Abstract
Significance:
Aquaporins and ion channels establish and regulate gradients of calcium, sodium, potassium, chloride, water, and protons in the epidermis. These elements have been found to play significant roles in skin biology and wound healing. In this study, we review our understanding of these channels and ion gradients, with a special emphasis on their role in acute wound healing.
Recent Advances:
Specifically, we assess the temporal and spatial arrangements of ions and their respective channels in the intact skin and during wound and healing to provide a novel perspective of the role of ionic gradients through the various stages of wound healing.
Critical Issues:
The roles of gradients of ions and channels in wound healing are currently not well understood. A collective analysis of their traits and arrangements in the skin during wound healing may provide a new perspective and understanding of the functionality of gradients of ions and channels in skin biology and wound healing.
Future Directions:
It is important to elucidate how the gradients of ions and ion channels regulate and facilitate wound healing. A better understanding of the ionic environments may identify novel therapeutic targets and improved strategies to promote wound healing and possibly treat other cutaneous diseases.
Keywords: ion gradients, ion channels, epidermis, wound healing, wound age, wound types

Kevin Mai, DO
SCOPE AND SIGNIFICANCE
Hemostasis, inflammation, proliferation, and maturation are the typical phases of wound healing. Each phase is processed by different cell types and molecular mediators.1–3 Specifically, the ionic environment has been shown to be critical in facilitating wound healing processes, such as cellular differentiation, migration, and proliferation. Recent discoveries have identified unique gradients of water and ions, including calcium, potassium, sodium, chloride, and protons in the epidermis.4–7 In this review, we attempt to draw connections between ion channels and gradients to suggest possible roles the ionic environment has in cutaneous wound healing.
TRANSLATIONAL RELEVANCE
A precise medical approach to wound healing will require the development of phase-specific strategies to address the different physiological elements associated with each phase of wound healing. Given that ion gradients play a major role in skin homeostasis and wound healing,2,8 the development of therapeutic approaches that restore epidermal ion gradients will be an important step in the adoption of a phase-specific precision medicine approach to wound healing. A better understanding of the ionic environment behind wound healing may bridge the application of translational research into clinical operations.
CLINICAL RELEVANCE
Skin wounds are associated with high morbidity and pose a significant economic burden on both patients and society. Each year, over 7.75 million people in the United States suffer from skin wounds.1 With the rising population, prevalence and financial burden of skin wounds are expected to increase. In this review, we discuss changes in the gradients of ions and channels in wound healing and possible clinical implications.
GRADIENTS OF IONS AND EXPRESSION OF ION CHANNELS AND PUMPS
Ion gradients in the epidermis and their changes following injury
Calcium gradient
Calcium ions have been known to regulate the differentiation of keratinocytes in vitro. However, the localization of these cations in epithelial tissues was not well characterized until G.K. Menon et al. demonstrated the existence of a calcium gradient using ion capture cytochemistry.9 Under homeostatic conditions, the calcium gradient peaks in the outer stratum granulosum and has the lowest level in the basal layer (Fig. 1).4,10 Although how the calcium gradient is maintained in our epidermis is still not well understood, there is evidence to suggest that the stratum corneum as well as the tight junctions present in the stratum granulosum are crucial in the conservation of the gradient.10 Calcium plays an important role in maintaining the protein- and lipid-rich epidermal barrier, while also governing barrier-related processes such as keratinocyte terminal differentiation.4,10,11
Figure 1.
Gradients of ions and AQP3 in the epidermis. The gradients of ions and AQP3 are depicted as triangles with both dark shade and width to show high level, whereas lighter shade and decreased width show lower level. Those triangles are for illustrative purpose and do not have exact quantitative comparison across ions and the AQP3. AQP3, aquaporin 3.
The maintenance of a calcium gradient across the healthy epidermis allows for perturbations in this gradient to be recognized as disruptions of epidermal integrity. Physical or chemical injury to the epidermis disrupts the calcium gradients, which has been linked to increased lipid synthesis and lamellar body formation and secretion.11 Protein Kinase C (PKC) delta controls changes in intracellular calcium concentrations following epithelial ion gradient disruption,12 a mechanism by which disruption of extracellular calcium levels can trigger an intracellular response. The transmembrane calcium concentration gradient that forms following extracellular disruption of calcium levels in wounds leads to intracellular changes that initiate healing. Such changes further support the association between disruption of the calcium gradient and skin wound healing (Fig. 2).
Figure 2.
Injury disrupts ionic gradients in the epidermis. Epidermal wound healing and restoration of ionic gradients, which in turn affect migration, proliferation, and differentiation of keratinocytes. The milieu established by the new epidermis modulates local environment for wound healing processes in dermis.
Reconstruction of the gradient has also been shown to be crucial in wound healing. Since it is unclear how the gradient is restored, it may be important to first look at sources of epidermal calcium. Platelets, for example, house a considerable amount of calcium.13 The extensive role of platelets in the hemostasis phase of wound healing suggests an important contribution to the initial charge of the calcium gradient. Other important sources include respective ion channels.
Potassium gradient
Within the skin, potassium participates in wound healing through moderating terminal differentiation of keratinocytes and stratum corneum barrier function.14 In contrast to the calcium gradient, potassium levels peak in the stratum spinosum and fall to their lowest point in the stratum granulosum (Fig. 1).6 It has been proposed that injuries that disrupt the potassium gradient are a secondary outcome to keratinocyte ion pumps becoming senescent to prevent further loss of ions through a breached barrier.14
Akin to the calcium gradient, the potassium gradient shares a comparable increase in lamellar body secretion following gradient disruption.11,14,15 How these gradients interact to facilitate these wound-healing processes may be explained by their corresponding ion channels. Although potassium channels amass in the epidermal layer, the majority are activated following a rise in extracellular calcium.16 Hence, a rise in extracellular calcium following skin barrier disruption results in rising potassium. In turn, these ionic modifications induce the hyperpolarization of less differentiated keratinocytes.14 Aside from their role in suppressing the differentiation of keratinocytes, potassium also increases the rate of calcium influx.14 With this cycle, potassium may function as an intermediate and feedback inhibitor in restoring the calcium gradient.
