Abstract
Vagus nerve stimulation (VNS) can modulate vagal activity and neuro-immune communication. Human and animal studies have provided growing evidence that VNS can produce analgesic effects in addition to alleviating refractory epilepsy and depression. The vagus nerve (VN) projects to many brain regions related to pain processing, which can be affected by VNS. In addition to neural regulation, the anti-inflammatory property of VNS may also contribute to its pain-inhibitory effects. To date, both invasive and noninvasive VNS devices have been developed, with noninvasive devices including transcutaneous stimulation of auricular VN or carotid VN that are undergoing many clinical trials for chronic pain treatment. This review aimed to provide an update on both preclinical and clinical studies of VNS in the management for chronic pain, including fibromyalgia, abdominal pain, and headaches. We further discuss potential underlying mechanisms for VNS to inhibit chronic pain.
Keywords: Vagus nerve, Electric stimulation, Neuromodulation, Chronic pain, Analgesia
Introduction
Chronic pain persists beyond the normal time that tissues take to heal following an injury and is not simply an accompanying symptom of other diseases [1]. Chronic pain is often resulted from peripheral tissue damage and persistent inflammation or pathological adaptations in the peripheral or central nervous system [2]. Unfortunately, current pain management interventions are unsatisfactory, causing inadequate pain relief and multiple individual and societal burdens [3–6]. At present, oral analgesics are usually the first treatment given and play a vital role in chronic pain management. Commonly used drugs include nonsteroidal anti-inflammatory drugs (NSAIDs), opioids, antidepressants, and antiepileptics. However, side effects need to be taken seriously as the drugs are used longer or at higher doses, especially in the elderly. NSAIDs can increase the risk of gastrointestinal toxicities and serious thrombotic cardiovascular events [7]. Long-term use of codeine carries an increased risk of cardiovascular events in older patients [8]. Respiratory depression, nausea, sedation, cognitive impairment, and addictive potential are also concerns with chronic opioid use. Antidepressants and antiepileptics can induce dizziness, somnolence, and cognitive dysfunction as well. Recently, a growing number of interventional pain procedures have been used, such as block injections, denervation surgery, implantable drug delivery systems, and spinal cord stimulation. Nevertheless, block injections can be used only a limited number of times per year. Other surgical treatments are risky and expensive, and all require strict indications. Therefore, it is necessary to explore more effective treatments for chronic pain conditions with fewer adverse effects.
The vagus nerve (VN, cranial nerve X) is the longest and most widely distributed cranial nerve, which courses from the medulla to the distal third of the colon. The VN regulates multiple systems including cardiovascular, respiratory, immune, endocrine, and autonomic systems, and hence plays an important role in maintaining homeostasis [9]. VN stimulation (VNS) is a neuromodulatory therapy by electrical stimulation of the VN, which has been an effective treatment of refractory epilepsy and depression. In the past 2 decades, it has also demonstrated pain-inhibitory efficacy in multiple preclinical and clinical studies [10–13]. Mechanistically, VNS may control pain by alleviating inflammation and modulating activities of neurons in the pain pathways.
Compared with the broad and multidisciplinary indications mentioned in the previous literature, the review presented here focuses exclusively on the application of VNS in the field of chronic pain. Thus, in this narrative review, we aimed to give an up-to-date review of animal and clinical research studies, supporting the utility of VNS for the treatment of multiple chronic pain conditions. Furthermore, we illustrate the anatomic features of the VN and the history of developing VNS technology. Finally, potential mechanisms which may underlie VNS-induced pain inhibition will be discussed.
Methods
We performed a comprehensive literature search of PubMed/MEDLINE database to retrieve articles published in English language using a combination of keywords such as “vagus nerve stimulation” or “electrical auricular puncture” or “chronic pain” or “pain management” or “headache.” All types of studies were included in this review such as systematic review, randomized trials, descriptive and analytic studies, review articles, and protocols.
Anatomy and Physiology of the VN
The VN arises from the lateral aspect of the medulla and exits the skull through the jugular foramen with its field of distribution extending beyond the head and neck to the thorax and abdomen. It is a mixed nerve and consists of sensory, motor, and parasympathetic fibers and contains approximately 80% afferent fibers and 20% efferent fibers [14, 15]. There are three types of VN fibers which vary in myelination, size, and conduction speed. Each type of fiber has its own special physiological property. Specifically, myelinated A-fibers are composed of small and large fibers. The small fibers are visceral afferent fibers and the large are both afferent and efferent somatic fibers [16]. B-fibers provide efferent sympathetic and parasympathetic preganglionic innervation. Small unmyelinated C-fibers, making up approximately 70% of all vagal fibers, convey visceral information from various visceral organs. It has been demonstrated that vagal C-fibers afferents are important for the antinociception effect produced by VNS in animal models [17].
The VN has four related nuclei in the medulla: the spinal nucleus of the trigeminal nerve, the nucleus of the solitary tract (NTS), the dorsal motor nucleus, and the nucleus ambiguous. The spinal nucleus of the trigeminal nerve receives sensory inputs from the external auditory meatus, the posterior fossa meninges, the larynx, and the upper esophagus. As the main target of VNS, NTS receives sensory inputs from the pharynx, epiglottis, and most visceral organs. The dorsal motor nucleus is the origin of general visceral efferent preganglionic parasympathetic fibers innervating most thoracic and abdominal organs. The nucleus ambiguous gives rise to efferent fibers innervating the stylopharyngeus muscle and the striated muscles of the palate, larynx, and pharynx.
The afferent pathway of VN starts from the vagal afferents innervating the cervical, thoracic, and abdominal organs, mainly passes through the NTS, and then terminates in higher brain regions. Many brain areas in this pathway are involved in pain processing, including the thalamus, hypothalamus, parabrachial nucleus, the periaqueductal gray (PAG), the amygdala, and locus coeruleus (LC) [18–21]. The descending vagal efferent fibers then convey regulatory information to various organs.
History of Developing VNS Technology
VNS devices are available in both implanted invasive and noninvasive forms and operate under various stimulation parameters including current intensity, frequency, pulse width, stimulation ON-time and OFF-time.
Implantable VNS (iVNS) was first developed by Jake Zabara in the 1980s and demonstrated promising antiepileptic effects in canine models [22]. With the success in increasing number of animal and clinical studies, iVNS was approved by the US Food and Drug Administration (FDA) for management of refractory epilepsy in 1997 [23–25]. Interestingly, it was found that epileptic patients also showed mood improvement after iVNS treatment, and iVNS was later approved to treat refractory depression in 2005 [26, 27]. More recently, the potential utility of iVNS has been extended to a diverse array of diseases including heart failure, rheumatoid arthritis, sepsis, obesity, and chronic pain [28–30]. Despite being well-established, this method remains expensive and has been associated with severe implantation complications such as nerve injuries. Moreover, stimulation is not restricted to afferent fibers of the cervical VN but may extend to efferent fibers of the VN as well, causing adverse effects such as bradycardia, cough, voice alteration, and hoarseness [31, 32].
To avoid surgical complications associated with iVNS, noninvasive form of transcutaneous VNS and minimally invasive form of percutaneous VNS were developed. Similar to iVNS, the transcervical VNS (tcVNS) stimulates VN fibers in the carotid sheath. The most widely used tcVNS device (gammaCore, electroCore LLC, Basking Ridge, NJ, USA) has received FDA approval for the treatment of acute cluster headache in 2017 and for acute migraine treatment and adjunctive cluster headache prevention in 2018. The transauricular VNS (taVNS) stimulates the auricular branch of the VN, which innervates the cavity of conchae and exclusively supplies the cymba conchae. The auricular branch of the VN joins the main bundle of the VN projecting to the NTS [33]. The NEMOS device (Cerbomed, Erlangen, Germany), one of the most widely used taVNS devices, received European certification as a treatment for epilepsy and depression in 2010, for chronic pain in 2012, and for anxiety in 2019 [34]. Commonly observed adverse events of noninvasive VNS (nVNS) are relatively mild, including local discomfort, skin irritation, transient muscle stiffness, and pain [35]. The percutaneous auricular VNS (paVNS) has features similar to acupuncture and can be performed with 2–3 miniature needle electrodes penetrating the skin in the vagally innervated regions of the out ear. Compared with the diffuse stimulation fields of taVNS, paVNS can precisely and specifically stimulate the local afferent auricular branch VN endings due to the small size of needle electrodes. The paVNS has been demonstrated to alleviate chronic pain, acute postoperative pain, and peripheral arterial disease [36, 37]. Minor side effects of paVNS mainly include local skin irritation and inadvertent bleeding [38].
Acupuncture is an established adjuvant analgesic method for the treatment of chronic pain and electrical stimulation of acupuncture points can enhance analgesic efficacy [36, 39, 40]. Almost all specific auricular acupuncture points used to treat pain conditions are situated in areas of external auricle receiving exclusively or mixed innervation by the auricular branch of the VN and cervical nerves. Consequently, the analgesic effects of auricular acupuncture may be explained by stimulation of the auricular branch of VN [41].
VNS for the Treatment of Chronic Pain Conditions
Over the past few decades, some animal and clinical studies have suggested that VNS may exert analgesic effects using certain defined parameters [11, 12, 39, 40, 42]. Recently, due to the convenience of using nVNS, an increasing number of studies have focused on the role of VNS in pain management as shown in Table 1.
Table 1.
