Abstract
Background
The SARS-CoV-2 pandemic represents one of the most challenging issues that have recently influenced everyday life in countries all over the world. Understanding the risk of this disease is of high importance in patients with multiple sclerosis (MS) as they represent a vulnerable population through their treatment with disease-modifying therapies (DMTs). Infective episodes may trigger relapses and lead to deterioration of the health condition.
Summary
Vaccination is an important preventive measure against infectious diseases. In MS patients, concerns have been raised about the effectiveness of vaccines in patients on various immunomodulatory drugs and about their possible adverse effects including impairment of neurological functions. The objectives of this article were to summarize the current knowledge on immune responses to the COVID-19 vaccines and their safety in MS patients and to provide practical guidance based on the data available to date.
Key Messages
Although MS is not associated with a higher risk of COVID-19, this infection can trigger relapses or pseudo-relapses. Vaccines against SARS-CoV-2 are recommended for all MS patients who are not in the active phase of the disease, despite the fact that there is still a lack of long-term reliable data on the effectiveness and safety of vaccines against COVID-19. Some DMTs can reduce vaccine humoral responses, but might still provide some protection and adequate T-cell response. To optimize the effectiveness of vaccination, the ideal timing of vaccine application and DMTs dosing regimen is crucial.
Keywords: Multiple sclerosis, SARS-CoV-2, COVID-19, Vaccine, Disease-modifying therapy
Introduction
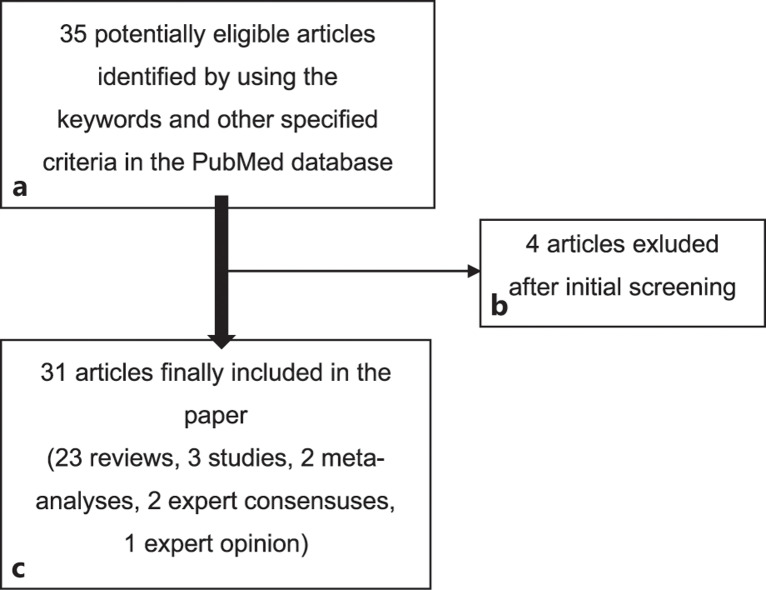
Multiple sclerosis (MS) is a chronic inflammatory degenerative disease of the central nervous system (CNS) characterized by early axonal damage that causes the most common nontraumatic disability in young adults worldwide. Global prevalence in 2020 reached almost 36 per 100,000 persons, and an estimated 2.8 million people are living with this condition around the world [1–4] (Fig. 1; Table 1).
Fig. 1.
Flowchart showing the article selection procedure.
Table 1.
Specification of the included articles and the summary of their results
| Article | Summary/key points concerning COVID-19 infection and/or vaccination in patients with MS | |
|---|---|---|
| 1 | Kelly et al. [5] Safety and Efficacy of COVID-19 Vaccines in Multiple Sclerosis Patients |
Non-live vaccines are likely safe and effective in MS patients on various DMTs. Demyelinating events are extremely rare with most COVID-19 vaccines and have mainly been reported with vector vaccines. Attenuated but partially protective vaccine responses are expected in patients taking S1P modulators and cell-depleting DMTs |
| 2 | Landtblom et al. [6] Multiple Sclerosis and COVID-19: The Swedish Experience |
Patients with anti-CD20 therapies have a higher risk of worse COVID-19 disease course; the recommendation of increased intervals between rituximab infusions to diminish the risk of severe COVID-19 infection and favor vaccine response |
| 3 | Coyle PK et al. [3] Vaccine Considerations for Multiple Sclerosis in the COVID-19 |
Infections can be associated with an increased risk of relapse or pseudo-relapses in MS patients. Both T and B lymphocytes of the adaptive immune system are integral to the successful generation of immunological memory production neutralizing antibodies. S1P, alemtuzumab, cladribine, and anti-CD20 therapies may impair the response to vaccination |
| 4 | Cabreira V et al. [7] Multiple Sclerosis, Disease-Modifying Therapies and COVID-19: A Systematic Review on Immune Response and Vaccination Recommendations |
DMT causing severe lymphopenia and hypogammaglobulinemia, such as anti-CD20 therapies, might be connected to increased hospitalization, worse outcomes, and a higher risk of reinfections of COVID-19. Clinical evidence does not support an increased risk of MS relapse of vaccination failure, but vaccination timing needs to be individually tailored |
| 5 | Sharifian-Dorche M et al. [8] COVID-19 and Disease-Modifying Therapies in Patients with Demyelinating Diseases of the Central Nervous System: A Systematic Review |
The risk of mortality of COVID-19 in MS patients is similar to non-MS population; relatively higher mortality was seen among patients on anti-CD20 therapies or patients without medication. For patients on teriflunomide, fingolimod, siponimod, and anti-CD20 monoclonal antibodies, attenuation of the COVID-19 post-vaccine response is possible |
| 6 | Toscano S et al. [9] Multiple Sclerosis, COVID-19 and Vaccines: Making the Point |
All MS patients should be advised on COVID-19 risks and preventive measures, and all should be recommended to vaccinate as soon as possible. Individual benefit/risk ratio should always be evaluated in the management of patients with MS, particularly for those treated with immune-depleting DMTs |
| 7 | Smith TE et al. [10] Infection Mitigation Strategies for Multiple Sclerosis Patients on Oral and Monoclonal Disease-Modifying Therapies |
All MS patients should be vaccinated against COVID-19 as soon as possible unless not otherwise contraindicated. To enhance the effectiveness of vaccination, the guidelines recommend getting fully vaccinated 2–4 weeks before starting anti-CD20 therapy, S1P modulators, and cladribine, and 4 weeks before starting alemtuzumab. For those already on a DMT, the NMSS recommends getting vaccinated 24 weeks or more after the last alemtuzumab dose and 12 weeks after the last ocrelizumab or rituximab dose. If this coordination is not possible, it is preferable to schedule vaccination regardless, which likely provides at least partial protection |
| 8 | Iannetta M et al. [11] B- and T-Cell Responses After SARS-CoV-2 Vaccination in Patients With Multiple Sclerosis Receiving Disease-Modifying Therapies: Immunological Patterns and Clinical Implications |
The evaluation of T-cell responses, anti-S titers, and peripheral blood lymphocyte absolute count in patients with MS on DMTs (ocrelizumab, fingolimod, natalizumab) can help better characterize the immunological response after SARS-CoV-2 vaccination |
| 9 | Inshasi J et al. [12] Expert Consensus and Narrative Review on the Management of Multiple Sclerosis in the Arabian Gulf in the COVID-19 Era: Focus on Disease-Modifying Therapies and Vaccination against COVID-19 |
MS appears not to be a risk factor for severe adverse COVID-19 outcomes per se in the absence of advanced disability or a progressive phenotype. All currently available DMTs except alemtuzumab can be started safely at this time (with individual consideration for alemtuzumab). There is no need to alter the administration of INFβ, teriflunomide, dimethyl fumarate, GA, natalizumab, fingolimod, or cladribine tablets for vaccination against COVID-19. Delay new starts on other DMTs for up to 6 weeks after completion of vaccination (allow white cell counts to recover where applicable) |
| 10 | Gold R et al. [13] Vaccination in Multiple Sclerosis Patients Treated with Highly Effective Disease-Modifying Drugs: An Overview with Consideration of Cladribine Tablets |
