Abstract
Objective
The objective of this study is to conduct a bibliometric analysis of the present status, areas of focus, and upcoming developments in the research of anesthetic drugs and their impact on immune function, along with other related research domains.
Methods
From January 1, 2008 to June 9, 2023, A thorough exploration of anesthetic drug-related literature pertaining to immune function was carried out through the utilization of the Web of Science. The bibliometric analysis was predominantly executed by means of CiteSpace, GraphPad Prism 8.0, and the acquisition of data regarding the country, institution, author, journal, and keywords associated with each publication.
Results
This study analyzed a comprehensive total of 318 publications, consisting of 228 articles and 90 reviews, to determine the publication output of anesthetic drugs on immune function. Notably, China exhibited the highest publication output with (109, 34.28%) articles. Among the institutions analyzed, Harvard University was found to be the most productive with (12, 3.77%) publications. The study findings indicate that Buggy, Donal J (5, 1.57%) and Yuki, Koichi (5, 1.57%) had the highest publication records. Anesthesiology was the most frequently cited journal with a total of (206) citations. The results also revealed that “surgery” was the most frequently used keyword, appearing (48 times), followed by “general anesthesia” (41 times) and “breast cancer” (37 times). The study has identified several current areas of interest, with a particular emphasis on “metastasis”, “inflammation”, “recurrence”, “anesthesia technique”, and “induction”. It is anticipated that forthcoming research endeavors will concentrate on exploring the impacts of isoflurane, sevoflurane, and ketamine on immune function.
Conclusion
This study provided a thorough analysis of the research trends and developments in investigating the impact of anesthetic drugs on immune function, incorporating pertinent research and collaborative entities such as authors, institutions, and countries.
Keywords: anesthetic drugs, immune function, microenvironment, immunotherapy, targeted therapy
Introduction
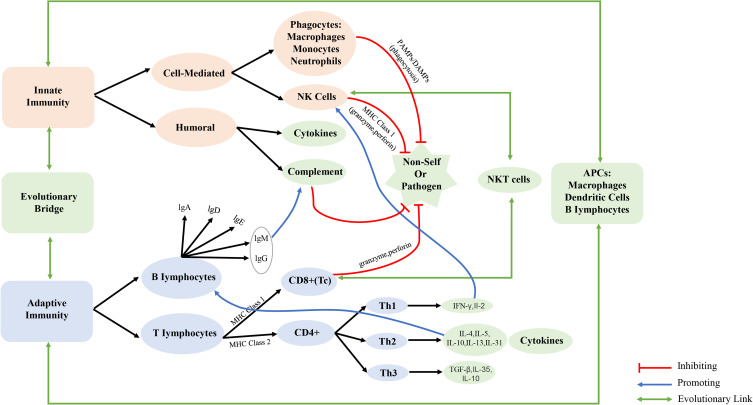
The immune system is made up of innate and adaptive components. The innate immune system is comprised of host defenses that have survived evolutionary change through time and respond swiftly to infections without the need for a specific antigen trigger. Adaptive immunity, on the other hand, recognizes specific antigens and then stores antigen-specific immunologic “memory” to protect the host from future shocks brought on by the same pathogen (Figure 1).1,2 The rapid growth of immunology has drawn increased attention to its impact on human homeostasis, causing the anesthesiology community, both domestically and globally, to examine the effect of anesthesia on immune function more closely.3 Yokoyama et al found a noteworthy reduction in catecholamine levels and natural killer cell activity in the plasma of participants, concomitant with an elevation in the CD4+/CD8+ ratio, following epidural space or stellate ganglion blockade.4 In contrast to the group receiving general anesthesia, the concentrations of tumor necrosis factor TNF-α and interleukin IF-6 exhibited only marginal elevation post-surgery during total hip arthroplasty under regional anesthesia, while the cortisol level demonstrated a significant decrease.5 In 1981, Shapiro et al6 discovered the involvement of anesthetics in the progression and metastasis of cancer. Novac et al7 discovered that NLRP3 and cytokine concentrations in the serum were greater after minimally invasive surgery (MIS) than before MIS in both groups of patients (Midazolam+Fentanyl - Group 1, Propofol+Fentanyl - Group 2). Presently, numerous research groups are directing their attention towards the impact of anesthetic drugs on immune function, postoperative mortality, and recurrence rate of cancer surgery patients.8–12 A comprehensive investigation of the effect of anesthetic drugs on immune function holds significant implications for perioperative medication in patients.
Figure 1.