Sodium and chloride gradient
In the skin, sodium and chloride through sweat serve as osmotic buffers and thermoregulators, among other things.17 These ions are involved in skin wounds and share a synonymous distribution of their gradients from the center of the stratum corneum to its outermost apical surface (Fig. 1).7 Their spatial distribution along the external layer of the epidermis suggests sweat glands may play a role in wound healing. Sweat, in this context, may be a key source and vehicle for transferring sodium and chloride to the outer epidermis where the gradients can be reestablished following wound induction. This is supported by Watabe et al.'s study where increased sodium was found in the stratum corneum of hidrotic skin compared to anhidrotic skin.18 Losing these gradients following wound induction may be attributed to damage or loss of these glandular units.
The purpose of these gradients, especially their location in the outer epidermis, can be explained by the role of sodium and chloride as osmotic elements.17 An osmotic drive in the outer epidermis aids in maintaining epidermal hydration by preventing excessive passive water loss through evaporation. This is supported by experiments where anhidrotic skin was less hydrated than hidrotic skin.18 This is especially important in wound healing since an adequately hydrated environment helps facilitate the process.
pH gradient
The pH gradient (i.e., proton gradient), also termed the “acid mantle,” establishes a low pH of 4–6 localized in the stratum corneum (Fig. 1).5 The skin's pH is linked to various functions ranging from antimicrobial defense to lipid synthesis.19 When skin is wounded, the acid mantle is lost, and the epithelial pH increases.3,20 In fact, it is within a higher pH (∼7.4) where keratinocyte growth and proliferation thrive.21 These studies suggest that the pH gradient is a vital intermediate in signaling wound healing and epithelial reorganization.
The loss of this gradient may be attributed to factors like lactate, which helps maintain the epidermal pH. An important source of epidermal lactate is keratinocytes.22 Hence, the loss of keratinocytes in wounds has been associated with the loss of the pH gradient.20 The reepithelization of wounds has been noted to lower the epidermal pH, which further emphasizes keratinocytes as an important source of lactate.20,23 Furthermore, there is evidence to suggest that lactate has proangiogenic activity and can be used therapeutically to accelerate the healing process. In Porporato et al.’s, lactate has been demonstrated to promote ischemic and excisional wounds in mice models, and inhibition of its uptake by cells can significantly delay ischemic reperfusion.24 Lactate's role in wound healing may be contributed by its role in proangiogenic activities as well as its contribution to the pH gradient.
In place of the vertical pH gradient lost following wound induction, a horizontal gradient is created. Studies measuring the pH across wounds have found that the center of wounds typically has the highest pH compared to wound borders.20,23 This arrangement provides some insight into the directionality in restoring the acid mantle.
Expressions of ion channels, pumps, exchangers, and aquaporins in the epidermis
Ion channels, pumps, exchangers, and aquaporins are expressed differently in different layers of the epidermis and have distinct gradients of expression levels across the epidermis. Many of these molecules have been found highly expressed in the basal level of the epidermis (Fig. 3).8,14,25–29
Figure 3.
Expression of Orai1, AQP3, TRPV3, NKA, ENaC, L-Type VGCC, and CFTR across the epidermis. Orai1 protein has been found mostly expressed among the stratum basale with a slighter expression in higher layers. AQP3 expression is high among the stratum basale and spinosum layer and decreases in upper layers with total absence in the stratum corneum. TRPV3 has been found to be detected strongly in the basal layer of the epidermis. NKA are expressed preferentially at the stratum germinativum compared to the upper layers of the epidermis. ENaC has been found to be expressed throughout the epidermis, except for the stratum corneum, and with a higher level of expression among differentiated keratinocytes. The α1C subunit of L-Type VGCC is expressed throughout the epidermis with a higher expression in apical layers. CFTR channels are expressed uniformly across the epidermis. ENaC, epithelial sodium channel; NKA, Na/K+ ATPase; TRPV, transient receptor potential vanilloid; CFTR, cystic fibrosis transmembrane conductance regulator; VGCC, voltage-gated calcium channels.
Calcium channels
Transient receptor potential vanilloid (TRPV) channels are calcium-permeable nonselection cation channels widely expressed across mammalian tissues, especially the skin. Activation of these channels allows for the transmembrane flux of calcium and the depolarization of cells. Due to their mechanical and thermosensory attributes, they have been heavily studied in skin wound healing.29–31
TRPV1, TRVP2, TRVP3, and TRVP4 channels are found expressed among basal and suprabasal keratinocytes (Fig. 3).26,29 Channels such as TRPV3 have been found to be strongly expressed among basal keratinocytes. TRPV1 channels are associated with cell death due to their induction of mitochondrial damage and calcium influx.30 Because of their low distribution within the dermis, these channels may hold less influence on the calcium gradient.29 Like TRPV1, strong activation of TRPV3 is associated with cell death.29 However, with moderate activation, TRPV3 promotes keratinocyte proliferation through calcium/calmodulin-dependent protein kinase II-induced nuclear factor kappa B.32
There also exists clinical data that suggest significant upregulation of TRPV3 expression in keratinocytes isolated from psoriatic lesions as well as the epidermis of burn scars with pruritis. Furthermore, TRPV3 antagonists have been speculated to play a role in the attenuation of psoriatic symptoms.33 Although the clinical use of TRPV disruptors is possible, there has been no clinical evidence or trial so far suggesting this use. The apoptotic role of TRPV3 suggests some involvement in disrupting the calcium gradient by killing cells regulating the gradient. TRPV4 channels are involved in organizing actin junctions through a Rho-mediated process.34 In contrast, TRPV2 channels play a unique role in scar formation through transforming growth factor-β1 and α-smooth muscle actin-mediated contractions in dermal fibroblast.31
Orai1 is an important pore subunit for a store-operated calcium channel. Orai1 channels are calcium influx channels that are mainly expressed in the stratum basale (Fig. 3). These calcium channels may play an important role in the skin, serving as a key intermediate in keratinocyte differentiation, proliferation, and polarized motility.8 Upon the endoplasmic reticulum depletion of calcium storage, stromal interaction molecule1 activates and recruits Orai1 to the endoplasmic reticulum-plasma membrane junction. Orai1 then enhances focal adhesive turnover through an epidermal-growth-factor receptor-PKC β-calpain-focal adhesion kinase-mediated pathway.8
α1C subunit of L-type voltage-gated calcium channels (VGCC) are expressed throughout the epidermal keratinocytes of human skin with a higher level of expression among apical layers. Denda et al. demonstrated that topical application of calcium on the skin after stratum corneum disruption resulted in the delayed recovery of the barrier. However, when nifedipine and verapamil, blockers of VGCC, were topically applied, the removal of that delay was seen. VGCC's effect on barrier recovery was further evidenced in the delay of barrier recovery upon application of S-(-)-BAY K8644, an opener of VGCC.35 These results suggest that VGCC plays an important role in skin barrier homeostasis and blocking these channels may reduce the amount of time required for skin recovery.
