Abstract
Background
Early diagnosis of late-onset sepsis (LOS) and necrotizing enterocolitis (NEC) by monitoring heart rate characteristics (HRC) of preterm infants might reduce the risk of death and morbidities. We aimed to systematically assess the effects of HRC monitoring on death, LOS, and NEC.
Methods
A systematic search was performed in MEDLINE, Embase, Cochrane Library, and Web of Science.
Results
Fifteen papers were included in this review. Three of these papers reported results from the only identified randomized controlled trial (RCT). This RCT showed that HRC monitoring resulted in a small but significant reduction in mortality (absolute risk reduction 2.1% [95% confidence interval 0.01–4.14]) without any differences in neurodevelopmental impairment. The risk of bias was rated high due to performance and detection bias and failure to correct for multiple testing. Most diagnostic cohort studies showed high discriminating accuracy in predicting LOS but lacked sufficient quality and generalizability. No studies for the detection of NEC were identified.
Conclusion
Supported by multiple observational cohort studies, the RCT identified in this systematic review showed that HRC monitoring as an early warning system for LOS might reduce the risk of death in preterm infants. However, methodological weaknesses and limited generalizability do not justify implementation of HRC in clinical care. A large international RCT is warranted.
Keywords: Preterm infants, Heart rate, Sepsis, Monitoring
Introduction
Due to advances in neonatal care, the overall mortality among preterm infants has declined significantly over the past decades [1, 2]. In addition, there has been a shift in mortality cause from respiratory failure toward infectious diseases such as late-onset sepsis (LOS) and necrotizing enterocolitis (NEC) [2]. Early diagnosis and treatment of LOS and NEC have been suggested to prevent clinical deterioration with a positive effect on intact survival in this vulnerable population.
However, one of the difficulties clinicians face in daily practice is that most preterm infants suffering from sepsis or NEC show nonspecific or unclear clinical symptoms with potential delay in antibiotic treatment, resulting in clinical deterioration with possible fatal outcome. A real-time noninvasive continuous monitor system using an algorithm detecting changes in heart rate characteristics (HRC) has been suggested as an early predictor of various pathologies including sepsis and NEC [3–5]. Starting antibiotics earlier based on HRC data might have a positive effect on the outcome of preterm infants. Several reports on the predictive power of HRC have resulted in a commercially available bedside monitoring tool HeRO monitor (Medical Predictive Science Corporation [MSCP]; Charlottesville, VA, USA), providing real-time data on HRC variability [6, 7]. Although this modality is increasingly being implemented in daily care in many neonatal units across the world, no systematic review exists summarizing the diagnostic accuracy and the effect of the use of HRC monitoring in preterm infants with sepsis or NEC. The aim of this systematic review was to identify, appraise, and summarize all available evidence on the effect of all HRC monitoring on the prediction of LOS and NEC in preterm infants, its impact on associated mortality, neonatal morbidity, and the rate of antibiotic use.
Methods
Protocol and Registration
The protocol for this systematic review was registered prospectively with PROSPERO (ID number CRD42021234320). The manuscript was written in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses of Diagnostic Test Accuracy studies (PRISMA-DTA) checklist (online suppl. 1; for all online suppl. material, see https://doi.org/10.1159/000531118).
Search Methods for Identification of Studies
The electronic search for relevant articles was accomplished on November 18, 2022, by a medical information specialist including the MEDLINE and Embase via the Ovid platform, the Cochrane Library, and Web of Science databases. The search included terms for “preterm infants,” “heart rate characteristics,” and synonyms (online suppl. 2). No language restrictions were applied, but the search was restricted for study design. Publications before the year 2000 were not included in this review because we anticipated that the digital technology was not available at that time. Reference lists of the identified studies were screened for other eligible studies.
Criteria for Considering Studies for This Review
Studies eligible for inclusion in this review were systematic reviews, randomized controlled trials (RCTs), cohort studies, case-control studies, crossover studies, case series. Studies were eligible if they (a) included preterm infants, (b) compared the use of HRC (both the commercially available HeRO monitor as well as noncommercially available analyses) versus standard monitoring, with (c) LOS and NEC-related mortality and morbidity as outcome measurements. Fetal heart studies investigating the effects of antenatal HRC and postnatal outcomes as hypoxic ischemic encephalopathy, asphyxia, or intraventricular hemorrhage were excluded. In addition, animal studies and case reports were excluded.
Selection of Studies, Data Collection, and Analysis
Studies eligible for this review were divided into three groups: “not relevant,” “maybe,” and “relevant.” Two authors (H.K. and C.L.) selected the articles independently and sorted them by classification. Any differences between the two independent classifications were resolved by discussion and consensus. Of the articles belonging to the “relevant” and “maybe” articles, an abstract review or a full-text review was done for further evaluation. The primary outcome was mortality until hospital discharge. Secondary outcomes were LOS (culture proven and clinical sepsis), NEC, cost of hospitalization, length of hospital stay, use and duration of antibiotics, and long-term neurodevelopmental outcome. The use of HRC monitoring is considered a treatment, and its effects for dichotomous outcomes were expressed as absolute risk reduction (ARR) with 95% confidence intervals (CIs). Numbers needed to monitor (NNTM) were defined as the number of patients who need to be monitored to prevent one adverse event. NNTM with 95% CI in case of significance were calculated using graphpad.com [8].
Assessment of Risk of Bias
Risk of bias classification in RCTs was done using the standard domain questions of the Cochrane Neonatal Review Group “risk of bias” tool. Risk of bias of the cohort studies was determined by appraising the studies with the QUADAS-2 tool for the quality assessment of diagnostic accuracy studies. Quality assessment of included studies was performed by two authors (H.K. and C.L.). To summarize the quality of these studies, risk of bias of all items was classified into three groups: “low,” “high,” and “unclear” risk of bias. The authors of this review were not blinded to the names of the authors of the included articles while assessing the risk of bias.
