Abstract
Introduction
We present the largest series of paediatric intracranial empyemas occurring after COVID-19 infection to date, and discuss the potential implications of the pandemic on this neurosurgical pathology.
Methods
Patients admitted to our centre between January 2016 and December 2021 with a confirmed radiological diagnosis of intracranial empyema were retrospectively reviewed, excluding non-otorhinological source cases. Patients were grouped according to onset before or after onset of the COVID-19 pandemic and COVID-19 status. A literature review of all post-COVID-19 intracranial empyemas was performed. SPSS v27 was used for statistical analysis.
Results
Sixteen patients were diagnosed with intracranial empyema: n = 5 prior to 2020 and n = 11 after, resulting in an average annual incidence of 0.3% prior to onset of the pandemic and 1.2% thereafter. Of those diagnosed since the pandemic, 4 (25%) were confirmed to have COVID-19 on recent PCR test. Time from COVID-19 infection until empyema diagnosis ranged from 15 days to 8 weeks. Mean age for post-COVID-19 cases was 8.5 years (range: 7–10 years) compared to 11 years in non-COVID cases (range: 3–14 years). Streptococcus intermedius was grown in all cases of post-COVID-19 empyema, and 3 of 4 (75%) post-COVID-19 cases developed cerebral sinus thromboses, compared to 3 of 12 (25%) non-COVID-19 cases. All cases were discharged home with no residual deficit.
Conclusion
Our post-COVID-19 intracranial empyema series demonstrates a greater proportion of cerebral sinus thromboses than non-COVID-19 cases, potentially reflecting the thrombogenic effects of COVID-19. Incidence of intracranial empyema at our centre has increased since the start of the pandemic, causes of which require further investigation and multicentre collaboration.
Keywords: COVID-19, Intracranial empyemas, Subdural empyemas, Empyema, Paediatric neurosurgery
Introduction
Intracranial empyemas (IEs) are serious, though relatively rare, neurosurgical pathologies. Epidemiologically, IEs most commonly affect children and young adults [1], with an estimated prevalence of 1 in 193,000 [2]. Males are more commonly affected, with a ratio of 3:1 male to female [1, 3]. In most cases, early diagnosis and adequate treatment result in good outcomes [1–3].
The aetiology of IEs is multifactorial, with between 40 and 80% occurring as a result of otorhinologic infections, usually from the paranasal sinuses, with the remainder occurring following head trauma or cranial surgery [4]. In those cases of empyema secondary to paranasal sinusitis, the most common microorganisms reported include anaerobic and microaerophilic streptococci such as those from the Streptococcus milleri group: Streptococcus anginosus or intermedius [3]. However, a number of infections reported are polymicrobial [3, 5, 6].
Patients with SEs may present with a number of symptoms, including headache, nausea, vomiting, focal neurology, and meningism, typically with a history of recent sinusitis [3]. Complications from SEs are numerous and include seizures, cerebral abscesses, cortical venous thromboses, cerebral sinus thromboses, sepsis, and hydrocephalus [3], with poorer outcomes in older patients [6], and up to half of all patients suffering from long term neurological deficits [5].
The novel coronavirus SARS-COV-2, or COVID-19, was first identified in Wuhan, China, in December 2019, subsequently resulting in over 440 million infections worldwide and near 6 million deaths worldwide at the time of writing [7]. COVID-19 has been associated with a number of immunological sequelae and various secondary bacterial infections. While most secondary infections have been of respiratory origin [8], a number of patients presenting with neurological symptoms have been found to have CNS involvement. It is postulated that endothelial cell damage, subsequent inflammatory response, and thromboses can lead to brain injury as a result of COVID-19 infection [9, 10]. Separately, a more typical meningitis has also been described across several case reports [11, 12]. Subdural empyemas have been reported following COVID-19 infection in a small number of adults [13, 14], with three paediatric cases also described [14–16]. In this article, we report a marked increase in the incidence of IE since the beginning of the pandemic in our region, as well as a series of paediatric patients with subdural empyema directly following COVID-19 infection.
