Abstract
Matrix-assisted ionization (MAI)-mass spectrometry (MS) eliminates the need for high voltage, a heat source, lasers, and compressed gases in the ionization process and uses minimal solvents in sample preparation, thus making MAI ideal for field-portable mass spectrometers. The broad applicability of MAI is demonstrated by simple, rapid, and robust positive and negative detection mode analyses of low and high mass compounds including some pesticides, dyes, drugs, lipids, and proteins (186 Da to 8.5 kDa) from various materials including urine, biological tissue sections, paper, and plant material on a low pumping capacity, single-quadrupole mass spectrometer. Different sample introduction methods are applicable, including the use of a pipet tip or glass melting point tube, allowing integration of sample preparation with sample introduction for increased analytical utility and ease of operation, even when sampling directly from surfaces.
Graphical Abstract

Recent trends toward miniaturization of chemical analysis instrumentation, in particular mass spectrometry (MS), have the potential to enhance in-field chemical analyses, including environmental monitoring,1,2 point-of-care clinical diagnostics,3 and forensic investigations.4 Potential examples include on-site detection of pesticides used extensively in modern-day farming and point-of-care diagnostic testing (e.g., drug levels). Additionally, portable or lower cost mass spectrometers are anticipated to have value in diverse areas such as forensic chemical analysis of, for example, documents to identify possible fraudulent ink insertion,5 as well as being a laboratory tool to rapidly access synthetic reaction products.6
A means of converting compounds to gas-phase ions is a requirement of MS. Various ionization methods to achieve this have been explored for “portable” applications. Typically, mass spectrometric analysis employs matrix-assisted laser desorption/ionization (MALDI), electrospray ionization (ESI), atmospheric pressure chemical ionization (APCI), or variants thereof.7,8 ESI-MS has been studied for use in clinical diagnostics but requires the analyte be in a “sprayable” solvent and uses high voltage and compressed gases for desolvation. With ESI, sample workup (e.g., desalting) of biological samples, such as urine, is typically required to produce appropriate conditions.9,10 ESI, MALDI, and APCI methodologies require high voltages, heat, laser, or compressed gas for volatile and nonvolatile analyte conversion to gas-phase ions for analysis by MS. Additionally, vacuum exposure, typically used with MALDI and laserspray ionization (LSI), can have negative effects on sample integrity and preservation. These complexities are a serious limitation for field-portable and miniaturized mass spectrometers11,12 and increases the cost of the instrumentation and expertise necessary for operation.
Matrix-assisted ionization (MAI) is a new ionization method for use in MS capable of producing abundant highly charged ions similar to those obtained from solution by ESI.13,14 Minimal sample preparation and ease of sample introduction to the mass spectrometer inlet orifice makes MAI simple to use and fast.15 The robustness of MAI has been demonstrated by its applicability to tissue13,16 and blood13 samples, as well as the quantification of hydrocodone directly from infant urine.17
MAI typically operates from the solid state,18 requiring a matrix that sublimes when exposed to subatmospheric pressure in the inlet of a mass spectrometer without the application of high voltages, laser irradiation, or heat, although temperature can be used to regulate the duration of the ionization process.15,18 Charge separation may occur through the same process that produces triboluminescence19 upon crystal fracturing.13,14,20 Thus, more than 40 compounds have been shown to produce gas-phase ions of nonvolatile compounds when exposed to the low-pressure conditions of a mass spectrometer inlet at near ambient temperature.18 A heated nitrogen gas stream over the matrix/analyte sample outside of the mass spectrometer inlet can also be used with MAI.21 Parameters shown to affect analytical performance of the new ionization processes20 are the type of matrix,14,18 temperature,15,17,18 pressure,14,18 voltages throughout the mass spectrometer,15,16,22,23 and collisions to aid charge separation and desolvation.20
Previous studies demonstrated MAI to be operational with MS and MS/MS on a variety of mass spectrometers including, but not limited to, the SYNAPT G2 with ion mobility,15,16 LTQ-Velos linear ion trap,13,17 Orbitrap Exactive,22,24 and 9.4 T Apex Fourier transform ion cyclotron resonance (FTICR).25 Here, a QDa mass spectrometer with low pumping capacity is converted to operate with MAI. Quick, easy, and robust detection and characterization of compounds including drugs, dyes, some pesticides, lipids, and proteins are demonstrated from solution samples and solid material surfaces.
