Abstract
Background:
The human exposome, defined as “…everything that is not the genome”, comprises all chemicals in the body interacting with life processes. The exposome drives genes x environment (GxE) interactions that can cause long-term latency and chronic diseases. The exposome constantly changes in response to external exposures and internal metabolism. Different types of compounds are found in different biological media.
Objective:
Measure polar volatile organic compounds (PVOCs) excreted in urine to document endogenous metabolites and exogenous compounds from environmental exposures.
Methods:
Use headspace collection and sorbent tube thermal desorption coupled with bench-top gas chromatography – mass spectrometry (GC-MS) for targeted and non-targeted approaches. Identify and categorize PVOCs that may distinguish among healthy and affected individuals.
Results:
Method is successfully demonstrated to tabulate a series of 28 PVOCs detected in human urine across 120 samples from 28 human subjects. Median concentrations range from below detect to 165 ng/ml. Certain PVOCs have potential health implications.
Conclusions:
Headspace collection with sorbent tubes is an effective method for documenting PVOCs in urine that are otherwise difficult to measure. This methodology can provide probative information regarding biochemical processes and adverse outcome pathways (AOPs) for toxicity testing.
Keywords: biomarkers, urinary metabolites, gas chromatography/mass spectroscopy, human exposome, adverse outcome pathways, non-targeted analysis
Introduction:
Recent theoretical and scientific developments have improved our understanding of the processes underlying environmentally-related human disease [1, 2]. These advancements have implicated the interaction between an individual’s internal and external environment as an aggressive driver of disease, and have helped motivate innovative research endeavors that aim to discover synergies between environmental exposure and genetics in order to explain a larger percentage of disease etiology [3–5]. Although current epidemiological research suggests that environmental factors have the potential to account for 70–90% of disease etiology and much of the variability of disease within populations [6, 7], very little is known about the processes governing gene-environment interactions and critical infrastructure is needed to begin building this knowledge base [8].
Generally speaking, research efforts focusing on gene-environment interactions fit within the scope of the study of the human exposome, a comprehensive field that incorporates and relies on sub-fields such as genomics, metabolomics, lipidomics, epigenomics, and proteomics. The concept of the exposome was first proposed by Christopher Wild in 2005 as the cumulative exogenous (environmental) and endogenous exposure that an individual acquires over the course of his or her lifetime [1]. More recently, investigators entrenched in exposomics have expanded upon Wild’s concept and have redefined the exposome as “the cumulative measure of environmental influences and associated biological responses throughout the lifespan, including exposures from the environment, diet, behavior, and endogenous processes” [9]. This redefinition is an appeal to exposure scientists, toxicologists, and environmental epidemiologists to help elucidate the exposome in a collective effort to better understand the underpinnings of human disease. Academic consideration of the exposome was initially slow, but now, exposomics is increasingly integrated into collaborative research centers and large-scale studies, such as the HELIX project, the Children’s Health Exposure Analysis Resource, and the HERCULES Exposome Research Center are leading centers for this work [8, 10, 11]. Of additional interest, a series of studies have expanded upon the exposome concept and have invoked the Environmentally Wide Association Study (EWAS) concept [12–14].
As a bridge between environmental exposures, endogenous processes, and disease states, biomarker research is poised to play an integral role in mapping the exposome. However, if biomonitoring is to be effectively implemented, researchers must first develop sensitive and specific methodologies to measure a broad suite of biomarkers, and then establish parameters that describe their prevalence in the general, non-remarkable population [15–18]. Once an assemblage of appropriate and measurable biomarkers has been established and the normal ranges have been determined, this ensemble may then be used to track biological perturbations and resolve critical links between environmental exposures and health outcomes.
Numerous polar volatile organic compounds (PVOCs) are present in biological fluids; they are commonly diet-derived and/or formed as by-products of normal metabolic processes. The presence of PVOCs in biological fluids may also indicate environmental exposure or metabolism [19, 20] as well as disease states [21].
Urine is a concentrated and well-studied biological fluid and has the potential to be an ideal and accessible matrix for monitoring and evaluating PVOC biomarkers [22–24]. There are several advantages to analyzing PVOCs in urine compared to other biological matrices: for example, sample collection is non-invasive, specimens are easy to collect and store, compounds in urine tend to be fairly concentrated, urine samples can be abundantly supplied, and many compounds of interest are concentrated in the kidney before excretion [25]. However, urine is an extremely complex biological matrix, and there can be enormous within- and between individuals variation of the compounds found in urine. Some causes of variation are diet, overall health, occupation, location, gender, race, genotype, physical activity, the frequency of habits such as smoking and drinking, and water consumption [26]. Although water consumption may not disrupt the actual occurrence of urinary metabolites, a high-water content in urine may be a large barrier to compound detection as the water dilutes the sample. In addition, high water content adds to a PVOC’s preference for polar solutions. Indeed this is a serious analytical challenge because PVOCs dissolved in human biological samples are inherently difficult to extract as they have a chemical affinity for the liquid phase and preferentially stay in the aqueous media. Several extraction methods have been developed to cope with this natural affinity, and the advantages and disadvantages of the most popular extraction methods are discussed in Pleil et al. [26]. The primary disadvantages of most extraction methods are their time-consuming, complicated procedures and the required use of expensive and advanced equipment. Thus, high water content in human urine presents unique challenges to extracting and quantifying urinary PVOCs.
Although urinary biomarkers have the potential to characterize baseline levels of PVOCs, reconstruct environmental exposures, and/or evaluate metabolic disorders, the aforementioned challenges of using urine have limited scientific efforts to characterize urinary PVOCs in the general population. Establishing baseline levels of PVOCs in the general, non-remarkable population is a critical step to mapping the urinary exposome and subsequently evaluating environmentally related disease. Currently, there is little information regarding the type and quantity of PVOC compounds found in urine; furthermore, the data is limited in such a way that baseline levels across diverse populations have not yet been established.
This paper presents a novel approach to measuring PVOCs in urine. The overarching analytical method was adapted from Pleil et al. [26], where a similar methodology was used to measure PVOCs from exhaled breath condensate (EBC). Our impetus for adapting a passive diffusion transfer method to urine is to develop an extraction and detection method that can be executed quickly and with relative ease in most analytical labs. A secondary goal of developing a detection method for urinary PVOCs was to identify endogenous volatiles commonly found in human urine across a diverse population and to augment the database on baseline levels of PVOCs. Ultimately, understanding the biomarker content, distributions, patterns, and typical levels in different media will allow researchers to assess individuals with outlier responses, develop knowledge of adverse outcome pathways (AOPs), and develop intervention strategies to protect public and environmental health [27–30].