Potassium channels
Two of the major epidermal potassium channels that may be involved in maintaining the potassium gradient are Kcnh2 and Kcnj8 channels. Kcnh2 channels are voltage-activated potassium channels that hyperpolarize the plasma membrane by conducting K+ out of cells, thereby maintaining keratinocyte homeostasis.2 Kcnj8 channels differ in that they are inward rectifying potassium channels that maintain a membrane potential depolarization.36 Interestingly, wound healing can be promoted by Kcnj8 activation and Kcnh2 inhibition, favoring a net intracellular influx of potassium. The spatial organization of these channels in the skin has not been reported.
AQP3 channels
Aquaporins are involved in the functionality and regulation of ion channels. Aquaporin 3 (AQP3) channels transport both water and glycerol across the plasma membrane and are involved in keratinocyte migration.37 These channels are mostly expressed in stratum basale keratinocytes, with lower levels expressed in cells below the stratum corneum (Fig. 3).27 The epidermal aqueous gradient stretches from the stratum corneum to the stratum granulosum and seemingly aligns with AQP3 channels and other ion gradients.38
The transepithelial loss of water following barrier disruption results in profound disruption in the surrounding ionic environment and also unveils unique patterns of AQP3 expression in wound healing. This was characterized by Western blot analysis of intact keratinocytes from murine and human skin surrounding the wound, suggesting a unique topographic arrangement of AQP3 in burn wounds.14 These channels maintain a significantly higher level of expression along burn wound edges compared to burn wound centers and uninjured skin (Fig. 4).37 Further investigation with AQP3-null mice showed delayed healing of cutaneous wounds related to reduced water and glycerol transport, resulting in impaired keratinocyte migration and AQP-3 facilitated proliferation, respectively.39
Figure 4.
Potential Clinical Implications of Epidermal Ion Channels. Overview of how the discussed ion channels may be utilized clinically.
Na/K+ ATPase
Na/K+ ATPase (NKA) is expressed preferentially at the stratum germinativum compared to the upper layers of the epidermis and plays an important role in maintaining transepithelial potential (TEP) in normal epithelium.40 The role of TEP in the normal epidermis is not yet identified. However, there is evidence to suggest that it plays a crucial role in the differentiation process during wound healing.41
NKA's effect on the skin's TEP highlights sodium's influence on cellular migration. In wound healing, the distribution of NKA from the basal layer upwards forms a declining gradient (Fig. 3).25 The TEP is established by the sodium gradient that is formed across the epidermis. In wound healing, the TEP is crucial in establishing the epithelial electric field, which affects cellular orientation, protein distribution, and protein synthesis.25 Following wound induction, the TEP is disrupted, which induces a direct current toward the middle of the wound.
How sodium and its gradient influence the TEP in wound healing can be observed in their activity levels. The activity of NKA echoes that of the TEP in wound healing. Studies have shown that both the TEP and NKA increase over the course of wound healing.41 In contrast, inhibition of NKA activity has been linked with significant decreases in the TEP and cellular migration rate.25
Na+/H+ exchangers - NHE1
Epidermal intracellular pH induces proliferation and differentiation in multiple stem and pluripotent cells in the skin. Sodium-proton exchanger 1 (NHE1) is a glycoprotein found in the epidermis, which has a major role in regulating intracellular pH.21 NHE1, predominantly expressed in the granulosa layer,21,42 is a crucial antiporter that regulates the epidermal pH by extruding intracellular H+ ions in exchange for extracellular sodium,19 acidifying the lower stratum corneum.
When NHE1 was knocked out in mice, the epidermal pH increased.19 Its expression is also dependent on the epidermal pH. Several studies demonstrated that low epidermal pH downregulates expression of NHE1, while higher epidermal pH induces expression of NHE1.3,43 NHE1 also holds a cytosolic tail that binds calmodulin and calcineurin. Calcium binds these components to facilitate attachment.20 The loss of calcium would result in reduced NHE1 activity and loss of the acid mantle. The reliance on calcium suggests that the restoration of the pH gradient may also be dependent on the calcium gradient.
As mentioned previously, the center of wounds holds a higher epidermal pH compared to the margins. This is supported by the drastically increasing expression of NHE1 at the early wound margins (Fig. 5).3 This increasing expression at wound margins suggests that the pH gradient recovers in an inward manner. Akin to AQP3 and TRPV3 channels, this unique pattern may be utilized for diagnostic purposes (Fig. 4). The disarrangement of this exchanger can aid in understanding wound healing in pathologies with cutaneous pH disturbances like psoriasis and ichthyosis.21,44
Figure 5.
Epidermal NHE1 and pH in wound healing. Following wound insult, the center of wounds expresses a higher epidermal pH compared to the periphery of the wound. NHE1 is also found in a relatively higher expression along the wound periphery during the course of wound healing. NHE1, sodium-hydrogen exchanger 1.
ENaC channel
Epithelial sodium channels (ENaC) are expressed strongly in all epidermal layers, except for the stratum corneum. Its expression is increased in more differentiated keratinocytes, and it plays an important role in maintaining sodium homeostasis.45 In the maturation phase of wound healing after epithelialization, the skin barrier dysfunction typically results in sodium dysregulation due to wound dehydration, which causes chronic inflammation characteristic of pathologies such as hypertrophic scarring.46
It has been clinically observed that prevention of water loss by occlusive dressing has been beneficial to the wound healing process. Xu et al. further identified that ENaC responds to reduced hydration status in keratinocytes by activating fibroblasts through the proinflammatory cyclooxygenase 2 (COX-2)/prostaglandin E2 (PGE2) pathway.46,47 These results suggest that ENaC pathway is a potential therapeutic target for fibrosis.
