Significance
Transition-metal oxides are cost-effective and earth-abundant electrocatalysts for economically alkaline polymer electrolyte fuel cells and alkaline electrolysers. For transition-metal oxides, they usually exhibit different magnetic states, namely ferromagnetic (FM) ordered state, antiferromagnetic (AFM) ordered state, paramagnetic state, etc. The intrinsic connection between the magnetic state and performance of transition-metal oxides remains an open question. In this work, we show that FM ordering of transition-metal oxides is closely related with their metal–oxygen hybridization, a property that is considered to govern the performance of transition-metal oxides. Our work bridges the gap between the classic metal–oxygen hybridization theory and understanding of oxides from spin/magnetism perspective and helps transition magnetic oxides to break through their activity limits.
Keywords: electrocatalysis, magnetic ordering, metal–oxygen hybridization
Abstract
The efficiency of transition-metal oxide materials toward oxygen-related electrochemical reactions is classically controlled by metal–oxygen hybridization. Recently, the unique magnetic exchange interactions in transition-metal oxides are proposed to facilitate charge transfer and reduce activation barrier in electrochemical reactions. Such spin/magnetism-related effects offer a new and rich playground to engineer oxide electrocatalysts, but their connection with the classical metal–oxygen hybridization theory remains an open question. Here, using the MnxVyOz family as a platform, we show that ferromagnetic (FM) ordering is intrinsically correlated with the strong manganese (Mn)–oxygen (O) hybridization of Mn oxides, thus significantly increasing the oxygen reduction reaction (ORR) activity. We demonstrate that this enhanced Mn–O hybridization in FM Mn oxides is closely associated with the generation of active Mn sites on the oxide surface and obtaining favorable reaction thermodynamics under operating conditions. As a result, FM-Mn2V2O7 with a high degree of Mn–O hybridization achieves a record high ORR activity. Our work highlights the potential applications of magnetic oxide materials with strong metal–oxygen hybridization in energy devices.
Transition-metal oxides (1) have received intensive attention from both academia and industry because of their great potential as electrocatalysts for oxygen-related reactions (2) in economically alkaline polymer electrolyte fuel cells (3) and alkaline electrolysers (4). In the past decades, numerous investigations have been devoted to improving the performance of oxide electrocatalysts (5, 6) through nanostructuring (7, 8), defect introducing (9, 10), doping (11, 12), and/or phase transition (13, 14). Despite great progresses, current performance of oxide electrocatalysts has entered the bottleneck stage, and little room is left for further improvement through material/structure engineering.
Magnetic and spin effects hold promise for advancing oxygen electrocatalysis (15–26). It has been experimentally proved that the introduction of magnetic field can improve the catalytic efficiency by promoting gas/liquid diffusion (27). Besides, it has recently been revealed that the intrinsic activity of electrocatalysts is closely related to their internal spin polarization and spin/magnetic interactions (15, 28). Specifically, an electrocatalyst with sufficient spin polarization is able to donate/accept electrons with appropriate electrons’ spin (e-spin) from/to the reaction intermediates to balance the spin of oxygen molecules with a ground triplet state, and thus accelerating oxygen electrocatalysis by allowing spin conservation throughout the reaction (15, 16, 19, 20, 29, 30). For example, chiral molecules and biologically active centers ingeniously build spin-selective channels to exact e-spins with the “correct” orientation, resulting in ultrahigh reaction turnover frequencies (16, 17). Therefore, transition-metal oxides with intrinsic magnetic ordering are highly expected to achieve spin selectivity through unique magnetic exchange interactions (18–20).
On the other hand, during the past decades, intensive research efforts have been paid to explore electronic structure descriptors [eg filling (31, 32), p-band centre (33, 34), metal–oxygen hybridization (35–37), etc.] to provide a guideline for the design of highly active oxide materials. In particular, metal–oxygen hybridization (1, 38), which can well describe the charge transfer between active metal sites and oxygen intermediates, has been proved to govern the activity of oxide electrocatalysts. In view of recent advances in spin-enhanced oxygen electrocatalysis, revealing possible correlation among magnetic ordering, metal–oxygen hybridization, and resulting changes in electrochemical property of magnetic oxides will undoubtedly deepen the understanding of oxygen-related reactions; however, this remains largely unexplored.
Herein, using MnxVyOz family as a platform, we probe the intrinsic correlation among magnetic ordering, metal–oxygen hybridization, and oxygen reduction reaction (ORR) activity. We quantify the same Mn oxides with ferromagnetic (FM) and antiferromagnetic (AFM) orderings and show that FM ordering greatly enhances the manganese (Mn)–oxygen (O) hybridization, which accelerates the electron transfer between surface Mn sites and adsorbed oxygen species in the electrolyte, thereby facilitating the evolution of surface Mn ions to active Mn3+ species during the reaction, evidenced by quantitative cyclic voltammetry (CV), in situ X-ray absorption near edge structure (XANES), and in situ ultraviolet–visible (UV–Vis) spectroscopy. Moreover, we reveal that the enhanced Mn–O hybridization enables Mn oxide with FM ordering to achieve a favorable reaction path under working conditions, demonstrated by density functional theory (DFT) calculations. As a consequence, Mn2V2O7 with FM ordering affords a superior high ORR activity, outperforming the state-of-the-art Pt/C catalyst. More importantly, Mn2V2O7 exhibits a positive magnetic response under an external magnetic field.
Results
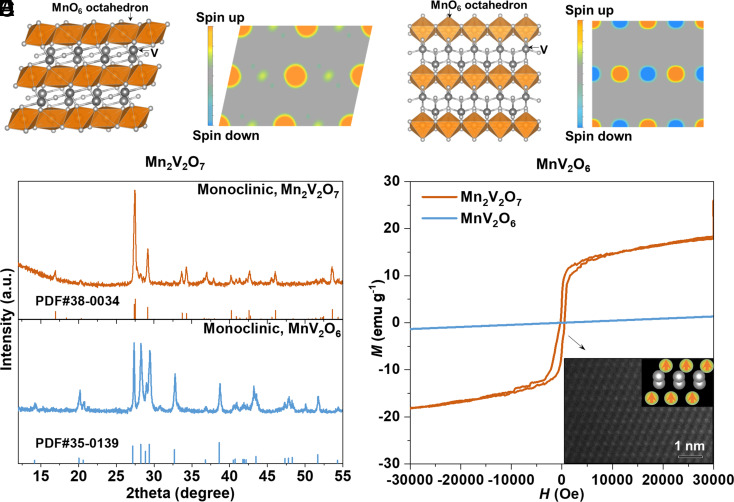
Magnetic Orderings of Mn2V2O7 and MnV2O6.