Herein we present a series of techniques that can be implemented in most environmental or biological analytical laboratories with standard glassware and analytical GC-MS instrumentation. This is done deliberately to make urine PVOCs analysis as accessible as possible. We acknowledge that more sophisticated methods and instruments could be employed for complex biological matrices. In fact, we have studied two-dimensional gas chromatography methods linked with time-of-flight (TOF) mass spectrometry (GCxGC-TOF/MS), as well as liquid chromatography linked with high-resolution mass-spectrometry (LC-HRMS) applications for other projects [31, 32]. More recently, we have also implemented synchronous SIM-Scan data acquisition using GC-MS [33]. However, for this initial exploratory development, we selected single-unit (1Da) resolution GC-MS instrumentation, which comprises most of standard analyses in commercial laboratories.
Clinical significance
Urine is regularly collected for clinical analysis of health state indicators such as glucose, ketone bodies, pH, proteins, bilirubin, specific gravity, creatinine, blood cells, etc. Expanding the use of this common biological medium beyond the standard diagnostic assays has clinical significance in exploring health state. PVOCs tend to be shorter-lived biomarkers of recent exposure and phase-1 metabolism, and so provide a different dimension to monitoring the changing human exposome. These measures could ultimately be incorporated into standard clinical practice for rapid screening or broader diagnostic tests.
Materials and Methods
Ex-R study design and biological sample collection
For the method presented here, we analyzed a series of human urine specimens that were collected as part of the “Pilot Study to Estimate Human Exposures to Pyrethroids using an Exposure Reconstruction Approach” (Ex-R study). Details regarding the Ex-R study are published elsewhere [34]. Briefly, the primary goals of the Ex-R study were to assess the variability of select pyrethroid metabolites in the urine samples of non-occupationally exposed adults, and to provide an enhanced characterization of pyrethroid exposure and metabolism using an exposure reconstruction approach. To accomplish these goals, the Ex-R study investigated the longitudinal exposures of 50 adults to pyrethroid insecticides over a six-week monitoring period. Environmental samples (solid food, drinking water, surface wipes, and vacuum dust), diaries (food, activity, and pesticide use), and urine samples were collected during sampling weeks between November 2009 and May 2011. To qualify for the Ex-R study, subjects had to be healthy adults (between the ages of 18 to 50), not pregnant, with no occupational exposure to pyrethroid insecticides, no pre-existing medical conditions that would affect urine output (i.e., kidney, liver, or heart disease), currently living in a residential home or apartment, able to provide their own transportation to and from the Ex-R study facilities, and could speak, read, and use English fluently [34].
Additional terms of the Ex-R study were to measure biomarkers from selected samples to supplement the pyrethroid metabolite data and to advance the understanding of systems biology with respect to cumulative exposures. The analyses presented in this study were performed, in part, to complement biomarker analyses and to assist the principle investigators with the Ex-R study to accurately assess between- and within-person variance components reflective of individual absorption, distribution, metabolism, and excretion parameters.
Subject work flow
For the analyses presented here, urine specimens were collected at the U.S. Environmental Protection Agency’s (EPA) National Health and Environmental Effects Research Laboratory (NHEERL) located on the campus of the University of North Carolina at Chapel Hill (UNC-CH). All biological samples and accompanying measurements (such as creatinine concentration and specific gravity measured in each urine void) were provided to this study by NHEERL investigators in accordance with UNC-CH Medical School Institutional Review Board (IRB # 09–0741). Volatile organics analyses of the biological specimens were also performed under these protocols.
Urine samples were single voids collected during a 24-hour sampling period on sampling weeks. Please see Morgan et al. for the detailed sampling schedule [34]. Participants collected their urine voids in separate 1 L polypropylene containers at their residences on weeks 1, 2, and 6 of the 6-week study. The time and date of each void was recorded and 8 mL aliquots of urine from each void were placed into separate 10 mL cryogenic vials with lids. All aliquots were stored in laboratory freezers (−80°C) until analysis. For our study, 120 human urine specimens were made available for testing representing 28 Ex-R study participants with about 4 repeats per person, selected at random.
Subjects were nominally healthy adults with unremarkable recent exposure history. The analytes presented in this study reflect compounds that were present at measurable levels in at least some of the urine samples.
Materials
Absorbent tubes were standard 3.5 (89 mm) length x .025” (6.4 mm) outside diameter filled with 350 ng 60–80 mesh Tenax® TA (Scientific Instrumentation Specialists, Inc., Ringoes, NJ). Neat standards with estimated 98% or better purity were purchased in Polystandard™ Kits in 2 mL vials (Accustandard, New Haven, CT). All standards were prepared using high purity de-ionized water. Glass sample bulbs were locally fabricated (75 mL volume, 130 mm length) and sealed with Teflon lined plastic caps. Phosphate Buffered Saline (PBS) was American Chemical Society (ACS) reagent grade and sterile (Gibco; DPBS 1x). Samples were transferred using glass syringes with Teflon plungers.
Sample preparation and PVOC extraction
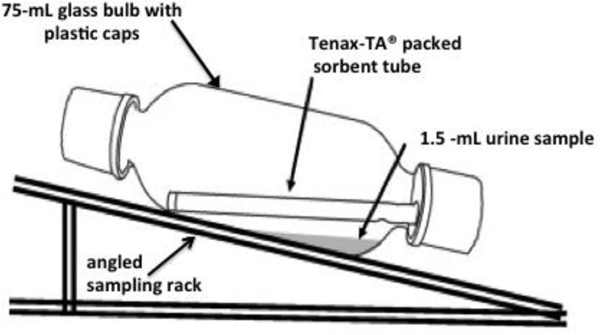
Tenax tubes were pre-cleaned using a Markes R-TC20, Multi-Tube Conditioning/Dry Purge Unit (Markes International, Ltd, Llantrisandt, UK) immediately prior to use with high-purity helium purge (100 mL/min) at 280 °C for 2 hours and 20 min. Glass bulbs were cleaned by hand using a glassware brush and water with inorganic soap and washed in a laboratory dishwasher for a more aggressive clean-up. Immediately prior to testing, glass bulbs were rinsed once with dichloromethane (DCM), allowed to dry, and rinsed at least three times with high-purity DI water. Glass bulbs were allowed to dry before samples were added. For spiked samples, we mixed primary standards in 25 mL vials using neat materials to achieve known dilutions in the ~6 μg/mL range. These were subsequently diluted to achieve specific levels (0–0.765 μg) in 1.5 mL aliquots. Glass bulbs were set at an angle of ~15° with respect to the horizontal to keep the aqueous sample from contacting the Tenax tube directly (see Figure 1). In previous studies [26] experiments were performed to determine general guidelines for sample preparation and assess recovery efficiencies. Through these preliminary experiments, desorption from an aqueous sample to Tenax® TA was conducted at room temperature and 24 hours was determined to be a reasonable desorption time. 1.5 mL proved to be the optimal sample volume for all aqueous samples, blanks, and spiked calibration samples. In this study, PBS was used as the base solution for all blanks and spiked samples as it is a suitable proxy for urine.
