Abstract
During the past decade, the outcomes of pediatric patients with acute myeloid leukemia (AML) have plateaued with 5-year event-free survival (EFS) and overall survival (OS) of approximately 46% and 64%, respectively. Outcomes are particularly poor for those children with high-risk disease, who have 5-year OS of 46%. Substantial survival improvements have been observed for a subset of patients treated with targeted therapies. Specifically, children with KMT2A-rearranged AML and/or FLT3 internal tandem duplication (FLT3-ITD) mutations benefitted from the addition of gemtuzumab ozogamicin (GO), an anti-CD33 antibody-drug conjugate, in the AAML0531 clinical trial (NCT00372593). Sorafenib also improved response and survival in children with FLT3-ITD AML in the AAML1031 clinical trial (NCT01371981). Advances in characterization of prognostic cytomolecular events have helped to identify patients at highest risk of relapse and facilitated allocation to consolidative hematopoietic stem cell transplant (HSCT) in first remission. Some patients clearly have improved survival with HSCT, although the benefit is largely unknown for most patients. Finally, data-driven refinements in supportive care recommendations continue to evolve with meaningful and measurable reductions in toxicity and improvements in EFS and OS. As advances in application of targeted therapies, risk stratification, and improved supportive care measures are incorporated into current trials and become standard-of-care, there is every expectation that we will see improved survival with a reduction in toxic morbidity and mortality. The research agenda of the COG Myeloid Diseases Committee continues to build upon experience and outcomes with an overarching goal of curing more children with AML.
INTRODUCTION
State of Disease: Clinical
Myeloid malignancies account for approximately 20% of all childhood leukemias and include four general categories defined by molecular pathology and treatment: (1) acute promyelocytic leukemia (APL), (2) myeloid leukemia of Down Syndrome (ML-DS), (3) chronic myeloid leukemia (CML) and (4) AML.1 The risk- and therapy-defining molecular events described over the past 10 years in childhood AML are different from adults with the same diagnosis.2 Historically, therapy for childhood AML was adapted from data generated from the older adult AML experience. Recent comprehensive genomic characterization of AML across the lifespan demonstrates that AML in older adults and that in infants and young children less than 3 years are unique and genomically defined entities with distinct pathology. AML in older children, adolescents and young adults share more similarities than differences that are driven by genomic alterations that transcend age alone and impart distinct genomically driven phenotype, pathology, and outcome.
Current Outcomes in Pediatric AML
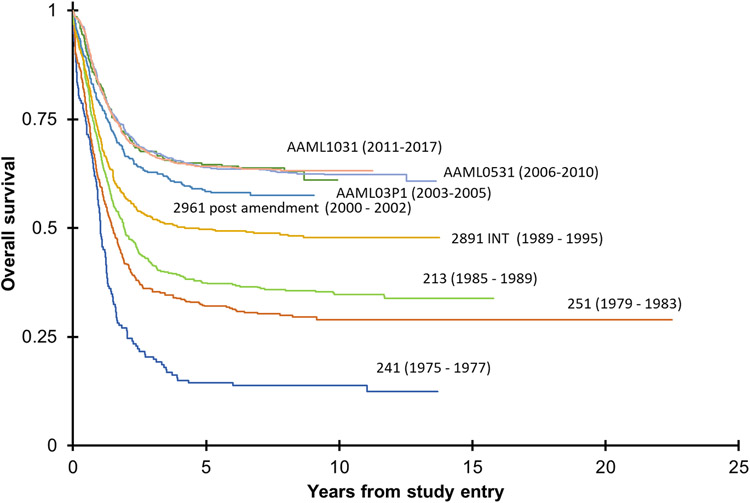
Clinical outcomes of children, adolescents, and young adults with AML have changed in the past 20 years with a 5-year overall survival (OS) of approximately 60% (Figure 1).1,3,4 Conventional chemotherapy regimens, reliant on high doses of cytarabine and anthracyclines, have changed little during this period. Progress has focused on the dose and schedule intensification of conventional therapy, improved identification of patients with higher risk of relapse, advances in HSCT, and enhanced supportive care. Major recent advances in elucidation of predictive biomarkers of treatment response and survival have led to further therapy refinements.5,6
Figure 1.
Overall survival on successive clinical trials since 1975.
Patients with nucleophosmin 1 (NPM1) mutations,7,8 CCAAT enhancer-binding protein alpha (CEBPA) biallelic mutations,9 and core-binding factor (CBF) leukemias (RUNX1::RUNXT1 or CBFB::MYH11 rearrangements) have more favorable outcomes.1,5,6,10 This is in stark contrast to outcomes for children with high-risk (HR) cytomolecular alterations who continue to have unacceptably poor survival despite maximally intensive initial and relapse therapy.3,4 The refractory nature of AML combined with challenges to conduct relapsed trials have limited or ability to identify new active agents for children. Outcomes in pediatric AML and related myeloid disorders will improve only if we develop treatment approaches that are informed by disease biology and incorporate novel agents relevant to the unique AML biology observed in infants, children and young adults.
State of Disease: Biological
Molecular targets
AML is most commonly the result of cumulative chromosomal or genomic events that combine to impair differentiation and increase proliferation and/or cell survival. Animal modeling support the two-hit hypothesis where 2 or more cooperating mutations are required for myeloid pathogenesis2,11-19. Class I mutations lead to an activation of signal transduction and proliferation. RAS pathway genes (NRAS, KRAS, PTPN11, and NF1) and receptor tyrosine kinases (FLT3 and KIT) are among the most common Class I mutations seen in childhood AML. Class II mutations may include small insertions or deletions in transcriptional regulators like CEBPA and RUNX1 or result from large chromosomal translocations that lead to a new fusion oncogene that impairs hematopoietic differentiation.14-19 The most common rearrangements in childhood AML are the core-binding factor translocations [t(8;21)(q22;q22)] and inv(16) that result in RUNX1::RUNX1T1 and CBFB::MYH11 fusion oncogenes, respectively. The KMT2A gene at 11q23 can rearrange to create novel fusion proteins with a number of partner genes. More recent data demonstrates that specific fusions in infant AML delivered in a context appropriate manner may indeed be sufficient for malignant transformation, where transduction of CBFA2T3::GLIS2 or NUP98::KDM5A fusion transcript into normal cord blood stem cells generated leukemia phenotypically similar to de novo disease.20
Recent advancements in next generation sequencing (NGS) have improved our understanding of the genomic landscape of AML. Several recent publications have identified cryptic or previously undetected translocations using NGS approaches. These include, for example, CBFA2T3::GLIS2, NUP98 family fusions, and MLLT10 rearrangements, all of which predict poor outcomes with conventional therapy.2,21-25 The COG Myeloid Disease Committee has contributed to major publications (Table 1) and has described AML-defining cytogenetic, molecular, and immunophenotypic changes (Table 2) that predict survival and offer unique vulnerabilities for therapeutic targeting (Table 3).
Table 1.
A summary of key publications from the past 10 years that inform the current standard of care and result from COG data and specimen requests, and utilize the COG Statistics and Data Center.
