Abstract
As a new enabling nanotechnology tool for wireless, target-specific, and long-distance stimulation of mechanoreceptors in vivo, here we present a hydrogel magnetomechanical actuator (h-MMA) nanoparticle. To allow both deep-tissue penetration of input signals and efficient force-generation, h-MMA integrates a two-step transduction mechanism that converts magnetic anisotropic energy to thermal energy within its magnetic core (i.e., Zn0.4Fe2.6O4 nanoparticle cluster) and then mechanical energy to induce the surrounding polymer (i.e., pNiPMAm) shell contraction, finally delivering forces to activate targeted mechanoreceptors. We show that h-MMAs enable on-demand modulation of Notch signaling in both fluorescence reporter cell lines and a xenograft mouse model, demonstrating the utility as a powerful in vivo perturbation approach for mechanobiology interrogation in a minimally invasive and untethered manner.
Keywords: magnetic nanoparticle cluster, magnetic hyperthermia effect, thermoresponsive hydrogel, mechanosensitive receptor, perturbation biology
Tools for manipulating signaling, activity, and function of specific cells in living organisms have enormous potential to promote novel perturbation biology approaches and unprecedented therapeutic strategies1–4. The past decades have witnessed a drastic expansion in physical perturbation methods including nano/micro-electrode arrays5,6, microfluidics7,8, optogenetics9–11, upconversion nanoparticles12–15, thermogenetic16–20, sonogenetics21,22, and mechanogenetics23,24, enabling neuromodulation, stem cell differentiation, immune cell activation, and cell migration and adhesion with high spatiotemporal precision. However, despite the potential, broad in vivo applications of these tools have been lagged by technical limitations. Electrode- or light-based techniques require device implementation into target tissues due to the low tissue penetration depth of input signals25,26. Mechanogenetic tools based on force exerted by single magnetic particles under magnetic field gradient only allow short-distance operation, incompatible with animal studies27,28. On the other hand, the use of alternating or rotational magnetic field with uniform strength offers long-range stimulation of targeted receptors by converting magnetic anisotropy energy to thermal energy or torque, as demonstrated in magneto-thermogenetic and m-Torquer regulation of specific channel proteins including TRPV and Piezo1, respectively29–32. The development of a new tool, that exploits the long-distance operation of the uniform magnetic field while converting the magnetic energy to a different form of physical cue beyond heat or torque, will greatly enhance its useability and applicability to diverse cell signaling processes.
We particularly sought to develop a hydrogel magnetomechanical actuator (h-MMA) nanoparticle that eventually exerts mechanical tensile stress to target proteins in response to oscillatory magnetic field stimulation, as many mechanosensitive proteins relay the signal by unfolding the force-sensing domain upon the application of mechanical pulling33–38. Since the direct conversion of magnetic anisotropy energy to mechanical pulling is difficult, we employed a two-step mechanism – magnetic-to-thermal and thermal-to-mechanical transductions, wherein each transduction could be more straightforward by using magnetic nanoparticles and thermosensitive hydrogel polymers, respectively (Fig. 1a)39–61. Specifically, we designed an h-MMA comprised of a magnetic nanoparticle cluster (MNC) core surrounded by a poly N-isopropylmetylacrylamide (pNiPMAm) shell layer. We chose the MNC core to maximize heat generation per particle, while effectively localizing the energy to its surrounding shell layer (detailed discussion in Fig. 2). We also chose pNiPMAm as the shell layer of h-MMA, because of its volume-phase-transition (VPT) behaviors ideally suited for the envisioned application, which include 1) drastic size reduction (up to 75 %) upon VPT, 2) tunable critical transition temperature (Tc) near the body temperature, and 3) facile bio-functionalization. To fabricate h-MMA, we first assembled 13 nm Zn-doped iron oxide (Zn0.4Fe2.6O4) nanoparticles into a superlattice via oil-in-water microemulsion entrapping followed by evaporating the low-boiling point solvent in the oil phase62–66. MNCs were coated with a thin SiO2 (~10 nm) layer and then pNiPMAm via the Stöber method and radical polymerization, respectively (Fig. 1b, See methods for details)67. Figure 1c shows representative transmission electron microscope (TEM) images and dynamic light scattering analysis (inset) of MNCs (TEM: 238 ± 28.2 nm, hydrodynamic size: 284 ± 45.1 nm), MNC@SiO2 (TEM: 260 ± 37.8 nm, hydrodynamic size: 302 ± 83.1 nm), and MNC@SiO2@pNiPMAm (TEM: 700 ± 50.2 nm, hydrodynamic size: 817 ± 198 nm), confirming the high quality of particles with respect to cluster, size and shape uniformity, and colloidal stability, where these parameters were important for the desired downstream application. To estimate the number density of Zn0.4Fe2.6O4 nanoparticles per MNC, we performed tilted-angle TEM analyses of MNCs along different superlattice crystallographic directions. MNCs have a face-centered-cubic (FCC) cluster with a lattice constant of 20.8 nm suggesting approximately 3,136 particles per MNC (Fig. 1d). The FCC-structured MNCs exhibit maximized nanoparticle packing density (4.44 × 105 particles per µm3) and therefore higher magnetization (1.249 pemu per MNC @500 Oe), compared to random aggregate counterparts (3.60 × 105 particles per µm3, 0.982 pemu per random aggregate @500 Oe) (Fig. S1). Accordingly, when assessed magnetic-to-thermal energy conversion capacity via calorimetric bulk solution heating measurement under the application of alternating magnetic fields (AMF at 500 kHz, 500 Oe), a single MNC particle generated approximately 6.83 pW of individual particle power loss (IPLP), 2800 and 1.34 times stronger than a single Zn0.4Fe2.6O4 nanoparticle (2.44 fW) and a random aggregate (5.08 pW), respectively (Fig. 1e, Fig. S2, Supplementary Note 1).
Figure 1. Design, synthesis, and characterization of hydrogel magnetomechanical actuator (h-MMA) nanoparticles.
