Amyotrophic lateral sclerosis (ALS), commonly known as Lou Gehrig’s disease, is a progressive neurodegenerative disorder that primarily affects motor neurons in the brain and spinal cord. As the disease progresses, the affected neurons degenerate, leading to muscle weakness and eventually paralysis and death by catastrophic respiratory failure (1). ALS is characterized by its clinical heterogeneity. Genetic mutations play a significant role in the etiology of ALS, which contributes to approximately 10% of all ALS cases (2).
Immune response is broadly recognized to influence the progression of ALS, supported by patient samples and animal models (3). However, the direct connection between genetic causes and the impaired immune system was still elusive. In this issue of PNAS, Chi et al. reported that several ALS-associated RNA/DNA-binding proteins and C9ORF72 repeat expansion influence the gene expression of components in the major histocompatibility complex II (MHC-II) antigen presentation pathway (4). This possibly leads to immune system dysfunction which can contribute to neurodegeneration in ALS.
There have been lots of discussions about the involvement of the immune system in the onset and progression of ALS. Neuroinflammation in the central nervous system (CNS), evidenced by the activation of microglia, astrocytes, and the alteration of cytokines, is recognized as a hallmark of ALS (5, 6). On the one hand, as the primary immune cells of the CNS, microglia are activated and exhibit a neuroprotective effect by producing anti-inflammatory cytokines and clearing cell debris (6). On the other hand, they also release proinflammatory cytokines and reactive oxygen species that damage neurons during disease progression (6). In addition to the increase of activated microglia in postmortem tissues, dysregulated inflammatory cytokines and elevated active state of T cells have been identified in the cerebrospinal fluid (CSF) of patients, suggesting the changes of immune response in the CNS (5).
There is also compelling evidence supporting altered peripheral immune response in ALS. Analyses of blood samples from ALS patients showed altered levels of cytokines, such as TNF-α, IL-6, and IL-1β (7). Moreover, a reduction in CD4+ T cell numbers and an elevation in the CD4+/CD8+ T cell ratio have been observed in patient blood samples (8). A subset of CD4+ T cells, Tregs, which suppresses immune response has also been found to be dysregulated in ALS, potentially leading to reduced ability to control inflammation (5, 8). Additionally, there is evidence suggesting the interaction of the central and peripheral immune system (9). The active T lymphocytes, especially CD4+ and CD8+ T cells, as well as natural killer (NK) cells have been reported to penetrate the blood–brain barrier in ALS patients, indicating an adaptive immune response (10–12).
“Chi et al. identified the MHC-II antigen presentation as a common pathway influenced by multiple ALS-linked mutant genes.”
Despite numerous observations of immune response changes in ALS, the direct link between genetic mutations and impaired immune functions remains elusive. Recently, accumulating evidence revealed a correlation between C9ORF72 repeat expansion and immune changes, especially due to the loss of function of the C9ORF72 protein (13). Two independent C9orf72 knockout mouse lines consistently exhibited deficits in the innate immune system, resulting in splenomegaly, lymphadenopathy, and elevated proinflammatory cytokines (14, 15). Within the CNS, C9ORF72 is highly expressed in microglia (15). Loss of C9orf72 results in the accumulation of lysosomes and increased proinflammatory microglia in mice (15). Although the absence of C9orf72 is not sufficient to induce neuronal loss or impaired motor function, it can exacerbate pathological and behavioral phenotypes in transgenic mice expressing the expanded repeats (16). This indicates that the immune defects caused by C9ORF72 haploinsufficiency can contribute to neurodegeneration.
