Abstract
Two membrane-bound, reductive dehalogenases that constitute a novel pathway for complete dechlorination of tetrachloroethene (perchloroethylene [PCE]) to ethene were partially purified from an anaerobic microbial enrichment culture containing Dehalococcoides ethenogenes 195. When titanium(III) citrate and methyl viologen were used as reductants, PCE-reductive dehalogenase (PCE-RDase) (51 kDa) dechlorinated PCE to trichloroethene (TCE) at a rate of 20 μmol/min/mg of protein. TCE-reductive dehalogenase (TCE-RDase) (61 kDa) dechlorinated TCE to ethene. TCE, cis-1,2-dichloroethene, and 1,1-dichloroethene were dechlorinated at similar rates, 8 to 12 μmol/min/mg of protein. Vinyl chloride and trans-1,2-dichloroethene were degraded at rates which were approximately 2 orders of magnitude lower. The light-reversible inhibition of TCE-RDase by iodopropane and the light-reversible inhibition of PCE-RDase by iodoethane suggest that both of these dehalogenases contain Co(I) corrinoid cofactors. Isolation and characterization of these novel bacterial enzymes provided further insight into the catalytic mechanisms of biological reductive dehalogenation.
Perchloroethylene (PCE) and trichloroethene (TCE) are federally regulated toxic chemicals which pose a risk to public health (12). Unfortunately, these widely used solvents are among the most common contaminants of groundwater (35). The oxidized nature of PCE and TCE makes them difficult to degrade via oxidative processes (11), and their densities and low water solubilities make them difficult to remove via pump-and-treat techniques in the common situation where neat solvent is present in the subsurface. For these reasons, there is growing interest in in situ, anaerobic biological alternatives for dealing with these compounds. Sequential reductive dechlorination of PCE and TCE to less-chlorinated ethenes has been widely observed under anaerobic conditions (1–3, 14, 16, 28, 33). This process can be cometabolically mediated by a variety of anaerobic bacteria, including methanogens and sulfate reducers (1, 14, 33). More recently, anaerobic enrichment cultures have been developed that are capable of growth with PCE as the terminal electron acceptor, in a process termed dehalorespiration (8, 10, 18, 19, 22, 24). Some of these cultures can completely dechlorinate PCE and TCE to the benign end products ethene and ethane (8, 10, 18, 22). A pure culture of the novel organism Dehalobacter restrictus has been isolated from an ethane-producing mixed culture (30). Independently, the novel bacterium Dehalospirillum multivorans was obtained in pure culture by enrichment from activated sludge exposed to PCE (29). Unfortunately, both of these organisms produce the toxic compound cis-dichloroethene (cis-DCE) as the terminal product. In contrast, an isolate capable of reducing chloroethenes to ethene, tentatively named Dehalococcoides ethenogenes 195, was recently isolated from an ethene-producing enrichment culture (23).
The identification of dehalorespiring bacteria has stimulated interest in isolating the enzymes responsible for catalyzing the dechlorination of PCE and TCE. Recently, a corrinoid enzyme, PCE-reductive dehalogenase (PCE-RDase), was isolated by Neumann et al. from Dehalospirillum multivorans (25, 26). This cytosolic enzyme reduces PCE or TCE to cis-DCE as the terminal product. The following two membrane-associated chloroaromatic reductive dehalogenases have also been identified: 3-chlorobenzoate-reductive dehalogenase and 3-chloro-4-hydroxybenzoate dehalogenase (9, 21, 27). One of these enzymes, 3-chlorobenzoate-reductive dehalogenase has been purified and characterized as a heme protein (9, 27). Experiments performed with cell extracts have indicated that this enzyme is also capable of slowly dechlorinating PCE to TCE and a DCE isomer, the final product. It has become evident that there are many newly identified and yet-to-be-discovered bacterial species that contain a variety of reductive dehalogenases involved in dehalorespiration. Yet to date, enzymes capable of reductively dehalogenating PCE or TCE to a benign end product have not been described. We report here the partial purification from an anaerobic mixed culture containing Dehalococcoides ethenogenes 195 of two novel dehalogenases that catalyze complete reductive dechlorination of PCE to the nontoxic terminal product ethene.
MATERIALS AND METHODS
Chemicals.
All buffers, salts, chlorinated solvents, and reagents were obtained from Sigma, Aldrich, Baker, or Fisher, except as noted below. A 29.2% acrylamide–0.8% bisacrylamide solution was obtained from Bio-Rad. Coomassie Plus protein assay reagent was obtained from Pierce.
Anaerobic bacterial culture.
Continuously maintained 10-liter PCE–methanol-fed anaerobic enrichment cultures were grown in 13.3-liter glass carboys in clear medium as described previously (31) except that hemin (0.05 g/liter) and cysteine (0.5 g/liter) were used instead of nitrilotriacetic acid, ferrous chloride, and sodium sulfide. The 10-liter cultures grown at USAF Armstrong Laboratory by J.K.M. and R.V.S. were started from a single 100-ml culture obtained from the original culture maintained by J.M.G. at Cornell University. The halorespiring characteristics of the cultures have been stable for years (10, 31), and the cultures at Armstrong Laboratory and Cornell University exhibit the same PCE utilization rate and product distribution. For protein purification, an anaerobic enrichment culture was used instead of a pure culture due to the slow growth, complex nutritional requirements, and low yield of biomass of the latter (23).