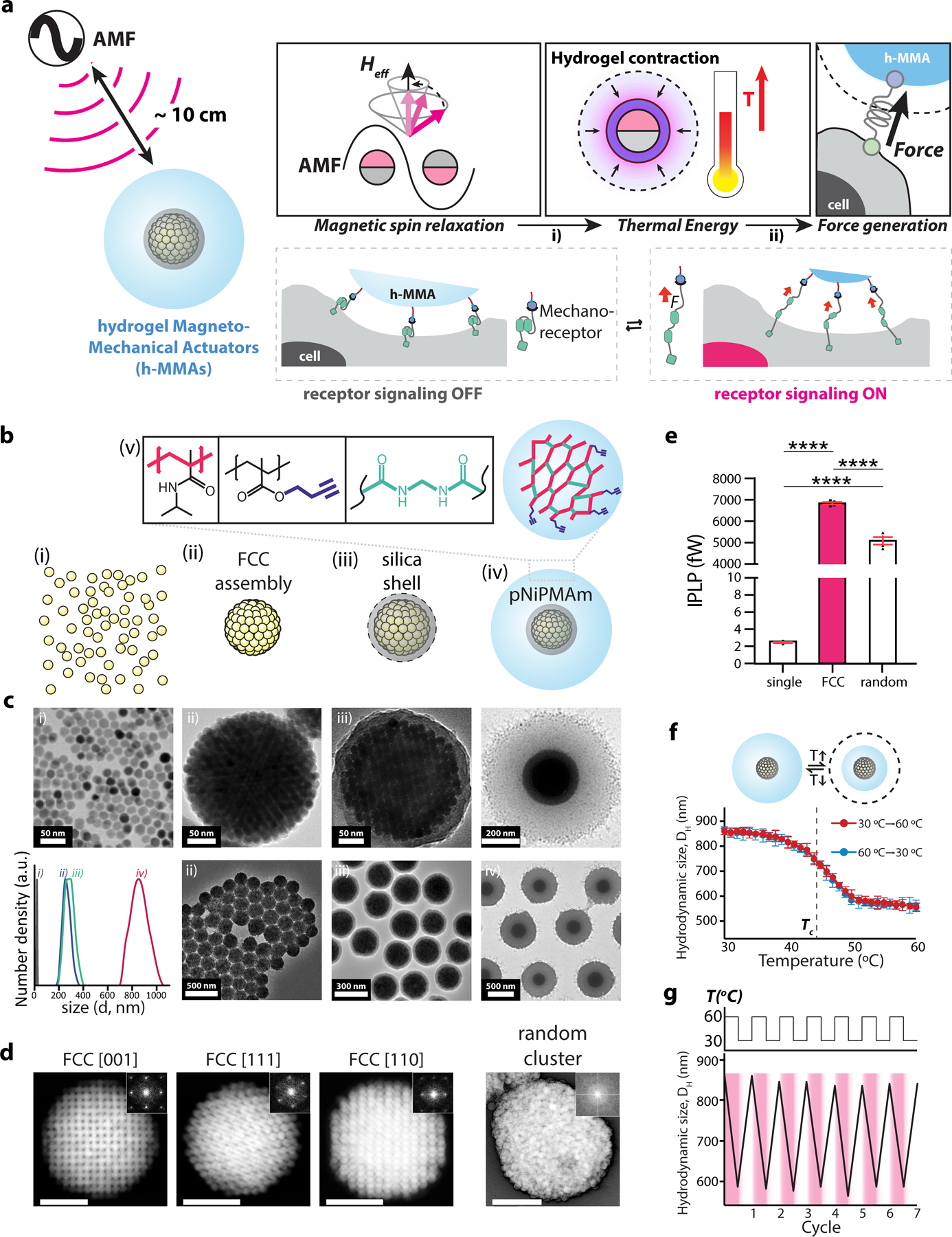
(a) Schematic illustration showing wireless control of targeted mechanoreceptors using h-MMAs. h-MMAs exert mechanical tensile stress to target proteins in response to alternating magnetic fields via a 2-step mechanism including i) magnetic-to-thermal and ii) thermal-to-mechanical transductions. (b, c) Schematics and TEM images of i) 13 nm Zn-doped iron oxide (Zn0.4Fe2.6O4) nanoparticles, ii) magnetic nanoparticle clusters (MNCs) in a face-centered cubic (FCC) superlattice structure, iii) MNC@SiO2, and iv) MNC@SiO2@pNiPMAm. v) Surface functionalization of h-MMAs with pNiPMAm polymer and alkyne functional group cross-linked by N, N’-methylenebisacrylamide. DLS spectra at each stage of h-MMA synthesis is also shown in the inset. (d) Scanning TEM images and reduced FFT images (insets) of an FCC cluster at different crystalline faces and of a random cluster. Scale bars = 100 nm. (e) Individual particle loss power (IPLP) values of single MNPs, FCC-structured MNCs, and random aggregates. Data are mean ± s.e.m. from n = 4 independent trials (****p<0.0001; one-way ANOVA followed by Tukey’s). (f) Temperature-dependent VPT of h-MMAs. The critical temperature (Tc) is marked with a black dotted line (43 ± 0.5 °C, n = 3). (g) Measurement of the hydrodynamic size of h-MMAs through temperature-controlled DLS measurement during 7 cycles of repeated heating and cooling.
Figure 2. Thermal polymerization of N-(2-aminoethyl)methacrylate (AEM) on MNC@SiO2 to investigate AMF-induced local heating effects by MNCs.
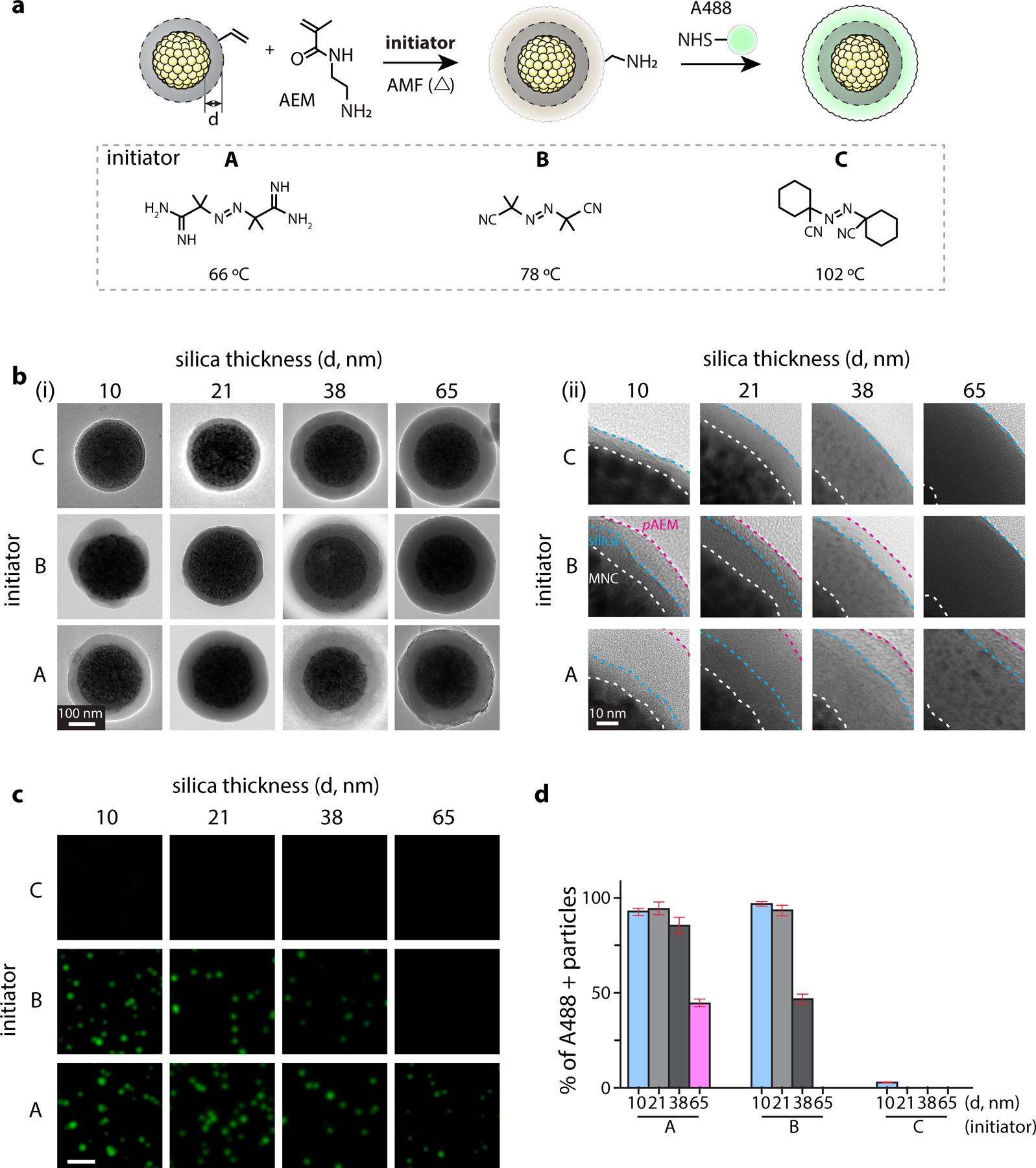
(a) (top) Schematic illustrations of radical polymerization of AEM and subsequent conjugation of amine-reactive fluorescence dyes within it. We varied the SiO2 layer thickness (d = 10, 21, 38, 65 nm) to investigate distance-dependent thermal decay from MNC surface. (bottom) Three thermo-labile azo-molecule initiators with varied decomposition temperatures for AEM polymerization: A, 2,2′-azobis(2-methylpropionamidine) dihydrochloride; B, 2,2′-azobis(2-methylpropionitrile); C, 1,1′-azobis(cyclohexanecarbonitrile). (b) Representative TEM images (Left: entire particle view, Right: zoom-in view) after AMF-stimulation in presence of respective MNC@SiO2 particles and initiators. Scale bar = 100 nm. Interface of MNC/SiO2, SiO2/pAEM, and AEM/vacuum (white dotted line) are marked by white, blue, magenta dotted lines, respectively. Scale bar = 10 nm. (c) Fluorescence signals of the Alexa488-positive particles prepared with radical polymerization conditions shown in Figure 2b and subsequent conjugation of A488–NHS. The images were acquired with a 488-nm laser and FITC emission filter. Scale bar = 2 µm. (d) Fraction analysis of A488-positive particles. Fluorescence-positive particles were identified by using a threshold calculated as mean ± 3×SD of basal signal intensity. Data are mean ± SD from n = 3 independent experiments.
