Abstract
Duodenal stenosis caused by upper tract urothelial carcinoma (UTUC) is rare. A 70-year-old male patient was diagnosed with a UTUC invading the duodenum 3 months before admission. Owing to duodenal stenosis, enteral nutrition was necessary. We performed pancreaticoduodenectomy with right nephroureterectomy and right hemicolectomy using a multi-disciplinary approach. Postoperative pathology revealed a UTUC invading the right kidney, duodenum, pancreas, and transverse colon. The patient underwent chemotherapy and immunotherapy after surgery, which improved his quality of life.
Keywords: Case report, upper tract urothelial carcinoma, duodenal stenosis, multidisciplinary discussion, surgery, invasion, chemotherapy, immunotherapy
Introduction
Urothelial carcinomas (UCs) are the fourth most common tumors. 1 However, upper tract UC (UTUCs) are uncommon, accounting for less than 5% of all UCs. 1 UTUCs occur most frequently in older people, and the incidence rate of UTUCs in men is three times higher than that in women. 2 Duodenal stenosis caused by UTUCs is rare; to the best of our knowledge, only five cases have been reported to date.3–7
There are no randomized controlled trial (RCT) data supporting the role of radical surgery in patients with locally advanced UTUCs. Therefore, individual evaluation is essential, and surgical treatment requires patient consent after a comprehensive evaluation. 8 We report this rare case to provide treatment reference for our colleagues.
The reporting of this case conforms to the CARE guidelines. 9
Case report
A 70-year-old male patient was hospitalized in another center owing to recurrent nausea and vomiting 3 months before presentation to our hospital. Computed tomography (CT) at the previous center suggested duodenal invasion and right hydronephrosis. Gastroscopy revealed duodenal obstruction, and pathological results suggested a malignant tumor originating from a ureteral epithelial carcinoma. Ureteroscopy revealed stenosis of the upper ureter and no cancer in the bladder. Positron emission tomography (PET)-CT showed that the tumor was locally invasive, and no bladder involvement or distant metastasis was observed. Owing to the duodenal obstruction, enteral nutrition was necessary. Tislelizumab with gemcitabine-platinum was administered for two courses (3 weeks for each course). However, conservative treatment did not resolve the duodenal stenosis, and the patient presented to our center for further treatment. He had a history of smoking, and drinking alcohol, but no family history of cancer.
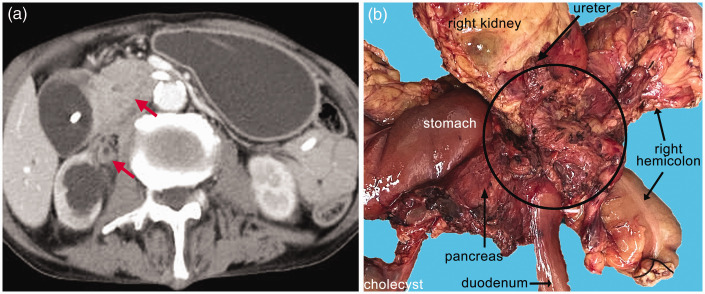
On admission, the patient was weak, and his body mass index (BMI) was only 17.5 kg/m2. Laboratory test results were as follows: leukocyte count: 13.2 × 109/L, neutrophil percentage: 80.4%, C-reactive protein: 27.5 mg/L, hemoglobin: 90 g/L, alanine aminotransferase: 111 U/L, aspartate aminotransferase: 82 U/L, albumin: 36.5 g/L, total bilirubin: 97.1 μmol/L, direct bilirubin: 71.2 μmol/L, urea: 20.38 mmol/L, creatinine: 140 μmol/L, serum amylase: 177 U/L, and carbohydrate antigen 199 (CA199): 156 kU/L. The concentrations of alpha-fetoprotein (AFP), prostate-specific antigen (PSA), and carcinoembryonic antigen (CEA) were within normal ranges. Abdominal contrast-enhanced CT showed that the tumor had caused right ureteral stenosis and renal pelvis dilation, and that the tumor invaded the duodenum (Figure 1a).
