Abstract
The cyclic β-(1,2)-glucans of Rhizobium meliloti and Agrobacterium tumefaciens play an important role during hypoosmotic adaptation, and the synthesis of these compounds is osmoregulated. Glucosyltransferase, the enzyme responsible for cyclic β-(1,2)-glucan biosynthesis, is present constitutively, suggesting that osmotic regulation of the biosynthesis of these glucans occurs through modulation of enzyme activity. In this study, we examined regulation of cyclic glucan biosynthesis in vitro with membrane preparations from R. meliloti. The results show that ionic solutes inhibit glucan synthesis, even when they are present at low concentrations (e.g., 10 mM). In contrast, neutral solutes (glucose, sucrose, and the compatible solutes glycine betaine and trehalose) were found to stimulate glucan synthesis in vitro when they were present at high concentrations (e.g., 1 M). Furthermore, high concentrations of these neutral solutes were shown to compensate for the inhibition of glucosyltransferase activity by ionic solutes. Consistent with their ionic character, the compatible solute potassium glutamate and the osmoprotectant choline chloride inhibited glucosyltransferase activity in vitro. The results suggest that intracellular ion concentrations, intracellular osmolarity, and intracellular concentrations of nonionic compatible solutes all act as important determinants of glucosyltransferase activity in vivo. Additional experiments were performed with an ndvA mutant defective for transport of cyclic glucans and an ndvB mutant that produces a C-terminal truncated glucosyltransferase. Cyclic β-(1,2)-glucan biosynthesis, although reduced, was found to be osmoregulated in both mutants. These results reveal that NdvA and the C terminus of NdvB are not required for osmotic regulation of cyclic β-(1,2)-glucan biosynthesis.
The cyclic β-(1,2)-glucans of Rhizobium meliloti and Agrobacterium tumefaciens are generally believed to play an important role during hypoosmotic adaptation. Consistent with this role, the synthesis of these molecules is osmoregulated, and the highest levels are produced during growth under low-osmolarity conditions (5, 17, 31, 42). These molecules are localized to the periplasm (5, 31, 41), where they appear to be predominant osmotic solutes, and it is within the periplasmic compartment that they become highly modified with phosphoglycerol and/or succinyl substituents (4, 6). Anionic glucans are thought to be the most effective periplasmic solutes because their associated counterions also contribute to periplasmic osmolarity.
Two chromosomal loci, ndvA and ndvB in R. meliloti and chvA and chvB in A. tumefaciens, are involved in cyclic β-(1,2)-glucan biosynthesis and transport (5, 12, 25, 38, 43). The chvA and chvB genes are functional and structural homologs of the ndvA and ndvB genes, respectively (12, 18). Studies with ndv and chv mutants have provided the most direct evidence that periplasmic cyclic β-(1,2)-glucans do indeed function during hypoosmotic adaptation. While wild-type R. meliloti grows over a broad osmolarity range (0 to 650 mM NaCl) (8, 28), ndv mutants are specifically impaired for growth in hypoosmotic media, although their growth is restored to wild-type levels when solutes (e.g., 100 mM NaCl) are added to the growth medium (13, 17, 42).
The ndvB and chvB genes have each been shown to encode a high-molecular-weight cytoplasmic membrane protein (molecular weight, approximately 319,000) that is involved in the biosynthesis of the cyclic β-(1,2)-glucans from UDP-glucose (20, 25, 43, 45). A number of studies (5, 10, 40, 44–46) have revealed that this protein becomes covalently linked to the glucan backbone during biosynthesis. Formation of this protein-oligosaccharide intermediate has been demonstrated in A. tumefaciens (45), R. meliloti (25, 45, 46), Rhizobium fredii (2), Rhizobium loti (27), and Rhizobium leguminosarum (15). As expected, this intermediate cannot be detected in membrane preparations obtained from ndvB or chvB mutants that produce severely truncated glucosyltransferases, and cyclic β-(1,2)-glucan biosynthesis cannot be detected with membrane preparations derived from these mutants (20, 25, 43).
A recent study by Castro et al. (14) provided strong evidence that the ndvB- and chvB-encoded glucosyltransferase is responsible for all stages of neutral cyclic β-(1,2)-glucan backbone biosynthesis. In this study, it was demonstrated that solubilized A. tumefaciens inner membrane protein, separated on native protein gels, was able to form the 319-kDa protein-linked oligosaccharide intermediate in situ. Cyclic β-(1,2)-glucan was also formed when the gel portion containing the 319-kDa protein intermediate was incubated with UDP-[14C]glucose, demonstrating that all three enzymatic activities required for neutral cyclic β-(1,2)-glucan backbone synthesis (initiation, elongation, and cyclization) are associated with this protein.
The ndvA gene encodes a 67-kDa protein with homology to a number of bacterial ATP-binding transport proteins belonging to the ABC transport superfamily (38). The NdvA protein is thought to be responsible for transport of cyclic glucans, since ndvA mutants do not produce extracellular cyclic β-(1,2)-glucans and have less than 15% of the wild-type levels of neutral periplasmic cyclic glucans, even though the 319-kDa-protein-linked oligosaccharide intermediate is readily detected (38).
As stated above, the highest levels of cyclic β-(1,2)-glucan are synthesized when cells are grown in low-osmolarity media. Thus, it has been of interest to determine the basis for this osmoregulated biosynthesis. It has been shown that membrane preparations from cells grown under hyperosmotic conditions catalyze in vitro synthesis of neutral cyclic β-(1,2)-glucans from UDP-glucose at rates similar to the rates obtained with membrane preparations from cells grown under hypoosmotic conditions (42). In addition, NdvB (ChvB) levels are similar within cells grown under high- and low-osmotic-strength conditions (42), and the expression of ndvB (chvB) does not appear to be induced further when cells are grown under low-osmolarity conditions (17, 42). These results suggest that osmotic regulation of cyclic β-(1,2)-glucan synthesis does not occur at the level of transcription or translation. Instead, cyclic β-(1,2)-glucan synthesis may be regulated through modulation of enzyme activity. Zorreguieta et al. (42) proposed that elevated cytoplasmic ionic strength leads to inhibition of cyclic β-(1,2)-glucan biosynthesis. This was suggested by their results which showed that cyclic β-(1,2)-glucan synthesis in vitro is inhibited by the presence of high concentrations (0.1 to 0.4 M) of ionic solutes.