Dehalogenase activity assays.
Activity assays were performed in 15-ml glass vials sealed by crimping 20-mm aluminum seals over Teflon-lined, butyl rubber septa. All additions were made inside a glove box containing a 96% nitrogen–4% hydrogen atmosphere. Titanium(III) citrate was prepared as described by Zehnder and Wuhrmann (37). The final aqueous volume of 2.0 ml contained 25 mM 1,3-bis(tris[hydroxymethyl]methylamino)propane (BTP) (pH 7), 150 mM NaCl, 2 mM titanium(III) citrate, 2 mM methyl viologen, and enzyme. Five microliters of a pentane solution (0.40% [vol/vol] in ethanol) was added as an internal standard. Chlorinated ethenes diluted in ethanol were injected to start the reaction. The assay vials were inverted and incubated at 35°C in a shaking water bath. The reaction was terminated by adding 0.2 ml of 10 N H2SO4. Control reaction mixtures contained all of the components except enzyme. None of the chlorinated ethenes was dehalogenated in the absence of enzyme. Headspace samples (500 μl) were injected onto a Hewlett-Packard model HP5890 Series II gas chromatograph containing a column (2.4 m by 3.2 mm) consisting of 1% SP-1000 on 60/80 Carbopack B coupled to a flame ionization detector. The initial temperature was 60°C; the column was kept at 60°C for 1 min, and then the temperature was raised to 210°C at a rate of 35°C per min and kept at 210°C for 5 min. Vapor-liquid partitioning of the analytes was taken into account, and masses are reported below as total masses in the assay vials. The rates were based on the total component masses in the assay vials. No attempts were made to ascertain limitations on observed enzymatic rates imposed by mass transfer between the liquid and vapor phases; therefore, the rates reported below were system dependent and most likely represent lower limits.
The gas chromatography system described above did not resolve the cis and trans isomers of 1,2-DCE. Headspace samples obtained at selected times were injected into a model HP5890 Series II gas chromatograph containing a GSQ column (0.53 mm by 30 m) connected to a flame ionization detector. The initial temperature was 50°C; the column was kept at 50°C for 2 min, and then the temperature was raised to 200°C at a rate of 25°C per min and kept at 200°C for 12 min. This system clearly resolved the cis and trans isomers of 1,2-DCE.
Purification of dehalogenases.
All procedures after cell harvesting were performed either in a Coy glove box containing a 96% nitrogen–4% hydrogen atmosphere with argon-sparged buffers or under a stream of argon. Seven liters of culture was harvested by tangential flow membrane filtration on a 0.45-μm-pore-size membrane filter (Millipore). The resulting cell paste (6 to 8 g, wet weight) was suspended to a concentration of 0.35 g/ml in lysis buffer containing 25 mM BTP, (pH 7), 2 mM cysteine, 2 mM Fe(NH4)2(SO4)2, 150 mM NaCl, and 1 mM phenylmethylsulfonyl fluoride. Cells were lysed with a French press at 62 MPa. The lysate was subjected to centrifugation at 105,000 × g for 1 h at 4°C. The supernatant was decanted, and the pellet consisting of cell walls and membranes was uniformly suspended with a tissue homogenizer to a concentration of 20 mg (wet weight) per ml in lysis buffer. The cell wall-membrane suspension (20 mg [wet weight] per ml) was incubated at 25°C for 30 min in lysis buffer containing 0.1% (vol/vol) Triton X-100. Detergent-solubilized protein was separated by centrifugation at 20,000 × g for 45 min at 4°C. The protein solution was diluted 1:1 with column buffer (lysis buffer without NaCl or phenylmethylsulfonyl fluoride) containing 1.0 M (NH4)2SO4 and applied at a rate of 10 ml/min to a POROS HP/M column (16 by 100 mm) in column buffer containing 0.5 M (NH4)2SO4 by using a Perseptive Biosystems BioCAD workstation. PCE-RDase and TCE-reductive dehalogenase (TCE-RDase) coeluted in 0.25 M (NH4)2SO4 in column buffer. The concentration of (NH4)2SO4 in the dehalogenase pool was increased to 0.6 M, and the dehalogenase pool was applied to a POROS PH/M column (4.6 by 100 mm) at a flow rate of 5 ml/min. PCE-RDase eluted early in a 0.5 to 0 M (NH4)2SO4 gradient, at 38 to 45 mS. After the gradient was completed, TCE-RDase was eluted in column buffer amended with 0.1% Triton X-100. Protein assays were performed by the Bradford procedure (4) by using Pierce Coomassie Plus reagent.
Native gel electrophoresis.
