Abstract
Introduction
Kidney stones (KSs) are associated with hematuria and renal failure and pose a significant clinical and public health concern. Diabetes is associated with a higher risk of KSs. In addition, α-Klotho (Klotho), as a novel antiaging protein, is associated with kidney disease, diabetes, and complications and may participate in the pathological mechanism of KSs. However, studies that used large population-based database research are limited. Therefore, this study aimed to investigate whether or not KS prevalence is associated with serum Klotho levels in diabetic adults in the USA.
Methods
This nationally representative cross-sectional study used data on diabetic adults in the USA aged 40–79 years from the National Health and Nutrition Examination Survey 2007–2016 cycles. Multivariate logistic regression models were used to calculate the association between Klotho and KS. Restricted cubic splines were used to further test for linearity and explore the shape of the dose-response association. Moreover, we performed stratified and interaction analyses to see if the relationship was stable in different subgroups.
Results
Among the 3,537 diabetic patients included in this study (mean age of 61.4 years, consisting of 51.3% males), 543 participants (15.4%) suffered from KS. In the fully adjusted model, Klotho was negatively associated with KS (OR = 0.72; 95% CI: 0.54–0.96; p = 0.027). A negative relationship was observed between the occurrence of KS and Klotho (nonlinear: p = 0.560). Some differences in the association between Klotho and KS were found in stratified analyses; however, these differences lacked statistical significance.
Conclusions
Serum Klotho was negatively associated with the incidence of KS; when ln-transformed Klotho concentration increased by 1 unit, the risk of KS was 28% lower.
Keywords: α-Klotho (Klotho), Kidney stones, Diabetes mellitus, National Health and Nutrition Survey
Introduction
Kidney stones (KSs) are mineral concretions in the renal calyces and pelvis that are free or attached to the renal papillae, which are formed when the urine is supersaturated relative to minerals. KS is a highly frequent disease with an increasing number of cases that have been seen in recent years, which poses an increasing threat to the economy and healthcare infrastructure worldwide [1, 2]. In a lifetime course, 10% of people will get KS, and 70% of those cases will reoccur [3]. Growing evidence has supported the idea that KS is a systemic disease rather than an isolated urinary metabolic disorder. KS was significantly correlated with a raised risk of various morbidities, including stroke, coronary heart disease, renal cell carcinoma, and end-stage renal disease [4, 5]. Diabetes mellitus (DM) is an important influencing factor, which is closely related to the development of KS [6, 7]. Previous investigations have suggested that individuals with DM are substantially more likely to have KS (including calcium oxalate (CaOx) and uric acid KS). KS disease may become more clinically concerning because of the rising DM prevalence, thereby stressing the importance of additional preventive measures [8]. Primary care physicians, nephrologists, and urologists can help reduce the condition’s prevalence and release the clinical and public health concern by recognizing and treating underlying risk factors for forming KSs.
In 1997, α-Klotho (Klotho) was first discovered in mice as an antiaging hormone, which was expressed abundantly in choroid plexus epithelial cells of the brain and distal convoluted tubules of the kidney [9, 10]. Moreover, Klotho serves as the co-receptor for the circulating hormone fibroblast growth factor 23 (FGF23), it also acts as an enzyme that alters the sugar chains of transient receptor potential vanilloid-5 (TRPV5, a calcium channel implicated in calcium reabsorption in the kidney) and regulates its activity [11]. Furthermore, Klotho controls phosphate/calcium metabolism and aging. Klotho has also been associated with numerous age-related diseases, such as kidney disease, DM, Alzheimer’s disease, chronic obstructive pulmonary disease, certain types of cancer, cardiovascular and cerebrovascular diseases and their complications, and mortality [12–14]. Klotho has been linked to DM, and it is expected to serve as a therapeutic target and a preclinical marker for the disease [15, 16]. Recently, Klotho has been reported to be associated with KS. According to Zhu et al. [17], as forerunner lesions of idiopathic CaOx stones, Klotho deficiency is one clinical characteristic of Randall’s plaques. They also discovered that the Klotho protein produced from HK-2 cells prevented hRIFs from differentiating into osteoblasts by blocking the Wnt-β-catenin pathway. In addition, in vitro CaOx KS model, Klotho overexpression prevented the CaOx-induced enhancement of crystal cell adhesion and apoptosis in HKC cells, whereas in vivo assays revealed that Klotho overexpression reduced renal oxidative injury, renal apoptosis, and crystal deposition that were caused by glyoxylate administration in mouse kidneys [18]. The Klotho gene demonstrated a protective effect, with multiple studies demonstrating a strong correlation between Klotho gene variations and urolithiasis susceptibility [19, 20]. However, most studies on the relationships between Klotho gene polymorphisms and KS were conducted in Asia and lacked information on multiethnicity, which might be considered a confounding factor.
Meanwhile, the protective benefits of the Klotho gene on KS have been proven, but limited information is known about the predictive capabilities of serum Klotho levels for the incidence of KS, especially in the US diabetic population. Accumulated evidence fundamentally validated the importance of Klotho to KS, and additional research into this connection may probably be justified. Therefore, in this study, we aimed to evaluate whether or not serum Klotho is associated with the incidence of KS in diabetics in a relatively large and nationally representative population aged 40–79 years in the USA.