Figure 1.
(a) The tumor caused right ureteral stenosis and renal pelvis dilation and invaded the duodenum (red arrows) and (b) Surgical specimen: the tumor is outlined by the circle.
After admission, a multidisciplinary discussion was performed, and the patient was diagnosed with locally advanced UTUC. The multidisciplinary team agreed that the tumor was resectable, and that surgical resection might improve the patient’s quality of life and potentially prolong his life. The team also suggested performing chemotherapy and immunotherapy after surgery. Therefore, we performed surgery on the 6th day after admission. Intraoperatively, we found that the tumor had invaded the duodenum, pancreatic head, transverse colon, and inferior vena cava. Therefore, we performed pancreaticoduodenectomy with right nephroureterectomy and right hemicolectomy. The following lymph nodes were excised during the operation: perigallbladder, periduodenal, peripancreatic, right hemicolonic, intra-hepatoduodenal ligamentary, and para-aortic (Figure 1b). The operation was successful.
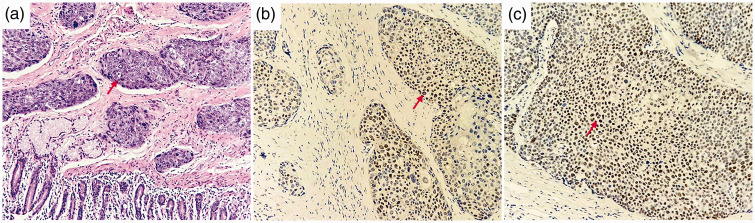
After surgery, parenteral nutrition, anti-infection, and other symptomatic treatments were administered. The patient recovered well and ate successfully on the 4th day after the operation. No surgery-related complications, such as biliary fistula, intestinal fistula, and pancreatic fistula occurred. The patient was discharged on the 18th postoperative day. Postoperative pathology indicated high-grade infiltrating UC of the right ureter as the primary tumor. The tumor invaded the right renal parenchyma, pelvic mucosa, duodenum, pancreas, and transverse colon. Immunohistochemical staining indicated GATA binding protein 3 (GATA3) and tumor protein 63 (P63) positivity (Figure 2). Additionally, 5 of 36 dissected peripheral lymph nodes were positive, with tumor metastases around the gallbladder, colon, pancreas, and para-aortic lymph nodes. Therefore, the tumor-node-metastasis (TNM) stage was T4N1M0. 8 Postoperatively, the patient received two courses of immunotherapy (tislelizumab 200 mg) and chemotherapy (cisplatin 50 mg and gemcitabine 1.2 g) every 3 weeks. However, he refused further treatment owing to severe myelosuppression. Four months after surgery, imaging revealed no tumor recurrence. However, 6 months after surgery, he was diagnosed with multiple liver metastases, and he died of cachexia 2 months later.
Figure 2.
Histopathology (HE staining) showing a high-grade infiltrating urothelial carcinoma of the right ureter with extensive scaling and necrosis (a, red arrow; ×200). Immunohistochemical staining is positive for GATA3 (b, red arrow) and P63 (c, red arrow; ×200). HE, hematoxylin and eosin; GATA 3, GATA binding protein 3; P63, tumor protein 63.
Discussion
Duodenal stenosis caused by UTUCs is rare. To the best of our knowledge, only five cases have been reported to date (Table 1)3–7 and all were elderly. Four of the five patients were men. This is because of the higher incidence of UTUCs in men. Radical surgery was performed in one case, and palliative gastrointestinal bypass surgery was performed in another two cases. The remaining two cases received conservative treatment. Four of the five patients received chemotherapy. Only one patient received immunotherapy because immunotherapy drugs for UTUCs became available only recently. Nephroureterectomy and regional lymph node dissection are the main surgical methods for locally advanced UTUCs. 10 Of the five previous cases, the patient who received radical surgery survived the longest. Therefore, it was reasonable to perform radical surgery in our case.
Table 1.