We next tested the capacity of h-MMA (i.e., MNC@SiO2@pNiPMAm) to transduce heat to mechanical motion by measuring its hydrodynamic size via dynamic light scattering (DLS) while gradually increasing the bulk solution temperature. h-MMA exhibited a clear VPT near 43 °C (i.e., Tc), where the hydrodynamic size gradually decreases from approximately 858 nm to 556 nm with increasing temperature from 30 to 60 °C, respectively, suggesting the collapse of the pNiPMAm layer to 64.8 % of the original size (Fig. 1f)9,58,60,68. h-MMA showed stable (variance of the size < 30 nm @ 30 °C with no aggregation), reversible, and sustained VPT behaviors during repeated temperature cycles between 30 °C to 60 °C, confirming the high performance of h-MMAs as thermal-to-mechanical transduction (Fig. 1g, Fig. S3). Previous studies have reported that nanoscale heating of the particle core leads to volume-phase transition of pNiPMAm and the collapse of hydrogel particles on the time scale of ~100 nanoseconds, which allows for h-MMA applications of pN force58,60.
Together, these two transduction experiments (i.e., AMF-to-bulk heating and bulk heating-to-h-MMA contraction) indicate that h-MMAs are capable of converting an AMF input to the mechanical output signal via bulk solution heating using high particle concentration. However, prolonged bulk heating can cause many undesired consequences including cell/tissue damage, nonspecific activation of thermosensitive receptors, and perturbation of extracellular environments. We previously showed that, at a low particle concentration (0.1 mg/ml), magnetic nanoparticle (i.e., 15 nm CoFe2O4@MnFe2O4) produced local and transient heating to its vicinity (< 10 nm) (Fig. S4), finally facilitating radical polymerization of vinyl monomers69. We hypothesized that the MNC core could induce local heating with more extended ranges, while minimally influencing bulk solution temperature. To test this hypothesis, we synthesized a set of MNC@SiO2 particles with varied SiO2 layer thickness (d) of approximately 10 (9.7 ± 2.5), 21 (21.4 ± 3.2), 38 (38.3 ± 3.8), and 65 (64.8 ± 5.5) nm, and induced radical polymerization of N-(2-aminoethyl)methacrylate (AEM) under AMF application (500 kHz at 500 Oe, 30 min ON / 30 min OFF cycle for 4 times; Fig. 2a, Fig. S6). We employed a series of thermo-labile azo-molecule radical initiators (initiators A-C) with varied degradation temperatures of 66, 78, and 102 °C at a given experimental condition, respectively (Fig. 2a)70,71. We then assessed poly-AEM (pAEM) formation on MNC@SiO2 particles by TEM (Fig. 2b). When d equals or below 38 nm and initiators A or B were used, we observed the formation of an additional contrasted layer on MNC@SiO2, presumably corresponding to pAEM. With the initiator C, however, we observed no changes compared with original MNC@SiO2 particles, suggesting that temperature reached 78 °C but was below 102 °C at d ≦ 38 nm. When d = 65 nm, we detected the contrasted layer for initiators A but not for B or C, indicating that the temperature at this distance range is approximately 66–78 °C. To test whether the contrasted layer in TEM corresponds to pAEM or not, we further reacted as-synthesized particles with amine-reactive fluorescence dyes (Alexa 488-NHS) and counted fluorescence-positive fractions for respective MNC@SiO2 and initiator combinations under fluorescence microscopy (Fig. 2c, d, Fig. S7). Since the original MNC@SiO2 particle has no amine functional group but pAEM does, positive fluorescence signals after Alexa 488-NHS treatment indicate the pAEM layer formation. We observed fluorescence-positive particles only from the samples with the silica thickness and initiator combinations that show additional contrasted layers under TEM, confirming the formation of pAEM shells (Fig. 2d, Fig. S7). During AMF stimulation, changes in bulk solution temperature was minimal (Fig. S5). While exact distance-dependent temperature decay profiles from the MNC core remained to be determined, these results confirm that AMF stimulation of MNC can induce significant local heating (> 60 °C) over 60 nm distance ranges from the MNC surface. These results also suggest that AMF stimulation of h-MMA (i.e., MNC@SiO2@pNiPMAm) can induce VPT (Tc = 43 °C) of a substantial portion of the thermoresponsive layer (i.e., pNiPMAm).
