Abstract
Anxiety is a critical component of the development and maintenance of drug addiction, however, anti-anxiety medications such as benzodiazepines and beta-blockers (β-adrenergic receptor antagonists) are not used for the treatment of substance use disorder, except for the management of the acute withdrawal syndrome. Preclinical studies have shown that beta blockers may reduce stress-induced relapse, however, the effect of beta blockers on the escalation and maintenance of drug intake has not been tested. To address this issue, we chronically administered the β-adrenergic receptor antagonist propranolol during the escalation or maintenance of cocaine intake in a model of extended access (6 h) to cocaine self-administration (0.5 mg/kg). The behavioral specificity of propranolol was tested using a non-drug reward (saccharin). Daily administration of propranolol (15 mg/kg) prevented the development of escalation of cocaine self-administration, and partially reversed self-administration after establishment of escalation of intake. Moreover, propranolol dose-dependently decreased the motivation for cocaine tested under a progressive ratio schedule of reinforcement during the development of escalation and after maintenance. Finally, propranolol administration had no effect on escalation and maintenance of saccharin self-administration. These results demonstrate that chronic treatment with propranolol provide therapeutic efficacy in reducing cocaine self-administration during the development and after establishment of escalation of cocaine self-administration in an animal model relevant to cocaine use disorder. These results suggest that beta blockers should be further investigated as a target for medication development for the treatment of cocaine use disorder.
Keywords: adrenergic beta-antagonist, motivation, norepinephrine, psychological stress, substance-related disorders, therapeutics
Graphical Abstract

1 |. INTRODUCTION
Anxiety disorders are often comorbid with substance use disorders (SUDs)1. While there is shared vulnerability between both disorders2,3, substances can also be used to self-medicate and reduce anxiety disorder symptoms4. Negative affect and anxiety occur increasingly during the withdrawal period after using drugs of abuse5, which during early discontinuation of cocaine use has been attributed to noradrenergic dysregulation6. Long access to drugs and negative emotional and psychological states of withdrawal often lead to drug seeking, escalation of consumption, and relapse7. Effective treatments for withdrawal symptoms are likely to reduce relapse of cocaine consumption8,9. Treatment for anxiety includes therapy (cognitive behavioral and/or exposure therapy) and/or medications (anxiolytic medications, antidepressants, and beta-blockers)10. Propranolol is a non-selective beta-adrenergic receptor (β-AR) antagonist (beta-blocker)11,9 that is primarily indicated to treat high blood pressure11 and also acts as an anxiolytic that is often used off label to treat social anxiety disorder11.
Alpha adrenoceptors affect smooth muscle contractions (α−1R and α−2R) and beta adrenoceptors (β-AR) affect the heart, bronchodilation, and lipolysis12. Preclinical studies have shown that norepinephrine antagonists reduce stress-induced reinstatement of cocaine seeking (β-AR antagonists: betaxol/ICI-118,55113), dependence-induced increases in self-administration (α−1R antagonist prazosin14), and stress-induced reinstatement of cocaine seeking (α−2R antagonist clonidine)15,16. Propranolol administered after systemic administration of cocaine reduces subsequent cocaine locomotor sensitization17. Propranolol has been shown to reduce cue- and drug-induced reinstatement of heroin seeking in male Sprague Dawley rats when administered immediately after conditioned stimulus presentation18. Early pre-treatment with the β-adrenergic antagonist propranolol decreased cocaine self-administration when given after daily short access to cocaine in rats19 and squirrel monkeys20. Taken together, these studies clearly show that the noradrenergic system can decrease cocaine self-administration, however, there is no evidence that β-AR antagonists also decrease cocaine self-administration when animals exhibit escalation of cocaine intake such as when they are given extended access to self-administration20,14.
Escalation of cocaine intake after extended access to cocaine self-administration is a well-established paradigm that leads to compulsive-like drug taking and seeking in rodents21. Rats that are allowed extended access to cocaine self-administration (6h daily), will escalate their intake over time, whereas those under a short access for cocaine self-administration (1h daily), will maintain a regular, lower, cocaine self-administration over time21, 22,14. The aim of this study was to test the efficacy and specificity of the ß-AR receptor antagonist propranolol in reducing cocaine intake and the motivation to seek cocaine. Our overall hypothesis was that propranolol pretreatment may reduce cocaine self-administration and the motivation to consume cocaine when given extended access to cocaine self-administration. The propranolol treatment was tested both as a prevention method by administering propranolol before each extended access session and as a treatment method after establishment of escalation of cocaine intake (Experiment 1). We tested the specificity of the effects observed on a model of escalation to a non-drug reward using saccharin self-administration (Experiment 2). Finally, we identified the range of efficacy using a dose-response experiment (Experiment 3–4).
2 |. MATERIALS AND METHODS
2.1 |. Experimental timeline
Cocaine (Experiment 1 and 3) or saccharin (Experiment 2 and 4) self-administration was performed with acquisition of self-administration followed by 2 phases. Phase 1 included long access sessions that allowed rats 6 hours of access to lever press to receive cocaine in operant chambers for 15 days (Figure 1A and B). During phase 1, rats were given pretreatment injections of saline or 15 mg/kg (sc) propranolol to determine an effect of propranolol on cocaine self-administration (Figure 1A and B). Phase 2 included post-escalation and drug reversal (Figure 1A and B). Post-escalation sessions gave animals 6 hours of access to cocaine self-administration for 9 days (Figure 1A and B). Drug reversal involved animals who were pretreated with saline during Phase 1 escalation being pretreated with propranolol during Phase 2 post-escalation and animals pretreated with propranolol during Phase 1 escalation being pretreated with saline during Phase 2 post-escalation (Figure1A).
FIGURE 1.
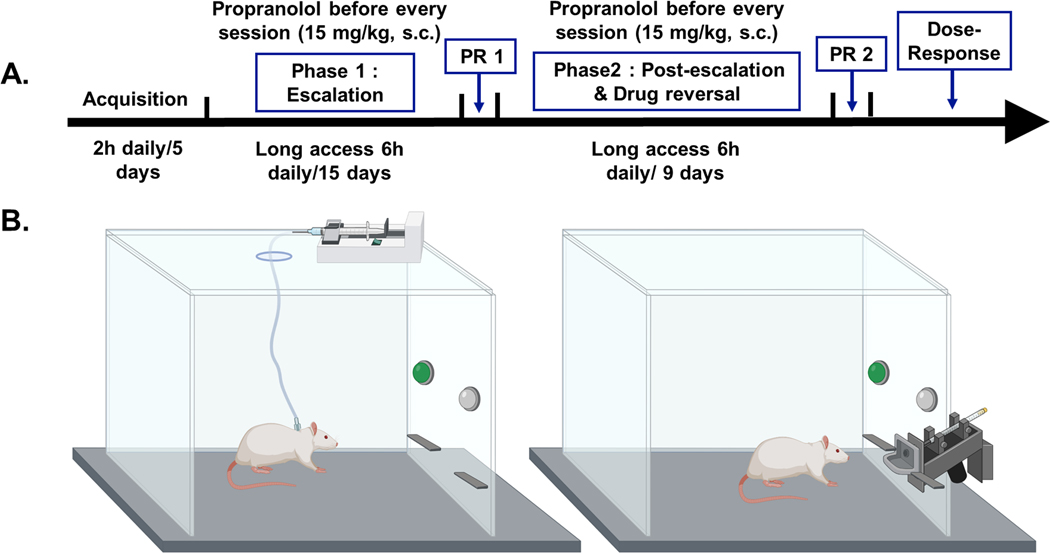
Experimental timeline and chamber set-up for cocaine and saccharin self-administration. (A) Cocaine self-administration timeline. Rats were allowed to lever press for cocaine SA initially for 2 h daily for 5 days. Next, during Phase 1 escalation, they were allowed to self-administer cocaine for 6 h daily for 15 days. This phase was followed by a progressive ratio test of motivation. Then, during Phase 2 post-escalation and drug reversal, the rats were allowed to SA cocaine for 6 h daily, for just 9 days. This phase was followed by a second progressive ratio test. Finally, dose–response tests for propranolol pretreatment were performed. Some rats were injected with 15 mg/kg, s.c. propranolol 30 min before each escalation and drug reversal session during Phases 1 and 2. (B) Chamber set-up for i.v. cocaine self-administration (left side) and saccharin liquid reward self-administration (right side). Chamber image created with BioRender.com by Alicia Avelar
2.2 |. Animals
Male and female Wistar rats, (220–250 g) were used for all experiments. In total 64 rats were utilized for this project: N = 28 for experiment 1, N = 21 for Experiment 2, N = 8 for experiment 3, and N = 7 for experiment 4) (Charles River, Hollister, CA). The animals were group-housed 2 same-sex animals/cage and maintained on a 12 h light/dark cycle with ad libitum access to food and water. All animal procedures were approved by The Scripps Research Institute Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines. Catheter failure and sickness let to removal of 2 rats from experiment 1 during phase 2. Additionally, one rat was excluded from the analysis in Experiment 1, because of a recording failure on multiple days.