Materials and Methods
Data Sources
National Health and Nutrition Survey (NHANES) is a national representative survey conducted by the National Center for Health Statistics (NCHS), which aims to assess the health or nutritional status of the noninstitutionalized US population. Moreover, NHANES collects demographic and in-depth health information through home visits, screening, and laboratory testing conducted by a mobile examination center. The survey’s design, methods, and data are available to the public. The NCHS Research Ethics Review Board approved the NHANES study protocol, and participants provided written informed consent at enrollment (the website is https://www.cdc.gov/nchs/nhanes/irba98.htm). Ethical approval and consent were not required as this study was based on publicly available de-identified data. Information on Klotho was only provided in the NHANES cycles from 2007 to 2016 for adults aged 40–79 years. NHANES data are available on the NHANES website (http://www.cdc.gov/nchs/nhanes.htm; accessed on December 19, 2022). This study complied with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Design and Population
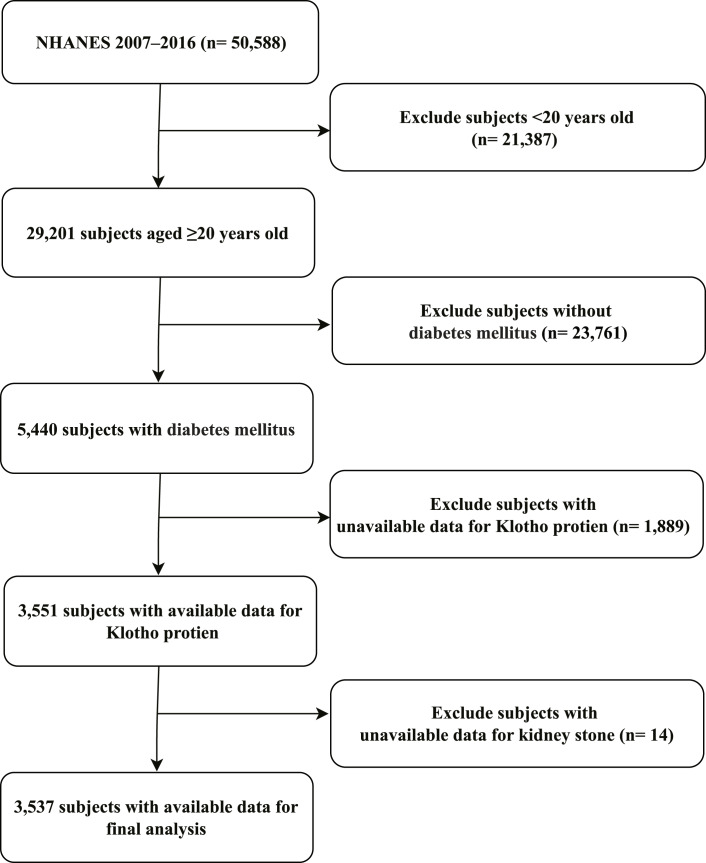
Among the 50,588 participants who participated in the 2007–2016 NHANES, 21,387 subjects who were younger than 20 years and 23,761 individuals without DM were initially excluded, thereby leading to a population of 5,440 patients with DM. Then, we further excluded 1,889 individuals who did not have complete laboratory data on Klotho and 14 with no available laboratory data on KS. Finally, a total of 3,537 DM subjects with complete serum Klotho and KS data were included in this study. Figure 1 shows the flowchart of the exclusion criteria.
Fig. 1.
Schematic representation of the participant selection process and distribution of participant groups. NHANES, National Health and Nutrition Examination Survey.
Diabetes Mellitus
The diagnostic criteria for DM were formulated based on the American Diabetes Association criteria [21], participants who met one of the following criteria were defined as DM: (1) self-reported physician diagnosis of diabetes; (2) receipt of oral glucose-lowering medicines or insulin; and (3) fasting plasma glucose level of at least 126 mg/dL, 75 g oral glucose tolerance test (OGTT) of at least 200 mg/dL (to convert glucose to millimoles per liter, multiply by 0.0555), or hemoglobin A1c (HbA1c) level of at least 6.5% (48 mmol/mol; to convert the HbA1c percentage of total hemoglobin to a proportion of total hemoglobin, multiply by 0.01).
Serum Klotho Protein
Serum samples were obtained and stored at −80°C until evaluation at the mobile examination center. Klotho in the Northwest Lipid Metabolism and Diabetes Research Laboratories of the Division of Metabolism, Endocrinology, and Nutrition at the University of Washington was quantified using a commercial ELISA kit made by IBL International, Japan [22]. For quality control, two samples were analyzed in each ELISA plate with low or high Klotho concentration. However, when the results were not within the standard deviation (SD) of 2 of the assigned value, the entire experiment was rejected and repeated. The lowest detection limit was 6 pg/mL, and no imputation was conducted given the final value of all samples exceeded this limit. Details of laboratory methodology, quality assurance, and monitoring can be found at the following link: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/SSKL_E.htm.
Kidney Stones
The incidence of KS was the study’s main outcome. KSs were identified based on the results from the questionnaire question “Have you ever had a KS?” from the Kidney Conditions Urology survey. One was considered to have a history of KS occurrence if they answered “yes.” Using the computer-assisted personal interview technology, trained interviewers asked questions about KS in the interviewees’ homes. This self-reported questionnaire’s validity and accuracy have been established, and it detected 97% of the males clinically diagnosed with KS [23].
Covariate
Herein, we used questionnaires, physical exams, and laboratory testing to gather covariate data. These following covariates were included: sex, age, race and ethnicity, educational level, marital status, physical activity, smoking status, alcohol drinking status, hypertension, chronic kidney disease (CKD), body mass index (BMI) (kg/m2), HbA1c (%), albumin (g/dL), triglycerides (mg/dL), total cholesterol (mg/dL), high-density lipoprotein (HDL) cholesterol (mg/dL), blood urea nitrogen (BUN, mg/dL), uric acid (μmol/L), serum calcium (mg/dL), serum phosphorus (mg/dL), and serum 25-hydroxyvitamin D3 (vitamin D, nmol/L).
In NHANES, information on self-reported race and ethnicity was derived from responses to survey questions on race and Hispanic ethnicity. As utilized by NHANES, we categorized the participants into the following six races and ethnicities: Mexican American, non-Hispanic white, non-Hispanic black, other Hispanic, and other races (including multiracial). Educational level was divided into three levels (high school or less, some college, and college or above). Marital status was classified into the following four groups: married, never married, living with a partner, and others (including widowed, divorced, or separated). Physical activity was coded as sedentary, moderate, and vigorous. Moderate were the participants reported to do any modest sports, fitness, or leisure activities that cause a slight increase in respirations or heart rate for at least 10 min constantly within the last 30 days; meanwhile, vigorous was defined as any intense sports, fitness, or entertainments that cause massive increases in respirations or pulse rate like running or basketball for at least 10 min continuously within the last 30 days. Smoking status was classified as follows: never smoked (or smoked <100 cigarettes), former smoker (smoked ≥100 cigarettes but already quit), and current smoker. Participants who answered “yes” to the following question were classified as alcohol drinkers, “In any year, have you had at least consumed any type of alcoholic beverage at least 12 times?” In addition, hypertension was defined as a systolic blood pressure value ≥140 mm Hg and/or a diastolic blood pressure value ≥90 mm Hg or a self-reported physician diagnosis of hypertension [24]. Moreover, systolic blood pressure and diastolic blood pressure were obtained from three consecutive blood pressure measurements and other methodological measurements. Furthermore, CKD was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2 [25]. Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation (GFR = 141·min [Scr/κ, 1]α × max [Scr/κ, 1] − 1.209 × 0.993 Age × 1.018 [if women] × 1.159 [if black]); κ was 0.7 and 0.9 for women and men, a was −0.329 and −0.411 for women and men, respectively, and min and max indicate the minimum of Scr/κ or 1 and the maximum of Scr/κ or 1, respectively. During the body examination, the subjects’ height and weight were recorded, and their BMI was estimated. HbA1c was measured using high-performance liquid chromatography methods. All the above variables were described on the NHANES website, which can be accessed at https://wwwn.cdc.gov/Nchs/Nhanes/continuousnhanes.