Results
Study Selection
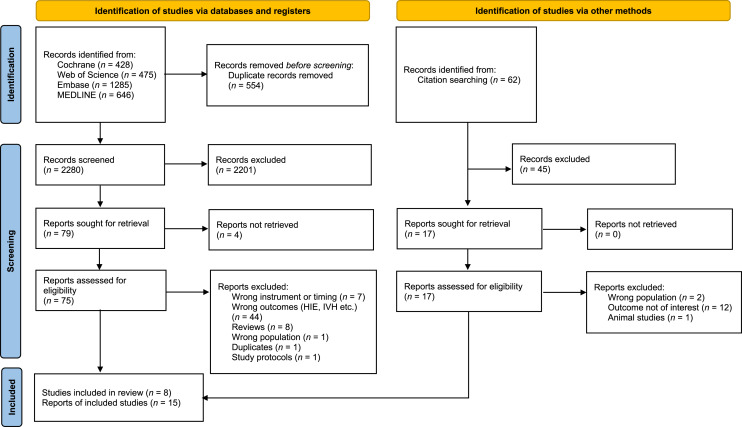
The literature search and citation searches generated a total of 2,896 references in the different databases (Fig. 1). After removing duplicates and excluding 2,280 references on title and/or abstract, 92 full texts were read, of which 77 were excluded for various reasons, such as reporting of a different outcome [9–11] or investigated other biomarkers and physiological signals in addition to HRC instead of standard monitoring [12–17].
Fig. 1.
Flow diagram search results November 18, 2022.
In total, fifteen eligible reports were identified. We identified three reports of a RCT [3, 18, 19]. Four other additional reports of this RCT were excluded from this review because these reports investigated subpopulations of preterm infants [5, 20] or a different primary outcome of interest [21, 22]. Of the twelve cohort studies [6, 7, 23–32], four were identified as duplicated cohort studies [23–27].
Study Characteristics
The fifteen selected studies published between 2001 and 2021 included a total of 8,230 infants (range 10–3,003 participants) (Table 1). All studies except two (one French [28] and one Swiss study [32]) were conducted in the USA. The majority of the studies included infants with a gestational age (GA) of less than 33 weeks and/or a birth weight (BW) of <1,500 g. Before the commercially available bedside HeRO monitor was approved by the FDA, the cohort studies performed their HRC analyses offline. These studies calculated the HRC index or sample asymmetry as a degree of reduced heart rate variability and transient decelerations [6, 7, 23–28]. Except for one study using Nevrokard aHRV software (Nevrokard Kiauta; Slovenia) [30], the studies published after 2010 used the HeRO monitor [3, 18, 19, 29, 31, 32]. The definition of LOS and the percentage of infants diagnosed with LOS were very heterogeneous between the studies.
Table 1.
Study and patient characteristics of included studies
| Author | Year of publication | Country of origin | Single/multicenter | Recruitment period | Inclusion criteria | Number included | GA weeks (SD), N/n | BW in kg (SD), N/n | Frequency HRC tool | Outcome of interest | Definition sepsis outcome | % of sepsis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Randomized trials | ||||||||||||
| Moorman et al. [18]† | 2011 | USA | Multi (9) | Apr 2004–May 2010 | GA <33 weeks/BW <1,500 g | 3,003 | 28 (3)/28 (3) | 1.0 (0.3)/1.0 (0.3) | 1 h | Days alive/ventilator free | Positive blood culture | 25 |
| Cohort studies | ||||||||||||
| Griffin et al. [7] | 2001 | USA | Single | Aug 1995–Apr 1999 | Risk factors for LOS | 89 | 26 (2)/28 (3) | 0.8 (0.4)/1.0 (0.3) | 6 h | Sepsis/sepsis like | Clinical suspicion sepsis, starting antibiotics | 71 |
| Griffin et al. [26]§ | 2005 | USA | Multi (2) | Sep 1999–Jul 2003 | All admissions | 1,022 | 31 (27–36)/33 (27–37) | 1.6 (1.0–2.7)/1.9 (0.9–2.8) | 6 h | Death, UTI, or sepsis | Antibiotics ≥5 days | 24 |
| Beuchee et al. [28] | 2009 | France | Single | Jun 2003–Jun 2004 | GA <33 weeks/PNA >72 h with apnea | 51 | 29 (27–30)/29 (27–30) | 1.1 (0.9–1.3)/1.2 (1.1–1.4) | 1 h | Sepsis | CRP >5 mg/L and positive blood culture | 21 |
| Lake et al. [29]‡ | 2014 | USA | Multi (9) | Apr 2004–May 2010 | GA <33 weeks/BW <1,500 g | 1,489 | 28 (3)/28 (3) | 1.0 (0.3)/1.0 (0.3) | 1 h | Sepsis | Positive blood culture | 26 |
| Bohanon et al. [30] | 2015 | USA | Single | Aug 2012–May 2013 | PNA <1 month/BW <1,000 g | 10 | 27 (1)/27 (1) | 0.7 (0.1)/0.6 (0.1) | 5 h | Sepsis | Sepsis continuum definition^ | 40 |
| Coggins et al. [31] | 2016 | USA | Single | Jan 2010–Jun 2012 | All admissions | 2,384 | 27 (25–35) | 0.9 (0.7–2.5) | At least once every 12 h | Sepsis | CDC definition of BSI | 2 |
| Rio et al. [32]⁺ | 2021 | Swiss | Single | Sep 2014–Dec 2018 | Proven LOS >72 h | 182 | 28 (4)/29 (8) | 1 (0.6)/1.1 (1.3) | 1 h | Sepsis | Positive blood culture | 32 |
HRC, heart rate characteristics, GA, gestational age; LOS, late-onset sepsis; PNA, postnatal age; BW, birth weight; SD, standard deviation; n/N, heart rate variability monitoring versus conventional monitoring; CDC, Centers for Disease Control and Prevention; BSI, bloodstream infection [33].
