Abstract
We used metalloregulated luciferase reporter fusions and spectroscopic quantification of soluble Hg(II) to determine that the hydroperoxidase-catalase, KatG, of Escherichia coli can oxidize monatomic elemental mercury vapor, Hg(0), to the water-soluble, ionic form, Hg(II). A strain with a mutation in katG and a strain overproducing KatG were used to demonstrate that the amount of Hg(II) formed is proportional to the catalase activity. Hg(0) oxidation was much decreased in stationary-phase cells of a strain lacking KatG, suggesting that the monofunctional hydroperoxidase KatE is less effective at this reaction. Unexpectedly, Hg(0) oxidation also occurred in a strain lacking both KatE and KatG, suggesting that activities other than hydroperoxidases may carry out this reaction. Two typical soil bacteria, Bacillus and Streptomyces, also oxidize Hg(0) to Hg(II). These observations establish for the first time that bacteria can contribute, as do mammals and plants, to the oxidative phase of the global Hg cycle.
Gaseous monatomic Hg(0) vapor can arise from a number of natural and anthropogenic sources (25). Over 95% of atmospheric mercury is Hg(0), although the portion which is free monatomic vapor or is adherent to airborne particulates likely varies extensively (8, 14). In order to enter the biosphere and ultimately the food chain, the relatively unreactive Hg(0) must be oxidized to reactive Hg(II), the form which avidly combines with sulfhydryl and imino-nitrogen ligands in proteins and other important biological molecules. The current view is that Hg(0) is oxidized to Hg(II) in the atmosphere as a result of its interaction with ozone in the presence of water (6, 13, 20, 21, 23, 24). In freshwater mercury cycling, oxidation of Hg(0) is assumed to be unimportant (28). It has also been known for over a decade that mammals (17, 22, 29) and plants (7) effectively oxidize monatomic Hg(0) vapor to Hg(II) using catalase and possibly other peroxidases. The two electron transfer from Hg(0) to Hg(II) occurs at the expense of hydrogen peroxide and is mediated by a high-spin Fe(IV) in the heme cofactor (29). This reaction is central to Hg(0) intoxication as it converts the relatively nonreactive gaseous form of mercury into the highly reactive and toxic water-soluble ionic form, Hg(II). The possibility that microorganisms might also be able to oxidize Hg(0) led us to explore this process in bacteria.
To address this question, we examined wild-type Escherichia coli and several derivatives with altered catalase activity. E. coli produces two hydroperoxidases (15). HPI, encoded by katG, is a bifunctional catalase-peroxidase. HPII, encoded by katE, is a monofunctional catalase (Table 1). These enzymes also differ in that KatG is produced predominantly in exponential phase and KatE is produced in stationary phase (19). In order to determine the generality of this process, we also asked whether common soil bacteria could effect this transformation.
TABLE 1.
Bacterial strains and their catalase activities
| Strain | Genotype and/or phenotype | Source | Catalase (min)a |
|---|---|---|---|
| MP180 | HfrH thi | Loewen | 2.5 |
| UM202 | HfrH thi katG::Tn10 | Loewen | >10 |
| JTG100 | btuB::Tn10 | Demple | 6.1 |
| JTG101 | TetsoxyRΔ | Demple | 6.2 |
| JTG314 | TetsoxyRΔ, overproduces KatG | Demple | 0.5 |
| GC4468 | Δlac U169rpsL | Schellhorn | 2.2, 0.9 |
| GC202 | Same as GC4468 but katG17::Tn10 | Schellhorn | 2.4, 1.7 |
| NC4468 | Same as GC4468 but Φ(katE::lacZ+)131 | Schellhorn | 1.4, 1.1 |
| NC202 | Same as GC4468 but Φ(katE::lacZ+)131 katG17::Tn10 | Schellhorn | >10, >10 |
| Bacillus subtilis PY79 | Prototroph, SPβ−, tetracycline resistant | Youngman | 0.53 |
| Streptomyces venezuelae | Prototroph | Westpheling | 3.9 |
The shorter the time, the greater the activity (see Materials and Methods). For the Schellhorn strains, the first number is the activity immediately before exposure to Hg(0) and the second is the activity after 90 min of exposure to Hg(0).