EXPERIMENTAL SECTION
Instrumentation, Materials, and Sample Introduction
A single-quadrupole ACQUITY QDa Detector mass spectrometer (QDa; Waters Corporation, Manchester, UK) designed for use as a liquid chromatography (LC) detector,26 and having an atmospheric pressure-ESI miniature Z-spray source, was converted to operate as a standalone MAI mass spectrometer. In brief, the commercial ESI source housing and the gas and sample skimmer cones were removed providing direct access to the 200 μm diameter inlet aperture which enables the pressure differential between ambient and the subatmospheric pressure of the mass spectrometer. The source block temperature was set at 50 °C unless otherwise stated. MAI matrixes 3-nitrobenzonitrile (3-NBN) and 1,2-dicyanobenzene (1,2-DCB) were dissolved in acetonitrile and 3:1 acetonitrile/water, respectively, at 0.1 mg μL−1 as previously reported.13,14,18 Analyte standard solutions were mixed 1:1 (v/v) with matrix solution producing a matrix/analyte molar ratio of approximately 70 000:1 prior to MAI-MS analysis via pipet tip, paper, syringe, and glass plate introduction. A 1 μL aliquot was air-dried (~30 s) on the material exterior producing matrix/analyte crystals which were then introduced to the mass spectrometer inlet orifice. A single acquisition used 5 pmol of analyte standard unless otherwise specified. The molecular weight (MW) shown in the text for all analytes is the theoretical monoisotopic mass unless otherwise noted. Details regarding purchased materials and analyte standard solution preparation, as well as additional instrument information regarding overriding the source, software used, size, weight, and power specifications, can be found in the Supporting Information.
Chemical Analyses
A leaf from a tulip tree (Liriodendron tulipifera) was spiked with a 1.95 μL aliquot of 20 pmol μL−1 paraquat dichloride pesticide solution resulting in a total of 7.3 ng of paraquat (10 ng of paraquat dichloride) deposited onto the leaf surface in an area of ~5 mm2. The solution on the leaf was allowed to air-dry for about 20 min until no solvent visibly remained. The rounded end of a glass melting point tube was rubbed along the leaf surface for <30 s in the area where the paraquat was deposited. Then, 1 μL of 3-NBN matrix solution was spotted onto the same end of the melting point tube and allowed to crystallize, and the crystals were introduced to the inlet aperture of the mass spectrometer. A 5 V cone voltage was employed.
Blue ink from a Bic (BIC USA Inc., Shelton, CT) multicolor pen was drawn onto a blank sheet of Boise X-9 nongloss multiuse copy paper (Boise White Paper, L.L.C., Boise, ID). The blue ink was allowed to dry for about 20 min. The melting point tube was rubbed along the blue ink marks and treated for analysis as described above for the leaf surface analysis. Crystal violet dye standard solution was analyzed with pipet tip introduction for comparison. Cone voltages of 0 and 80 V were used for the ink and standard samples.
Biological Analyses and Field-Portability
A 10 μm thick mouse brain tissue section was obtained as previously described.27 A 2.5 μL aliquot of 1,2-DCB matrix solution was dispensed onto and retrieved from the tissue surface ~5 times using a pipet tip for liquid extraction of lipid molecules, similar to discontinuous solvent-assisted ionization (SAI).28 Unlike SAI, the extraction solution was allowed to air-dry on the pipet tip resulting in the formation of matrix/analyte crystals which were introduced to the inlet aperture of the mass spectrometer. Fast polarity switching was employed to obtain both negative and positive ion spectra, with cone voltage switching between −40 and +40 V at a rate of 40 Hz.
The QDa mass spectrometer was transported to the Detroit Medical Center (DMC; Detroit, MI) for field-portable urine analysis in a clinical laboratory setting. Urine samples were obtained from the DMC and prepared as follows: undiluted urine was mixed 1:1 by volume with 3-NBN matrix solution, a 1 μL aliquot was allowed to air-dry and crystallize on the end of a pipet tip, and these matrix/analyte crystals were introduced to the inlet aperture of the mass spectrometer. Control human urine was spiked with hydrocodone drug solution to a final concentration of 6.0 μg mL−1 for analysis. Cone voltages of 5 and 80 V were used. Urine from an individual taking allergy medications was acquired and analyzed with a 5 V cone voltage.