Methodology
Electronic records of all patients who were admitted under the neurosurgical service at our centre, between January 2016 and December 2021 with a confirmed radiological diagnosis of IE were retrospectively reviewed. Cases occurring as a result of any non-otorhinological origin, e.g., post-surgical or post-meningitis empyemas were excluded. Data collected included (i) age, (ii) sex, (iii) preceding symptomatology, (iv) imaging, (v) microbiology reports. In cases occurring after January 2020, “COVID-19 status” was also collected. Admission protocol for our centre is to perform a COVID-19 PCR swab on admission, unless proof of a positive PCR within the last 90 days can be demonstrated. Cases were grouped according to whether they were “pre” or “post-pandemic,” with the distinguishing date being post-January 2020. To calculate annual incidence of IE, surgical admission data for the trust was reviewed; “number of patients admitted for operative intervention” was used as the denominator.
A review of the literature was subsequently performed using PubMed, Scopus, and Google Scholar. Search terms included “subdural,” “intracranial,” “empyema,” “coronavirus,” and “COVID-19.”
Microsoft Excel and SPSS v27 were used for data storage and analysis, respectively. Fisher’s exact and χ2 tests were used for comparison of categorical data, while independent sample t tests were used for comparison of means in parametric data.
Results
Sixteen cases of IE were identified between January 2016 and December 2021 at our centre. Between January 2016 and December 2019, 6 patients at our tertiary centre were diagnosed with IEs. One of these 6 cases occurred as a result of neonatal meningitis and was therefore excluded, giving a rate of 1.25 patients per year. During this same time period, the mean number of patients admitted under neurosurgery for operative intervention was n = 427; overall, this resulted in an average IE incidence per year of 0.3% prior to onset of the pandemic. Since the beginning of the pandemic in January 2020 up until December 2021, 11 patients were diagnosed with IEs, resulting in a rate of 5.5 per year. Mean number of surgical admissions since the onset of the pandemic was n = 446, therefore annual incidence of IE during that time was 1.2%.
COVID-19 PCR testing was undertaken at the time of admission for all patients presenting since the start of the pandemic, of which 100% were negative. Of these, 4 patients (1 male and 3 female) were admitted between October and December 2021, and despite negative PCR at admission, they had all recently tested positive for COVID-19 (as identified on recent positive PCR test or antibody serology) prior to admission. None of the non-COVID-19 cohort reported any history to suggest recent COVID-19 infection.
In the COVID-19-positive group, time from COVID-19 infection until empyema diagnosis ranged from 15 days to 8 weeks. Mean patient age for empyema cases following COVID-19 diagnosis was 8.5 years (range: 7–10 years) compared to 11 years in non-COVID cases (range: 3–14 years) (Table 1). Presenting symptoms were similar between those who had recently had COVID-19 and those who had not, typically consisting of headache, fever, nausea, lethargy, and photophobia with a background of recent sinusitis or otitis media. 100% of COVID-19-positive patients reported a history of frontal and maxillary sinusitis, compared to 58% of COVID-negative patients, wherein 42% presented following otological infections and subsequent mastoiditis as the source of their empyema. Functional endoscopic sinus surgery was required in 3 of 4 (75%) post-COVID empyema patients.
Table 1.
Subgroup comparison of empyema patients according to presentation pre- or post-pandemic, as well as COVID-19 status
| Pre-2020 (n = 5) | 2020 onwards (n = 11) | COVID negative (n = 12) | COVID positive (n = 4) | |
|---|---|---|---|---|
| Mean age, years | 11.4 | 9.9 | 11.0 | 8.5 |
| Male, n (%) | 3 (60) | 4 (36) | 6 (50) | 1 (25) |
| Frontal sinusitis, n (%) | 3 (60) | 8 (73) | 7 (58) | 4 (100) |
| Cerebral sinus thrombosis, n (%) | 1 (20) | 5 (45) | 3 (25) | 3 (75) |
All 4 post-COVID empyema patients had supratentorial empyemas, and the pathogenic organism in each case was Streptococcus intermedius. Three of 4 (75%) post-COVID patients developed cerebral sinus thromboses, compared to 3 of 13 (23%) of all non-COVID cases (Table 1). Mean admission length was 18.5 days (range: 12–25), and all were discharged home well for completion of their antibiotic therapy with no residual deficit. No geographical association was identified between any of the cases. A summary of the clinicopathological details for each post-COVID empyema patient can be found in Table 2, while illustrative imaging is demonstrated in Figure 1.
