Abstract
The assumption of the pineal hormone melatonin as a therapeutic use for COVID-19-affected people seems promising. Its intake has shown significant improvement in the patients’ conditions. Higher melatonin titers in children may provide a protective shield against this disease. The hormone melatonin works as an anti-inflammatory, antioxidant, immunomodulator, and strategically slows down the cytokine release which is observed in the COVID-19 disease, thereby improving the overall health of afflicted patients. The medical community is expected shortly to use remedial attributes like anti-inflammatory, antioxidant, antivirals, etc., of melatonin in the successful prevention and cure of COVID-19 morbidity. Thus, the administration of melatonin seems auspicious in the cure and prevention of this COVID-19 fatality. Moreover, melatonin does not seem to reduce the efficiency of approved vaccines against the SARS-CoV-2 virus. Melatonin increases the production of inflammatory cytokines and Th1 and enhances both humoral and cell-mediated responses. Through the enhanced humoral immunity, melatonin exhibits antiviral activities by suppressing multiple inflammatory products such as IL-6, IL1β, and tumor necrosis factor α, which are immediately released during lung injury of severe COVID-19. Hence, the novel use of melatonin along with other antivirals as an early treatment option against COVID-19 infection is suggested. Here, we have chalked out the invasion mechanisms and appropriate implications of the latest findings concerned with melatonin against the virus SARS-CoV-2. Nevertheless, within the setting of a clinical intervention, the promising compounds must go through a series of studies before their recommendation. In the clinical field, this is done in a time-ordered sequence, in line with the phase label affixed to proper protocol of trials: phase I–phase II and the final phase III. Nevertheless, while medical recommendations can only be made on the basis of reassuring evidence, there are still three issues worth considering before implementation: representativeness, validity, and lastly generalizability.
Keywords: Comorbidities, COVID-19, Cytokine, Melatonin, SARS-CoV-2, Therapeutic
Introduction
COVID-19, a devastating viral disease spread out throughout the world, first struck human society in December 2019 [1]. So far what has been observed is that children, as compared to elderly ones, and the nocturnal animal bats possess high levels of the pineal hormone melatonin, which may contribute to their high antiviral resistance. Precise discovery of the pineal hormone melatonin was made in 1958 by Lerner and his coworkers from the bovine pineal gland [2]. Melatonin, as we know, is generally involved in circadian rhythm entrainment and pigmentary aggregations [3–5]. Melatonin, which is also a well-known anti-oxidative and anti-inflammatory molecule, limits virus-related diseases and induces antiviral immuno-stimuli in COVID-19 patients who may even have comorbidities of diabetes and obesity [6–8]. Ancillary treatment involving the hormone melatonin along with an antiviral drug proves beneficial against general viruses, and thus could even counteract COVID-19 as well [9]. This review highlights about prominent benefits of melatonin in the medication of the COVID-19 fatal infection inferred by the virus SARS-CoV-2.
Invasion of Host Cells by SARS-CoV-2
SARS-CoV-2 virus is transmitted through respiratory drops and aerosols among the masses. Inside the host’s tissues, this virus attaches to targeted cell receptors and penetrates the cells of the host either through plasma membrane fusion or endocytosis. Coronaviruses are composed of four types of structural classes of proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins [10, 11]. Spike protein is composed of two active subunits (S1 and S2) in which S1 binds with the host cell membrane receptor while the S2 subunit plays an important role in the binding of viral and host cell membranes This protein protrudes from the viral cell membrane and plays a vital role in host cell binding and then enter into the cell [11].
The operative cell membrane receptor for SARS-CoV is ACE-2 which is characteristically expressed on epithelial cells of the lungs [12]. ACE-2 receptor is quite ubiquitous because of its fundamental role in many cardiovascular functions. S protein binds initially with this ACE-2 receptor and thus host cell intrusion by the virus begins [13–15]. With the coupling of the SARS-CoV-2 virus to ACE-2 receptor, the S protein now activates through dual cleavage of a protease, the first one while priming at S1/S2 cleavage site and the second while triggering at the location next after fusion protein within an S2 subunit [16–18]. First cleavage makes the S2 subunit stable at the binding site and further cleavage possibly stimulates the S protein leading to conformational changes that result in the host cell and viral membrane bindings [19].
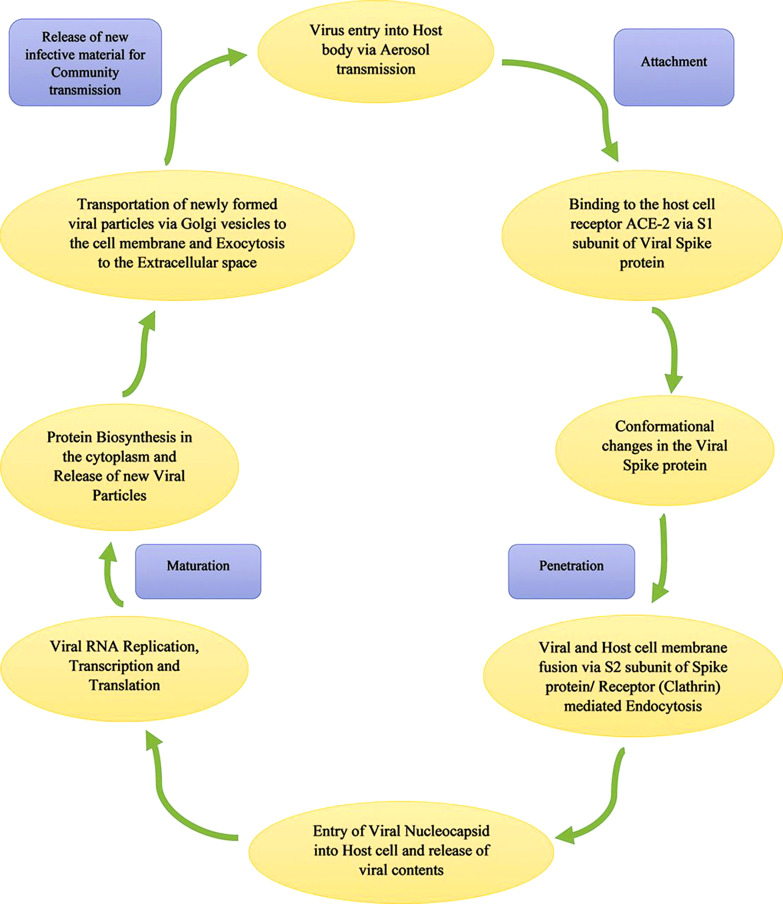
After cell membrane integration, the virus invades the alveolar epithelial cells of the respiratory tract to release viral contents inside the cells. Within the host cell, viral replication undergoes to form −ve strands of RNA by the pre-existing single-strands (ss) +ve RNA through transcription. This new −ve strand RNA serves as the template to form daughter strands of +ve RNAs. During translation, these new RNAs produce newer proteins in the cell cytoplasm [20–22]. N protein of the virus binds with these new RNAs and the protein M helps in channelizing into the endoplasmic reticulum. These nucleocapsids are enclosed within lumen of endoplasmic reticulum from where they are transported through Golgi vesicles to cell membrane and ultimately to the extracellular fluid space through exocytosis. These newly synthesized viral particles proceed to invade adjacent epithelial cells. Moreover, they produce virginal infective materials for mass community transmission through respiratory aerosols [11]. Life cycle of the SARS-CoV-2 is shown in Figure 1.