| Citation | Clinical Data Sets | Key Findings |
|---|---|---|
| Biomarkers | ||
| Ostronoff et al, 2014 | CCG-2941/2961 AAML03P1, AAML0531 |
The NUP98::NSD1 chimeric oncogene commonly co-occurs with the FLT3-ITD mutations, is common adolescents and predicts a poor survival when occurring alone or co-occurring with FLT3-ITD |
| Tarlock et al, 2014 | CCG-2861, -2891, -2961, POG-9421, AAML03P1 AAML0531 | The t(6;9)(p23;q34) translocation resulting in the DEK::NUP214 fusion product co-occurs commonly with the FLT3-ITD, and is an independent predictor of poor survival |
| Brodersen et al, 201662 | AAML0531 | A recurrent diagnostic immunophenotype (CD56+, HLA-DR+, CD38+) identifies a group of patients with a distinctively poor outcome. This is now called the RAM phenotype in current trials. |
| Pollard et al, 201630 | AAML0531 | Patients with higher CD33 expression have a reduction in relapse risk and improvement in EFS when treated with GO. |
| Tarlock et al, 201627 | AAML03P1, AAML0531 | GO reduces the relapse risk for patients with FLT3-ITD. |
| Lamba et al, 201763 | AAML0531 | A CD33 single nucleotide polymorphism (rs12459419 C>T) occurring in the splice enhancer region predicts EFS in patient treated with GO. This alternatively spliced CD33 isoform lacks expression of the antibody binding site for GO. |
| Bolouri et al, 20182 | AAML03P1, AAML0531 | This was the most comprehensive genomic profiling of childhood AML, and it demonstrated the differences in mutations across the lifespan. |
| Tarlock et al, 2019 64 | AAML0531 | Exon 17 and not exon 8 KIT mutation co-occurring with core-binding factor mutations predicts a higher rate of relapse following conventional therapy with or without GO. |
| Brodersen et al, 2020 65 | AAML0531 | Compared to standard morphology, difference-from normal flow cytometry was a better predictor of OS in children with AML. In fact, there was a significant false negative and false positive rate by morphology when confirmed by flow. |
| Tarlock et al, 2020 66 | AAML0531, AAML1031 | Colony-stimulating factor 3 receptor (CSF3R) activating mutations co-occur with CEBPA and t(8;21) mutations, but predict poor outcomes in the patients with a co-occurring CEBPA mutation. |
| Huang et al, 2021 | 2961, AAML03P1 AAML0531, AAML1031 | The co-occurrence of the CBFB-MYH11 re-arrangement and KIT mutations in AML in children predicts a higher risk for relapse. |
| Noort et al, 2021 | AAML03P1, AAML0531, AAML1031, and European samples* | Childhood AML with the NUP98::KDM5A fusion tends to occur in younger patient with a higher rate of acute megakaryocytic AML. OS and EFS is reduced. |
| Pollard, et al 202129 | AAML0531 | Gemtuzumab ozogamicin improves overall outcomes in pediatric KMT2A-rearranged AML and in high-risk vs. not high-risk fusions. |
| Tarlock et al 20219 | AAML03P1, AAML0531, AAML1031 | Described favorable CEBPA-bZIp mutations regardless of monoallelic or biallelic status. Described high relapse risk observed when co-occurring CSF3R mutation is present. |
| Umeda et al, 202267 | AAML1031 | A multi-group analysis identifying exon 13 tandem duplications in upstream binding transcription factor (UBTF)as a recurring mutation in AML that predict poor outcomes |
| Lamble et al, 202268 | AAML1031 | The authors establish that higher CD123 expression correlates with high risk cyto-molecular events in childhood AML. |
| Huang et al, 202269 | AAML03P1, AAML0531, AAML1031 | With diagnostic cytogenetics and RNA sequencing from more than 1500 patients, the authors identify a cytomolecular risk prediction model for childhood AML utilizing a leukemic stem cell signature based on AML cytomolecular subtypes. |
| Elsayed, et al, 202270 | AAML0531 | In this multi-group trial, the authors validate pharmacogenomic markers involved in cytarabine metabolism as prognostic for survival. |
| Gbadamosi et al, 202271 | AAML03P1, AAML0531 | The authors identified a scoring system best on expression of 10 genes involved in DNA damage response to predict survival following treatment that includes GO. |
| Marrero et al, 202372 | AAML1031, St Jude AML02 and AML08 | A multi-group analysis describing the effect of SAMHD1, which participates in cytarabine metabolism, polymorphisms on survival in childhood AML. |
| Farrar et al JCO 202373 | AAML03P1, AAML0531 AAML1031 |
Long noncoding RNA expression enhances predictive power of traditional cytogenetic and mutation defined risk and may be comparable to historical cytogenetic/molecular risk characteristics |
| Bertrums et al, 202374 | AAML03P1, AAML0531, AAML1031 | A multi-group analysis describing the poor outcomes associated with NUP98 fusions and describing the commonly occurring co-mutations. |
| Chisholm et al, 202375 | AAML0531, AAML1031 | Described cases of non-DS AMKL by molecular and cytogenetic subtypes and reported their corresponding EFS and OS |
| Lamble et al, 202376 | AAML03P1, AAML0531, AAML1031 | Described prognostic significance of CREBBP sequence variants and their impact on co-occurring mutations in AML . Demonstrated that such mutations are associated with similar high risk as that of CREBBP::KAT6A fusions |
| Therapy and Supportive Care | ||
| Gamis et a, 2014l4 | AAML0531 | GO improves event free survival in children with de novo AML when combined with conventional therapy. |
| Guest et al, 201777 | AAML03P1 and AAML0531 | GO was well tolerated in infant AML and was associated with favorable outcomes. |
| Taub et al, 201739 | AAML0431 | Use of high dose cytarabine early in treatment for DS- ML improved outcomes from historical studies with manageable toxicities. |
| Kutny et al, 201732 | AAML0631 | Arsenic consolidation allowed for cumulative reduction in anthracyclines with comparable outcomes in pediatric APL |
| Getz et al, 201956 | AAML0531 | Early treatment-related cardiotoxicity may be associated with decreased EFS and OS. |
| Getz et al, 202057 | AAML1031 | Dexrazoxane preserved cardiac function without compromising EFS and OS or increasing noncardiac toxicities. |
| Elgarten et al, 202178 | AAML0531, AAML1031 | Intensifying a second induction course does not improve survival in children with high-risk AML |
| Hitzler et al79 | AAML1531 | Dose reduction of cytarabine treatment for Downs Syndrome patients with ML-DS who were MRD negative at end induction lead to higher rates of relapse establishing importance of this treatment in ML-DS |
| Getz et al, 202257 | AAML0531, AAML1031 | Capizzi AraC can be eliminated in patients who low risk molecular/cytogenetic risk features who are MRD negative after Induction I |
| Pollard et al, 202280 | AAML0531, AAML1031 | The addition of sorafenib to conventional therapy improves induction response and survival in patient with FLT3-ITD with an allelic ratio greater than 0.4. |
| Kutny et al 202233 | AAML1331 | Arsenic and ATRA are sufficient treatment for standard-risk APL and can be a mainstay of therapy in high-risk APL |
Includes samples from IEOP (Associazione Italiana di Ematologia e Oncologia Pediatrica), BFM (Berlin-Frankfurt-Münster) group, CPH (Czech Pediatric Hematology Working Group), DCOG (Dutch Childhood Oncology Group) and LAME (Leucémie Aiquë Myéloblastique Enfant).
Table 2.
Risk Stratification for Pediatric Patients with Newly-Diagnosed AML Used in the COG AAML1831 Clinical Trial.
| Primary Risk Marker | Modifying Risk Marker | Comment |
|---|---|---|
| RUNX1::RUNXT1 and CBFB:MYH11 mutations* | MRD+ at EOI1 | End-induction 1 (EOI1) MRD-negative complete remission indicates that treatment de-escalation possible with 4 (not 5) cycles of chemotherapy without HSCT |
| KIT exon 17 mutation | Presence of CKIT mutation indicates need for 5 cycles of chemotherapy, but HSCT in CR1 not indicated | |
| Unfavorable cytogenetic and/or NGS marker | Presence of an unfavorable genetic marker (see below) supersedes risk group and these patients are recommended to receive HSCT in CR1 | |
| NPM1 or CEBPA bZIP mutation | MRD+ at EOI1 | MRD negative response indicates that treatment de-escalation possible with 4 (not 5) cycles of chemotherapy without HSCT |
| Unfavorable cytogenetic and/or NGS marker | Presence of any additional unfavorable genetic marker (see below) supersedes risk group and these patients are recommended to receive SCT. | |
| FLT3-ITD |
NPM1 or CEBPA bZIP mutation AND MRD+ at EOI1 |
Co-occurring NPM1 or CEBPA bZIP mutation allows 5 cycles of chemotherapy without HSCT only if MRD negative, otherwise HSCT recommended |
| Unfavorable cytogenetic and/or NGS marker | None | HSCT recommended if any of the following are present: t(3;21)(26.2;q22) RUNX1::MECOM t(3;5)(q25;q34) NPM1::MLF1 t(6;9)(p22.3;q34.1) DEK::NUP214 t(8;16)(p11.2;p13.3) KAT6A::CREBBP (if 90 days or older at diagnosis) t(16;21)(p11.2;q22.2) FUS::ERG inv(16)(p13.3q24.3) CBFA2T3::GLIS2 t(4;11)(q21;q23.3) KMT2A::MLLT2 t(6;11)(q27;q23.3) KMT2A::MLLT4 t(10;11)(p12.3;q23.3) KMT2A::MLLT10 t(10;11)(p12.1;q23.3) KMT2A::ABI1 t(11;19)(q23.3;p13.3) KMT2A::ENL 11p15 rearrangement (NUP98 with any partner gene) 12p13.2 rearrangement (ETV6 with any partner gene) deletion 12p to include 12p13.2 (loss of ETV6) monosomy 5/del(5q) to include 5q31 (loss of EGR1) monosomy 7 10p12.3 rearrangement (MLLT10 with any partner gene) |
previously referred to as core binding factor (CBF) AML, t(8;21) or inv(16)/t(16;16)
MRD measurable residual disease, HSCT hematopoietic stem cell transplant, NGS next generation sequencing
Table 3.
