Abstract
Significance:
Dihydrolipoamide dehydrogenase (DLDH) is a flavin-dependent disulfide oxidoreductase. The active form of DLDH is a stable homodimer, and its deficiencies have been linked to numerous metabolic disorders. A better understanding of redox and nonredox features of DLDH may reveal druggable targets for disease interventions or preventions.
Recent Advances:
In this article, the authors review the different roles of DLDH in selected pathological conditions, including its deficiency in humans, its role in stroke and neuroprotection, skin photoaging, Alzheimer's disease, and DLDH as a nondehydrogenating protein, and construction of genetically modified DLDH animal models for further studying the role of DLDH in specific pathological conditions. DLDH is also vulnerable to oxidative modifications in pathological conditions.
Critical Issues:
Novel animal models need to be constructed using gene knockdown techniques to investigate the redox- and nonredox roles of DLDH in related metabolic diseases. Specific small-molecule DLDH inhibitors need to be discovered. The relationship between modifications of specific amino acid residues in DLDH and given pathological conditions is an interesting area that remains to be comprehensively evaluated.
Future Directions:
Cell-specific or tissue-specific knockdown of DLDH creating specific pathological conditions will provide more insights into the mechanisms, whereby DLDH may have therapeutic values under a variety of pathological conditions. Antioxid. Redox Signal. 39, 794–806.
Keywords: BN-PAGE, dihydrolipoamide dehydrogenase, metabolic disease, mitochondria, oxidative stress, redox
Introduction
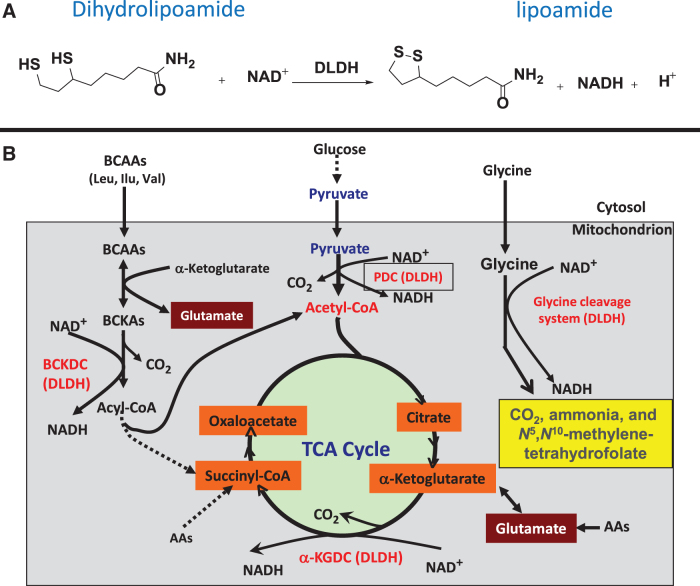
Dihydrolipoamide dehydrogenase (DLDH) is a flavin-dependent oxidoreductase catalyzing the oxidation of dihydrolipoamide to lipoamide with concurrent production of NADH at the expense of NAD+ (Fig. 1A) (Argyrou et al., 2002; Carothers et al., 1989; Williams, 1992). DLDH is involved in several key metabolic enzyme complexes, including pyruvate dehydrogenase complex (PDC), α-keto glutarate dehydrogenase complex (α-KGDC), branched chain α-keto acid dehydrogenase complex, and the glycine cleavage system (Fig. 1B) (Vettakkorumakankav and Patel, 1996; Yan et al., 2008).
FIG. 1.
Chemical reaction and metabolic pathways involving DLDH. (A) DLDH catalyzed oxidation of dihydrolipoamide to form lipoamide with concurrent generation of NADH at the expense of NAD+. (B) DLDH involved enzyme complexes or system in which DLDH serves as a subunit catalyzing the reaction shown in (A). DLDH, dihydrolipoamide dehydrogenase.
DLDH can also behave as an NADH-specific diaphorase, whereby it catalyzes the oxidation of NADH with reduction of NAD+ and ubiquinone (Patel and Harris, 1995; Xia et al., 2001). The active form of DLDH, when functioning as a dehydrogenase, is a homodimer with each monomer contributing key amino acid residues to the homodimeric functional activities (Argyrou and Blanchard, 2004; Brautigam et al., 2005; Vaubel et al., 2011). In this article, we overview the role of DLDH in health and disease by discussing selected paradigms.
Our Serendipitous Finding of DLDH
Between 2003 and 2005, the first author of this article was heavily involved in investigating protein carbonylation in aging and disease using both mouse and rat as animal models (Wu et al., 2016; Yan, 2009). A major technique used at the time was two-dimensional gel electrophoresis of oxidized proteins using the specific probe 2,4-dinitrophenyl hydrazine (DNPH) that specifically reacts with protein carbonyl groups to produce 2,4-dinitrophenyl hydrazones, which can be further identified by Western blot assays using anti-DNPH antibodies (Reznick and Packer, 1994; Yan, 2009; Yan et al., 1998; Yan et al., 1997a; Yan et al., 1997b).
Protein carbonyls can also be labeled by aldehyde-reactive probes linked to biotin (Wu et al., 2016). After labeling, the oxidized proteins were then separated by two-dimensional gel electrophoresis, followed by Western blotting detection of the oxidized proteins using anti-DNPH antibodies or streptavidin (if the labeling probe contains a biotin moiety) (Wu et al., 2016; Yan, 2009).
The method revealed numerous proteins that underwent oxidative carbonylation in aging or under pathological conditions, leading to identification of a large number of structural proteins or cytoskeleton proteins that cannot be readily characterized by their functions (Yan and Forster, 2011). This is a bottleneck for 2D proteomics as many of the identified proteins cannot be functionally analyzed within a short time. In fact, many identified carbonylated proteins cannot be readily analyzed if no methods are available at the time of identification. This is particularly true if the identified proteins are cytoskeleton proteins instead of enzymes. Therefore, it is difficult to know if the identified carbonylated proteins have any functional abnormalities due to carbonylation.
To avoid this problem, we tweaked our approach by analyzing functional activity first, and then determining whether any detectable changes in activities could be due to oxidative carbonylation or modifications. At the time of this approach pivot, we wanted to focus on mitochondrial NAD+/NADH-specific oxidoreductases using blue native polyacrylamide gel electrophoresis (BN-PAGE). We modified the original BN-PAGE protocol (Schagger, 1995) by performing nongradient gel electrophoresis to resolve whole mitochondrial proteins instead of only mitochondrial membrane proteins (Yan et al., 2007).
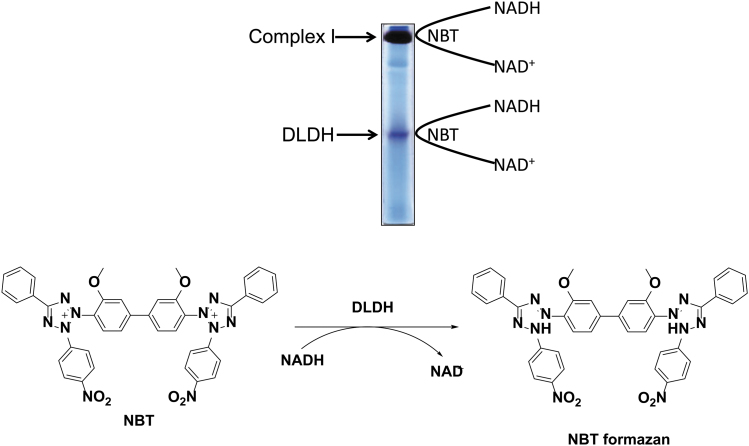
As a result, we observed two gel bands containing NADH dehydrogenating activity when the gel strip was incubated with a buffer containing NADH and the artificial electron acceptor nitroblue tetrazolium (Fig. 2). The upper band is obviously that of mitochondrial complex I (NADH-ubiquinone oxidoreductase), and the lower band was then identified to be that of DLDH (Yan et al., 2007). Since then, the first author of this article (L-J.Y.) at the University of North Texas Health Science Center at Fort Worth has been focusing on studying these two mitochondrial enzymes (Yan and Forster, 2009; Yan et al., 2008).
FIG. 2.
