 | 9,980 | 9,989 | 9,995 | 9,998 | 9,999 | 10,000 |
The EKE results are the fitted values.
Based on the numbers of estimated infested bundles the pest freedom was calculated (i.e. = 10,000 – number of infested bundles per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.38.
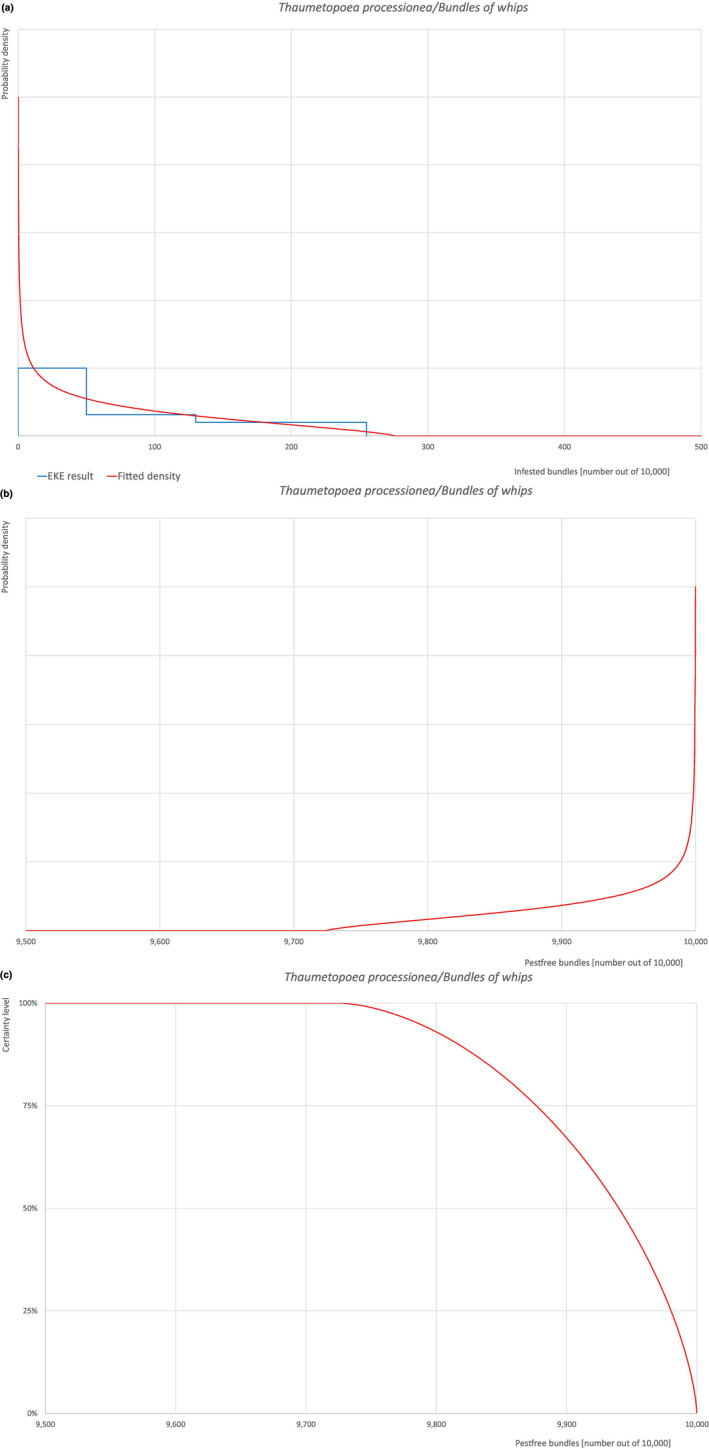
Figure A.19: (a) Elicited uncertainty of pest infestation per 10,000 bundles (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free bundles per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 bundles
A.7.6. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old with circumference below 80 mm at 1.2 m height
A.7.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare root plants/trees up to 7 years old
The scenario assumes that the nurseries are located in a pest‐free area for the whole period of plant development and the plant material taken to the nurseries originate only from pest‐free areas within the UK.
A.7.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare root plants/trees up to 7 years old
The scenario assumes that the nurseries are not in a pest‐free area and plant material taken to the nursery could originate from infested areas in the EU and in the UK. This scenario also assumes a high difficulty in eradicating the pest. Finally, it also assumes that there is no restriction of trade in smaller plants in infested areas and buffer zones.
A.7.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare root plants/trees up to 7 years old (Median)
The median is slightly skewed to the left (lower values) because the pest is of concern in the UK and measures are taken against this pest. However, the mean values are not lower because high‐pest pressure from the surroundings of the nurseries is assumed.
A.7.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The uncertainty is almost equally distributed around the median (the third quartile shows slightly less uncertainty) because measures are taken again the pest in the UK, and because of the ease of detection of the pest in this commodity.
A.7.6.5. Elicitation outcomes of the assessment of the pest freedom for Thaumetopoea processionea on bare root plants/trees up to 7 years old with circumference below 80 mm at 1.2 m height
The following Tables show the elicited and fitted values for pest infestation (Table A.39) and pest freedom (Table A.40).
Table A.39.
Elicited and fitted values of the uncertainty distribution of pest infestation by Thaumetopoea processionea per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.0 | 37.0 | 75.0 | 150.0 | 250.0 | ||||||||||
| EKE | 0.663 | 2.00 | 4.64 | 10.8 | 20.2 | 33.3 | 47.9 | 81.5 | 122 | 145 | 172 | 198 | 223 | 238 | 250 |
The EKE results is the BetaGeneral (0.82917, 1.5137, 0, 265) distribution fitted with @Risk version 7.6.
Table A.40.
The uncertainty distribution of plants free of Thaumetopoea processionea per 10,000 plants calculated by Table A.39
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,750 | 9,850 | 9,925 | 9,963 | 10,000 | ||||||||||
| EKE results | 9,750 | 9,762 | 9,777 | 9,802 | 9,828 | 9,855 | 9,878 | 9,918 | 9,952 | 9,967 | 9,980 | 9,989 | 9,995 | 9,998 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.40.
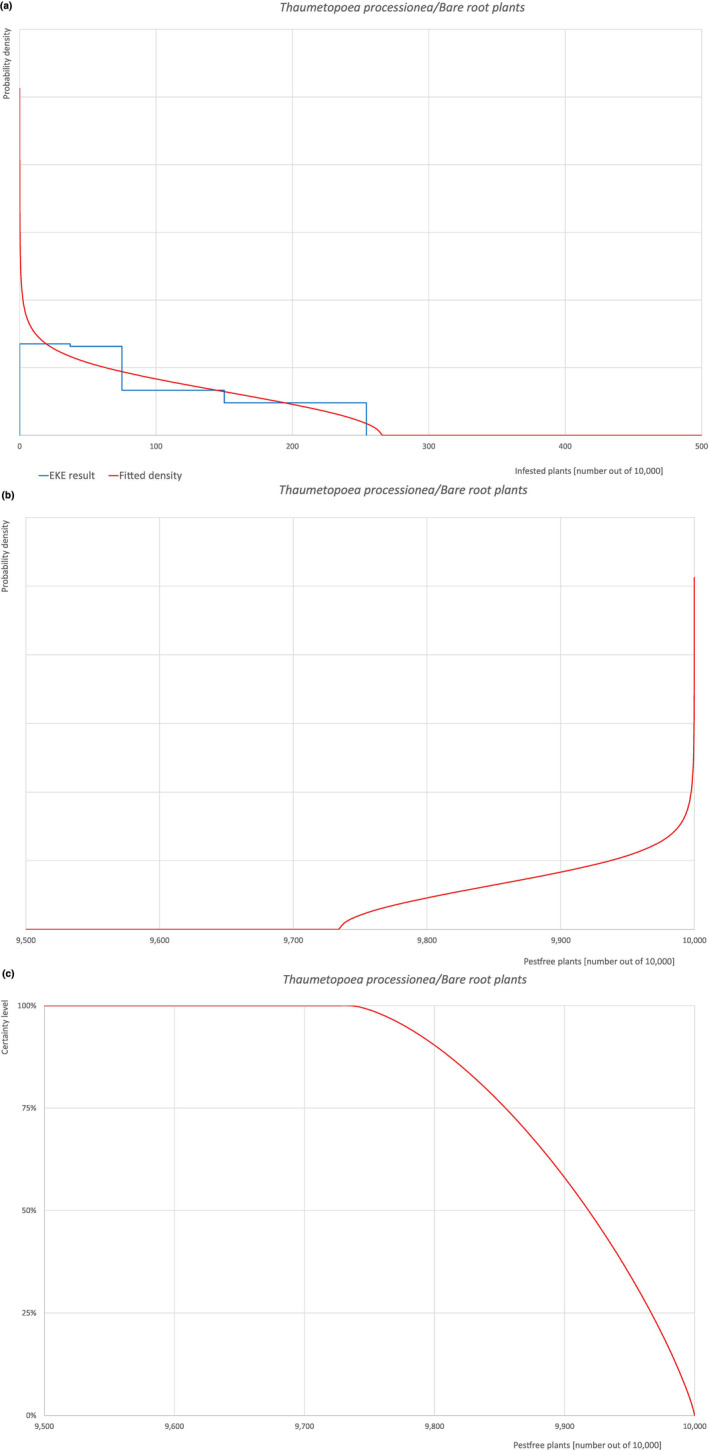
Figure A.20: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.7.7. Overall likelihood of pest freedom for plants in pots up to 15 years old with circumference below 80 mm at 1.2 m height
A.7.7.1. Reasoning for a scenario which would lead to a reasonably low number of infested plants in pots up to 15 years old
The scenario assumes that the nurseries are located in a pest‐free area for the whole period of plant development and the plant material taken to the nurseries originate only from pest‐free areas within the UK.
A.7.7.2. Reasoning for a scenario which would lead to a reasonably high number of infested plants in pots up to 15 years old
The scenario assumes that the nurseries are not in a pest‐free area and plant material taken to the nursery could originate from infested areas in the EU and in the UK. This scenario also assumes a high difficulty in eradicating the pest, and that there is no restriction of trade in smaller plants in infested areas and buffer zones. This scenario also assumes that these plants are traded throughout the year, including the period when leaves are present. In addition, the plants are denser and may have a higher oviposition rate compared to bare root plants. Finally, larvae may hide in foliage and be more difficult to detect.
A.7.7.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested plants in pots up to 15 years old (Median)
The median is slightly skewed to the left (lower values) because the pest is of concern in the UK and measures are taken against this pest. But mean values are not lower because plants can be traded throughout the year (plants with leaves), the oviposition rates can be high, and larvae may be difficult to detect hidden in the foliage.
A.7.7.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The uncertainty is almost equally distributed around the median (the third quartile shows slightly less uncertainty) because measures are taken again the pest in the UK, but trade in plants with leaves throughout the year is much riskier because of the difficulty in detecting signs of the pest and because of the increased rate of oviposition on larger plants with leaves.
A.7.7.5. Elicitation outcomes of the assessment of the pest freedom for Thaumetopoea processionea on plants in pots up to 15 years old with circumference below 80 mm at 1.2 m height
The following Tables show the elicited and fitted values for pest infestation (Table A.41) and pest freedom (Table A.42).
Table A.41.
Elicited and fitted values of the uncertainty distribution of pest infestation by Thaumetopoea processionea per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0.0 | 45.0 | 90.0 | 180.0 | 300.0 | ||||||||||
| EKE | 0.847 | 2.52 | 5.77 | 13.2 | 24.7 | 40.5 | 57.9 | 98.0 | 146 | 174 | 207 | 238 | 267 | 286 | 301 |
The EKE results is the BetaGeneral (0.84084, 1.5462, 0, 320) distribution fitted with @Risk version 7.6.
Table A.42.
The uncertainty distribution of plants free of Thaumetopoea processionea per 10,000 plants calculated by Table A.41
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,700 | 9,820 | 9,910 | 9,955 | 10,000 | ||||||||||
| EKE results | 9,699 | 9,714 | 9,733 | 9,762 | 9,793 | 9,826 | 9,854 | 9,902 | 9,942 | 9,960 | 9,975 | 9,987 | 9,994 | 9,997 | 9,999 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.42.
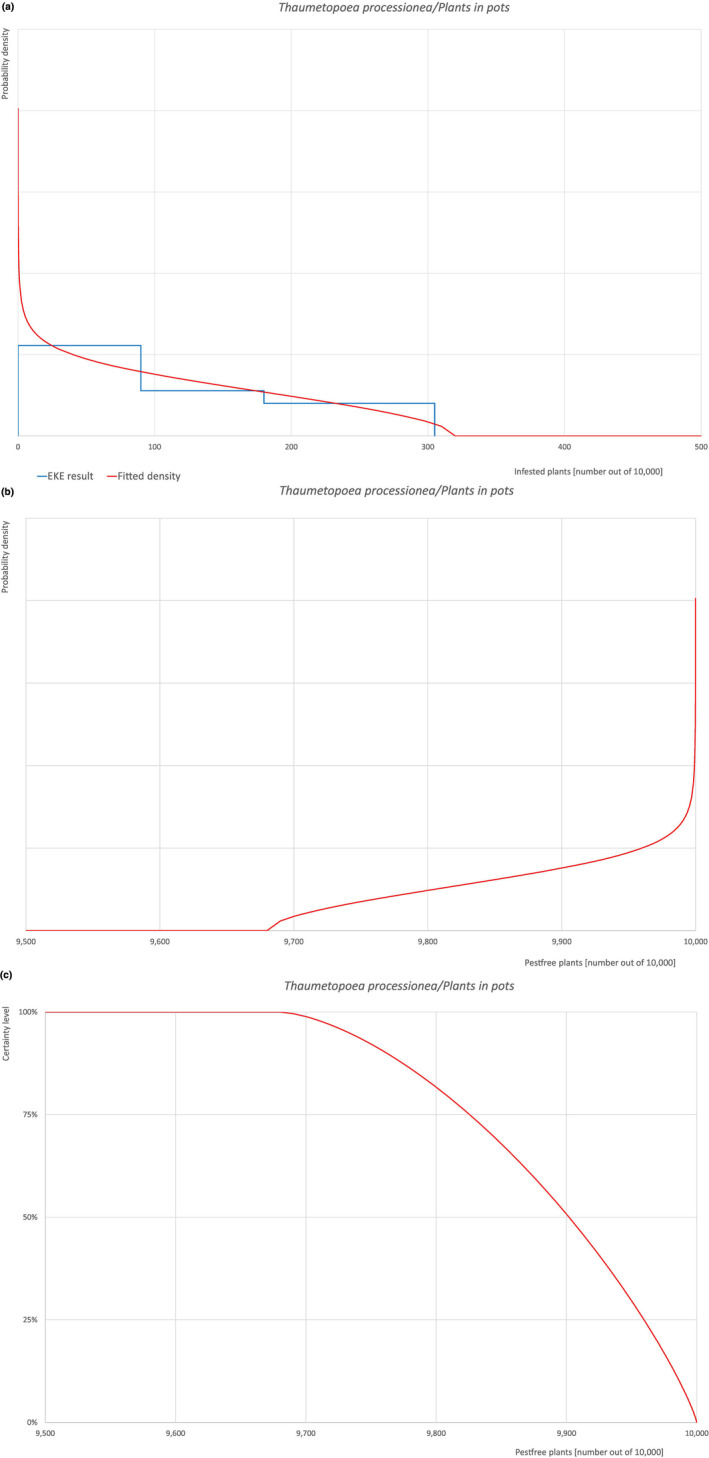
Figure A.21: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.7.8. Reference list
Baker R, Caffier D, Choiseul JW, De Clercq P, Dormannnsné‐Simon E, Gerowitt B, Karadjova OE, Lövei G, Lansink AO, Makowski D, Manceau C, Manici L, Perdikis D, Puglia AP, Schans J, Schrader G, Steffek R, Strömberg A, Tiilikkala K, van Lenteren JC and Vloutoglou I, 2009. Scientific opinion of the Panel of Plant Health on a pest risk analysis on Thaumetopoea processionea L., the oak processionary moth, prepared by the UK and extension of its scope to the EU territory. EFSA Journal 2009;7(6):1195, 63 pp. https://doi.org/10.2903/j.efsa.2009.1195
Basso A, Negrisolo E, Zilli A, Battisti A and Cerretti P, 2017. A total evidence phylogeny for the processionary moths of the genus Thaumetopoea (Lepidoptera: Notodontidae: Thaumetopoeinae). Cladistics, 33, 557–573. https://doi.org/10.7934/p2806
Battisti A, Avci M, Avtzis D, Mohamed Lahbib BJ, Berardi L, Wahiba B, Branco M, Chakali G El Alaoui El Fels MA, Frérot B, Hódar J, Ionescu‐Mălăncuş I, Ipekdal K, Larsson S, Traian M, Mendel Z, Meurisse N, Mirchev P, Nemer N and Zamoum M, 2015. Natural history of the processionary moths (Thaumetopoea spp.): new insights in relation to climate change. In Roques A (ed.), Processionary moths and climate change: an update. Springer Dordrecht, 15–81. https://doi.org/10.1007/978-94-017-9340-7_2
CABI (Centre for Agriculture and Bioscience International), online. Thaumetopoea processionea (oak processionary moth). Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.53502 [Accessed: 10 December 2022].
Csóka G, Hirka A, Szöcs L, Móritz N, Rasztovits E and Pödör Z, 2018. Weather‐dependent fluctuations in the abundance of the oak processionary moth, Thaumetopoea processionea (Lepidoptera: Notodontidae). European Journal of Entomology, 115, 249–255. https://doi.org/10.14411/eje.2018.024
Damestoy T, 2019. Interactions between oaks and the oak processionary moth, Thaumetopoea processionea L.: from trees to forest. Biodiversity and Ecology. Université de Bordeaux, 128 pp.
Damestoy T, Moreira X, Jactel H, Valdes‐Correcher E, Plomion C and Castagneyrol B, 2021. Growth and mortality of the oak processionary moth, Thaumetopoea processionea, on two oak species: direct and trait‐mediated effects of host and neighbour species. Entomologia Generalis, 41, 13–25. https://doi.org/10.1127/entomologia/2020/1005
DEFRA (Department for Environment, Food and Rural Affairs), online. UK risk register details for Thaumetopoea processionea. Available online: https://planthealthportal.defra.gov.uk/pests-and-diseases/uk-plant-health-risk-register/viewPestRisks.cfm?cslref=7319 [Accessed: 8 December 2022].
de Jong Y, Verbeek M, Michelsen V, de Place Bjørn P, Los W, Steeman F, Bailly N, Basire C, Chylarecki P, Stloukal E, Hagedorn G, Wetzel FT, Glöckler F, Kroupa A, Korb G, Hoffmann A, Häuser C, Kohlbecker A, Müller A, Güntsch A, Stoev P, and Penev L, online. Fauna Europaea ‐ all European animal species on the web. Biodiversity Data Journal. Available online: https://fauna-eu.org/ [Accessed: 8 December 2022].