A moving-boundary electrophoresis system with a trailing-phase pH of 7.4 (buffer system 12 of Chrambach [7]) was used to separate active PCE-RDase and TCE-RDase from contaminating proteins in a 6% polyacrylamide resolving gel with a 4% polyacrylamide stacking gel. The following modifications were used: the buffer concentrations were increased fivefold in order to raise the ionic strength to 50 mM, which increased the stability of the dehalogenases; and 0.05% Triton X-100 was included in the gel, the anolyte buffer, and the catholyte buffer. PCE-RDase from the 4.6- by 100-mm PH/M column was applied to lanes 1 and 2 of a four-lane native gel, and TCE-RDase from the 4.6- by 100-mm PH/M column was applied to lanes 3 and 4 of the native gel. After electrophoresis, lanes 2 and 3 were stained with Coomassie blue, and Rf values were calculated for the protein bands. Based on the Rf values, the protein bands from lanes 1 and 4 of the gel were excised and assayed with PCE and TCE, respectively. Sodium dodecyl sulfate (SDS) was added to a final concentration of 2% (wt/vol) upon completion of the assay. A headspace sample was removed for determination of products by gas chromatography. The SDS-protein solution was removed, concentrated, and subjected to denaturing gel electrophoresis in a 9% polyacrylamide gel (20).
Reversible inhibition of dehalogenases.
Light-reversible alkylation of corrinoids by iodoalkanes was based on the procedure of Brot and Weissbach (5). TCE-RDase and PCE-RDase were incubated with or without 0.5 mM iodoalkane and 2 mM titanium(III) citrate for 30 min in foil-wrapped clear vials. Portions of the enzyme solutions were injected into 15-ml amber vials and assayed as described above. The foil was removed from the vials containing the remaining iodoalkane-treated enzyme solutions, the vials were placed on ice and exposed to a 150-W flood lamp for 5 min, and equivalent quantities of the enzyme solutions were assayed. TCE-RDase solutions were incubated with vinyl chloride (VC) for 90 min. PCE-RDase solutions were incubated with PCE for 15 min.
RESULTS AND DISCUSSION
Culture description.
The anaerobic bacterial enrichment culture used in this work grows by utilizing high levels of PCE (0.5 mM) as the terminal electron acceptor with methanol as a source of electrons, as previously described (31). A pure culture capable of dechlorinating PCE to ethene was isolated from a 10−6 dilution derived from the enrichment culture and was tentatively named Dehalococcoides ethenogenes 195 (23). However, the enrichment culture was used in this study, because the strictly anaerobic pure culture has unidentified nutritional requirements that result in low yields of biomass, which prevented us from using it as a source of cells for purification and characterization of the dehalogenases.
Characteristics of cell lysate.
All of the dehalogenase activity was associated with the cell wall-membrane fraction after centrifugation of cell lysate for 1 h at 105,000 × g. In cell lysates or cell wall-membrane suspensions the dehalogenases reduced PCE and TCE by using hydrogen (4%, vol/vol) as an electron source through the action of membrane-bound hydrogenases. Addition of the cytosolic fraction to the membrane fraction did not increase the dehalogenase activity, but addition of methyl viologen did enhance activity. This indicated that oxidation of hydrogen by the hydrogenases was not rate limiting for dehalogenase activity, and therefore, the endogenous electron donor was associated with the membranes. As purification of the dehalogenases proceeded and hydrogenase activity declined, dechlorination activity required the addition of titanium(III) citrate as a reductant and methyl viologen as the electron carrier.
Pattern of dehalogenation of chlorinated ethenes by membranes.
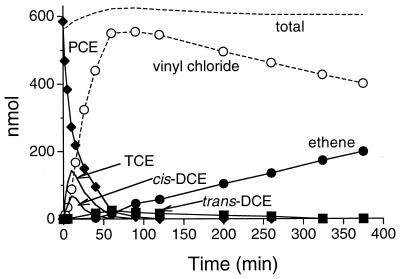
A time course demonstrating the sequential reductive dechlorination of PCE to ethene by membrane fragments is shown in Fig. 1. Dechlorination of approximately 600 nmol of PCE proceeded rapidly, while the accumulation of VC nearly mirrored the decrease in PCE. Both TCE and cis-DCE concentrations reached their maximum values simultaneously early in the time course and declined as the amount of PCE declined. The accumulation of 1,1-DCE exhibited a similar pattern, although the maximum amount of 1,1-DCE observed was only 3 nmol (4% of the amount of cis-DCE). trans-DCE exhibited a different pattern than the other DCE isomers; it was created and dechlorinated much more slowly, and its concentration reached a peak when PCE had nearly disappeared. The data indicated that trans-DCE was a minor component in the flux between TCE and VC. The pattern of accumulation and degradation of DCE isomers, VC, and ethene was similar when TCE was used as the initial substrate (data not shown). The same pattern of substrate utilization was observed with the enrichment culture (31), which supported the contention that all of the components necessary for dechlorination of PCE to ethene are present in the membranes.
FIG. 1.
Time course for PCE degradation by the membrane-bound dehalogenases. Assays were performed as described in the text, except that 600 nmol of PCE was added and allowed to equilibrate at 35°C for 20 min before the reaction was started by adding a cell wall-membrane suspension (2.6 mg of protein). 1,1-DCE was detected but did not accumulate to a significant extent.
Purification of PCE-RDase.
