Significance
Puberty is a key period when sex differences in anxiety emerge, but causal mechanisms are unknown. We show that androgens play a key role in programming behavioral responses to social defeat stress. Using calcium imaging we show that neural activity in the bed nucleus of the stria terminalis (BNST) is increased by social threats but not defensive responses like freezing. These responses are more generalized in males lacking pubertal androgen exposure. These observations contribute to recent discussions on the function of the extended amygdala and support the hypothesis that the BNST can encode immediate threats. Our findings highlight the importance of pubertal androgens in determining adult behavioral responses to social stress.
Keywords: puberty, androgens, stress, extended amygdala, anxiety
Abstract
Anxiety disorders are a major public health concern and current treatments are inadequate for many individuals. Anxiety is more common in women than men and this difference arises during puberty. Sex differences in physiological stress responses may contribute to this variability. During puberty, gonadal hormones shape brain structure and function, but the extent to which these changes affect stress sensitivity is unknown. We examined how pubertal androgens shape behavioral and neural responses to social stress in California mice (Peromyscus californicus), a model species for studying sex differences in stress responses. In adults, social defeat reduces social approach and increases social vigilance in females but not males. We show this sex difference is absent in juveniles, and that prepubertal castration sensitizes adult males to social defeat. Adult gonadectomy does not alter behavioral responses to defeat, indicating that gonadal hormones act during puberty to program behavioral responses to stress in adulthood. Calcium imaging in the medioventral bed nucleus of the stria terminalis (BNST) showed that social threats increased neural activity and that prepubertal castration generalized these responses to less threatening social contexts. These results support recent hypotheses that the BNST responds to immediate threats. Prepubertal treatment with the nonaromatizable androgen dihydrotestosterone acts in males and females to reduce the effects of defeat on social approach and vigilance in adults. These data indicate that activation of androgen receptors during puberty is critical for programming behavioral responses to stress in adulthood.
Anxiety disorders are one of the most frequently diagnosed categories of mental illness, with over 28% lifetime prevalence in the United States (1). Treatments are available but a large fraction of those seeking treatment do not improve (2). Determining the underlying mechanisms of symptoms has been an effective strategy for developing new treatments for many health conditions. Psychosocial stress is an important risk factor for anxiety disorders, and there is strong evidence for sex differences in stress responses. Sex differences in stress responses are thought to contribute to sex differences in the anxiety risk (3–5), as anxiety rates are higher in women vs. men (6–11). Sex differences in anxiety diagnoses emerge during puberty (12–15). Puberty is a key developmental stage characterized by changes in physiological stress responses (16) as well as cortical and subcortical reorganization (17–19). Preclinical studies show that during puberty, stressors have stronger behavioral effects on behavior in females than males (20–22). Although the cause of these differences is unknown, other studies show that gonadal hormones shape brain structure (23–26), function (27), and gene expression networks (28) that could influence stress responses. Human imaging studies show that neural circuits affected by anxiety change during puberty (29, 30), and gonadal hormones are thought to contribute to these changes (31). The extended amygdala, which includes the bed nucleus of the stria terminalis (BNST), is especially sensitive to steroid hormones (28).
Functional MRI studies in humans show that activity within the BNST is correlated with trait anxiety (32) and is increased in response to unpredictable threats (33). These studies have limited temporal resolution (~3 s) and participants must remain immobile during observations (34). Thus, it is unclear if BNST activity more closely tracts threats or behavioral responses to threats. In contrast in vivo calcium imaging has subsecond temporal resolution that can link neural activity more closely with specific behaviors (35). Fiber photometry allows for data collection in freely moving animals (36), expanding the behavioral repertoire that can be studied. Previous work in California mice (Peromyscus californicus) shows that social defeat stress has stronger effects on BNST structure and function in adult females than males (37, 38) and that these changes drive stress-induced social avoidance and social vigilance (39–41). It is unknown whether stress-induced increases in BNST activity occur during proximity to social threats or during avoidance of a threat. It is also unknown what causes these sex differences. In adults, sex differences in behavioral responses to defeat are independent of gonadal hormones (42, 43), suggesting that sex differences are organized during development. California mice are an ideal species for studying sex differences in stress responses (44) and have a slower pace of development compared to other rodents (45, 46). Here, we use a combination of pubertal hormone manipulations, calcium imaging, and immunohistochemistry to demonstrate that androgens act during puberty to reduce behavioral and neural responses to social defeat stress, thus serving as a key mechanism regulating developmental programming.
Results
Late Onset of Pubertal Development in California Mice.
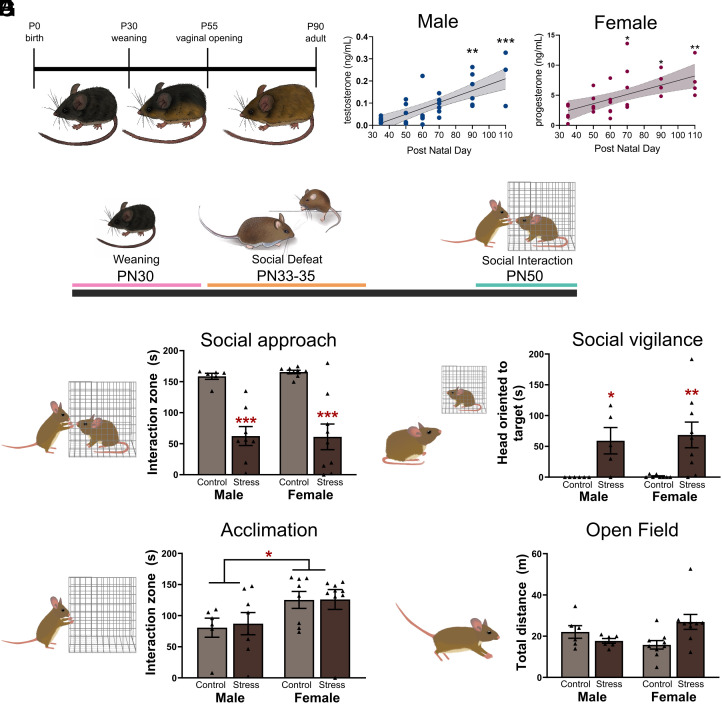
Pubertal development is multifaceted (47, 48), so we used complementary methods to assess this process in California mice (Fig. 1A and SI Appendix, Fig. S1A). In males, testosterone levels did not increase significantly from postnatal day (PN)35 until PN90 (Fig. 1B, F5,28 = 6.51, P < 0.01, Cohen’s d = 1.9) while testes weight did not increase significantly until PN70 (SI Appendix, Fig. S1A, F1,21 = 112.3, P < 6.9e−11) In females, progesterone levels did not increase from PN35 levels until PN70 (Fig. 1B, F5.26 = 2.56, P = 0.05, d = 1.7) while uterine weight increased at PN50 (SI Appendix, Fig. S1B, F1,32 = 6.99, P = 0.01). In both males and females, there was no evidence for preputial separation or vaginal opening (external genitalia) before PN50 (SI Appendix, Fig. S1 C and D).
Fig. 1.
Puberty in California mice is a period of sexual differentiation of behavioral stress responses. (A) Summary of pubertal development in California mice. (B) In males, testosterone levels are not increased until PN90 while in females, progesterone levels increase at PN70 (n = 4 to 5 per time point). (C) Prepubertal California mice were exposed to social defeat or control conditions after weaning and tested as juveniles at PN50. (D and E) Social defeat reduced social approach and increased social vigilance in both males and females (n = 6 to 8 per group). (F) Females investigated an empty cage during the acclimation phase more than males. (G) There were no differences in locomotor behavior in the open-field phase. *P < 0.05, **P < 0.01, ***P < 0.001 vs. PN 35. *P < 0.05, **P < 0.01, ***P < 0.001 vs. same sex control. †P < 0.05 vs. males. Data available at DOI: 10.6084/m9.figshare.23681493 (49).
Juvenile Male and Female California Mice Reduce Social Approach and Increase Social Vigilance after Defeat.
California mice are weaned at PN30, after which we waited 3 d (PN34-36) before randomly assigning juveniles to social defeat stress or control conditions. In adults, sex differences in the effects of social defeat endure for several weeks (50). Here, mice were tested 2 wk after the completion of social defeat in a social interaction test at PN50 (Fig. 1C), before the onset of puberty. In contrast to adults, social defeat reduced social approach (Fig. 1D, F1,27 = 47.09, P < 0.001) in both males (P < 0.001, Cohen’s d = 3.0) and females (P < 0.001, d = 2.4). Defeat also increased social vigilance (Fig. 1E, Kruskal–Wallis = 16.33, P < 0.001) in males (P = 0.03, d = 1.6) and females (P = 0.02, d = 1.8). These results contrast with observed sex differences in adults, which show that social defeat decreases social approach (38, 42, 43, 51) and increases social vigilance (40) in females but not males. During the acclimation phase, females spent more time investigating an empty cage than males (Fig. 1F, F1,27 = 6.64, P = 0.016) with no effects of stress. There were no differences in locomotor behavior in the open-field phase (Fig. 1G), time spent in the center (SI Appendix, Fig. S2A), or vigilance behavior during the acclimation phase (SI Appendix, Fig. S2B). Together with previous work, these results suggest that developmental changes during puberty contribute to sex differences in stress response in adults.