Fig. 1.
An outline of the SARS-CoV-2 virus life cycle (Source: Parasher [20]).
Melatonin
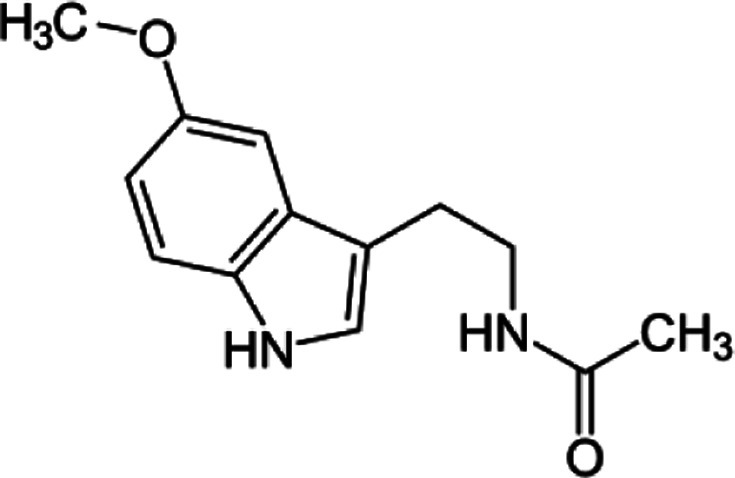
(Melatonin)
[N-Acetyl-5-methoxytryptamine, MF: C13H16N2O2, FW: 232.278]
Melatonin shows its photoperiodic, circadian effects, and several chronobiological processes through the pharmacologically specific, high-affinity receptors [23–29]. Generally synthesized at night hours, melatonin may act as a signal of darkness to the body [3, 30, 31]. Vanecek [24] has also reported that the hormone melatonin shows its effects through the distinguished high-affinity receptors present on the cell membrane that is coupled with the GTP binding proteins. Three types of melatonin receptors do exist: Mel1A, Mel1B, and Mel1C.
According to Sack et al. [32], the administration of melatonin hormone can commence circadian rhythms in blind people who have independent rhythms. Melatonin inhibits cellular cAMP accumulation. It regulates the transcription factors (third messengers), i.e., cAMP binding protein gets phosphorylated and expression of c-Fos occurs. Although the phenomenon of the molecular mechanisms of melatonin-induced effects is still in its infancy, it could engage minimum of two analogous transduction mechanisms: one controlling phospholipid metabolism along with [Ca+2 ]i. transport and the next blocking adenylyl cyclase [24].
Melatonin is formed in a biosynthetic pathway from an essential amino acid L-Tryptophan, via 5-hydroxylation by tryptophan-5-hydroxylase enzyme into 5-hydroxytryptophan, which is decarboxylated by another enzyme aromatic amino acid decarboxylase to 5-hydroxytryptamine (serotonin) that is converted to N-acetylserotonin by the enzyme serotonin N-acetyltransferase (SNAT/AA-NAT – arylalkylamine N-acetyltransferase), whereafter N-acetylserotonin is ultimately converted into the hormone melatonin (N-acetyl-5-methoxytryptamine) by the enzyme hydroxyindole-O-methyltransferase (HIOMT, also known as acetylserotonin methyltransferase) [3, 28, 33–35].
Potential Clinical Applications of Melatonin
COVID-19 disease spread throughout the world where millions of people showed COVID positive results and over 6.5 million people have lost their lives due to this disease [1]. To avoid emergencies, treatment of COVID-19 must start shortly after diagnosis. Compounds that can relieve inflammation and oxidative damage can reduce the rates and deaths by infection [36]. Physicians and scientists have been trying for early breakthroughs in COVID-19 prevention [9]. This disease, as described before, develops as the virus SARS-CoV-2 clings to receptors of angiotensin-converting enzyme 2 (ACE2) in lungs’ epithelial cells, causing a pro-inflammatory response which often leads to cytokine gust and acute respiratory distress syndrome [6, 37]. Further pro-oxidant response stimulates reactive oxygen species (ROS) mediated injury to air sacs in the lungs [38].
Melatonin has been used in the treatment of respiratory syncytial virus (RSV) morbidity, by activating toll-like receptor (TLR3), RSV leads to signal cascade and this initiates nuclear factor kappa B (NF-κB) activity which is a transcription factor upregulating the formation of cytokines that are pro-inflammatory. RSV-infected macrophages show a reduced TLR3-guided downstream expression of the gene when treated with this pineal hormone melatonin [39]. Melatonin characteristically inhibits the activity of NF-κB, thereby diminishing the response of hyperinflammation caused by such infectious viruses in our respiratory system [40]. In the case of mice, having infected with influenza A, when they were treated with melatonin, then they showed decreased tumor necrosis factor (TNF)-α producing CD8 cells in spleen and lungs, and this can profoundly diminish the lethality of our lungs injury [41].
Through the murine models, it appears that melatonin possesses protective functions against the Ebola virus, which is the causing factor for acute vascular endothelial rupture that results in multi-organ hemorrhage. Such fatal consequences occur because of an excessive rise in inflammatory cytokines and chemokines like interferon-α, TNF-α, IL-6, IL-8, monocyte chemoattractant protein-1, and tissue factors that lead to fibrinolysis and coagulation irregularities [42]. Melatonin neutralizes ROS and weakens this cytokine surge that is correlated with infection by the virus while increasing the TH type 2-produced cytokines, interferon-gamma response, and natural killer cell activity to combat the Ebola viral infection mechanisms. Thus, melatonin reduces pro-inflammatory conditions, induces autogenous antioxidants, counterbalances ROS of viral infections, and upgrades mitochondrial functioning, thereby stopping injury to endothelial layers which can cause putrefying severance and distributed intravascular clottings [43–45]. Melatonin plays a preventive role in hemorrhagic fevers as seen in patients who had significantly reduced blood plasma melatonin titers than the control people [46–49]. Melatonin increases the production of inflammatory cytokines and Th1-type in rheumatoid arthritis and enhances both humoral and cell-mediated responses [50]. However, in the case of rheumatoid arthritis, administration of exogenous melatonin hardly exerts any significant therapeutic benefits [51]. Melatonin when added with vaccines against diarrhea for cattles increased the production of IFN-α and IFN-β and stimulated the production of T lymphocytes [52]. This indirectly implicates a stronger effect of melatonin on COVID vaccination and efficacy.