Table 2.
Summary of patients diagnosed with IE after recent COVID-19 infection
| Patient | Age | Sex | Symptoms | Location | Thrombus | COVID-19 interval | Outcome |
|---|---|---|---|---|---|---|---|
| 1 | 7 | F | Headache, irritable, cough | Fronto-temporal extradural | Nil | 8 weeks prior to empyema diagnosis | Admission: 14 days |
| Full recovery | |||||||
| 2 | 7 | F | Fever, photophobia, headache | Frontal extradural + subdural | Superior sagittal sinus CVST | 5 weeks prior to empyema diagnosis | Admission: 23 days |
| Full recovery | |||||||
| 3 | 13 | F | Headache, forehead swelling | Bifrontal extradural and subcutaneous | Superior sagittal sinus CVST | 3 weeks prior to empyema diagnosis | Admission: 25 days |
| Readmitted for further bone debridement. Full recovery | |||||||
| 4 | 10 | M | Periorbital swelling, fever, headache | Right orbit + bifrontal subdural | Superior sagittal sinus CVST | 15 days prior to empyema diagnosis | Admission: 12 days |
| Readmitted for further ENT surgery. Full recovery |
CVST, cerebral venous sinus thrombosis.
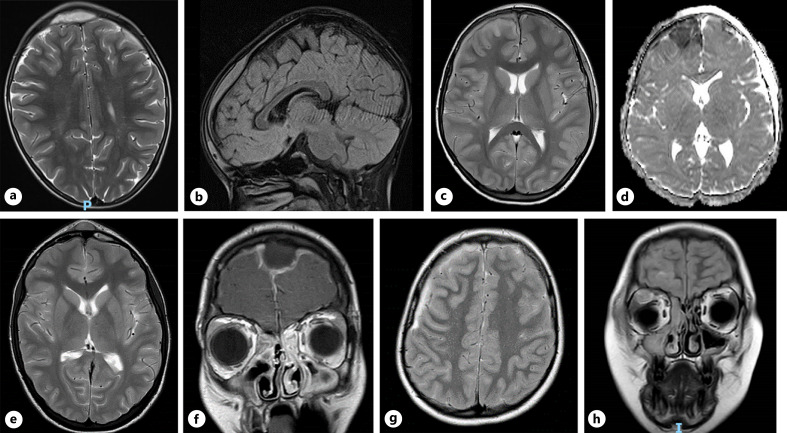
Fig. 1.
Patient 1: axial preoperative T2 (a) and sagittal FLAIR MRI sequences (b), demonstrating midline frontal extradural empyema. Patient 2: axial preoperative T2 MRI sequence, demonstrating right frontal subdural empyema (c) and underlying restricted diffusion on DWI (d). Patient 3: axial preoperative T2 sequence MRI, demonstrating frontal extradural collection, frontal sinusitis, and midline subgaleal extension (e); confirmed on coronal FLAIR (f). Patient 4: axial preoperative T2 sequence MRI highlighting right-sided frontal subdural empyema (g), with evidence of right-sided orbital abscess on preoperative coronal FLAIR (h).
Discussion
IEs are associated with significant morbidity and usually occur as a result of severe sinus infection [6]. Both the literature [2–4] and our own historical data suggest these collections are extremely rare, which makes the short timeframe over which the series of patients in this article was presented particularly alarming. Causes for such an increase in incidence are unclear, though a distinguishing feature of n = 4 of our cases is that of recent COVID-19 infection.
Literature Review
A literature review identified 608 relevant articles using the above search terms. Of these, 5 papers described purulent intracranial infections directly associated with COVID-19 infections: 2 adult case reports and 3 paediatric [13–17]. Clinical data for these reports are summarised in Table 3. Patients ranged between 13 and 65 years of age, 4 of whom were male. Presenting symptoms included headache, fevers, confusion, visual disturbance, nausea, and lethargy. One patient initially developed hydrocephalus secondary to meningitis while in ICU being treated for COVID-19 pneumonia; only on internalisation of a ventriculoperitoneal shunt and subsequent deterioration was the empyema diagnosed [14]. Across 3 patients, the COVID-19 diagnosis preceded identification of empyema diagnosis by 20–54 days [13, 14, 17]; in 2 patients, the diagnoses of empyema and COVID-19 were concurrent [15, 16]. Empyemas reported in the literature were convexity (n = 3), parafalcine (n = 1), and intraventricular (n = 1) [13–17] (Table 3).