2.3 |. Intrajugular catheter surgery (Experiments 1 and 3)
Rats, adapted to the vivarium for 1 week were anesthetized with isoflurane inhalation (5% induction, 2–3% maintenance) and were implanted with a silastic catheter into the right external jugular vein under aseptic conditions23, 24. After the surgery, rats were injected with flunixin (2.5 mg/kg, sc; Butler Schein, Dublin, OH, USA) and cefazolin (160 mg/kg, im; Butler Schein, Dublin, OH, USA) and allowed to recover for 7– 10 days prior the start of self-administration training. The catheters were flushed daily with 0.1 ml of sterile heparinized saline solution (10 U/ml of heparin sodium: American Pharmaceutical Partners, Schaumburg, IL, USA) in 0.9% bacteriostatic sodium chloride (Hospira, Lake Forest, IL, USA) post-surgery and for the duration of the experiment.
2.4 |. Operant chambers
Self-administration occurred in operant conditioning chambers (Med Associates, St. Albans, VT, USA) in a sound-attenuating, ventilated environmental cubicles containing two retractable levers. Cocaine was delivered through a plastic catheter tubing that connected the animal to an infusion pump (experiments 1 and 3). Saccharin was delivered into a drinking cup located between the levers (experiment 2 and 4). Each active lever press resulted in an infusion by activation of the infusion pump for 4 seconds and the delivery of 0.1 ml of cocaine (0.5 mg/kg, iv) or 0.1 mL saccharin solution (0.2% w/v25). During that time a cue light located above the active lever, was illuminated, and lasted throughout a time-out period (20 s) during which the pressing on the active lever did not have scheduled consequences. Pressing the inactive lever was recorded but did not have any scheduled consequence (experiments 1 and 3) or delivered regular drinking water (experiments 2 and 4).
2.5 |. Cocaine self-administration (experiments 1 and 3)
Rats were trained to self-administer cocaine (0.5 mg/kg per 0.1 ml infusion) for 2 h sessions for 5 consecutive days under a fixed-ratio1 (FR1) schedule of reinforcement. Rats were then divided into two groups, balanced by the number of rewards per session on the last three days of training and switched to 6 h daily access, 7 days/week. To examine the effect of propranolol on the establishment of escalation (phase 1), one group of rats received the β-adrenergic antagonist propranolol (15 mg/kg, sc) 30 min before the start of each 6 h session. Since the half-life of propranolol is 2 – 3 h in Wistar rats26, it is expected that most of the session is under the influence of propranolol. The control group received saline injections 30 before the session. The escalation sessions continued for 15 sessions by which time a stable escalation of cocaine intake was achieved in the control group (Phase 1: Escalation).
To examine the effect of propranolol after establishment of cocaine self-administration (phase 2) the treatment was switched: half the rats from the saline group (phase 1) then received propranolol instead of saline (phase 2), and the previous propranolol group (phase 1) was switched to saline treatment (Phase 2: Post escalation & drug reversal). On the day following the end of each phase (phase 1 and phase 2), rats were tested under a progressive ratio schedule of reinforcement (PR) to evaluate their motivation for cocaine. Only rats that acquired self-administration of cocaine, at least 10 infusions during each of the last 2 days of acquisition and at least 20 infusions during the 1st 6h session, were used in experiments 1 and 3.
2.6 |. Saccharin self-administration (experiments 2 and 4)
To test the effect of propranolol on a non-drug reward we administered pretreatment of propranolol on saccharin self-administration using a protocol that leads to an escalation of saccharin intake similar to the escalation of cocaine in terms of magnitude of behavioral output. Rats underwent similar experimental procedures and conditions as described for cocaine except that they did not undergo surgery and a valid lever press resulted on the delivery of 0.1 ml saccharin solution (0.2% w/v) into a small drinking cup25. Rats acquired saccharin self-administration in 30 min sessions over 5 days. They were then switched to 60 min sessions under an FR1 schedule of reinforcement for 15 days of Phase 1. Phase 2 involved reversing treatment similar to the cocaine procedure. Self-administration sessions for saccharin were run 7 days/week. Only rats that acquired self-administration of saccharin (if they had at least 10 doses of saccharin during each of the last 2 days of acquisition and at least 20 doses during the 1st 60 min session), were included in experiments 3 and 4.
2.7 |. Progressive ratio schedule of reinforcement (experiments 1 and 2)
To measure the motivation to seek cocaine or saccharin, the rats were tested under a progressive ratio (PR) schedule of reinforcement on the day following the end of each phase. The number of responses required to obtain a reward was determined by the progression defined by Richardson and Roberts27. This led to the following progression of response requirements per reward: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 60325,27,28. The last lever press requirement to receive reward that was achieved by an animal during a PR session is the breakpoint and is used as a measure of motivation.
2.8 |. Dose-response effect of propranolol on cocaine and saccharin self-administration. (Experiments 3 and 4)
To further confirm our results and identify the dose-range of efficacy we performed a dose-response for propranolol on cocaine (Experiment 3) and saccharin (Experiment 4) self-administration. The animals from the saline/saline groups were used for our dose-response experiments. After the second progressive ratio test, these rats had 4 days off and then were allowed to re-baseline self-administration for 3 days before participating in the dose-response experiments. Four different doses of propranolol (0 mg/kg, 5 mg/kg, 15 mg/kg, and 40 mg /kg sc) were administered after stabilization of escalation of cocaine or saccharin self-administration using a Latin square design. The 40 mg/kg dose was chosen as the maximum dose as higher doses can produce non-specific effects29,30. On test day, propranolol or saline was administered 30 min prior the 6 h self-administration session. Each self-administration session was separated by 2 days washout, where the rats remained in their home cages. And their catheters were flushed with 0.1 ml of sterile heparinized saline solution to maintain patency.
2.9 |. Drug treatment
Cocaine HCl (National Institute on Drug Abuse, Bethesda, MD) was dissolved in 0.9% in sterile saline at a dose of 0.5 mg/kg/infusion and administered intravenously. Propranolol (15mg/kg sc, Sigma-Aldrich) was dissolved in sterile 0.9% saline. Saccharin, sodium salt hydrate 98 % (Sigma-Aldrich), was dissolved in tap water (0.2% w/v).
2.10 |. Data analysis
Statistical analysis was performed with Prism 8 (GraphPad). Values are represented as the mean ± SEM. Cocaine or saccharin self-administration over the sessions in each phase, were analyzed using two-way repeated measure analyses of variance (RMANOVA). Sidak’s multiple comparisons tests were used when applicable. Lever press data were analyzed using nonparametric (Mann-Whitney) tests. PR breakpoints were analyzed using nonparametric (Mann-Whitney or Kruskal-Wallis) or parametric (unpaired t-test) tests. Dose response effects in experiments 2 and 4 were analyzed using one-way ANOVAs with post hoc Dunnett’s multiple comparisons test when significant main effects were found with ANOVA.
3 |. RESULTS
3.1 |. Cocaine self-administration (experiments 1 and 3)
3.1 a. |. Propranolol effects on escalation of cocaine self-administration.
