Abstract
Background
Maternal nutrition during pregnancy is known to have an effect on fetal growth and development. It is recommended that women increase their calcium intake during pregnancy and lactation, although the recommended dosage varies among professionals. Currently, there is no consensus on the role of routine calcium supplementation for pregnant women other than for preventing or treating hypertension.
Objectives
To determine the effect of calcium supplementation on maternal, fetal and neonatal outcomes (other than for preventing or treating hypertension) as well as any possible side effects.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30th September 2014).
Selection criteria
We considered all published, unpublished and ongoing randomised controlled trials (RCTs) comparing maternal, fetal and neonatal outcomes in pregnant women who received calcium supplementation versus placebo or no treatment. Cluster‐RCTs were eligible for inclusion but none were identified. Quasi‐RCTs and cross‐over studies were not eligible for inclusion.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy.
Main results
Twenty‐five studies met the inclusion criteria, but only 23 studies contributed data to the review. These 23 trials recruited 18,587 women, with 17,842 women included in final analyses. There were no statistically significant differences between women who received calcium supplementation and those who did not in terms of reducing preterm births less than 37 weeks' gestation (risk ratio (RR) 0.86, 95% confidence interval (CI) 0.70 to 1.05; 13 studies, 16,139 women; random‐effects model) or less than 34 weeks' gestation (RR 1.04, 95% CI 0.80 to 1.36; four trials, 5669). Most studies were of low risk of bias. We conducted sensitivity analysis for the outcome of preterm birth less than 37 weeks by removing two trials with unclear risk of bias for allocation concealment; the results then favoured treatment with calcium supplementation (RR 0.80, 95% CI 0.65 to 0.99; 11 trials, 15,379 women). There was no significant difference in infant low birthweight between the two treatment groups (RR 0.93, 95% CI 0.81 to 1.07; six trials, 14,162 infants; random‐effects model). However, when compared to the control group, women in the calcium supplementation group gave birth to slightly heavier birthweight infants (mean difference 56.40, 95% CI 13.55 to 99.25; 21 trials, 9202 women; random‐effects model).
Three outcomes were chosen for assessment with the GRADE software: preterm birth less than 37 weeks; preterm birth less than 34 weeks; and low birthweight less than 2500 g. Evidence for these outcomes was assessed as of moderate quality.
Authors' conclusions
This review indicates that there are no clear additional benefits to calcium supplementation in prevention of preterm birth or low infant birthweight. While there was a statistically significant difference of 56 g identified in mean infant birthweight, there was significant heterogeneity identified, and the clinical significance of this difference is uncertain.
Keywords: Female; Humans; Infant, Newborn; Pregnancy; Dietary Supplements; Dietary Supplements/adverse effects; Infant, Low Birth Weight; Pregnancy Outcome; Prenatal Nutritional Physiological Phenomena; Birth Weight; Calcium, Dietary; Calcium, Dietary/administration & dosage; Calcium, Dietary/adverse effects; Pre‐Eclampsia; Pre‐Eclampsia/prevention & control; Premature Birth; Premature Birth/prevention & control; Randomized Controlled Trials as Topic; Sensitivity and Specificity
Plain language summary
Effect of taking extra calcium (other than preventing or treating high blood pressure) during pregnancy for improving maternal and infant health
Maternal nutrition during pregnancy is known to have a significant effect on fetal growth and development. In our review, regular intake of extra calcium tablets during pregnancy did not improve the number of preterm births or other infant outcomes, except for a slight increase in infant birthweight in the group of women who received calcium supplementation. Most studies included in this review were assessed as of low risk of bias, and evidence for specific outcomes was graded as of moderate quality, Taking calcium supplementation did not appear to have any obvious side effects. Our review included 25 randomised controlled studies, but only 23 studies involving 18,587 women contributed outcome data. The majority of the evidence was based on fewer numbers of studies.
Summary of findings
Summary of findings for the main comparison. Calcium supplementation versus placebo or no treatment (maternal outcomes) for preventing or treating hypertension) for improving pregnancy and infant outcomes.
| Calcium supplementation versus placebo or no treatment for improving pregnancy and infant outcomes | ||||||
| Patient or population: healthy pregnant women receiving calcium supplementation vs placebo or no treatment Settings: trials located in Australia, Guatemala, India (3), Iran, and the USA (3). A multi‐centre study took place in Argentina, Egypt, India, Peru, South Africa, United Kingdom and Vietnam. Intervention: calcium supplementation versus placebo or no treatment (maternal outcomes) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Calcium supplementation versus placebo or no treatment (maternal outcomes) | |||||
| Preterm birth (a) Birth prior to 37 weeks | Study population | RR 0.86 (0.7 to 1.05) | 16139 (13 studies) | ⊕⊕⊕⊝ moderate1 | We conducted sensitivity analysis by removing 2 trials with unclear risk of bias for allocation concealment; the results then favoured treatment with calcium supplementation (RR 0.80, 95% CI 0.65 to 0.99; 11 trials, 15379 women). | |
| 105 per 1000 | 90 per 1000 (73 to 110) | |||||
| Moderate | ||||||
| 100 per 1000 | 86 per 1000 (70 to 105) | |||||
| Preterm birth (b) Birth prior to 34 weeks | Study population | RR 1.04 (0.8 to 1.36) | 5669 (4 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 36 per 1000 | 38 per 1000 (29 to 49) | |||||
| Moderate | ||||||
| 30 per 1000 | 31 per 1000 (24 to 41) | |||||
| Low birthweight (< 2500 g) | Study population | RR 0.93 (0.81 to 1.07) | 14162 (6 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 116 per 1000 | 108 per 1000 (94 to 125) | |||||
| Moderate | ||||||
| 86 per 1000 | 80 per 1000 (70 to 92) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Wide confidence interval crossing the line of no effect. (‐1) 2 Statistical heterogeneity (I2 > 60%). (‐1)
Background
Description of the condition
Calcium metabolism
Calcium is an essential mineral for many of the body's processes (Trichopoulou 1990). Calcium is a key and important intracellular component for maintaining cell membranes, and has a role in nerve cell function, muscle contraction, enzyme and hormone actions, and is essential for bone mineralisation. Maternal nutrition during pregnancy has a significant effect on fetal growth and development (Luke 1994; Susser 1991). Calcium is transported across the placenta by an active transport process, and is important in many developmental functions, including skeletal development (McGuire 2007).
During pregnancy and lactation women require an increase in their calcium intake (Cross 1995; Ritchie 1998). This is not only to maintain maternal calcium balance and bone density, but also to meet the demands of the growing fetus/infant.
Description of the intervention
Calcium supplementation
The recommendations for calcium intake during pregnancy and lactation vary from 600 mg to 1425 mg per day, up to 600 mg higher than in non‐pregnant women (Prentice 1994). Approximately 200 mg of calcium per day is secreted into breast milk (Prentice 1994).
The increase in calcium requirements may be met through dietary intake. However, supplementation of calcium during pregnancy and lactation has been recommended by some, at doses between 300 mg and 2000 mg per day (Belizan 1991; Koo 1999; Raman 1978). For this review, we have arbitrarily divided calcium supplementation into low dose (less than 1000 mg per day) and high dose (1000 mg or more per day) (Jarjou 2006; Kalkwarf 1997;Prentice 1995; Raman 1978; Villar 1990).
Calcium tablets are inexpensive and readily available. However, side effects have been reported, including difficulty in swallowing, an increase in urinary stones and urinary tract infection, as well as reduced absorption of other minerals such as iron, zinc and magnesium (Hallberg 1992; McGuire 2007).
The effect of calcium supplementation on weight is unclear, with some studies identifying a reduction in body weight, possibly through the combination of calcium with fatty acids which are subsequently not absorbed by the body (Heaney 2002; Sampath 2008; Trowman 2006; Yanovski 2009).
How the intervention might work
During pregnancy and lactation, maternal bone mineral density decreases in multiple sites of the body such as the lumbar spine, femoral neck, hip and wrist. However, this is quickly reversed after cessation of breastfeeding (Cross 1995; Kalkwarf 1997; Laskey 1999; Prentice 1995; Sowers 1993; Sowers 1995). Inadequate intake of calcium may harm both the woman and her fetus. Maternal risks of inadequate calcium intake include osteopenia, osteoporosis, tremor, paraesthesia, muscle cramps and tetany (muscle spasm and twitching). Potential problems for the fetus/infant include delayed fetal growth, low birthweight and poor bone mineralisation (Inzucchi 1999; Koo 1999). It is unclear whether calcium supplementation may help women and babies avoid the complications associated with inadequate calcium intake.
Why it is important to do this review
Current approach to calcium supplementation in pregnancy
Currently, there is no consensus on the role of routine calcium supplementation for pregnant women.
A Cochrane review evaluating calcium supplementation for the prevention of pre‐eclampsia identified a significant beneficial effect, almost halving the risk of women developing pregnancy‐induced hypertension (Hofmeyr 2014). However, the effect of calcium supplementation on other pregnancy and infant outcomes remains uncertain, with some studies identifying a beneficial effect on fetal growth and bone mineralisation (Chan 2006; Chang 2003; Janakiraman 2003), although this is not universal (Jarjou 2006; Prentice 1995). Calcium also plays a role in smooth muscle function, being important in muscle contraction. Some studies have suggested that calcium supplementation may contribute to altered muscle tone and may therefore contribute to the risk of preterm birth (Hofmeyr 2014), although the precise effect is unclear (Belizan 1991; Carroli 1994; Lopez‐Jaramillo 1989; Villar 1990; Villar 1998). While there is a clear benefit of calcium supplementation in the prevention of hypertension during pregnancy, the effect on other outcomes requires further evaluation.
Objectives
To determine the effect of calcium supplementation on maternal, fetal and neonatal outcomes (other than for preventing or treating hypertension), including the occurrence of side effects.
Methods
Criteria for considering studies for this review
Types of studies
We included all published, unpublished and ongoing simple and randomised controlled trials (RCTs) comparing maternal, fetal, and neonatal outcomes in pregnant women who received calcium supplementation compared with placebo or no treatment. Cluster‐RCTs were eligible for inclusion but none were identified. Quasi‐RCTs and cross‐over studies were not eligible for inclusion.
Types of participants
Pregnant women who received any calcium supplementation compared with placebo or no treatment.
Types of interventions
Calcium supplementation during pregnancy compared with placebo or no treatment.
Types of outcome measures
Primary outcomes
Maternal outcomes
Preterm birth less than 37 weeks' gestation
Infant outcomes
Low birthweight (less than 2500 g)
Secondary outcomes
Maternal outcomes
Preterm birth less than 34 weeks' gestation
Maternal weight gain
Maternal bone mineral density (BMD) measured by dual‐energy x‐ray absorptiometry (osteopenia is classified as BMD between ‐1 and ‐2.5 SD; osteoporosis is classified as BMD less than ‐2.5 SD)
Leg cramps
Backache
Tetany
Incidence of fracture
Duration of breastfeeding
Tremor
Paraesthesia
Mother admitted to an intensive care unit
Maternal death
Mode of birth (vaginal birth, Instrumental vaginal birth, caesarean section)
Postpartum haemorrhage
Fetal and neonatal outcomes
Stillbirth or fetal death (fetus died in uterus after 20 weeks' gestation or during labour and delivery)
Neonatal death (baby died in first 28 days of life)
Perinatal mortality (stillbirth and neonatal death)
Admission to neonatal intensive care unit
Birthweight
Birth length
Head circumference
Intrauterine growth restriction
Neonatal BMD (measured by single‐photon absorptiometry or dual‐energy x‐ray absorptiometry)
Osteopenia
Rickets
Fracture
Adverse outcomes
Side effects of calcium supplementation
Compliance
Satisfaction (as defined by the trial authors)
Urinary stones
Urinary tract infection
Nephrocalcinosis
Impaired renal function (as defined by the trial authors)
Maternal anaemia (as defined by the trial authors)
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 September 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeBuppasiri 2011.
For this update, the following methods were used for assessing the 19 reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
For this update the quality of the evidence was assessed using the GRADE approach (Schunemann 2009). We assessed the quality of the body of evidence relating to the following outcomes.
Preterm birth < 37 weeks
Preterm birth < 34 weeks
Low birthweight (< 2500 g)
GRADE profiler (GRADEpro 2014) was used to import data from Review Manager 5.3 (RevMan 2014) and create a ’Summary of findings’ table, or a summary of the intervention effect and a measure of quality for each of the above outcomes. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference as outcomes were measured in the same way between trials. In future updates, we may use the standardised mean difference to combine trials that measure the same outcome but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We have not included any cluster‐randomised trials in this update. If in future updates we include cluster‐randomised trials in the analyses, we will adjust their sample sizes using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We have not included cross‐over trials in this review and do not consider this design appropriate to answer the review's questions.
Other unit of analysis issues
We have not included multiple pregnancies in this review because multiple pregnancies may have an effect on the outcomes of interest, such as preterm birth, and so studies including multiple pregnancies are not considered eligible for inclusion.
In studies that had more than two treatment groups, we divided the placebo arm between the two treatment arms. Specifically, for the trial Belizan 1983, in Analysis 2.4 and Analysis 2.9, the placebo arm was halved to enable inclusion of data for treatment groups one and two.
2.4. Analysis.
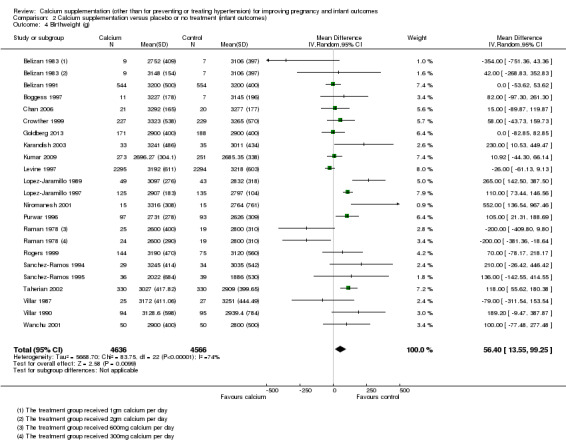
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 4 Birthweight (g).
2.9. Analysis.
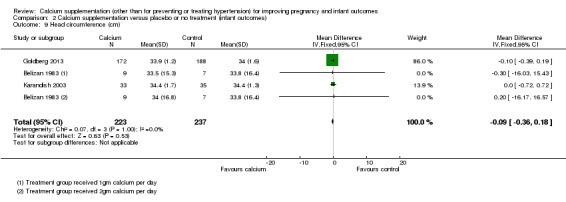
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 9 Head circumference (cm).
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. When we identified substantial heterogeneity (above 30%), we explored it by performing pre‐specified subgroup analysis.
Assessment of reporting biases
Where there were 10 or more studies in the meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we performed exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. Where we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We carried out the following subgroup analyses for maternal primary outcomes.
Preterm birth < 37 weeks by dose of calcium (low dose or less than 1000 mg/day versus high dose or 1000 mg/day or more)
Preterm birth < 37 weeks by gestational week started to take calcium (before 20 weeks versus 20 weeks or more)
Preterm birth < 37 weeks by type of calcium (carbonate versus lactate versus gluconate)
We carried out the following subgroup analyses for Infant primary outcomes.
Low birthweight < 2500 g by gestational week started to take calcium (before 20 weeks versus 20 weeks or more)
Low birthweight < 2500 g by type of calcium (gluconate versus carbonate)
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to carry out the following sensitivity analysis to explore the effect of trial quality assessed by concealment of allocation, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Preterm birth < 37 weeks (adequate allocation concealment versus unclear or no allocation concealment)
Preterm birth < 34 weeks (adequate allocation concealment versus unclear or no allocation concealment)
Low birthweight (< 2500 g)
We conducted the above sensitivity analysis, apart from the low birthweight outcome because there were no trials at unclear or low risk of bias for allocation concealment contributing data to this outcome.
Results
Description of studies
Results of the search
The original search yielded 72 trial reports (Buppasiri 2011). After exploring the contents and grouping for duplicates, we included data from 21 trials (54 reports). One further trial that was otherwise eligible for inclusion specifically focused on maternal blood lead levels and did not report any other outcomes, and so has not contributed data to the review (Ettinger 2009). We have provided details of this study in Characteristics of included studies but we have not referred to this study in the discussion of included studies below. We excluded 14 trials and four trials were 'Awaiting classification' because only the abstracts were available (Almirante 1998; Chames 2002;Galimberti 2001) ‐ we tried to contact the authors but unfortunately full papers were not available.
For this update, a search in September 2014 identified another 19 reports for eligibility assessment. Three new trials were eligible for inclusion (Goldberg 2013; Herrera 2006; Kumar 2009). Four new reports (related to three trials), one of which requires translation (Zheng 2000), were abstracts and were added to Studies awaiting classification. Six reports were excluded, three of which were duplicates for studies in awaiting classification (Almirante 1998; Chames 2002; Galimberti 2001) and have now been excluded because we have not had replies from authors at the second round of queries. Finally, six further new reports were additional publications for already included studies.
At this update, we now have 25 included studies (63 reports), but only 23 studies contribute outcome data (Herrera 2006 and Ettinger 2009 contribute no outcome data). There are 20 excluded studies (21 reports), and three abstracts that remain in Studies awaiting classification (we have attempted to contact authors but have had no replies). We should note that the newly included Goldberg 2013 reports on the same trial as the previously included Jarjou 2006. Jarjou 2006 analyses a subset of women and reports specific outcomes not included in the much later report on the full sample. We have kept these data separate for clarity.
Included studies
For more information about included studies, see:Characteristics of included studies.
Design
All included studies were reported as randomised controlled trials (RCTs), and one trial (Villar 2006) was stratified by country.
Sample size
The total number of participants included in the 23 trials (of the 25 included trials) that contributed data to this review was 18,587 pregnant women, but only 17,842 were included in final analyses. Ettinger 2009 and Herrera 2006 did not contribute to outcome data for this review. Missing data amounted to 4.01% overall (745 in 17,842). The sample size varied from 23 to 8325 participants per trial.
Setting
The 25 included trials took place in various countries: Argentina, Australia, Columbia, Egypt, Ecuador, Gambia, Guatemala, Hong Kong, India, Iran, Mexico, South Africa, United States and Vietnam.
Participants
This review includes data for 18,578 pregnant women. Three trials (Chan 2006; Herrera 2006; Villar 1990) included only adolescent pregnant women (309 women, mean age 17.0 years), but the remaining trials were not restricted to adolescents. Two trials (Jarjou 2006; Raman 1978) included only pregnant women from low socioeconomic groups. The largest study (Villar 2006 with 8325 women) recruited only pregnant women who received less than 600 mg dietary calcium per day. One study (Lopez‐Jaramillo 1997) included pregnant women who had lived at an altitude of 2800 m for a period of at least one year. One study (Sanchez‐Ramos 1994) enrolled pregnant women who had normotension but positive roll‐over and angiotensin tests.
Interventions
Calcium supplementation was used in the treatment groups in all trials and compared with placebo or no treatment control groups. Various types of calcium were used such as calcium carbonate, calcium gluconate, calcium lactate and combined calcium. Calcium carbonate was prescribed in most studies (in 17 of the 23 trials). Calcium lactate was prescribed in one trial and calcium gluconate was prescribed in one trial. Combined calcium supplementation was prescribed in two trials and three trials did not specify the type of calcium used. For timing of calcium supplementation; 11 trials (Belizan 1991; Boggess 1997; Crowther 1999; Goldberg 2013; Jarjou 2006; Karandish 2003; Lopez‐Jaramillo 1989; Purwar 1996; Sanchez‐Ramos 1995; Taherian 2002; Villar 1990) started calcium supplementation at 20 weeks' gestational age (or after) until delivery. Five trials (Belizan 1983; Chan 2006; Kumar 2009; Levine 1997; Villar 2006) started calcium supplementation at gestational age less than 20 weeks until delivery. Timing was unclear in the remaining studies. For dosage of calcium, 14 trials (Belizan 1991; Boggess 1997; Crowther 1999; Goldberg 2013; Jarjou 2006; Karandish 2003; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Purwar 1996; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Villar 1990; Villar 2006) prescribed 1000 mg/d or more (range 1000 to 2000 mg/d). Three trials (Raman 1978; Rogers 1999; Taherian 2002) prescribed calcium less than 1000 mg/day (range 300 mg to 600 mg). In the Taherian 2002 study, calcium supplementation (Caltrate) was prescribed 600 mg at 22 to 32 weeks' gestational age and then 1200 mg from 32 weeks until delivery.
Outcomes
The primary outcomes or objectives of 16 of the 23 trials that contributed data to this review were incidence of pregnancy induced hypertension or changes in blood pressure, which were not relevant to this review. However, these studies also reported other outcome data relevant to this review, e.g. preterm birth, maternal weight gain, gestational age, birthweight, birth length, and we have therefore included these data. Thirteen trials with a total of 16,139 participants (Belizan 1991; Boggess 1997; Crowther 1999; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Purwar 1996; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Taherian 2002; Villar 1990; Villar 2006; Wanchu 2001) evaluated the effect of calcium supplementation on preterm birth before 37 weeks. Four trials, with 5669 participants (Crowther 1999; Kumar 2009; Levine 1997; Wanchu 2001) evaluated the effect of calcium supplementation on preterm birth before 34 weeks. Six of the trials with 14,162 participants (Crowther 1999; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Villar 1990; Villar 2006) evaluated the effect of calcium supplementation on low birthweight (less than 2500 g). Seven trials (Belizan 1991; Crowther 1999; Levine 1997; Villar 1987; Villar 1990; Villar 2006; Wanchu 2001) evaluated side effects of calcium supplementation. For further details, seeCharacteristics of included studies.