Table 3.
Results of a literature review of all IEs associated with COVID-19 infection
| Report | Age | Sex | Symptoms | Location | Thrombus | COVID-19 interval | Outcome |
|---|---|---|---|---|---|---|---|
| Charlton et al. [17], 2021 | 65 | M | Headache, scalp tenderness | Right frontal convexity | Bilateral + saddle pulmonary embolisms | 54 days prior to empyema diagnosis | Discharged to inpatient rehabilitation |
| Meguins et al. [13], 2021 | 49 | M | “Locked in” syndrome (quadriplegia, absent respiratory effort) | 4th ventricle with associated ventriculitis | Not reported | 20 days prior to empyema diagnosis | Remained as inpatient; died 14 weeks later |
| Haroon et al. [14], 2021 | 13 | F | Fever, seizure, visual blurring, vomiting | Parafalcine | Not reported | Only tested positive 3 months post-washout | Full recovery |
| Ljubimov et al. [15], 2022 | 15 | M | Pyrexia, nausea, vomiting, left-sided hemiplegia | Right convexity | No thrombus, INR of 2.0 requiring reversal | Co-infection confirmed on admission | Full recovery |
| Boateng et al. [16], 2021 | 13 | M | Fever, headache, hemiplegia | Right convexity | R internal jugular/sigmoid | Positive on admission | Full recovery |
Effect of COVID-19
While children are increasingly recognised as experiencing a milder manifestation of COVID-19 [18], each of these cases experienced significant sinusitis before subsequently developing evidence of intracranial infection. All 4 post-COVID-19 empyema patients presented at least 18 months into the pandemic, by which time the infrastructure for timely PCR testing was established. This is highly relevant, as the empyema patients presenting since 2020, but not confirmed as previously having had COVID-19, may very well have previously been exposed to the virus but not had access to confirmatory testing. Although PCR testing at the time of admission was negative for all patients, antibody testing was not routinely undertaken and therefore “previous exposure” could not be confirmed or excluded.
The immunomodulatory effects of COVID-19 are becoming increasingly recognised [19], and while Streptococcus intermedius is commonly identified in SEs, the increase in incidence following COVID-19 infection reported here is noteworthy. Whether this relationship is coincidental, or occurs secondarily to a post-viral immune dysregulation thus permitting CNS bacterial penetration, remains to be seen. Both “delta” and “omicron” COVID-19 variants also became more prevalent during 2021; whether differences in the immunological impact of these variants could explain the increase in post-COVID-19 empyema incidence is speculative, as variant genotyping was unfortunately unavailable for each of these cases. Of note, 1 of 4 patients presented here was also found to have an orbital abscess. Published case reports describe severe orbital cellulitis or orbital abscesses in conjunction with COVID-19 infection [20, 21], but whether these too are directly linked to COVID-19 co-infection is unclear.
Societal Effects
An alternative explanation for this increase in IE incidence could be secondary to a societal response to COVID-19, as opposed to the virus itself. While global lockdowns have had a significant effect on mitigating COVID-19 transmission, delayed consequences of such measures are now being reported. “Lockdown” measures were legally enforced from March 26, 2020 in the UK, with the cessation of various measures occurring sporadically over the subsequent 12 months. Social distancing and widespread mask wearing resulted in the decline of several respiratory pathogens [22, 23], incurring a so-called “immunity debt” in the youngest members of the population by limiting their exposure to the usual battery of endemic pathogens [24]. This phenomenon has been linked to the marked rise seen in interseasonal respiratory syncytial virus cases [25] worldwide, among other non-COVID viral and bacterial diseases [24, 26]. Whether an “immunity debt” could also be responsible for the sudden increase in the incidence of sinusitis and subsequent empyemas described here is as yet unconfirmed.