†Original RCT reported in three reports, the original RCT [18], subgroup of patients with LOS [3], and long-term neurodevelopmental outcome [19].
‡Reports on the standard treatment arm of the RCT [18].
⁺Case-control study design.
^Sepsis continuum definition according to the International Pediatric Sepsis Consensus Conference 2002 [34].
Description of RCT
The identified RCT was conducted in nine neonatal intensive care units in the USA [18]. This study randomized a total of 3,003 patients between 2004 and 2010. After enrolling the first 257 patients, the inclusion criteria were adjusted (infants with BW <1,500 g to infants born <33 weeks of GA) in order to exclude more mature infants. Primary outcome for this trial was survival without mechanical ventilation. Fourteen infants were excluded, of whom 11 were randomized in the intervention arm, leaving 2,989 infants for the final analysis [18]. Two additional manuscripts of this RCT were included, reporting outcomes of a subgroup analysis of patients (n = 700) diagnosed with late-onset neonatal sepsis [3] and long-term follow-up outcomes (n = 628) at 2 years of corrected age (CA) [19].
Description of the Cohort Studies
Seven cohort studies reporting their results in twelve manuscripts and including a total of 5,227 participants were identified in this review [6, 7, 23–32]. Data of subpopulations from one large cohort of preterm infants [26] were used in several publications comparing either HRC to laboratory results [27], clinical signs [6], for deriving and validating HRC as a prediction model for LOS and sepsis-like illness [23], different aspects of HRC [24], or other outcomes [25]. Furthermore, one cohort study reanalyzed data from patients included in the previously mentioned RCT [18], receiving conventional monitoring [29]. There was a wide range of included infants (10–2,384 infants). The cohort studies described in this review included patients with a mean GA of less than 30 weeks, except for one study including all admissions to the neonatal intensive care unit as reflected by the higher mean GA [26]. No studies were identified with the focus of early detection of NEC. All cohort studies investigated LOS as the outcome of interest. However, the definition of sepsis and the baseline risk for sepsis (71-2%) differed considerably between the studies (Table 1). Although sepsis was defined in the studies as having at least one positive blood culture, different international accepted criteria were used [33, 34]. Some included clinical signs of infections in the definition of sepsis [7], others duration of antibiotic therapy [26] or biomarkers [28].
Risk of Bias and Quality of Evidence
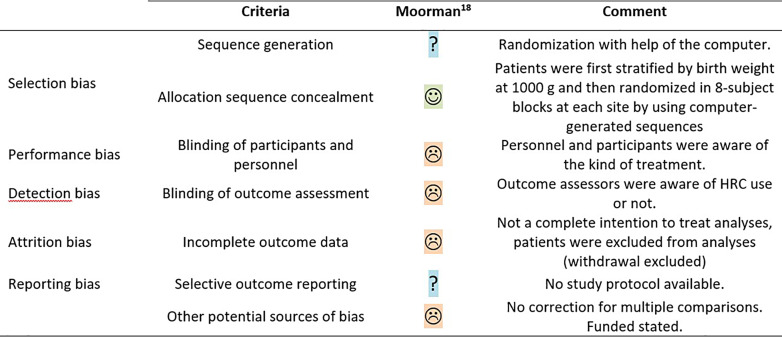
The methodological quality assessed with the Cochrane Collaboration’s risk of bias tool for the RCT was judged as low (Fig. 2) [18]. Given the intervention of this trial, performance and detection bias were deemed unavoidable. A small percentage of randomized participants were excluded from the primary outcome analysis, leading to potential attrition bias, and since no protocol was published, selective outcome reporting could not be excluded. Finally, no correction for multiple comparison analyses was applied on the secondary outcomes and exploratory subgroup analyses.
Fig. 2.
Methodological quality of the RCTs following the Cochrane Collaboration’s tool for assessing risk of bias. ☺, low risk; ☹, high risk; ?, unclear risk.
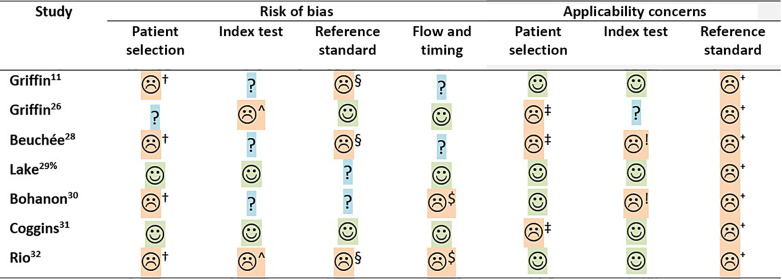
The risk of bias assessed with the QUADAS-2 tool for the individual cohort studies is shown in Figure 3. Most cohort studies included a consecutive number of patients, based on predetermined eligibility criteria. However, two studies were deemed to have a high risk of selection bias for either including a selective population of preterm infants with a central venous line admitted for more than 2 weeks or including infants with severe bradycardia once every hour [7, 28]. One study had a high risk of bias in patient selection due to the case-control design [30], and one study did not report if patients were excluded [27]. Only two studies stated clearly that the index test was blinded to the clinical staff [7, 29]. All studies, except two, had a high or unclear risk of bias in the domain of the reference test [26, 31], whereas all studies except two had a low risk of bias for flow and timing [28, 30]. Concerns on applicability were high in three studies for patient selection and in most studies high or unclear for the index test and reference test [26, 28, 31]. Concerns of applicability for the reference standard were mainly due to unclear description, a composite outcome of sepsis, urinary tract infection, and death, or including only culture-positive infectious episodes.