Gonadal Hormones Reduce Susceptibility to Defeat in Adult Males.
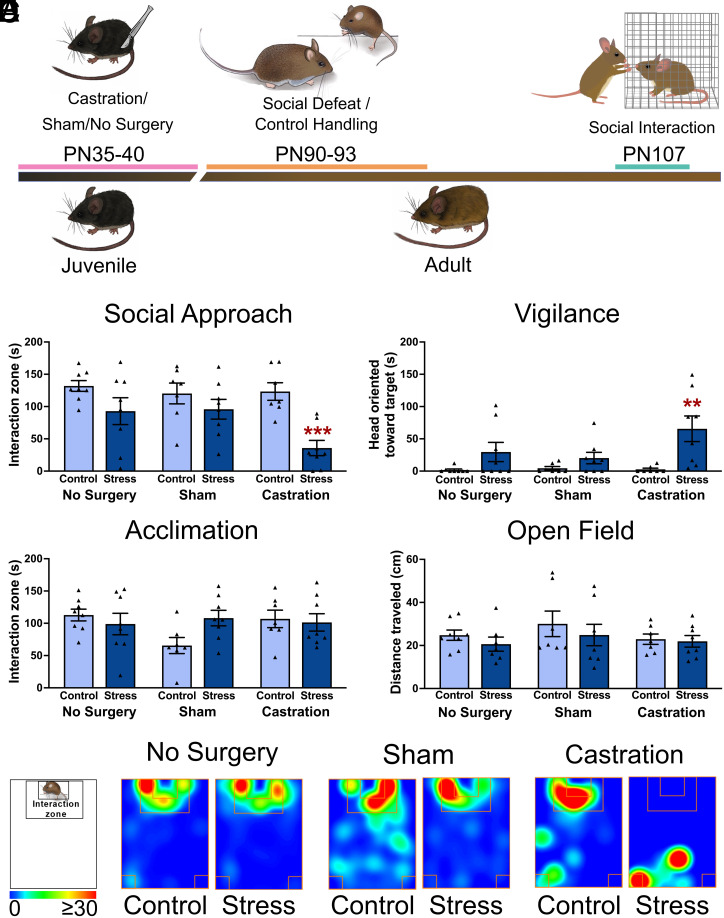
To assess the impact of gonadal hormones during puberty, males were randomly assigned to prepubertal castration, sham surgery, or no-surgery between PN35-40 (Fig. 2A). Gonadectomy affected social approach only in males that were exposed to social defeat as adults (Fig. 2B, trt*stress F2,41 = 3.32, P < 0.05). Castrated males exposed to defeat had reduced social approach vs. controls (P < 0.0001, d = 2.6), whereas there was no effect of defeat in sham or no-surgery males. Similarly, social defeat increased social vigilance in castrated (Fig. 2C, Mann–Whitney U = 23.5, P < 0.01, d = 1.6) but not sham or no-surgery mice. There were no differences in behavior during the acclimation (Fig. 2D and SI Appendix, Fig. S3A) or open-field phases (Fig. 2E and SI Appendix, Fig. S3B). The results contrast sharply with previous results that showed no effect of adult castration or ovariectomy on stress-induced social avoidance in California mice (43) and point to gonadal hormones acting during puberty as a key mechanism of sexual differentiation. These data suggest that pubertal hormones play an organizing role in the brain to diminish the effects of social defeat on social approach and social vigilance in males. The BNST plays a key role in modulating stress-induced social avoidance and social vigilance in males and females (38, 40, 41). Since prepubertal castration did not affect behavior in unstressed mice, we used fiber photometry to assess the effects of castration on BNST neural activity in males exposed to defeat.
Fig. 2.
Prepubertal castration increases sensitivity to social defeat in adulthood. (A) Timeline of prepubertal castration and behavioral testing in adults. (B and C) Social defeat decreased social approach and increased social vigilance in castrated males but not sham or no-surgery controls (n = 7 to 8 per group). (D and E) No differences were observed during the acclimation or open-field phases. (F) Representative heatmaps for the interaction phase showing reduced time spent in the interaction zone forcastrated males exposed to social defeat. **P < 0.01, ***P < 0.001 vs control. Data available at DOI: 10.6084/m9.figshare.22782959 (52).
Calcium Imaging of BNST Activity in Castrated and Intact Males.
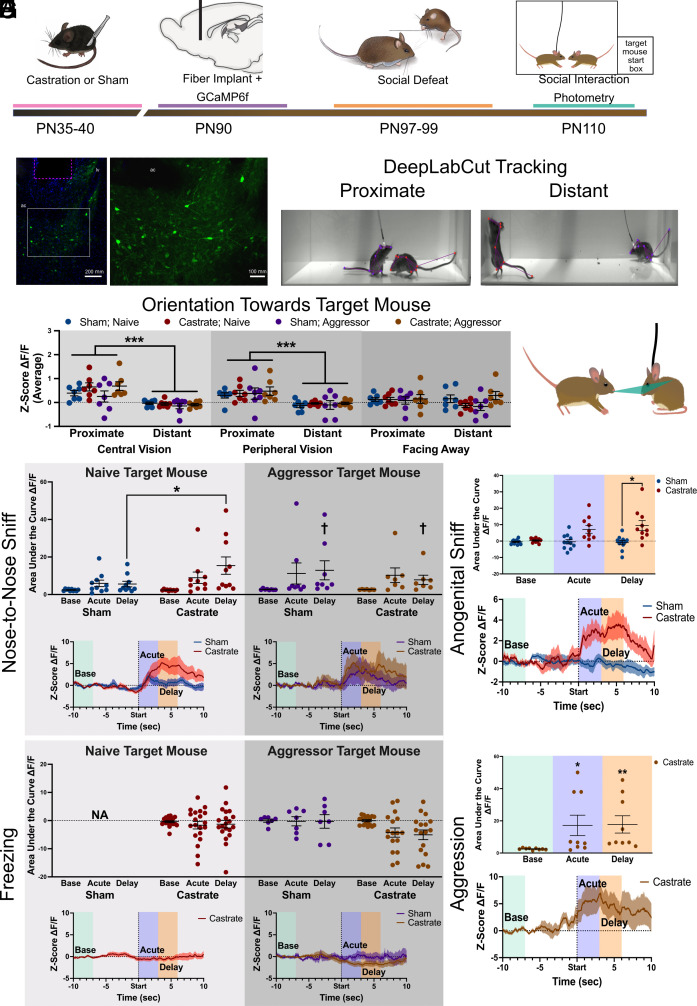
Previous immediate early gene analyses shows that social stress increases neural activity within the BNST (53, 54). These analyses provide a snapshot of overall activity during about 1 hr, so it is impossible to determine whether increased activity occurs during brief episodes of contact with social threats or during periods of avoidance. We used the subsecond temporal resolution of GCaMP6f calcium imaging to evaluate activity in the ventral BNST during discrete behavioral episodes and to determine how this activity was modulated by prepubertal castration (Fig. 3 A and B). Using DeepLabCut (Fig. 3 C and D) we determined orientation (55) and distance between focal mice and target mice with (aggressor) or without (naïve) prior experience winning aggressive encounters. Increased GCaMP6f fluorescence response (ΔF/F) in BNST was observed when focal mice were within one body length (8 cm) of target mice and within the central (Fig. 3D, 0° ≥ 40°, β = −0.438, z = −9.783, P > 0.001) or peripheral visual fields (40° ≥ 100°, β = −0.486, z = −10.356, P > 0.001) but not if the focal mouse was facing away from target mice (100° ≥ 180°). We then examined ΔF/F in specific behavioral contexts.
Fig. 3.
Prepubertal castration increases BNST neural activity and sensitivity to social defeat in adulthood. (A) Experimental timeline for fiber photometry observations of GCaMP6f in the ventral BNST of prepubertally castrated or sham surgery California mice. (B) Photomicrographs of GCaMP in BNST at low (B) and high (B’) magnification. The magenta box indicates position of the fiber. (C) Representative images showing DeepLabCut tracking of mice in proximate and distant conditions. (D) DeepLabCut tracking of body midpoint and nose of the focal mouse was used to determine the orientation of the focal mouse to the target mouse nose (green triangle). GCaMP6f signals were significantly stronger when the focal mouse was within 8 cm of the target mouse but only when the target mouse was in central vision (0° ≥ 40°) or peripheral vision (40° ≥ 100°). (E) Nose-to-nose sniffing with a naive target mouse induced a delayed increase in ΔF/F in castrated but not sham males. In contrast nose-to-nose sniffing with an aggressor target mouse increased ΔF/F in both sham and castrated males. (F) There were no changes in ΔF/F following bouts of freezing. (G) Anogenital sniffing of naive target mice increased ΔF/F in castrated but not sham males. (H) When castrated males were attacked by aggressor target mice, increased ΔF/F was observed. Sham males were not attacked enough for analysis. *P < 0.05, **P < 0.01 vs sham. ***P < 0.001 vs proximate, †P < 0.05 vs. baseline. ac= anterior commissure. Data available at DOI: 10.6084/m9.figshare.23664543 (56).