An additional effect of the COVID-19 pandemic on society was that of patients delaying seeking access to healthcare. Months of “lockdown” and media emphasis on only seeking healthcare support when absolutely necessary may have skewed public perception of the definition of “absolutely necessary.” The overall increase in number of IEs since the onset of the pandemic may represent a group of what were initially cases of simple sinusitis that in other circumstances would have been treated earlier in the community, but due to the pandemic resulted in a delay in treatment and eventual deterioration. This phenomenon has been recognised [27] elsewhere and highlights how anticipating the psychological impact of such a global event is crucial in order to better tackle any future pandemics that may occur. The increase in incidence of IEs before and after January 2020 (0.3% vs. 1.2%), in which 7 of 11 (63.6%) cases did not occur following COVID-19 infection, suggests that the increase may not be a direct pathophysiological effect of the virus itself.
Cerebral Venous Sinus Thrombosis
Cerebral venous sinus thromboses (CVST) were diagnosed in 3 of 4 patients in this series, and while CVST is a recognised complication of SEs, the prevalence reported in the literature is less so at approximately 10% [28]. In our own data, 3 of 12 (25%) empyema patients without a history of COVID-19 infection developed sinus thromboses, which is also less than the 75% of those who were recently COVID-19 positive in this series. The relationship between COVID-19 and hypercoagulability are well described, with deep vein thromboses and pulmonary embolisms frequently reported 29. CVST has also been identified in isolation as a result of COVID-19 infection in several patients [30, 31], with a mortality of 36.4% [31]. CVST in itself is a marker of poor prognosis in SE [28], though fortunately good outcomes were demonstrated across all 3 patients in this series. While this series is small, the difference in prevalence of CVST in patients with recent COVID-19 infection compared to the literature suggests yet more investigations are required to determine whether a viral-induced hypercoagulability is responsible here.
This series serves as a reminder of the importance of adequately investigating patients presenting with persistent sinusitis, particularly those with an evolving history of meningism or neurological features. In light of this series, greater attention may also be paid to any history of recent COVID-19 infection. Early involvement of all relevant specialities was key to the positive outcomes seen here. Close co-operation between neurosurgery, ENT, and anaesthetics resulted in prompt surgical intervention and insertion of appropriate IV access, while early referral to infectious diseases, neurology, and haematology allowed for continual tailoring of both antimicrobial and anticoagulant treatment.
Limitations
This article has several limitations, primarily the retrospective nature of the data. Our series is also limited by its small sample size, which prohibits any robust statistical comparison between groups. To counter this, we hope to share our experience with other centres in an attempt to discern whether the increase in incidence of IEs seen here was reproduced elsewhere. Construction and maintenance of a prospective database to monitor the incidence of IEs across the UK, or even internationally, may permit more robust statistical analysis and ultimately shine light on an underlying explanation for the increase in incidence observed here. Greater detail regarding the COVID-19 diagnoses featured in this series would also be a beneficial addition to this work. Viral genotyping would enable us to determine whether subtypes of COVID-19 had varying impact on the likelihood of developing an IE, and patient antibody status could potentially identify previous infections that have subsequently led to empyema in those without a positive PCR. Unfortunately, neither of these technologies were readily available to the clinical team at the time of admission.
Conclusion
Our series of paediatric IEs occurring after COVID-19 infection highlight another potential non-respiratory sequelae of the virus. Our series demonstrates a greater proportion of cerebral sinus thromboses than in non-COVID-19 cases, potentially indicative of the thrombogenic effects of COVID-19 itself. Overall, incidence of IE at our centre has increased markedly since the start of the pandemic: the causes of which are likely multifactorial and require further investigation. Collaborative work with other neurosurgical centres is necessary to compare their experience during the pandemic.
Statement of Ethics
Ethical approval for this study was not required in view of the retrospective nature of the study and all the procedures being performed were part of the routine care. This study protocol was reviewed by the Alder Hey Children’s Hospital NHS Foundation Trust neurosurgical department in research and audit meeting and received approval on December 12, 2021. Written informed consent was obtained from the participants’ parent/legal guardian/next of kin to participate in the study. All data collected and used in the study were fully anonymised prior to analysis and submission for publication.
Conflict of Interests Statement
The authors have no relevant financial or non-financial interests to disclose.
Funding Sources
No external funding was received for this study.