RESULTS AND DISCUSSION
Sample Introduction and Acquisition Conditions
A brief description of sample introduction results on the QDa mass spectrometer are provided here. More detailed information regarding ESI source removal and sample introduction methods are provided in the Supporting Information. Removing the ESI source for MAI use takes less than 30 s (Video S-1). Initial MAI experiments on a QDa mass spectrometer (Figure S-1) involved removal of the ESI source housing leaving access to the sample and gas skimmer cones (Figure S-1I). To improve analytical performance, these cones were also removed providing direct and easy access to the 200 μm inlet aperture (Figures S-1II and S-1III). Despite the limited mass-to-charge (m/z) range of the instrument (30 to 1250 m/z), the production of multiply charged ions by MAI allowed mass spectra of larger analytes to be obtained, e.g., ubiquitin protein (MW 8565 Da) (Figure S-2A). Software deconvolution of detected ubiquitin charge states (+7 to +14) determined an average mass of 8565 Da for the protein (Figure S-2B). No evidence of sample carryover between sample acquisitions was observed during any studies described, including after introductions of 22.5 pmol of analytes buspirone and leucine-enkephalin (Figure S-3), an amount more than four times the 5 pmol amount typically analyzed in a single sample acquisition.
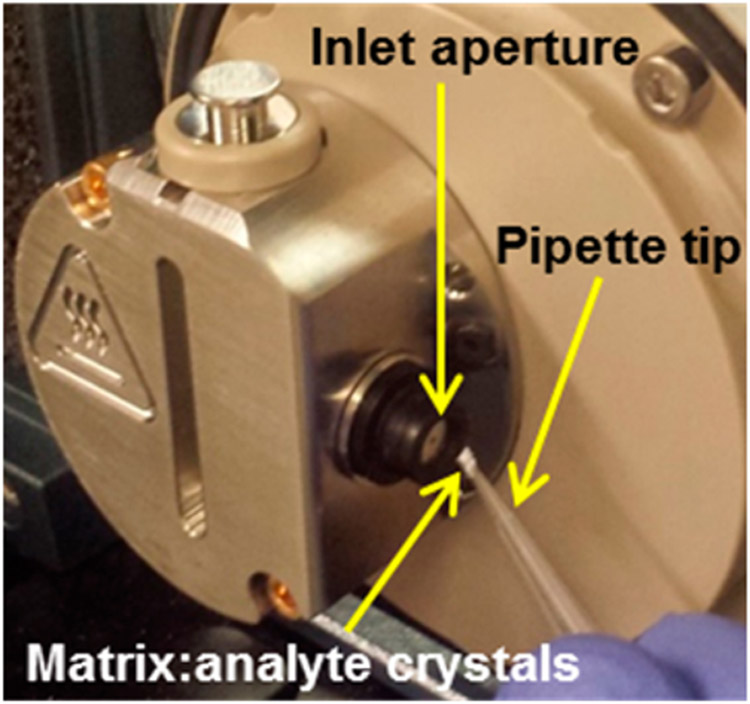
Various methods for introducing matrix/analyte to the mass spectrometer inlet aperture have been developed, including the use of a pipet tip (Figure 1), syringe, glass melting point tube, glass microscopy slide, and paper with various sensitivities for different analytes (Table S-1, Figure S-4). Pipet tip and glass melting point tube introduction methods were used for the remainder of this study because of their utility and convenience for introducing matrix/analyte crystals into the subatmospheric pressure to perform MAI.
Figure 1.
MAI pipet tip introduction of matrix/analyte crystals into the inlet aperture of a QDa mass spectrometer with overridden source housing, high voltages, desolvation gas, and skimmer assembly removed. Details are provided in the Supporting Information.