Figure 1:
Schematic of 75 mL glass bulb arrangement for the passive absorption of PVOCs from urine onto Tenax-TA packed sorbent tubes.
All urine samples, PBS blanks, and calibration standards were prepared and analyzed identically. To evaluate PVOCs as urinary biomarkers, we explore a passive diffusion transfer method to capture polar volatile organic carbons excreted in human urine. The core study design was presented previously [26, 35] and adapted here to be relevant and appropriate for human urine. The passive diffusion transfer method is as follows:
Tenax tubes are cleaned and conditioned with ultra-high purity helium at 290 °C for 2 hours and 20 minutes prior to exposure.
Each 75 ml glass bulb is washed and then heated to 70 °C to remove residual compounds.
Once cooled to room temperature, glass bulbs are rinsed with DCM, allowed to dry, rinsed again with high-purity deionized water, and allowed to dry thoroughly before samples are added.
Once bulbs are thoroughly dry, they are placed on an inclined rack, and a clean Tenax absorbent tube is inserted into each bulb.
Urine samples and calibration standards are stored at −20°C and thawed to room temperature before testing.
Urine samples (1.5 mL) are transferred to individual bulbs via glass syringe.
Blanks are prepared by injecting, via glass syringe, 1.5 mL of PBS into individual bulbs. Blanks are tested in duplicate.
Calibration standards are prepared by injecting, via syringe, 130 μl of the standard mixture (765 μg/mL) into glass bulbs containing 1.5 mL of PBS. Calibration standards were tested in duplicate.
Bulbs are angled approximately 15° with respect to the horizontal, and care was taken to keep the aqueous solution from contacting the Tenax tube directly.
Bulbs are sealed and left at room temperature for 24 hours.
Tenax tubes are removed, capped, and stored for subsequent analysis.
The exposed Tenax tubes were placed into an Ultra TD autosampler coupled with a Unity thermal desorber (Markes International, Ltd. Llantrisant, UK) equipped with a water management secondary trap. The autosampler/desorber was configured to dry-purge each Tenax tube sample for 10 min with helium at room temperature to remove residual adsorbed water vapor. Each tube was subsequently desorbed at 260 °C, and analytes were refocused on a secondary multipurpose trap at 0 °C with subsequent ballistic heating to 280 °C for injection. After desorption, samples were automatically injected into a gas chromatography-mass spectrometry (GC/MS) system (6890N GC; 59731 MS; Agilent, Palo Alto, CA). Chromatographic separation was accomplished with a crossbond 5% di-phenyl (Rxi-5ms, 60 m x 0.25 mm ID) with a 0.25 μm stationary phase column (Restek, Bellefonte, PA). The GC oven was programmed with an initial temperature of 40 °C for 2 minutes, followed by a 10 °C/minute ramp to 250 °C where it was held for 8 minutes.
Discovery and Targeted Analyses
In the discovery phase of our analyses, the MS was operated in scan mode to discover urinary PVOCs that successfully passed through the column and created an ion in the MS source. We scanned from 33 m/z (mass to charge ratio) to 350 m/z in default 0.1 m/z increments. We used aggressive manual integration based on an approximate signal to noise ratio S/N=3 of the extracted ion chromatograms (EICs) to identify the most prevalent urinary PVOCs in the headspace of our samples. Approximately 10 urine samples were selected at random for discovering prevalent urinary PVOCs in our participant population. From these samples, 28 PVOCs from human urine were selected for targeted analyses. We selected 14 PVOCs from the exhaled breath condensate (EBC) method [26, 35] and 14 additional PVOCs detected in urine samples during the development phase. We define detection of PVOCs from urine samples by the ability to integrate peaks and identify PVOCs with elution time, primary and confirmatory ions, and certified standards. Table 1 presents specific PVOCs measured in targeted analyses and their associated retention times, primary and confirmatory ions, and R2 values from multipoint calibration curves. Additional compound identification information, including DTXSID obtained through EPA Chemistry Dashboard searches, can be found in Appendix B.
Table 1.
GC/MS Parameters for PVOCs in Urine
| Elution order | Compound Name | Retention Time (min) | SIM Ion (Da) | Confirmation Ions (Da) | R2 |
|---|---|---|---|---|---|
| 1 | 1-Propanol | 5.016 | 59 | 31, 42 | 0.993 |
| 2 | 2-Methyl-propanal | 5.042 | 72 | 43 | 0.989 |
| 3 | 2-Butanone | 5.427 | 72 | 43, 39 | 0.998 |
| 4 | 2 - Ethoxy-2-methyl propane | 5.756 | 59 | 87 | 0.994 |
| 5 | 2-Methyl-1-propanol | 5.809 | 43 | 33 | 0.994 |
| 6 | Amylene hydrate | 6.001 | 59 | 73 | 0.991 |
| 7 | Isovaleraldehyde | 6.245 | 44 | 58 | 0.995 |
| 8 | 1-Butanol | 6.351 | 56 | 41 | 0.995 |
| 9 | 2-Pentanone | 6.766 | 43 | 86 | 0.989 |
| 10 | Pentanal | 6.939 | 44 | 58 | 0.993 |
| 11 | 3-Methyl-1-butanol | 7.642 | 55 | 7 | 0.997 |
| 12 | 4-Methyl-2-pentanone | 7.759 | 43 | 58, 100 | 0.997 |
| 13 | Dimethyl disulfide | 7.957 | 94 | 79 | 0.987 |
| 14 | 3-Methyl-3-pentanol | 8.01 | 73 | 55 | 0.994 |
| 15 | Pyrrole | 8.071 | 67 | 39 | 0.992 |
| 16 | 4-Methyl-2-pentanol | 8.106 | 45 | 69 | 0.993 |
| 17 | 1-Pentanol | 8.274 | 42 | 55 | 0.988 |
| 18 | Hexanal | 8.986 | 56 | 72 | 0.987 |
| 19 | Furfural | 9.742 | 96 | 95 | 0.990 |
| 20 | 2-Ethyl-1-butanol | 9.837 | 43 | 70 | 0.984 |
| 21 | 1-Hexanol | 10.42 | 56 | 43, 69 | 0.984 |
| 22 | 4-Heptanone | 10.54 | 71 | 114 | 0.999 |
| 23 | Allyl isothiocyanate | 10.824 | 99 | 39 | 0.996 |
| 24 | Heptanal | 11.145 | 70 | 43 | 0.999 |
| 25 | 1-Heptanol | 12.501 | 70 | 56 | 0.992 |
| 26 | Benzaldehyde | 12.527 | 106 | 105 | 0.997 |
| 27 | Octanal | 13.205 | 43 | 57 | 0.996 |
| 28 | Carvone | 17.76 | 82 | 54 | 0.983 |
In the targeted phase of our analyses, the MS was operated in Selected Ion Monitoring (SIM) mode with 11 or less ions per group. Dwell times were adjusted to achieve 1.8–2.2 SIM scans per second (typically ~ 70 ms/ion) to optimize chromatographic peak shape [36]. Standard autotune procedures with perfluoro tributyl amine (PFTBA) (Agilent, Palo Alto, CA) were employed for the MS detector. Individual compounds were quantified using peak areas of EICs. Primary ions were used for quantification of the target peaks, while secondary ions were used to validate the compound’s presence. As an additional layer of confidence in our findings, the ratio of primary to secondary ions from the sample was then compared to the analogous ion-ratio in the certified standard. To validate the presence of our compounds, ion ratios from our samples had to be within 25% of the standard values.