Activation of ENaC is heavily dependent on proteolytic activities in the cell and its long opening and closing time are inconsistent with direct sensing mechanisms. Instead, Nax was proposed as the sodium sensing channel in human skin keratinocytes.46 Nax functions to maintain sodium homeostasis using prostasin, a protease critical for activation of ENaC. Prostasin knockdown cells result in a block of sodium flux and suppression of downstream inflammatory genes COX-2, interleukin (IL)-1B, and IL-8.46,47 In vivo knockdown of Nax in animal models also showed delayed stratification of keratinocytes and less stratified cell layers overall. These results suggest that inhibition of Nax channels may have possible therapeutic value in conditions characterized by excessive scarring and inflammation such as atopic dermatitis and hypertrophic scarring.
CFTR channel
In exploring the chloride channel cystic fibrosis transmembrane conductance regulator (CFTR)'s role in wound healing, conditions with impaired CFTR channel, such as cystic fibrosis, provide valuable insight into the channel's functionality. Impairment of CFTR has been associated with delayed wound healing and impaired cellular migration. This may be due to the loss of synergistic relationship between epidermal sodium and chloride channels. In human skin, CFTR channels have been found to be expressed in keratinocytes uniformly across the layers of the epidermis.48 Activation of epidermal sodium channels is dependent on chloride channels.48 Hence, impaired wound healing in this context supports the theory that it may be due to the loss of sodium's and chloride's collaborative efforts in creating an osmotically driven water flux.
Voltage-gated sodium channels
Voltage-gated sodium channels Nav, along with sodium-calcium exchanger are heavily expressed in intraepidermal free nerve endings and have an implied functional role in neuropathic pain.49 P.Zhao et al. demonstrated through real-time reverse transcriptase-polymerase chain reaction analysis that Nax is also expressed variably throughout the layers of stratum basalis to stratum granulosum. Furthermore, the upregulation of these channels in the painful skin of patients with complex regional pain syndrome and postherpetic neuralgia suggests a role of keratinocyte sodium channels in pain states.49 Further studies in epidermal signaling pathway may be useful in development of peripheral analgesics without affecting the central nervous system.
Channels that are differentially expressed by wound type and wound age
The channels that encompass different phases of wound healing can be identified through their level of activity (Fig. 6). In embryonic mice, Zhang et. al demonstrated that both Kcnh2 and Kcnj8 channels held the highest level of expression immediately following skin incisions.2 The peak and gradual reduction of these channels within a 24-h window coincide with the homeostatic phase of wound healing. Inhibiting TRPV2 channels in keratinocytes of rat skin wounds reduced the standard rise in intracellular calcium. This was shown to be crucial in myofibroblast differentiation, dermal contraction, and scar formation.31
Figure 6.
Example of changes in ion channels and water channels in different phases of wound healing. Changes in expression of ion channels and water channels in skin wound healing. Those changes may serve as potential markers for identifying wound healing phases.
These functions of TRPV2 correspond with that of the remodeling phase that predominates the end of wound healing. Furthermore, ENaC's function as an inducer of inflammatory cytokines such as COX-2, IL-1B, and IL-8 also corresponds to that of the remodeling phase of wound healing, specifically after epithelization.46 Although no channel has been identified as a key marker for the inflammatory phase, calcium has been shown to peak during this interval.50 Since the TEP does not recover until roughly day four, and Kcnj8/Kcnh2 channels fall within 24 h, a low NKA and Kcnj8/Kcnh2 activity also corresponds with the inflammatory phase.2,25
Multiple channels appear to be involved with the proliferative phase of healing. Ishida et al. showed that AQP3-positive cells started gradually increasing after day 3 of wounds on human skin. The expression peaked on day 7 before decreasing thereafter.51 Despite the variations in age among sample subjects, AQP channel expression patterns remained consistent. On the contrary, the use of NHE1 expression in differentiating the proliferative phase may be inferior to the use of AQP3 channels. Haverkampf et al. showed that, although the rise in NHE1 expression aligns with the proliferative phase, the variability held a degree of inconsistency.3 Finally, NKA activity correlated with the proliferative phase through their interconnection with the TEP. Following wound injury, the TEP has been shown to form on day 4 before peaking by day 8 and gradually returning to the basal level thereafter.25
Channels may also aid in differentiating types of wounds (Fig. 7). AQP3 channels, for example, may identify burn wound edges. Since TRPV channel activity consistently rose as temperatures increased in cultured human keratinocytes, they may be useful in monitoring the effectiveness of cooling treatments for burn wounds.26 In addition, TRPV3 offers a method of outlining burn wounds as their expression levels were found scattered along burn wound edges (Fig. 4).26 Orai1 channels corresponded with ultraviolet B (UVB) damage since orai1 mRNA expression in human keratinocytes increased after UVB induction.52 Differentiating UVB-induced wounds would help identify photosensitive disorders such as porphyria cutanea tarda or polymorphous light eruption.
Figure 7.
Functional test of ion channels using pharmacological agents. An overview of functional studies utilizing ion channel targeting agents.
The different expressivity of these channels over the course of wound healing provides a novel perspective into the order by which the ion gradients are restored. Potassium may be one of the early gradients recovered. Its early reestablishment may be the foundation necessary to restore the other gradients. The high expressions of both AQP3 and NHE1 channels in the proliferative phase suggest that the acid mantle requires the reestablishment of an aqueous source. The establishment of a chronological order of ion channel repair in wound healing opens more doors for a precision medicine approach through a phase-specific focus. Before making this conclusion, it is important to identify whether this order is consistent among all types of skin wounds.