No trials reported the effect of calcium supplementation on leg cramps, backache, tetany, tremor, paraesthesia, osteopenia, osteoporosis, fracture in pregnant women, duration of breastfeeding or postpartum haemorrhage, and no trials reported on fetal or neonatal osteopenia, rickets and fracture.
Excluded studies
We excluded 20 trials from this review. The reasons for exclusion include: participants, interventions and methodology were not appropriate and there was insufficient information for inclusion. For more information, seeCharacteristics of excluded studies. For more information about the studies which we have not yet assessed for inclusion, seeCharacteristics of studies awaiting classification.
Risk of bias in included studies
The number of participants in trials ranged from 23 to 8325 per trial.The risk of bias in included studies varied. The overall missing data (lost to final analysis) were 4.01% (745 in 17842) ranging from (0% to 68.1%). Seven of the 23 trials contributing data had no missing data. Ten of the 23 trials had missing data less than 10%. Only one trial had a very high rate of missing data (68.1%). The largest trial had 0.16% missing data. The majority of included studies used methods of sequence generation and allocation concealment which we assessed as being at low risk of bias and overall, the included studies were assessed as low risk of bias for other domains of methodological quality. For an overview of review authors' judgments about each 'Risk of bias' item for individual included studies, seeFigure 1 and Figure 2.
1.
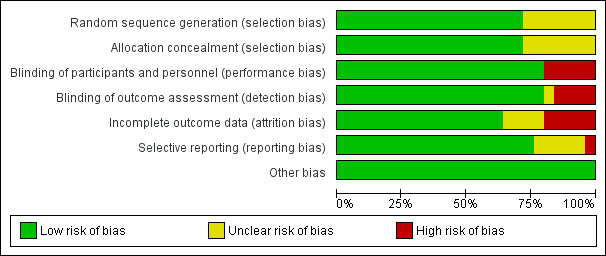
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
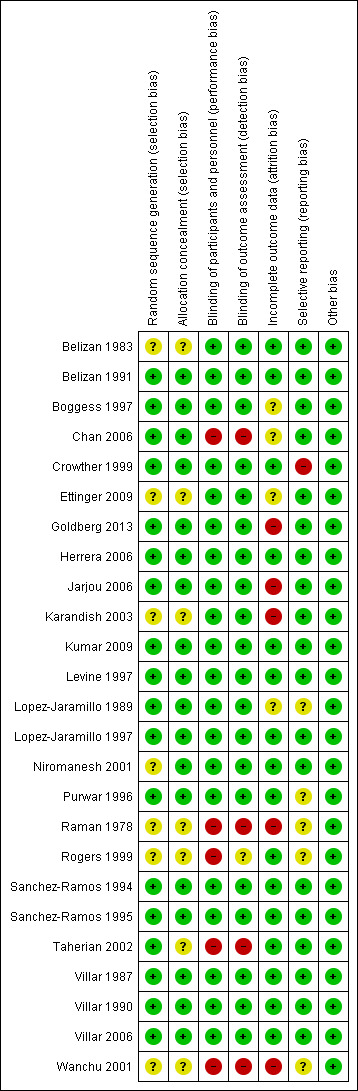
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
All studies included in this review were reported as being RCTs. Sample size calculation was clearly stated in only one trial (Crowther 1999). However, the two largest trials (Levine 1997; Villar 2006) had good methodological quality. Sequence generation was clearly described in 18 trials rated as 'low risk of bias' (Belizan 1991; Boggess 1997; Chan 2006; Crowther 1999; Goldberg 2013; Herrera 2006; Jarjou 2006; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Lopez‐Jaramillo 1997; Purwar 1996; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Taherian 2002; Villar 1987; Villar 1990; Villar 2006). The remaining studies did not describe how the randomisation sequence was generated and were assessed as of unclear risk (Belizan 1983; Ettinger 2009; Karandish 2003; Niromanesh 2001;Raman 1978; Rogers 1999; Wanchu 2001).
Adequate allocation concealment was reported in 18 trials, and these were rated as 'low risk of bias' (Belizan 1991; Boggess 1997; Chan 2006; Crowther 1999; Goldberg 2013; Herrera 2006; Jarjou 2006; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Lopez‐Jaramillo 1997; Niromanesh 2001; Purwar 1996; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Villar 1987; Villar 1990; Villar 2006). The remaining studies did not describe allocation concealment.
Blinding
Most of studies were considered to be of low risk of performance bias and detection bias. Double‐blinding was reported in 20 studies (Belizan 1983; Belizan 1991; Boggess 1997; Crowther 1999; Ettinger 2009; Goldberg 2013; Herrera 2006; Jarjou 2006; Karandish 2003; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Lopez‐Jaramillo 1997; Niromanesh 2001; Purwar 1996; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Villar 1987; Villar 1990; Villar 2006). One study (Chan 2006) was unable to blind because the groups consumed different food. The four trials using 'no treatment' as the control group were unable to blind the participants (Raman 1978; Rogers 1999; Taherian 2002; Wanchu 2001).
Incomplete outcome data
Most studies reported incomplete outcome data. Intention‐to‐treat (ITT) analyses was used in 11 trials assessed as of low or unclear risk of attrition bias (Belizan 1983; Belizan 1991; Crowther 1999; Kumar 2009; Lopez‐Jaramillo 1989; Niromanesh 2001; Rogers 1999; Taherian 2002; Villar 1987; Villar 1990; Villar 2006). Goldberg 2013 also used ITT analysis, but the attrition rate was 20%; the trial was assessed as high risk of bias. Four additional trials did not use ITT analyses and were assessed as high risk of bias for incomplete outcome data (Jarjou 2006; Karandish 2003: Raman 1978; Wanchu 2001). The remaining trials did not conduct ITT analyses but were assessed as unclear or low risk for attrition bias (Boggess 1997; Chan 2006; Herrera 2006; Lopez‐Jaramillo 1997; Purwar 1996; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; The rate of losses to follow‐up varied from 0% to 68.1%.
Selective reporting
We did not have the protocols for all the included studies; therefore we could not fully address selective reporting. Where trials specified their intended outcomes for analyses and then also presented relevant data for all of these outcomes, we assessed the trial as of low risk of bias.
Other potential sources of bias
None identified.
Effects of interventions
See: Table 1
Comparison: Calcium supplementation versus placebo or no treatment
Primary outcomes
Maternal outcomes
1. Preterm birth less than 37 weeks' gestation
Thirteen trials (Belizan 1991; Boggess 1997; Crowther 1999; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Purwar 1996; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Taherian 2002; Villar 1990; Villar 2006; Wanchu 2001) with data for 16,139 women. There were 8074 women who received calcium supplementation and 8065 women who received placebo or no treatment. Meta‐analysis evaluating the effect of calcium supplementation versus placebo or no treatment on preterm birth before 37 weeks revealed that there was no statistically significant difference between the two groups (average risk ratio (RR) 0.86, 95% confidence interval (CI) 0.70 to 1.05; random‐effects model; Analysis 1.1). However, there was substantial heterogeneity between trials (Tau² = 0.05; Chi² = 25.60, df = 11 (P = 0.007); I² = 57%). Therefore, we explored the source of heterogeneity by subgroup analyses stratified by total dose of calcium per day (less than 1000 mg/day or 1000 mg/day or more), starting time of calcium supplementation (before or after 20 weeks) and type of calcium (calcium carbonate, lactate and gluconate).
1.1. Analysis.
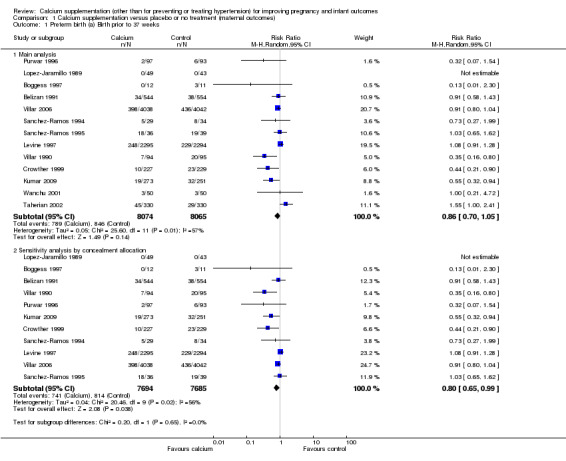
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 1 Preterm birth (a) Birth prior to 37 weeks.
For total dose of calcium per day, there appeared to be a difference between subgroups (Test for subgroup differences: Chi² = 6.93, df = 1 (P = 0.008), I² = 85.6%; Analysis 1.2). However, only one study was included in the low‐dose subgroup (Taherian 2002), while 12 studies were in the high‐dose subgroup, so this apparent difference between groups may have occurred by chance.
1.2. Analysis.
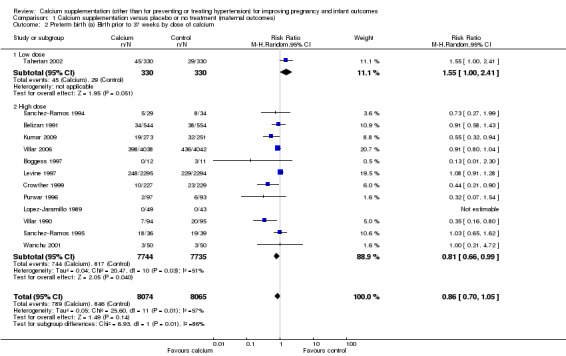
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 2 Preterm birth (a) Birth prior to 37 weeks by dose of calcium.
For the starting time of calcium supplementation, we found that there was no statistically significant differences between subgroups for women who started calcium before 20 weeks and for women who started calcium at 20 weeks or more (Analysis 1.3).
1.3. Analysis.
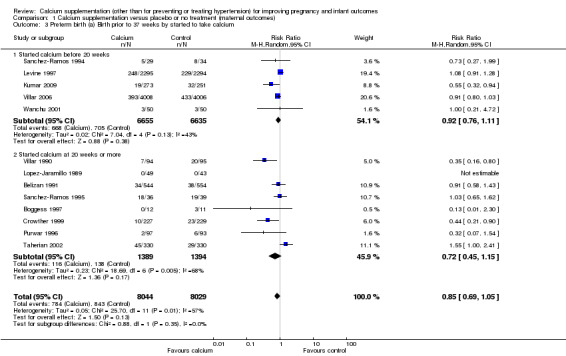
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 3 Preterm birth (a) Birth prior to 37 weeks by started to take calcium.
For type of calcium, there was no statistically significant difference between subgroups when women received calcium carbonate or calcium gluconate; however only one trial gave calcium gluconate to 92 women and in this study there was no preterm birth before 37 weeks in either the treatment or placebo group (Analysis 1.4).
1.4. Analysis.
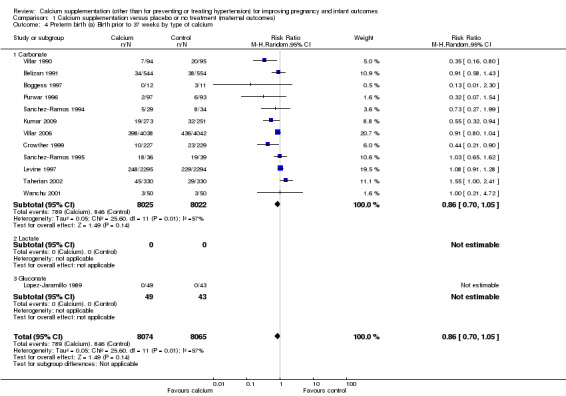
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 4 Preterm birth (a) Birth prior to 37 weeks by type of calcium.
We also conducted sensitivity analyses by removing two included trials (Taherian 2002; Wanchu 2001) whose allocation of concealment was unclear. The results then favoured treatment with calcium supplementation (RR 0.80, 95% CI 0.65 to 0.99; 11 trials, 15,379 women; random‐effects model; Analysis 1.1). There was significant heterogeneity for this outcome (Tau² = 0.04; Chi² = 20.46, df = 9 (P = 0.02); I² = 56%).
To investigate possible publication bias we generated a funnel plot (seeFigure 3). Visual examination of the funnel plot suggested there might be some asymmetry and the possibility of publication bias. However, substantial heterogeneity (as is found with this outcome), reporting bias and chance can each contribute to funnel plot asymmetry (Sterne 2011). Further, there were only 13 trials included in the analysis, and for outcomes with heterogeneity the minimum of recommendation of 10 trials may not be adequate (Sterne 2011). We therefore concluded that there was no strong evidence of publication bias for the outcome of preterm birth before 37 weeks.
3.
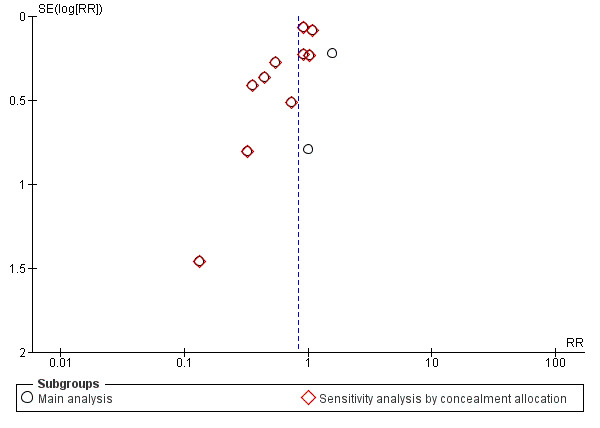
Funnel plot of comparison: 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), outcome: 1.1 Preterm birth (a) Birth prior to 37 weeks.
Infant outcomes
1. Low birthweight (less than 2500 g)
There was no statistically significant protective effect of calcium supplementation on low birthweight (six trials, Crowther 1999; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Villar 1990; Villar 2006, with 14,162 women); (RR 0.93, 95% CI 0.81 to 1.07; random‐effects model). However, there was significant heterogeneity between trials (Tau² = 0.01; Chi² = 10.61, df = 4 (P = 0.03); I² = 62%; Analysis 2.1). Women from these trials all received a high dose.
2.1. Analysis.
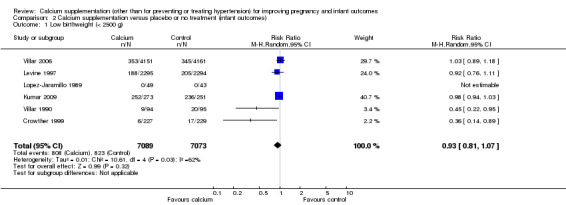
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 1 Low birthweight (< 2500 g).
We planned to carry out subgroup analyses for starting time and for type of calcium supplementation. There was some evidence that the starting time of supplementation was associated with different treatment effects (Test for subgroup differences: Chi² = 8.77, df = 1 (P = 0.003), I² = 88.6%). In two studies supplementation started early and there was no evidence of a significant difference between treatment and control groups (RR 0.98, 95% CI 0.94 to 1.03; three trials, 13,425 women), whereas the treatment appeared to have a significant effect in studies where supplementation started after 20 weeks' gestation (RR 0.41, 95% CI 0.23 to 0.73; three trials, 737 women). However, as a total of only six studies contributed estimable data to this subgroup analysis, these differences may have occurred by chance (Analysis 2.2). For type of calcium supplementation, most studies (Crowther 1999; Kumar 2009; Levine 1997; Villar 1990; Villar 2006) used calcium carbonate and one trial (Lopez‐Jaramillo 1989) used calcium gluconate (Analysis 2.3).
2.2. Analysis.
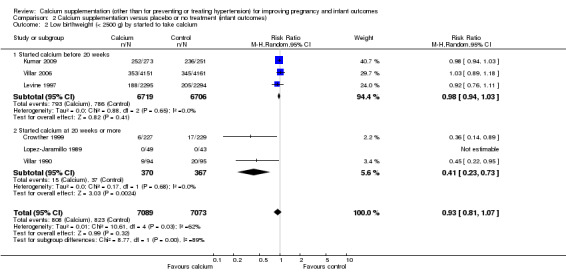
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 2 Low birthweight (< 2500 g) by started to take calcium.
2.3. Analysis.
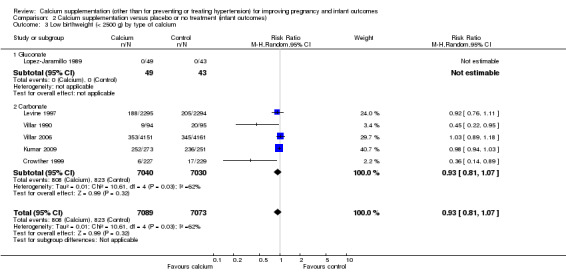
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 3 Low birthweight (< 2500 g) by type of calcium.
We did not conduct sensitivity analyses because all included trials for this outcome were rated as 'low risk of bias' for allocation of concealment.
We did not investigate publication bias for this outcome because the number of included trials was insufficient (six trials).
Secondary outcomes
Maternal outcomes
1. Preterm birth less than 34 weeks' gestation
There was no statistically significant difference in birth prior to 34 weeks between calcium supplementation versus placebo or no treatment (four trials, Crowther 1999; Kumar 2009; Levine 1997; Wanchu 2001, 5669 women) (RR 1.04, 95% CI 0.80 to 1.36) (Analysis 1.5). We did not perform subgroup analysis for this outcome as there was no evidence of substantial heterogeneity (I² = 0%).
1.5. Analysis.
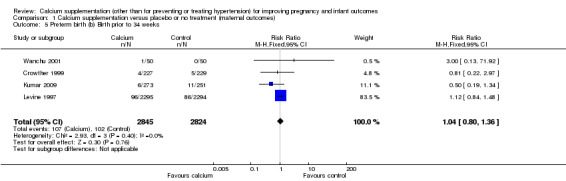
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 5 Preterm birth (b) Birth prior to 34 weeks.
We performed a sensitivity analyses and removed one included trial (Wanchu 2001) that had 'unclear' risk of bias for allocation concealment. The result did not change (RR 1.03, 95% CI 0.79 to 1.35, three trials, 5569 women) (Analysis 1.6).
1.6. Analysis.
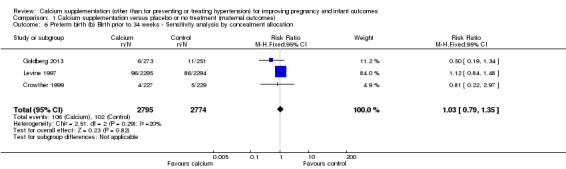
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 6 Preterm birth (b) Birth prior to 34 weeks ‐ Sensitivity analysis by concealment allocation.
2. Maternal weight gain
Three trials (Lopez‐Jaramillo 1989; Lopez‐Jaramillo 1997; Villar 1987, 404 women) evaluated the effect of calcium supplementation on maternal weight gain. There was no statistically significant difference between treatment versus placebo or no treatment. We found no statistically significant difference between groups (mean difference (MD) ‐29.46 g per week, 95% CI ‐119.80 to 60.89 g per week; random‐effects model) (Analysis 1.7). There was also substantial heterogeneity between trials (Tau² = 5007.60, I² = 80%).
1.7. Analysis.
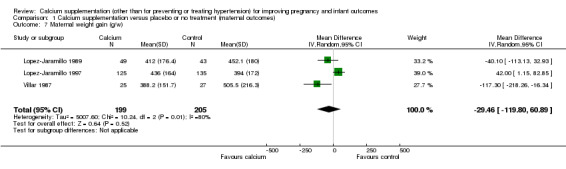
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 7 Maternal weight gain (g/w).
3. Maternal bone mineral density (BMD)
There was only one trial, involving 273 women (Raman 1978) that evaluated the effect of calcium supplementation and placebo on BMD. The author used radiographic density calculated and expressed in terms of aluminium equivalents as defined by Williams and Mason (Williams 1962).
We have presented the data for this outcome separately for treatment arms receiving different doses of supplementation.
In calcium 300 mg:
first phalanx: there was no statistically significant difference between treatment versus placebo or no treatment (62 women, MD ‐0.07 g/cm², 95% CI ‐0.29 to 0.15 g/cm² (Analysis 1.8));
second metacarpal: there was no statistically significant difference between treatment versus placebo or no treatment (62 women, MD 0.19 g/cm², 95% CI ‐0.02 to 0.40 g/cm² (Analysis 1.9));
fourth metacarpal: there was no statistically significant difference between treatment versus placebo or no treatment (62 women, MD 0.06 g/cm², 95% CI ‐0.17 to 0.29 g/cm² (Analysis 1.10)).