MATERIALS AND METHODS
Bacterial strains.
Bacterial strains used are listed in Table 1. Strains were taken from storage at −70°C and grown on Luria agar plates. The luciferase reporter plasmid was pCC306 (4), which carries a merR-merOP-merT′-luxAB transcriptional fusion and KanR on a P15A replicon.
Growth conditions.
For every experiment, each strain was inoculated directly from the freezer stock to a Luria agar plate and incubated for 8 to 16 h at 37°C. Fresh growth on the plates was then inoculated into either Luria broth containing 25 μg of kanamycin per ml (for luciferase assays) or M63 medium containing 1% Casamino Acids [for direct Hg(0) oxidation assays] and grown overnight. Overnight cultures were subcultured in the same medium and incubated with aeration (300 rpm) at 37°C, while turbidity and catalase activity were periodically monitored (see below). We found it best to grow strains MP180, UM202, JTG100, JTG101, and JTG314 to mid-exponential phase and then subculture them (at a 1:14.5 dilution) and let them grow again to mid-exponential phase in order to minimize carryover of KatE from overnight cultures. Strains GC4468 and NC4468 were grown until their respective catalase activities were optimum (exponential phase); catalase activity in GC202 was optimum in late-exponential to early-stationary phase. Strain NC202, which lacks catalase activity, was grown to stationary phase. The Bacillus and Streptomyces strains had optimum catalase activity in mid-exponential and late-stationary phases, respectively. When catalase activity was at maximum, an aliquot of each culture was assayed for Hg(0) oxidation by one of two methods (see below).
Catalase activity.
In initial work we monitored the decrease in the optical density of a standard H2O2 solution at 240 nm effected by French press-derived cell extracts and found this correlated well with expected activities based on genotype. However, the need to expose the cells to Hg(0) as soon as their catalase activity was at maximum led us to employ a more rapid assay which could be used with intact cells. In this method (1a), a hemostat was used to dip 6-mm Whatman 31 paper discs (Schleicher & Schuell) into the cell culture. Each disc was placed immediately at the bottom of a 30-ml Corex tube containing 15 ml of 0.5% hydrogen peroxide (Sigma Chemical Co., St. Louis, Mo.) at 37°C. The bubbles of O2 gas formed by disproportionation of H2O2 lifted the paper disc from the bottom of the Corex tube to the surface of the H2O2 solution. The time required for the disc to rise was proportional to the amount of catalase on the disc and hence in the cells. Reaction rates were standardized by comparison to purified catalase (Sigma no. C-30). Each value reported below (Table 1) is based on measurements of three separate discs for each culture at each time point in the growth curve; standard deviations for three runs on the same culture were typically ca. 2 to 5%. The assay is only semiquantitative but very rapid and reproducible and much less subjective than the conventional “many bubbles versus fewer bubbles” plate or slide assays.
Quantification of Hg(0) oxidation. (i) Indirectly, by using a luciferase reporter.
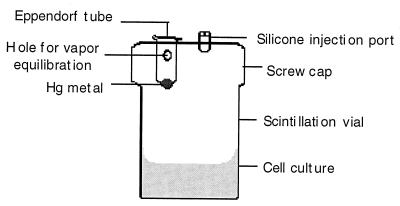
The culture was diluted 1:100 into luciferase assay medium (Luria broth containing kanamycin [25 mg/ml] and 0.1% decanal [Sigma Chemical Co.]). For Hg(II) induction, mercuric chloride (J. T. Baker Inc., Phillipsburg, N.J.) was added to a final concentration of 0.5 μM in a 2.0-ml culture in a 25-ml plastic scintillation vial. For Hg(0) induction, the culture was transferred by syringe into a sealed reaction vessel (Fig. 1) containing a suspended 5-μl droplet of liquid Hg(0). Reaction vessels had been preequilibrated for 24 h at 24°C to establish a Hg(0)-saturated atmosphere (0.1 μM at 25°C [5]). Light emission over time at 24°C was monitored by using a liquid scintillation spectrometer (model LS3801; Beckman Instruments Inc., Irvine, Calif.).