Statistical Analyses
Concentrations and distributions of Klotho were collected and assessed. A Shapiro-Wilk statistical test was used to confirm whether or not continuous variables have a normal distribution. We used natural logarithmic transformation (ln) to create a normal distribution given that the serum Klotho levels had an asymmetry distribution to the right. Continuous variables with normal distribution were expressed as mean ± SD and contrasted through a one-way analysis of variance. The median (interquartile range) presented a skewed distribution of continuous data, and the Kruskal-Wallis H test was employed to evaluate the results. Variables that were categorical or dichotomous were presented as absolute values (percentages) and contrasted using χ2 statistics. In addition, given that the percentage of missing data was small (the missing rate varied from 0% to 6.19%) for any variable, no imputation method was used.
We calculated odds ratios (ORs) and 95% confidence intervals (CIs) through logistic regression to assess the prevalence of KS associated with serum Klotho. Participants were divided into five groups based on Klotho quintile amounts: Q1 (181.7–619.9 pg/mL; n = 708), Q2 (620.0–742.3 pg/mL; n = 705), Q3 (743.5–867.0 pg/mL; n = 709), Q4 (867.4–1,053.0 pg/mL; n = 707), Q5 (1,054.0–5,038.3 pg/mL; n = 708), and the lowest quintile served as the reference group. We also conducted a logistic analysis and used Klotho as continuous and categorical variables. Model 1 was adjusted for sociodemographic variables (sex, age, race and ethnicity, educational level, and marital status). Model 2 was further adjusted for physical activity, smoking status, and alcohol drinking status. Model 3 was further adjusted for hypertension, CKD, BMI, and HbA1c. Model 4 was further adjusted for albumin, triglycerides, total cholesterol, HDL, BUN, and uric acid. Model 5 was fully adjusted, similar to model 4, and additional adjustments for calcium, phosphorus, and vitamin D. We employed restricted cubic splines to test for linearity to further investigate the shape of the dose-response relationship between Klotho levels and KS incidence. The smooth curve fitting graph was established and adjusted based on the covariables contained in model 5. Four knots (at the 5th, 35th, 65th, and 95th percentiles) of Klotho level distribution were used. Moreover, logistic regression models were used to conduct interaction and subgroup analyses based on age, sex, race and ethnicity, educational level, marital status, physical activity, hypertension, CKD, smoking status, alcohol drinking status, BMI, and HbA1c.
Given that the sample size was determined solely based on the data provided, no a priori estimate of statistical power was made. All analyses were performed using the statistical software packages R 4.2.2 and Free Statistics software version 1.7 [26]. A descriptive study was conducted on all participants. A p value of <0.05 indicated significance by two-tailed testing.
Results
Participants and Demographic Characteristics
A total of 3,537 diabetic patients with available data on serum Klotho and KS were included in the analysis, and 543 participants (15.4%) suffered from KS. The median Klotho concentration was 804.5 pg/mL (Q1 = 645.7 and Q3 = 1,013.0). Table 1 presents the clinical and biochemical features of the study population based on Klotho levels. Participants’ mean age ± SD was 61.4 ± 10.1 years, 1,814 (51.3%) were men, and most of them were self-reported as non-Hispanic white (1,199, 33.9%). At baseline, participants with higher Klotho concentration had a higher HbA1c and total cholesterol, lower uric acid, serum phosphorus, vitamin D, and BUN levels and were more likely to have never smoked, not drunk alcohol, and not have CKD.
Table 1.
Baseline characteristics of the study participants
| Variables | Total (n = 3,537) | Klotho levels quartiles (pg/mL) | p value | ||||
|---|---|---|---|---|---|---|---|
| Q1 (n = 708) | Q2 (n = 705) | Q3 (n = 709) | Q4 (n = 707) | Q5 (n = 708) | |||
| Age, years | 61.4±10.1 | 62.8±10.2 | 62.2±10.0 | 61.7±10.1 | 60.6±9.8 | 59.5±10.0 | <0.001 |
| Sex, n (%) | 0.693 | ||||||
| Male | 1,814 (51.3) | 366 (51.7) | 375 (53.2) | 367 (51.8) | 353 (49.9) | 353 (49.9) | |
| Female | 1,723 (48.7) | 342 (48.3) | 330 (46.8) | 342 (48.2) | 354 (50.1) | 355 (50.1) | |
| Race and ethnicity, n (%) | <0.001 | ||||||
| Mexican American | 715 (20.2) | 137 (19.4) | 144 (20.4) | 131 (18.5) | 159 (22.5) | 144 (20.3) | |
| Other Hispanic | 458 (12.9) | 77 (10.9) | 73 (10.4) | 103 (14.5) | 95 (13.4) | 110 (15.5) | |
| Non-Hispanic white | 1,199 (33.9) | 246 (34.7) | 286 (40.6) | 247 (34.8) | 234 (33.1) | 186 (26.3) | |
| Non-Hispanic black | 835 (23.6) | 182 (25.7) | 137 (19.4) | 157 (22.1) | 149 (21.1) | 210 (29.7) | |
| Other race | 330 (9.3) | 66 (9.3) | 65 (9.2) | 71 (10.0) | 70 (9.9) | 58 (8.2) | |
| Educational level, n (%) | 0.642 | ||||||
| High school or less | 2,096 (59.3) | 435 (61.6) | 426 (60.4) | 409 (57.9) | 420 (59.4) | 406 (57.3) | |
| Some college | 901 (25.5) | 164 (23.2) | 179 (25.4) | 183 (25.9) | 189 (26.7) | 186 (26.3) | |