Summary of clinical studies of VNS for the treatment of chronic pain
| Author (year) | Pain syndrome | Model, sample size | Device type | Study design | Stimulation schedule | Follow-up | Efficacy | Tolerability |
|---|---|---|---|---|---|---|---|---|
| Goadsby et al. [43] (2014) | Migraine | Human, n = 30 | tcVNS | Open-label observational cohort study | Two 90 s doses at a 15 min interval applied at the right neck | 6 weeks | Pain-free rate was 22% at 2 h after tcVNS | Well-tolerated |
| Barbanti et al. [44] (2015) | Migraine | Human, n = 50 | tcVNS | Open-label observational cohort study | Two 120 s doses at a 3 min interval applied at the right neck | 2 weeks | Pain-free rate was 22.9% at 2 h after treatment | Well-tolerated |
| Silberstein et al. [45] (2016) | Chronic migraine | Human, n = 59 | tcVNS | RCT | Two 120 s doses at a 5–10 min interval applied unilaterally three times/day | Randomized phase: 2 months Open-label phase: 6 months | Randomized phase: no difference in reduction of headache days Open-label phase: significant difference in headache days compared to baseline in tcVNS group | Well-tolerated |
| Najib et al. [46] (2022) | Chronic migraine | Human, n = 113 | tcVNS | RCT | Randomized phase: 12 weeks | The percentage of participants with a ≥50% reduction in migraine days was greater in the tcVNS group than the sham group (p = 0.0481) | Well-tolerated | |
| Nesbitt et al. [47] (2015) | Cluster headache | Human, n = 19 | tcVNS | Open-label observational cohort study | Acute treatment: three consecutive 120 s doses applied unilaterally | 13 months | Acute treatment: 47% of all attacks could be aborted within an average of 11±1 min of initial device application Prevention: 24 h attack frequency reduction from a mean of 4.5–2.6 (p < 0.0005) | Well-tolerated |
| Prevention: two or three consecutive 120 s doses applied unilaterally twice/day | ||||||||
| Gaul et al. [48] (2016) | Cluster headache | Human, n = 97 | tcVNS | RCT | Three 120 s doses at a 5-min interval applied at the right side of the neck twice/day | Randomized phase: 4 weeks | Significant reduction in weekly cluster attacks and improvement of at least 50% response rate in tcVNS group | Well-tolerated |
| Open-label phase: 4 weeks | ||||||||
| Straube et al. [49] (2015) | Chronic migraine | Human, n = 46 | taVNS | RCT | A total of 4 h per day without specific distribution applied to the left ear | 3 months | Significant reduction in headache days in both 1 HZ and 25 HZ group while 1 HZ group has more profound effect | Well-tolerated |
| Zhang et al. [50] (2021) | Chronic migraine | Human, n = 70 | taVNS | RCT | 30 min for each session applied at left ear and complete 12 sessions during 4 weeks | 4 weeks | Significant reduction in number of migraine days, pain intensity, and migraine attack time in taVNS group | Well-tolerated |
| Silberstein et al. [51] (2016) | Cluster headache | Human, n = 150 | tcVNS | RCT | Three consecutive 120 s doses applied to the right side of the neck | Randomized phase: 1 month Open-label phase: 3 months | Randomized phase: significant difference of response rate only in eCH treated with tcVNS | Well-tolerated |
| Open-label phase: response rates were similar in eCH and cCH cohort | ||||||||
| Goadsby et al. [52] (2018) | Cluster headache | Human, n = 102 | tcVNS | RCT | Three consecutive 120 s doses applied unilaterally | Randomized phase: 2 weeks | Randomized phase: a higher response rate with tcVNS than with sham in the eCH subgroup (p < 0.01) | Well-tolerated |
| Open-label phase: 2 weeks | ||||||||
| Lange et al. [53] (2011) | Fibromyalgia | Human, n = 14 | iVNS | Open-label observational cohort study | 250 μS 20 Hz pulses with 30 s ON and 5 min OFF. Current intensity: 0.75–2 mA | 3-month study of iVNS with follow-up at 5, 8, and 11 months after stimulation initiation | At the end of 3 months, 5 patients had become MCID+, 2 no longer meeting criteria for widespread pain or tenderness criteria for fibromyalgia | Chest pain Dyspepsia |
| Kutlu et al. [54] (2020) | Fibromyalgia | Human, n = 60 | taVNS | RCT | 30 min per day applied to both ears | 5 weekdays for 4 weeks | taVNS did not give additional benefit together with exercise | Well-tolerated |
| Muthulingam et al. [55] (2021) | Chronic pancreatitis | Human, n = 28 | tcVNS | RCT, crossover study | One 120 s doses applied bilaterally three times a day | 2-week tcVNS followed by 2-week sham stimulation or vice versa | No differences in pain scores were seen in response to 2 weeks tcVNS as compared to sham treatment | Acute pancreatitis Worsening of pain |
| Farmer et al. [56] (2020) | Esophageal pain | Human, study 1: n = 15 study 2: n = 18 | taVNS | RCT, crossover study | Study 1: 30 min tcVNS during acid infusion | 120 min after completion of the acid infusion | The development of acid-induced esophageal hypersensitivity was prevented and reversed with tVNS in comparison to sham | Well-tolerated |
| Study 2: 30 min tcVNS after acid infusion | ||||||||
| Shi et al. [57] (2021) | Irritable bowel syndrome | Human, n = 42 | taVNS | RCT | 30 min stimulation applied bilaterally twice a day | 4 weeks | taVNS decreased VAS pain score and improved anxiety and depression | Well-tolerated |
| Venborg et al. [58] (2021) | Polymyalgia rheumatica | Human, n = 15 | tcVNS | Open-label, proof-of-concept experimental pilot study | Day1–4: three consecutive 120 s doses applied bilaterally | 5 days | A 14% reduction in the pain score for the hips was shown on day 5 in comparison with baseline (p < 0.05), while global pain score had no change | None noted |
| Day 5: one 120 s stimulation | ||||||||
| Aranow et al. [59] (2021) | Systemic lupus erythematosus | Human, n = 18 | taVNS | RCT, pilot study | 5 min stimulation per day applied to the left ear for 4 consecutive days | 12 days | Subjects receiving taVNS achieved a significantly greater reduction in their pain compared with sham group | Well-tolerated |
cCH, chronic cluster headache; eCH, episodic cluster headache; iVNS, invasive vagus nerve stimulation; MCID, minimal clinically important difference; RCT, randomized controlled trial; taVNS, transauricular vagus nerve stimulation; tcVNS, transcervical vagus nerve stimulation.
Chronic Primary Headache
The primary aim of treatment for chronic headache disorders was to prevent acute attack. The first-line treatment is pharmacological therapy. Onabotulinumtoxin A and topiramate are the only currently available therapies with high-quality evidence for the preventive therapy of chronic migraine [60]. As for chronic cluster headache, verapamil, lithium, and topiramate are effective in reducing the frequency of attacks. However, drugs have adverse effects and contraindications. Ideal therapy providing rapid symptom relief with minimal adverse events needs further investigat. There is evidence in support of the use of VNS for the acute and preventive treatment in chronic headache.
iVNS: during clinical trials of VNS for epilepsy treatment, many patients reported a reduction in headache frequency and intensity. For example, Kirchner found that 1 patient with a long history of chronic tension-type headache showed an 80% reduction of pain severity after VNS [11]. Later, several small case series also demonstrated significant improvements in chronic migraine and chronic cluster headache after VSN treatment [61, 62]. However, since both epilepsy and antiepileptic medications may affect pain thresholds, it was difficult to ascertain and generalize the VNS effects on primary headache at that time [63, 64]. The first study that focused on using VNS for headache treatment was conducted in 2003 and yielded promising results. Seven patients with migraine unresponsive to pharmacological treatments were treated with iVNS. Six months later, 1 patient reported a greater than 50% reduction in headache frequency, duration, and severity; 4 patients showed a 75% or greater improvement on the migraine disability assessment questionnaire scale [10]. To date, as refractory epilepsy and depression remain the primary indication for iVNS, the randomized controlled study of iVNS for headache treatment has not yet been carried out.
tcVNS: owing to the convenience and good safety profile, a growing number of studies have been conducted to evaluate the utility of nVNS in headache management. For migraine attack, Goadsby et al. [43] enrolled 30 participants to assess the effect of tcVNS for acute treatment of migraine and the pain-free rate at 2 h after tcVNS treatment for all moderate or severe attack was 22%. Barbanti et al. [44] found that the pain relief rate (≥50% reduction in visual analog scale [VAS] score) was 64.6% and 39.6% of patients achieved pain-free status at 2 h after treatment in their multicenter study. Interestingly, the tcVNS also had a prophylactic effect in chronic migraine. Silberstein et al. [45] conducted the first double-blinded, randomized controlled pilot trial to study the prophylactic effect of tcVNS on chronic migraine. After 2-months randomized phase, tcVNS failed to significantly decrease headache days compared to baseline and the mean reduction of headache days did not significantly differ between sham and tcVNS groups. However, patients in tcVNS group showed less headache days after 6-months open-label phase. Previous studies also suggested that the longer VNS treatment was associated with greater therapeutic effects [48, 65, 66]. Most recently, Najib et al. [46] conducted a multicenter trial to evaluate the efficacy and safety of tcVNS for migraine prevention and mean reduction in monthly migraine days were also similar in two groups. Nevertheless, the percentage of participants with a ≥50% reduction in migraine days was greater in tcVNS group and patients with aura responded preferentially.
VNS is also practical and effective as an acute and preventive treatment in chronic cluster headache. Silberstein et al. [51]and Goadsby et al. [52] both found that tcVNS induced the significantly higher response rate of episodic cluster headache, but the acute treatment was not effective for chronic cluster headache during randomized phase. However, findings after the 3-month open-label phase in the study of Silberstein et al. [51] showed that the response rate was similar between these two cohorts, indicating further benefit with continued use of tcVNS. Nesbitt et al. [47] studied 19 patients with cluster headache treated with tcVNS and found that 15 patients reported an overall improvement in their condition from baseline. Importantly, prophylactic use of tcVNS significantly reduced the mean 24-h attack frequency from 4.5 to 2.6 h. Gaul et al. [48] also conducted a large randomized trial to determine if adjunctive prophylactic tcVNS is effective for chronic cluster headache. Compared to the standard treatment alone, combining tcVNS with the treatment induced a significantly greater reduction in the number of attacks per week. Prophylactic tcVNS was also associated with a significantly decreased consumption of abortive medications and a greater improvement of life quality. Subsequently, they further demonstrated that adding tcVNS to standard treatments led to rapid, significant, and sustained reduction of attack frequency within 2 weeks [67].