Clinical significance of channels in wound healing
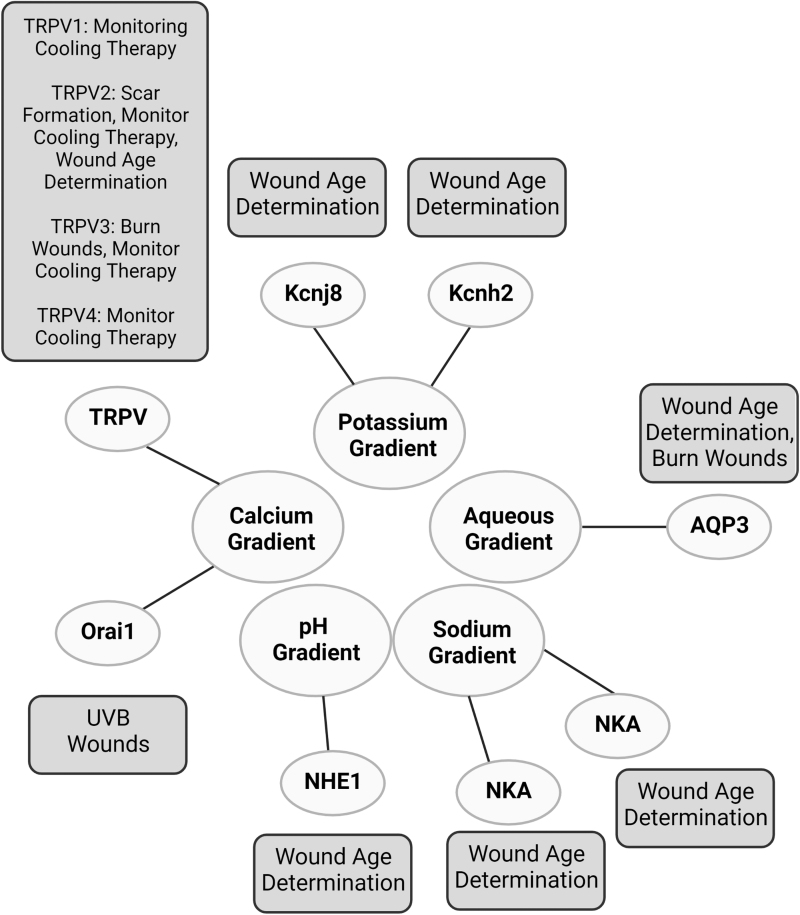
Understanding the distribution and significance of these gradients and channels across wound healing will be key in conceptualizing their therapeutic possibilities (Fig. 8). By correlating treatment with ionic nuances across the course of wound healing, a precision medicine approach can be implemented. For example, a net inward flow of potassium through Kcnj8 activation and Kcnh2 inhibition has been shown to augment wound healing.2 A deeper analysis demonstrated that this process predominates the homeostatic phase of healing. Establishing the time course and order of these ion and gradient activities in wound healing guides therapeutic targeting. Discerning when to target AQP3 channels will be beneficial in pathologies like psoriasis, whereas understanding the role of TRPV2 channels in dermal contraction and scar formation within the maturation phase will be valuable for individuals susceptible to hypertrophic or keloid scars.31
Figure 8.
Selected Examples of Ion and Water Channels in epidermis and their implications in injuries and diseases. This figure depicts the associations between ion and aqueous gradients with their respective channels, pumps and exchangers.
Therapeutic possibilities
The role these ion channels hold in differentiating the age and type of wounds opens the discussion for many therapeutic possibilities. Manipulating function of these channels in specific phases or types of wounds may affect wound healing. For example, wound healing has been shown to be augmented by the net inward potassium flow through Kcnj8 activation and Kcnh2 inhibition.2
For the proliferative phase, AQP3 channels may be a potential target. The use of histone deacetylase inhibitors has shown to induce AQP3 expression in basal keratinocytes.53 This would be useful for those with abnormally low AQP3 expression such as in psoriasis. For the maturation phase, TRPV2 channels may be a viable target due to their position in dermal contraction and scar formation.31 This would be valuable in individuals susceptible to hypertrophic or keloid scars, especially when the current standard treatment calls for corticosteroids, which with long-term use can lead to skin atrophy.54
The same concept could be employed for channels associated with specific types of wounds. The TRPV1 selective antagonist, capsazepine, has been shown to protect keratinocytes when exposed to elevated temperatures. However, in the presence of the nonselective antagonist, ruthenium red, the range of reduced keratinocyte death was decreased.26 The reduced effectiveness when utilizing nonselective antagonists may be due to the protective role TRPV4 holds in skin barrier homeostasis.32 Since TRPV3 expression was found to be scattered along burn wound edges, therapeutic targeting of this region may be an added benefit.26
Periplocin has been shown to augment Na+/K+ ATPase-mediated healing in skin wounds. This promotes proliferation and migration, specifically in fibroblast cells, which increase dermal and epidermal thickening.55 However, participation of a TEP-dependent mechanism has not yet been explored in the setting of periplocin. Due to the shared fate of increased cellular migration, a TEP-mediated role is not impractical when considering these pharmacological agents. This would also support the theory of pharmacological modification of wound fields discussed by Nuccitelli et al.56
Conclusion
We attempt to provide an overview of ionic gradients, expression of ion channels aquaporins in the epidermis, and their changes in skin wounds (Fig. 9). The ionic environments and their correct evolution in healing are expected to be critical to essential cell behaviors, such as cell proliferation, migration, differentiation, adhesion, and correct tissue formation. An understanding of ion channels in skin wounds may eventually serve as a foundation for the future development of therapeutic strategies.
Figure 9.
Epidermal Distribution of Ion Channels and Pumps. An overview of the distribution of the discussed ion channels and pumps across the epidermis.
To modulate ionic gradients and ion channels in skin biology and acute wound healing, it is essential to investigate the prevalence of abnormal ionic environments, ionic gradients, and ion channel variability in wound healing. The epidermis is expected to play a central role in regulating local ionic gradients spatiotemporally. How does epidermal regulation of ionic environments interact with the dermis underneath, the infiltration of immune cells, and the growth of new vessels and innervation?
There exists ample evidence of communication between epidermal keratinocytes and dermal fibroblasts, especially in wound healing. For example, several studies have shown keratinocyte production of TGF-B, IL-1, and ET-1 increases production of col-I from fibroblasts. Vice versa, keratinocyte cadherin expression is upregulated in the presence of fibroblasts. Despite these robust studies of dermal and epidermal communication, the study of ion channels in this context is yet not well characterized.57 One aspect of changing ionic environment at wounds, wound electric fields have been demonstrated to play a critical role in guiding epithelial migration and growth.58 One of the goals is to understand what ionic environment is most beneficial for wound healing, and how to efficiently promote the restoration of the ion gradients to heal a wound. While ion channels hold substantial potential for diagnosing and treating skin wounds, there is still much to uncover.