Intriguingly, the most robust changes in ΔF/F occurred after close contact with aggressor target mice. After engaging in nose-to-nose sniffing with aggressors, both castrated and sham males showed a delayed increase in BNST ΔF/F (Fig. 3E, β = 10.22, z = 2.05, P = 0.04). In contrast, after engaging in nose-to-nose sniffing with nonaggressive naive mice, castrated males showed a larger increase in BNST ΔF/F than sham males (Fig. 3E, β = 13.2, z = 2.23, P = 0.03). These results suggest that BNST ΔF/F is enhanced by social threats and that this response is more generalized in prepubertally castrated males. There were no acute or delayed changes in ΔF/F following bouts of freezing (Fig. 3F), which was robustly induced by aggressor targets (SI Appendix, Fig. S4) and to a lesser extent by naive targets. We next examined bouts of anogenital sniffing, which were less frequent and primarily limited to nonaggressive naive target mice (SI Appendix, Fig. S4). Castrated mice also showed a larger delayed increase in ΔF/F in BNST than shams (Fig. 3G, β = 6.12, z = 2.06, P = 0.04). Finally, when castrated males were attacked by aggressor target mice, there were acute (Fig. 3H, β = 14.66, z = 2.56, P = 0.01) and delayed (β = 15.25, z = 2.67, P = 0.008) increases in ΔF/F. To assess the impact of defeat stress on BNST activity, we performed calcium imaging before and after social defeat stress in intact adult male mice (Fig. 4A).
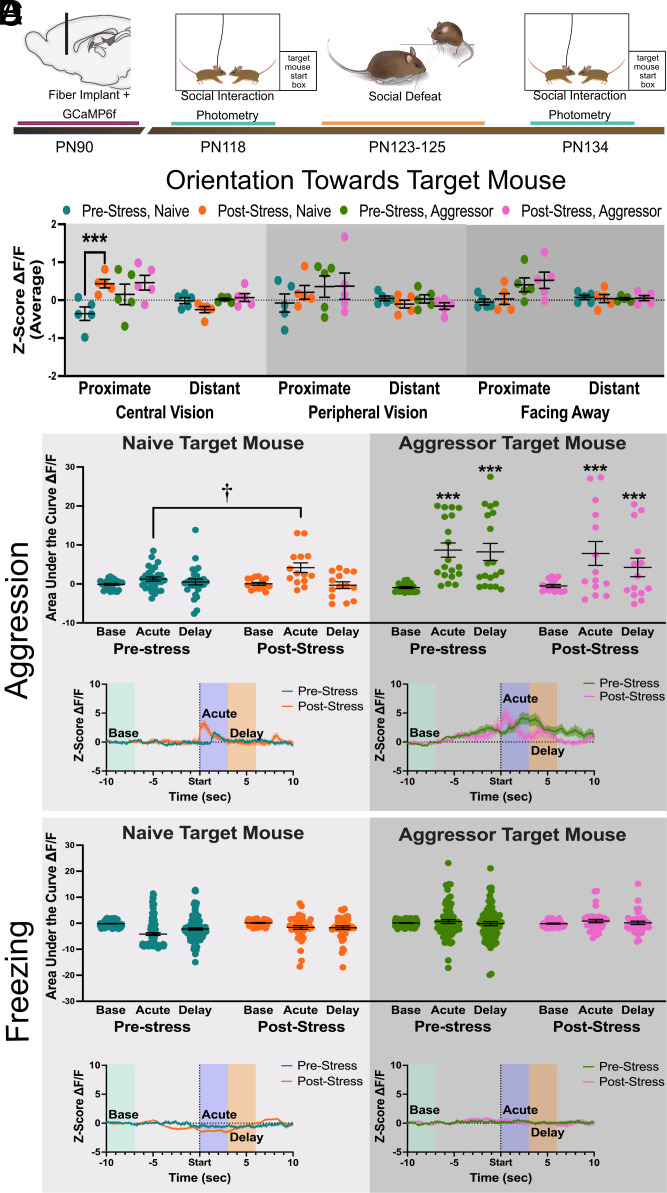
Fig. 4.
Exposure to aggressor target increases BNST activity in pre- and post-stress intact males. (A) Timeline of surgery and behavioral testing in adults. (B) DeepLabCut tracking of body orientation of the focal mouse showed that social defeat increased GCaMP6f signals to aggression received by naive target mice in the center of the visual field compared to prestress. (C) Aggression received from naive target mouse induced an acute increase in ΔF/F in poststress but not prestress males. In contrast aggression received from the aggressor target mice increased acute and delayed ΔF/F in both pre- and post-stress males. (D) There were no changes in ΔF/F following bouts of freezing. †P < 0.05 vs. pre-stress, ***P < 0.001 vs. baseline. Data available at DOI: 10.6084/m9.figshare.23983032 (57).
Male California mice are aggressive in novel environments (58), and we found that during prestress testing aggression was initiated by focal mice (SI Appendix, Fig. S4). Both aggression-naive and experienced aggressor target mice responded by attacking focal mice (SI Appendix, Fig. S4). Although social defeat stress increased ΔF/F when naive target mice were in the center of the visual field and within 8 cm (Fig. 4B, β = 0.73, z = 5.33, P < 0.001), other analyses suggested that the effects of stress were less robust. Before stress, there was no increase in ΔF/F when focal mice were attacked by naive target mice. After stress, attacks by naive target mice induced an acute increase in ΔF/F (Fig. 4C, β = 2.81, P = 0.045) with no change in activity during the delayed (3 to 6 s) period. More robust increases in ΔF/F were observed when focal mice received aggression from experienced aggressors. Pre- and post-stress mice exhibited acute (Fig. 4C, β = 9.63, z = 3.89, P < 0.001) and delayed (Fig. 4c, β = 9.16, z = 3.70 P < 0.001) increases in ΔF/F after attacks by experienced aggressors. There were no changes in ΔF/F during bouts of freezing (Fig. 4D) and no changes in ΔF/F when prestress focal mice engaged in anogenital sniffing with naive target mice (SI Appendix, Fig. S5). In this study, stress-induced acute increases in ΔF/F after attacks by naive target mice, but attacks by experienced aggressors induced both acute and delayed increased in ΔF/F. Importantly, increased ΔF/F induced by aggressors were not stress dependent. To assess the extent to which prepubertal castration impacted neuronal activity in other circuits modulating social approach, we used c-fos immunohistochemistry.
Gonadal Hormones Reduce Neural Activity in PVN Oxytocin Neurons.
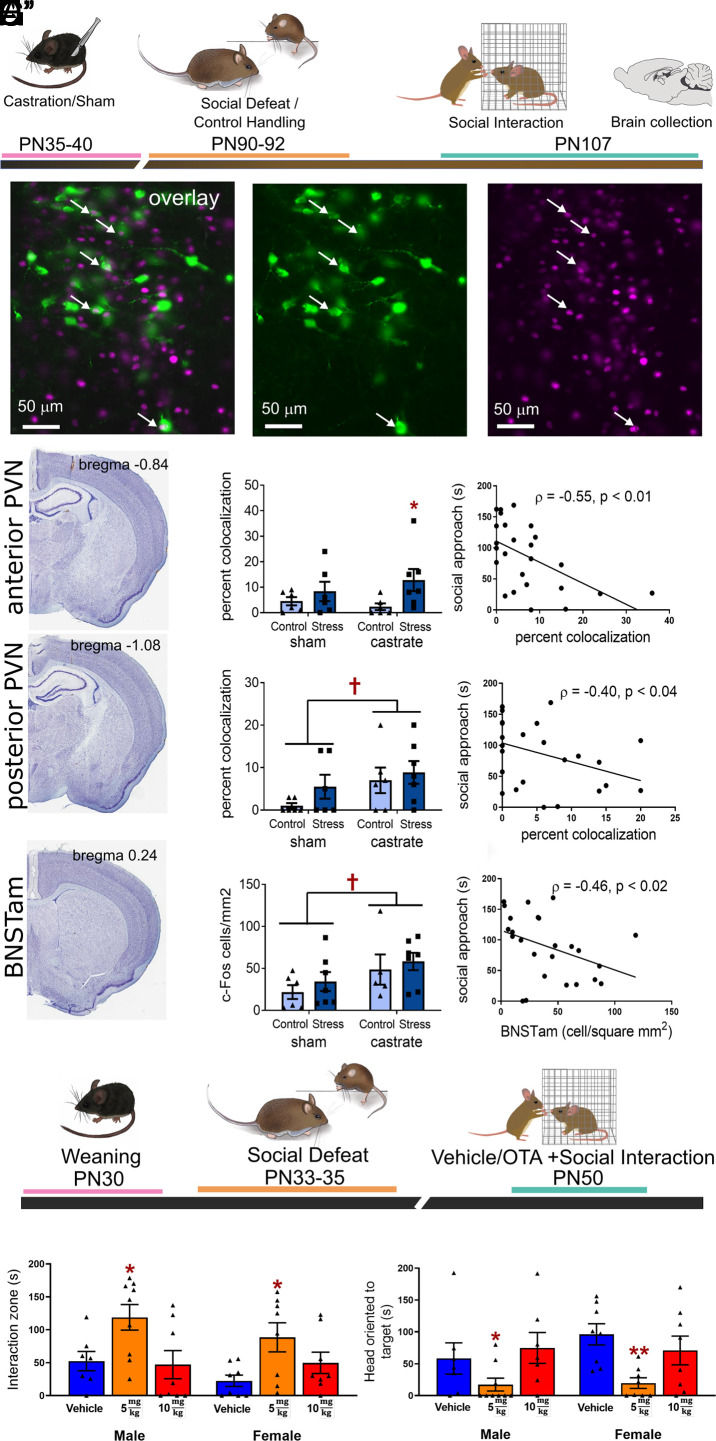
Social defeat stress increases c-fos expression in oxytocin neurons in the Paraventricular Nucleus of the Hypothalamus (PVN) (37), a response that has been associated with heightened immediate early gene expression in the anteromedial BNST (40). We used oxytocin/c-fos immunohistochemistry (Fig. 5 A and B) to examine anterior and posterior PVN, which differ in connectivity (59) and stress sensitivity (37). In anterior PVN, prepubertal castration increased oxytocin/c-fos colocalizations when compared to sham (Fig. 5C', Mann–Whitney U = 38, P = 0.01) mice that had undergone social defeat. Oxytocin/c-fos colocalizations were negatively correlated with social approach (Fig. 5C", Spearman ρ = −0.55, P < 0.01) and positively correlated with social vigilance (SI Appendix, Fig. S6A, ρ = 0.59, P < 0.01). In the posterior PVN castration increased oxytocin/c-fos colocalizations regardless of stress status (Fig. 5D', Mann–Whitney U = 113.5, P = 0.04) and oxytocin/c-fos colocalizations were negatively correlated with social approach (Fig. 5D", ρ = −0.41, P = 0.04) but not social vigilance (SI Appendix, Fig. S6B). We also examined the effects of prepubertal castration on c-fos in the anteromedial BNST, where oxytocin induces social avoidance and social vigilance (41). Prepubertal castration increased the number of c-fos positive neurons in anteromedial BNST (Fig. 5E', S5D, F1,21 = 4.51, P = 0.046), regardless of stress status. The number of c-fos cells was negatively correlated with social approach (Fig. 5E", ρ = −0.46, P = 0.02) but not social vigilance (SI Appendix, Fig. S6C).