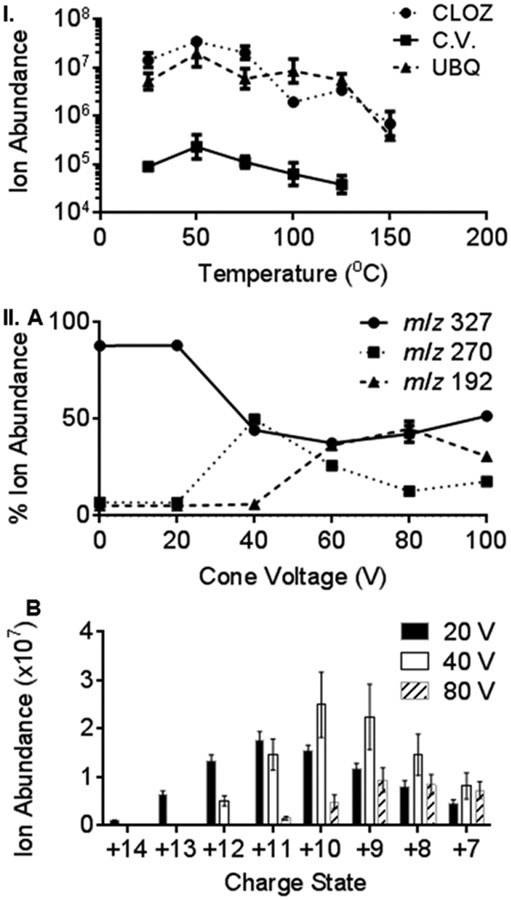
The optimal QDa mass spectrometer source block temperature was determined (Figure 2I) using the analytes clozapine drug (MW 326 Da), crystal violet dye (MW 372 Da), and ubiquitin protein by introducing the matrix (3-NBN)/analyte crystals using the pipet tip method. Source block temperatures from room temperature operation to 150 °C were studied in increments of 25 °C. The ion abundance of the +1 charge state for the drug clozapine and dye crystal violet was monitored, and all detected ubiquitin charge states were summed. For all three compounds, the highest analyte ion abundances were observed at 50 °C with higher temperatures providing a decrease in ion abundance (Figures 2I and S-5-S-7). These results are in accord with previous MAI studies using 3-NBN matrix on the Z-Spray source of the SYNAPT G2 where best analyte ion abundances were observed near room temperature.13-18 No significant differences in charge state distributions were observed with increased temperatures (Figures S-5-S-7). Additionally, higher source block temperatures decreased the duration of the ionization event from >5 min at 25 °C to as short as ~15 s at 150 °C (Figures S-8-S-10). This trend was previously observed on the SYNAPT G2,15 which has similar inlet design but higher pumping capacity. While shorter ion duration is advantageous for rapid analyses, increased chemical background was observed at higher source block temperatures with all studied analytes (Figures S-5-S-7).
Figure 2.
MAI ion abundance as a function of (I) inlet temperature, using analytes clozapine drug (CLOZ), crystal violet dye (C.V.), and ubiquitin protein (UBQ) and (II) cone voltage, monitoring (A) CLOZ fragment ions and (B) UBQ charge state ions. All data displayed as mean values of triplicate measurements using pipet tip introduction.
The source cone voltage was also studied from 0 to 100 V in increments of 20 V for clozapine drug and ubiquitin protein using the MAI matrix 3-NBN (Figure 2II). The default cone voltage using ESI on the QDa mass spectrometer is 15 V, but cone voltage optimization has allowed higher detected ion abundances.26 Using MAI, clozapine showed extensive fragmentation (Figure 2II.A) when the cone voltage was increased past 20 V, which was consistent with previous ESI-MS/MS reports.29 At 40 V, approximately equal ion abundances were observed for the intact [M + H]+ and the fragment ion, m/z 270. Above 40 V, the relative ion abundance of m/z 270 decreased. At 60 and 80 V, a lower mass fragment ion, m/z 192, was approximately equal in intensity to the [M + H]+. Interestingly, at 100 V, the [M + H]+ ion was again the base peak in the mass spectrum (Figure S-11I). The highest average clozapine [M + H]+ ion abundance was obtained at a cone voltage of 0 V, and the lowest was obtained at 40 V, with values of 3.36 × 107 and 4.46 × 106, respectively.
The ion abundance of each detected ubiquitin charge state (+7 to +14) was monitored, and mass spectra (Figure S-11II), extracted ion abundances (Table S-2), and mean ion abundances (Figure S-12) at all cone voltages are provided. Average ubiquitin abundances at three representative cone voltages (20, 40, and 80 V) are plotted (Figure 2II.B). No ubiquitin fragmentation was observed, similar to ESI literature where in-source acceleration voltages of >100 V were required to produce ubiquitin fragment ions.30 The summed ion abundance of all detected ubiquitin charge states steadily increased from 0 to 40 V and decreased from 40 to 100 V, so that a cone voltage of 40 V gave the highest average ubiquitin ion abundance (1.39 × 108). At a cone voltage of 0 V, the lowest average ubiquitin ion abundance (6.98 × 106) was obtained. Thus, 40 V may provide sufficient collisional activation energy for desolvation of the hypothesized matrix/analyte clusters.13-18,20-25,28,31 A shift from high (+14) to low (+7) charge states was observed as cone voltage was increased. The highest charge state, +14 (m/z 613), was observed only at 20 V. Previous MAI experiments using the 3-NBN matrix on a SYNAPT G2 yielded ubiquitin charge states ranging from +11 (m/z 780) to +5 (m/z 1715).31 Due to the limited upper mass range of the QDa mass spectrometer (m/z 1250), the lowest observable ubiquitin charge state was +7 (m/z 1224).