TAKE-HOME MESSAGE
The epidermis has unique concentration gradients of ions.
Ion channels and pumps are expressed differentially in different layers of the epidermis.
Injuries or diseases disrupt the gradients of ions and expression pattern of ion channels and pumps.
Wound healing reestablished the ion gradients and expression pattern of ion channels and pumps, which in turn contribute to proper wound healing.
The roles of ion gradients and differential expression of ion channels/pumps warrant further investigation, which will help elucidate the mechanisms of wound healing, and may suggest a new therapeutic approach.
Abbreviations and Acronyms
- AQP3
aquaporin 3
- CFTR
cystic fibrosis transmembrane conductance regulator
- COX-2
proinflammatory cyclooxygenase 2
- ENaC
epithelial sodium channel
- IL
interleukin
- NHE1
sodium-hydrogen exchanger 1
- NKA
Na/K+ ATPase
- PGE2
prostaglandin E2
- PKC
protein kinase C
- TEP
transepithelial potential
- TRPV
transient receptor potential vanilloid
- UVB
ultraviolet B
- VGCC
voltage-gated calcium channels
AUTHOR DISCLOSURE AND GHOSTWRITING
The authors declare no conflict of interest. All authors wrote, critically reviewed, and revised the article. No ghostwriter was utilized to produce this article.
ACKNOWLEDGMENTS AND FUNDING SOURCES
Research work in the Zhao Laboratory at UC Davis has been supported by a DARPA grant (D20AC00003, Program Leader: Marco Rolando, University of California Santa Cruz), an AFOSR MURI grant (FA9550-16-1-0052, DURIP – FA9550-22-1-0149) Program Leader: Wolfgang Losert, University of Maryland), and NIH 1R01EY019101. Work in the Zhao Laboratory is also supported by NEI Core Grant (P-30 EY012576), and generous donation from the Burns family, and Mr. and Mrs. Meyers. EM is supported by NIH 5K24AR077313.
The authors thank Dr. Reid for critical reading, comments, and suggestions.
Figures were created with BioRender.com
ABOUT THE AUTHORS
Kevin Mai, DO (First Author), is a medical student at the Western University of Health Sciences, College of Osteopathic Medicine of the Pacific, in Pomona, CA.
Emanual Maverakis, MD, is a professor in the Department of Dermatology, University of California Davis, School of Medicine. His laboratory specializes in skin transcriptomics.
Jung Li is a medical student at Des Moines University College of Osteopathic Medicine, in Des Moines, Iowa.
Min Zhao, MD, PhD (Last Author), is a professor of the Department of Ophthalmology & Vision Science, and Department of Dermatology, University of California Davis, School of Medicine. His laboratory has continuous research interest and focuses on electric signals produced by fluxes of ions at wounds. His laboratory demonstrated the time courses and ionic components at wounds in cornea and skin, discovered genetic and molecular mechanisms for cells to sense electric fields, and is investigating possible approaches to enhance wound healing and regeneration using electrical signaling.
REFERENCES
- 1. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341(10):738–46; doi: 10.1056/nejm199909023411006 [DOI] [PubMed] [Google Scholar]
- 2. Zhang W, Bei M. Kcnh2 and Kcnj8 interactively regulate skin wound healing and regeneration. Wound Repair Regen 2015;23(6):797–806; doi: 10.1111/wrr.12347 [DOI] [PubMed] [Google Scholar]
- 3. Haverkampf S, Heider J, Weiß KT, et al. NHE1 expression at wound margins increases time-dependently during physiological healing. Exp Dermatol 2017;26(2):124–126; doi: 10.1111/exd.13097 [DOI] [PubMed] [Google Scholar]
- 4. Menon GK, Elias PM, Feingold KR. Integrity of the permeability barrier is crucial for maintenance of the epidermal calcium gradient. Br J Dermatol 1994;130(2):139–147; doi: 10.1111/j.1365-2133.1994.tb02892.x [DOI] [PubMed] [Google Scholar]
- 5. Ohman H, Vahlquist A. In vivo studies concerning a pH gradient in human stratum corneum and upper epidermis. Acta Derm Venereol 1994;74(5):375–379; doi: 10.2340/0001555574375379 [DOI] [PubMed] [Google Scholar]
- 6. Denda M, Hosoi J, Asida Y. Visual imaging of ion distribution in human epidermis. Biochem Biophys Res Commun 2000;272(1):134–137; doi: 10.1006/bbrc.2000.2739 [DOI] [PubMed] [Google Scholar]
- 7. Warner RR, Myers MC, Taylor DA. Electron probe analysis of human skin: Element concentration profiles. J Invest Dermatol 1988;90(1):78–85; doi: 10.1111/1523-1747.ep12462576 [DOI] [PubMed] [Google Scholar]
- 8. Vandenberghe M, Raphaël M, Lehen'kyi V, et al. ORAI1 calcium channel orchestrates skin homeostasis. Proc Natl Acad Sci U S A 2013;110(50):E4839–E4848; doi: 10.1073/pnas.1310394110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol 1985;84(6):508–512; doi: 10.1111/1523-1747.ep12273485 [DOI] [PubMed] [Google Scholar]
- 10. Kurasawa M, Maeda T, Oba A, et al. Tight junction regulates epidermal calcium ion gradient and differentiation. Biochem Biophys Res Commun 2011;406(4):506–511; doi: 10.1016/j.bbrc.2011.02.057 [DOI] [PubMed] [Google Scholar]
- 11. Menon GK, Price LF, Bommannan B, et al. Selective obliteration of the epidermal calcium gradient leads to enhanced lamellar body secretion. J Invest Dermatol 1994;102(5):789–795; doi: 10.1111/1523-1747.ep12377921 [DOI] [PubMed] [Google Scholar]
- 12. Ahn BK, Jeong SK, Kim HS, et al. Rottlerin, a specific inhibitor of protein kinase C-delta, impedes barrier repair response by increasing intracellular free calcium. J Invest Dermatol 2006;126(6):1348–1355; doi: 10.1038/sj.jid.5700244 [DOI] [PubMed] [Google Scholar]