The available evidence indicates reduced vaccination efficacy on treatment with MS drugs acting on the S1P receptor, natalizumab, and B-cell-depleting therapies. Vaccine efficacy may be enhanced with optimized timing of application. Protective antiviral antibody titers can be elicited by immunization with SARS-CoV-2 vaccines being treated with oral cladribine |
| 11 | Beard K et al. [2] Insight in Booster COVID-19 Vaccine and Disease-Modifying Therapy in Multiple Sclerosis |
The authors strongly recommend a third vaccine to boost the weak antibody responses to all patients receiving anti-CD20 therapy. Preferentially, a third vaccine dose would be given 12 weeks, or more, after their last infusion to allow B cells to repopulate prior to vaccination. A similar booster vaccine recommendation applies to cladribine treatment |
| 12 | Bhise V et al. [14] Potential Risks and Benefits of Multiple Sclerosis Immune Therapies in the COVID-19 Era: Clinical and Immunological Perspectives |
MS patients are not at higher risk for contracting COVID-19 as far as the disease itself is concerned, but they could be at higher risk for worse outcomes if exposed to the virus while on immunosuppressive therapy Immunomodulatory agents such as IFNβ, GA, and dimethyl fumarate are less likely to affect adversely the course of COVID-19 as long as significant lymphopenia is not present The timing of vaccination may play an important role in some DMTs |
| 13 | Wu X et al. [15] Response of COVID-19 Vaccination in Multiple Sclerosis Patients Following Disease-Modifying Therapies: A Meta-Analysis |
It seems that routine serological monitoring may be required for patients with MS on anti-CD20 therapies and S1P modulators after SARS-CoV-2 vaccination; the benefits of a booster dose are highlighted. The effect of cellular response and optimal interval from the last anti-CD20 treatment to vaccination should be further addressed |
| 14 | Golshani M et al. [16] Multiple Sclerosis Patients and Disease-Modifying Therapies: Impact on Immune Responses against COVID-19 and SARS-CoV-2 Vaccination |
All MS patients with no additional contraindications are recommended to receive approved SARS-CoV-2 vaccines. However, live-attenuated vaccines are not recommended. COVID-19-infected MS patients under treatment with S1P or anti-CD20 therapies may show a lower immune response against the virus. MS patients treated with cladribine, fingolimod, ocrelizumab, or rituximab generated lower anti-spike/anti-RBD IgG responses, but protective levels of CD4+ and CD8+ T-cell responses following COVID-19 vaccination. Therefore, a time window for vaccination due to B-cell-depletion and attenuated humoral responses by anti-CD20 antibodies is suggested |
| 15 | Reder AT et al. [17] T cell Responses to COVID-19 Infection and Vaccination in Patients with Multiple Sclerosis Receiving Disease-Modifying Therapy |
Innate, humoral, and T-cell immune responses combat COVID-19 and generate protective immunity. Assays detecting cytokine expression by T cells show an association between SARS-CoV-2-specific T-cell responses and milder/asymptomatic COVID-19 and protective immune memory COVID-19 infection is less severe in patients with MS receiving IFN-beta and GA, but slightly more severe with anti-CD20 therapies. Responses to COVID-19 vaccination are normal with most DMTs, but some anti-CD20 therapies reduce B-cell responses, and some S1P modulators reduce B- and T- cell responses |
| 16 | Sellner J et al. [18] Multiple Sclerosis and SARS-CoV-2 Vaccination: Considerations for Immune-Depleting Therapies |
Neurologists must balance the risk of SARS-CoV-2 infection and its potentially life-threatening course against a possible progression of the underlying condition. It seems that not all DMTs are associated with an increased risk of severe SARS-CoV-2 infection in patients with MS. Vaccination against the virus may be an elegant strategy to overcome the potential hazards associated with immune-depleting agents and the subsequent restricted use of these therapies |
| 17 | Kim E et al. [19] Vaccination of Multiple Sclerosis Patients during the COVID-19 Era: Novel Insights into Vaccine Safety and Immunogenicity |
There is a growing body of evidence that supports the safety, immunogenicity, and efficacy of COVID-19 vaccines. It seems that the majority of MS patients will likely benefit from vaccinations; patients on DMTs may need to carefully weigh the risks and benefits of pursuing or forgoing vaccination |
| 18 | Hollen C et al. [20] Multiple Sclerosis Management During the COVID-19 Pandemic |
Most patients with MS do not need major revisions to their treatment plan due to COVID-19 risk. However, individuals who are older, more disabled, and on more potent therapies may need to consider strategies for decreasing their overall risk |
| 19 | Yamout BI et al. [21] MENACTRIMS Practice Guideline for COVID-19 Vaccination in Patients with Multiple Sclerosis |
COVID-19 vaccination is recommended for all MS patients, and currently available vaccines are safe and effective. Attenuated but potentially partially protective vaccine response is expected in MS patients taking S1P modulators and B-cell-depleting therapies. Other DMTs are not expected to significantly impact the efficacy of COVID-19 vaccines. Coordinating vaccine timing with dosing regimens for some therapies may optimize vaccine efficacy |
| 20 | Witman Tsur S et al. [4] Current Immunological and Clinical Perspective on Vaccinations in Multiple Sclerosis Patients: Are They Safe after All? |
The timing of the vaccine needs to be carefully planned if MS patients are on immune therapies that may blunt the response to the vaccine, such as alemtuzumab, cladribine, or ocrelizumab Vaccination is likely to be effective in subjects on beta-IFNs, GA, dimethyl fumarate, teriflunomide, and natalizumab. In patients treated with S1P modulators, especially with severe lymphopenia, the response to the vaccine is likely to be adequate. While initially any vaccination was considered a potential immune trigger of exacerbation in MS, over the years, most vaccines were proven safe |
| 21 | Al Jumah M et al. [22] Managing Multiple Sclerosis in the COVID-19 Era: A Review of the Literature and Consensus Report from a Panel of Experts in Saudi Arabia |
The next treatment in an ongoing course of immune-depleting therapy should be delayed, where possible, until the patient has recovered from COVID-19 The benefit of all approved COVID-19 vaccines appears to outweigh any possible risks of vaccination. Vaccines for COVID-19 are not live, and there is thus no reason to suggest a safety issue with any of the new generations of vaccines in MS patients Some DMTs may diminish the immune response to vaccines, although recent experiences show an adequate serological response to other vaccines in patients treated with DMTs, such as cladribine tablets New treatment should be delayed for 2–4 weeks to allow vaccination to proceed. Where treatment is already ongoing, one of these vaccines should be performed immediately without disruption of treatment (first-line DMTs, natalizumab, fingolimod), when lymphocytes have recovered sufficiently (cladribine tablets, alemtuzumab) or 4 months after the last dose (ocrelizumab) |
| 22 | Immovilli P et al. [1] Multiple Sclerosis Treatment in the COVID-19 Era: A Risk-Benefit Approach |