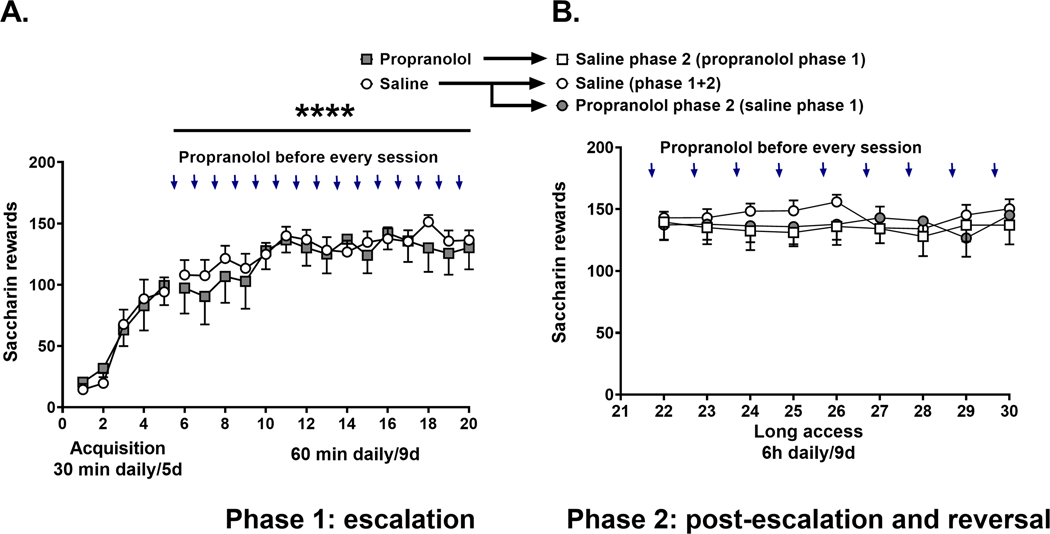
We tested the effect of propranolol pretreatment on escalation of cocaine self-administration of rats in 2 phases. During phase 1 (Figure 2A) rats were trained to self-administer cocaine (0.5 mg/kg per 0.1 ml infusion) for 2 h sessions for 5 consecutive days under a fixed-ratio 1 (FR1) schedule of reinforcement (Figure 2A). Next, rats self-administered cocaine during 6-hour long access sessions (Figure 2A) for 15 days. Some rats were pretreated with saline and others with 15 mg/kg (sc) propranolol 30 minutes before each daily long access session (Figure 2A). Using a repeated measures analysis of variance (RMANOVA) test we found that there was a significant effect of time (F (5.422, 135.5) = 4.678, p = 0.0004), propranolol pretreatment (F (1, 25) = 6.235, p = 0.0195), and interaction (F (23, 575) = 4.442, p < 0.0001) on long access sessions of cocaine self-administration (Figure 2A). During phase 2 post-escalation and drug reversal rats were allowed to self-administer cocaine for 6-hour long access sessions for 9 days (Figure 2B). At this point rats maintained stable high levels of cocaine self-administration (2-way RMANOVA (factor time), F (4.905, 112.8) = 1.103, p = 0.3624). Phase 2 had 3 groups, including: saline only during phase 1 and 2, saline during phase 2 with propranolol pretreatment during phase 1, and propranolol during phase 2 with saline pretreatment during phase 1 (Figure 2B). Despite a trend for decreased cocaine self-administration in the propranolol phase 2 group compared to the Saline phase 1 + 2 group, there was no significant effect of propranolol pretreatment during phase 2 (RMANOVA F (2,23) = 1.323, p = 0.2859). These results suggest that propranolol pretreatment does not reverse cocaine self-administration in rats after stabilization of cocaine escalation (post-escalation). An alternative hypothesis, tested in experiments 3 and 4 is that higher doses of propranolol may be required in the post-escalation state.
FIGURE 2.
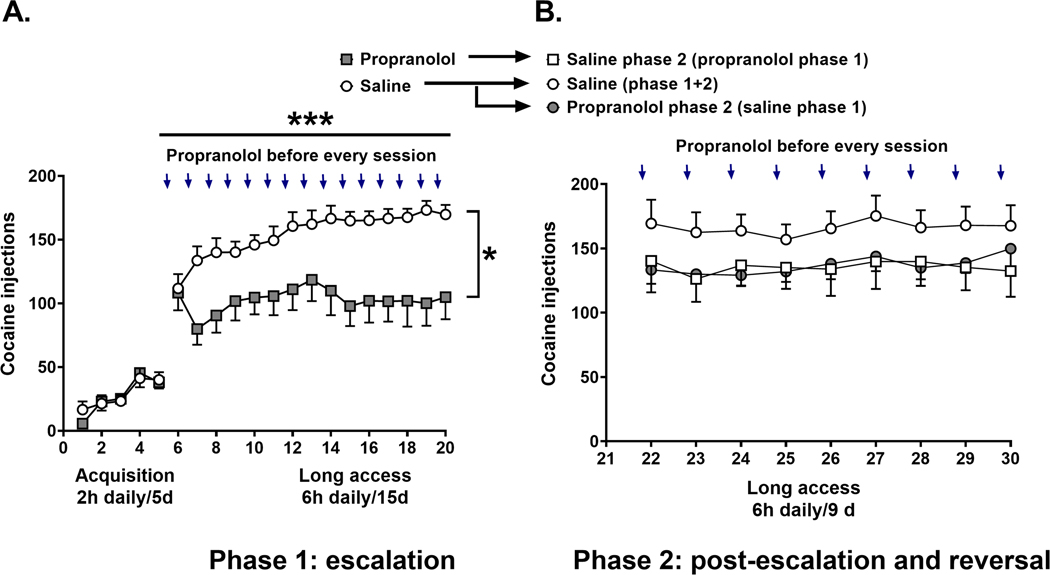
Propranolol pretreatment reduces cocaine self-administration in rats during escalation. (A) The left panel represents the 5 days of cocaine acquisition 2 h/day/5 days. The open circles represent the pattern of cocaine self-administration in the control group that received saline 30 min before each cocaine self-administration session (6 h/day/15 days) (N = 18). Filled squares represent the pattern of cocaine in rats that received propranolol (15 mg/kg, s.c) 30 min before each cocaine self-administration session (6 h/day/15 days) (N = 10). (B) The open circles represent the control group that received saline during the escalation (Phase 1) and continued to receive saline in Phase 2 (N = 10). The filled circles represent the group that received saline during the escalation (Phase 1) and was switched to propranolol in Phase 2 (N = 10). The open squares represent the group that received propranolol during the escalation (Phase 1) and was switched to saline in Phase 2 (N = 6). Results are expressed as mean ± SEM of number of cocaine self-administration injections acquired by animals. *p = 0.0195 treatment effect; ***p = 0.0004 time effect
3.1 b. |. Propranolol effects on lever pressing for cocaine.
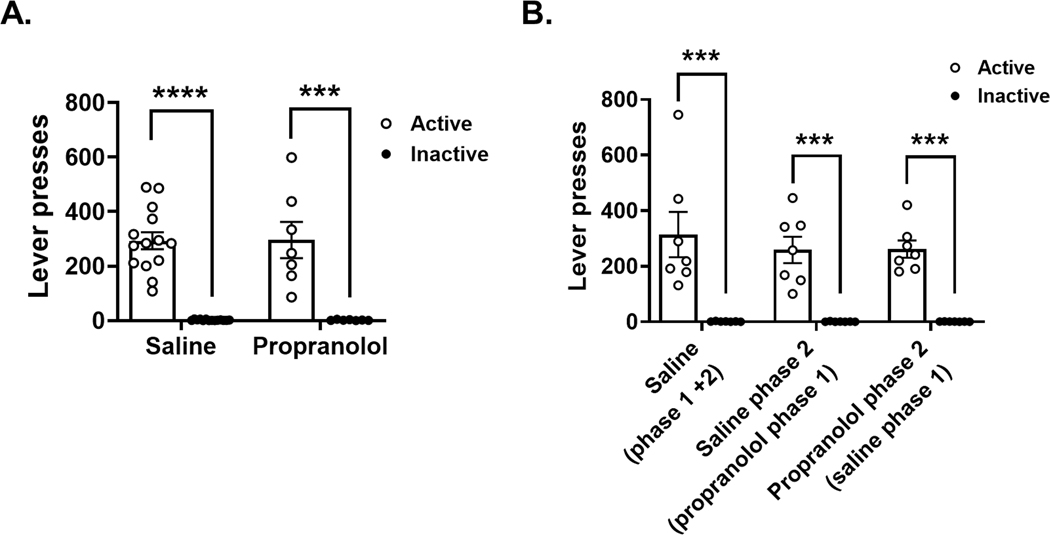
We compared active vs. Inactive lever presses during cocaine self-administration to determine if rats could accurately discriminate between an active lever press resulting in cocaine (iv) administration and an inactive lever press resulting in no drug administration. During phase 1 escalation (Figure 3A) and phase 2 post-escalation and drug reversal (Figure 3B) rats pressed the active lever significantly more than the inactive lever during both phase 1 (Mann-Whitney tests, Saline p < 0.000001 and Propranolol p = 0.00008) and phase 2 (Mann-Whitney tests, Saline (phase 1 + 2) p = 0.00004, Saline phase 2 (propranolol phase 1) p = 0.00008, and Propranolol phase 2 (saline phase 1) p = 0.0006). These results support that the rats were able to discriminate between active and inactive levers and that saline and propranolol pretreatments did not alter that discrimination.
FIGURE 3.