The purpose of the present study was to further explore the regulation of cyclic β-(1,2)-glucan biosynthesis in vitro. We examined the effects of a number of solutes on glucosyltransferase activity and found that ionic solutes inhibit glucan synthesis, even when they are present at low concentrations (e.g., 10 mM). Conversely, we found that high concentrations (e.g., 1 M) of neutral solutes are not inhibitory and instead stimulate glucan synthesis in vitro. Furthermore, we found that the presence of high concentrations of neutral solutes can compensate for the inhibition of glucosyltransferase activity caused by ionic solutes. Finally, we found that neither NdvA nor the C-terminal portion of NdvB is required for osmotic regulation of cyclic β-(1,2)-glucan biosynthesis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
R. meliloti 102F34 (17) was the wild-type strain used for all experiments. LI1 (18) is an ndvA::Tn5 mutant of 102F34, and TY24 (25) is an ndvB::Tn5 mutant of 102F34 that produces a truncated glucosyltransferase. The site of the Tn5 insertion in TY24 was determined by sequencing a PCR product containing the ndvB-Tn5 juncture. This region of the TY24 genome was amplified with a primer specific to the Tn5 inverted repeat and a primer corresponding to nucleotides 4146 to 4165 of the ndvB gene. The site of the Tn5 insertion was determined to be nucleotide 5421 of the ndvB gene. This insertion results in a truncated glucosyltransferase lacking the C-terminal 1,140 amino acids of NdvB, which represents 40% of the protein (data not shown).
Cultures used for membrane preparation were grown in YM medium (30) or GMS medium (8) at 30°C with aeration. Cultures used for isolation of cyclic β-(1,2)-glucans were grown in GMS medium. In order to examine the effects of growth in high-osmotic-strength medium, 0.4 M NaCl was added to basal GMS medium. In some experiments, the compatible solute glycine betaine was added to a final concentration of 10 mM. For growth of mutants, neomycin sulfate was added to a final concentration of 10 μg/ml. Growth of all cultures was monitored at 650 nm.
Preparation of the membrane fraction.
Cultures were harvested during the mid- to late logarithmic growth phase (A650, 0.5 to 0.7) by centrifugation at 10,000 × g for 10 min at 4°C. Cells were washed twice with YM salts (30), once with ice-cold buffer A [6 mM MgSO4, 5 mM 2-mercaptoethanol, 50 mM 3-(N-morpholino)propanesulfonic acid (pH 7.2), 1 mM dithiothreitol], and resuspended in buffer A to 1/150th of the original culture volume. The cell suspension was passaged twice through a French pressure cell at 6,000 to 8,000 lb/in2. The cell extract was centrifuged at 12,000 × g for 15 min to pellet the unbroken cells. The supernatant was then centrifuged at 100,000 × g for 90 min at 4°C to pellet the membrane fraction. The supernatant (nonmembrane fraction) was decanted, and the membrane pellet was resuspended in buffer A by homogenization in 1/1,500th of the original culture volume. Aliquots of the membrane fraction were stored at −20°C for up to 1 month. Each aliquot was thawed only once, just prior to use. The protein concentration of the membrane fraction was determined by a modification of the method of Lowry et al. (24).
Assay for glucosyltransferase activity.
The standard reaction mixture used for the glucosyltransferase activity assay contained 50 mM 3-(N-morpholino)propanesulfonic acid (pH 7.2), 10 mM MgSO4, 5 mM 2-mercaptoethanol, 0.8 mM UDP-[1-3H]glucose (2,400 cpm/nmol), and membrane fraction (40 μg of protein) in a total volume of 100 μl. After incubation at 37°C for 30 min (a period during which glucosyltransferase activity was linear), 0.6 ml of 40% (vol/vol) ethanol was added to stop the reaction. The mixture was vortexed and centrifuged at 12,500 × g for 5 min at room temperature. A 0.5-ml portion of the supernatant was applied to a 1-ml DEAE-cellulose column (type DE52; Whatman Inc., Clifton, N.J.) that previously had been equilibrated with 10 mM Tris-HCl (pH 7.4) containing 7% (vol/vol) 1-propanol. The column was washed with 1.5 ml of 25% (vol/vol) ethanol, and the flowthrough and wash volumes were combined as the neutral fraction (under the conditions used UDP-glucose was adsorbed onto the column). Since in vitro synthesis of anionic cyclic glucans has never been detected, only neutral products were examined. A 0.5-ml aliquot of the neutral fraction was counted in 5 ml of Ecoscint H scintillation solution (National Diagnostics, Atlanta, Ga.) with a model LS1701 liquid scintillation counter (Beckman Instruments, Fullerton, Calif.). In some cases, the remainder of the neutral product was passed over a Sephadex G-50 gel filtration column as described below to confirm that it eluted at the same position as the cyclic β-(1,2)-glucan standard. In each experiment assays were performed in duplicate. Activities were calculated as nanomoles of UDP-glucose converted to neutral product. The basal activity was the level of activity in the standard assay mixture described above with no additional salts or solutes.
Large-scale isolation of cell-associated cyclic β-(1,2)-glucans.
Cultures grown in 1 liter of GMS medium (both low and high osmolarity) to the mid-logarithmic phase (A650, 0.5 to 0.7) were harvested by centrifugation at 4°C for 10 min at 10,000 × g. The cell pellets were extracted with 30 ml of 70% (vol/vol) ethanol at 70°C for 30 min. After centrifugation, the supernatant was removed and concentrated under a vacuum. The concentrated supernatant was applied to a Sephadex G-50 gel filtration column (1 by 56 cm). The column was eluted at room temperature with 0.15 M ammonium acetate (pH 7.0) containing 7% 1-propanol at a flow rate of 15 ml/h. Fractions were assayed for total carbohydrate content by the phenol-sulfuric acid method (24).
Chemicals.
Most chemicals were purchased from Sigma Chemical Company (St. Louis, Mo.) or Fisher Scientific (Pittsburgh, Pa.). UDP-[1-3H]glucose was purchased from New England Nuclear (Boston, Mass.).
RESULTS
Glucosyltransferase activity is optimally stimulated by magnesium sulfate.
Although it has previously been shown that magnesium or manganese ions are required for glucosyltransferase activity during biosynthesis of cyclic β-(1,2)-glucans (44, 46), this requirement has not been carefully examined. Initially, we confirmed this requirement by demonstrating that in vitro glucosyltransferase activity was essentially undetectable (the level of activity was less than 10% of basal level of activity) when either magnesium was omitted from the assay mixture or EDTA (10 mM) was added to the assay mixture (data not shown). This requirement was further investigated by comparing the abilities of several magnesium and manganese salts to stimulate enzyme activity when they were present at a concentration of 10 mM in the reaction mixture. For these experiments, membranes were pelleted and washed before use to remove the magnesium sulfate present in the buffer used for membrane preparation and resuspension. While both magnesium and manganese were capable of stimulating activity, the level of glucosyltransferase activity was dependent on the salt used. Manganese salts were less effective than their magnesium counterparts at stimulating glucosyltransferase activity. Magnesium sulfate had the greatest stimulatory effect (1.31 nmol of UDP-glucose was incorporated into neutral product), while magnesium chloride, a compound used in other studies (44, 46), was the least effective magnesium salt for stimulating activity (0.80 nmol of UDP-glucose was incorporated into neutral product). Magnesium acetate had an intermediate effect (1.05 nmol of UDP-glucose was incorporated into neutral product). Additional experiments showed that a magnesium sulfate concentration between 10 and 20 mM was optimal (data not shown); therefore, 10 mM magnesium sulfate was used in all assays, and the level of activity observed with this standard reaction mixture was defined as the basal activity level.