We next examined whether h-MMAs can be used for the envisioned application: target-specific and long-range stimulation of mechanosensitive receptors in cells. As an initial study, we applied h-MMAs to control Notch1 signaling in a cell culture model. We previously showed that Notch1 is a true mechanoreceptor, where mechanogenetic stimulation of Notch1 resulted in its cell surface activation and downstream signaling24,72,73. We hypothesized that, when targeted to Notch1, h-MMAs can provide the same function while allowing long-range stimulation. To allow bio-targeting, fluorescence imaging, and minimal nonspecific binding, we conjugated h-MMAs with single-stranded oligonucleotides, fluorescence dyes (Alexa 488), and polyethylene glycol (PEG), respectively, via click chemistry (see Method sections for details). We then treated a fluorescence reporter U2OS cell line expressing SNAP-Notch1-Gal4 and UAS-H2B-mCherry with benzylguanine-functionalized oligonucleotides bearing complementary sequences (BG-DNA) and oligo-conjugated h-MMAs, sequentially (Fig. 3a). Robust green fluorescence signals were seen at the cell membrane under confocal fluorescence microscopy, suggesting the surface labeling of cells with h-MMA (Fig. S6). Control groups without BG-DNA, using h-MMAs without azide oligonucleotides, or using U2OS cells not expressing SNAP-Notch1 showed negligible fluorescence, confirming target-specific h-MMA labeling (Fig. S8). We then applied AMF (500 kHz at 500 Oe, 30 s ON / 2 min OFF cycle for 15 times, Fig. S9–11, S15) and measured reporter mCherry signals of the cells 24 hr post-stimulation (Fig. 3a). Cells treated with h-MMA and AMF stimulation showed robust nuclear mCherry signals (56.8% mCherry-positive cell fraction; 13.9-fold mean fluorescence intensity) comparable to the cells with Notch receptor-ligand engagement, while those treated with h-MMA (1.38 %; 0.63-fold) or AMF (1.29 %; 0.80-fold) alone showed negligible nuclear fluorescence (Fig. 3b–d, Fig. S12–15) 72,74. The pNiPMAm particle collapse in response to alternating magnetic field stimulation exerts > 13 pN force per receptor, which is adequate to mechanically activate Notch19,24,73. To directly assess surface activation of Notch, we performed a Western immunoblotting assay that detects cleaved Notch intracellular domain (NICD). Cells treated with both h-MMA and AMF stimulation produced a significantly increased amount of NICD (6.60-fold) compared to the control groups (Fig. 3e). In vitro AMF stimulation resulted in minimal bulk solution heating (< 1℃), which has no effect on cell signaling or on cell viability (Fig. S16, 17). These results demonstrate the capacity of h-MMA for specific and targeted stimulation of cells expressing mechanoreceptors.
Figure 3. AMF-induced activation of Notch signaling in a cell line model using h-MMA nanoparticles.
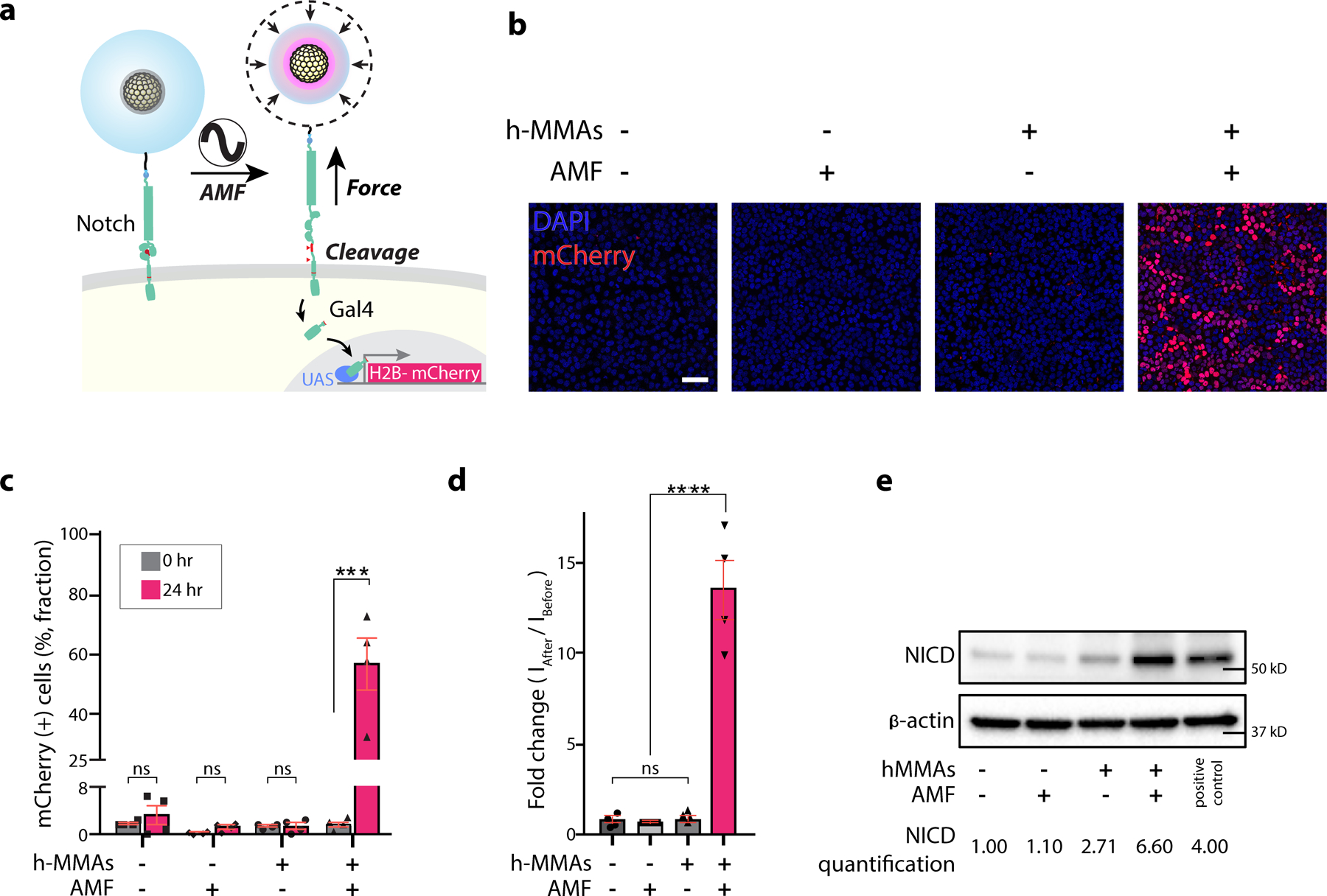
(a) A schematic illustration of h-MMAs induced activation of U2OS reporter cells expressing SNAP-Notch1-Gal4 and H2B-mCherry. h-MMAs specifically label Notch1 receptors via SNAP-benzylguanine (BG) chemistry. AMF stimulation (50 kHz at 500 Oe, 30 s ON / 2 min OFF cycle for 15 times) induced VPT and exerted mechanical force to Notch1. Mechanical stimulation of Notch1 triggers enzymatic cleavages of Notch1 and downstream transcription of H2B-mCherry. (b) Representative confocal fluorescence images of the reporter cells treated with h-MMA nanoparticles and AMF. Cells treated with no h-MMA or AMF were used as controls. Scale bar = 100 µm. (c) Percentage of the mCherry-positive cells with or without AMF stimulation or h-MMA treatment. Fluorescence-positive fractions were measured at (0 hr) and 24-hr post stimulation. Data are mean ± SD from n = 4 biological replicates (ns, non-significant; ***p < 0.001; two-tailed Student’s t-test). (d) Normalized fold-change in mCherry fluorescence signal observed at 24-hr post-AMF stimulation as compared to the baseline level for respective experimental conditions. Data are mean ± SD from n = 4 biological replicates (ns, non-significant; ****p < 0.0001; two-tailed Student’s t-test). (e) Immunoblot analysis of cleaved Notch intracellular domain (NICD). β-actin levels represent the loading control. The number below the gel images indicates the relative NICD band intensity. The intensity of each NICD band relative to the respective β-actin band was quantified and normalized to that of the control groups treated with no h-MMAs or AMF.