- 13. Skaer RJ, Peters PD, Emmines JP. The localization of calcium and phosphorus in human platelets. J Cell Sci 1974;15(3):679–682. [DOI] [PubMed] [Google Scholar]
- 14. Mauro T, Bench G, Sidderas-Haddad E, et al. Acute barrier perturbation abolishes the Ca2+ and K+ gradients in murine epidermis: Quantitative measurement using PIXE. J Invest Dermatol 1998;111(6):1198–1201; doi: 10.1046/j.1523-1747.1998.00421.x [DOI] [PubMed] [Google Scholar]
- 15. Denda M, Tsutsumi M, Inoue K, et al. Potassium channel openers accelerate epidermal barrier recovery. Br J Dermatol 2007;157(5):888–893; doi: 10.1111/j.1365-2133.2007.08198.x [DOI] [PubMed] [Google Scholar]
- 16. Mauro T, Dixon DB, Komuves L, et al. Keratinocyte K+ channels mediate Ca2+-induced differentiation. J Invest Dermatol 1997;108(6):864–870; doi: 10.1111/1523-1747.ep12292585 [DOI] [PubMed] [Google Scholar]
- 17. Baker LB, Wolfe AS. Physiological mechanisms determining eccrine sweat composition. Eur J Appl Physiol 2020;120(4):719–752; doi: 10.1007/s00421-020-04323-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Watabe A, Sugawara T, Kikuchi K, et al. Sweat constitutes several natural moisturizing factors, lactate, urea, sodium, and potassium. J Dermatol Sci 2013;72(2):177–182; doi: 10.1016/j.jdermsci.2013.06.005 [DOI] [PubMed] [Google Scholar]
- 19. Proksch E. pH in nature, humans and skin. J Dermatol 2018;45(9):1044–1052; doi: 10.1111/1346-8138.14489 [DOI] [PubMed] [Google Scholar]
- 20. Rinnerthaler M, Richter K. The Influence of Calcium on the Skin pH and epidermal barrier during aging. Curr Probl Dermatol 2018;54:79–86; doi: 10.1159/000489521 [DOI] [PubMed] [Google Scholar]
- 21. Charruyer A, Ghadially R. Influence of pH on skin stem cells and their differentiation. Curr Probl Dermatol 2018;54:71–78; doi: 10.1159/000489520 [DOI] [PubMed] [Google Scholar]
- 22. Halprin KM, Ohkawara A. Lactate production and lactate dehydrogenase in the human epidermis. J Invest Dermatol 1966;47(3):222–229; doi: 10.1038/jid.1966.133 [DOI] [PubMed] [Google Scholar]
- 23. Schneider LA, Korber A, Grabbe S, et al. Influence of pH on wound-healing: a new perspective for wound-therapy? Arch Dermatol Res 2007;298(9):413–420; doi: 10.1007/s00403-006-0713-x [DOI] [PubMed] [Google Scholar]
- 24. Porporato PE, Payen VL, De Saedeleer CJ, et al. Lactate stimulates angiogenesis and accelerates the healing of superficial and ischemic wounds in mice. Angiogenesis 2012;15(4):581–592; doi: 10.1007/s10456-012-9282-0 [DOI] [PubMed] [Google Scholar]
- 25. Moulin VJ, Dubé J, Rochette-Drouin O, et al. Electric potential across epidermis and its role during wound healing can be studied by using an. Adv Wound Care (New Rochelle) 2012;1(2):81–87; doi: 10.1089/wound.2011.0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Radtke C, Sinis N, Sauter M, et al. TRPV channel expression in human skin and possible role in thermally induced cell death. J Burn Care Res 2011;32(1):150–159; doi: 10.1097/BCR.0b013e318203350c [DOI] [PubMed] [Google Scholar]
- 27. Straseski JA, Gibson AL, Thomas-Virnig CL, et al. Oxygen deprivation inhibits basal keratinocyte proliferation in a model of human skin and induces regio-specific changes in the distribution of epidermal adherens junction proteins, aquaporin-3, and glycogen. Wound Repair Regen 2009;17(4):606–616; doi: 10.1111/j.1524-475X.2009.00515.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park CW, Kim HJ, Choi YW, et al. TRPV3 Channel in Keratinocytes in Scars with Post-Burn Pruritus. Int J Mol Sci 2017;18(11):2425; doi: 10.3390/ijms18112425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Facer P, Casula MA, Smith GD, et al. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol 2007;7:11; doi: 10.1186/1471-2377-7-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim SR, Kim SU, Oh U, et al. Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+-mediated mitochondrial damage and cytochrome c release. J Immunol 2006;177(7):4322–4329; doi: 10.4049/jimmunol.177.7.4322 [DOI] [PubMed] [Google Scholar]
- 31. Ishii T, Uchida K, Hata S, et al. TRPV2 channel inhibitors attenuate fibroblast differentiation and contraction mediated by keratinocyte-derived TGF-β1 in an in vitro wound healing model of rats. J Dermatol Sci 2018;90(3):332–342; doi: 10.1016/j.jdermsci.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 32. Wang Y, Li H, Xue C, et al. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol Toxicol 2021;37(2):313–330; doi: 10.1007/s10565-020-09536-2 [DOI] [PubMed] [Google Scholar]
- 33. Broad LM, Mogg AJ, Eberle E, et al. TRPV3 in drug development. Pharmaceuticals (Basel) 2016;9(3):55; doi: 10.3390/ph9030055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sokabe T, Tominaga M. The TRPV4 cation channel: A molecule linking skin temperature and barrier function. Commun Integr Biol 2010;3(6):619–621; doi: 10.4161/cib.3.6.13461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Denda M, Fujiwara S, Hibino T. Expression of voltage-gated calcium channel subunit alpha1C in epidermal keratinocytes and effects of agonist and antagonists of the channel on skin barrier homeostasis. Exp Dermatol 2006;15(6):455–460; doi: 10.1111/j.0906-6705.2006.00430.x [DOI] [PubMed] [Google Scholar]