In a recent meta-analysis, Lai et al. [68] analyzed six randomized controlled trials on the effects of tcVNS on primary headache disorders and showed that tcVNS is effective for acute relief of both migraine and cluster headache. The gammaCore device has received FDA approval for the treatment of acute cluster headache treatment in 2017 and for acute migraine treatment and adjunctive cluster headache prevention in 2018.
taVNS: there are only a few clinical studies examining the effects of taVNS on headache. Straube et al. [49] included 46 patients with chronic migraine treated with 1 Hz or 25 Hz of the taVNS. Both groups showed significant decreases in the number of headache days. Interestingly, 1 Hz taVNS induced a significant greater headache days reduction than 25 Hz stimulation did, which is the most frequently used VNS frequency. Headache intensity was not significantly changed, and there were no group differences. In a functional magnetic resonance imaging (fMRI) study, Zhang et al. [50] reported that the migraine patients showed a significant reduction in migraine days, migraine attack times, and pain intensity 4 weeks after taVNS.
In summary, VNS has demonstrated reproducible inhibitory effects on primary headache disorders. More randomized controlled trials are needed to further explore the optimal stimulation parameter for each type of nVNS to inhibit primary headache disorders.
Chronic Widespread Pain
VNS was also shown to modulate nociception and other clinical pain conditions. Chronic widespread pain is defined as pain involving three or more body quadrants and axial bones, accompanied by significant affective disorders and dysfunction [69]. Fibromyalgia is the most common chronic widespread pain and characterized by fatigue, sleep disorders, cognitive dysfunction, and depression. The pathogenesis of fibromyalgia has been linked to central sensitization and other factors, such as inflammatory, genetic, and psychosocial factors. Current therapies include pharmacotherapy (anticonvulsants, serotonin and norepinephrine [NE] reuptake inhibitors, tricyclics) and non-pharmacological therapies (education, exercise, cognitive behavioral therapy).
Since the stimulation of vagal afferents may reduce pain through modulating descending serotonergic and noradrenergic neurons which play an important role in central sensitization, Lange et al. [53] conducted the first open-label study to assess the efficacy, safety, and tolerability of iVNS in 14 fibromyalgia patients. Most patients tolerated implantation well, and the adverse effects of iVNS were comparable to those reported in using iVNS to treat other diseases. Importantly, 5 patients no longer met the diagnostic criteria for fibromyalgia after 11 months of treatment. The invasive nature of iVNS limited conducting further research in a larger cohort. Accordingly, Kutlu et al. [54] examined the impact of taVNS in conjunction with exercise on pain intensity and life quality in 60 patients with fibromyalgia. Combined use of taVNS significantly reduced the VAS score averages, but there is a no significant difference between the control (exercise only) and treatment group. Veterans with Gulf War illness treated with tcVNS also reported no improvement in either widespread pain or migraine frequency or severity relative to veterans who received sham tcVNS [70]. Further studies in larger sample size and with other stimulation parameters are needed to investigate the effects of nVNS on chronic widespread pain.
Chronic Visceral Pain
Chronic Pancreatitis
Chronic abdominal pain is the primary symptom of chronic pancreatitis. It has been demonstrated that chronic pancreatitis patients have abnormal pain processing in central nervous system [71]. Sustained pancreatic nociceptive afferent inputs can lead to central sensitization over time. This may help explain why patients respond inadequately to therapies directed against pathophysiological changes of the pancreas [72]. Currently, the use of analgesics for chronic pancreatitis pain is still based on the 1986 World Health Organization analgesic ladder for cancer pain. Antioxidants and pregabalin may also have some benefits to pain relief [73, 74].
In a small randomized crossover study, Muthulingam et al. [55] evaluated the effects of 2-week tcVNS treatment on pain symptoms and quality of life in patients with chronic pancreatitis. Both tcVNS and control groups induced significant improvements in pain scores when compared to their baselines, respectively. However, no significant difference was found between the two groups, and no change in the cardiac vagal tone was observed. The negative results may be partial due to insufficient activation of parasympathetic nervous system and treatment time. Later, Muthulingam et al. [75] conducted another trial using fMRI in chronic pancreatitis patients to investigate if tcVNS changes functional connections of limbic structures. Compared with sham treatment, 2 weeks of tcVNS reduced functional connection of limbic structures, suggesting a brain modulatory effect by tcVNS which may partially underlie the beneficial effect of tcVNS in these patients.
Functional Gastrointestinal Disorders
Symptomatic treatment of abdominal pain of functional gastrointestinal disorders may be accomplished with several classes of medication, including antispasmodics, peppermint oil, tricyclic antidepressants, and selective serotonin reuptake inhibitors [76]. Cognitive behavioral therapy and exercise also help patients reduce and manage pain symptoms [77, 78].
Visceral hypersensitivity is an important cause of chronic abdominal pain in functional gastrointestinal disorders [79]. Increased parasympathetic tone played an important role in visceral and esophageal pain hypersensitivity. Physiologically increasing of parasympathetic tone with deep breathing prevented the development of esophageal pain hypersensitivity, and this effect was antagonized by atropine [80]. In a randomized crossover trial, Farmer et al. [56] found that taVNS prevented the development and even reversed the established hypersensitivity in an acid-induced esophageal pain model by increasing parasympathetic tone. Correspondingly, the decreased pain thresholds at 60 min and 120 min after acid infusion were increased by taVNS treatment.
Irritable bowel syndrome is another common functional gastrointestinal disorder characterized by abdominal pain and altered bowel habits in the absence of demonstrable organic disease. In a randomized trial of 42 patients with irritable bowel syndrome, 4 weeks of taVNS substantially ameliorated abdominal pain and constipation. Compared with sham stimulation, the VAS pain score was decreased by 64% after taVNS treatment. In addition, taVNS decreased the levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and serotonin (5-HT) in circulation and enhanced vagal activity assessed by the spectral analysis of heart rate variability. The vagal nerve activity was negatively correlated with the VAS score. These interesting findings suggested the autonomic and immune modulation involved in the improvement symptoms with the taVNS [57].
Chronic Neuropathic Pain
Trigeminal Allodynia
Trigeminal neuralgia is characterized by touch-evoked brief shock-like paroxysmal pain in one or more divisions of the trigeminal nerve. First-line treatment is sodium channel blockers, either carbamazepine or oxcarbazepine. Patients who fail medical management due to persistent pain or unacceptable side effects have options like open surgery and radiotherapy.
Oshinsky et al. [81] explored the effect of tcVNS for treatment of chronic trigeminal allodynia in a rat model induced by repeatedly infusing prostaglandin E2 onto the dura. They showed that periorbital sensitivity significantly decreased for up to 3.5 h after 2 min of tcVNS. Additionally, tcVNS blocked or even reversed the increased level of extracellular glutamate in the trigeminal nucleus caudalis. There was no significant difference in the levels of the inhibitory neurotransmitter gamma aminobutyric acid, 5-HT, and NE. Mechanistically, the suppression of increased glutamate level in the trigeminal nucleus caudalis induced by glyceryl trinitrate may be an important mechanism for nVNS to inhibit trigeminal allodynia. Clinical studies are needed to validate these preclinical findings of nVNS in patients with trigeminal neuralgia.
Diabetic Peripheral Neuropathic Pain
Painful neuropathy is a frequent comorbidity of diabetes, and the pathophysiology is not yet fully elucidated and the treatment remains unsatisfactory. Current treatments are purely symptomatic and the most commonly recommended first-line agents include anticonvulsants (gabapentin and pregabalin), serotonin-noradrenaline reuptake inhibitors, and tricyclic antidepressants. Unfortunately, the therapeutic effect may offset by intolerable adverse effects.
Previous animal study showed that 30 min taVNS per day for consecutive 5 weeks exerted an antidiabetic effect in Zucker diabetic fatty (ZDF, fa/fa) rats and triggered the secretion of melatonin, suggesting a potentially beneficial effect of taVNS in diabetic neuropathy [82]. Wang et al. [83] found that 30 min taVNS treatment per day for consecutive 28 days elevated plasma melatonin concentration, upregulated the expression of melatonin receptors in the amygdala, and alleviated thermal hyperalgesia in ZDF rats. Intraperitoneally injected luzindole, a melatonin receptor antagonist, attenuated the pain inhibitory effects of taVNS. Accordingly, the mechanisms of taVNS on inhibiting neuropathic pain may involve the release of extrapineal melatonin and increased expression of central melatonin receptors. Li et al. [84] also showed that similar taVNS treatment inhibited pain hypersensitivity in ZDF rats as detected by both thermal hyperalgesia and mechanical allodynia in the hind paw. Importantly, this pain inhibitory effect of taVNS was found to be due to an elevated plasma 5-HT concentration and increased expression of central 5-HT receptor type 1A (5-HT1AR) in the hypothalamus. 5-HT1AR plays an important role in descending pain inhibitory pathway. Both 5-HT1AR and melatonin receptor type 1 are expressed in the ventromedial hypothalamic nucleus. In addition, both 5-HT and melatonin are biochemically derived from tryptophan and were elevated by taVNS. It is possible that they may work synergistically in mediating pain inhibition from VNS. At present, there is a lack of clinical study to determine whether nVNS can alleviate pain in diabetic patients.
Chronic Musculoskeletal Pain
Chronic musculoskeletal pain is defined as persistent or recurrent pain that arises as part of a disease process directly affecting bones, joints, muscles, or related soft tissues [85]. For the treatment of chronic musculoskeletal pain, it is necessary not only to treat symptomatically but also to actively treat the primary diseases. Commonly used analgesics include NSAIDs, muscle relaxants, anticonvulsants, antidepressants, and opioids. Non-pharmacological therapies, including exercise, physical therapy, and interventional procedures, can alleviate pain to some extent.
Frøkjaer et al. [86] found that combined taVNS and physiological modulation (deep breathing) of vagal tone could reduce somatic pain sensitivity and increase musculoskeletal pain thresholds in healthy subjects. The anti-inflammatory of VNS may also contribute to its promising effect in chronic musculoskeletal pain.