To demonstrate in vivo translation of these successful in vitro experiments, we next generated a xenograft mouse model implanted with fluorescence reporter Notch1-U2OS cells described above (Fig. 4a). To establish a bilateral tumor model, 4 × 106 SNAP-Notch1-Gal4 and UAS-H2B-mCherry expressing U2OS cells were subcutaneously implanted into both sides. Mice were provided with the doxycycline (Dox) diet (2 mg/mL) for 8 days to induce robust Notch1 expression in the xenografts (Fig. S18). We injected BG-conjugated h-MMAs intratumorally to the left xenograft site locally, while 30 cycles of AMF stimulation (30 s ON / 2 min OFF for total 75 min) were applied to both sites (Fig. 4a, Fig. S19). After 24 hrs, we sacrificed mice and extracted both tumor masses for immunofluorescence analysis. To detect nuclear mCherry expression, tumors were cryosectioned, immunostained with anti-mCherry antibodies, and imaged under confocal fluorescence microscopy. Consistent with the in vitro results, we observed robust mCherry fluorescence signals from the tumor treated with h-MMA and AMF but negligible signals from control groups without Dox, BG-h-MMAs, or applied AMF (Fig. 4b–d), supporting the capacity of h-MMAs for target- and AMF-specific modulation of mechanoreceptors in vivo. To test whether h-MMA causes side effects, we evaluated tissue inflammation and toxicity by immunohistochemical analyses against Iba1. No cytotoxicity or tissue inflammation was seen due to h-MMAs and/or AMF stimulation in vivo (Fig. S20, 21). While promising, biodistribution and systematic clearance of h-MMA have remained to be investigated for its potential clinical uses.
Figure 4. Minimally invasive remote control of cells expressing Notch receptors using h-MMA nanoparticles.
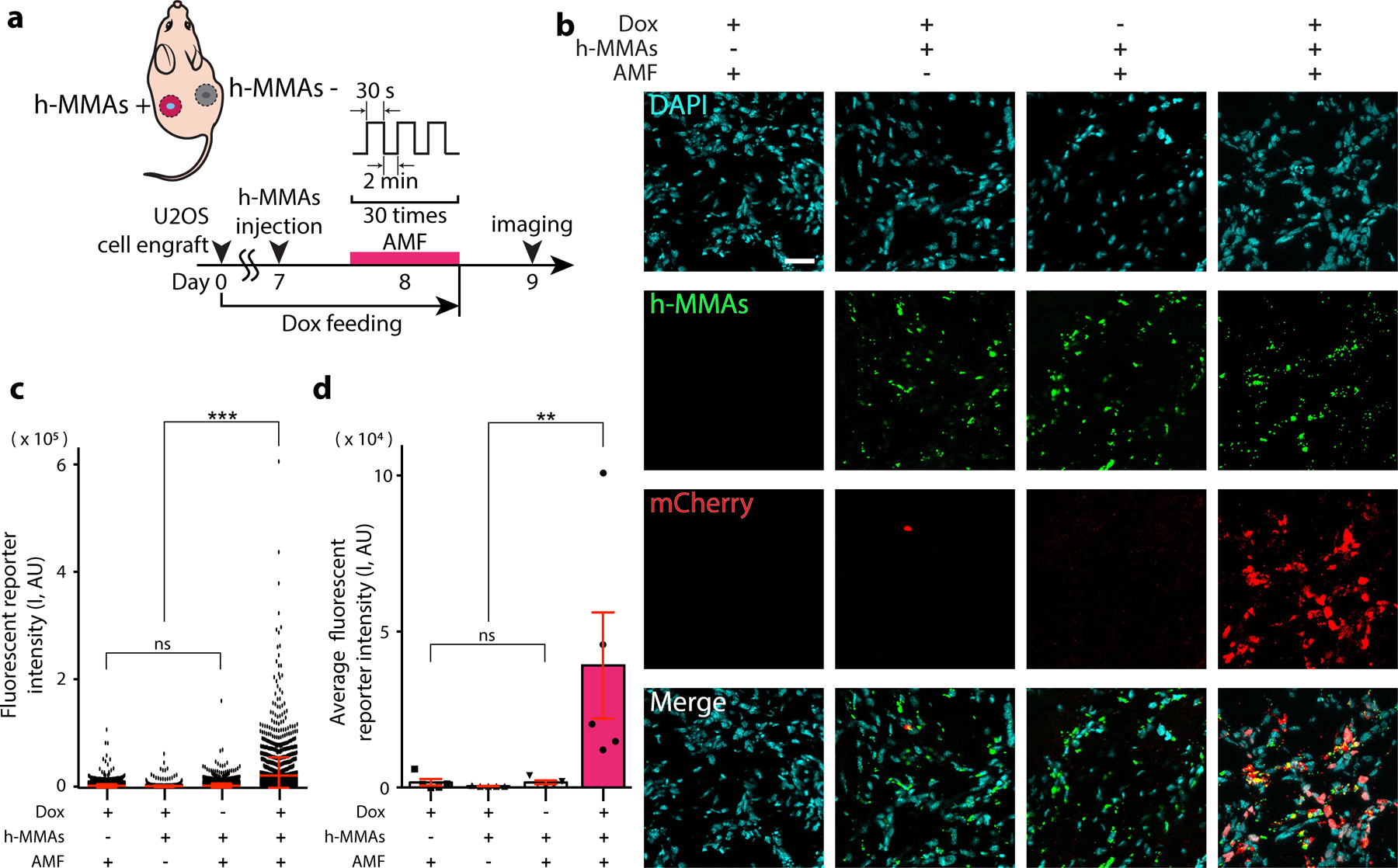
(a) A schematic illustration of AMF-induced h-MMA stimulation using a xenograft mouse model. Fluorescent reporter Notch1-U2OS cells were implanted on both sides of the mice on day 0. Mice were provided with a doxycycline (Dox, 2 mg/ml) diet for Notch receptor expression. One week later, h-MMAs were directly injected into the center of the xenograft, and AMF was applied to the xenograft position. On Day 9, mice were sacrificed and cryosectioned tissues were prepared for immunohistochemical analyses. (b) Representative confocal fluorescence images of tumor sections with AMF and h-MMA treatment. Substantial mCherry (red) expression was observed only in the presence of h-MMAs (green), Dox treatment, and AMF. DAPI (blue), Scale bar = 40 µm. Tumors with no AMF or h-MMA treatments were used as negative controls. (c) Quantification of nuclear mCherry signal per single cell in the representative slices where h-MMAs were localized. Data are mean ± s.e.m. from n = 2,300 cells from 5 animals (ns, non-significant; ***p<0.005; one-way ANOVA followed by Tukey’s). (d) Quantification of average nuclear mCherry fluorescence intensity. Data are mean ± SD from n = 5 animals (ns, non-significant; **p<0.005; one-way ANOVA followed by Tukey’s).