| College or above | 536 (15.2) | 107 (15.2) | 100 (14.2) | 115 (16.3) | 98 (13.9) | 116 (16.4) | |
| Marital status, n (%) | 0.449 | ||||||
| Married | 2,044 (57.8) | 389 (54.9) | 434 (61.6) | 413 (58.3) | 412 (58.3) | 396 (55.9) | |
| Never married | 303 (8.6) | 57 (8.1) | 56 (8.0) | 57 (8.1) | 61 (8.6) | 72 (10.2) | |
| Living with a partner | 137 (3.9) | 31 (4.4) | 27 (3.8) | 23 (3.2) | 27 (3.8) | 29 (4.1) | |
| Other | 1,051 (29.7) | 231 (32.6) | 187 (26.6) | 215 (30.4) | 207 (29.3) | 211 (29.8) | |
| Physical activity, n (%) | 0.130 | ||||||
| Sedentary | 2,362 (66.8) | 494 (69.8) | 460 (65.2) | 466 (65.8) | 460 (65.1) | 482 (68.1) | |
| Moderate | 918 (26.0) | 167 (23.6) | 192 (27.2) | 203 (28.7) | 186 (26.3) | 170 (24.0) | |
| Vigorous | 256 (7.2) | 47 (6.6) | 53 (7.5) | 39 (5.5) | 61 (8.6) | 56 (7.9) | |
| HbA1c, n (%) | <0.001 | ||||||
| <7.0 | 2,025 (57.4) | 499 (65.8) | 401 (59.4) | 362 (56.7) | 387 (55.8) | 376 (49.3) | |
| ≥7.0 | 1,502 (42.6) | 259 (34.2) | 274 (40.6) | 277 (43.3) | 306 (44.2) | 386 (50.7) | |
| BMI, n (%) | 0.495 | ||||||
| <25.0 kg/m2 | 425 (12.3) | 95 (12.9) | 75 (11.4) | 78 (12.5) | 74 (10.8) | 103 (13.6) | |
| ≥25.0 kg/m2 | 3,037 (87.7) | 644 (87.1) | 582 (88.6) | 548 (87.5) | 611 (89.2) | 652 (86.4) | |
| Smoking status, n (%) | 0.008 | ||||||
| Never | 1,708 (48.3) | 320 (45.3) | 307 (43.5) | 350 (49.4) | 372 (52.7) | 359 (50.7) | |
| Former | 1,218 (34.5) | 255 (36.1) | 280 (39.7) | 233 (32.9) | 226 (32.0) | 224 (31.6) | |
| Current | 608 (17.2) | 131 (18.6) | 118 (16.7) | 126 (17.8) | 108 (15.3) | 125 (17.7) | |
| Alcohol drinker, n (%) | 2,146 (64.7) | 466 (69.8) | 437 (65.7) | 408 (61.4) | 421 (63.6) | 414 (62.8) | 0.016 |
| Hypertension, n (%) | 2,592 (73.3) | 579 (76.2) | 509 (75.1) | 468 (73.1) | 498 (71.7) | 538 (70.4) | 0.074 |
| CKD, n (%) | 614 (17.4) | 202 (28.6) | 146 (20.7) | 109 (15.4) | 75 (10.6) | 82 (11.6) | <0.001 |
| KS, n (%) | 543 (15.4) | 127 (17.9) | 96 (13.6) | 124 (17.5) | 100 (14.1) | 96 (13.6) | 0.035 |
| Uric acid, μmol/L | 343.9±93.3 | 364.2±103.2 | 354.1±91.6 | 343.9±92.1 | 334.7±83.1 | 322.6±90.1 | <0.001 |
| Albumin, g/dL | 4.2±0.3 | 4.1±0.4 | 4.2±0.3 | 4.2±0.3 | 4.2±0.3 | 4.1±0.4 | 0.005 |
| HDL, mg/dL | 48.2±15.2 | 49.3±16.9 | 47.2±13.9 | 48.7±15.8 | 47.6±13.4 | 48.3±15.6 | 0.064 |
| Total cholesterol, mg/dL | 187.3±46.9 | 183.0±48.4 | 184.2±44.8 | 186.6±45.1 | 188.3±42.3 | 194.2±52.3 | <0.001 |
| Calcium, mg/dL | 9.4±0.4 | 9.4±0.4 | 9.4±0.4 | 9.4±0.4 | 9.4±0.4 | 9.5±0.4 | 0.206 |
| Phosphorus, mg/dL | 3.7±0.6 | 3.8±0.7 | 3.7±0.6 | 3.7±0.6 | 3.7±0.5 | 3.7±0.6 | 0.008 |
| Vitamin D, nmol/La | 55.0 (37.6, 73.0) | 57.2 (37.4, 77.2) | 58.6 (41.1, 76.5) | 53.6 (38.0, 70.8) | 52.9 (37.2, 71.1) | 51.9 (35.8, 68.1) | <0.001 |
| BUN, mg/dLa | 14.0 (11.0, 18.0) | 16.0 (12.0, 21.0) | 15.0 (12.0, 19.0) | 14.0 (11.0, 19.0) | 13.5 (11.0, 17.0) | 13.0 (10.0, 16.0) | <0.001 |
| Triglycerides, mg/dLa | 155.0 (104.0, 236.0) | 153.0 (104.8, 225.2) | 155.0 (107.8, 237.0) | 152.0 (102.0, 233.0) | 155.5 (109.0, 239.8) | 159.0 (99.0, 246.5) | 0.638 |
CKD, chronic kidney disease; BMI, body mass index; HDL, high-density lipoprotein; HbA1c, hemoglobin A1c; BUN, blood urea nitrogen.
aVitamin D, BUN, and triglycerides expressed as median (IQR) and rest are expressed as mean ± SD.
Associations between Serum Klotho and KSs
Findings from the multivariable logistic proportional hazards’ regression for the association between serum Klotho levels and the incidence of KS are shown in Table 2. When Klotho was analyzed as a continuous variable, a significant independent negative association was discovered between serum Klotho and the risk of KS in the non-adjusted crude model (OR: 0.73, 95% CI: 0.56–0.94; p = 0.017); meanwhile, further adjustment did not significantly affect the results. With increased quintiles of Klotho levels, the incidence of KS decreased, and the OR of quintile 5 was lower than that of quintile 1 (OR: 0.72, 95% CI: 0.54–0.96). After adjusting for sociodemographic variables (sex, age, race and ethnicity, educational level, and marital status), physical activity, smoking status, alcohol drinking status, hypertension, CKD, BMI, and HbA1c in model 3, the association between Klotho and KS was marginally significant (OR: 0.76, 95% CI: 0.57–1.01; p = 0.058). Nevertheless, the negative correlation between serum Klotho and KS is in a reasonable direction despite not being statistically significant. Model 5 was further adjusted for albumin, triglycerides, total cholesterol, HDL, BUN, uric acid, calcium, phosphorus, and vitamin D. In the fully adjusted model 5, when ln-transformed Klotho concentration increased by 1 unit, the risk of KS was 28% lower (model 5, OR = 0.72; 95% CI: 0.54–0.96; p = 0.027). Restricted cubic spline of the association between ln-transformed Klotho and KS is shown in online supplementary material (for all online suppl. material, see https://doi.org/10.1159/000531045). Serum Klotho levels and the incidence of KS had a negative association when all potential confounders were taken into account (nonlinearity: p = 0.560).