Effects of growth medium osmolarity on both in vivo and in vitro cyclic glucan synthesis.
Zorreguieta et al. (42) have shown that while the in vivo accumulation of cell-associated cyclic glucan by A. tumefaciens is reduced by 85 to 95% when cells are grown under high-osmolarity conditions, the level of glucosyltransferase activity within membrane preparations derived from these cells is reduced by only 20 to 50% (compared to levels found in membrane preparations derived from cells grown under low-osmolarity conditions). Similar experiments were conducted in the present study to determine if the in vitro glucosyltransferase activity in R. meliloti membrane preparations is similarly affected by the osmolarity of the growth medium. R. meliloti cells were grown in GMS medium, a low-osmolarity defined medium, or GMS medium containing 0.4 M NaCl. In some experiments, 10 mM glycine betaine was added to the high-osmotic-strength medium because it has been shown to be a compatible solute for R. meliloti and is accumulated intracellularly when cells are grown under high-osmolarity conditions (29, 32, 34). One-half of each culture was used for isolation of cell-associated cyclic β-(1,2)-glucans, and the other half was used to isolate membranes for in vitro assays of glucosyltransferase activity.
The amount of cell-associated cyclic glucan isolated from each culture, as well as the level of in vitro glucosyltransferase activity of membrane preparations from each culture, are shown in Table 1. The results show that cyclic glucan accumulation was repressed almost 70% in cells grown under high-osmolarity conditions compared to cells grown under low-osmolarity conditions. However, addition of 10 mM glycine betaine to the high-osmolarity growth medium partially restored the level of cyclic glucan accumulated to 50% of the level seen in cells grown under low-osmolarity conditions. In contrast to the previous study of Zorreguieta et al. (42) performed with A. tumefaciens, there was no difference in glucosyltransferase activity in membrane preparations from R. meliloti cells grown under the different osmotic conditions. These results suggest that the level of NdvB protein present is not affected by the growth medium osmolarity and that osmotic regulation of cyclic β-(1,2)-glucan biosynthesis must occur at the level of enzyme activity. This possibility was further investigated by examining the effects of a variety of solutes (both ionic and nonionic) on in vitro glucosyltransferase activity in R. meliloti membrane preparations.
TABLE 1.
Effect of growth under different osmotic conditions on in vivo and in vitro cyclic glucan synthesis
| Growth medium | Cell-associated cyclic glucana | Glucosyltransferase activityb |
|---|---|---|
| GMS | 30.8 | 1.38 |
| GMS + 0.4 M NaCl | 9.7 | 1.42 |
| GMS + 0.4 M NaCl + 10 mM betaine | 15.8 | 1.31 |
Cell-associated cyclic glucans were extracted from cell pellets with 70% ethanol, as described in the text. Glucan levels were determined by the phenol-sulfuric acid method after fractionation of the ethanolic extracts by Sephadex G-50 column chromatography. Results are expressed as micrograms of glucose equivalents per milligram of total cell protein.
Activity is expressed as nanomoles of UDP-glucose converted to neutral product in a standard in vitro reaction mixture containing 40 μg of membrane protein.
Inhibition of in vitro glucosyltransferase activity by ionic solutes.
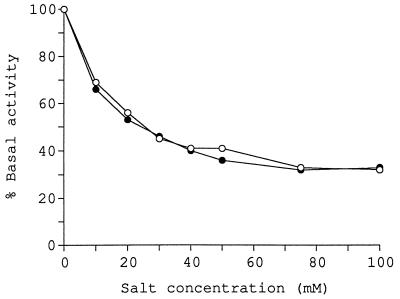
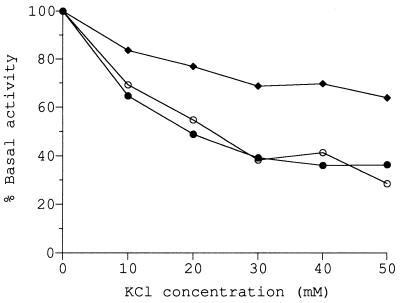
The effects of potassium chloride and sodium chloride on glucosyltransferase activity in membrane preparations are shown in Fig. 1. The presence of either salt inhibited activity, even at concentrations as low as 10 mM. At a concentration of 50 mM, approximately 60% inhibition of enzyme activity was observed with both salts.
FIG. 1.
Inhibition of glucosyltransferase activity by potassium chloride and sodium chloride. Assays were performed in the presence of potassium chloride (•) or sodium chloride (○) at the concentrations indicated. The basal level of activity was 1.02 nmol of UDP-glucose converted to neutral product under standard reaction conditions.
Because both sodium chloride and potassium chloride had almost identical effects on enzyme activity, the effects of lithium chloride and ammonium chloride were also examined to determine whether inhibition was due to the type of anion or cation present. The results revealed that lithium chloride and ammonium chloride were both as effective as potassium chloride and sodium chloride in inhibiting glucosyltransferase activity in membrane preparations (data not shown). There are several possible explanations for these results: (i) anion concentration (Cl− in this case) is an important determinant in enzyme inhibition; (ii) various monovalent cations all have similar effects on enzyme activity; or (iii) overall ionic strength in general is an important determinant of enzyme activity.
To distinguish among the possibilities listed above, the effects of several potassium and sodium salts were examined at a concentration of 50 mM. As shown in Table 2, the level of inhibition varied and was dependent on the anion present in the assay. Phosphate and chloride salts were most effective at inhibiting enzyme activity, while acetate and sulfate salts were least effective. The degree of inhibition was similar whether the potassium or sodium salt was used.
TABLE 2.