In summary, we developed a novel in vivo perturbation platform based on h-MMA nanoparticles. We demonstrated that h-MMA nanoparticles effectively convert magnetic anisotropy energy into mechanical tensile stress to the tethered target molecules via two-step processes involving magnetic-to-thermal and thermal-to-mechanical energy transductions. A similar two-step transduction approach using hydrogel optomechanical actuator nanoparticles for controlling mechanoreceptors in vitro has been reported previously9, but our h-MMA enabled robust deep-tissue stimulation of mechanoreceptors in living organisms using non-invasive and biologically transparent AMF-input. We showed a proof-of-concept study to regulate Notch receptors and downstream synthetic transcription signals, but this generalizable technique can be used to control and understand diverse mechanosensitive receptors in living organisms.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by Institute for Basic Science (IBS-R026-D1) (M.K. and J.C.), by National Research Foundation of Korea (NRF-2021R1F1A1063378) (M.K.), and by the National Institute of Health and the National Institute of General Medical Science (R35GM134948) (Y.J.).
ABBREVIATIONS
- h-MMA
magnetomechanical actuator
- m-Torquer
torque-generating magnetic nanoparticle
- MNC
magnetic nanoparticle cluster
- pNiPMAm
poly N-isopropylmetylacrylamide
- VPT
volume-phase-transition
- TEM
transmission electron microscope
- FCC
face-centered-cubic
- AMF
alternating magnetic fields
- IPLP
individual particle power loss
- DLS
dynamic light scattering
- FFT
Fast Fourier Transform
- AEM
N-(2-aminoethyl)methacrylate
- pAEM
poly-AEM
- BG
Benzylguanine
- NICD
Notch intracellular domain
- PEG
polyethylene glycol
- Dox
doxycycline
- IHC
immunohistochemistry
- ROI
region of interest
Footnotes
ASSOCIATED CONTENT
Supporting Information
Experimental setup for chemical synthesis of hydrogel magnetomechanical actuators (h-MMAs), characterization of h-MMAs, cell line generation and tissue culture, receptor-specific labeling of h-MMAs, in vitro experiments including h-MMA labeling, AMF stimulation, immunofluorescence staining, and immunoblotting assay, in vivo experiment including xenograft generation, in vivo h-MMA delivery and AMF stimulation, immunohistochemistry (IHC), and statistical analyses; Figures S1 – S21; Supplementary Note 1 for the calculation of IPLP values of h-MMA nanoparticles.
Notes
The authors declare no competing financial interests.
REFERENCES
- 1.Deisseroth K Optogenetics. Nat. Methods 2011, 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toettcher JE; Voigt CA; Weiner OD; Lim WA The Promise of Optogenetics in Cell Biology: Interrogating Molecular Circuits in Space and Time. Nat. Methods 2011, 8, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parnas O; Jovanovic M; Eisenhaure TM; Herbst RH; Dixit A; Ye CJ; Przybylski D; Platt RJ; Tirosh I; Sanjana NE; Shalem O; Satija R; Raychowdhury R; Mertins P; Carr SA; Zhang F; Hacohen N; Regev A A Genome-wide CRISPR Screen in Primary Immune Cells to Dissect Regulatory Networks. Cell 2015, 162 (3), 675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gero M The Optogenetic Catechism. Science 2009, 326 (5951), 395. [DOI] [PubMed] [Google Scholar]
- 5.Cogan SF Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng 2008, 10, 275. [DOI] [PubMed] [Google Scholar]
- 6.Guosong H; Lieber CM Novel Electrode Technologies for Neural Recordings. Nat. Rev. Neurosci 2019, 20 (6), 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sonnen KF; Merten CA Microfluidics as an Emerging Precision Tool in Developmental Biology. Dev. Cell 2019, 48 (3), 293. [DOI] [PubMed] [Google Scholar]
- 8.Sonnen KF; Lauschke VM; Uraji J; Falk HJ; Petersen Y; Funk MC; Beaupeux M; François P; Merten CA; Aulehla A Modulation of Phase Shift between Wnt and Notch Signaling Oscillations Controls Mesoderm Segmentation. Cell 2018, 172 (5), 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z; Liu Y; Chang Y; Seyf HR; Henry A; Mattheyses AL; Yehl K; Zhang Y; Huang Z; Salaita K Nanoscale Optomechanical Actuators for Controlling Mechanotransduction in Living Cells. Nat. Methods 2016, 13 (2), 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tischer D; Weiner OD Illuminating Cell Signalling with Optogenetic Tools Nat. Rev. Mol. Cell Biol 2014, 15 (8), 551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K; Cui B Optogenetic Control of Intracellular Signaling Pathways. Trends Biotechnol 2015, 33 (2), 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.All AH; Zeng X; Teh DBL; Yi Z; Prasad A; Ishizuka T; Thakor N; Hiromu Y; Liu X Expanding the Toolbox of Upconversion Nanoparticles for in vivo Optogenetics and Neuromodulation. Adv. Mater 2019, 31, 1803474. [DOI] [PubMed] [Google Scholar]
- 13.Chen S; Weitemier AZ; Zeng X; He L; Wang X; Tao Y; Huang AJY; Hashimotodani Y; Kano M; Iwasaki H; Parajuli LK; Okabe S; Teh DBL; All AH; Tsutsui-Kimura I; Tanaka KF; Liu X; McHugh TJ Near-Infrared Deep Brain Stimulation via Upconversion Nanoparticle–Mediated Optogenetics. Science 2018, 359, 679. [DOI] [PubMed] [Google Scholar]
- 14.Yi Z; All AH; Liu X Upconversion Nanoparticle-Mediated Optogenetics. Adv. Exp. Med. Biol 2021, 1293, 641. [DOI] [PubMed] [Google Scholar]
- 15.Ao Y; Zeng K; Yu B; Miao Y; Hung W; Yu Z; Xue Y; Tan TTY; Xu T; Zhen M; Yang X; Zhang Y; Gao S An Upconversion Nanoparticle Enables Near Infrared-Optogenetic Manipulation of the Caenorhabditis Elegans Motor Circuit. ACS Nano 2019, 13 (3), 3373. [DOI] [PubMed] [Google Scholar]
- 16.Chen R; Romero G; Christiansen MG; Mohr A; Anikeeva P Wireless Magnetothermal Deep Brain Stimulation. Science 2015, 347 (6229), 1477. [DOI] [PubMed] [Google Scholar]
- 17.Su H; Brockman JM; Duan Y; Sen N; Chhabra H; Bazrafshan A; Blanchard AT; Meyer T; Andrews B; Doye JPK; Ke Y; Dyer RB; Salaita K Massively Parallelized Molecular Force Manipulation with On-Demand Thermal and Optical Control. J. Am. Chem. Soc 2021, 143 (46), 19466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwizera EA; Stewart S; Mahmud MM; He X Magnetic Nanoparticle-Mediated Heating for Biomedical Applications. J. Heat Transfer 2022, 144 (3), 030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero G; Christiansen MG; Barbosa LS; Garcia F; Anikeeva P Localized Excitation of Neural Activity via Rapid Magnetothermal Drug Release. Adv. Funct. Mater 2016, 26, 6471. [Google Scholar]