Melatonin can significantly decrease the viral load in blood, death rate, and lethality of the disease, e.g., as in encephalitis and myocarditis [39]. The decreased anti-inflammatory response occurs because of melatonin-mediated down-regulation of TNF-α of the central nervous system [53]. Increased volumes of cytokine IL-1B also determine the shielding effect of melatonin against central nervous system attacking viruses [54, 55]. Melatonin administration reduces the death rate caused by these viruses and remarkably postpones the beginning of the disease; this supporting evidence goes in favor of melatonin to be used in the treatment of many viral diseases [39].
Melatonin against COVID-19
Ample evidences for the promising use of melatonin as an adjuvant in the treatment of COVID-19 now exist [56]. Acute oxidative stress and inflammatory responses are caused due to the SARS-CoV-2 viral infection. Administration of melatonin may suppress quite a few of these reactions. SARS-CoV-2 penetrates the epithelial cells of alveoli by attaching with ACE2, this is assisted by S1 and S2 subunits of spike protein present on the virus [57]. S1 facilitates binding with ACE2, while S2 directs merging of the virus with plasma membrane [58]. Surface expression and retention of ACE2 in cell membranes are controlled by calmodulin. Melatonin inhibits the binding of ACE2 receptors with the virus SARS-CoV-2 by blocking calmodulin. Splitting of polyproteins of the virus is regulated by SARS-CoV-2 protease (chymotrypsin-like protease), but this enzyme is inhibited by melatonin [59]. SARS-CoV-2 couples with ACE2 thereby producing angiotensin II and inhibits the shielding effects of angiotensin 1–7 [60]. On the contrary, providing a rescue, melatonin comes as a significant blocker of angiotensin II activation and induces angiotensin 1–7 action [61].
Activation of NOD-like receptors family pyrin group containing 3 (NLRP3) initiates pro-inflammatory cytokines release such as IL-18 and IL-1B. Pieces of evidence show that melatonin functions as a blocker of NLRP3 inflammasome, thereby shutting down pyroptosis and finally showing an anti-inflammatory effect [62]. Induction of a cytokine surge by SARS-CoV-2 infection leads to IL-1B, IL-6, IL-17, C-reactive protein, and TNF-α upregulations because there is a rise in the activation of macrophages, mast cells, and neutrophils [8, 63]. Melatonin inhibits signaling of NF-κB, downregulates inducible nitric oxide synthase and cyclooxygenase-2, and inhibits TLR4 activation. Blocking of TLR4 reduces the levels of TNF-α, IL-1B, IL-6, and IL-8 [64]. Melatonin by reducing pro-inflammatory cytokines shows anti-inflammatory effects, the elevation of anti-inflammatory cytokines like IL-10 and inhibition of NF-κB. It even changes the hyperinflammatory glycolytic macrophages into anti-inflammatory macrophages which go through the process of oxidative phosphorylation, this further downregulates cytokine production. Moreover, this pineal hormone activates sirtuin 1 protein, which obstructs the formation of hyperinflammatory macrophages. By inhibiting chymotrypsin type protease and calmodulin, melatonin diminishes virus entry and multiplication in host cell [59].
The hormone melatonin is likely to exert antioxidative actions against SARS-CoV-2 virus by the direct destruction of oxygen and nitrogen-based-free radicals, inactivation of pro-oxidant enzymes, homeostasis regulation of mitochondria and activation of antioxidant enzymes like catalase, superoxide dismutase (SOD), and GSH [38, 65–68]. Even regulates autophagy, endoplasmic reticulum stress, and apoptosis through its antioxidant properties [69]. Melatonin helps in the reduction of severe lung oxidative destructions by suppressing ROS and reestablishment of SOD and GSH titers in the pulmonary organs of RSV-infected mice [41, 70]. Melatonin antagonizes ROS injury by preserving the inner membrane of mitochondria and stimulating the electron transport chain to enhance mitochondrial citric acid cycles and production of ATPs, thereby decreasing electrons leakage and ROS formation [65].
Preliminary pieces of evidence of successful melatonin treatments for COVID-19 patients having respiratory problems do exist [36–38]. Having anti-inflammatory and ROS-scavenging properties, melatonin is projected and even used in the treatment of varied viral infections which are causing excessive immunoinflammatory responses [42, 43, 71, 72]. Most viruses, inclusive of those that are causing cytokine storms, lead to a decrease in pineal melatonin production, which adversely hits the patient’s immune system [73]. SARS-CoV-2 disrupts the host’s mechanism of melatonin production to avoid self-disruption and begins multiplying inside the patient’s respiratory tract cells. Viruses diminish the anti-inflammatory effects of melatonin by repressing the expression of the gene of several melatonin synthesizing enzymes and also deplete tryptophan which is the antecedent for melatonin. These melatonin depleting phenomena lead to enhanced lethality of several viral ailments [38]. Melatonin is a tryptophan-derived hormone produced in the pineal gland and cells of the immune system. It is a promising treatment option to suppress the lethality of COVID-19 manifestations due to its known protective antioxidant, immunomodulatory and anti-inflammatory qualities [43, 62, 74, 75].
SARS-CoV-2 causing this pandemic may never disappear totally and will keep on appearing as novel strains through mutations. Being immunomodulatory, antioxidant, anti-inflammatory, and antiviral, melatonin can be the right choice for therapeutic use alone or in combination with other drugs to ameliorate the consequences of COVID-19 infections [76–79]. Being a hydroxyl radicals scavenger and stimulator of antioxidative enzymes like glutathione peroxidase (GSH) and SOD, the hormone melatonin even protects against oxidative damage of the cells significantly [9]. It also controls cognitive decay following SARS-CoV-2 infection and thus could serve as the “silver bullet” against the current COVID-19 pandemic even in high doses. Today our prime social responsibility is to combat this disease along with all the safety measures [80, 81].