1.8. Analysis.
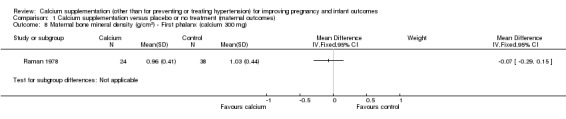
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 8 Maternal bone mineral density (g/cm2) ‐ First phalanx (calcium 300 mg).
1.9. Analysis.
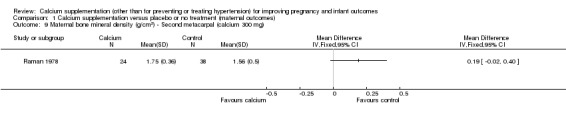
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 9 Maternal bone mineral density (g/cm2) ‐ Second metacarpal (calcium 300 mg).
1.10. Analysis.
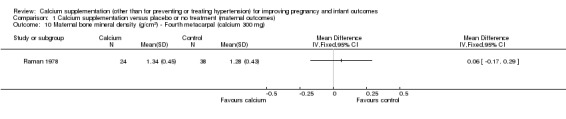
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 10 Maternal bone mineral density (g/cm2) ‐ Fourth metacarpal (calcium 300 mg).
In calcium 600 mg:
first phalanx: there was no statistically significant difference between treatment versus placebo or no treatment (63 women, MD 0.09 g/cm², 95% CI ‐0.10 to 0.28 g/cm² (Analysis 1.11));
second metacarpal: there was no statistically significant difference between treatment versus placebo or no treatment (63 women, MD 0.14 g/cm², 95% CI ‐0.11 to 0.39 g/cm² (Analysis 1.12));
fourth metacarpal: there was no statistically significant difference between treatment versus placebo or no treatment (63 women, MD 0.07 g/cm², 95% CI ‐0.13 to 0.27 g/cm² (Analysis 1.13)).
1.11. Analysis.
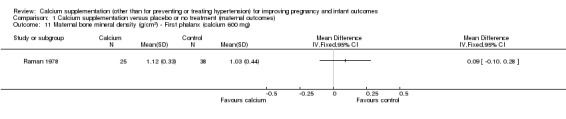
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 11 Maternal bone mineral density (g/cm2) ‐ First phalanx (calcium 600 mg).
1.12. Analysis.
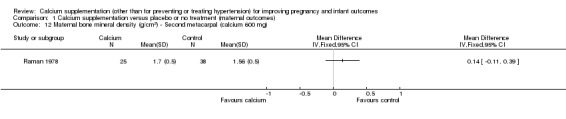
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 12 Maternal bone mineral density (g/cm2) ‐ Second metacarpal (calcium 600 mg).
1.13. Analysis.
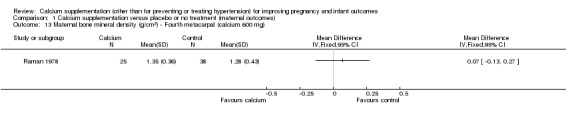
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 13 Maternal bone mineral density (g/cm2) ‐ Fourth metacarpal (calcium 600 mg).
4. Maternal death
Two trials involving 8974 women (Goldberg 2013; Villar 2006) reported this outcome. Although there appeared to be fewer deaths in the group receiving calcium supplements compared with controls (two versus seven), the difference between groups was not statistically significant (RR 0.29, 95% CI 0.06 to 1.38; Analysis 1.14).
1.14. Analysis.
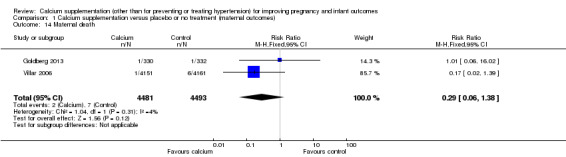
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 14 Maternal death.
5. Maternal admission to intensive care unit
Only one trial with 8312 women reported on this outcome (Villar 2006). There was no statistically significant difference between treatment and control groups (RR 0.84, 95% CI 0.66 to 1.07) (Analysis 1.15).
1.15. Analysis.
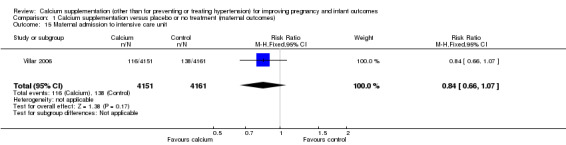
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 15 Maternal admission to intensive care unit.
6. Mode of birth ‐ vaginal birth, Instrumental vaginal birth, caesarean section (non‐prespecified outcome)
Vaginal birth: eight trials involving 6916 women (Belizan 1991; Crowther 1999; Levine 1997; Purwar 1996; Rogers 1999; Sanchez‐Ramos 1995 ; Villar 1990; Wanchu 2001) reported on this outcome. There was no statistically significant difference between treatment and control groups (RR 1.01, 95% CI 0.99 to 1.03) (Analysis 1.16).
Instrumental birth: two trials involving 675 women (Crowther 1999; Rogers 1999) reported on this outcome. There was no statistically significant difference between treatment and control groups (RR 0.89, 95% CI 0.66 to 1.20) (Analysis 1.17).
Caesarean section: nine trials involving 7440 women (Belizan 1991; Crowther 1999; Kumar 2009; Levine 1997; Purwar 1996; Rogers 1999; Sanchez‐Ramos 1995; Villar 1990; Wanchu 2001) reported on this outcome. There was no statistically significant difference between treatment and control groups (RR 0.99, 95% CI 0.89 to 1.10) (Analysis 1.18).
1.16. Analysis.
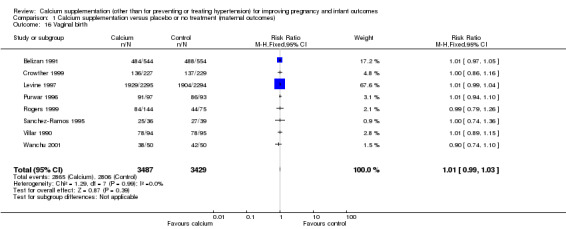
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 16 Vaginal birth.
1.17. Analysis.
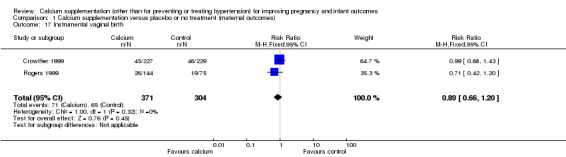
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 17 Instrumental vaginal birth.
1.18. Analysis.
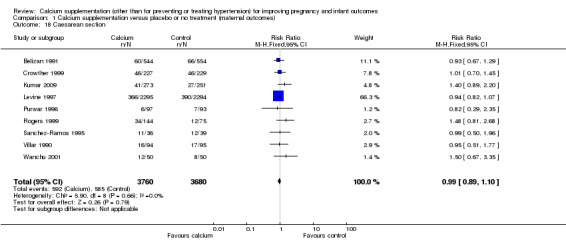
Comparison 1 Calcium supplementation versus placebo or no treatment (maternal outcomes), Outcome 18 Caesarean section.
7. Postpartum haemorrhage (non‐prespecified outcome)
Data were not available for this outcome.
Data were not available for the following maternal secondary outcomes: leg cramps; backache; tetany (muscle spasm and twitching); incidence of fracture; duration of breastfeeding; tremor; paraesthesia.
Fetal and neonatal outcomes
1. Perinatal mortality
Eight trials (15,785 women) reported perinatal mortality (Belizan 1991; Goldberg 2013; Levine 1997; Lopez‐Jaramillo 1997; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Taherian 2002; Villar 2006). There was no statistically significant difference between the groups (RR 0.87, 95% CI 0.72 to 1.06) (Analysis 2.5).
2.5. Analysis.
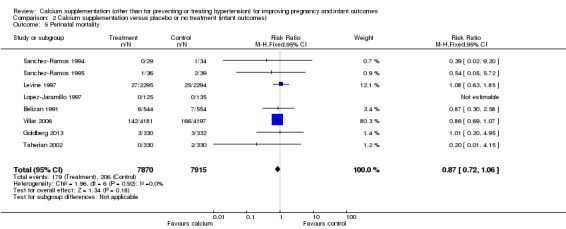
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 5 Perinatal mortality.
2. Stillbirth or fetal death
Six trials (Crowther 1999; Goldberg 2013; Kumar 2009; Levine 1997; Taherian 2002; Villar 2006) involving 15,269 women reported stillbirth or fetal death separately. There was no statistically significant difference between the groups (RR 0.91, 95% CI 0.72 to 1.14) (Analysis 2.6).
2.6. Analysis.
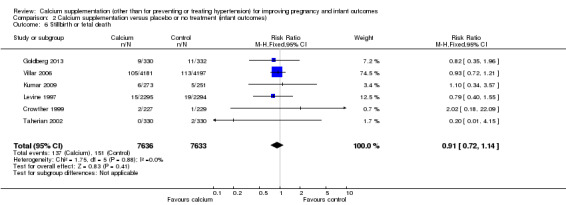
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 6 Stillbirth or fetal death.
3. Neonatal death
Data were not available for this outcome.
4. Admission to neonatal intensive care unit
Admission to neonatal intensive care unit was reported in four trials involving 14,062 women (Belizan 1991; Levine 1997; Sanchez‐Ramos 1994; Villar 2006). There was no statistically significant difference between the groups (RR 1.05, 95% CI 0.94 to 1.18; I² = 0%) (Analysis 2.7).
2.7. Analysis.
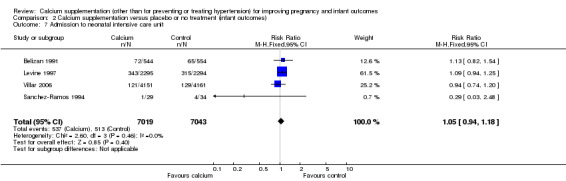
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 7 Admission to neonatal intensive care unit.
5. Birthweight
Mean birthweight (g) was reported in 21 trials involving 9202 women (Belizan 1983; Belizan 1991; Boggess 1997; Chan 2006; Crowther 1999; Goldberg 2013; Karandish 2003; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Lopez‐Jaramillo 1997; Niromanesh 2001; Purwar 1996; Raman 1978; Rogers 1999; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Taherian 2002; Villar 1987; Villar 1990; Wanchu 2001). (In the trials by Belizan 1983 and Raman 1978 data were reported separately for women receiving different doses of calcium; in the meta‐analysis we have therefore included findings for different doses separately as there was some heterogeneity between the different treatment arms; in both cases we divided the control group between the two entries to avoid double counting.) There was a statistically significant difference in birthweight between the groups (MD 56.40, 95% CI 13.55 to 99.25); Tau² = 5668.70, I² = 74%; random‐effects model) (Analysis 2.4) with the women in the calcium supplementation group on average having heavier babies than those in the control group.
6. Birth length
Birth length was reported in seven trials (6389 women) (Belizan 1983; Belizan 1991; Goldberg 2013; Karandish 2003; Levine 1997; Raman 1978; Villar 1990). There was no statistically significant difference between the groups (MD ‐0.09, 95% CI ‐0.25 to 0.06) (Analysis 2.8).
2.8. Analysis.
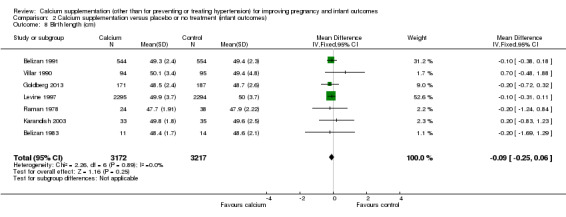
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 8 Birth length (cm).
7. Head circumference
Three trials involving 460 women reported head circumference (Belizan 1983; Goldberg 2013; Karandish 2003) (again data for the two treatment arms of the Belizan 1983 trial were entered separately with the control group shared between entries). There was no statistically significant difference between the groups (MD ‐0.09, 95% CI ‐0.36 to 0.18) (Analysis 2.9).
8. Intrauterine growth restriction
Intrauterine growth restriction was reported in six trials involving 1701 women (Kumar 2009; Purwar 1996; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Taherian 2002; Villar 1990). There was no statistically significant difference between the groups (RR 0.83, 95% CI 0.61 to 1.13) (Analysis 2.10).
2.10. Analysis.
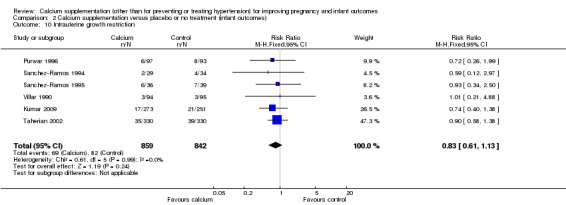
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 10 Intrauterine growth restriction.
9. Neonatal BMD
We presented the data for this outcome separately as subgroups (with subtotals only) due to the different definition of this outcome as defined by authors (Analysis 2.11):
2.11. Analysis.
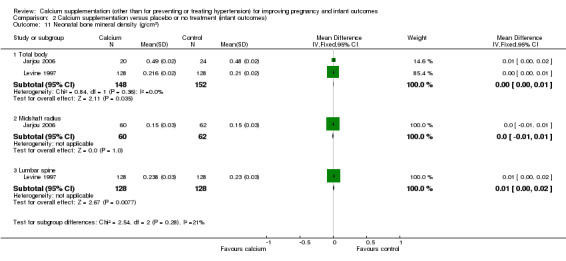
Comparison 2 Calcium supplementation versus placebo or no treatment (infant outcomes), Outcome 11 Neonatal bone mineral density (g/cm2).
total body: there was no statistically significant difference between treatment versus placebo or no treatment in two trials, 300 women (Jarjou 2006; Levine 1997; MD 0.00 g/cm², 95% CI 0.00 to 0.01 g/cm²; I² = 0%);
midshaft radius: there was no statistically significant difference between treatment versus placebo or no treatment in one trial involving 122 women (Jarjou 2006; MD 0.00 g/cm², 95% CI ‐0.01 to 0.01 g/cm²);
lumbar spine 1 to 4: there was no statistically significant difference between treatment versus placebo or no treatment in one trial involving 256 women (Levine 1997; MD 0.01 g/cm², 95% CI 0.00 to 0.02 g/cm²).
We have excluded the data from the Raman 1978 trial from our meta‐analysis because they were skewed but they have been presented separately in an additional table (seeTable 2).
1. Neonatal bone density (Skewed data).
| Study | Outcome | Treatment | Control | ||||
| Mean | SD | Total | Mean | SD | Total | ||
| Raman 1978 (Ca 300 mg) | Ulna | 1.19 | 0.81 | 24 | 0.64 | 0.26 | 38 |
| Raman 1978 (Ca 300 mg) | Fibula | 1.12 | 0.6 | 24 | 0.65 | 0.41 | 38 |
| Raman 1978 (Ca 300 mg) | Midshaft radius | 1.17 | 0.62 | 24 | 0.08 | 0.4 | 38 |
| Raman 1978 (Ca 300 mg) | Tibia | 0.91 | 0.35 | 24 | 0.58 | 0.41 | 38 |
| Raman 1978 (Ca 600 mg) | Ulna | 1.03 | 0.53 | 25 | 0.64 | 0.26 | 38 |
| Raman 1978 (Ca 600 mg) | Midshaft radius | 1.17 | 0.65 | 25 | 0.08 | 0.4 | 38 |
| Raman 1978 (Ca 600 mg) | Tibia | 1.11 | 0.82 | 25 | 0.58 | 0.41 | 38 |
| Raman 1978 (Ca 600 mg) | Fibula | 1.51 | 0.61 | 25 | 0.65 | 0.41 | 38 |
The standard deviation (SD) was imputed from the standard error of a mean (SEM).
Data were not available for the following secondary fetal and neonatal outcomes: osteopenia; rickets; fracture.
Adverse outcomes, compliance and maternal satisfaction
1. Side effects of calcium supplementation
Four trials reported side effects of calcium supplementation (Belizan 1991; Villar 1987; Villar 2006; Wanchu 2001). We have presented the data for this outcome separately as subgroups (with subtotals only) due to the different definitions of this outcome in the trials (Analysis 3.1).
3.1. Analysis.
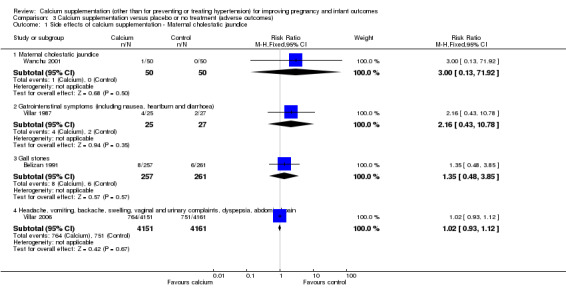
Comparison 3 Calcium supplementation versus placebo or no treatment (adverse outcomes), Outcome 1 Side effects of calcium supplementation ‐ Maternal cholestatic jaundice.
Maternal cholestatic jaundice: there was no statistically significant difference between the groups in one trial involving 100 women (Wanchu 2001) (RR 3.00, 95% CI 0.13 to 71.92).
Gastrointestinal symptoms consisting of nausea, heartburn and diarrhoea: there was no statistically significant difference between the groups in one trial involving 52 women (Villar 1987) (RR 2.16, 95% CI 0.43 to 10.78).
Gall stones: there was no statistically significant difference between the groups in one trial involving 518 women (Belizan 1991) (RR 1.35, 95% CI 0.48 to 3.85).
Headache, vomiting, backache, swelling, vaginal and urinary complaints, dyspepsia, abdominal pain: there was no statistically significant difference between the groups in one trial involving 8312 women (Villar 2006) (RR 1.02, 95% CI 0.93 to 1.12).
2. Urinary stones
Three trials involving 13,419 women reported this outcome (Belizan 1991; Levine 1997; Villar 2006). There was no statistically significant difference between the groups (RR 1.11, 95% CI 0.48 to 2.54; I² = 39%) (Analysis 3.2).
3.2. Analysis.
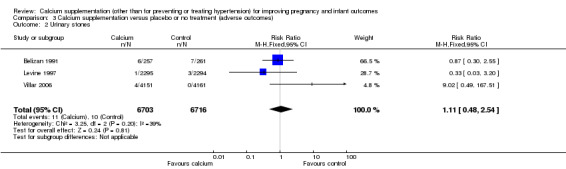
Comparison 3 Calcium supplementation versus placebo or no treatment (adverse outcomes), Outcome 2 Urinary stones.
3. Urinary tract infection
Three trials involving 1743 women reported this outcome (Belizan 1991; Crowther 1999; Villar 1990). There was no statistically significant difference between the groups (RR 0.95, 95% CI 0.69 to 1.30; I² = 0%) (Analysis 3.3).
3.3. Analysis.
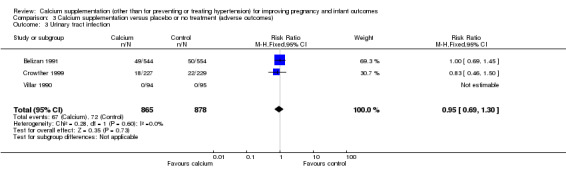
Comparison 3 Calcium supplementation versus placebo or no treatment (adverse outcomes), Outcome 3 Urinary tract infection.
5. Renal colic
This outcome was reported in one trial with 8312 women (Villar 2006). There was no evidence of a statistically significant difference between groups (RR 1.67, 95% CI 0.40 to 6.99) (Analysis 3.4).
3.4. Analysis.
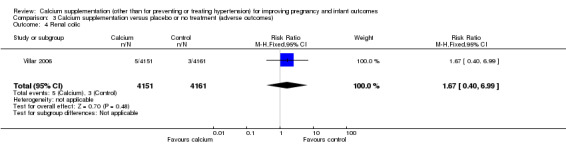
Comparison 3 Calcium supplementation versus placebo or no treatment (adverse outcomes), Outcome 4 Renal colic.
5. Impaired renal function
There was no statistically significant difference between the groups for this outcome in one trial, involving 4589 women (Levine 1997) (RR 0.91, 95% CI 0.51 to 1.64) (Analysis 3.8) (Analysis 3.5).
3.5. Analysis.
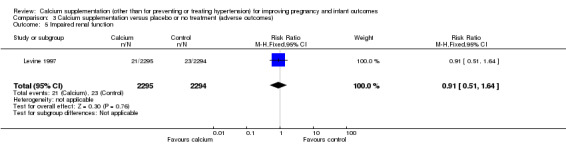
Comparison 3 Calcium supplementation versus placebo or no treatment (adverse outcomes), Outcome 5 Impaired renal function.
6. Maternal anaemia
One trials, involving 1098 women, reported this outcome (Belizan 1991). There was no statistically significant difference between the groups (RR 1.04, 95% CI 0.9 to 1.22) (Analysis 3.6).
3.6. Analysis.
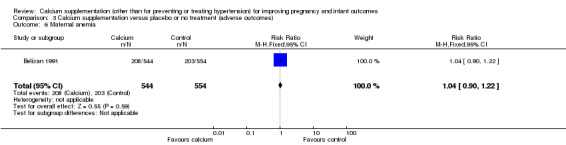
Comparison 3 Calcium supplementation versus placebo or no treatment (adverse outcomes), Outcome 6 Maternal anemia.