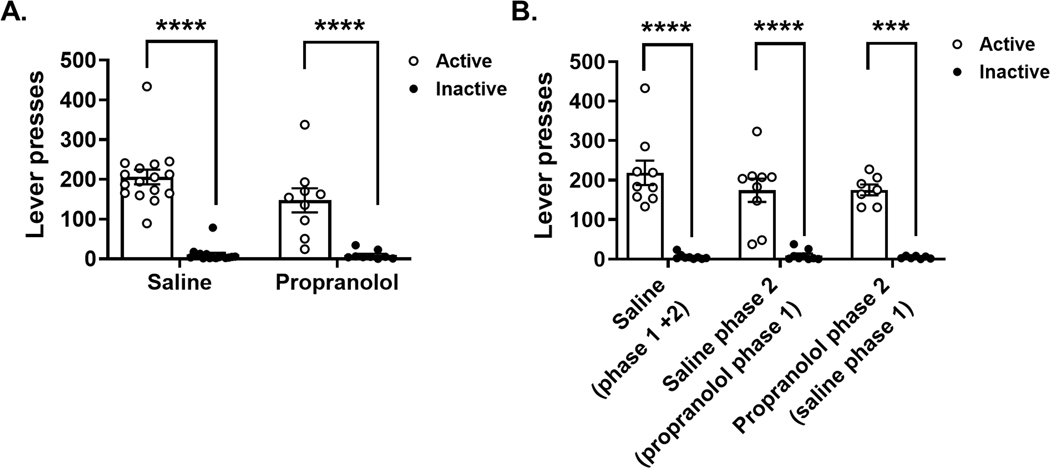
Cocaine self-administration lever presses during Phase 1 escalation and Phase 2 post-escalation and drug reversal. Open circles represent presses on the active lever and black circles represent presses on the inactive lever. (A) The left graph represents the escalation period (Phase 1). The saline group (N = 16) as well as the propranolol group (N = 9) show higher lever presses for the positive lever then for the negative lever, which indicates a good lever discrimination (Mann–Whitney tests, saline p < 0.000001 and propranolol p = 0.00008). (B) The right graph represents the post-escalation period where drug treatment was reversed (Phase 2). The first group of columns represents the saline group (N = 9). The middle two columns represent the group that received propranolol in Phase 1 and were switched to saline in Phase 2 (N = 9). The next group of columns represents saline group from Phase 1 that was switch to propranolol in Phase 2 (N = 7). The positive lever presses were higher than the negative lever presses for all groups during Phase 2. Results are expressed as mean ± SEM of number of lever presses per animal in Phases 1 and 2 (Mann–Whitney tests, saline [Phase 1 + 2] p = 0.00004, saline Phase 2 [propranolol Phase 1] p = 0.00008 and propranolol Phase 2 [saline phase 1] p = 0.0006). ****p < 0.0001 lever press type effect
3.1 c. |. Propranolol effects on the motivation for cocaine
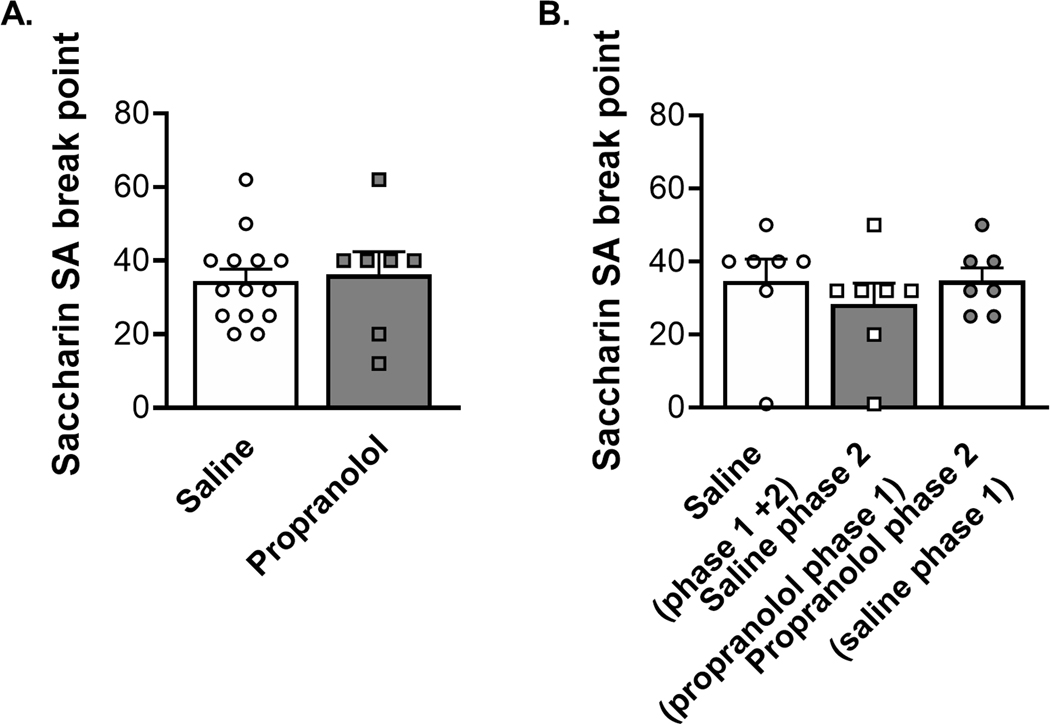
We employed progressive ratio tests to get breakpoints to measure motivation of the rats to work for cocaine30. Propranolol pretreatment significantly reduced breakpoint for cocaine self-administration during phase 1 escalation (Figure 4A; Mann-Whitney test, p = 0.0146). During phase 2 post-escalation and drug reversal rats who received propranolol pretreatment during phase 1 had significantly lower breakpoints compared to rats who received saline pretreatment during phase 1 and 2 (Figure 4B; Kruskal-Wallis test p = 0.0275, Dunn’s multiple comparison test p = 0.015) These results suggest a long-lasting effect of propranolol pretreatment during phase 1 that reduced motivation of rats to work for cocaine.
FIGURE 4.
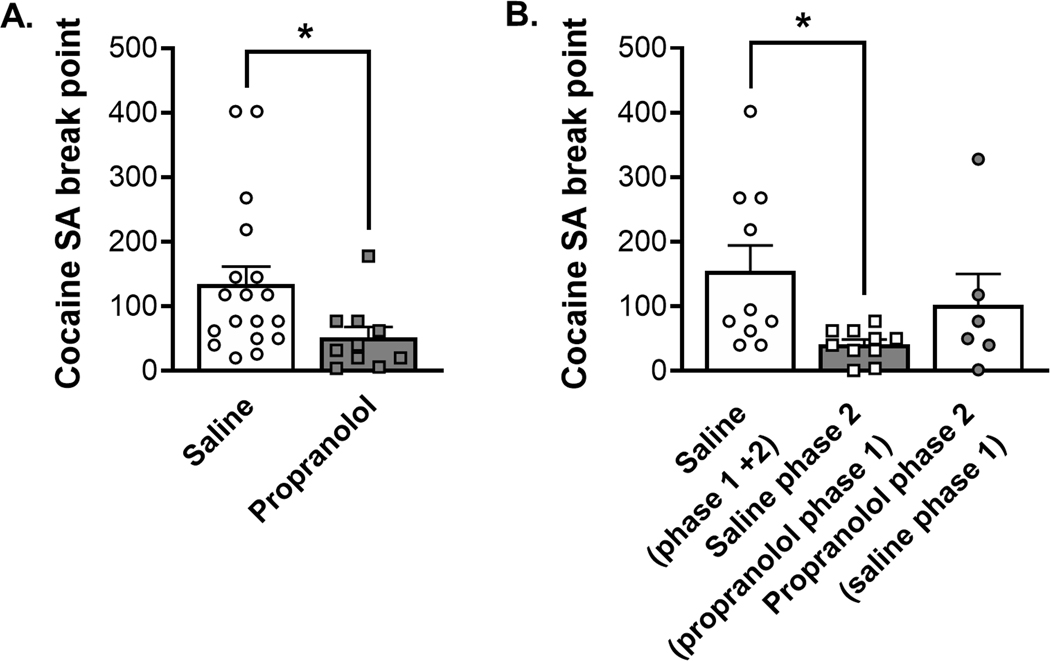
Propranolol pretreatment reduces breakpoint during progressive ratio self-administration tests of motivation to consume cocaine. (A) The left graph represents the first PR test which followed the escalation period (Phase 1), the saline group is indicated by opened circles and white column (N = 18) and the propranolol group represented by filled square and grey column (N = 10). Propranolol decreased progressive ratio breakpoint by the end of Phase 1 escalation self-administration (Mann–Whitney test, p = 0.0146). (B) The right graph represents the second progressive ratio test at the end of Phase 2 (stabilization and drug reversal). The open symbols represent saline pretreatment during Phase 2, and grey-filled symbols represent propranolol pretreatment during Phase 2. The control group that received saline during the escalation (Phase 1) and continued to receive saline in Phase 2 has a white column indicating saline pretreament during Phase 1 (N = 10). The group that received saline during the escalation (Phase 1) and was switch to propranolol in Phase 2 is shown with a white column and grey-filled symbols (N = 6). The opened squares represent the group that received propranolol during the escalation (Phase 1) and were switched to saline in Phase 2 (N = 10). Propranolol decreased progressive ratio breakpoint by the end of Phase 2 post-escalation and drug reversal when given during Phase 1 (Kruskal–Wallis test p = 0.0275, Dunn’s multiple comparison test p = 0.015), suggesting a lasting effect of propranolol pretreatment on motivation to consume cocaine. Data are expressed as mean ± SEM of the breakpoint achieved during the PR. *p < 0.05, compared to control rats that received saline
3.2 |. Saccharin self-administration (experiments 2 and 4)
3.2 a. |. Propranolol effects on saccharin self-administration
To control for non-specific effects of propranolol we tested the effect of propranolol on saccharin self-administration. Lever pressing for saccharin increased significantly over the sessions (Figure 5A; RMANOVA (factor time) F (5.731, 108.9) = 11.58, p < 0.0001). Propranolol did not have any effect on saccharin self-administration (RMANOVA (factor treatment) F (1, 19) = 0.1493, p = 0.7035), and there was no interaction between propranolol treatment and saccharin intake over the long access sessions (RMANOVA (interaction treatment x time) F (14, 266) = 1.162, p = 0.3048). Propranolol pretreatment had no effect on phase 2 post-escalation and drug reversal (Figure 5B RMANOVA (factor treatment) F (2, 18) = 0.2294, p = 0.79). These results support that propranolol pretreatment does not reduce escalation of self-administration of a non-drug reward.