Manganese-based oxides are one of the most active materials towards ORR (39). In this work, we investigated the MnxVyOz family, in which edge-sharing magnetic [MnO6] octahedron layers with identical Mn2+ magnetic moment of 4.58 μB are separated by the nonmagnetic [VOn] layers. Mn2V2O7 and MnV2O6 are two typical oxides in MnxVyOz family with C2/m space group (Fig. 1 A and C), exhibiting FM and AFM orderings, respectively. That is, the e-spins of adjacent Mn ions in each [MnO6] layer of Mn2V2O7 and MnV2O6 are in the same and opposite directions, respectively (Fig. 1 B and D).
Fig. 1.
Magnetic structure of Mn2V2O7 and MnV2O6. (A) Crystal structure of Mn2V2O7. (B) Spin distribution map of Mn2V2O7 (010) plane. (C) Crystal structure of MnV2O6. (D) Spin distribution map of MnV2O6 (100) plane. (E) XRD patterns of Mn2V2O7 and MnV2O6. (F) Magnetic hysteresis loops of Mn2V2O7 and MnV2O6, with the Inset showing the atomic-scale high-angle annular dark-field scanning transition electron microscopic image of Mn2V2O7.
We experimentally investigated the magnetic properties of Mn2V2O7 and MnV2O6 using vibrating sample magnetometer. Note that these two oxides were fabricated by cation exchange method (40) using the same sacrificial template to make sure that they possess similar morphology and surface area (Fig. 1E and SI Appendix, Figs. S1–S4). As-fabricated Mn2V2O7 shows an alternating Mn and V atomic layer structure (Fig. 1 F, Inset). Moreover, as illustrated in Fig.1F, Mn2V2O7 exhibits obvious hysteresis in the range of ±10,000 Oe with a saturation magnetization of ~18.3 emu g−1, confirming its long-range FM ordering of Mn2V2O7, while MnV2O6 shows a typical AFM behaviour with a tiny magnetic susceptibility (~4.4 × 10−5 emu g−1 Oe−1).
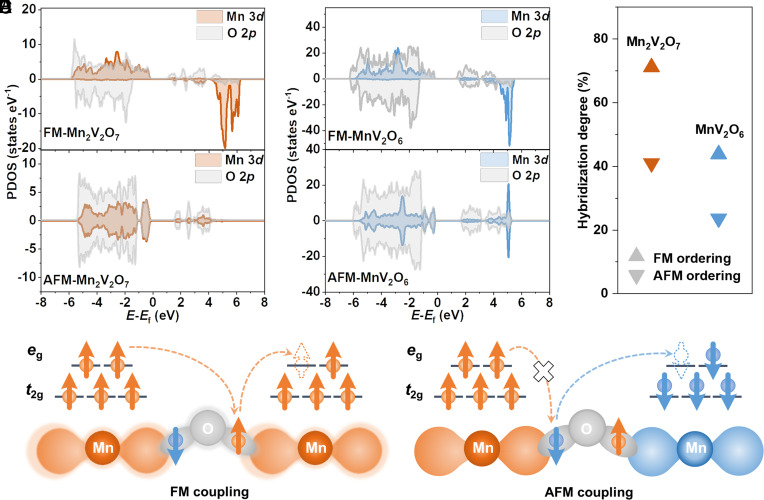
Correlation between Magnetic Ordering and Mn–O Hybridization.
To better understand the relationship between magnetic ordering and Mn–O hybridization, we calculated the electronic states of Mn-3d and O-2p of Mn2V2O7 and MnV2O6 with both FM and AFM orderings (SI Appendix, Fig. S5). As shown in Fig. 2A, the projected Mn-3d orbitals of FM-Mn2V2O7 are highly spin-polarized, where all the occupied states are in the same spin channel. More importantly, a strong hybridization between the Mn-3d and O-2p orbitals is observed near the Fermi level. In contrast, for AFM-Mn2V2O7, the Mn-3d states in valence and conduction bands are symmetrically distributed. A similar case is also observed in MnV2O6 with FM and AFM orderings (Fig. 2B).
Fig. 2.
Correlation between magnetic ordering and metal–oxygen hybridization. (A and B) PDOS of Mn2V2O7 and MnV2O6 with FM and AFM orderings, respectively. (C) Hybridization degrees of Mn2V2O7 and MnV2O6 with FM and AFM orderings. (D and E) Schematic diagrams of FM and AFM interactions, respectively.
Moreover, we quantified the hybridization degree of Mn-3d and O-2p orbitals in Mn2V2O7 and MnV2O6 by analysing their projected density of states (PDOS, SI Appendix, Figs. S6 and S7). Since highly spin-polarized states are observed in manganese oxides with FM ordering–the electronic states near the Fermi level are mainly contributed by Mn-3d and O-2p in spin-up channel; here, we only considered the electronic states in the spin-up channel to quantify metal–oxygen hybridization (41, 42). Specifically, the hybridization degree between Mn-3d and O-2p orbital is defined as: (38, 43), where and are the integration of the overlapping O-2p with Mn-3d states and the total O-2p states, respectively, in the spin-up channel from –6 to 0 eV. Note that both FM and AFM ordering were considered for the same oxide to focus on the effect of magnetic ordering on metal–oxygen hybridization and to exclude the influence of other structural factors (1, 32), such as metal valence state, crystal structure, etc. Impressively, the hybridization degree of Mn-3d and O-2p orbitals in Mn2V2O7 increases from ~41% for AFM to ~71% for FM ordering (Fig. 2C). A similar enhancement of Mn–O orbital hybridization is also found in MnV2O6 from AFM to FM ordering (Fig. 2C). These collective results reveal a clear correlation between magnetic ordering (FM/AFM) and Mn–O hybridization, which can explain that FM exchange interaction in Mn oxides promotes delocalization of parallel spins in the Mn–O bonds and facilitates easy electron transition between interconnected Mn and O ions (SI Appendix, Figs. S6 and S7 and Table S1), thus enhancing Mn–O hybridization, and vice versa (19, 22, 38, 44, 45) (Fig. 2D). In contrast, AFM exchange interaction in Mn oxides inhibits electron transition between interconnected Mn and O ions, thereby reducing Mn–O hybridization, and vice versa (Fig. 2E).