Fig. 5.
Sexual differentiation of oxytocin-dependent circuits of social avoidance occurs during puberty. (A) Experimental timeline for immunohistochemistry analyses in prepubertally castrated or intact California mice. (B) Overlay of oxytocin (green) and c-fos (magenta) immunostaining in the PVN. Arrows indicate colocalizations in oxytocin (B’) and c-fos (B”) images. (C) In the anterior PVN, social defeat increased colocalizations (n = 6 to 7 per group) in prepubertally castrated males but not intact males (C’) and that social approach was negatively correlated with oxytocin/c-fos colocalizations (C”). (D) In the posterior PVN, castration increased oxytocin/c-fos colocalizations regardless of stress status (D’) and social approach was negatively correlated with colocalizations. (E) In anteromedial BNST, where oxytocin receptors drive social avoidance, prepubertal castration increased c-fos immunoreactivity regardless of stress status (E’) and c-fos positive cells were negatively correlated with social approach (E”). (F) Experimental timeline for examining effects of oxytocin receptors on social behavior. (G and H) In both males and females exposed to social defeat, an i.p. injection of 5 mg/kg of the oxytocin receptor antagonist L368,899 increased social approach and decreased social vigilance (n = 7 to 9 per group). *, **P < 0.05, control/vehicle. †P < 0.05 vs. intact. Data available at DOI: 10.6084/m9.figshare.23929644 (60).
We also tested whether defeat-induced social avoidance and social vigilance in juvenile mice were dependent on oxytocin receptors (Fig. 5F) as in adults (40). In both males and females, treatment with 5 mg/kg i.p. (intraperitoneal) of the oxytocin receptor antagonist L-368,899 30 min before testing increased social approach (Fig. 5G, main effect of dose F2,42 = 8.42, P < 0.001) and decreased social vigilance (Fig. 5H, Kruskal–Wallis = 15.36, P < 0.009). There were no differences in behavior during the acclimation (SI Appendix, Figs. S5F and S6E) or open-field (SI Appendix, Figs. S5H and S6G) phases. Together, these results suggest that stress-induced social avoidance and social vigilance in both male and female juvenile mice is dependent on oxytocin receptor activation, as seen in adult females. Additionally, the results indicate that male pubertal hormones program these circuits to be less active in adulthood.
Androgens Reverse the Effects of Prepubertal Castration on Behavior.
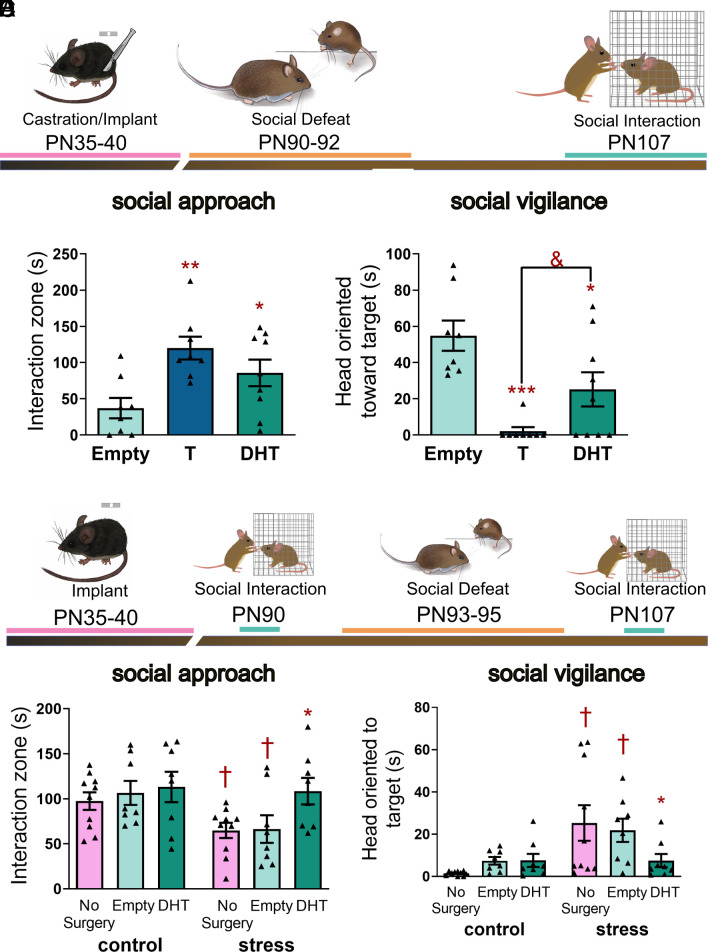
To test the impact of androgen replacement at puberty, juvenile males were prepubertally castrated and randomly assigned to receive a silastic implant containing testosterone, the nonaromatizable androgen dihydrotestosterone (DHT), or sealant only (Fig. 6A). These implants produce plasma hormone levels within the physiological range of adult male California mice (61). Between PN90-92, mice were exposed to defeat stress and then 2 wk later tested in a social interaction test. Results demonstrated that hormone replacement altered social approach (Fig. 6B, F2,22 = 6.2, P < 0.01) and social vigilance (Fig. 6C, Kruskal–Wallis = 13.13, P < 0.001). For social approach, both testosterone (P = 0.002, d = 1.7) and DHT (P = 0.002, d = 1.0) treatment yielded significantly higher levels of social approach compared to males treated with empty implants. Similarly for social vigilance, both testosterone- (P < 0.001, d = 3.1) and DHT (P = 0.049, d = 1.1)-treated males had lower social vigilance than males treated with empty implants. There was a nonsignificant trend for testosterone-treated males to have lower social vigilance than DHT-treated males (P = 0.078, d = 1.0). During the acclimation phase, testosterone and DHT treatment increased approach to an empty cage (SI Appendix, Fig. S7A, F2,22 = 5.02, P < 0.02) and there were no differences in behavior during the open-field phase (SI Appendix, Fig. S7B and C). We also tested whether DHT treatment at puberty could impact female behavior (Fig. 6D). In social interaction tests performed before stress exposure, there were no differences in social approach (Fig. 6E), social vigilance (Fig. 6F), acclimation (SI Appendix, Fig. S7D), or open-field behavior (SI Appendix, Fig. S7 E and F). Importantly, after social defeat, hormone treatment altered social approach (Fig. 6E, F2,23 = 3.76, P = 0.04) with DHT increasing social approach vs. empty implants (P = 0.02, d = 0.1). Effects on social vigilance were weaker (Kruskal–Wallis = 5.04, P = 0.08), with a post hoc test indicating lower levels of social vigilance in DHT-treated mice vs. empty implant (Mann-Whitney = 11, P = 0.028, d = 1.2). After stress exposure, DHT treatment increased approach to an empty cage (SI Appendix, Fig. S7D, F2,23 = 5.32, P = 0.01) while there were no differences in the open-field phase (SI Appendix, Fig. S7 E and F)
Fig. 6.