Inhibition of glucosyltransferase activity by potassium and sodium salts
| Salt addeda | % Inhibition of enzyme activityb |
|---|---|
| Potassium acetate | 41.2 ± 3.1 |
| Potassium chloride | 56.6 ± 4.4 |
| Potassium phosphate | 70.2 ± 1.8 |
| Potassium sulfate | 47.7 ± 2.5 |
| Sodium acetate | 42.5 ± 2.2 |
| Sodium chloride | 59.6 ± 5.5 |
| Sodium phosphate | 69.3 ± 2.2 |
| Sodium sulfate | 44.8 ± 2.9 |
Potassium and sodium salts were added at a final concentration of 50 mM to an otherwise standard assay mixture.
Data are the averages from five experiments ± standard errors.
It was found (see above) that different magnesium salts did not stimulate glucosyltransferase activity equally. The results suggested that while the presence of magnesium stimulated activity, the presence of acetate, chloride, and sulfate ions inhibited activity to various degrees, with chloride ions appearing to have the most inhibitory effect. This is consistent with the results shown in Table 2 and suggests that both the anion and the overall ion concentrations in the reaction mixture are important in regulating enzyme activity.
Effects of increasing concentrations of neutral solutes on in vitro glucosyltransferase activity.
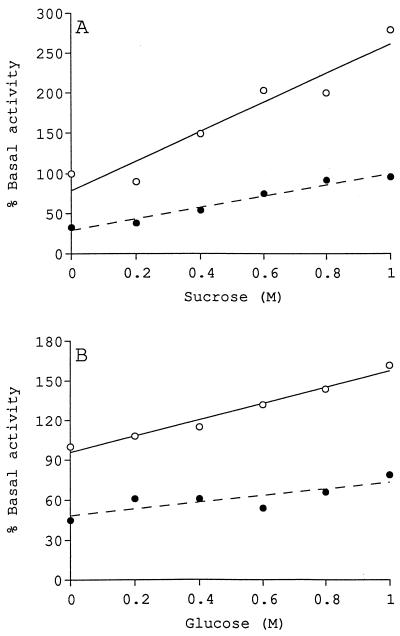
During adaptation to high-osmotic-strength environments, the osmolarity of the cytoplasm must increase. Thus, in addition to cytoplasmic ionic strength, cytoplasmic osmolarity may also influence glucosyltransferase activity. The effect of increased osmolarity on glucosyltransferase activity was initially examined by using sucrose and glucose as the solutes. These solutes were chosen because they are nonionic and can be used at high concentrations (up to a final concentration of 1 M in reaction mixtures). The results of these experiments are shown in Fig. 2. As the osmolarity of the reaction mixture was increased with neutral solutes, enzyme activity also increased. Sucrose was more effective than glucose at stimulating enzyme activity. As the sucrose concentration was increased to 1 M, the level of activity increased to 270% of the basal level (Fig. 2A), while the presence of 1 M glucose increased the level of activity to 170% of the basal level (Fig. 2B). The neutral products formed in the presence of 1 M sucrose and 1 M glucose were chromatographed on a Sephadex G-50 gel filtration column, and both were found to elute at the same volume as the cyclic β-(1,2)-glucan standard (data not shown).
FIG. 2.
Stimulation of glucosyltransferase activity by sucrose or glucose. Assays were performed in the absence (○) or presence (•) of 50 mM potassium chloride. (A) Sucrose. The basal level of activity was 1.02 nmol of UDP-glucose converted to neutral product under standard reaction conditions. (B) Glucose. The basal level of activity was 0.85 nmol of UDP-glucose converted to neutral product under standard reaction conditions.
The effects of high concentrations of neutral solutes in the presence of potassium chloride were also examined to determine whether the stimulatory effects of high concentrations of glucose or sucrose could compensate for the inhibitory effects of moderate concentrations of ionic solutes. Figure 2 shows the effects of increasing glucose and sucrose concentrations in the presence of 50 mM potassium chloride. The results reveal that in the presence of potassium chloride alone, enzyme activity was reduced to 30 to 45% of the basal level of activity, but as increasing concentrations of sucrose (Fig. 2A) or glucose (Fig. 2B) were added, enzyme activity increased. Indeed, the presence of 1 M glucose restored activity to 80% of the basal level, while the presence of 1 M sucrose restored enzyme activity to 100% of the basal level.
Effects of compatible solutes and osmoprotectants on glucosyltransferase activity.
The effects of the compatible solutes glycine betaine, trehalose, and potassium glutamate and the osmoprotectant choline chloride were examined. All three compatible solutes have been shown to accumulate in R. meliloti when cells are grown in high-osmotic-strength media (32, 37). Furthermore, R. meliloti also has the ability to convert choline to glycine betaine during growth under high-osmolarity conditions (32, 36). Based on previous analyses of R. meliloti cells grown in high-osmolarity media, it can be estimated that compatible solutes are accumulated intracellularly at concentrations as high as several hundred millimolar (1, 9, 22, 37). Thus, the effects of compatible solutes on in vitro glucosyltransferase activity were examined over a concentration range of 0 to 1 M.
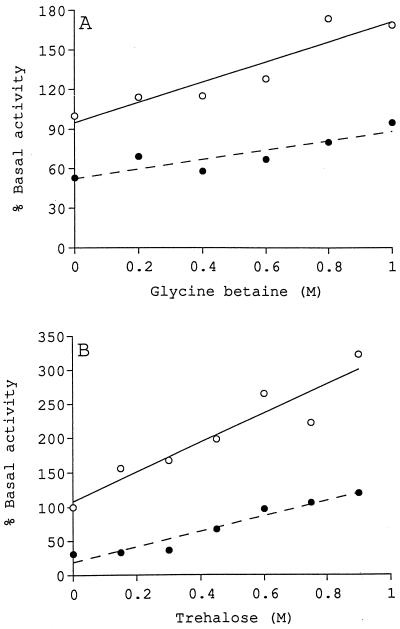
Glucosyltransferase activity increased linearly as the concentrations of glycine betaine and trehalose were increased, as shown in Fig. 3A and B, respectively. Trehalose was more effective at stimulating enzyme activity than glycine betaine. The presence of 1 M glycine betaine increased the level of activity to 180% of the basal level (Fig. 3A), while the presence of 0.9 M trehalose increased the level of activity to approximately 300% of the basal level (Fig. 3B). The neutral products formed in the presence of 1 M glycine betaine or 0.9 M trehalose were found to elute from a Sephadex G-50 gel filtration column at the same volume as the cyclic β-(1,2)-glucan standard (data not shown).
FIG. 3.
Stimulation of glucosyltransferase activity by the compatible solutes glycine betaine and trehalose. Assays were performed in the absence (○) or presence (•) of 50 mM potassium chloride. (A) Glycine betaine. The basal level of activity was 0.81 nmol of UDP-glucose converted to neutral product under standard reaction conditions. (B) Trehalose. The basal level of activity was 0.88 nmol of UDP-glucose converted to neutral product under standard reaction conditions.