7. Compliance
Data were not available for this outcome.
8. Satisfaction
Data were not available for this outcome.
Discussion
Summary of main results
Calcium supplementation did not reduce preterm birth. Dosage, prescription timing and the type of calcium supplementation did not effect this outcome. Calcium supplementation did not decrease the rate of low birthweight. Timing of supplementation and the type of calcium supplementation did not show any clear protective effect for low birthweight. No trial reported the effect of low‐dose calcium supplementation (less than 1000 mg) on low birthweight babies. There was no evidence that calcium supplementation had any effect on maternal weight gain during pregnancy. There was no evidence to support the benefit of calcium supplementation in increasing bone mineral density in pregnant women but in infants, there was a statistically significant difference between treatment and placebo or no treatment in total body and tibial bone mineral density. While there was a statistically significant increase in birthweight in the calcium supplementation group, there was also high heterogeneity among the studies, so the results for this outcome should be interpreted with caution. Additionally, the 56 g increase in birthweight might not be clinically important. There was no evidence that calcium supplementation reduced the rate of intrauterine growth restriction, perinatal mortality, stillbirth or fetal death rate. Calcium supplementation also did not increase birth length or fetal head circumference. We found no evidence to show that calcium supplementation was associated with side effects such as postpartum haemorrhage, cholestatic jaundice, gall stones, gastrointestinal symptoms, headache, urinary stones, urinary tract infection or impaired renal function.
Overall completeness and applicability of evidence
Missing data amounted to 4.01% overall (745 in 17,842). One small trial showed a marked loss of follow‐up (68.1%, Raman 1978). The loss to follow‐up rates in most trials were less than 20%. Most trials prespecified outcomes in included studies especially the primary outcomes, but no data were reported for some of our secondary outcomes. As we mentioned above, the primary objectives of most of the included studies were incidence of pregnancy‐induced hypertension or changes in blood pressure, which were not relevant to this review. However, these studies also had other outcomes relevant to this review, e.g. preterm birth, maternal weight gain, gestational age, birthweight, birth length and therefore, we have included them.
The largest trial in this review (Villar 2006) recruited pregnant women from a population who received less than 600 mg of dietary calcium per day. The other two big trials (Belizan 1991; Levine 1997) did not limit daily calcium intake. In addition, there were variations between trials in terms of duration of supplementation. The subgroup analysis to assess the effect on preterm delivery before 37 weeks of calcium supplementation before versus after 20 weeks' gestation revealed no protective effect on either group. There were too few studies to assess other types of calcium prescribed or other outcomes of interest such as preterm delivery before 34 weeks, maternal bone mineral density, and major fetal outcomes. This may be evidence that routine calcium supplementation in pregnant women for preventing preterm birth and low birthweight is not warranted.
The largest trial in this review (Villar 2006) recruited pregnant women from a population who received less than 600 mg of dietary calcium per day. The other two big trials (Belizan 1991; Levine 1997) did not limit daily calcium intake. In addition, there were variations between trials in terms of duration of supplementation.
The main analysis to assess the effect on preterm delivery before 37 and 34 weeks did not show significant benefit, but in a sensitivity analysis of 11 low risk of bias trials (Belizan 1991; Boggess 1997; Crowther 1999; Kumar 2009; Levine 1997; Lopez‐Jaramillo 1989; Purwar 1996; Sanchez‐Ramos 1994; Sanchez‐Ramos 1995; Villar 1990; Villar 2006), there was a statistically significant benefit of calcium supplementation in reducing preterm delivery less than 37 weeks. Type of calcium supplementation and timing for prescribing did not make any differences.
Hofmeyr 2014 found a reduction in preterm birth for women receiving high‐dose calcium supplementation (11 trials, 15,275 women; risk ratio (RR) 0.76, 95% confidence interval (CI) 0.60 to 0.97; I² = 60%). Hofmeyr 2014 also found a reduction in the risk of developing pre‐eclampsia for women receiving supplementation (13 trials, 15,730 women; RR 0.45, 95% Cl 0.31 to 0.65; I² = 70%). There were eight trials in common for the preterm outcome for this and the Hofmeyr review. However, results for the preterm birth prior to 37 weeks outcome in this systematic review did not reach statistical significance until two trials were removed during sensitivity analysis (see Analysis 1.1). Inclusion criteria between the reviews differed, and therefore the results were also different.
Quality of the evidence
Most of the studies (17 of the 25 trials) were at low risk of bias for both sequence generation and allocation concealment, seeFigure 1 and Figure 2. Seven trials did not describe the methods of sequence generation or allocation concealment clearly. Three outcomes were chosen for assessment with GRADE software for quality: low birthweight (less than 2500 g), preterm birth less than 37 weeks and preterm birth less than 34 weeks. Evidence for each outcome was considered to be of moderate quality.
Potential biases in the review process
We followed methods set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) to try to reduce bias in the review process.
Agreements and disagreements with other studies or reviews
A Cochrane review by Hofmeyr 2014 entitled 'Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems' showed that routine calcium supplementation during pregnancy reduced the risk of pre‐eclampsia and preterm birth. As discussed above, some of our results differ from Hofmeyr 2014 due to differing inclusion criteria.
Authors' conclusions
Implications for practice.
This review found that calcium supplementation did not reduce preterm birth less than 37 weeks. There is not enough evidence to assess dosage, timing and type of calcium supplementation on pregnancy outcomes other than pregnancy‐induced hypertension. The review by Hofmeyr 2014 shows a significant protective effect of calcium supplementation on pre‐eclampsia/eclampsia, and reduced preterm birth but our review reveals no additional benefits of calcium supplementation. The discrepancy result might be due to inadequate sample size. Therefore, calcium supplementation during pregnancy would be primarily considered to prevent pre‐eclampsia.
Implications for research.
Large multicentre trials to detect the benefit of calcium supplementation on preterm birth as the primary outcome are needed to provide more solid evidence.
In addition, the results from this review found that there are a few short‐term additional benefits of calcium supplementation (other than pre‐eclampsia prevention) other than slight increases fetal birthweight and neonatal bone mineral density. There are limited data to assess its long‐term benefits such as osteoporosis in later life. Further research might be needed to provide evidence regarding long‐term benefits.
What's new
| Date | Event | Description |
|---|---|---|
| 30 September 2014 | New citation required but conclusions have not changed | Review updated. |
| 30 September 2014 | New search has been performed | Search updated and 19 new reports were assessed for eligibility. Three new trials were included (Goldberg 2013; Herrera 2006; Kumar 2009). Four reports were abstracts added to awaiting classification, one of which requires translation (Zheng 2000). Six reports were duplicates for already included studies. Six reports were excluded. Methods updated and 'Summary of findings' table added. |
Acknowledgements
The authors would like to thank Professor Caroline Crowther, Phillippa Middleton, Ruth Martis and the SEA‐ORCHID project for supporting a fellowship for Pranom Buppasiri, enabling her to complete this systematic review.
We would like to thank Dr. Reza Navaei for translating Karandish 2003.
We also thank the Thai Senior Researcher Fund for support during the development of the review.
Nancy Medley's work was financially supported by the UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization. The named authors alone are responsible for the views expressed in this publication.
Data and analyses
Comparison 1. Calcium supplementation versus placebo or no treatment (maternal outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Preterm birth (a) Birth prior to 37 weeks | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Main analysis | 13 | 16139 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.05] |
| 1.2 Sensitivity analysis by concealment allocation | 11 | 15379 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.65, 0.99] |
| 2 Preterm birth (a) Birth prior to 37 weeks by dose of calcium | 13 | 16139 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.05] |
| 2.1 Low dose | 1 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.00, 2.41] |
| 2.2 High dose | 12 | 15479 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.66, 0.99] |
| 3 Preterm birth (a) Birth prior to 37 weeks by started to take calcium | 13 | 16073 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.69, 1.05] |
| 3.1 Started calcium before 20 weeks | 5 | 13290 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.76, 1.11] |
| 3.2 Started calcium at 20 weeks or more | 8 | 2783 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.45, 1.15] |
| 4 Preterm birth (a) Birth prior to 37 weeks by type of calcium | 13 | 16139 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.05] |
| 4.1 Carbonate | 12 | 16047 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.05] |
| 4.2 Lactate | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Gluconate | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Preterm birth (b) Birth prior to 34 weeks | 4 | 5669 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.80, 1.36] |
| 6 Preterm birth (b) Birth prior to 34 weeks ‐ Sensitivity analysis by concealment allocation | 3 | 5569 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 7 Maternal weight gain (g/w) | 3 | 404 | Mean Difference (IV, Random, 95% CI) | ‐29.46 [‐119.80, 60.89] |
| 8 Maternal bone mineral density (g/cm2) ‐ First phalanx (calcium 300 mg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 Maternal bone mineral density (g/cm2) ‐ Second metacarpal (calcium 300 mg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Maternal bone mineral density (g/cm2) ‐ Fourth metacarpal (calcium 300 mg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Maternal bone mineral density (g/cm2) ‐ First phalanx (calcium 600 mg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Maternal bone mineral density (g/cm2) ‐ Second metacarpal (calcium 600 mg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13 Maternal bone mineral density (g/cm2) ‐ Fourth metacarpal (calcium 600 mg) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Maternal death | 2 | 8974 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.38] |
| 15 Maternal admission to intensive care unit | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.07] |
| 16 Vaginal birth | 8 | 6916 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.99, 1.03] |
| 17 Instrumental vaginal birth | 2 | 675 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.66, 1.20] |
| 18 Caesarean section | 9 | 7440 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.10] |
Comparison 2. Calcium supplementation versus placebo or no treatment (infant outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Low birthweight (< 2500 g) | 6 | 14162 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 2 Low birthweight (< 2500 g) by started to take calcium | 6 | 14162 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 2.1 Started calcium before 20 weeks | 3 | 13425 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.94, 1.03] |
| 2.2 Started calcium at 20 weeks or more | 3 | 737 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.73] |
| 3 Low birthweight (< 2500 g) by type of calcium | 6 | 14162 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 3.1 Gluconate | 1 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Carbonate | 5 | 14070 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 4 Birthweight (g) | 21 | 9202 | Mean Difference (IV, Random, 95% CI) | 56.40 [13.55, 99.25] |
| 5 Perinatal mortality | 8 | 15785 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.06] |
| 6 Stillbirth or fetal death | 6 | 15269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.14] |
| 7 Admission to neonatal intensive care unit | 4 | 14062 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.94, 1.18] |
| 8 Birth length (cm) | 7 | 6389 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.25, 0.06] |
| 9 Head circumference (cm) | 3 | 460 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.36, 0.18] |
| 10 Intrauterine growth restriction | 6 | 1701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.13] |
| 11 Neonatal bone mineral density (g/cm2) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Total body | 2 | 300 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [0.00, 0.01] |
| 11.2 Midshaft radius | 1 | 122 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.01, 0.01] |
| 11.3 Lumbar spine | 1 | 256 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [0.00, 0.02] |
Comparison 3. Calcium supplementation versus placebo or no treatment (adverse outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Side effects of calcium supplementation ‐ Maternal cholestatic jaundice | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Maternal cholestatic jaundice | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 1.2 Gatrointenstinal symptoms (including nausea, heartburn and diarrhoea) | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.43, 10.78] |
| 1.3 Gall stones | 1 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.48, 3.85] |
| 1.4 Headache, vomiting, backache, swelling, vaginal and urinary complaints, dyspepsia, abdominal pain | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.93, 1.12] |
| 2 Urinary stones | 3 | 13419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.48, 2.54] |
| 3 Urinary tract infection | 3 | 1743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.69, 1.30] |
| 4 Renal colic | 1 | 8312 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.40, 6.99] |
| 5 Impaired renal function | 1 | 4589 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.51, 1.64] |
| 6 Maternal anemia | 1 | 1098 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.22] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Belizan 1983.
| Methods | Type of study: simple randomisation into 3 groups. Method of treatment allocation: not stated. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: yes. Losses to follow‐up: 0%. | |
| Participants | Location: outpatient clinic of Guatemala Social Security Hospital.
Time frame: not stated.
Eligible criteria: age 20 to 35 years, single fetus, without evidence of previous pathology and certain date, not receiving any medical treatment during recruitment.
Total recruited: 36 pregnant women. Treatment group 1, n = 11, treatment group 2, n = 11, placebo group, n = 14. |
|
| Interventions |
Started treatment at 15 weeks until delivery. |
|
| Outcomes |
|
|
| Notes | The authors did not mention how many tablets were provided in calcium 1 g, 2 g and placebo group. Missing data = 0%. For data in Analysis 2.4 and Analysis 2.9 the placebo n was halved to enable inclusion of data for treatment groups 1 and 2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After the patients volunteers to participate the trial. Simple randomisation were used to devise patient into 3 groups, receive 1, 2 g calcium comparing with placebo." Comment: method of random sequence generation was not clearly described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients were unaware of group status. Study drug and placebo were the same size and weight and had the same organoleptic characteristics. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only 3 obstetrics and gynaecology residents were in charge of measuring BP, after standardisation with double auricular stethoscope. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all enrolled participants were analysed. MIssing data = 0%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Belizan 1991.
| Methods | Type of study: multicentre, double‐blind, randomised controlled trial.
Method of treatment allocation: randomisation was conducted at each hospital by a random‐generator program. A complete set of numbered, sealed, opaque envelopes containing the randomisation codes was sent to each of 3 hospitals.
Placebo: yes, starch tablets.
Sample size calculation: not stated.
Intention‐to‐treat analyses: yes.
Losses to follow‐up: 2.3%. 27 women were lost to follow‐up after randomisation (14 in the calcium group, 13 in the placebo group) but before they started treatment, and therefore were not included in the followed up analyses. Follow‐up was incomplete for 52 women in the calcium group and 46 in placebo group because of change of hospital, physician or residence. |
|
| Participants | Location: the women enrolled from 3 affiliated hospitals of Centro Rosario de Estudios Perinatales, Rosario, Argentina (2 were public hospitals, the another was a private hospital). Time frame: January 1987 to September 1989. Eligible criteria: GA < 20 weeks and confirmed by ultrasound, nulliparous, singleton pregnancy, BP < 140/90 mmHg. No evidence of present or past disease from clinical examination or laboratory tests, not taking any medications and had normal glucose tolerance test. Exclusion criteria: gestational date estimated from LMP and ultrasonography different by more than 10 days. Total recruited: 1194 pregnant women; treatment group, n = 593, control group, n = 601. A total of 579 women in the calcium group and 588 in the placebo group were included in final analyses. | |
| Interventions | 2 g calcium/d (4 tablets/day; each calcium tablet contained 500 mg calcium carbonate and granulated starch). Compared with placebo tablet. Started treatment at 20 weeks. | |
| Outcomes |
|
|
| Notes | Treatment group, n = 593, control group, n = 601. For final analysis, treatment group, n = 579, 588 in placebo group but for other pregnancy outcomes other than pregnancy hypertension, n = 544 in calcium group and n = 554 in control group. Missing data 27 in 1194 = 2.3%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomised at each hospital by a random‐generator program." |
| Allocation concealment (selection bias) | Low risk | Quote: "A complete set of numbered, sealed, opaque envelopes containing the randomisation codes was sent to each of three hospitals." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The calcium tablets were specially prepared by a local pharmaceutical company; the placebo tablets contained lactose and granules starch and were identical to the calcium tablets with respect to weight, size, flavour and colour. The nurses and physicians responsible for prenatal care were all unaware of the women's treatment status. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The nurses and physicians responsible for prenatal care were all unaware of the women's treatment status and were also responsible for distribution the bottle of medications, taking BP and collection of blood and urine samples at every visit. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Follow‐up was incomplete 52 in treatment group and 46 in placebo group because of change of hospital, physician, or residence. Nonetheless, all were included in analyses up to time when they were lost to follow‐up. For the subgroup with incomplete follow‐up, information about delivery was available for 17 in calcium group and 12 in placebo group." Missing data 27 in 1194 = 2.3%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Boggess 1997.
| Methods | Type of study: double‐blind randomised controlled trial. Method of treatment allocation: a computer‐generated random number table was used. Using a randomisation schedule in a block of 10. All containers and tablets were prepared and dispensed by the University Drug Pharmacy. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: no (23 women were randomised and 18 of them completed study). Of the 5 who did not complete the study, 3 developed preterm labour and 1 was non compliance, and 1 self‐discontinued study medication due to side effects. Losses to follow‐up: 5 in 23 = 21.7%. | |
| Participants | Location: University of Washington Medical Center Women's Clinic. Time frame: not stated. Eligible criteria: age 18 to 35 years who received antenatal care. Exclusion criteria: BP > 140/90 mmHg at 24 weeks, smoking or used illicit drugs, multiple gestation, had history of cardiovascular, renal, or endocrine disorder, hypertension prior to pregnancy, or calcium supplementation. Total recruited: 23 pregnant women; calcium group, n = 12, placebo group, n = 11. | |
| Interventions | 1.5 g/d of calcium carbonate. Compared with placebo tablets. Started treatment at 28 to 31 weeks. | |
| Outcomes | Hemodynamic function measurement. | |
| Notes | 1. GA was reported as median and range. We changed them into mean and SD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The study patients were assigned in a double‐blind fashion to receive orally either 1.5 element calcium as calcium carbonate or placebo daily, using a randomisation schedule in blocks of ten developed by a computer‐generated random number table." |
| Allocation concealment (selection bias) | Low risk | Quote: "All containers and tablets were prepared and dispensed by the University Drug Pharmacy." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment versus placebo. Quote: "Placebo tablets were same size, weight, colour, and organoleptic characteristics." All participants were blinded to intervention. All containers and tablets were prepared and dispensed by the University Drug Pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All containers and tablets were prepared and dispensed by the University Drug Pharmacy. The investigators were blinded to outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Twenty‐three women were randomised and 18 of them completed study. Of the five who failed to complete the study; three developed preterm labour (all placebo), one was noncompliance (placebo), one discontinued due to side effects (calcium)." Missing data = 5 in 23 = 21.7%; relevant data such as preterm birth were added back into the analysis. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Chan 2006.
| Methods | Type of study: computer‐generated randomisation. Method of treatment allocation: the pregnant women were randomly assigned to 1 of 3 groups: control, orange juice fortified with calcium, and dairy. Computer‐generated randomisation was kept in envelopes. Placebo: no. Sample size calculation: not stated. Intention‐to‐treat analyses: no. Losses to follow‐up at delivery 8.3% : in control group, missing data at delivery = 0, missed 6 months visit, n = 3 and umbilical cord not collected, n = 2. In orange juice plus calcium missing data = 3, failed to meet required 4 servings, n = 12, misses 6 month visit, n = 3,and mothers blood was not collected, n = 3 and umbilical blood was not collected, n = 3. In daily group, missing data at delivery = 3, missed at 6‐month visit, n = 2, mother's blood was not collected, n = 3, and umbilical blood was not collected, n = 5. | |
| Participants | Location: University of Utah Teen Mother and Child Program. Time frame: not stated. Eligible criteria: healthy adolescent (15 to 17 years old) pregnant women. GA < 20 weeks by last normal menstrual period. Exclusion criteria: hypertension, diabetes, renal or liver diseases, used alcohol, tobacco or medications that would effect Ca metabolism during pregnancy. Total recruited: 72 healthy pregnant adolescents. | |
| Interventions | There were 3 groups. Group 1; control (consumed usual diet) n = 23. Group 2; orange juice fortified with calcium consumed at least 4 servings of orange juice plus calcium (more than 1200 mg Ca) n = 24. Group 3; dairy (consumed at least 4 servings of dairy product (Ca more than 1200 mg) e.g., milk, yogurt, cheese, n = 25. Started treatment at 20 weeks. | |
| Outcomes |
|
|
| Notes | Missing data at delivery 6 in 72 = 8.3%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Pregnant mothers were randomly assigned to one of three groups; control, orange juice fortified with calcium, and dairy. Computer‐generated randomisation was kept in sealed envelopes." |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated randomisation was kept in sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The control group consumed their usual diet while the orange juice plus calcium group were counselled to consume at least four servings of orange juice plus calcium (more than 1200 mg Ca) so that their Ca intake would be similar to the dairy group.The dairy group was counselled to consume at least four servings of dairy products (more than 1200 mg Ca) daily. Dairy products consisted of milk, yogurt, and cheese." Comment: it was impossible to blind because of the different kinds of food. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Could not blind participants and investigators due to different intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the authors displayed a flow chart of participants. Missing data in control group = 13%, in orange juice plus calcium = 12.5 %, in daily product 8%. Missing data at delivery 6 in 72 = 8.3%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Crowther 1999.
| Methods | Type of study: placebo‐controlled double‐blind trial.
Method of treatment allocation: the randomisation schedule was prepared by the drug company with stratification made by centre using variable blocks.
Placebo: yes, starch tablets.
Sample size calculation: a study of 948 women was estimated to have an 80% probability of detecting differences in the rate of preterm birth at P = 0.05 and an 88% power to detect a 50% difference with the rate of PIH with the same significance level.