Risk factors for higher COVID-19 mortality in MS patients were age, comorbidity, progressive disease course, and anti-CD20 therapy Risk factors for severe COVID-19 were older age, male gender, higher disability, comorbidities, and methylprednisolone in the month before infection, anti-CD20 therapies, and progressive MS course Reduced COVID-19 vaccine responses were observed in patients with S1P modulators (reduced humoral and cellular response) and anti-CD20 therapies (reduced humoral response), and appropriate timing is recommended |
| 23 | Muñoz-Jurado A et al. [23] SARS-CoV-2 Infection in Multiple Sclerosis Patients: Interaction with Treatments, Adjuvant Therapies, and Vaccines against COVID-19 |
B-cell-depletion therapies may cause patients to have a lower probability of generating a detectable neutralizing antibody titer |
| 24 | Rieckmann P et al. [24] Expert Opinion on COVID-19 Vaccination and the Use of Cladribine Tablets in Clinical Practice |
All people with MS being treated with cladribine tablets should be vaccinated against COVID-19 as soon as possible, including those who have already experienced infection with SARS-CoV-2. People with MS should be prioritized for COVID-19 vaccination on an individual basis depending on risk factors (age, disability, comorbidities). The risk of COVID-19 outweighs the risks of vaccination in MS patients treated with cladribine. Cladribine treatment is unlikely to have a major impact on vaccine responses, and the length of protection is currently unclear Treatment initiation with cladribine tablets and long-term stability of MS should be prioritized over vaccination. Delay treatment of re-treatment with cladribine tablets until 2–4 weeks after completing COVID-19 vaccination, if possible If already undergoing a course of cladribine tablets, patients should receive COVID-19 vaccination when available and offered, regardless of lymphocyte counts of timing or the subsequent dose Any approved non-live COVID-19 vaccines are safe for use in people with MS on cladribine tablets There is currently no available evidence to indicate that vaccination against COVID-19 will lead to an MS relapse or permanent disease worsening |
| 25 | Tornatore C et al. [25] Vaccine Response in Patients With Multiple Sclerosis Receiving Teriflunomide |
Most DMTs, including teriflunomide, do not increase the risk of more severe COVID-19 symptoms and do not seem to increase the severity of COVID-19 symptoms or contribute to poorer outcomes. The risk of serious outcomes may be higher for untreated patients of those on anti-CD20 therapies. Some DMTs might reduce the efficacy of vaccines, but most of them incl. teriflunomide do not probably have a negative impact on the immune response to vaccination |
| 26 | Giovannoni G et al. [26] Cladribine Tablets for Relapsing-Remitting Multiple Sclerosis: A Clinician's Review |
Immunization against COVID-19 is strongly recommended for all MS patients regardless of age and comorbidities. Results of previous studies showed high levels of antibodies after the second vaccine dose in healthy patients, untreated MS patients, and patients under cladribine treatment |
| 27 | Inshasi JS et al. [27] Position of Cladribine Tablets in the Management of Relapsing-Remitting Multiple Sclerosis: An Expert Narrative Review From the United Arab Emirates |
Current data support the safety of cladribine tablets in patients who contract COVID-19, and receipt of this treatment per se should not represent a barrier to vaccination against COVID-19 |
| 28 | Moser T et al. [28] Real-World Evidence for Cladribine Tablets in Multiple Sclerosis: Further Insights into Efficacy and Safety |
Humoral responses to SARS-COV-2 vaccines were unimpaired in cladribine patients, and postvaccination seropositivity was independent of lymphocyte counts and age. Antibody titers remained sustained for 6 months; similarly, cladribine appears not to affect pretreatment antibody levels to common pathogens and this treatment was not associated with worse or fatal COVID-19 courses. Existing literature findings suggest no negative impact of cladribine on vaccine response and COVID-19 outcomes |
| 29 | Louapre C et al. [29] Anti-CD20 Therapies Decrease Humoral Immune Response to SARS-CoV-2 in Patients with Multiple Sclerosis or Neuromyelitis Optica Spectrum Disorders |
SARS-CoV-2 antibody response was decreased in patients with MS or NMO-SD treated with anti-CD20 therapies. Monitoring long-term risk of reinfection and specific vaccination strategies in this population may be warranted |
| 30 | Gombolay GY et al. [30] Immune Responses to SARS-CoV-2 Vaccination in Multiple Sclerosis: A Systematic Review/Meta-Analysis |
Antibody responses are decreased in S1P modulators and anti-CD20 therapies; however, cellular responses were positive in most anti-CD20 therapies with decreased T-cell responses in S1P modulators. mRNA vaccines had increased seroconversion rates compared to non-RNA vaccines |
| 31 | Pugliatti M et al. [31] Anti-SARS-CoV-2 Vaccination in People with Multiple Sclerosis: Lessons Learnt a Year in |
The potential consequences of SARS-CoV-2 infection in MS patients outweigh the risk of vaccination Currently, EU-approved COVID-19 vaccines produce high immunogenicity associated with favorable safety profile in the MS population. The data support not delaying vaccination or stopping MS treatment during the COVID-19 pandemic. Clinicians should discuss COVID-19 vaccination timing with patients to maximize the effectiveness of the vaccine, taking into consideration their risk |
NMSS; National Multiple Sclerosis Society; IFN, interferon; GA, glatiramer acetate; NMO-SD, neuromyelitis optica spectrum disorders.
Although the exact cause of MS remains unclear, environmental, genetic, and epigenetic factors are believed to play a key role in the pathogenesis of the disease. In previous studies, a link between viral and bacterial infections and the risk of relapses or disease progression has been shown [2, 3, 16, 32].
The clinical symptoms of MS result from immune-mediated mechanisms that are complex and include various interactions among immune cells, cytokines, chemokines, other molecules, and tissue structures. In short, autoreactive CNS-directed B and T cells activated in the periphery penetrate into the CNS, cause inflammatory damage to the myelin sheath, and increase the permeability of the blood-brain barrier [4, 33].
In recent years, treatment possibilities for MS patients have significantly improved with the development of several immunomodulatory drugs called disease-modifying therapies (DMTs). This treatment suppresses or alters the number and/or function of lymphocyte subpopulations to prevent further myelin damage and disability progression [3, 13, 26]. The immunosuppressive effect varies by drugs based on their mechanisms of action. This makes some MS patients more vulnerable to infections and may weaken their immune response to vaccination [4, 14, 16, 17].
An overview of the most frequently used DMTs, their dosing, and mechanisms of action are presented in Table 2. The aims of this article were to summarize the published literature on immune responses to COVID-19 vaccination and its safety and to provide guidance on COVID-19 immunization in patients with MS using various medications.