Melatonin may lower COVID-19 infection severity by targeting human physiological processes [73]. Based on practical pieces of evidence, Kleszczyński et al. [70] have encouraged physicians to test the potential role of melatonin against this infection. The hormone regulates COVID-19-associated proteins directly or indirectly through G-Protein coupled receptors and nuclear melatonin receptors, thereby demanding the need for placebo-controlled randomized clinical trials of melatonin against these virus infections [82]. Melatonin may enhance the efficacy of COVID-19 vaccination [83]. Even reduces the ROS-stimulated damage, cytokine-induced lymphopenia and inflammation in COVID-19 like infections. Potential benefits do exist in melatonin use for COVID-19 treatment at the earliest possible [9]. Several findings reiterate the idea that exogenous melatonin administration may boost the efficacy of immune response and duration of immunity conferred by COVID-19 vaccination [84, 85] by increasing the peripheral blood IgG-expressing B cells and CD4+ T cells [86]. Moreover, additional implications of melatonin may effectively diminish sepsis, mortality and thrombosis in COVID-19 subjects [87]. As mentioned earlier, this disease establishes as SARS-CoV-2 binds with ACE2 receptors in respiratory tract epithelial cells. This initiates a pro-inflammatory effect that generally leads to a cytokine storm and proliferous beginning of acute respiratory distress syndrome [6, 37]. Figures 2–4 give the concepts and outlines for controlling the COVID-19 infection.
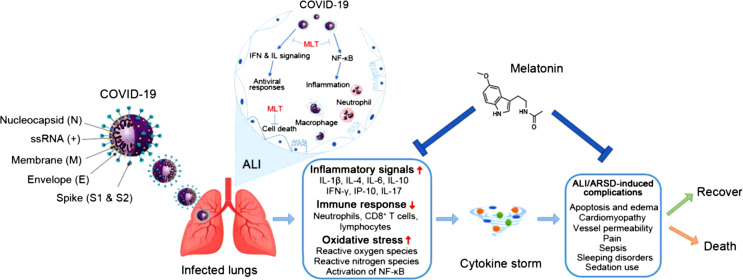
Fig. 2.
COVID-19 pathology – cytokine storm leading to death could effectively be reduced by the administration of melatonin (Source: Vlachou et al. [88]). The figure shows the probable mechanistic routes, where melatonin could deplete the infection process. Severe oxidation, inflammation, and a hyperactive immune response contribute to COVID-19 pathology, leading to a cytokine surge that causes acute lung injury/acute respiratory distress syndrome (ALI/ARDS) and frequent deaths, which can be significantly lowered by the administration of melatonin.
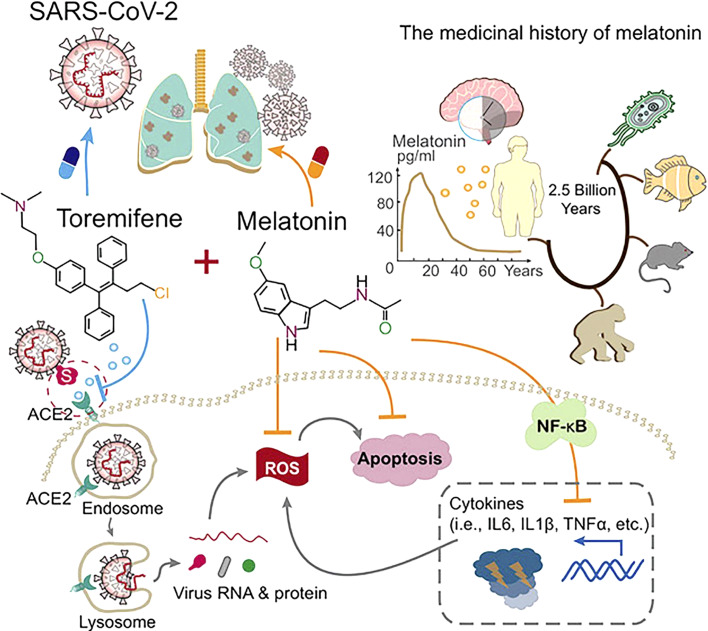
Fig. 4.
Humoral immunity and melatonin – a therapeutic approach with toremifene (Source: Cheng et al. [89]) – melatonin is evolutionarily conserved from bacteria to humans. Melatonin suppresses inflammatory pathways that include IL-6, IL1β, and TNFα, which are released during lung injury of severe COVID-19 infection. Toremifene which is a selective estrogen receptor modulator and FDA-approved to treat advanced breast cancer has shown several antiviral activities such as against the Ebola virus, MRES-CoV, SARS-CoV-1, and SARS-CoV-2. Hence, the synergistic antiviral and anti-inflammatory effects of melatonin and toremifene offer a promising treatment approach for COVID-19.
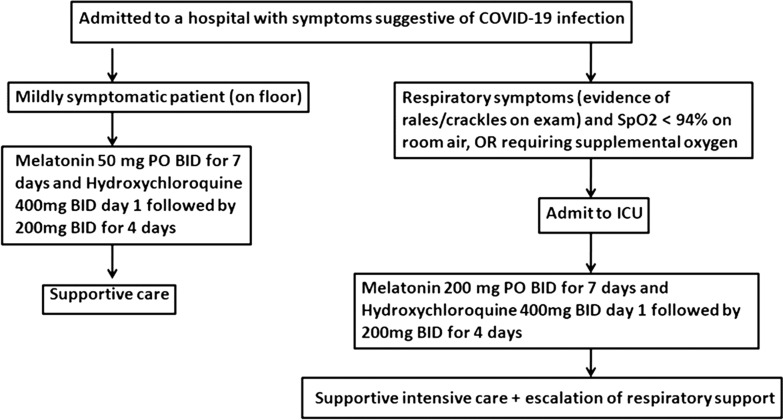
Fig. 3.
Adjuvant therapeutic action of melatonin during COVID-19 infection (Source: Reiter et al. [76]). Melatonin was found to curtail the toxicity of hydroxychloroquine and increase its potency. This can be an exploratory protocol, a preliminary phase I/II study.
Early Intervention in COVID-19 by Melatonin
Potential benefits do exist in the earliest use of melatonin for COVID-19 treatment. Melatonin possesses reasonable features of minimizing inflammation and most probably repressing the cytokine surge caused by SARS-CoV-2. If administered at the early stages of infection, it can dispense benefits at a tolerable safety profile and at a reduced cost. Even though this hormone fights early virus multiplications, its administration in patients with COVID-19 infection is not destined to be used as a remedy but rather as an assistive envoy that prepares the body to smartly defeat virus infections. Melatonin can augment drug efficacy and diminish toxicity and thus appears appropriate to be used together with other treatments for COVID-19 infection [9]. In case studies where the body’s immunity is repressed, melatonin stimulates the immune response, and in patients having inflammation, melatonin shows an immunosuppressive effect [88]. Also now it seems quite clear that in COVID-19, the oxidative and inflammatory effects due to the virus get eliminated by melatonin which also escalates the immunity of the patient to tactfully counter the infection and recover effectively within a short recovery period [85, 90]. Along with an antiviral, melatonin serves for a speedy recovery of patients afflicted with COVID-19 disease and can be best administered as chronotherapy in such patients [81].
