Abstract
Purpose
Recent studies have expanded the scope of research on the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet beyond its impact on cognitive performance. These investigations have specifically explored its potential to provide protection against cardiometabolic diseases and associated risk factors, including obesity and dyslipidemia.
Methods
We systematically summarized and evaluated all existing observational and trial evidence for the MIND diet in relation to cardiometabolic diseases and their risk factors in adults. PubMed, Embase, CINAHL and Cochrane Library databases were systematically searched to extract original studies on humans published until September 2023, without date restrictions. A total of 491 studies were initially retrieved, out of which 23 met the eligibility criteria and were included in the final review. Duplicated and irrelevant studies were screened out by five independent reviewers using the Rayyan platform. Quality assessment was ascertained using the Newcastle-Ottawa scale for observational studies and the Cochrane risk-of-bias tool (RoB 2) for randomized trials.
Results
Across the different study designs, the MIND diet was generally associated with an improvement in anthropometric measures and other cardiometabolic outcomes, such as blood pressure, glycemic control, lipid profile, inflammation and stroke. The effects of the MIND eating pattern on some cardiovascular diseases are less conclusive.
Conclusion
The findings of this systematic review support the recommendation of the MIND diet as a strategy to reduce cardiometabolic risk in adults. Further well-designed and long-term studies are warranted.
Keywords: MIND diet, dietary patterns, cardiometabolic diseases, systematic review
Introduction
Cardiometabolic diseases are rapidly growing as a global health concern,1,2 comprising a variety of conditions including cardiovascular disease (CVD), diabetes mellitus (DM), dyslipidemia, hypertension and non-alcoholic fatty liver disease (NAFLD).3 According to the International Diabetes Federation (IDF), more than one in ten adults are now living with diabetes globally, and this number will continue to rise.4 Since the beginning of the century, the prevalence of diabetes in adults has increased more than threefold, from an estimated 151 million in the year 2000 to 537 million today. This occurs in parallel with increases in the rates of other cardiometabolic diseases. For one, CVD remains the leading cause of mortality worldwide.5 From 271 million in 1990 to 523 million in 2019, prevalent cases of CVD have almost doubled over the last 30 years.6
A substantial body of evidence exists to support the role of a healthy diet in the prevention and control of these cardiometabolic conditions.7–10 In many countries around the world, dietary guidelines are shifting their focus from nutrient-based recommendations in support of dietary-based recommendations.11–16 This shift in dietary recommendations carries forward the emphasis that nutrients and foods are not consumed in isolation, but rather in various combinations over time.17 Dietary patterns account for the synergistic and/or antagonistic effects of these combinations in the diet as a whole.18 Furthermore, the overall influence of diet on cardiometabolic disease is more likely to be caused by the combined effects of dietary components, rather than those of a single nutrient or food.
Dietary patterns rich in plant foods, such as fruits, vegetables, wholegrains, nuts, seeds and legumes, are increasingly recommended as a strategy to lower the risk of cardiometabolic diseases.10 The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets are examples of such patterns.19,20 These diets also limit red meats, sweets, sweetened beverages and processed foods that are often associated with the Western diet, and typically linked with chronic disease.21 Several systematic reviews and meta-analyses have reported significant cardiometabolic benefits related to the adherence to the Mediterranean and DASH diets, including lower risks of DM and CVD, as well as improved blood pressure and lipid-lowering effects.22–30
In 2015, the Mediterranean and DASH diets were combined into a hybrid diet tailored specifically for the protection of the brain.31 Termed the Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND), the diet emphasizes ten brain-healthy foods, namely green leafy vegetables, other vegetables, berries, nuts, beans, whole grains, fish, poultry, olive oil, and wine (Table 1).32 On the MIND diet, five brain-unhealthy foods (butter and margarine, cheese, red meat and products, fast fried foods, pastries and sweets) should be consumed in limited amounts. Hence, similar to the Mediterranean and DASH diets, the MIND diet highlights plant-based foods with limited intake of animal and saturated fat foods. It uniquely specifies the consumption of green leafy vegetables and berries, but does not emphasize other types of fruits. There are no recommendations on the consumption of potatoes and dairy products, nor does the diet recommend more than one fish meal per week.32 These features were based on a comprehensive literature review, which found that of all vegetables and fruits, green leafy vegetables and berries provided the greatest level of neuroprotection.31,33–36
Table 1.
MIND Diet Components and Servings
| Diet Component | Servings |
|---|---|
| Brain-healthy foods | |
| Green leafy vegetables | ≥6 servings per week |
| Other vegetables | ≥1 serving per day |
| Berries | ≥2 servings per week |
| Nuts | ≥5 servings per week |
| Beans | >3 meals per week |
| Whole grains | ≥3 servings per day |
| Fish (not fried) | ≥1 meal per week |
| Poultry (not fried) | ≥2 meals per week |
| Olive oil | Primary oil used |
| Wine | 1 glass per day |
| Brain-unhealthy foods | |
| Butter, margarine | <1 tablespoon per day |
| Cheese | <1 serving per week |
| Red meat and products | <4 meals per week |
| Fast fried foods | <1 time per week |
| Pastries and sweets | <5 servings per week |
Note: Data from Morris et al.32
While the MIND diet has extensively been studied in relation to cognitive performance,37,38 recent investigations examined its protective capacity on cardiometabolic diseases and their risk factors, such as obesity, inflammation and dyslipidemia.39–42 It is hypothesized that the MIND diet may have positive effects on the prevention or management of cardiometabolic diseases. This is because the MIND diet includes many components of the Mediterranean and DASH diets, which have known cardiometabolic benefits.22–30 Given the rapidly increasing aging population, there is a pressing need to address the alarming rise in the rates of cardiometabolic diseases, which are common among older adults. To our best knowledge, there is currently no comprehensive review summarizing studies on the associations of the MIND diet with cardiometabolic diseases nor any of their risk factors. Therefore, the aim of this review is to systematically summarize and evaluate all existing observational and trial evidence for the MIND diet in relation to cardiometabolic diseases and their risk factors in adults.
Methods
The current systematic review was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.43
Search Strategy
A literature search was conducted in PubMed, Embase, CINAHL and Cochrane Library databases until September 2023, with no date restrictions. The following keywords were employed in the search: “cardiovascular disease” OR “dyslipidemia” OR “non-insulin dependent diabetes mellitus” OR “abdominal obesity” OR “hypertension” OR “nonalcoholic fatty liver” OR “inflammation” OR “insulin resistance” OR “anthropometry” OR “metabolic syndrome” OR “cardiometabolic risk factor” OR “liver function test” OR “lipid profile” OR “glycemic control” OR “body composition” in all-fields keywords AND “Mediterranean-DASH intervention for neurodegenerative delay” OR “MIND diet” in all-fields keywords. No limits were applied to ensure the collection of all possible research papers. Details on the search strategy used are included in Supplementary Tables 1–5.
Eligibility Criteria
All original articles published in English language were included. Included studies were observational studies (cross-sectional, case-control and cohort studies) and interventional studies (randomized controlled trials) addressing the association of the MIND diet with cardiometabolic diseases (CVD, DM, stroke, dyslipidemia, obesity, hypertension, NAFLD) and their risk factors (anthropometric measurements, blood pressure, lipid profile, glycemia, insulin resistance, inflammation). The excluded studies were published in other languages, performed on other populations (eg, children), or examined other outcomes (eg, cognition). Furthermore, studies that investigated the association of other dietary patterns (eg, Mediterranean diet or DASH diet) with cardiometabolic diseases and their risk factors were also excluded.
Selection of Studies
The extracted studies were transferred to the Rayyan platform,44 and duplicates were removed prior to screening. The titles and abstracts of the remaining records were screened by five independent reviewers to identify articles potentially eligible for inclusion in the systematic review. In case of exclusion of any study, the reasons for exclusion were documented. The full texts of the screened studies were then critically reviewed separately by each of the five reviewers for eligibility and data extraction. Any discrepancy in evaluation between the reviewers was resolved through meetings and discussions.
Data Extraction
Data extraction of selected studies was performed and cross-checked by all five reviewers. Data from each study were extracted and categorized as follows: first author and year of publication, country, study design, follow-up duration (if applicable), sample size and characteristics, dietary assessment method and MIND diet range/categories. In studies where some components of the MIND diet were not included in the final calculation of its score, these components were also documented. Moreover, any outcome measure related to cardiometabolic diseases and their risk factors were extracted. Finally, covariates and findings accompanied by odds/hazard ratios, confidence intervals, or other indicators of association and their p-values, if available, were extracted. Results of the fully adjusted models of the studies were used to evaluate the relationship of the MIND diet with cardiometabolic parameters.
Quality Assessment
Selected studies were assessed for methodological quality by five independent reviewers. The quality and risk of bias of the included observational studies (cross-sectional, case-control and cohort studies) were assessed using the Newcastle-Ottawa quality assessment tool.45 Three main domains were assessed to determine the quality and risk of bias: selection of the exposed and non-exposed groups; comparability of groups on the basis of the design or analysis controlling for confounders; and the assessment of either outcome (cross-sectional and cohort studies) or exposure (case-control). A star system was applied to classify the articles as good, moderate, or poor quality. Studies with a total score of 6 or higher were classified as high quality. The quality of the randomized controlled trials (RCTs) was evaluated using the Cochrane Collaboration’s tool46 focusing on bias in selection, performance, detection, attrition, and reporting. In addition, bias in the matching of control and treatment groups regarding age, education and anthropometric indices were examined.