Fig. 3.
Methodological quality of observational studies following QUADAS-2 statement. ☺, low risk; ☹, high risk; ?, unclear risk. †Selected patient group/case-control design; ^threshold adapted; §reference test not likely to classify all sepsis episodes; $not all participants included in analyses; ‡all admissions or exclusively with bradycardia; !not continuously monitored; ⁺reference test not including clinical suspected sepsis episodes; and %quality assessment mainly reported in the original RCT [18].
The overall summary per domain showed that the majority of cohort studies had a high or unclear risk of bias for every domain, except for the flow and timing domain. Especially, the applicability of the index and reference tests in these studies was of concern (Fig. 3).
Outcomes of the RCT
The primary outcome, number of days alive and ventilator free in 120 days after randomization, showed no difference between the intervention group and the control group (96 days vs. 94 days) (Table 2). However, compared to the control group, mortality was significantly reduced in the group allocated to HRC monitoring with an ARR of 2.07% (95% CI 0.01–4.14) and an NNTM of 49 (95% CI 24–15,484). No differences were seen in other outcomes (including the rate of proven sepsis), except that more blood cultures were drawn in the HRC monitoring group compared to the control group (1.8 vs. 1.6 blood cultures per month, p = 0.05). In the subgroup analysis of infants with a BW <1,000 g, the positive effect of HRC on mortality incidence was more distinct with an ARR of 4.34% (95% CI 0.71–7.97) and an NNTM of 24 (95% CI 13–141) (Table 2). The secondary analyses in a subgroup (n = 700) of patients who were diagnosed with LOS showed a similar significant risk reduction in hospital mortality in favor of HRC monitoring (15.2% vs. 21.9%; ARR 7.91% [95% CI 2.04–13.79]; NNTM 13 [95% CI 7–49]) [3]. Seventy percent of all sepsis episodes were caused by Gram-positive bacteria, mainly the coagulase-negative staphylococci (CONS), with an equal distribution of pathogens between the two groups. This specific organism was the only pathogen related to mortality that reached a significant mortality reduction when comparing the HRC group to the control group (6.3% in the HRC group vs. 18.1% in the control group; p < 0.05) [3]. Furthermore, although the overall group did not show a difference in duration of antibiotic treatments (15.7 days vs. 15.0 days; p = 0.31), the subgroup analysis of infants diagnosed with LOS revealed that compared to infants in the control group, the infants in the HRC monitoring group received significantly longer antibiotic treatments (32.1 vs. 29.0 days, p = 0.047).
Table 2.
Outcomes RCT HRC versus conventional monitoring
| Outcome | n/N HRC arm | n/N standard arm | ARR (95% CI) | NNTM (95% CI) | p value |
|---|---|---|---|---|---|
| Mortality at hospital discharge, n/N (%) | 122/1,500 (8.1) | 152/1,489 (10.2) | 2.07 (0.01–4.14) | 49 (24–15,483) | 0.05 |
| Mortality at 18–22 months CA, n/N (%) | 79/327 (24.2) | 99/309 (32.0) | 7.88 (0.91–14.85) | 13 (7–110) | 0.03 |
| Late-onset septicemia, n/N (%) | 358/1,500 (23.9) | 379/1,489 (25.5) | 1.59 (−1.50–4.68) | – | 0.34 |
| Death or NDI, n/N (%) | 127/321 (39.6) | 136/306 (44.4) | 4.88 (−2.84–12.60) | – | 0.22 |
| Death or CP (GMFCS level 2–5), n/N (%) | 102/327 (31.2) | 112/309 (36.2) | 5.05 (−2.29–12.40) | – | 0.18 |
| NDI in survivors, n/N (%) | 53/247 (21.5) | 40/210 (19.1) | −2.41 (−4.97–9.79) | – | 0.56 |
| CP in survivors (GMFCS level 2–5, n/N (%) | 23/246 (9.4) | 13/210 (6.2) | −3.16 (−1.73–8.04) | – | 0.22 |
| Deafness survivors, n/N (%) | 11/248 (4.4) | 1/210 (0.5) | −3.96 (−6.69 to −1.23) | −26 (−15 to −81) | 0.01 |
| Infants with antibiotic treatment, n/N (%) | 1,020/1,500 (68) | 980/1,489 (65) | −2.18 (−5.56–1.19) | – | 0.21 |
| Duration of antibiotic treatment, mean (SD) | 15.7 (19.4) | 15.0 (19.1) | 0.31 | ||
| Days in NICU, mean (SD) | 59.6 (33.7) | 58.7 (34.5) | 0.47 |
NDI, neurodevelopmental impairment, defined as having one of the following cognitive or language composite score on the Bayley Scales of Infant and Toddler Development third edition of <70, GMFCS of >1, hearing impairment, or bilateral visual impairment.
GMFCS, Gross Motor Function Classification System; HRC, heart rate characteristics; ARR, absolute risk reduction; RR, relative risk; NNTM, numbers needed to monitor; CI, confidence interval; SD, standard deviation, NICU, neonatal intensive care unit.