Chemical Analyses
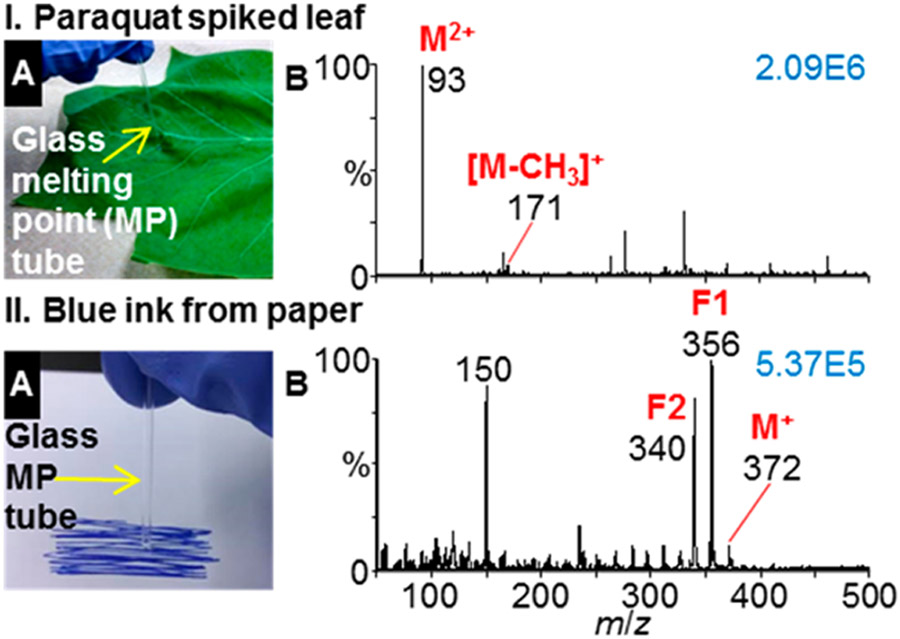
Pesticide standards of paraquat (MW 186 Da), metolachlor (MW 284 Da), and foramsulfuron (MW 452 Da) were ionized and detected (Figure S-13) using MAI with the QDa mass spectrometer. The ion abundances observed for metolachlor and foramsulfuron were lower than for paraquat, a dication salt, and as a result the chemical background for these compounds were greater than observed with paraquat. A surface sampling method was also developed to demonstrate detection of a pesticide from plant tissue. A dry glass melting point tube was established as a disposable tool for both surface sampling and introduction to the mass spectrometer inlet (Figures 3 and S-14). Using the melting point tube as a swab eliminates contact of the analyzed surface with harmful solvents or chemicals. Following the surface swab, 3-NBN matrix solution was placed on the melting point tube for matrix/analyte crystallization. The Food and Agriculture Organization of the United Nations has established an acceptable daily intake (ADI) for paraquat of 4000 ng kg−1 per day by humans.32 Detection of 7.3 ng of paraquat (10 ng of paraquat dichloride) applied to a leaf in an area of ~5 mm2 was achieved using a dry glass melting point tube for surface swabbing and matrix/analyte introduction to the inlet orifice of the mass spectrometer using 5 V cone voltage (Figure 3I). The mass spectrum contains the highly abundant intact M2+ (m/z 93) paraquat peak, as well as the [M − CH3]+ (m/z 171) fragment ion, similar to the literature.33 ESI-MS is the typical method for paraquat analysis but requires extraction from tissue.34
Figure 3.
MAI surface analyses using a glass melting point tube for sampling and matrix/analyte introduction. (I) Leaf spiked with 7.3 ng of paraquat pesticide ([C12H14N2]2+, MW 186 Da) acquired with 5 V cone voltage and (II) blue ink from paper acquired at 80 V cone voltage: (A) sampling method and (B) mass spectrum. Characteristic crystal violet dye ([C25H30N3]+, MW 372 Da) fragment ions labeled as F1 and F2.34 Ion abundances displayed in blue in top right of spectra.