Autoimmune Diseases
Rheumatoid arthritis is a chronic autoimmune, inflammatory disease characterized by inflammation in the musculoskeletal joints, resulting in cartilage and bone degradation. Preclinical data confirmed that vagotomy exacerbated arthritis in mice [87]. Koopman et al. [29] first found that iVNS could improve patient’s assessment of pain. Polymyalgia rheumatica is an inflammatory rheumatic disease of unknown etiology, characterized by chronic muscle pain and morning stiffness in the shoulders, pelvic girdle, and neck. Venborg et al. [58] conducted an open-label, proof-of-concept pilot study to investigate the effect of acute (20 min) and chronic (5 days) tcVNS. A significant reduction of VAS pain score in the hip from baseline was found on day 5, but the global VAS score showed no significant change. tcVNS induced a significant increase in cardiac vagal tone at 20 min after initial stimulations compared with baseline. VNS has also been considered for treatment of systemic lupus erythematosus, a chronic autoimmune inflammatory disease characterized by musculoskeletal pain and fatigue [59]. Subjects receiving taVNS for 4 consecutive days achieved a significantly greater reduction in their pain and fatigue, and these effects remained during the whole study period of 12 days. Interestingly, the change of reported pain from baseline correlated significantly with the change in fatigue.
Osteoarthritis
Krusche-Mandl et al. [88] found electrical auricular acupuncture significantly decreased pain scores and increased the pain-free walking time in patients with knee osteoarthritis. Patients in their study received continuous low-frequency electrical auricular acupuncture for 96 h per week and the treatment lasted 6 weeks. Moreover, acupuncture showed a continuing effect and pain scores further decreased during follow-up. Accordingly, taVNS may also be a potential non-pharmacological strategy for this disease.
Chronic Low Back Pain
The abnormalities of elements comprising the lumbar spine (e.g., soft tissue, vertebrae, zygapophyseal and sacroiliac joints, intervertebral discs, and neurovascular structures) can contribute to low back pain. The management of chronic low back pain is extremely challenging. Pharmacological treatments should begin with NSAIDs or muscle relaxants, and then tramadol or duloxetine for next step. Opioids are recommended to use only for low back pain refractory to other treatments [89]. Non-pharmacological approaches such as exercise and physical therapy are beneficial. However, this patient group has only small functional improvements from the use of the medication [90]. Some interventional procedures including radiofrequency and spinal nerve stimulation may be effective, but the invasive and risky nature limits their use.
Sator-Katzenschlager et al. found continuous auricular electroacupuncture (48 h per week for 6 weeks) relieves pain more effectively than conventional manual auricular acupuncture in chronic low back pain patients treated with standardized analgesic therapy and the effect lasted for 3 months after the treatment ended [36]. One pilot study found taVNS combined with mindful mediation also improved back pain severity and pressure pain threshold, especially for those with greater negative affect [91]. Thus, VNS may be a promising treatment strategy for chronic low back pain.
Mechanisms Underlying the Pain Modulation of VNS
The experience of pain depends on peripheral sensory inputs, spinal transmission, and brain processing. Though the exact mechanisms by which VNS inhibits chronic pain remain to be determined, investigators have proposed several working hypotheses based on findings in animal studies and clinical trials.
Pain Pathways Modulation
Spinal Ascending Input
VNS was shown to inhibit the spinal nociceptive transmission as suggested by its inhibitory effect on responses of spinal nociceptive neurons in the spinothalamic tract and spinoreticular tract [92–96]. Chandler et al. [97] showed that VNS inhibited the spinothalamic tract neurons below C3 spinal level, but excited neurons between C1 and C3, indicating that neurons in high cervical segments may play a critical role in VNS-induced pain inhibition. Moreover, Lyubashina et al. [98] found that continuous cervical VNS suppressed electrically evoked firing rates in 48% spinal trigeminal neurons, and more than 80% of responders showed a carryover inhibition at 5 min after terminating VSN in an animal model of headache.
Brain Modulation
VNS modulates pain through multiple pain-processing structures in the brain (shown in Fig. 1). Animal experiments using local anesthetics to inhibit neuronal activities in NTS, nucleus raphe magnus, LC, and PAG suggested that these structures play important roles in the pain-inhibitory effects of VNS [99–102]. An increasing number of clinical trials using fMRI also confirmed this notion. For example, migraineurs treated with 1 Hz taVNS showed a significant deactivation of signals at the LC, parabrachial nucleus, and NTS. Moreover, taVNS increased resting state functional connectivity between the LC and left secondary somatosensory cortex, mygdala, anterior cingulate cortex, and hippocampus. The increased resting state functional connectivity between the left LC and left secondary somatosensory cortex was negatively associated with the frequency of migraine attacks [103]. Compared with 20 Hz, 1 Hz taVNS produced greater increases of connections between PAG and middle cingulate cortex, left insula, and anterior cingulate cortex [104]. Zhang et al. [50] also found that taVNS increased the connectivity between the thalamus subregion and anterior cingulate cortex in migraine patients.
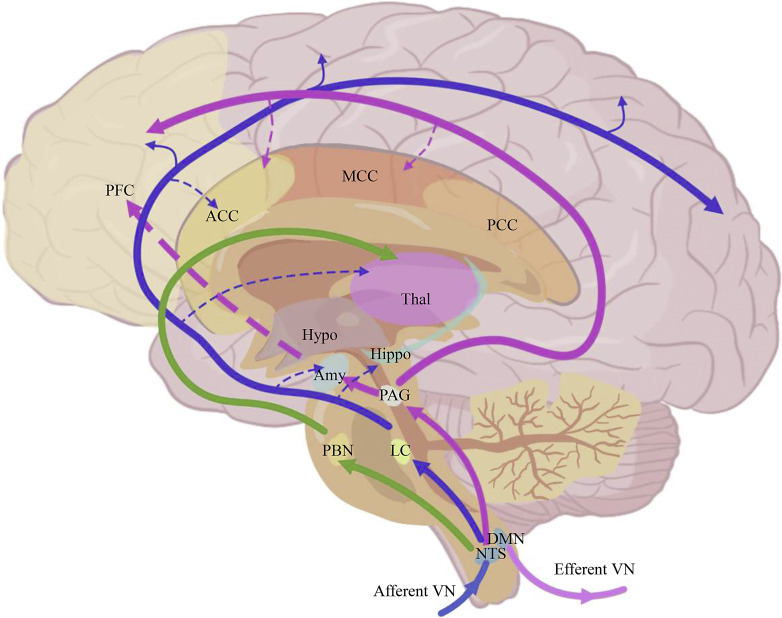
Fig. 1.
Neural pathways involved in pain modulation by vagus nerve stimulation (VNS). Afferent vagal fibers project to the brain mainly via the nucleus tractus solitarius, and then reach higher brain structures that are associated with pain perception, such as the amygdala, hippocampus, hypothalamus, and anterior cingulate cortex. VNS can modulate the activities and connection of these brain areas. The activities of PAG and LC, and their connection with higher brain areas are modulated by vagal activity to regulate the descending pain inhibition systems. The dotted lines represent increased connections by VNS. ACC: anterior cingulate cortex; Amy: amygdala; DMN, dorsal motor nucleus; Hippo, hippocampus; Hypo, hypothalamus; LC, locus coeruleus; MCC, middle cingulate cortex; NTS, nucleus of the solitary tract; PAG, periaqueductal gray; PBN, parabrachial nucleus; PCC, posterior cingulate cortex; PFC, prefrontal cortex; Thal, thalamus.
VNS can activate the descending pain inhibition system. The intrathecal administration of opioid receptor antagonist naloxone attenuated cervical VNS-produced inhibition of the tail flick reflex. Intrathecal injection of both phentolamine and methysergide also significantly attenuated inhibition of tail flick reflex by cervical VNS [39]. Collectively, these results suggest that cervical VNS activated spinal opioidergic system and descending serotonergic and noradrenergic systems to inhibit spinal nociceptive processing. Intravenous injection of 5-HT also stimulated vagal afferents and inhibited the tail flick reflex by activating descending inhibitory systems [105]. Hu et al. [106] provided evidence of activation of δ-opioid receptors in mediating the therapeutic action of iVNS in headache. Recently, Laura et al. [107] tested the effects of short-term tcVNS on the descending pain inhibitory system, quantified by the nociceptive flexor (RIII) reflex in twenty-seven healthy subjects. However, they did not observe the activation of the descending pain inhibition pathway during and up to an hour after tcVNS. Conversely, De Icco et al. [108] found a statistically significant increase of RIII reflex threshold at 5 and 30 min after 4 min of tcVNS in healthy participants. Descending pain inhibition may be a possible mechanism for pain control of VNS, further studies are needed to provide more details, especially the effects of long-term VNS on descending pain modulation system.
Cortical spreading depression (CSD) was suggested to be the main mechanism underlying migraine aura and a trigger for headache [109]. Both iVNS and tcVNS significantly suppressed spreading depression susceptibility in the occipital cortex in rats. The electrical stimulation threshold to evoke the CSD was elevated by more than 2-fold, and this effect could persist for more than 3 h [110]. VNS may suppress CSD through inhibition of glutamate release, the neurotransmitter critical for CSD initiation and spread [111]. Morais found that VNS inhibited CSD through central mechanisms which involve NTS and subcortical neuromodulatory centers that provide serotonergic and norepinephrinergic innervations to the cortex. Most recently, Liu reported that tcVNS suppressed CSD susceptibility in an intensity-dependent manner, with two 2-min nVNS applied at 5 min apart being the most effective paradigm [112]. Accordingly, central mechanisms including spinal and supraspinal modulation may be a key factor in pain control by VNS.
Anti-Inflammatory Effect
Inflammation exerts a pivotal role in pain perception with glial, immune cells, and pro-inflammatory cytokines implicated in chronic pain states [113]. Multiple studies have discovered that the VN was involved in the inflammatory response, and VNS may control pain through alleviating inflammation and neuroinflammation [114]. The tcVNS can cause a greater decrease of cytokines levels (TNF, IL-1β, IL-8), as well as a larger increase of IL-10 in healthy volunteers [115]. Chaudhry et al. [116] found that adjunctive tcVNS significantly reduced the IL-1β plasma levels and the number of severe attacks per month in migraineurs. Although the precise anti-inflammatory mechanism of VNS is not fully elucidated, it may achieve the effect through the cholinergic anti-inflammatory pathway (CAP), hypothalamic-pituitary-adrenal axis pathway, and the release of specialized proresolving mediators (SPMs) (shown in Fig. 2).