Table 2.
Associations between serum Klotho protein and KS in the multiple regression model
| Variable | ln Klotho (n = 3,537) | Klotho levels quintiles (pg/mL) | |||||
|---|---|---|---|---|---|---|---|
| Q1 (n = 708) | Q2 (n = 705) | Q3 (n = 709) | Q4 (n = 707) | Q5 (n = 708) | |||
| OR (95% CI) | p value | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Unadjusted | 0.73 (0.56–0.94) | 0.017 | 1.00 (ref) | 0.72 (0.54–0.96) | 0.97 (0.74–1.27) | 0.75 (0.57–1.00) | 0.72 (0.54–0.96) |
| Model 1 | 0.76 (0.58–1.00) | 0.050 | 1.00 (ref) | 0.68 (0.51–0.91) | 0.95 (0.72–1.25) | 0.74 (0.56–0.99) | 0.75 (0.56–1.01) |
| Model 2 | 0.76 (0.57–1.00) | 0.048 | 1.00 (ref) | 0.76 (0.56–1.02) | 0.94 (0.71–1.26) | 0.76 (0.57–1.03) | 0.79 (0.59–1.06) |
| Model 3 | 0.76 (0.57–1.01) | 0.058 | 1.00 (ref) | 0.79 (0.59–1.06) | 0.96 (0.71–1.29) | 0.78 (0.58–1.05) | 0.80 (0.59–1.07) |
| Model 4 | 0.75 (0.57–1.00) | 0.049 | 1.00 (ref) | 0.78 (0.58–1.06) | 0.98 (0.72–1.31) | 0.78 (0.58–1.06) | 0.79 (0.58–1.06) |
| Model 5 | 0.72 (0.54–0.96) | 0.027 | 1.00 (ref) | 0.79 (0.58–1.06) | 0.95 (0.70–1.28) | 0.77 (0.57–1.05) | 0.76 (0.56–1.03) |
Model 1: adjusted for sociodemographic variables (sex, age, race and ethnicity, educational level, and marital status).
Model 2: adjusted for model 1 + physical activity, smoking status, and alcohol drinking status.
Model 3: adjusted for model 2 + hypertension, CKD, BMI, and HbA1c.
Model 4: adjusted for model 3 + albumin, triglycerides, total cholesterol, HDL, BUN, and uric acid.
Model 5: adjusted for model 4 + calcium, phosphorus, and vitamin D.
OR, odds ratio; 95% CI, 95% confidence interval.
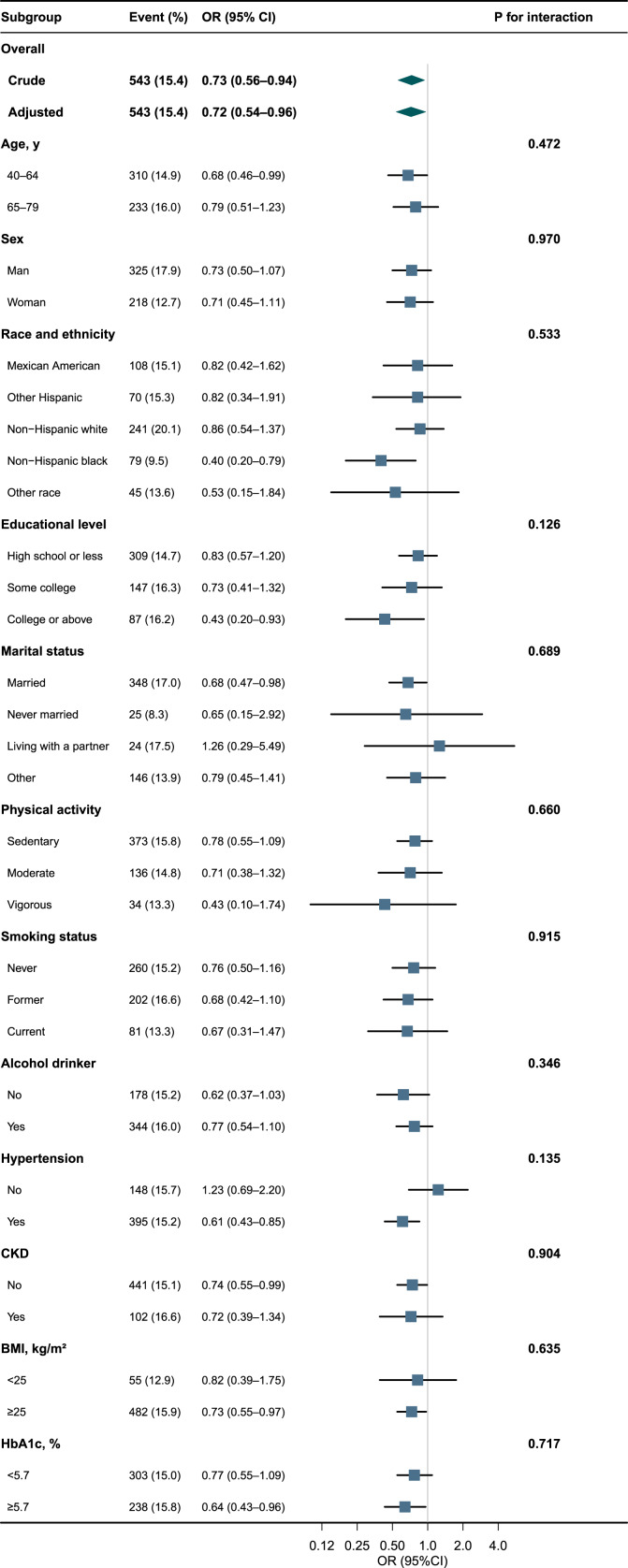
Subgroup Analyses
Herein, we conducted stratified and interaction analyses to ascertain if the association between serum Klotho protein and KS incidence was consistent across several subgroups (Fig. 2). Consistent results were observed when the analysis was stratified by age, sex, race and ethnicity, educational level, marital status, physical activity, smoking status, alcohol drinking status, hypertension, CKD, BMI, and HbA1c. We stratified the study population based on HbA1c levels using a cutoff point of 5.7% (which was chosen as close to the median HbA1c value in the entire sample) to evaluate the contribution of glycemic control. As shown in Figure 2, Klotho was associated with KS among participants with better glycemic control (OR: 0.64, 95% CI: 0.43–0.96), overweight or obese (OR: 0.73, 95% CI: 0.55–0.97), non-CKD (OR: 0.74, 95% CI: 0.55–0.99), hypertension (OR: 0.61, 95% CI: 0.43–0.85), married (OR: 0.68, 95% CI: 0.47–0.98), with college or above educational level (OR: 0.43, 95% CI: 0.20–0.93), and non-Hispanic black (OR: 0.40, 95% CI: 0.20–0.79). However, we did not find statistically significant interactions in stratified analyses for the investigation of effect modification.