Five cases of duodenal stenosis caused by UTUCs.
| Authors, Year | Sex | Age (years) | Surgery | Chemotherapy | Immunotherapy | Survival time |
|---|---|---|---|---|---|---|
| Takiuchi et al., 20173 | M | 66 | radical surgery | gemcitabine + carboplatin | no | >8 months |
| Ando et al., 20194 | M | 83 | short-circuit surgery | no | no | >2 months |
| Stroman et al., 20155 | M | 61 | no | gemcitabine and cisplatin | no | >6 months |
| Motoo et al., 20217 | M | 59 | short-circuit surgery | gemcitabine and cisplatin | pembrolizumab | >5 months |
| Nakai et al., 20156 | F | 68 | no | yes, but no details | no | no details |
M, male; F, female.
The TNM stage of locally advanced UTUCs is pT3/T4N0M0 or pT3/T4NxM0, or pTanyN+M0. 8 Therefore, this case constituted locally advanced UTUCs. In a previous study, neoadjuvant chemotherapy and subsequent radical surgery resulted in higher overall survival (OS) in patients with locally advanced UTUCs compared with surgical treatment alone. 11 A phase III prospective randomized trial revealed that gemcitabine-platinum combination chemotherapy improved disease-free survival in patients with locally advanced UTUCs after radical surgery. 12
Immune checkpoint inhibitors can be used to treat UTUCs. 13 Tislelizumab was approved for advanced UC by the National Medical Products Administration of China. Owing to duodenal stenosis, our patient could not eat, and his quality of life was poor. After a multidisciplinary discussion, the expert team agreed that the tumor was resectable, and surgical resection might improve the patient’s quality of life and potentially prolong his life. For these reasons, we decided to perform surgical treatment. The patient had undergone chemotherapy and immunotherapy before admission; therefore, repeat neoadjuvant chemotherapy was not performed. Nephroureterectomy with bladder cuff excision is the standard treatment for high-risk UTUCs. 14 We made the emergency intraoperative decision not to perform bladder cuff excision for the following reasons: First, this was a locally advanced UTUC. Second, intraoperative pathology indicated negative resection margins for the ureter. Third, bladder cuff excision requires another lower abdominal incision. Fourth, the patient was elderly, and he may not have tolerated expanded surgery.
The most common complications after pancreaticoduodenectomy with right hemicolectomy are pancreatic fistula, hemorrhage, anastomotic leak, and surgical site infections. 15 The major complications following nephroureterectomy are hemorrhage, ileus, and venous thrombosis. 16 However, none of these complications occurred in this case. The patient was able to eat on the 4th day after surgery, and his serum bilirubin concentration had returned to normal at the time of discharge. Therefore, our treatment significantly improved his quality of life.
In conclusion, multidisciplinary discussion and individual evaluation were necessary in the treatment of this patient. We hope that the details of our treatment process can provide a reference for our colleagues, should they encounter similar cases.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605231206958 for Duodenal stenosis caused by locally advanced upper tract urothelial carcinoma: a case report by Jun Lu, Weijiang Zhou, Huadong He, Jun Chen, Jian Wu, Lixin Zhou and Xiao Xu in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605231206958 for Duodenal stenosis caused by locally advanced upper tract urothelial carcinoma: a case report by Jun Lu, Weijiang Zhou, Huadong He, Jun Chen, Jian Wu, Lixin Zhou and Xiao Xu in Journal of International Medical Research
Author contributions: JL and WZ made substantial contributions to managing the report and writing the manuscript. JC made substantial contributions to the acquisition of the clinical data. JW made substantial contributions to the pathological analysis. LZ, XX, and HH were the main surgeons during the diagnosis and treatment of the patient. All authors read and approved the final manuscript.
The authors declare that there is no conflict of interest.
Funding: This work was supported by National Natural Science Funds for Distinguished Young Scholars of China [grant number 81625003].
ORCID iDs: Lixin Zhou https://orcid.org/0000-0003-0561-8080
Data availability statement
All data generated during this study are included in the published article.