Statistical Analyses
Blank corrections for urine samples were performed by subtracting the mean value of batch blanks from the raw area counts of the urine samples from that batch. Sample concentrations were extrapolated from a 6-point standard curve that ranged from 0 to 0.765 μg/μL. The standard curve was used to assure linearity of the response over the concentration range of interest. Sensitivity was determined by the repeat analysis of blank samples, and the analytical limit of detection (LOD) was calculated (for each analyte) as 3 times the standard deviation of ten blank values as shown in column 2 of Table 3. Although dependent on the specific analyte, LOD’s ranged from 0.024 (allyl isocyanate) to 8.34 ng/ml (1-hexanal); these values were dependent upon the variability of baseline blanks. The effect of LOD upon the overall interpretation of results is discussed in detail below.
Table 3.
Summary statistics (using lognormal distributions) of 28 PVOCs (ng/ml) detected in human urine across n = 120 samples from 28 human subjects
| Compound | LOD | # Imputed | GM | GSD | 95% FR | Min | p25 | median | p75 | p95 | p99 | Max |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Isovaleraldehyde | 0.379 | 0 | 3.798 | 2.374 | 29.66 | 0.662 | 2.000 | 4.149 | 6.490 | 14.70 | 36.71 | 39.32 |
| 2-Pentanone | 0.293 | 0 | 173.6 | 3.133 | 87.93 | 1.626 | 81.46 | 162.7 | 410.4 | 967.5 | 1513 | 2105 |
| 4-Methyl-2-pentanone | 0.125 | 0 | 4.323 | 2.612 | 43.13 | 0.208 | 2.387 | 4.424 | 8.224 | 16.67 | 20.66 | 26.03 |
| 2-Butanone | 0.605 | 1 | 69.27 | 2.389 | 30.38 | 7.037 | 35.70 | 64.64 | 118.1 | 301.5 | 452.6 | 465.8 |
| Pyrrole | 0.083 | 1 | 15.96 | 3.060 | 80.21 | 0.842 | 7.891 | 15.40 | 29.60 | 116.3 | 167.6 | 259.9 |
| 4-Heptanone | 0.417 | 1 | 62.02 | 5.505 | 801.0 | 0.701 | 21.38 | 70.24 | 214.5 | 731.8 | 2578 | 3678 |
| Dimethyl disulfide | 0.026 | 2 | 3.388 | 2.795 | 56.18 | 0.088 | 2.072 | 3.668 | 6.745 | 18.15 | 21.27 | 22.36 |
| Carvone | 0.614 | 5 | 8.430 | 9.183 | 5954 | 0.027 | 2.078 | 6.617 | 20.75 | 540.9 | 4129 | 5665 |
| 1-Pentanol | 0.138 | 6 | 0.754 | 2.697 | 48.85 | 0.056 | 0.344 | 0.816 | 1.597 | 3.118 | 3.417 | 19.03 |
| Pentanal | 0.660 | 8 | 5.657 | 2.987 | 72.90 | 0.324 | 3.017 | 6.130 | 10.69 | 27.80 | 108.9 | 116.6 |
| Allyl isothiocyanate | 0.024 | 16 | 0.611 | 16.95 | 6.58E+4 | <LOD | 0.097 | 0.596 | 2.940 | 98.71 | 732.7 | 735.1 |
| 2-Methyl-1-propanol | 1.402 | 24 | 6.176 | 7.680 | 2955 | <LOD | 1.761 | 4.402 | 39.23 | 103.0 | 344.0 | 1007 |
| Hexanal | 8.342 | 28 | 21.63 | 2.598 | 42.20 | <LOD | 10.10 | 22.17 | 46.70 | 86.83 | 113.5 | 118.5 |
| 3-Methyl-1-butanol | 0.237 | 37 | 0.525 | 4.539 | 376.1 | <LOD | <LOD | 0.452 | 1.633 | 7.618 | 15.94 | 28.09 |
| 2-Methyl-propanal | 1.782 | 40 | 3.632 | 3.019 | 76.00 | <LOD | <LOD | 3.514 | 7.909 | 18.05 | 40.10 | 43.53 |
| 2 - Ethoxy-2-methyl propane | 0.027 | 54 | 0.676 | 22.78 | 2.10E+5 | <LOD | 0.071 | 0.316 | 14.99 | 51.76 | 113.1 | 162.5 |
| 1-Propanol | 1.249 | 63 | 0.894 | 11.95 | 1.67E+4 | <LOD | <LOD | <LOD | 3.567 | 93.45 | 221.8 | 320.5 |
| Furfural | 0.401 | 63 | 0.413 | 2.423 | 32.10 | <LOD | <LOD | 0.407 | 0.745 | 1.705 | 2.584 | 3.114 |
| Benzaldehyde | 1.374 | 68 | 1.010 | 4.365 | 322.6 | <LOD | <LOD | <LOD | 2.405 | 15.01 | 21.15 | 23.62 |
| 1-Hexanol | 0.160 | 71 | 0.062 | 8.476 | 4351 | <LOD | <LOD | <LOD | 0.232 | 1.246 | 5.048 | 211.4 |
| Amylene hydrate | 3.210 | 74 | 6.763 | 4.242 | 288.5 | <LOD | <LOD | 6.469 | 21.98 | 58.87 | 173.5 | 435.6 |
| 2-Ethyl-1-butanol | 0.092 | 82 | 0.057 | 5.926 | 1070 | <LOD | <LOD | <LOD | 0.155 | 1.320 | 1.893 | 4.158 |
| Heptanal | 3.090 | 86 | 2.613 | 1.605 | 6.383 | <LOD | <LOD | <LOD | 3.497 | 5.624 | 7.760 | 8.236 |
| 4-Methyl-2-pentanol | 0.065 | 105 | 0.004 | 17.19 | 6.95E+4 | <LOD | <LOD | <LOD | <LOD | 0.453 | 2.397 | 4.622 |
| 1-Heptanol | 0.177 | 107 | 0.012 | 11.62 | 1.50E+4 | <LOD | <LOD | <LOD | <LOD | 0.727 | 2.064 | 7.511 |
| 1-Butanol | 1.360 | 112 | 0.004 | 69.14 | 1.63E+7 | <LOD | <LOD | <LOD | <LOD | 5.952 | 28.03 | 538.8 |
| Octanal | 18.01 | 114 | 6.957 | 2.007 | 15.34 | <LOD | <LOD | <LOD | <LOD | 21.45 | 33.99 | 43.82 |
| 3-Methyl-3-pentanol | 0.065 | 118 | 0.000 | 645.1 | 1.03E+11 | <LOD | <LOD | <LOD | <LOD | <LOD | 0.296 | 4.970 |
Abbreviations: FR: fold range; GM: geometric mean; GSD: geometric standard deviation; LOD: limit of detection; Max: maximum; Min: minimum; p25: 25th percentile; p75: 75th percentile; p95 95th percentile; p99: 99th percentile
Values below the LOD of each analyte were treated as left-censored data; we used multiple ordered value imputation to replace left-censored data with specific values calculated from a QQ-plot [37]. Furthermore, all distributions were assumed to be lognormal a-priori in accordance to previous work exploring biomarkers measurements [38] and were confirmed as such using statistical procedures developed specifically for this purpose [39].