Current early-phase clinical trials for children with relapsed/refractory AML.
| Targeted therapy |
Target | Chemotherapy combination |
Age |
Clinicaltrials.gov identifier |
Study group | Notes |
|---|---|---|---|---|---|---|
| Small molecule inhibitors | ||||||
| venetoclax | BCL-2 | cytarabine +/− idarubicin | 2 - 20 years | NCT03194932 | SJCRH | ALL also eligible |
| venetoclax | BCL-2 | FLA + GO | 29 days - 21 years | NCT05183035 | LLS PedAL/EuPAL | CD33+ AML |
| selinexor, venetoclax | XPO1, BCL-2 | FLA/FLAG | ≤ 30 years | NCT04898894 | SJCRH | ALAL also eligible |
| gilteritinib | FLT3 | gilteritinib, FLAG | 6 months - 21 years | NCT04240002 | Astellas | FLT3-ITD or mutation |
| quizartinib | FLT3 | FLA + etoposide | 1 month - 21 years | NCT03793478 | ITCC/COG | FLT3-ITD or mutation |
| pexidartinib | FLT3 | 3 - 35 years | NCT02390752 | NCI | ALL and solid tumor also eligible | |
| MRX-2843 | FLT3 | ≥ 12 years | NCT04872478 | Meryx | ALL and MPAL also eligible | |
| enasidenib | IDH2 | 2 - 18 years | NCT04203316 | COG | IDH2 mutation | |
| pevonedistat | NEDD8 | azacytidine + FLA | 1 month - 21 years | NCT03813147 | COG/PEP-CTN | active, not recruiting |
| ALRN-6924 | MDM2/MDMX | +/− cytarabine | 1 - 21 years | NCT03654716 | DFCI | CNS and solid tumors; lymphoma and leukemia also eligible |
| idasanutlin | MDM2 | FLA or venetoclax | ≤ 30 years | NCT04029688 | Hoffmann-La Roche | ALL and solid tumor also eligible |
| revumenib (SNDX-5613) | menin | ≥ 30 days | NCT04065399 | Syndax | KMT2A rearrangement, NUP98 rearrangement, or NPM1 mutation; KMT2A-rearranged ALL also eligible | |
| revumenib | menin | FLA | ≥ 30 days | NCT05326516 | Syndax | KMT2A rearrangement, NUP98 rearrangement, or NPM1 mutation; KMT2A-rearranged ALL or MPAL also eligible |
| revumenib | menin | decitabine + cedazuridine (ASTX727) + venetoclax | ≥ 12 years | NCT05360160 | MDACC | MPAL also eligible |
| niclosamide | CREB | cytarabine | 2 - 25 years | NCT05188170 | Stanford University | ALAL also eligible |
| Antibody and cellular immunotherapies | ||||||
| GO | CD33 | Liposomal daunorubicin and cytarabine | ≤ 21 years | NCT04915612 | MDACC | CD33+ AML |
| CD33 CAR T cells | CD33 | fludarabine + cyclophosphamide LD | 1 - 35 years | NCT03971799 | CIBMTR multi-site | CD33+ AML |
| CD33xCD3 bispecific antibody | CD33xCD3 | 2 - 21 years | NCT05077423 | Y-mAbs Therapeutics, COG/PEP-CTN | study terminated early due to financial decision by sponsor | |
| CD33 CAR T cells (DARIC) | CD33 | rapamycin (activates DARIC) | ≤ 30 years | NCT05105152 | SCH | study terminated before expansion cohort due to financial decision by sponsor |
| flotetuzumab | CD123xCD3 | NCT04158739 | COG/PEP-CTN | active, not recruiting | ||
| CD123CART | CD123 | fludarabine + cyclophosphamide LD, rituximab for T cell termination | ≤ 21 years | NCT04318678 | SJCRH | CD123+ AML/MDS; ALL and BPDCN also eligible |
| CD123CART | CD123 | LD chemotherapy (fludarabine, cyclophosphamide) | 1 - 29 years | NCT04678336 | CHOP | CD123+ AML |
| SAR443579 (NK cell engager) | CD123 | ≥ 12 years | NCT05086315 | Sanofi | B-ALL and high-risk MDS also eligible | |
| CLL-1 CAR T cells | CLL-1 (CLEC12A, CD371) | fludarabine + cyclophosphamide LD | ≤ 75 years | NCT04219163 | BCM/TCH | CLL-1+ AML |
| CIML NK cells | AML cells | FLAG or fludarabine + cyclophosphamide LD | ≥ 1 year | NCT03068819 | WUSTL | post-HSCT relapse |
| CIML NK cells | AML cells | fludarabine + cyclophosphamide LD | ≥ 1 year | NCT04024761 | DFCI | post-HSCT relapse |
ALAL = acute leukemia of ambiguous lineage, BCM/TCH = Baylor College of Medicine/Texas Children’s Hospital, BPDCN = blastic plasmacytoid dendritic cell neoplasm, CHOP = Children's Hospital of Philadelphia, CREB = cAMP response element binding protein, CIBMTR = Center for International Blood and Marrow Transplant Research, CIML = cytokine-induced memory-like, COG = Children's Oncology Group, DARIC = dimerizing agent-regulated immune-receptor complex, DFCI = Dana-Farber Cancer Institute, FLA = fludarabine/cytarabine, FLAG = fludarabine/cytarabine + g-csf, HSCT = hematopoietic stem cell transplant, ITCC = Innovative Therapies for Childhood Cancer consortium, LD = lymphodepleting chemotherapy, LLS PedAL/EuPAL = Leukemia & Lymphoma Society Pediatric Acute Leukemia and European Pediatric Acute Leukemia consortium, MDS = myelodysplastic syndrome, MPAL = mixed phenotypic acute leukemia, NCI = National Cancer Institute, SCH = Seattle Children's Hospital, SJCRH = St Jude Children's Research Hospital, WUSTL = Washington University in St Louis.
Recent Findings
Clinical Achievements
De novo AML
Starting with AAML03P1 (NCT00070174) and AAML0531, COG trials adopted the Medical Research Council (MRC) chemotherapy backbone, which continues to be the foundation for trial objectives that utilize both randomized comparisons as well as comparisons informed by historical datasets.4,26 In a randomized comparison, COG AAML0531 demonstrated that the addition of the anti-CD33 antibody-drug conjugate GO to the MRC backbone significantly improved EFS, relapse rate (RR), and disease-free survival (DFS) in children with high CD33 expression, FLT3-ITD mutations, and those with KMT2A rearrangements.27-30 The successor AAML1031 randomized phase 3 trial (NCT01371981) investigated whether the addition of bortezomib to the MRC backbone could improve survival3. While bortezomib did not improve outcomes, AAML1031 results led to many other important findings that are summarized in Table 1.
Acute Promyelocytic Leukemia
Acute promyelocytic leukemia (APL) accounts for 10-20% of cases of childhood acute myeloid leukemia and is characterized by the recurrent t(15;17) resulting in a PML::RARA oncoprotein. Historically, APL had poor cure rates with high rates of relapse and early death from complications of the disease and treatment. Presenting white blood cell (WBC>10,000 cells/uL) count has consistently proven to be a risk marker for survival with APL. Outcomes improved with treatment including all-trans retinoic acid (ATRA)31 and arsenic trioxide (ATO). The COG AAML0631 (NCT00866918) trial incorporated an ATO consolidation cycle while decreasing total anthracycline dose and demonstrated very high cure rates for both patients with standard risk (SR) APL and those with HR APL.32 The 3 year event free survival was 95% for SR APL and 83% for HR APL. Relapse rate was low at 4% and similar between the two risk groups, but survival was lower in the HR APL group due to increased number of early death events, predominately from coagulopathy.
The most recent COG AAML1331 phase 3 clinical trial (NCT02339740) for children with newly-diagnosed APL used an ATRA and ATO based treatment regimen with a non-inferiority comparison to AAML0631. All patients received daily dosing of ATRA and ATO in induction until achievement of hematologic remission and then intermittent ATRA and ATO treatments during 4 consolidation cycles. There was no maintenance therapy. Patients with HR APL received 4 doses of idarubicin during induction only. Cytotoxic chemotherapy was thus able to be eliminated for patients with SR APL and was significantly reduced for those with HR APL. Patients with SR APL had 2-year EFS and OS rates of 98% and 99% with one death during induction and one relapse. Patients with HR APL had a 2-year EFS and OS rates of 96% and 100% with no deaths and two relapses.33 Eliminating maintenance therapy also shortened treatment duration to approximately 9 months. Detailed and intensive supportive care to manage coagulopathy and differentiation syndrome resulted in low rates of early death.