Table 2.
Disease-modifying therapies for multiple sclerosis treatment. Based on Witman 2021 [4], Margoni 2022 [33], Sharifian-Dorche 2021 [8], Kelly 2021 [5], Inshasi 2021 [27], Moser 2022 [28]
| Generic drug name | Brand names, route of administration, dosing | Mechanism of action |
|---|---|---|
| Interferon beta, peginterferon beta-1a | Avonex IM once weekly, Rebif SC three times a week, Extavia, and Betaferon SC every other day Plegridy SC or IM every 14 days |
Increases the expression of anti-inflammatory cells, increases the number of CD56 natural killer cells, increases nerve growth factor production, decreases the expression of pro-inflammatory cytokines, decreases the number of inflammatory cells crossing the blood-brain barrier |
| Glatiramer acetate | Copaxone SC daily or three times a week (different doses) | Prevents T-cell response against myelin and activation of T-helper type-2 cells to secrete anti-inflammatory cytokines reducing CNS inflammatory demyelination (by competing with myelin antigens for the interaction with major histocompatibility complex type-2 molecules on antigen-presenting cells) |
| Teriflunomide | Aubagio orally once daily | Reduces replication of autoreactive lymphocytes as a selective inhibitor of dihydroorotate dehydrogenase (key mitochondrial enzyme in the de novo pyrimidine synthesis) |
| Dimethyl fumarate | Tecfidera orally twice daily | Immunomodulation and neuroprotection probably via inhibition of the Nrf-2 protein ultimately inhibiting inflammatory cascades |
| Fingolimod | Gilenya orally once daily | Prevents egress of T cells from lymph nodes (by modulation of the receptor for sphingosine-1-phosphate), decreases the number of pathogenic lymphocytes migrating to the CNS, reduces central inflammation |
| Siponimod | Mayzent orally once daily (after titration period) | Prevents egress of T cells from lymph nodes (by modulation of the receptor for sphingosine-1-phosphate), decreases the number of pathogenic lymphocytes migrating to the CNS, reduces central inflammation |
| Ozanimod | Zeposia orally once daily (after titration period) | Prevents egress of T cells from lymph nodes (by modulation of the receptor for sphingosine-1-phosphate), decreases the number of pathogenic lymphocytes migrating to the CNS, reduces central inflammation |
| Cladribine | Mavenclad orally (in cycles) | Inhibits DNA synthesis and repair in highly dividing cells inducing B- and T-cell apoptosis (acts as a purine nucleoside analog). Its effect on T cells is less pronounced and short-lived compared to B cells |
| Rituximab | MabThera IV every 6–9 months (after initial 2 doses) | Targets CD20 on the surface of B cells causing prolonged selective B-cell lymphopenia (as a chimeric monoclonal antibody) |
| Ocrelizumab | Ocrevus IV every 6 months (after initial 2 doses) | Targets CD20 on the surface of B cells causing prolonged selective B-cell lymphopenia (as a humanized monoclonal antibody) |
| Ofatumumab | Kesimpta SC every 4 weeks (after an initial dosing regimen) | Targets CD20 on the surface of B cells causing prolonged selective B-cell lymphopenia (as a fully human monoclonal antibody) |
| Natalizumab | Tysabri IV monthly | Blocks the leukocyte interaction with vascular cell adhesion molecules, prevents leukocyte migration to the CNS (as a humanized monoclonal antibody against alpha-4 integrin on the surface of leukocytes) |
| Alemtuzumab | Lemtrada IV cycles | Causes immunomodulation of T and B lymphocytes and generalized lymphopenia with a more prolonged effect on T cells (acts as a humanized monoclonal antibody that targets CD52 receptors on the surface of mature lymphocytes, natural killers, and macrophages) |
IM, intramuscular; SC, subcutaneous; IV, intravenous; CNS, central nervous system.
Methods
A literature search was conducted on December 1, 2022, in PubMed database. The authors searched for open-access articles in English (except for case reports) published as of January 1, 2021, using the keywords: multiple sclerosis AND vaccin* AND covid AND (disease-modifying OR DMT). Thus, 35 papers were discovered. These articles were screened by title and abstract, and four of them (Margoni et al. [33], Kulikowska et al. [35], Cencioni et al. [54], and Hauer and Sellner [55]) were excluded as the COVID-19 infection or vaccinations were mentioned only marginally. The remaining 31 articles were reviewed to receive up-to-date knowledge and recommendations on the topic. The selection procedure is shown in Figure 1, and the list of articles and the summary of their results are stated in Table 1. In addition, the most relevant cross-references were studied, too.
SARS-CoV-2 Pathogenesis and Neurotropism
COVID-19 disease is caused by SARS-CoV-2, which is an enveloped single-stranded RNA virus composed of four major structural proteins: envelope, membrane, nucleocapsid, and spike (S) proteins and other non-structural proteins which are essential in virus replication, viral pathogenesis, and immunomodulation [16, 34]. The virus is capable of infecting cells in several human organs such as respiratory system, gastrointestinal tract, heart, kidneys, and CNS [14].
Entrance into a host cell is enabled by the connection between the angiotensin-converting enzyme 2 receptor expressed on the surface of target cells and viral S protein [14, 35]. After the incubation period of 2–14 days, symptoms of the disease such as fever, cough, headache, hyposmia, digestive disorders, and dyspnea may occur. The most severe course of the disease is expected in the elderly and people with comorbidities. These may develop interstitial pneumonia, acute respiratory distress syndrome, and multi-organ failure [1, 35]. Life-threatening conditions as a result of SARS-CoV-2 are supposed to be caused by the immune system’s overactivity leading to a cytokine storm [1, 9, 35].
The immune reaction to COVID-19 consists of local inflammatory responses and the release of proinflammatory cytokines like tumor necrosis factor-α, interleukins IL1β and IL-6, and numerous chemokines. Innate immune system activation (e.g., macrophages, dendritic cells, natural killer cells), together with antigen-specific cytotoxic T cells and neutralizing antibodies produced by B cells, represents the complex immune reaction that usually leads to the disease control [3, 7, 16, 35]. However, in severe forms of COVID-19, dysregulation of the immune system and cytokine release syndrome play their role. The cytokine storm is characterized by the increase of inflammatory cytokines and decreased lymphocyte count which may result in hypercoagulation, vascular damage, and other complicating or even life-threatening conditions [16, 35].
In addition, neurological complications such as ischemic stroke, cerebral venous thrombosis, encephalopathy, demyelination, or Guillain-Barre syndrome can be associated with SARS-CoV-2 infection. That indicates possible neuroinvasiveness of the virus [23, 32, 35]. Moreover, several cases of newly diagnosed MS following COVID-19 as well as relapses or pseudo-relapses of MS triggered by SARS-CoV-2 infection have been described in several research papers [4, 23, 32].
COVID-19 in People with MS
Based on existing literature, MS has been associated with a generally increased risk of infections, including pulmonary, urinary, skin, and opportunistic ones, and a higher hospitalization rate. These findings could be connected to the disease-related dysregulation of the immune system itself, but also to the effect of immunomodulatory/immunosuppressive medication used for MS treatment [3, 9, 16].