Melatonin can also act against the virus itself. Seeing the safe profile of melatonin, it can be applied as a precautionary measure and therapeutic agent against COVID-19 and should be started seriously even before the clinical trials due to the emergency of the pandemic [91, 92]. Patients having comorbidities must receive treatment at the earliest possible. Melatonin would be an effective first-line, safe and cheap remedy for COVID-19, specifically in critical and high-risk susceptible populations. CDC-USA has charted out the patients at exposure for acquiring serious cases of COVID-19, like – cancer, diseases of kidney, COPD, cardiac anomalies, history of smoking, etc [9, 93]. Moreover, being highly safe, accessible in abundance, and even cheap at cost, Cross et al. [9] have recommended the administration of melatonin about 2.5–10 mg dose before sleep at night to all adults infected with SARS-CoV-2 immediately on the very first day of diagnosis, especially for the patients having comorbidities and higher mortality risk. Ample shreds of evidence as anti-cancerous [94], antioxidant, anti-inflammatory [95], and immunomodulatory prove that the effects of melatonin through the specific nuclear and plasma membrane melatonin receptors [96] can significantly deplete the gravity of symptoms and motility to cells caused by viral diseases when started as an early remedy. The pineal product melatonin strategically slows down the cytokine surge seen in COVID-19 subjects, reduces oxidized destruction to upgrade the resistive capabilities of patients and thus provides an increased life span. Synergistic antiviral and anti-inflammatory effects of melatonin and toremifene seem promising in the protection against COVID-19 through the enhanced humoral immunity by inhibition of IL-6, IL1β, and TNFα release (Fig. 4) [89].
Summary and Conclusion
Already there have been retrospective studies for the administration of pineal hormone melatonin in the therapy for COVID-19 with other types of drugs like antivirals, but it needs to be escalated to reach larger geographical scales, and even to the vaccinated people as an adjuvant alone or in combination with other COVID-19 therapeutic medicines. The combo effects of anti-inflammation and antioxidation by melatonin provide an effective treatment for SARS-CoV-2. This pineal product disrupts all the chronological stages of the viral life cycle that includes cell entry, replication, and deleterious downstream signaling cascades. Melatonin stimulates the production of inflammatory cytokines and Th1 and intensifies both humoral and cell-mediated responses. Melatonin exhibits multiple antiviral activities through the increased humoral immunity. Melatonin significantly interrupts at each level of the virus life cycle to support the immune system of the host and squelch a harmful overreaction. The safety parameters of melatonin have been verified in many human studies. However, in order to ascertain further confirmations, we need to evaluate first of all safety and then efficacy of the pineal hormone melatonin against COVID-19. Its effects, when administered to COVID-19 patients, definitely require the rigorous clinical studies as the pandemic has recently shown its devastating effects in the year 2020-21.
Conflict of Interest Statement
The authors report no conflict of interest.
Funding Sources
No funding was received.
Author Contributions
Major contributions to the conception, analysis, acquisition, design, and interpretation of information were performed by Dr M. Mubashshir. Compilation of the pieces of literature was jointly done by Dr. T. Negi and Ms R.B. Sharma. The best possible chronological sequence of events and findings was laid down by the dual collaboration of Dr. Rawal and Dr. H. Khatoon. Major scientific and language upgradations were together achieved by Dr. V. Laxmi and Mr. O. Dubey. The first draft was cross-examined for its proper citations and references by Dr. N. Singhvi and Ms. G. Negi. The final critical revisions of the manuscript that came through the above team of experts were checked twice by Prof N. Ahmad and Prof M. Ovais. All the authors have read and approved the manuscript.
Funding Statement
No funding was received.
References
- 1. WHO: Situation Report-1 ; 2020. (https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200121-sitrep-1-2019-ncov.pdf).
- 2. Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J Am Chem Soc. 1958 May;80(10):2587. 10.1021/ja01543a060. [DOI] [Google Scholar]
- 3. Arendt J. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev Reprod. 1998 Jan 1;3:13–22. 10.1530/ror.0.0030013. [DOI] [PubMed] [Google Scholar]
- 4. Sköld HN, Amundsen T, Svensson PA, Mayer I, Bjelvenmark J, Forsgren E. Hormonal regulation of female nuptial coloration in a fish. Horm Behav. 2008 Sep 1;54(4):549–56. 10.1016/j.yhbeh.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 5. Ovais M, Srivastava SK, Sumoona S, Mubashshir M. Evidence for the presence of novel β-melatonin receptors along with classical α-melatonin receptors in the fish Rasbora daniconius (Ham.). J Recept Signal Transduct Res. 2015 Jul 4;35(4):238–48. 10.3109/10799893.2014.951896. [DOI] [PubMed] [Google Scholar]
- 6. Zhang R, Wang X, Ni L, Di X, Ma B, Niu S, et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020 Jun 1;250:117583. 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El-Missiry MA, El-Missiry ZM, Othman AI. Melatonin is a potential adjuvant to improve clinical outcomes in individuals with obesity and diabetes with coexistence of Covid-19. Eur J Pharmacol. 2020 Sep 5;882:173329. 10.1016/j.ejphar.2020.173329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Öztürk G, Akbulut KG, Güney Ş. Melatonin, aging, and COVID-19: could melatonin be beneficial for COVID-19 treatment in the elderly? Turk J Med Sci. 2020;50(6):1504–12. 10.3906/sag-2005-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cross KM, Landis DM, Sehgal L, Payne JD. Melatonin for the early treatment of COVID-19: a narrative review of current evidence and possible efficacy. Endocr Pract. 2021 Aug 1;27(8):850–5. 10.1016/j.eprac.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.. Bosch BJ, Van der Zee R, De Haan CA, Rottier PJ. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003 Aug 15;77(16):8801–11. 10.1128/jvi.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). Treasure island (FL): StatPearls Publishing; 2022. [PubMed] [Google Scholar]