The long-term follow-up report in the subset of patients with a BW <1,000 g (n = 628 of 884 [72%] participants) showed that there was no difference in the composite outcome death or neurodevelopmental impairment (NDI) at 18–22 months CA [19]. No differences were found between the two groups in the separate NDI domains, but mortality at 2 years CA remained significantly lower in the HRC group compared with the control group (24.2% vs. 32.0%; ARR 7.88% [95% CI 0.91–14.9]; NNTM 13 [95% CI 7–110]) [19]. Among the survivors, deafness was significantly higher in the HRC group compared to the standard monitoring group (4.4% vs. 0.5%; ARR −3.96% [95% CI: −6.69 to −1.23]; NNTM −26 [95% CI: −15 to −81]) in favor of the standard monitoring group, which means that for every 26 infants monitored for changes in HRC, one infant will be diagnosed witdeafness.
Outcomes in Cohort Studies
The first cohort study, investigating HRC as a potential tool for early detection of sepsis, compared the Neonatal Therapeutic Intervention Scoring System and the Neonatal Acute Physiology score obtained over 24 h of epochs with HRC. This study showed a high discriminating accuracy (area under the receiver operator characteristic curve [AUROC] 0.90, p < 0.0001) [7]. The results of the cohort study by Griffin et al. [6, 23, 24, 26, 27] showed that the discriminating power to detect sepsis of a demographic prediction model [23], using clinical symptoms [6], laboratory tests [27], or a composite outcome of sepsis, urinary tract infection, and death [26], significantly increased when HRC parameters were added (AUROC 0.77, 0.70, and 0.82, respectively). The final report investigated another HRC analysis, called sample asymmetry analysis, showing that it could be a useful new technique for early detection of LOS [24].
Beuchée et al. [28] showed that abnormal HRC parameters were strongly associated with the risk of sepsis (AUROC 0.88; 95% CI 0.80–0.94). Lake et al. [18] investigated the control group of the previously published HRC randomized trial and showed that when HRC was added to a standard risk factor model, the predictive performance of sepsis increased (AUROC 0.78; 95% CI: 0.75–0.80) [29]. Bohanon et al. [30] showed that sepsis alters HRC with significant changes in time domain indices. No formal discriminating power was calculated in this cohort.
Finally, a single center large retrospective cohort study investigated the HRC monitoring index of all admitted infants, showing that elevated HRC scores had limited ability to detect a sepsis [31]. In the 48-h period before confirmed sepsis, the majority of the infants did not have elevated HRC scores, including those with a severe inflammatory response. Seventeen (37%) of the infants with a confirmed sepsis showed a mild elevated score and 5 (11%) a high score on the monitor. Furthermore, the results report a large number of false negative high scores of the HRC monitor.
Discussion
This systematic review identified, appraised, and summarized eight studies, published in fifteen reports [3, 6, 7, 18, 19, 23–32]. The only large multicenter RCT (n = 3,003) conducted to date showed that using HRC monitoring to detect sepsis in an early stage of the disease reduced mortality but at the cost of a significant increase in the use of antibiotics [3, 18, 19]. However, there are a number of methodological concerns that weaken the validity of the reported results and raise the question if the randomized evidence is strong enough to justify implementation of HRC monitoring in routine clinical care. First, the risk of bias was scored as high because the intervention (HRC monitoring) was not blinded, which makes the study more subjective to performance and detection bias [35]. The impact of the bias caused by an unblinded intervention on the results of a RCT might differ between outcomes. Some outcomes are more depending on medical interpretation, such as sepsis or dependency of mechanical ventilation than others such as mortality. But even for the outcome mortality, performance and detection bias have been reported [36]. Since blinding is not feasible, future studies investigating HRC monitoring might overcome this potential bias and strengthen the results of the trial by assessing whether the cause of death is related to the intervention allocation by an independent assessor blinded to the study allocation. Second, the reported mortality reduction with HRC monitoring at 120 days and 18–22 months follow-up was modest (2.1% ARR) and a high NNTM with a very wide CI (48, 95% CI: 24–15,484), leading to uncertainty on true clinical effect size and relevance outside the realm of a randomized trial [18]. A subgroup analysis of infants with a BW <1,000 g showed a larger effect on mortality reduction (ARR 4.4%, 95% CI: 0.7–8.0) but without a significant interaction term [18]. Mortality, however, was not the primary outcome of this trial, and no correction for multiple testing was performed, thereby increasing the chance of a false-positive finding [37, 38]. Another aspect jeopardizing the generalizability of this trial is the microorganism causing the septicemia-related mortality. Of all blood cultures (n = 9,882) taken in this trial, 70% of the sepsis episodes were caused by Gram-positive bacteria, and in particular CONS (50%). Benefit of HRC monitoring, in terms of mortality reduction, was only seen in sepsis caused by CONS [3]. Given the fact that overall the mortality rate of sepsis caused by a CONS is relatively low (0.3–1.6%), it remains unknown how generalizable the effect on mortality reduction is outside daily clinical practice [39, 40]. Concerning the effects of HRC monitoring on long-term neurodevelopmental outcomes, it is important to mention that only a subset of the participants (884 of the 3,003 participants, 29%) in this RCT were included in the post hoc analysis report. Of these infants, the outcome death or NDI was assessed in 628 (72%), showing no significant differences in this composite outcome. When analyzing the separate components of the combined outcome, a reduced risk of death at 18–22 months was found [19]. The finding of an increased risk of deafness in survivors is concerning and unexplained. Although the duration of antibiotic treatment and number of septic episodes were not different between the group of infants with HRC monitoring compared to non-monitoring, it is unknown if this difference is caused by an increased rate of aminoglycoside use, an antibiotic known for its ototoxicity [41].