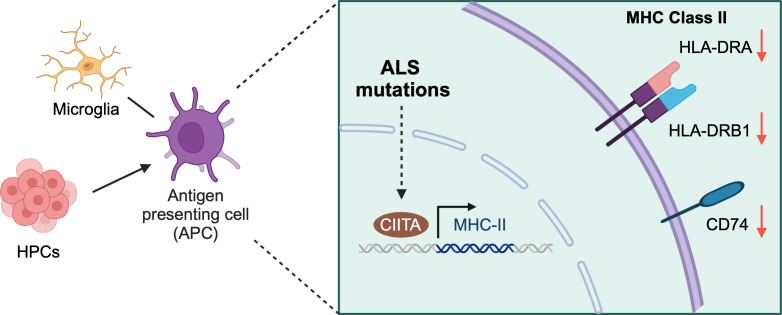
Chi et al. previously revealed that a group of ALS-associated RNA/DNA-binding proteins, including FUS, EWSR1, TAF15, and MATR3, are components of U1 snRNP and RNAP II machinery (17). Many studies focused on their functions in neurons (18), although these proteins are expressed broadly in many cell types, including immune cells. In this work, Chi et al. found that many immune-related proteins were down-regulated after knocking out FUS, TAF15, or MATR3 in HeLa cells using quantitative mass spectrometry analysis (4). Among the down-regulated genes affected by all the three RNA/DNA-binding proteins, a significant portion has functions related to antigen presentation. Interestingly, HLA-DRA and HLA-DRB1, the major components of the HLA-DR (Human Leukocyte Antigen–DR isotype) complex which functions in MHC-II antigen presentation (19), were the top down-regulated proteins. CD74 was known as the HLA-DR gamma chain, which stabilizes HLA-DR and chaperons the complex to the endosomal system for antigen processing (20). Related to the reduction of HLA-DR components, the CD74 mRNA level was also decreased in all the lines knocking out FUS, TAF15, or MATR3. The authors further validated the findings in the human HMC-3 microglia cell line. The downregulation of the MHC-II pathway was evident in cells with FUS, EWSR1, TAF15, or MATR3 knockdown. Altogether, this indicates that these RNA/DNA-binding proteins play important roles in regulating HLA-DR mediated MHC-II antigen presentation to T cells. The authors further demonstrated that the reduction in the components of the MHC-II pathway is due to the downregulation of CIITA, the major transcription factor regulating gene expression of HLA-DR and related factors (Fig. 1).
Fig. 1.
ALS mutations result in the decreased expression of components in the MHC-II pathway. Microglia serve as the antigen-presenting cells in the central nerve system, and HPCs can give rise to antigen-presenting cells in the peripheral system. In both cell types, FUS and C9ORF72 mutations lead to decreased expression of HLA-DRA, HLA-DRB1, and CD74, the key components of the MHC-II pathway through the regulation of transcriptional factor CIITA. Such alterations in the pathway could interrupt immune response. Created with BioRender.
To further study the effect of disease-causative mutations on the MHC-II pathway, Chi et al. used the FUSR495X mutation as an example. Two cell types were used: HMC-3 microglia and human embryonic stem cell (ES)-differentiated hematopoietic progenitor cells (HPCs) that can give rise to various types of immune cells. The R495X heterozygous mutation was introduced to endogenous FUS by CRISPR editing in both systems. The authors found a reduction of HLA-DRA, HLA-DRB1, and CD74 expression levels along with the decreased CIITA in FUSR495X mutant cells. Interestingly, they did not find similar changes in the FUSR495X ES cells before differentiation to HPCs, suggesting that the reduction of MHC-II-associated genes is cell type specific. Additionally, Chi et al. found the reduction of the same panel of genes in induced pluripotent stem cell (iPSC)-differentiated HPCs derived from C9ORF72-ALS patients. This suggests that the disruptions in the MHC-II pathway in HPCs could be a common feature in ALS associated with FUS and C9ORF72 mutations. This finding also unveils a novel potential mechanism underlying altered immune responses in C9ORF72-ALS/FTD.