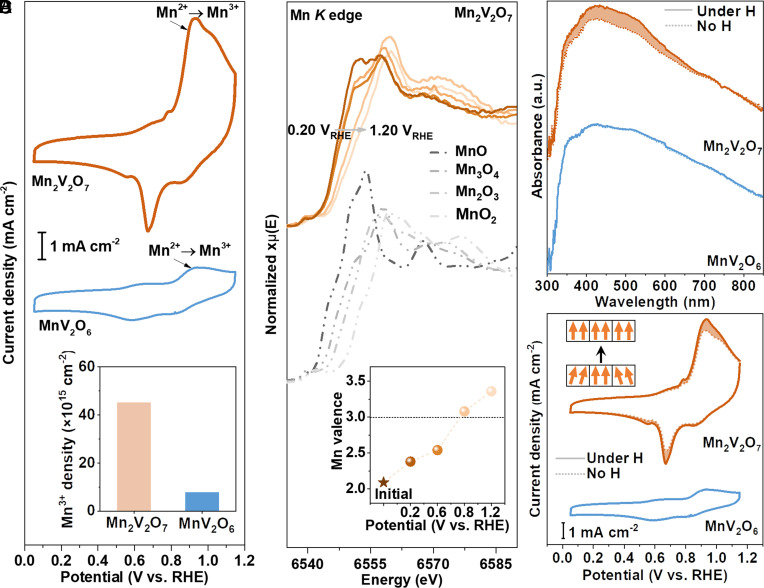
Monitoring In Situ Evolution of Mn Sites.
It has been recently revealed that the metal sites in most transition-metal oxides undergo dynamical changes, and the newly formed metal sites induced by the applied voltage and electrolyte serve as the “real” active sites in electrochemical reactions (46, 47). It is expected that the strong metal–oxygen hybridization in FM oxides will enable the metal sites to rapidly donate/accept electrons from/to adsorbed oxygen species in the electrolyte, thereby facilitating the formation of active metal sites in electrochemical reactions (31, 36, 48, 49). Here, we monitored the dynamic changes of Mn sites in fabricated Mn2V2O7 and MnV2O6 by CV in argon-saturated 1.0 M KOH at potentials from 0 to 1.20 V versus reversible hydrogen electrode (RHE). It is worth noticing that the experimentally fabricated Mn2V2O7 and MnV2O6 afford FM and AFM orderings, respectively, as aforementioned (Fig. 1F). As illustrated in Fig. 3A, the two oxides show quite different redox characteristics, although they exhibit the same initial Mn valence states of +2. More specifically, the anodic and cathodic peak intensities of FM-Mn2V2O7 are much higher than those of AFM-MnV2O6, especially the anodic Mn2+→Mn3+ peak at 0.92 VRHE. This result indicates a greatly enhanced transformation of Mn2+ to Mn3+ on the surface of FM-Mn2V2O7 as the applied anodic potential increases.
Fig. 3.
In situ evolution of Mn sites on Mn2V2O7 and MnV2O6. (A) CV curves of Mn2V2O7 and MnV2O6 in Ar-saturated 1.0 M KOH at a scan rate of 50 mV s−1, with the Inset showing Mn3+ site densities of Mn2V2O7 and MnV2O6 at ~1.20 VRHE. (B) In situ XANES spectra of Mn2V2O7 at different potentials, with the Inset showing the corresponding Mn valences. (C) In situ UV–Vis spectra of Mn2V2O7 at 0.9 VRHE and MnV2O6 at 1.0 VRHE under magnetic field (H) and with no H. (D) CV curves of Mn2V2O7 and MnV2O6 in Ar-saturated 1.0 M KOH at a scan rate of 50 mV s−1 under magnetic field (H) and with no H. The Inset schematic diagram shows that the spins in each domain of Mn2V2O7 tend to align under the external magnetic field.
Notably, Mn3+ sites with eg electron-filling of ~1 are the active sites of Mn oxides towards ORR (32); however, Mn3+ is energetically unstable on the surface of Mn oxides due to the single eg orbital occupying (50, 51). We quantified the Mn3+ site densities on FM-Mn2V2O7 and AFM-MnV2O6 based on a detailed analysis of the CV curves (SI Appendix, Note S1, Figs. S8 and S9, and Tables S2 and S3). It is exciting to find that the site density of Mn3+ on FM-Mn2V2O7 is ~6 times higher than that on AFM-MnV2O6 at the ORR onset potential (Fig. 3 A, Inset). This finding is supported by the in situ Mn K-edge XANES that the measured average Mn valence of FM-Mn2V2O7 is beyond 3+ at 1.20 VRHE due to the formation of large amounts of Mn3+ during the reaction (Fig. 3B and SI Appendix, Figs. S10 and S11 and Table S4). In contrast, AFM-MnV2O6 exhibits a significantly lowered Mn valence state under identical working conditions (SI Appendix, Fig. S12 and Table S5). Such substantial increase of Mn3+ on FM-Mn2V2O7 is in line with our expectation that the strong Mn–O hybridization in FM-Mn2V2O7 accelerates the electron transfer of surface Mn sites to adsorbed oxygen species through well-mixed Mn-3d and O-2p orbitals (Mn2+–O2− ↔ Mn(2+δ)+–O(2−δ)−) (36), thereby facilitating the evolution of Mn2+ to Mn3+ under working conditions (we have excluded the possibility of facilitated evolution of Mn2+ to Mn3+ on FM-Mn2V2O7 by the enhanced electrical conductivity of FM-Mn2V2O7 compared with that of AFM-MnV2O6, SI Appendix, Fig. S13).
To further confirm the critical role of magnetic ordering in generating active Mn sites in the electrochemical reaction, the response of FM-Mn2V2O7 and AFM-MnV2O6 under a magnetic field was monitored by in situ UV–Vis spectroscopy, which is sensitive to the changes of valence electron in transition-metal-based materials (50). Spectral changes of FM-Mn2V2O7 and AFM-MnV2O6 were recorded at varying applied potentials. As illustrated in Fig. 3C and SI Appendix, Fig. S14, the UV–Vis spectra of AFM-MnV2O6 remain unchanged under the magnetic field, while the absorption peak of FM-Mn2V2O7 increases by 10% under the magnetic field. According to the literature (50, 52), the peaks of Mn oxides at 350~390 nm and 470~510 nm are assigned to the charge-transfer transition of O2– → Mn3+ and the d–d transition of Mn3+, respectively. The enhanced absorption peaks of FM-Mn2V2O7 under the magnetic field indicate that the implementation of field increases the amounts of Mn3+ on the surface of FM-Mn2V2O7. This finding is further confirmed by the CV analysis that the peak current density of FM-Mn2V2O7 increases by 18% under the magnetic field (Fig. 3D). Correspondingly, the Mn3+ site density on FM-Mn2V2O7 is increased by 11% under the magnetic field (SI Appendix, Fig. S15 and Table S2). These results are reasonable that the applied magnetic field can align the spins of the individual magnetic domains within a single FM electrocatalyst (Fig. 3 D, Inset) and promotes the FM exchange interactions (15, 20), thereby enhancing electron delocalization and the evolution of magnetic metal centres.