Therefore, at the beginning of the COVID-19 pandemic, MS patients were supposed to represent the vulnerable group for the severe course of this infection [3, 9, 23]. Surprisingly, recent studies indicate that most patients with MS treated with DMTs do not have a greater risk of acquiring COVID-19 or having a more severe form of the disease [4, 9].
However, some authors have found that anti-CD20 therapy may increase the odds of developing COVID-19 and may be connected to severe infection [3]. These findings were confirmed by other studies, and it seems that history of treatment with anti-CD20 therapies, recent methylprednisolone use, presence of comorbidities, no MS treatment, older age, longer disease course, higher disability, and progressive form of MS are associated with severe COVID-19 course [1, 9, 20, 32, 36]. Similar study results showed that the risk of hospitalization, intensive care unit admission, and death after COVID-19 in patients with MS were higher in people with advanced disabilities and underlying comorbidities including obesity [1, 29]. The risk of hospitalization was also higher in patients on anti-CD20 drugs when compared to patients on interferon or healthy control group [3, 14]. Heterogenous outcomes were found in several studies with patients on natalizumab and fingolimod [9].
In general, the most recent data do not support the increased risk of worse COVID-19 outcomes related to patients on DMTs [6, 29, 36]. Some studies even suggested the potential beneficial effect of INF and glatiramer acetate that were associated with a lower risk of contraction in COVID-19 [1, 6, 9, 14, 16, 17]. Several authors agreed that teriflunomide could have a beneficial effect against COVID-19 too, since it may prevent an excessive host immune response [23].
Immune Response to SARS-CoV-2 Infection
SARS-CoV-2 infects humans via the respiratory system and attaches to the ACE-2 receptor. This interaction stimulates innate and adaptive immune responses in order to destroy the virus. The process is very complex and includes antigen-presenting cells and CD4+ and CD8+ T-cell activation, B-cell stimulation, increase in anti-body-producing cells, and finally immunoglobulin (Ig) membrane, G, and A production. However, not all patients show this immune response pattern, but some of them may remain IgM seronegative [34].
It seems that T cells play a critical role in the control of infection [18, 30], while neutralizing antibodies may confer protection against reinfection [7]. The duration of this protection is still unclear [15]. Severe cases of COVID-19 are supposed to be caused by harmful immune dysregulation represented by hyperinflation, cytokine storm, lymphopenia, and neutrophilia [16, 18, 34]
MS and Vaccination against COVID-19
Several vaccines against COVID-19 have been developed and licensed for use by regulatory authorities around the globe. The first vaccines to be approved were those from Pfizer-BioNTech (BNT162b2, Comirnaty), Moderna (mRNA-1273, Spikevax), Oxford/AstraZeneca (ChAdOx1 nCoV-19 or AZD1222, Vaxzevria), and Janssen (Ac26COV2.S, Jcovden). These were followed by many other vaccines and even more that are currently under trial [5, 21].
Some approaches used in vaccine development against COVID are based on novel technological platforms, and their mechanisms of action differ from traditional ones [37]. Nucleic acid vaccines contain a specific antigen-coding DNA or mRNA that tells the human organism to produce the virus‘s distinctive S protein which induces the immune response. For example, vaccines developed by Pfizer/BioNTech and Moderna are based on this principle (using mRNA included in a lipid particle) [4, 37].
Vector vaccines, such as those by Janssen and Oxford/AstraZeneca, use a modified version of an adenovirus to deliver the SARS-CoV-2 gene coding the targeted viral antigen into the recipients’ cells. The genetic information provides the instructions for specific viral protein production within the host cells. These proteins are capable of generating both humoral and cellular immune responses [31, 38].
However, some vaccines are based on well-known immunization strategies, such as delivering the whole inactivated SARS-CoV-2 virus into the human body (CoronaVax by Sinovac or vaccine by Valneva) or introducing only harmless protein fragments into the vaccinated person (e.g., Nuvaxovid subunit vaccine by Novavax) [37–39]. Several live-attenuated vaccines are also being developed in order to induce long-lasting immunity, but are still in clinical trials (e.g., COVI-VAC) and are unlikely to be used in patients with MS [31, 39].
By December 2022, there are 50 vaccines approved by regulatory agencies or national authorities and more than 240 vaccine candidates worldwide [39]. The European Medicines Agency (EMA) authorized 7 vaccines that can be used in the European Union (Comirnaty, Spikevax, Vaxzevria, Jcovden, Nuvaxovid, COVID-19 Vaccine Valneva, and VidPrevtyn Beta by Sanofi/GSK) and several adapted version of these vaccines to provide broader protection against different virus variants [39, 40].
In short, regardless of the vaccine type (viral vector, based on mRNA, recombinant, live-attenuated, etc.), they work by introducing the antigen or the genetic information for the antigen into the human body. Thus, immune responses toward the antigens are generated [25].
Coordinated interactions between T and B cells of the adaptive immune system are essential to the successful generation of immunological memory and production of neutralizing antibodies following recognition of vaccine antigens by innate immune cells [3, 21]. The effect of the vaccination is usually measured according to IgG antibody titers. Cellular immune functions (antigen-specific B- and T-cell-based immune responses) are evaluated in a smaller number of trials as it is more complicated due to difficulties in standardization, biological variability, and technical complexity [25, 41, 42].
The rapid development of COVID-19 vaccines has raised questions regarding both their immunogenicity and safety in patients with MS. Although we are lacking robust data on COVID-19 vaccination, some conclusions based on previous vaccine studies and currently available research on COVID-19 vaccines can be drawn [5, 11, 21, 25].
A few DMTs can negatively influence vaccine efficacy. According to some authors, significantly reduced humoral responses were reported in patients receiving anti-CD20 therapies (ocrelizumab and rituximab) and fingolimod compared with untreated patients, while people on teriflunomide developed similar antibody levels to untreated patients [15, 19, 25, 36]. Some research papers also indicated that cladribine, alemtuzumab, and natalizumab may impair the immune response [21, 32, 36], but the findings are not consistent [23, 28, 30].
Higher antibody response was observed in mRNA vaccines when compared to non-mRNA ones [30]. Several studies reported higher antibody levels in patients who received mRNA-1273 vaccine (Moderna) versus BNT162b2 (Pfizer-BioNTech) [25].
In conclusion, it is believed that interferon, glatiramer acetate, dimethyl fumarate, teriflunomide, and natalizumab may not suppress the immune response to vaccination, whereas DMTs causing sequestration or depletion of T and/or B cells (S1P modulators, cladribine, alemtuzumab, and anti-CD20 therapies) may reduce vaccine efficacy [9, 12, 21, 24]. According to the most recent meta-analysis, it seems that only S1P modulators and anti-CD20 therapies may significantly blunt the antibody response, but patients on these medications still benefit from COVID-19 vaccination [23, 30].
Interestingly, in a study measuring also cell-based responses 1 month after the second dose of vaccine, a robust cellular response appeared even in patients on anti-CD20 therapy, although their COVID-19 antibody titers were poor [43]. Similar findings were reported by other studies that observed robust cellular response to the COVID-19 vaccine in the majority of patients on teriflunomide and other DMTs, except for S1P modulators [28, 44]. S1P modulators reduce both B- and T-cell responses, while anti-CD20 therapies reduce B-cell-mediated response [15, 17].