- 20.Rosenfeld D; Senko AW; Moon J; Yick I; Varnavides G; Gregureć D; Koehler F; Chiang PH; Christiansen MG; Maeng LY; Widge AS; Anikeeva P Transgene-Free Remote Magnetothermal Regulation of Adrenal Hormones. Sci. Adv 2020, 6, eaaz3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maresca D; Lakshmanan A; Abedi M; Bar-Zion A; Farhadi A; Lu GJ; Szablowski JO; Wu D; Yoo S; Shapiro MG Biomolecular Ultrasound and Sonogenetics. Annu. Rev. Chem. Biomol. Eng 2018, 9, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan CH; Wei KC; Chiu NH; Liao EC; Wang HC; Wu RY; Ho YJ; Chan HL; Wang TSA; Huang YZ; Hsieh TH; Lin CH; Lin YC; Yeh CK Sonogenetic-Based Neuromodulation for the Amelioration of Parkinson’s Disease. Nano Lett 2021, 21 (14), 5967. [DOI] [PubMed] [Google Scholar]
- 23.Zhu L; Wu Y; Yoon CW; Wang Y Mechanogenetics for Cellular Engineering and Cancer Immunotherapy. Curr. Opin. Biotechnol 2020, 66, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo D; Southard KM; Kim JW; Lee HJ; Farlow J; Lee JU; Litt DB; Haas T; Alivisatos AP; Cheon J; Gartner ZJ; Jun YW A Mechanogenetic Toolkit for Interrogating Cell Signaling in Space and Time. Cell 2016, 165, 1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perlmutter JS; Mink JW Deep Brain Stimulation. Annu. Rev. Neurosci 2006, 29, 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montgomery KL Yeh AJ; Ho JS; Tsao V; Iyer SM; Grosenick L; Ferenczi EA; Tanabe Y; Deisseroth K; Delp SL; Poon ASY Wirelessly Powered, Fully Internal Optogenetics for Brain, Spinal and Peripheral Circuits in Mice. Nat. Methods 2015, 12 (10), 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JW; Seo D; Lee JU; Southard KM; Lim Y; Kim D; Gartner ZJ; Jun YW; Cheon J Single-Cell Mechanogenetics Using Monovalent Magnetoplasmonic Nanoparticles. Nat. Protoc 2017, 12, 1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwak M; Gu W; Jeong H; Lee H; Lee JU; An M; Kim YH; Lee JH; Cheon J; Jun YW Small, Clickable, and Monovalent Magnetofluorescent Nanoparticles (MFNs) Enable Mechanogenetic Regulation of Receptors in a Crowded Live Cell Microenvironment. Nano Lett 2019, 19, 3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanley SA; Kelly L; Latcha KN; Schmidt SF; Yu X; Nectow AR; Sauer J; Dyke JP; Dordick JS; Friedman JM Bidirectional Electromagnetic Control of the Hypothalamus Regulates Feeding and Metabolism. Nature 2016, 531 (7596), 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munshi R; Qadri SM; Zhang Q; Rubio IC; Pino P; Pralle A Magnetothermal Genetic Deep Brain Stimulation of Motor Behaviors in Awake, Freely Moving Mice. Elife 2017, 6, e27069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JU; Shin W; Lim Y; Kim J; Kim WR; Kim H; Lee JH; Cheon J Non-Contact Long-Range Magnetic Stimulation of Mechanosensitive Ion Channels in Freely Moving Animals. Nat. Mater 2021, 20, 1029. [DOI] [PubMed] [Google Scholar]
- 32.Shin W; Jeong S; Lee JU; Jeong SY; Shin J; Kim HH; Cheon J; Lee JH Magnetogenetics with Piezo1 Mechanosensitive Ion Channel for CRISPR Gene Editing. Nano Lett 2022, 22 (18), 7415. [DOI] [PubMed] [Google Scholar]
- 33.Wang X; Ha TJ Defining Single Molecular Forces Required to Activate Integrin and Notch Signaling. Science 2013, 340 (6135), 991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jo MH; Li J; Jaumouillé V; Hao Y; Coppola J; Yan J; Waterman CM; Springer TA; Ha TJ Single-Molecule Characterization of Subtype-Specific β1 Integrin Mechanics. Nat. Commun 2022, 13 (1), 7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dutta PK; Zhang Y; Blanchard AT; Ge C; Rushdi M; Weiss K; Zhu C; Ke Y; Salaita K Programmable Multivalent DNA-Origami Tension Probes for Reporting Cellular Traction Forces. Nano Lett 2018, 18, 4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramey-Ward AN; Su H; Salaita K Mechanical Stimulation of Adhesion Receptors Using Light-Responsive Nanoparticle Actuators Enhances Myogenesis. ACS Appl. Mater. Interfaces 2020, 12 (32), 35903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong J; Ge C; Jothikumar P; Yuan Z; Liu B; Bai K; Li K; Rittase W; Shinzawa M; Zhang Y; Palin A; Love P; Yu X; Salaita K; Evavold BD; Singer A; Zhu C A TCR Mechanotransduction Signaling Loop Induces Negative Selection in the Thymus. Nat. Immunol 2018, 19, 1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y; Li Z; Ju LA Tensile and Compressive Force Regulation on Cell Mechanosensing. Biophys. Rev 2019, 11, 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Périgo EA; Hemery G; Sandre O; Ortega D; Garaio E; Plazaola F; Teran FJ Fundamentals and Advances in Magnetic Hyperthermia. Appl. Phys. Rev 2015, 2, 041302. [Google Scholar]
- 40.Sebesta C; Hinojosa DT; Wang B; Asfouri J; Li Z; Duret G; Jiang K; Xiao Z; Zhang L; Zhang Q; Colvin VL; Goetz SM; Peterchev AV; Dierick HA; Bao G; Robinson JT Subsecond Multichannel Magnetic Control of Select Neural Circuits in Freely Moving Flies. Nat. Mater 2022, 21, 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosensweig RE Heating Magnetic Fuid with Alternating Magnetic Field. J. Magn. Magn. Mater 2002, 252, 370. [Google Scholar]
- 42.Lee JH; Jang JT; Choi JS; Moon SH; Noh SH; Kim JW; Kim JG; Kim IS; Park KI; Cheon J Exchange-Coupled Magnetic Nanoparticles for Efficient Heat Induction. Nat. Nanotechnol 2011, 6, 418. [DOI] [PubMed] [Google Scholar]
- 43.Jang JT; Nah H; Lee JH; Moon SH; Kim MG; Cheon J Critical Enhancements of MRI Contrast and Hyperthermic Effects by Dopant-Controlled Magnetic Nanoparticles. Angew. Chem. Int. Ed. Engl 2009, 48, 1234. [DOI] [PubMed] [Google Scholar]
- 44.Cardellini A; Fasano M; Bigdeli MB; Chiavazzo E; Asinari P Thermal Transport Phenomena in Nanoparticle Suspensions. J. Phys. Condens. Matter 2016, 28, 483003. [DOI] [PubMed] [Google Scholar]
- 45.Obaidat IM; Issa B; Haik Y Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5 (1), 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shubitidze F; Kekalo K; Stigliano R; Baker I Magnetic Nanoparticles with High Specific Absorption Rate of Electromagnetic Energy at Low Field Strength for Hyperthermia Therapy. J. Appl. Phys 2015, 117, 094302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cobianchi M; Guerrini A; Avolio M; Innocenti C; Corti M; Arosio P; Orsini F; Sangregorio C; Lascialfari A Experimental Determination of the Frequency and Feld Dependence of Specific Loss Power in Magnetic Fluid Hyperthermia. J. Magn. Magn. Mater 2017, 444, 154. [Google Scholar]