FIGURE 5.
Propranolol does not reduce saccharin self-administration. Saccharin is an artificial sweetener that can be considered a natural or non-drug reward. In fact, our data show that rats will press levers to self-administer saccharin, evidence that saccharin is behaviourally reinforcing. (A) The left panel represents saccharin acquisition 30 min daily per 5 days. The open circles represent the pattern of saccharin self-administration in the control group that received saline 30 min before each saccharin self-administration session (60 min/day/9 days) (N = 14). The arrows indicate that propranolol (15 mg/kg, s.c) was administered 30 min before a saccharin self-administration session (grey filled squares, n = 7). There was a significant effect of time (RMANOVA F[5.731, 108.9] = 11.58, p < 0.0001) but not propranolol pretreatment on escalation of saccharin self-administration (F[1, 19] = 0.1493, p = 0.7035). (B) Arrows in the right panel indicate that rats received propranolol 30 min before each saccharin self-administration session (60 min/day/9 days). The open circles represent the control group that received saline during the escalation (Phase 1) and continued to receive saline in Phase 2 (N = 7). The open squares represent the group that received propranolol during the escalation (Phase 1) and saline in Phase 2 (N = 7). The grey-filled circles represent the group that received saline during the escalation (Phase 1) and were switched to propranolol in Phase 2 (N = 7). Propranolol pretreatment did not alter post-escalation or drug reversal of saccharin self-administration (RMANOVA F[2, 18] = 0.2294, p = 0.79). Results are expressed as mean ± SEM of number of saccharin self-administration rewards acquired by animals.
3.2 b. |. Propranolol effects on lever pressing for saccharin.
We compared active vs. inactive lever presses during saccharin self-administration to determine if rats could accurately discriminate between an active lever press resulting in liquid saccharin administration and an inactive lever press resulting in no saccharin administration. During phase 1 escalation (Figure 6A) and phase 2 post-escalation and drug reversal (Figure 6B) rats pressed the active lever significantly more than the inactive lever during both phase 1 (Mann-Whitney tests, Saline p < 0.000001 and Propranolol p = 0.0006) and phase 2 (Mann-Whitney test for each treatment group, Saline (phase 1 + 2) p = 0.0006, Saline phase 2 (propranolol phase 1) p = 0.0006, and Propranolol phase 2 (saline phase 1) p = 0.0006). These results support that the rats were able to discriminate between active and inactive levers and that saline and propranolol pretreatments did not alter that discrimination.
FIGURE 6.
Saccharin self-administration lever presses during Phase 1 escalation and Phase 2 post-escalation and drug reversal. Open circles represent presses on the active lever, and black circles represent presses on the inactive lever. (A) The left graph represents the escalation period (Phase 1); the saline group (N = 14) and the propranolol group (N = 7) show higher lever presses for the positive lever and then for the negative lever, which indicates a good lever discrimination. Propranolol pretreatment did not alter Phase 1 active or inactive lever presses (Mann–Whitney tests, saline p < 0.000001 and propranolol p = 0.0006). (B) The right graph represents the post-escalation period where drug treatment was reversed (Phase 2). The first group of columns represents the saline group (N = 7). The middle 2 columns represent the group that received propranolol in Phase 1 and was switched to saline in Phase 2 (N = 7). The next group of columns represents saline group from Phase 1 that was switch to propranolol in Phase 2 (N = 7). In all three groups, the lever presses for the positive lever were higher than negative lever presses, indicating a good discrimination of the two levers. Propranolol pretreatment did not alter active or inactive lever presses (Mann–Whitney test for each treatment group, saline [Phase 1 + 2] p = 0.0006, saline phase 2 [propranolol Phase 1] p = 0.0006 and propranolol Phase 2 [saline Phase 1] p = 0.0006). Results are expressed as mean ± SEM of number of lever presses per animal in Phases 1 and 2. Lever press type effect ***p = 0.0006, ****p < 0.000001
3.2 c. |. Propranolol treatment effects on the motivation for saccharin
We employed progressive ratio tests to get breakpoints to measure motivation of the rats to work for liquid saccharin. Propranolol pretreatment did not alter breakpoint for saccharin self-administration during phase 1 escalation (Figure 7A; unpaired t test, t (19) = 0.2884, p = 0.77) or phase 2 post-escalation and drug reversal (Figure 7B; Kruskal-Wallis test, p = 0.38). These results support that propranolol pretreatment did not alter motivation to work and consume liquid saccharin.
FIGURE 7.
Propranolol pretreatment does not alter breakpoint during progressive ratio self-administration tests of motivation to consume saccharin. (A) The left graph represents the first progressive ratio test, which occurred at the end of Phase 1. The saline group is indicated by opened circles (N = 14) and the propranolol group represented by grey squares (N = 7). Propranolol did not alter progressive ratio breakpoint by the end of Phase 1 escalation SA (unpaired t test, p = 0.77). (B) The right graph represents the progressive ratio test at the end of Phase 2 (post-escalation and drug reversal). The open circle represents the control group that received saline during the escalation (Phase 1) and continued to receive saline in Phase 2 (N = 7). The open squares represent the group that received propranolol during the escalation (Phase 1, grey column) and was switched to saline in Phase 2 (N = 7). The grey-filled circles represent the group that received saline during the escalation (Phase 1, white column) and was switched to propranolol in Phase 2 (N = 7). Propranolol did not alter progressive ratio breakpoint by the end of Phase 2 post-escalation and drug reversal whether animals were given ropranolol during Phase 1 or 2 (Kruskal–Wallis test, p = 0.38), supporting that propranolol does not reduce motivation to consume non-drug rewards. Results are expressed as mean ± SEM of number of saccharin SA acquired by animals.
3.3 |. Dose-response study for propranolol on cocaine and saccharin self-administration. (Experiments 3 and 4)
We tested for a dose-response effect of propranolol pretreatment using 3 doses (5, 15, and 40 mg/kg sc) prior to cocaine and saccharin self-administration (Figure 8A and 8B). We found that the highest dose (40 mg/kg) of propranolol administered as pretreatment reduced cocaine injections received by rats during self-administration (Figure 8A One-way ANOVA F (3, 28) = 5.057, p = 0.0063; Dunnett’s multiple comparisons test p = 0.002). Conversely, none of the propranolol doses had an effect on number of liquid saccharin rewards consumed by rats (Figure 8B One-way ANOVA F (3, 24) = 2.298, p = 0.103). Our dose-response data support that high dose propranolol is effective to reduce cocaine, but not saccharin rewards received by rats. Therefore, propranolol pretreatment may be useful to reduce drug consumption but not consumption of more natural/non-drug rewards.
FIGURE 8.