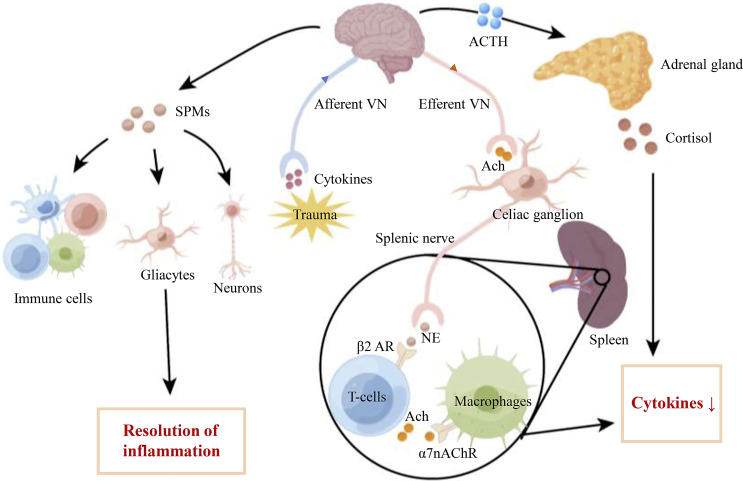
Fig. 2.
Schematic illustration of the anti-inflammatory effect of the vagus nerve stimulation (VNS). The activation of VN may induce anti-inflammatory effects through the CAP, the hypothalamic-pituitary-adrenal axis pathway, and the production of SPMs. VNS activates VN efferents to release acetylcholine (Ach) in the coeliac ganglia and make a synapse with the sympathetic splenic nerve. Norepinephrine released from the splenic nerve binds to the β2 adrenergic receptor of T cells that release Ach. Ach then binds to the α7 nicotinic Ach receptor expressed on macrophages, leading to a decrease of cytokines production. VNS also stimulates the HPAA, causing the brain to release adrenocorticotropic hormone, which acts on the adrenal glands and then promotes cortisol production. Furthermore, VNS may induce the production of SPMs acting on their receptors which are expressed on immune cells, gliacytes, and neurons to modulate inflammation and neuroinflammation. Ach: acetylcholine; ACTH: adrenocorticotrophin hormone; NE: norepinephrine; SPMs: specialized proresolving mediators; VN: vagus nerve; β2 AR: β2 adrenergic receptor; α7nAChR: α7 nicotinic Ach receptor; HPAA, hypothalamic-pituitary-adrenal axis.
Increasing experimental evidence suggested that VNS might modulate the immune response and systemic inflammation through the CAP [117–120]. The classical CAP modulates inflammatory responses through splenic nerve of the celiac ganglia to release NE. Then, NE bounds to the β2 adrenergic receptor on the T lymphocytes and the release of acetylcholine (Ach) increased. ACh then binds to the alpha7 nicotinic Ach receptor (α7nAChR), and inhibits the release of pro-inflammatory cytokines such as TNF-α from macrophages in the spleen. Later, Matteoli et al. [121] showed that the anti-inflammatory effect of VN in the intestine is through muscularis resident macrophages expressing α7nAChR, which is independent of the spleen and T cells. Rawat et al. [122] found that taVNS upregulated the CAP by increasing the expression of α7nAChR. Except for peripheral regulation, VNS can exert the anti-inflammatory effect through central α7nAChR-dependent mechanism [123, 124]. Wang et al. [125] demonstrated that taVNS reversed the downregulated expression of α7nAChR on the hippocampus to relieve neuroinflammation. Jiang et al. [126] showed that iVNS significantly decreased the levels of TNF-α, IL-1β, and IL-6 through elevating the expression of α7nAchR on microglia.
SPMs, including lipoxins, resolvins, protectins, and maresins, are endogenous lipid mediators generated during the resolution phase of inflammation [127]. Due to their potent proresolving and anti-inflammatory effects, several studies examined the effects of SPMs in inhibiting inflammatory pain and neuropathic pain in rodent models. Intrathecal injection of resolvin D1 attenuated chronic pancreatitis-induced mechanical allodynia and reduced the expressions of TNF-α, IL-1β, and IL-6 in thoracic spinal dorsal horn [128]. Intraplantar pretreatment of mice with resolvin E1 reduced carrageenan-induced neutrophil infiltration, paw edema, and pro-inflammatory cytokine expression and relieve the inflammation pain [129]. Intrathecal administration of lipoxin A4 decreased the pro-inflammatory cytokines (TNF-α, IL-1β), increased the expression of anti-inflammatory cytokines (IL-10), and alleviated neuropathic pain in the rat model with low back pain [130]. Recently, Serhan et al. [131] found that VNS could increase SPMs including resolvins 1, protectins 1/neuroprotectin 1, maresins 1, and resolvins 5 in human. Although the pain-inhibitory effect of SPMs is also related to other mechanisms, its direct anti-inflammatory action still plays a vital role [132]. VNS may increase plasma levels of adrenocorticotropic hormone and corticosterone through the hypothalamic-pituitary-adrenal axis pathway, which could also mediate some of its pain inhibitory and anti-inflammatory effects [133].
Limitations and Future Directions
As an alternative, non-pharmacological intervention with fewer side effects, VNS is a promising neuromodulation method to treat chronic pain syndromes. However, there exits several limitations in current VNS studies for chronic pain management. First, most of the studies were conducted for a short period of time, leading to negative results in some studies during the randomized phase [45, 51]. A longer intervention may have more pronounced therapeutic effect. By the way, with the extension of treatment time, one thing that needs to be noted is therapeutic compliance of patients. The second problem is the small sample size, especially in clinical trials for chronic pain conditions other than headaches. Third, very few studies have been done to explore the characteristics of suitable populations for VNS.
Therefore, it is necessary to conduct more preclinical and clinical trials in the future. To begin with, current conclusions about the use of VNS for chronic pain syndromes mentioned above need to be validated in more and larger randomized controlled studies. Other types of chronic pain such as chronic cancer pain and postoperative pain may also benefit from this technique. Additionally, the relationship between stimulation schedule or stimulation parameters and therapeutic effects needs to be elucidated, which will help optimize the use of VNS in individual pain condition. Equally important is the selection of patients who are likely to respond better to this autonomic intervention. More research studies are needed to determine the optimal indication. At last, although several hypotheses of analgesic effects of VNS have been proposed, the precise mechanism is still unclear and the exact neural pathway needs further investigation.
Conclusion
The VN represents the most extensive neural system connecting organs and the brain and plays a critical role in homeostats and maintaining our physiology under a balanced condition. VN exerts an anti-nociceptive and anti-inflammatory effect within the viscera. Current preclinical and clinical VNS studies have demonstrated clinical beneficial effects for the use of VNS in primary headache disorders. With its good safety profile and demonstrated efficacy in previous trials, extending VNS in management of other clinical pain conditions is promising.
Conflict of Interest Statement
The authors have declared that no competing interest exists.
Funding Sources
This work was supported by grants from the National Natural Science Foundation of China (81771181, 81571065, 82171217); the Beijing Natural Science Foundation (7202053).
Author Contributions
Y.W. defined the topic of review. P.S. prepared the manuscript, and HL created the figures. J.J., Y.G., and X.C. aided in manuscript review and figure design. All authors read and approved the final manuscript.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81771181, 81571065, 82171217); the Beijing Natural Science Foundation (7202053).