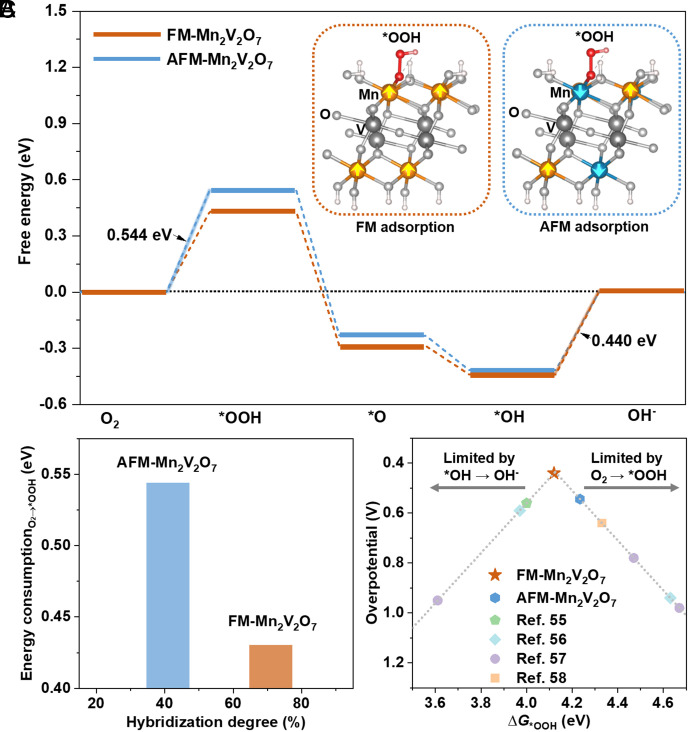
Magnetic Ordering Adjusted Reaction Thermodynamics.
The above results reveal that FM and AFM orderings with different hybridization degrees of Mn–O orbitals have different affinity for oxygen species (53), which will undoubtedly affect the adsorption of oxygen intermediates. To further investigate the magnetic effect on reaction thermodynamics, we constructed Mn2V2O7 with FM and AFM orderings (SI Appendix, Note S2 and Tables S6 and S7) and rationally exposed Mn3+ sites on the surface of Mn2V2O7 (Inset of Fig. 4 A and SI Appendix, Note S3, Figs. S16–S18, and Table S8). We calculated ORR free-energy diagrams on the Mn sites of FM- and AFM-Mn2V2O7 (we experimentally exclude V ions as the ORR active sites on Mn2V2O7, SI Appendix, Fig. S19). As illustrated in Fig. 4A and SI Appendix, Table S9, the potential-limiting step of AFM-Mn2V2O7 is the first reaction step to convert the adsorbed O2 to *OOH, while the oxygen intermediates bind more strongly on FM-Mn2V2O7 to promote the formation of *OOH, and the potential-limiting step transfers to *OH desorption.
Fig. 4.
Magnetic ordering regulated ORR thermodynamics on Mn2V2O7. (A) ORR free-energy diagrams of Mn2V2O7 with FM and AFM orderings at 1.23 VRHE, with the Inset showing atomic configurations of *OOH adsorbed on Mn2V2O7 (001) surface with FM and AFM orderings. The highlight indicates the potential-limiting step. (B) Correlation between energy consumption of O2 → *OOH and hybridization degree. (C) Volcano plot of DFT calculated theoretical overpotential as a function of ΔG*OOH. The dashed lines were plotted by the equation (54); η = 1.23 V−{min (4.92 eV−ΔG*OOH, ΔG*OH)}/e, where ΔG*OOH = ΔG*OH + 3.33 eV, and ΔG*OH is the adsorption free energy of *OH intermediate. For comparison, the data points of other transition-metal oxide catalysts are plotted according to the reported works (55–58).
Note that the conversion of triplet O2 to the *OOH intermediate (O2 → *OOH) is generally considered as the main limitation of ORR on oxide materials (59, 60). A stronger Mn–O hybridization with FM ordering is expected to stabilize *OOH, thus increasing the driving force for O2 → *OOH (32). As shown in Fig. 4B, FM-Mn2V2O7 shows a ~30% increase in Mn–O hybridization compared with AFM-Mn2V2O7; correspondingly, the energy consumption of on FM-Mn2V2O7 is reduced by ~0.1 eV compared with that on AFM-Mn2V2O7. This finding demonstrates that the strong Mn–O hybridization by FM ordering promotes the formation of *OOH and greatly enhances the intrinsic activity of each Mn active site on FM-Mn2V2O7.
Moreover, we correlated the calculated overpotentials of Mn2V2O7 and other reported electrocatalysts with their adsorption-free energies of *OOH intermediate (ΔG*OOH). As shown in Fig. 4C, a typical volcano plot was delivered, where FM-Mn2V2O7 lies at the volcano peak. Our calculation results highlight the key role of FM ordering with strong Mn–O hybridization in optimizing reaction thermodynamics of magnetic transition-metal oxides.
Magnetic Effect on ORR Activity.