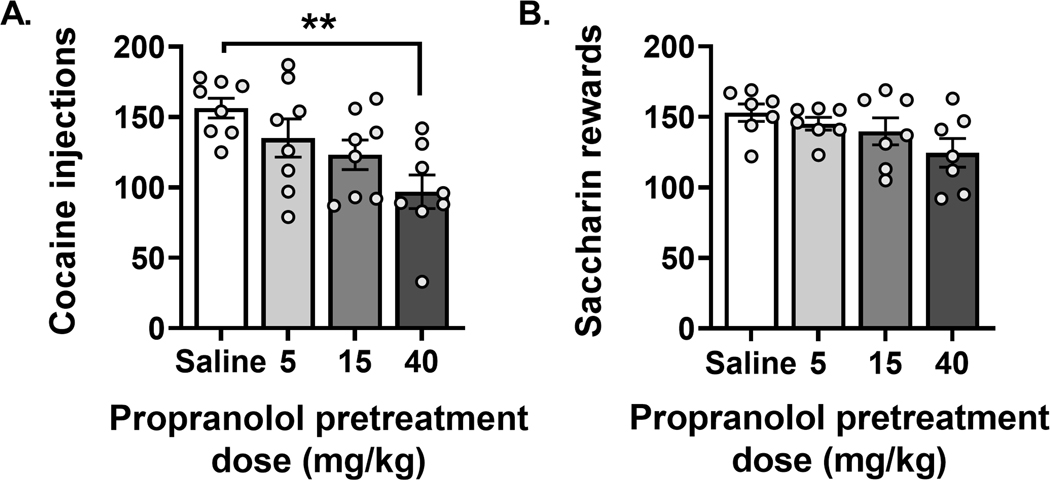
Propranolol pretreatment dose dependently reduced cocaine but not saccharin selfadministration. Three doses of ropranolol (5, 15 and 40 mg/kg) or saline were administered (sc) 30 min before self-administration session using a Latin square design procedure to determine which dose animals received. The doses were tested on rats that achieved a stable self-administration of cocaine or saccharin. High dose ropranolol (40 mg/kg) reduced cocaine self-administration A (N = 8, one-way ANOVA F[3, 28] = 5.057, p = 0.0063; Dunnett’s multiple comparisons test **p = 0.002) but had no effect on saccharin self-administration B (N = 7, one-way ANOVA F[3, 24] = 2.298, p = 0.103). Data are expressed as mean ± SEM of self-administration compared to control rats that received saline.
4 |. DISCUSSION
This study revealed the efficacy of chronic propranolol treatment in preventing escalation of cocaine intake (Figure 2) and reducing cocaine seeking post-escalation (Figure 4) in a model of extended access to cocaine self-administration in rats. Propranolol had no effect on lever discrimination during self-administration (Figure 3). Discontinuation of the propranolol treatment was not associated with a significant rebound increase in cocaine self-administration, and initiation of propranolol treatment after establishment of cocaine escalation was established and maintained during phase 2 had no effect on cocaine intake and seeking. Dose-response curves for propranolol pretreatment showed reduced cocaine self-administration with increasing propranolol dose, with only the highest dose (40 mg/kg propranolol) reaching significance (Figure 8). All effects of the propranolol pretreatment were selective for cocaine and were not reproduced when using saccharin as a non-drug natural reward for self-administration. Overall, the main conclusions of the paper were not changed after analyzing sex-specific effects (see supplementary materials and Fig. S1, S2, S3, S4, S5 for details).
Propranolol was effective in reducing cocaine intake during escalation and post-escalation; however, the dose-response post-escalation suggests that animals may require higher doses of propranolol to obtain the same decreasing effect on cocaine intake. Indeed, while the 15 mg/kg dose reduced intake by ~20%, only the higher dose (40 mg/kg) significantly reduced cocaine intake. This possible decreased potency may be due to low statistical power to detect the effect or could be due to neuroadaptations in the norepinephrine system. For instance, escalation of cocaine self-administration may lead to increased norepinephrine levels which may compete with propranolol and reduce its potency. Moreover, chronic cocaine exposure may lead to upregulation31, 32, 33 or downregulation34, 35 of norepinephrine receptors depending on the exposure level and brain regions which may further explain the decreased potency of propranolol post-escalation (Fig. S6).
Since propranolol is a beta blocker that reduces physiological symptoms related to anxiety36 it is possible that propranolol also reduces some of the negative emotional states experienced during withdrawal therefore reducing the motivation to take cocaine. Indeed, animals with greater anxiety-like behavior also show greater cocaine reward39 and motivation for self-administering cocaine38, 39. Further studies are needed to test whether the effect of propranolol in decreasing escalation of cocaine intake is mediated via a decrease in anxiety-like behaviors or other mechanisms.
The model of extended access to cocaine is known to produce a robust increase in self-administration, which has face validity for compulsive drug intake in humans21,14. In the present study, we demonstrate that treatment with the β-AR antagonist propranolol before cocaine self-administration sessions can prevent escalation of cocaine intake (phase 1)21. Propranolol pre-treatment after the escalation was established and stabilized (Phase 2) did not affect cocaine self-administration, indicating that propranolol effects are restricted to the period when escalation occurs. Escalation of drug self-administration leads to drug dependence40 and perhaps once a rat is dependent on cocaine from phase 1 escalation of intake, then their intake can no longer be influenced by pretreatment of propranolol during phase 2. However, propranolol does reduce cocaine self-administration when administered as pretreatment during phase 1 which results in lower cocaine intake and blunted/attenuation of escalation of cocaine self-administration which may be possible due to the rat not yet being dependent on cocaine. Indeed, previous work has shown that propranolol reduces cocaine self-administration in rats19 and squirrel monkeys20. In those studies, however, acute propranolol pretreatment was performed only after the establishment of stable intake with short access to cocaine, which was 3h/day for rats19 and 100 min/day for squirrel monkeys20. Short access to cocaine, in rats, is known to result in stable cocaine self-administration with no escalated pattern20, 21. Furthermore, it is known that motivation for cocaine is increased after escalated self-administration during extended access to cocaine41,14. In contrast to these observed reductions in cocaine intake after establishment of stable self-administration in the models of short access to cocaine, chronic propranolol treatment had no effect on cocaine intake after escalation and stabilization of self-administration with our model of long access to cocaine. This is important as it plays a role during the period when the switch between controlled to uncontrolled use is thought to occur (escalation period). In another study, pretreatment with disulfiram (an inhibitor of dopamine β-hydroxylase, the enzyme that converts dopamine into norepinephrine) 2 h before cocaine self-administration sessions had no effect on maintenance responding for cocaine. In this experiment the treatment was acute after a stabilized 2 h daily cocaine self-administration42, 43. In conditions where we can suppose no dependence for cocaine because of the short access to the drug, acute propranolol can reduce self-administration. Since the propranolol pretreatment was given subcutaneously it is likely causing both central and peripheral effects to reduce motivation and intake during cocaine self-administration. Further studies using beta blockers that do not cross the blood-brain barrier should be performed to test this hypothesis.
Propranolol pretreatment during the escalation phase (Figure 1A), which is known to increase the motivation for cocaine, was sufficient to reduce the motivation for cocaine even 10 days after the treatment, indicating that propranolol might have sustained efficacy or that it prevents the development of motivated cocaine seeking behavior. Wee et al14 previously looked at effects of norepinephrine on motivation for cocaine. In that study, adrenergic receptor antagonists were administered before testing motivation for cocaine in a model of extended access to cocaine. They showed that prazosin (α1 receptor antagonist) but not betaxolol (β1-noradrenergic receptor antagonist) or UK14304 (α2-noradrenergic receptor agonist) reduced the breakpoint for cocaine. The selective dopamine β-hydroxylase inhibitor nepicastat, the enzyme that converts dopamine (DA) to norepinephrine (NE) was also shown to reduce the breakpoint for cocaine after a stable 2h daily cocaine self-administration43. Fewer activated α-adrenergic receptors and lower concentration of synthesized NE are both conditions that reduce motivation to consume cocaine. A study comparing the effects of β1 and β2-AR antagonists and agonists demonstrated that injection into the ventral Bed Nucleus of the Stria Terminalis (BNST) with a β2-AR specific antagonist ICI-118,551 prevented footshock-induced reinstatement of cocaine seeking while a β2-AR specific agonist clenbuterol induced reinstatement of cocaine seeking.44 β2-ARs have also been shown as important for stress induced reinstatement of cocaine conditioned place preference behavior in mice, where β2-AR antagonism by ICI-118,551 (ip) prevents reinstatement of cocaine conditioned place preference after extinction of that behavior.19,45 This suggests that the β2-AR antagonism by the nonspecific β-AR antagonist propranolol may be responsible for the propranolol reduction in escalation of cocaine self-administration that we observed. Another possible mechanism to explain the effect of propranolol on cocaine intake is if propranolol increases the half-life of cocaine. While we are not aware of any study reporting increased half-life of cocaine after propranolol treatment and it is unlikely that propranolol altered cocaine metabolism since cocaine is metabolized by a different set of enzymes in the liver (carboxylesterase type 1 (hCE1), carboxylesterase type 2 (hCE2), and cytochrome P450 enzymes)46, 47, further studies should be performed to evaluate the effect of chronic propranolol treatment on the metabolism of cocaine.