Results
Selection of Studies
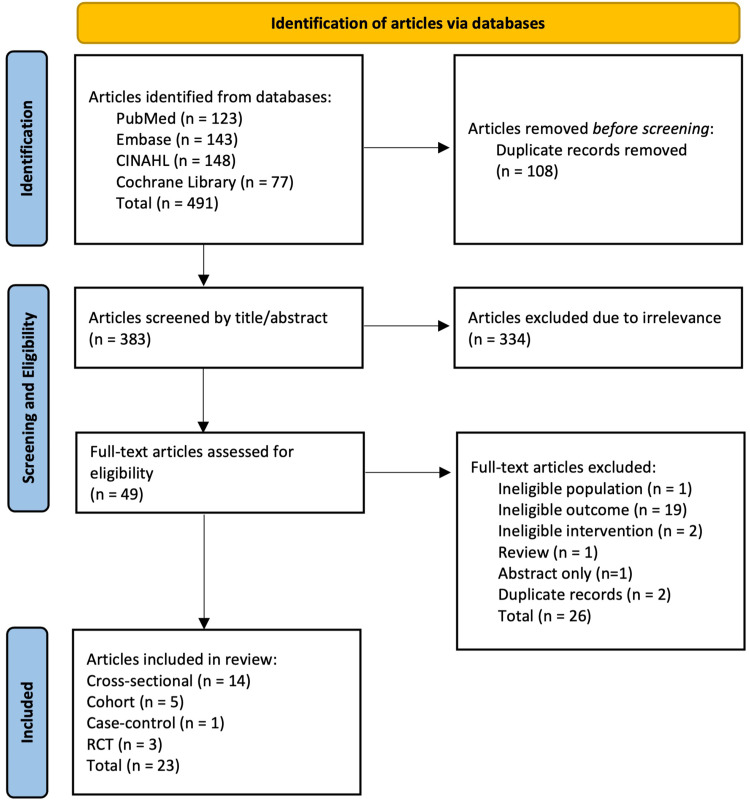
As guided by the search strategy, 491 records were first retrieved, while 383 remained after removing duplicates. During the first screening phase, records were screened by title and abstract, and only 49 publications were relevant to the topic and scope of this review. An additional 26 articles were excluded after full-text screening as they did not meet the eligibility criteria. Finally, 23 articles (14 cross-sectional, 5 cohort, 1 case-control and 3 RCTs) were included in this review (Figure 1).
Figure 1.
Flow diagram of the identified and screened articles on the MIND diet in relation to cardiometabolic diseases and their risk factors.
Characteristics of Included Studies
Studies included in this review varied in terms of key characteristics as shown in Table 2. Variation was observed in study design, sample size, MIND diet scoring, dietary assessment methods and sample characteristics. The majority of the studies found on the MIND diet in relation to cardiometabolic diseases were observational,39,41,42,47–63 except for three RCTs.40,64,65 All studies were published within the past six years (2018–2023). Twelve of the studies were conducted in Iran,39–41,49,53,54,57–59,61–63 one of which was an RCT.40 The rest of the studies spanned across Egypt,65 China,42,51,64 the United States48,55,56,60 and Australia.47,50,52
Table 2.
Characteristics of the Included Studies on the Associations of the MIND Diet with Cardiometabolic Diseases and Their Risk Factors
| Reference | Country/Study Design/Follow-Up Length | Sample Size and Characteristics | Dietary Assessment Method and MIND Diet Range/Categories | Outcome Measure(s) | Results | Covariates | |
|---|---|---|---|---|---|---|---|
| Cross-sectional studies (n=14) | |||||||
| [56] | Holthaus et al (2023) | USA | n=163 adults (95 women) Age: 33.8 ± 6.0 years |
Monthly and yearly DHQII MIND diet score: 0-15 |
- Visceral adipose tissue - WC - TG - SBP - DBP - HDL-C - FBS |
- Inverse association between the MIND diet and WC (p=0.009), TG (p=0.002), SBP (p=0.001), DBP (p<0.001), and FBS (p=0.02). - Positive association between MIND diet adherence and HDL-C (p=0.007). |
Age, sex, income, physical activity. |
| [57] | Zare et al (2023) | Iran | n=60 adults with T2DM Age: ≥60 years |
MIND dietary score questionnaire MIND diet score: 0-14 Wine excluded |
- FBS - HbA1C - TG - Chol - LDL-C - HDL-C - CRP - SBP - DBP |
- Inverse correlation between MIND diet score and blood pressure (r= −0.449 for SBP, r= −0.414 for DBP; p<0.001). | None. |
| [58] | Khadem et al (2023) | Iran | n=229 overweight and obese women (BMI ≥ 25 kg/m2) Age: 18–48 years (36.2 ± 8.3 years) |
147-item semi-quantitative FFQ; nutritionist administered MIND diet score: 0-14 Wine excluded |
- Metabolically unhealthy overweight/obesity | - No significant association between overweight/obesity phenotypes and the MIND score (OR: 1.63; 95%CI 0.79−3.33, p=0.18). | Age, energy intake, BMI, physical activity, marital status. |
| [59] | Fateh et al (2023) | Iran | n=1328 adults (51.60% female) Age: 46.2 ± 7.9 years |
168-item FFQ; dietitian administered MIND diet score: 0-13 Wine and olive oil excluded |
- Serum TG - Serum HDL-C - General obesity |
- No significant association between the highest MIND diet score and increased serum TG (OR: 0.60; 95%CI 0.43-1.11), reduced serum HDL-C (OR: 0.79; 95%CI 0.47−1.07), and general obesity (OR: 0.78; 95%CI 0.51-1.10) in the fully adjusted model. | Age, gender, energy intake, physical activity, SES, smoking, BMI. |
| [60] | Song et al (2023) | USA | n=6887 adults attending NHANES 2003-2006 Age: 20–85 years |
FFQ MIND diet score 0-15: - MDS-low (<7.5) - MDS-medium (7.5–8.0) - MDS-high (≥8.5) |
- HTN - SBP - ASCVD in HTN patients |
- Positive association between MDS-high group and lower odds of HTN (OR: 0.76; 95%CI 0.58-0.97) and decreased SBP (β=−0.41, p=0.033). - Positive association between MDS-high group and lower odds of ASCVD in HTN participants (OR: 0.80; 95% CI 0.51−0.97). |
Age, sex, race/ethnicity, smoking status, BMI, physical activity, DM, DLP, energy intake. |
| [61] | Ardekani et al (2023) | Iran | n=339 obese adults Age: 20–50 years |
168-item FFQ and food diaries MIND diet score: 0-14 Wine excluded |
- SBP - DBP - FBS - TC - LDL-C - HDL-C - TG - Insulin - HOMA-IR - QUICKI |
- No significant association between MIND diet score and QUICKI in the fully adjusted model. - No significant association between MIND diet score and other cardiometabolic risk factors. |
Age, sex, SES, physical activity, energy intake. |
| [47] | Gauci et al (2022) | Australia | n=141 (71 women) healthy middle-aged adults Age: 52.8 ± 6.9 years |
Multiple 24-hour recalls (2-4) using ASA24 MIND diet score: 0-15 |
- Blood pressure - Blood lipids - Blood glucose - Anthropometric measures (BMI, WC) - MetSSS |
- MIND diet adherence was negatively correlated with waist circumference (β=−0.28; p<0.01) and positively correlated with HDL-C (β=0.19; p<0.05). - MIND diet was not significantly related to MetSSS. |
Age, gender, energy intake. |
| [48] | Walker et al (2021) | USA | n=2,512; metabolically healthy Age: 66 ± 9 years (44.8% male) |
Harvard semi-quantitative FFQ MIND diet score: 0-15 |
- Prevalent DM - TC/HDL-C - SBP - BMI |
- Negative correlations (p <0.0001) between cumulative MIND diet score with TC/HDL-C (β=−0.14), BMI (β=−0.11) and DM status (β=−0.08). - MIND diet was not significantly related to SBP. |
Age, sex, energy intake. |
| [41] | Khatibi et al (2021) | Iran | n=263 overweight and non-menopause women with a BMI ranging from 25 to 40 kg/m2. Age: >18 years |
147-item semi-quantitative FFQ; self-administered MIND diet score: 0-15 |
- FBS - TG - TC - LDL-C - HDL-C - Weight - WC - WHR - BMI |
- Significant interaction between MIND diet and genotype for metabolic dyslipidemia; subjects with dominant allele had a lower odds of dyslipidemia (β=−0.25±132; OR: 0.77; 95% CI 0.60–1.00; p=0.05). | Age, energy intake, BMI, physical activity. |
| [49] | Mohammad-pour et al (2020) | Iran | n=836 Iranian adults (70% female) with no pre-existing conditions Age: 20-59 years (47.7 ± 10 years) |
168-item FFQ; dietitian administered via face-to-face interview MIND diet score: 0-14 - T1: <6 - T2: 6.5-7.5 - T3: >8 Wine excluded |
- MetS - Anthropometric measurements (BMI, WC) - Blood pressure - FBS - Lipid profile |
- No association between MIND diet score and MetS (OR: 0.88; 95%CI 0.62–1.24). - MIND diet score was inversely associated with reduced HDL-C (OR: 0.59; 95%CI 0.41–0.85; p=0.008) and general obesity (OR 1.19 0.80–1.78; 95% CI 0.80–1.78; p=0.02). |
Age, gender, marital status, physical activity, education status, occupation, smoking, energy intake, BMI. |
| [50] | Wong et al (2020) | Australia | n=5,376 adults (2,749 men) with 295 CVD (191 IHD, 50 HF and 85 cerebrovascular disease) cases. Age: ≥18 years |
Repeated 24 hr recall: Face-to-face multiple pass 24 h recall + second 24 h recall conducted over the phone Consumption of 15 food components separated into quintiles. MIND diet score ranged from 15-75 and was distributed into tertiles for analysis. |
CVD including: - IHD, CHD and angina - HF - Cerebrovascular disease |
- Third tertile (highest score) of the MIND score had an OR of 0.37 (95%CI 0.13-1.02; p-trend=0.044) for HF compared to the first tertile. | Age, sex, education, marital status, energy intake, BMI, SES, smoking, physical activity. |
| [39] | Aminianfar et al (2020) | Iran | n=6,724 adults Mean age: 36.8 ± 8.08 years |
106-item DS-FFQ MIND diet score: 0-13 - T1: 0.5-<6 - T2: 6–7 - T3: >7–11.5 Wine and olive oil excluded |
- General obesity - Central obesity |
- No significant association between adherence to MIND diet and odds of general and central obesity. - High adherence to MIND diet (T3) negatively associated with abdominal obesity in women (OR: 0.81; 95%CI 0.67−0.98; p-trend=0.03). |