Androgen treatment at puberty reduces effects of social defeat on social approach and social vigilance in males and females. (A) Experimental timeline for surgery, social defeat, and social interaction testing in males. (B) Castrated males treated with DHT or testosterone (T) implants had higher social approach than males treated with empty implants (n = 7 to 8 per group). (C) Treatment with DHT or T also lowered social vigilance. (D) Experimental timeline for surgery, behavior testing, and social defeat in female California mice. (E) Hormone treatment had no effect on social approach before stress exposure but after stress exposure, DHT-treated females showed more social approach than females treated with empty implants or no-surgery controls (n = 8 to 10 per group). (F) Similarly, there were no differences in social vigilance in before stress, but after stress, social vigilance increased in no-surgery control and empty implant females but not DHT females. *P < 0.05, **P < 0.01, ***P < 0.001 vs. empty implant. &P < 0.078 vs T. †P < 0.05 vs. control (pre-stress). Data available at DOI: 10.6084/m9.figshare.23664546 (62).
Discussion
Sex differences in stress sensitivity emerge at puberty in both humans (63, 64) and other animals (20–22), coinciding with an increased incidence of anxiety in women (10, 12, 14, 15). However, little is known about the underlying mechanisms. Our study fills a gap in knowledge by establishing a causal link between androgens and reduced impact of social stress on approach and vigilance behaviors. Previous studies showed that social stress increased neural activity within the BNST, but the precise timing of this activity in relation to threats and defensive responses was unknown. Here, calcium imaging data showed that ventral BNST neural activity increased when focal mice interacted with aggressor target mice, but no changes were observed during freezing. Prepubertal castration generalized BNST responses to less threatening social interactions with nonaggressive mice. These data inform current debates on BNST function (65) and are consistent with reports of increased reactivity of the BNST in humans diagnosed with anxiety disorders (66). Our findings show that androgens play an organizational role during puberty to attenuate behavioral and neural responses of the BNST to social stress in adulthood.
BNST Neurons Respond to Social Threats.
Immediate early gene analyses in rodents (37, 67–70) and neuroimaging work in primates (71) and humans (32) show that stressful social contexts increase neural activity within the BNST. However, these approaches have coarse temporal resolution, impeding the assessment of how threat proximity drives BNST responses. This is reflected in alternative hypotheses for BNST function. Early work suggested that the BNST encodes diffuse or remote threats (72) while more recent work suggests that the BNST is highly responsive to immediate threats (65). Our calcium imaging data show that ventral BNST calcium transients were reliably triggered when receiving aggressive attacks while activity was no different from baseline during bouts of freezing or when aggressive target mice were more than 8 cm away from focal mice. Across both studies, our results support the hypothesis that ventral BNST responds to immediate threats.
Prepubertally castrated males exposed to defeat exhibited more freezing and heightened BNST calcium transients in the presence of naive target mice. Prepubertal castration also increased avoidance and social vigilance toward novel naive target mice, similar to stressed adult females (43, 73). These data suggest that male gonadal hormones act during puberty to reduce negative valence assessments of novel social contexts, an important function of the BNST (74, 75). Thus, androgens may impede overgeneralization of threat, which is a key characteristic of anxiety disorders (76). Our results align with imaging studies in nonhuman primates (77) and humans (66) reporting stronger BNST responses in individuals with elevated anxiety-related behaviors. Intriguingly, androgens can reduce neuronal excitability in both adult (78) and pubertal rodents (79–81). These findings are in line with our observations that prepubertal castration led to increased c-fos/oxytocin colocalizations in the PVN and c-fos expression in anteromedial BNST. Oxytocin neurons in the PVN project to the BNST (82), suggesting that these outcomes could be functionally linked. Our results suggest that androgens acting during puberty may reduce the excitability of circuits that are affected by social defeat. Defeat stress increased acute BNST responses in intact males after attacks by naive target mice. At first glance, this outcome appears inconsistent with observations in the castration study that implicate gonadal hormones in preventing the generalization of threat responses. In the prestress social interaction test, intact focal mice were attacked by naive target mice in the testing arena. After defeat stress, focal mice were tested in the same arena. Focal mice may have learned that naive target mice or the testing location were threatening, which may explain the effect of defeat stress on acute BNST responses. Future work is needed to assess the extent to which androgens modulate the perception of threat.
An open question is which BNST cell types respond to social threats. This is further complicated by the fact that the BNST exhibits heterogeneity in neural responses, even within genetically defined cell populations (83–85). This variation could be explained by projection-specific populations within a cell type (86). This is supported by optogenetic studies showing the projection-specific effects of BNST neurons on behavior (87–90). Future research considering genetic and projection-specific populations during neuronal recording of activity in different social contexts will be informative. Future studies could also consider whether effects of pubertal hormones are dependent on prenatal or neonatal (91, 92) surges in testosterone.
Activation of Androgen Receptors during Puberty Blunts Stress-Induced Social Avoidance.
In adult hamsters, dominant males have more androgen receptor expression in the medial amygdala (MEA) than subordinates, and pharmacological inhibition of AR in the MEA of dominant males increases sensitivity to social stress (93). Similarly, testosterone can exert acute anxiolytic effects in a variety of behavioral assays (94–96). Interestingly, in adult California mice, androgens do not affect social approach regardless of stress exposure even though they blunt acute corticosterone responses to defeat (43). Here, we show that androgens act during puberty to influence how social behavior is impacted by stress, suggesting that even in species where androgens do not have an overt role in regulating stress sensitivity in adults, androgens can program behavioral responses during adolescence. This may be particularly relevant for humans, where the relationship between gonadal hormones and stress responses in adults can be inconsistent (97–99). Although pubertal DHT and testosterone treatment had similar effects on social approach in stressed males, the effect of DHT on social vigilance was weaker. Similar effects were observed in females. These results suggest that estrogen receptors may play a complementary role in the pubertal organization of stress-induced behavior. Estrogens can enhance anxiety-related behaviors by activating Esr1 or exerting anxiolytic effects via Esr2 (100). Both receptors are present in the BNST, and future studies should consider the possible developmental effects of these receptors on stress-induced social vigilance.
Our studies provide strong evidence for a key role for pubertal hormone action, but they have a few limitations. Silastic implants released hormones both during puberty and adulthood. We did not test whether effects of pubertal androgen exposure were maintained in the absence of androgens in adults, as has been done for sexual behavior in hamsters (101). However, our previous work clearly demonstrates that adult gonadal hormones have little impact on stress-induced social avoidance (43, 50). Although testosterone reduces the impact of stress on social behaviors, social defeat still has strong effects on male California mice. Social defeat induces deficits in reversal learning in male but not female California mice (102), and future work is needed to assess whether this sex difference is mediated by androgens. Social defeat also induces a conditioned defeat phenotype in both males and females (73), similar to hamsters (103). Thus, the extent to which androgens blunt the effects of social defeat on behavior is limited.
Functional Implications.
Steroid hormones alter brain development during puberty (23, 25, 27), and our work indicates that sex differences in the effects of stress on social behavior are affected by pubertal androgen exposure. Increased sensitivity to social stress coincided with more generalized reactivity of BNST neurons during social engagement. Importantly, the effects of pubertal androgens on social behavior were only apparent following exposure to social defeat. This demonstrates that organizational effects of pubertal hormones can cause latent vulnerabilities that are only revealed after stressful social experiences. Our results suggest that it will be worthwhile to consider whether testosterone levels during adolescence predict behavioral or neural responses to stressors in adulthood. Overall, our research sheds light on how androgens shape the complex interplay between brain circuits and behavioral sensitivity to stress.
Materials and Methods
Animals.
All studies were conducted with California mice (P. californicus) raised in a colony at UC Davis. Mice were housed in same sex groups (2–4) in clear polypropylene cages with Sani-Chip bedding (Harlan Laboratories, Indianapolis, IN, USA), Nestlets (Ancare, Bellmore, NY, USA), and Enviro-Dri (Eco-bedding, Fibercore, Cleveland, OH, USA). Mice were kept on a 16L:8D light cycle and had ad libitum access to food and water. All procedures were approved by the Institutional Animal Care and Use Committee at the University of California, Davis and in accordance with NIH guidelines.
Puberty Quantification.
For measures of first vaginal opening, preputial separation, weight, and coat color mice were briefly anesthetized (>1 min isoflurane) before being weighed, photographed, and assessed for vaginal opening/preputial separation. A separate set of mice were euthanized (https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf) (104) to determine uterine or testes size. Trunk blood was collected and plasma was obtained and frozen for hormone assays (SI Appendix, Supplementary Methods). We also quantified the transition from the juvenile pelage (dark gray) to adult (brown) adult pelage (105) by quantifying digital images in ImageJ as previously described (106). All quantifications were done using nonexperimental, unstressed mice.
Juvenile Social Defeat.
Male and female juveniles (PN34-36) underwent 3 consecutive days of social defeat stress (or control handling) as described previously (50). During each episode of defeat, the focal mouse was placed in the home cage of a novel male–female adult resident pair. The opposite sex resident mouse was removed from the cage prior to the start of the defeat session. Each session of defeat lasted for 7 min or until the test mouse received 7 bites. Control mice were placed into a clean, empty cage for 7 min across 3 consecutive days.