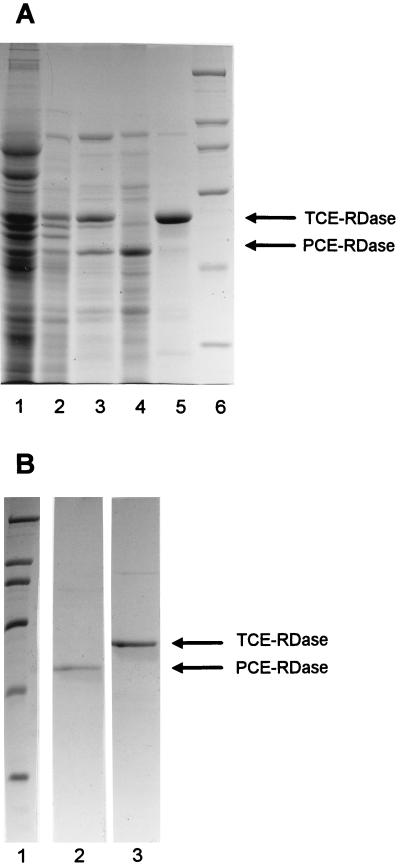
PCE-RDase was solubilized from bacterial membranes with 0.1% Triton X-100 and was purified 75-fold by hydrophobic interaction chromatography (Table 1). The 4.6- by 100-mm PH/M column separated PCE-RDase from TCE-RDase, as shown in Fig. 2A, lanes 4 and 5. One peak of PCE-RDase activity was found at each step in the purification process, suggesting that only one such enzyme was present in the mixed culture. Attempts to further purify PCE-RDase by other liquid chromatography techniques (e.g., ion-exchange, gel filtration, dye affinity, hydroxyapatite, chromatofocusing, or immobilized metal affinity chromatography) or by precipitation (e.g., precipitation with ammonium sulfate, organic solvents, or polyethylene glycol 3400) resulted in no further increase in specific activity due to lability of the activity under the conditions employed, failure to obtain an increase in purity, or both. However, a combination of preparative native gel electrophoresis and denaturing polyacrylamide gel electrophoresis (PAGE) resulted in positive identification of the protein responsible for dehalogenation of PCE. The instability of PCE-RDase at pH values above 8 or below 5 necessitated the use of an electrophoresis system operating at neutral pH. Native PAGE in which a neutral buffer system (7) was used in the presence of 0.05% Triton X-100 separated active PCE-RDase from contaminating proteins. The protein band from the native gel exhibiting strong PCE-RDase activity was eluted and subjected to SDS-PAGE. A band migrating at a molecular weight of 51,000 represented the majority of the protein (Fig. 2B, lane 2). Attempts to determine the molecular weight of native PCE-RDase by gel filtration chromatography were unsuccessful, as the enzyme appeared to form small aggregates which had a range of molecular weights. PCE-RDase appears to be quite specific; neither TCE, the DCE isomers, nor VC was dechlorinated by the enzyme.
TABLE 1.
Partial purification of PCE-RDase
| Purification step | Total protein (mg) | Total activity (μmol of TCE/min)b | Sp act (μmol of TCE/min/mg of protein) | Yield (%) | Purifi- cation (fold) |
|---|---|---|---|---|---|
| Cell membranesa | 292 | 79.5 | 0.27 | 100 | 1.0 |
| Triton X-100 extract | 87 | 89.4 | 1.03 | 112 | 3.8 |
| HP/M POROS column, step elution | 3.2 | 11.6 | 3.63 | 15 | 13 |
| PH/M POROS column, gradient elution | 0.65 | 13.3 | 20.5 | 17 | 75 |
Accurately determining total activity in cell lysates was complicated by the presence of other enzymes competing for the reduced methyl viologen. This was also observed in cell membrane suspensions but to a much lesser extent; therefore, yield and purification values were determined relative to the cell membrane data.
Dehalogenase activities were assayed as described in the text by using 5 μmol of PCE. For steps in which TCE-RDase was present (all of the steps except the PH/M POROS column step) the amount of TCE produced was calculated by adding the amounts of all products.
FIG. 2.
Identification of PCE-RDase and TCE-RDase by SDS-PAGE. (A) SDS–7.5% polyacrylamide gel (20) containing preparations obtained from purification steps. Lane 1, membrane suspension; lane 2, 0.1% Triton X-100 extract; lane 3, 0.25 M ammonium sulfate eluate of dehalogenases from the 16- by 100-mm HP/M POROS column; lane 4, PCE-RDase which eluted early in the 0.5 to 0 M ammonium sulfate gradient from the 4.6- by 100-mm PH/M POROS column; lane 5, TCE-RDase eluted by 0.1% Triton X-100 from the 4.6- by 100-mm PH/M POROS column; lane 6, molecular weight standards (molecular weights, 205,000, 116,000, 97,400, 66,000, 45,000, and 29,000). (B) SDS–9% polyacrylamide gel containing PCE-RDase and TCE-RDase extracted from a native 6% polyacrylamide gel (see text). Lane 1, molecular weight standards (see above); lane 2, protein band from the native gel which had PCE-RDase activity; lane 3, protein band from the native gel which had TCE-RDase activity.
Purification of TCE-RDase.