Age, gender, energy intake, marital status, education, smoking status, family size, home ownership, physical activity, family size, breakfast skipping. |
| [42] | Chan et al (2019) | China | n=2,646 community-dwelling Chinese older adults (1332 men and 1314 women) Age: >65 years |
280-item FFQ; self-administered MIND diet score: 0-9 - T1: 2-4 - T2: 4.5-5 - T3: 5.5-7.5 Lack of sufficient information on 6 components: olive oil, fish, beans, poultry, red meats and products, fast fried foods. |
- hsCRP | - Higher MIND diet score was associated with lower hsCRP in men (T3 vs T1: OR: 0.68; 95%CI 0.52−0.90). - No associations found in women. |
Baseline age, BMI, energy intake, current smoking status, current alcohol use, physical activity, number of diseases. |
| [51] | Woo et al (2018) | China | n=2,090 (993 men and 1097 women) Mean age: 51 years |
280 food item FFQ; self-administered (past 12 months prior to the interview) MIND Diet Score: 0-9 Lack of sufficient information on 6 components: olive oil, fish, beans, poultry, red meats and products, fast fried foods. |
- ABI reflecting atherosclerosis (ABI Score >0.9 indicates better vascular health) | - Low MIND diet scores associated with having ABI <0.9 (OR: 2.6; 95%CI 1.24−5.42). | Age, BMI, smoking, drinking, PASE score, energy intake, education level, medical history of HTN, DM and heart disease, season of blood taking, vitamin D supplementation, CRP, homocysteine (blood), tryptophan (blood). |
| Cohort studies (n=5) | |||||||
| Golzarand et al (2023) | Iran Mean follow-up of 5.9 years |
n=1299 adults Age: ≥19 years old |
168 item FFQ; administered by qualified nutritionists MIND diet score: 0-13 - T1: <7.5 - T2: 7.5-8.5 - T3: >8.5 Olive oil and wine excluded |
Metabolically unhealthy phenotypes (1 out of the 4): - FBS ≥ 100 mg/dL - HDL-C < 50 mg/dL in women and < 40 mg/dL in men - TG ≥ 150 mg/dL - SBP ≥ 130 mmHg - DBP ≥ 85 mmHg - or on medication for any of the above conditions |
- Higher MIND scores were associated with 47% (HR: 0.53; 95%CI 0.34–0.83) lower risk of the metabolically unhealthy normal weight phenotype. - Higher MIND scores were associated with reduced risk of metabolically unhealthy overweight/obesity phenotypes (HR: 0.57; 95%CI 0.43–0.74). |
Age, sex, WC, changes in weight from baseline, smoking, energy intake. | |
| Razmpoosh et al (2022) | Iran Median follow-up of 7.4 years |
n=2706 adults without HTN at baseline. Age: 20-79 years Mean age: 37.9 ± 12.5 years |
Semi-quantitative FFQ; dietitian administered MIND diet score: 0-13 Wine and olive oil excluded |
- HTN | - No significant associations between MIND diet scores and risk of HTN. | Age, sex, physical activity, education, family history of CVD, smoking, BMI, CKD, DM, pre-HTN, DLP, aspirin intake, energy intake, caffeine intake, olive intake. | |
| [55] | Tison et al (2022) | USA Prospective Cohort |
n=8,750 adults (4,916 women) without diabetes at baseline. Age: ≥45 years Mean age (SD): 63.2 (8.5) |
Block98 107-item FFQ; self-administered MIND diet score: - Q5: 9.5-13.5 - Q4: 8.5-9.0 - Q3: 7.5-8.0 - Q2: 6.0-7.0 - Q1: 2.0-5.5 |
- Type 2 DM | - High MIND diet scores were associated with lower DM incidence (Q1 vs. Q5 RR: 1.33). | Energy intake, age, race, sex, income, region, education, smoking, physical activity, alcohol consumption, WC. |
| [52] | Livingstone et al (2022) | Australia ≤19 years |
n=10,009 participants (52% females) randomly selected from areas in Australia. Age: ≥25 (51.8 ± SD 14.3 years) |
74 food item semi-quantitative FFQ; self-administered MIND diet score: 0-13 Butter/margarine and olive oil excluded |
- Nonfatal cardiovascular events (MI and Stoke) | - Positive association between MIND diet and nonfatal CVD events (MI and stroke) (HR: 1.19; 95%CI 1.05−1.34). Significance lost after exclusion of deaths in the first 2 years of follow-up. | Age, sex, education, smoking, HTN, DM, physical activity. |
| [53] | Golzarand et al (2022) | Iran 10.6-years |
n=2863 healthy adults Age: ≥19 years |
168-item FFQ; dietitian administered MIND diet score: 0-13 - T1: ≤7.5 - T2: 8-9 - T3: ≥9.0 Olive oil and wine excluded |
- Cardiovascular events - CHD - Stroke |
- Negative association between MIND diet and the incidence of CVD (HR: 0.84; 95%CI 0.74−0.96). | Age, gender, BMI, smoking, SES, energy intake, DM, HTN. |
| Case-control studies (n=1) | |||||||
| [54] | Salari-Moghaddam et al (2022) | Iran | n=388 193 hospitalized stroke cases 195 hospital-based controls Age: >45 years |
168-item FFQ; dietitian administered MIND diet score: 0-14 Wine excluded |
- Stroke | - MIND diet score was inversely associated with the odds of stroke (T3 vs T1: OR 0.41; 95%CI 0.18−0.94). | Age, sex, energy intake, physical activity, smoking, HTN, DM, dyslipidemia, heart disease, BMI. |
| Randomized controlled trials (n=3) | |||||||
| [64] | Yau et al (2022) | China Single blind RCT (4-week intervention) |
n=72; older adults (76.4% women) with hypertension. MIND group: n= 23 MIND + FB group: n= 25 Control group: n= 24 Age: 66 ± 9.7 years |
Harvard semi-quantitative FFQ MIND diet score: 0-15 |
- SBP - Lipid panel (TC, HDL-C, TG, and LDL-C) - Glucose - BMI - WHR - BFP |
- WHR decreased by 0.03 (p=0.050) in the MIND group - TC and LDL-C significantly decreased by 0.60 and 0.33 mmol/L (p<0.01) in the MIND group, respectively. - TG and glucose levels were significantly lower by 0.28 mmol/L and 0.68 mmol/L (p<0.05) in the MIND group, respectively. - MIND diet was not significantly related to SBP, HDL-C and BFP. |
Adjustment was done for SBP analysis only: age, WHR, and SBP at baseline. |
| [40] | Arjmand et al (2022) | Iran Single-blind RCT (3 months) |
n=37 healthy overweight and obese women with MMSE ≥24 Calorie-restricted MIND diet group (n = 22) Calorie-restricted control group (n = 15) Age: 40-60 years |
168-item semi-quantitative FFQ MIND diet score: 0-14 Wine excluded |
Anthropometric parameters: - PBF - FFM - BMI - WC |
- Significant reduction in weight (−3.98±−0.29), BMI (−1.55±−0.11), PBF (-5.16±-0.82), and WC (−3.54±0.56) in the MIND diet group. |
None. |
| [65] | Elsayed et al (2022) | Egypt RCT (12 weeks) |
n=60 postmenopausal women, with mild cognitive impairment (MCI) Aerobic exercise + MIND-low calorie diet group (n= 30) MIND-low calorie diet control group (n = 30) Mean age: Exposure: 65.39 ± 2.83 Control: 65.13 ± 3.17 |
Perceived Dietary Adherence Questionnaire | Anthropometric measurements: - Weight - BMI |
- The control group (participants on MIND diet only) had lower body weight and BMI after 12 weeks of intervention (p<0.05). | None. |
Abbreviations: ABI, Ankle-Brachial Index; ASA24, Automated Self-Administered 24-Hour Dietary Assessment Tool; ASCVD, Atherosclerotic Cardiovascular Disease; BFP, Body Fat Percentage; BMI, Body Mass Index; CHD, Coronary Heart Disease; CKD, Chronic Kidney Disease; CVD, Cardiovascular Disease; DHQII, Dietary History Questionnaire Version II; DM, Diabetes mellitus; DS-FFQ, Dish-based Semi-quantitative Food Frequency Questionnaire; FFM, Fat Free Mass; FFQ, Food Frequency Questionnaire; Forest Bathing, FB; FBS, Fasting Blood Sugar; Glu, Glucose; HDL-C, High-Density Lipoprotein Cholesterol; HF, Heart Failure; hsCRP, High-Sensitivity C-Reactive Protein; HTN, Hypertension; IHD, Ischemic Heart Disease; LDL-C, Low-Density Lipoprotein Cholesterol; MetSSS, Metabolic syndrome severity score; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; MMSE, Mini-Mental State Examination; OR, Odds Ratio; PASE, Physical Activity Scale for Elderly; PBG, Percent Body Fat; QUICKI, Quantitative Insulin-Sensitivity Check Index; RCT, Randomized Controlled Trial; SBP, Systolic Blood Pressure; SES, Socioeconomic Status; TC, Total Cholesterol; TC/HDL-C, Total cholesterol to HDL-cholesterol ratio; TEE, Total Energy Expenditure; TG, Triglycerides; WC, Waist Circumference; WHR, Waist-to-Hip ratio.