Findings from previous work reported that that high-spiked samples (at 0.8 μg/mL) were well within the linear range of the analytical method and that room-air contamination was a negligible contributor to background levels. Method sensitivity was also evaluated previously by calculating extraction efficiency (EEf) using blank corrected values [26]. Briefly, aldehydes were captured by the Tenax tubes with EEfs greater than 95%. For the n-alcohols, extraction efficiencies were lower compared to the aldehydes and efficiencies were proportionate to the molecular weight of the alcohol (greater extraction efficiency as molecular weight increases). EEfs ranged from 42% to 78% for the C3 to C5 n- alcohols and from 88% to 94% for the heavier n-alcohols and for the branched alcohols. Certainly, EEF is an important parameter and needs to be explored further for different compounds and scenarios. However, we note that the EEf is only an indicator that a compound could be extracted from the medium, it does not affect quantitation as the blanks, controls, calibration and real-world samples are all processed identically and therefore this parameter divides out of the concentration calculations.
Results
Measurement parameters of PVOCs in urine
In the discovery phase of this study, 28 PVOCs were detected in human urine that could be measured and verified with certified standards. Blank controls (n=10) and human urine samples obtained from Ex-R study participants (n=120 across 28 participants) were then probed for the presence of the 28 PVOCs using the methodology described previously. When using aggressive manual integration, trace levels of PVOC analytes were found in all blank controls. Overall, background levels were very low for all analytes (geometric means of <3.5 ng/mL), with the exception of hexanal and octanal (geometric means of 13.5 ng/mL and 8.05 ng/mL, respectively). Similar findings were reported previously when developing this method for EBC [26].
Human subjects
Physical characteristics of participants in the urinary PVOC method development study and the full Ex-R cohort are reported in Table 2. Characteristics of the full cohort were originally reported in Morgan et al. [34] and are reported here for comparison. With regards to physical characteristics (age, weight, and height), average and range statistics demonstrate good representation among participants in our study when compared to the full Ex-R cohort. We note that the random selection procedure slightly under represented non-Hispanic White females. All samples were collected with informed consent.
Table 2.
Characteristics of the full Ex-R cohort and the subset of Ex-R subjects used in the urinary PVOC method development study: (for age, weight, height: mean, standard deviation, and range).
| Subset of Ex-R cohort for urinary PVOC method development | Full Ex-R cohort | |||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Characteristic | All | Females | Males | All | Females | Males |
| Sex (number) | 28 | 13 | 15 | 50 | 30 | 20 |
| Age (years) | 31.1 ±7.2 (19–47) | 33.4 ±6.6 (23–46) | 29.3 ±7.3 (19–47) | 33.4 ±9.1 (19–50) | 34.8 ±9.4 (21–50) | 31.3 ±8.5 (19–48) |
| Weight (Kg) | 84.4 ±19.1 (50.3–130) | 80.7 ±19.9 (57.6–130) | 88.2 ±18.4 (50.3–129) | 82.2 ±18.9 (48.1–130) | 75.6 ±17.9 (48.1–130) | 92.0 ±16.2 (57.6–130) |
| Height (cm) | 172 ± 9.9 (157–191) | 166 ±8.9 (157–191) | 179 ±6.9 (166–188) | 170 ±8.5 (149–191) | 165 ±5.8 (149–191) | 178 ±5.3 (166–188) |
| Race | ||||||
| Non-Hispanic White | 13 (46%) | 4 (14%) | 9 (32%) | 25 (56%) | 14 (31%) | 11 (25%) |
| Non-Hispanic Black | 6 (22%) | 4(14%) | 2 (7%) | 11 (25%) | 9 (20%) | 2 (4%) |
| Hispanic | 4 (14%) | 2 (7%) | 2 (7%) | 6 (13%) | 3 (7%) | 3 (7%) |
| Asian | 2 (7%) | 1 (4%) | 1 (4%) | 2 (4%) | 1 (2%) | 1 (2%) |
| Native American | 0 | 0 | 0 | 1 (2%) | 0 (0%) | 1 (2%) |
| Unknown | 3 (11%) | 2 (7%) | 1 (4%) | - | - | - |
Descriptive PVOCs statistics
Descriptive statistics for PVOCs measured from human urine samples are reported in Table 3. Notably, 12 PVOCs were consistently detected in all samples (i.e., less than 20% of samples required imputation), 23 out of 28 PVOCs were measured in urine samples at the 75th percentile, and all PVOCs were detectable at the 99th percentile value. These results are encouraging as PVOC levels may be influenced by the water content of urine, which can vary based on participant hydration status and other physiological factors. More sensitive instrumentation with lower LODs may detect these PVOCs more frequently.
Table 3 shows how specific LODs actually affect the value of the data. For example, isovaleraldyhyde (row 1) has a minimum real-world value at 0.662 ng/ml, well above the LOD = 0.379 ng/ml, the compound furfural (row 18) has a median value at 0.407 ng/ml with a similar LOD = 0.401 ng/ml and the compound 4-methyl-2-pentanol (row 24) can only be quantified at the 95th percentile at 0.453 ng/ml despite a very sensitive LOD = 0.065 ng/ml. As such, the importance of the method is better described by how much of the real-world distribution can be quantified rather than by the calculated method LOD.