BN-PAGE analysis of whole mitochondrial proteins. Shown is rat brain mitochondria preparation resolved by nongradient BN-PAGE (9%). Upon completion of gel electrophoresis, the gel strip was incubated in a solution containing NADH and NBT that serves as an artificial electron acceptor (reaction shown below the gel image). Among numerous NADH-dependent oxidoreductases, only complex I and DLDH can be detected by this method of BN-PAGE. The detailed procedures for enzymes activity staining are given in references (Yan and Forster, 2009; Yan et al., 2007). BN-PAGE, blue native polyacrylamide gel electrophoresis; NBT, nitroblue tetrazolium.
It should be stressed that if only mitochondrial membrane proteins were analyzed by BN-PAGE, DLDH would have not been found, as DLDH itself is not a membrane protein (Yan et al., 2007). It is also possible that the sample preparation procedure in the original BN-PAGE protocol could lead to loss of DLDH due to dissociation from its parent complexes such as PDC and α-KGDC, both of which are known to associate with mitochondrial membranes (Carothers et al., 1989; Williams, 1992).
Structure and Function of DLDH
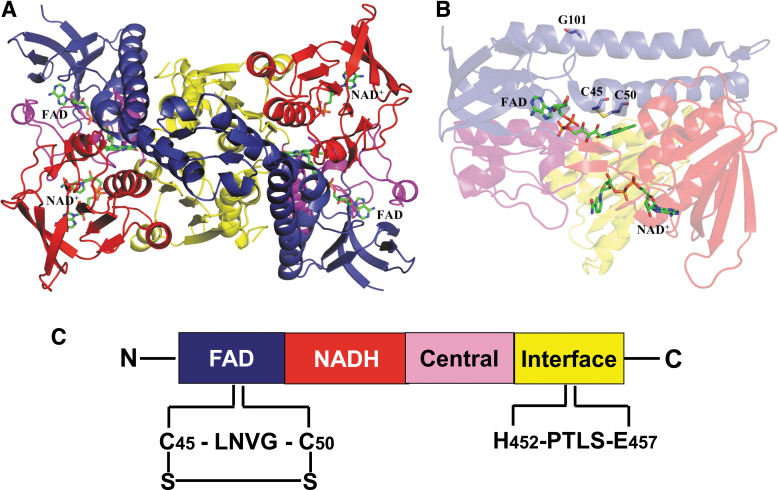
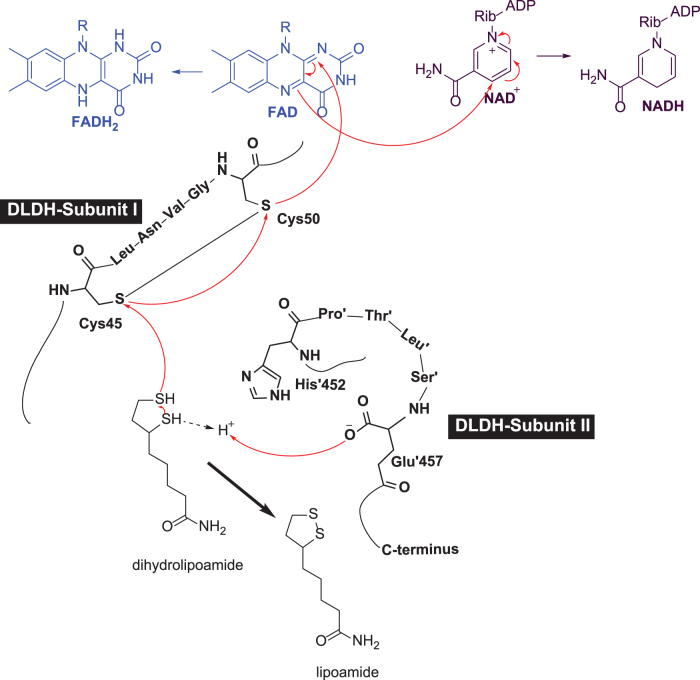
Mammalian DLDH monomer has ∼475 amino acid residues with very slight variation from rodents to humans. The molecular weight of the monomer is ∼51 kDa (Carothers et al., 1989; Jentoft et al., 1992). In rat, each DLDH monomer has 10 cysteine residues (Yan et al., 2013a), including cysteine 45 (Cys45) and cysteine 50 (Cys50) at the active center, which transfers electrons from the substrate dihydrolipoamide to NAD+ via flavin adenine dinucleotide (FAD) (Fig. 3A, B). As shown in Figure 3C, each monomer contains four domains: the FAD binding domain, the NAD+ binding domain, the central domain, and the interface domain (Brautigam et al., 2005; Jentoft et al., 1992).
FIG. 3.
A structural model for DLDH. (A) Shows the structure of DLDH homodimer containing a tightly bound FAD molecule and a loosely bound NAD molecule. The two cysteine residues at the active center of the enzyme are shown in (B). FAD-binding domain, the NAD+-binding domain, the central domain, and the interface domain are shown in blue, red, magenta, and yellow, respectively. C45, C50, G101, FAD, and NAD+ are shown as stick model. Images (A, B) were created by Pymol (www.pymol.org) using data from the Protein Data Bank (PDB ID: 1ZMC) (Brautigam et al., 2005). C shows the four domains contained in each monomer from N-terminal to C-terminal and the key amino acid residues involved in NAD-dependent oxidation of dihydrolipoamide. C45, cysteine 45; C50, cysteine 50; FAD, flavin adenine dinucleotide; G101, glycine residue number 101; LNVG, leucine-asparagine-valine-glycine; PTLS, proline-threonine-leucine-serine.
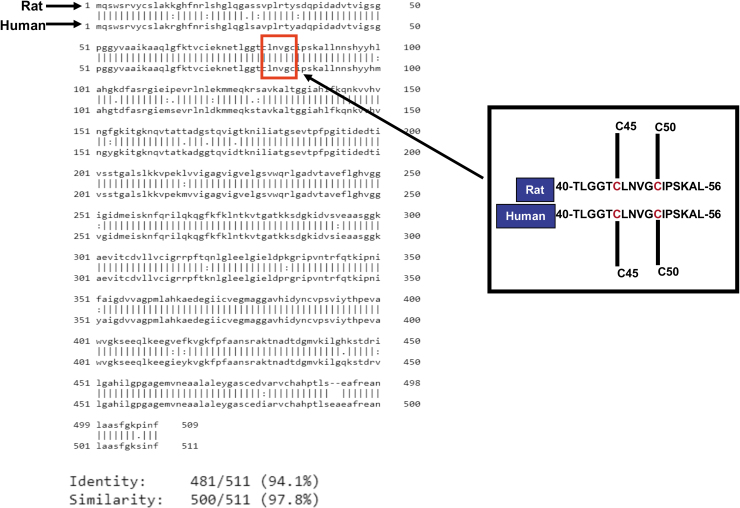
The two Cys residues of the CXXXXC active site interact with the FAD cofactor, while the C-terminal interface domain with the conserved H452XXXXE457 also participates in the catalytic activity of the homodimeric enzyme. As shown in Figure 4, there is a 97.8% similarity and 94.1% identity between rat and human DLDH amino acid sequences (Fig. 4, the left panel).
FIG. 4.
Comparison of amino acid sequences of rat and human DLDH. The left panel shows whole sequence alignment, and the right panel shows segments containing the two cysteine residues at the active center of DLDH. As can be seen from the alignment, this segment of amino acid residues is identical between rat and human. Sequence alignment was performed using the website tool EMBOSS Needle (ClustalW2<Multiple Sequence Alignment<EMBL-EBI). Data were derived from National Center for Biotechnology Information database (GenBank IDs for rat and human are AAH62069.1 and AAB01381.1, respectively) (Feigenbaum and Robinson, 1993; Strausberg et al., 2002).
Moreover, the primary peptide sequences containing Cys45 and Cys50 from amino acid residues 40 to 56 in mature form of DLDH are identical (Fig. 4, right panel). These data indicate that DLDH is highly conserved in evolution. Figure 5 shows that electrons from the substrate dihydrolipoamide are transferred to reduce the Cys45–Cys50 disulfide. The electron from reduced Cys50 are transferred to the isoalloxazine ring of FAD and further to NAD+, generating NADH to complete the catalytic reaction and to restore the Cys45–Cys50 disulfide of the enzyme.
FIG. 5.
Diagram depicting the catalytic mechanism of DLDH. Electrons from the substrate dihydrolipoamide are transferred to cys-45, cys-50, then to FAD, and finally to NAD+ with the generation of lipoamide and NADH.