- 12. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003 Nov 27;426(6965):450–4. 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y, Guo Y, Pan Y, Zhao ZJ. Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophysical Res Commun. 2020 Apr 23;525(1):135–40. 10.1016/j.bbrc.2020.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020 Apr 16;181(2):281–92.e6. 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020 Apr;5(4):562–9. 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun. 2020 Mar 27;11(1):1620. 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li T, Zhang Y, Fu L, Yu C, Li X, Li Y, et al. siRNA targeting the leader sequence of SARS-CoV inhibits virus replication. Gene Ther. 2005 May;12(9):751–61. 10.1038/sj.gt.3302479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Belouzard S, Chu VC, Whittaker GR. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A. 2009 Apr 7;106(14):5871–6. 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012 Jun 20;4(6):1011–33. 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2021 May 1;97(1147):312–20. 10.1136/postgradmedj-2020-138577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997 Jan 1;48:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang N, Shen HM. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci. 2020;16(10):1724–31. 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dubocovich ML, Takahashi JS. Use of 2-[125I] iodomelatonin to characterize melatonin binding sites in chicken retina. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3916–20. 10.1073/pnas.84.11.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vanecek J. Cellular mechanisms of melatonin action. Physiol Rev. 1998 Jan 7;78(3):687–721. 10.1152/physrev.1998.78.3.687. [DOI] [PubMed] [Google Scholar]
- 25. Reppert SM, Weaver DR, Rivkees SA, Stopa EG. Putative melatonin receptors in a human biological clock. Science. 1988 Oct 7;242(4875):78–81. 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- 26. Teh MT, Sugden D. The putative melatonin receptor antagonist GR128107 is a partial agonist on Xenopus laevis melanophores. Br J Pharmacol. 1999 Mar;126(5):1237–45. 10.1038/sj.bjp.0702404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Masana MI, Dubocovich ML. Melatonin receptor signaling: finding the path through the dark. Sci STKE. 2001 Nov 6;2001(107):pe39. 10.1126/stke.2001.107.pe39. [DOI] [PubMed] [Google Scholar]
- 28. Arendt J. The pineal gland and pineal tumours. In: Feingold KR, Anawalt B, Boyce A, editors. Endotext. South dartmouth (MA): MDText.com, Inc.; 2011. [Google Scholar]
- 29. Farce A, Chugunov AO, Logé C, Sabaouni A, Yous S, Dilly S, et al. Homology modeling of MT1 and MT2 receptors. Eur J Med Chem. 2008 Sep 1;43(9):1926–44. 10.1016/j.ejmech.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 30. Arendt J. Melatonin and the mammalian pineal gland: Springer Science & Business Media; 1994 Dec 31. [Google Scholar]
- 31. Filadelfi AM, Castrucci AM. Comparative aspects of the pineal/melatonin system of poikilothermic vertebrates. J Pineal Res. 1996 May;20(4):175–86. 10.1111/j.1600-079x.1996.tb00256.x. [DOI] [PubMed] [Google Scholar]
- 32. Sack RL, Brandes RW, Kendall AR, Lewy AJ. Entrainment of free-running circadian rhythms by melatonin in blind people. N Engl J Med Overseas Ed. 2000 Oct 12;343(15):1070–7. 10.1056/nejm200010123431503. [DOI] [PubMed] [Google Scholar]
- 33. Ekstrzm PE, Meissl HI. The pineal organ of teleost fishes. Rev Fish Biol Fish. 1997 Jun;7(2):199–284. 10.1023/a:1018483627058. [DOI] [Google Scholar]
- 34. Kim JS, Coon SL, Blackshaw S, Cepko CL, Møller M, Mukda S, et al. Methionine adenosyltransferase: adrenergic-cAMP mechanism regulates a daily rhythm in pineal expression. J Biol Chem. 2005 Jan 7;280(1):677–84. 10.1074/jbc.M408438200. [DOI] [PubMed] [Google Scholar]
- 35. Chowdhury I, Sengupta A, Maitra SK. Melatonin: fifty years of scientific journey from the discovery in bovine pineal gland to delineation of functions in human. Indian J Biochem Biophys. 2008;45(5):289–304. [PubMed] [Google Scholar]
- 36. Salles C. Correspondence COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020 Jul 7;253:117716. 10.1016/j.lfs.2020.117716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Simko F, Hrenak J, Dominguez-Rodriguez A, Reiter RJ. Melatonin as a putative protection against myocardial injury in COVID-19 infection. Expert Rev Clin Pharmacol. 2020 Sep 1;13(9):921–4. 10.1080/17512433.2020.1814141. [DOI] [PubMed] [Google Scholar]
- 38. Tan DX, Hardeland R. Targeting host defense system and rescuing compromised mitochondria to increase tolerance against pathogens by melatonin may impact outcome of deadly virus infection pertinent to COVID-19. Molecules. 2020 Sep 25;25(19):4410. 10.3390/molecules25194410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boga JA, Coto-Montes A, Rosales-Corral SA, Tan DX, Reiter RJ. Beneficial actions of melatonin in the management of viral infections: a new use for this “molecular handyman”. Rev Med Virol. 2012 Sep;22(5):323–38. 10.1002/rmv.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Silvestri M, Rossi GA. Melatonin: its possible role in the management of viral infections-a brief review. Ital J Pediatr. 2013 Dec;39:61–5. 10.1186/1824-7288-39-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang SH, Cao XJ, Liu W, Shi XY, Wei W. Inhibitory effect of melatonin on lung oxidative stress induced by respiratory syncytial virus infection in mice. J Pineal Res. 2010 Mar;48(2):109–16. 10.1111/j.1600-079X.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 42. Tan DX, Korkmaz A, Reiter RJ, Manchester LC. Ebola virus disease: potential use of melatonin as a treatment. J Pineal Res. 2014 Nov;57(4):381–4. 10.1111/jpi.12186. [DOI] [PubMed] [Google Scholar]
- 43. Anderson G, Maes M, Markus RP, Rodriguez M. Ebola virus: melatonin as a readily available treatment option. J Med Virol. 2015 Apr;87(4):537–43. 10.1002/jmv.24130. [DOI] [PubMed] [Google Scholar]
- 44. Srinivasan V, Mohamed M, Kato H. Melatonin in bacterial and viral infections with focus on sepsis: a review. Recent Pat Endocr Metab Immune Drug Discov. 2012 Jan 1;6(1):30–9. 10.2174/187221412799015317. [DOI] [PubMed] [Google Scholar]