Eight cohort studies investigating 5,227 participants were included, published in twelve reports. In summary, all but one report concluded that HRC alone or in combination with other prognostic factors had modest to good discriminating power with an AUROC varying between 0.78 and 0.90. However, the same discriminating power was shown only using clinical, laboratory, and radiographic information [42]. The results of these studies should be interpreted with caution given the great clinical heterogeneity found between these HRC studies and the low methodological quality of the studies as assessed with the QUADAS-2 tool. The number of participants, GA, and baseline risk for sepsis varied greatly between the different studies, as well as the definitions of outcome measurements. Given this heterogeneity, performing a meta-analysis for diagnostic accuracy of HRC monitoring was not possible. No specific domain of the QUADAS-2 was at risk for bias, but the applicability was judged low with concerns on the index and reference test for most studies (Fig. 3). Furthermore, most studies provided the sensitivity of the HRC monitor, but a major limitation of the identified studies was failing to report false-positive results. False-positive results caused by specific medications and surgery might not been handled in a similar way in the different studies. The one study reporting the false-positive results in their cohort showed a high rate of false positives and false negatives [31]. The discrepancy between this study and the other observational cohort studies might be caused by the low rate of LOS in the study of Coggins and thus a low a priori probability of the outcome of interest or because the lack of a universally generalizable and validated definition for sepsis in preterm infants [43, 44].
An important limitation of this review might be publication bias. Due to the clinical heterogeneity in the eligible studies, no meta-analyses or funnel plots could be performed. Second, we only included studies comparing HRC monitoring versus conventional monitoring for LOS reported as a binary outcome. Studies investigating the predictive value of HRC for deterioration of a disease, for example, from a conservative-treated NEC to a NEC needing surgical intervention, were excluded, as were studies investigating a combination of HRC, clinical scores, and biomarkers to increase the diagnostic accuracy of sepsis in preterm infants [5, 44, 45]. Given the similarities between LOS and NEC, this review focused on both disease entities. Unfortunately, as another limitation of this review, we were unable to identify studies investigating the use of HRC monitoring for the early detection of NEC. Although speculative, it might be well that this is caused by publication bias since an early detection of NEC might even be more complicated given its multifactorial nature. Finally, no formal GRADE assessment was performed since only one RCT was identified [46]. The applicability of this review was deemed low for several reasons, especially because most studies did not include clinically suspected sepsis but only culture-proven sepsis.
To refute or support the results and conclusion of this review, a well-designed adequately powered large RCT would be appropriate. To increase generalizability of such a trial, a multicenter and international setting would be needed given the great differences in antimicrobial stewardship programs and the incidence and resistance patterns of microorganisms between centers and countries [47]. This future adequately powered trial should allocate preterm infants <30 weeks of GA to HRC monitoring versus standard monitoring using a strict treatment protocol. This trial should also collect data on the core outcomes and the number of false positives, number of sepsis workups, and the number and duration of antibiotic prescriptions. Since blinding is not feasible, study outcome assessors should be kept unaware of the intervention. Since this would be impossible for the primary outcome death, an independent data monitoring committee should be installed, assessing the causes of death and its relation to the allocation while remaining blinded to treatment allocation.
The results of this systematic review show that HRC monitoring might be a potential and helpful tool for the early detection of late-onset neonatal sepsis in preterm infants. However, the reported benefit on mortality should be interpreted with caution given the methodological weaknesses, the uncertainty of clinical relevance, and the concerns on generalizability. Before implementing HRC monitoring as standard of care in daily practice, we recommend conducting an international RCT.
Statement of Ethics
An ethics statement is not applicable because this study is based exclusively on published literature. Ethical approval and consent were not required as this study was based on publicly available data.
Conflict of Interest Statement
The authors have no possible conflicts of interest to declare.
Funding Sources
No funding relevant to this study was received for the preparation of data or the manuscript.
Author Contributions
H.J. Koppens contributed to the design of the study; data collection, analyses, and interpretation; and writing of the manuscript. W. Onland and C.A. Lutterman contributed to the design of the study, data analyses and interpretation, and writing of the manuscript. D.H. Visser contributed to the design of the study, data interpretation, and writing of the manuscript. N.P. Denswil contributed to the design of the study and writing of the manuscript. A.H. van Kaam contributed to the design of the study, interpretation of the analyses, and writing of the manuscript. H.J. Koppens wrote the first draft of the manuscript. All authors approved the final version to be published.
Funding Statement
No funding relevant to this study was received for the preparation of data or the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. Further inquiries can be directed to the corresponding author.
Supplementary Material
Supplementary Material
References
- 1. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates. 1993-2012. JAMA. 2015;314(10):1039–51. 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van Beek PE, Groenendaal F, Broeders L, Dijk PH, Dijkman KP, van den Dungen FAM, et al. Survival and causes of death in extremely preterm infants in The Netherlands. Arch Dis Child Fetal Neonatal Ed. 2021;106(3):251–7. 10.1136/archdischild-2020-318978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fairchild KD, Schelonka RL, Kaufman DA, Carlo WA, Kattwinkel J, Porcelli PJ, et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatr Res. 2013;74(5):570–5. 10.1038/pr.2013.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Metzler M, Govindan R, Al-Shargabi T, Vezina G, Andescavage N, Wang Y, et al. Pattern of brain injury and depressed heart rate variability in newborns with hypoxic ischemic encephalopathy. Pediatr Res. 2017;82(3):438–43. 10.1038/pr.2017.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stone ML, Tatum PM, Weitkamp JH, Mukherjee AB, Attridge J, McGahren ED, et al. Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J Perinatol. 2013;33(11):847–50. 10.1038/jp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griffin MP, Lake DE, O’Shea TM, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatr Res. 2007;61(2):222–7. 10.1203/01.pdr.0000252438.65759.af. [DOI] [PubMed] [Google Scholar]
- 7. Griffin MP, Moorman JR. Toward the early diagnosis of neonatal sepsis and sepsis-like illness using novel heart rate analysis. Pediatrics. 2001;107(1):97–104. 10.1542/peds.107.1.97. [DOI] [PubMed] [Google Scholar]
- 8. Software G ; 2020. Available from: https://www.graphpad.com/quickcalcs/catMenu/.