TCE-RDase was purified 24-fold by detergent solubilization and hydrophobic interaction chromatography, as shown in Fig. 2A, lane 5, and Table 2. A single peak of TCE-RDase activity was identified at each step in the purification process, suggesting that only one such enzyme was present in the mixed culture. When a large mass (40 μg) of the partially purified TCE-RDase was applied to an SDS-polyacrylamide gel, numerous faint bands were observed (Fig. 2A, lane 5). Other chromatographic techniques (see above) resulted in no increase in specific activity of TCE-RDase. Again, native gel electrophoresis was used to positively identify the active protein. The active protein band eluted from the native gel gave one predominant band at 61 kDa and a faint band at 110 kDa, as determined by SDS-PAGE (Fig. 2A, lane 5; Fig. 2B, lane 3). The 110-kDa protein eluted as a major peak between PCE-RDase and TCE-RDase on the 4.6- by 100-mm PH/M column and exhibited no dehalogenase activity by itself. As in the PCE-RDase study, attempts to determine the native molecular weight of TCE-RDase by gel filtration chromatography were unsuccessful, possibly due to aggregation or mixed populations of detergent micelles and enzyme.
TABLE 2.
Partial purification of TCE-RDase
| Purification step | Total protein (mg) | Total activity (μmol of VC/min)a | Sp act (μmol of VC/min/mg of protein) | Yield (%) | Purifi- cation (fold) |
|---|---|---|---|---|---|
| Cell membranes | 292 | 149 | 0.51 | 100 | 1.0 |
| Triton X-100 extract | 87 | 82.4 | 0.95 | 55 | 1.9 |
| HP/M POROS column, step elution | 3.2 | 15.1 | 4.7 | 10 | 9.3 |
| PH/M POROS column, gradient elution | 0.48 | 5.82 | 12.1 | 4 | 24 |
TCE-RDase activity was assayed for 10 min by using 5 μmol of cis-DCE as the substrate. No ethene was produced during the assay; therefore, activity is expressed as micromoles of VC produced per minute.
TCE-RDase was assayed with cis-DCE to prepare the purification table, and activity is reported in Table 2 as micromoles of VC produced. TCE-RDase was also assayed with TCE or VC as the starting substrate, and the purification and yield values were similar. The specific activity for TCE degradation was 43% of the specific activity when cis-DCE was used. More dramatically, the specific activity when VC was the starting substrate was only 0.3% of the specific activity obtained with cis-DCE.
TCE-RDase exhibited a broad substrate range that included TCE, cis-DCE, trans-DCE, 1,1-DCE, and VC. Low rates of PCE dechlorination were observed, but this activity was most likely attributable to contamination with PCE-RDase (Fig. 2A, lane 5). The rates for all of the DCE isomers were determined in parallel experiments. The rates of reduction of the other DCE isomers relative to the rate of reduction of cis-DCE were 72% for 1,1-DCE and 3.7% for trans-DCE.
Pattern of dehalogenation of TCE by TCE-RDase.
A time course for dechlorination of TCE by TCE-RDase is shown in Fig. 3. The occurrence between TCE and ethene of intermediates whose concentrations were far greater than the concentration of enzyme indicated that the products dissociated from TCE-RDase after each two-electron reduction cycle. The pattern produced by TCE-RDase was similar to the pattern observed for the membranes and the mixed culture (31); TCE and cis-DCE were dechlorinated rapidly, trans-DCE was degraded slowly, and VC accumulated and was slowly degraded to ethene. However, there was one striking difference; much more trans-DCE accumulated. After 30 min nearly all of the TCE was degraded and the products were equally distributed between the trans-DCE and VC pools. The rapid production of VC from TCE presumably occurred via cis-DCE rather than trans-DCE, since cis-DCE is dechlorinated 27 times faster. Thus, it appears that cis-DCE and trans-DCE were produced in approximately equal proportions by partially purified TCE-RDase. It is conceivable that a second catalyst which preferentially dechlorinates trans-DCE was separated from TCE-RDase during the purification. However, cis-DCE was degraded about 30- to 50-fold faster than trans-DCE whether crude cell lysate or partially purified TCE-RDase was used as the catalyst, a fact which argues against the existence of a second catalyst. Alternatively, TCE-RDase may have been altered during the purification process, resulting in the loss of its apparent regioselectivity for production of the cis-DCE isomer. This could be a rather subtle alteration of the enzyme considering that all three DCE isomers were produced from TCE whether membranes or partially purified TCE-RDase was used; only the proportions changed.
FIG. 3.
Time course of TCE degradation by TCE-RDase. Assays were performed as described in the text by using 20 μg of TCE-RDase. 1,1-DCE was detected but did not accumulate to a significant extent.
The estimated reduction potentials for the chlorinated ethenes are as follows: TCE to cis-DCE, 0.54 V; TCE to trans-DCE, 0.53 V; TCE to 1,1-DCE, 0.50 V; cis-DCE to VC, 0.36 V; trans-DCE to VC, 0.37 V; 1,1-DCE to VC, 0.40 V; and VC to ethene, 0.49 V (34). Thus, using free energies derived from the reduction potentials, one would expect the order of reduction rates to be TCE > VC > DCEs. However, the observed order of reduction rates was cis-DCE > 1,1-DCE > TCE > trans-DCE > VC. This suggests that kinetic factors, rather than thermodynamic factors, control the competence of each substrate for reductive dechlorination by TCE-RDase.
Roles of corrinoids in the dehalogenases.