Our experiments have construct validity since the rats are capable of learning which lever is associated with a drug that reinforces their lever pressing behavior. The observed effects are unlikely to be attributed to a nonspecific effect of propranolol on learning and discrimination of the operandum as rats pretreated with propranolol could discriminate between the active and inactive levers and because propranolol didn’t affect responding for the natural reward, under both a fixed ratio and progressive ratio schedule of reinforcement. These results are consistent with previous work showing that manipulation of the norepinephrine system has no effects on natural rewards. For instance, transgenic mice lacking dopamine beta-hydroxylase (DBH), the enzyme responsible for synthesizing norepinephrine (NE), showed a lack of reward behavior for cocaine in a conditioned place preference (CPP) paradigm, without affecting the preference for food36 or reward for food in squirrel monkeys20. Also, pretreatment with the DBH inhibitor nepicastat had no effect on food self-administration35.
Dose-response curves of propranolol pretreatment showed that only the highest dose (40 mg/kg propranolol) reduced cocaine self-administration in rats after stabilization of escalation of cocaine intake (Figure 8A). None of the doses of propranolol changed saccharin self-administration although a trend for a decrease was observed at the highest dose (Figure 8B). The fact that a higher dose of propranolol (40 mg/kg) was required to decrease cocaine intake after stabilization of cocaine intake compared to during the escalation of intake (15 mg/kg) suggests a decreased involvement of the beta-adrenergic system once stabilization of escalation has occurred. Moreover, previous studies showed that lower doses of propranolol (5 and 10 mg/kg, ip) reduces cocaine self-administration in a short access model19 and in a cocaine vs. food choice procedure in a short access model48 further suggesting that propranolol may have higher efficacy in reducing cocaine self-administration in rats with limited access to cocaine and before escalation of cocaine. The latter study also showed that propranolol increased breaking point for cocaine while we observed a decrease in breaking point in rats given extended access to cocaine, also suggesting that the role of beta-adrenergic system may differ in rats with limited vs. extended access to cocaine.
There is some evidence that administering beta blockers, such as propranolol, to humans who have used cocaine or other psychostimulants can cause increases in vasoconstriction and blood pressure due to unopposed alpha adrenoceptor activation49. This is an important concern, due to increased risk of cardiovascular issues and death from using propranolol to treat humans who use cocaine and other psychostimulants, but unopposed alpha effects are also rarely occurring or at least rarely reported and therefore may be important to consider, but not common enough to exclude the usage of propranolol treatments in all patients who use cocaine49. However, those cocaine users with higher heart rates and blood pressure may have less risk of worsening cardiovascular function and death by using treatment drugs that are mixed alpha and beta adrenoceptor antagonists such as labetalol or carvedilol49. Additionally, it may be safest to use propranolol treatment for humans who are actively participating in a treatment program with a good success rate of preventing relapse. Propranolol has been shown to effectively reduce cocaine use in humans, especially those experiencing severe withdrawal50. Overall, our results demonstrate that beta blockers prevent escalation of cocaine intake and reduce motivation for cocaine in rats given extended access to cocaine, this effect is dose-dependent, but limited in rats with a previous history of escalation of cocaine intake.
Supplementary Material
ACKNOWLEDGEMENT
This work was funded by the NIH (U01DA043799), the Preclinical Addiction Research Consortium, the Dutch Research Council (NOW: 019-163LW.011), and the NIAAA TSRI T32 Postdoctoral Fellowship: 5T32AA007456.
REFERENCES
- 1.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004. Aug;61(8):807–16. doi: 10.1001/archpsyc.61.8.807. PMID: 15289279. [DOI] [PubMed] [Google Scholar]
- 2.Swendsen J, Le Moal M. Individual vulnerability to addiction. Ann N Y Acad Sci. 2011. Jan;1216:73–85. doi: 10.1111/j.1749-6632.2010.05894.x. PMID: 21272012. [DOI] [PubMed] [Google Scholar]
- 3.George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010. Nov;35(2):232–47. doi: 10.1016/j.neubiorev.2010.05.002. Epub 2010 May 20. PMID: 20493211; PMCID: PMC2955797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady KT, Haynes LF, Hartwell KJ, Killeen TK. Substance use disorders and anxiety: a treatment challenge for social workers. Soc Work Public Health. 2013;28(3–4):407–423. Doi: 10.1080/19371918.2013.774675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koob GF. The dark side of emotion: the addiction perspective. Eur J Pharmacol. 2015. Apr 15;753:73–87. doi: 10.1016/j.ejphar.2014.11.044. Epub 2015 Jan 9. PMID: 25583178; PMCID: PMC4380644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, Price LH. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatry. 1994. Sep;51(9):713–9. doi: 10.1001/archpsyc.1994.03950090045007. PMID: 8080348. [DOI] [PubMed] [Google Scholar]
- 7.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007. Aug;164(8):1149–59. doi: 10.1176/appi.ajp.2007.05030503. PMID: 17671276; PMCID: PMC2837343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fitzgerald PJ. Elevated Norepinephrine may be a Unifying Etiological Factor in the Abuse of a Broad Range of Substances: Alcohol, Nicotine, Marijuana, Heroin, Cocaine, and Caffeine. Subst Abuse. 2013. Oct 13;7:171–83. doi: 10.4137/SART.S13019. PMID: 24151426; PMCID: PMC3798293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sofuoglu M, Kosten TR. Novel approaches to the treatment of cocaine addiction. CNS Drugs. 2005;19(1):13–25. doi: 10.2165/00023210-200519010-00002. PMID: 15651902. [DOI] [PubMed] [Google Scholar]
- 10. https://www.nimh.nih.gov/health/topics/anxiety-disorders .
- 11.Emilien G, Maloteaux JM. Current therapeutic uses and potential of beta-adrenoceptor agonists and antagonists. Eur J Clin Pharmacol. 1998. Feb;53(6):389–404. Doi: 10.1007/s002280050399. PMID: 9551698. [DOI] [PubMed] [Google Scholar]
- 12.Farzam K, Kidron A, Lakhkar AD. Adrenergic Drugs. [Updated 2022 Jul 3]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534230/ [PubMed] [Google Scholar]
- 13.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002. Jul 1;22(13):5713–8. doi: 10.1523/JNEUROSCI.22-13-05713.2002. PMID: 12097523; PMCID: PMC6758192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wee S, Mandyam CD, Lekic DM, Koob GF. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008. Apr;18(4):303–11. doi: 10.1016/j.euroneuro.2007.08.003. Epub 2007 Oct 24. PMID: 17920248; PMCID: PMC2376122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000. Aug;23(2):138–50. Doi: 10.1016/S0893-133X(99)00158-X. PMID: 10882840. [DOI] [PubMed] [Google Scholar]
- 16.Koob GF. A role for brain stress systems in addiction. Neuron. 2008. Jul 10;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. PMID: 18614026; PMCID: PMC2748830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernardi RE, Lattal KM. Post-conditioning propranolol disrupts cocaine sensitization. Pharmacology, Biochemistry, and Behavior. 2012. Oct;102(4):515–519. DOI: 10.1016/j.pbb.2012.06.015. PMID: 22750295; PMCID: PMC3423590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Huang S, Yang C, Wu F, Zheng Q, Yan H, Yan J, Luo Y, Galaj E. Blockade of β-Adrenergic Receptors by Propranolol Disrupts Reconsolidation of Drug Memory and Attenuates Heroin Seeking. Front Pharmacol. 2021. May 25;12:686845. Doi: 10.3389/fphar.2021.686845. PMID: 34113256; PMCID: PMC8185332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris GC, Hedaya MA, Pan WJ, Kalivas P. beta-adrenergic antagonism alters the behavioural and neurochemical responses to cocaine. Neuropsychopharmacology. 1996. Mar;14(3):195–204. Doi: 10.1016/0893-133X(95)00089-V. PMID: 8866703. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg SR, and Gonzalez FA (1976). Effects of propranolol on behavior maintained under fixed-ratio schedules of cocaine injection or food presentation in squirrel monkeys. J. Pharmacol. Exp. Ther 198, 626–634. [PubMed] [Google Scholar]