Sample size varied between 37 to 10,009 participants among the different studies. The mean age of participants was above 35 years in most studies. Most studies included both males and females, except for four studies that included only women.40,41,58,65 Additionally, there were some discrepancies in the study population of the included reports. Some were conducted on healthy adults,47–49,53,56,59 three studies included overweight or obese women,40,41,58 while one included post-menopausal women with mild cognitive impairment (MCI).65 Additionally, one study recruited older adults with hypertension,64 while another was conducted on individuals with diabetes.57 The only case-control study included hospitalized stroke cases and hospitalized controls.54
For the assessment of dietary intake, most studies used Food Frequency Questionnaires (FFQs) to determine MIND diet scores. Two studies used multiple 24-hour dietary recalls.47,50 The range for the MIND diet scores varied between studies. Only six studies used the full score of 15, as they included all MIND diet components.41,47,48,56,60,64 Six studies excluded wine from the score, using a total score of 14.40,49,54,57,58,61 Another five studies excluded wine and olive oil, resulting in a total MIND diet score of 13.39,53,59,62,63 Two studies used a score of 9 due to lack of sufficient information on other MIND diet components.42,51 One study used a score range of 15–75, as the consumption of 15 food components were separated into quintiles and given a score of five for each.50 Finally, one study did not use a score, since the MIND diet was delivered as an intervention.65
The main confounders adjusted for in most studies included age, sex, energy intake, body mass index (BMI), marital status, physical activity, education status, occupation, smoking, socio-economic status, alcohol use, number of diseases and medical history of diseases (hypertension, diabetes, dyslipidemia, and heart disease). Two RCTs and one cross-sectional study did not adjust for any confounders.40,57,65
Quality of Articles
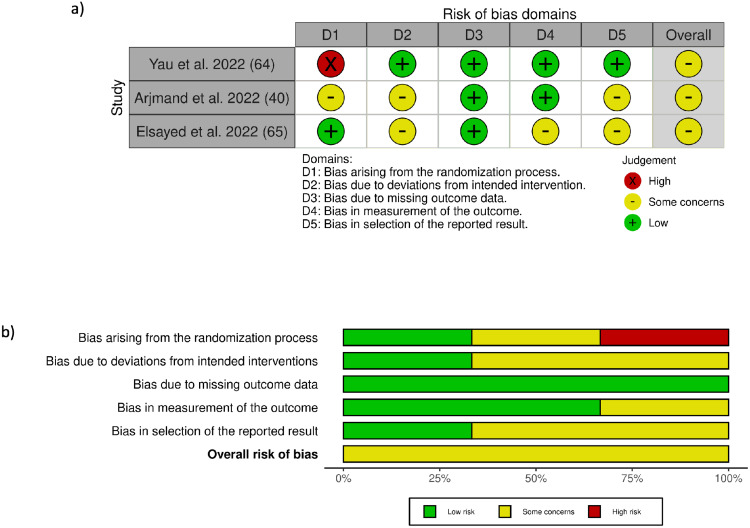
All included observational studies, except one,57 were rated as moderate to good quality (Supplementary Tables 6–8). Cohort studies were of moderate to good quality, with quality assessment scores ranging from five to eight (out of nine representing the lowest degree of bias) (Supplementary Table 6). The single case-control study included in this review was of moderate quality with a score of five (out of nine representing the lowest degree of bias) (Supplementary Table 7). The quality assessment scores of the fourteen cross-sectional studies ranged between three and six (out of seven representing the lowest degree of bias) (Supplementary Table 8). The main factors impairing quality of these observational studies were the self-administered questionnaires, self-reported exposures or outcomes, selection bias, confounding bias (due to lack of adjustment of important covariates) and non-response bias related to lack of information regarding certain domains. As for the randomized controlled trials, the overall risk-of-bias judgment for all RCTs had some concerns (Figure 2; Supplementary Tables 9–11) Reasons limiting the quality of these RCTs were lack of blinding due to the nature of the interventions and the significant differences between trial groups.
Figure 2.
(a) Risk of bias summary of studies examining the effects of the MIND diet. (b) Risk of bias graph of studies examining the effects of the MIND diet.
Relationship Between MIND Diet and Cardiometabolic Diseases
Cross-Sectional Studies
The relationship between the MIND diet and cardiometabolic diseases was investigated in 14 cross sectional studies (Table 2).39,41,42,47–49,51,56–61 Seven of these studies showed no significant association between the MIND diet and cardiometabolic disease or Metabolic Syndrome (MetS).39,47,49,50,58,59,61 MetS is defined as a cluster of cardiometabolic risk factors such as central obesity, hypertension, dyslipidemia and impaired glucose tolerance.66 MIND diet adherence was negatively correlated with waist circumference, BMI, total cholesterol (TC) to high density lipoprotein cholesterol (HDL-C) ratio (TC/HDL-C) and diabetes status,39,47,48,56 and positively correlated with HDL-C (p<0.05).49,56 Additionally, lower scores in the MIND pattern were positively associated with low ankle brachial index (ABI), which indicates atherosclerosis.51 Some studies had gender specific outcomes, whereby a higher adherence to the MIND diet was negatively associated with obesity, but only in women (OR: 0.81; P<0.03).39 In another study, the inflammatory biomarker high sensitivity C-reactive protein (hs-CRP) was lower among men adhering to the MIND diet.42 One study reported a significant interaction between MIND diet and the CAV1 rs3807992 polymorphism for metabolic dyslipidemia.41
Cohort and Case-Control Studies
A total of five cohort studies tested the association between the MIND diet and cardiometabolic disease over time.52,53,55,62,63 One study showed that a higher MIND diet score was associated with lower incidence of diabetes,55 while another showed an inverse association between the MIND diet and incident CVD.53 According to Golzarand et al, as the consumption of MIND diet components increased, the risk of CVD decreased.53 Whole grains, green leafy vegetables, and beans reduced the risk of CVD by 60%, 45%, and 65%, respectively.53 On the other hand, Livingstone et al reported a positive association between the MIND diet and nonfatal CVD events (MI and stroke) (HR: 1.20; 95% CI: 1.05–1.36).52 However, this association lost its significance after excluding deaths that occurred in the first 2 years of follow-up. In addition, one study did not find an association between higher MIND diet scores and hypertension risk.62 In another study, there was a reduced risk of metabolically unhealthy phenotypes (such as elevated lipid profile, blood pressure or blood glucose) with higher MIND diet scores.63 One case control study conducted in Iran included participants aged over 45 years that were recruited as hospitalized stroke cases and hospital-based controls. The study found that the MIND diet score was inversely associated with the odds of stroke (T3 vs T1 OR: 0.41; 95% CI: 0.18–0.94).54
Randomized Controlled Trials
The effect of the MIND diet intervention on cardiometabolic diseases or their risk factors was reported in three single-blind randomized controlled trials conducted on three different populations.40,64,65 After a four-week intervention on older adults with hypertension, the MIND diet decreased waist-to-hip ratio (WHR) by 0.03 (p=0.050) compared to the control group.64 Total cholesterol and LDL-C decreased by 0.60 mmol/L and 0.33 mmol/L in the MIND diet group (compared to 0.57 and 0.28 mmol/L in the control group), respectively. Additionally, blood glucose levels decreased significantly by 0.68 mmol/L in the MIND diet group.64 In a 3-month trial on healthy overweight and obese women, the effects of a calorie-restricted MIND diet and a calorie-restricted control diet on anthropometric measures were investigated. The MIND diet group participants experienced a significant reduction in weight (–3.98±–0.29), BMI (–1.55±–0.11), percentage of body fat (–5.16±–0.82), and waist circumference (–3.54±0.56).40 Furthermore, a trial on 60 postmenopausal women with mild cognitive impairment (MCI) also showed that participants following the MIND diet significantly reduced their body weight and BMI after 12 weeks of intervention (p<0.05).65
Discussion
This is the first systematic review to assess the relationship between the MIND diet and cardiometabolic diseases and their risk factors. Overall, the included studies indicated that adherence to the MIND diet was associated with favorable cardiometabolic outcomes in adults. Across all the different study designs, a desirable significant effect was observed on obesity and anthropometric indicators, including waist circumference,40,47,48,56 WHR64 and BMI.40,48,65 Significant improvements were also found in blood pressure,57 glycemic outcomes,48,56 HDL,47–49,56 triglycerides,56,64 and total cholesterol.64 Moreover, the MIND diet was found to reduce inflammatory markers,42 as well as the incidence of DM,55 CVD,53 stroke54 and atherosclerosis.51 However, no significant effect was found for systolic blood pressure,64 metabolic syndrome,47,49 HDL,64 body fat percent (BFP),64 and other markers of cardiovascular disease risk in some studies.50
Anthropometric Measurements
The studies included in this systematic review reported on several anthropometric measures. In RCTs, the MIND diet was associated with a reduction in waist circumference, BMI, WHR and weight, whereas effects on BFP were conflicting.40,64,65 Yau et al reported no effect of the dietary intervention on BFP in comparison to the control group, which may be due to lower levels of body fat at baseline. Cross-sectional investigations also revealed negative correlations with waist circumference,47,56 general obesity39,49 and BMI,48 but no association with abdominal obesity.49 As cross-sectional studies are observational in nature and report on current intake, the discrepancy in abdominal obesity may be explained by the energy-restricted interventions employed in the RCTs. Nonetheless, previous evidence indicates that increased consumption of MUFAs in the form of olive oil and nuts, as in the MIND diet, is not related to visceral fat deposition or weight gain.67–71 The overall findings of beneficial anthropometric effects are in line with other dietary patterns rich in MIND diet components, such as the Mediterranean and DASH diets.72–75
Lipid Profile
In the present systematic review, the MIND diet was generally associated with a beneficial effect on lipid biomarkers. Of the included RCTs, Yau et al reported a reduction in triglycerides, TC and LDL-C in the MIND diet group.64 In cross-sectional studies, MIND diet was positively associated with HDL-C47,49,56 and negatively related to TC/HDL-C ratio.48 Similar findings have been reported for the DASH diet, which shares many components with the MIND eating pattern.23,75 The results for these biomarkers were comparable across different study designs, except for TG. In a cross-sectional analysis, Holthaus et al reported an inverse association of TG with high MIND diet scores,56 while Mohammadpour et al reported no significant association between a higher MIND diet score and the odds of high serum TG.49 This may be explained by the high intake of red meat and margarine by participants in the highest tertile (T3) of the MIND diet, apart from a high intake of brain healthy foods. The intake of these foods should be limited to a certain number of servings per day in the MIND eating pattern.