The cofactor requirements for enzymatic reductive dechlorination were partially determined by inhibition experiments performed with iodoalkanes. TCE-RDase lost 93% of its activity upon incubation with 1-iodopropane and titanium(III) citrate in the dark. Subsequent exposure to light restored 80% of the original activity. This indicated that a corrinoid cofactor containing Co(I) was involved in dechlorination by the TCE-RDase (5, 25). This cofactor requirement was also observed for the recently isolated cytosolic PCE-RDase from Dehalospirillum multivorans, which reduces PCE or TCE to cis-DCE (26).
Similar analyses were performed with the PCE-RDase isolated in this study. Incubation of PCE-RDase with titanium(III) citrate and 1-iodopropane had no effect on activity. In contrast, incubation of PCE-RDase with iodoethane and titanium(III) citrate resulted in a loss of 85% of the initial activity. After irradiation with light 83% of the initial activity was recovered. This suggests that PCE-RDase also contains a corrinoid with cobalt(I). The difference between the reactivity of PCE-RDase with iodoethane and the reactivity of PCE-RDase with 1-iodopropane implies that the substrate binding pocket of PCE-RDase may be too small to accommodate the three-carbon compound 1-iodopropane.
The results of the iodoalkane inhibition experiments which suggested that corrinoids are present in PCE-RDase and TCE-RDase were consistent with previous observations about the nutritional requirements of the hydrogen-utilizing enrichment cultures. High concentrations of vitamin B12 (50 μg/liter) were found to greatly stimulate dechlorination activity in these cultures (22). Therefore, it was reasonable to expect a priori that corrinoids might be cofactors for the dehalogenases.
It has previously been observed that transition metal cofactors (vitamin B12, heme, and coenzyme F430) reductively dechlorinate PCE and TCE (15). A comparison of reductive dechlorination by vitamin B12 (cyanocobalamin) and TCE-RDase revealed similarities and differences. Both TCE-RDase and free cyanocobalamin dechlorinate VC much more slowly than they dechlorinate TCE or the DCE isomers. However, TCE-RDase dechlorinates TCE, cis-DCE, and 1,1-DCE at similar rates, whereas cyanocobalamin dechlorinates TCE 50- to 100 times faster than it dechlorinates the DCE isomers (15). The other major difference between cyanocobalamin and TCE-RDase is the absolute rate of degradation of chlorinated ethenes. For example, TCE is dechlorinated at a rate of 3 nmol/min/μmol of cyanocobalamin (6) and at a rate of 300 μmol/min/μmol of TCE-RDase monomer; the enzyme is 5 orders of magnitude faster than cyanocobalamin. The differences between free cyanocobalamin and TCE-RDase are probably due to one or more factors relating to the protein environment of the corrinoid in TCE-RDase. For instance, the enzyme may affect the geometry of the corrinoid (36), the ligation of the cobalt may be different, additional redox cofactors may be present, amino acid side chains may be involved in the active site, or a combination of these factors may occur.
Other inhibitors of the dehalogenases.
TCE-RDase was 50% inhibited by 2.5 mM sodium cyanide or 7 mM sodium azide. PCE-RDase was 20% inhibited by 20 mM sodium cyanide. Both enzymes were completely inhibited by 2 mM sodium sulfite or sodium dithionite. The dehalogenases were not inhibited by 2 mM sodium sulfate, sodium selenate, sodium sulfide, or 100% carbon monoxide. Incubation of the dehalogenases with the metal chelators EDTA (5 mM), bathophenanthroline disulfonate, and 2,2-dipyridyl for 30 min had no effect on activity. TCE-RDase was completely inhibited by 1 mM cuprous chloride, while PCE-RDase was only slightly inhibited. A similar pattern was observed with 5 mM zinc chloride, although TCE-RDase was not fully inhibited. The inhibition by cyanide and the lack of inhibition by carbon monoxide were consistent with the presence of a B12 cofactor (13).
The pattern of inhibition by the other reagents may indicate the presence of one or more metal centers, in addition to B12, that are tightly bound by the enzymes or inaccessible to large chelating agents or both. By analogy to the PCE-RDase from Dehalospirillum multivorans (26), these putative metal centers may be iron-sulfur clusters. The effects of EDTA and azide on PCE-RDase and TCE-RDase contrast with the effects of these reagents on the dehalogenase from Dehalospirillum multivorans (25); this fact does not exclude the possibility that iron-sulfur clusters are present in the former but does emphasize the uniqueness of each dehalogenase. The presence of iron-sulfur clusters in PCE-RDase and TCE-RDase would be consistent with the observation that ferrous ammonium sulfate and cysteine stabilized the activity of these enzymes.
Alternatively, the inhibition of the dehalogenases by copper and zinc may result from interaction with amino acid residues (e.g., cysteine, methionine, or histidine residues) important for the catalytic activity of TCE-RDase (32). Similarly, sulfite (a product of the oxidation of dithionite) is known to form S-sulfonate derivatives of cysteine residues (17), and thiosulfate might react analogously, which could account for the inhibition of the dehalogenases by those reagents.