EFSA PLH Panel (EFSA Panel on Plant Health), Bragard C, Baptista P, Chatzivassiliou E, Di Serio F, Jaques Miret JA, Justesen AF, MacLeod A, Magnusson CS, Milonas P, Navas‐Cortes JA, Parnell S, Potting R, Reignault PL, Stefani E, Thulke H‐H, Van der Werf W, Vicent Civera A, Yuen J, Zappalà L, Battisti A, Mas H, Rigling D, Faccoli M, Gardi C, Iacopetti G, Mikulová A, Mosbach‐Schulz O, Stergulc F, Streissl F and Gonthier P, 2023. Scientific Opinion on the commodity risk assessment of Fagus sylvatica plants from the UK. EFSA Journal 2023;21(7):8118, 151 pp. https://doi.org/10.2903/j.efsa.2023.8118
EPPO (European and Mediterranean Plant Protection Organization), online_a. Thaumetopoea processionea (THAUPR), Categorization. Available online: https://gd.eppo.int/taxon/THAUPR/categorization [Accessed: 10 December 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_b. Thaumetopoea processionea (THAUPR). Distribution details in United Kingdom. Available online: https://gd.eppo.int/taxon/THAUPR/distribution/GB [Accessed: 10 December 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_c. First report of Thaumetopoea processionea in Jersey. EPPO Reporting Service no. 10–2022. Num. article: 2022/213. Available online: https://gd.eppo.int/reporting/article-7444 [Accessed: 8 December 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_d. Thaumetopoea processionea (THAUPR), Distribution Available online: https://gd.eppo.int/taxon/THAUPR/distribution [Accessed: 10 December 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_e.Thaumetopoea processionea. EPPO datasheet. Available online. https://gd.eppo.int/taxon/THAUPR/datasheet [Accessed 8 December 2022].
EPPO (European and Mediterranean Plant Protection Organization), online_f. Thaumetopoea processionea (THAUPR), Hosts. Available online: https://gd.eppo.int/taxon/THAUPR/hosts [Accessed: 10 December 2022].
Evans HF, 2008. Oak processionary moth Pest Risk Analysis. Revision June 2008. Forest Research, Tree Health Division. 30 pp.
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en [Accessed: 22 December 2022].
Forestry Commission, online. Oak processionary moth (Thaumetopoea processionea) ‐ Life cycle. Edinburgh, UK: Forestry Commission, Plant Health Service. Available online: https://www.forestresearch.gov.uk/tools-and-resources/fthr/pest-and-disease-resources/oak-processionary-moth-thaumetopoea-processionea/opm-manual-4-biology-and-life-cycle/ [Accessed: 8 December 2022].
GBIF (Global Biodiversity Information Facility) Secretariat, online. GBIF BackBone Taxonomy. Available online: https://www.gbif.org/ [Accessed: 10 December 2022].
GOV.UK (the UK government), online. Guidance Managing oak processionary moth in England. Updated on 11 April 2023. Available online: https://www.gov.uk/guidance/managing-oak-processionary-moth-in-england [Accessed: 21 April 2023].
Groenen F and Meurisse N, 2012. Historical distribution of Thaumetopoea processionea in Europe suggests recolonization instead of expansion. Agricultural and Forest Entomology, 14, 147–155. https://doi.org/10.1111/j.1461-9563.2011.00552.x
Marzano M, Ambrose‐Oji B, Hall C and Moseley D, 2020. Pests in the City: managing public health risks and social values in response to Oak Processionary Moth (Thaumetopoea processionea) in the United Kingdom. Forests, 11, 199. https://doi.org/10.3390/f11020199
Rahlenbeck S and Utikal J, 2015. The oak processionary moth: a new health hazard? British Journal of General Practice, 65, 435–436. https://doi.org/10.3399/bjgp15X686341
Stigter H, Geraedts WHJM and Spijkers HCP, 1997. Thaumetopoea processionea in the Netherlands: Present status and management perspectives (Lepidoptera: Notodontidae). Proceedings of the Section Experimental and Applied Entomology of the Netherlands Entomological Society (N.E.V.), 3–16.
Suprunenko YF, Castle MD, Webb CR, Branson J, Hoppit A and Gilligan CA, 2022. Estimating expansion of the range of oak processionary moth (Thaumetopoea processionea) in the UK from 2006 to 2019. Agricultural and Forest Entomology, 10 pp. https://doi.org/10.1111/afe.12468
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Zielonka M, 2020. Pest case studies ‐ On the oak processionary moth Thaumetopoea processionea (Lepidoptera: Thaumetopoeidae). Harper Adams University, 9 pp.
A.8. Trinophylum cribratum
A.8.1. Organism information
| Taxonomic information |
Current valid scientific name: Trinophylum cribratum Synonyms: Callidium impressipenne Name used in the EU legislation: – Order: Coleoptera Family: Cerambycidae Common name: deodar longicorn bast‐eater; deodar longicorn beetle Name used in the Dossier: Trinophylum cribratum |
|
| Group | Insects | |
| EPPO code | – | |
| Regulated status | Trinophylum cribratum is neither regulated in the EU nor listed by EPPO. | |
| Pest status in the UK |
Trinophylum cribratum is present in the UK territory since 1947 (Gilmour, 1948; Uhthoff‐Kaufmann, 1990; Twinn and Harding, 1999). It was probably introduced from India before the Second World War. Although reported as a very local and rare saproxylic species in central and southern England (Dossier Section 5.0) is now considered as ‘an established indigenous beetle’ (Uhthoff‐Kaufmann, 1990). |
|
| Pest status in the EU |
Trinophylum cribratum is absent in the EU (GBIF, online; de Jong et al., online). Some recent findings in Croatia (Lovric, 2021) are not confirmed by reliable identification. |
|
| Host status on Quercus sp. | Quercus robur and Q. cerris are hosts of Trinophylum cribratum (Gilmour, 1948; Uhthoff‐Kaufmann, 1990; Twinn and Harding, 1999). | |
| PRA information | No Pest Risk Assessment is available. | |
| Other relevant information for the assessment | ||
| Biology |
Trinophylum cribratum is a polyphagous longhorn beetle belonging to the subfamily Cerambycinae; it is native to Asia, where it is known from northern India and Pakistan (Gahan, 1906), and was accidentally introduced to Europe (England) in the first half of the last century. The species is univoltine; adults (11–13 mm length) start to fly in June and may be observed until September; they usually rest during the day and have crepuscular habits, sometimes attracted by light. After mating, the females search for freshly felled or severely declining trees to lay eggs in the bark crevices; no information about the number of eggs laid is available (Stebbing, 1914; Uhthoff‐Kaufmann, 1990). The young larvae develop during summer under the bark by initially feeding only on phloem, but as they grow also the sapwood is affected; according to Stebbing (1914), the larvae need cambium ‘in state of considerable freshness’ to develop. The larvae tunnel irregular galleries filled with sawdust and excrements until late autumn, then they overwinter at full grown stage. Pupation and adult flight may be observed in late spring‐early summer of the next year (Stebbing, 1914; Uhthoff‐Kaufmann, 1990). Adults are considered quite good fliers (Uhthoff‐Kaufmann, 1990) but no specific information about the flight distance is available. Dunn et al. (2016) studied the dispersal behaviour of adults of some Cerambycinae beetle species similar to Trinophylum cribratum (e.g. Phymatodes sp. and Neoclytus sp.) and found a flight distance of about 40 m; however this should be taken as a general reference, since the active dispersal of saproxylic insects is very difficult to measure (Dunn et al., 2016). Considering the pest biology as described above, human assisted spread is mostly via infested wood with/without bark and possibly adult beetle hitch‐hiking. |
|
| Symptoms | Main type of symptoms |
No specific external symptoms on living trees are known. Declining/dead trees or infested logs show dense irregular larval galleries filled with frass in the phloem/sapwood and elliptic exit holes of adult on the bark; however, these also are no specific symptoms, since similar species of longhorn beetles (e.g. Phymatodes testaceus) are often abundantly found in logs infested by Trinophylum cribratum (Uhthoff‐Kaufmann, 1990). |
| Presence of asymptomatic plants | No information on the presence of asymptomatic plants was found. | |
| Confusion with other pests | While symptoms under bark can easily be confused with those caused by other cerambycids (see above), the adults of Trinophylum cribratum are quite easy to recognise by experts by using morphological keys. | |
| Host plant range | Trinophylum cribratum is a polyphagous beetle feeding on both conifers and deciduous trees. Stebbing (1914) and Pierce (1917) only mention the deodar (Cedrus deodara) as host plant. According to Uhthoff‐Kaufmann (1990), however, the host plant range of T. cribratum is much wider, including unspecified ‘Indian oaks and other native hardwoods’ in Asia, and several important tree genera and species in England, such as Betula, Crataegus, Fagus, Fraxinus, Juglans, Larix, Malus, Pinus sylvestris, Platanus, Pyrus, Pyracantha, Quercus cerris and Q. robur. | |
| Reported evidence of impact |
Trinophylum cribratum is a longicorn beetle developing in the cambium and sapwood of seriously weakened and declining standing trees, windthrows, freshly felled trees, logs and firewood. It is considered a serious pest of deodar in Northern India, attacking standing trees weakened by forest fire, storms and heavy snow, often in association with bark beetles and buprestid beetles (Stebbing, 1914). |
|
|
In England, damage by T. cribratum is mostly recorded from wood merchants' yards, where the beetle was found on seasoned oak logs also infested by Phymatodes testaceus. Highly infested timber is only merchantable as firewood or chips (Uhthoff‐Kaufmann, 1990), but no detailed data on the economic impact of the pest is available. Damage on standing trees is only occasionally reported and the impact seems to be negligible (Uhthoff‐Kaufmann, 1990; Dossier Section 3.0). |
||
| Evidence that the commodity is a pathway |
There is no evidence that plants for planting may be a pathway for Trinophylum cribratum. However, the Panel cannot exclude that commodities with relatively large diameter (e.g. > 5 cm diameter) can be infested by this pest as it occurs for other longhorn beetles. |
|
| Surveillance information | Trinophylum cribratum is not under official surveillance, as does not meet criteria of quarantine pest for Great Britain (Dossier Section 5.0). | |
A.8.2. Possibility of pest presence in the nursery
A.8.2.1. Possibility of entry from the surrounding environment
Trinophylum cribratum is present in the UK in central and southern England as a very local and rare species, possibly found on wood merchants' yards or on declining/dead standing trees. Natural spread of the pest is by adults flying in search of suitable wood material to reproduce.
T. cribratum is a saproxylic beetle living on Quercus robur and Q. cerris and able to reproduce also on Betula spp., Crataegus spp, Fagus spp., Fraxinus spp., Juglans spp., Larix spp., Malus spp., Pinus spp., Platanus spp., Pyrus spp., Pyracantha spp. Many of these species, mostly Quercus spp. and Fagus spp., are present within 2 km from the nurseries. Moreover, the woodlands may be at the border of the nurseries (Dossier Section 3.0). Cedrus deodara, an important host of T. cribratum, is most likely present as ornamental tree in private gardens in the same area. The presence of declining or dead host trees suitable for the reproduction of the pest in the area cannot be excluded.
Uncertainties:
-
–
The possibility of presence of the pest on declining trees in the surrounding area of nurseries.
-
–
No information on the possible presence of infested wood (mostly logs and firewood) in the merchants' yards in the surrounding area.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for T. cribratum to enter the nurseries from surrounding environment. In the surrounding area, suitable hosts are present and adults can enter the nurseries by flight.
A.8.2.2. Possibility of entry with new plants/seed
The starting materials are either seeds or seedlings. Seeds are certified and coming from the UK. Seedlings are obtained either from the UK or the EU (mostly the Netherlands) (Dossier Section 3.0). The material mentioned above is not a pathway for the pest.
In addition to Quercus robur plants, the nurseries also produce other plants (Dossier Section 6.0). Out of them, there are many suitable hosts for the pest (such as Betula spp., Crataegus spp., Fagus spp., Juglans spp., Pinus spp., Quercus spp., etc.). There is no information on how and where the plants are produced.
The nurseries are using virgin peat or peat‐free compost (a mixture of coir, tree bark, wood fibre, etc.) as a growing media (Dossier Section 1.0). The growing media is certified and heat‐treated by commercial suppliers during production to eliminate pests and diseases (Dossier Section 3.0). Soil and growing media are not pathways for T. cribratum.
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that it is possible for the pest to enter the nurseries via plants of other species used for plant production in the area, if they have larger diameter (e.g. more than 5 cm). The entry of the pest with seeds and the growing media the Panel also considers as not possible.
A.8.2.3. Possibility of spread within the nursery
Quercus plants are either grown in containers (cells, pots, tubes, etc.) outdoors/in the open air or in field. Cell grown trees may be grown in greenhouses, however most plants will be field grown, or field grown in containers (Dossier Section 1.0). There are no mother plants present in the nurseries (Dossier Section 3.0).
Pruning residues are removed from the nursery to reduce the number of over wintering sites for pests and diseases (Dossier Section 1.0).
The pest cannot infest healthy and vigorous plants and the phytosanitary condition of growing material is continuously monitored in the nurseries so that declining/dead trees are unlikely to be found there.
Uncertainties:
-
–
None.
Taking into consideration the above evidence and uncertainties, the Panel considers that the spread of T. cribratum within the nurseries although unlikely cannot be excluded due to the presence of old hosts.
A.8.3. Information from interceptions
In the EUROPHYT/TRACES‐NT database there are no records of notification of Quercus plants for planting neither from the UK nor from other countries due to the presence of T. cribratum between the years 1995 and December 2022 (EUROPHYT, online; TRACES‐NT, online).
A.8.4. Evaluation of the risk mitigation measures
In the table below, all risk mitigation measures currently applied in the UK are listed and an indication of their effectiveness on T. cribratum is provided. The description of the risk mitigation measures currently applied in the UK is provided in the Table 6.
| N | Risk mitigation measure | Effect on the pest | Evaluation and uncertainties |
|---|---|---|---|
| 1 | Registration of production sites | Yes |
The registration and the release of UK plant passport should have an effect on warranting pest‐free plant material, although the pest in not a quarantine one in the UK.
Uncertainties:
|
| 2 | Physical separation | No |
As the production is not carried out in separate areas, the possibility that the pest can move from the outside to the nurseries and from one tree species to another within the nurseries is concrete. |
| 3 | Certified plant material | No |
The pest is not known to be present in the EU. It is not expected that seedlings are pathway for the pest. Seeds are not pathway. |
| 4 | Growing media | No |
The pest is not affected by the growing medium as in the nurseries all the stages develop above ground. |
| 5 | Surveillance, monitoring and sampling | Yes |
Regular surveys are carried out during the production by visual inspection of the plants.
Uncertainties:
|
| 6 | Hygiene measures | No |
Weeding and disinfection are not relevant for this pest. |
| 7 | Removal of infested plant material | Yes |
The removal of infested plants and pruning residues either healthy or infested will have a positive effect on the pest.
Uncertainties:
|
| 8 | Irrigation water | No |
Water is not relevant for this pest. |
| 9 | Application of pest control products | Yes |
The pest is very difficult to control with pesticides at the larval stage, as this stage is protected under the bark or inside the wood. Pest control products can only have a very limited effect on controlling adults after emergence. Physical measures like removing wilting branches could have an effect.
Uncertainties:
|
| 10 | Measures against soil pests | No |
Soil is not relevant for this pest. |
| 11 | Inspections and management of plants before export | Yes |
Inspections carried out before export will be visual and should have an effect on warranting that commodities are free of the pest. However, the detection of signs could be difficult in big trees.
Uncertainties:
|
| 12 | Separation during transport to the destination | Yes |
The separation of the plants during the transport could have a limited effect on reducing the possibility that pest is moving among plants only if the transport happens when adults are emerging, between June and September. Separation is not affecting the larvae as they are hidden under the bark or inside the wood. Separation is not affecting eggs as they are not mobile.
Uncertainties:
|
A.8.5. Overall likelihood of pest freedom for bare root plants/trees up to 7 years old
A.8.5.1. Reasoning for a scenario which would lead to a reasonably low number of infested bare root plants/trees up to 7 years old
This scenario assumes that the pest only attacks very declining trees or recent dead ones, and this kind of trees are not expected to be present within the nursery. The scenario also assumes a very low pressure of the pest in the area where nurseries are located.
A.8.5.2. Reasoning for a scenario which would lead to a reasonably high number of infested bare root plants/trees up to 7 years old
This scenario assumes that the pest is present in the surroundings of the nurseries, although a low abundance is expected. This scenario also assumes that the pest mainly attacks very declining trees or recent dead ones, and although this kind of trees are not expected to be present within the nursery, pruning and potting could cause stress or weakness on some trees that could be attractive for the pest. The scenario envisages that commodity can be traded at any time of the year, and during the summer some adult could be present in the plants and associated to the commodity as a hitchhiker. Finally, this scenario contemplates the possibility that declining branches can be colonised and unnoticed during inspections.
A.8.5.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested bare root plants/trees up to 7 years old (Median)
The median is skewed to the left (lower values) because the pest mainly attacks very declining trees or recent dead ones, and this kind of trees are not expected to be present within the nursery. Moreover, the abundance of the pest is expected to be low in the surroundings.
A.8.5.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because the pest mainly attacks very declining trees or recent dead ones not expected to be present within the nursery, and because a low pest pressure in the surroundings is expected.
A.8.5.5. Elicitation outcomes of the assessment of the pest freedom for Trinophylum cribratum on bare root plants/trees up to 7 years old
The following Tables show the elicited and fitted values for pest infestation (Table A.43) and pest freedom (Table A.44).
Table A.43.
Elicited and fitted values of the uncertainty distribution of pest infestation by Trinophylum cribratum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 1 | 1 | 2 | 5 | ||||||||||
| EKE | 0.0176 | 0.0436 | 0.0874 | 0.177 | 0.302 | 0.467 | 0.646 | 1.06 | 1.60 | 1.94 | 2.39 | 2.89 | 3.48 | 3.97 | 4.50 |
The EKE results is the BetaGeneral (1.0126, 3.9819, 0, 6.55) distribution fitted with @Risk version 7.6.
Table A.44.
The uncertainty distribution of plants free of Trinophylum cribratum per 10,000 plants calculated by Table A.43
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,996 | 9,998 | 9,999 | 10,000 | 10,000 | ||||||||||
| EKE results | 9,995.5 | 9,996.0 | 9,996.5 | 99,97.1 | 99,97.6 | 9,998.1 | 9,998.4 | 9,998.9 | 9,999.4 | 9,999.5 | 9,999.7 | 9,999.8 | 9,999.91 | 9,999.96 | 9,999.98 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.44.