Ethics statement
The requirement for ethical approval was waived for this study because of the retrospective design, which did not impact the management of the patient’s care. We have obtained the patient’s written informed consent for publication of this report. We have also obtained patient consent for treatment. All patient details have been de-identified.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2022. CA Cancer J Clin 2022; 72: 7–33. [DOI] [PubMed] [Google Scholar]
- 2.Shariat SF, Favaretto RL, Gupta A, et al. Gender differences in radical nephroureterectomy for upper tract urothelial carcinoma. World J Urol 2011; 29: 481–486. [DOI] [PubMed] [Google Scholar]
- 3.Takiuchi D, Morimoto O, Wada R, et al. A case of urothelial carcinoma who underwent pancreaticoduodenectomy and was diagnosed with groove pancreatitis and preoperatively suffered from duodenal stenosis. Gan To Kagaku Ryoho 2017; 44: 2003–2005. [PubMed] [Google Scholar]
- 4.Ando T, Watanabe K, Takahashi K, et al. Duodenal and rectal obstructions due to urothelial cancer infiltration from recurrent renal pelvic cancer in the bladder wall: an autopsy case. Urol Case Rep 2019; 27: 100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stroman LA, Sharma N, Sullivan M. Upper ureteric transitional cell carcinoma, extending to the renal pelvis, presenting as duodenal obstruction. BMJ Case Rep 2015; 2015: bcr2015210028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakai Y, Isayama H, Takahara N, et al. Endoscopic ultrasound-guided fine-needle aspiration for duodenal obstruction without a discrete mass. Dig Dis Sci 2015; 60: 1502–1504. [DOI] [PubMed] [Google Scholar]
- 7.Motoo I, Ando T, Mihara H, et al. Endoscopic ultrasound-guided fine needle aspiration for the diagnosis of duodenal stenosis due to urothelial carcinoma. Intern Med 2021; 60: 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niegisch G, Gerullis H, Lin SW, et al. A real-world data study to evaluate treatment patterns, clinical characteristics and survival outcomes for first- and second-line treatment in locally advanced and metastatic urothelial cancer patients in Germany. J Cancer 2018; 9: 1337–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 10.Ibilibor C, Kennady EH, Greene KL. The role of surgery for locally advanced urothelial cancers. Curr Opin Urol 2022; 32: 614–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu D, Hu J, He T, et al. Effect of neoadjuvant chemotherapy on locally advanced upper tract urothelial carcinoma: a pooled analysis. Transl Androl Urol 2020; 9: 2094–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birtle A, Johnson M, Chester J, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): a phase 3, open-label, randomised controlled trial. Lancet 2020; 395: 1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Califano G, Ouzaid I, Verze P, et al. Immune checkpoint inhibition in upper tract urothelial carcinoma. World J Urol 2021; 39: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 14.Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009; 115: 1224–1233. [DOI] [PubMed] [Google Scholar]
- 15.Das B, Fehervari M, Hamrang-Yousefi S, et al. Pancreaticoduodenectomy with right hemicolectomy for advanced malignancy: a single UK hepatopancreaticobiliary centre experience. Colorectal Dis 2023; 25: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kocher NJ, Canes D, Bensalah K, et al. Incidence and preoperative predictors for major complications following radical nephroureterectomy. Transl Androl Urol 2020; 9: 1786–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605231206958 for Duodenal stenosis caused by locally advanced upper tract urothelial carcinoma: a case report by Jun Lu, Weijiang Zhou, Huadong He, Jun Chen, Jian Wu, Lixin Zhou and Xiao Xu in Journal of International Medical Research
Supplemental material, sj-pdf-2-imr-10.1177_03000605231206958 for Duodenal stenosis caused by locally advanced upper tract urothelial carcinoma: a case report by Jun Lu, Weijiang Zhou, Huadong He, Jun Chen, Jian Wu, Lixin Zhou and Xiao Xu in Journal of International Medical Research
Data Availability Statement
All data generated during this study are included in the published article.