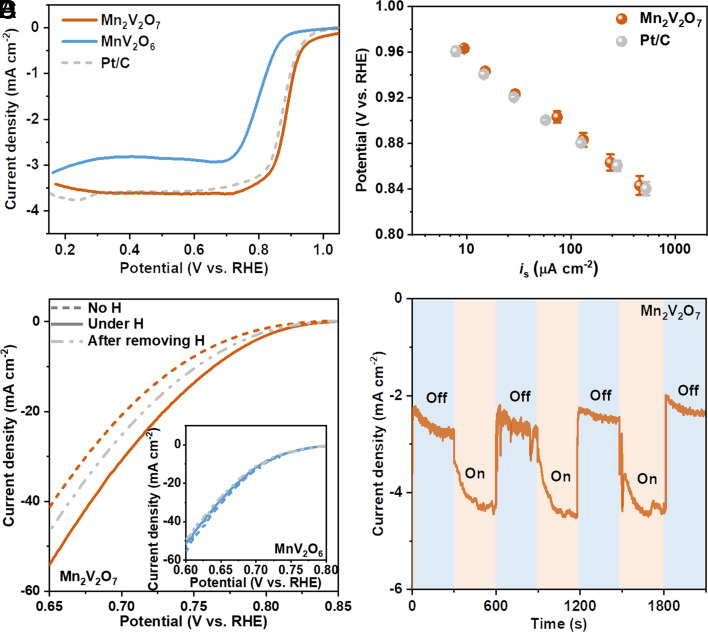
We then proceeded to measure the ORR performance of the experimentally fabricated FM-Mn2V2O7 and AFM-MnV2O6 using a rotating disk electrode in O2-saturated 1.0 M KOH (SI Appendix, Fig. S20). The state-of-the-art Pt/C was included for reference purposes (SI Appendix, Fig. S21). As expected, FM-Mn2V2O7 shows far better ORR activity than AFM-MnV2O6 (Fig. 5A and SI Appendix, Fig. S22), which should be ascribed to the more Mn3+ active sites (Fig. 3 A and B) and more favorable reaction thermodynamics (Fig. 4) on the former. More impressively, FM-Mn2V2O7 exhibits a half-wave potential of 0.89 VRHE, which is even 0.01 V better than that of the benchmark Pt/C catalyst. To better evaluate the intrinsic ORR activity of FM-Mn2V2O7 and Pt/C electrocatalysts, their specific surface areas were carefully assessed (SI Appendix, Fig. S23). As shown in Fig. 5B, the normalized activity of FM-Mn2V2O7 with respect to its specific surface area is ~74 μA cm−2 at 0.90 VRHE, superior to that of the Pt/C catalyst (~57 μA cm−2) (SI Appendix, Note S4). Such excellent performance of FM-Mn2V2O7 is consistent with the above calculation result that the intrinsic activity of FM-Mn2V2O7 locates at the top of the ORR activity volcano plot (Fig. 4C). Moreover, we note that FM-Mn2V2O7 is among the most active ORR catalysts in alkaline reported so far (SI Appendix, Table S10). Besides, the FM-Mn2V2O7 catalyst retains its crystal structure without noticeable structural reconstruction during the ORR process (SI Appendix, Note S5 and Fig. S24).
Fig. 5.
Magnetic effect on ORR activity. (A) ORR polarization curves of Mn2V2O7, MnV2O6 and Pt/C in O2-saturated 1.0 M KOH at a scan rate of 5 mV s−1 with a rotating rate of 1,600 rpm. (B) Specific activities (is) of Mn2V2O7 and Pt/C. Error bars represent SDs from at least three independent repeated measurements. (C) LSV curves of Mn2V2O7 and MnV2O6 (Inset) in O2-saturated 1.0 M KOH at a scan rate of 5 mV s−1 with no magnetic field (H), under magnetic field and after removing magnetic field (~1 T). (D) Magneto-chronoamperometry experiment of Mn2V2O7 in O2-saturated 1.0 M KOH at 0.78 VRHE.
To further confirm the critical role of magnetic ordering in ORR performance, FM-Mn2V2O7 and AFM-MnV2O6 were loaded onto Teflon-treated carbon fiber paper and their linear sweep voltammetry (LSV) curves were measured under a magnetic field of ~1 T (SI Appendix, Fig. S25). As shown in Fig. 5C, the current density of FM-Mn2V2O7 increases under the external magnetic field, and decreases after the magnetic field was removed (LSV curve does not fully recover to the initial state due to the remanence in FM-Mn2V2O7), demonstrating the ORR activity enhancement by the external magnetic field. Moreover, FM-Mn2V2O7 shows excellent stability under the magnetic field (SI Appendix, Fig. S26). In contrast, AFM-MnV2O6 exhibits negligible magnetic response (Fig. 5 C, Inset), which is consistent with previous reports (15, 30) that the magnetic susceptibility of AFM material is too low, and the applied magnetic field is not strong enough to overcome the AFM coupling.
Notably, we exclude that the activity enhancement of FM-Mn2V2O7 originates from magnetohydrodynamic effect (27) on mass transport (e.g., O2 or solution ion). Specifically, under the same magnetic field conditions, the performance enhancements of AFM-MnV2O6 and nonmagnetic Pt/C catalyst are not evident (Inset of Fig. 5 C and SI Appendix, Figs. S27 and S28). Besides, in the absence of magnetic field, the LSV curve of FM-Mn2V2O7 remains almost unchanged when the electrolyte was stirred to promote mass transport (SI Appendix, Fig. S29). We note that although magnetic field has been reported to enhance mass transport (27), Teflon-treated carbon fiber paper used here can greatly promote O2 transportation during ORR. Therefore, in our case, the performance enhancement of FM-Mn2V2O7 is not caused by magnetic field-promoted mass transport.
Moreover, the response of FM-Mn2V2O7 under the magnetic field was further verified by cyclic ON–OFF test. As shown in Fig. 5D, the magnetic perturbation was immediate and evident when the magnetic field was applied, and the current density increases by 61%. These results are in good agreement with the above experimentally observations that the external magnetic field can promote the conversion of Mn ions to active Mn3+ on FM-Mn2V2O7 (Fig. 3 C and D). Furthermore, it is found that applying a magnetic field increases the reactivity of per active site on FM-Mn2V2O7 (SI Appendix, Fig. S30), which is consistent with the calculated favorable thermodynamics of FM-Mn2V2O7 (Fig. 4).
Discussion
In conclusion, we have found a correlation among magnetic ordering, metal–oxygen hybridization, and electrocatalytic ORR activity of Mn oxides. We show that the optimum transition-metal oxide electrocatalyst should exhibit FM ordering with strong metal–oxygen hybridization, which is conductive to generate large amounts of active sites on the catalyst surface and achieve favourable reaction thermodynamics under working conditions. We emphasize that for the electrocatalyst near the apex of the activity volcano plot, FM ordering can help the electrocatalyst to break the activity limit to climb to or even beyond the apex of the volcano plot. Our work bridges the gap between the classic theory of metal–oxygen hybridization and the new understanding of magnetic oxides from spin and magnetism perspective, which unambiguously can further advance oxygen electrocatalysis.