Discussion
Several compounds that were identified using this method were ubiquitous in the patient population and may have utility as biomarkers. Table 4 highlights these compounds and their potential associations with health outcomes. 2-Pentanone, 4-heptanone, 2-butanone, and 4-methyl-2-pentanone have all been found consistently in urine from healthy individuals [40]. While the presence of these PVOCs in urine at normal levels may be benign, increases or decreases in the concentrations of these compounds that deviate from expected values may indicate the presence of a disease state. Furthermore, if the unremarkable population already has high variability, such PVOCs may be more difficult to use as biomarkers on an individual basis. Instead, patterns of certain PVOCs may become important as bioindicators of adverse health outcomes, or individual subjects may indeed be showing pre-clinical effects.
Table 4.
Characteristics and health associations of 12 PVOCs commonly detected in human urine. RCC: renal cell carcinoma; iMR: idiopathic membranous nephropathy; ADHD: attention-deficit/hyperactivity disorder.
| Compound | Associations with health outcomes | Source |
|---|---|---|
| Isovaleraldehyde (3-Methylbutanal) | Increased in urinary incontinence | [49] |
| 2-Pentanone | Showed differences in RCC compared to controls; increased in overweight/obese children; increased in iMR; present in most urine samples from healthy individuals; lung cancer | [40, 50–53] |
| 4-Methyl-2-pentanone | Present in most urine samples from healthy individuals; solvent exposure from paint, varnish, rubber cements, and adhesives | [40, 54] |
| 2-Butanone | Present in most urine samples from healthy individuals; lung cancer | [40, 53] |
| Pyrrole | Decreased in patients with mesangial proliferative glomerulonephritis; increased in patients with psychiatric disorders | [42, 44] |
| 4-Heptanone | Decreased in RCC; increased in iMR; present in most urine samples from healthy individuals; increased in diabetic patients | [40, 42, 45, 52] |
| Dimethyl disulfide | Generated by E. coli-increased in urinary tract infection; decreased in cancer patients | [23, 55] |
| Carvone | Derived from caraway seeds, considered beneficial for health; tumor inhibitor | [56] |
| 1-Pentanol | Most likely from solvent exposure; known neurotoxicant in humans | [57] |
| Pentanal | Increased in patients with prostate cancer | [58] |
| Allyl isothiocyanate | Ingested in cruciferous vegetables and has anti-cancer properties: inhibits bladder cancer | [47, 48] |
| 2-Methyl-1-propanol (Isobutanol) | Elevated in diabetes mellitus | [46, 59] |
Gaining insight from the variability of certain compounds within- and between-subjects is beyond the scope of this discovery study and would require many more samples and subjects. However, for now, the initial results indicate which compounds may have potential as pre-clinical biomarkers.
Some of the PVOCs, such as 2-pentanone and 4-heptanone, have been reported as biomarkers for many different diseases. For example, 4-heptanone has been previously shown to be increased in the blood and breath of patients with renal disease [41], but decreased levels of this PVOC have been observed in urine samples from such patients [42]. Decreased levels in urine suggest that patients are not able to excrete 4-heptanone, and the compound remains in other tissues to be released in blood and breath [42]. Increases in 4-heptanone and 2-pentanone have been identified in idiopathic membranous nephropathy (iMR), an autoimmune disease of the kidney, which may indicate the presence of oxidative stress. Elevation in 2-pentanone may be due to increased fatty acid β-oxidation [43]. Interestingly, elevated pyrrole levels have been associated with psychiatric disorders, such as schizophrenia, ADHD, and bipolar disorders. Elevated levels of pyrrole have also been shown to coordinate with increases in histamine in these individuals [44].
The presence of exogenous compounds in urine, such as the metabolites of phthalates, may not necessarily be disease indicators but byproducts of treatment and exposure. For example, 4-heptanone may be of exogenous origins [45], although it has been labeled as a biomarker for many diseases. 4-Heptanone is a known metabolite of di-(2-ethylhexyl)-phthalate, which is a common plasticizer [46]. While 4-heptanone has been shown to be increased in urine samples from diabetic patients, this may be due to exposure to plastic materials during hemodialysis [45]. Therefore, it is important to consider both endogenous and exogenous sources of urinary biomarkers, as individuals with increased urinary 4-heptanone levels may be experiencing plasticizer exposure instead of an endogenous change in metabolism.
In contrast, the elevation of some PVOCs in urine may not indicate an adverse health outcome, but can be advantageous. For example, allyl isothiocyanate has been shown to inhibit the growth of bladder cancer. Allyl isothiocyanate is found in cruciferous vegetables, and increased levels of this PVOC in urine may indicate ingestion of a healthy diet that will help prevent cancer growth [47, 48].
Conclusions
The PVOCs in urine represent a part of the human exposome reflecting recent metabolism, lipid peroxidation and oxidative stress. Understanding which compounds are commonly found, as well as their distribution, patterns and concentrations in relatively healthy humans is important for assessing statistical variances in individuals. Herein we presented a relatively straightforward methodology for documenting PVOCs in urine samples that can be quickly adapted by any laboratory that can make standard VOCs measurements in air. We find that many of the compounds are ubiquitous across subjects and as such likely represent common pathways of human metabolism. We further propose that individuals who deviate statistically from what is considered the unremarkable distribution, as indicated by the QQ-plots, should be considered as having a perturbation of their metabolism. Such observations could be critical in developing AOPs through more detailed assessments of recent activity, exposures, and exogenous biomarkers, however will require further detailed study.
The basic understanding gleaned from this work could be expanded in future work using more sophisticated analytical instrumentation and methods to confirm identities of compounds and to achieve a much more detailed assessment by greatly improving specificity and sensitivity. Recently, we have evaluated methodology to streamline the GC-MS methods shown here by implementing simultaneous targeted (SIM) and non-targeted (scan) analyses (SIM/scan mode), which could become an efficient augmentation to this work [33]. Furthermore, we have explored the advantages of using high-resolution mass spectrometry (HR-MS) to help refine the complexity of biomarker media [60] and the application of new non-targeted discovery software tools to parse out complex patterns from GC-MS results [32]. Also, we recommend that work continues for understanding the within- and between-person variability of biomarkers in humans to help assess the statistical likelihood of health related outliers and attendant risk [61]. In recent work, we have found that standard summary statistics of case-control or longitudinal biomarker studies may suffer from ambiguity resulting from unanticipated individual variance of response, and so we caution that future work considers testing data subsets for grouped responses at the individual level [62].
In conclusion, continuing to document subsets of biological exposome data is a crucial endeavor in understanding human systems biology. More sophisticated correlations with exogenous exposures and other external stressors will then allow us to assess the subtle biochemical perturbations that could lead to long-term health effects, identify AOPs in human physiology, and ultimately inform effective strategies protective of public health.