- 48.Mohapatra J; Xing M; Liu JP Inductive Thermal Effect of Ferrite Magnetic Nanoparticles. Materials 2019, 12 (19), 3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rytov RA; Bautin VA; Usov NA Towards Optimal Thermal Distribution in Magnetic Hyperthermia. Sci. Rep 2022, 12, 3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christiansen MG; Senko AW; Chen R; Romero G; Anikeeva P Magnetically Multiplexed Heating of Single Domain Nanoparticles. Appl. Phys. Lett 2014, 104, 213103. [Google Scholar]
- 51.Ovejero JG; Armenia I; Serantes D; Veintemillas-Verdaguer S; Zeballos N; López-Gallego F; Grüttner C; Fuente JM; Morales MP; Grazu V Selective Magnetic Nanoheating: Combining Iron Oxide Nanoparticles for Multi-Hot-Spot Induction and Sequential Regulation. Nano Lett 2021, 21, 7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stolarczyk JK; Deak A; Brougham DF Nanoparticle Clusters: Assembly and Control Over Internal Order, Current Capabilities, and Future Potential. Adv. Mater 2016, 28, 5400. [DOI] [PubMed] [Google Scholar]
- 53.Singamaneni S; Bliznyuk VN; Binek C; Tsymbal EY Magnetic Nanoparticles: Recent Advances in Synthesis, Self-Assembly and Applications. J. Mater. Chem 2011, 21, 16819. [Google Scholar]
- 54.Polo-Corrales L; Rinaldia C Monitoring Iron Oxide Nanoparticle Surface Temperature in an Alternating Magnetic Field Using Thermoresponsive Fluorescent Polymers. J. Appl. Phys 2012, 111, 07B334. [Google Scholar]
- 55.Guibert C; Dupuis V; Peyre V; Fresnais J Hyperthermia of Magnetic Nanoparticles: Experimental Study of the Role of Aggregation. J. Phys. Chem. C. Nanomater. Interfaces 2015, 119 (50), 28148. [Google Scholar]
- 56.Serantes D; Simeonidis K; Angelakeris M; Chubykalo-Fesenko O; Marciello M; Morales MDP; Baldomir D; Martinez-Boubeta C Multiplying Magnetic Hyperthermia Response by Nanoparticle Assembling. J. Phys. Chem. C Nanomater. Interfaces 2014, 118 (11), 5927. [Google Scholar]
- 57.Sakellari D; Brintakis K; Kostopoulou A; Myrovali E; Simeonidis K; Lappas A; Angelakeris M Ferrimagnetic Nanocrystal Assemblies as Versatile Magnetic Particle Hyperthermia Mediators. Mater. Sci. Eng. C 2016, 58, 187. [DOI] [PubMed] [Google Scholar]
- 58.Zhao J; Su H; Vansuch GE; Liu Z; Salaita K; Dyer RB Localized Nanoscale Heating Leads to Ultrafast Hydrogel Volume-Phase Transition. ACS Nano 2019, 13 (1), 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dong Y; Bazrafshan A; Pokutta A; Sulejmani F; Sun W; Combs JD; Clarke KC; Salaita K Chameleon-Inspired Strain-Accommodating Smart Skin. ACS Nano 2019, 13, 9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su H; Liu Z; Liu Y; Ma VP; Blanchard A; Zhao J; Galior K; Dyer RB; and Salaita K Light-Responsive Polymer Particles as Force Clamps for the Mechanical Unfolding of Target Molecules. Nano Lett 2018, 18, 2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang H; Delikanli S; Zeng H; Ferkey DM; Pralle A Remote Control of Ion Channels and Neurons through Magnetic-Field Heating of Nanoparticles. Nat. Nanotechnol 2010, 5, 602. [DOI] [PubMed] [Google Scholar]
- 62.Nai J; Wang S; Lou XWD Ordered Colloidal Clusters Constructed by Nanocrystals with Valence for Efficient CO2 Photoreduction. Sci. Adv 2019, 5, eaax5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teich EG; Anders GV; Klotsa D; Dshemuchadse J; Glotzer SC Clusters of Polyhedra in Spherical Confinement. Proc. Natl. Acad. Sci. U. S. A 2016, 113 (6), E669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ganesan V; Lahiri BB; Louis C; Philip J; Damodaran SP Size-Controlled Synthesis of Superparamagnetic Magnetite Nanoclusters for Heat Generation in an Alternating Magnetic Field. J. Mol. Liq 2019, 281, 315. [Google Scholar]
- 65.Wang Y; Xu F; Zhang C; Lei D; Tang Y; Xu H; Zhang Z; Lu H; Du X; Yang GY High MR Sensitive Fluorescent Magnetite Nanocluster for Stem Cell Tracking in Ischemic Mouse Brain. Nanomedicine 2011, 7, 1009. [DOI] [PubMed] [Google Scholar]
- 66.Isojima T; Suh SK; Vander Sande JB; Hatton TA Controlled Assembly of Nanoparticle Structures: Spherical and Toroidal Superlattices and Nanoparticle-Coated Polymeric Beads. Langmuir 2009, 25 (14), 8292. [DOI] [PubMed] [Google Scholar]
- 67.Fu R; Jin X; Liang J; Zheng W; Zhuang J; Yang W Preparation of Nearly Monodispersed Fe3O4/SiO2 Composite Particles from Aggregates of Fe3O4 Nanoparticles. J. Mater. Chem 2011, 21, 15352. [Google Scholar]
- 68.Ramey-Ward AN; Su H; Salaita K Mechanical Stimulation of Adhesion Receptors Using Light-Responsive Nanoparticle Actuators Enhances Myogenesis. ACS Appl. Mater. Interfaces 2020, 12 (32), 35903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lim Y; Noh SH; Shin TH; Lee JU; Lungerich D; Lee JH; Cheon J Magnetothermally Activated Nanometer-level Modular Functional Group Grafting of Nanoparticles. Nano Lett 2021, 21, 3649. [DOI] [PubMed] [Google Scholar]
- 70.Azo Polymerization Initiators Comprehensive Catalog. FUJIFILM Wako Pure Chemical Corporation.
- 71.Riedinger A; Guardia P; Curcio A; Garcia MA; Cingolani R; Manna L; Pellegrino T Subnanometer Local Temperature Probing and Remotely Controlled Drug Release Based on Azo-Functionalized Iron Oxide Nanoparticles. Nano Lett 2013, 13, 2399. [DOI] [PubMed] [Google Scholar]
- 72.Kopan R; Ilagan XG The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell 2009, 137 (2) 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gordon WR; Zimmerman B; He L; Miles LJ; Huang J; Tiyanont K; McArthur DG; Aster JC; Perrimon N; Loparo JJ; Blacklow SC Mechanical Allostery: Evidence for a Force Requirement in the Proteolytic Activation of Notch. Dev. Cell 2015, 33 (6), 729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tagami S; Okochi M; Yanagida K; Ikuta A; Fukumori A; Matsumoto N; Ishizuka-Katsura Y; Nakayama T; Itoh N; Jiang J; Nishitomi K; Kamino K; Morihara T; Hashimoto R; Tanaka T; Kudo T; Chiba S; Takeda M Regulation of Notch Signaling by Dynamic Changes in the Precision of S3 Cleavage of Notch-1. Mol. Cell Biol 2008, 28, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