Overview of innate and adaptive immunities including evolutionary links between the two. APCs indicates antigen presenting cells; CD4+, cluster of differentiation 4 positive; CD8+, cluster of differentiation 8 positive; IFN-γ, interferon gamma; IgA, G, D, E, M, immunoglobulin A, G, D, E, M; IL, interleukin; MHC, major histocompatibility class; NK, natural killer; NKT, natural killer T cell; reg, regulatory T cell; Th1, T helper type 1; Th2, T helper type 2; Tc, cytotoxic T cell; TGFβ, transforming growth factor beta.
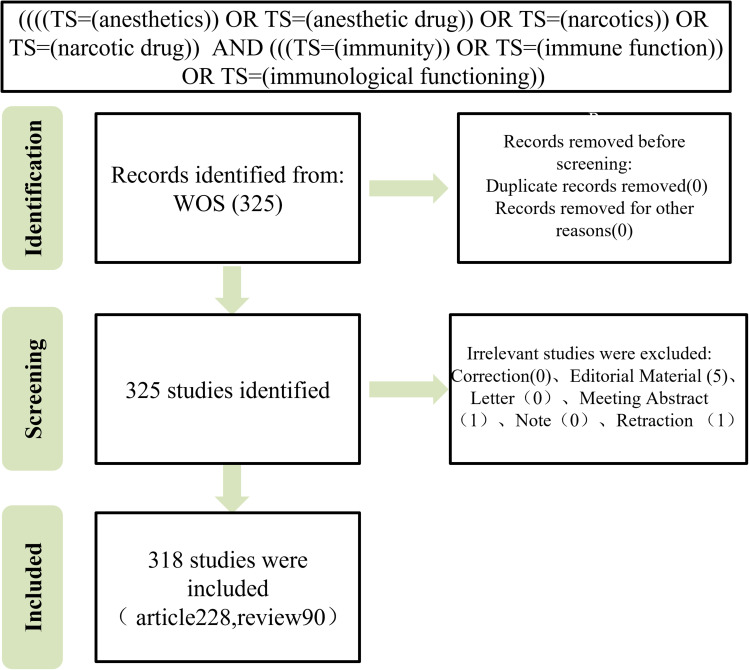
Figure 2.
Flowchart depicting the process of publication selection.
Bibliometric analyses are widely utilized in academia for the purpose of quantitatively evaluating published research and predicting future trends in scientific inquiry.13 This type of research employs mathematical and statistical tools to explicitly and statistically analyze the scientific movement, including the interrelationships between countries, institutions, authors, journals, and keywords.14 Additionally, bibliometric analysis has been applied to mesenchymal stem cells on immunomodulatory functions,15 single-cell sequencing on immune cells.16 However, a bibliometric analysis of anesthetics drugs’ impact on immune function has not been reported in the literature thus far. In the current study, bibliometric analysis was utilized to assess the research status of anesthetics drugs’ impact on immune function over the past a decade, as well as identify the research focus and emerging topics.
Materials and Methods
Literature Searching
The present study utilized the Web of Science (WOS) core collection as the primary database. Search strategy: (((TS = (anesthetics)) OR TS = (narcotics)) OR TS = (narcotics)) OR TS = (narcotic drug)) AND (((TS = (immunity)) OR TS = (immune function)) OR TS = (immunological functioning))). English language articles or reviews published between January 1, 2008 and June 9, 2023 were included in this study.
Method
The articles obtained from the WOS search were exported in plain text format as a “txt” file that encompassed the complete record and references, and was titled “download anesthetics on immune function” and saved. The software tool, CiteSpace V (6.2.R4), was employed to conduct a visual analysis of the literature, compute centrality, and generate co-maps of countries/regions, institutions, authors, published journals, and literature citations. Additionally, co-maps, cluster maps, time maps, and emergent maps of keywords were drawn using the same software tool. The study employs a specific parameter setting, wherein the time partition spans from January 1, 2008, to June 9, 2023, with a time slice of 1 year. The “Pruning” option is utilized to verify “Pathfinder” and “Pruning sliced networks”, while the remaining options retain their default settings. The visual atlas employs a node and font size to signify frequency, with the line (Edge) between nodes representing the relationship between them. The thickness of the line indicates the tightness of the relationship, while the color of the node and line signifies the distance of the year, with red denoting closer proximity.