As can be seen in Figure 3B, the substrate and the NAD+ molecules are located on different domains of the enzyme. Hence, dihydrolipoamide and NAD+ cannot really “see” each other. Therefore, the enzyme operates like a mini-electron transport system (Fig. 5). Conceivably, disruption of this mini-electron transport system by any means can impair the enzymatic function, leading to accumulation of the upstream metabolic intermediates such as pyruvate or lactate (Broxton et al., 2022).
DLDH Deficiency
Only few cases of DLDH deficiency have been reported in humans (Ambrus, 2019; Hong et al., 1996; Liu et al., 1993; Odievre et al., 2005; Shany et al., 1999; Staretz-Chacham et al., 2021; Szabo et al., 2019). DLDH deficiency may be manifested by several disorders such as maple syrup urine disease (Ambrus, 2019; Chuang et al., 2006; Hengeveld and de Kok, 2002), the early onset of neurological disease, hepatic disease, and myopathic abnormalities (Quinonez and Thoene, 1993). If untreated, the early onset of neurological disease and hepatic disorders caused by DLDH deficiency can lead to death, and myopathic presentation can be reflected by muscle weakness and increased levels of creatinine and lactate (Quinonez and Thoene, 1993).
For the purpose of diagnosis, genetic analysis such as DLD gene sequencing instead of DLDH enzymatic activity measurements is often the primary approach to making sure DLDH deficiency is indeed the cause of the observed metabolic abnormalities (Quinonez and Thoene, 1993). Updated information in reference (Quinonez and Thoene, 1993) such as metabolic abnormalities in DLDH deficiency and levels of certain metabolites representing DLDH deficiency may help design additional animal models for investigating DLDH deficiency and metabolic diseases (Table 1).
Table 1.
Metabolic Abnormalities Associated with Dihydrolipoamide Dehydrogenase Deficiency
| Metabolite | Presentation |
Normal | ||
|---|---|---|---|---|
| Neurologic | Hepatic | Myopathic | ||
| Plasma lactate | ↑ | ↑ | Normal to ↑ | <2.2 μM |
| Urine α-ketoglutarate | Normal to ↑ | Typically normal | Normal to ↑ | • Neonates: 4–524 mmol/mol creatinine • Children: 36–117 mmol/mol creatinine • Adults: 4–74 mmol/mol creatinine |
| Urine branched-chain ketoacids | Absent to ↑ | Typically absent | Absent to ↑ | • Neonates: <7 mmol/mol creatinine • All other ages: not detectable |
| Plasma leucine | Normal to ↑ | Typically normal | Normal to ↑ | • Infants: 46–147 μM • Children: 30–246 μM • Adolescents-adults: 86–206 μM |
| Plasma isoleucine | Normal to ↑ | Typically normal | Normal to ↑ | • Infants: 12–77 μM • Children: 6–122 μM • Adolescents-adults: 34–106 μM |
| Plasma valine | Normal to ↑ | Typically normal | Normal to ↑ | • Infants: 79–217 μM • Children: 132–480 μM • Adolescents-adults: 155–343 μM |
| Plasma allo-isoleucine | Normal to ↑ | Typically normal | Normal to ↑ | <5 μM |
This table was reproduced from reference (Quinonez and Thoene, 1993).
DLDH and Ischemic Stroke
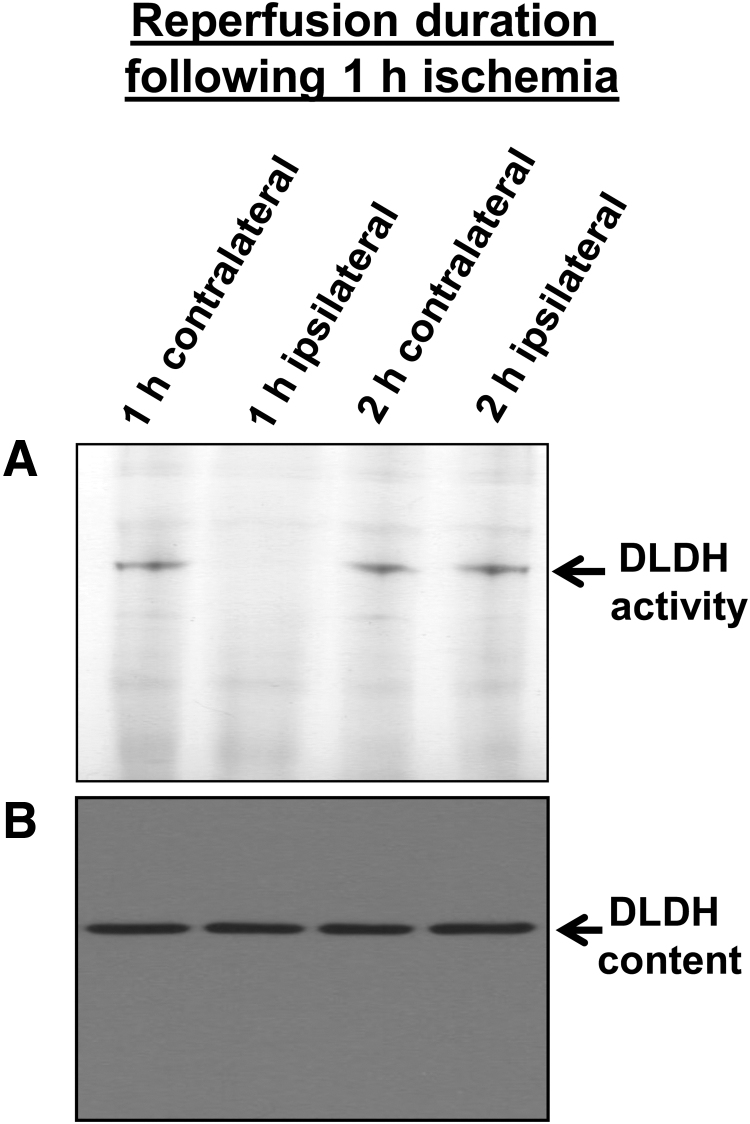
During our investigation of the role of DLDH in stroke and neuroprotection in an animal model of middle cerebral artery occlusion, we have found that upon 1 h ischemia and 1 h reperfusion, DLDH activity was nearly completely lost while DLDH protein content did not change when analyzed by BN-PAGE (Fig. 6A, B) (Wu et al., 2017). However, the enzyme's activity showed a quick recovery beyond 2 h's reperfusion after 1 h ischemia of the brain (Fig. 6A). At the end of 24 h reperfusion, the infarction area was much larger than that of control with a full recovery of DLDH activity (Wu et al., 2017).
FIG. 6.
BN-PAGE measurement of DLDH diaphorase activities (A) and Western blot assay of DLDH protein content (B) after ischemic stroke. Rat brain underwent 1 h ischemia followed by 1 or 2 h reperfusion. As can be seen in this figure, upon a 1-h reperfusion, no DLDH activity could be detected (A, lane 2 from the left), but protein content did not change (B). However, DLDH activity was fully recovered after a 2-h reperfusion. This figure was reproduced with copyright permission from reference (Wu et al., 2017).
Such studies indicate that there was a short period loss of DLDH activity after ischemic stroke. It was previously shown that DLDH is a source of reactive oxygen species (ROS) (Bunik and Brand, 2018; Goncalves et al., 2016; Kareyeva et al., 2012; Quinlan et al., 2014), and that slowing down ROS generation during reperfusion can mitigate tissue injury (Jaeschke, 2003; Sehirli et al., 2003). Our results further implied that ROS generation due to the quick recovery of DLDH activity upon reperfusion could be associated with cerebral ischemic injury, and that DLDH underwent reversible modifications during this ischemia–reperfusion process. Therefore, if DLDH's loss of function is prolonged to prevent its ROS production after ischemia reperfusion, the infarction size in the brain after ischemia–reperfusion might be decreased.
This reasoning led to our extensive studies of DLDH preconditioning and postconditioning for neuroprotection against ischemic stroke (Sumien et al., 2020; Wu et al., 2018; Wu et al., 2017). We took the advantage that 5-methoxyindole-2-carboxylic acid (MICA) is a reversible inhibitor of DLDH (Hanson et al., 1969; Haramaki et al., 1997). When MICA was administered to rats either via intraperitoneal injection or via diet, the brain showed mitigated infarction areas upon stroke (Wu et al., 2017). Our studies further elucidated the mechanisms of DLDH inactivation, which was caused by protein sulfenic acid that could be labeled by a sulfenic acid-reactive probe followed by Western blot assays using antibodies recognizing the probe (Wu et al., 2017).