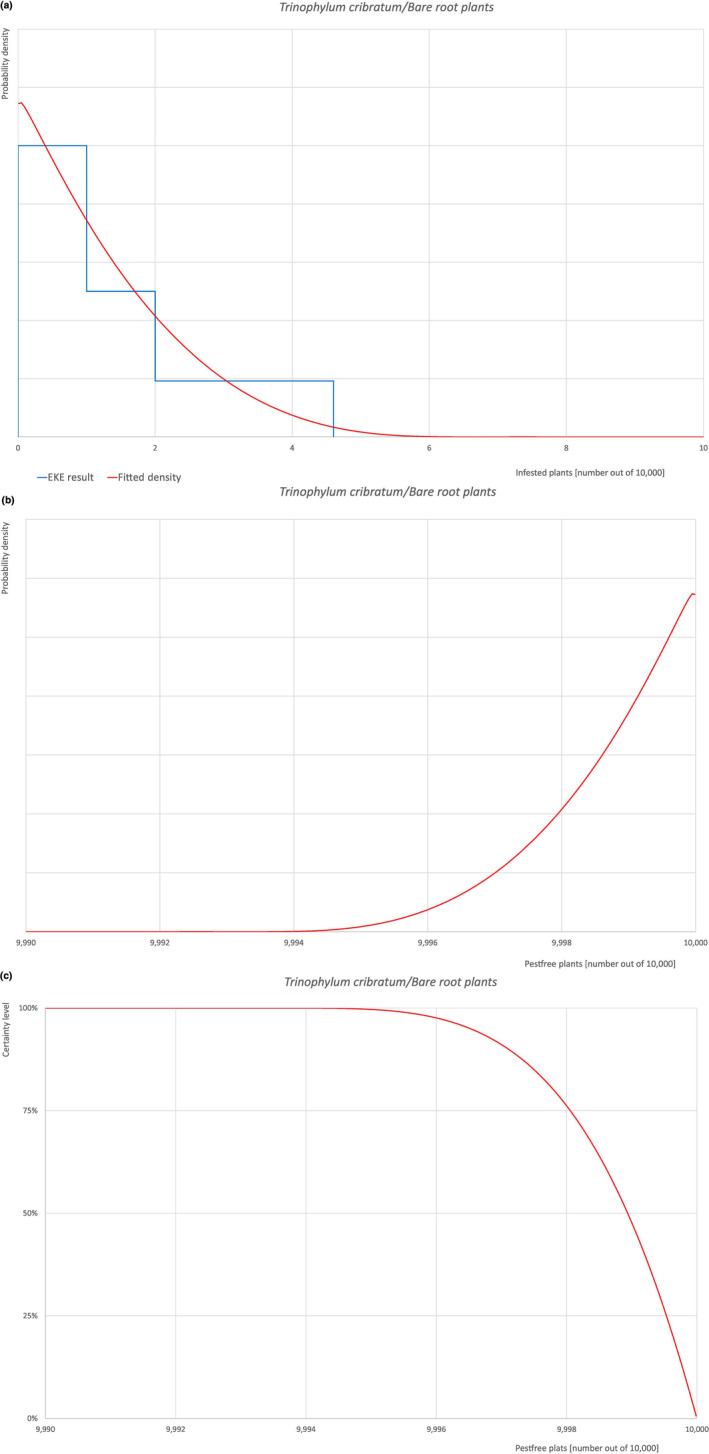
Figure A.22: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.8.6. Overall likelihood of pest freedom for plants in pots up to 15 years old
A.8.6.1. Reasoning for a scenario which would lead to a reasonably low number of infested plants in pots up to 15 years old
This scenario assumes that the pest only attacks very declining trees or recent dead ones, and this kind of trees are not expected to be present within the nursery. The scenario also assumes a very low pressure of the pest in the area where nurseries are located.
A.8.6.2. Reasoning for a scenario which would lead to a reasonably high number of infested plants in pots up to 15 years old
This scenario assumes that the pest is present in the surroundings of the nurseries, although a low abundance is expected. This scenario also assumes that the pest mainly attacks very declining trees or recent dead ones, and although this kind of trees are not expected to be present within the nursery, pruning and potting could cause stress or weakness on some trees that could be attractive for the pest. The scenario envisages that commodity can be traded at any time of the year, and during the summer some adult could be present in the plants and associated to the commodity as a hitchhiker. Finally, this scenario contemplates the possibility that declining branches can be colonised and unnoticed during inspections.
A.8.6.3. Reasoning for a central scenario equally likely to over‐ or underestimate the number of infested plants in pots up to 15 years old (Median)
The median is skewed to the left (lower values) because the pest mainly attacks very declining trees or recent dead ones, and this kind of trees are not expected to be present within the nursery. Moreover, the abundance of the pest is expected to be low in the surroundings.
A.8.6.4. Reasoning for the precision of the judgement describing the remaining uncertainties (1st and 3rd quartile/interquartile range)
The Panel assumes a high uncertainty in the first quartile, and a medium uncertainty above the median, because the pest mainly attacks very declining trees or recent dead ones not expected to be present within the nursery, and because a low pest pressure in the surroundings is expected.
A.8.6.5. Elicitation outcomes of the assessment of the pest freedom for Trinophylum cribratum on plants in pots up to 15 years old
The following Tables show the elicited and fitted values for pest infestation (Table A.45) and pest freedom (Table A.46).
Table A.45.
Elicited and fitted values of the uncertainty distribution of pest infestation by Trinophylum cribratum per 10,000 plants
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elicited values | 0 | 1 | 3 | 5 | 9 | ||||||||||
| EKE | 0.0119 | 0.0412 | 0.106 | 0.271 | 0.547 | 0.958 | 1.43 | 2.59 | 4.05 | 4.92 | 5.95 | 6.95 | 7.90 | 8.51 | 9.00 |
The EKE results is the BetaGeneral (0.73889, 1.5253, 0, 9.6) distribution fitted with @Risk version 7.6.
Table A.46.
The uncertainty distribution of plants free of Trinophylum cribratum per 10,000 plants calculated by Table A.45
| Percentile | 1% | 2.5% | 5% | 10% | 17% | 25% | 33% | 50% | 67% | 75% | 83% | 90% | 95% | 97.5% | 99% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Values | 9,991 | 9,995 | 9,998 | 9,999 | 10,000 | ||||||||||
| EKE results | 9,991.0 | 9,991.5 | 9,992.1 | 9,993.0 | 9,994.0 | 9,995.1 | 9,996.0 | 9,997.4 | 9,998.6 | 9,999.04 | 9,999.45 | 9,999.73 | 9,999.89 | 9,999.96 | 9,999.99 |
The EKE results are the fitted values.
Based on the numbers of estimated infested plants the pest freedom was calculated (i.e. = 10,000 – number of infested plants per 10,000). The fitted values of the uncertainty distribution of the pest freedom are shown in Table A.46.
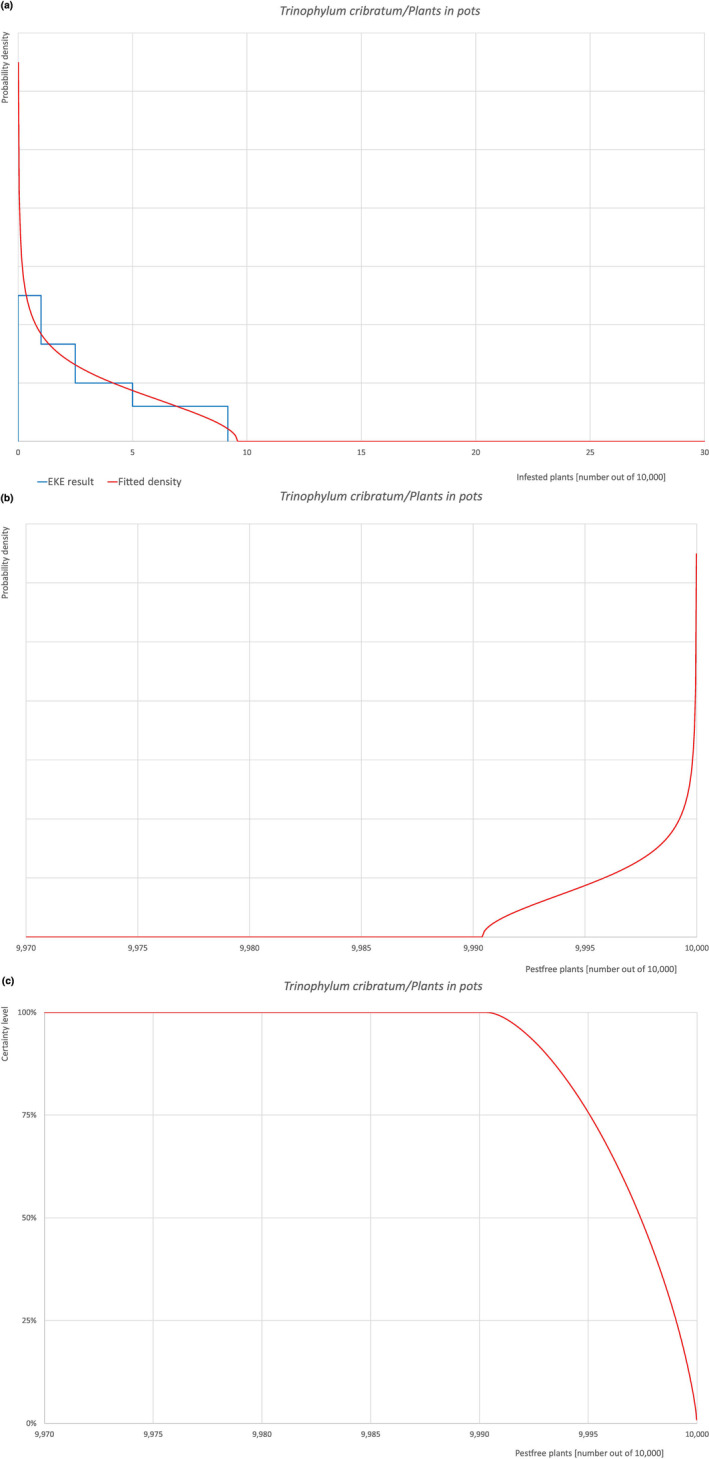
Figure A.23: (a) Elicited uncertainty of pest infestation per 10,000 plants (histogram in blue – vertical blue line indicates the elicited percentile in the following order: 1%, 25%, 50%, 75%, 99%) and distributional fit (red line); (b) uncertainty of the proportion of pest‐free plants per 10,000 (i.e. = 1 – pest infestation proportion expressed as percentage); (c) descending uncertainty distribution function of pest infestation per 10,000 plants
A.8.7. Reference list
de Jong Y, Verbeek M, Michelsen V, de Place Bjørn P, Los W, Steeman F, Bailly N, Basire C, Chylarecki P, Stloukal E, Hagedorn G, Wetzel FT, Glöckler F, Kroupa A, Korb G, Hoffmann A, Häuser C, Kohlbecker A, Müller A, Güntsch A, Stoev P, and Penev L, online. Fauna Europaea ‐ all European animal species on the web. Biodiversity Data Journal. Available online: https://fauna-eu.org/ [Accessed: 31 December 2022].
Dunn E, Hough‐Goldstein J, Hanks L, Millar J and D'Amico V, 2016. Range of attraction of pheromone lures and dispersal behaviour of cerambycid beetles. Annals of the Entomological Society of America, 1–9. https://doi.org/10.1093/aesa/saw055
EUROPHYT (European Union Notification System for Plant Health Interceptions), online. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en [Accessed: 22 December 2022].
Gahan CJ, 1906. Coleoptera – vol. I. Cerambycidae. In: Bingham CT (ed.), The Fauna of British India including Ceylon and Burma. Taylor and Francis, London, 347 pp.
GBIF (Global Biodiversity Information Facility) Secretariat, online. GBIF BackBone Taxonomy. Available online: https://www.gbif.org/ [Accessed: 31 December 2022].
Gilmour EF, 1948. Trinophylum cribratum Bates (Col., Cerambycidae) new to Britain. The Entomologist's Monthly Magazine, 84, 12–16.
Lovric V, 2021. Catch analysis of non‐target entomofauna of beetles (Coleptera) in the pheromone monitoring system of NP Paklenica. University of Zagreb, Faculty of Forestry and Wood Technology, Thesis, 59 pp. (in Croatian).
Pierce WD, 1917. A manual of dangerous insects likely to be introduced in the United States through importations. USDA, 328 pp.
Stebbing EP, 1914. Indian forest insects of economic importance. Coleoptera. Eyre and Spottiswood, London, 804 pp.
TRACES‐NT, online. TRAde Control and Expert System. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
Twinn PGF and Harding PT, 1999. Provisional atlas of the longhorn beetles (Coleoptera, Cerambycidae) of Britain. Institute of Terrestrial Ecology, Biological Record Centre, 100 pp.
Uhthoff‐Kaufmann RR, 1990. The distribution of the genera Trinophylum Bates, Gracilia Serv., Aromia Serv., and Hylotrupes Serv. (Col.: Cerambycidae) in the British Isles. Entomologist's Record and Journal of Variation, 102, 267–274.
Appendix B – Web of Science All Databases Search String
1.
In the Table B.1, the search string for Quercus robur used in Web of Science is reported. Totally, 284 papers were retrieved. Titles and abstracts were screened, and 31 pests were added to the list of pests (see Appendix F).
Table B.1.