Author Contributions
B.J.H. and J.C.D. were responsible for the conception of analysis, data collection, data analysis, and data interpretation, as well as the writing and revision of the manuscript and manuscript submission. K.A. was responsible for data collection and analysis. R.D., A.N., A.K., S.T.K., B.C., D.H., H.G., W.D., J.E., C.P., B.P., A.S., and C.M. were responsible for writing and revising the manuscript. All authors critically reviewed and revised the draft of the manuscript. All authors had access to the underlying data and verified the findings. All authors have seen and approved the final version. The corresponding author had full access to all data and the final responsibility to submit for publication.
Funding Statement
No external funding was received for this study.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further enquiries regarding access to the datasets generated and/or analysed during the current study may be directed to the corresponding author.
References
- 1. De Bonis P, Anile C, Pompucci A, Labonia M, Lucantoni C, Mangiola A. Cranial and spinal subdural empyema. Br J Neurosurg. 2009;23(3):335–40. 10.1080/02688690902939902. [DOI] [PubMed] [Google Scholar]
- 2. Nathoo N, Nadvi SS, van Dellen JR, Gouws E. Intracranial subdural empyemas in the era of computed tomography: a review of 699 cases. Neurosurgery. 1999;44(3):529–35; discussion 535-6. 10.1097/00006123-199903000-00055. [DOI] [PubMed] [Google Scholar]
- 3. Osborn MK, Steinberg JP. Subdural empyema and other suppurative complications of paranasal sinusitis. Lancet Infect Dis. 2007;7(1):62–7. 10.1016/S1473-3099(06)70688-0. [DOI] [PubMed] [Google Scholar]
- 4. Silverberg AL, DiNubile MJ. Subdural empyema and cranial epidural abscess. Med Clin North Am. 1985;69(2):361–74. 10.1016/s0025-7125(16)31048-3. [DOI] [PubMed] [Google Scholar]
- 5. French H, Schaefer N, Keijzers G, Barison D, Olson S. Intracranial subdural empyema: a 10-year case series. Ochsner J. 2014;14(2):188–94. [PMC free article] [PubMed] [Google Scholar]
- 6. Dill SR, Cobbs CG, McDonald CK. Subdural empyema: analysis of 32 cases and review. Clin Infect Dis. 1995;20(2):372–86. 10.1093/clinids/20.2.372. [DOI] [PubMed] [Google Scholar]
- 7. World Health Organisation . WHO coronavirus (COVID-19) dashboard. 2022; updated 2022 Mar 1; https://covid19.who.int (accessed March 08, 2022) [Google Scholar]
- 8. Farrell JM, Zhao CY, Tarquinio KM, Brown SP. Causes and consequences of COVID-19-associated bacterial infections. Front Microbiol. 2021;12:682571. 10.3389/fmicb.2021.682571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boldrini M, Canoll PD, Klein RS. How COVID-19 affects the brain. JAMA Psychiatry. 2021;78(6):682–3. 10.1001/jamapsychiatry.2021.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kantonen J, Mahzabin S, Mäyränpää MI, Tynninen O, Paetau A, Andersson N, et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020;30(6):1012–6. 10.1111/bpa.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naz S, Hanif M, Haider MA, Ali MJ, Ahmed MU, Saleem S. Meningitis as an initial presentation of COVID-19: a case report. Front Public Health. 2020;8:474. 10.3389/fpubh.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duong L, Xu P, Liu A, et al. Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles. Brain Behav Immun. 2020;87:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meguins L, Rocha AS, Laurenti MR, de Morais DF. Ventricular empyema associated with severe pyogenic meningitis in COVID-19 adult patient: case report. Surg Neurol Int. 2021;12:346. 10.25259/SNI_514_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haroon K, Reza MA, Taher T, Alam MS, Haque RU, Ahmed MF, et al. Acute subdural empyema in the young COVID-19 patient- A case report. Bangla J Neurosurg. 2021;10(2):206–9. 10.3329/bjns.v10i2.53776. [DOI] [Google Scholar]