On PN50, mice underwent a social interaction test. The social interaction test had 3 phases: open-field, acclimation, and interaction. In the open-field phase, the mouse was placed into an open area and allowed to explore it for 3 min. In the acclimation phase, an empty cage was placed at one end of the arena and the mouse was allowed to explore for 3 min. In the interaction phase, a caged, unknown adult conspecific replaced the empty cage at one end of the arena, and the mouse was allowed to explore for 3 min. Time in the interaction zone (8 cm from the cage) is defined as “social approach” and was scored with AnyMaze software (43). The duration of social vigilance behavior was hand scored from a video recording. Social vigilance was defined as any time the test mouse was sitting still, head oriented toward the target mouse, while outside of the interaction zone (40).
Prepubertal Castration.
Male juveniles were randomly assigned to castration, sham surgery, or no-surgery control between PN35 and PN40 (61). During castration, mice were anesthetized with isoflurane and treated with 0.1 mg/kg buprenorphine and 5 mg/kg of carprofen. Nonsurgery controls were included to control for possible effects of early life exposure to the anesthesia isoflurane (107). These mice received no manipulations until the start of social defeat stress/control handling. At PN90-92, all mice underwent 3 d of either social defeat or control handling. At PN106, mice were tested in a social interaction test. Brains were collected 1 h after the social interaction test.
Calcium Imaging of the BNST.
In the first study, male juveniles were randomly assigned to castration or sham surgery after weaning. As adults, all mice received an injection of AAV9.Syn.GCaMP6s at a rate of 100 nL/min for a total volume of 500 nL in the ventral BNST (AP +0.45mm, ML ±1.0mm, DV −5.6 mm). The virus was allowed to diffuse for 10 min before the needle was withdrawn. An optical fiber (Doric) with a 2.5-mm core and 0.66 NA threaded through a ceramic ferrule was implanted at the injection site and the ferrule was secured to the skull with a layer of C&B Metabond (Parkell), followed by a layer of dental cement to form a thick headcap. Mice were housed two per cage with a clear, perforated acrylic divider that allowed auditory, tactile, and olfactory contact.
Mice recovered for 1 wk before undergoing 3 consecutive days of social defeat stress. Ten days later, mice began 3 consecutive days of patch cord habituation in which the patch cord was gently coupled to their optical fiber implant. The mouse then explored a novel cage for 10 min. The next day mice were tested in a social interaction test with freely moving target mice. The focal mouse was placed into an empty area (51 × 25.4 × 76 cm) attached to a small box (13 × 10 × 18 cm) with a sliding door for 6 min. Photometry recording was performed with an isosbestic channel of 405 nm and an excitatory channel of 470 nm, both set to 50 μW output. Photometry data from the acclimation period were excluded from analysis due to initial bleaching. Next, an unfamiliar, nonaggressive adult male target mouse was introduced into the arena through the sliding door. Mice were allowed to freely interact for 3 min. The target mouse was removed, and then a sexually experienced, aggressive male (from a previous social defeat episode) was introduced into the arena for 3 min. After testing brains were collected to confirm viral expression and placement of the fiber. In a second study, we examined calcium transients in intact adult males before and after stress. Surgery for GCaMP expression and fiber implantation were performed as described above. After 4 wk of recovery, all mice were habituated and tested with freely moving target mice (naive and aggressive) as in the first study. Five days later, mice were exposed to three episodes of social defeat and then tested again in a social interaction test 1 wk after the last episode of defeat.
We used Deeplabcut to quantify distance and orientation of the focal mouse in relation to target mice with precision that matched the high temporal resolution of photometry data (108–110). Video was taken from the side view at a rate of 30 fps. In addition, training was performed to track the nose, right ear, left ear, body midpoint, front leg, back leg, tail base, and tail tip for both the test mouse and stimulus mouse. The tracking for the nose and body midpoint was used in order to determine proximity, defined as times when the body midpoint of the two mice was within 8 cm (the approximate length of a California mouse) of each other. We determined the direction the test mouse was facing in relation to the stimulus mouse using their coordinate points obtained from the deeplabcut tracking (test mouse nose, body midpoint, and target mouse nose). We binned angles as within the test mouse’s central vision (0° ≥ 40°), peripheral vision (40° ≥ 100°), or facing away from the stimulus mouse (100° ≥ 180°) (55). All scripts are deposited at https://github.com/bctrainorlab/behavioral_quantification. Nose-to-nose sniffing, anogenital sniffing, freezing, aggressive behavior directed towards focal mice, and aggressive behavior directed towards target mice were scored by an observer without knowledge of the treatment group using BORIS.
Pubertal Hormone Manipulation Studies.
Male juveniles were castrated and received a subcutaneous silastic implant (i.d. 0.04 in, o.d. 0.085 in) containing 1 mm crystalline testosterone, DHT, or Dowsil Sealant (3145 RTV MIL-A-46146). These implants yield hormone levels that are within the physiological range of intact male California mice (111) and continued to release hormones into adulthood. At adulthood, mice underwent 3 d social defeat and were tested in a social interaction test 2 wk later.
Intact females were randomly assigned to receive a DHT or empty implant at PN35-PN40 as described for males. At PN90, all mice were tested in a social interaction test. Three days later females were exposed to 3 d of social defeat on consecutive days. One week later, females were tested again in a social interaction test. This within-subjects design produces behavioral effects similar to the between-subjects design used for males (112).
Immunohistochemistry.
Sections from castrated or intact males were stained for either oxytocin/c-fos (PVN) or c-fos (anteromedial BNST) as previously described (37). Full methods are described in SI Appendix, Supplementary Methods.
Juvenile Social Defeat + OTA (Oxytocin Antagonist) Injections.
Male and female juveniles (PN34-36) underwent 3 consecutive days of social defeat stress. At P50 mice were tested in a social interaction test. Thirty minutes before the start of the social interaction test, each mouse received an i.p. injection of 5 mg/kg or 10 mg/kg of the OTA L-368,899 (40), or an injection of sterile phosphate-buffered saline.
Statistics.
Statistical analyses for experiments (excluding fiber photometry experiments) were performed using R statistical software. Normality was assessed via QQPlot. A Flinger–Killen test was used to assess the homogeneity of variance. Steroid hormone data were log transformed for analysis. Hormone and most behavioral and cell count data were analyzed using ANOVA with planned comparisons for comparing treatment groups with controls. Social vigilance and oxytocin/c-fos colocalization data had heterogeneous variability, so nonparametric analyses were used. Full methods of photometry data analyses are described in SI Appendix, Supplementary Methods.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
Thanks to C. Dong and J. Roshgadol for assistance with fiber photometry, A. Bentz for helpful discussions, N. Duque-Wilckens for drawings. This work was supported by NIH F32MH125597 to E.C.W., NIH P51OD011107 to A.S.F., NIH P51OD011107 to A.K., NIH U01NS120820 to L.T., NSF IOS1937335, NIH R01MH121829, and a UC Davis Academic Senate Grant to B.C.T.
Author contributions
E.C.W. and B.C.T. designed research; E.C.W., P.X.L., H.C.Z., A.S.G., A.A.L., Z.D.P., S.S., H.I.C., A.V.R., T.D., A.K., C.C., and B.C.T. performed research; E.C.W., H.C.Z., L.T., and A.S.F. contributed new reagents/analytic tools; E.C.W., H.C.Z., A.S.F., P.X.L, and B.C.T. analyzed data; and E.C.W. and B.C.T. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Behavior, photometry, cell counts data have been deposited in Figshare (10.6084/m9.figshare.23681493 (49); 10.6084/m9.figshare.22782959 (52); 10.6084/m9.figshare.23664543 (56); 10.6084/m9.figshare.23983032 (57); 10.6084/m9.figshare.23929644 (60); and10.6084/m9.figshare.23664546 (62)).