The immune response of T cells to vaccination seems to be more important than antibodies’ level in the prevention of death, hospitalization, and severe SARS-CoV-2 infection [34]. Some authors described a better vaccine response in anti-CD20-treated patients who did not have a complete B-cell depletion at the time of vaccination and in patients on S1P modulators with high lymphocyte count [1, 44].
Based on these findings, recommendations were issued to increase the efficacy of vaccination, as timing can positively influence vaccine efficacy [1, 13, 19, 21, 24, 25]. See Table 3.
Table 3.
General guidance for the COVID-19 vaccination and timing recommendations according to National Multiple Sclerosis Society and European Multiple Sclerosis Platform
| DMT | The interval from the last dose of MS treatment to the first vaccine dose | Next treatment dose after full COVID-19 vaccinationa | Starting a new MS treatment after full COVID-19 vaccinationa |
|---|---|---|---|
| Beta-interferons | NA | No delay | No delay |
| Glatiramer acetate | NA | No delay | No delay |
| Teriflunomide, fumarates, natalizumab | NA | No delay | No delay |
| S1P receptor modulators (fingolimod, siponimod, ozanimod, ponesimod) | NA | No delay | At least 2 (to 4b) weeks |
| Alemtuzumab | At least 24 weeks | At least 4 weeks | At least 4 weeks |
| Oral cladribine | NA | 2–4 weeks | At least 2 (to 4b) weeks |
| Anti-CD20 therapies (rituximab, ocrelizumab) | At least 12 weeksb | At least 4 weeks | At least 2 (to 4b) weeks |
| Ofatumumab | At least 4 weeks | At least 4 weeks | At least 2 weeks |
| High-dose steroids | 3–5 days | NA | NA |
Based on National Multiple Sclerosis Society 2022 [50], European Multiple Sclerosis Platform 2022 [51].
DMTs, disease-modifying therapies; NA, non-applicable; NMSS, National Multiple Sclerosis Society; EMSP, European Multiple Sclerosis Platform.
aFully vaccinated means two doses of the mRNA (Pfizer-BioNTech or Moderna) or one dose of the vector vaccine (Janssen) according to NMSS and one dose of Janssen vaccine and two doses of any other type of vaccine according to EMSP.
bOnly EMSP recommendations.
Despite the lack of robust data on humoral responses of MS patients to vaccination, an additional dose is recommended for those who are considered to be severely immunosuppressed. Although the threshold antibody level to determine sufficient immunity following vaccination is unknown, blunted immune response is likely in persons treated with anti-CD20 therapies, S1P modulators, and alemtuzumab [1, 2, 11, 15, 16, 20, 22].
Some authors suggest that IgG humoral response assessment is crucial for determining the vaccination status of immune-compromised patients to evaluate the necessity of additional vaccine doses [13, 45]. Decisions concerning vaccination schedules should be made in cooperation with the patient and the physician. If possible, Pfizer/BioNTech or Moderna vaccine should be preferred for booster vaccination [31].
Safety of COVID-19 Vaccines in Patients with MS
Vaccines against COVID were developed in a relatively short time period, and their safety and tolerability are still being monitored. According to a meta-analysis [46], the local side effects such as pain, redness, and swelling at the vaccination site together with systemic events (fever, fatigue, headache, chill, nausea, vomiting, and arthralgia) were reported in all four types of vaccines assessed (adenovirus vector-based, mRNA, subunit, and inactivated). Serious adverse events following COVID-19 vaccination are rare but may occur, too. Cases of severe allergic reactions, myocarditis, and pericarditis and neurological disorders (incl. Bell’s palsy, Guillain-Barré syndrome, or new manifestations of demyelinating diseases) were reported [38, 47].
Several types of thrombosis have been documented after mRNA vaccines, but more frequently after the adenovirus vector vaccines [46]. This even led to a short-term withdrawal of the Oxford/AstraZeneca (Vaxzevria) and Janssen (currently Jcovden) vaccines in 2021 [38]. Very soon, the regulatory authorities declared that the benefits of these vaccines far outweigh their possible risks. However, several updates of the product information of both vaccines were followed [48].
In patients with MS, similar mild adverse effects (such as local pain, fatigue, muscle or joint pain, headache, and low-grade fever) following COVID-19 vaccination commonly occurred [23]. Fever following the vaccine administration may temporarily worsen the disease symptoms [21, 23].
Although new demyelination and MS relapses were also reported after vaccination, the rate is similar to the incidence of non-vaccinated persons. Of note, more than half of the patients had a history of immune-mediated diseases. The incidence was very low, and causality could not be confirmed [48]. All in all, no increased risk of relapse activity was observed [15, 24, 31, 45, 49].
Live-attenuated vaccines, in general, should be avoided in persons on immunomodulatory or immunosuppressive drugs, but they may be administered before starting this type of treatment [16, 18, 32]. For example, varicella-zoster virus seronegative patients are strongly advised to be vaccinated 4–6 weeks prior to initiation of cladribine, S1P modulators, and alemtuzumab treatment [4, 10, 13, 26, 27].
According to general recommendations, all MS patients in a stable phase of the disease with no additional contraindications should be vaccinated against COVID-19. Available studies show that vaccines are safe and do not increase the short-term risk of MS relapse [1, 9, 13, 21, 31, 50–52].
Treatment with most DMTs should not be stopped or interrupted for vaccination. Especially in the case of fingolimod and natalizumab, the drug discontinuation may increase the risk of severe MS rebound [5, 12, 20].
Vaccination is of utmost importance in high-risk groups, i.e., patients with progressive MS, with higher levels of disability, persons of older age, with obesity, and other comorbidities. These patients should be vaccinated as soon as possible [21]. In conclusion, the benefits of vaccination far outweigh its potential adverse events and risk of COVID-19 disease consequences, but the timing with respect to DMTs and MS stability should be considered for each patient [20, 22, 32, 53].
Discussion
At present, there is no indication that MS disease per se would be associated with a higher risk of contracting COVID-19 or of a more severe course of the disease. This applies even to patients with immunomodulatory treatment. Only the patients on anti-CD20 therapies seem to be more susceptible to having a severe case of COVID-19 [6, 12, 19, 53].
Although long-term reliable data on the efficacy and safety of COVID-19 vaccines in both the general population and people with MS are lacking so far [4], guidelines for healthcare providers were developed in many countries. According to recent literary sources, non-live SARS-CoV-2 vaccines are recommended for all MS patients who are not in an active phase of the disease [21, 50, 51].
Some DMTs, especially S1P modulators and B-cell-depleting therapies, can reduce vaccine humoral responses, but might still provide some protection, and adequate T-cell response remains possible. Coordinating vaccination timing with dosing regimens of some DMTs may play a crucial role in optimizing vaccine efficacy [15, 19, 21, 31].