- 15. Ljubimov V, Babadjouni R, Ha J, Krutikova VO, Koempel JA, Chu J, et al. Adolescent subdural empyema in setting of COVID-19 infection: illustrative case. J Neurosurg Case Lessons. 2022;3(4):CASE21506. 10.3171/CASE21506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boateng G, Ristagno EH, Levy E, Kahoud R, Thacker PG, Setter DO, et al. A complicated presentation of pediatric COVID-19 with necrotizing pneumonia and pulmonary artery pseudoaneurysms. Pediatr Pulmonol. 2021;56:4042–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Charlton M, Nair R, Gupta N. Subdural empyema in adult with recent SARS-CoV-2 positivity case report. Radiol Case Rep. 2021;16(12):3659–61. 10.1016/j.radcr.2021.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Antonelli M, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5(10):708–18. 10.1016/S2352-4642(21)00198-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ingraham NE, Lotfi-Emran S, Thielen BK, Techar K, Morris RS, Holtan SG, et al. Immunomodulation in COVID-19. Lancet Respir Med. 2020;8(6):544–6. 10.1016/s2213-2600(20)30226-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carvalho V, Vergínio VEO, Brito GC, Pereira-Stabile CL, Stabile GAV. Coronavirus disease 2019 as a possible cause of severe orbital cellulitis. J Craniofac Surg. 2021;32(8):e795–8. 10.1097/SCS.0000000000007826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turbin RE, Wawrzusin PJ, Sakla NM, Traba CM, Wong KG, Mirani N, et al. Orbital cellulitis, sinusitis and intracranial abnormalities in two adolescents with COVID-19. Orbit. 2020;39(4):305–10. 10.1080/01676830.2020.1768560. [DOI] [PubMed] [Google Scholar]
- 22. Tanislav C, Kostev K. Fewer non-COVID-19 respiratory tract infections and gastrointestinal infections during the COVID-19 pandemic. J Med Virol. 2022;94(1):298–302. 10.1002/jmv.27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eyre T, Peters L, Andersson MI, Peniket A, Eyre DW. Reduction in incidence of non-COVID-19 respiratory virus infection amongst haematology inpatients following UK social distancing measures. Br J Haematol. 2021;195(2):194–7. 10.1111/bjh.17651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen R, Ashman M, Taha MK, Varon E, Angoulvant F, Levy C, et al. Pediatric Infectious Disease Group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity gap? Infect Dis Now. 2021;51(5):418–23. 10.1016/j.idnow.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Foley DA, Phuong LK, Peplinski J, Lim SM, Lee WH, Farhat A, et al. Examining the interseasonal resurgence of respiratory syncytial virus in Western Australia. Arch Dis Child. 2022;107(3):e7. 10.1136/archdischild-2021-322507. [DOI] [PubMed] [Google Scholar]
- 26. Hodjat P, Christensen PA, Subedi S, Bernard DW, Olsen RJ, Long SW. The reemergence of seasonal respiratory viruses in houston, Texas, after relaxing COVID-19 restrictions Microbiol Spectr. 2021;9(9):e0043021. 10.1128/Spectrum.00430-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shukla P, Lee M, Whitman SA, Pine KH. Delay of routine health care during the COVID-19 pandemic: a theoretical model of individuals’ risk assessment and decision making. Soc Sci Med. 2022;307:115164. 10.1016/j.socscimed.2022.115164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Konar S, Gohil D, Shukla D, Sadashiva N, Uppar A, Bhat DI, et al. Predictors of outcome of subdural empyema in children. Neurosurg Focus. 2019;47(2):E17. 10.3171/2019.5.FOCUS19268. [DOI] [PubMed] [Google Scholar]
- 29. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–15. 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abouhashem S, Eldawoody H, Taha MM. Cerebral venous sinus thrombosis in patients with COVID-19 infection. Interdiscip Neurosurg. 2021;24:101091. 10.1016/j.inat.2021.101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nwajei F, Anand P, Abdalkader M, Andreu Arasa VC, Aparicio HJ, Behbahani S, et al. Cerebral venous sinus thromboses in patients with SARS-CoV-2 infection: three cases and a review of the literature. J Stroke Cerebrovasc Dis. 2020;29(12):105412. 10.1016/j.jstrokecerebrovasdis.2020.105412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further enquiries regarding access to the datasets generated and/or analysed during the current study may be directed to the corresponding author.