Characterization of crystal violet dye in blue ink from the surface of paper was also demonstrated using the melting point tube surface sampling strategy and in-source fragmentation (Figure 3II). MAI-MS of the surface ink sample in the absence of applied cone voltage yields primarily the crystal violet cation (m/z 372) (Figure S-15). Using a cone voltage of 80 V produced a mass spectrum containing two characteristic crystal violet fragment ions (m/z 340 and 356), consistent with the literature.35 The MS and fragmentation MS spectra of the blue ink were nearly identical to crystal violet results obtained at both 0 and 80 V cone voltages (Figure S-15).
Biological Analyses and Field-Portability
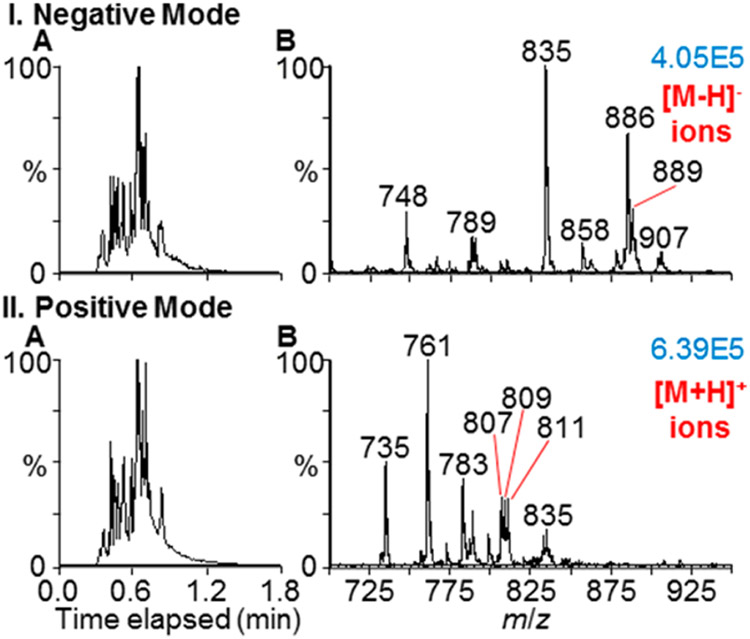
MAI-MS was also adapted for the detection of lipids (Figure 4) from a mouse brain tissue section using the matrix 1,2-DCB. An aliquot of matrix solution was used for the extraction of lipid analytes from the mouse brain tissue, similar to previous work.28 Upon introduction of the matrix/analyte crystals to the inlet of the mass spectrometer, nearly identical total ion chronograms (TICs) were obtained in both the negative and positive ion modes in a single sample introduction lasting approximately 1 min with 50 °C source block temperature. Lipids were tentatively assigned (Figure S-16) using MS lipid analysis reported in the literature.36-40 Negative mode lipid assignments may include phosphatidylethanolamines (PEs), phosphatidylglycerols (PGs), phosphatidylserines (PSs), phosphatidylinositols (PIs), phosphatidylcholines (PCs), and sulfatides (STs). Positive mode lipid assignments may include phosphatidic acids (PAs), PGs, PSs, and PIs. The matrix 1,2-DCB18 produced both negative and positive gas-phase ions simultaneously, providing support for charge separation due to crystal fracturing occurring in the subatmospheric pressure of the instrument.13,14,20
Figure 4.
Mouse brain tissue surface analysis using the MAI pipet tip introduction, 1,2-DCB matrix, and rapid voltage (−40 and +40 V) switching. Lipids detected in (I) negative and (II) positive ion modes. (A) Total ion chronograms and (B) mass spectra. Tentative lipid assignments available in Figure S-16. Ion abundance displayed in blue in top right corner of each spectrum.
As a first test of field-portability, the mass spectrometer and all necessary accessories were transported to a clinical laboratory at the Detroit Medical Center (DMC) for in-field use (Figure S-17). The instrument was fully functional within 15 min of being plugged into a 120 V electrical outlet. Hydrocodone spiked at 6.0 μg mL−1 to a control urine sample was characterized within 5 min of instrument operation (Figure S-18). Although urine is a salty biological matrix, sufficient analyte precursor ion abundance (9.63E4) using MAI allowed detection of intact hydrocodone [M + H]+ and fragment ions, m/z 199 and 171, by simple addition of matrix solution to the spiked urine. The observed fragment ions agree with LC-ESI-MS/MS41 and MAI-MS/MS from urine.17 Undiluted urine obtained from an individual taking allergy medication was analyzed using the matrix 3-NBN by the DMC personnel. Protonated fexofenadine (MW 501 Da), an active ingredient in the medication, was detected (Figure S-19).