This oxidative modification of cysteine residues by ROS or reactive nitrogen species (RNS) is a reversible process (Yan et al., 2013a; Yan et al., 2012), thus explaining the loss and recovery of DLDH activities observed during cerebral ischemia reperfusion. Nevertheless, the sulfenylated Cys residues are unknown, since the Cys residues in the active site motif could not be identified as sulfenylated using mass spectrometry. Nonetheless, prolonged sulfenation of the enzyme active center's cysteine upon MICA inhibition could be one of the mechanisms underlying neuroprotection of MICA-induced DLDH inhibition.
Moreover, we found that DLDH inhibition by MICA involves activation of the nuclear factor-erythroid factor 2-related factor 2 (Nrf2) signaling pathway, leading to upregulation of NAD(P)H quinone dehydrogenase 1 (NQO1) that protected the brain against oxidative damage (Wu et al., 2017). We later found that MICA administered after ischemic stroke is also neuroprotective, and the underlying mechanisms also involve activation of the Nrf2 signaling pathway (Wu et al., 2018). The potential mechanism by which DLDH sulfenation and MICA inhibition activate the Nrf2 signaling pathway is shown in Figure 7. It is likely that DLDH inhibition caused a stress response that triggers Nrf2 translocation to the nucleus.
FIG. 7.
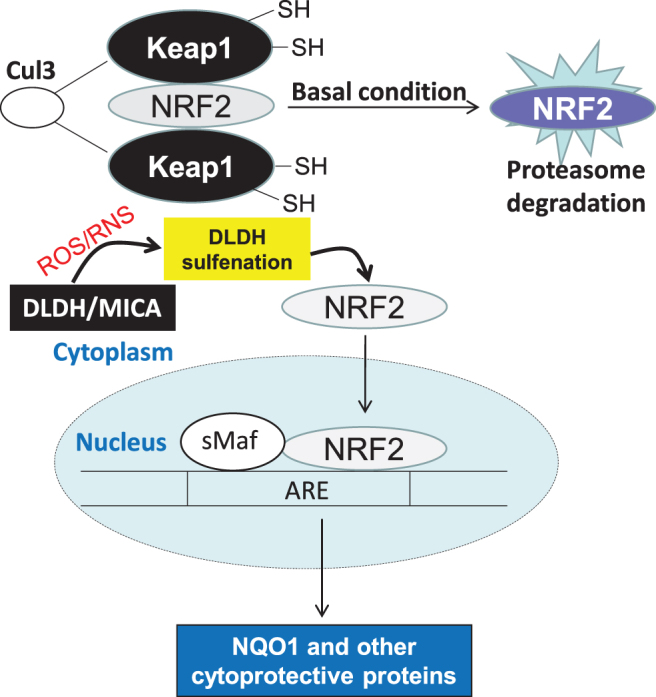
A schematic diagram depicting the likely mechanisms that trigger Nrf2 activation and translocation into the nucleus. DLDH inhibition by MICA and DLDH sulfenation could cause a stress condition inducing Nrf2 activation that plays a protective role in ischemic stroke injury. MICA, 5-methoxyindole-2-carboxylic acid; Nrf2, nuclear factor-erythroid factor 2-related factor 2.
DLDH and Alzheimer's Disease
It has been reported that DLDH is a risk factor for Alzheimer's disease (AD) in an Ashkenzai Jewish population, and this DLDH genotype of risk only appears to be affecting male population in this ethnic group (Brown et al., 2007; Brown et al., 2004). In Caenorhabditis elegans, suppression of DLDH activity has been shown to be neuroprotective against β amyloid toxicity (Ahmad and Ebert, 2021).
Suppression of DLDH activity in the same C. elegans AD model can also induce human tau protein phosphorylation via elevated whole-body glucose content (Ahmad, 2018). While the results of such studies demonstrate that lowering energy metabolism is neuroprotective in AD (Ahmad and Ebert, 2021), how exactly DLDH is involved in the pathogenesis of AD is not completely understood. In addition, a comprehensive evaluation of the role of DLDH in a variety of rodent models of AD has yet to be performed.
DLDH and Skin Photoaging
In studies focusing on tricarboxylic acid (TCA) cycle enzymes and skin photoaging, it was found that DLDH in α-keto glutarate dehydrogenase in the TCA cycle is the only enzyme whose expression was decreased in the skin upon ultraviolet (UV) irradiation (Moon et al., 2015; Sun et al., 2018). This decline in DLDH protein expression can be corrected by natural antioxidants such as paeonol extracted from Paeonia suffruticosa Andr. (Sun et al., 2018). The increased DLDH expression upon phytochemical stimulation further activates the Nrf2 signaling pathway that leads to increased antioxidant protein expression such as heme oxygenase-1 and NQO1 (Sun et al., 2018). Therefore, DLDH in the TCA cycle could be a major target for antiskin photoaging therapy.
In addition, as recently reported, the role of transketolase in light stress resistance may be mediated by DLDH (Chen et al., 2022) as DLDH expression was downregulated by transketolase knockdown. Furthermore, it has been found that downregulation of DLDH by UVA can inhibit melanoma progression that is mediated by oxidative stress and dysregulated enzyme metabolism (Yumnam et al., 2021).
Nondehydrogenating Functions of DLDH
Enzymatically active DLDH is a stable homodimer (Yan et al., 2007). When the homodimer is unstabilized or disrupted, however, each DLDH monomer can act as a serine protease (Babady et al., 2007; Jeffery, 2011). Such findings demonstrate that DLDH, when existing as a monomer, has a moonlighting function. In addition, DLDH has been shown to possess other nondehydrogenating functions, which include adherence to metal-oxide surfaces (Dayan et al., 2019b), TiO2 binding (Dayan et al., 2017), attenuation of radiation toxicity (Alzahrani and Ebert, 2019; Alzahrani and Ebert, 2018), and ROS production (Starkov, 2008; Starkov et al., 2004; Tahara et al., 2007). It has been suggested that DLDH's DNA binding and ROS production are involved in cellular oxidative damage and cell death (Dayan et al., 2020; Dayan et al., 2019a; Dayan et al., 2019b).
Furthermore, DLDH modified by a small peptide such as arginine-glycine-aspartic acid has been shown to have a variety of applications such as inhibition of tumor growth, enhancement of cancer cell apoptosis, and photodynamic treatment of melanoma cells, acting as a tool for coupling DLDH to cell surface integrins (Fleminger and Dayan, 2021). For detailed DLDH nondehydrogenating functional mechanisms, readers may refer to a recent excellent review article by Fleminger and Dayan (2021).
DLDH Oxidative Modifications
As a redox-sensitive enzyme (Yan et al., 2013a; Yan et al., 2012; Yan et al., 2008), DLDH is susceptible to oxidative modifications by other sources of ROS and RNS (Gutierrez-Correa, 2010; Gutierrez-Correa and Stoppani, 2002; Gutierrez-Correa and Stoppani, 1999; Gutierrez-Correa and Stoppani, 1995; Gutierrez Correa and Stoppani, 1993; Lee et al., 2009). The enzyme can be attacked by both endogenous reactive species generated within mitochondria (Yan et al., 2013a) and those nonmitochondrial sources (Yan et al., 2012). We have found that DLDH can undergo reversible modifications by mitochondrial hydrogen peroxide generated from complex III instead of complex I (Yan et al., 2013a).
In addition, in vitro studies indicate that DLDH is vulnerable to reversible modifications by Angeli's salt via nitroxyl hydride (HNO) modifications of cysteines' sulfur group (Yan et al., 2012). In addition to these reversible modifications, others have reported that DLDH can also undergo irreversible modifications such as carbonylation (Frohnert and Bernlohr, 2013; Hussain et al., 2006) and nitrosylation (Foster and Stamler, 2004).
It should be pointed out that the relationship between oxidation of specific amino acid residues and given pathological conditions remains to be comprehensively investigated. It should also be noted that while most post-translational modifications of DLDH are conceivably deleterious, modifications that are beneficial under particular pathological conditions have also been reported in our investigation of DLDH and stroke neuroprotection, suggesting that DLDH sulfenation was neuroprotective (Wu et al., 2017).