Glycemic Control and Type 2 Diabetes
Seven studies evaluated the MIND diet and its effects on glycemia.48,49,55–57,61,64 The RCT by Yau et al showed a reduction in glucose levels in the MIND group, and these results were supported by some of the observational research included in this review. A cohort study showed that high MIND diet scores were associated with lower incidence of diabetes.55 In two cross-sectional studies in the USA, the MIND diet was negatively correlated with DM48 and FBS,56 respectively. However, no association was found with fasting blood glucose in three Iranian studies.49,57,61 Published literature on the effect of the Mediterranean and DASH diets on glycemic measures has been somewhat inconsistent. While Mediterranean diets have shown beneficial effects on glycemic profile,76,77 some meta-analyses of DASH did not find a significant association with blood glucose.75,78
Blood Pressure and Hypertension
Nine observational studies and one RCT included in the systematic review reported on blood pressure.47–49,56,57,60,61,63,64 Some observational studies showed that blood pressure was inversely related to the MIND diet,56,57,60 while others did not.48,61 For instance, Mohammadpour et al found no association between elevated blood pressure and the MIND eating pattern.49 This could be explained by the baseline value of blood pressure in tertile 3, which was already much lower than tertile 1 and 2. Further, the MIND diet was not associated with SBP in the RCT. In addition, a cohort study did not find an association between higher MIND diet scores and hypertension risk.62 Comparing to the two dietary patterns that the MIND diet is derived from, while the DASH diet has been studied extensively for its beneficial effects on blood pressure,22,79,80 the evidence on the Mediterranean diet and improved blood pressure outcomes is compelling,81–83 but not conclusive.84,85 Further trials and longitudinal studies are needed before the MIND diet can be recommended as an intervention for controlling blood pressure.
Cardiovascular Disease
Several observational studies reported on the association of the MIND diet with cardiovascular diseases, such as coronary heart disease (CHD), heart failure, stroke, as well as atherosclerosis.50–54 An inverse association between the MIND diet and stroke was reported in one cohort53 and one case-control study.54 Although the association between the MIND diet and stroke has not been widely studied, these findings are in agreement with the protective effects of the Mediterranean and DASH diets, from which the MIND diet is derived.86–88 In the same cohort, MIND diet was also inversely related to coronary heart disease.53 One cross-sectional study reported a positive association between low MIND diet score and low ankle-brachial index (ABI), which is an indication of atherosclerosis.51 This is in line with evidence on the Mediterranean and DASH dietary patterns, which have been strongly linked to lower risks of CVD.28,89 In another cross-sectional study, the MIND diet was found to be unrelated to CVDs, including ischemic heart disease, CHD, angina, heart failure and cerebrovascular disease.50 However, upon exclusion of participants who misreported their energy intake, an inverse association was found between the MIND diet and heart failure only. The lack of a relationship with most CVDs that was observed in this study may be partly explained by the narrow range of MIND diet scores that were present in the sample, which may have affected the ability to detect the associations.
Inflammation
One cross-sectional study in this review found an association between the MIND diet and reduced hsCRP in men, suggesting a protective effect against inflammation.42 This finding is generally in line with observations from the literature, in which similar diets of higher quality, such as the Mediterranean and DASH diets, were associated with a lower level of CRP.90–92 The lack of an observed association in women in the study by Chan et al may be partly explained by the incomplete dietary assessment in the study. The maximum score of MIND adherence was 9 instead of 15, as the authors reported insufficient information to determine the use of olive oil and the consumption of fish, beans, poultry, red meat and products, and fast fried foods.42 Western dietary patterns characterized by high intake of red meats and fried foods have been previously related to increased levels of CRP.93 A conclusion on the effect of the MIND diet on inflammation in women cannot be drawn without such crucial information on their dietary intake. Furthermore, the gender difference may also be explained by the heterogeneity in the dietary patterns of men in the study. Hence, the benefits of the MIND diet components were more easily detected in men than in women, ultimately showing a reduction in their CRP.42
Mechanisms
The MIND eating pattern is derived from the Mediterranean and DASH diets to include components with the strongest evidence for cognitive health. Diabetes and hypertension are risk factors for Alzheimer’s disease, and the pathogenesis for neurocognitive disorders lies within similar pathways as cardiometabolic diseases.94 Therefore, it is reasonable to presume that similar to the Mediterranean95–97 and DASH eating patterns,22 the MIND diet would also have cardiometabolic benefits.
One proposed mechanism behind the association between the MIND diet with cardiometabolic diseases could be attributed to its components that are rich in antioxidants and anti-inflammatory molecules.32 The MIND diet consists of ten brain-healthy foods (green leafy vegetables, other vegetables, berries, nuts, beans, whole grains, fish, poultry, olive oil, and wine), which are known to have cardiometabolic protective properties.32 A 3-year prospective cohort study found that a high adherence to the healthful plant-based diet index (hPDI), which is rich in whole grains, fruits, vegetables, nuts, legumes, vegetable oils, and tea/coffee, was inversely associated with T2DM (HR: 0.55; 95% CI 0.51–0.59, p<0.001) in American adults.98 This negative association could be explained by the high content of bio-active substances known as phytochemicals found in plants, mainly the green leafy vegetables.99,100 The MIND diet is rich in phytochemicals, a group of more than 5000 compounds that have been established to reduce the risk of different chronic diseases.100
Furthermore, the MIND diet specifies high consumption of berries, which are rich in specific phytochemicals termed flavonoids.32 Flavonoids are known to play a significant role in cardiometabolic health by improving oxidative stress, insulin sensitivity, glucose tolerance, inflammatory status, lipid metabolism, and adipocyte differentiation.101–104 Moreover, in-vitro and in-vivo studies have revealed that flavonoids can be promising anti-diabetic phytochemicals.105 Another potential mechanism appears to be linked to the role of the MIND diet in upregulating genes involved in mitochondrial respiration and oxidative phosphorylation.106 Mitochondrial dysfunction is associated with a reduction in these processes and leads to an increase in reactive oxygen species, which are implicated in the progression of cardiometabolic diseases, inflammation and insulin resistance.107,108
The MIND diet was originally developed for cognitive health and has been extensively studied in observational research for its benefits on cognition via mechanisms similar to those discussed above.37,109 Recently, the first RCT comparing the MIND diet to a control diet with mild caloric restriction showed no significant changes in cognition and brain structure between the two groups.110 Additionally, the incidence of adverse events was similar in both groups and was not associated with the diets. Although comparable outcomes were observed across both groups, the findings highlight the MIND diet as a potential dietary pattern that could be adopted to reduce risk factors associated with the pathogenesis of cardiometabolic and cognitive diseases. Given the observed null association, it may be conceivable that individuals in the control group enhanced their diets, as indicated by comparable weight loss observed across both groups. Additionally, there is a possibility that repeated testing improved cognitive markers, and an extended period greater than three years would be required to observe positive effects related to diet. Further, long-term trials are necessary to validate the findings of the MIND diet observed in various prospective studies.
Strengths
The current systematic review is the first to summarize all available evidence on the association of the MIND diet with cardiometabolic diseases. An extensive search of four databases was conducted by 5 independent reviewers, which may help to reduce publication bias. Each article was carefully reviewed and critically appraised using well-established tools. Studies included in the review spanned across 5 different countries (USA, Australia, Iran, China, Egypt), improving the generalizability and applicability of the findings. The prospective cohorts all had large sample sizes (>2000 participants), which enhances the precision of the estimates. The overall quality of the observational studies was judged as moderate to good, and one RCT had a low risk of bias. Most of the included studies used validated dietary assessment tools, mainly food frequency questionnaires. Another strength is the adjustment for key confounders (eg, energy intake and BMI) by many of the included studies, which helps to illustrate the independent relationship between the MIND diet and cardiometabolic diseases. Furthermore, as an a priori dietary pattern, the MIND diet can be easily compared with other studies, an added strength to this review.