References
- 1. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. 2015;156(6):1003–7. 10.1097/j.pain.0000000000000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Descalzi G, Ikegami D, Ushijima T, Nestler EJ, Zachariou V, Narita M. Epigenetic mechanisms of chronic pain. Trends Neurosci. 2015;38(4):237–46. 10.1016/j.tins.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cherubino P, Sarzi-Puttini P, Zuccaro SM, Labianca R. The management of chronic pain in important patient subgroups. Clin Drug Investig. 2012;32(Suppl 1):35–44. 10.2165/11630060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73. 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Mil Med. 2016;181(5):397–9. [DOI] [PubMed] [Google Scholar]
- 6. Macfarlane GJ, Beasley M, Jones GT, Stannard C. The epidemiology of regular opioid use and its association with mortality: prospective cohort study of 466 486 UK biobank participants. EClinicalMedicine. 2020;21:100321. 10.1016/j.eclinm.2020.100321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286(8):954–9. 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 8. Solomon DH, Rassen JA, Glynn RJ, Garneau K, Levin R, Lee J, et al. The comparative safety of opioids for nonmalignant pain in older adults. Arch Intern Med. 2010;170(22):1979–86. 10.1001/archinternmed.2010.450. [DOI] [PubMed] [Google Scholar]
- 9. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1–3):1–17. 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 10. Multon S, Schoenen J. Pain control by vagus nerve stimulation: from animal to man.and back. Acta Neurol Belg. 2005;105(2):62–7. [PubMed] [Google Scholar]
- 11. Kirchner A, Birklein F, Stefan H, Handwerker HO. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology. 2000;55(8):1167–71. 10.1212/wnl.55.8.1167. [DOI] [PubMed] [Google Scholar]
- 12. Ness TJ, Fillingim RB, Randich A, Backensto EM, Faught E. Low intensity vagal nerve stimulation lowers human thermal pain thresholds. Pain. 2000;86(1–2):81–5. 10.1016/s0304-3959(00)00237-2. [DOI] [PubMed] [Google Scholar]
- 13. Randich A, Gebhart GF. Vagal afferent modulation of nociception. Brain Res Brain Res Rev. 1992;17(2):77–99. 10.1016/0165-0173(92)90009-b. [DOI] [PubMed] [Google Scholar]
- 14. Hatton KW, McLarney JT, Pittman T, Fahy BG. Vagal nerve stimulation: overview and implications for anesthesiologists. Anesth Analg. 2006;103(5):1241–9. 10.1213/01.ane.0000244532.71743.c6. [DOI] [PubMed] [Google Scholar]
- 15. Nemeroff CB, Mayberg HS, Krahl SE, McNamara J, Frazer A, Henry TR, et al. VNS therapy in treatment-resistant depression: clinical evidence and putative neurobiological mechanisms. Neuropsychopharmacology. 2006;31(7):1345–55. 10.1038/sj.npp.1301082. [DOI] [PubMed] [Google Scholar]
- 16. Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part I. Headache. 2016;56(1):71–8. 10.1111/head.12647. [DOI] [PubMed] [Google Scholar]
- 17. Ren K, Zhuo M, Randich A, Gebhart GF. Vagal afferent stimulation-produced effects on nociception in capsaicin-treated rats. J Neurophysiol. 1993;69(5):1530–40. 10.1152/jn.1993.69.5.1530. [DOI] [PubMed] [Google Scholar]
- 18. Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153(1):1–26. 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- 19. Morest DK. Experimental study of the projections of the nucleus of the tractus solitarius and the area postrema in the cat. J Comp Neurol. 1967;130(4):277–300. 10.1002/cne.901300402. [DOI] [PubMed] [Google Scholar]
- 20. Cechetto DF. Central representation of visceral function. Fed Proc. 1987;46(1):17–23. [PubMed] [Google Scholar]
- 21. Chakravarthy K, Chaudhry H, Williams K, Christo PJ. Review of the uses of vagal nerve stimulation in chronic pain management. Curr Pain Headache Rep. 2015;19(12):54. 10.1007/s11916-015-0528-6. [DOI] [PubMed] [Google Scholar]
- 22. Zabara J. Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia. 1992;33(6):1005–12. 10.1111/j.1528-1157.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 23. Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: preliminary results. Epilepsia. 1990;31(Suppl 2):S40–3. 10.1111/j.1528-1157.1990.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 24. Uthman BM, Wilder BJ, Penry JK, Dean C, Ramsay RE, Reid SA, et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology. 1993;43(7):1338–45. 10.1212/wnl.43.7.1338. [DOI] [PubMed] [Google Scholar]
- 25. Morris GL, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(16):1453–9. 10.1212/WNL.0b013e3182a393d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harden CL, Pulver MC, Ravdin LD, Nikolov B, Halper JP, Labar DR. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93–9. 10.1006/ebeh.2000.0046. [DOI] [PubMed] [Google Scholar]
- 27. Cristancho P, Cristancho MA, Baltuch GH, Thase ME, O’Reardon JP. Effectiveness and safety of vagus nerve stimulation for severe treatment-resistant major depression in clinical practice after FDA approval: outcomes at 1 year. J Clin Psychiatry. 2011;72(10):1376–82. 10.4088/JCP.09m05888blu. [DOI] [PubMed] [Google Scholar]
- 28. De Ferrari GM, Crijns HJGM, Borggrefe M, Milasinovic G, Smid J, Zabel M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32(7):847–55. 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- 29. Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113(29):8284–9. 10.1073/pnas.1605635113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang DW, Yin YM, Yao YM. Vagal modulation of the inflammatory response in sepsis. Int Rev Immunol. 2016;35(5):415–33. 10.3109/08830185.2015.1127369. [DOI] [PubMed] [Google Scholar]
- 31. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014;1(2):64–73. 10.1007/s40473-014-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liporace J, Hucko D, Morrow R, Barolat G, Nei M, Schnur J, et al. Vagal nerve stimulation: adjustments to reduce painful side effects. Neurology. 2001;57(5):885–6. 10.1212/wnl.57.5.885. [DOI] [PubMed] [Google Scholar]
- 33. Ellrich J. Transcutaneous auricular vagus nerve stimulation. J Clin Neurophysiol. 2019;36(6):437–42. 10.1097/WNP.0000000000000576. [DOI] [PubMed] [Google Scholar]
- 34. Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part II. Headache. 2016;56(2):259–66. 10.1111/head.12650. [DOI] [PubMed] [Google Scholar]
- 35. Mertens A, Raedt R, Gadeyne S, Carrette E, Boon P, Vonck K. Recent advances in devices for vagus nerve stimulation. Expert Rev Med Devices. 2018;15(8):527–39. 10.1080/17434440.2018.1507732. [DOI] [PubMed] [Google Scholar]
- 36. Sator-Katzenschlager SM, Scharbert G, Kozek-Langenecker SA, Szeles JC, Finster G, Schiesser AW, et al. The short- and long-term benefit in chronic low back pain through adjuvant electrical versus manual auricular acupuncture. Anesth Analg. 2004;98(5):1359–64 table of contents. 10.1213/01.ane.0000107941.16173.f7. [DOI] [PubMed] [Google Scholar]
- 37. Sator-Katzenschlager SM, Szeles JC, Scharbert G, Michalek-Sauberer A, Kober A, Heinze G, et al. Electrical stimulation of auricular acupuncture points is more effective than conventional manual auricular acupuncture in chronic cervical pain: a pilot study. Anesth Analg. 2003;97(5):1469–73. 10.1213/01.ANE.0000082246.67897.0B. [DOI] [PubMed] [Google Scholar]
- 38. Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, et al. Current directions in the auricular vagus nerve stimulation II: an engineering perspective. Front Neurosci. 2019;13:772. 10.3389/fnins.2019.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ren K, Randich A, Gebhart GF. Vagal afferent modulation of a nociceptive reflex in rats: involvement of spinal opioid and monoamine receptors. Brain Res. 1988;446(2):285–94. 10.1016/0006-8993(88)90887-6. [DOI] [PubMed] [Google Scholar]
- 40. Busch V, Zeman F, Heckel A, Menne F, Ellrich J, Eichhammer P. The effect of transcutaneous vagus nerve stimulation on pain perception: an experimental study. Brain Stimul. 2013;6(2):202–9. 10.1016/j.brs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 41. Usichenko T, Hacker H, Lotze M. Transcutaneous auricular vagal nerve stimulation (taVNS) might be a mechanism behind the analgesic effects of auricular acupuncture. Brain Stimul. 2017;10(6):1042–4. 10.1016/j.brs.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 42. Ren K, Randich A, Gebhart GF. Spinal serotonergic and kappa opioid receptors mediate facilitation of the tail flick reflex produced by vagal afferent stimulation. Pain. 1991;45(3):321–9. 10.1016/0304-3959(91)90057-5. [DOI] [PubMed] [Google Scholar]
- 43. Goadsby PJ, Grosberg BM, Mauskop A, Cady R, Simmons KA. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34(12):986–93. 10.1177/0333102414524494. [DOI] [PubMed] [Google Scholar]
- 44. Barbanti P, Grazzi L, Egeo G, Padovan AM, Liebler E, Bussone G. Non-invasive vagus nerve stimulation for acute treatment of high-frequency and chronic migraine: an open-label study. J Headache Pain. 2015;16:61. 10.1186/s10194-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Silberstein SD, Calhoun AH, Lipton RB, Grosberg BM, Cady RK, Dorlas S, et al. Chronic migraine headache prevention with noninvasive vagus nerve stimulation: the EVENT study. Neurology. 2016;87(5):529–38. 10.1212/WNL.0000000000002918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Najib U, Smith T, Hindiyeh N, Saper J, Nye B, Ashina S, et al. Non-invasive vagus nerve stimulation for prevention of migraine: the multicenter, randomized, double-blind, sham-controlled PREMIUM II trial. Cephalalgia. 2022;42(7):560–9. 10.1177/03331024211068813. [DOI] [PubMed] [Google Scholar]
- 47. Nesbitt AD, Marin JCA, Tompkins E, Ruttledge MH, Goadsby PJ. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84(12):1249–53. 10.1212/WNL.0000000000001394. [DOI] [PubMed] [Google Scholar]
- 48. Gaul C, Diener H-C, Silver N, Magis D, Reuter U, Andersson A, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia. 2016;36(6):534–46. 10.1177/0333102415607070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Straube A, Ellrich J, Eren O, Blum B, Ruscheweyh R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain. 2015;16:543. 10.1186/s10194-015-0543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Huang Y, Li H, Yan Z, Zhang Y, Liu X, et al. Transcutaneous auricular vagus nerve stimulation (taVNS) for migraine: an fMRI study. Reg Anesth Pain Med. 2021;46(2):145–50. 10.1136/rapm-2020-102088. [DOI] [PubMed] [Google Scholar]