The effects of increasing concentrations of glycine betaine and trehalose in the presence of 50 mM potassium chloride were also examined. These experiments revealed that high concentrations of glycine betaine (Fig. 3A) and trehalose (Fig. 3B) compensated for inhibition by potassium chloride and restored activity to 95 and 120%, respectively, of the basal level under standard assay conditions. These results are similar to those obtained with sucrose and glucose.
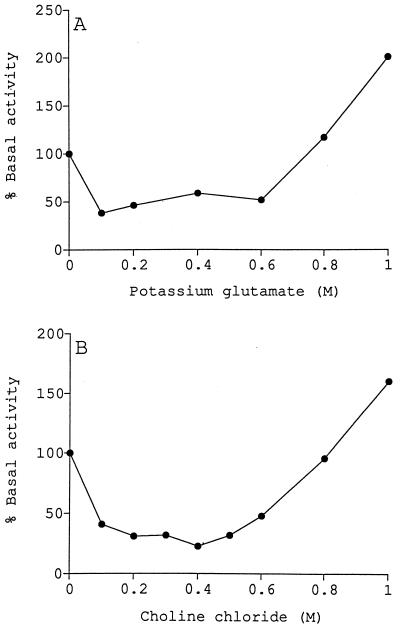
The results observed when increasing levels of potassium glutamate or choline chloride were added to glucosyltransferase assay mixtures were very different from the results observed in the presence of high concentrations of glycine betaine or trehalose. When potassium glutamate was added to the reaction mixtures at concentrations up to 0.6 M, enzyme activity was inhibited 50 to 60% (Fig. 4A). However, at higher concentrations, the amount of neutral product formed appeared to increase. A similar result was obtained when choline chloride was added to the reaction mixtures in increasing concentrations (Fig. 4B). At a concentration of 0.5 M, choline chloride inhibited enzyme activity by approximately 75%. However, at concentrations above 0.5 M, the amount of neutral product formed again appeared to increase. At a choline chloride concentration of 1.5 M the amount of neutral product formed was more than 10 times the basal amount (data not shown).
FIG. 4.
Effect of potassium glutamate or choline chloride on the production of neutral products from UDP-glucose. Assays were performed in the presence of potassium glutamate (A) or choline chloride (B) at the concentrations indicated. The basal levels of activity were 0.82 and 1.05 nmol of UDP-glucose converted to neutral product under standard reaction conditions for the assays performed with potassium glutamate and choline chloride, respectively. Sephadex G-50 column chromatography revealed that the primary neutral product formed in the presence of high potassium glutamate and choline chloride concentrations eluted later than cyclic β-(1,2)-glucan, suggesting that a membrane-associated enzyme distinct from glucosyltransferase was being stimulated.
The neutral products formed in reaction mixtures containing 1 M potassium glutamate or 1.5 M choline chloride were chromatographed on a Sephadex G-50 gel filtration column to determine if their elution profiles were similar to the elution profile of the cyclic β-(1,2)-glucan standard. The majority of the neutral product formed in both of the reactions eluted in a distinct peak later than the cyclic glucan peak (data not shown). Less than 10% of the neutral product formed in the presence of 1 M potassium glutamate and less than 4% of the neutral product formed in the presence of 1.5 M choline chloride eluted as expected for cyclic β-(1,2)-glucan. These levels represent 18 and 28%, respectively, of the level of neutral product observed in the cyclic glucan peak obtained with standard reaction mixtures in the absence of additional solutes. These results reveal that the presence of high concentrations of potassium glutamate and choline chloride inhibit glucosyltransferase activity and suggest that the neutral product formed in the presence of high concentrations of these solutes results from stimulation of another, as-yet-unidentified enzyme present in the membrane preparations.
Cyclic glucan biosynthesis is osmotically regulated in an ndvA mutant and in a truncated ndvB mutant.
It has previously been shown that the ndvA::Tn5 mutant LI1 (which is defective for transport of cyclic glucans) and the ndvB::Tn5 mutant TY24 (which produces a truncated NdvB glucosyltransferase lacking the C-terminal 1,140 amino acids) synthesize cyclic β-(1,2)-glucans, although at reduced levels compared to the wild type (7, 25). It had not been determined previously, however, whether cyclic glucan biosynthesis is subject to osmotic regulation in these mutants. To address this issue, we isolated cyclic glucans from the wild type and each mutant after growth in GMS medium (low osmolarity) and GMS medium containing 0.4 M NaCl (high osmolarity). The results revealed that although overall cyclic glucan levels were reduced in the mutants compared to the wild type, biosynthesis of cyclic glucans was still subject to osmotic regulation. When TY24 cells were grown in GMS medium, the level of cyclic glucan produced was 21 μg of glucose equivalents/mg of total cell protein. When TY24 cells were grown in GMS medium containing 0.4 M NaCl, the amount of cyclic glucan was reduced nearly 50%, to 12.2 μg of glucose equivalents/mg of total cell protein. This osmotic effect was more severe in LI1, which exhibited a nearly 70% reduction in cyclic glucan levels when cells were grown under high-osmolarity conditions. When LI1 cells were grown in GMS medium, the amount of cyclic glucan produced was 10.3 μg of glucose equivalents/mg of total cell protein, compared to 3.3 μg of glucose equivalents/mg of total cell protein produced by LI1 cells grown in GMS medium containing 0.4 M NaCl.
Glucosyltransferase activity in TY24 and LI1 membrane preparations is subject to ionic inhibition.
The effect of increasing concentrations of potassium chloride on glucosyltransferase activity in TY24 and LI1 membrane preparations was also examined. As shown in Fig. 5, in vitro glucosyltransferase activity was inhibited in LI1 membrane preparations to the same extent that it was inhibited in membrane preparations from the wild-type parent strain. Although glucosyltransferase activity in TY24 membrane preparations was also found to be subject to ionic inhibition, the degree of inhibition was less than the degree of inhibition observed with membrane preparations from wild-type and LI1 cells. At a potassium chloride concentration of 50 mM, membrane preparations from the wild type and LI1 showed 65 to 70% inhibition of glucosyltransferase activity, while only 40% inhibition was observed with TY24 membrane preparations.
FIG. 5.
Inhibition of glucosyltransferase activity in wild-type, TY24, and LI1 membrane preparations by potassium chloride. Assays with membrane preparations from the wild-type (•), TY24 (⧫), and LI1 (○) strains were performed in the presence of potassium chloride at the concentrations indicated. The basal levels of activity for wild-type, TY24, and LI1 membrane preparations were 1.24, 0.29, and 1.32 nmol of UDP-glucose converted to neutral product under standard reaction conditions, respectively.