Supplementary Material
Acknowledgements:
The authors are grateful to Adam Biales, Andrew Lindstrom, Karen Oliver, and Michael Madden of US EPA, and William Funk of Northwestern University School of Medicine for expert advice. We are also grateful to Marsha Morgan of US EPA for organizing the original Ex-R study. This work was reviewed according to US EPA protocols and approved for publication. Reference to manufacturers and trade names are not recommendations for use.
Footnotes
Disclosure:
The authors assert that they have no conflicts of interest.
Supplemental Files:
Two supplemental files are provided separately. File #1 shows the graphical results of multiple value imputation for the urinary PVOCs. File #2 is a spreadsheet showing the identification information including names, database codes, retention times and primary ions for all identified compounds.
References
- 1.Wild CP, Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev, 2005. 14(8): p. 1847–50. [DOI] [PubMed] [Google Scholar]
- 2.Willett WC, Balancing Life-Style and Genomics Research for Disease Prevention. Science, 2002. 296. [DOI] [PubMed] [Google Scholar]
- 3.Heindel JJ and Vandenberg LN, Developmental origins of health and disease: a paradigm for understanding disease etiology and prevention. Current opinion in pediatrics, 2015. 27(2): p. 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lioy PJ and Rappaport SM, Exposure science and the exposome: an opportunity for coherence in the environmental health sciences. Environmental health perspectives, 2011. 119(11): p. a466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vineis P et al. , The exposome in practice: design of the EXPOsOMICS project. International journal of hygiene and environmental health, 2017. 220(2): p. 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rappaport SM, et al. , The blood exposome and its role in discovering causes of disease. Environmental health perspectives, 2014. 122(8): p. 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter DJ, Gene–environment interactions in human diseases. Nature Reviews Genetics, 2005. 6(4): p. 287. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y, et al. , The Exposome: Embracing the Complexity for Discovery in Environmental Health. Environ Health Perspect, 2016. 124(8): p. A137–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller GW and Jones DP, The nature of nurture: refining the definition of the exposome. Toxicol Sci, 2014. 137(1): p. 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pleil JD and Stiegel MA, Evolution of environmental exposure science: using breath-borne biomarkers for “discovery” of the human exposome. Anal Chem, 2013. 85(21): p. 9984–90. [DOI] [PubMed] [Google Scholar]
- 11.Vrijheid M, et al. , The human early-life exposome (HELIX): project rationale and design. Environ Health Perspect, 2014. 122(6): p. 535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel CJ, Bhattacharya J, and Butte AJ, An environment-wide association study (EWAS) on type 2 diabetes mellitus. PloS one, 2010. 5(5): p. e10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lind PM, et al. , An environmental wide association study (EWAS) approach to the metabolic syndrome. Environment international, 2013. 55: p. 1–8. [DOI] [PubMed] [Google Scholar]
- 14.Bell SM and Edwards SW, Identification and prioritization of relationships between environmental stressors and adverse human health impacts. Environmental health perspectives, 2015. 123(11): p. 1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Athersuch TJ and Keun HC, Metabolic profiling in human exposome studies. Mutagenesis, 2015. 30(6): p. 755–762. [DOI] [PubMed] [Google Scholar]
- 16.Pleil JD, Categorizing biomarkers of the human exposome and developing metrics for assessing environmental sustainability. Journal of Toxicology and Environmental Health, Part B, 2012. 15(4): p. 264–280. [DOI] [PubMed] [Google Scholar]
- 17.Hubal EAC, Biologically relevant exposure science for 21st century toxicity testing. Toxicological sciences, 2009. 111(2): p. 226–232. [DOI] [PubMed] [Google Scholar]
- 18.de Lacy Costello B, et al. , A review of the volatiles from the healthy human body. Journal of breath research, 2014. 8(1): p. 014001. [DOI] [PubMed] [Google Scholar]
- 19.Altemose B, et al. , Association of air pollution sources and aldehydes with biomarkers of blood coagulation, pulmonary inflammation, and systemic oxidative stress. Journal of Exposure Science and Environmental Epidemiology, 2017. 27(3): p. 244. [DOI] [PubMed] [Google Scholar]
- 20.Amann A, et al. , The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. Journal of breath research, 2014. 8(3): p. 034001. [DOI] [PubMed] [Google Scholar]
- 21.Kwak J and Preti G, Challenges in the Investigation of Volatile Disease Biomarkers in Urine. 2013, Elsevier: Amsterdam, The Netherlands. p. 394–404. [Google Scholar]
- 22.Coca S, et al. , Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney international, 2008. 73(9): p. 1008–1016. [DOI] [PubMed] [Google Scholar]
- 23.Silva C, Passos M, and Câmara J, Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. British journal of cancer, 2011. 105(12): p. 1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stiegel M, et al. , Kidney injury biomarkers and urinary creatinine variability in nominally healthy adults. Biomarkers, 2015. 20(6–7): p. 436–452. [DOI] [PubMed] [Google Scholar]
- 25.Mills GA and Walker V, Headspace solid-phase microextraction profiling of volatile compounds in urine: application to metabolic investigations. J Chromatogr B Biomed Sci Appl, 2001. 753(2): p. 259–68. [DOI] [PubMed] [Google Scholar]
- 26.Pleil JD, et al. , Volatile polar metabolites in exhaled breath condensate (EBC): collection and analysis. Journal of breath research, 2008. 2(2): p. 026001. [DOI] [PubMed] [Google Scholar]
- 27.Kleinstreuer NC, et al. , Adverse outcome pathways: from research to regulation scientific workshop report. Regulatory Toxicology and Pharmacology, 2016. 76: p. 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Villeneuve DL, et al. , Adverse outcome pathway (AOP) development I: strategies and principles. Toxicological Sciences, 2014. 142(2): p. 312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ankley GT, et al. , Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environmental Toxicology and Chemistry, 2010. 29(3): p. 730–741. [DOI] [PubMed] [Google Scholar]
- 30.Angrish MM, et al. , Taxonomic applicability of inflammatory cytokines in adverse outcome pathway (AOP) development. Journal of Toxicology and Environmental Health, Part A, 2016. 79(4): p. 184–196. [DOI] [PubMed] [Google Scholar]