- 45. Gómez-Moreno G, Guardia J, Ferrera MJ, Cutando A, Reiter RJ. Melatonin in diseases of the oral cavity. Oral Dis. 2010 Apr;16(3):242–7. 10.1111/j.1601-0825.2009.01610.x. [DOI] [PubMed] [Google Scholar]
- 46. Ma Y, Tang K, Song C, Fan L, Zhang Y, Zhang C, et al. The reduced level of plasma melatonin in HFRS patients is correlated with disease severity and stage. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2018 Nov 1;34(11):1027–31. [PubMed] [Google Scholar]
- 47. San‐Miguel B, Crespo I, Vallejo D, Álvarez M, Prieto J, González-Gallego J, et al. Melatonin modulates the autophagic response in acute liver failure induced by the rabbit hemorrhagic disease virus. J Pineal Res. 2014 Apr;56(3):313–21. 10.1111/jpi.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tunon MJ, San-Miguel B, Crespo I, Laliena A, Vallejo D, Alvarez M, et al. Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J Pineal Res. 2013 Oct;55(3):221–8. 10.1111/jpi.12063. [DOI] [PubMed] [Google Scholar]
- 49. Laliena A, San Miguel B, Crespo I, Alvarez M, González-Gallego J, Tuñón MJ. Melatonin attenuates inflammation and promotes regeneration in rabbits with fulminant hepatitis of viral origin. J Pineal Res. 2012 Oct;53(3):270–8. 10.1111/j.1600-079X.2012.00995.x. [DOI] [PubMed] [Google Scholar]
- 50. Cutolo M, Maestroni GJ. The melatonin-cytokine connection in rheumatoid arthritis. Ann Rheum Dis. 2005 Aug 1;64(8):1109–11. 10.1136/ard.2005.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maestroni GJ, Otsa K, Cutolo M. Melatonin treatment does not improve rheumatoid arthritis. Br J Clin Pharmacol. 2008 May;65(5):797–8. 10.1111/j.1365-2125.2007.03088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang YX, Yang GH, Zhang LL, Wang J, Wang JF. Melatonin as immune potentiator for enhancing subunit vaccine efficacy against bovine viral diarrhea virus. Vaccines. 2021 Sep 18;9(9):1039. 10.3390/vaccines9091039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Valero N, Bonilla E, Pons H, Chacin-Bonilla L, Añez F, Espina LM, et al. Melatonin induces changes to serum cytokines in mice infected with the Venezuelan equine encephalomyelitis virus. Trans R Soc Trop Med Hyg. 2002 May 1;96(3):348–51. 10.1016/s0035-9203(02)90121-5. [DOI] [PubMed] [Google Scholar]
- 54. Bonilla E, Valero N, Chacín-Bonilla L, Pons H, Larreal Y, Medina-Leendertz S, et al. Melatonin increases interleukin-1beta and decreases tumor necrosis factor alpha in the brain of mice infected with the Venezuelan equine encephalomyelitis virus. Neurochem Res. 2003 May;28(5):681–6. 10.1023/a:1022897314108. [DOI] [PubMed] [Google Scholar]
- 55. Valero N, Espina LM, Mosquera J. Melatonin decreases nitric oxide production, inducible nitric oxide synthase expression and lipid peroxidation induced by Venezuelan encephalitis equine virus in neuroblastoma cell cultures. Neurochem Res. 2006 Jul;31(7):925–32. 10.1007/s11064-006-9098-7. [DOI] [PubMed] [Google Scholar]
- 56. Brown GM, Pandi-Perumal SR, Pupko H, Kennedy JL, Cardinali DP. Melatonin as an add-on treatment of COVID-19 infection: current status. Diseases. 2021 Sep 20;9(3):64. 10.3390/diseases9030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Martín Giménez VM, Prado N, Diez E, Manucha W, Reiter RJ. New proposal involving nanoformulated melatonin targeted to the mitochondria as a potential COVID-19 treatment. Nanomedicine. 2020 Oct;15(29):2819–21. 10.2217/nnm-2020-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Acuña-Castroviejo D, Escames G, Figueira JC, de la Oliva P, Borobia AM, Acuña-Fernández C. Clinical trial to test the efficacy of melatonin in COVID-19. J Pineal Res. 2020 Oct;69(3):e12683. 10.1111/jpi.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cardinali DP. High doses of melatonin as a potential therapeutic tool for the neurologic sequels of covid-19 infection. Melatonin Res. 2020 Jun 15;3(3):311–7. 10.32794/mr11250064. [DOI] [Google Scholar]
- 60. Hojyo S, Uchida M, Tanaka K, Hasebe R, Tanaka Y, Murakami M, et al. How COVID-19 induces cytokine storm with high mortality. Inflamm Regen. 2020 Dec;40:37–7. 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Campos LA, Cipolla-Neto J, Amaral FG, Michelini LC, Bader M, Baltatu OC. The angiotensin-melatonin axis. Int J Hypertens. 2013 Oct;2013:521783. 10.1155/2013/521783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shneider A, Kudriavtsev A, Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int Rev Immunol. 2020 Jul 3;39(4):153–62. 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- 63. Bahrampour Juybari K, Pourhanifeh MH, Hosseinzadeh A, Hemati K, Mehrzadi S. Melatonin potentials against viral infections including COVID-19: current evidence and new findings. Virus Res. 2020 Oct 2;287:198108. 10.1016/j.virusres.2020.198108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hardeland R. Melatonin and inflammation: story of a double-edged blade. J Pineal Res. 2018 Nov;65(4):e12525. 10.1111/jpi.12525. [DOI] [PubMed] [Google Scholar]
- 65. Zhang HM, Zhang Y. Melatonin: a well-documented antioxidant with conditional pro-oxidant actions. J Pineal Res. 2014 Sep;57(2):131–46. 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 66. Chitimus DM, Popescu MR, Voiculescu SE, Panaitescu AM, Pavel B, Zagrean L, et al. Melatonin’s impact on antioxidative and anti-inflammatory reprogramming in homeostasis and disease. Biomolecules. 2020 Aug 20;10(9):1211. 10.3390/biom10091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adamczyk-Sowa M, Sowa P, Adamczyk J, Niedziela N, Misiolek H, Owczarek M, et al. Effect of melatonin supplementation on plasma lipid hydroperoxides, homocysteine concentration and chronic fatigue syndrome in multiple sclerosis patients treated with interferons-beta and mitoxantrone. J Physiol Pharmacol. 2016 Apr 1;67(2):235–42. [PubMed] [Google Scholar]
- 68. Herrera EA, González-Candia A. Comment on Melatonin as a potential adjuvant treatment for COVID-19. Life Sci. 2020 Jul 7;253:117739. 10.1016/j.lfs.2020.117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Banerjee A, Czinn SJ, Reiter RJ, Blanchard TG. Crosstalk between endoplasmic reticulum stress and anti-viral activities: a novel therapeutic target for COVID-19. Life Sci. 2020 Aug 15;255:117842. 10.1016/j.lfs.2020.117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kleszczyński K, Slominski AT, Steinbrink K, Reiter RJ. Clinical trials for use of melatonin to fight against COVID-19 are urgently needed. Nutrients. 2020 Aug 24;12(9):2561. 10.3390/nu12092561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Elmahallawy EK, Luque JO, Aloweidi AS, Gutierrez-Fernandez J, Sampedro-Martinez A, Rodriguez-Granger J, et al. Potential relevance of melatonin against some infectious agents: a review and assessment of recent research. Curr Med Chem. 2015 Nov 1;22(33):3848–61. 10.2174/0929867322666150827093730. [DOI] [PubMed] [Google Scholar]