String for Quercus robur
| Web of Science All databases |
TOPIC: (“Quercus robur” OR “common oak” OR “English oak” OR “pedunculate oak” OR “Quercus accessiva” OR “Quercus accomodata” OR “Quercus acutiloba” OR “Quercus afghanistanensis” OR “Quercus alligata” OR “Quercus amoenifolia” OR “Quercus apula” OR “Quercus arenaria” OR “Quercus argentea” OR “Quercus assimilis” OR “Quercus asturica” OR “Quercus atrosanguinea” OR “Quercus aurea” OR “Quercus australis” OR “Quercus banatica” OR “Quercus batavica” OR “Quercus bavarica” OR “Quercus belgica” OR “Quercus castanoides” OR “Quercus commiserata” OR “Quercus concordia” OR “Quercus croatica” OR “Quercus cunisecta” OR “Quercus cupressoides” OR “Quercus cupulatus” OR “Quercus danubialis” OR “Quercus discredens” OR “Quercus emarginulata” OR “Quercus esthonica” OR “Quercus ettingeri” OR “Quercus femina” OR “Quercus filicifolia” OR “Quercus foemida” OR “Quercus frutetorum” OR “Quercus grecescui” OR “Quercus haerens” OR “Quercus hentzei” OR “Quercus hispanica” OR “Quercus hodginsii” OR “Quercus hohenackeri” OR “Quercus immodica” OR “Quercus implicata” OR “Quercus kunzei” OR “Quercus laciniata” OR “Quercus lanuginosa” OR “Quercus lentula” OR “Quercus longaeva” OR “Quercus longipedunculata” OR “Quercus louettii” OR “Quercus lucorum” OR “Quercus ludens” OR “Quercus lugdunensis” OR “Quercus macroloba” OR “Quercus madritensis” OR “Quercus microcarpa” OR “Quercus montivaga” OR “Quercus natalis” OR “Quercus nescensis” OR “Quercus ochracea” OR “Quercus oelandica” OR “Quercus pectinata” OR “Quercus pedunculata” OR “Quercus petropolitana” OR “Quercus pilosa” OR “Quercus pilosula” OR “Quercus plebeia” OR “Quercus pluriceps” OR “Quercus pseudopeduncula” OR “Quercus pyrenaica” OR “Quercus quaerens” OR “Quercus rossica” OR “Quercus rostanii” OR “Quercus salicifolia” OR “Quercus scandica” OR “Quercus schlosseriana” OR “Quercus scotica” OR “Quercus scythica” OR “Quercus semipinnata” OR “Quercus similata” OR “Quercus stilbophylla “OR “Quercus tanaicensis” OR “Quercus tephrochlamys” OR “Quercus tholeyroniana” OR “Quercus tomentosa” OR “Quercus transiens” OR “Quercus tristis” OR “Quercus urbica” OR “Quercus vallicola” OR “Quercus verecunda” OR “Quercus versatilis” OR “Quercus vialis” OR “Quercus volhynica” OR “Quercus vulgaris” OR “Quercus wolgensis”) AND TOPIC: (pathogen* OR pathogenic bacteria OR fung* OR oomycet* OR myce* OR bacteri* OR virus* OR viroid* OR insect$ OR mite$ OR phytoplasm* OR arthropod* OR nematod* OR disease$ OR infecti* OR damag* OR symptom* OR pest$ OR vector OR hostplant$ OR “host plant$” OR host OR “root lesion$” OR decline$ OR infestation$ OR damage$ OR symptom$ OR dieback* OR “die back*” OR “malaise” OR aphid$ OR curculio OR thrip$ OR cicad$ OR miner$ OR borer$ OR weevil$ OR “plant bug$” OR spittlebug$ OR moth$ OR mealybug$ OR cutworm$ OR pillbug$ OR “root feeder$” OR caterpillar$ OR “foliar feeder$” OR virosis OR viroses OR blight$ OR wilt$ OR wilted OR canker OR scab$ OR rot OR rots OR rotten OR “damping off” OR “damping‐off” OR blister$ OR “smut” OR mould OR mold OR “damping syndrome$” OR mildew OR scald$ OR “root knot” OR “root‐knot” OR rootknot OR cyst$ OR “dagger” OR “plant parasitic” OR “parasitic plant” OR “plant$parasitic” OR “root feeding” OR “root$feeding”) NOT TOPIC: (“winged seeds” OR metabolites OR *tannins OR climate OR “maple syrup” OR syrup OR mycorrhiz* OR “carbon loss” OR pollut* OR weather OR propert* OR probes OR spectr* OR antioxidant$ OR transformation OR RNA OR DNA OR “Secondary plant metabolite$” OR metabol* OR “Phenolic compounds” OR Quality OR Abiotic OR Storage OR Pollen* OR fertil* OR Mulching OR Nutrient* OR Pruning OR drought OR “human virus” OR “animal disease*” OR “plant extracts” OR immunological OR “purified fraction” OR “traditional medicine” OR medicine OR mammal* OR bird* OR “human disease*” OR biomarker$ OR “health education” OR bat$ OR “seedling$ survival” OR “anthropogenic disturbance” OR “cold resistance” OR “salt stress” OR salinity OR “aCER method” OR “adaptive cognitive emotion regulation” OR nitrogen OR hygien* OR “cognitive function$” OR fossil$ OR *toxicity OR Miocene OR postglacial OR “weed control” OR landscape) NOT TOPIC: (“Abdera biflexuosa” OR “Abdera quadrifasciata” OR “Abortiporus biennis” OR “Absidia caerulea” OR “Absidia californica” OR “Absidia cylindrospora” OR “Absidia glauca” OR “Absidia spinosa” OR “Acalles ptinoides” OR “Acalles roboris” OR “Acanthochermes quercus” OR “Acanthococcus aceris” OR “Acanthococcus roboris” OR “Acanthosoma haemorrhoidale” OR “Acaricalus halli” OR “Aceria ilicis” OR “Aceria quercinus” OR “Acleris ferrugana” OR “Acleris literana” OR “Acleris rhombana” OR “Acremonium bacillisporum” OR “Acremonium charticola” OR “Acremonium strictum” OR “Acrobasis repandana” OR “Acrobasis sodalella” OR “Acrobasis tumidana” OR “Acrocercops brongniardella” OR “Acrogenospora sphaerocephala” OR “Acronicta aceris” OR “Acronicta alni” OR “Acronicta auricoma” OR “Acronicta impleta” OR “Acronicta leporina” OR “Acronicta psi” OR “Acronicta tridens” OR “Actinocladium rhodosporum” OR “Aderus oculatus” OR “Aenetus virescens” OR “Aglia tau” OR “Agrilus angustulus” OR “Agrilus biguttatus” OR “Agrilus laticornis” OR “Agrilus pannonicus” OR “Agrilus sulcicollis” OR “Agrilus viridis” OR “Agriopis aurantiaria” OR “Agriopis leucophaearia” OR “Agriopis marginaria” OR “Agrochola helvola” OR “Agrochola litura” OR “Agrochola macilenta” OR “Agrypnus murinus” OR “Alcis jubata” OR “Alcis repandata” OR “Alebra albostriella” OR “Aleimma loeflingiana” OR “Aleurocystidiellum disciforme” OR “Allantus togatus” OR “Allokermes galliformis” OR “Allygus mixtus” OR “Allygus modestus” OR “Alosterna tabacicolor” OR “Alsophila aescularia” OR “Alternaria alternata” OR “Altica oleracea” OR “Altica quercetorum” OR “Alysidium resinae” OR “Amanita excelsa var. spissa” OR “Amanita muscaria” OR “Amanita phalloides” OR “Ampedus balteatus” OR “Ampedus cardinalis” OR “Ampedus cinnabarinus” OR “Ampedus elongantulus” OR “Ampedus nigerrimus” OR “Ampedus nigrinus” OR “Ampedus pomorum” OR “Ampedus praeustus” OR “Ampedus ruficeps” OR “Ampedus rufipennis” OR “Ampedus sanguineus” OR “Ampedus sanguinolentus” OR “Amphiporthe raveneliana” OR “Amphipyra berbera” OR “Amphipyra berbera ssp. svenssoni” OR “Amphipyra pyramidea” OR “Amphisphaeria bufonia” OR “Amphisphaeria fallax” OR “Amphisphaeria multipunctata” OR “Amphitetranychus savenkoae” OR “Amphitetranychus viennensis” OR “Anaglyptus mysticus” OR “Anaplodera sexguttata” OR “Anavirga laxa” OR “Ancylis mitterbacheriana” OR “Andricus albopunctatus” OR “Andricus amenti” OR “Andricus anthracina” OR “Andricus aries” OR “Andricus callidoma” OR “Andricus clemantinae” OR “Andricus corruptrix” OR “Andricus curvator” OR “Andricus fecundator” OR “Andricus foecundatrix” OR “Andricus gemmeus” OR “Andricus glandulae” OR “Andricus grossulariae” OR “Andricus inflator” OR “Andricus kollari” OR “Andricus legitimus” OR “Andricus lignicola” OR “Andricus lignicolus” OR “Andricus lucidus” OR “Andricus malpighii” OR “Andricus nudus” OR “Andricus paradoxus” OR “Andricus quadrilineatus” OR “Andricus quercuscalicis” OR “Andricus quercuscorticis” OR “Andricus quercusradicis” OR “Andricus quercusramuli” OR “Andricus rhizomae” OR “Andricus seminationis” OR “Andricus sieboldi” OR “Andricus solitarius” OR “Andricus testaceipes” OR “Aneurus avenius” OR “Aneurus laevis” OR “Angustimassarina quercicola” OR “Anisandrus dispar” OR “Anisandrus maiche” OR “Anisostomula areola” OR “Anisostomula cookeana” OR “Anisota virginiensis” OR “Anitys rubens” OR “Anobium punctatum” OR “Anoplophora chinensis” OR “Anorthoa munda” OR “Antheraea paphia” OR “Antheraea pernyi” OR “Antheraea polyphemus” OR “Antheraea roylei” OR “Antheraea yamamai” OR “Anthina flammea” OR “Antrodia albida” OR “Apethymus filiformis” OR “Apethymus serotinus” OR “Aphelonyx cerricola” OR “Aphis fabae” OR “Aphrophora alni” OR “Aphthona melancholica” OR “Apiognomonia errabunda” OR “Apiognomonia quercina” OR “Discula umbrinella” OR “Apiognomonia platani” OR “Apiognomonia veneta” OR “Apiosporium quercicola” OR “Apocheima hispidaria” OR “Apoda limacodes” OR “Aporia crataegi” OR “Aposphaeria protea” OR “Apple proliferation group phytoplasmas” OR “Arachnophora fagicola” OR “Aradus corticalis” OR “Aradus depressus” OR “Arboridia ribauti” OR “Archarius pyrrhoceras” OR “Archiearis parthenias” OR “Archips argyrospila” OR “Archips crataegana” OR “Archips crataeganus” OR “Archips podanus” OR “Archips rosana” OR “Archips xylosteana” OR “Archips xylosteanus” OR “Arctornis l‐nigrum” OR “Arge rustica” OR “Argyresthia glaucinella” OR “Argyresthia retinella” OR “Armillaria cepistipes” OR “Armillaria gallica” OR “Armillaria mellea” OR “Armillaria novae‐zelandiae” OR “Armillaria ostoyae” OR “Arnoldiola gemmae” OR “Arnoldiola libera” OR “Arnoldiola quercicola” OR “Arnoldiola quercus” OR “Arthrobotrys superba” OR “Ascochyta quercus” OR “Ascodichaena rugosa” OR “Asemum striatum” OR “Aspergillus versicolor” OR “Asperisporium robur” OR “Cercospora querci var. robur” OR “Aspidiotus nerii” OR “Asterodiaspis bella” OR “Asterodiaspis changbaishanensis” OR “Asterodiaspis minor” OR “Asterodiaspis minus” OR “Asterodiaspis quercicola” OR “Asterodiaspis variolosa” OR “Asteromella quercifolii” OR “Asteroscopus sphinx” OR “Astrosphaeriella applanata” OR “Athelia arachnoidea” OR “Athelicium hallenbergii” OR “Attactagenus plumbeus” OR “Attelabus nitens” OR “Aulacorthum solani” OR “Aureobasidium pullulans” OR “Auricularia auricula‐judae” OR “Auricularia mesenterica” OR “Automeris coresus” OR “Automeris rubrescens” OR “Automeris zephyria” OR “Automeris zozine” OR “Bactrodesmium pusillum” OR “Bactrodesmium submoniliforme” OR “Bactrodesmium traversianum” OR “Barbatosphaeria barbirostris” OR “Beauveria bassiana” OR “Bena bicolorana” OR “Bena prasinana” OR “Bionectria ochroleuca” OR “Biorhiza pallida” OR “Biscogniauxia mediterranea” OR “Nummularia clypeus” OR “Biscogniauxia repanda” OR “Bispora betulina” OR “Biston betularia” OR “Biston strataria” OR “Bitylenchus maximus” OR “Blastobasis lacticolella” OR “Blastobasis phycidella” OR “Boarmia roboraria” OR “Boidinia furfuracea” OR “Boletus edulis” OR “Borkhausenia fuscescens” OR “Bostrichus capucinus” OR “Botryobasidium subcoronatum” OR “Botryohypochnus isabellinus” OR “Botryosphaeria dothidea” OR “Botryosphaeria quercuum” OR “Melanops tulasnei” OR “Botryosphaeria stevensii” OR “Diplodia quercina” OR “Botrytis cinerea” OR “Bourdotigloea multifurcata” OR “Brachionycha sphinx” OR “Brachyalara straminea” OR “Brachyneura quercina” OR “Brachysporiella laxa” OR “Brachysporium bloxami” OR “Brachysporium britannicum” OR “Brachysporium dingleyae” OR “Brachysporium fusiforme” OR “Cryptadelphia fusiformis” OR “Brachysporium nigrum” OR “Brenneria goodwinii” OR “Brenneria roseae subsp. roseae” OR “Brevicellicium olivascens” OR “Bryobia praetiosa” OR “Bryobia rubrioculus” OR “Bucculacus kaweckii” OR “Bucculatrix ulmella” OR “Bulgaria inquinans” OR “Bursaphelenchus mucronatus” OR “Bursaphelenchus xylophilus” OR “Byssomerulius corium” OR “Cabera pusaria” OR “Cacoecimorpha pronubana” OR “Cacumisporium capitulatum” OR “Caliroa annulipes” OR “Caliroa cerasi” OR “Caliroa cinxia” OR “Caliroa varipes” OR “Callidium violaceum” OR “Callimorpha dominula” OR “Callirhytis bella” OR “Callirhytis erythrocephala” OR “Callirhytis glandium” OR “Calliteara pudibunda” OR “Callophrys rubi” OR “Calocera cornea” OR “Calocera glossoides” OR “Calocybe carnea” OR “Caloptilia alchimiella” OR “Caloptilia leucapennella” OR “Caloptilia rhodinella” OR “Caloptilia robustella” OR “Caloptilia sulphurella” OR “Calosphaeria dryina” OR “Calycellina punctata” OR “Calycina citrina” OR “Camarosporium quercus” OR “Cameraria hamadryadella” OR “Campaea margaritata” OR “Camposporium cambrense” OR “Campylomma verbasci” OR “Candidatus Phytoplasma asteris” OR “Candidatus Phytoplasma fraxini” OR “Capitotricha bicolor” OR “Capsodes flavomarginatus” OR “Carcina quercana” OR “Cardiophorus erichsoni” OR “Cardiophorus gramineus” OR “Cardiophorus ruficollis” OR “Carpatolechia decorella” OR “Cassida hemisphaerica” OR “Catephia alchymista” OR “Catocala nymphagoga” OR “Catocala promissa” OR “Catocala sponsa” OR “Caudospora taleola” OR “Diaporthe taleola” OR “Hercospora taleola” OR “Cenococcum graniforme” OR “Ceraceomerulius serpens” OR “Cerambyx cerdo” OR “Cerambyx scopolii” OR “Cerastis leucographa” OR “Ceratocystis fagacearum” OR “Ceratocystis grandicarpa” OR “Ophiostoma grandicarpum” OR “Ceratocystis moniliformis” OR “Ceratocystis variospora” OR “Ceratocystis virescens” OR “Ceratophorum helicosporum” OR “Ceriporia purpurea” OR “Ceriporia reticulata” OR “Ceriporia viridans” OR “Cerylon fagi” OR “Cerylon ferrugineum” OR “Cerylon histeroides” OR “Cetrelia cetrarioides” OR “Chaetomium aureum” OR “Chaetomium cochlioides” OR “Chaetomium globosum” OR “Chaetomium homopilatum” OR “Chaetopsis grisea” OR “Chaetopyrena quercicola” OR “Chaetosphaerella fusca” OR “Chaetosphaeria myriocarpa” OR “Chloridium clavaeforme” OR “Chalara angustata” OR “Chalara breviclavata” OR “Chalastospora gossypii” OR “Chalciporus piperatus” OR “Cheirospora botryospora” OR “Chionaspis salicis” OR “Chloridium botryoideum var. botryoideum” OR “Chloridium lignicola” OR “Chloridium pachytrachelum” OR “Chloridium preussii” OR “Chloridium virescens” OR “Chloridium virescens var. caudigerum” OR “Chloridium virescens var. chlamydosporum” OR “Chloridium virescens var. virescens” OR “Chlorociboria aeruginascens” OR “Chloroclysta miata” OR “Chloroclysta siterata” OR “Choristoneura diversana” OR “Choristoneura hebenstreitella” OR “Chrecidus quercipodus” OR “Chrysomphalus aonidum” OR “Chyliza leptogaster” OR “Ciboria candolleana” OR “Sclerotinia candolleana” OR “Cicadetta montana” OR “Cirrenalia lignicola” OR “Cis pygmaeus” OR “Cladosporium agoseridis” OR “Cladosporium bruhnei” OR “Davidiella allicina” OR “Cladosporium cladosporioides” OR “Cladosporium epiphyllum” OR “Cladosporium fumago” OR “Cladosporium herbarum” OR “Cladosporium licheniphilum” OR “Cladosporium macrocarpum” OR “Clasterosporium atrum” OR “Clathrospora diplospora” OR “Clavaria gibbsiae” OR “Clavulina rugosa” OR “Cleorodes lichenaria” OR “Clepsis rurinana” OR “Clitocybe brunneoceracea” OR “Clitocybe spinulosa” OR “Clonostachys rosea” OR “Clytra quadripunctata” OR “Clytus arietis” OR “Coccomyces coronatus” OR “Coccomyces delta” OR “Coccomyces dentatus” OR “Coccomyces tumidus” OR “Coccus hesperidum” OR “Codinaea britannica” OR “Codinaea fertilis” OR “Codinaea simplex” OR “Coeliodes dryados” OR “Coeliodes erythroleucos” OR “Coeliodes nigritarsis” OR “Coeliodes rana” OR “Coeliodes ruber” OR “Coeliodes transversealbofasciatus” OR “Coleophora anatipennella” OR “Coleophora currucipennella” OR “Coleophora flavipennella” OR “Coleophora ibipennella” OR “Coleophora kuehnella” OR “Coleophora lutipennella” OR “Colletotrichum acutatum” OR “Colletotrichum fioriniae” OR “Collybia dryophila” OR “Colocasia coryli” OR “Colotois pennaria” OR “Colpoma quercinum” OR “Conostroma didymum” OR “Coltricia perennis” OR “Comibaena bajularia” OR “Common oak ringspot‐associated emaravirus” OR “Comstockaspis perniciosa” OR “Coniella quercicola” OR “Coniothecium quercinum” OR “Coniothyrium carteri” OR “Coniothyrium microscopicum” OR “Coniothyrium quercinum” OR “Conistra erythrocephala” OR “Conistra ligula” OR “Conistra rubiginea” OR “Conistra vaccinii” OR “Conopalpus testaceus” OR “Contarinia quercina” OR “Copaxa mannana” OR “Coprinopsis atramentaria” OR “Coprinus comatus” OR “Cordana pauciseptata” OR “Corticium odoratum” OR “Cortinarius flexipes” OR “Cortinarius saniosus” OR “Corynesporopsis quercicola” OR “Coryneum kunzei” OR “Coryneum neesii” OR “Coryneum umbonatum” OR “Corythucha arcuata” OR “Cosmia pyralina” OR “Cosmia trapezina” OR “Cossonus parallelepipedus” OR “Cossus cossus” OR “Crassa unitella” OR “Craterellus cornucopioides” OR “Craterellus tubaeformis” OR “Crepidotus chimonphilus” OR “Criconema annuliferum” OR “Criconemoides pleriannulatus” OR “Crocallis elinguaria” OR “Cronartium quercuum” OR “Uredo quercus” OR “Crossonema menzeli” OR “Cryphonectria parasitica” OR “Endothia parasitica” OR “Cryptadelphia obovata” OR “Cryptadelphia polyseptata” OR “Cryptarcha nitidissima” OR “Cryptoblabes bistriga” OR “Cryptocephalus bipunctatus” OR “Cryptocephalus parvulus” OR “Cryptocephalus punctiger” OR “Cryptocephalus pusillus” OR “Cryptocephalus querceti” OR “Cryptocephalus sexpunctatus” OR “Cryptocoryneum condensatum” OR “Cryptosporiopsis melanigena” OR “Curculio elephas” OR “Curculio glandium” OR “Curculio pyrrhoceras” OR “Curculio venosus” OR “Curculio villosus” OR “Cyanosporus caesius” OR “Spongiporus caesius” OR “Cybosia mesomella” OR “Cyclocybe parasitica” OR “Cyclophora linearia” OR “Cyclophora porata” OR “Cyclophora punctaria” OR “Cyclophora puppillaria” OR “Cydia fagiglandana” OR “Cydia splendana” OR “Cylindrium aeruginosum” OR “Cylindrium clandestinum” OR “Cylindrocladiella parva” OR “Cylindrosporium kelloggii” OR “Cyllecoris histrionicus” OR “Cymatophorima diluta” OR “Cynips agama” OR “Cynips disticha” OR “Cynips divisa” OR “Cynips longiventris” OR “Cynips quercusfolii” OR “Cystopezizella cupulincola” OR “Cystotheca lanestris” OR “Cytospora chrysosperma” OR “Cytospora intermedia” OR “Cytospora quercus” OR “Cytospora sacculus” OR “Dacrymyces minor” OR “Dacrymyces stillatus” OR “Daedalea quercina” OR “Daldinia childiae” OR “Daldinia concentrica” OR “Daldinia decipiens” OR “Daldinia pyrenaica” OR “Daldinia vernicosa” OR “Dasineura dryophila” OR “Dasineura libera” OR “Dasineura pallasi” OR “Dasineura panteli” OR “Dasineura squamosa” OR “Dasycera oliviella” OR “Dasychira georgiana” OR “Dasytes aeratus” OR “Datana ministra” OR “Deileptenia ribeata” OR “Amphiporthe leiphaemia” OR “Dendrostoma leiphaemia” OR “Diaporthe leiphaemia” OR “Discula quercina” OR “Fusicoccum quercinum” OR “Dendrothele commixta” OR “Dendrothele tetracornis” OR “Denticollis linearis” OR “Deporaus betulae” OR “Armillaria tabescens” OR “Desarmillaria tabescens” OR “Diadegma anurum” OR “Diaporthe foeniculacea” OR “Diaporthe helianthi” OR “Diaporthe insularis” OR “Diaporthe leucospermi” OR “Diaporthe padi var. patria” OR “Diaporthe rudis” OR “Diaspidiotus alni” OR “Diaspidiotus osborni” OR “Diaspidiotus ostreaeformis” OR “Diaspidiotus wuenni” OR “Diaspidiotus zonatus” OR “Diatrype flavovirens” OR “Diatrype stigma” OR “Diatrypella aspera” OR “Diatrypella pulvinata” OR “Diatrypella quercina” OR “Actinopelte dryina” OR “Dicarpella dryina” OR “Tubakia dryina” OR “Tubakia suttoniana” OR “Dichomera saubinetii” OR “Dichomitus campestris” OR “Dichonia aprilina” OR “Dichostereum rhodosporum” OR “Dictyochaeta querna” OR “Dicycla oo” OR “Didymella nigricans” OR “Digitodesmium elegans” OR “Dinoptera collaris” OR “Diphyllaphis mordvilkoi” OR “Diplodia corticola” OR “Diplodia quercus” OR “Diplodia seriata” OR “Dirphiopsis trisignata” OR “Discosia artocreas” OR “Dissoleucas niveirostris” OR “Ditiola peziziformis” OR “Ditula angustiorana” OR “Diurnea fagella” OR “Diurnea lipsiella” OR “Diurnea phryganella” OR “Dorcatoma chrysomelina” OR “Dorcatoma flavicornis” OR “Dorytomus rubrirostris” OR “Dothidea noxia” OR “Dothiorella quercina” OR “Drepana binaria” OR “Drepana falcataria” OR “Drepanothrips reuteri” OR “Drymonia dodonaea” OR “Drymonia ruficornis” OR “Dryobotodes eremita” OR “Dryocoetinus villosus” OR “Dryocyba carri” OR “Dryophilocoris flavoquadrimaculatus” OR “Dryophthorus corticalis” OR “Dwayaangam cornuta” OR “Dyseriocrania subpurpurella” OR “Dystebenna stephensi” OR “Eacles imperialis” OR “Eacles oslari” OR “Ectoedemia albifasciella” OR “Ectoedemia atrifrontella” OR “Ectoedemia heckfordi” OR “Ectoedemia heringi” OR “Ectoedemia longicaudella” OR “Ectoedemia quinquella” OR “Ectoedemia subbimaculella” OR “Ectropis consonaria” OR “Ectropis crepuscularia” OR “Edwardsiana diversa” OR “Edwardsiana flavescens” OR “Edwardsiana frustrator” OR “Edwardsiana plebeja” OR “Edwardsiana rosae” OR “Eichleriella subleucophaea” OR “Elasmostethus interstinctus” OR “Elasmucha grisea” OR “Elateroides dermestoides” OR “Electrophaes corylata” OR “Elegia similella” OR “Elongisporangium anandrum” OR “Enargia paleacea” OR “Enchnoa infernalis” OR “Endophragmia glanduliformis” OR “Endophragmia hyalosperma” OR “Endophragmiella corticola” OR “Endophragmiella fallacia” OR “Endophragmiella ovoidea” OR “Endophragmiella pallescens” OR “Endothia fluens” OR “Endothia gyrosa” OR “Endotricha flammealis” OR “Ennomos alniaria” OR “Ennomos autumnaria” OR “Ennomos erosaria” OR “Ennomos quercinaria” OR “Eotetranychus carpini” OR “Eotetranychus pruni” OR “Eotetranychus tiliarium” OR “Eotetranychus uncatus” OR “Epagoge grotiana” OR “Epicoccum italicum” OR “Epicoccum nigrum” OR “Epione repandaria” OR “Epirrita autumnata” OR “Epirrita christyi” OR “Epirrita dilutata” OR “Epitrimerus cristatus” OR “Epuraea guttata” OR “Erannis defoliaria” OR “Eremotes ater” OR “Eriocrania subpurpurella” OR “Eriogaster lanestris” OR “Eriophyes ilicis” OR “Eriophyes quercinus” OR “Erwinia billingiae” OR “Erysiphe alphitoides” OR “Microsphaera alphitoides” OR “Microsphaera quercina” OR “Oidium quercinum” OR “Erysiphe communis” OR “Erysiphe hypophylla” OR “Microsphaera hypophylla” OR “Erysiphe japonica” OR “Erysiphe penicillata” OR “Microsphaera penicillata” OR “Erysiphe quercicola” OR “Eriotremex formosanus” OR “Esperia sulphurella” OR “Etheirodon fimbriatus” OR “Eudemis porphyrana” OR “Eudemis profundana” OR “Euepixylon udum” OR “Eulecanium cerasorum” OR “Eulecanium ciliatum” OR “Eulecanium douglasi” OR “Eulecanium tiliae” OR “Euophryum confine” OR “Eupeodes lapponicus” OR “Eupithecia abbreviata” OR “Eupithecia dodoneata” OR “Eupithecia exiguata” OR “Eupithecia irriguata” OR “Eupithecia orphnata” OR “Eupithecia virgaureata” OR “Euplexia lucipara” OR “Euproctis chrysorrhoea” OR “Euproctis similis” OR “Eupsilia transversa” OR “Eupterycyba jucunda” OR “Eurhadina concinna” OR “Eurhadina kirschbaumi” OR “Eurhadina pulchella” OR “Eurhadina ribauti” OR “Eutypa lata” OR “Eutypella cerviculata” OR “Euwallacea fornicatus” OR “Euwallacea fornicatus sensu lato” OR “Euwallacea fornicatus sensu stricto” OR “Exidia glandulosa” OR “Exidia truncata” OR “Exidiopsis novae‐zelandiae” OR “Exochalara longissima” OR “Fagocyba carri” OR “Fagocyba cruenta” OR “Favolaschia calocera” OR “Favonius quercus” OR “Fenestella phaeospora” OR “Fistulina hepatica” OR “Foaiella danesii” OR “Fomes annosus” OR “Fomes connatus” OR “Fomes fomentarius” OR “Fomitiporia mediterranea” OR “Fomitiporia robusta” OR “Phellinus robustus” OR “Fomitopsis pinicola” OR “Fusarium culmorum” OR “Fusarium lateritium” OR “Gibberella baccata” OR “Fusarium oxysporum” OR “Fusarium sambucinum” OR “Fusarium solani” OR “Haematonectria haematococca” OR “Neocosmospora solani” OR “Fuscoporia ferrea” OR “Fuscoporia ferruginosa” OR “Fuscoporia wahlbergii” OR “Dothiorella advena” OR “Fusicoccum advenum” OR “Fusicoccum quercus” OR “Fusidium griseum” OR “Elfvingia applanata” OR “Ganoderma applanatum” OR “Ganoderma australe” OR “Ganoderma resinaceum” OR “Garnaudia elegans” OR “Gasterocercus depressirostris” OR “Gastrallus immarginatus” OR “Geosmithia langdonii” OR “Geotrichum candidum” OR “Geotrichum clavatum” OR “Gibbsiella quercinecans” OR “Globisporangium debaryanum” OR “Gloeocystidiellum porosum” OR “Gloeosporidina moravica” OR “Gloniopsis curvata” OR “Gloniopsis praelonga” OR “Gnomonia setacea” OR “Gnomoniella fasciculata” OR “Gnorimus variabilis” OR “Gonimbrasia tyrrhea” OR “Gonioctena decemnotata” OR “Gonocerus acuteangulatus” OR “Gonodera luperus” OR “Gonytrichum caesium” OR “Gonytrichum caesium var. chloridioides” OR “Gonytrichum caesium var. subglobosum” OR “Gonytrichum chlamydosporium var. chlamydosporium” OR “Gonytrichum chlamydosporium var. simile” OR “Gracilia minuta” OR “Grammoptera abdominalis” OR “Grammoptera ruficornis” OR “Grammoptera ustulata” OR “Graphiphora augur” OR “Graphium penicillioides” OR “Grifola frondosa” OR “Griposia aprilina” OR “Grosmannia olivacea” OR “Guignardia cookeana” OR “Guignardia punctoidea” OR “Gymnoascus reessii” OR “Gymnopilus junonius” OR “Collybia fusipes” OR “Gymnopus fusipes” OR “Gynaephora selenitica” OR “Gynanisa maja” OR “Gypsonoma dealbana” OR “Gyrothrix citricola” OR “Hadrobregmus denticollis” OR “Hainesia lythri” OR “Halyomorpha halys” OR “Hapalopilus croceus” OR “Hapalopilus rutilans” OR “Haplographium catenatum” OR “Harpiphorus lepidus” OR “Harpocera thoracica” OR “Hebeloma cavipes” OR “Hebeloma crustuliniforme” OR “Hebeloma mesophaeum” OR “Hebeloma sacchariolens” OR “Hebeloma velutipes” OR “Helicogloea aquilonia” OR “Helicosporium pallidum” OR “Helicosporium vegetum” OR “Helicotylenchus digonicus” OR “Helicotylenchus paxilli” OR “Helicotylenchus pseudorobustus” OR “Heliococcus bohemicus” OR “Heliozela sericiella” OR “Helminthosporium macrocarpum” OR “Helminthosporium quercinum” OR “Hemicrepidius hirtus” OR “Hemileuca grotei” OR “Hemileuca maia” OR “Hemileuca slosseri” OR “Hemithea aestivaria” OR “Hericium coralloides” OR “Herminia grisealis” OR “Heterobasidion annosum” OR “Heterogenea asella” OR “Heterosporium proteus” OR “Holocerina smilax” OR “Hoplocallis microsiphon” OR “Hoplocallis picta” OR “Hoplocallis ruperti” OR “Hoplochaetaphis zachvatkini” OR “Hoplotylus femina” OR “Hormiactis candida” OR “Humicola grisea” OR “Hylesinus crenatus” OR “Hylobius abietis” OR “Hymenochaete cinnamomea” OR “Hymenochaete rubiginosa” OR “Hymenoscyphus fructigenus” OR “Hyphoderma cremeoalbum” OR “Hyphoderma macedonicum” OR “Hyphoderma praetermissum” OR “Hyphoderma setigerum” OR “Hyphodontia alutaria” OR “Hyphodontia nespori” OR “Hyphodontia quercina” OR “Hyphodontia sambuci” OR “Hypholoma fasciculare” OR “Hypochnicium punctulatum” OR “Hypocrea aureoviridis” OR “Hypocrea gelatinosa” OR “Hypocrea lixii” OR “Hypocrea sinuosa” OR “Hypocrea strictipilosa” OR “Hypomecis punctinalis” OR “Hypomecis roboraria” OR “Hypomyces rosellus” OR “Hyponectria cookeana” OR “Hypospilina pustula” OR “Hypoxylon bipapillatum” OR “Hypoxylon fragiforme” OR “Hypoxylon howeanum” OR “Hypoxylon porphyreum” OR “Hypulus quercinus” OR “Hysterium pulicare” OR “Hysterium vulgare” OR “Hysterobrevium curvatum” OR “Hysterographium mori” OR “Iassus lanio” OR “Icerya purchasi” OR “Ilyonectria robusta” OR “Imbrasia alcinoe” OR “Incrucipulum capitatum” OR “Incurvaria masculella” OR “Inocybe bongardii” OR “Inocybe geophila” OR “Inocybe sindonia” OR “Inonotus hispidus” OR “Polyporus hispidus” OR “Involvulus caeruleus” OR “Ischnodes sanguinicollis” OR “Ischnomera caerulea” OR “Ischnomera sanguinicollis” OR “Issus coleoptratus” OR “Issus muscaeformis” OR “Endophragmia verruculosa” OR “Ityorhoptrum verruculosum” OR “Janus cynobati” OR “Jodia croceago” OR “Jodis lactearia” OR “Judolia cerambyciformis” OR “Kermes cordiformis” OR “Kermes quercus” OR “Kermes roboris” OR “Kermes williamsi” OR “Kirschsteiniothelia aethiops” OR “Korynetes caeruleus” OR “Kretzschmaria deusta” OR “Kuwania rubra” OR “Lacanobia contigua” OR “Lacanobia thalassina” OR “Laccaria echinospora” OR “Laccaria laccata var. pallidifolia” OR “Laccaria tetraspora f. major” OR “Lachnus acutihirsutus” OR “Lachnus crassicornis” OR “Lachnus longirostris” OR “Lachnus pallipes” OR “Lachnus roboris” OR “Lacon querceus” OR “Laeticorticium roseum” OR “Laetiporus sulphureus” OR “Polyporus sulphureus” OR “Lampronia oehlmaniella” OR “Lamprotettix nitidulus” OR “Laothoe populi” OR “Lasiorhynchites cavifrons” OR “Lasiorhynchites olivaceus” OR “Lasiosphaeria capitata” OR “Lasiosphaeria caudata” OR “Lateriramulosa uni” OR “Ledomyia lugens” OR “Ledra aurita” OR “Leiopus nebulosus” OR “Lenzites betulina” OR “Lepidosaphes malicola” OR “Lepidosaphes ulmi” OR “Lepraria lobificans” OR “Leptodontium elatius” OR “Leptographium flavum” OR “Leptographium tardum” OR “Leptographium vulnerum” OR “Leptosphaeria alcides f. quercina” OR “Leptothyrium botryoides” OR “Leptura aurulenta” OR “Leptura scutellata” OR “Leratiomyces erythrocephalus” OR “Lestodiplosis quercina” OR “Lestodiplosis roburella” OR “Limoniscus violaceus” OR “Lindbergina aurovittata” OR “Lithophane ornithopus” OR “Lithophane socia” OR “Lobesia reliquana” OR “Lomographa cararia” OR “Longidorus cylindricaudatus” OR “Longidorus elongatus” OR “Longidorus intermedius” OR “Longidorus juglandicola” OR “Longidorus macrosoma” OR “Longidorus poessneckensis” OR “Longidorus uroshis” OR “Lonsdalea britannica” OR “Lophocampa catenulata” OR “Lophodermium petiolicola” OR “Lophodermium punctiforme” OR “Lucanus cervus” OR “Luperus flavipes” OR “Luperus longicornis” OR “Lycia hirtaria” OR “Lycia pomonaria” OR “Lyctus brunneus” OR “Lyctus linearis” OR “Lymantor coryli” OR “Lymantria dispar” OR “Lymantria monacha” OR “Lymexylon navale” OR “Macrodiplosis dryobia” OR “Macrodiplosis pustulans” OR “Macrodiplosis pustularis” OR “Macrodiplosis roboris” OR “Macrodiplosis volvens” OR “Macrolabis quercicola” OR “Macrophoma nitens” OR “Macrosiphum euphorbiae” OR “Macrosporium commune” OR “Magdalis cerasi” OR “Malacosoma neustria” OR “Malacosoma parallela” OR “Malthinus frontalis” OR “Marssonia matteiana” OR “Marssonina martinii” OR “Marsupiomyces epidermoidea” OR “Megacoelum infusum” OR “Megalopyge chrysocoma” OR “Megalopyge lanata” OR “Meganola strigula” OR “Megapenthes lugens” OR “Megaplatypus mutatus” OR “Megathrips lativentris” OR “Melanaspis obscura” OR “Melandrya caraboides” OR “Melangyna cincta” OR “Melanophila acuminata” OR “Melasis buprestoides” OR “Meliniomyces bicolor” OR “Meliniomyces variabilis” OR “Meloidogyne ardenensis” OR “Meloidogyne chitwoodi” OR “Meloidogyne hapla” OR “Meloidogyne mali” OR “Melolontha hippocastani” OR “Melolontha melolontha” OR “Chaetosphaeria pulviscula” OR “Melanomma pulvis” OR “Menispora caesia” OR “Menispora ciliata” OR “Menispora glauca” OR “Meripilus giganteus” OR “Mesites tardii” OR “Mesocriconema curvatum” OR “Mesocriconema solivagum” OR “Mesocriconema xenoplax” OR “Mesoneura opaca” OR “Mesosa nebulosa” OR “Metriotes lutarea” OR “Microcyclospora quercina” OR “Microdiplodia iliceti” OR “Microsphaera alni var. extensa” OR “Microsphaera alphitoides var. alphitoides” OR “Microsphaera extensa var. extensa” OR “Microstroma album” OR “Microstroma quercinum” OR “Microthelia incrustans” OR “Microthyrium microscopicum” OR “Minucia lunaris” OR “Mirandina corticola” OR “Mollisia sericeomarginata” OR “Moma alpium” OR “Hyaloceras monochaetum var. gallicola” OR “Monochaetia monochaeta” OR “Monochaetia monochaeta var. gallicola” OR “Monocillium mucidum” OR “Monodictys castaneae” OR “Monodictys lepraria” OR “Monodictys paradoxa” OR “Monodiplosis liebeli” OR “Monostichella moravica” OR “Moristroma quercinum” OR “Moritziella corticalis” OR “Mortierella alpina” OR “Mortierella exigua” OR “Mortierella fatshederae” OR “Mortierella gamsii” OR “Mortierella humilis” OR “Mortierella hyalina” OR “Mortierella macrocystis” OR “Mortierella parvispora” OR “Mortierella turficola” OR “Mortierella verticillata” OR “Mortierella zonata” OR “Mucor abundans” OR “Mucor hiemalis” OR “Mucor plumbeus” OR “Mucor racemosus” OR “Mycelium radicis” OR “Mycena corticola” OR “Mycena inclinata” OR “Mycena parsonsii” OR “Mycetochara humeralis” OR “Mycetophagus piceus” OR “Mycoacia uda” OR “Mycocalicium subtile” OR “Mycosphaerella maculiformis” OR “Mycosphaerella punctiformis” OR “Phyllosticta maculiformis” OR “Ramularia endophylla” OR “Sphaerella punctiformis” OR “Mycosphaerella septorispora” OR “Mycosphaerelloides madeirae” OR “Mythimna turca” OR “Myxosporium roumeguerei” OR “Myzocallis bella” OR “Myzocallis boerneri” OR “Myzocallis castanicola” OR “Myzocallis komareki” OR “Myzocallis schreiberi” OR “Myzocallis taurica” OR “Myzocallis walshii” OR “Nacerdes melanura” OR “Nacophora quernaria” OR “Naemospora croceola” OR “Nathrius brevipennis” OR “Naucoria salicis” OR “Nectria cinnabarina” OR “Nectria grammicospora” OR “Nectria inventa” OR “Nectria peziza” OR “Nemania diffusa” OR “Nemania serpens” OR “Nemania serpens var. serpens” OR “Neocladophialophora quercina” OR “Neocoenorrhinus germanicus” OR “Neocoenorrhinus interpunctatus” OR “Neocoenorrhinus minutus” OR “Neocoenorrhinus pauxillus” OR “Neocosmospora euwallaceae” OR “Neocosmospora ipomoeae” OR “Neocucurbitaria quercina” OR “Pyrenochaeta quercina” OR “Neofusicoccum australe” OR “Neofusicoccum luteum” OR “Botryosphaeria parva” OR “Neofusicoccum parvum” OR “Neolamproconium silvestre” OR “Neolygus viridis” OR “Nectria ditissima” OR “Nectria galligena” OR “Neonectria ditissima” OR “Nephopterix tumidella” OR “Neuroterus albipes” OR “Neuroterus albipes albipes” OR “Neuroterus anthracinus” OR “Neuroterus aprilinus” OR “Neuroterus numismalis” OR “Neuroterus politus” OR “Neuroterus quercusbaccarum” OR “Neuroterus saliens” OR “Neuroterus tricolor” OR “Neurotoma mandibularis” OR “Noctua fimbriata” OR “Nola confusalis” OR “Notodonta dromedarius” OR “Nummularia succenturiata” OR “Nycteola degenerana” OR “Nycteola revayana” OR “Obolarina dryophila” OR “Obrium cantharinum” OR “Ochropacha duplaris” OR “Ocneria prolai” OR “Odinia maculata” OR “Odontopera bidentata” OR “Oecophora bractella” OR “Oedocephalum glomerulosum” OR “Oemona hirta” OR “Oidium dubium” OR “Oligonychus bicolor” OR “Oligonychus brevipodus” OR “Oligonychus buschi” OR “Oligonychus coffeae” OR “Oligonychus longiclavatus” OR “Oligonychus pritchardi” OR “Oligonychus propetes” OR “Oncopodiella cubispora” OR “Oncopodiella felis” OR “Operophtera brumata” OR “Ophiostoma minus” OR “Ophiostoma novo” OR “Ophiostoma kubanicum” OR “Ophiostoma piceae” OR “Ophiostoma quercus” OR “Ophiostoma