- 36. Delaney JT, Muhammad R, Blair MA, et al. A KCNJ8 mutation associated with early repolarization and atrial fibrillation. Europace 2012;14(10):1428–1432; doi: 10.1093/europace/eus150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sebastian R, Chau E, Fillmore P, et al. Epidermal aquaporin-3 is increased in the cutaneous burn wound. Burns 2015;41(4):843–847; doi: 10.1016/j.burns.2014.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. von Zglinicki T, Lindberg M, Roomans GM, et al. Water and ion distribution profiles in human skin. Acta Derm Venereol 1993;73(5):340–343. [PubMed] [Google Scholar]
- 39. Hara-Chikuma M, Verkman AS. Roles of aquaporin-3 in the epidermis. J Invest Dermatol 2008;128(9):2145–2151, doi: 10.1038/jid.2008.70 [DOI] [PubMed] [Google Scholar]
- 40. Abe Y, Nishizawa M. Electrical aspects of skin as a pathway to engineering skin devices. APL Bioeng 2021;5(4):041509; doi: 10.1063/5.0064529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dubé J, Rochette-Drouin O, Lévesque P, et al. Restoration of the transepithelial potential within tissue-engineered human skin in vitro and during the wound healing process in vivo. Tissue Eng Part A 2010;16(10):3055–3063; doi: 10.1089/ten.TEA.2010.0030 [DOI] [PubMed] [Google Scholar]
- 42. Behne MJ, Meyer JW, Hanson KM, et al. NHE1 regulates the stratum corneum permeability barrier homeostasis. Microenvironment acidification assessed with fluorescence lifetime imaging. J Biol Chem 2002;277(49):47399–47406; doi: 10.1074/jbc.M204759200 [DOI] [PubMed] [Google Scholar]
- 43. Hachem JP, Behne M, Aronchik I, et al. Extracellular pH Controls NHE1 expression in epidermis and keratinocytes: implications for barrier repair. J Invest Dermatol 2005;125(4):790–797; doi: 10.1111/j.0022-202X.2005.23836.x [DOI] [PubMed] [Google Scholar]
- 44. Ohman H, Vahlquist A. The pH gradient over the stratum corneum differs in X-linked recessive and autosomal dominant ichthyosis: a clue to the molecular origin of the “acid skin mantle”? J Invest Dermatol 1998;111(4):674–677; doi: 10.1046/j.1523-1747.1998.00356.x [DOI] [PubMed] [Google Scholar]
- 45. Guitard M, Leyvraz C, Hummler E. A nonconventional look at ionic fluxes in the skin: lessons from genetically modified mice. News Physiol Sci 2004;19:75–79; doi: 10.1152/nips.01503.2003 [DOI] [PubMed] [Google Scholar]
- 46. Xu W, Hong SJ, Zhong A, et al. Sodium channel Nax is a regulator in epithelial sodium homeostasis. Sci Transl Med 2015;7(312):312ra177; doi: 10.1126/scitranslmed.aad0286 [DOI] [PubMed] [Google Scholar]
- 47. Xu W, Hong SJ, Zeitchek M, et al. Hydration status regulates sodium flux and inflammatory pathways through epithelial sodium channel (ENaC) in the skin. J Invest Dermatol 2015;135(3):796–806; doi: 10.1038/jid.2014.477 [DOI] [PubMed] [Google Scholar]
- 48. Dong J, Jiang X, Zhang X, et al. Dynamically regulated CFTR expression and its functional role in cutaneous wound healing. J Cell Physiol 2015;230(9):2049–2058; doi: 10.1002/jcp.24931 [DOI] [PubMed] [Google Scholar]
- 49. Zhao P, Barr TP, Hou Q, et al. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: evidence for a role in pain. Pain 2008;139(1):90–105; doi: 10.1016/j.pain.2008.03.016 [DOI] [PubMed] [Google Scholar]
- 50. Njau SN, Epivatianos P, Tsoukali-Papadopoulou H, et al. Magnesium, calcium and zinc fluctuations on skin induced injuries in correlation with time of induction. Forensic Sci Int 1991;50(1):67–73; doi: 10.1016/0379-0738(91)90135-6 [DOI] [PubMed] [Google Scholar]
- 51. Ishida Y, Kuninaka Y, Furukawa F, et al. Immunohistochemical analysis on aquaporin-1 and aquaporin-3 in skin wounds from the aspects of wound age determination. Int J Legal Med 2018;132(1):237–242; doi: 10.1007/s00414-017-1725-0 [DOI] [PubMed] [Google Scholar]
- 52. Oh SJ. The Effects of UVB on ORAI1 Channel and the Role of ORAI1 Channel on the UVB-Induced Epidermal Hyperplasia and Upregulation of TSLP and COX-2. Graduate School, Yonsei University: South Korea; 2016. [Google Scholar]
- 53. Choudhary V, Olala LO, Kagha K, et al. Regulation of the Glycerol Transporter, Aquaporin-3, by Histone Deacetylase-3 and p53 in Keratinocytes. J Invest Dermatol 2017;137(9):1935–1944; doi: 10.1016/j.jid.2017.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kolbe L, Kligman AM, Schreiner V, et al. Corticosteroid-induced atrophy and barrier impairment measured by non-invasive methods in human skin. Skin Res Technol 2001;7(2):73–77; doi: 10.1034/j.1600-0846.2001.70203.x [DOI] [PubMed] [Google Scholar]
- 55. Chen L, Jiang P, Li J, et al. Periplocin promotes wound healing through the activation of Src/ERK and PI3K/Akt pathways mediated by Na/K-ATPase. Phytomedicine 2019;57:72–83; doi: 10.1016/j.phymed.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 56. Nuccitelli R, Nuccitelli P, Ramlatchan S, et al. Imaging the electric field associated with mouse and human skin wounds. Wound Repair Regen 2008;16(3):432–441; doi: 10.1111/j.1524-475X.2008.00389.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Russo B, Brembilla NC, Chizzolini C. Interplay between keratinocytes and fibroblasts: A systematic review providing a new angle for understanding skin fibrotic disorders. Front Immunol 2020;11:648; doi: 10.3389/fimmu.2020.00648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature 2006;442(7101):457–460; doi: 10.1038/nature04925 [DOI] [PubMed] [Google Scholar]