- 51. Silberstein SD, Mechtler LL, Kudrow DB, Calhoun AH, McClure C, Saper JR, et al. Non-invasive vagus nerve stimulation for the ACute treatment of cluster headache: findings from the randomized, double-blind, sham-controlled ACT1 study. Headache. 2016;56(8):1317–32. 10.1111/head.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goadsby PJ, de Coo IF, Silver N, Tyagi A, Ahmed F, Gaul C, et al. Non-invasive vagus nerve stimulation for the acute treatment of episodic and chronic cluster headache: a randomized, double-blind, sham-controlled ACT2 study. Cephalalgia. 2018;38(5):959–69. 10.1177/0333102417744362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lange G, Janal MN, Maniker A, Fitzgibbons J, Fobler M, Cook D, et al. Safety and efficacy of vagus nerve stimulation in fibromyalgia: a phase I/II proof of concept trial. Pain Med. 2011;12(9):1406–13. 10.1111/j.1526-4637.2011.01203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kutlu N, Özden AV, Alptekin HK, Alptekin JÖ. The impact of auricular vagus nerve stimulation on pain and life quality in patients with fibromyalgia syndrome. BioMed Res Int. 2020;2020:8656218. 10.1155/2020/8656218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muthulingam JA, Olesen SS, Hansen TM, Brock C, Drewes AM, Frøkjær JB. Cervical transcutaneous vagal neuromodulation in chronic pancreatitis patients with chronic pain: a randomised sham controlled clinical trial. PLoS One. 2021;16(2):e0247653. 10.1371/journal.pone.0247653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Farmer AD, Albusoda A, Amarasinghe G, Ruffle JK, Fitzke HE, Idrees R, et al. Transcutaneous vagus nerve stimulation prevents the development of, and reverses, established oesophageal pain hypersensitivity. Aliment Pharmacol Ther. 2020;52(6):988–96. 10.1111/apt.15869. [DOI] [PubMed] [Google Scholar]
- 57. Shi X, Hu Y, Zhang B, Li W, Chen JD, Liu F. Ameliorating effects and mechanisms of transcutaneous auricular vagal nerve stimulation on abdominal pain and constipation. JCI Insight. 2021;6(14):e150052. 10.1172/jci.insight.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Venborg J, Wegeberg A-M, Kristensen S, Brock B, Brock C, Pfeiffer-Jensen M. The effect of transcutaneous vagus nerve stimulation in patients with polymyalgia rheumatica. Pharmaceuticals. 2021;14(11):1166. 10.3390/ph14111166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Aranow C, Atish-Fregoso Y, Lesser M, Mackay M, Anderson E, Chavan S, et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis. 2021;80(2):203–8. 10.1136/annrheumdis-2020-217872. [DOI] [PubMed] [Google Scholar]
- 60. Sun-Edelstein C, Rapoport AM. Update on the pharmacological treatment of chronic migraine. Curr Pain Headache Rep. 2016;20(1):6. 10.1007/s11916-015-0533-9. [DOI] [PubMed] [Google Scholar]
- 61. Hord ED, Evans MS, Mueed S, Adamolekun B, Naritoku DK. The effect of vagus nerve stimulation on migraines. J Pain. 2003;4(9):530–4. 10.1016/j.jpain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 62. Sadler RM, Purdy RA, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalalgia. 2002;22(6):482–4. 10.1046/j.1468-2982.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 63. Velioglu SK, Gedikli O, Yıldırım M, Ayar A. Epilepsy may cause increased pain sensitivity: evidence from absence epileptic WAG/Rij rats. Epilepsy Behav. 2017;75:146–50. 10.1016/j.yebeh.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 64. Ruiz L, Ferrandi D. Topiramate in migraine progression. J Headache Pain. 2009;10(6):419–22. 10.1007/s10194-009-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aaronson ST, Carpenter LL, Conway CR, Reimherr FW, Lisanby SH, Schwartz TL, et al. Vagus nerve stimulation therapy randomized to different amounts of electrical charge for treatment-resistant depression: acute and chronic effects. Brain Stimul. 2013;6(4):631–40. 10.1016/j.brs.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 66. Dodick DW, Silberstein SD, Reed KL, Deer TR, Slavin KV, Huh B, et al. Safety and efficacy of peripheral nerve stimulation of the occipital nerves for the management of chronic migraine: long-term results from a randomized, multicenter, double-blinded, controlled study. Cephalalgia. 2015;35(4):344–58. 10.1177/0333102414543331. [DOI] [PubMed] [Google Scholar]
- 67. Gaul C, Magis D, Liebler E, Straube A. Effects of non-invasive vagus nerve stimulation on attack frequency over time and expanded response rates in patients with chronic cluster headache: a post hoc analysis of the randomised, controlled PREVA study. J Headache Pain. 2017;18(1):22. 10.1186/s10194-017-0731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lai YH, Huang YC, Huang LT, Chen RM, Chen C. Cervical noninvasive vagus nerve stimulation for migraine and cluster headache: a systematic review and meta-analysis. Neuromodulation. 2020;23(6):721–31. 10.1111/ner.13122. [DOI] [PubMed] [Google Scholar]
- 69. Butler S, Landmark T, Glette M, Borchgrevink P, Woodhouse A. Chronic widespread pain-the need for a standard definition. Pain. 2016;157(3):541–3. 10.1097/j.pain.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 70. Natelson BH, Stegner AJ, Lange G, Khan S, Blate M, Sotolongo A, et al. Vagal nerve stimulation as a possible non-invasive treatment for chronic widespread pain in Gulf Veterans with Gulf War Illness. Life Sci. 2021;282:119805. 10.1016/j.lfs.2021.119805. [DOI] [PubMed] [Google Scholar]
- 71. Kleeff J, Whitcomb DC, Shimosegawa T, Esposito I, Lerch MM, Gress T, et al. Chronic pancreatitis. Nat Rev Dis Primers. 2017;3:17060. 10.1038/nrdp.2017.60. [DOI] [PubMed] [Google Scholar]
- 72. Singh VK, Yadav D, Garg PK. Diagnosis and management of chronic pancreatitis: a review. JAMA. 2019;322(24):2422–34. 10.1001/jama.2019.19411. [DOI] [PubMed] [Google Scholar]
- 73. Olesen SS, Bouwense SAW, Wilder-Smith OHG, van Goor H, Drewes AM. Pregabalin reduces pain in patients with chronic pancreatitis in a randomized, controlled trial. Gastroenterology. 2011;141(2):536–43. 10.1053/j.gastro.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 74. Rustagi T, Njei B. Antioxidant therapy for pain reduction in patients with chronic pancreatitis: a systematic review and meta-analysis. Pancreas. 2015;44(5):812–8. 10.1097/MPA.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 75. Muthulingam JA, Hansen TM, Olesen SS, Drewes AM, Frøkjær JB. Two-week cervical vagus nerve stimulation in chronic pancreatitis patients induces functional connectivity changes of limbic structures. Neuromodulation. 2022;25(3):471–8. 10.1111/ner.13482. [DOI] [PubMed] [Google Scholar]
- 76. Ruepert L, Quartero AO, de Wit NJ, van der Heijden GJ, Rubin G, Muris JW. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;2011(8):CD003460. 10.1002/14651858.CD003460.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Webb AN, Kukuruzovic RH, Catto-Smith AG, Sawyer SM. Hypnotherapy for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2007;4:CD005110. 10.1002/14651858.CD005110.pub2. [DOI] [PubMed] [Google Scholar]
- 78. Defrees DN, Bailey J. Irritable bowel syndrome: epidemiology, pathophysiology, diagnosis, and treatment. Prim Care. 2017;44(4):655–71. 10.1016/j.pop.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 79. Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, et al. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19(1 Suppl l):62–88. 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 80. Botha C, Farmer AD, Nilsson M, Brock C, Gavrila AD, Drewes AM, et al. Preliminary report: modulation of parasympathetic nervous system tone influences oesophageal pain hypersensitivity. Gut. 2015;64(4):611–7. 10.1136/gutjnl-2013-306698. [DOI] [PubMed] [Google Scholar]
- 81. Oshinsky ML, Murphy AL, Hekierski H, Cooper M, Simon BJ. Noninvasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155(5):1037–42. 10.1016/j.pain.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang S, Zhai X, Li S, McCabe MF, Wang X, Rong P. Transcutaneous vagus nerve stimulation induces tidal melatonin secretion and has an antidiabetic effect in Zucker fatty rats. PLoS One. 2015;10(4):e0124195. 10.1371/journal.pone.0124195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang S, Li S, Zhai X, Rong P, He J, Liu L, et al. Transcutaneous auricular vagal nerve stimulation releases extrapineal melatonin and reduces thermal hypersensitivity in Zucker diabetic fatty rats. Front Neurosci. 2022;16:916822. 10.3389/fnins.2022.916822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Li S, Sun C, Rong P, Zhai X, Zhang J, Baker M, et al. Auricular vagus nerve stimulation enhances central serotonergic function and inhibits diabetic neuropathy development in Zucker fatty rats. Mol Pain. 2018;14:1744806918787368. 10.1177/1744806918787368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Treede RD, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. 2019;160(1):19–27. 10.1097/j.pain.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 86. Frøkjaer JB, Bergmann S, Brock C, Madzak A, Farmer AD, Ellrich J, et al. Modulation of vagal tone enhances gastroduodenal motility and reduces somatic pain sensitivity. Neurogastroenterol Motil. 2016;28(4):592–8. 10.1111/nmo.12760. [DOI] [PubMed] [Google Scholar]
- 87. van Maanen MA, Lebre MC, van der Poll T, LaRosa GJ, Elbaum D, Vervoordeldonk MJ, et al. Stimulation of nicotinic acetylcholine receptors attenuates collagen-induced arthritis in mice. Arthritis Rheum. 2009;60(1):114–22. 10.1002/art.24177. [DOI] [PubMed] [Google Scholar]
- 88. Krusche-Mandl I, Kaider A, Starlinger J, Preschitz M, Schuster R, Kefurt R, et al. Implementation of electrical auricular acupuncture and low frequency modulated electric current therapy in pain management of patients with knee osteoarthritis: a randomized pilot trial. J Clin Med. 2019;8(8):1229. 10.3390/jcm8081229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Qaseem A, Wilt TJ, McLean RM, Forciea MA; Clinical Guidelines Committee of the American College of Physicians, Denberg TD, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American college of physicians. Ann Intern Med. 2017;166(7):514–30. 10.7326/M16-2367. [DOI] [PubMed] [Google Scholar]