Overall, the glucosyltransferase activity in TY24 membrane preparations was much lower than the glucosyltransferase activity in membrane preparations from wild-type and LI1 cells. The basal level of activity in TY24 membrane preparations was only 23% of the wild-type basal level of activity. This does not correlate with the in vivo results, which showed that the level of cyclic glucan produced by TY24 cells grown under low-osmolarity conditions was 75% of the level produced by wild-type cells under the same growth conditions. One possible explanation for this discrepancy between the in vivo and in vitro results is that the protein-glucan intermediate formed by the truncated glucosyltransferase of TY24 may be unstable under the conditions used for the in vitro assays. This explanation was suggested by the study of Ielpi and coworkers in which a UDP-[14C]glucose-labeled protein-glucan intermediate could not be detected when TY24 membrane preparations were used (25). Instability of the protein-glucan intermediate formed by the truncated glucosyltransferase may also explain why glucosyltransferase activity in TY24 membrane preparations appears to be less sensitive to ionic inhibition than glucosyltransferase activity in wild-type or LI1 membrane preparations.
DISCUSSION
It has been suggested by Zorreguieta et al. (42) that cyclic β-(1,2)-glucan biosynthesis in vivo may be regulated at the posttranslational level by cytoplasmic levels of K+, since K+ ions (in the form of potassium glutamate) have been shown to accumulate in a variety of bacteria (including R. meliloti) during growth under high-osmotic-strength conditions (16, 32, 37). These researchers showed that relatively high concentrations (0.1 to 0.4 M) of potassium chloride and sodium chloride strongly inhibited in vitro glucosyltransferase activity in A. tumefaciens membrane preparations, as well as the formation of the protein-glucan intermediate. They also showed that the in vitro enzyme activity in membranes prepared from A. tumefaciens cells grown under high-osmotic-strength conditions was 20 to 50% lower than the in vitro enzyme activity in membranes prepared from cells grown under low-osmolarity conditions. However, this moderate difference in activity was not enough to account for the high degree of inhibition (85 to 95%) of cyclic β-(1,2)-glucan production in vivo in cells grown under high-osmolarity conditions (42).
The results of the present study obtained with R. meliloti are consistent with the previous findings of Zorreguieta and coworkers (42) and provide clear evidence that the NdvB glucosyltransferase of R. meliloti is constitutively expressed regardless of growth medium osmolarity. In fact, the results of the present study are more striking than those reported previously because we found no difference in glucosyltransferase activity in membranes prepared from R. meliloti cells grown under high- and low-osmotic-strength conditions. We also found that in vitro glucosyltransferase activity is extremely sensitive to ionic strength conditions and is inhibited by a variety of salts at concentrations as low as 10 mM. Chloride and phosphate salts were found to be most inhibitory, suggesting that the level of inhibition depends not only on ion concentration but also on the identity of the anion.
In studies performed with A. tumefaciens membrane preparations, Zorreguieta et al. (42) found that high concentrations (0.1 to 0.4 M) of nonionic solutes had no effect on in vitro glucosyltransferase activity. In contrast to these previous studies, our results show that in vitro enzyme activity is stimulated by high concentrations of nonionic solutes, including the compatible solutes glycine betaine and trehalose. Furthermore, high concentrations of these nonionic solutes are able to compensate, in part, for the inhibition of glucosyltransferase activity caused by ionic solutes. It should be noted that potassium glutamate and choline chloride were found to inhibit glucosyltransferase activity, although they act as a compatible solute and an osmoprotectant, respectively, for R. meliloti (3, 23, 32, 36). However, it may be concluded that this inhibition is due to the ionic nature of these compounds.
Our results suggest that intracellular ion concentrations, intracellular osmolarity, and intracellular concentrations of nonionic compatible solutes all act as important determinants of glucosyltransferase activity in vivo. During growth under high-osmolarity conditions, R. meliloti has been shown to accumulate K+ ions in the form of potassium glutamate (3, 32). The nature of the nonionic compatible solutes accumulated by R. meliloti and their intracellular concentrations depend on their availability in the environment, as well as the degree of osmotic stress. Thus, the relative levels of ionic compatible solutes (e.g., potassium glutamate) and nonionic compatible solutes (e.g., glycine betaine or trehalose) within the cell may vary greatly.
The findings described above are fully consistent with our analyses of cell-associated cyclic β-(1,2)-glucan levels within cells grown in low- and high-osmolarity media. For example, as shown in Table 1, R. meliloti cultures grown under high-osmolarity conditions accumulated only 31% as much cyclic glucan as cultures grown under low-osmolarity conditions. However, when 10 mM glycine betaine was added to high-osmolarity cultures, the level of cyclic glucan accumulation increased to approximately 50% of the level found in cultures grown under low-osmolarity conditions. A similar effect has been reported for the peanut rhizobia (21). These results suggest that high intracellular concentrations of glycine betaine stimulate glucosyltransferase activity within cells that are also likely to have elevated levels of intracellular potassium glutamate. It should be noted that there were no differences in glucosyltransferase activity in membrane preparations from these cultures. Thus, the presence of glycine betaine does not result in higher levels of the NdvB protein within R. meliloti membranes.
The results presented here offer an explanation for the finding of Breedveld and Miller that cyclic β-(1,2)-glucans can be detected within mature alfalfa nodules infected with R. meliloti (5). Although nodules are thought to provide a relatively high-osmolarity environment for bacteroids (3), synthesis of cyclic glucans still occurs. In view of the results presented here, this is not surprising. Medicago sativa, the host of R. meliloti, has been shown to accumulate glycine betaine (35), and R. meliloti bacteroids have also been shown to accumulate glycine betaine during osmotic stress (19). In addition, trehalose is found in nodules (39), where it is synthesized by the bacteroids (33, 39). Thus, the presence of these nonionic compatible solutes should explain the ability of R. meliloti bacteroids to produce cyclic glucans in the osmotic environment of the root nodule.
While cyclic β-(1,2)-glucan biosynthesis is osmotically regulated in members of the family Rhizobiaceae, a recent study by Briones and coworkers (11) suggests that this is not the case for members of the genus Brucella, which have been shown to synthesize cyclic glucans with structures essentially identical to the structures of the cyclic glucans synthesized by members of the genera Rhizobium and Agrobacterium. Interestingly, cyclic glucan biosynthesis by Brucella spp. proceeds via formation of a protein-glucan intermediate (11), and a Brucella abortus gene which complements an R. meliloti ndvB mutant has been cloned (26). It should be noted that although cyclic glucan biosynthesis in Brucella spp. appears not to be osmotically regulated, the growth conditions examined by Briones and coworkers (11) were limited, and cells were grown in complex media. Thus, the accumulation of nonionic compatible solutes from the growth medium should have occurred and may have stimulated cyclic glucan biosynthesis, as shown in the present study for R. meliloti.