Fig. 2.
Associations between serum Klotho and KSs in different subgroups. Except for the stratification component itself, each stratification factor was adjusted for sex, age, race and ethnicity, educational level, marital status, physical activity, smoking status, alcohol drinking status, hypertension, CKD, BMI, HbA1c, albumin, triglycerides, total cholesterol, HDL, BUN, uric acid, calcium, phosphorus, and vitamin D. OR, odds ratio; 95% CI, 95% confidence interval
Discussion
This nationally representative cross-sectional study of 3,537 diabetic patients from the 2007–2016 NHANES evaluates the association between serum Klotho levels and the incidence of KS. Based on our knowledge, this is the first study to investigate the relationship between serum Klotho levels and the prevalence of KS in a significant, unselected sample of patients with DM recruited from the general population. After adjustment for potential confounders, elevated serum Klotho counts were independently associated with lower odds of KS in the diabetic population, and the association was independent of glycemic control and CKD. Some differences in the association between Klotho and KS were found in stratified analyses; however, these differences were not statistically significant.
One prospective study of participants with primary hyperoxaluria type 1 found that those with KS had lower levels of Klotho than individuals without nephrocalcinosis and CaOx KS [27]. Klotho gene G395A single-nucleotide polymorphisms were linked to the formation of renal stones among North Indian patients with KS [28]. Moreover, a meta-analysis involving five distinct research studies revealed that Klotho prevented the development of urinary stones [20]. All studies were conducted in Asian countries, and only a small number focused on the US population, which lessens their external validity. Our study, which focused on patients from the US population and used estimations from a nationally representative sample, indicated that serum Klotho was independently associated with a decreased risk of KS, which was consistent with prior research’s credible findings. Conversely, a recent case-control study by Litvinova et al. [29] that included 50 calcium urolithiasis patients found no statistically significant correlation between the Klotho gene and calcium urolithiasis in the Russian population. The disparities in results that were obtained may be due to the disparate baseline features of the research populations, thereby presuming that the limited sample size was sufficient.
Klotho proteins may be involved in the pathogenic process of calcium KS. Salloum et al. [30] reported that a deficiency of Klotho protein causes phenotypes in the 129S1/SvlmJ mouse strain, which appeared as increased renal calcification. Meanwhile, according to Frick et al. [31], Klotho was decreased in genetic hypercalciuric stone-forming rats. When 1,25(OH)2D3 (1,25D) is administered, Klotho is inhibited in the genetic hypercalciuric stone-forming rats based on the RNA expression of the kidney calcium transfer components. Klotho acted as an activator of the kidney calcium reabsorption channel TRPV5, and its fall would reduce the tubular reabsorption of the 1,25D-induced release of calcium from the bones [32]. In addition, Zhu et al. [17] reported that Randall’s plaques had Klotho deficit as one pathological feature. Klotho secreted from HK-2 cells prevented the osteogenic development of human renal interstitial fibroblasts by deactivating the Wnt-β-catenin pathway. Ahmatjan et al. [18] established an in vitro CaOx KS model and demonstrated that Klotho protein inhibits HKC cells’ response to oxidative stress via the Keap1-Nrf2-ARE signaling pathway, reduces CaOx crystal adhesion and apoptosis, and lowers the incidence of KS.
Moreover, Klotho is crucial for maintaining the balance of calcium and phosphorus, and it reduces the production of calcitriol by blocking 1α-hydroxylase activity [11, 33]. Klotho may participate in systemic calcium homeostasis through its triple action on the kidneys, gut, and bone and prevents KS from developing. Body calcium balance is carefully regulated, and tiny modifications in renal calcium reabsorption might cause excessive urine calcium excretion and encourage KS development. The epithelium calcium channel TRPV5 is the rate-limiting stage of active calcium reabsorption in the kidney. The external sugar residues on TRPV5 are hydrolyzed by Klotho, which traps the channel in the plasma membrane. This keeps the kidney’s calcium permeability and long-lasting calcium channel function intact. Furthermore, Klotho opens a cell surface channel through the hydrolysis of the extracellular N-linked oligosaccharides. The active calcium reabsorption modifies the total calcium excretion into urine, which affects the development of KS [34]. About 20% of the calcium that individuals consume is absorbed through their net intestinal absorption. Calcitriol, which increases calcium transport via both genomic and non-genomic pathways, is primarily responsible for controlling the intestinal calcium absorption capacity [35]. Klotho modulates the level of serum calcitriol by regulating the renal expression of important enzymes involved in the metabolism of vitamin D. In addition, as the co-receptor for the circulating hormone FGF23, Klotho stimulates osteoblastic cell proliferation and inhibits bone mineralization, which promotes the mobilization of calcium and phosphate [11, 36]. Each hormone may not be sufficient to determine biological and clinical results alone because of the complexity of the homeostasis mechanisms of endocrine systems. Besides, Klotho has recently been demonstrated that it is inversely correlated with uric acid [37]. Since elevated uric acid has also been linked to an increase in the risk of calcium and uric acid KS, it suggests another possible mechanism of Klotho in reducing the incidence of KS [38]. However, the physiology of renal stones in DM patients has not been fully understood; Klotho protein may be involved in the pathogenic mechanism, and further research will be necessary to identify the primary mechanism.