FIG. 1.
Reaction vessel for measurement of Hg(0) oxidation. See Materials and Methods for complete description.
(ii) Directly, by wet-ashing and cold-vapor atomic absorption.
For direct quantification by wet-ashing and cold-vapor atomic absorption, we employed either the scintillation vial reaction vessel (Fig. 1) or a variant based on a 50-ml Erlenmeyer flask fitted with a similar screw cap. Either 2.0 ml (for scintillation vials) or 6.0 ml (for Erlenmeyer flasks) of each culture was injected into a reaction vessel (Fig. 1) preequilibrated so as to have an atmosphere of 0.275 μM mercury vapor at 37°C (5), and incubation was continued at 37°C, with shaking at 75 rpm. At the indicated time points a 0.5-ml sample was removed through the injection port with a hypodermic needle, vortexed in an Eppendorf tube to blow off any unoxidized Hg(0) and immediately transferred into 1 ml of concentrated sulfuric acid (Leeman Labs, Lowell, Mass.) in a closed digestion vessel and held at 25°C. Subsequent procedures are a variation of a protocol supplied by the manufacturer of the PS200 automated Hg analyzer used (Leeman Labs). After all samples were taken, 0.5 ml of concentrated nitric acid (Trace Element Grade; Baker) was added to each sample, which was then heated in the closed digestion vessel at 80°C for 30 min. After cooling to room temperature, 4 ml of 5% potassium permanganate (Leeman Labs) and 2 ml of 5% potassium persulfate (Leeman Labs) were added to the sample. The sample was incubated at 30°C for 1.5 h. One milliliter of 12% hydroxylamine sulfate–12% sodium chloride (Leeman Labs) was added, and the samples were shaken until the purple color disappeared (ca. 15 to 20 s), indicating complete oxidation of sample carbon. The soluble Hg(II) content was determined by using a cold-vapor atomic absorption spectrometer (model PS200; Leeman Labs), which was calibrated daily with mercury standards. Medium blanks and Hg standards in medium were also assayed routinely to define matrix effects for correction of experimental Hg(II) values.
RESULTS
Detection of Hg(II) by a mer-lux transcriptional fusion.
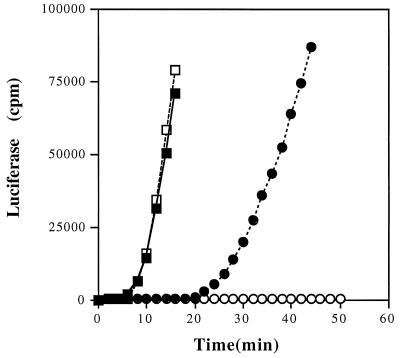
Since it is well established that ionic Hg(II) is required for MerR-mediated induction of transcription in the mer operon (26), we initially made use of this fact to detect the oxidation of Hg(0) to Hg(II) in real time in a growing culture. We used a mer-lux transcriptional fusion (4) to compare the ability of an exponentially growing E. coli strain having or lacking hydroperoxidase I (KatG, the product of the katG gene) to induce mer transcription when exposed either to ionic Hg(II) or to monatomic vapor Hg(0). We found that ionic Hg(II) induces MerR-dependent luciferase expression equally well in exponential-phase E. coli strains with or without hydroperoxidase I (KatG) (Fig. 2). However, cells lacking KatG were impaired in their ability to induce luciferase when exposed to Hg(0), although the wild type readily did so. This observation demonstrates that Hg(0) can pass through the cell wall and be oxidized by catalase, which is a cytosolic enzyme in E. coli. The lag time before the beginning of luciferase induction by Hg(0) may reflect the time necessary to achieve an intracellular Hg(II) concentration sufficient for mer operon induction, which in other work has been shown to be ca. 0.01 μM (4).