CONCLUSION
No high voltages, high heat, laser, complicated source designs, compressed gas, or extensive sample preparation are required for ionization by MAI achieving high ion abundance in positive and negative mode without metal cation adduction. This ionization method minimizes the footprint, energy consumption, cost, expertise, and equipment necessary for a field-portable mass spectrometer. The work reported here suggests that rapid, in-field MAI-MS characterization is potentially feasible for environmental monitoring, point-of-care diagnostic testing, and forensic applications. Compared to ESI based methods, MAI is more environmentally friendly,42,43 as less waste is produced and introduced to the environment. For relatively pure samples, intentional in-source fragmentation can be used for compound characterization, and analyte ions are produced even directly from biological samples such as urine and tissue. With further improvements to instrumentation and procedures used with MAI, we anticipate that this approach will expand the use of MS analyses outside the traditional analytical laboratory environment.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful for support from NSF CHE-1411376, DuPont Young Professor Award, Eli Lilly Young Investigator Award, Waters Center of Innovation Award, WSU Schaap Faculty Award (to S.T.), WSU Thomas C. Rumble Fellowship (to C.D.F.), and NIH DA011322 and DA021696 (to K.M.). The authors would like to thank Dr. Bruce Aoki and David Johnson (Waters) for their aid in overriding the source housing of the QDa mass spectrometer and Professor Charles N. McEwen (USciences) for his comments.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Levedev AT Annu. Rev. Anal. Chem 2013, 6, 163–189. [DOI] [PubMed] [Google Scholar]
- (2).Wright S; Malcolm A; Wright C; O’Prey S; Crichton E; Dash N; Moseley RW; Zaczek W; Edwards P; Fussell RJ; Syms RR A. Anal. Chem 2015, 87, 3115–3122. [DOI] [PubMed] [Google Scholar]
- (3).Ferreira CR; Yannell KE; Jarmusch AK; Pirro V; Ouyang Z; Cooks RG Clin. Chem 2016, 62, 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Wells JM; Roth MJ; Keil AD; Grossenbacher JW; Justes DR; Patterson GE; Barket DJ Jr. J. Am. Soc. Mass Spectrom 2008, 19, 1419–1424. [DOI] [PubMed] [Google Scholar]
- (5).Weyermann C; Marquis R; Mazzella W; Spengler BJ Forensic Sci. 2007, 52, 216–220. [DOI] [PubMed] [Google Scholar]
- (6).Mei Y; Dissanayake P; Allen MJ J. Am. Chem. Soc 2010, 132, 12871–12873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Takats Z; Wiseman JM; Gologan B; Cooks RG Science 2004, 306, 471–473. [DOI] [PubMed] [Google Scholar]
- (8).McEwen CN; McKay RG; Larsen BS Anal. Chem 2005, 77, 7826–7831. [DOI] [PubMed] [Google Scholar]
- (9).Cech NB; Enke CG Mass Spectrom. Rev 2001, 20, 362–387. [DOI] [PubMed] [Google Scholar]
- (10).Chen J; Narayan SB; Edinger AL; Bennet MJ J. Chromatogr. B: Anal. Technol. Biomed. Life Sci 2012, 883–884, 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ouyang Z; Cooks RG Annu. Rev. Anal. Chem 2009, 2, 187–214. [DOI] [PubMed] [Google Scholar]
- (12).Yang Q; Wang H; Maas JD; Chappell WJ; Manicke NE; Cooks RG; Ouyang Z Int. J. Mass Spectrom 2012, 312, 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Inutan ED; Trimpin S Mol. Cell. Proteomics 2013, 12, 792–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Trimpin S; Inutan ED J. Am. Soc. Mass Spectrom 2013, 24, 722–732. [DOI] [PubMed] [Google Scholar]