Myeloid Leukemia of Down Syndrome (ML-DS)
ML-DS evolves from a subclone of the preleukemic neonatal disorder transient abnormal myelopoiesis (TAM) as a result of co-operation between somatic mutations of GATA1 and mutations in genes encoding cohesin complex components, epigenetic modifiers and signal transducers including RAS pathway genes.34,35 Persistence of TAM blasts for longer than 3 months after diagnosis (detectable by flow cytometry or PCR) was found to correlate with a three-fold increased risk of progression to ML-DS.36,37
There has been significant progress in the treatment and survival of children with ML-DS. Reduction of treatment intensity, from A2971 (NCT00003593)38 and AAAML0431 (NCT00369317),39 reduced the cumulative anthracycline dose from 320 to 240 mg/m2. Despite this reduction of treatment intensity, 5-year EFS was 89.9% and OS 93%. Flow cytometric measurable residual disease (MRD) correlated with outcome and 5-year DFS was 92.7% for MRD-negative patients. Treatment-related mortality (TRM) remained <1% while the majority of ≥ grade3 febrile neutropenic episodes (30%) and microbiologically confirmed infections at sterile sites (23% of patients) were associated with the high dose cytarabine/asparaginase course.
AAML1531 (NCT02521493) introduced a MRD-based risk stratification of treatment intensity for ML-DS based on flow cytometric MRD after the first course of induction therapy.40 The 85% of patients with negative MRD received 1 less high dose cytarabine/asparaginase course compared to AAML0431. Interim analysis, however, revealed that 2-year EFS among MRD-negative (standard risk) patients (85.6%) was inferior to the predecessor study AAML0431 (93.5%)39 due to an increased relapse rate and prompted the closure of the standard risk arm. Standard risk patients who relapsed were more likely to have a complex karyotype and had a low probability of survival (1-year OS 16.7%). Data evaluating the intensification of treatment for patients with positive MRD are not yet available.
Three successive trials (A297138, AAML0431,39 and AAML153140), each implementing a reduction of treatment intensity for ML-DS, confirm that TRM is no longer a dominant cause of treatment failure. Additionally, given the unsatisfactory outcome of patients after relapse of ML-DS41, future trials must focus on prevention of relapse instead emerges as a priority given the unsatisfactory outcome of patients after relapse of ML-DS (with the possible exception of those achieving a second remission prior to HSCT.41 Finally, flow cytometric MRD did not identify a favorable prognostic group for treatment de-intensification. A clinically applicable risk stratification of ML-DS therefore remains to be established.
Chronic Myeloid Leukemia
Like other myeloid diseases, the relative rarity of CML in children compared to adults means that management recommendations are derived from adults. Younger patients, however, often have a more aggressive clinical presentation. Tyrosine kinase inhibitors (TKIs) are highly effective in inducing deep molecular remissions. The current standard of care in children is continuous TKI though transition to adult oncology care, where trials without TKI are standard practice.42 Prolonged exposure to TKIs in children during growth and development can have profound effects on bone growth and metabolism and endocrine function.43,44 The current COG AAML18P1 (NCT03817398) pilot trial focuses on identifying children who can stop TKI without disease recurrence.
Relapsed/refractory AML
Although clinical outcomes for pediatric patients with newly-diagnosed AML have improved due to advances in cytomolecular- and MRD-based risk stratification and enhanced supportive care, nearly half of children continue to relapse and have dismal long-term clinical outcomes.2,45 Importantly, the prognostic significance of some cytomolecular alterations appears to be maintained at relapse. For example, recent studies have reported 4-year 80% OS in patients with relapsed AML harboring RUNX1::RUNXT1 or CBFB::MYH11 rearrangements.46,47 However, the duration of first remission is the most robust prognosticator in children with relapsed AML. Survival is particularly poor for children who relapse at <12 months from initial AML diagnosis (20-30% OS versus 50-60% for relapse ≥12 months).46,48
While no uniform salvage therapy approach(es) for first AML relapse in children has/have been adopted due to differing strategies by pediatric oncology cooperative groups and lack of universally-defined response criteria, consolidation of second remission with allogeneic HSCT (or second HSCT) remains the standard-of-care. Most first relapse regimens include fludarabine with cytarabine (FLA), and/or anthracycline chemotherapy depending on prior cumulative dose exposure with or without GO.47,49 The COG AAML1421 phase 1/2 trial of liposomal daunorubicin/cytarabine (cycle 1) and FLA (cycle 2) for pediatric patients with first relapse of AML (n=37) reported a 81% CR/CRp/CRi with 80% MRD negative. The 2-year OS was 53%. These promising data led to an FDA label extension for pediatric patients >=1 year and to frontline investigation of liposomal daunorubicin/cytarabine in the randomized AAML1831 phase 3 trial. Current and recent pediatric trials are also exploring venetoclax-based salvage therapies given their emerging success.50 Approaches to second or greater AML relapse are far more variable and have included phase 1 clinical trial investigation of new agents when available.51,52
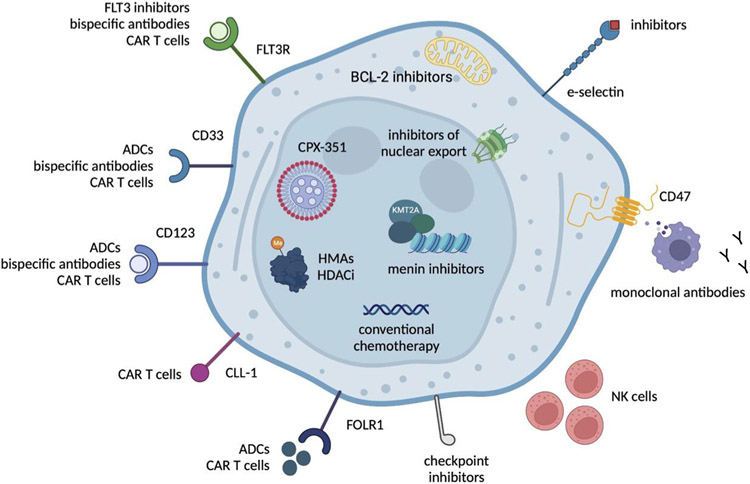
COG and other international consortia have focused upon defining the genetic and immunophenotypic landscape of childhood AML, aligning these biologic characteristics with clinical outcomes data, and investigating precision medicine approaches for relevant high-risk subtypes. Current high-priority pediatric AML targets for which there are active and developing clinical trials are summarized in Figure 2. Several other targeted inhibitors in combination with chemotherapy are also under current investigation via early-phase clinical trials (Table 3). The APAL2020SC Screening Trial (NCT04726241), sponsored by the Leukemia & Lymphoma Society and conducted through COG, provides an opportunity for broad, centralized screening of genomic and cell surface targets for rapid eligibility determination for targeted therapy trials.
Figure 2. Therapeutic targets in relapsed pediatric AML and precision medicine therapies under current or planned clinical investigation.
ADCs = antibody-drug conjugates, CAR = chimeric antigen receptor, FLT3R = FLT3 receptor, FOLR1 = folate receptor 1, HDACi = histone deacetylase inhibitors, HMAs = hypomethylating agents, KMT2A = lysine methyltransferase 2A, NK = natural killer. Figure was created using BioRender.com.
Supportive Care
Advances in supportive care have contributed to improved survival outcomes in COG pediatric AML trials. The COG Myeloid Disease Committee has led and facilitated supportive care research in two primary ways. First, COG phase 3 AML trials were used as a platform for secondary data analyses and embedded supportive care studies. Second, collaboration with the COG Cancer Control and Supportive Care (CCSC) Committee has yielded highly impactful supportive care interventional trials. Secondary data analyses of COG trial data provided supporting data for two randomized clinical through the COG CCSC Committee 53. Specifically, a recently published randomized clinical trial of levofloxacin versus no prophylaxis in pediatric patients with high-risk neutropenia and demonstrated that levofloxacin was associated with a decreased risk of bacteremia in patients with acute leukemia 54. A second COG trial demonstrated that, in pediatric patients with AML, caspofungin reduced the risk of invasive fungal infections relative to fluconazole 55. These two trials have been practice defining for the pediatric AML community and set the foundation for subsequent trials testing novel antibiotics and antifungals.