To our knowledge, it is the largest study to date investigating the association between serum Klotho concentrations and incidence of KS performed in an unselected sample of middle-aged and older US diabetic individuals, thereby encompassing both sexes and participants from various ethnic backgrounds. Most previous studies conducted in Asian countries indicated that Klotho has a protective effect on the formation of KS, which is consistent with our findings. Our study focused on the participants from the US nationally representative estimated population and may complement previous works. Nevertheless, previous studies did not look into the association and interaction between serum Klotho levels and KS in various subgroups. Our subgroup analysis showed that patients without CKD and with better glycemic control had a lower risk of KS. Nonetheless, this finding should be cautiously interpreted given the limited sample size of individuals in our investigation, and further well-designed prospective research in this field is required.
However, the present study has some limitations, which need to be considered. First, we were unable to infer causality from the results because of the cross-sectional nature of our analysis [39]. Given the complexity of this correlation, additional longitudinal studies and investigations into the interactions between serum Klotho and KS at the tissue level may shed light on Klotho’s potential to forecast clinically relevant patient outcomes. Second, the accuracy of the commercially available ELISA kit to quantify Klotho has also come under scrutiny [22]. Although the assay used in the present investigation would be less specific than labor-intensive immunoprecipitant immunological blot assay techniques, commercially available ELISA kits would be ideal for extensive epidemiological research [40]. Finally, the association between Klotho and KS may also be altered by additional potentially confounding variables. Therefore, future multicenter cohort studies will be needed to confirm these findings by including more possible confounders and using standardized and consistent measures.
Conclusion
In this large population-based cross-sectional study including 3,537 US diabetic patients aged 40–79 years from 2007 to 2016 NHANES, serum Klotho was negatively associated with the risk of KS. Serum Klotho and the incidence of KS had a negative association; the risk of KS decreased by 28% with each unit elevation in ln-transformed Klotho concentration.
Acknowledgments
We gratefully thank Jie Liu, PhD (Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital), for his helpful review and comments regarding the manuscript. We also appreciate Dr. Lijie Gao, PhD (Department of Rehabilitation, Southern Medical University of Zhujiang Hospital), for providing assistance in revising this manuscript.
Statement of Ethics
The NCHS Research Ethics Review Board approved the NHANES study protocol, and participants provided written informed consent at enrollment (the website is https://www.cdc.gov/nchs/nhanes/irba98.htm). Ethical approval and consent were not required as this study was based on publicly available de-identified data.
Conflict of Interest Statement
The remaining authors declare that they have no conflict of interest.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Yuxiuzi Xiao: conceptualization, formal analysis, and writing – original draft. Zuomiao Xiao: writing – review and editing – and project administration. All authors contributed to and approved the final manuscript.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability Statement
NHANES data used in this work are publicly available. All raw data are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/). Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Tang J, Mettler P, McFann K, Chonchol M. The association of prevalent kidney stone disease with mortality in US adults: the National Health and Nutrition Examination Survey III, 1988–1994. Am J Nephrol. 2013;37(5):501–6. 10.1159/000350691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abufaraj M, Xu T, Cao C, Waldhoer T, Seitz C, D’Andrea D, et al. Prevalence and trends in kidney stone among adults in the USA: analyses of national health and nutrition examination survey 2007–2018 data. Eur Urol Focus. 2021 Nov;7(6):1468–75. 10.1016/j.euf.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 3. Finkielstein VA, Goldfarb DS. Strategies for preventing calcium oxalate stones. CMAJ. 2006 May 9;174(10):1407–9. 10.1503/cmaj.051517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khan SR, Pearle MS, Robertson WG, Gambaro G, Canales BK, Doizi S, et al. Kidney stones. Nat Rev Dis Primers. 2016 Feb 25;2(1):16008. 10.1038/nrdp.2016.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wigner P, Grębowski R, Bijak M, Szemraj J, Saluk-Bijak J. The molecular aspect of nephrolithiasis development. Cells. 2021 Jul 29;10(8):1926. 10.3390/cells10081926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin BB, Huang RH, Lin BL, Hong YK, Lin ME, He XJ. Associations between nephrolithiasis and diabetes mellitus, hypertension and gallstones: a meta-analysis of cohort studies. Nephrology. 2020 Sep;25(9):691–9. 10.1111/nep.13740. [DOI] [PubMed] [Google Scholar]
- 7. Mao Y, Hu W, Liu L, Liu Q. Association between gestational diabetes mellitus and future risk of kidney stones. Front Public Health. 2022;10:843383. 10.3389/fpubh.2022.843383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donate-Correa J, Martín-Núñez E, Delgado NP, de Fuentes MM, Arduan AO, Mora-Fernández C, et al. Implications of Fibroblast growth factor/Klotho system in glucose metabolism and diabetes. Cytokine Growth Factor Rev. 2016 Apr;28:71–7. 10.1016/j.cytogfr.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 9. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997 Nov 6;390(6655):45–51. 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 10. Lim K, Groen A, Molostvov G, Lu T, Lilley KS, Snead D, et al. α-Klotho expression in human tissues. J Clin Endocrinol Metab. 2015 Oct;100(10):E1308–18. 10.1210/jc.2015-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Neyra JA, Hu MC, Moe OW. Klotho in clinical nephrology: diagnostic and therapeutic implications. Clin J Am Soc Nephrol. 2020 Dec 31;16(1):162–76. 10.2215/CJN.02840320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kale A, Shelke V, Sankrityayan H, Dagar N, Gaikwad AB. Klotho restoration via ACE2 activation: a potential therapeutic strategy against acute kidney injury-diabetes comorbidity. Biochim Biophys Acta Mol Basis Dis. 2022 Aug 28;1868(12):166532. 10.1016/j.bbadis.2022.166532. [DOI] [PubMed] [Google Scholar]