FIG. 2.
Induction of mer-lux fusions by Hg(II) or by Hg(0) in the wild type (MP180) and in a katG mutant (UM202), each carrying pCC306 (4). Each value is the average of two independent determinations from three separate cultures. —▪—, wild type with Hg(II); —□—, katG with Hg(II); —•—, wild type with Hg(0); —○—, katG with Hg(0). Standard errors ranged from 3 to 16% but are not shown for clarity.
Direct detection of Hg(II).
While this indirect reporter activation was quite striking, we wanted to confirm that the response we saw really did result from the accumulation of ionic Hg(II) in the culture solution and not from some other undefined effect of Hg(0) on the cells. To do this we grew the cultures while exposing them to Hg(0) and periodically inserted a needle through the injection port of the reaction vessel (Fig. 1), withdrew samples, vortexed them to blow off any dissolved, but unoxidized, Hg(0) and subjected them to total Hg analysis.
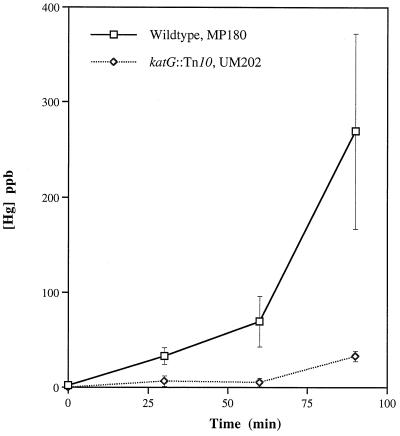
Wild-type E. coli produced stably dissolved Hg(II) as soon as exposure to Hg(0) was begun, accumulating concentrations as high as 40 ppb (200 nM) in only 30 min at 37°C, well within the range of the Kapp for Hg(II) induction of the mer-lux fusion (10 nM) (4). The absence of the katG gene product (hydroperoxidase I) almost completely eliminates the ability of the log-phase cells to oxidize Hg(0) (Fig. 3). However, by this technique, a small amount of Hg(0) oxidation can be detected (above the background oxidation in the medium, which has been subtracted in all figures) as the katG mutant cells enter stationary phase (at 90 min). The possible basis for this early-stationary-phase Hg(0) oxidation is considered below.
FIG. 3.
Hg(0) oxidation by wild-type E. coli (MP180) and a strain lacking KatG (UM202). Each value is the average of results from three experiments; standard errors are indicated by vertical bars. See Table 1 for complete genotypes.
KatG overexpression enhances Hg(0) oxidation.
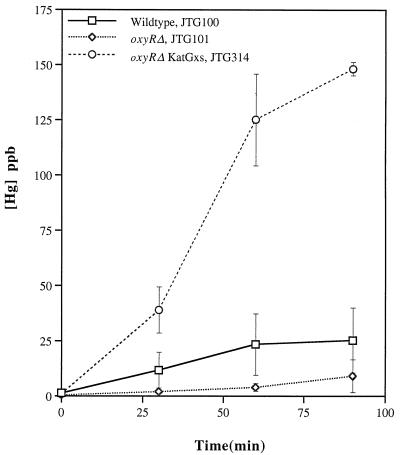
Our initial data implicated KatG as capable of Hg(0) oxidation, so we asked whether overexpression of this enzyme further accelerates this process. Greenberg and Demple (12) had previously isolated a second-site suppressor of an oxyR mutant in which expression of the katG gene is independent of the OxyR activator. This strain, JTG314, has considerably higher catalase activity than either of its two antecedent strains, JTG100 and JTG101 (Table 1), and its ability to oxidize Hg(0) is also correspondingly greater (Fig. 4). Greenberg and Demple (12) had also derived a strain which overexpressed KatE. However, we found that phenotype to be unstable (data not shown), so we took a different approach to assessing whether KatE can oxidize Hg(0).
FIG. 4.
Hg(0) oxidation by E. coli strains with altered expression of katG. Each value is the average of results from three experiments; standard errors are indicated by vertical bars. See Table 1 for complete genotypes.