Limitations
The review has several limitations. For one, out of twenty-three studies included, fourteen were cross-sectional in design, which limits temporality and causality. Second, some studies had small sample sizes (<100 participants), which might affect their overall power. Third, many studies excluded some MIND diet components from their score due to lack of sufficient information from the participants. Most importantly, eight studies excluded olive oil, a key component of the MIND diet. Given the established protective effects of dietary patterns high in olive oil on cardiometabolic outcomes, the findings from these studies should be interpreted with caution. Some components of the MIND diet require assessment of weekly consumption, which could not be applied to studies that used 24-hour recalls. However, the collection of multiple 24-hour recalls on non-consecutive days may be used to estimate usual dietary intakes in group settings.111 In addition, while most studies employed validated FFQs, this method of dietary assessment is prone to recall bias or reporting errors. In addition, the heterogeneity among the studies, such as in their designs, populations and outcomes, makes it difficult to compare results. Lastly, although most studies used multivariable models, the possibility of residual confounding cannot be fully ruled out.
Conclusion
In conclusion, the MIND diet appears to be associated with improvements in cardiometabolic parameters, including anthropometric measures, lipid profile, inflammation and incidence of stroke. However, due to heterogeneity among the included studies, the results should be interpreted with caution. Nonetheless, given the demonstrated effectiveness of the MIND diet in managing Alzheimer’s disease, which shares similar pathways and risk factors with cardiometabolic diseases, this healthful eating pattern may have the potential to play a role in disease risk reduction. With an increase in the aging population and the prevalence of multiple comorbidities, including cognitive and cardiometabolic diseases, healthy dietary patterns like the MIND diet should be promoted as a strategy for prevention. Further well-designed and long-term studies are warranted to confirm these findings.
Acknowledgment
We thank Qatar National Library for providing open access funding for the publishing of this review.
Funding Statement
This work has not received any funding.
Ethics Policies
This research did not require ethical approval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing or conflicts of interest for this study.
References
- 1.Sattar N, Gill JMR, Alazawi W. Improving prevention strategies for cardiometabolic disease. Nat Med. 2020;26(3):320–325. doi: 10.1038/s41591-020-0786-7 [DOI] [PubMed] [Google Scholar]
- 2.Miranda JJ, Barrientos-Gutiérrez T, Corvalan C, et al. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat Med. 2019;25(11):1667–1679. doi: 10.1038/s41591-019-0644-7 [DOI] [PubMed] [Google Scholar]
- 3.Ndisang JF, Rastogi S. Cardiometabolic diseases and related complications: current status and future perspective. Biomed Res Int. 2013;2013:467682. doi: 10.1155/2013/467682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun H, Saeedi P, Karuranga S, et al. IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183(109119):109119. doi: 10.1016/j.diabres.2021.109119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The global burden of cardiovascular diseases and risk: a compass for future health. J Am Coll Cardiol. 2022;80(25):2361–2371. doi: 10.1016/j.jacc.2022.11.005 [DOI] [PubMed] [Google Scholar]
- 6.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update From the GBD 2019 Study. J Am Coll Cardiol. 2020;76(25):2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badimon L, Chagas P, Chiva-Blanch G. Diet and cardiovascular disease: effects of foods and nutrients in classical and emerging cardiovascular risk factors. Curr Med Chem. 2019;26(19):3639–3651. doi: 10.2174/0929867324666170428103206 [DOI] [PubMed] [Google Scholar]
- 8.Papamichou D, Panagiotakos DB, Itsiopoulos C. Dietary patterns and management of type 2 diabetes: a systematic review of randomised clinical trials. Nutr Metab Cardiovasc Dis. 2019;29(6):531–543. doi: 10.1016/j.numecd.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 9.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383(9933):1999–2007. doi: 10.1016/S0140-6736(14)60613-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petersen KS, Flock MR, Richter CK, Mukherjea R, Slavin JL, Kris-Etherton PM. Healthy dietary patterns for preventing cardiometabolic disease: the role of plant-based foods and animal products. Curr Dev Nutr. 2017;1(12):cdn.117.001289. doi: 10.3945/cdn.117.001289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snetselaar LG, de Jesus JM, DeSilva DM, Stoody EE. Dietary Guidelines for Americans, 2020–2025: understanding the scientific process, guidelines, and key recommendations. Nutr Today. 2021;56(6):287–295. doi: 10.1097/NT.0000000000000512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wingrove K, Lawrence MA, McNaughton SA. Dietary patterns, foods and nutrients: a descriptive analysis of the systematic reviews conducted to inform the Australian Dietary Guidelines. Nutr Res Rev. 2021;34(1):117–124. doi: 10.1017/S0954422420000190 [DOI] [PubMed] [Google Scholar]
- 13.Kromhout D, Spaaij CJK, de Goede J, Weggemans RM. The 2015 Dutch food-based dietary guidelines. Eur J Clin Nutr. 2016;70(8):869–878. doi: 10.1038/ejcn.2016.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinho-Gomes AC, Kaur A, Scarborough P, Rayner M. Are the eatwell guide and nutrient profiling models consistent in the UK? Nutrients. 2021;13(8):2732. doi: 10.3390/nu13082732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slater JJ, Mudryj AN. Are we really “eating well with Canada’s food guide”? BMC Public Health. 2018;18(1):652. doi: 10.1186/s12889-018-5540-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Thani M, Al Thani AA, Al-Chetachi W, et al. Adherence to the Qatar dietary guidelines: a cross-sectional study of the gaps, determinants and association with cardiometabolic risk amongst adults. BMC Public Health. 2018;18(1):503. doi: 10.1186/s12889-018-5400-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3–9. doi: 10.1097/00041433-200202000-00002 [DOI] [PubMed] [Google Scholar]
- 18.van Dam RM. New approaches to the study of dietary patterns. Br J Nutr. 2005;93(5):573–574. doi: 10.1079/BJN20051453 [DOI] [PubMed] [Google Scholar]
- 19.Widmer RJ, Flammer AJ, Lerman LO, Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128(3):229–238. doi: 10.1016/j.amjmed.2014.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Filippou CD, Tsioufis CP, Thomopoulos CG, et al. Dietary Approaches to Stop Hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11(5):1150–1160. doi: 10.1093/advances/nmaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopp W. How Western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab Syndr Obes. 2019;12:2221–2236. doi: 10.2147/DMSO.S216791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiavaroli L, Viguiliouk E, Nishi SK, et al. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11(2):338. doi: 10.3390/nu11020338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113(1):1–15. doi: 10.1017/S0007114514003341 [DOI] [PubMed] [Google Scholar]
- 24.Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases—Incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29(4):611–618. doi: 10.1016/j.nut.2012.12.018 [DOI] [PubMed] [Google Scholar]
- 25.Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr. 2017;147(6):1174–1182. doi: 10.3945/jn.116.242552 [DOI] [PubMed] [Google Scholar]
- 26.Franquesa M, Pujol-Busquets G, García-Fernández E, et al. Mediterranean diet and cardiodiabesity: a systematic review through evidence-based answers to key clinical questions. Nutrients. 2019;11(3):655. doi: 10.3390/nu11030655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez-González MA, Gea A, Ruiz-Canela M. The Mediterranean diet and cardiovascular health. Circ Res. 2019;124(5):779–798. doi: 10.1161/CIRCRESAHA.118.313348 [DOI] [PubMed] [Google Scholar]
- 28.Rosato V, Temple NJ, La Vecchia C, Castellan G, Tavani A, Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2019;58(1):173–191. doi: 10.1007/s00394-017-1582-0 [DOI] [PubMed] [Google Scholar]
- 29.Esposito K, Maiorino MI, Bellastella G, Chiodini P, Panagiotakos D, Giugliano D. A journey into a Mediterranean diet and type 2 diabetes: a systematic review with meta-analyses. BMJ Open. 2015;5(8):e008222. doi: 10.1136/bmjopen-2015-008222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martín-Peláez S, Fito M, Castaner O. Mediterranean diet effects on type 2 diabetes prevention, disease progression, and related mechanisms. a review. Nutrients. 2020;12(8):2236. doi: 10.3390/nu12082236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015;11(9):1015–1022. doi: 10.1016/j.jalz.2015.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015;11(9):1007–1014. doi: 10.1016/j.jalz.2014.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006;67(8):1370–1376. doi: 10.1212/01.wnl.0000240224.38978.d8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang JH, Ascherio A, Grodstein F. Fruit and vegetable consumption and cognitive decline in aging women. Ann Neurol. 2005;57(5):713–720. doi: 10.1002/ana.20476 [DOI] [PubMed] [Google Scholar]