Recent published secondary data analyses from COG AML clinical trials have reported clinically important cardiac toxicity outcomes during and shortly after completion of front link therapy. Analyses of AAML0531 data demonstrated a higher rate of left ventricular systolic dysfunction (LVSD) than previously reported and that early LVSD was associated with decreased survival outcomes.56 Secondary data analyses from the AAML1031 trial demonstrated a cardioprotective effect of dexrazoxane with compromising survival outcomes.57 These data to the required inclusion of dexrazoxane as a supportive care measure in the standard chemotherapy arm of AAML1831 and embedded cardiotoxicity studies.
Embedded supportive care studies included an analysis of quality of life (QoL) in the recent AAML1031 clinical trial. This study included 505 guardians and 348 children who completed a series of QoL surveys that revealed an association between adverse event frequency and lower QoL.58 This work has set the foundation for an ongoing embedded study of neurocognitive function in the current AAML1831 trial. Secondary data analyses of multiple COG trials have demonstrated a consistent disparity in outcomes by race and ethnicity, serving as proxies for systemic racism and barriers to optimal care. Work by many investigators, including the Myeloid Diseases Disparity Committee, seeks to identify modifiable drivers of these disparities.59 Moving forward, the COG Myeloid Diseases Committee will continue to utilize a two-pronged strategy of advancing supportive care with specific emphases on infection prevention and management, cardio-oncology, neurological outcomes, and addressing disparities.
Biological Achievements
Cytogenetic and molecular landscape in Childhood AML
The procurement of bone marrow samples for correlative biologic studies on serial COG trials has resulted in invaluable access to diagnostic and remission specimens that have further informed our understanding of disease biology and prognostic features. Major findings from AML biology-defining studies incorporating COG samples are included in Table 1.
Risk Classification
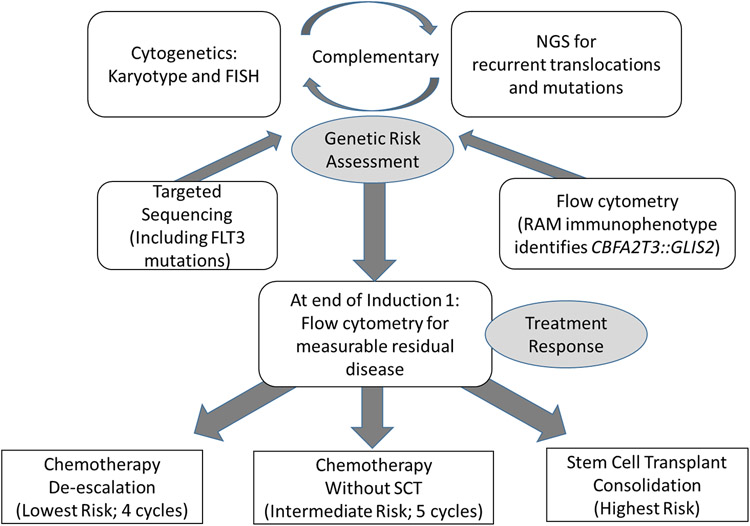
Risk group assignments in childhood AML links specific disease characteristics to the risk of induction failure or relapse. Two general categories of data is used to determine risk status which include (1) Cytomolecular (CM) characteristics and (2) response to induction therapy. The assessment of response occurs after the initial induction regimen using a validated multiparameter flow cytometry (MPF) assay capable of reliably detecting malignant population at a sensitivity of 0.01-0.1%. With the exception of CM favorable risk (Table 2) AML, the assessment of response by MPF is a reliable and significant predictor of event-free and overall survival. The accurate assessment of risk for relapse in children with AML requires the interpretation and integration of cytogenetic and FISH data, with both traditional sequencing and next-generation sequencing along with immunophenotyping for initial phenotype and end of induction response assessment (Figure 3).
Figure 3. Flow diagram for diagnostic and end of induction response assessments to accurately risk stratify patients in the AAML1831 Phase 3 trial.
Historically, risk assignment was based on cytogenetics alone. High risk features including the presence of monosomy 5/5q deletion and monosomy 7 have long been identified as prognostic features predicting a high risk of relapse, while CBF fusions have been considered a more favorable biomarker in all COG de novo AML studies. FLT3-ITD mutations, detected by polymerase chain reaction, were added as a high-risk variant during AAML0531. The AAML1031 trial was the first to incorporate response assessed by MPF into risk classification, and incorporated NPM1 and CEBPA mutations as favorable risk biomarkers. Improvements in cytogenetic and molecular diagnostic techniques have significantly expanded the number of risk stratifying lesions. Advances in next generation sequencing have been essential in validating traditional cytomolecular techniques as well as identifying cryptic risk-defining lesions not detected through traditional methods. In the current Phase 3 study AAML1831, COG incorporates a more comprehensive list of risk defining events using the diagnostic approach described in Figure 3. The complexity of risk classification reflects the heterogeneity of childhood AML and the necessity for all future trials to be informed by, and evaluated according to, standards developed for children.
Key Trials to be Pursued
Treatment Approach for AML
Recent successes in incorporating targeted therapies such as GO and/or sorafenib to intensive chemotherapy regimens have improved survival in subsets of patients, but with additive toxicity. Future strategies will continue to capitalize on identification of relevant therapeutic targets and the incorporation of novel therapies, such as menin inhibition for patients with KMT2A or NUP98 rearrangements. Based upon FDA approval and wide clinical use of venetoclax in adults with AML, but a paucity of data in children, future trials will also aim to elucidate the therapeutic benefit of BCL-2 inhibition in pediatric AML. The COG Myeloid Diseases Committee anticipates that the successor phase 3 trial to AAML1831 will incorporate a design that tests targeted agents involving approximately 45% of patients with relevant genetic alterations. For the remaining 55% of patients who lack a known driver variant directly responsive to targeted therapies, candidate interventions are under consideration. However, future trials will need to balance efforts to utilize a dose and dosing schedule that will limit potential for toxicity, while maximizing efficacy of the targeted agent. The COG Myeloid Diseases Committee also plans to continue its focus upon reducing anthracycline-induced cardiotoxicity of through incorporation of cardioprotective strategies such as dexrazoxane and, possibly, liposomal anthracycline chemotherapeutics (eg, CPX-351). Early phase clinical trials will also investigate new immunotherapies that target critical cell surface targets, such as CD123, CD33, and FOLR1, with a continued emphasis on identifying novel targets through correlative biology studies.
Treatment approach for APL
APL is now one of the most curable forms of childhood cancer with the utilization of ATRA and ATO-based therapy. However, ATO infusions in medical settings create a significant burden of care for patients and their families. An oral formulation of ATO administered at home has the potential to significantly improve quality of life for patient. There are promising trials showing efficacy of oral arsenic compounds. Thus, it is a goal of the COG myeloid committee to evaluate oral ATO in pediatric patients with APL to determine if similar drug exposure (through pharmacokinetic analysis) is achieved with oral versus IV formulations of ATO.
Treatment approach for ML-DS
Building on the successful reduction of TRM in past ML-DS trials, the major objective of future trials is the prevention of relapse events. This goal can be accomplished by developing a clinically applicable risk stratification of ML-DS (similar to non-DS AML) by correlating the molecular subtypes of ML-DS with outcomes, implementing molecular MRD assays based upon measuring the size of cell clones with patient-specific GATA1 and co-operating mutations by error-corrected NGS, and by the introduction of new drugs into the treatment of ML-DS (e.g., liposomal cytarabine/daunorubicin, GO, inhibitors of pathways activated in ML-DS blasts60). Judicious inclusion of patients with ML-DS in early phase trials is essential to assure access to new forms of AML therapy also for this vulnerable group.
Summary and Future Directions
During the past decade, the COG Myeloid Disease Committee has improved outcomes for some children with AML via therapeutic clinical trials, increased understanding of the cytomolecular features of pediatric AML and correlation with clinical outcomes, and intercalation of critical supportive care measures. The continued evaluation of targeted strategies requires an expert and experienced network of collaborating pediatric institutions joined as the COG with aspirations for more global trials to better study children the growing number of rare molecular subsets. Effective development of new precision medicine approaches for children with relapsed AML and other acute leukemias will also be further facilitated by efforts of the ACCELERATE Pediatric Strategy Forum6 and the recently-created international consortium created by the Leukemia & Lymphoma Society to facilitate international collaborations involving the COG.61 This innovative international cooperative infrastructure has successfully engaged academic pediatric oncologists, federal regulatory agencies, and pharmaceutical companies to (1) standardize relapse definitions, response criteria, and outcomes reporting, (2) hasten pediatric-specific drug development and clinical investigation, and (3) increase enrollment efficiency of rare high-risk subtypes of childhood acute leukemias within specific trials. Taken together, the COG Myeloid Diseases Committee continues to leverage critical lessons learned, new biologic understanding, and access to new chemotherapies and targeted therapies with an overarching goal of curing more children with AML.