Results
Analysis of Publication Trend
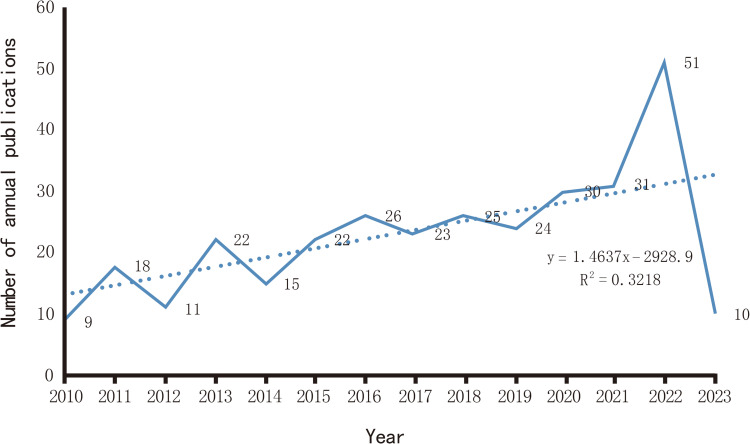
After excluding meeting sets, news, retractions, editorials, and letters, a total of 318 documents were included in this study, comprising 228 articles (71.70%) and 90 reviews (28.30%), as determined through a search of WOS (Figure 2). The annual number of publications in this field has exhibited a steady increase from 2010 to the present, with peaks observed in 2020 (30 articles), 2021 (31 articles), and 2022 (51 articles). These findings suggest that the academic community has shown a significant interest in this field, with a growing number of scholars expected to engage in research in this area in 2023 (Figure 3).
Figure 3.
The yearly quantity of publications pertaining to the impact of anesthetic drugs on immune function.
Distribution Analysis of Country/Region, Institution and Author
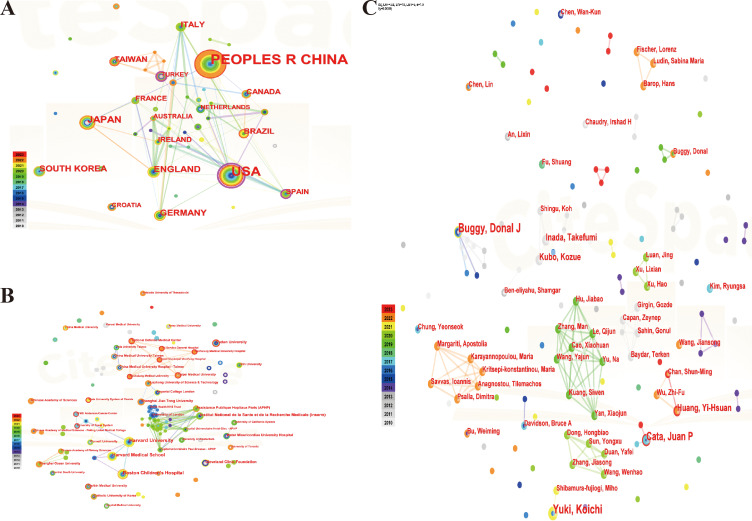
A comprehensive analysis of the papers published by 46 countries revealed that 82 instances of cooperation occurred among these countries/regions. Notably, China emerged as the most prolific publisher with (109, 34.28%), followed by the United States (71, 22.33%), Japan (22, 6.92%) and the United Kingdom (17, 5.35%). The concept of centrality was employed to gauge the significance of nodes within the network, with a centrality value exceeding 0.1 indicating a higher degree of cooperation and contribution among nodes. Centrality ranked first in the United States (0.68), followed by Spain (0.27) and Turkey (0.23), and fifth in the United Kingdom, indicating that the main research countries in this field are China, the United States and Japan, which cooperate closely. The centrality of China is less than 0.1, indicating that China has a high number of documents issued, but there is a lack of deepening cooperation with international countries, which should be further strengthened in the future (Figure 4A), (Table 1). In this field, 292 institutions published papers and 410 inter-agency collaborations were established. The top five universities, based on the number of papers issued, were Harvard University (12), Boston Children’s Hospital (10), Harvard Medical School (9), National Institute of Health and Medicine (Inserm) (7), and Fudan University (6). The National Institute of Health and Medical Research (Inserm) and Florida State University System Center were ranked first with a centrality score of 0.01 (Figure 4B), (Table S1). The authors who issued the highest number of papers were Buggy, Donal J (5), Yuki, Koichi (5), Cata, Juan P (4), Inada, Takefumi (3), and Kubo, Kozue (3). The centrality rankings for all authors were found to be less than 0.1, suggesting that there is a need to improve collaboration between the teams of these authors (Figure 4C), (Table S2).
Figure 4.
Distribution analysis of countries, institutions and authors. (A).co-occurrence map of countries; (B). co-occurrence map of institutions; (C). co-occurrence map of authors.
Table 1.