Finally, our studies with the ndvA mutant LI1 revealed that osmotic regulation of cyclic glucan biosynthesis does not require that cyclic glucans be transported to the periplasm or the extracellular medium. Indeed, cyclic glucan biosynthesis in LI1 was osmotically regulated in a manner similar to that found in the wild-type parent strain. It is interesting that cyclic glucan biosynthesis is also osmotically regulated in mutant TY24. This mutant synthesizes a truncated NdvB glucosyltransferase which lacks the C-terminal 1,140 amino acids, corresponding to 40% of the protein. Breedveld and coworkers (7) have previously shown that the structure of the cyclic glucan produced by TY24 is indistinguishable from the structure of the cyclic glucan produced by the wild-type parent strain. This leads to speculation concerning the role of the C terminus of NdvB. The C terminus is apparently not required for synthesis of the cyclic glucan backbone but is perhaps involved in stabilization of the protein-glucan intermediate, as suggested by Ielpi et al. (25). Instability of the protein-glucan intermediate could explain the reduced levels of cyclic glucan produced by this mutant. More studies are needed to clarify the role of the C terminus of NdvB.
ACKNOWLEDGMENT
This work was supported by grant MCB-9505706 from the National Science Foundation.
REFERENCES
- 1.Bernard T, Pocard J-A, Perroud B, Le Rudulier D. Variations in the response of salt-stressed Rhizobium strains to betaines. Arch Microbiol. 1986;143:359–364. [Google Scholar]
- 2.Bhagwat A A, Keister D L. Synthesis of β-glucans by Bradyrhizobium japonicum and Rhizobium fredii. Can J Microbiol. 1992;38:510–514. [Google Scholar]
- 3.Botsford J L, Lewis T A. Osmoregulation in Rhizobium meliloti: production of glutamic acid in response to osmotic stress. Appl Environ Microbiol. 1990;56:488–494. doi: 10.1128/aem.56.2.488-494.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breedveld M W, Hadley J A, Miller K J. A novel cyclic β-1,2-glucan mutant of Rhizobium meliloti. J Bacteriol. 1995;177:6346–6351. doi: 10.1128/jb.177.22.6346-6351.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breedveld M W, Miller K J. Cyclic β-glucans of members of the family Rhizobiaceae. Microbiol Rev. 1994;58:145–161. doi: 10.1128/mr.58.2.145-161.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breedveld M W, Miller K J. Synthesis of glycerophosphorylated cyclic (1,2)-β-glucans in Rhizobium meliloti strain 1021 after osmotic shock. Microbiology. 1995;141:583–588. doi: 10.1099/13500872-141-3-583. [DOI] [PubMed] [Google Scholar]
- 7.Breedveld M W, Yoo J S, Reinhold V N, Miller K J. Synthesis of glycerophosphorylated cyclic β-(1,2)-glucans by Rhizobium meliloti ndv mutants. J Bacteriol. 1994;176:1047–1051. doi: 10.1128/jb.176.4.1047-1051.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breedveld M W, Zevenhuizen L P T M, Zehnder A J B. Osmotically-induced oligo- and polysaccharide synthesis by Rhizobium meliloti SU-47. J Gen Microbiol. 1990;136:2511–2519. [Google Scholar]
- 9.Breedveld M W, Zevenhuizen L P T M, Zehnder A J B. Osmotically-regulated trehalose accumulation and cyclic β-(1,2)-glucan excretion by Rhizobium leguminosarum biovar trifolii TA-1. Arch Microbiol. 1991;156:501–506. [Google Scholar]
- 10.Breedveld M W, Zevenhuizen L P T M, Zehnder A J B. Synthesis of cyclic β-(1,2)-glucans by Rhizobium leguminosarum biovar trifolii TA-1: factors influencing excretion. J Bacteriol. 1992;174:6336–6342. doi: 10.1128/jb.174.20.6336-6342.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Briones G, Inon de Iannino N, Steinberg M, Ugalde R A. Periplasmic cyclic 1,2-β-glucan in Brucella spp. is not osmoregulated. Microbiology. 1997;143:1115–1124. doi: 10.1099/00221287-143-4-1115. [DOI] [PubMed] [Google Scholar]
- 12.Cangelosi G A, Martinetti G, Leigh J A, Lee C C, Theines C, Nester E W. Role of Agrobacterium tumefaciens ChvA protein in export of β-1,2-glucan. J Bacteriol. 1989;171:1609–1615. doi: 10.1128/jb.171.3.1609-1615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cangelosi G A, Martinetti G, Nester E W. Osmosensitivity phenotypes of Agrobacterium tumefaciens mutants that lack periplasmic β-1,2-glucan. J Bacteriol. 1990;172:2172–2174. doi: 10.1128/jb.172.4.2172-2174.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro O A, Zorreguieta A, Ielmini V, Vega G, Ielpi L. Cyclic β-(1,2)-glucan synthesis in Rhizobiaceae: roles of the 319-kilodalton protein intermediate. J Bacteriol. 1996;178:6043–6048. doi: 10.1128/jb.178.20.6043-6048.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castro O, Zorreguieta A, Semino C, Ielpi L. Biosynthesis of cyclic β-(1,2)-glucans in Rhizobium leguminosarum biovars viciae, phaseoli and trifolii. Arch Microbiol. 1995;163:454–462. [Google Scholar]
- 16.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dylan T, Helinski D R, Ditta G S. Hypoosmotic adaptation in Rhizobium meliloti requires β-1,2-glucan. J Bacteriol. 1990;172:1400–1408. doi: 10.1128/jb.172.3.1400-1408.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dylan T, Ielpi L, Stanfield S, Kashyap L, Douglas C, Yanofsky M, Nester E, Helinski D R, Ditta G. Rhizobium meliloti genes required for nodule development are related to chromosomal virulence genes in Agrobacterium tumefaciens. Proc Natl Acad Sci USA. 1986;83:4403–4407. doi: 10.1073/pnas.83.12.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fougere F, Le Rudulier D. Glycine betaine biosynthesis and catabolism in bacteroids of Rhizobium meliloti: effect of salt stress. J Gen Microbiol. 1990;136:2503–2510. doi: 10.1099/00221287-136-1-157. [DOI] [PubMed] [Google Scholar]