Supporting Information
References
- 1.Kessler R. C., et al. , Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Bruce S. E., et al. , Influence of psychiatric comorbidity on recovery and recurrence in generalized anxiety disorder, social phobia, and panic disorder: A 12-year prospective study. AJP 162, 1179–1187 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bangasser D. A., Cuarenta A., Sex differences in anxiety and depression: Circuits and mechanisms. Nat. Rev. Neurosci. 22, 674–684 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Wellman C. L., et al. , Sex differences in risk and resilience: Stress effects on the neural substrates of emotion and motivation. J. Neurosci. 38, 9423–9432 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinton E. A., Li D. C., Allen A. G., Gourley S. L., Social isolation in adolescence disrupts cortical development and goal-dependent decision-making in adulthood, despite social reintegration. eNeuro 6, ENEURO.0318-19.2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., et al. , Global, regional and national burden of anxiety disorders from 1990 to 2019: Results from the Global Burden of Disease Study 2019. Epidemiol. Psychiatric Sci. 30, e36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollingworth S. A., Burgess P. M., Whiteford H. A., Affective and anxiety disorders: Prevalence, treatment and antidepressant medication use. Aust. N. Z. J. Psychiatry 44, 513–519 (2010). [DOI] [PubMed] [Google Scholar]
- 8.Hunt C., Issakidis C., Andrews G., DSM-IV generalized anxiety disorder in the Australian National Survey of Mental Health and Well-Being. Psychol. Med. 32, 649–659 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Ma X., et al. , Generalized anxiety disorder in China: Prevalence, sociodemographic correlates, comorbidity, and suicide attempts. Perspect. Psychiatric Care 45, 119–127 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Munk-Jørgensen P., et al. , Prevalence of generalized anxiety disorder in general practice in Denmark, Finland, Norway, and Sweden. PS 57, 1738–1744 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Remes O., Brayne C., van der Linde R., Lafortune L., A systematic review of reviews on the prevalence of anxiety disorders in adult populations. Brain Behav. 6, e00497 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beesdo K., et al. , Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Arch. Gen. Psychiatry 64, 903–912 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Dahl R. E., Adolescent brain development: A period of vulnerabilities and opportunities. Keynote address. Ann. N. Y. Acad. Sci. 1021, 1–22 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Davey C. G., Yücel M., Allen N. B., The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosci. Biobehav. Rev. 32, 1–19 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Wesselhoeft R., Pedersen C. B., Mortensen P. B., Mors O., Bilenberg N., Gender–age interaction in incidence rates of childhood emotional disorders. Psychol. Med. 45, 829–839 (2015). [DOI] [PubMed] [Google Scholar]
- 16.DePasquale C. E., Herzberg M. P., Gunnar M. R., The pubertal stress recalibration hypothesis: Potential neural and behavioral consequences. Child Dev. Perspect. 15, 249–256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goddings A.-L., Beltz A., Peper J. S., Crone E. A., Braams B. R., Understanding the role of puberty in structural and functional development of the adolescent brain. J. Res. Adolesc. 29, 32–53 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Chini M., Hanganu-Opatz I. L., Prefrontal cortex development in health and disease: Lessons from rodents and humans. Trends Neurosci. 44, 227–240 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Caballero A., Orozco A., Tseng K. Y., Developmental regulation of excitatory-inhibitory synaptic balance in the prefrontal cortex during adolescence. Semin. Cell Dev. Biol. 118, 60–63 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asgari P., McKinney G., Hodges T. E., McCormick C. M., Social instability stress in adolescence and social interaction in female rats. Neuroscience 477, 1–13 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Graf A., et al. , Acute and long-term sex-dependent effects of social instability stress on anxiety-like and social behaviours in Wistar rats. Behav. Brain Res. 438, 114180 (2023). [DOI] [PubMed] [Google Scholar]
- 22.Bourke C. H., Neigh G. N., Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm. Behav. 60, 112–120 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmed E. I., et al. , Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat. Neurosci. 11, 995–997 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delevich K., Piekarski D., Wilbrecht L., Neuroscience: Sex hormones at work in the neocortex. Curr. Biol. 29, R122–R125 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Drzewiecki C. M., Willing J., Juraska J. M., Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: A role for pubertal onset. Synapse 70, 361–368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz K. M., Sisk C. L., The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci. Biobehav. Rev. 70, 148–158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delevich K., Thomas A. W., Wilbrecht L., Adolescence and “late blooming” synapses of the prefrontal cortex. Cold Spring Harb. Symp. Quant. Biol. 83, 37–43 (2019), 10.1101/sqb.2018.83.037507. [DOI] [PubMed] [Google Scholar]
- 28.Gegenhuber B., Wu M. V., Bronstein R., Tollkuhn J., Gene regulation by gonadal hormone receptors underlies brain sex differences. Nature 606, 153–159 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyer A. E., et al. , Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. Am. J. Psychiatry 169, 205–212 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clauss J. A., Blackford J. U., Behavioral inhibition and risk for developing social anxiety disorder: A meta-analytic study. J. Am. Acad. Child Adolesc. Psychiatry 51, 1066–1075.e1 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorn L. D., Hostinar C. E., Susman E. J., Pervanidou P., Conceptualizing puberty as a window of opportunity for impacting health and well-being across the life span. J. Res. Adolesc. 29, 155–176 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Somerville L. H., et al. , Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cereb. Cortex 23, 49–60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figel B., et al. , Phasic amygdala and BNST activation during the anticipation of temporally unpredictable social observation in social anxiety disorder patients. Neuroimage Clin. 22, 101735 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glover G. H., Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 22, 133–139 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong C., et al. , Fluorescence imaging of neural activity, neurochemical dynamics, and drug-specific receptor conformation with genetically encoded sensors. Annu. Rev. Neurosci. 45, 273–294 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siciliano C. A., Tye K. M., Leveraging calcium imaging to illuminate circuit dysfunction in addiction. Alcohol 74, 47–63 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinman M. Q., et al. , Sex-specific effects of stress on oxytocin neurons correspond with responses to intranasal oxytocin. Biol. Psychiatry 80, 406–414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg G. D., et al. , Sex differences in stress-induced social withdrawal: Role of brain derived neurotrophic factor in the bed nucleus of the stria terminalis. Front. Behav. Neurosci. 7, 223 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duque-Wilckens N., et al. , Extra-hypothalamic oxytocin neurons drive stress-induced social vigilance and avoidance. Proc Natl. Acad. Sci. U.S.A. 117, 26406–26413 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duque-Wilckens N., et al. , Oxytocin receptors in the anteromedial bed nucleus of the stria terminalis promote stress-induced social avoidance in female California mice. Biol. Psychiatry 83, 203–213 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luo P. X., et al. , Oxytocin receptor behavioral effects and cell types in the bed nucleus of the stria terminalis. Horm. Behav. 143, 105203 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trainor B. C., et al. , Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (peromyscus californicus). PLoS One 6, e17405 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trainor B. C., et al. , Sex differences in stress-induced social withdrawal: Independence from adult gonadal hormones and inhibition of female phenotype by corncob bedding. Horm. Behav. 63, 543–550 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuske J. X., Trainor B. C., “Mean girls: Social stress models for female rodents” in Current Topics in Behavioral Neurosciences, Miczek K.A., Sinha R., Eds. (Springer, Cham, 2021), pp. 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu X., Arumugam R., Zhang N., Lee M. M., Androgen profiles during pubertal Leydig cell development in mice. Reproduction 140, 113–121 (2010). [DOI] [PubMed] [Google Scholar]
- 46.Deboer M. D., Li Y., Puberty is delayed in male mice with dextran sodium sulfate colitis out of proportion to changes in food intake, body weight, and serum levels of leptin. Pediatr. Res. 69, 34–39 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendle J., Beltz A. M., Carter R., Dorn L. D., Understanding puberty and its measurement: Ideas for research in a new generation. J. Res. Adolesc. 29, 82–95 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Shirtcliff E. A., Dahl R. E., Pollak S. D., Pubertal development: Correspondence between hormonal and physical development: hormonal correlates of pubertal stage. Child Dev. 80, 327–337 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trainor B., Wright E., Figure 1 and Figures S1 and S2: Behavior and physiology data. Figshare. 10.6084/m9.figshare.23681493. Accessed 1 October 2023. [DOI]
- 50.Trainor B. C., et al. , Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PLoS One 6, e17405 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenberg G. D., Steinman M. Q., Doig I. E., Hao R., Trainor B. C., Effects of social defeat on dopamine neurons in the ventral tegmental area in male and female California mice. Eur. J. Neurosci. 42, 3081–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright E., Trainor B., Fig 2, SFig 3.xlsx. Figshare. 10.6084/m9.figshare.22782959. Accessed 2 October 2023. [DOI]
- 53.Numa C., et al. , Social defeat stress-specific increase in c-Fos expression in the extended amygdala in mice: Involvement of dopamine D1 receptor in the medial prefrontal cortex. Sci. Rep. 9, 16670 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kollack-Walker D., Watson A., Differential expression of c- fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J. Neuroendocrinol. 11, 547–559 (1999). [DOI] [PubMed] [Google Scholar]