Chi and colleagues have identified MHC-II antigen presentation as a common pathway influenced by multiple ALS-linked mutant genes. This study reveals novel molecular mechanisms on how disease-causative mutations can lead to perturbed gene expression related to immune cell function. The disruption of the MHC-II pathway will likely influence the immune response and therefore contribute to the observed neuroinflammation in both mouse models and patients. The findings of this research underscore the importance of potential non-cell-autonomous toxicity in neurodegeneration and warrant more consideration for therapy design. The finding also sparks new intriguing questions. For instance, how do mutations in these diverse genes all lead to alterations in the MHC-II pathway? Can similar defects be detected in animal models or patient samples? What is the functional outcome of the reduced MHC-II pathway in immune cells and how this affects neurons? Is the restoration of CIITA expression sufficient to rescue the immune cell defects induced by the disease-associated mutations? Answering these questions could further improve our understanding on the molecular mechanism of immune dysregulation and its contribution to the disease etiology of ALS and provide insights on the development of biomarkers and therapeutic strategies.
Acknowledgments
We thank NIH for funding support (RF1NS113820, RF1NS127925, and R01AG078948). Z.Z. was a recipient of the Milton Safenowitz Post-Doctoral Fellowship from the ALS Association, the Toffler Scholar Award, and the Postdoc Development Grant from the Muscular Dystrophy Association.
Author contributions
Z.Z. and S.S. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
See companion article, “Causal ALS genes Impact the MHC class II antigen presentation pathway,” 10.1073/pnas.2305756120.
References
- 1.Kiernan M. C., et al. , Amyotrophic lateral sclerosis. Lancet 377, 942–955 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Renton A. E., Chiò A., Traynor B. J., State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 17, 17–23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao W., Beers D. R., Appel S. H., Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J. Neuroimmune Pharmacol. 8, 888–899 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chi B., et al. , Causal ALS genes impact the MHC class II antigen presentation pathway. Proc. Natl. Acad. Sci. U.S.A. 120, e2305756120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans M. C., Couch Y., Sibson N., Turner M. R., Inflammation and neurovascular changes in amyotrophic lateral sclerosis. Mol. Cellular Neurosci. 53, 34–41 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Lall D., Baloh R. H., Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia. J. Clin. Invest. 127, 3250–3258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Y., et al. , Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: A meta-analysis study. Sci. Rep. 7, 9094 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mantovani S., et al. , Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J. Neuroimmunol. 210, 73–79 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Berriat F., Lobsiger C. S., Boillée S., The contribution of the peripheral immune system to neurodegeneration. Nat. Neurosci. 26, 942–954 (2023). [DOI] [PubMed] [Google Scholar]
- 10.Kawamata T., Akiyama H., Yamada T., McGeer P. L., Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am. J. Pathol. 140, 691–707 (1992). [PMC free article] [PubMed] [Google Scholar]
- 11.Murdock B. J., et al. , Correlation of peripheral immunity with rapid amyotrophic lateral sclerosis progression. JAMA Neurol. 74, 1446–1454 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masrori P., Beckers J., Gossye H., Van Damme P., The role of inflammation in neurodegeneration: Novel insights into the role of the immune system in C9orf72 HRE-mediated ALS/FTD. Mol. Neurodegeneration 17, 22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lall D., Baloh R. H., Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia. J. Clin. Inves. 127, 3250–3258 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudria-Lopez E., et al. , Full ablation of C9orf72 in mice causes immune system-related pathology and neoplastic events but no motor neuron defects. Acta Neuropathol. 132, 145–147 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Rourke J. G., et al. , C9orf72 is required for proper macrophage and microglial function in mice. Science 351, 1324–1329 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Q., et al. , Reduced C9ORF72 function exacerbates gain of toxicity from ALS/FTD-causing repeat expansion in C9orf72. Nat. Neurosci. 23, 615–624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chi B., et al. , Interactome analyses revealed that the U1 snRNP machinery overlaps extensively with the RNAP II machinery and contains multiple ALS/SMA-causative proteins. Sci. Rep. 8, 8755 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz J. C., Cech T. R., Parker R. R., Biochemical properties and biological functions of FET proteins. Annu. Rev. Biochem. 84, 355–379 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum J. S., Wearsch P. A., Cresswell P., Pathways of antigen processing. Annu. Rev. Immunol. 31, 443–473 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schröder B., The multifaceted roles of the invariant chain CD74—More than just a chaperone. Biochim. Biophys. Acta. 1863, 1269–1281 (2016). [DOI] [PubMed] [Google Scholar]