Intention‐to‐treat analyses: yes. Pre‐calculation samples needed 948 women to be recruited in trial but because of shortage of funds, but only 456 pregnant women were recruited. Losses to follow‐up: 0%. |
|
| Participants | Location: 5 Australian Medical Centres.
Time frame: August 1992 to December 1996. Eligible criteria: nulliparous, singleton pregnancy, at less than 24 weeks, with normal BP at trial entry (< 140/90 mmHg) and expected to birth at 5 collaborating centres were expected for trial but recruitment to the trial was stopped by the steering group without knowledge of study outcomes after 456 women were randomised when the limited financial resources available for the trial were exhausted. Exclusion criteria: used of antihypertensive or medical disorder where calcium supplementation was contraindicated such as renal failure, hyperparathyroidism or renal calculi. Total recruitments: 948 pregnant women planned to be recruited. Data were analysed when pregnancy outcome data were available for all 456 women recruited. Of 456, 227 were assigned to calcium group, 229 were in placebo group. |
|
| Interventions | Women were asked to take 3 tablets daily orally, equivalent 1.8 g calcium or placebo (calcium carbonate, 600 mg of elemental calcium per tablet). Started treatment at 20 weeks until delivery. Compared with 3 tablets of placebo tablets. | |
| Outcomes | 1. Incidence of PIH. 2. Pregnancy outcomes; preterm birth, premature rupture of membrane, birthweight. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation schedule was prepared by the drug company with stratification made by centre using variable blocks." |
| Allocation concealment (selection bias) | Low risk | Quote: "A study number was given by the central randomisation office. This corresponded to a sealed treatment pack held at the collaboration centre and were provided by Lederle Laboratories." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Women who gave consent were enrolled into the trial by telephoning the central randomization number in the Maternal Perinatal Clinic Trial Unit . The randomization schedule was prepared by the drug company with stratification made by center using variable blocks." All women and staff were blind to group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Women received antenatal care and postnatal care from attending obstetrics care. Data were collected from case notes by research assistants and checked by senior obstetrician, all blinded to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "948 women were expected for trial but recruitment to the trial was stopped by the steering group without knowledge of study outcomes after 456 women were randomised when the limitation financial resource available for the trial were exhaust. Data were performing when pregnancy outcome data were available for all 456 women recruited. Of 456: 227 were assigned to calcium group, 229 were placebo group." Comment: the number of participants in treatment and control groups was equal. Missing data = 0%. |
| Selective reporting (reporting bias) | High risk | Trial stopped before enrolment complete due to a shortage of research funds. |
| Other bias | Low risk | None identified. |
Ettinger 2009.
| Methods | Type of study: Double‐blind, randomised, placebo‐controlled trial. Method of treatment allocation:not stated Placebo: yes Intention‐to‐treat basis: yes |
|
| Participants | Location: Mexican Social Security Institute, Mexico city, Mexico. Time frame: 2001‐2003 670 women were randomised in the first trimester of pregnancy. 334 to the treatment group, and 336 to the placebo group. |
|
| Interventions | 1200 mg calcium daily (two 600‐mg calcium carbonate tablets) versus placebo. | |
| Outcomes | Maternal blood lead levels at first, second, and third trimester. | |
| Notes | We have not included outcome data from this study as the trial specifically focused on the effects of calcium supplementation on blood lead levels. The study does not address any of the review's primary or secondary outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women were randomly assigned to received 1200 mg calcium (2 tabs of 600 mg calcium carbonate tablets) or placebo. Study described as double‐blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled trial. Study described as double‐blind. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 670 randomised. 565 women completed follow‐up. 557 included in the analysis (83%). Lost to follow‐up in placebo 18%, calcium group 14%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Goldberg 2013.
| Methods | Type of study: double‐blind, randomised, parallel, placebo‐controlled trial.
Method of treatment allocation: stratified by clinic to receive calcium supplementation or placebo. The assignment within each stratum was by random permuted block of 4 in each week each clinic. The allocation sequence was generated by using random‐number tables. The code was held by a member of the trial team who was not directly involve in data collection and had no contact with participant. Placebo: yes, starch tablets. Sample size calculation: at 5% significance and 80% power, a sample size needed 260 participants per group. Loss to follow‐up: 330 randomised to intervention and 332 to the control group. Data for 260 women in the intervention group and 265 in the control group at delivery. Attrition accounted for in study flowchart with numbers given for maternal (2), fetal (10), neonatal or infant death (25); exclusion after randomisation for incorrect GA (70) or multiple pregnancy (11) missing data for BP at 36 weeks' gestation (1) and women moving away or withdrawal (18). ITT and per protocol analyses undertaken. 3 women unaccounted for in flowchart. Jarjou 2006 presents data for a subset of the women in reported in Goldberg 2013, for the outcome of neonatal bone density. |
|
| Participants | Location: 3 antenatal clinics, covering cluster of villages in different geographic regions in West Kiangin Gambia.
Time frame: May 1995 ‐ March 2000. Eligible criteria: healthy pregnant women with no medical history affecting calcium metabolism presenting for prenatal care at 1 of 3 outpatient clinics. Singleton only, gestation 18‐22 weeks. Exclusion criteria: any complications of pregnancy. Total recruitments: 662 pregnant women were recruited, 330 in treatment group, 332 in placebo group. Data for 525, with loss to groups comparable. |
|
| Interventions | 1500 mg calcium (3 chewable calcium carbonate tablets, each consist of 500 mg elemental calcium) versus 3 chewable placebo in similar shape, colour, and taste, from 20 weeks' gestation until delivery. | |
| Outcomes | BP at 36 weeks' gestation. Maternal BP in first year postpartum. Infant growth measures collected at 2, 13 and 52 weeks. Breast milk calcium concentration, neonatal bone mineral density at age < 1 year. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Double‐blind, randomised, parallel, placebo‐controlled trial.The assignment within each stratum was by random permuted block of 4 in each week at each clinic. The allocation sequence was generated by using random‐number tables. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was generated by using random‐number tables. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment group and placebo group received identical tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | BP, anthropometric measurements were measured by trained field staff using a standard protocol. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 330 randomised to intervention and 332 to the control group. Data for 260 women in the intervention group and 265 in the control group at delivery. Attrition in both arms approximately 20%. Authors accounted for women lost in flowchart and conducted ITT analysis where possible. For infant outcomes, the denominator varies. Many infants without data for birth measurements because their mothers spent the traditional 8‐day confinement period away from their village. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes are reported. |
| Other bias | Low risk | None detected. |
Herrera 2006.
| Methods | Randomised controlled trial. Study described as placebo‐controlled and double‐blind. | |
| Participants | Location: 3 clinical settings in Santander de Quilichao and Cali, Columbia. Healthy pregnant adolescent women < 19 yrs, between 17 and 19 weeks pregnant. Primigravidas only. No medical complications at trial entry. Women were recruited from clinics while attending outpatient prenatal care. Sample size calculation determined 26 women needed per group. 52 women randomised; data for 48. 2 women from each arm lost to follow‐up. |
|
| Interventions | Oral calcium 600 mg (1 capsule twice daily) versus oral placebo 600 mg (twice daily). | |
| Outcomes | Concentrations of plasma ionised calcium and concentration of the free intracellular calcium concentration only. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence. Randomisation for all 3 sites conducted centrally. |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted centrally. Allocation concealed in sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Women and staff were unaware of treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff blind to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 (8.4%) women lost to follow‐up in each treatment group. Authors state that these exclusions did not modify the results. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes are reported. |
| Other bias | Low risk | None detected. |
Jarjou 2006.
| Methods | This study reports on a subset of women. The entire sample is reported in Goldberg 2013 above. Jarjou 2006 and Goldberg 2013 do not report the same outcomes, so there is no duplication of participants in this review's analysis. Type of study: a randomised, double‐blind, placebo‐controlled study. Method of treatment allocation: participants were randomly assigned in double‐blind fashion to receive calcium or placebo by using block of 4 from published sets of tables in each month and thereby to minimise the potential for seasonal confounding. The code was held by a member of study team who was not directly involved with the collection of data in the field or laboratory and who had no contact with the study participants. Stratification: not stated. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: no, final analyses were 61 from 77 in treatment group, 64 from 78 in control group. Losses to follow‐up: 19.3 % (30 in 155). |
|
| Participants | Location: Gambian women, in rural village of Keneba and Manduar, in the province of West King.
Time frame: May 1995 to June 1999.
Eligible criteria: nulliparity with no history of any medical condition known to affect calcium or bone metabolism, normal single viable pregnancy with known menstrual period date (LMP), registering at antenatal clinic before 20 weeks of gestation and intended to undergo delivery at the same institution, normal glucose tolerance test and willing to participate in trial, first antenatal visit BP below 140/90 mmHg and free of any underlying medical disorders, based on a comprehensive medical examination and routine laboratory tests. Exclusion criteria: had history or evidence of renal disease, collagen vascular disease, chronic hypertension and endocrinological disease or if they took any medication. Total recruited: 155 pregnant women. Treatment group, n = 77 and control group, n = 78 women. In the final analysis only 125 mother‐infant pairs were analysed (61 in the treatment group, 64 in the control group). |
|
| Interventions | 1500 mg of calcium (3 chewable tablets of calcium carbonate per day, 500 mg of elemental calcium). 3 tablets of placebo (contained microcrystalline cellulose and lactose) per day, same shape, taste and texture. The study started from 20 weeks until delivery. | |
| Outcomes |
|
|
| Notes | Lost to follow‐up of infants outcomes: 16 in treatment group; only 61 infants were analysed, 14 in control group; only 64 infants were analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned in double‐blind fashion to receive calcium or placebo by using block of 4 to ensure that equal numbers of subjects were allocated to supplement and placebo groups in each month and thereby to minimize the potential for seasonal confounding. Randomization was achieved by using published sets of tables." |
| Allocation concealment (selection bias) | Low risk | Quote: "The code was held by a member of study team who was not directly involved with the collection of data in the field or laboratory and who had no contact with the study participants." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects were randomly assigned in double‐blind fashion to receive calcium or placebo by using block of 4. Treatment drug and placebo (contained microcrystalline cellulose and lactose) per day, same shape, taste and texture)." Women and staff blind to group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The code was held by a member of study team who was not directly involved with the collection of data in the field or laboratory and who had no contact with the study participants." Anthropometric measurements collected by medical staff. Breast‐milk calcium and phosphorus was collected and sent to laboratory centre. Maternal urine calcium also sent to central laboratory. Matenal calcium intake assessed by field workers. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "There were no significant differences between the 2 groups in supplementation period, or compliance." Flowchart reports attrition due to maternal (1), fetal or neonatal death (5), multiple pregnancy (4), miscalculation of gestation at trial entrance (19) and lost contact (1). Missing data 30 in 155 = 19.3%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Karandish 2003.
| Methods | Type of study: double‐blind, placebo‐controlled, randomised clinical trial. Method of treatment allocation: no data. Placebo: yes (starch tablets). Intention‐to‐treat analyses: no, the initial number of participants were 77 pregnant women but by the end of study 68 participants remained. Losses to follow‐up: 11.7 % (9 in 77). |
|
| Participants | Location: 2 prenatal clinics in county of Ahvaz, Iran. Time frame: no data. Eligible criteria: pregnant women between the ages of 18 to 35. Pregnant women in their third trimester before week 28 of their pregnancies. No history of abortion or stillbirth. Not suffering from any metabolic or chronic diseases. Not having previous history of giving birth to twins. Not being on any other supplements with the exception of iron and folic acid. Total recruited 77 pregnant women, treatment group, n = 33, placebo group, n = 35. |
|
| Interventions | 1000 mg of calcium (2 capsules of 500 mg calcium carbonate) compared with placebo. The study started from 28th‐30th week gestation until delivery. | |
| Outcomes | Anthropometric parameters of neonates including weight, head circumference and length. | |
| Notes | This paper was written in Farsi. Dr Reza Navaei kindly translated it to English using the Cochrane Pregnancy and Childbirth Group's translation form. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Double‐blind, placebo‐controlled, randomised clinical trial were mentioned but there was no detail of sequencing generation. |
| Allocation concealment (selection bias) | Unclear risk | No detail in allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomised controlled trial. Placebo made by Manufacturer as the calcium capsules. Blinding of participants and staff. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was double blind. Patients and clinic staff were unaware of type of medicine. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The initial participants were 77 pregnant women but the final analyses were 68 women. No details about 9 women who dropped out from the study. |
| Selective reporting (reporting bias) | Low risk | The preplanned outcomes were reported. |
| Other bias | Low risk | None identified. |
Kumar 2009.
| Methods | Type of study: double‐blinded randomised trial with simple randomisation sequence developed manually. The blinding of study participants and investigators was done by assigning coded numbers to the package. Method of treatment allocation: the packages were distributed to the participants using the random number in sequence. Placebo: yes. Intention‐to‐treat analyses: no, only participants 524 who delivered in hospital. Losses to follow‐up: 28 from 552 = 5.1% (17 in treatment group and 11 in placebo group). |
|
| Participants | Location: At Lok Nayak Hospital, New Delhi, India. Time frame: January 2005 and December 2007. Eligible criteria: healthy normotensive primigravidas with non complicated singleton pregnancy, 12‐25 weeks' gestation Exclusion criteria: multiple pregnancy, polyhydramnios, fetal malformation, diabetes, chronic hypertension, renal disease, cardiovascular disease, urolithiasis or BP > 140/90 mmHg. Total recruited 552 pregnant women, treatment group, n = 290, placebo group, n = 262. |
|
| Interventions | Oral calcium carbonate 4 tablets daily (500 mg each) compared with placebo, 4 tablets daily, from GA 12‐25 week until delivery. | |
| Outcomes | 1. BP. 2. Maternal and neonatal outcomes including: pre‐eclampsia, preterm delivery, induction of labour, CS, fetal distress during labour, meconium during labour, GA at delivery, gestational duration at delivery (wk; < 32, 32 to 36, 37 to 40, > 40), birthweight g, birthweight (kg < 2, 2 to 2.5, 2.5 to 4), small‐for‐gestational age, stillbirth. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Double‐blinded randomised trial with simple randomisation sequence developed manually. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed by assigning treatment packages with code, which was unbroken until the end of the study. The packages were distributed to the participants using the random number in sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The blinding of study participants and investigators was done by assigning coded numbers to the package. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All women were followed up in prenatal clinic in a routine manner. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up: 28 from 552 = 5.1% (17 in treatment group and 11 in placebo group). |
| Selective reporting (reporting bias) | Low risk | The preplanned outcomes were reported. |
| Other bias | Low risk | None identified. |
Levine 1997.
| Methods | Type of study: double‐blind, computer‐generated simple randomisation sequence. Method of treatment allocation: packages of study tablets were prepared and numbered by manufacturer and then shipped to the medical centre. Stratification: not stated. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: yes. Losses to follow‐up: 253 in 4589 women (5.5%); 132 in the calcium group, 121 in the placebo group. |
|
| Participants | Location: The Calcium for Preeclampsia Prevention (CPEP) Trial at 5 U.S medical centres. Time frame: not stated. Eligibility criteria: nulliparity, normal single viable pregnancy with known menstrual period date (LMP), registering at antenatal clinic before 11 to 21 weeks of gestation and intended to undergo delivery at the same institution, normal glucose tolerance test and willing to participate in trial, BP below 134/84 mmHg and free of any underlying medical disorders, based on a comprehensive medical examination and routine laboratory tests. Exclusion criteria: taking medication, had bad obstetrical conditions, pre‐existing disease, elevated serum concentration of creatinine (> 1.0 mg/dL) or calcium (> 10.6 mg /dlL, pregnant women with renal disease, haematuria, or history of urolithiasis in themselves or in first‐degree relative and who report frequently use of calcium supplementation or antacid. Total recruited: 4589 pregnant women; treatment group n = 2295, control group n = 2294. |
|
| Interventions | 2 g of calcium (4 chewable tablets of calcium carbonate per day, 500 mg of elemental calcium), start at 13 to 20 weeks until delivery, 2 tablets with morning meal and 2 tablets with evening meal. Compared with 4 tablets of placebo (contained lactose and granulated starch) per day, same size, weight and colour. | |
| Outcomes |
|
|
| Notes |
Koo 1999 was another subset report of Levine 1997. Total recruited: 289 pregnant women. 13 refused consent; only 256 women and 256 infants were included (128 in each group).
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Package of study tablets were prepared and numbered by manufacturer according to a computer‐generated simple randomisation sequence developed by statisticians." |
| Allocation concealment (selection bias) | Low risk | Quote: "Package of study tablets were prepared and numbered by manufacturer according to a computer‐generated simple randomisation sequence developed by statisticians and then were shipped to the medical centres. Upon enrolment, each woman was assigned the next number packages of medication at the centre and thus was randomised automatically to receive calcium or placebo according to the pre assigned random sequence." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind, calcium supplementation versus placebo. Package of study tablets were prepared and numbered by manufacturer according to a computer‐generated simple randomisation sequence developed by statisticians. Women and staff blind to allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All pregnant women received routines prenatal care. Staff blind to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of 4589 women enrolled in the study, 253 women (5.5%) were lost to follow‐up; 132 in the calcium group, 121 in the placebo group." Missing data 253 in 4589 = 5.5%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Lopez‐Jaramillo 1989.
| Methods | Type of study: prospective, randomised, double‐blind, controlled clinical trial. Method of treatment allocation: table of random numbers. The containers and the calcium tablets for both groups were prepared in Facultad de Quimica y Farmacia. Stratification: not stated. Placebo: yes, start tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: yes. Losses to follow‐up: 13.2%. Only women with no missing values for any of the covariate and outcome variables were included in the analysis. 49 in calcium group and 43 in placebo group. 6 women in calcium group and 8 in placebo group were eliminated from analysis because they were delivered before 38 weeks. |
|
| Participants | Location: antenatal outpatient clinic in the Hospital Gineco‐Obsterica Isdro Aroya in Quito, Ecuador. Time frame: 30 months during 1984‐1986. Eligible criteria: nulliparity, age < 25 years, certain LMP, registration at antenatal clinic for the first prenatal visit before 24 weeks' gestation and residency in Quito (2800 m altitude) for a period of at least 1 year before conception, BP < 120/80 mmHg and free for of any underlying medical disorders based on a comprehensive medical student examination and routine laboratory tests. Exclusion criteria: had history of cardiovascular, renal or endocrinological disease or if they took any type of drug or vitamin/mineral preparation. Total recruited: 106 pregnant women; n = 55 in treatment group, n = 51 in control group. |
|
| Interventions | 2000 mg of calcium (4 tablets of calcium gluconate daily, 500 mg of elemental calcium) compared with 4 tablets of placebo per day, same size, weight and colour. Started treatment at 23 weeks until delivery. | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a table of random numbers each patients was assigned independently in sequence to one of two treatment regimens." |
| Allocation concealment (selection bias) | Low risk | Quote: "The containers and the calcium tablets for both groups were prepared in Facultad de Quimica y Farmacia." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment versus placebo. All participants were blinded to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The placebo group also received 4 tablets daily of the same size, weight, colour and organoleptic characteristics as calcium tablets. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "106 women satisfactory met the subject selection criteria. Only women with no missing values for any of the covariate and outcome variables were included in these statistical analysis. 49 in the calcium supplemented group and 43 in the placebo group. Six women in calcium supplement group and eight in the placebo group were eliminated from analysis because they were delivery before 38 weeks." Missing data 14 in 106 = 13.2%. |
| Selective reporting (reporting bias) | Unclear risk | Comment: not all enrolled participants were analysed. |
| Other bias | Low risk | None identified. |
Lopez‐Jaramillo 1997.
| Methods | Type of study: a prospective, randomised, double‐blind, controlled clinical trial. Sequence according to random numbers table. Method of treatment allocation: used a table of random numbers to assign each patient independently in sequence to 1 of 2 treatment regimens. Treatment assignment was double‐blind, with composition of tablets unknown to the patients and to all clinical personnel involved in the study. The containers and tablets were prepared in the Facultad de Quimica y Farmacia, Universidad Central del Ecuador. Stratification: not stated. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: no. Losses to follow‐up: yes, 14 in 274 = 5.1%. |
|
| Participants | Location: Hospital Gineco‐Obstetrico Isidro Ayora in Quito, Ecuador. Time frame: 56‐month period between 1990 to 1995. Eligible criteria: age < 17.5 years, nulliparity, normal single viable pregnancy with known menstrual period date (LMP), registering at antenatal clinic before 20 weeks of gestation, residency in Quito (2800 m altitude) for a period of at least 1 year before conception, BP < 120/80 mmHg and free from any underlying medical disorders, based on a comprehensive medical examination and laboratory test. Exclusion criteria: had history of cardiovascular, renal or endocrinological disease or if they took any type of drugs or vitamin/mineral preparations. Total recruited: 274 pregnant teenagers were randomised; 14 women failed to complete the protocol (3 changed residence, 7 changed to a private hospital, 2 changed to hospital of social insurance, 2 by non‐compliance to treatment); only 260 completed the study, 125 girls received 2000 mg calcium, 135 girls in control group. |
|
| Interventions | 2 g calcium (4 tablets of calcium carbonate per day, 500 mg of elemental calcium) compared with 4 tablets of placebo (contained lactose and granulated starch) per day, same size, weight, colour and organoleptic characteristics as calcium tablets. | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a table of random numbers to assigned each patient independently in sequence to one of two treatment regimens." |
| Allocation concealment (selection bias) | Low risk | Quote: "The containers and tablets were prepared in the Facultad de Quimica y Farmacia,Universidad Central del Ecuador." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "We used a table of random numbers to assigned each patient independently in sequence to one of two treatment regimens." Quote: "Treatment assignment was double‐blind, with the composition of the tablets unknown to the patients and to all clinical personnel involve in the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Treatment assignment was double‐blind, with the composition of the tablets unknown to the patients and to all clinical personnel involve in the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "274 teenagers were randomised and then 14 women failed to completed the protocol (3 changed the residence, 7 changed to the private hospital, 2 changed to hospital of social insurance, 2 by non‐compliance to treatment) then only 260 completed the study; 125 girls received 2000 mg calcium, 135 girls in the control group." Missing data 5.1%. The authors did not provide information about how many people were missing in each group. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Niromanesh 2001.