- 35.Willis LM, Shukitt-Hale B, Joseph JA. Recent advances in berry supplementation and age-related cognitive decline. Curr Opin Clin Nutr Metab Care. 2009;12(1):91–94. doi: 10.1097/MCO.0b013e32831b9c6e [DOI] [PubMed] [Google Scholar]
- 36.Devore EE, Kang JH, Breteler MMB, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72(1):135–143. doi: 10.1002/ana.23594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kheirouri S, Alizadeh M. MIND diet and cognitive performance in older adults: a systematic review. Crit Rev Food Sci Nutr. 2022;62(29):8059–8077. doi: 10.1080/10408398.2021.1925220 [DOI] [PubMed] [Google Scholar]
- 38.van den Brink AC, Brouwer-Brolsma EM, Berendsen AAM, van de Rest O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease-a review. Adv Nutr. 2019;10(6):1040–1065. doi: 10.1093/advances/nmz054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aminianfar A, Hassanzadeh Keshteli A, Esmaillzadeh A, Adibi P. Association between adherence to MIND diet and general and abdominal obesity: a cross-sectional study. Nutr J. 2020;19(1):15. doi: 10.1186/s12937-020-00531-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arjmand G, Abbas-Zadeh M, Fardaei M, Eftekhari MH. The Effect of Short-term Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet on hunger hormones, anthropometric parameters, and brain structures in middle-aged overweight and obese women: a randomized controlled trial. Iran J Med Sci. 2022;47(5):422–432. doi: 10.30476/IJMS.2021.90829.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khatibi N, Mirzababaei A, Shiraseb F, Abaj F, Koohdani F, Mirzaei K. Interactions between caveolin 1 polymorphism and the Mediterranean and Mediterranean-DASH Intervention for Neurodegenerative Delay diet (MIND) diet on metabolic dyslipidemia in overweight and obese adult women: a cross-sectional study. BMC Res Notes. 2021;14(1):364. doi: 10.1186/s13104-021-05777-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan R, Yu B, Leung J, Lee JSW, Woo J. Association of dietary patterns with serum high-sensitivity C-reactive protein level in community-dwelling older adults. Clin Nutr ESPEN. 2019;31:38–47. doi: 10.1016/j.clnesp.2019.03.004 [DOI] [PubMed] [Google Scholar]
- 43.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. doi: 10.1186/s13643-021-01626-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1). doi: 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lo CK, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45. doi: 10.1186/1471-2288-14-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 47.Gauci S, Young LM, Arnoldy L, et al. The association between diet and cardio-metabolic risk on cognitive performance: a cross-sectional study of middle-aged Australian adults. Front Nutr. 2022;9:862475. doi: 10.3389/fnut.2022.862475 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Walker ME, O’Donnell AA, Himali JJ, et al. Associations of the Mediterranean-dietary approaches to stop hypertension intervention for neurodegenerative delay diet with cardiac remodelling in the community: the Framingham Heart Study. Br J Nutr. 2021;126(12):1888–1896. doi: 10.1017/S0007114521000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohammadpour S, Ghorbaninejad P, Janbozorgi N, Shab-Bidar S. Associations between adherence to MIND diet and metabolic syndrome and general and abdominal obesity: a cross-sectional study. Diabetol Metab Syndr. 2020;12(1):101. doi: 10.1186/s13098-020-00611-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong MMH, Grech A, Louie JCY. Dietary patterns and cardiovascular disease in Australian adults: findings from the 2011–12 Australian Health Survey. Nutr Metab Cardiovasc Dis. 2020;30(5):738–748. doi: 10.1016/j.numecd.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 51.Woo J, Yu BWM, Chan RSM, Leung J. Influence of dietary patterns and inflammatory markers on atherosclerosis using ankle brachial index as a surrogate. J Nutr Health Aging. 2018;22(5):619–626. doi: 10.1007/s12603-018-1031-7 [DOI] [PubMed] [Google Scholar]
- 52.Livingstone KM, Milte CM, Torres SJ, et al. Nineteen-year associations between three diet quality indices and all-cause and cardiovascular disease mortality: the Australian diabetes, obesity, and lifestyle study. J Nutr. 2022;152(3):805–815. doi: 10.1093/jn/nxab386 [DOI] [PubMed] [Google Scholar]
- 53.Golzarand M, Mirmiran P, Azizi F. Adherence to the MIND diet and the risk of cardiovascular disease in adults: a cohort study. Food Funct. 2022;13(3):1651–1658. doi: 10.1039/D1FO02069B [DOI] [PubMed] [Google Scholar]
- 54.Salari-Moghaddam A, Nouri-Majd S, Shakeri F, et al. The association between adherence to the MIND diet and stroke: a case-control study. Nutr Neurosci. 2022;25(9):1956–1961. doi: 10.1080/1028415X.2021.1918982 [DOI] [PubMed] [Google Scholar]
- 55.Tison SE, Shikany JM, Long DL, et al. Differences in the Association of Select Dietary Measures With Risk of Incident Type 2 Diabetes. Diabetes Care. 2022;45(11):2602–2610. doi: 10.2337/dc22-0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holthaus TA, Sethi S, Cannavale CN, et al. MIND dietary pattern adherence is inversely associated with visceral adiposity and features of metabolic syndrome. Nutr Res. 2023;116:69–79. doi: 10.1016/j.nutres.2023.06.001 [DOI] [PubMed] [Google Scholar]
- 57.Zare S, Arjmand G, Eftekhari MH, Zare M. Adherence to Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) dietary pattern in elderly with type 2 diabetes and the correlation with cognitive functions and metabolic profile. Int J Nutr Sci. 2023;8(2):102–108. [Google Scholar]
- 58.Khadem A, Shiraseb F, Mirzababaei A, Noori S, Mirzaei K. Association of Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet and metabolically unhealthy overweight/obesity phenotypes among Iranian women: a cross sectional study. BMC Endocr Disord. 2023;23(1):84. doi: 10.1186/s12902-023-01333-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fateh HL, Muhammad SS, Kamari N. Associations between adherence to MIND diet and general obesity and lipid profile: a cross-sectional study. Front Nutr. 2023;10:1078961. doi: 10.3389/fnut.2023.1078961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song Y, Chang Z, Cui K, et al. The value of the MIND diet in the primary and secondary prevention of hypertension: a cross-sectional and longitudinal cohort study from NHANES analysis. Front Nutr. 2023;10. doi: 10.3389/fnut.2023.1129667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ardekani AM, Vahdat S, Hojati A, et al. Evaluating the association between the Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet, mental health, and cardio-metabolic risk factors among individuals with obesity. BMC Endocr Disord. 2023;23(1). doi: 10.1186/s12902-023-01284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Razmpoosh E, Moslehi N, Abdollahi S, Soltani S, Mirmiran P, Azizi F. The Mediterranean, DASH, and MIND diets and the incident of hypertension over a median follow-up of 7.4 years in the Tehran Lipid and Glucose Study. BMC Public Health. 2022;22(1). doi: 10.1186/s12889-022-14843-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Golzarand M, Moslehi N, Mirmiran P, Azizi F. Adherence to the DASH, MeDi, and MIND diet scores and the incidence of metabolically unhealthy phenotypes. Obes Res Clin Pract. 2023;17(3):226–232. doi: 10.1016/j.orcp.2023.04.001 [DOI] [PubMed] [Google Scholar]
- 64.Yau KY, Law PS, Wong CN. Cardiac and mental benefits of Mediterranean-DASH intervention for neurodegenerative delay (MIND) diet plus forest bathing (FB) versus MIND diet among older Chinese adults: a randomized controlled pilot study. Int J Environ Res Public Health. 2022;19(22):14665. doi: 10.3390/ijerph192214665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elsayed MM, Rabiee A, El Refaye GE, Elsisi HF. Aerobic exercise with Mediterranean-DASH intervention for neurodegenerative delay diet promotes brain cells’ longevity despite sex hormone deficiency in postmenopausal women: a randomized controlled trial. Oxid Med Cell Longev. 2022;2022:4146742. doi: 10.1155/2022/4146742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fahed G, Aoun L, Bou Zerdan M, et al. Metabolic syndrome: updates on pathophysiology and management in 2021. Int J Mol Sci. 2022;23(2):786. doi: 10.3390/ijms23020786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bes-Rastrollo M, Sánchez-Villegas A, de la Fuente C, de Irala J, Martinez JA, Martínez-González MA. Olive oil consumption and weight change: the SUN prospective cohort study. Lipids. 2006;41(3):249–256. doi: 10.1007/s11745-006-5094-6 [DOI] [PubMed] [Google Scholar]
- 68.Martínez-González MA, Bes-Rastrollo M. Nut consumption, weight gain and obesity: epidemiological evidence. Nutr Metab Cardiovasc Dis. 2011;21 Suppl 1:S40–S45. doi: 10.1016/j.numecd.2010.11.005 [DOI] [PubMed] [Google Scholar]
- 69.Calder PC, Harvey DJ, Pond CM, Newsholme EA. Site-specific differences in the fatty acid composition of human adipose tissue. Lipids. 1992;27(9):716–720. doi: 10.1007/BF02536031 [DOI] [PubMed] [Google Scholar]
- 70.Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120(9 Suppl 1):S10–S16; discussion S16–S17. doi: 10.1016/j.amjmed.2007.06.006 [DOI] [PubMed] [Google Scholar]
- 71.Prasad DS, Kabir Z, Dash AK, Das BC. Abdominal obesity, an independent cardiovascular risk factor in Indian subcontinent: a clinico epidemiological evidence summary. J Cardiovasc Dis Res. 2011;2(4):199–205. doi: 10.4103/0975-3583.89803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Funtikova AN, Benítez-Arciniega AA, Gomez SF, Fitó M, Elosua R, Schröder H. Mediterranean diet impact on changes in abdominal fat and 10-year incidence of abdominal obesity in a Spanish population. Br J Nutr. 2014;111(8):1481–1487. doi: 10.1017/S0007114513003966 [DOI] [PubMed] [Google Scholar]