ACKNOWLEDGEMENTS
We are sincerely grateful to the patients and families who participate in Children’s Oncology Group clinical trials and the treating physicians and medical teams involved in their clinical care and clinical research. None of this work would have been possible without the expert and tireless support of Mary Beth Sullivan, Jeanette Cassar, Gabriel Luevanos, Monica Curtain, Robert Gerbing, James Wang, and Wendy Lee from the Children’s Oncology Group. These studies were supported by National Institutes of Health/National Cancer Institute (NIH/NCI) awards U10CA098413, U10CA098543, U10CA180899, U10CA180886, and U24CA196173. Addition support includes:
TMC is supported by the Evans Family Endowed Chair for Pediatric Cancer
TAA is supported by NIH/NCI U10CA180899
SKT is a Scholar of the Leukemia and Lymphoma Society and holds the Joshua Kahan Endowed Chair in Pediatric Leukemia Research.
JP previously received funding from the St. Baldrick’s Foundation and CureSearch that supported research described in this paper.
RA is supported by the Mai and Harry West Endowed Chair in Pediatric Research.
SM Target Pediatric AML, the Andrew McDonough B+ Foundation, St Baldrick's Foundation, the COG Foundation
EAK received funding from the Leukemia Research Foundation of Delaware.
Glossary
- AML
acute myeloid leukemia
- APL
acute promyelocytic leukemia
- ATO
arsenic trioxide
- ATRA
all-trans retinoic acid
- CBF
core binding factor
- CM
cytomolecular
- CML
chronic myeloid leukemia
- CBF
core-binding factor
- CR
Complete Response
- CRp
complete response without platelet recovery
- CRi
complete response with incomplete count recovery
- CCSC
Cancer Control and Supportive Care
- COG
Children’s Oncology Group
- DFS
disease-free survival
- EFS
event free survival
- FLA
fludarabine and cytarabine
- GO
gemtuzumab ozogamicin
- HR
high risk
- HSCT
hematopoietic stem cell transplant
- ITD
internal tandem duplication
- LVSD
left ventricular systolic dysfunction
- MLDS
myeloid leukemia of Down syndrome
- MRC
Medical Research Council
- MRD
minimal residual disease
- MPF
multiparameter flow cytometry
- NGS
next generation sequencing
- OS
overall survival
- PCR
polymerase chain reaction
- QoL
quality of life
- RR
relapse rate
- SR
standard risk
- TAM
transient abnormal myelopoiesis
- TRM
treatment-related mortality
Footnotes
DISCLOSURE OF FINANCIAL RELATIONSHIPS
TMC – consulted for Day One Biopharmaceuticals
SKT receives/d research funding for unrelated studies from Beam Therapeutics, Incyte Corporation, and Kura Oncology, has consulted for bluebird bio and Jazz Pharmaceuticals, has received travel support from Amgen, and serves/d on scientific advisory boards of Aleta Biotherapeutics, Kura Oncology, and Syndax Pharmaceuticals. She has also served an uncompensated Advisor for the LLS PedAL/EuPAL consortium.
JP receives research funding from Astellas Pharma Inc. and Servier Pharmaceuticals for unrelated research.
The remaining authors declare no conflicts of interest.
REFERENCES
- 1.Gamis AS, Alonzo TA, Perentesis JP, Meshinchi S, Committee COGAML. Children's Oncology Group's 2013 blueprint for research: acute myeloid leukemia. Pediatr Blood Cancer. Jun 2013;60(6):964–71. doi: 10.1002/pbc.24432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolouri H, Farrar JE, Triche T, Jr., et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat Med. Jan 2018;24(1):103–112. doi: 10.1038/nm.4439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aplenc R, Meshinchi S, Sung L, et al. Bortezomib with standard chemotherapy for children with acute myeloid leukemia does not improve treatment outcomes: a report from the Children's Oncology Group. Haematologica. Jul 2020;105(7):1879–1886. doi: 10.3324/haematol.2019.220962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gamis AS, Alonzo TA, Meshinchi S, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children's Oncology Group trial AAML0531. J Clin Oncol. Sep 20 2014;32(27):3021–32. doi: 10.1200/JCO.2014.55.3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative Efforts Driving Progress in Pediatric Acute Myeloid Leukemia. J Clin Oncol. Sep 20 2015;33(27):2949–62. doi: 10.1200/JCO.2015.62.8289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson ADJ, Zwaan CM, Kolb EA, et al. Paediatric Strategy Forum for medicinal product development for acute myeloid leukaemia in children and adolescents: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration. Eur J Cancer. Sep 2020;136:116–129. doi: 10.1016/j.ejca.2020.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostronoff F, Othus M, Kantarjian HM, et al. A model for prediction of FLT3-ITD and NPM1 (without FLT3-ITD) positivity in patients with newly diagnosed acute myeloid leukaemia. Br J Haematol. Oct 2013;163(1):130–2. doi: 10.1111/bjh.12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostronoff F, Othus M, Lazenby M, et al. Prognostic significance of NPM1 mutations in the absence of FLT3-internal tandem duplication in older patients with acute myeloid leukemia: a SWOG and UK National Cancer Research Institute/Medical Research Council report. J Clin Oncol. Apr 1 2015;33(10):1157–64. doi: 10.1200/JCO.2014.58.0571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarlock K, Lamble AJ, Wang YC, et al. CEBPA-bZip mutations are associated with favorable prognosis in de novo AML: a report from the Children's Oncology Group. Blood. Sep 30 2021;138(13):1137–1147. doi: 10.1182/blood.2020009652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimwade D, Walker H, Oliver F, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood. Oct 1 1998;92(7):2322–33. [PubMed] [Google Scholar]
- 11.Faber ZJ, Chen X, Gedman AL, et al. The genomic landscape of core-binding factor acute myeloid leukemias. Nat Genet. Dec 2016;48(12):1551–1556. doi: 10.1038/ng.3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Rooij JD, Branstetter C, Ma J, et al. Pediatric non-Down syndrome acute megakaryoblastic leukemia is characterized by distinct genomic subsets with varying outcomes. Nat Genet. Mar 2017;49(3):451–456. doi: 10.1038/ng.3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radtke I, Mullighan CG, Ishii M, et al. Genomic analysis reveals few genetic alterations in pediatric acute myeloid leukemia. Proc Natl Acad Sci U S A. Aug 4 2009;106(31):12944–9. doi: 10.1073/pnas.0903142106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sohal J, Phan VT, Chan PV, et al. A model of APL with FLT3 mutation is responsive to retinoic acid and a receptor tyrosine kinase inhibitor, SU11657. Blood. Apr 15 2003;101(8):3188–97. doi: 10.1182/blood-2002-06-1800 [DOI] [PubMed] [Google Scholar]
- 15.Yuan Y, Zhou L, Miyamoto T, et al. AML1-ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc Natl Acad Sci U S A. Aug 28 2001;98(18):10398–403. doi: 10.1073/pnas.171321298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly LM, Kutok JL, Williams IR, et al. PML/RARalpha and FLT3-ITD induce an APL-like disease in a mouse model. Proc Natl Acad Sci U S A. Jun 11 2002;99(12):8283–8. doi: 10.1073/pnas.122233699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grisolano JL, O'Neal J, Cain J, Tomasson MH. An activated receptor tyrosine kinase, TEL/PDGFbetaR, cooperates with AML1/ETO to induce acute myeloid leukemia in mice. Proc Natl Acad Sci U S A. Aug 5 2003;100(16):9506–11. doi: 10.1073/pnas.1531730100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stubbs MC, Kim YM, Krivtsov AV, et al. MLL-AF9 and FLT3 cooperation in acute myelogenous leukemia: development of a model for rapid therapeutic assessment. Leukemia. Jan 2008;22(1):66–77. doi: 10.1038/sj.leu.2404951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zuber J, Radtke I, Pardee TS, et al. Mouse models of human AML accurately predict chemotherapy response. Genes Dev. Apr 1 2009;23(7):877–89. doi: 10.1101/gad.1771409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Q, Hadland B, Smith JL, et al. CBFA2T3-GLIS2 model of pediatric acute megakaryoblastic leukemia identifies FOLR1 as a CAR T cell target. J Clin Invest. Nov 15 2022;132(22)doi: 10.1172/JCI157101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiba N, Yoshida K, Hara Y, et al. Transcriptome analysis offers a comprehensive illustration of the genetic background of pediatric acute myeloid leukemia. Blood Adv. Oct 22 2019;3(20):3157–3169. doi: 10.1182/bloodadvances.2019000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiba N, Ichikawa H, Taki T, et al. NUP98-NSD1 gene fusion and its related gene expression signature are strongly associated with a poor prognosis in pediatric acute myeloid leukemia. Genes Chromosomes Cancer. Jul 2013;52(7):683–93. doi: 10.1002/gcc.22064 [DOI] [PubMed] [Google Scholar]