roboris” OR “Ophiostoma valachicum” OR “Ophiostoma pluriannulatum” OR “Ophiostoma pseudokarelicum” OR “Ophiostoma querci” OR “Ophiostoma solheimii” OR “Ophiostoma sparsiannulatum” OR “Ophiostoma villosum” OR “Opilio mollis” OR “Orchestes fagi” OR “Orchestes hortorum” OR “Orchestes pilosus” OR “Orchestes quercus” OR “Orgyia antiqua” OR “Orgyia recens” OR “Orientus ishidae” OR “Orsodacne cerasi” OR “Orthosia cerasi” OR “Orthosia cruda” OR “Orthosia gothica” OR “Orthosia incerta” OR “Orthosia miniosa” OR “Orthosia munda” OR “Orthotylus nassatus” OR “Orthotylus prasinus” OR “Orthotylus tenellus” OR “Otiorhynchus auropunctatus” OR “Otiorhynchus rugosotriatus” OR “Otiorhynchus singularis” OR “Otiorhynchus sulcatus” OR “Ourapteryx sambucaria” OR “Oxythrips quercicola” OR “Pammene albuginana” OR “Pammene argyrana” OR “Pammene fasciana” OR “Pammene germmana” OR “Pammene giganteana” OR “Pammene splendidulana” OR “Pamphilius sylvarum” OR “Pamphilius varius” OR “Pandemis cerasana” OR “Pandemis corylana” OR “Panonychus ulmi” OR “Pantoea agglomerans” OR “Pantoea cedenensis” OR “Pappia fissilis” OR “Paracolax tristalis” OR “Paradarisa consonaria” OR “Paradarisa extersaria” OR “Paralongidorus maximus” OR “Paralongidorus milanis” OR “Paraphaeosphaeria neglecta” OR “Pararoussoella quercina” OR “Parasola kuehneri” OR “Paratrichodorus pachydermus” OR “Paratrichodorus tunisiensis” OR “Paratylenchus projectus” OR “Paratylenchus straeleni” OR “Parauncinula septata” OR “Parectropis similaria” OR “Parthenolecanium corni” OR “Parthenolecanium rufulum” OR “Paxillus ammoniavirescens” OR “Paxillus involutus” OR “Pealius quercus” OR “Pechipogo strigilata” OR “Penicillium aurantiogriseum” OR “Penicillium citreonigrum” OR “Penicillium citrinum” OR “Penicillium daleae” OR “Penicillium frequentans” OR “Penicillium herqueri” OR “Penicillium janczewskii” OR “Penicillium luteum” OR “Penicillium minioluteum” OR “Penicillium purpurogenum” OR “Penicillium purpurogenum var. rubri” OR “Penicillium spinulosum” OR “Peniophora cinerea” OR “Peniophora limitata” OR “Peniophora pseudoversicolor” OR “Peniophora quercina” OR “Peniophora rufomarginata” OR “Pentarthrum huttoni” OR “Pentatoma rufipes” OR “Perenniporia fraxinea” OR “Perenniporia japonica” OR “Peribatodes ilicaria” OR “Periclista albida” OR “Periclista lineolata” OR “Periclista pubescens” OR “Periconia cambrensis” OR “Periconia digitata” OR “Peridea anceps” OR “Perisomena caecigena” OR “Pestalotiopsis biciliata” OR “Pestalotiopsis monochaeta” OR “Pezicula alba” OR “Cryptosporiopsis quercina” OR “Pezicula cinnamomea” OR “Cryptosporiopsis radicicola” OR “Pezicula radicicola” OR “Pezicula sporulosa” OR “Phaeoacremonium inflatipes” OR “Phaeoacremonium rubrigenum” OR “Phaeoacremonium viticola” OR “Phaeobotryon quercicola” OR “Phaeostalagmus cyclosporus” OR “Phalera bucephala” OR “Phanerochaete martelliana” OR “Phanerochaete sordida” OR “Phanerochaete velutina” OR “Phellinus ferruginosus” OR “Fomes igniarius” OR “Phellinus igniarius” OR “Phenacoccus aceris” OR “Phialea sydowiana” OR “Phialocephala dimorphospora” OR “Phigalia pilosaria” OR “Phlebia albomellea” OR “Phlebia livida” OR “Phlebia radiata” OR “Phlebia rufa” OR “Phlebiopsis crassa” OR “Phlebiopsis ravenelii” OR “Phloeophagus lignarius” OR “Phlogophora meticulosa” OR “Phloiophilus edwardsi” OR “Phloiotrya vaudoueri” OR “Phobetron hipparchia” OR “Phoma carteri” OR “Querciphoma carteri” OR “Phoma cava” OR “Phoma innumerabilis” OR “Phoma pomorum var. pomorum” OR “Phomopsis glandicola” OR “Phomopsis quercella” OR “Phomopsis quercina” OR “Phragmocephala elliptica” OR “Phycita roborella” OR “Phylacteophaga froggatti” OR “Microsphaera alni” OR “Phyllactinia alnicola” OR “Phyllactinia corylea” OR “Phyllactinia guttata” OR “Phyllactinia suffulta” OR “Phyllactinia roboris” OR “Phyllobius argentatus” OR “Phyllobius calcaratus” OR “Phyllobius glaucus” OR “Phyllobius maculicornis” OR “Phyllobius oblongus” OR “Phyllobius pyri” OR “Phyllobius roboretanus” OR “Phyllobius viridiaeris” OR “Phyllocoptes roboris” OR “Phyllodiplosis cocciferae” OR “Phyllonorycter delitella” OR “Phyllonorycter distentella” OR “Phyllonorycter hamadryadella” OR “Phyllonorycter harrisella” OR “Phyllonorycter heegeriella” OR “Phyllonorycter hortella” OR “Phyllonorycter ilicifoliella” OR “Phyllonorycter kuhlweiniella” OR “Phyllonorycter lautella” OR “Phyllonorycter messaniella” OR “Phyllonorycter muelleriella” OR “Phyllonorycter parisiella” OR “Phyllonorycter quercifoliella” OR “Phyllonorycter roboris” OR “Phyllosticta associata” OR “Phyllosticta concentrica” OR “Phyllosticta ilicicola” OR “Phyllosticta ilicina” OR “Phyllosticta quercicola” OR “Phyllosticta quercus” OR “Phylloxera coccinea” OR “Phylloxera confusa” OR “Phylloxera corticalis” OR “Phylloxera foae” OR “Phylloxera glabra” OR “Phylloxera italica” OR “Phylloxera quercus” OR “Phylus melanocephalus” OR “Phymatodes testaceus” OR “Physarum cinereum” OR “Physatocheila dumetorum” OR “Physisporinus lineatus” OR “Phytocoris dimidiatus” OR “Phytocoris reuteri” OR “Phytophthora cactorum” OR “Phytophthora cambivora” OR “Phytophthora cinnamomi” OR “Phytophthora citricola” OR “Phytophthora cryptogea” OR “Phytophthora europaea” OR “Phytophthora gallica” OR “Phytophthora gonapodyides” OR “Phytophthora kernoviae” OR “Phytophthora multivora” OR “Phytophthora plurivora” OR “Phytophthora pseudosyringae” OR “Phytophthora psychrophila” OR “Phytophthora quercina” OR “Phytophthora ramorum” OR “Phytophthora syringae” OR “Phytophthora uliginosa” OR “Pilophorus perplexus” OR “Piptoporus quercinus” OR “Pityohyphantes phrygianus” OR “Placynthiella dasaea” OR “Plagionotus arcuatus” OR “Plagiotrochus australis” OR “Plagiotrochus coriaceus” OR “Plagiotrochus quercusilicis” OR “Plagodis dolabraria” OR “Plagodis pulveraria” OR “Platypus apicalis” OR “Platypus cylindrus” OR “Platypus quercivorus” OR “Platyrhinus resinosus” OR “Platystomos albinus” OR “Plenodomus gallarum” OR “Pleurophragmium rousselianum” OR “Pleurothecium recurvatum” OR “Podalia albescens” OR “Podoxyphium yuccae” OR “Poecilium alni” OR “Poecilocampa populi” OR “Poecilothrips albopictus” OR “Pogonocherus hispidulus” OR “Pogonocherus hispidus” OR “Polia nebulosa” OR “Polydrusus cervinus” OR “Polydrusus flavipes” OR “Polydrusus formosus” OR “Polydrusus marginatus” OR “Polydrusus mollis” OR “Polydrusus pterygomalis” OR “Polydrusus tereticollis” OR “Polygonum aviculare” OR “Polyploca ridens” OR “Polyporus brumalis” OR “Polyporus dryadeus” OR “Polyporus gayanus” OR “Polyporus leptocephalus” OR “Polyporus squamosus” OR “Polyporus tuberaster” OR “Polyporus zonatus” OR “Polyscytalum neofecundissimum” OR “Polystepha malpighii” OR “Polystepha quercus” OR “Porostereum crassum” OR “Porostereum spadiceum” OR “Povolnya leucapennella” OR “Pratylenchus crenatus” OR “Pratylenchus penetrans” OR “Pratylenchus pratensis” OR “Pratylenchus thornei” OR “Prionus coriarius” OR “Prionychus ater” OR “Prionychus melanarius” OR “Pristiphora armata” OR “Procraerus tibialis” OR “Profenusa pygmaea” OR “Proliferodiscus tricolor” OR “Psallus albicinctus” OR “Psallus ambiguus” OR “Psallus confusus” OR “Psallus mollis” OR “Psallus perrisi” OR “Psallus quercus” OR “Psallus variabilis” OR “Psallus varians” OR “Psallus wagneri” OR “Pseudatemelia subochreella” OR “Pseudaulacaspis pentagona” OR “Pseudeparius sepicola” OR “Pseudocistela ceramboides” OR “Pseudococcus viburni” OR “Pseudocraterellus undulatus” OR “Pseudoinonotus dryadeus” OR “Pseudoips fagana” OR “Pseudoips praninana” OR “Pseudoips prasinana” OR “Pseudomonas daroniae” OR “Pseudomonas dryadis” OR “Pseudomonas kirkiae” OR “Pseudomonas syringae pv. syringae” OR “Pseudospiropes obclavatus” OR “Pseudospiropes simplex” OR “Pseudotelphusa paripunctella” OR “Pseudotelphusa scalella” OR “Pseudotrichoconis echinophila” OR “Pseudovalsa longipes” OR “Coryneum depressum” OR “Pseudovalsa umbonata” OR “Psoricoptera gibbosella” OR “Psylliodes cuprea” OR “Psylliodes picina” OR “Ptilinus pectinicornis” OR “Ptilodon capucina” OR “Ptinus palliatus” OR “Ptinus subpilosus” OR “Ptycholoma lecheana” OR “Ptycholoma lecheanum” OR “Pulcherricium caeruleum” OR “Pulvinaria kuwacola” OR “Pulvinaria vitis” OR “Pyrochroa coccinea” OR “Pyrochroa serraticornis” OR “Pyrrhidium sanguineum” OR “Pythium aphanidermatum” OR “Pythium undulatum” OR “Pythium vanterpoolii” OR “Pythium vexans” OR “Quadraspidiotus zonatus” OR “Quercusia quercus” OR “Quernaspis lepineyi” OR “Raffaelea quercivora” OR “Rahnella victoriana” OR “Ramichloridium schulzeri” OR “Ramularia vizellae” OR “Reticularia lycoperdon” OR “Rhabdomiris striatellus” OR “Rhagium bifasciatum” OR “Rhagium inquisitor” OR “Rhagium mordax” OR “Rhinocladiella atrovirens” OR “Rhinocladiella quercus” OR “Rhizochaete filamentosa” OR “Rhogogaster scalaris” OR “Rhopalomesites tardyi” OR “Rhycaphytoptus massalongoianus” OR “Rhyncaphytoptus farkaschi” OR “Rhynchaenus avellanae” OR “Rhynchaenus erythropus” OR “Rhynchaenus pilosus” OR “Rhynchaenus quercus” OR “Rhynchites aeneovirens” OR “Rhynchites cavifrons” OR “Rhynchites germanicus” OR “Rhynchites interpunctatus” OR “Rhynchites sericeus” OR “Rhyncolus lignarius” OR “Rhynophytoptus massalongoianus” OR “Ribautiana debilis” OR “Ribautiana scalaris” OR “Ribautiana tenerrima” OR “Ribautiana ulmi” OR “Mycena austrororida” OR “Roridomyces austrororidus” OR “Rosellinia corticium” OR “Rosellinia desmazieresii” OR “Rosellinia desmazieri” OR “Rosellinia glandiformis” OR “Rosellinia quercina” OR “Rosellinia subsimilis” OR “Rosellinia thelena” OR “Rotylenchus robustus” OR “Rugonectria sinica” OR “Russula amoenolens” OR “Russula ionochlora” OR “Russula sororia” OR “Rutpela maculata” OR “Rutstroemia firma” OR “Saccosoma farinaceum” OR “Saccosoma floccosum” OR “Saperda scalaris” OR “Saturnia lindia” OR “Saturnia pavonia” OR “Schiffermuelleria grandis” OR “Schizophyllum commune” OR “Schizopora paradoxa” OR “Xylodon paradoxus” OR “Schizotetranychus garmani” OR “Scleroderma cepa” OR “Scleroderma verrucosum” OR “Sclerotinia pseudotuberosa” OR “Scolytus intricatus” OR “Scolytus multistriatus” OR “Scolytus rugulosus” OR “Scolytus scolytus” OR “Scutellonema bradys” OR “Sebacina novae” OR “Seimatosporium quercina” OR “Selenia dentaria” OR “Selenia lunularia” OR “Selenia tetralunaria” OR “Septonema binum” OR “Septonema chaetospira” OR “Septonema secedens” OR “Septoria ocellata” OR “Septoria quercicola” OR “Septoria quercina” OR “Septotrullula bacilligera” OR “Serraca punctinalis” OR “Sibine trimacula” OR “Sillia ferruginea” OR “Sistotremastrum niveocremeum” OR “Skeletocutis nivea” OR “Sordaria macrospora” OR “Sorocybe resinae” OR “Spadicoides grovei” OR “Speira cohaerens” OR “Speudotettix subfusculus” OR “Sphaerotheca lanestris” OR “Sphaerulina myriadea” OR “Sphaerulina quercicola” OR “Sphinginus lobatus” OR “Sphinx ligustri” OR “Spilosoma lutea” OR “Spilosoma luteum” OR “Spongipellis spumeus” OR “Sporidesmium adscendens” OR “Sporidesmium coronatum” OR “Sporidesmium folliculatum” OR “Sporoschisma juvenile” OR “Sporoschisma mirabile” OR “Sporothrix aurorae” OR “Sporothrix brunneoviolacea” OR “Sporothrix cryptarchum” OR “Ophiostoma dentifundum” OR “Sporothrix dentifunda” OR “Sporothrix eucastaneae” OR “Sporothrix inflata” OR “Ceratocystis prolifera” OR “Sporothrix prolifera” OR “Sporothrix stenoceras” OR “Sporothrix undulata” OR “Stachybotrys alternans” OR “Stauropus fagi” OR “Steccherinum ochraceum” OR “Stenocorus meridianus” OR “Stenolechia gemmella” OR “Stenoscelis hylastoides” OR “Stenurella melanura” OR “Stereum gausapatum” OR “Stereum hirsutum” OR “Stereum rugosum” OR “Sterocorynes truncorum” OR “Sterrhopterix fusca” OR “Stigmella atricapitella” OR “Stigmella basiguttella” OR “Stigmella dorsiguttella” OR “Stigmella eberhardi” OR “Stigmella roborella” OR “Stigmella ruficapitella” OR “Stigmella samiatella” OR “Stigmella suberivora” OR “Stigmella svenssoni” OR “Stomaphis quercus” OR “Stomaphis wojciechowskii” OR “Strangalia attenuata” OR “Stromatoscypha fimbriata” OR “Strophedra nitidana” OR “Strophosoma melanogrammum” OR “Strophosomus capitatus” OR “Strophosomus melanogrammus” OR “Stypella subhyalina” OR “Subulicystidium longisporum” OR “Sympodiella foliicola” OR “Sympodiella quercina” OR “Synanthedon vespiformis” OR “Syndemis musculana” OR “Synergus clandestinus” OR “Taeniolella dichotoma” OR “Taeniolella exilis” OR “Taeniolina scripta” OR “Tapesia melaleucoides” OR “Taphrina caerulescens” OR “Taphrorhychus villifrons” OR “Taphrorychus bicolor” OR “Targionia vitis” OR “Teleiodes flavimaculella” OR “Teleiodes luculella” OR “Temnocerus longiceps” OR “Temnocerus nanus” OR “Tetranychus urticae” OR “Tetratoma desmaresti” OR “Tetrops praeustus” OR “Thamnotettix dilutior” OR “Thaumatotibia leucotreta” OR “Thaumetopoea processionea” OR “Thelaxes dryophila” OR “Thelaxes suberi” OR “Thelonectria brayfordii” OR “Thrips major” OR “Thrips minutissimus” OR “Thyridium vestitum” OR “Ticogloea guttulata” OR “Tiliacea aurago” OR “Tillus elongatus” OR “Tischeria dodonaea” OR “Tischeria ekebladella” OR “Tobacco mosaic virus” OR “Tobacco necrosis virus” OR “Tomentella brevispina” OR “Tomentella bryophila” OR “Tomentella crinalis” OR “Tomentella neobourdotii” OR “Tomentella puberula” OR “Tomentella punicea” OR “Tomentella rubiginosa” OR “Tomentella sublilacina” OR “Tomentellopsis submollis” OR “Tomentellopsis zygodesmoides” OR “Tomoxia bucephala” OR “Torostoma apicale” OR “Tortricodes alternella” OR “Tortrix viridana” OR “Torula herbarum” OR “Trachodes hispidus” OR “Trametes hirsuta” OR “Trametes ochracea” OR “Trametes suaveolens” OR “Trametes velutina” OR “Coriolus versicolor” OR “Trametes versicolor” OR “Trametes zonata” OR “Trechispora farinacea” OR “Trechispora microspora” OR “Tremella mesenterica” OR “Tremex columba” OR “Tremex fuscicornis” OR “Tremex magus” OR “Triaxomasia caprimulgella” OR “Trichiura crataegi” OR “Trichius fasciatus” OR “Trichoderma koningii” OR “Trichoderma polysporum” OR “Trichoderma lignorum” OR “Trichoderma viride” OR “Trichodorus californicus” OR “Trichodorus gilanensis” OR “Trichodorus similis” OR “Trichodorus variopapillatus” OR “Trichodorus viruliferus” OR “Tricholoma sulphureum” OR “Trichothecium roseum” OR “Trigonaspis megaptera” OR “Trigonaspis synaspis” OR “Trimmatostroma betulinum” OR “Trinodes hirtus” OR “Trinophylum cribratum” OR “Trioza remota” OR “Trisateles emortualis” OR “Trogoxylon impressum” OR “Troposporella fumosa” OR “Trypodendron domesticum” OR “Trypodendron signatum” OR “Tubakia americana” OR “Tuberculatus annulatus” OR “Tuberculatus borealis” OR “Tuberculatus eggleri” OR “Tuberculatus maculipennis” OR “Tuberculatus moerickei” OR “Tuberculatus neglectus” OR “Tuberculatus pallescens” OR “Tuberculatus querceus” OR “Tuberculoides annulatus” OR “Tuberculoides borealis” OR “Tuberculoides neglectus” OR “Typhlocyba quercus” OR “Tyromyces chioneus” OR “Umbelopsis isabellina” OR “Umbelopsis nana” OR “Umbelopsis ramanniana” OR “Umbelopsis vinacea” OR “Uraba lugens” OR “Usnea distensa” OR “Usnea molliuscula” OR “Ustulina vulgaris” OR “Valdensia heterodoxa” OR “Valsa ambiens” OR “Valsa ceratosperma” OR “Valsaria rubricosa” OR “Valsella quercicola” OR “Verticillium dahliae” OR “Virgariella atra” OR “Virgariella ovoidea” OR “Volvopluteus gloiocephalus” OR “Vuilleminia comedens” OR “Watsonalla binaria” OR “Xanthia aurago” OR “Xenoacremonium falcatum” OR “Xenocriconemella macrodora” OR “Xenodiplosis laeviusculi” OR “Xenoseimatosporium quercinum” OR “Xerocomellus chrysenteron” OR “Xerocomellus cisalpinus” OR “Xestia triangulum” OR “Xestia xanthographa” OR “Xestobium rufovillosum” OR “Xiphinema americanum sensu stricto” OR “Xiphinema belmontense” OR “Xiphinema citricolum” OR “Xiphinema diversicaudatum” OR “Xiphinema floridae” OR “Xiphinema georgianum” OR “Xiphinema index” OR “Xiphinema laevistriatum” OR “Xiphinema naturale” OR “Xiphinema oxycaudatum” OR “Xiphinema pachtaicum” OR “Xiphinema plesiopachtaicum” OR “Xiphinema rivesi” OR “Xiphinema setariae” OR “Xiphinema simile” OR “Xiphinema tarjanense” OR “Xiphydria longicollis” OR “Xylaria hypoxylon” OR “Xyleborinus attenuatus” OR “Xyleborinus saxeseni” OR “Xyleborus dispar” OR “Xyleborus dryographus” OR “Xyleborus monographus” OR “Xylena exsoleta” OR “Xyletinus longitarsis” OR “Xylobolus frustulatus” OR “Xylobolus princeps” OR “Xylodiplosis nigritarsis” OR “Xylosandrus compactus” OR “Xylosandrus germanus” OR “Xylota sylvarum” OR “Ypsolopha alpella” OR “Ypsolopha lucella” OR “Ypsolopha parenthesella” OR “Ypsolopha sylvella” OR “Ypsolopha ustella” OR “Zanclognatha strigilata” OR “Zeiraphera isertana” OR “Zeuzera pyrina” OR “Zignoella fallax” OR “Zygina angusta” OR “Zygina flammigera” OR “Zygina suavis” OR “Zygina tiliae” OR “Zygorhynchus moelleri”) |
Appendix C – Plant taxa reported to be present in the nurseries of Quercus robur
1.