- 13. Kresovich JK, Bulka CM. Low serum klotho associated with all-cause mortality among a nationally representative sample of American adults. J Gerontol A Biol Sci Med Sci. 2022 Mar 3;77(3):452–6. 10.1093/gerona/glab308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang K, Mao Y, Lu M, Liu X, Sun Y, Li Z, et al. Association between serum Klotho levels and the prevalence of diabetes among adults in the United States. Front Endocrinol. 2022;13:1005553. 10.3389/fendo.2022.1005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Landry T, Shookster D, Huang H. Circulating α-klotho regulates metabolism via distinct central and peripheral mechanisms. Metabolism. 2021 Aug;121:154819. 10.1016/j.metabol.2021.154819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hwang HJ, Kim N, Herman AB, Gorospe M, Lee JS. Factors and pathways modulating endothelial cell senescence in vascular aging. Int J Mol Sci. 2022 Sep 4;23(17):10135. 10.3390/ijms231710135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu Z, Ruan S, Jiang Y, Huang F, Xia W, Chen J, et al. α-Klotho released from HK-2 cells inhibits osteogenic differentiation of renal interstitial fibroblasts by inactivating the Wnt-β-catenin pathway. Cell Mol Life Sci. 2021 Dec;78(23):7831–49. 10.1007/s00018-021-03972-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahmatjan B, Ruotian L, Rahman A, Bin M, Heng D, Yi H, et al. Klotho inhibits the formation of calcium oxalate stones by regulating the Keap1-Nrf2-ARE signaling pathway. Int Urol Nephrol. 2023;55(2):263–76. 10.1007/s11255-022-03398-9. [DOI] [PubMed] [Google Scholar]
- 19. Lanka P, Devana SK, Singh SK, Sapehia D, Kaur J. Klotho gene polymorphism in renal stone formers from Northwestern India. Urolithiasis. 2021 Jun;49(3):195–9. 10.1007/s00240-020-01226-2. [DOI] [PubMed] [Google Scholar]
- 20. Mohammadi A, Shabestari AN, Baghdadabad LZ, Khatami F, Reis LO, Pishkuhi MA, et al. Genetic polymorphisms and kidney stones around the globe: a systematic review and meta-analysis. Front Genet. 2022;13:913908. 10.3389/fgene.2022.913908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association . 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2021. Diabetes Care. 2021 Jan;44(Suppl 1):S15–33. 10.2337/dc21-S002. [DOI] [PubMed] [Google Scholar]
- 22. Pedersen L, Pedersen SM, Brasen CL, Rasmussen LM. Soluble serum Klotho levels in healthy subjects. Comparison of two different immunoassays. Clin Biochem. 2013 Aug;46(12):1079–83. 10.1016/j.clinbiochem.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 23. Weinberg AE, Patel CJ, Chertow GM, Leppert JT. Diabetic severity and risk of kidney stone disease. Eur Urol. 2014 Jan;65(1):242–7. 10.1016/j.eururo.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elliott WJ. Systemic hypertension. Curr Probl Cardiol. 2007 Apr;32(4):201–59. 10.1016/j.cpcardiol.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 25. Kidney Disease: Improving Global Outcomes KDIGO Glomerular Diseases Work Group . KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021 Oct;100(4s):S1–276. 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 26. Ruan Z, Lu T, Chen Y, Yuan M, Yu H, Liu R, et al. Association between psoriasis and nonalcoholic fatty liver disease among outpatient US adults. JAMA Dermatol. 2022 Jul 1;158(7):745–53. 10.1001/jamadermatol.2022.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jayachandran M, Yuzhakov SV, Kumar S, Larson NB, Enders FT, Milliner DS, et al. Specific populations of urinary extracellular vesicles and proteins differentiate type 1 primary hyperoxaluria patients without and with nephrocalcinosis or kidney stones. Orphanet J Rare Dis. 2020 Nov 11;15(1):319. 10.1186/s13023-020-01607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aziz MS, Aamir AU, Khan A, Khan Z, Shah SQ, Safi SZ, et al. Investigation of klotho G395A and C1818T polymorphisms and their association with serum glucose level and risk of type 2 diabetes mellitus. Genes. 2022 Aug 26;13(9):1532. 10.3390/genes13091532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Litvinova MM, Khafizov K, Korchagin VI, Speranskaya AS, Asanov AY, Matsvay AD, et al. Association of CASR, CALCR, and ORAI1 genes polymorphisms with the calcium urolithiasis development in Russian population. Front Genet. 2021;12:621049. 10.3389/fgene.2021.621049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Salloum JS, Garsetti DE, Rogers MB. Genetic background influences the impact of KLOTHO deficiency. Physiol Genomics. 2020 Oct 1;52(10):512–6. 10.1152/physiolgenomics.00094.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Frick KK, Asplin JR, Favus MJ, Culbertson C, Krieger NS, Bushinsky DA. Increased biological response to 1,25(OH)(2)D(3) in genetic hypercalciuric stone-forming rats. Am J Physiol Ren Physiol. 2013 Mar 15;304(6):F718–26. 10.1152/ajprenal.00645.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frick KK, Asplin JR, Krieger NS, Culbertson CD, Asplin DM, Bushinsky DA. 1,25(OH)2D3-enhanced hypercalciuria in genetic hypercalciuric stone-forming rats fed a low-calcium diet. Am J Physiol Ren Physiol. 2013 Oct 15;305(8):F1132–8. 10.1152/ajprenal.00296.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rodríguez M. FGF23: is it another biomarker for phosphate-calcium metabolism? Adv Ther. 2020 May;37(Suppl 2):73–9. 10.1007/s12325-019-01181-4. [DOI] [PubMed] [Google Scholar]
- 34. de Groot T, Bindels RJ, Hoenderop JG. TRPV5: an ingeniously controlled calcium channel. Kidney Int. 2008 Nov;74(10):1241–6. 10.1038/ki.2008.320. [DOI] [PubMed] [Google Scholar]
- 35. Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol Rev. 2016 Jan;96(1):365–408. 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hu L, Napoletano A, Provenzano M, Garofalo C, Bini C, Comai G, et al. Mineral bone disorders in kidney disease patients: the ever-current to pic. Int J Mol Sci. 2022/10/13/;23(20):12223. 10.3390/ijms232012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee HJ, Choi JY, Lee J, Kim D, Min JY, Min KB. Association between serum uric acid and α-klotho protein levels in the middle-aged population. Aging. 2022 Mar 29;14(6):2537–47. 10.18632/aging.203987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramos GK, Goldfarb DS. Update on uric acid and the kidney. Curr Rheumatol Rep. 2022;24(5):132–8. 10.1007/s11926-022-01069-3. [DOI] [PubMed] [Google Scholar]
- 39. Wang X, Cheng Z. Cross-sectional studies: strengths, weaknesses, and recommendations. Chest. 2020 Jul;158(1s):S65–s71. 10.1016/j.chest.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 40. Barker SL, Pastor J, Carranza D, Quiñones H, Griffith C, Goetz R, et al. The demonstration of αKlotho deficiency in human chronic kidney disease with a novel synthetic antibody. Nephrol Dial Transplant. 2015;30(2):223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
NHANES data used in this work are publicly available. All raw data are available on the NHANES website (https://www.cdc.gov/nchs/nhanes/). Further inquiries can be directed to the corresponding author.