More specifically, in patients treated with anti-CD20 drugs and alemtuzumab, a reconstitution period following the last treatment dose before vaccination and a time window prior to the next DMT administration after vaccination should be considered [1, 16, 50, 51]. Further studies with larger sample sizes and real-world data are needed to provide knowledge on the efficacy of booster vaccine doses, and the long-term safety profile of currently available and future vaccines in order to optimize the success of treatment strategies and vaccination in MS patients. Moreover, questions regarding innate and adaptive immune responses after SARS-CoV-2 infection and COVID-19 vaccination and persistence of the protection remain to be elucidated.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received to assist with the preparation of this manuscript.
Author Contributions
E.P.: literature search, interpretation of the data, and drafting the manuscript. P.M.: literature search, data interpretation, and manuscript contribution and revision. M.K.: manuscript revision and proofreading. R.M.: idea for the article and supervision. All authors read and approved the final manuscript.
Funding Statement
No funding was received to assist with the preparation of this manuscript.
References
- 1. Immovilli P, Morelli N, Terracciano C, Rota E, Marchesi E, Vollaro S, et al. Multiple sclerosis treatment in the COVID-19 era: a risk-benefit approach. Neurol Int. 2022 Apr 15;14(2):368–77. 10.3390/neurolint14020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beard K, Sriwastava S. Insight in booster COVID-19 vaccine and disease modifying therapy in multiple sclerosis. J Neurol Sci. 2021 Nov 15;430:120034. 10.1016/j.jns.2021.120034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coyle PK, Gocke A, Vignos M, Newsome SD. Vaccine considerations for multiple sclerosis in the COVID-19 era. Adv Ther. 2021 Jul;38(7):3550–88. 10.1007/s12325-021-01761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Witman Tsur S, Adrian Zaher E, Tsur M, Kania K, Kalinowska-Łyszczarz A. Current immunological and clinical perspective on vaccinations in multiple sclerosis patients: are they safe after all? Int J Mol Sci. 2021 Apr 8;22(8):3859. 10.3390/ijms22083859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelly H, Sokola B, Abboud H. Safety and efficacy of COVID-19 vaccines in multiple sclerosis patients. J Neuroimmunol. 2021 Jul 15;356:577599. 10.1016/j.jneuroim.2021.577599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Landtblom AM, Berntsson SG, Boström I, Iacobaeus E. Multiple sclerosis and COVID-19: the Swedish experience. Acta Neurol Scand. 2021 Sep;144(3):229–35. 10.1111/ane.13453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cabreira V, Abreu P, Soares-Dos-Reis R, Guimarães J, Sá MJ. Multiple sclerosis, disease-modifying therapies and COVID-19: a systematic review on immune response and vaccination recommendations. Vaccines. 2021 Jul 11;9(7):773. 10.3390/vaccines9070773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharifian-Dorche M, Sahraian MA, Fadda G, Osherov M, Sharifian-Dorche A, Karaminia M, et al. COVID-19 and disease-modifying therapies in patients with demyelinating diseases of the central nervous system: a systematic review. Mult Scler Relat Disord. 2021 May;50:102800. 10.1016/j.msard.2021.102800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Toscano S, Chisari CG, Patti F. Multiple sclerosis, COVID-19 and vaccines: making the point. Neurol Ther. 2021 Dec;10(2):627–49. 10.1007/s40120-021-00288-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith TE, Kister I. Infection mitigation strategies for multiple sclerosis patients on oral and monoclonal disease-modifying therapies. Curr Neurol Neurosci Rep. 2021 May 19;21(7):36. 10.1007/s11910-021-01117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iannetta M, Landi D, Cola G, Campogiani L, Malagnino V, Teti E, et al. B- and T-cell responses after SARS-CoV-2 vaccination in patients with multiple sclerosis receiving disease modifying therapies: immunological patterns and clinical implications. Front Immunol. 2022 Jan 17;12:796482. 10.3389/fimmu.2021.796482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inshasi J, Alroughani R, Al-Asmi A, Alkhaboury J, Alsalti A, Boshra A, et al. Expert consensus and narrative review on the management of multiple sclerosis in the arabian gulf in the COVID-19 era: focus on disease-modifying therapies and vaccination against COVID-19. Neurol Ther. 2021 Dec;10(2):539–55. 10.1007/s40120-021-00260-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gold R, Fätkenheuer G, Hartung HP, Kleinschnitz C, Marks R, Maschke M, et al. Vaccination in multiple sclerosis patients treated with highly effective disease-modifying drugs: an overview with consideration of cladribine tablets. Ther Adv Neurol Disord. 2021;14:17562864211019598. 10.1177/17562864211019598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhise V, Dhib-Jalbut S. Potential risks and benefits of multiple sclerosis immune therapies in the COVID-19 era: clinical and immunological perspectives. Neurotherapeutics. 2021 Jan;18(1):244–51. 10.1007/s13311-021-01008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu X, Wang L, Shen L, Tang K. Response of COVID-19 vaccination in multiple sclerosis patients following disease-modifying therapies: a meta-analysis. eBioMedicine. 2022 Jul;81:104102. 10.1016/j.ebiom.2022.104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Golshani M, Hrdý J. Multiple sclerosis patients and disease modifying therapies: impact on immune responses against COVID-19 and SARS-CoV-2 vaccination. Vaccines. 2022 Feb 11;10(2):279. 10.3390/vaccines10020279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reder AT, Stuve O, Tankou SK, Leist TP. T cell responses to COVID-19 infection and vaccination in patients with multiple sclerosis receiving disease-modifying therapy. Mult Scler. 2022 Nov 28;135245852211342. 10.1177/13524585221134216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sellner J, Rommer PS. Multiple sclerosis and SARS-CoV-2 vaccination: considerations for immune-depleting therapies. Vaccines. 2021 Jan 28;9(2):99. 10.3390/vaccines9020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim E, Haag A, Nguyen J, Kesselman MM, Demory Beckler M. Vaccination of multiple sclerosis patients during the COVID-19 era: novel insights into vaccine safety and immunogenicity. Mult Scler Relat Disord. 2022 Nov;67:104172. 10.1016/j.msard.2022.104172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hollen C, Bernard J. Multiple sclerosis management during the COVID-19 pandemic. Curr Neurol Neurosci Rep. 2022 Aug;22(8):537–43. 10.1007/s11910-022-01211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yamout BI, Zakaria M, Inshasi J, Al-Jumah M, Zeineddine M, Dahdaleh M, et al. MENACTRIMS practice guideline for COVID-19 vaccination in patients with multiple sclerosis. Mult Scler Relat Disord. 2021 Nov;56:103225. 10.1016/j.msard.2021.103225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al Jumah M, Abulaban A, Aggad H, Al Bunyan R, AlKhawajah M, Al Malik Y, et al. Managing multiple sclerosis in the Covid19 era: a review of the literature and consensus report from a panel of experts in Saudi Arabia. Mult Scler Relat Disord. 2021 Jun;51:102925. 10.1016/j.msard.2021.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Muñoz-Jurado A, Escribano BM, Agüera E, Caballero-Villarraso J, Galván A, Túnez I. SARS-CoV-2 infection in multiple sclerosis patients: interaction with treatments, adjuvant therapies, and vaccines against COVID-19. J Neurol. 2022 Sep;269(9):4581–603. 