In summary, we identified two membrane-bound reductive dehalogenases which constitute a novel catabolic pathway for complete dechlorination of PCE to ethene. Membranes prepared from a mixed culture exhibited a pattern of substrate utilization similar to the patterns exhibited by the mixed culture (31) and the isolated dechlorinating organism Dehalococcoides ethenogenes 195 (22). The 61-kDa TCE-RDase appears to be a corrinoid-containing enzyme that catalyzes dechlorination of all chlorinated ethenes except PCE. The 51-kDa PCE-RDase specifically reduces PCE to TCE at a high rate and also appears to contain a corrinoid. Both PCE-RDase and TCE-RDase of strain 195 share certain properties with the membrane-bound halobenzoate-reductive dehalogenases and the cytosolic PCE-RDase from Dehalospirillum multivorans, while other properties are unique. Further study of PCE-RDase and TCE-RDase should contribute to our understanding of enzymatic reductive dechlorination of toxic chlorinated ethenes.
ACKNOWLEDGMENTS
This work was performed while R.V.S. held a National Research Council-U.S. Air Force Postdoctoral Research Associateship. J.K.M. and D.R.B. were funded in part by the Strategic Environmental Research and Development Program of the Department of Defense, the Department of Energy, and the U. S. Environmental Protection Agency. J.M.G. and S.H.Z. were supported by the U. S. Air Force Armstrong Laboratory, Environmental Quality Directorate, Tyndall Air Force Base, Fla.
REFERENCES
- 1.Bagley D M, Gossett J M. Tetrachloroethene transformation to trichloroethene and cis-1,2-dichloroethene by sulfate-reducing enrichment cultures. Appl Environ Microbiol. 1990;56:2511–2516. doi: 10.1128/aem.56.8.2511-2516.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrio-Lage G, Parsons F Z, Nassar R S. Kinetics of the depletion of trichloroethene. Environ Sci Technol. 1987;21:366–370. doi: 10.1021/es00158a005. [DOI] [PubMed] [Google Scholar]
- 3.Bouwer E J, McCarty P L. Transformation of 1- and 2-carbon halogenated aliphatic organic compounds under methanogenic conditions. Appl Environ Microbiol. 1983;45:1286–1294. doi: 10.1128/aem.45.4.1286-1294.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Brot B, Weissbach H. Enzymatic synthesis of methionine: chemical alkylation of the enzyme-bound cobamide. J Biol Chem. 1965;240:3064–3070. [PubMed] [Google Scholar]
- 6.Burris D R, Delcomyn C A, Smith M H, Roberts A L. Reductive dechlorination of tetrachloroethylene and trichloroethylene catalyzed by vitamin B12 in homogeneous and heterogeneous systems. Environ Sci Technol. 1996;30:3047–3052. [Google Scholar]
- 7.Chrambach A. The practice of quantitative gel electrophoresis. Deerfield Beach, Fla: VCH Publishers; 1985. [Google Scholar]
- 8.deBruin W P, Kotterman M J J, Posthumus J A, Schraa G, Zehnder A J B. Complete biological reductive transformation of tetrachloroethene to ethane. Appl Environ Microbiol. 1992;58:1996–2000. doi: 10.1128/aem.58.6.1996-2000.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeWeerd K A, Suflita J M. Anaerobic aryl reductive dehalogenation of halobenzoates by cell extracts of “Desulfomonile tiedjei.”. Appl Environ Microbiol. 1990;56:2999–3005. doi: 10.1128/aem.56.10.2999-3005.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiStefano T D, Gossett J M, Zinder S H. Reductive dechlorination of high concentrations of tetrachloroethene to ethene by an anaerobic enrichment culture in the absence of methanogenesis. Appl Environ Microbiol. 1991;57:2287–2292. doi: 10.1128/aem.57.8.2287-2292.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensley B D. Biochemical diversity of trichloroethylene metabolism. Annu Rev Microbiol. 1991;45:283–299. doi: 10.1146/annurev.mi.45.100191.001435. [DOI] [PubMed] [Google Scholar]
- 12.Federal Register. Environmental Protection Agency, National Primary and Secondary Drinking Water Regulations. Fed Regist. 1989;54:22062–22160. [Google Scholar]
- 13.Firth R A, Hill H A O, Pratt J M, Thorp R G, Williams R J P. The chemistry of vitamin B12. Part XI. Some further formation constants. J Chem Soc A. 1969;1969:381–386. [Google Scholar]
- 14.Freedman D L, Gossett J M. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol. 1989;55:2144–2151. doi: 10.1128/aem.55.9.2144-2151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gantzer C J, Wackett L P. Reductive dechlorination catalyzed by bacterial transition-metal coenzymes. Environ Sci Technol. 1991;25:715–722. [Google Scholar]
- 16.Gibson S A, Sewell G W. Stimulation of reductive dechlorination of tetrachloroethene in anaerobic aquifer microcosms by addition of short-chain organic acids and alcohols. Appl Environ Microbiol. 1992;58:1392–1393. doi: 10.1128/aem.58.4.1392-1393.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glazer A N, DeLange R J, Sigman D S. Modification of protein side-chains: group-specific reagents. In: Work T S, Work E, editors. Laboratory techniques in biochemistry and molecular biology. Chemical modification of proteins: selected methods and analytical procedures. New York, N.Y: American Elsevier Publishing Co. Inc.; 1975. pp. 108–109. [Google Scholar]