| Methods | Type of study: double‐blind, placebo‐controlled clinical trial. Randomly assigned to 1 of 2 treatments. Method of treatment allocation: the manufacturing company coded the tablets. The hospital pharmacy dispensed the tablet among the participants. Stratification: not stated. Placebo: yes, starch tablet. Sample size calculation: not stated. Intention‐to‐treat analyses: yes. Losses to follow‐up: 0%. |
|
| Participants | Location: Mirza‐Kochak‐Khan Gynecology Hospital, Tehran, Iran. Time frame: not stated. Eligible criteria: high risk for pre‐eclampsia, positive roll‐over test, GA 28 to 32 weeks, BP < 140/90 mmHg. Exclusion criteria: negative for roll‐over test and had any chronic condition such as diabetes, renal diseases, cardiovascular disease, hypertension, and severe anaemia. Total recruited: 30 women at high risk of pre‐eclampsia (15 in the calcium group, 15 in the control group). |
|
| Interventions | 2 g of calcium (4 tablets of 500 mg orally every 6 hours). Compared with placebo. Started treatment at 28 to 32 weeks. | |
| Outcomes |
|
|
| Notes | No details about the type of calcium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Thirty women at high risk of preeclampsia were randomly assigned to 2 g of calcium daily intake and placebo regimen." Comment: the method of sequence generation was not described. |
| Allocation concealment (selection bias) | Low risk | Quote: "The manufactory company coded the tablets. The hospital pharmacy dispensed the tablet among the subjects." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:" Randomization and blinding of subjects and investigator were managed by providing coded tablets of the same packaging and physical characteristics for both calcium and placebo tablets." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote:" Randomization and blinding of subjects and investigator were managed by providing coded tablets of the same packaging and physical characteristics for both calcium and placebo tablets." Blood pressure and proteinuria were evaluated in each visit. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There was no loss to follow up in the course of study." Missing data = 0%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Purwar 1996.
| Methods | Type of study: randomised double‐blind placebo‐controlled trial. Method of treatment allocation: the women were assigned randomly in a double‐blind fashion to 1 of 2 treatment groups (calcium/placebo) using computer‐generated random number table. All the containers and tablets were specially prepared by local Universal pharmaceutical, Nagpur. Stratification: not stated. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: no. Losses to follow‐up: yes, 11 in 201 = 5.5%. |
|
| Participants | Location: the Government Medical College and Hospital, Nagpur, India. Time frame: October 1,1993 to December 31, 1994. Eligible criteria: nulliparity, normal single viable pregnancy with known menstrual period date (LMP), registering at antenatal clinic before 20 weeks of gestation and intending to undergo delivery at the same institution, normal glucose tolerance test < 140 mg/dl and willing to participate in trial, first antenatal visit below 140/90 mmHg and free of any underlying medical disorders, based on a comprehensive medical examination and routine laboratory tests. Exclusion criteria: renal disease, collagen vascular disease, chronic hypertension, endocrinological disease or if on any medication. Total recruited: 201 pregnant women; treatment group, n = 103, control group, n = 98. Final number for analysis (treatment group n = 97, control group n = 93). |
|
| Interventions | 2 g calcium (4 tablets of 500 mg calcium carbonate). Placebo (4 tablets of placebo) same size, weight and colour. | |
| Outcomes |
|
|
| Notes | Missing data 11 in 201 = 5.5%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The women were assigned randomly in a double‐blind fashion at 20 weeks gestation to 1 of 2 treatment groups (calcium/placebo) using computer‐generated random number table." |
| Allocation concealment (selection bias) | Low risk | Quote: "All the containers and tablets used were specially prepared for the study by local Universal Pharmaceutical Pvt Ltd, Nagpur." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The calcium supplemented group received 4 tablets of calcium (500 mg of elemental calcium each) for total 2 g and the placebo group received 4 tablets of the same size, weight, and colour." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blood pressure were measured by one physician specially trained. Any ante/intrapartum maternal and fetal complications were recorded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 98 women randomly assigned to the placebo groups and 103 in calcium groups. Eleven women (5.47%) were lost to follow‐up after randomisation (5 in the placebo group and 6 in the calcium group). The total of 93 women in the placebo group and 98 women in the calcium group were included in the fin final analysis." Missing data 11 in 201 = 5.5%. |
| Selective reporting (reporting bias) | Unclear risk | None identified. There were inconsistent missing data. The number lost to follow‐up is 11 from 201; t200 participants should have remained in the final analysis, but the given number included in final analysis was 201 participants. |
| Other bias | Low risk | None identified. |
Raman 1978.
| Methods | Type of study: pregnant women were assigned by strict rotation to 1 of 3 groups. Method of treatment allocation: not clearly stated. Stratification: not stated. Placebo: no (no treatment). Sample size calculation: not stated. Intention‐to‐treat analyses: no. Losses to follow‐up: 186 in 273 = 68.1%. |
|
| Participants | Location: India, poor segment of the population. Time frame: not stated. Eligible criteria: pregnant women who were in low‐economic status and had regularly consumed supplements were enrolled. Exclusion criteria: pregnant women suffered from complications such as toxaemia, hypertension and diabetes. Total recruited: 273 pregnant women divided into 3 groups. |
|
| Interventions | Calcium lactate was given in tablet form supplying 150 mg of elemental calcium per tablet. Started treatment at 18 to 22 weeks until delivery. Group 1: control, n = 38 no treatment. Group 2: n = 25 received calcium 300 mg/d. Group 3: n = 24 received calcium 600 mg /d. |
|
| Outcomes |
|
|
| Notes | Comment: only 87 participants completed data: 38, 25, 24 participants in 3 groups respectively, high rate of losses to follow‐up 186 in 273 = 68.1%. For data in Analysis 2.4 the placebo n was halved to enable inclusion of data for treatment groups 1 and 2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the women were assigned by strictly rotation to one of three groups". Comment: method of sequence generation was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment versus no treatment. The participants were not blinded. The intervention was divided into 2 groups which unequal dosage. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unable to blind outcomes assessment due to different dosage and treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 273 mothers registered, data completed in all aspect, could be obtained in 87 subjects (38, 25, 24 respectively)". Comment: high rate of loss to follow‐up. Missing data 186 in 273 = 68.1%. (in treatment group (calcium 300 mg/d) = 72.5%; in treatment group (calcium 600 mg/d) = 73.6%; in control group = 58.2%). |
| Selective reporting (reporting bias) | Unclear risk | Unclear. |
| Other bias | Low risk | None identified. |
Rogers 1999.
| Methods | Type of study: randomised trial. Method of treatment allocation: unsealed envelopes. Stratification: not stated. Placebo: no (no treatment). Sample size calculation: not stated. Intention‐to‐treat analyses: yes. Losses to follow‐up: 18 in 237 =7.6%. | |
| Participants | Location: Chinese Women's Hospital, Hong Kong. Time frame: July 1992 to December 1994. Eligible criteria: normotensive, MAP > 80 and < 106 mmHg, second trimester, singleton and used cutoff value 60 mmHg of left lateral position. Exclusion criteria: MAP < 60 mmHg. Total recruited: 500 pregnant women (131 patients were excluded only 369 patients were randomised),154 in calcium group, 132 in low‐dose aspirin, 83 in control group. | |
| Interventions | Compared 3 groups of total 369 patients.
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was into three groups: control, low‐dose aspirin, and calcium supplementation in a ratio of 1:2:2 using five unsealed envelopes." Of 500 nulliparous women screened, 369 were randomised; 154 were in calcium group, 132 were in low‐dose aspirin and 83 as control group." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was into three groups; control, low‐dose aspirin, and calcium supplementation in a ratio of 1:2:2 using five unsealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The participants received different interventions. Could not blind both participants and assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "All randomisation, data collection and data entry were undertaken by the same research assistant with the exception of outcome data, which were entered by the first two authors. The research assistant was therefore blind to the outcome group." It is unclear whether the authors collecting data would have been aware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Fifty (10%) patients eventually delivered in other hospitals and were therefore not subjected to analysis, as they could not reliably be classified into the 3 outcomes groups. 144, 118, and 75 were in calcium group, low‐dose aspirin, and control groups respectively were included in final analysis." Missing data 18 in 237 = 7.6%. |
| Selective reporting (reporting bias) | Unclear risk | None identified. |
| Other bias | Low risk | None identified. |
Sanchez‐Ramos 1994.
| Methods | Type of study: randomised double‐blind, placebo‐controlled clinical trial. Method of treatment allocation: women with positive angiotensin test were randomised by means of a computer‐generated list. Calcium and placebo tablets were provided by pharmaceutical company. Stratification: not stated. Placebo: yes, placebo. Sample size calculation: not stated. Intention‐to‐treat analyses: no. Losses to follow‐up: 4 in 67 = 6.0%. Post randomised exclusion: 6 in 67 = 8.9 % did not comply fully with the protocol; 4 were excluded from analysis after randomisation because of a lack of information, 1 was admitted to another hospital (in placebo group), another woman refused to participate after 1 week of trial (in calcium group). |
|
| Participants | Location: University of Florida Health Science Center, Jacksonville, Florida. Time frame: January 1, 1989 to July 30, 1993. Eligible criteria: normotensive, nulliparous with increased risk of PIH with positive angiotensin sensitivity test only who were positive roll‐over test (women supine diastolic BP value were more than 20 mmHg higher than those obtained on her side) received angiotensin infusion at 24 to 28 weeks. Exclusion criteria: participants with conditions known to increase the incidence of PIH, including history or evidence of renal disease, collagen vascular disease, diabetes mellitus, chronic hypertension and multiple pregnancy. Total recruited: 281 pregnant women were positive roll‐over test; 67 women positive angiotensin sensitivity test; 33 received calcium, 34 received placebo. Final analyses, calcium group, n = 29, control group, n = 34. |
|
| Interventions | 2 g of calcium carbonate, compared with placebo (contained starch and were identical to calcium tablets with respected to weight, size, flavour and appearance). | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women with positive angiotensin test were randomised by means of a computer‐generated list to receive either 2 g/day of elemental calcium or matching placebo." |
| Allocation concealment (selection bias) | Low risk | Quote: "Calcium and placebo tablets were provided by pharmaceutical company." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment versus placebo. All participants were blinded to intervention. Placebo contained starch and were identical to calcium tablets with respect to weight, size, flavour and appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators were blinded to treatment. Placebo tablets contained starch and were identical to calcium tablets with respect to weight, size, flavour and appearance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Six women (8.9%) did not comply fully the protocol; of these, four were excluded from analysis after randomisation because lack of information. One had a single follow‐up prenatal visited and refused to continue participating in the study. She was admitted to another hospital at 35 weeks' gestation with severe preeclampsia and required labour induction (in placebo group). Another woman refused to participate after one week of trial (in calcium group)." Missing data 4 in 67 = 6%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Sanchez‐Ramos 1995.
| Methods | Type of study: computer‐generated list of random numbers. Method of treatment allocation: the randomisation list was maintain by pharmaceutical personnel. The drug and placebo were delivered by pharmacy to antepartum ward, where a nurse administered the medication. Stratification: not stated. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: no, because of a decline in perinatal research support, the study was terminated 3 cases earlier than suggested by power analysis. Losses to follow‐up: 0%. |
|
| Participants | Location: University Medical Center, Jacksonville, Florida, USA. Time frame: July 1990 ‐ January 1993. Eligible criteria: nulliparity, 24 to 36 weeks' gestation, mild pre‐eclampsia and no evidence of severe pre‐eclampsia within 48 hours of admission. Exclusion criteria: proteinuria > 5 g/d, platelet count < 100,000, oliguria (urine < 500 mL/d), pulmonary oedema, elevated liver enzyme > 200 U/L, microangiopathic haemodynamic anaemia, fetal growth retardation, known sensitivity to calcium, chronic hypertension, chronic renal disease, diabetes mellitus,or calcium supplement before admission. Total recruited: 75 eligible participants; 36 in treatment group, 39 in control group (because of a decline in perinatal research support, the study was terminated 3 cases earlier than suggested by power analysis). |
|
| Interventions | 2 g of calcium/d (4 tablets of calcium carbonate per day, 500 mg of elemental calcium). Placebo (4 tablets of placebo per day, same size, weight and colour). | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned using a computer‐generated list of random number to receive either calcium or matching placebo." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation list was maintained by pharmacy personnel; the drug and placebo were delivered by pharmacy to antepartum ward, where nurse administered the medication." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment versus placebo. All participants were blinded to intervention. Placebo tablets contained starch and were identical to calcium tablets with respect to weight, size, flavour and appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators were blinded to treatment. Placebo tablets contained starch and were identical to calcium tablets with respect to weight, size, flavour and appearance. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "During the studied period, 75 eligible subjects were identified and invited to participate; 36 (48%) were assigned to calcium and 39 (52%) to placebo. Because of decline in perinatal research support, the study was terminated three cases earlier than suggested by power analysis." Missing data = 0%. |
| Selective reporting (reporting bias) | Low risk | Trial was stopped before complete enrolment due to decline of research fund. |
| Other bias | Low risk | None identified. |
Taherian 2002.
| Methods | Type of study: randomised controlled study. Method of treatment allocation: the sampling method was non probability convenience. Used table of random numbers to assign each case independently to 1 of 3 groups. Stratification: not stated. Placebo: no (no treatment). Sample size calculation: not stated. Intention‐to‐treat analyses: yes. Losses to follow‐up: 0%. |
|
| Participants | Location: Isfahan Health Centre, Iran. Time frame: April 1998 to March 2001. Eligible criteria: nulliparity, single gestation, first antenatal visit before 20 weeks of gestation, BP < 130/80 mmHg and no proteinuria by urine dipstick. Exclusion criteria: had history of cardiovascular, renal disease or endocrinologic problem, medical or obstetric complications and those with known hazardous condition (multiple gestation, hydatidiform mole). Total recruited: 990 healthy pregnant women (n = 330 participants/group). |
|
| Interventions | Group 1: received 75 mg aspirin /day, n = 330. Group 2: received 500 mg calcium carbonate/day, n = 330. Group 3: no treatment as control group, n = 330. Started treatment at 20 weeks until delivery. | |
| Outcomes |
|
|
| Notes | Comment: the results were reported as mean and 95% CI; we changed them into SD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The sampling method was non probability convenience. We used table of random numbers to assign each case independently to one of three groups." |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Group 1 received 75 mg aspirin; group 2 received 500 mg oral calcium‐D daily; and the control group 3 received no medication at all." Comment: it was impossible to blind because the difference between drug and no treatment in control group. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote:" All cases received prenatal care according to the approved model, BP, body weight and maternal height were measured." Comment: assessors were not blinded because the participants received different treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all enrolled participants were included in the analyses. Missing data = 0%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Villar 1987.
| Methods | Type of study: double‐blinded, randomised controlled clinical trial. Method of treatment allocation: the women were assigned randomly in the double‐blind fashion at 26 weeks' gestation to 1 of 2 treatment groups, using a randomisation schedule prepared in advance for the complete population. Stratification: not stated. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: yes. Losses to follow‐up: 0%. |
|
| Participants | Location: The Johns Hopkins Hospital in Baltimore and Perinatal Study Center of Rosario, Argentina. Time frame: 1983 to 1985. Eligible criteria: nulliparous, singleton, known LMP, age 18 to 30 years, free from any underlying medical disorders, negative roll‐over test. Exclusion criteria: history of cardiovascular or renal disease or taking any drug. Total recruited: 52 pregnant women: *18 white: 9 in calcium group, 9 in placebo group; *34 black women: 16 in calcium group, 18 in placebo group. Total in calcium group, n = 25; in placebo group, n = 27. |
|
| Interventions | 3 tablets of calcium carbonate (500 mg each). Compared with 3 placebo tablets with same size, weight, size, colour and organoleptic characteristics. Started treatment at 26 weeks. | |
| Outcomes |
|
|
| Notes | Comment: the authors provide only mean birthweight but not SD. SDs in both groups were imputed by mean. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The women were assigned randomly in the double blind fashion at 26 weeks gestation to one of two treatment groups, using a randomisation schedule prepared in advance for the complete population." |
| Allocation concealment (selection bias) | Low risk | Quote: "The same randomization code, standardization process, and tablets were used in both populations and code was kept in central allocation (Baltimore). Random number in closed envelopes and corresponding medication were distributing to the two hospitals at the beginning. All containers and tablets were prepared by pharmaceutical." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The women were assigned randomly in the double blind fashion at 26 weeks gestation to one of two treatment groups, using a randomisation schedule prepared in advance for the complete population." Quote: "Placebo same size, weight, size, colour and organoleptic characteristic." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All BP value were measured by one nurse‐midwife and one physician especially recruited and trained for the study." Comment: not specifically stated that outcome assessors were blinded, but placebo and treatment were identical. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all enrolled participants were included in the analyses. Missing data = 0%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Villar 1990.
| Methods | Type of study: double‐blind, randomised placebo‐controlled clinical trial. Method of treatment allocation: computer‐generated list of random number. Opaque envelopes with the bottle number were located at the clinic and the project co‐ordinator was in charge of the administration of the treatment assigned. Stratification: not stated. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: yes. Losses to follow‐up: 0%. |
|
| Participants | Location: Adolescent Pregnancy Clinic of the Johns Hopkins Hospital in Baltimore. Time frame: 1985 to1988. Eligible criteria: age < 17 year, GA < 20 week, singleton pregnancy, certain LMP, free from any underlying medical disorders determined by history, physical examination, and laboratory tests. Exclusion criteria: underlying medical disorders determined by history, physical examination, and laboratory tests. Total recruited: 190 adolescent pregnant women; 95 in the calcium group, 95 in the placebo group. |
|
| Interventions | 2 g of calcium (4 tablets of calcium carbonate per day, 500 mg of elemental calcium) compared with 4 tablets of placebo (contained lactose and granulated starch) per day, same size, weight and colour. Started treatment at 20 weeks until delivery. | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐ generated list of random number was used to allocate the corresponding treatments." |
| Allocation concealment (selection bias) | Low risk | Quote: "Opaque envelope with the bottle number were locate at the clinic and project coordinator was in charge of the administration of the treatment assigned." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treatment versus placebo. All participants were blinded to interventions. Placebo was the same size, weight, colour, and had the same organoleptic characteristics as the calcium tablets. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo was he same size, weight, colour, and had the same organoleptic characteristics as the calcium tablets. The investigators were blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all enrolled participants were analysed. Missing data = 0%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Villar 2006.