Nonmitochondrial or Extracellular DLDH in Mammalian Cells
There have been scarce reports regarding the existence of nonmitochondrial DLDH in mammalian tissues. We have found incidentally, via mass spectrometry peptide sequencing of a serum protein band separated by BN-PAGE, that DLDH exists in rat serum (Yan et al., 2013b). This isoform of DLDH (if we can name so) is different from mitochondrial DLDH, in that this serum DLDH is vulnerable to experimental procedures, including ammonium sulfate extraction, gel electrophoresis, and column chromatography, as the enzyme activity is lost after these experimental procedures. Enzymatic activity assays also indicate the existence of DLDH in mouse and human serum (Yan et al., 2013b).
Moreover, serum DLDH does not seem to possess diaphorase activities (i.e., NADH oxidation). Our study indicates that unlike mitochondrial DLDH that is highly stable, serum DLDH is labile. In spite of these findings, the function of this serum protein remains presently unknown. In addition, although our results agree with those reported in the 1970s and 1980s that there existed a serum DLDH (Melancon et al., 1980; Pelley and Bradley, 1989; Pelley et al., 1976), that whether a different gene other than the one encoding mitochondrial DLDH encodes serum DLDH also remains to be determined.
Nevertheless, it is known that the DLDH encoding gene can yield three alternatively spliced isoforms of DLDH (https://www.uniprot.org/uniprotkb/P09622/entry). Therefore, it is possible that serum DLDH may be one of the three isoforms. In addition, why serum DLDH has a poor stability remains to be investigated. Is lack of FAD the cause of the poor stability? This is possible. However, the FAD content in serum DLDH has yet to be quantitated.
Genetic Manipulation of DLDH for Studies of DLDH Function in Health and Disease
As a critical enzyme involved in several metabolic pathways, knockout of DLDH encoding gene in rodents would be conceivably lethal. In mouse, a global knockout of DLDH indeed yields embryonic lethality, and no viable pups could be obtained (Johnson et al., 1997). Nonetheless, a heterogeneous mouse model of DLDH (Dldh+/−) with only 50% DLDH expressed when compared with wildtype animals is viable and normal with no apparent disease phenotypes (Johnson et al., 1997). The same DLDH deficient mouse model, however, shows vulnerability to neurotoxic challenges (Klivenyi et al., 2004).
We have found that this DLDH deficient mouse model also exhibited increased cerebral damage upon stroke challenge (Li et al., 2015). What surprised us is that a global DLDH overexpression in a transgenic mouse model is also deleterious as the transgenic mouse brain also shows increased damage after stroke (Li et al., 2015). These results indicate that DLDH overexpression in animals is not beneficial to ischemic challenge, at least in the brain.
One explanation for this is that the overexpressed DLDH cannot be incorporated into the respective DLDH-containing enzyme complexes as other subunits, such as E1 and E2 in the respective enzymes' complexes, are not proportionally overexpressed, leading to free or noncomplexed DLDH intracellularly. This noncomplexed DLDH could be toxic to cells.
Our own studies also demonstrate that either knockout or knock-in of DLDH is harmful to the brain. Therefore, future studies may need to take pathway-specific or cell-specific approaches to understand the role of DLDH under specific pathological conditions. Design of cell-specific or tissue-specific transgenic DLDH animal models may also be used to investigate the role of DLDH under physiological conditions or in any given pathological conditions. For example, such genetic approaches may be used to test whether DLDH itself, when not in its respective enzyme complexes, can serve as a suitable therapeutic target or an antioxidant as has been suggested previously (Ahmad and Ebert, 2021; Chang et al., 2022; Dayan et al., 2019a; Igamberdiev et al., 2004; Kliukiene et al., 1997; Yang et al., 2019).
DLDH Autoantibodies in the Serum of Cancer Patients and Other Diseases
It has been reported that DLDH abnormalities occur in certain cancer cells (Qi and Zhu, 2023), and that DLDH in certain diseases and cancers can induce immune response as anti-DLDH antibodies can be detected in the serum of the patients. For example, primary biliary cirrhosis has been associated with the production of DLDH autoantibodies (Dubel et al., 1999; Yeaman et al., 2000), and DLDH is a major autoantigen in hepatitis C virus infection (Wu et al., 2002).
Evidence has also been presented that in both the sera of ovarian cancer patients and endometrial cancer patients, anti-DLDH antibodies were detected (Yoneyama et al., 2015; Yoneyama et al., 2014). These DLDH antibodies may be a biomarker for such cancers or at least cancer-driven disruption of DLDH involved enzyme complexes. These studies also indicate that free DLDH, not DLDH in each respective enzyme complex, is immunogenic. Nonetheless, how DLDH induces immune responses resulting in the formation of anti-DLDH antibodies remains unclear. Further studies will need to be conducted to screen anti-DLDH antibodies in different patients with different types of cancers to see if this is a universal phenomenon for all types of cancers.
Summary
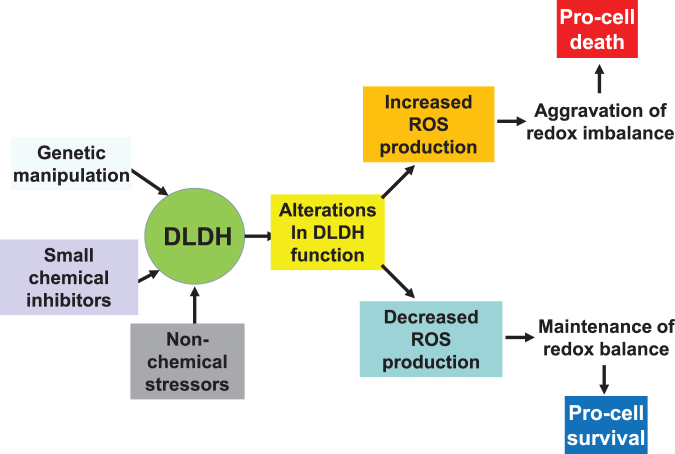
In this article, after review of DLDH structure and function, we overviewed the role of DLDH in several pathological conditions, including DLDH deficiency and metabolic manifestations in humans, DLDH in ischemic stroke and neuroprotection, and role of DLDH in photo light-induced skin abnormalities. The roles and features of DLDH as a nondehydrogenating protein were also discussed; as well as DLDH autoantibodies in certain diseases and cancers. It should be pointed out that depending on modes of functional manipulation, duration and magnitude of functional modulation, and the physiological or pathological conditions, DLDH may be manipulated for either beneficial (prosurvival) or detrimental (prodeath) purposes via modulation of redox balance and oxidative stress (Fig. 8).
FIG. 8.
Scheme depicting modulation of DLDH functions that can lead to either prodeath or prosurvival consequences via DLDH ROS production that affects cellular redox balance and oxidative stress. ROS, reactive oxygen species.
To further explore DLDH as a therapeutic target in a given pathological condition, novel animal models would need to be constructed, which appears to be challenging, given that DLDH is a housekeeping oxidoreductase. In this regard, tissue-specific or cell-specific knockdown of DLDH using small RNA interference techniques (Ahmad, 2018; Broxton et al., 2022) should be a promising approach. Inhibition of DLDH function by DLDH inhibitors such as MICA (Bauman and Pease, 1969; Bauman and Hill, 1968) and valproic acid (Luis et al., 2007) can also offer plausible approaches. Overall, while DLDH structure and physiological function have been well characterized, its roles in numerous pathological conditions such as metabolic disorders, including hypertension, diabetes, and dyslipidemia, are yet to be comprehensively investigated.
Acknowledgments
L-J.Y. would like to thank his postdocs, students, and collaborators who were involved in studies discussed in this article.
Abbreviations Used
- α-KGDC
α-ketoglutarate dehydrogenase complex
- AD
Alzheimer's disease
- BN-PAGE
blue native polyacrylamide gel electrophoresis
- DLDH
dihydrolipoamide dehydrogenase
- DNPH
2,4-dinitrophenyl hydrazine
- FAD
flavin adenine dinucleotide
- G101
glycine residue number 101
- HNO
nitroxyl hydride
- LNVG
leucine-asparagine-valine-glycine
- MICA
5-methoxyindole-2-carboxylic acid
- NBT
nitroblue tetrazolium
- NQO1
NAD(P)H quinone dehydrogenase 1
- Nrf2
nuclear factor-erythroid factor 2-related factor 2
- PDC
pyruvate dehydrogenase complex
- PTLS
proline-threonine-leucine-serine
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TCA
tricarboxylic acid
- UV
ultraviolet
Author Disclosure Statement
No competing financial interests exist.