10.1007/s00415-022-11237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rieckmann P, Centonze D, Giovannoni G, Hua LH, Oreja-Guevara C, Selchen D, et al. Expert opinion on COVID-19 vaccination and the use of cladribine tablets in clinical practice. Ther Adv Neurol Disord. 2021;14:17562864211058298. 10.1177/17562864211058298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tornatore C, Wiendl H, Lublin AL, Geertsen SS, Chavin J, Truffinet P, et al. Vaccine response in patients with multiple sclerosis receiving teriflunomide. Front Neurol. 2022;13:828616. 10.3389/fneur.2022.828616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Giovannoni G, Mathews J. Cladribine tablets for relapsing-remitting multiple sclerosis: a clinician’s review. Neurol Ther. 2022 Jun;11(2):571–95. 10.1007/s40120-022-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inshasi JS, Alfahad S, Alsaadi T, Hassan A, Zein T, Mifsud VA, et al. Position of cladribine tablets in the management of relapsing-remitting multiple sclerosis: an expert narrative review from the United Arab Emirates. Neurol Ther. 2021 Dec;10(2):435–54. 10.1007/s40120-021-00243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moser T, Ziemssen T, Sellner J. Real-world evidence for cladribine tablets in multiple sclerosis: further insights into efficacy and safety. Wien Med Wochenschr. 2022 Nov;172(15–16):365–72. 10.1007/s10354-022-00931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Louapre C, Collongues N, Stankoff B, Giannesini C, Papeix C, Bensa C, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020 Sep 1;77(9):1079. 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gombolay GY, Dutt M, Tyor W. Immune responses to SARS-CoV-2 vaccination in multiple sclerosis: a systematic review/meta-analysis. Ann Clin Transl Neurol. 2022 Aug;9(8):1321–31. 10.1002/acn3.51628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pugliatti M, Hartung HP, Oreja-Guevara C, Pozzilli C, Airas L, Alkhawajah M, et al. Anti-SARS-CoV-2 vaccination in people with multiple sclerosis: lessons learnt a year in. Front Immunol. 2022 Oct 17;13:1045101. 10.3389/fimmu.2022.1045101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bellucci G, Rinaldi V, Buscarinu MC, Reniè R, Bigi R, Pellicciari G, et al. Multiple sclerosis and SARS-CoV-2: has the interplay started? Front Immunol. 2021 Sep 27;12:755333. 10.3389/fimmu.2021.755333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Margoni M, Preziosa P, Filippi M, Rocca MA. Anti-CD20 therapies for multiple sclerosis: current status and future perspectives. J Neurol. 2022 Mar;269(3):1316–34. 10.1007/s00415-021-10744-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Saad N, Moussa S. Immune response to COVID-19 infection: a double-edged sword. Immunol Med. 2021 Jul 3;44(3):187–96. 10.1080/25785826.2020.1870305. [DOI] [PubMed] [Google Scholar]
- 35. Kulikowska J, Kulczyńska-Przybik A, Mroczko B, Kułakowska A. The significance of COVID-19 immunological status in severe neurological complications and multiple sclerosis-A literature review. Int J Mol Sci. 2021 May 31;22(11):5894. 10.3390/ijms22115894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sormani MP, De Rossi N, Schiavetti I, Carmisciano L, Cordioli C, Moiola L, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021 Apr;89(4):780–9. 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. COVID-19 Vaccine Tracker . Vaccines types. [Internet] [cited 2022 Dec 02]. Available from: https://covid19.trackvaccines.org/vaccine-types/.
- 38. Mascellino MT, Di Timoteo F, De Angelis M, Oliva A. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect Drug Resist. 2021 Aug;14:3459–76. 10.2147/IDR.S315727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.COVID19 Vaccine Tracker, Vaccines candidates in clinical trials. [Internet] [cited 2022 Dec 02]. Available from: https://covid19.trackvaccines.org/vaccines/.
- 40. European Medicines Agency . COVID-19 vaccines: authorised. [Internet] [cited 2022 Dec 10]. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#adapted-covid-19-vaccines-section.
- 41. Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021 Feb 5;371(6529):eabf4063. 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Limaye S. Abnormal laboratory results: tests for cell-mediated immunity. Aust Prescr. 2010 Jun 1;33(3):84–7. 10.18773/austprescr.2010.041. [DOI] [Google Scholar]
- 43. Apostolidis SA, Kakara M, Painter MM, Goel RR, Mathew D, Lenzi K, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med. 2021 Nov;27(11):1990–2001. 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Achiron A, Dolev M, Menascu S, Zohar DN, Dreyer-Alster S, Miron S, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult Scler. 2021;27(6):864–70. 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dreyer-Alster S, Menascu S, Mandel M, Shirbint E, Magalashvili D, Dolev M, et al. COVID-19 vaccination in patients with multiple sclerosis: safety and humoral efficacy of the third booster dose. J Neurol Sci. 2022 Mar 15;434:120155. 10.1016/j.jns.2022.120155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharif N, Alzahrani KJ, Ahmed SN, Dey SK. Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021 Oct 11;12:714170. 10.3389/fimmu.2021.714170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ismail II, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J Neuroimmunol. 2022 Jan;362:577765. 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. European Medicine Agency . COVID-19 Vaccine Jannsen: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. [Internet] [cited 2022 Dec 06]. Available from: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-provide-further-context-risk-very-rare-blood-clots-low-blood). [Google Scholar]
- 49. Czarnowska A, Tarasiuk J, Zajkowska O, Wnuk M, Marona M, Nowak K, et al. Analysis of side effects following vaccination against COVID-19 among individuals with multiple sclerosis treated with DMTs in Poland. Front Neurol. 2022 Jun 14;13:913283. 10.3389/fneur.2022.913283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. National Multiple Sclerosis Society . COVID-19 vaccine guidance for people living with MS. [Internet] [cited 2022 Dec 02]. Available from: https://www.nationalmssociety.org/coronavirus-covid-19-information/covid-19-vaccine-guidance. [Google Scholar]
- 51.European Multiple Sclerosis Platform, Updated global COVID-19 advice for people with MS. [Internet] [cited 2022 Dec 02]. Available from: https://emsp.org/news/global-covid-19-advice-for-people-with-ms/.
- 52. Ghaderi S, Berg-Hansen P, Bakken IJ, Magnus P, Trogstad L, Håberg SE. Hospitalization following influenza infection and pandemic vaccination in multiple sclerosis patients: a nationwide population-based registry study from Norway. Eur J Epidemiol. 2020 Apr;35(4):355–62. 10.1007/s10654-019-00595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Monschein T, Hartung HP, Zrzavy T, Barnett M, Boxberger N, Berger T, et al. Vaccination and multiple sclerosis in the era of the COVID-19 pandemic. J Neurol Neurosurg Psychiatry. 2021 Oct;92(10):1033–43. 10.1136/jnnp-2021-326839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cencioni MT, Genchi A, Brittain G, de Silva TI, Sharrack B, Snowden JA, et al. Immune reconstitution following autologous hematopoietic stem cell transplantation for multiple sclerosis: a review on behalf of the EBMT Autoimmune Diseases Working Party. Front Immunol. 2022;12:813957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hauer L, Sellner J. Diroximel fumarate as a novel oral immunomodulating therapy for relapsing forms of multiple sclerosis: a review on the emerging data. Drug Des Devel Ther. 2022;16:3915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]