- 9. Fairchild KD, Sinkin RA, Davalian F, Blackman AE, Swanson JR, Matsumoto JA, et al. Abnormal heart rate characteristics are associated with abnormal neuroimaging and outcomes in extremely low birth weight infants. J Perinatol. 2014;34(5):375–9. 10.1038/jp.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vergales BD, Zanelli SA, Matsumoto JA, Goodkin HP, Lake DE, Moorman JR, et al. Depressed heart rate variability is associated with abnormal EEG, MRI, and death in neonates with hypoxic ischemic encephalopathy. Am J Perinatol. 2014;31(10):855–62. 10.1055/s-0033-1361937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tuzcu V, Nas S, Ulusar U, Ugur A, Kaiser JR. Altered heart rhythm dynamics in very low birth weight infants with impending intraventricular hemorrhage. Pediatrics. 2009;123(3):810–5. 10.1542/peds.2008-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cabrera-Quiros L, Kommers D, Wolvers MK, Oosterwijk L, Arents N, van der Sluijs-Bens J, et al. Prediction of late-onset sepsis in preterm infants using monitoring signals and machine learning. Crit Care Explor. 2021;3(1):e0302. 10.1097/CCE.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zeigler AC, Ainsworth JE, Fairchild KD, Wynn JL, Sullivan BA. Sepsis and mortality prediction in very low birth weight infants: analysis of HeRO and nSOFA. Am J Perinatol. 2023;40(4):407–14. 10.1055/s-0041-1728829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leon C, Carrault G, Pladys P, Beuchee A. Early detection of late onset sepsis in premature infants using visibility graph analysis of heart rate variability. IEEE J Biomed Health Inform. 2021;25(4):1006–17. 10.1109/JBHI.2020.3021662. [DOI] [PubMed] [Google Scholar]
- 15. Kurul Ş, van Ackeren N, Goos TG, Ramakers CRB, Been JV, Kornelisse RF, et al. Introducing heart rate variability monitoring combined with biomarker screening into a level IV NICU: a prospective implementation study. Eur J Pediatr. 2022;181(9):3331–8. 10.1007/s00431-022-04534-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fairchild KD, Lake DE, Kattwinkel J, Moorman JR, Bateman DA, Grieve PG, et al. Vital signs and their cross-correlation in sepsis and NEC: a study of 1,065 very-low-birth-weight infants in two NICUs. Pediatr Res. 2017;81(2):315–21. 10.1038/pr.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sullivan BA, Nagraj VP, Berry KL, Fleiss N, Rambhia A, Kumar R, et al. Clinical and vital sign changes associated with late-onset sepsis in very low birth weight infants at 3 NICUs. J Neonatal Perinatal Med. 2021;14(4):553–61. 10.3233/NPM-200578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. J Pediatr. 2011;159(6):900–6.e1. 10.1016/j.jpeds.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schelonka RL, Carlo WA, Bauer CR, Peralta-Carcelen M, Phillips V, Helderman J, et al. Mortality and neurodevelopmental outcomes in the heart rate characteristics monitoring randomized controlled trial. J Pediatr. 2020;219:48–53. 10.1016/j.jpeds.2019.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. King WE, Carlo WA, O’Shea TM, Schelonka RL; HRC neurodevelopmental follow-up investigators . Heart rate characteristics monitoring and reduction in mortality or neurodevelopmental impairment in extremely low birthweight infants with sepsis. Early Hum Dev. 2021;159:105419. 10.1016/j.earlhumdev.2021.105419. [DOI] [PubMed] [Google Scholar]
- 21. Swanson JR, King WE, Sinkin RA, Lake DE, Carlo WA, Schelonka RL, et al. Neonatal intensive care unit length of stay reduction by heart rate characteristics monitoring. J Pediatr. 2018;198:162–7. 10.1016/j.jpeds.2018.02.045. [DOI] [PubMed] [Google Scholar]
- 22. King WE, Carlo WA, O’Shea TM, Schelonka RL, HRC neurodevelopmental follow-up investigators . Multivariable predictive models of death or neurodevelopmental impairment among extremely low birth weight infants using heart rate characteristics. J Pediatr. 2022;242:137–44.e4. 10.1016/j.jpeds.2021.11.026. [DOI] [PubMed] [Google Scholar]
- 23. Griffin MP, O’Shea TM, Bissonette EA, Harrell FE Jr, Lake DE, Moorman JR. Abnormal heart rate characteristics preceding neonatal sepsis and sepsis-like illness. Pediatr Res. 2003;53(6):920–6. 10.1203/01.PDR.0000064904.05313.D2. [DOI] [PubMed] [Google Scholar]
- 24. Kovatchev BP, Farhy LS, Cao H, Griffin MP, Lake DE, Moorman JR. Sample asymmetry analysis of heart rate characteristics with application to neonatal sepsis and systemic inflammatory response syndrome. Pediatr Res. 2003;54(6):892–8. 10.1203/01.PDR.0000088074.97781.4F. [DOI] [PubMed] [Google Scholar]
- 25. Griffin MP, O’Shea TM, Bissonette EA, Harrell FE Jr, Lake DE, Moorman JR. Abnormal heart rate characteristics are associated with neonatal mortality. Pediatr Res. 2004;55(5):782–8. 10.1203/01.PDR.0000119366.21770.9E. [DOI] [PubMed] [Google Scholar]
- 26. Griffin MP, Lake DE, Bissonette EA, Harrell FE Jr, O’Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116(5):1070–4. 10.1542/peds.2004-2461. [DOI] [PubMed] [Google Scholar]