- 73.Agnoli C, Sieri S, Ricceri F, et al. Adherence to a Mediterranean diet and long-term changes in weight and waist circumference in the EPIC-Italy cohort. Nutr Diabetes. 2018;8(1):1–10. doi: 10.1038/s41387-018-0023-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soltani S, Shirani F, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) diet on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Obes Rev. 2016;17(5):442–454. doi: 10.1111/obr.12391 [DOI] [PubMed] [Google Scholar]
- 75.Lari A, Sohouli MH, Fatahi S, et al. The effects of the Dietary Approaches to Stop Hypertension (DASH) diet on metabolic risk factors in patients with chronic disease: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2021;31(10):2766–2778. doi: 10.1016/j.numecd.2021.05.030 [DOI] [PubMed] [Google Scholar]
- 76.Kalkuz S, Demircan A. Effects of the Mediterranean diet adherence on body composition, blood parameters and quality of life in adults. Postgrad Med J. 2021;97(1154):798–802. doi: 10.1136/postgradmedj-2020-138667 [DOI] [PubMed] [Google Scholar]
- 77.Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract. 2010;89(2):97–102. doi: 10.1016/j.diabres.2010.04.019 [DOI] [PubMed] [Google Scholar]
- 78.Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition. 2013;29(7–8):939–947. doi: 10.1016/j.nut.2012.12.021 [DOI] [PubMed] [Google Scholar]
- 79.Moore TJ, Conlin PR, Ard J, Svetkey LP. DASH (Dietary Approaches to Stop Hypertension) diet is effective treatment for stage 1 isolated systolic hypertension. Hypertension. 2001;38(2):155–158. doi: 10.1161/01.HYP.38.2.155 [DOI] [PubMed] [Google Scholar]
- 80.Francisco SC, Araújo LF, Griep RH, et al. Adherence to the Dietary Approaches to Stop Hypertension (DASH) and hypertension risk: results of the Longitudinal Study of Adult Health (ELSA-Brasil). Br J Nutr. 2020;123(9):1068–1077. doi: 10.1017/S0007114520000124 [DOI] [PubMed] [Google Scholar]
- 81.Toledo E, Hu FB, Estruch R, et al. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med. 2013;11(1):207. doi: 10.1186/1741-7015-11-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. Am J Clin Nutr. 2004;80(4):1012–1018. doi: 10.1093/ajcn/80.4.1012 [DOI] [PubMed] [Google Scholar]
- 83.Ahmed FS, Wade AT, Guenther BA, Murphy KJ, Elias MF. Adherence to a Mediterranean diet associated with lower blood pressure in a US sample: findings from the Maine-Syracuse Longitudinal Study. J Clin Hypertens. 2020;22(12):2276–2284. doi: 10.1111/jch.14068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pitsavos C, Chrysohoou C, Panagiotakos DB, Lentzas Y, Stefanadis C. Abdominal obesity and inflammation predicts hyper- tension among prehypertensive men and women: the ATTICA Study. Heart Vessels. 2008;23(2):96–103. doi: 10.1007/s00380-007-1018-5 [DOI] [PubMed] [Google Scholar]
- 85.Núñez-Córdoba JM, Valencia-Serrano F, Toledo E, Alonso A, Martínez-González MA. The Mediterranean diet and incidence of hypertension: the Seguimiento Universidad de Navarra (SUN) Study. Am J Epidemiol. 2009;169(3):339–346. doi: 10.1093/aje/kwn335 [DOI] [PubMed] [Google Scholar]
- 86.Saulle R, Lia L, De Giusti M, La Torre G. A systematic overview of the scientific literature on the association between Mediterranean Diet and the Stroke prevention. Clin Ter. 2019;170(5):e396–e408. doi: 10.7417/CT.2019.2166 [DOI] [PubMed] [Google Scholar]
- 87.Kontogianni MD, Panagiotakos DB. Dietary patterns and stroke: a systematic review and re-meta-analysis. Maturitas. 2014;79(1):41–47. doi: 10.1016/j.maturitas.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 88.Feng Q, Fan S, Wu Y, et al. Adherence to the dietary approaches to stop hypertension diet and risk of stroke: a meta-analysis of prospective studies. Medicine. 2018;97(38):e12450. doi: 10.1097/MD.0000000000012450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwingshackl L, Bogensberger B, Hoffmann G. Diet quality as assessed by the healthy eating index, alternate healthy eating index, dietary approaches to stop hypertension score, and health outcomes: an updated systematic review and meta-analysis of cohort studies. J Acad Nutr Diet. 2018;118(1):74–100.e11. doi: 10.1016/j.jand.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 90.Sureda A, Bibiloni MDM, Julibert A, et al. Adherence to the mediterranean diet and inflammatory markers. Nutrients. 2018;10(1):62. doi: 10.3390/nu10010062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soltani S, Chitsazi MJ, Salehi-Abargouei A. The effect of dietary approaches to stop hypertension (DASH) on serum inflammatory markers: a systematic review and meta-analysis of randomized trials. Clin Nutr. 2018;37(2):542–550. doi: 10.1016/j.clnu.2017.02.018 [DOI] [PubMed] [Google Scholar]
- 92.Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24(9):929–939. doi: 10.1016/j.numecd.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 93.Christ A, Lauterbach M, Latz E. Western diet and the immune system: an inflammatory connection. Immunity. 2019;51(5):794–811. doi: 10.1016/j.immuni.2019.09.020 [DOI] [PubMed] [Google Scholar]
- 94.Edwards GA, Gamez N, Escobedo G, Calderon O, Moreno-Gonzalez I. Modifiable risk factors for Alzheimer’s disease. Front Aging Neurosci. 2019;11:146. doi: 10.3389/fnagi.2019.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grosso G, Mistretta A, Frigiola A, et al. Mediterranean diet and cardiovascular risk factors: a systematic review. Crit Rev Food Sci Nutr. 2014;54(5):593–610. doi: 10.1080/10408398.2011.596955 [DOI] [PubMed] [Google Scholar]
- 96.Guasch-Ferré M, Willett WC. The Mediterranean diet and health: a comprehensive overview. J Intern Med. 2021;290(3):549–566. doi: 10.1111/joim.13333 [DOI] [PubMed] [Google Scholar]
- 97.AlAufi NS, Chan YM, Waly MI, Chin YS, Mohd Yusof BN, Ahmad N. Application of Mediterranean diet in cardiovascular diseases and Type 2 diabetes mellitus: motivations and challenges. Nutrients. 2022;14(13):2777. doi: 10.3390/nu14132777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Satija A, Bhupathiraju SN, Rimm EB, et al. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: results from three prospective cohort studies. PLoS Med. 2016;13(6):e1002039. doi: 10.1371/journal.pmed.1002039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134(12 Suppl):3479S–3485S. doi: 10.1093/jn/134.12.3479S [DOI] [PubMed] [Google Scholar]
- 100.Liu RH. Health-promoting components of fruits and vegetables in the diet. Adv Nutr. 2013;4(3):384S–392S. doi: 10.3945/an.112.003517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mahmoud AM, Hernández Bautista RJ, Sandhu MA, Hussein OE. Beneficial effects of citrus flavonoids on cardiovascular and metabolic health. Oxid Med Cell Longev. 2019;2019:5484138. doi: 10.1155/2019/5484138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li C, Schluesener H. Health-promoting effects of the citrus flavanone hesperidin. Crit Rev Food Sci Nutr. 2017;57(3):613–631. doi: 10.1080/10408398.2014.906382 [DOI] [PubMed] [Google Scholar]
- 103.Millar CL, Duclos Q, Blesso CN. Effects of dietary flavonoids on reverse cholesterol transport, HDL metabolism, and HDL function. Adv Nutr. 2017;8(2):226–239. doi: 10.3945/an.116.014050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rees A, Dodd GF, Spencer JPE. The effects of flavonoids on cardiovascular health: a review of human intervention trials and implications for cerebrovascular function. Nutrients. 2018;10(12):1852. doi: 10.3390/nu10121852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gandhi GR, Vasconcelos ABS, Wu DT, et al. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: a systematic review of in vitro and in vivo studies. Nutrients. 2020;12(10):2907. doi: 10.3390/nu12102907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang EY, Kim DY, Kim HK, et al. Modified Korean MIND Diet: a nutritional intervention for improved cognitive function in elderly women through mitochondrial respiration, inflammation suppression, and amino acid metabolism regulation. Mol Nutr Food Res. 2023:e2300329. doi: 10.1002/mnfr.202300329 [DOI] [PubMed] [Google Scholar]
- 107.San-Millán I. The key role of mitochondrial function in health and disease. Antioxidants. 2023;12(4):782. doi: 10.3390/antiox12040782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Avram VF, Merce AP, Hâncu IM, et al. Impairment of mitochondrial respiration in metabolic diseases: an overview. Int J Mol Sci. 2022;23(16):8852. doi: 10.3390/ijms23168852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang L, Tao Y, Chen H, et al. Mediterranean-Dietary Approaches to Stop Hypertension Intervention for Neurodegenerative Delay (MIND) diet and cognitive function and its decline: a prospective study and meta-analysis of cohort studies. Am J Clin Nutr. 2023;118(1):174–182. doi: 10.1016/j.ajcnut.2023.04.025 [DOI] [PubMed] [Google Scholar]
- 110.Barnes LL, Dhana K, Liu X, et al. Trial of the MIND diet for prevention of cognitive decline in older persons. N Engl J Med. 2023;389(7):602–611. doi: 10.1056/NEJMoa2302368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ma Y, Olendzki BC, Pagoto SL, et al. Number of 24-hour diet recalls needed to estimate energy intake. Ann Epidemiol. 2009;19(8):553–559. doi: 10.1016/j.annepidem.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]