| Methods | Type of study: multicentre, randomised, placebo‐controlled, double‐blind trial. Central randomisation at WHO Geneva. Method of treatment allocation: computer‐generated random number blocking with randomly varying groups of 6 to 8 women and were used to restrict randomisation in the strata (country). The technique consisted of allocating consecutively numbered treatment boxes for each woman. Randomisation codes remained at the WHO Clinical trial Unit until the time of analysis and were not available to any person until the analyses were completed. Boxes and tablets were prepared and numbered by Magistra SA, GENEVA and were shipped to each centre. Stratification: yes, by country. Placebo: yes, starch tablets. Sample size calculation: not stated. Intention‐to‐treat analyses: yes. Losses to follow‐up: 13 in 8325 = 0.16%. Before treatment started, 2 women in the calcium group were not pregnant. 2 women in the placebo group were excluded from the analyses. 143 (3.4%) in calcium group (4157‐143) were lost to follow‐up and no delivery information; 4008 pregnancies available for analyses. 155 (3.7%) in placebo group (4168‐155) lost to follow‐up and no delivery information; 4006 pregnancies available for analyses of preterm labour and 4161 pregnancies available for analyses of PIH (final analyses) of preterm labour and 4151 pregnancies available for analyses of PIH (final analyses). Post randomised exclusion: 4 in calcium were not pregnant, 5 in placebo group. |
|
| Participants | Location: Rosiario, Argentina; Assiut , Egypt; Nagpur and Vellor, India; Lima, Peru; Johannesburg, South Africa; Ho Chiminh City, Vietnam; where population intake calcium < 600 mg/d. Time frame: November 2001 to July 2003. Eligible criteria: healthy nulliparity, normal single viable pregnancy with known menstrual period date (LMP), registering at antenatal clinic before 20 weeks of gestation. Exclusion criteria: BP > 140/90 mmHg, had history or evidence of chronic hypertension, renal disease, signs and symptoms of nephrolithiasis, parathyroid disease and disease that require digoxin, phenytoin, or tetracycline therapy. Total recruited: 8325 pregnant women were randomised, treatment group, n = 4157, control group, n = 4168. |
|
| Interventions | 1.5 g of calcium carbonate (1 x 500 mg tablet, 3 times per day at meal time), chewable tablets started at 20 weeks until delivery, and > 3 hours after any iron supplement. Compared with 3 tablets of placebo (contained lactose, sorbitol, cellulose plus other calcium free ingredient) per day, same form, colour and taste. | |
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Central randomisation at WHO Geneva. Computer‐generated random number blocking with randomly varying groups of 6 to 8 women and were used to restrict randomisation in the strata (country)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The technique consisted of allocating consecutively numbered treatment boxes for each woman. Randomization codes remained at the WHO Clinical trial Unit until the time of analysis and were not available to any person until the analyses were completed. Boxes and tablets were prepared and numbered by Magistra SA, GENEVAand were shipped to each centre." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Women were assigned randomly to receive calcium tablets or placebo.They were identical in form, color and taste." Randomisation code kept until study end. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Women were assigned randomly to receive calcium tablets or placebo.They were identical in form, color and taste." Blood pressure was recorded by trained nurses and doctors. Randomisation code kept until study end. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of 8325 women assigned randomly to group, 4157 were assigned to the calcium group and 4168 were assigned to the placebo group. Nine women (5 in the placebo group; 4 in calcium group) were determined to not be pregnant, and 2 women from each group who were lost to follow‐up before starting any treatment were excluded from all analyses. Delivery information was unavailable for 143 (3.4%) in the placebo group; therefore, they did not contribute to the preterm analyses, but the available data were included in the analyses for other outcomes. Thus, 4151 women in the calcium group and 4161 women in the placebo group contribute to the final analyses." Missing data 13 in 8325 = 0.16%. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Wanchu 2001.
| Methods | Type of study: randomly assigned to 2 treatment groups. Method of treatment allocation: not stated. Stratification: not stated. Placebo: no (no treatment). Sample size calculation: not stated. Intention‐to‐treat analyses: no. Losses to follow‐up: 20 in 120 = 16.7%. |
|
| Participants | Location: Post Graduate Institute of Medical Education and Research, Chandigarh. Time frame: not stated. Eligible criteria: uncomplicated normotensive primigravida with singleton pregnancy, GA < 20 weeks. Exclusion criteria: multiple pregnancy, molar pregnancy, hydramnios, congenital malformation, chronic hypertension, chronic renal disease, diabetes mellitus and those already on calcium supplementation. Total recruited: 120 pregnant women were enrolled, 100 who completed the protocol were analysed. 50 participants in treatment group, 50 participants in control group. | |
| Interventions | 2 g of calcium (4 tablets of calcium carbonate). Compared with no treatment. Started treatment at 20 weeks. | |
| Outcomes |
|
|
| Notes | No restriction was put on dietary calcium intake in either group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to ether group of two treatment groups." Comment: the method of sequence generation was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Comment: the method of allocation concealment was not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment versus no treatment. Could not blind participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Could not blind investigator due to different treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "120 pregnant women were enrolled in the study, 100 women who completed the protocol were analysed." Missing data = 20 in 120 = 16.7%. |
| Selective reporting (reporting bias) | Unclear risk | None identified. |
| Other bias | Low risk | None identified. |
BP: blood pressure Ca: calcium CI: confidence interval CS: caesarean section g: gram g/d: grams per day GA: gestational age ITT: intention‐to‐treat IUGR: intrauterine growth restriction LMP: last menstrual period MAP: mean arterial pressure NICU: neonatal intensive care unit mg: milligram mL/d: millilitres per day mmHg: millimetres mercury PIH: pregnancy‐induced hypertension PROM: preterm rupture of the membranes SD: standard deviation U/L: units per litre WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Almirante 1998 | This trial was reported in abstract form only. We emailed the authors for further information at the last publication of the review (2011) and have had no reply. The study has been excluded for insufficient information. |
| Asemi 2012 | The intervention was not relevant. The intervention was a combination of calcium and vitamin D supplementation. The study population was at risk for pre‐eclampsia. The intervention took place in the third trimester. For these reasons the trial was excluded. |
| Chames 2002 | This trial was reported in abstract form only. We emailed the authors for further information at the last publication of the review (2011) and have had no reply. The study has been excluded for insufficient information. |
| Diogenes 2013 | The intervention was not relevant. The intervention for this trial was a combination of calcium and vitamin D. |
| Duggin 1974 | The outcomes were not relevant and allocation to groups was not random. 7 primiparas (aged 16‐19 years) who were at 32‐36 weeks' gestation were included. The participants were divided into control participants (patient 1‐4) and supplemented participants (patients 5‐7) to measure metabolic balance. |
| Felix 1991 | There was no random allocation to groups in this trial (women allocated to groups in sequence). The aim of the study was to examine the hypotensive effects of calcium in Andean women. |
| Galimberti 2001 | This trial was reported in abstract form only. We emailed the authors for further information at the last publication of the review (2011) and have had no reply. The study has been excluded for insufficient information. |
| Hammar 1981 | The participants were not appropriate. This study was aimed to determine the effect of calcium treatment in pregnant women who suffered from leg cramps; 42 pregnant women who suffered from leg cramps with gestational age 21‐38 weeks were included. |
| Janakiraman 2003 | This was a cross‐over study examining bone resorption among pregnant women during received calcium supplementation. 32 pregnant women gestational age 25‐35 weeks participated in the study for 20 days. Each women received 1200 mg calcium supplement for 10 days and multivitamin without calcium for 10 days. N‐telopeptides of type I collagen (NTX), a biomarker of bone resorption were measured. |
| Kalkwarf 1997 | The intervention was not appropriate. The study aimed to examine the effect of calcium supplementation on bone density in postpartum period. The randomised, placebo‐controlled trial of 1 g calcium supplementation was conducted in 97 lactating and 99 non‐lactating women a mean 16 ± 2 days postpartum (the study of lactation). The other trial (the study of weaning) 95 lactating women who weaned their infants in 2 months after enrolment and 92 non‐lactating women were enrolled 5.6 ± 0.8 months postpartum. |
| Kent 1995 | The participants were not appropriate. This was a study focusing on the postpartum period and women were not randomised to receive calcium until 36 weeks' gestation. This trial aimed to study the effect of an oral calcium supplement on regional bone loss in normal lactation women. 79 pregnant women at gestational age 36 weeks were randomised to received placebo or 500 mg twice daily of calcium through to 24 weeks' lactation. |
| Liu 2011 | The intervention was not appropriate. The participants were provided calcium supplementation until 6 weeks postpartum and measured bone mineral density post‐treatment. |
| Lopez‐Jaramillo 1990 | The participants were not appropriate.The study aimed to examine the effect of calcium supplementation on risk of PIH in pregnant women who had a positive roll‐over test. |
| Mahomed 2000 | The participants were not appropriate. The study aimed to examine the effect of calcium supplementation on risk of PIH and preterm labour in twin pregnancy. |
| Mazurkevich 2013 | This intervention was not relevant. The intervention in this study was a combination of calcium carbonate and cholecalciferol. |
| Mukherjee 1997 | The participants were not appropriate. The study aimed to examine the effect of calcium supplementation in reducing leg cramps in homogeneous Chinese population. All pregnant women who suffered from leg cramps during January 1994 and May 1995 were enrolled to received either calcium gluconate 600 mg twice daily or 2 multivitamin tablets twice daily. |
| Odendaal 1974 | The participants were not appropriate. Calcium was supplemented only when participants suffered from leg cramps, which not relevant to the objective of the review. |
| Prentice 1995 | This study did not examine calcium supplementation amongst pregnant women. 60 Gambian mothers consuming a low‐calcium were randomised to receive calcium supplement or placebo from 10 days to 78 weeks postpartum. |
| Qui 1999 | The intervention was not relevant. Calcium was supplemented from 20 weeks' gestation to postpartum 45 days. |
| Robinson 1947 | The intervention was not appropriate. Calcium was given to treat women with leg cramps, which was not relevant to the objective of the review. |
g: grams PIH: pregnancy‐induced hypertension
Characteristics of studies awaiting assessment [ordered by study ID]
Aghamohammady 2010.
| Methods | Randomised controlled trial. |
| Participants | Healthy pregnant women age > 35. Nulliparous only, between 15‐20 weeks' gestation. |
| Interventions | 2 g daily elemental calcium versus 2 g daily placebo. |
| Outcomes | Preterm delivery, pre‐eclampsia. |
| Notes | This trial is reported in abstract form only with no outcome data. We have emailed the authors for additional information and outcome data. |
Sulovic 2013.
| Methods | Randomised controlled trial. |
| Participants | Healthy nulliparous pregnant women between 14‐23 weeks' gestation. |
| Interventions | Daily treatment with 2 g elemental calcium versus daily treatment with placebo. |
| Outcomes | Incidence of pre‐eclampsia, PIH, preterm deliveries, small for gestational age births, fetal or neonatal deaths. |
| Notes | This trial is reported in abstract form only. We have emailed the authors for additional outcome data. |
Zheng 2000.
| Methods | Randomised controlled trial. |
| Participants | Pregnant women with gestation 20‐34 weeks. |
| Interventions | Osteoform capsules 2 tablets daily (dosage not stated) vs no treatment. |
| Outcomes | Serum calcium level, symptoms, PIH, IUGR. |
| Notes | This trial is reported in Chinese language only and has been sent for translation. |
Ca: Calcium g/d: grams per day IUGR: intrauterine growth restriction PIH: pregnancy‐induced hypertension
Differences between protocol and review
We used stillbirth or fetal death as the same outcome, these were listed as separate outcomes in the protocol.
We added mode of birth (vaginal birth, instrumental birth, caesarean section), postpartum haemorrhage as secondary maternal outcomes.
We deleted limb pain from the list of neonatal outcomes and osteopenia and osteoporosis from the list of maternal outcomes.
We have modified the wording in the methods sections for Assessment of risk of bias in included studies and Assessment of reporting biases to update them with the current methods being used by the Cochrane Pregnancy and Childbirth Group. The quality of the evidence was assessed using the GRADE approach (Schunemann 2009) in order to assess the quality of the body of evidence relating key outcomes.
Contributions of authors
P Buppasiri (PB) developed the protocol. P Lumbiganon (PL) and J Thinkhamrop (JT) edited and commented on the protocol. C Ngamjarus (CN) commented on the protocol.
For the review, PB and JT independently extracted the data. CN conducted the statistical analysis and summarised the results. PB drafted the review. All review authors commented and finalised the review.
For the updated review, PB and Nancy Medley (NM) independently extracted new data and updated the review. NM added GRADE summary. All review authors commented and finalised the update review.
Sources of support
Internal sources
Faculty of Medicine, Khon Kaen University, Thailand.
Faculty of Public Health, Khon Kaen University, Thailand.
External sources
Thailand Research Fund / Senior Research Scholar, Thailand.
SEA‐ORCHID Project, Thailand.
Department of Nutrition for Health and Development, WHO, Switzerland.
UNDP‐UNFPA‐UNICEF‐WHO‐World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), Department of Reproductive Health and Research (RHR), World Health Organization, Switzerland.
Declarations of interest
Malinee Laopaiboon received an honorarium from the Thailand Research Fund which is a non‐profit organisation.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Belizan 1983 {published data only}
- Belizan JM, Villar J, Zalazar A, Rojas L, Chan D, Bryce GF. Preliminary evidence of the effect of calcium supplementation on blood pressure in normal pregnant women. American Journal of Obstetrics and Gynecology 1983;146:175‐80. [DOI] [PubMed] [Google Scholar]
Belizan 1991 {published data only}
- Belizan JM, Villar J, Bergel E, Pino A, Fulvio S, Galliano SV, et al. Long term effect of calcium supplementation during pregnancy on the blood pressure of offspring: follow up of a randomized controlled trial. BMJ 1997;315:281‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belizan JM, Villar J, Gonzalez L, Campodonico L, Bergel E. Calcium supplementation to prevent hypertensive disorders of pregnancy. New England Journal of Medicine 1991;325:1399‐405. [DOI] [PubMed] [Google Scholar]
- Bergel E, Gibbons L, Rasines MG, Luetich A, Belizan JM. Maternal calcium supplementation during pregnancy and dental caries of children at 12 years of age: follow‐up of a randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica 2010;89(11):1396‐402. [DOI] [PubMed] [Google Scholar]
- Stephens IF. Effect of calcium supplementation during pregnancy on blood pressure of offspring; Authors cannot be sure of effect's generalisability to all children aged 5‐9 [letter; comment]. BMJ 1998;316(7126):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Belizan JM, Repke JT. Does calcium supplementation reduce pregnancy‐induced hypertension and prematurely?. Proceedings of International Symposium on Advances in the Prevention of Low Birthweight; 1988 May 8‐11; Cape Cod, Massachusetts, USA. 1988.
Boggess 1997 {published data only}
- Boggess KA. A randomised controlled trial of the effect of third trimester calcium supplementation on maternal haemodynamic function. Obstetrics & Gynecology 1997;90:157‐61. [DOI] [PubMed] [Google Scholar]
Chan 2006 {published data only}
- Chan GM. Effects of dairy foods on mothers and their newborns. ClinicalTrials.gov (http://clinicaltrials.gov/) [accessed 21 June 2007] 2007.
- Chan GM. The effects of dietary milk on adolescent pregnant mothers and their newborn [abstract]. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco, CA, USA. 2006.
- Chan GM, McElllgot K, McNaught T, Gill G. Effects of dietary calcium intervention on adolescent mothers and newborns. Obstetrics & Gynecology 2006;108:565‐71. [DOI] [PubMed] [Google Scholar]
Crowther 1999 {published data only}
- Crowther C, Hiller J, Pridmore B, Bryce R, Duggan P, Hague W, et al. Calcium supplementation in nulliparous women for the prevention of pregnancy included hypertension, pre‐eclampsia and preterm birth: an Australian randomised trial. 2nd Annual Congress of the Perinatal Society of Australia & New Zealand; 1998 March 30‐April 4; Alice Springs, Australia. 1998:101.
- Crowther CA, Hiller JE, Pridmore B, Bryce R, Duggan P, Hague WM, et al. Calcium supplementation in nulliparous women for the prevention of pregnancy‐induced hypertension, preeclampsia and preterm birth: an Australian randomized trial Fracog and the ACT study group. Australian and New Zealand Journal of Obstetrics and Gynaecology 1999;39(1):12‐8. [DOI] [PubMed] [Google Scholar]
- Griffith EC, Crowther CA, Hiller JE, Wilson KJ, ACT Study Group. Leg cramps in pregnancy: ineffectiveness of calcium supplementation. 2nd Annual Congress of the Perinatal Society of Australia & New Zealand; 1998 March 30‐April 4; Alice Springs, Australia. 1998:99.
- Hiller JE, Crowther CA, Moore VA, Willson K, Robinson JS. Calcium supplementation in pregnancy and its impact on blood pressure in children and women: follow up of a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2007;47(2):115‐21. [DOI] [PubMed] [Google Scholar]
Ettinger 2009 {published data only}
- Ettinger AS, Lamadrid H, Mercado A, Kordas K, Peterson K, Hu H, et al. Effect of calcium on bone resorption and bone mineral density in pregnancy: a randomized control trial. American Journal of Epidemiology 2011;173(Suppl):S236. [Google Scholar]
- Ettinger AS, Lamadrid‐Figueroa H, Tellez‐Rojo MM, Mercado‐Garcia A, Peterson KE, Schwartz J, et al. Effect of calcium supplementation on blood lead levels in pregnancy: a randomized placebo‐controlled trial. Environmental Health Perspectives 2009;117(1):26‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2013 {published data only}
- Goldberg GR, Jarjou LM, Cole TJ, Prentice A. Randomized, placebo‐controlled, calcium supplementation trial in pregnant Gambian women accustomed to a low calcium intake: effects on maternal blood pressure and infant growth. American Journal of Clinical Nutrition 2013;98(4):972‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrera 2006 {published data only}
- Herrera JA, Arevalo‐Herrera M, Villegas A, Herrera S, Villalba M, Bromet A. Calcium oral supplementation in adolescent pregnant women. Colombia Medica 2006;37(2 Suppl 1):15‐20. [Google Scholar]
Jarjou 2006 {published data only}
- Hawkesworth S, Sawo Y, Fulford AJ, Goldberg GR, Jarjou LM, Prentice A, et al. Effect of maternal calcium supplementation on offspring blood pressure in 5‐ to 10‐y‐old rural Gambian children. American Journal of Clinical Nutrition 2010;92(4):741‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesworth S, Walker CG, Sawo Y, Fulford AJ, Jarjou LM, Goldberg GR, et al. Nutritional supplementation during pregnancy and offspring cardiovascular disease risk in The Gambia. American Journal of Clinical Nutrition 2011;94(6 Suppl):1853S‐1860S. [DOI] [PubMed] [Google Scholar]
- Jarjou L, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, et al. Randomized, placebo‐controlled, calcium supplementation study in pregnant Gambian women: effects on breast‐milk calcium concentrations and infant birth weight, growth, and bone mineral accretion in the first year of life. American Journal of Clinical Nutrition 2006;83(3):657‐66. [DOI] [PubMed] [Google Scholar]
- Jarjou LM, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake. American Journal of Clinical Nutrition 2010;92(2):450‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjou LM, Prentice A, Bennett J. Impact of calcium supplementation in the preceding pregnancy on the human milk calcium concentration of Gambian women. Advances in Experimental Medicine and Biology 2004;54:347‐9. [DOI] [PubMed] [Google Scholar]
- Jarjou LMA, Bennett J, Laidlow A, Goldberg GR, Prentice A. Changes in bone turnover and calciotropic hormones in lactating Gambian women supplemented with calcium during pregnancy. Journal of Human Lactation 2007;23:86‐7. [Google Scholar]
- Jarjou LMA, Sawo Y, Goldberg GR, Laskey MA, Cole TJ, Prentice A. Persistent effects of calcium supplementation during pregnancy on maternal bone mineral content: a follow‐up study in Gambian women. Proceedings of the 16th ISRHML Conference "Breastfeeding and the Use of Human Milk. Science and Practice"; 2012 Sept 27th‐Oct 1st; Trieste, Italy. 2012:Abstract no.:105.
- Jarjou MA, Yankuba S, Goldberg R, Laskey MA, Cole J, Prentice A. Unexpected long‐term effects of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake: a follow‐up study. American Journal of Clinical Nutrition 2013;98(3):723‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A, Jarjou LM, Goldberg GR, Bennett J, Cole TJ, Schoenmakers I. Maternal plasma 25‐hydroxyvitamin D concentration and birthweight, growth and bone mineral accretion of Gambian infants. Acta Paediatrica 2009;98(8):1360‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karandish 2003 {published data only}
- Karandish M, Djazayery A, Mahmoudi M, Behrooz A. The effect of calcium supplementation during pregnancy on birth weight. Medical Journal of Reproduction and Infertility 2003;4(3):184. [Google Scholar]
Kumar 2009 {published data only}
- Kumar A, Devi SG, Batra S, Singh C, Shukla DK. Calcium supplementation for the prevention of pre‐eclampsia. International Journal of Gynecology & Obstetrics 2009;104(1):32‐6. [DOI] [PubMed] [Google Scholar]
Levine 1997 {published data only}
- Harrison‐Hohner J, Coste S, Dorato V, Curet LB, McCarron D, Hatton D. Prenatal calcium supplementation and postpartum depression: an ancillary study to a randomized trial of calcium for prevention of preeclampsia. Archives of Women's Mental Health 2001;3:141‐6. [Google Scholar]
- Hatton DC, Harrison‐Hohner J, Coste S, Reller M, McCarron D. Gestational calcium supplementation and blood pressure in the offspring. American Journal of Hypertension 2003;16:801‐5. [DOI] [PubMed] [Google Scholar]
- Koo WWK, Walters JC, Esterlitz J, Levine RJ, Bush AJ, Sibai B. Maternal calcium supplementation and fetal bone mineralization. Obstetrics & Gynecology 1999;94:577‐82. [DOI] [PubMed] [Google Scholar]
- Levine RJ. Calcium for preeclampsia prevention (CPEP): a double‐blind, placebo‐controlled trial in healthy nulliparas. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S2. [DOI] [PubMed] [Google Scholar]
- Levine RJ, CPEP Study Group. The trial of calcium for preeclampsia prevention (CPEP). 8th World Congress of the International Society for the Study of Hypertension in Pregnancy; 1992 November 8‐12; Buenos Aires, Argentina. 1992:94.