Funding Information
L-J.Y. was supported in part by the National Institute of Neurological Disorders and Stroke (R01NS079792) and UNTHSC (seed grants: RI6044, RI10015, and RI10039).
/doi/suppl/10.1089/ars.2022.0181/suppl_file/Suppl_DataS1.pptx
References
- Ahmad W. Dihydrolipoamide dehydrogenase suppression induces human tau phosphorylation by increasing whole body glucose levels in a C. elegans model of Alzheimer's Disease. Exp Brain Res 2018;236(11):2857–2866; doi: 10.1007/s00221-018-5341-0 [DOI] [PubMed] [Google Scholar]
- Ahmad W, Ebert PR. Suppression of a core metabolic enzyme dihydrolipoamide dehydrogenase (DLD) protects against amyloid beta toxicity in C. elegans model of Alzheimer's disease. Genes Dis 2021;8(6):849–866; doi: 10.1016/j.gendis.2020.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzahrani S, Ebert PR. Oxygen and arsenite synergize phosphine toxicity by distinct mechanisms. Toxicol Sci 2018;167(2):419–425; doi: 10.1093/toxsci/kfy248 [DOI] [PubMed] [Google Scholar]
- Alzahrani SM, Ebert PR. Attenuation of radiation toxicity by the phosphine resistance factor dihydrolipoamide dehydrogenase (DLD). Sci Rep 2019;9(1):6455; doi: 10.1038/s41598-019-42678-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrus A. An updated view on the molecular pathomechanisms of human dihydrolipoamide dehydrogenase deficiency in light of novel crystallographic evidence. Neurochem Res 2019;44(10):2307–2313; doi: 10.1007/s11064-019-02766-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyrou A, Blanchard JS. Flavoprotein disulfide reductases: Advances in chemistry and function. Prog Nucleic Acid Res Mol Biol 2004;78:89–142; doi: 10.1016/S0079-6603(04)78003-4 [DOI] [PubMed] [Google Scholar]
- Argyrou A, Blanchard JS, Palfey BA. The lipoamide dehydrogenase from Mycobacterium tuberculosis permits the direct observation of flavin intermediates in catalysis. Biochemistry 2002;41(49):14580–14590; doi: 10.1021/bi020376k [DOI] [PubMed] [Google Scholar]
- Babady NE, Pang YP, Elpeleg O, et al. Cryptic proteolytic activity of dihydrolipoamide dehydrogenase. Proc Natl Acad Sci U S A 2007;104(15):6158–6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman N, Hill CJ. Inhibition of gluconeogenesis and alpha-keto oxidation by 5-methoxyindole-2-carboxylic acid. Biochemistry 1968;7(4):1322–1327. [DOI] [PubMed] [Google Scholar]
- Bauman N, Pease BS. Effects of 5-methoxyindole-2-carboxylic acid on carbohydrate metabolism. Biochem Pharmacol 1969;18(5):1093–1101. [DOI] [PubMed] [Google Scholar]
- Brautigam CA, Chuang JL, Tomchick DR, et al. Crystal structure of human dihydrolipoamide dehydrogenase: NAD+/NADH binding and the structural basis of disease-causing mutations. J Mol Biol 2005;350(3):543–552. [DOI] [PubMed] [Google Scholar]
- Brown AM, Gordon D, Lee H, et al. Association of the dihydrolipoamide dehydrogenase gene with Alzheimer's disease in an Ashkenazi Jewish population. Am J Med Genet B Neuropsychiatr Genet 2004;131(1):60–66. [DOI] [PubMed] [Google Scholar]
- Brown AM, Gordon D, Lee H, et al. Testing for linkage and association across the dihydrolipoyl dehydrogenase gene region with Alzheimer's disease in three sample populations. Neurochem Res 2007;32(4–5):857–869. [DOI] [PubMed] [Google Scholar]
- Broxton CN, Kaur P, Lavorato M, et al. Dichloroacetate and thiamine improve survival and mitochondrial stress in a C. elegans model of dihydrolipoamide dehydrogenase deficiency. JCI Insight 2022;7(20):15622; doi: 10.1172/jci.insight.156222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunik VI, Brand MD. Generation of superoxide and hydrogen peroxide by side reactions of mitochondrial 2-oxoacid dehydrogenase complexes in isolation and in cells. Biol Chem 2018;399(5):407–420; doi: 10.1515/hsz-2017-0284 [DOI] [PubMed] [Google Scholar]
- Carothers DJ, Pons G, Patel MS. Dihydrolipoamide dehydrogenase: Functional similarities and divergent evolution of the pyridine nucleotide-disulfide oxidoreductases. Arch Biochem Biophys 1989;268(2):409–425. [DOI] [PubMed] [Google Scholar]
- Chang LC, Chiang SK, Chen SE, et al. Targeting 2-oxoglutarate dehydrogenase for cancer treatment. Am J Cancer Res 2022;12(4):1436–1455. [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang T, Zeng S, et al. Transketolase in human Muller cells is critical to resist light stress through the pentose phosphate and NRF2 pathways. Redox Biol 2022;54:102379; doi: 10.1016/j.redox.2022.102379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang DT, Chuang JL, Wynn RM. Lessons from genetic disorders of branched-chain amino acid metabolism. J Nutr 2006;136(1 Suppl):243S–249S; doi: 10.1093/jn/136.1.243S [DOI] [PubMed] [Google Scholar]
- Dayan A, Babin G, Ganoth A, et al. The involvement of coordinative interactions in the binding of dihydrolipoamide dehydrogenase to titanium dioxide—Localization of a putative binding site. J Mol Recognit 2017;30(8):2617; doi: 10.1002/jmr.2617 [DOI] [PubMed] [Google Scholar]
- Dayan A, Fleminger G, Ashur-Fabian O. Targeting the Achilles' heel of cancer cells via integrin-mediated delivery of ROS-generating dihydrolipoamide dehydrogenase. Oncogene 2019a;38(25):5050–5061; doi: 10.1038/s41388-019-0775-9 [DOI] [PubMed] [Google Scholar]
- Dayan A, Lamed R, Benayahu D, et al. RGD-modified dihydrolipoamide dehydrogenase as a molecular bridge for enhancing the adhesion of bone forming cells to titanium dioxide implant surfaces. J Biomed Mater Res A 2019b;107(3):545–551; doi: 10.1002/jbm.a.36570 [DOI] [PubMed] [Google Scholar]
- Dayan A, Yeheskel A, Lamed R, et al. Dihydrolipoamide dehydrogenase moonlighting activity as a DNA chelating agent. Proteins 2020. [Epub ahead of print]; doi: 10.1002/prot.25991 [DOI] [PubMed] [Google Scholar]
- Dubel L, Tanaka A, Leung PS, et al. Autoepitope mapping and reactivity of autoantibodies to the dihydrolipoamide dehydrogenase-binding protein (E3BP) and the glycine cleavage proteins in primary biliary cirrhosis. Hepatology 1999;29(4):1013–1018; doi: 10.1002/hep.510290403 [DOI] [PubMed] [Google Scholar]
- Feigenbaum AS, Robinson BH. The structure of the human dihydrolipoamide dehydrogenase gene (DLD) and its upstream elements. Genomics 1993;17(2):376–381; doi: 10.1006/geno.1993.1335 [DOI] [PubMed] [Google Scholar]
- Fleminger G, Dayan A. The moonlighting activities of dihydrolipoamide dehydrogenase: Biotechnological and biomedical applications. J Mol Recognit 2021;34(11):e2924; doi: 10.1002/jmr.2924 [DOI] [PubMed] [Google Scholar]
- Foster MW, Stamler JS. New insights into protein S-nitrosylation. Mitochondria as a model system. J Biol Chem 2004;279(24):25891–25897 [DOI] [PubMed] [Google Scholar]
- Frohnert BI, Bernlohr DA. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Adv Nutr 2013;4(2):157–163; doi: 10.3945/an.112.003319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves RL, Bunik VI, Brand MD. Production of superoxide/hydrogen peroxide by the mitochondrial 2-oxoadipate dehydrogenase complex. Free Radic Biol Med 2016;91:247–255; doi: 10.1016/j.freeradbiomed.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Correa J. Trypanosoma cruzi dihydrolipoamide dehydrogenase as target of reactive metabolites generated by cytochrome c/hydrogen peroxide (or linoleic acid hydroperoxide)/phenol systems. Free Radic Res 2010;44(11):1345–1358. [DOI] [PubMed] [Google Scholar]