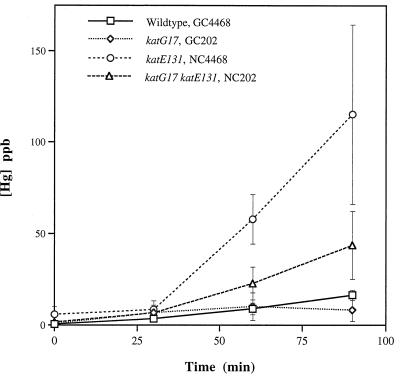
Oxidation of Hg(0) by KatE.
Mukhopadhyay and Schellhorn (18) constructed a well-defined set of transposon-knockout mutations in katG, katE, and in both genes, in the same genetic background (18). We used these strains to ask whether the stationary-phase catalase, KatE, can carry out this reaction (Fig. 5). All strains were grown to the point of their optimum catalase activity (see Materials and Methods) and then immediately exposed to Hg(0); catalase activity was also monitored throughout the period of Hg(0) exposure (Table 1) and did not decline for any of the strains which had either intact catalase gene. The parental strain (GC4468) had a maximum catalase activity between that of the other two wild-type strains examined here (MP180 and JTG100) (Table 1) and oxidized Hg(0) at a rate comparable to that of JTG100 (Fig. 4) rather than to that of MP180 (Fig. 3). Interestingly, the katE mutant (which produces only KatG) had greater Hg(0) oxidizing activity than the wild-type parental strain. The katG mutant had much lower Hg(0)-oxidizing activity than the wild type, suggesting that KatE does not oxidize Hg(0) as well as KatG does, and all strains which oxidized Hg(0) exhibited a lag period of ca. 25 min. The double-mutant strain had no detectable catalase activity; thus, it was surprising that this strain oxidized Hg(0) at a higher rate than the wild-type parental strain. We will discuss below the possible bases for each of these results.
FIG. 5.
Hg(0) oxidation by E. coli strains lacking KatG, KatE, or both. Each value is the average of results from four experiments; standard errors are indicated by vertical bars. See Table 1 for complete genotypes.
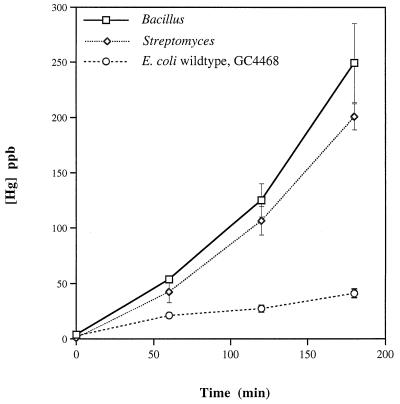
Hg(0) oxidation by soil bacteria.
E. coli is not a major component of the microbiota of most terrestrial ecosystems, so we moved beyond this mammalian flora bacterium to assess the Hg(0)-oxidizing capacity of two well-defined strains representative of soil bacteria (Fig. 6). The prototroph Bacillus subtilis PY79 had strong catalase activity and correspondingly strong Hg(0) oxidation activity. The prototroph Streptomyces venezuelae had somewhat less catalase activity but oxidized mercury at a high rate (Fig. 6). Both carried out the reaction at a higher rate and to a greater final extent than the wild-type E. coli strain (GC4468) used for comparison in these experiments.
FIG. 6.
Hg(0) oxidation by typical soil bacteria. Each value is average of results from three experiments; standard errors are indicated by vertical bars. See Table 1 for complete genotypes.
DISCUSSION
Our observations indicate, firstly, that bacterial hydroperoxidases can oxidize volatile, monatomic mercury vapor, Hg(0), to soluble, ionic mercuric ion, Hg(II), even as their mammalian and plant counterparts can. While there are differences among strains in the absolute amounts of Hg(0) oxidized, the wild-type strains of all three genera examined readily carry out the reaction. We also found that monatomic Hg(0) vapor is more susceptible to this oxidation process than is a 5-μl droplet of Hg(0) liquid metal immersed in the bacterial culture suspension (25a). This is very likely a result of coating the surface of the liquid Hg(0) droplet with thiol-containing medium constituents, which limits the evaporation of Hg(0) atoms from the droplet surface and consequently limits the Hg(0) available for oxidation by either biotic or abiotic processes (1).