- Levine RJ, Esterlitz JR, Raymond EG, DerSimonian R, Hauth JC, Ben Curet L. Trial of calcium for preeclampsia prevention (CPEP): rationale, design, and methods. Controlled Clinical Trials 1996;17(5):442‐69. [DOI] [PubMed] [Google Scholar]
- Levine RJ, Hauth JC, Curet LB, Sibai BM, Catalano PM, Morris CD, et al. Trial of calcium to prevent preeclampsia. New England Journal of Medicine 1997;337:69‐76. [DOI] [PubMed] [Google Scholar]
Lopez‐Jaramillo 1989 {published data only}
- Lopez‐Jaramillo P, Narvaez M, Weigel RM, Yepez R. Calcium supplementation reduces the risk of pregnancy‐induced hypertension in an Andes population. British Journal of Obstetrics and Gynaecology 1989;96:648‐55. [PubMed] [Google Scholar]
- Lopez‐Jaramillo P, Narvaez M, Yepez R. Effect of calcium supplementation on the vascular sensitivity to angiotensin II in pregnant women. American Journal of Obstetrics and Gynecology 1987;156:261‐2. [DOI] [PubMed] [Google Scholar]
- Navaez M, Lopez‐Jaramillo P, Weigel M. Calcium (Ca++) supplementation reduces the risk for pregnancy induced hypertension (PIH). 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:180‐1.
Lopez‐Jaramillo 1997 {published data only}
- Lopez‐Jaramillo P, Delgado F, Jacome P, Teran E, Ruano C, Rivera J. Calcium supplementation and risk of preeclampsia in Ecuadorian pregnancy teenagers. Obstetrics & Gynecology 1997;90:162‐7. [DOI] [PubMed] [Google Scholar]
Niromanesh 2001 {published data only}
- Niromanesh S, Laghaii S, Mosavi‐Jarrahi A. Supplementation in prevention of pre‐eclampsia. International Journal of Gynecology & Obstetrics 2001;74:17‐21. [DOI] [PubMed] [Google Scholar]
Purwar 1996 {published data only}
- Purwar M, Kulkarni H, Motghare V, Dhole S. Calcium supplementation and prevention of pregnancy induced hypertension. Journal of Obstetrics and Gynaecology Research 1996;22:425‐30. [DOI] [PubMed] [Google Scholar]
- Purwar M, Motghare V, Kulkarni H. Calcium supplementation and prevention of pregnancy induced hypertension: randomized double blind controlled trial [abstract]. Journal of Clinical Epidemiology 1996;49 Suppl 1:28S. [DOI] [PubMed] [Google Scholar]
Raman 1978 {published data only}
- Raman L, Rajalakshmi K. Effect of calcium supplementation to undernourished mothers during pregnancy on bone density of the neonates. American Journal of Clinical Nutrition 1978;31:466‐9. [DOI] [PubMed] [Google Scholar]
Rogers 1999 {published data only}
- Rogers MS, Fung HYM, Hung CY. Calcium and low dose aspirin prophylaxis in women at high risk of pregnancy induced hypertension. Hypertension in Pregnancy 1999;18:165‐72. [DOI] [PubMed] [Google Scholar]
Sanchez‐Ramos 1994 {published data only}
- Sanchez‐Ramos L, Briones DK, Kaunitz AM, Delvalle GO, Gaudier FL, Walker CD. Prevention of pregnancy induced hypertension by calcium supplementation in angiotensin II sensitive patients. Obstetrics & Gynecology 1994;84:349‐53. [PubMed] [Google Scholar]
- Sanchez‐Ramos L, Valle GO, Briones D, Walker RN, Delke I, Gaudier F. Prevention of preeclampsia by calcium supplementation in angiotensin‐sensitive patients. American Journal of Obstetrics and Gynecology 1994;170:408. [PubMed] [Google Scholar]
Sanchez‐Ramos 1995 {published data only}
- Sanchez‐Ramos L, Adair CD, DelValle GO, Gaudier F, Delke I. Calcium supplementation in mild preeclampsia remote from term: a prospective randomized double‐blind clinical trial. American Journal of Obstetrics and Gynecology 1993;168:385. [DOI] [PubMed] [Google Scholar]
- Sanchez‐Ramos L, Adair CD, Kaunitz AM, Briones DK, Valle GO, Delke I. Calcium supplementation in mild preeclampsia remote from term: a randomized double clinical trial. Obstetrics & Gynecology 1995;85:915‐8. [DOI] [PubMed] [Google Scholar]
Taherian 2002 {published data only}
- Taherin AA, Taherian A, Shirvani A. Prevention of preeclampsia with low‐dose aspirin or calcium supplementation. Archives of Iranian Medicine 2002;5:151‐6. [Google Scholar]
Villar 1987 {published data only}
- Repke JT, Villar J, Anderson C, Pareja G, Dubin N, Belizan JM. Biochemical changes associated with blood pressure reduction induced by calcium supplementation during pregnancy. American Journal of Obstetrics and Gynecology 1989;160:684‐90. [DOI] [PubMed] [Google Scholar]
- Villar J, Repke J, Belizan JM, Pareja G. Calcium supplementation reduces blood pressure during pregnancy; results of a randomized control clinical trial. Obstetrics & Gynecology 1987;70:317‐22. [PubMed] [Google Scholar]
Villar 1990 {published data only}
- Repke J, Villar J, Bergel E, Belizan JM. The effect of iron absorption in patients receiving calcium supplementation. Proceedings of 9th Annual Meeting of the Society of Perinatal Obstetricians; 1989 Feb 1‐4; New Orleans, Louisiana, USA. 1989:512.
- Villar J, Belizan JM, Repke J. The effect of calcium supplementation on the incidence of hypertensive disorders of pregnancy and prematurity (Study 1). 7th World Congress of Hypertension in Pregnancy; 1990 October; Perugia, Italy. 1990:54.
- Villar J, Repke JT. Calcium supplementation during pregnancy may reduce preterm in high‐risk populations. American Journal of Obstetrics and Gynecology 1990;163:1124‐31. [DOI] [PubMed] [Google Scholar]
Villar 2006 {published data only}
- Abalos E, Merialdi M, Wojdyla D, Carroli G, Campodonico L, Yao SE, et al. Effects of calcium supplementation on fetal growth in mothers with deficient calcium intake: a randomised controlled trial. Paediatric and Perinatal Epidemiology 2010;24(1):53‐62. [DOI] [PubMed] [Google Scholar]
- Abdel‐Aleem H, Merialdi M, Elsnosy ED, Elsedfy GO, Abdel‐Aleem MA, Villar J. The effect of calcium supplementation during pregnancy on fetal and infant growth: a nested randomized controlled trial within WHO calcium supplementation trial. Journal of Maternal‐Fetal & Neonatal Medicine 2009;22(2):94‐100. [DOI] [PubMed] [Google Scholar]
- Carroli G, Merialdi M, Wojdyla D, Abalos E, Campodonico L, Yao SE, et al. Effects of calcium supplementation on uteroplacental and fetoplacental blood flow in low‐calcium‐intake mothers: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2010;202(1):45.e1‐45.e9. [DOI] [PubMed] [Google Scholar]
- Hofmeyr GJ, Mlokoti Z, Nikodem VC, Mangesi L, Ferreira S, Singata M, et al. Calcium supplementation during pregnancy for preventing hypertensive disorders is not associated with changes in platelet count, urate, and urinary protein: a randomized control trial. Hypertension in Pregnancy 2008;27(3):299‐304. [DOI] [PubMed] [Google Scholar]
- Villar J, Abdel‐Aleem, Merialdi M, Mathai M, Ali MM, Zavaleta N, et al. World Health Organization randomized trial of calcium supplementation among low calcium intake pregnant women. American Journal of Obstetrics and Gynecology 2006;194(3):639‐49. [DOI] [PubMed] [Google Scholar]
- Villar J, Aleem HA, Merialdi M, Mathai M, Ali M, Zavaleta N, et al. WHO randomized trial of calcium supplementation among low calcium intake pregnant women [abstract]. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Villar J, Sun W, Merialdi M, Abdel‐Aleem H, Mathai M, et al. Blood pressure dynamics during pregnancy and spontaneous preterm birth. American Journal of Obstetrics and Gynecology 2007;197(2):162.e1‐162.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wanchu 2001 {published data only}
- Wanchu M, Malhotra S, Khular M. Calcium supplementation in pre‐eclampsia. Journal of the Association of Physician of India 2001;49:795‐8. [PubMed] [Google Scholar]
References to studies excluded from this review
Almirante 1998 {published data only}
- Almirante CY. Calcium supplementation during pregnancy in prevention of EPH gestosis. Prenatal and Neonatal Medicine 1998;3 Suppl 1:24. [Google Scholar]
Asemi 2012 {published data only}
- Asemi Z, Tabassi Z, Heidarzadeh Z, Khorammian H, Sabihi SS, Samimi M. Effect of calcium‐vitamin D supplementation on metabolic profiles in pregnant women at risk for pre‐eclampsia: a randomized placebo‐controlled trial. Pakistan Journal of Biological Sciences 2012;15(7):316‐24. [DOI] [PubMed] [Google Scholar]
Chames 2002 {published data only}
- Chames M, Liu H, Bendich A, Bogden J, Sibai B, Prada J. A randomised trial of calcium supplementation effects on blood lead levels in pregnancy. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S137. [Google Scholar]
Diogenes 2013 {published data only}
- Diogenes ME, Bezerra FF, Rezende EP, Taveira MF, Pinhal I, Donangelo CM. Effect of calcium plus vitamin D supplementation during pregnancy in Brazilian adolescent mothers: a randomized, placebo‐controlled trial. American Journal of Clinical Nutrition 2013;98(1):82‐91. [DOI] [PubMed] [Google Scholar]
- Diogenes MEL, Bezerra FF, Rezende EP, Taveira MF, Pinhal I, Donangelo CM. Maternal vitamin D status of adolescent mothers at mid‐pregnancy influence bone mineral content of their newborns. FASEB Journal 2011;25:603.19. [Google Scholar]
Duggin 1974 {published data only}
- Duggin GG, Dale NE, Lyneham RC, Evans RA, Tiller DJ. Calcium balance in pregnancy. Lancet 1974;2:926‐7. [DOI] [PubMed] [Google Scholar]
Felix 1991 {published data only}
- Felix C, Jacome P, Lopez A, Moya W, Narvaez M, Lopez‐Jaramillo P. The hypotensive effect of calcium supplementation during normal pregnancy in Andean women is not related to vascular production of prostacyclin by umbilical arteries. Journal of Obstetrics and Gynaecology 1991;11:93‐6. [Google Scholar]
Galimberti 2001 {published data only}
- Galimberti D, Joao M, Bernacchi S, Gimenez S, Carames V. IGF‐1 and bone turnover in pregnant women with low calcium intake [abstract]. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):17. [Google Scholar]
Hammar 1981 {published data only}
- Hammar M, Larsson L, Tegler L. Calcium treatment of leg cramps in pregnancy. Effect on clinical symptoms and total serum and ionized serum calcium concentration. Acta Obstetricia et Gynecologica Scandinavica 1981;60(4):345‐7. [DOI] [PubMed] [Google Scholar]
Janakiraman 2003 {published data only}
- Janakiraman V, Eittinger A, Mercado‐Garcia A, Hu H, Hernandez‐Avila M. Calcium supplementations and bone resorption in pregnancy; a randomized cross over trial. American Journal of Preventive Medicine 2003;24:260‐4. [DOI] [PubMed] [Google Scholar]
Kalkwarf 1997 {published data only}
- Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M. The effect of calcium supplementation on bone density during lactation and after weaning. New England Journal of Medicine 1997;337:523‐8. [DOI] [PubMed] [Google Scholar]
Kent 1995 {published data only}
- Kent GN, Price RI, Gutteridge DH, May KD, Allen JR, Smith M, et al. Site specific reduction in bone loss by calcium supplementations in normal lactation. Osteoporosis International 1995;5:315. [Google Scholar]
Liu 2011 {published data only}
- Liu Z, Qiu L, Chen YM, Su YX. Effect of milk and calcium supplementation on bone density and bone turnover in pregnant Chinese women: a randomized controlled trial. Archives of Gynecology and Obstetrics 2011;283:205‐11. [DOI] [PubMed] [Google Scholar]
Lopez‐Jaramillo 1990 {published data only}
- Lopez‐Jaramillo P, Narvaez M, Felix C, Lopez A. Dietary calcium supplementation and prevention of pregnancy induced hypertension. Lancet 1990;335:293. [DOI] [PubMed] [Google Scholar]
Mahomed 2000 {unpublished data only}
- Mahomed K, Marume A, Hammond N, Madzima M. Calcium supplementation for the prevention of pregnancy induced hypertension and preterm labour in twin pregnancy: a randomised controlled trial. Personal communication 1998.
Mazurkevich 2013 {published data only}
- Mazurkevich M, Doronin G, Firsova T. State of placental complex during physiological pregnancy during correction of mineral insufficiency. Journal of Perinatal Medicine 2013;41(Suppl 1):Abstract no:1213. [Google Scholar]
Mukherjee 1997 {published data only}
- Mukherjee J, Jong A, Wu MY, Tsim YL. Leg cramps in pregnancy and calcium supplementation. Acta Obstetricia et Gynecologica Scandinavica 1997;76:89. [Google Scholar]
Odendaal 1974 {published data only}
- Odendaal HJ. Calcium for treatment of leg cramps during pregnancy. South African Medical Journal 1974;48:780‐1. [PubMed] [Google Scholar]
Prentice 1995 {published data only}
- Prentice A, Jarjou LM, Cole TJ, Stirling DM, Dibba B, Fairweather‐Tait S. Calcium requirements of lactating Gambian mothers: effects of a calcium supplement on breast‐milk calcium concentration, maternal bone mineral content, and urinary calcium excretion. American Journal of Clinical Nutrition 1995;62:58‐67. [DOI] [PubMed] [Google Scholar]
Qui 1999 {published data only}
- Qiu L, Su Y, Peng Y. Effects of different levels of calcium intake on bone of pregnant women. Chung‐Hua Yu Fang i Hsueh Tsa Chih [Chinese Journal of Preventive Medicine] 1999;33:369‐71. [PubMed] [Google Scholar]
Robinson 1947 {published data only}
- Robinson M. Cramps in pregnancy. Journal of Obstetrics and Gynaecology of the British Commonwealth 1947;54:826‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aghamohammady 2010 {published data only}
- Aghamohammadi A, Rajabi A. The effect of calcium supplementation during pregnancy on preterm delivery and preeclampsia in nulliparous beyond age 35. Acta Obstetricia et Gynecologica Scandinavica 2012;91(Suppl 159):60. [Google Scholar]
- Aghamohammady A. The effect of calcium supplementation during pregnancy on preterm delivery in primiparous beyond age 35. Journal of Maternal‐Fetal and Neonatal Medicine 2010;23(S1):100. [Google Scholar]
Sulovic 2013 {published data only}
- Sulovic N, Kontic‐Vucinic O, Sulovic L, Relic G, Nebojsa R. Did calcium management prevent preeclampsia?. Journal of Perinatal Medicine 2013;41(Suppl 1):Abstract no:454. [Google Scholar]
Zheng 2000 {published data only}
- Zheng QS, Zhang YP. Clinical experience with calcium supplementation in pregnancy. Journal of Practical Obstetrics and Gynecology 2000;16(2):102‐3. [Google Scholar]
Additional references
Carroli 1994
- Carroli G, Duley L, Belizan JM, Villar J. Calcium supplementation during pregnancy: a systematic review of randomized controlled trials. British Journal of Obstetrics and Gynaecology 1994;101:753‐8. [DOI] [PubMed] [Google Scholar]
Chang 2003
- Chang SC, O'Brien KO, Nathanson MS, Caulfield LE, Mancini J, Witter FR. Fetal femur length is influenced by maternal dairy intake in pregnant African American adolescents. American Journal of Clinical Nutrition 2003;77:1248‐54. [DOI] [PubMed] [Google Scholar]
Cross 1995
- Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation and postweaning: a longitudinal study. American Journal of Clinical Nutrition 1995;61:514‐23. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- McMaster University. GRADEpro. [Computer program on www.gradepro.org]. Version 2014. McMaster University, 2014.
Hallberg 1992
- Hallberg L, Rossander‐Hulten L, Brune M, Gleerup A. Calcium and iron absorption: mechanism of action and nutritional importance. European Journal of Clinical Nutrition 1992;46:317‐27. [PubMed] [Google Scholar]
Heaney 2002
- Heaney RP, Davies KM, Barger‐Lux J. Calcium and weight: clinical studies. Journal of the American of Nutrition 2002;21:152S‐155S. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2014
- Hofmeyr GJ, Lawrie TA, Atallah ÁN, Duley L, Torloni MR. Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001059.pub4] [DOI] [PubMed] [Google Scholar]
Inzucchi 1999
- Inzucchi SE, Burrow GN. Endocrine disorder in pregnancy. Medicine of the Fetus and Mother. 2nd Edition. Philadelphia: Lippincott Williams & Wilkins, 1999. [Google Scholar]
Koo 1999
- Koo WWK, Walters JC, Esterlitz J, Levine RJ, Bush AJ, Sibai B. Maternal calcium supplementation and fetal bone mineralization. Obstetrics & Gynecology 1999;94:577‐82. [DOI] [PubMed] [Google Scholar]
Laskey 1999
- Laskey MA, Prentice A. Bone mineral change during and after lactation. Obstetrics & Gynecology 1999;94:608‐15. [DOI] [PubMed] [Google Scholar]
Luke 1994
- Luke B. Nutrition influences fetal growth. Clinical Obstetrics and Gynecology 1994;37:538‐49. [DOI] [PubMed] [Google Scholar]
McGuire 2007
- McGuire M, Beeman KA. Nutritional Sciences, from Fundamentals to Food. Australia: Thomson Wardworth, 2007. [Google Scholar]
Prentice 1994
- Prentice A. Maternal calcium requirements during pregnancy and lactation. American Journal of Clinical Nutrition 1994;59(2 Suppl):477S‐4783S. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ritchie 1998
- Ritchie LD, Fung EB, Halloran BP, Turnlund JR, Loan MD, Cann CE, et al. A longitudinal study of calcium homeostasis during human pregnancy and lactation and after resumption of menses. American Journal of Clinical Nutrition 1998;67:693‐701. [DOI] [PubMed] [Google Scholar]
Sampath 2008
- Sampath V, Havel PJ, King JC. Calcium supplementation does not alter lipid oxidation or lipolysis in overweight or obese women. Obesity 2008;16:2400‐4. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: Von der Evidenz zur Empfehlung. Beschreibung des Systems und Losungsbeitrag zur Ubertragbarkeit von Studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [PUBMED: 19839216] [DOI] [PubMed] [Google Scholar]
Sowers 1993
- Sowers MF, Corton G, Shapiro B, Jannausch ML, Crutchfield M, Smith ML, et al. Changes in bone density with lactation. JAMA 1993;269:3130‐5. [PubMed] [Google Scholar]
Sowers 1995
- Sowers MF, Randolph J, Shapiro B, Jannausch M. A prospective study of bone density and pregnancy after an extended period of lactation with bone loss. Obstetrics & Gynecology 1995;85:285‐9. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne J, Sutton A, Ioannidis J, Terrin N, Jones D, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:4002. [DOI] [PubMed] [Google Scholar]
Susser 1991
- Susser M. Maternal weight gain, infant birth weight, and diet: causal sequence. American Journal of Clinical Nutrition 1991;53:1384‐96. [DOI] [PubMed] [Google Scholar]
Trichopoulou 1990
- Trichopoulou A, Vassilakou T. Recommended dietary intakes in the European community member state: an overview. European Journal of Clinical Nutrition 1990;44(Suppl 2):51‐125. [PubMed] [Google Scholar]
Trowman 2006
- Trowman R, Dumville JC, Hahn S, Torgerson DJ. A systematic review of the effects of calcium supplementation on body weight. British Journal of Nutrition 2006;95:1033‐8. [DOI] [PubMed] [Google Scholar]
Villar 1998
- Villar J, Gulmezoglu M, Onis M. Nutritional and antimicrobial interventions to prevent preterm birth: an overview of randomized controlled trials. Obstetrical & Gynecological Survey 1998;53:575‐85. [DOI] [PubMed] [Google Scholar]
Williams 1962
- William DE, Mason RL. Bone density measurements in vivo. Science 1962;138:39. [DOI] [PubMed] [Google Scholar]
Yanovski 2009
- Yanovski JA, Parikh SJ, Yanoff LB, Benkinger BI, Calis KA, Reynolds JC, et al. Effects of calcium supplementation on boy weight and adiposity in overweight and obese adult: a randomized clinical trial. Annals of Internal Medicine 2009;150:821‐9, W145‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Buppasiri 2011
- Buppasiri P, Lumbiganon P, Thinkhamrop J, Ngamjarus C, Laopaiboon M. Calcium supplementation (other than for preventing or treating hypertension) for improving pregnancy and infant outcomes. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007079.pub2] [DOI] [PubMed] [Google Scholar]