- 55.Samonds J. M., Choi V., Priebe N. J., Mice discriminate stereoscopic surfaces without fixating in depth. J. Neurosci. 39, 8024–8037 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright E., Trainor B., Fig 3.xlsx. Figshare. 10.6084/m9.figshare.23664543. Accessed 2 October 2023. [DOI]
- 57.Wright E., Trainor B., Fig 4.xlsx. Figshare. 10.6084/m9.figshare.23983032. Accessed 1 October 2023. [DOI]
- 58.Bester-Meredith J. K., Marler C. A., Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus). Horm. Behav. 40, 51–64 (2001). [DOI] [PubMed] [Google Scholar]
- 59.Lewis E. M., et al. , Parallel social information processing circuits are differentially impacted in Autism. Neuron 108, 659–675.e6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trainor B., Wright E., Figure 5 and Figure S6. Figshare. 10.6084/m9.figshare.23929644. Accessed 1 October 2023. [DOI]
- 61.Trainor B. C., Marler C. A., Testosterone promotes paternal behaviour in a monogamous mammal via conversion to oestrogen. Proc. R Soc. Lond. Ser. B Biol. Sci. 269, 823–829 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright E., Fig 6, SFig 7.xlsx. Figshare. 10.6084/m9.figshare.23664546. Accessed 2 October 2023. [DOI]
- 63.Hostinar C. E., Johnson A. E., Gunnar M. R., Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev. Sci. 18, 281–297 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wright E. C., Hostinar C. E., Trainor B. C., Anxious to see you: Neuroendocrine mechanisms of social vigilance and anxiety during adolescence. Eur. J. Neurosci. 52, 2516–2529 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shackman A. J., Fox A. S., Contributions of the central extended amygdala to fear and anxiety. J. Neurosci. 36, 8050–8063 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clauss J. A., Avery S. N., Benningfield M. M., Blackford J. U., Social anxiety is associated with BNST response to unpredictability. Depress Anxiety 36, 666–675 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kollack-Walker S., Newman S. W., Mating and agonistic behavior produce different patters of Fos immunolabeling in the male Syrian hamster brain. Neuroscience 66, 721–736 (1995). [DOI] [PubMed] [Google Scholar]
- 68.Nasanbuyan N., et al. , Oxytocin-oxytocin receptor systems facilitate social defeat posture in male mice. Endocrinology 159, 763–775 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Newman E. L., et al. , Fighting females: Neural and behavioral consequences of social defeat stress in female mice. Biol. Psychiatry 86, 657–668 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martinez M., Phillips P. J., Herbert J., Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur. J. Neurosci. 10, 20–33 (1998). [DOI] [PubMed] [Google Scholar]
- 71.Fox A. S., et al. , Intergenerational neural mediators of early-life anxious temperament. Proc. Natl. Acad. Sci. U.S.A. 112, 9118–9122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davis M., Walker D. L., Miles L., Grillon C., Phasic vs. sustained fear in rats and humans: Role of the extended amygdala in fear vs. anxiety. Neuropsychopharmacology 35, 105–135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steinman M. Q., et al. , Hypothalamic vasopressin systems are more sensitive to the long term effects of social defeat in males versus females. Psychoneuroendocrinology 51, 122–134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Flanigan M. E., Kash T. L., Coordination of social behaviors by the bed nucleus of the stria terminalis. Eur. J. Neurosci. 55, 2404–2420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lebow M. A., Chen A., Overshadowed by the amygdala: The bed nucleus of the stria terminalis emerges as key to psychiatric disorders. Mol. Psychiatry 21, 450–463 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lissek S., et al. , Classical fear conditioning in the anxiety disorders: A meta-analysis. Behav. Res. Therapy 43, 1391–1424 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Fox A. S., Shelton S. E., Oakes T. R., Davidson R. J., Kalin N. H., Trait-like brain activity during adolescence predicts anxious temperament in primates. PLOS One 3, e2570 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Williams E. S., et al. , Androgen-dependent excitability of mouse ventral hippocampal afferents to nucleus accumbens underlies sex-specific susceptibility to stress. Biol. Psychiatry 87, 492–501 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harley C. W., Malsbury C. W., Squires A., Brown R. A., Testosterone decreases CA1 plasticity in vivo in gonadectomized male rats. Hippocampus 10, 693–697 (2000). [DOI] [PubMed] [Google Scholar]
- 80.Hebbard P. C., King R. R., Malsbury C. W., Harley C. W., Two organizational effects of pubertal testosterone in male rats: Transient social memory and a shift away from long-term potentiation following a tetanus in hippocampal CA1. Exp. Neurol. 182, 470–475 (2003). [DOI] [PubMed] [Google Scholar]
- 81.Moradpour F., Fathollahi Y., Naghdi N., Hosseinmardi N., Javan M., Prepubertal castration causes the age-dependent changes in hippocampal long-term potentiation. Synapse 67, 235–244 (2013). [DOI] [PubMed] [Google Scholar]
- 82.Knobloch H. S., et al. , Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73, 553–566 (2012). [DOI] [PubMed] [Google Scholar]
- 83.Yu W., Caira C. M., Del R Rivera Sanchez N., Moseley G. A., Kash T. L., Corticotropin-releasing factor neurons in the bed nucleus of the stria terminalis exhibit sex-specific pain encoding in mice. Sci. Rep. 11, 12500 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodriguez-Romaguera J., et al. , Prepronociceptin-expressing neurons in the extended amygdala encode and promote rapid arousal responses to motivationally salient stimuli. Cell Rep. 33, 108362 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang B., Karigo T., Anderson D. J., Transformations of neural representations in a social behaviour network. Nature 608, 741–749 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaouane N., Ada S., Hausleitner M., Haubensak W., Dorsal bed nucleus of the stria terminalis-subcortical output circuits encode positive bias in pavlovian fear and reward. Front. Neural. Circuits 15, 772512 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian G., et al. , An extended amygdala-midbrain circuit controlling cocaine withdrawal-induced anxiety and reinstatement. Cell Rep. 39, 110775 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S.-Y., et al. , Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jennings J. H., et al. , Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giardino W. J., et al. , Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat. Neurosci. 21, 1084–1095 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Corbier P., Edwards D. A., Roffi J., The neonatal testosterone surge: A comparative study. Arch. Int. Physiol. Biochim. Biophys. 100, 127–131 (1992). [DOI] [PubMed] [Google Scholar]
- 92.Lenz K. M., McCarthy M. M., Organized for sex–Steroid hormones and the developing hypothalamus: Steroids and the developing hypothalamus. Eur. J. Neurosci. 32, 2096–2104 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cooper M. A., et al. , Gonadal steroid hormone receptors in the medial amygdala contribute to experience-dependent changes in stress vulnerability. Psychoneuroendocrinology 129, 105249 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Aikey J. L., Nyby J. G., Anmuth D. M., James P. J., Testosterone rapidly reduces anxiety in male house mice (Mus musculus). Horm. Behav. 42, 448–460 (2002). [DOI] [PubMed] [Google Scholar]
- 95.Frye C. A., Seliga A. M., Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn. Affective Behav. Neurosci. 1, 371–381 (2001). [DOI] [PubMed] [Google Scholar]
- 96.Carrier N., Kabbaj M., Extracellular signal-regulated kinase 2 signaling in the hippocampal dentate gyrus mediates the antidepressant effects of testosterone. Biol. Psychiatry 71, 642–651 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Seeman T. E., Singer B., Wilkinson C. W., McEwen B., Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology 26, 225–240 (2001). [DOI] [PubMed] [Google Scholar]
- 98.Uhart M., Chong R., Oswald L., Lin P., Wand G., Gender differences in hypothalamic–pituitary–adrenal (HPA) axis reactivity. Psychoneuroendocrinology 31, 642–652 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Kirschbaum C., Kudielka B. M., Gaab J., Schommer N. C., Hellhammer D. H., Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Med. 61, 154–162 (1999). [DOI] [PubMed] [Google Scholar]
- 100.Borrow A. P., Handa R. J., Estrogen receptors modulation of anxiety-like behavior. Vitam. Horm. 103, 27–52 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schulz K. M., et al. , Gonadal hormones masculinize and defeminize reproductive behaviors during puberty in the male Syrian hamster. Horm. Behav. 45, 242–249 (2004). [DOI] [PubMed] [Google Scholar]
- 102.Laredo S. A., et al. , Effects of defeat stress on behavioral flexibility in males and females: Modulation by the mu-opioid receptor. Eur. J. Neurosci. 41, 434–441 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huhman K. L., et al. , Conditioned defeat in male and female Syrian hamsters. Horm. Behav. 44, 293–299 (2003). [DOI] [PubMed] [Google Scholar]
- 104.Leary S., et al. , AVMA Guidelines for the Euthanasia of Animals: 2020 Edition (AVMA, 2020), pp. 1–121. [Google Scholar]
- 105.Collins H. H., Studies of the pelage phases and of the nature of color variations in mice of the genus Peromyscus. J. Exp. Zool. 38, 45–107 (1923). [Google Scholar]
- 106.Ounpraseuth S., et al. , A method to quantify mouse coat-color proportions. PLOS One 4, e5414 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Murphy K. L., Baxter M. G., Long-term effects of neonatal single or multiple isoflurane exposures on spatial memory in rats. Front. Neurol. 4, 87 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lauer J., et al. , Multi-animal pose estimation and tracking with DeepLabCut. Nat. Methods 19, 496–504 (2023), 10.1101/2021.04.30.442096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nath T., et al. , Using DeepLabCut for 3D markerless pose estimation across species and behaviors. Nat. Protoc. 14, 2152–2176 (2019). [DOI] [PubMed] [Google Scholar]
- 110.Mathis A., et al. , DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Trainor B. C., Bird I. M., Alday N. A., Schlinger B. A., Marler C. A., Variation in aromatase activity in the medial preoptic area and plasma progesterone is associated with the onset of paternal behavior. NEN 78, 36–44 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Minie V. A., et al. , Enriched laboratory housing increases sensitivity to social stress in female California mice (Peromyscus californicus). Appl. Animal Behav. Sci. 241, 105381 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Behavior, photometry, cell counts data have been deposited in Figshare (10.6084/m9.figshare.23681493 (49); 10.6084/m9.figshare.22782959 (52); 10.6084/m9.figshare.23664543 (56); 10.6084/m9.figshare.23983032 (57); 10.6084/m9.figshare.23929644 (60); and10.6084/m9.figshare.23664546 (62)).