Secondly, in E. coli, KatG is clearly able to effect Hg(0) oxidation and apparently does so even more strongly in a strain where it is the only functional catalase (NC4468) (Fig. 5). The elevated Hg(0) oxidation by this strain lacking KatE might arise from an increase in the intracellular concentrations of the cosubstrate for the oxidation, H2O2. The H2O2 concentration fluctuates as a function of growth phase even in wild-type cells (10, 11). By the same argument, if the [H2O2] were also higher in the katG strain, its inability to oxidize Hg(0) suggests even more strongly that KatE is not able to carry out this reaction. We did attempt to measure the [H2O2] in these strains but the reported levels (ca. 0.2 μM) (10, 11) are nearly at the limit of detection by standard reagents, and our measurements were not reproducible.
Variations in available H2O2 may also underlie the differences in the abilities of JTG100 and JTG101 to oxidize Hg(0). Although their pre-Hg(0)-exposure catalase activities are similar (Table 1), the wild-type strain (JTG100) oxidized more Hg(0) than the oxyR mutant (JTG101) (Fig. 4). During Hg(0) exposure, increases in [H2O2] might arise from direct effects of Hg(II) on the electron transport pathway (16) or from Hg(II)-mediated activation of the superoxide stress response (9), including superoxide dismutase, whose product is H2O2. Such increases in cellular [H2O2] could induce KatG in JTG100 but not in JTG101, which lacks a functional OxyR protein. Moreover, the need to build up a minimum [H2O2] before the rate of Hg(0) oxidation can increase might result in the lag period observed in Fig. 5.
Surprisingly, in the katEG strain (NC202), Hg(0) is oxidized by an activity which does not disproportionate H2O2 and, hence, is not detectable in the catalase assay we used. Such an activity might result from derepression of an enzyme provoked by the absence of both hydroperoxidases and exposure to Hg(0). Alternatively, it might be the result of an additional mutation in the katEG strain, the presence of which is discernible only under the rather unusual conditions of this assay. The slight increase in Hg(0) oxidation during early stationary phase in the katG strain UM202 (Fig. 3) might also be attributable to derepression of some other non-catalase-oxidizing activity.
Thirdly, at the maximum rate observed here (Fig. 3) (strain MP180, between 60 and 90 min), 1.0 ml (ca. 108) of wild-type E. coli cells oxidized 1,000 pmol of Hg(0) to Hg(II) in 30 min, for a rate of 33 pmol/min · ml of cells−1. The rate of oxidation by the medium alone (not shown) was approximately 28-fold less at 1.2 pmol/min · ml of medium−1. We assume that Hg(0) oxidation by the medium alone arose from ozone present in the vessel headspace (13). The medium alone plateaued at ca. 25 ppb after 60 min of exposure; in contrast, the reactions containing cells were only beginning to fall off slightly at 90 min. We expect that formation of Hg(II) by the cells should plateau near 1 to 2 μM, a level of Hg(II) which is toxic for bacterial cells lacking the mercury resistance (mer) locus. In this regard, it is notable that, although quite brisk, this Hg(0) oxidation by bacterial catalase is much slower than the MerA-mediated reduction of Hg(II) to Hg(0) effected by bacteria carrying the entire set of mer operon structural genes. A fully induced, log-phase culture expressing MerA can reduce >90% of 10 μM Hg(II) in ca. 10 min at 37°C (27) or ca. 1,000 pmol/min · ml of cells−1.