Materials and Methods
Synthesis of Mn2V2O7 and MnV2O6 Catalysts.
Mn2V2O7 and MnV2O6 were synthesized via a facile cation exchange method (40, 55) using ZnO as sacrificial templates. Specifically, ZnO nanosheets were first fabricated on a fluorine-doped tin oxide (FTO) substrate. Then, as-fabricated ZnO nanosheets were exchanged with Mn and V precursors in the gas phase (SI Appendix, Fig. S1). In the cation exchange reaction, the FTO-supported ZnO nanosheets were placed in the center of the tube, and the manganese chloride (MnCl2) and the treated vanadium chloride (VCl3) were placed at 4.5 and 9.5 cm upstream of the tube center, respectively. For Mn2V2O7, VCl3 was deliquesced at 100 °C for 50 min before use, and the furnace was heated to and held at 525 °C for 30 min in 50 s.c.c.m nitrogen gas flow. For MnV2O6, VCl3 was deliquesced at 60 °C for 70 min before use, and the furnace was heated to and held at 495 °C for 30 min in 50 s.c.c.m nitrogen gas flow.
Materials Characterization.
X-ray diffraction (XRD) characterization was carried out on a Bruker D8 Advance diffractometer with Cu Kα radiation. Scanning electron microscopy (SEM) was performed on a Hitachi S-4800 SEM. High-angle annular dark-field scanning transition electron microscopy image was collected on a JEOL ARM200F microscope with a STEM aberration corrector operated at 200 kV. The convergent semiangle and collection angle were 21.5 and 200 mrad, respectively. XANES measurements were performed at a Stanford Synchrotron Radiation Light Source. X-ray photoelectron spectroscopy data was recorded using a Kα Thermo fisher spectrometer (Thermo Fisher Scientific). Brunauer–Emmett–Teller surface area was determined from nitrogen adsorption data measured at 77 K on a Microporous instrument Tristar 3000.
Magnetic Property Measurements.
Magnetic properties of Mn2V2O7 and MnV2O6 were measured with a superconducting quantum interference device magnetometer (MPMS XL-7T, Quantum Design). The magnetic hysteresis loop measurements were recorded at room temperature in fields between −30,000 and +30,000 Oe.
In Situ Spectroscopic Characterizations.
In situ Mn and V K-edge XANES spectra during ORR were recorded using a homemade electrochemical cell with polyimide film windows, and the valence analysis was conducted by the linear combination fitting method. In situ UV–Vis spectra were performed on a Hitachi U-3010 with a homemade photoelectrochemical cell, with catalysts fabricated directly on a FTO substrate as the working electrode, a graphite rod as the counter electrode, and a saturated calomel as the reference electrode.
Electrochemical Characterizations.
The electrocatalytic performance of the oxide catalysts was measured on a WaveDriver 20 electrochemical workstation (Pine Research Instrument). The reference electrode was a saturated calomel and the counter electrode was a graphite rod. The catalyst ink was prepared by dispersing 5 mg catalyst, 5 mg carbon powder (Vulcan XC 72), and 50 μL Nafion solution (5 wt%) in 1 mL deionized water. Afterwards, the ink solution was sonicated for 30 min to get a uniform suspension. The well-dispersed catalyst ink was dropped onto a polished glassy-carbon rotating disk electrode (0.196 cm2) to maintain a catalyst mass-loading of 0.25 mg cm−2 for all measurements. A Pt/C (20 wt%) catalyst was used as a reference. Potentials were calibrated to RHE in the high-purity H2-saturated 1.0 M KOH solution (61) (SI Appendix, Fig. S31). The CV curves were recorded at a scan rate of 50 mV s−1. The LSV curves were recorded at a scan rate of 5 mV s−1 at 1,600 rpm until the CV signals were stable (at least 20 sweeps).
For LSV curves under a magnetic field, a Hall Effect Test System (CH-100) was used to apply the magnetic field (SI Appendix, Fig. S25). The LSV curve was first recorded in the absence of an applied magnetic field (marked as “no H”). Then, an external magnetic field of 1T was applied to magnetize the electrode for 10 min, and the LSV curve was recorded under the field (marked as “under H”). Finally, the magnetic field was removed, and the LSV curve was remeasured (marked as “after removing H”).
Computational Methods.
The spin-polarized DFT calculations were implemented in Vienna Ab initio Simulation Package (62) using the projector augmented wave (63) pseudopotential and Perdew–Burke–Ernzerhof (64) exchange-correlation functional. In bulk and surface calculations, convergence conditions were set as 0.02 eV Å−1 for force on each atom and 10–5 eV for energy. A plane wave kinetic energy cut-off of 500 eV was adopted. Mn2V2O7 and MnV2O6 bulks (SI Appendix, Fig. S5) with a monoclinic structure of space group C2/m, and Mn2V2O7 (001) surface (SI Appendix, Fig. S16) with a vacuum layer of 15 Å were studied. The Brillouin zone was sampled by the Monkhorst–Pack scheme, namely, a 3×4×2 k-point for the MnV2O6 bulk, a 5×4×6 k-point for the Mn2V2O7 bulk, and a 5×5×1 k-point for the Mn2V2O7 (001). The Mn2V2O7 (001) slab (a = 6.54 Å, b = 8.97 Å) contains 50 atoms including 12 H atoms. During surface optimizations, the bottom H–O–Mn layers were fixed while other atomic layers were fully relaxed. FM and AFM structures were constructed by setting the Mn atomic spins. The Hubbard U approach (65) was used to describe strongly correlated d-electron systems, and here, an effective Coulomb parameter (Ueff) value of 4.0 eV was applied to Mn 3d states and Ueff = 3.1 eV for V 3d states (66). The Gibbs free-energy change (ΔG) was obtained by
| [1] |
where ΔE is the reaction energy calculated by DFT, ΔZPE and ΔS are the changes in zero-point energy and entropy, respectively, which are related to vibrational frequency, T is the temperature at 298.15 K, e is the elementary charge, and U is the electrode potential. The entropies of gas phase molecules were taken from the National Institute of Standards and Technology database (67).
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
T.L. acknowledged funding from the National Natural Science Foundation of China (52071231 and 51722103) and the Natural Science Foundation of Tianjin city (19JCJQJC61900). Z.P. Hu acknowledged funding from the National Natural Science Foundation of China (21933006 and 21773124) and the Fundamental Research Funds for the Central Universities Nankai University (No. 63213042, 63221346, and ZB22000103). Calculations were performed on Supercomputing Center of Nankai University and TianHe-1A at the National Supercomputer Center, Tianjin.