- Gutierrez Correa J, Stoppani AO. Inactivation of lipoamide dehydrogenase by cobalt(II) and iron(II) Fenton systems: Effect of metal chelators, thiol compounds and adenine nucleotides. Free Radic Res Commun 1993;19(5):303–314. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Correa J, Stoppani AO. Inactivation of heart dihydrolipoamide dehydrogenase by copper Fenton systems. Effect of thiol compounds and metal chelators. Free Radic Res 1995;22(3):239–250. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Correa J, Stoppani AO. Inactivation of myocardial dihydrolipoamide dehydrogenase by myeloperoxidase systems: Effect of halides, nitrite and thiol compounds. Free Radic Res 1999;30(2):105–117. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Correa J, Stoppani AO. Myeloperoxidase-generated phenothiazine cation radicals inactivate Trypanosoma cruzi dihydrolipoamide dehydrogenase. Rev Argent Microbiol 2002;34(2):83–94. [PubMed] [Google Scholar]
- Hanson RL, Ray PD, Walter P, et al. Mode of action of hypoglycemic agents. I. Inhibition of gluconeogenesis by quinaldic acid and 5-methoxyindole-2-carboxylic acid. J Biol Chem 1969;244(16):4351–4359. [PubMed] [Google Scholar]
- Haramaki N, Han D, Handelman GJ, et al. Cytosolic and mitochondrial systems for NADH- and NADPH-dependent reduction of alpha-lipoic acid. Free Radic Biol Med 1997;22(3):535–542. [DOI] [PubMed] [Google Scholar]
- Hengeveld AF, de Kok A. Structural basis of the dysfunctioning of human 2-oxo acid dehydrogenase complexes. Curr Med Chem 2002;9(4):499–520. [DOI] [PubMed] [Google Scholar]
- Hong YS, Kerr DS, Craigen WJ, et al. Identification of two mutations in a compound heterozygous child with dihydrolipoamide dehydrogenase deficiency. Hum Mol Genet 1996;5(12):1925–1930. [DOI] [PubMed] [Google Scholar]
- Hussain SN, Matar G, Barreiro E, et al. Modifications of proteins by 4-hydroxy-2-nonenal in the ventilatory muscles of rats. Am J Physiol Lung Cell Mol Physiol 2006;290(5):L996–L1003. [DOI] [PubMed] [Google Scholar]
- Igamberdiev AU, Bykova NV, Ens W, et al. Dihydrolipoamide dehydrogenase from porcine heart catalyzes NADH-dependent scavenging of nitric oxide. FEBS Lett 2004;568(1-3):146–150. [DOI] [PubMed] [Google Scholar]
- Jaeschke H. Role of reactive oxygen species in hepatic ischemia-reperfusion injury and preconditioning. J Invest Surg 2003;16(3):127–140. [PubMed] [Google Scholar]
- Jeffery CJ. Proteins with neomorphic moonlighting functions in disease. IUBMB Life 2011;63(7):489–494; doi: 10.1002/iub.504 [DOI] [PubMed] [Google Scholar]
- Jentoft JE, Shoham M, Hurst D, et al. A structural model for human dihydrolipoamide dehydrogenase. Proteins 1992;14(1):88–101. [DOI] [PubMed] [Google Scholar]
- Johnson MT, Yang HS, Magnuson T, et al. Targeted disruption of the murine dihydrolipoamide dehydrogenase gene (Dld) results in perigastrulation lethality. Proc Natl Acad Sci U S A 1997;94(26):14512–14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareyeva AV, Grivennikova VG, Vinogradov AD. Mitochondrial hydrogen peroxide production as determined by the pyridine nucleotide pool and its redox state. Biochim Biophys Acta 2012;1817(10):1879–1885. [DOI] [PubMed] [Google Scholar]
- Kliukiene R, Maroziene A, Nivinskas H, et al. The protective effects of dihydrolipoamide and glutathione against photodynamic damage by Al-phtalocyanine tetrasulfonate. Biochem Mol Biol Int 1997;41(4):707–713; doi: 10.1080/15216549700201751 [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Starkov AA, Calingasan NY, et al. Mice deficient in dihydrolipoamide dehydrogenase show increased vulnerability to MPTP, malonate and 3-nitropropionic acid neurotoxicity. J Neurochem 2004;88(6):1352–1360; doi: 10.1046/j.1471-4159.2003.02263.x [DOI] [PubMed] [Google Scholar]
- Lee HM, Reed J, Greeley GHJr., et al. Impaired mitochondrial respiration and protein nitration in the rat hippocampus after acute inhalation of combustion smoke. Toxicol Appl Pharmacol 2009;235(2):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Luo X, Wu J, et al. Mitochondrial dihydrolipoamide dehydrogenase is upregulated in response to intermittent hypoxic preconditioning. Int J Med Sci 2015;12(5):432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TC, Kim H, Arizmendi C, et al. Identification of two missense mutations in a dihydrolipoamide dehydrogenase-deficient patient. Proc Natl Acad Sci U S A 1993;90(11):5186–5190; doi: 10.1073/pnas.90.11.5186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis PB, Ruiter JP, Aires CC, et al. Valproic acid metabolites inhibit dihydrolipoyl dehydrogenase activity leading to impaired 2-oxoglutarate-driven oxidative phosphorylation. Biochim Biophys Acta 2007;1767(9):1126–1133. [DOI] [PubMed] [Google Scholar]
- Melancon SB, Fontaine G, Geoffroy G, et al. Correlation between serum lipoamide dehydrogenase activity and phosphatidylcholine therapy in Friedreich's ataxia. Can J Neurol Sci 1980;7(4):413–416. [DOI] [PubMed] [Google Scholar]
- Moon E, Park HM, Lee CH, et al. Dihydrolipoyl dehydrogenase as a potential UVB target in skin epidermis; using an integrated approach of label-free quantitative proteomics and targeted metabolite analysis. J Proteomics 2015;117:70–85; doi: 10.1016/j.jprot.2014.12.016 [DOI] [PubMed] [Google Scholar]
- Odievre MH, Chretien D, Munnich A, et al. A novel mutation in the dihydrolipoamide dehydrogenase E3 subunit gene (DLD) resulting in an atypical form of alpha-ketoglutarate dehydrogenase deficiency. Hum Mutat 2005;25(3):323–324; doi: 10.1002/humu.9319 [DOI] [PubMed] [Google Scholar]
- Patel MS, Harris RA. Mammalian alpha-keto acid dehydrogenase complexes: Gene regulation and genetic defects. FASEB J 1995;9(12):1164–1172. [DOI] [PubMed] [Google Scholar]
- Pelley JW, Bradley CA. Lipoamide dehydrogenase in serum: A potential diagnostic tool. Ann N Y Acad Sci 1989;573(1):404–404. [Google Scholar]
- Pelley JW, Little GH, Linn TC, et al. Lipoamide dehydrogenase in serum: A preliminary report. Clin Chem 1976;22(2):275–277. [PubMed] [Google Scholar]
- Qi H, Zhu D. Oncogenic role of copper-induced cell death-associated protein DLD in human cancer: A pan-cancer analysis and experimental verification. Oncol Lett 2023;25(5):214; doi: 10.3892/ol.2023.13800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan CL, Goncalves RL, Hey-Mogensen M, et al. The 2-oxoacid dehydrogenase complexes in mitochondria can produce superoxide/hydrogen peroxide at much higher rates than complex I. J Biol Chem 2014;289(12):8312–8325; doi: 10.1074/jbc.M113.545301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinonez SC, Thoene JG. Dihydrolipoamide Dehydrogenase Deficiency. In: GeneReviews((R)). (Adam MP, Everman DB, Mirzaa GM, et al. eds.) University of Washington: Seattle, WA; 1993. [PubMed] [Google Scholar]
- Reznick AZ, Packer L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol 1994;233:357–363. [DOI] [PubMed] [Google Scholar]
- Schagger H. Quantification of oxidative phosphorylation enzymes after blue native electrophoresis and two-dimensional resolution: normal complex I protein amounts in Parkinson's disease conflict with reduced catalytic activities. Electrophoresis 1995;16(5):763–770. [DOI] [PubMed] [Google Scholar]
- Sehirli AO, Sener G, Satiroglu H, et al. Protective effect of N-acetylcysteine on renal ischemia/reperfusion injury in the rat. J Nephrol 2003;16(1):75–80. [PubMed] [Google Scholar]
- Shany E, Saada A, Landau D, et al. Lipoamide dehydrogenase deficiency due to a novel mutation in the interface domain. Biochem Biophys Res Commun 1999;262(1):163–166; doi: 10.1006/bbrc.1999.1133 [DOI] [PubMed] [Google Scholar]