In summary, we have described here a microbial mercury fixation process in which the relatively inert, gaseous form of the element Hg(0) is converted to a biologically available, reactive, ionic form of Hg(II). Our observations suggest that there are differences in the abilities of bacterial hydroperoxidases to oxidize Hg(0), just as is the case with the eukaryotic heme hydroperoxidases: bovine liver catalase, lactoperoxidase, and horseradish peroxidase (22). The degree to which reactions such as these figure in the global mercury cycle has not been explicitly assessed. However, the rapidity of the process observed in the laboratory suggests that the contribution might be considerable and have significant environmental consequences. For example, in sulfate-rich sediments, it is Hg(II), which is the substrate for formation of monomethyl- and dimethylmercury by sulfate-reducing bacteria in lake sediments (2, 3). Hg(0) oxidation by respiring microbes in adjacent niches might facilitate the biomagnification of this neurotoxic organomercurial by providing a source of mercury in a reactive, methylatable form. Our current efforts are directed towards assessment of Hg(0) oxidation in natural soil and freshwater microcosms.
ACKNOWLEDGMENTS
We gratefully acknowledge the assistance of Fan Lee in developing the catalase assay and the technical support staff of Leeman Laboratories in implementing the PS200 protocols. We appreciate the gift of strains from Bruce Demple, Peter Loewen, Herb Schellhorn, Janet Westpheling, and Phil Youngman. J.A.M. used the luciferase reporter, and T.S. and K.P. used cold-vapor atomic absorption to detect Hg(0) oxidation. T.S. and A.O.S. cowrote the paper.
This work was supported by EPA grant number R82-0779-010 and DOE grant ER62358 to A.O.S.
REFERENCES
- 1.Bloom, N. Personal communication.
- 1a.Choinsk J S, Patterson J W. A simple and inexpensive way for studying the kinetics of the enzyme catalase. J Biol Educ. 1996;27:7–9. [Google Scholar]
- 2.Compeau G, Bartha R. Methylation and demethylation of mercury under controlled redox, pH, and salinity conditions. Appl Environ Microbiol. 1984;48:1203–1207. doi: 10.1128/aem.48.6.1203-1207.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compeau G C, Bartha R. Sulfate-reducing bacteria: principal methylators of mercury in anoxic estuarine sediment. Appl Environ Microbiol. 1985;50:498–502. doi: 10.1128/aem.50.2.498-502.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condee C W, Summers A O. A mer-lux transcriptional fusion for real-time examination of in vivo induction kinetics and promoter response to altered superhelicity. J Bacteriol. 1992;174:8094–8101. doi: 10.1128/jb.174.24.8094-8101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dean J A, editor. Lange’s handbook of chemistry. New York, N.Y: McGraw-Hill; 1973. [Google Scholar]
- 6.DeMagalhaes M E A, Tubino M. A possible path for mercury in biological systems—the oxidation of metallic mercury by molecular oxygen in aqueous-solutions. Sci Total Environ. 1995;170:229–239. doi: 10.1016/0048-9697(95)04711-5. [DOI] [PubMed] [Google Scholar]
- 7.Du S-H, Fang S C. Catalase activity of C3 and C4 species and its relationship to mercury vapor uptake. Environ Exp Bot. 1983;23:347–353. [Google Scholar]
- 8.Fitzgerald W F, Mason R P. Biogeochemical cycling of mercury in the marine environment. In: Sigel A, Sigel H, editors. Mercury and its effects on environment and biology. 1st ed. Vol. 34. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 53–111. [Google Scholar]