Top 10 Countries/Regions for Publication /Centrality
| No. | Publication | Country | Centrality | Country |
|---|---|---|---|---|
| 1 | 109 | PEOPLES R CHINA | 0.68 | USA |
| 2 | 71 | USA | 0.27 | SPAIN |
| 3 | 22 | JAPAN | 0.23 | TURKEY |
| 4 | 17 | ENGLAND | 0.07 | AUSTRALIA |
| 5 | 15 | GERMANY | 0.06 | ENGLAND |
| 6 | 14 | SOUTH KOREA | 0.06 | ITALY |
| 7 | 11 | TAIWAN | 0.05 | GERMANY |
| 8 | 11 | ITALY | 0.05 | CANADA |
| 9 | 11 | BRAZIL | 0.02 | IRELAND |
| 10 | 10 | CANADA | 0.02 | NETHERLANDS |
Analysis of Frequently Cited Journals
This study’s literature incorporates sources from 293 journals, with Anesthesiology (206 citations), Anesth Analg (202 citations), and Brit J Anaesth (179 citations) being the most frequently cited. The majority of the top ten journals belong to JCR area II, with a greater number of publishers hailing from the United States and United Kingdom, such as Nature and Lancet. These findings suggest a high level of research quality and proficiency within this field (Figure S1A), (Table S3). As for the top ten cited papers in this field. According to the research conducted by Kim et al,12 the administration of local anesthetics, specifically lidocaine, results in an increase in the activity of natural killer (NK) cells. Conversely, anesthetics such as propofol and locoregional anesthesia, which mitigate surgery-induced neuroendocrine responses by suppressing the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS), may lead to reduced immunosuppression and lower rates of cancer recurrence in comparison to the use of volatile anesthetics and opioids. The findings of Sekandarzad et al13 suggest that volatile anesthetics may promote tumor formation, while preclinical and emerging clinical evidence suggests that propofol may possess tumor protective properties. The perioperative phase in cancer patients presents a distinctive setting in which surgical stress response can result in immune suppression. The utilization of regional anesthesia techniques, in conjunction with multimodal analgesia consisting of NSAIDs, opioids, and local anesthetics, serves as a crucial component of balanced anesthesia by preventing the pathophysiologic consequences of pain and neuroendocrine stress response. It is imperative to recognize that perioperative anesthesia and analgesia can intensify immunosuppression in immunocompromised cancer patients, wherein the NK plays a pivotal role in anti-tumor immunity. In their study, Cho et al14 conducted a comparison of the effects of two distinct anesthesia and analgesia techniques on the NK cell cytotoxicity (NKCC) in patients undergoing breast cancer surgery. The results indicated that the utilization of Propofol anesthesia in conjunction with postoperative ketorolac analgesia yielded a favorable impact on immune function by preserving NKCC, as opposed to the use of sevoflurane anesthesia and postoperative fentanyl analgesia in the same patient population (Figure S1B), (Table 2).
Table 2.
Top 10 Most Cited Publications
| Authors | Title | First Author | Journal | Year | Citations |
|---|---|---|---|---|---|
| Biki et al17 | Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis | Biki B | Anesthesiology | 2008 | 760 |
| Snyder et al18 | Effect of anaesthetic technique and other perioperative factors on cancer recurrence | Snyder GL | Brit J Anaesth | 2010 | 653 |
| Myles et al19 | Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial | Myles PS | BMJ-Brit Med J | 2011 | 330 |
| Sessler et al20 | Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial | Sessler DI | Lancet | 2019 | 265 |
| Kim et al21 | Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence | Kim R | J Transl Med | 2018 | 244 |
| Gupta et al22 | Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in Central Sweden | Gupta A | Brit J Anaesth | 2011 | 208 |
| Stollings et al23 | Immune modulation by volatile anesthetics | Stollings LM | Anesthesiology | 2016 | 206 |
| Sekandarzad et al24 | Perioperative anesthesia care and tumor progression | Sekandarzad MW | Anesth Analg | 2017 | 153 |
| Cho et al25 | The effects of perioperative anesthesia and analgesia on immune function in patients undergoing breast cancer resection: a prospective randomized study | Cho JS | Int J Med Sci | 2017 | 85 |