- 27. Griffin MP, Lake DE, Moorman JR. Heart rate characteristics and laboratory tests in neonatal sepsis. Pediatrics. 2005;115(4):937–41. 10.1542/peds.2004-1393. [DOI] [PubMed] [Google Scholar]
- 28. Beuchée A, Carrault G, Bansard JY, Boutaric E, Bétrémieux P, Pladys P. Uncorrelated randomness of the heart rate is associated with sepsis in sick premature infants. Neonatology. 2009;96(2):109–14. 10.1159/000208792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lake DE, Fairchild KD, Moorman JR. Complex signals bioinformatics: evaluation of heart rate characteristics monitoring as a novel risk marker for neonatal sepsis. J Clin Monit Comput. 2014;28(4):329–39. 10.1007/s10877-013-9530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bohanon FJ, Mrazek AA, Shabana MT, Mims S, Radhakrishnan GL, Kramer GC, et al. Heart rate variability analysis is more sensitive at identifying neonatal sepsis than conventional vital signs. Am J Surg. 2015;210(4):661–7. 10.1016/j.amjsurg.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coggins SA, Weitkamp JH, Grunwald L, Stark AR, Reese J, Walsh W, et al. Heart rate characteristic index monitoring for bloodstream infection in an NICU: a 3-year experience. Arch Dis Child Fetal Neonatal Ed. 2016;101(4):F329–32. 10.1136/archdischild-2015-309210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rio L, Ramelet AS, Ballabeni P, Stadelmann C, Asner S, Giannoni E. Monitoring of heart rate characteristics to detect neonatal sepsis. Pediatr Res. 2022;92(4):1070–4. 10.1038/s41390-021-01913-9. [DOI] [PubMed] [Google Scholar]
- 33. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–32. 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 34. Goldstein B, Giroir B, Randolph A; International Consensus Conference on Pediatric Sepsis . International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 35. Beyer-Westendorf J, Buller H. External and internal validity of open label or double-blind trials in oral anticoagulation: better, worse or just different? J Thromb Haemost. 2011;9(11):2153–8. 10.1111/j.1538-7836.2011.04507.x. [DOI] [PubMed] [Google Scholar]
- 36. Martin GL, Trioux T, Gaudry S, Tubach F, Hajage D, Dechartres A. Association between lack of blinding and mortality results in critical care randomized controlled trials: a meta-epidemiological study. Crit Care Med. 2021;49(10):1800–11. 10.1097/CCM.0000000000005065. [DOI] [PubMed] [Google Scholar]
- 37. Vickerstaff V, Ambler G, Omar RZ. A comparison of methods for analysing multiple outcome measures in randomised controlled trials using a simulation study. Biom J. 2021;63(3):599–615. 10.1002/bimj.201900040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Farcomeni A. A review of modern multiple hypothesis testing, with particular attention to the false discovery proportion. Stat Methods Med Res. 2008;17(4):347–88. 10.1177/0962280206079046. [DOI] [PubMed] [Google Scholar]
- 39. Isaacs D, Australasian, Study Group For Neonatal Infections . A 10 year, multicentre study of coagulase negative staphylococcal infections in Australasian neonatal units. Arch Dis Child Fetal Neonatal Ed. 2003;88(2):F89–93. 10.1136/fn.88.2.f89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B; Israel Neonatal Network . Pathogen-specific early mortality in very low birth weight infants with late-onset sepsis: a national survey. Clin Infect Dis. 2005;40(2):218–24. 10.1086/426444. [DOI] [PubMed] [Google Scholar]
- 41. Borradori C, Fawer CL, Buclin T, Calame A. Risk factors of sensorineural hearing loss in preterm infants. Biol Neonate. 1997;71(1):1–10. 10.1159/000244391. [DOI] [PubMed] [Google Scholar]
- 42. Fischer JE, Harbarth S, Agthe AG, Benn A, Ringer SA, Goldmann DA, et al. Quantifying uncertainty: physicians’ estimates of infection in critically ill neonates and children. Clin Infect Dis. 2004;38(10):1383–90. 10.1086/420741. [DOI] [PubMed] [Google Scholar]
- 43. Wynn JL. Defining neonatal sepsis. Curr Opin Pediatr. 2016;28(2):135–40. 10.1097/MOP.0000000000000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kurul Ş, Simons SHP, Ramakers CRB, De Rijke YB, Kornelisse RF, Reiss IKM, et al. Association of inflammatory biomarkers with subsequent clinical course in suspected late onset sepsis in preterm neonates. Crit Care. 2021;25(1):12. 10.1186/s13054-020-03423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Badke CM, Carroll MS, Weese-Mayer DE, Sanchez-Pinto LN. Association between heart rate variability and inflammatory biomarkers in critically ill children. Pediatr Crit Care Med. 2022;23(6):e289–94. 10.1097/PCC.0000000000002936. [DOI] [PubMed] [Google Scholar]
- 46. GRADE handbook for grading quality of evidence and strength of recommendations. In: Schünemann H, Brożek JGG, Oxman A, editors. The GRADE working group; 2013. [Google Scholar]
- 47. Mukhopadhyay S, Sengupta S, Puopolo KM. Challenges and opportunities for antibiotic stewardship among preterm infants. Arch Dis Child Fetal Neonatal Ed. 2019;104(3):F327–32. 10.1136/archdischild-2018-315412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files. Further inquiries can be directed to the corresponding author.