- 20.Geremia R A, Cavaignac S, Zorreguieta A, Toro N, Olivares J, Ugalde R A. A Rhizobium meliloti mutant that forms ineffective pseudonodules in alfalfa produces exopolysaccharide but fails to form β-(1,2)-glucan. J Bacteriol. 1987;169:880–884. doi: 10.1128/jb.169.2.880-884.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghittoni N E, Bueno M A. Peanut rhizobia under salt stress: role of trehalose accumulation in strain ATCC 51466. Can J Microbiol. 1995;41:1021–1030. [Google Scholar]
- 22.Gloux K, Le Rudulier D. Transport and catabolism of proline betaine in salt-stressed Rhizobium meliloti. Arch Microbiol. 1989;151:143–148. [Google Scholar]
- 23.Gonzalez-Gonzalez R, Botsford J L, Lewis T. Osmoregulation in Rhizobium meliloti: characterization of enzymes involved in glutamate synthesis. Can J Microbiol. 1990;36:469–474. [Google Scholar]
- 24.Hanson R S, Phillips J A. Chemical composition. In: Gerhardt P, Murray R G E, Costilow R N, Nester E W, Wood W A, Krieg N R, Philips G B, editors. Manual of methods for general bacteriology. Washington, D.C: American Society for Microbiology; 1981. pp. 328–364. [Google Scholar]
- 25.Ielpi L, Dylan T, Ditta G S, Helinski D R, Stanfield S W. The ndvB locus of Rhizobium meliloti encodes a 319-kDa protein involved in the production of β-1,2-glucan. J Biol Chem. 1990;265:2843–2851. [PubMed] [Google Scholar]
- 26.Inon de Iannino N, Briones G, Tolmasky M E, Ugalde R A. Abstracts of the 96th General Meeting of the American Society for Microbiology 1996. Washington, D.C: American Society for Microbiology; 1996. Molecular cloning of a Brucella abortus gene that complements an ndvB deficient mutant of Rhizobium meliloti, abstr. H-137; p. 507. [Google Scholar]
- 27.Lepek V, Navarro de Navarro Y, Ugalde R A. Synthesis of β-(1,2)-glucan in Rhizobium loti. Expression of Agrobacterium tumefaciens chvB virulence region. Arch Microbiol. 1990;155:35–41. [Google Scholar]
- 28.Le Rudulier D, Bernard T. Salt tolerance in Rhizobium: a possible role for betaines. FEMS Microbiol Rev. 1986;39:67–72. [Google Scholar]
- 29.Le Rudulier D, Bernard T, Pocard J-A, Goas G. Enhancement of osmotolerance in Rhizobium meliloti by glycine betaine and proline betaine. C R Acad Sci. 1983;297:155–160. [Google Scholar]
- 30.Miller K J, Gore R S, Johnson R, Benesi A J, Reinhold V N. Cell-associated oligosaccharides of Bradyrhizobium spp. J Bacteriol. 1990;172:136–142. doi: 10.1128/jb.172.1.136-142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller K J, Kennedy E P, Reinhold V N. Osmotic adaptation by Gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986;231:48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- 32.Miller K J, Wood J M. Osmoadaptation by rhizosphere bacteria. Annu Rev Microbiol. 1996;50:101–136. doi: 10.1146/annurev.micro.50.1.101. [DOI] [PubMed] [Google Scholar]
- 33.Salminen S O, Streeter J G. Enzymes of α,α-trehalose metabolism in soybean nodules. Plant Physiol. 1986;81:538–541. doi: 10.1104/pp.81.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sauvage D, Hamelin J, Larher F. Glycine betaine and other structurally related compounds improve the salt tolerance of Rhizobium meliloti. Plant Sci Lett. 1983;31:291–302. [Google Scholar]
- 35.Sethi J K, Carew D P. Growth and betaine formation in Medicago sativa tissue cultures. Phytochemistry. 1974;13:321–324. [Google Scholar]
- 36.Smith L T, Pocard J-A, Bernard T, Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988;170:3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith L T, Smith G M, D’Souza M, Pocard J-A, Le Rudulier D, Madkour M A. Osmoregulation in Rhizobium meliloti: mechanism and control by other environmental signals. J Exp Zool. 1994;268:162–165. [Google Scholar]
- 38.Stanfield S W, Ielpi L, O’Brochta D, Helinski D R, Ditta G S. The ndvA gene product of Rhizobium meliloti is required for β-1,2-glucan production and has homology to the ATP-binding export protein HlyB. J Bacteriol. 1988;170:3523–3530. doi: 10.1128/jb.170.8.3523-3530.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Streeter J G. Accumulation of α,α-trehalose by Rhizobium bacteria and bacteroids. J Bacteriol. 1985;164:78–84. doi: 10.1128/jb.164.1.78-84.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williamson G, Damani K, Devenney P, Faulds C B, Morris V J, Stevens B J H. Mechanism of action of cyclic β-1,2-glucan synthetase from Agrobacterium tumefaciens: competition between cyclization and elongation reactions. J Bacteriol. 1992;174:7941–7947. doi: 10.1128/jb.174.24.7941-7947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zevenhuizen L P T M, Scholten-Koerselman H J. Surface carbohydrates of Rhizobium. I. β-1,2-glucans. Antonie Leeuwenhoek. 1979;45:165–175. doi: 10.1007/BF00418581. [DOI] [PubMed] [Google Scholar]
- 42.Zorreguieta A, Cavaignac S, Geremia R A, Ugalde R A. Osmotic regulation of β-1,2-glucan synthesis in members of the family Rhizobiaceae. J Bacteriol. 1990;172:4701–4704. doi: 10.1128/jb.172.8.4701-4704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zorreguieta A, Geremia R A, Cavaignac S, Cangelosi G A, Nester E W, Ugalde R A. Identification of the product of an Agrobacterium tumefaciens chromosomal virulence gene. Mol Plant-Microbe Interact. 1988;1:121–127. doi: 10.1094/mpmi-1-121. [DOI] [PubMed] [Google Scholar]
- 44.Zorreguieta A, Tolmasky M E, Staneloni R J. The enzymatic synthesis of β-1,2-glucans. Arch Biochem Biophys. 1985;238:368–372. doi: 10.1016/0003-9861(85)90176-6. [DOI] [PubMed] [Google Scholar]
- 45.Zorreguieta A, Ugalde R A. Formation in Rhizobium and Agrobacterium spp. of a 235-kilodalton protein intermediate in β-d-1,2-glucan synthesis. J Bacteriol. 1986;167:947–951. doi: 10.1128/jb.167.3.947-951.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zorreguieta A, Ugalde R A, Leloir L F. An intermediate in cyclic β-1,2-glucan biosynthesis. Biochem Biophys Res Commun. 1985;126:352–357. doi: 10.1016/0006-291x(85)90613-8. [DOI] [PubMed] [Google Scholar]