- 23.Hollink IH, van den Heuvel-Eibrink MM, Arentsen-Peters ST, et al. NUP98/NSD1 characterizes a novel poor prognostic group in acute myeloid leukemia with a distinct HOX gene expression pattern. Blood. Sep 29 2011;118(13):3645–56. doi: 10.1182/blood-2011-04-346643 [DOI] [PubMed] [Google Scholar]
- 24.Harrison CJ, Hills RK, Moorman AV, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol. Jun 1 2010;28(16):2674–81. doi: 10.1200/JCO.2009.24.8997 [DOI] [PubMed] [Google Scholar]
- 25.von Neuhoff C, Reinhardt D, Sander A, et al. Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. J Clin Oncol. Jun 1 2010;28(16):2682–9. doi: 10.1200/JCO.2009.25.6321 [DOI] [PubMed] [Google Scholar]
- 26.Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children's Oncology Group. Cancer. Feb 1 2012;118(3):761–9. doi: 10.1002/cncr.26190 [DOI] [PubMed] [Google Scholar]
- 27.Tarlock K, Alonzo TA, Gerbing RB, et al. Gemtuzumab Ozogamicin Reduces Relapse Risk in FLT3/ITD Acute Myeloid Leukemia: A Report from the Children's Oncology Group. Clin Cancer Res. Apr 15 2016;22(8):1951–7. doi: 10.1158/1078-0432.CCR-15-1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollard JA, Alonzo TA, Loken M, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. Apr 19 2012;119(16):3705–11. doi: 10.1182/blood-2011-12-398370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollard JA, Guest E, Alonzo TA, et al. Gemtuzumab Ozogamicin Improves Event-Free Survival and Reduces Relapse in Pediatric KMT2A-Rearranged AML: Results From the Phase III Children's Oncology Group Trial AAML0531. J Clin Oncol. Oct 1 2021;39(28):3149–3160. doi: 10.1200/JCO.20.03048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard JA, Loken M, Gerbing RB, et al. CD33 Expression and Its Association With Gemtuzumab Ozogamicin Response: Results From the Randomized Phase III Children's Oncology Group Trial AAML0531. J Clin Oncol. Mar 1 2016;34(7):747–55. doi: 10.1200/JCO.2015.62.6846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregory J, Kim H, Alonzo T, et al. Treatment of children with acute promyelocytic leukemia: results of the first North American Intergroup trial INT0129. Comparative Study Randomized Controlled Trial. Pediatr Blood Cancer. Dec 2009;53(6):1005–10. doi: 10.1002/pbc.22165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kutny MA, Alonzo TA, Gerbing RB, et al. Arsenic Trioxide Consolidation Allows Anthracycline Dose Reduction for Pediatric Patients With Acute Promyelocytic Leukemia: Report From the Children's Oncology Group Phase III Historically Controlled Trial AAML0631. J Clin Oncol. Sep 10 2017;35(26):3021–3029. doi: 10.1200/JCO.2016.71.6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kutny MA, Alonzo TA, Abla O, et al. Assessment of Arsenic Trioxide and All-trans Retinoic Acid for the Treatment of Pediatric Acute Promyelocytic Leukemia: A Report From the Children's Oncology Group AAML1331 Trial. JAMA Oncol. Jan 1 2022;8(1):79–87. doi: 10.1001/jamaoncol.2021.5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Labuhn M, Perkins K, Matzk S, et al. Mechanisms of Progression of Myeloid Preleukemia to Transformed Myeloid Leukemia in Children with Down Syndrome. Cancer cell. Aug 12 2019;36(2):123–138 e10. doi: 10.1016/j.ccell.2019.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida K, Toki T, Okuno Y, et al. The landscape of somatic mutations in Down syndrome-related myeloid disorders. Nature genetics. Nov 2013;45(11):1293–9. doi: 10.1038/ng.2759 [DOI] [PubMed] [Google Scholar]
- 36.Flasinski M, Scheibke K, Zimmermann M, et al. Low-dose cytarabine to prevent myeloid leukemia in children with Down syndrome: TMD Prevention 2007 study. Blood advances. Jul 10 2018;2(13):1532–1540. doi: 10.1182/bloodadvances.2018018945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamato G, Deguchi T, Terui K, et al. Predictive factors for the development of leukemia in patients with transient abnormal myelopoiesis and Down syndrome. Leukemia. May 2021;35(5):1480–1484. doi: 10.1038/s41375-021-01171-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sorrell AD, Alonzo TA, Hilden JM, et al. Favorable survival maintained in children who have myeloid leukemia associated with Down syndrome using reduced-dose chemotherapy on Children's Oncology Group trial A2971: a report from the Children's Oncology Group. Cancer. Oct 1 2012;118(19):4806–14. doi: 10.1002/cncr.27484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taub JW, Berman JN, Hitzler JK, et al. Improved outcomes for myeloid leukemia of Down syndrome: a report from the Children's Oncology Group AAML0431 trial. Blood. Jun 22 2017;129(25):3304–3313. doi: 10.1182/blood-2017-01-764324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hitzler J. High-dose AraC is essential for the treatment of ML-DS independent of postinduction MRD: results of the COG AAML1531 trial. Blood. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raghuram N, Nakashima K, Rahman S, et al. Survival Outcomes of Children with Relapsed or Refractory Myeloid Leukemia Associated with Down syndrome. submitted 2023; [DOI] [PubMed] [Google Scholar]
- 42.Hijiya N, Millot F, Suttorp M. Chronic myeloid leukemia in children: clinical findings, management, and unanswered questions. Pediatr Clin North Am. Feb 2015;62(1):107–19. doi: 10.1016/j.pcl.2014.09.008 [DOI] [PubMed] [Google Scholar]
- 43.Athale U, Hijiya N, Patterson BC, et al. Management of chronic myeloid leukemia in children and adolescents: Recommendations from the Children's Oncology Group CML Working Group. Pediatr Blood Cancer. Sep 2019;66(9):e27827. doi: 10.1002/pbc.27827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hijiya N, Schultz KR, Metzler M, Millot F, Suttorp M. Pediatric chronic myeloid leukemia is a unique disease that requires a different approach. Blood. Jan 28 2016;127(4):392–9. doi: 10.1182/blood-2015-06-648667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rubnitz JE, Kaspers GJL. How I treat pediatric acute myeloid leukemia. Blood. 2021;138(12):1009–1018. doi: 10.1182/blood.2021011694 [DOI] [PubMed] [Google Scholar]
- 46.Rasche M, Zimmermann M, Steidel E, et al. Survival Following Relapse in Children with Acute Myeloid Leukemia: A Report from AML-BFM and COG. Cancers (Basel). May 12 2021;13(10)doi: 10.3390/cancers13102336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaspers GJ, Zimmermann M, Reinhardt D, et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol. Feb 10 2013;31(5):599–607. doi: 10.1200/JCO.2012.43.7384 [DOI] [PubMed] [Google Scholar]
- 48.Niktoreh N, Lerius B, Zimmermann M, et al. Gemtuzumab ozogamicin in children with relapsed or refractory acute myeloid leukemia: a report by Berlin-Frankfurt-Munster study group. Haematologica. Jan 2019;104(1):120–127. doi: 10.3324/haematol.2018.191841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper TM, Absalon MJ, Alonzo TA, et al. Phase I/II Study of CPX-351 Followed by Fludarabine, Cytarabine, and Granulocyte-Colony Stimulating Factor for Children With Relapsed Acute Myeloid Leukemia: A Report From the Children's Oncology Group. J Clin Oncol. Jul 1 2020;38(19):2170–2177. doi: 10.1200/JCO.19.03306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karol SE, Alexander TB, Budhraja A, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose-escalation study. Lancet Oncol. Apr 2020;21(4):551–560. doi: 10.1016/S1470-2045(20)30060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egan G, Tasian SK. Relapsed pediatric acute myeloid leukaemia: state-of-the-art in 2023. Haematologica. Mar 2 2023;doi: 10.3324/haematol.2022.281106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasche M, Steidel E, Zimmermann M, et al. Second Relapse of Pediatric Patients with Acute Myeloid Leukemia: A Report on Current Treatment Strategies and Outcome of the AML-BFM Study Group. Cancers (Basel). Feb 14 2021;13(4)doi: 10.3390/cancers13040789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sung L, Lange BJ, Gerbing RB, Alonzo TA, Feusner J. Microbiologically documented infections and infection-related mortality in children with acute myeloid leukemia. Blood. Nov 15 2007;110(10):3532–9. doi: 10.1182/blood-2007-05-091942 [DOI] [PubMed] [Google Scholar]