- 18.Holliger C, Schraa G, Stams A J M, Zehnder A J B. A highly purified enrichment culture couples the reductive dechlorination of tetrachloroethene to growth. Appl Environ Microbiol. 1993;59:2991–2997. doi: 10.1128/aem.59.9.2991-2997.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holliger C, Schumacher W. Reductive dehalogenation as a respiratory process. Antonie Leeuwenhoek. 1994;66:239–246. doi: 10.1007/BF00871642. [DOI] [PubMed] [Google Scholar]
- 20.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 21.Loffler F E, Sanford R A, Tiedje J M. Initial characterization of a reductive dehalogenase from Desulfitobacterium chlororespirans Co23. Appl Environ Microbiol. 1996;62:3809–3813. doi: 10.1128/aem.62.10.3809-3813.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maymó-Gatell X, Tandoi V, Gossett J M, Zinder S H. Characterization of an H2-utilizing enrichment culture that reductively dechlorinates tetrachloroethene to vinyl chloride and ethene in the absence of methanogenesis and acetogenesis. Appl Environ Microbiol. 1995;61:3928–3933. doi: 10.1128/aem.61.11.3928-3933.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maymó-Gatell X, Chien Y, Gossett J M, Zinder S H. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science. 1997;276:1568–1571. doi: 10.1126/science.276.5318.1568. [DOI] [PubMed] [Google Scholar]
- 24.Mohn W W, Tiedje J M. Evidence for chemiosmotic coupling of reductive dechlorination and ATP synthesis in Desulfomonile tiedjei. Arch Microbiol. 1991;157:1–6. [Google Scholar]
- 25.Neumann A, Wohlfarth G, Diekert G. Properties of tetrachloroethene and trichloroethene dehalogenase of Dehalospirillum multivorans. Arch Microbiol. 1995;163:276–281. [Google Scholar]
- 26.Neumann A, Wohlfarth G, Diekert G. Purification and characterization of tetrachloroethene reductive dehalogenase from Dehalospirillum multivorans. J Biol Chem. 1996;271:16515–16519. doi: 10.1074/jbc.271.28.16515. [DOI] [PubMed] [Google Scholar]
- 27.Ni S, Fredrickson J K, Xun L. Purification and characterization of a novel 3-chlorobenzoate-reductive dehalogenase from the cytoplasmic membrane of Desulfomonile tiedjei DCB-1. J Bacteriol. 1995;177:5135–5139. doi: 10.1128/jb.177.17.5135-5139.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons F, Wood P R, DeMarco J. Transformation of tetrachloroethene and trichloroethene in microcosms and groundwater. J Am Water Works Assoc. 1984;76(2):56–59. [Google Scholar]
- 29.Scholz-Muramatsu H, Neumann A, Meßmer M, Moore E, Diekert G. Isolation and characterization of Dehalospirillum multivorans gen. nov., sp. nov., a tetrachloroethene-utilizing, strictly anaerobic bacterium. Arch Microbiol. 1995;163:48–56. [Google Scholar]
- 30.Schumacher W, Holliger C. The proton/electron ratio of the menaquinone-dependent electron transport from dihydrogen to tetrachloroethene in “Dehalobacter restrictus.”. J Bacteriol. 1996;178:2328–2333. doi: 10.1128/jb.178.8.2328-2333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tandoi V, DiStefano T D, Bowser P A, Gossett J M, Zinder S H. Reductive dehalogenation of chlorinated ethenes and halogenated ethanes by a high-rate anaerobic enrichment culture. Environ Sci Technol. 1994;28:973–979. doi: 10.1021/es00054a033. [DOI] [PubMed] [Google Scholar]
- 32.Vallee B L, Wacker W E C. Inhibition of metalloproteins by chelating agents and metals. In: Neurath H, editor. The proteins: composition, structure and function. 2nd ed. V. Metalloproteins. New York, N.Y: Academic Press; 1970. pp. 143–144. [Google Scholar]
- 33.Vogel T M, McCarty P L. Biotransformation of tetrachloroethylene to trichloroethylene, dichloroethylene, vinyl chloride, and carbon dioxide under methanogenic conditions. Appl Environ Microbiol. 1985;49:1080–1083. doi: 10.1128/aem.49.5.1080-1083.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogel T M, Criddle C S, McCarty P L. Transformations of halogenated aliphatic compounds. Environ Sci Technol. 1987;21:722–736. doi: 10.1021/es00162a001. [DOI] [PubMed] [Google Scholar]
- 35.Westrick J J, Mello J W, Thomas R F. The groundwater supply survey. J Am Water Works Assoc. 1984;76(5):52–59. [Google Scholar]
- 36.Wirt M D, Kumar M, Wu J-J, Scheuring E M, Ragsdale S W, Chance M R. Structural and electronic factors in heterolytic cleavage: formation of the Co(I) intermediate in the corrinoid/iron-sulfur protein from Clostridium thermoaceticum. Biochemistry. 1995;34:5269–5273. doi: 10.1021/bi00015a042. [DOI] [PubMed] [Google Scholar]
- 37.Zehnder A J B, Wuhrmann K. Titanium(III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976;194:1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]