- 72. Paemanee A, Hitakarun A, Roytrakul S, Smith DR. Screening of melatonin, α-tocopherol, folic acid, acetyl-L-carnitine and resveratrol for anti-dengue 2 virus activity. BMC Res Notes. 2018 Dec;11(1):307–7. 10.1186/s13104-018-3417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Anderson G, Reiter RJ. Melatonin: roles in influenza, Covid-19, and other viral infections. Rev Med Virol. 2020 May;30(3):e2109. 10.1002/rmv.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tan DX, Hardeland R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: focus on COVID-19. Melatonin Res. 2020 Mar 23;3(1):120–43. 10.32794/mr11250052. [DOI] [Google Scholar]
- 75. Hardeland R, Tan DX. Protection by melatonin in respiratory diseases: valuable information for the treatment of COVID-19. Melatonin Res. 2020 Jun 15;3(3):264–75. 10.32794/mr11250061. [DOI] [Google Scholar]
- 76. Reiter RJ, Abreu-Gonzalez P, Marik PE, Dominguez-Rodriguez A. Therapeutic algorithm for use of melatonin in patients with COVID-19. Front Med. 2020;7:226. 10.3389/fmed.2020.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reiter RJ, Sharma R, Ma Q, Dominquez-Rodriguez A, Marik PE, Abreu-Gonzalez P. Melatonin inhibits COVID-19-induced cytokine storm by reversing aerobic glycolysis in immune cells: a mechanistic analysis. Med Drug Discov. 2020 Jun;6:100044. 10.1016/j.medidd.2020.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Reiter RJ, Sharma R, Ma Q, Liu C, Manucha W, Abreu-Gonzalez P, et al. Plasticity of glucose metabolism in activated immune cells: advantages for melatonin inhibition of COVID-19 disease. Melatonin Res. 2020 Jun 15;3(3):362–79. 10.32794/mr11250068. [DOI] [Google Scholar]
- 79. Anderson GM. Fluvoxamine, melatonin and COVID-19. Psychopharmacology. 2021 Feb;238(2):611. 10.1007/s00213-020-05753-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Cardinali DP, Brown GM, Pandi-Perumal SR. Can melatonin be a potential “silver bullet” in treating COVID-19 patients? Diseases. 2020 Dec;8(4):44. 10.3390/diseases8040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Cardinali DP, Brown GM, Reiter RJ, Pandi-Perumal SR. Elderly as a high-risk group during COVID-19 pandemic: effect of circadian misalignment, sleep dysregulation and melatonin administration. Sleep Vigil. 2020 Dec;4(2):81–7. 10.1007/s41782-020-00111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Reynolds JL, Dubocovich ML. Melatonin multifaceted pharmacological actions on melatonin receptors converging to abrogate COVID-19. J Pineal Res. 2021 Aug;71(1):e12732. 10.1111/jpi.12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wichniak A, Kania A, Siemiński M, Cubała WJ. Melatonin as a potential adjuvant treatment for COVID-19 beyond sleep disorders. Int J Mol Sci. 2021 Aug 11;22(16):8623. 10.3390/ijms22168623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kow CS, Ramachandram DS, Hasan SS. Melatonin: revisited role as vaccine adjuvant during outbreaks of COVID-19 caused by the delta variant. J Neuroimmune Pharmacol. 2021 Nov 30;17(3–4):425–6. 10.1007/s11481-021-10036-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Maestroni G. Exogenous melatonin as potential adjuvant in anti-SarsCov2 vaccines. J Neuroimmune Pharmacol. 2020 Dec;15(4):572–3. 10.1007/s11481-020-09956-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ramos A, Míguez MP, Morgado S, Sanchez-Correa B, Gordillo JJ, Casado JG, et al. Melatonin enhances responsiveness to Dichelobacter nodosus vaccine in sheep and increases peripheral blood CD4 T lymphocytes and IgG-expressing B lymphocytes. Vet Immunol Immunopathol. 2018 Dec 1;206:1–8. 10.1016/j.vetimm.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 87. Hasan ZT, Atrakji DMQYMAA, Mehuaiden AK. The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients. Int J Infect Dis. 2022 Jan 1;114:79–84. 10.1016/j.ijid.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sehirli AO, Sayiner S, Serakinci N. Role of melatonin in the treatment of COVID-19; as an adjuvant through cluster differentiation 147 (CD147). Mol Biol Rep. 2020 Oct;47(10):8229–33. 10.1007/s11033-020-05830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cheng F, Rao S, Mehra R. COVID-19 treatment: combining anti-inflammatory and antiviral therapeutics using a network-based approach. Cleve Clin J Med. 2020 Jun 30. 10.3949/ccjm.87a.ccc037. [DOI] [PubMed] [Google Scholar]
- 90. Maestroni GJ. The immunoneuroendocrine role of melatonin. J Pineal Res. 1993 Jan;14(1):1–10. 10.1111/j.1600-079x.1993.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 91. Tesarik J. Melatonin attenuates growth factor receptor signaling required for SARS-CoV-2 replication. Melatonin Res. 2020 Oct 9;3(4):534–7. 10.32794/mr11250077. [DOI] [Google Scholar]
- 92. Tesarik J. Melatonin to reduce death toll due to COVID-19: from innate to adaptive immune response. Glob J Med Res. 2020 Jan;20(8):5–7. 10.34257/gjmrkvol20is8pg5. [DOI] [Google Scholar]
- 93. Simko F, Reiter RJ. Is melatonin deficiency a unifying pathomechanism of high risk patients with COVID-19? Life Sci. 2020 Sep 9;256:117902. 10.1016/j.lfs.2020.117902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Mubashshir M, Ahmad N, Sköld HN, Ovais M. An exclusive review of melatonin effects on mammalian melanocytes and melanoma. Exp Dermatol. 2023;32(4):324–30. 10.1111/exd.14715. [DOI] [PubMed] [Google Scholar]
- 95. Maity J, Dey T, Banerjee A, Chattopadhyay A, Das AR, Bandyopadhyay D. Melatonin ameliorates myocardial infarction in obese diabetic individuals: the possible involvement of macrophage apoptotic factors. J Pineal Res. 2023 Mar;74(2):e12847. 10.1111/jpi.12847. [DOI] [PubMed] [Google Scholar]
- 96. Ekmekcioglu C. Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother. 2006 Apr 1;60(3):97–108. 10.1016/j.biopha.2006.01.002. [DOI] [PubMed] [Google Scholar]