- 21.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998. Oct 9;282(5387):298–300. Doi: 10.1126/science.282.5387.298. PMID: 9765157. [DOI] [PubMed] [Google Scholar]
- 22.Kitamura O, Wee S, Specio SE, Koob GF, and Pulvirenti L. (2006). Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology 186, 48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- 23.de Guglielmo G, Kallupi M, Sedighim S, Newman AH, George O. Dopamine D3 Receptor Antagonism Reverses the Escalation of Oxycodone Self-administration and Decreases Withdrawal-Induced Hyperalgesia and Irritability-Like Behavior in Oxycodone-Dependent Heterogeneous Stock Rats. Front Behav Neurosci. 2020. Jan 14;13:292. doi: 10.3389/fnbeh.2019.00292. PMID: 31992976; PMCID: PMC6971096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedighim Sharona, Tieu Lani, George Olivier 2020. Intravenous Jugular Catheterization for Rats. protocols.io [Google Scholar]
- 25.Kallupi M, Scuppa G, de Guglielmo G, Calò G, Weiss F, Statnick MA, et al. (2017). Genetic Deletion of the Nociceptin/Orphanin FQ Receptor in the Rat Confers Resilience to the Development of Drug Addiction. Neuropsychopharmacology 42, 695–706. doi: 10.1038/npp.2016.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asdaq SM, Inamdar MN. Pharmacodynamic and Pharmacokinetic Interactions of Propranolol with Garlic (Allium sativum) in Rats. Evid Based Complement Alternat Med. 2011;2011:824042. Doi: 10.1093/ecam/neq076. Epub 2011 Jun 16. PMID: 21792365; PMCID: PMC3137651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson NR, and Roberts DC (1996). Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. Journal of Neuroscience Methods 66, 1–11. [DOI] [PubMed] [Google Scholar]
- 28.De Guglielmo G, Melis M, De Luca MA, Kallupi M, Li HW, Niswender K, et al. (2015). PPARγ activation attenuates opioid consumption and modulates mesolimbic dopamine transmission. Neuropsychopharmacology 40, 927–937. Doi: 10.1038/npp.2014.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenton L, Boon F, and Cain DP (2008). Combined but not individual administration of beta-adrenergic and serotonergic antagonists impairs water maze acquisition in the rat. Neuropsychopharmacology 33, 1298–1311. doi: 10.1038/sj.npp.1301518. [DOI] [PubMed] [Google Scholar]
- 30.Robinson MJF, Ross EC, and Franklin KBJ (2011). The effect of propranolol dose and novelty of the reactivation procedure on the reconsolidation of a morphine place preference. Behav. Brain Res 216, 281–284. doi: 10.1016/j.bbr.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee SP, Sharma VK, Kung-Cheung LS, Chanda SK, Riggi SJ. Cocaine and D-amphetamine induce changes in central beta-adrenoceptor sensitivity: effects of acute and chronic drug treatment. Brain Res. 1979. Oct 12;175(1):119–30. doi: 10.1016/0006-8993(79)90518-3. PMID: 226224. [DOI] [PubMed] [Google Scholar]
- 32.Seidler FJ, Slotkin TA. Fetal cocaine exposure causes persistent noradrenergic hyperactivity in rat brain regions: effects on neurotransmitter turnover and receptors. J Pharmacol Exp Ther. 1992. Nov;263(2):413–21. PMID: 1331397. [PubMed] [Google Scholar]
- 33.Henderson MG, McConnaughey MM, McMillen BA. Long-term consequences of prenatal exposure to cocaine or related drugs: effects on rat brain monoaminergic receptors. Brain Res Bull. 1991. Jun;26(6):941–5. doi: 10.1016/0361-9230(91)90261-h. PMID: 1657320. [DOI] [PubMed] [Google Scholar]
- 34.Lidow MS, Trakht T, Howard RL. Cocaine-induced alterations in the density of monoaminergic receptors in the embryonic guinea pig cerebral wall. Synapse. 1999. Jun 1;32(3):225–37. doi: . PMID: 10340632. [DOI] [PubMed] [Google Scholar]
- 35.Farfel GM, Kleven MS, Woolverton WL, Seiden LS, Perry BD. Effects of repeated injections of cocaine on catecholamine receptor binding sites, dopamine transporter binding sites and behavior in rhesus monkey. Brain Res. 1992. Apr 24;578(1–2):235–43. doi: 10.1016/0006-8993(92)90252-5. PMID: 1380862. [DOI] [PubMed] [Google Scholar]
- 36.Srinivasan AV. Propranolol: A 50-Year Historical Perspective. Ann Indian Acad Neurol. 2019. Jan-Mar;22(1):21–26. doi: 10.4103/aian.AIAN_201_18. PMID: 30692755; PMCID: PMC6327687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelloux Y, Costentin J, Duterte-Boucher D. Anxiety increases the place conditioning induced by cocaine in rats. Behav Brain Res. 2009. Feb 11;197(2):311–6. doi: 10.1016/j.bbr.2008.08.029. Epub 2008 Aug 29. PMID: 18793676. [DOI] [PubMed] [Google Scholar]
- 38.Homberg JR, van den Akker M, Raasø HS, Wardeh G, Binnekade R, Schoffelmeer AN, de Vries TJ. Enhanced motivation to self-administer cocaine is predicted by self-grooming behaviour and relates to dopamine release in the rat medial prefrontal cortex and amygdala. Eur J Neurosci. 2002. May;15(9):1542–50. doi: 10.1046/j.1460-9568.2002.01976.x. PMID: 12028365. [DOI] [PubMed] [Google Scholar]
- 39.Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ, Dalley JW, Belin D. High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology (Berl). 2012. Jul;222(1):89–97. doi: 10.1007/s00213-011-2626-4. Epub 2012 Jan 14. PMID: 22245944. [DOI] [PubMed] [Google Scholar]
- 40.Edwards and Koob, 2013 BehavPharm Edwards S, Koob GF. Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol. 2013. Sep;24(5–6):356–62. doi: 10.1097/FBP.0b013e3283644d15. PMID: 23839030; PMCID: PMC3866817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paterson NE, and Markou A. (2003). Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport 14, 2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- 42.Schroeder JP, Cooper DA, Schank JR, Lyle MA, Gaval-Cruz M, Ogbonmwan YE, Pozdeyev N, Freeman KG, Iuvone PM, Edwards GL, Holmes PV, Weinshenker D. Disulfiram attenuates drug-primed reinstatement of cocaine seeking via inhibition of dopamine β-hydroxylase. Neuropsychopharmacology. 2010. Nov;35(12):2440–9. doi: 10.1038/npp.2010.127. Epub 2010 Aug 25. PMID: 20736996; PMCID: PMC2956132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder JP, Epps SA, Grice TW, and Weinshenker D. (2013). The selective dopamine β-hydroxylase inhibitor nepicastat attenuates multiple aspects of cocaine-seeking behavior. Neuropsychopharmacology 38, 1032–1038. doi: 10.1038/npp.2012.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961. Sep 29;134(3483):943–4. doi: 10.1126/science.134.3483.943. PMID: 13714876. [DOI] [PubMed] [Google Scholar]
- 45.Picetti R, Ho A, Butelman ER, and Kreek MJ (2010). Dose preference and dose escalation in extended-access cocaine self-administration in Fischer and Lewis rats. Psychopharmacology 211, 313–323. doi: 10.1007/s00213-010-1899-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.Propranolol. [Updated 2017 Jan 15]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548218/# [PubMed] [Google Scholar]
- 47.Jastrzębska J, Daniel WA. Cocaine-Induced Time-Dependent Alterations in Cytochrome P450 and Liver Function. Int J Mol Sci. 2023. Jan 13;24(2):1632. doi: 10.3390/ijms24021632. PMID: 36675146; PMCID: PMC9866935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry AN, Westenbroek C, Jagannathan L, Becker JB. The Roles of Dopamine and α1-Adrenergic Receptors in Cocaine Preferences in Female and Male Rats. Neuropsychopharmacology. 2015. Nov;40(12):2696–704. Doi: 10.1038/npp.2015.116. Epub 2015 Apr 22. PMID: 25900120; PMCID: PMC4864645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards JR, Hollander JE, Ramoska EA, Fareed FN, Sand IC, Izquierdo Gómez MM, Lange RA. β-Blockers, Cocaine, and the Unopposed α-Stimulation Phenomenon. J Cardiovasc Pharmacol Ther. 2017. May;22(3):239–249. doi: 10.1177/1074248416681644. Epub 2016 Dec 14. PMID: 28399647. [DOI] [PubMed] [Google Scholar]
- 50.Kampman KM. New medications for the treatment of cocaine dependence. Psychiatry (Edgmont). 2005. Dec;2(12):44–8. PMID: 21120115; PMCID: PMC2994240. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