- 90. Patrick N, Emanski E, Knaub MA. Acute and chronic low back pain. Med Clin North Am. 2014;98(4):777–89, xii. 10.1016/j.mcna.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 91. Meints SM, Garcia RG, Schuman-Olivier Z, Datko M, Desbordes G, Cornelius M, et al. The effects of combined respiratory-gated auricular vagal afferent nerve stimulation and mindfulness meditation for chronic low back pain: a pilot study. Pain Med. 2022;23(9):1570–81. 10.1093/pm/pnac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ammons WS, Blair RW, Foreman RD. Vagal afferent inhibition of primate thoracic spinothalamic neurons. J Neurophysiol. 1983;50(4):926–40. 10.1152/jn.1983.50.4.926. [DOI] [PubMed] [Google Scholar]
- 93. Thies R, Foreman RD. Inhibition and excitation of thoracic spinoreticular neurons by electrical stimulation of vagal afferent nerves. Exp Neurol. 1983;82(1):1–16. 10.1016/0014-4886(83)90238-8. [DOI] [PubMed] [Google Scholar]
- 94. Ammons WS, Blair RW, Foreman RD. Vagal afferent inhibition of spinothalamic cell responses to sympathetic afferents and bradykinin in the monkey. Circ Res. 1983;53(5):603–12. 10.1161/01.res.53.5.603. [DOI] [PubMed] [Google Scholar]
- 95. Hobbs SF, Oh UT, Chandler MJ, Foreman RD. Cardiac and abdominal vagal afferent inhibition of primate T9-S1 spinothalamic cells. Am J Physiol. 1989;257(4 Pt 2):R889–95. 10.1152/ajpregu.1989.257.4.R889. [DOI] [PubMed] [Google Scholar]
- 96. Chandler MJ, Hobbs SF, Bolser DC, Foreman RD. Effects of vagal afferent stimulation on cervical spinothalamic tract neurons in monkeys. Pain. 1991;44(1):81–7. 10.1016/0304-3959(91)90152-N. [DOI] [PubMed] [Google Scholar]
- 97. Chandler MJ, Zhang J, Qin C, Foreman RD. Spinal inhibitory effects of cardiopulmonary afferent inputs in monkeys: neuronal processing in high cervical segments. J Neurophysiol. 2002;87(3):1290–302. 10.1152/jn.00079.2001. [DOI] [PubMed] [Google Scholar]
- 98. Lyubashina OA, Sokolov AY, Panteleev SS. Vagal afferent modulation of spinal trigeminal neuronal responses to dural electrical stimulation in rats. Neuroscience. 2012;222:29–37. 10.1016/j.neuroscience.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 99. Randich A, Aicher SA. Medullary substrates mediating antinociception produced by electrical stimulation of the vagus. Brain Res. 1988;445(1):68–76. 10.1016/0006-8993(88)91075-x. [DOI] [PubMed] [Google Scholar]
- 100. Randich A, Ren K, Gebhart GF. Electrical stimulation of cervical vagal afferents. II. Central relays for behavioral antinociception and arterial blood pressure decreases. J Neurophysiol. 1990;64(4):1115–24. 10.1152/jn.1990.64.4.1115. [DOI] [PubMed] [Google Scholar]
- 101. Ren K, Randich A, Gebhart GF. Electrical stimulation of cervical vagal afferents. I. Central relays for modulation of spinal nociceptive transmission. J Neurophysiol. 1990;64(4):1098–114. 10.1152/jn.1990.64.4.1098. [DOI] [PubMed] [Google Scholar]
- 102. Nishikawa Y, Koyama N, Yoshida Y, Yokota T. Activation of ascending antinociceptive system by vagal afferent input as revealed in the nucleus ventralis posteromedialis. Brain Res. 1999;833(1):108–11. 10.1016/s0006-8993(99)01521-8. [DOI] [PubMed] [Google Scholar]
- 103. Zhang Y, Liu J, Li H, Yan Z, Liu X, Cao J, et al. Transcutaneous auricular vagus nerve stimulation at 1 Hz modulates locus coeruleus activity and resting state functional connectivity in patients with migraine: an fMRI study. Neuroimage Clin. 2019;24:101971. 10.1016/j.nicl.2019.101971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cao J, Zhang Y, Li H, Yan Z, Liu X, Hou X, et al. Different modulation effects of 1 Hz and 20 Hz transcutaneous auricular vagus nerve stimulation on the functional connectivity of the periaqueductal gray in patients with migraine. J Transl Med. 2021;19(1):354. 10.1186/s12967-021-03024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Meller ST, Lewis SJ, Ness TJ, Brody MJ, Gebhart GF. Vagal afferent-mediated inhibition of a nociceptive reflex by intravenous serotonin in the rat. I. Characterization. Brain Res. 1990;524(1):90–100. 10.1016/0006-8993(90)90496-x. [DOI] [PubMed] [Google Scholar]
- 106. Hu B, Akerman S, Goadsby PJ. Characterization of opioidergic mechanisms related to the anti-migraine effect of vagus nerve stimulation. Neuropharmacology. 2021;195:108375. 10.1016/j.neuropharm.2020.108375. [DOI] [PubMed] [Google Scholar]
- 107. Alt LK, Wach K, Liebler EJ, Straube A, Ruscheweyh R. A randomized sham-controlled cross-over study on the short-term effect of non-invasive cervical vagus nerve stimulation on spinal and supraspinal nociception in healthy subjects. Headache. 2020;60(8):1616–31. 10.1111/head.13891. [DOI] [PubMed] [Google Scholar]
- 108. De Icco R, Martinelli D, Bitetto V, Fresia M, Liebler E, Sandrini G, et al. Peripheral vagal nerve stimulation modulates the nociceptive withdrawal reflex in healthy subjects: a randomized, cross-over, sham-controlled study. Cephalalgia. 2018;38(10):1658–64. 10.1177/0333102417742347. [DOI] [PubMed] [Google Scholar]
- 109. Ayata C. Cortical spreading depression triggers migraine attack: pro. Headache. 2010;50(4):725–30. 10.1111/j.1526-4610.2010.01647.x. [DOI] [PubMed] [Google Scholar]
- 110. Chen SP, Ay I, Lopes de Morais A, Qin T, Zheng Y, Sadeghian H, et al. Vagus nerve stimulation inhibits cortical spreading depression. Pain. 2016;157(4):797–805. 10.1097/j.pain.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20(3):221–7. 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 112. Liu TT, Morais A, Takizawa T, Mulder I, Simon BJ, Chen SP, et al. Efficacy profile of noninvasive vagus nerve stimulation on cortical spreading depression susceptibility and the tissue response in a rat model. J Headache Pain. 2022;23(1):12. 10.1186/s10194-022-01384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Tal M. A role for inflammation in chronic pain. Curr Rev Pain. 1999;3(6):440–6. 10.1007/s11916-999-0071-4. [DOI] [PubMed] [Google Scholar]
- 114. Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853–9. 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 115. Lerman I, Hauger R, Sorkin L, Proudfoot J, Davis B, Huang A, et al. Noninvasive transcutaneous vagus nerve stimulation decreases whole blood culture-derived cytokines and chemokines: a randomized, blinded, healthy control pilot trial. Neuromodulation. 2016;19(3):283–90. 10.1111/ner.12398. [DOI] [PubMed] [Google Scholar]
- 116. Chaudhry SR, Lendvai IS, Muhammad S, Westhofen P, Kruppenbacher J, Scheef L, et al. Inter-ictal assay of peripheral circulating inflammatory mediators in migraine patients under adjunctive cervical non-invasive vagus nerve stimulation (nVNS): a proof-of-concept study. Brain Stimul. 2019;12(3):643–51. 10.1016/j.brs.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 117. Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96. 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vida G, Peña G, Deitch EA, Ulloa L. α7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J Immunol. 2011;186(7):4340–6. 10.4049/jimmunol.1003722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lendvai IS, Maier A, Scheele D, Hurlemann R, Kinfe TM. Spotlight on cervical vagus nerve stimulation for the treatment of primary headache disorders: a review. J Pain Res. 2018;11:1613–25. 10.2147/JPR.S129202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. van Maanen MA, Stoof SP, Larosa GJ, Vervoordeldonk MJ, Tak PP. Role of the cholinergic nervous system in rheumatoid arthritis: aggravation of arthritis in nicotinic acetylcholine receptor α7 subunit gene knockout mice. Ann Rheum Dis. 2010;69(9):1717–23. 10.1136/ard.2009.118554. [DOI] [PubMed] [Google Scholar]
- 121. Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63(6):938–48. 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 122. Rawat JK, Roy S, Singh M, Guatam S, Yadav RK, Ansari MN, et al. Transcutaneous vagus nerve stimulation regulates the cholinergic anti-inflammatory pathway to counteract 1, 2-dimethylhydrazine induced colon carcinogenesis in Albino wistar rats. Front Pharmacol. 2019;10:353. 10.3389/fphar.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pavlov VA, Parrish WR, Rosas-Ballina M, Ochani M, Puerta M, Ochani K, et al. Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav Immun. 2009;23(1):41–5. 10.1016/j.bbi.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89(2):337–43. 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 125. Wang JY, Zhang Y, Chen Y, Wang Y, Li SY, Wang YF, et al. Mechanisms underlying antidepressant effect of transcutaneous auricular vagus nerve stimulation on CUMS model rats based on hippocampal α7nAchR/NF-κB signal pathway. J Neuroinflammation. 2021;18(1):291. 10.1186/s12974-021-02341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Jiang Y, Li L, Liu B, Zhang Y, Chen Q, Li C. Vagus nerve stimulation attenuates cerebral ischemia and reperfusion injury via endogenous cholinergic pathway in rat. PLoS One. 2014;9(7):e102342. 10.1371/journal.pone.0102342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhang LY, Jia MR, Sun T. The roles of special proresolving mediators in pain relief. Rev Neurosci. 2018;29(6):645–60. 10.1515/revneuro-2017-0074. [DOI] [PubMed] [Google Scholar]
- 128. Quan-Xin F, Fan F, Xiang-Ying F, Shu-Jun L, Shi-Qi W, Zhao-Xu L, et al. Resolvin D1 reverses chronic pancreatitis-induced mechanical allodynia, phosphorylation of NMDA receptors, and cytokines expression in the thoracic spinal dorsal horn. BMC Gastroenterol. 2012;12:148. 10.1186/1471-230X-12-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16(5):592–7 1p following 597. 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Miao GS, Liu ZH, Wei SX, Luo JG, Fu ZJ, Sun T. Lipoxin A4 attenuates radicular pain possibly by inhibiting spinal ERK, JNK and NF-κB/p65 and cytokine signals, but not p38, in a rat model of non-compressive lumbar disc herniation. Neuroscience. 2015;300:10–8. 10.1016/j.neuroscience.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 131. Serhan CN, de la Rosa X, Jouvene CC. Cutting edge: human vagus produces specialized proresolving mediators of inflammation with electrical stimulation reducing proinflammatory eicosanoids. J Immunol. 2018;201(11):3161–5. 10.4049/jimmunol.1800806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Tao X, Lee MS, Donnelly CR, Ji RR. Neuromodulation, specialized proresolving mediators, and resolution of pain. Neurotherapeutics. 2020;17(3):886–99. 10.1007/s13311-020-00892-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Hosoi T, Okuma Y, Nomura Y. Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am J Physiol Regul Integr Comp Physiol. 2000;279(1):R141–7. 10.1152/ajpregu.2000.279.1.R141. [DOI] [PubMed] [Google Scholar]