Table C.1.
Plant taxa reported in the Dossier Sections 6.0 to be present in the nurseries of Quercus robur
| Number | Plant taxa | Number | Plant taxa |
|---|---|---|---|
| 1 | Abelia | 292 | Lavatera |
| 2 | Abies alba | 293 | Leucanthemum |
| 3 | Abies concolor | 294 | Leucothoe |
| 4 | Abies fraserii | 295 | Leycesteria |
| 5 | Abies grandis | 296 | Leymus |
| 6 | Abies koreana | 297 | Liatris |
| 7 | Abies nobilis | 298 | Ligularia |
| 8 | Abies nordmanniana | 299 | Ligustrum |
| 9 | Abies procera | 300 | Ligustrum ovalifolium |
| 10 | Acacia | 301 | Ligustrum ovalifolium ‘Aureum' |
| 11 | Acanthus | 302 | Ligustrum vulgare |
| 12 | Acer | 303 | Liquidambar |
| 13 | Acer campestre | 304 | Liquidambar styr. ‘Slender Silhouette’ |
| 14 | Acer campestre ‘Elsrijk’ | 305 | Liquidambar styraciflua |
| 15 | Acer campestre fastigiata | 306 | Liquidambar styraciflua ‘Lane Roberts’ |
| 16 | Acer campestre ‘Streetwise’ | 307 | Liquidambar styraciflua ‘Worplesdon’ |
| 17 | Acer capillipes | 308 | Liriodendron tulipifera |
| 18 | Acer cappodocicum ‘Rubrum' | 309 | Liriope |
| 19 | Acer davidii | 310 | Lithodora |
| 20 | Acer davidii ‘George Forrest’ | 311 | Lobelia |
| 21 | Acer griseum | 312 | Lonicera |
| 22 | Acer lobelii | 313 | Lonicera nitida |
| 23 | Acer macrocarpa | 314 | Lonicera periclymenum |
| 24 | Acer palmatum | 315 | Lupinus |
| 25 | Acer palmatum ‘Atropurpureum' | 316 | Luzula |
| 26 | Acer palmatum ‘Red Wings’ | 317 | Lysimachia |
| 27 | Acer pensylvanicum | 318 | Magnolia |
| 28 | Acer platanoides | 319 | Magnolia ‘Galaxy’ |
| 29 | Acer platanoides ‘Columnare’ | 320 | Magnolia grandiflora ‘Ferruginea’ |
| 30 | Acer platanoides ‘Crimson King’ | 321 | Magnolia kobus |
| 31 | Acer platanoides ‘Crimson Sentry’ | 322 | Mahonia |
| 32 | Acer platanoides ‘Deborah’ | 323 | Malus |
| 33 | Acer platanoides ‘Emerald Queen’ | 324 | Malus ‘Adirondack’ |
| 34 | Acer platanoides ‘Globosum' | 325 | Malus ‘Comtesse de Paris’ |
| 35 | Acer platanoides ‘Perfect Upright’ | 326 | Malus ‘Evereste’ |
| 36 | Acer platanoides ‘Princeton Gold’ | 327 | Malus ‘Freja’ |
| 37 | Acer pseudoplatanus | 328 | Malus hupehensis |
| 38 | Acer pseudoplatanus ‘Erectum' | 329 | Malus ‘Mokum' |
| 39 | Acer pseudoplatanus purpurea | 330 | Malus sylvestris |
| 40 | Acer rubrum | 331 | Malus trilobata |
| 41 | Acer rubrum ‘Karpick’ | 332 | Malus tschonoskii |
| 42 | Acer rubrum ‘October Glory’ | 333 | Matteuccia |
| 43 | Acer tataricum subsp. ginnala | 334 | Maytenus boaria |
| 44 | Acer × freemanii ‘Armstrong’ | 335 | Meconopsis |
| 45 | Acer × freemanii ‘Autumn Blaze’ | 336 | Metasequoia glyptostroboides |
| 46 | Achillea | 337 | Miscanthus |
| 47 | Acorus | 338 | Molinia |
| 48 | Actaea | 339 | Monarda |
| 49 | Aesculus hippocastanum ‘Baumannii’ | 340 | Myrtus |
| 50 | Aesculus indica | 341 | Nandina |
| 51 | Aesculus × carnea ‘Briotii’ | 342 | Nemesia |
| 52 | Agapanthus | 343 | Nepeta |
| 53 | Agastache | 344 | Nothofagus antarctica |
| 54 | Ajuga | 345 | Nothofagus |
| 55 | Akebia | 346 | Nyssa sylvatica |
| 56 | Alchemilla | 347 | Olea europea |
| 57 | Allium | 348 | Olearia |
| 58 | Alnus | 349 | Ophiopogon |
| 59 | Alnus cordata | 350 | Osmanthus |
| 60 | Alnus glutinosa | 351 | Osmunda |
| 61 | Alnus glutinosa ‘Laciniata’ | 352 | Ostrya carpinifolia |
| 62 | Alnus incana | 353 | Pachysandra |
| 63 | Alnus incana ‘Aurea’ | 354 | Pachystegia |
| 64 | Alnus rubra | 355 | Paeonia |
| 65 | Alnus spaethii | 356 | Panicum |
| 66 | Alstroemeria | 357 | Parrotia persica ‘Vanessa’ |
| 67 | Amelanchier | 358 | Paulownia tomentosa |
| 68 | Amelanchier canadensis | 359 | Pennisetum |
| 69 | Amelanchier grandiflora ‘Ballerina’ | 360 | Penstemon |
| 70 | Amelanchier lamarckii | 361 | Perovskia |
| 71 | Amelanchier lamarckii ‘Robin Hill' | 362 | Persicaria |
| 72 | Ammonophylla | 363 | Philadelphus |
| 73 | Anemanthele | 364 | Phlomis |
| 74 | Anemone | 365 | Phlox |
| 75 | Aquilegia | 366 | Phormium |
| 76 | Araucaria araucana | 367 | Photinia |
| 77 | Arbutus | 368 | Photinia × fraseri ‘Red Robin’ |
| 78 | Arbutus unedo | 369 | Phygelius |
| 79 | Armeria | 370 | Physocarpus |
| 80 | Artemisia | 371 | Physostegia |
| 81 | Arum | 372 | Picea abies |
| 82 | Aruncus | 373 | Picea omorika |
| 83 | Asplenium | 374 | Picea orientalis |
| 84 | Astelia | 375 | Picea pungens glauca |
| 85 | Aster | 376 | Picea sitchensis |
| 86 | Astilbe | 377 | Pinus |
| 87 | Astrantia | 378 | Pinus nigra |
| 88 | Athyrium | 379 | Pinus nigra var. austriaca |
| 89 | Aucuba | 380 | Pinus peuce |
| 90 | Baptisia | 381 | Pinus pinaster |
| 91 | Berberis | 382 | Pinus pungens glauca |
| 92 | Berberis darwinii | 383 | Pinus radiata |
| 93 | Berberis thunbergii | 384 | Pinus sylvestris |
| 94 | Berberis thunbergii f. atropurpurea | 385 | Pittosporum |
| 95 | Bergenia | 386 | Platanus orientalis digitalis |
| 96 | Betula | 387 | Platanus × hispanica louisalead |
| 97 | Betula albosinensis ‘Fascination’ | 388 | Platanus |
| 98 | Betula albosinensis ‘Hillier’ | 389 | Polemonium |
| 99 | Betula albosinensis ‘Red Panda’ | 390 | Polygonatum |
| 100 | Betula ‘Edinburgh’ | 391 | Polypodium |
| 101 | Betula ermanii | 392 | Polystichum |
| 102 | Betula lenta | 393 | Populus |
| 103 | Betula nigra | 394 | Populus nigra ‘Italica’ |
| 104 | Betula papyrifera var. kenaica | 395 | Populus nigra |
| 105 | Betula pendula | 396 | Populus tremula |
| 106 | Betula pendula ‘Dalecarlica’ | 397 | Potentilla |
| 107 | Betula pendula fastigiata ‘Obelisk’ | 398 | Primula |
| 108 | Betula pendula ‘Zwitsers Glory’ | 399 | Prunus |
| 109 | Betula pubescens | 400 | Prunus ‘Accolade’ |
| 110 | Betula utilis ‘Jermyns’ | 401 | Prunus ‘Amanogawa’ |
| 111 | Betula utilis var. jacquemontii | 402 | Prunus avium |
| 112 | Blechnum | 403 | Prunus avium ‘Landscape Bloom' |
| 113 | Brachyglottis | 404 | Prunus avium ‘Plena’ |
| 114 | Brunnera | 405 | Prunus campanulata |
| 115 | Buddleja | 406 | Prunus cera |
| 116 | Buxus | 407 | Prunus cerasifera |
| 117 | Buxus sempervirens | 408 | Prunus cerasifera ‘Nigra’ |
| 118 | Calamagrostis | 409 | Prunus cerasifera ‘Pissardii’ |
| 119 | Calluna | 410 | Prunus ‘Ichiyo’ |
| 120 | Campanula | 411 | Prunus ‘Kanzan’ |
| 121 | Carex | 412 | Prunus ‘Kursar’ |
| 122 | Carpinus | 413 | Prunus lau.’Rotund’ |
| 123 | Carpinus betulus | 414 | Prunus laurocerasus |
| 124 | Carpinus betulus ‘Cube Head’ | 415 | Prunus laurocerasus ‘Magnoliifolia’ |
| 125 | Carpinus betulus ‘Pleached’ | 416 | Prunus ‘Litigiosa’ |
| 126 | Carpinus betulus ‘Fastigiata’ | 417 | Prunus lusitanica |
| 127 | Carpinus betulus ‘Lucas’ | 418 | Prunus maackii ‘Amber Beauty’ |
| 128 | Carpinus betulus ‘Streetwise’ | 419 | Prunus ‘Mount Fuji’ |
| 129 | Caryopteris | 420 | Prunus padus |
| 130 | Castanea | 421 | Prunus padus ‘Select’ |
| 131 | Castanea sativa | 422 | Prunus ‘Pandora’ |
| 132 | Castanea sativa ‘Anny's Summer Red’ | 423 | Prunus sargentii |
| 133 | Catalpa bignoniodes | 424 | Prunus sargentii ‘Rancho’ |
| 134 | Ceanothus | 425 | Prunus serrula |
| 135 | Cedrus atlantica ‘Glauca’ | 426 | Prunus ‘Shirofugen’ |
| 136 | Cedrus atlantica | 427 | Prunus ‘Snow Goose’ |
| 137 | Cedrus deodara | 428 | Prunus spinosa |
| 138 | Cedrus libani | 429 | Prunus ‘Spire’ |
| 139 | Celtis australis | 430 | Prunus ‘Sunset Boulevard’ |
| 140 | Centaurea | 431 | Prunus ‘Tai‐haku' |
| 141 | Centranthus | 432 | Prunus × schmittii |
| 142 | Ceratostigma | 433 | Prunus × sub. ‘Autumnalis Rosea’ |
| 143 | Cercidiphyllum japonicum | 434 | Prunus × subhirtella ‘Autumnalis’ |
| 144 | Cercis canadensis | 435 | Prunus yedoensis |
| 145 | Cercis silaquastrum | 436 | Pseudotsuga menziesii |
| 146 | Chaenomeles | 437 | Pterocarya stenoptera ‘Fern Leaf’ |
| 147 | Chamaecyparis | 438 | Pulmonaria |
| 148 | Chamaecyparis lawsoniana | 439 | Pyracantha |
| 149 | Choisya | 440 | Pyrus |
| 150 | Cistus | 441 | Pyrus calleryana ‘Chanticleer’ |
| 151 | Clematis | 442 | Pyrus calleryana ‘Red Spire’ |
| 152 | Convolvulus | 443 | Pyrus communis |
| 153 | Coprosma | 444 | Quercus |
| 154 | Coreopsis | 445 | Quercus castaneifolia ‘Green Spire’ |
| 155 | Cornus | 446 | Quercus cerris |
| 156 | Cornus kousa var. chinensis | 447 | Quercus frainetto ‘Hungarian Crown’ |
| 157 | Cornus sanguinea | 448 | Quercus ilex |
| 158 | Cortaderia | 449 | Quercus palustris |
| 159 | Corydalis | 450 | Quercus palustris ‘Green Pillar’ |
| 160 | Corylus | 451 | Quercus petraea |
| 161 | Corylus avellana | 452 | Quercus robur |
| 162 | Corylus colurna | 453 | Quercus robur ‘Fastigiata Koster’ |
| 163 | Cosmos | 454 | Quercus rubra |
| 164 | Cotinus | 455 | Quercus × bimundorum ‘Crimson Spire’ |
| 165 | Cotoneaster | 456 | Rhamnus |
| 166 | Cotoneaster bullatus | 457 | Rhamnus cathartica |
| 167 | Cotoneaster franchettii | 458 | Rhamnus frangula |
| 168 | Cotoneaster horizontalis | 459 | Rhus |
| 169 | Cotoneaster lacteus | 460 | Ribes |
| 170 | Cotoneaster simonsii | 461 | Robinia ‘Casque Rouge/Bessoniana’ |
| 171 | Crataegus | 462 | Robinia pseudoacacia |
| 172 | Crataegus laevigata ‘Pauls Scarlet’ | 463 | Robinia |
| 173 | Crataegus lavallei ‘Carreri’ | 464 | Rosa |
| 174 | Crataegus monogyna | 465 | Rosa arvensis |
| 175 | Crataegus persimilis ‘Prunifolia’ | 466 | Rosa canina |
| 176 | Crocosmia | 467 | Rosa rubiginosa |
| 177 | Cryptomeria japonica | 468 | Rosa rugosa |
| 178 | Cupressocyparis | 469 | Rosa rugosa ‘Alba’ |
| 179 | Cupressocyparis leylandii | 470 | Rosa rugosa rubra |
| 180 | Cupressus | 471 | Rosa spinosissima |
| 181 | Cupressus macrocarpa | 472 | Rosmarinus |
| 182 | Cynoglossum | 473 | Rudbeckia |
| 183 | Cytisus | 474 | Salix |
| 184 | Dahlia | 475 | Salix alba |
| 185 | Daphne | 476 | Salix alba ‘Britzensis’ |
| 186 | Davidia involucrata | 477 | Salix aurita |
| 187 | Delosperma | 478 | Salix babylonica pendula |
| 188 | Delphinium | 479 | Salix caprea |
| 189 | Deschampsia | 480 | Salix cinerea |
| 190 | Deutzia | 481 | Salix pentandra |
| 191 | Dicentra | 482 | Salix viminalis |
| 192 | Diervilla | 483 | Salvia |
| 193 | Digitalis | 484 | Sambucus |
| 194 | Doronicum | 485 | Sambucus nigra |
| 195 | Dryopteris | 486 | Sanguisorba |
| 196 | Echinacea | 487 | Santolina |
| 197 | Echinops | 488 | Sarcococca confusa |
| 198 | Elaeagnus | 489 | Scabiosa |
| 199 | Epimedium | 490 | Schizostylis |
| 200 | Eremurus | 491 | Sedum |
| 201 | Erigeron | 492 | Senecio |
| 202 | Eriophorum | 493 | Sequoia sempervirens |
| 203 | Eriostemon | 494 | Sequoiadendron giganteum |
| 204 | Eryngium | 495 | Sesleria |
| 205 | Erysimum | 496 | Sorbaria |
| 206 | Escallonia | 497 | Sorbus |
| 207 | Eucalyptus | 498 | Sorbus aria |
| 208 | Eucalyptus glaucescens | 499 | Sorbus aria ‘Majestica’ |
| 209 | Eucalyptus gunnii | 500 | Sorbus arnoldiana ‘Golden Wonder’ |
| 210 | Euonymus | 501 | Sorbus aucuparia |
| 211 | Euonymus europaeus | 502 | Sorbus aucuparia ‘Aspleniifolia’ |
| 212 | Euonymus europaeus ‘Red Cascade’ | 503 | Sorbus aucuparia ‘Cardinal Royal' |
| 213 | Euonymus japonicus ‘Bravo’ | 504 | Sorbus aucuparia ‘Sheerwater Seedling’ |
| 214 | Euphorbia | 505 | Sorbus aucuparia ‘Streetwise’ |
| 215 | Exochorda | 506 | Sorbus ‘Autumn Spire’ |
| 216 | Fagus | 507 | Sorbus commixta ‘Embley’ |
| 217 | Fagus aspelenifolia | 508 | Sorbus commixta ‘Olympic Flame’ |
| 218 | Fagus sylvatica | 509 | Sorbus ‘Glowing Pink’ |
| 219 | Fagus sylvatica ‘Atropurpurea’ | 510 | Sorbus ‘Hemsleyi John Bond’ |
| 220 | Fagus sylvatica ‘Dawyck’ | 511 | Sorbus intermedia |
| 221 | Fagus sylvatica ‘Dawyck Gold’ | 512 | Sorbus intermedia ‘Browers’ |
| 222 | Fagus sylvatica ‘Dawyck Purple’ | 513 | Sorbus ‘John Mitchell' |
| 223 | Fagus sylvatica ‘Purpurea’ | 514 | Sorbus ‘Sunshine’ |
| 224 | Fargesia | 515 | Sorbus torminalis |
| 225 | Fatsia | 516 | Sorbus × thuringiaca ‘Fastigiata’ |
| 226 | Festuca | 517 | Spiraea |
| 227 | Filipendula | 518 | Stachys |
| 228 | Foeniculum | 519 | Stachyurus |
| 229 | Forsythia | 520 | Stewartia pseudocamellia |
| 230 | Fraxinus angustifolia | 521 | Stipa |
| 231 | Fraxinus americana | 522 | Symphiocarpus |
| 232 | Fruit Trees | 523 | Symphoricarpos |
| 233 | Fuchsia | 524 | Symphytum |
| 234 | Galium | 525 | Syringa |
| 235 | Garrya | 526 | Taxodium dist. ‘Nutans’ |
| 236 | Gaultheria procumbens | 527 | Taxodium distichum |
| 237 | Gaultheria shallon | 528 | Taxus |
| 238 | Gaura | 529 | Taxus baccata |
| 239 | Genista | 530 | Tellima |
| 240 | Geranium | 531 | Thalictrum |
| 241 | Geum | 532 | Thuja |
| 242 | Ginkgo biloba | 533 | Thuja plicata |
| 243 | Ginkgo biloba ‘Globosum' | 534 | Thuja plicata ‘Fastigiata’ |
| 244 | Ginkgo biloba ‘Saratoga’ | 535 | Thymus |
| 245 | Gleditsia triacanthos ‘Skyline’ | 536 | Tiarella |
| 246 | Griselinia | 537 | Tilia |
| 247 | Hakonechloa | 538 | Tilia cordata |
| 248 | Halesia carolina | 539 | Tilia cordata ‘Corzam' |
| 249 | Halimium | 540 | Tilia cordata ‘Greenspire’ |
| 250 | Hebe | 541 | Tilia cordata ‘Streetwise’ |
| 251 | Hedera | 542 | Tilia cordata ‘Winter Orange’ |
| 252 | Helenium | 543 | Tilia ‘Harold Hillier’ |
| 253 | Helichrysum | 544 | Tilia henryana |
| 254 | Helleborus | 545 | Tilia oliveri |
| 255 | Hemerocallis | 546 | Tilia petolaris ‘Chelsea Sentinel' |
| 256 | Heuchera | 547 | Tilia platanoides |
| 257 | Heucherella | 548 | Tilia platyphyllos |
| 258 | Hippophae | 549 | Tilia platyphyllos ‘Aurea’ |
| 259 | Hippophae rhamnoides | 550 | Tilia platyphyllos ‘Princes Street’ |
| 260 | Hippophae salicifolia ‘Streetwise’ | 551 | Tilia platyphyllos ‘Streetwise’ |