Author contributions
T.L. designed research; J.L., C.Z., E.Z., and J.M. performed research; Y.C. contributed new reagents/analytic tools; H.L., Z.H., and T.L. analyzed data; and T.L. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Contributor Information
Hui Liu, Email: hui_liu@tju.edu.cn.
Zhenpeng Hu, Email: zphu@nankai.edu.cn.
Tao Ling, Email: lingt04@tju.edu.cn.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
Supporting Information
References
- 1.Hwang J., et al. , Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Jiao Y., Zheng Y., Jaroniec M., Qiao S. Z., Design of electrocatalysts for oxygen- and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 44, 2060–2086 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Wang Y. J., Qiao J., Baker R., Zhang J., Alkaline polymer electrolyte membranes for fuel cell applications. Chem. Soc. Rev. 42, 5768–5787 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Subbaraman R., et al. , Trends in activity for the water electrolyser reactions on 3d M(Ni Co, Fe, Mn) hydr(oxy)oxide catalysts. Nat. Mater. 11, 550–557 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Guo J., et al. , Direct seawater electrolysis by adjusting the local reaction environment of a catalyst. Nat. Energy 8, 264–272 (2023). [Google Scholar]
- 6.Du K., et al. , Interface engineering breaks both stability and activity limits of RuO2 for sustainable water oxidation. Nat. Commun. 13, 5716 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mistry H., Varela A. S., Kuhl S., Strasser P., Cuenya B. R., Nanostructured electrocatalysts with tunable activity and selectivity. Nat. Rev. Mater. 1, 16009 (2016). [Google Scholar]
- 8.Cheng F., et al. , Rapid room-temperature synthesis of nanocrystalline spinels as oxygen reduction and evolution electrocatalysts. Nat. Chem. 3, 79–84 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Xie J., et al. , Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 25, 5807–5813 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Ling T., et al. , Engineering surface atomic structure of single-crystal cobalt (II) oxide nanorods for superior electrocatalysis. Nat. Commun. 7, 12876 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng J. W. D., et al. , Gold-supported cerium-doped NiOx catalysts for water oxidation. Nat. Energy 1, 16053 (2016). [Google Scholar]
- 12.Li N., et al. , Influence of iron doping on tetravalent nickel content in catalytic oxygen evolving films. Proc. Natl. Acad. Sci. U.S.A. 114, 1486–1491 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y., et al. , High phase-purity 1T’-MoS2- and 1T’-MoSe2-layered crystals. Nat. Chem. 10, 638–643 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Bajdich M., García-Mota M., Vojvodic A., Nørskov J. K., Bell A. T., Theoretical investigation of the activity of cobalt oxides for the electrochemical oxidation of water. J. Am. Chem. Soc. 135, 13521–13530 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Garcés-Pineda F. A., Blasco-Ahicart M., Nieto-Castro D., López N., Galán-Mascarós J. R., Direct magnetic enhancement of electrocatalytic water oxidation in alkaline media. Nat. Energy 4, 519–525 (2019). [Google Scholar]
- 16.Mtangi W., et al. , Control of electrons’ spin eliminates hydrogen peroxide formation during water splitting. J. Am. Chem. Soc. 139, 2794–2798 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiao Y., Sharpe R., Lim T., Niemantsverdriet J. W. H., Gracia J., Photosystem II acts as a spin-controlled electron gate during oxygen formation and evolution. J. Am. Chem. Soc. 139, 16604–16608 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Sun Z., et al. , Regulating the spin state of Fe(III) enhances the magnetic effect of the molecular catalysis mechanism. J. Am. Chem. Soc. 144, 8204–8213 (2022). [DOI] [PubMed] [Google Scholar]
- 19.Ren X., et al. , Spin-polarized oxygen evolution reaction under magnetic field. Nat. Commun. 12, 2608 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu T., et al. , Spin pinning effect to reconstructed oxyhydroxide layer on ferromagnetic oxides for enhanced water oxidation. Nat. Commun. 12, 3634 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gracia J., Spin dependent interactions catalyse the oxygen electrochemistry. Phys. Chem. Chem. Phys. 19, 20451–20456 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Gracia J., Sharpe R., Munarriz J., Principles determining the activity of magnetic oxides for electron transfer reactions. J. Catal. 361, 331–338 (2018). [Google Scholar]
- 23.Li J., et al. , Spin effect on oxygen electrocatalysis. Adv. Energy Sustainability Res. 2, 2100034 (2021). [Google Scholar]
- 24.Baryshnikov G., Minaev B., Agren H., Theory and calculation of the phosphorescence phenomenon. Chem. Rev. 117, 6500–6537 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Melander M., Laasonen K., Jonsson H., Effect of magnetic states on the reactivity of an FCC(111) iron surface. J. Phys. Chem. C 118, 15863–15873 (2014). [Google Scholar]
- 26.Li Z., et al. , V “Bridged” Co-O to eliminate charge transfer barriers and drive lattice oxygen oxidation during water-splitting. Adv. Funct. Mater. 31, 2008822 (2020). [Google Scholar]
- 27.Zhang Y., et al. , Recent advances in magnetic field-enhanced electrocatalysis. ACS Appl. Energy Mater. 3, 10303–10316 (2020). [Google Scholar]
- 28.Hunt C., et al. , Quantification of the effect of an external magnetic field on water oxidation with cobalt oxide anodes. J. Am. Chem. Soc. 144, 733–739 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Sun Y., et al. , Spin-related electron transfer and orbital interactions in oxygen electrocatalysis. Adv. Mater. 32, 2003297 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Chen R. R., et al. , Antiferromagnetic inverse spinel oxide LiCoVO4 with spin-polarized channels for water oxidation. Adv. Mater. 32, 1907976 (2020). [DOI] [PubMed] [Google Scholar]
- 31.Suntivich J., May K. J., Gasteiger H. A., Goodenough J. B., Shao-Horn Y., A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles. Science 334, 1383–1385 (2011). [DOI] [PubMed] [Google Scholar]
- 32.Suntivich J., et al. , Design principles for oxygen-reduction activity on perovskite oxide catalysts for fuel cells and metal-air batteries. Nat. Chem. 3, 546–550 (2011). [DOI] [PubMed] [Google Scholar]