- Staretz-Chacham O, Pode-Shakked B, Kristal E, et al. The effects of a ketogenic diet on patients with dihydrolipoamide dehydrogenase deficiency. Nutrients 2021;13(10):3523; doi: 10.3390/nu13103523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci 2008;1147:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA, Fiskum G, Chinopoulos C, et al. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci 2004;24(36):7779–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausberg RL, Feingold EA, Grouse LH, et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci U S A 2002;99(26):16899–16903; doi: 10.1073/pnas.242603899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumien N, Huang R, Chen Z, et al. Effects of dietary 5-methoxyindole-2-carboxylic acid on brain functional recovery after ischemic stroke. Behav Brain Res 2020;378:112278; doi: 10.1016/j.bbr.2019.112278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Du J, Hwang E, et al. Paeonol extracted from Paeonia suffruticosa Andr. ameliorated UVB-induced skin photoaging via DLD/Nrf2/ARE and MAPK/AP-1 pathway. Phytother Res 2018;32(9):1741–1749; doi: 10.1002/ptr.6100 [DOI] [PubMed] [Google Scholar]
- Szabo E, Wilk P, Nagy B, et al. Underlying molecular alterations in human dihydrolipoamide dehydrogenase deficiency revealed by structural analyses of disease-causing enzyme variants. Hum Mol Genet 2019;28(20):3339–3354; doi: 10.1093/hmg/ddz177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara EB, Barros MH, Oliveira GA, et al. Dihydrolipoyl dehydrogenase as a source of reactive oxygen species inhibited by caloric restriction and involved in Saccharomyces cerevisiae aging. FASEB J 2007;21(1):274–283. [DOI] [PubMed] [Google Scholar]
- Vaubel RA, Rustin P, Isaya G. Mutations in the dimer interface of dihydrolipoamide dehydrogenase promote site-specific oxidative damages in yeast and human cells. J Biol Chem 2011;286(46):40232–40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettakkorumakankav NN, Patel MS. Dihydrolipoamide dehydrogenase: Structural and mechanistic aspects. Indian J Biochem Biophys 1996;33(3):168–176. [PubMed] [Google Scholar]
- Williams CH Jr. Lipoamide Dehydrogenase, Glutathione Reductase, Thioredoxin Reductase, and Mercuric Ion Reductase—A Family of Flavoenzyme Transhydrogenases. In: Chemistry and Biochemistry of Flavoenzymes. (Muller F ed.) CRC Press: Boca Raton; 1992; pp. 121–212. [Google Scholar]
- Wu J, Jin Z, Yang X, et al. Post-ischemic administration of 5-methoxyindole-2-carboxylic acid at the onset of reperfusion affords neuroprotection against stroke injury by preserving mitochondrial function and attenuating oxidative stress. Biochem Biophys Res Commun 2018;497(1):444–450; doi: 10.1016/j.bbrc.2018.02.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Li R, Li W, et al. Administration of 5-methoxyindole-2-carboxylic acid that potentially targets mitochondrial dihydrolipoamide dehydrogenase confers cerebral preconditioning against ischemic stroke injury. Free Radic Biol Med 2017;113:244–254; doi: 10.1016/j.freeradbiomed.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Luo X, Jing S, et al. Two-dimensional gel electrophoretic detection of protein carbonyls derivatized with biotin-hydrazide. J Chromatogr B Analyt Technol Biomed Life Sci 2016;1019:128–131; doi: 10.1016/j.jchromb.2015.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YY, Hsu TC, Chen TY, et al. Proteinase 3 and dihydrolipoamide dehydrogenase (E3) are major autoantigens in hepatitis C virus (HCV) infection. Clin Exp Immunol 2002;128(2):347–352; doi: 10.1046/j.1365-2249.2002.01827.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Bjornstedt M, Nordman T, et al. Reduction of ubiquinone by lipoamide dehydrogenase. An antioxidant regenerating pathway. Eur J Biochem 2001;268(5):1486–1490. [DOI] [PubMed] [Google Scholar]
- Yan LJ. Analysis of oxidative modification of proteins. Curr Protoc Protein Sci 2009;Chapter 14:Unit 14. [DOI] [PubMed] [Google Scholar]
- Yan LJ, Forster MJ. Resolving mitochondrial protein complexes using nongradient blue native polyacrylamide gel electrophoresis. Anal Biochem 2009;389(2):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Forster MJ. Chemical probes for analysis of carbonylated proteins: A review. J Chromatogr B Analyt Technol Biomed Life Sci 2011;879(17–18):1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Liu L, Forster MJ. Reversible inactivation of dihydrolipoamide dehydrogenase by Angeli's salt. Sheng Wu Wu Li Hsueh Bao 2012;28(4):341–350. [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Lodge JK, Traber MG, et al. Comparison between copper-mediated and hypochlorite-mediated modifications of human low density lipoproteins evaluated by protein carbonyl formation. J Lipid Res 1997a;38(5):992–1001. [PubMed] [Google Scholar]
- Yan LJ, Lodge JK, Traber MG, et al. Apolipoprotein B carbonyl formation is enhanced by lipid peroxidation during copper-mediated oxidation of human low-density lipoproteins. Arch Biochem Biophys 1997b;339(1):165–171; doi: 10.1006/abbi.1996.9867 [DOI] [PubMed] [Google Scholar]
- Yan LJ, Orr WC, Sohal RS. Identification of oxidized proteins based on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, immunochemical detection, isoelectric focusing, and microsequencing. Anal Biochem 1998;263(1):67–71. [DOI] [PubMed] [Google Scholar]
- Yan LJ, Sumien N, Thangthaeng N, et al. Reversible inactivation of dihydrolipoamide dehydrogenase by mitochondrial hydrogen peroxide. Free Radic Res 2013a;47(2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Thangthaeng N, Forster MJ. Changes in dihydrolipoamide dehydrogenase expression and activity during postnatal development and aging in the rat brain. Mech Ageing Dev 2008;129:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Thangthaeng N, Sumien N, et al. Serum dihydrolipoamide dehydrogenase is a labile enzyme. J Biochem Pharmacol Res 2013b;1(1):30–42. [PMC free article] [PubMed] [Google Scholar]
- Yan LJ, Yang SH, Shu H, et al. Histochemical staining and quantification of dihydrolipoamide dehydrogenase diaphorase activity using blue native PAGE. Electrophoresis 2007;28(7):1036–1045. [DOI] [PubMed] [Google Scholar]
- Yang X, Song J, Yan LJ. Chronic inhibition of mitochondrial dihydrolipoamide dehydrogenase (DLDH) as an approach to managing diabetic oxidative stress. Antioxidants (Basel) 2019;8(2):32; doi: 10.3390/antiox8020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman SJ, Kirby JA, Jones DE. Autoreactive responses to pyruvate dehydrogenase complex in the pathogenesis of primary biliary cirrhosis. Immunol Rev 2000;174:238–249; doi: 10.1034/j.1600-0528.2002.00021h.x [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Kojima S, Kodani Y, et al. Proteomic identification of autoantibodies in sera from patients with ovarian cancer as possible diagnostic biomarkers. Anticancer Res 2015;35(2):881–889. [PubMed] [Google Scholar]
- Yoneyama K, Shibata R, Igarashi A, et al. Proteomic identification of dihydrolipoamide dehydrogenase as a target of autoantibodies in patients with endometrial cancer. Anticancer Res 2014;34(9):5021–5027. [PubMed] [Google Scholar]
- Yumnam S, Kang MC, Oh SH, et al. Downregulation of dihydrolipoyl dehydrogenase by UVA suppresses melanoma progression via triggering oxidative stress and altering energy metabolism. Free Radic Biol Med 2021;162:77–87; doi: 10.1016/j.freeradbiomed.2020.11.037 [DOI] [PubMed] [Google Scholar]