- 9.Fuentes A M, Amabile-Cuevas C F. Mercury can induce multiple antibiotic resistance in Escherichia coli, through the partial activation of SoxR, a redox-sensing regulatory protein. FEMS Microbiol Lett. 1996;154:385–388. doi: 10.1111/j.1574-6968.1997.tb12671.x. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Flecha B, Demple B. Homeostatic regulation of intercellular hydrogen peroxide concentration in aerobically growing Escherichia coli. J Bacteriol. 1997;179:382–388. doi: 10.1128/jb.179.2.382-388.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Flecha B, Demple B. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J Biol Chem. 1995;270:13681–13687. doi: 10.1074/jbc.270.23.13681. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg J T, Demple B. Overproduction of peroxide-scavenging enzymes in Escherichia coli suppresses spontaneous mutagenesis and sensitivity to redox-cycling agents in oxyR− mutants. EMBO J. 1988;7:2611–2617. doi: 10.1002/j.1460-2075.1988.tb03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iverfeldt A, Lindqvist O. Atmospheric oxidation of elemental mercury by ozone in the aqueous phase. Atmos Environ. 1986;20:1567–1573. [Google Scholar]
- 14.Kim K-H, Hanson P J, Barnett M O, Lindberg S E. Biogeochemistry of mercury in the air-soil-plant system. In: Sigel A, Sigel H, editors. Mercury and its effects on environment and biology. 1st ed. Vol. 34. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 185–212. [Google Scholar]
- 15.Loewen P C, Switala J, Triggs-Raine B L. Catalases HPI and HPII in Escherichia coli are induced independently. Arch Biochem Biophys. 1985;243:144–149. doi: 10.1016/0003-9861(85)90782-9. [DOI] [PubMed] [Google Scholar]
- 16.Lund B, Miller D M, Woods J S. Studies on Hg(II)-induced hydrogen peroxide formation and oxidative stress in rat kidney mitochondria. Biochem Pharmacol. 1993;45:2017–2024. doi: 10.1016/0006-2952(93)90012-l. [DOI] [PubMed] [Google Scholar]
- 17.Magos L, Clarkson T W. Role of catalase in the oxidation of mercury vapor. Biochem Pharmacol. 1978;27:1373–1377. doi: 10.1016/0006-2952(78)90122-3. [DOI] [PubMed] [Google Scholar]
- 18.Mukhopadhyay S, Schellhorn H E. Identification and characterization of hydrogen peroxide-sensitive mutants of Escherichia coli: genes that require OxyR for expression. J Bacteriol. 1997;179:330–338. doi: 10.1128/jb.179.2.330-338.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulvey M R, Switala J, Borys A, Loewen P C. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990;172:6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munthe J. The aqueous oxidation of elemental mercury by ozone. Atmos Environ. 1992;26:1461–1468. [Google Scholar]
- 21.Munthe J, McElroy W J. Some aqueous reactions of potential importance in the atmospheric chemistry of mercury. Atmos Environ. 1992;26A:553–557. [Google Scholar]
- 22.Ogata M, Aikoh H. Mechanism of metallic mercury oxidation in vitro by catalase and peroxidase. Biochem Pharmacol. 1984;33:493–496. doi: 10.1016/0006-2952(84)90246-6. [DOI] [PubMed] [Google Scholar]
- 23.Pleijel K, Munthe J. Modelling the atmospheric mercury cycle—chemistry in fog droplets. Atmos Environ. 1995;29:1441–1457. [Google Scholar]
- 24.Schroeder W, Yarwood G, Niki H. Transformation processes involving mercury species in the atmosphere—results from a literature survey. Water Air Soil Pollut. 1991;56:653–666. [Google Scholar]
- 25.Sigel A, Sigel H, editors. Mercury and its effects on environment and biology. Vol. 34. New York, N.Y: Marcel Dekker, Inc.; 1997. [Google Scholar]
- 25a.Smith, T. Unpublished data.
- 26.Summers A O. Untwist and shout: a heavy metal-responsive transcriptional regulator. J Bacteriol. 1992;174:3097–3101. doi: 10.1128/jb.174.10.3097-3101.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Summers A O, Silver S. Mercury resistance in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1972;112:1228–1236. doi: 10.1128/jb.112.3.1228-1236.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandal G M, Fitzgerald W F, Rolfhus K R, Lamborg C H. Modelling the elemental mercury cycle in Pallette Lake, Wisconsin, USA. Water Air Soil Pollut. 1995;80:529–538. [Google Scholar]
- 29.Wigfield D C, Tse S. Kinetics and mechanism of the oxidation of mercury by peroxidase. Can J Chem. 1985;63:2940–2944. [Google Scholar]