- 33.Grimaud A., et al. , Activating lattice oxygen redox reactions in metal oxides to catalyse oxygen evolution. Nat. Chem. 9, 457–465 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Hwang J., et al. , Tuning perovskite oxides by strain: Electronic structure, properties, and functions in (electro)catalysis and ferroelectricity. Mater. Today 31, 100–118 (2019). [Google Scholar]
- 35.Huang Z.-F., et al. , Chemical and structural origin of lattice oxygen oxidation in Co-Zn oxyhydroxide oxygen evolution electrocatalysts. Nat. Energy 4, 329–338 (2019). [Google Scholar]
- 36.Suntivich J., et al. , Estimating hybridization of transition metal and oxygen states in perovskites from O K-edge X-ray absorption spectroscopy. J. Phys. Chem. C 118, 1856–1863 (2014). [Google Scholar]
- 37.Hong W. T., et al. , Charge-transfer-energy-dependent oxygen evolution reaction mechanisms for perovskite oxides. Energy Environ. Sci. 10, 2190–2200 (2017). [Google Scholar]
- 38.Grisolia M. N., et al. , Hybridization-controlled charge transfer and induced magnetism at correlated oxide interfaces. Nat. Phys. 12, 484–492 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aoki Y., et al. , In situ activation of a manganese perovskite oxygen reduction catalyst in concentrated alkaline media. J. Am. Chem. Soc. 143, 6505–6515 (2021). [DOI] [PubMed] [Google Scholar]
- 40.Ling T., Jaroniec M., Qiao S.-Z., Recent progress in engineering the atomic and electronic structure of electrocatalysts via cation exchange reactions. Adv. Mater. 32, 2001866 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Pickett W. E., Singh D. J., Electronic structure and half-metallic transport in the La1-xCaxMnO3 system. Phys. Rev. B 53, 1146–1160 (1996). [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharjee S., Waghmare U. V., Lee S.-C., An improved d-band model of the catalytic activity of magnetic transition metal surfaces. Sci. Rep. 6, 35916 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H., et al. , Metal–oxygen hybridization determined activity in spinel-based oxygen evolution catalysts: A case study of ZnFe2–xCrxO4. Chem. Mater. 30, 6839–6848 (2018). [Google Scholar]
- 44.Goodenough J. B., Theory of the role of covalence in the perovskite-type manganites [La, M(II)]MnO3. Phys. Rev. 100, 564–573 (1955). [Google Scholar]
- 45.Goodenough J. B., Loeb A. L., Theory of ionic ordering, crystal distortion, and magnetic exchange due to covalent forces in spinels. Phys. Rev. 98, 391–408 (1955). [Google Scholar]
- 46.Kang J., et al. , Valence oscillation and dynamic active sites in monolayer NiCo hydroxides for water oxidation. Nat. Catal. 4, 1050–1058 (2021). [Google Scholar]
- 47.Chung D. Y., et al. , Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 5, 222–230 (2020). [Google Scholar]
- 48.Forslund R. P., et al. , Exceptional electrocatalytic oxygen evolution via tunable charge transfer interactions in La0.5Sr1.5Ni1-xFexO4±δ Ruddlesden-Popper oxides. Nat. Commun. 9, 3150 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tesch M. F., et al. , Evolution of oxygen-metal electron transfer and metal electronic states during manganese oxide catalyzed water oxidation revealed with in situ soft X-ray spectroscopy. Angew. Chem. Int. Ed. Engl. 58, 3426–3432 (2019). [DOI] [PubMed] [Google Scholar]
- 50.Jin K., et al. , Mechanistic investigation of water oxidation catalyzed by uniform, assembled MnO nanoparticles. J. Am. Chem. Soc. 139, 2277–2285 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Takashima K., Hashimoto R., Nakamura, inhibition of charge disproportionation of MnO2 electrocatalysts for efficient water oxidation under neutral conditions. J. Am. Chem. Soc. 134, 18153–18156 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Takashima T., Yamaguchi A., Hashimoto K., Irie H., Nakamura R., In situ UV-vis absorption spectra of intermediate species for oxygen-evolution reaction on the surface of MnO2 in neutral and alkaline media. Electrochemistry 82, 325–327 (2014). [Google Scholar]
- 53.Hong W. T., et al. , Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 8, 1404–1427 (2015). [Google Scholar]
- 54.Xu H., Cheng D., Cao D., Zeng X. C., A universal principle for a rational design of single-atom electrocatalysts. Nat. Catal. 1, 339–348 (2018). [Google Scholar]
- 55.Li Y. J., et al. , Multiscale structural engineering of Ni-doped CoO nanosheets for zinc-air batteries with high power density. Adv. Mater. 30, 1804653 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Mu C., et al. , Rational design of spinel cobalt vanadate oxide Co2VO4 for superior electrocatalysis. Adv. Mater. 32, 1907168 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Tian Y., et al. , Engineering crystallinity and oxygen vacancies of Co(II) oxide nanosheets for high performance and robust rechargeable Zn–air batteries. Adv. Funct. Mater. 31, 2101239 (2021). [Google Scholar]
- 58.Bian J., et al. , Mg doped perovskite LaNiO3 nanofibers as an efficient bifunctional catalyst for rechargeable zinc-air batteries. ACS Appl. Energy Mater. 2, 923–931 (2019). [Google Scholar]
- 59.Song J., et al. , A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49, 2196–2214 (2020). [DOI] [PubMed] [Google Scholar]
- 60.Li H., et al. , Analysis of the limitations in the oxygen reduction activity of transition metal oxide surfaces. Nat. Catal. 4, 463–468 (2021). [Google Scholar]
- 61.Liang Y., et al. , Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 10, 780–786 (2011). [DOI] [PubMed] [Google Scholar]
- 62.Kresse G., Furthmüller J., Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996). [Google Scholar]
- 63.Blöchl P. E., Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994). [DOI] [PubMed] [Google Scholar]
- 64.Perdew J. P., Burke K., Ernzerhof M., Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996). [DOI] [PubMed] [Google Scholar]
- 65.Dudarev S. L., Botton G. A., Savrasov S. Y., Humphreys C. J., Sutton A. P., Electron-energy-loss spectra and the structural stability of nickel oxide: An LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998). [Google Scholar]
- 66.Yan Q., et al. , Mn2V2O7: An earth abundant light absorber for solar water splitting. Adv. Energy Mater. 5, 1401840 (2015). [Google Scholar]
- 67.Johnson R. D. III, Data from “NIST computational chemistry comparison and benchmark database.” https://cccbdb.nist.gov/. Deposited 22 May 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
All study data are included in the article and/or SI Appendix.