| 261 | Hosta | 552 | Tilia tomentosa ‘Brabant’ |
| 262 | Houttuynia | 553 | Tilia × euchlora |
| 263 | Hydrangea | 554 | Tilia × europaea ‘Pallida’ |
| 264 | Hypericum | 555 | Trachelospermum |
| 265 | Iberis | 556 | Trachycarpus fortunei |
| 266 | Ilex | 557 | Tradescantia |
| 267 | Ilex aquifolium | 558 | Tricyrtis |
| 268 | Ilex aquifolium ‘Marijo’ | 559 | Trollius |
| 269 | ilex crenata | 560 | Tsuga heterophylla |
| 270 | Ilex × altaclarensis ‘James G. Esson’ | 561 | Ulex |
| 271 | Ilex × altaclerensis ‘Golden King’ | 562 | Ulex europaeus |
| 272 | Ilex × koehneana ‘Chestnut Leaf’ | 563 | Ulmus |
| 273 | Imperata | 564 | Ulmus ‘Columnella’ |
| 274 | Iris | 565 | Ulmus ‘Fiorente’ |
| 275 | Jasminum | 566 | Ulmus glabra |
| 276 | Juglans nigra | 567 | Ulmus ‘New Horizon’ |
| 277 | Juglans regia | 568 | Ulmus ‘Rebona’ |
| 278 | Juniperus | 569 | Ulmus ‘San Zenobi’ |
| 279 | Juniperus communis | 570 | Uncinia |
| 280 | Knautia | 571 | Verbena |
| 281 | Kniphofia | 572 | Veronica |
| 282 | Koelreuteria paniculata | 573 | Viburnum |
| 283 | Laburnum | 574 | Viburnum lantana |
| 284 | Laburnum anagyroides | 575 | Viburnum opulus |
| 285 | Lamium | 576 | Vinca |
| 286 | Larix | 577 | Weigela |
| 287 | Larix decidua | 578 | Wisteria sinensis |
| 288 | Larix kaempferi | 579 | x Cupressocyparis leylandii |
| 289 | Larix × decidua | 580 | Yucca |
| 290 | Larix × eurolepsis | 581 | Yucca filamentosa |
| 291 | Lavandula | 582 | Zelkova serrata ‘Green Vase’ |
Appendix D – Water used for irrigation
1.
All mains water used meets the UK standard Water Supply (Water quality) regulation 2016 and the WHO/EU potable water standards, (Drinking water Directive (98/83/EC and the revised Drinking Water Directive 2020/2184) which includes a total freedom from both human and plant pathogens (Article 2–(7)). All mains water conducting pipework fully complies with the UK Water Supply (Water Fittings) regulations of 1999 and the amendments of 2019. Irrigation water used is not stored in any open tanks where air borne contamination could take place and is entirely isolated from any outside exposure (Dossier Section 3.0).
Bore hole water supply: in some cases, where the underlying geology permits, nurseries can draw water directly from bore holes drilled into underground aquafers. The water that fills these aquafers is naturally filtered through the layers of rock (e.g. limestone) over long periods of time, many millennia in some cases. The water from such supplies is generally of such high quality that it is fit for human consumption with little to no further processing and is often bottled and sold as mineral water (Dossier Section 3.0).
Rainwater or freshwater watercourse supply: some nurseries contributing to this application for both environmental and efficiency reasons use a combination of rain capture systems or abstract directly from available watercourses. All water is passed through a sand filtration system to remove contaminants and is contained in storage tanks prior to use. One nursery that operates this approach is currently in the process of installing additional nanobubble technology to treat the water (Dossier Section 3.0).
Appendix E – List of pests that can potentially cause an effect not further assessed
1.
Table E.1.
List of potential pests not further assessed
| N | Pest name | EPPO code | Group | Pest present in the UK | Present in the EU | Quercus confirmed as a host (reference) | Pest can be associated with the commodity | Impact | Justification for inclusion in this list |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Coniothyrium quercinum | CONIQU | Fungi | Yes | Limited | Quercus, Q. robur (Farr and Rossman, online) | Uncertain | No data | Uncertainty about impact and about association with the commodities. |
| 2 | Dothidea noxia | Fungi | Yes | Limited | Quercus (Dossier) | Yes | No data | Uncertainty about impact. | |
| 3 | Fuscoporia wahlbergii | Fungi | Yes | Limited | Quercus robur (Plant Pest Information Network New Zealand, online) | Yes | No data | Uncertainty about impact. | |
| 4 | Gibbsiella quercinecans | GIBSQU | Bacteria | Yes | Limited | Quercus, Q. robur (Biota of New Zealand, online) | Yes | No data | Uncertainty about impact. |
| 5 | Huntiella moniliformis | CERAMO | Fungi | Yes | Limited | Quercus robur (Farr and Rossman, online) | Uncertain | No data | Uncertainty about impact and about association with the commodities. |
| 6 | Kermes williamsi | Insects | Yes | No | Quercus (Database of Insects and their Food Plants, online) | Yes | No data | Uncertainty about impact. | |
| 7 | Lonsdalea britannica | LNSDQB | Bacteria | Yes | No data | Quercus robur (Dossier) | Yes | No data | Uncertainty about presence in the EU. |
| 8 | Phaeobotryon quercicola | Fungi | Yes | Limited | Quercus, Q. robur (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. | |
| 9 | Polyporus gayanus | Fungi | Yes | No | Quercus (Dossier) | Uncertain | No data | Uncertainty about impact. | |
| 10 | Pseudomonas daroniae | Bacteria | Yes | No data | Quercus robur (Dossier) | Yes | No data | Uncertainty about presence in the EU. | |
| 11 | Pseudomonas dryadis | Bacteria | Yes | No data | Quercus robur (Dossier) | Yes | No data | Uncertainty about presence in the EU. | |
| 12 | Pseudomonas kirkiae | Bacteria | Yes | No data | Quercus robur (Dossier) | Yes | No data | Uncertainty about presence in the EU. | |
| 13 | Sporothrix dentifunda | Fungi | Yes | Limited | Quercus robur (Farr and Rossman, online) | Yes | No data | Uncertainty about impact. |
Appendix F – Excel file with the pest list of Quercus robur
1.
Appendix F is available under the Supporting Information section on the online version of the scientific output.
Supporting information
Excel file with the pest list of Quercus robur
Suggested citation: EFSA PLH Panel (EFSA Panel on Plant Health) , Bragard, C ., Baptista, P ., Chatzivassiliou, E ., Di Serio, F ., Jaques Miret, J. A ., Justesen, A. F ., MacLeod, A ., Magnusson, C. S ., Milonas, P ., Navas‐Cortes, J. A ., Parnell, S ., Potting, R ., Reignault, P. L ., Stefani, E ., Thulke, H.‐H ., Van der Werf, W ., Civera Vicent, A ., Yuen, J ., … Gonthier, P . 2023. Commodity risk assessment of Quercus robur plants from the UK. EFSA Journal, 21(10), 1–242. 10.2903/j.efsa.2023.8314
Requestor European Commission
Question number EFSA‐Q‐2022‐00461
Panel members Claude Bragard, Paula Baptista, Elisavet Chatzivassiliou, Francesco Di Serio, Paolo Gonthier, Josep Anton Jaques Miret, Annemarie Fejer Justesen, Alan MacLeod, Christer Sven Magnusson, Panagiotis Milonas, Juan A Navas‐Cortes, Stephen Parnell, Roel Potting, Philippe L Reignault, Emilio Stefani, Hans‐Hermann Thulke, Wopke Van der Werf, Antonio Vicent Civera, Jonathan Yuen and Lucia Zappalà.
Declarations of interest If you wish to access the declaration of interests of any expert contributing to an EFSA scientific assessment, please contact interestmanagement@efsa.europa.eu.
Acknowledgements The Scientific Opinion was prepared in cooperation with the Università degli studi di Padova, Dipartimento Agronomia, Animali, Alimenti, Risorse Naturali e Ambiente (Italy) under the EFSA Art. 36 Framework Partnership Agreement ‘GP/EFSA/ALPHA/2019/01’ – Lot 3 ‐ taxa traded mostly as plants for planting for forestry purposes.
EFSA may include images or other content for which it does not hold copyright. In such cases, EFSA indicates the copyright holder and users should seek permission to reproduce the content from the original source.
Adopted: 19 September 2023
Notes
Regulation (EU) 2016/2031 of the European Parliament of the Council of 26 October 2016 on protective measures against pests of plants, amending Regulations (EU) 228/2013, (EU) 652/2014 and (EU) 1143/2014 of the European Parliament and of the Council and repealing Council Directives 69/464/EEC, 74/647/EEC, 93/85/EEC, 98/57/EC, 2000/29/EC, 2006/91/EC and 2007/33/EC. OJ L 317, 23.11.2016, pp. 4–104.
Commission Implementing Regulation (EU) 2018/2019 of 18 December 2018 establishing a provisional list of high risk plants, plant products or other objects, within the meaning of Article 42 of Regulation (EU) 2016/2031 and a list of plants for which phytosanitary certificates are not required for introduction into the Union, within the meaning of Article 73 of that Regulation C/2018/8877. OJ L 323, 19.12.2018, pp. 10–15.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, pp. 1–24.
Commission Implementing Regulation (EU) 2019/2072 of 28 November 2019 establishing uniform conditions for the implementation of Regulation (EU) 2016/2031 of the European Parliament and the Council, as regards protective measures against pests of plants, and repealing Commission Regulation (EC) No 690/2008 and amending Commission Implementing Regulation (EU) 2018/2019. OJ L 319, 10.12.2019, p. 1–279.
In accordance with the Agreement on the withdrawal of the United Kingdom of Great Britain and Northern Ireland from the European Union and the European Atomic Energy Community, and in particular Article 5(4) of the Protocol on Ireland/Northern Ireland in conjunction with Annex 2 to that Protocol, for the purposes of this Opinion, references to Member States include the United Kingdom in respect of Northern Ireland.
Plant Health (Amendment etc.) (EU Exit) Regulations 2020 of 14 December 2020, No. 1482, 80 pp. Available online: https://www.legislation.gov.uk/uksi/2020/1482/contents/made
Plant Health (Phytosanitary Conditions) (Amendment) (EU Exit) Regulations 2020, No. 1527, 276 pp. Available online: https://www.legislation.gov.uk/uksi/2020/1527/contents/made
References
- Biota of New Zealand , online. Available online: https://biotanz.landcareresearch.co.nz/ [Accessed: 14 July 2023].
- CABI (Centre for Agriculture and Bioscience International) , online. CABI Crop Protection Compendium Available online: https://www.cabi.org/cpc/ [Accessed: 01 December 2022].
- Database of Insects and their Food Plants , online. Available online: http://dbif.brc.ac.uk/hosts.aspx [Accessed: 14 July 2023].
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2018. Guidance on quantitative pest risk assessment. EFSA Journal 2018;16(8):5350, 86 pp. 10.2903/j.efsa.2018.5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA PLH Panel (EFSA Panel on Plant Health) , 2019. Guidance on commodity risk assessment for the evaluation of high risk plants dossiers. EFSA Journal 2019;17(4):5668, 20 pp. 10.2903/j.efsa.2019.5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPPO (European and Mediterranean Plant Protection Organization) , 2020. EPPO Technical Document No. 1081, EPPO Study on the risk of bark and ambrosia beetles associated with imported non‐coniferous wood. EPPO Paris Available online: https://www.eppo.int/RESOURCES/eppo_publications
- EPPO (European and Mediterranean Plant Protection Organization) , online. EPPO Global Database. Available online: https://gd.eppo.int/ [Accessed: 1 December 2022].
- EPPO (European and Mediterranean Plant Protection Organization) Bulletin , 2017. Commodity‐specific phytosanitary measures, PM 8/5 (1). Quercus, 47, 452–460. 10.1111/epp.12412 [DOI] [Google Scholar]
- EUROPHYT (European Union Notification System for Plant Health Interceptions) , online. Available online: https://food.ec.europa.eu/plants/plant-health-and-biosecurity/europhyt_en [Accessed: 22 December 2022].
- FAO (Food and Agriculture Organization of the United Nations) , 1995. ISPM (International standards for phytosanitary measures) No 4. Requirements for the establishment of pest free areas. Available online: https://www.ippc.int/en/publications/614/
- FAO (Food and Agriculture Organization of the United Nations) , 2017. ISPM (International standards for phytosanitary measures) No. 5. Glossary of phytosanitary terms. FAO, Rome. Available online: https://www.ippc.int/en/publications/622/
- FAO (Food and Agriculture Organization of the United Nations) , 2019. ISPM (International standards for phytosanitary measures) No 36. Integrated measures for plants for planting. FAO, Rome. Available online: https://www.ippc.int/en/publications/636
- Farr DF and Rossman AY, online. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://data.nal.usda.gov/dataset/united-states-national-fungus-collections-fungus-host-dataset [Accessed: 1 December 2022].
- Ferris H, online. Nemaplex (The Nematode‐Plant Expert Information System). Available online: http://nemaplex.ucdavis.edu/Nemabase2010/PlantNematodeHostStatusDDQuery.aspx [Accessed: 1 December 2022].
- Forestry Commission , online. Available online: https://forestry.maps.arcgis.com/apps/webappviewer/index.html?id=c647b00b75d34647aeb5a9d07eca9785 [Accessed: 18 April 2023].
- Kottek M, Grieser J, Beck C, Rudolf B and Rubel F, 2006. World map of Köppen‐ Geiger climate classification updated. Meteorologische Zeitschrift, 15, 259–263. [Google Scholar]
- Plant Pest Information Network New Zealand , online. Available online: https://www.mpi.govt.nz/resources-and-forms/registers-and-lists/plant-pest-information-network/ [Accessed: 14 July 2023].
- TRACES‐NT , online. TRAde control and expert system. Available online: https://webgate.ec.europa.eu/tracesnt [Accessed: 22 December 2022].
- Xu YM and Zhao ZQ, 2019. Longidoridae and Trichodoridae (Nematoda: Dorylaimida and Triplonchida). Fauna of New Zealand, 79, 149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Excel file with the pest list of Quercus robur