- (15).Woodall DW; Wang B; Inutan ED; Narayan SB; Trimpin S Anal. Chem 2015, 87, 4667–4674. [DOI] [PubMed] [Google Scholar]
- (16).Inutan ED; Wager-Miller J; Narayan SB; Mackie K; Trimpin S Int. J. Ion Mobility Spectrom 2013, 16, 145–159. [Google Scholar]
- (17).Chakrabarty S; DeLeeuw JL; Woodall DW; Jooss K; Narayan SB; Trimpin S Anal. Chem 2015, 87, 8301–8306. [DOI] [PubMed] [Google Scholar]
- (18).Trimpin S; Lutomski CA; El-Baba TJ; Woodall DW; Foley CD; Manly CD; Wang B; Liu C; Harless BM; Kumar R; Imperial LF; Inutan ED Int. J. Mass Spectrom 2015, 377, 532–545. [Google Scholar]
- (19).Sweeting LM Chem. Mater 2001, 13, 854–870. [Google Scholar]
- (20).Trimpin SJ Am. Soc. Mass Spectrom 2016, 27, 4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Chakrabarty S; Pagnotti VS; Inutan ED; Trimpin S; McEwen CN J. Am. Soc. Mass Spectrom 2013, 24, 1102–1107. [DOI] [PubMed] [Google Scholar]
- (22).Fenner MA; McEwen CN Int. J. Mass Spectrom 2015, 378, 107–112. [Google Scholar]
- (23).Pagnotti VS; Chakrabarty S; Wang B; Trimpin S; McEwen CN Anal. Chem 2014, 86, 7343–7350. [DOI] [PubMed] [Google Scholar]
- (24).Chubatyi ND; McEwen CN J. Am. Soc. Mass Spectrom 2015, 26, 1649–1656. [DOI] [PubMed] [Google Scholar]
- (25).Wang B; Tisdale E; Trimpin S; Wilkins CL Anal. Chem 2014, 86, 6792–6796. [DOI] [PubMed] [Google Scholar]
- (26).Spaggiari D; Mehl F; Desfontaine V; Perrenoud AG; Fekete S; Rudaz S; Guillarme DJ Chromatogr. A 2014, 1371, 244–256. [DOI] [PubMed] [Google Scholar]
- (27).Trimpin S; Herath TN; Inutan ED; Wager-Miller J; Kowalski P; Claude E; Walker JM; Mackie K Anal. Chem 2010, 82, 359–367. [DOI] [PubMed] [Google Scholar]
- (28).Wang B; Dearring CL; Wager-Miller J; Mackie K; Trimpin S Eur. Mass Spectrom 2015, 21, 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Weinmann W; Müller C; Vogt S; Frei AJ Anal. Toxicol 2002, 26, 303–307. [DOI] [PubMed] [Google Scholar]
- (30).Loo JA; Edmonds CG; Smith RD Anal. Chem 1993, 65, 425–438. [DOI] [PubMed] [Google Scholar]
- (31).Trimpin S; Inutan ED Anal. Chem 2013, 85, 2005–2009. [DOI] [PubMed] [Google Scholar]
- (32).Food and Agriculture Organization of the United Nations. www.fao.org/docrep/006/y5221e/y5221e0k.htm (Accessed Dec. 6, 2015). [Google Scholar]
- (33).Ariffin MM; Anderson RAJ Chromatogr. B: Anal. Technol. Biomed. Life Sci 2006, 842, 91–97. [DOI] [PubMed] [Google Scholar]
- (34).Wang Z; Wang Z; Xing JJ Anal. Toxicol 2011, 35, 23–27. [DOI] [PubMed] [Google Scholar]
- (35).Dowling G; Mulder PPJ; Duffy C; Regan L; Smyth MR Anal. Chim. Acta 2007, 586, 411–419. [DOI] [PubMed] [Google Scholar]
- (36).Murphy RC Mass Spectrometry of Phospholipids: Tables of Molecular and Product Ions; Illuminati Press: Denver, 2002. [Google Scholar]
- (37).Laskin J; Heath BS; Roach PJ; Cazares L; Semmes OJ Anal. Chem 2012, 84, 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Pulfer M; Murphy RC Mass Spectrom. Rev 2003, 22, 332–364. [DOI] [PubMed] [Google Scholar]
- (39).Wiseman JM; Ifa DR; Song Q; Cooks RG Angew. Chem., Int. Ed 2006, 45, 7188–7192. [DOI] [PubMed] [Google Scholar]
- (40).Jackson SN; Wang H-YJ; Woods AS; Ugarov M; Egan T; Schultz JA J. Am. Soc. Mass Spectrom 2005, 16, 133–138. [DOI] [PubMed] [Google Scholar]
- (41).Coles R; Kushnir MM; Nelson GJ; McMillin GA; Urry FM J. Anal. Toxicol 2007, 31, 1–14. [DOI] [PubMed] [Google Scholar]
- (42).Anastas P; Eghbali N Chem. Soc. Rev 2010, 39, 301–312. [DOI] [PubMed] [Google Scholar]
- (43).Liu P; Forni A; Chen H Anal. Chem 2014, 86, 4024–4032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.