- 54.Alexander S, Fisher BT, Gaur AH, et al. Effect of Levofloxacin Prophylaxis on Bacteremia in Children With Acute Leukemia or Undergoing Hematopoietic Stem Cell Transplantation: A Randomized Clinical Trial. JAMA. Sep 11 2018;320(10):995–1004. doi: 10.1001/jama.2018.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fisher BT, Zaoutis T, Dvorak CC, et al. Effect of Caspofungin vs Fluconazole Prophylaxis on Invasive Fungal Disease Among Children and Young Adults With Acute Myeloid Leukemia: A Randomized Clinical Trial. JAMA. Nov 5 2019;322(17):1673–1681. doi: 10.1001/jama.2019.15702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Getz KD, Sung L, Ky B, et al. Occurrence of Treatment-Related Cardiotoxicity and Its Impact on Outcomes Among Children Treated in the AAML0531 Clinical Trial: A Report From the Children's Oncology Group. J Clin Oncol. Jan 1 2019;37(1):12–21. doi: 10.1200/JCO.18.00313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Getz KD, Sung L, Alonzo TA, et al. Effect of Dexrazoxane on Left Ventricular Systolic Function and Treatment Outcomes in Patients With Acute Myeloid Leukemia: A Report From the Children's Oncology Group. J Clin Oncol. Jul 20 2020;38(21):2398–2406. doi: 10.1200/JCO.19.02856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagarajan R, Gerbing R, Alonzo T, et al. Quality of life in pediatric acute myeloid leukemia: Report from the Children's Oncology Group. Cancer Med. Aug 2019;8(9):4454–4464. doi: 10.1002/cam4.2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Children's Oncology G, Aplenc R, Alonzo TA, et al. Ethnicity and survival in childhood acute myeloid leukemia: a report from the Children's Oncology Group. Blood. Jul 1 2006;108(1):74–80. doi: 10.1182/blood-2005-10-4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grimm J, Bhayadia R, Gack L, Heckl D, Klusmann JH. Combining LSD1 and JAK-STAT inhibition targets Down syndrome-associated myeloid leukemia at its core. Leukemia. May 24 2022;doi: 10.1038/s41375-022-01603-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrew D Hughes SKT. In Which Trial Do I Belong? The PedAL APAL2020SC and EuPAL 2021 Registry Sorting Hats for Relapsed/Refractory Pediatric Acute Leukemias. Hematologist 2023. [Google Scholar]
- 62.Eidenschink Brodersen L, Alonzo TA, Menssen AJ, et al. A recurrent immunophenotype at diagnosis independently identifies high-risk pediatric acute myeloid leukemia: a report from Children's Oncology Group. Leukemia. Oct 2016;30(10):2077–2080. doi: 10.1038/leu.2016.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamba JK, Chauhan L, Shin M, et al. CD33 Splicing Polymorphism Determines Gemtuzumab Ozogamicin Response in De Novo Acute Myeloid Leukemia: Report From Randomized Phase III Children's Oncology Group Trial AAML0531. J Clin Oncol. Aug 10 2017;35(23):2674–2682. doi: 10.1200/JCO.2016.71.2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tarlock K, Alonzo TA, Wang YC, et al. Functional Properties of KIT Mutations Are Associated with Differential Clinical Outcomes and Response to Targeted Therapeutics in CBF Acute Myeloid Leukemia. Clin Cancer Res. Aug 15 2019;25(16):5038–5048. doi: 10.1158/1078-0432.CCR-18-1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brodersen LE, Gerbing RB, Pardo ML, et al. Morphologic remission status is limited compared to DeltaN flow cytometry: a Children's Oncology Group AAML0531 report. Blood Adv. Oct 27 2020;4(20):5050–5061. doi: 10.1182/bloodadvances.2020002070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tarlock K, Alonzo T, Wang YC, et al. Prognostic impact of CSF3R mutations in favorable risk childhood acute myeloid leukemia. Blood. Apr 30 2020;135(18):1603–1606. doi: 10.1182/blood.2019004179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Umeda M, Ma J, Huang BJ, et al. Integrated Genomic Analysis Identifies UBTF Tandem Duplications as a Recurrent Lesion in Pediatric Acute Myeloid Leukemia. Blood Cancer Discov. May 5 2022;3(3):194–207. doi: 10.1158/2643-3230.BCD-21-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lamble AJ, Eidenschink Brodersen L, Alonzo TA, et al. CD123 Expression Is Associated With High-Risk Disease Characteristics in Childhood Acute Myeloid Leukemia: A Report From the Children's Oncology Group. J Clin Oncol. Jan 20 2022;40(3):252–261. doi: 10.1200/JCO.21.01595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang BJ, Smith JL, Farrar JE, et al. Integrated stem cell signature and cytomolecular risk determination in pediatric acute myeloid leukemia. Nat Commun. Sep 19 2022;13(1):5487. doi: 10.1038/s41467-022-33244-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Elsayed AH, Cao X, Mitra AK, et al. Polygenic Ara-C Response Score Identifies Pediatric Patients With Acute Myeloid Leukemia in Need of Chemotherapy Augmentation. J Clin Oncol. Mar 1 2022;40(7):772–783. doi: 10.1200/JCO.21.01422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gbadamosi MO, Shastri VM, Elsayed AH, et al. A ten-gene DNA-damage response pathway gene expression signature predicts gemtuzumab ozogamicin response in pediatric AML patients treated on COGAAML0531 and AAML03P1 trials. Leukemia. Aug 2022;36(8):2022–2031. doi: 10.1038/s41375-022-01622-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marrero RJ, Cao X, Wu H, et al. SAMHD1 Single Nucleotide Polymorphisms Impact Outcome in Children with Newly Diagnosed Acute Myeloid Leukemia. Blood Adv. Jan 23 2023;doi: 10.1182/bloodadvances.2022009088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Farrar JE, Smith JL, Othus M, et al. Long Noncoding RNA Expression Independently Predicts Outcome in Pediatric Acute Myeloid Leukemia. J Clin Oncol. Jun 1 2023;41(16):2949–2962. doi: 10.1200/JCO.22.01114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bertrums EJM, Smith JL, Harmon L, et al. Comprehensive molecular and clinical characterization of NUP98 fusions in pediatric acute myeloid leukemia. Haematologica. Feb 23 2023;doi: 10.3324/haematol.2022.281653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chisholm KM, Smith J, Heerema-McKenney AE, et al. Pathologic, cytogenetic, and molecular features of acute myeloid leukemia with megakaryocytic differentiation: A report from the Children's Oncology Group. Pediatr Blood Cancer. May 2023;70(5):e30251. doi: 10.1002/pbc.30251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lamble AJ, Hagiwara K, Gerbing RB, et al. CREBBP alterations are associated with a poor prognosis in de novo AML. Blood. Apr 27 2023;141(17):2156–2159. doi: 10.1182/blood.2022017545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guest EM, Aplenc R, Sung L, et al. Gemtuzumab ozogamicin in infants with AML: results from the Children's Oncology Group trials AAML03P1 and AAML0531. Blood. Aug 17 2017;130(7):943–945. doi: 10.1182/blood-2017-01-762336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Elgarten CW, Wood AC, Li Y, et al. Outcomes of intensification of induction chemotherapy for children with high-risk acute myeloid leukemia: A report from the Children's Oncology Group. Pediatr Blood Cancer. Dec 2021;68(12):e29281. doi: 10.1002/pbc.29281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hitzler J, Alonzo T, Gerbing R, et al. High-dose AraC is essential for the treatment of ML-DS independent of postinduction MRD: results of the COG AAML1531 trial. Blood. Dec 9 2021;138(23):2337–2346. doi: 10.1182/blood.2021012206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pollard JA, Alonzo TA, Gerbing R, et al. Sorafenib in Combination With Standard Chemotherapy for Children With High Allelic Ratio FLT3/ITD+ Acute Myeloid Leukemia: A Report From the Children's Oncology Group Protocol AAML1031. J Clin Oncol. Jun 20 2022;40(18):2023–2035. doi: 10.1200/JCO.21.01612 [DOI] [PMC free article] [PubMed] [Google Scholar]