- 31.Phillips KA, et al. , Suspect screening analysis of chemicals in consumer products. Environmental science & technology, 2018. 52(5): p. 3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobus JR, et al. , Integrating tools for non-targeted analysis research and chemical safety evaluations at the US EPA. Journal of exposure science & environmental epidemiology, 2017: p. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geer Wallace MA, et al. , Calibration and performance of synchronous SIM/scan mode for simultaneous targeted and discovery (non-targeted) analysis of exhaled breath samples from firefighters. Journal of Chromatography A, 2017. 1516(Supplement C): p. 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan MK, et al. , Temporal variability of pyrethroid metabolite levels in bedtime, morning, and 24-h urine samples for 50 adults in North Carolina. Environ Res, 2016. 144(Pt A): p. 81–91. [DOI] [PubMed] [Google Scholar]
- 35.Hubbard HF, et al. , Application of novel method to measure endogenous VOCs in exhaled breath condensate before and after exposure to diesel exhaust. J Chromatogr B Analyt Technol Biomed Life Sci, 2009. 877(29): p. 3652–8. [DOI] [PubMed] [Google Scholar]
- 36.Pleil JD, Vossler TL, McKlenny WA, Oliver KD, Optimizing sensitivity of SIM mode of GC/MS analysis for EPA’s TO-14 air toxics method. Journal of the Air & Waste Management Association, 1991. 41(3): p. 287–293. [Google Scholar]
- 37.Pleil JD, Imputing defensible values for left-censored ‘below level of quantitation’(LoQ) biomarker measurements. Journal of breath research, 2016. 10(4): p. 045001. [DOI] [PubMed] [Google Scholar]
- 38.Pleil JD et al., Estimating common parameters of lognormally distributed environmental and biomonitoring data: Harmonizing disparate statistics from publications. Journal of Toxicology and Environmental Health, Part B, 2014. 17(6): p. 341–368. [DOI] [PubMed] [Google Scholar]
- 39.Pleil JD, QQ-plots for assessing distributions of biomarker measurements and generating defensible summary statistics. Journal of breath research, 2016. 10(3): p. 035001. [DOI] [PubMed] [Google Scholar]
- 40.Mochalski P and Unterkofler K, Quantification of selected volatile organic compounds in human urine by gas chromatography selective reagent ionization time of flight mass spectrometry (GC-SRI-TOF-MS) coupled with head-space solid-phase microextraction (HS-SPME). Analyst, 2016. 141(15): p. 4796–4803. [DOI] [PubMed] [Google Scholar]
- 41.Halliwell B, Gutteridge JM, and Cross CE, Free radicals, antioxidants, and human disease: where are we now? The Journal of laboratory and clinical medicine, 1992. 119(6): p. 598–620. [PubMed] [Google Scholar]
- 42.Wang D, et al. , Urinary volatile organic compounds as potential biomarkers for renal cell carcinoma. Biomedical reports, 2016. 5(1): p. 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yancey M et al. , Urinary profiles of organic acids and volatile metabolites during the starvation process in rats. Journal of Chromatography B: Biomedical Sciences and Applications, 1986. 382: p. 3–18. [DOI] [PubMed] [Google Scholar]
- 44.Mikirova N, Cross-Sectional Analysis of Pyrroles in Psychiatric Disorders: Association With Nutritional and Immunological Markers. Journal of Orthomolecular Medicine, 2015. 30(1). [Google Scholar]
- 45.Wahl HG, et al. , 4-Heptanone is a metabolite of the plasticizer di (2-ethylhexyl) phthalate (DEHP) in haemodialysis patients. Nephrology dialysis transplantation, 2004. 19(10): p. 2576–2583. [DOI] [PubMed] [Google Scholar]
- 46.Amann A and Smith D, Volatile biomarkers: non-invasive diagnosis in physiology and medicine. 2013: Newnes. [Google Scholar]
- 47.Zhang Y, Allyl isothiocyanate as a cancer chemopreventive phytochemical. Molecular nutrition & food research, 2010. 54(1): p. 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhattacharya A, et al. , The principal urinary metabolite of allyl isothiocyanate, N-acetyl-S-(N-allylthiocarbamoyl) cysteine, inhibits the growth and muscle invasion of bladder cancer. Carcinogenesis, 2011. 33(2): p. 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandey SK, et al. , Major odorants released as urinary volatiles by urinary incontinent patients. Sensors, 2013. 13(7): p. 8523–8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monteiro M, et al. , GC‐MS metabolomics-based approach for the identification of a potential VOC-biomarker panel in the urine of renal cell carcinoma patients. Journal of cellular and molecular medicine, 2017. 21(9): p. 2092–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cozzolino R, et al. , Urinary volatile organic compounds in overweight compared to normal-weight children: results from the Italian I. Family cohort. Scientific Reports, 2017. 7(1): p. 15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang M, et al. , Urinary Volatile Organic Compounds as Potential Biomarkers in Idiopathic Membranous Nephropathy. Medical Principles and Practice, 2017. 26(4): p. 375–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antón AP., et al., Headspace-programmed temperature vaporization-mass spectrometry for the rapid determination of possible volatile biomarkers of lung cancer in urine. Analytical and bioanalytical chemistry, 2016. 408(19): p. 5239–5246. [DOI] [PubMed] [Google Scholar]
- 54.Wong KL, B12 4-Methyl-2~ Pentanone. Spacecraft Maximum Allowable Concentrations for Selected Airborne Contaminants, 2000. 4: p. 240. [Google Scholar]
- 55.Ratiu I-A, et al. , The effect of growth medium on an Escherichia coli pathway mirrored into GC/MS profiles. Journal of breath research, 2017. 11(3): p. 036012. [DOI] [PubMed] [Google Scholar]
- 56.Craig WJ, Health-promoting properties of common herbs. The American journal of clinical nutrition, 1999. 70(3): p. 491s–499s. [DOI] [PubMed] [Google Scholar]
- 57.Grandjean P and Landrigan PJ, Developmental neurotoxicity of industrial chemicals. The Lancet, 2006. 368(9553): p. 2167–2178. [DOI] [PubMed] [Google Scholar]
- 58.Khalid T, et al. , Urinary volatile organic compounds for the detection of prostate cancer. PloS one, 2015. 10(11): p. e0143283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liebich HM, et al. , Gas-chromatographic and mass-spectrometric detection of low-molecular-weight aliphatic alcohols in urine of normal individuals and patients with diabetes mellitus. Clinical chemistry, 1975. 21(9): p. 1294–1296. [PubMed] [Google Scholar]
- 60.Pleil JD and Isaacs KK, High-resolution mass spectrometry: basic principles for using exact mass and mass defect for discovery analysis of organic molecules in blood, breath, urine and environmental media. J Breath Res, 2016. 10(1): p. 012001. [DOI] [PubMed] [Google Scholar]
- 61.Pleil JD and Sobus JR, Estimating lifetime risk from spot biomarker data and intraclass correlation coefficients (ICC). Journal of Toxicology and Environmental Health, Part A, 2013. 76(12): p. 747–766. [DOI] [PubMed] [Google Scholar]
- 62.Stiegel MA., et al., Linking physiological parameters to perturbations in the human exposome: Environmental exposures modify blood pressure and lung function via inflammatory cytokine pathway. Journal of Toxicology and Environmental Health, Part A, 2017. 80(9): p. 485–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.