| Mortezaee et al26 | Enriched cancer stem cells, dense stroma, and cold immunity: Interrelated events in pancreatic cancer | Mortezaee K | J Biochem Mol Toxicol | 2021 | 55 |
Analysis of Keywords
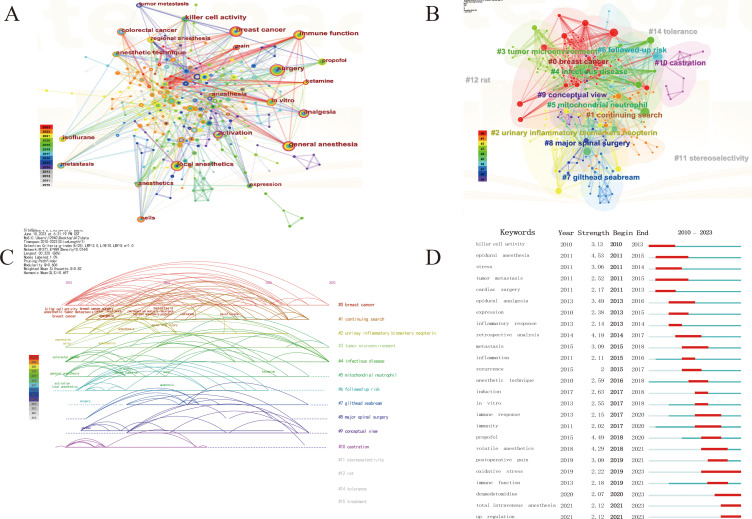
A quantitative investigation of keyword co-occurrence, which pertains to the manifestation of literature characteristic information, can be effectively utilized through keyword co-occurrence analysis to identify research hotspots within this domain. This study contained a total of 371 keywords, with surgery, general anesthesia, breast cancer, local anesthetics, and immune mechanism function being the top five keywords in frequency (occurring 48, 41, 37, 30, and 27 times, respectively). The top ten keywords in centrality were immune activation (0.22), general anesthesia (0.18), surgery (0.13), immune function (0.13), anesthesia (0.13), breast cancer (0.11), local anesthetics (0.1), local killer cell activity (0.1), anesthesia (0.1), and anesthetics (0.1) (Figure 5A), (Table S4). CiteSpace can cluster keywords based on the log-likelihood ratio algorithm based on keyword co-occurrence. Keyword clustering is a network cluster that forms interconnected network clusters for keywords with similar research topics in the research field, and each cluster is identified by header words that appear at high frequencies in the article. Clusters are numbered starting at 0, and the smaller the number, the more keywords are included. In this study, a cluster module value (Modularity, Q-value) of 0.606 > 0.3 means that the cluster structure is significant; a cluster mean profile value (Silhouette, S-value) of 0.82 > 0.7 means that the clusters are credible. The top 10 clustering labels were “breast cancer”, “continuing search”, “urinary inflammatory biomarkers neopterin”, “tumor microenvironment”, “infectious disease”, “mitochondrial neutrophil”, “subsequent risk”, “gilthead seabream”, “major spinal surgery”, and “concept view”. These clusters are closely connected and intertwined, indicating that there is a close connection between the clusters (Figure 5B), (Table S5). The timeline graph utilizes the historical process as its foundation, denoting significant keywords from the past on the axis. The size of the nodes represents their frequency, while the color of the nodes indicates their temporal distance.27 The visualization illustrates the evolution of important keywords over time. Between 2001 and 2015, numerous researchers conducted comprehensive and systematic investigations of this disease from various perspectives. The mechanism of action pertains to the activity of killer cells, impact on immune function, and apoptosis. The disease is centered on the metastasis and recurrence of breast and rectal cancer, while the side effects are associated with acute lung injury resulting from the use of local anesthetic propofol. Symptoms, on the other hand, are related to the stress response triggered by anesthesia technique, leading to painless or painful surgery. Notably, research hotspots from 2016 to the present encompass the effects of isoflurane, sevoflurane, and ketamine on immune function (Figure 5C). An emergent word is a term that suddenly gains prominence within a specific time frame and can be utilized to identify fluctuations in subject headings, keyword usage, and trends.28 Notably, the research frontiers from 2010 to 2015 encompassed a range of topics, including “killer cell activity”, “epidural anesthesia”, “stress”, “tumor metastasis”, “cardiac surgery”, “expression”, “inflammatory response”, and “retrospective analysis”. In contrast, the research frontiers from 2016 to 2023 focused on areas such as “metastasis”, “inflammation”, “recurrence”, “anesthesia technique”, “induction”, “in vitro experimentation”, “immune response”, “propofol”, “volatile anesthetics”, “postoperative pain”, “oxidative stress”, “immune function”, “dexmedetomidine”, and “total intravenous anesthesia”. (Figure 5D).
Figure 5.
Analysis of keywords. (A). Co-occurrence of keywords; (B). Clustering map of keywords; (C). Timeline graph of keywords; (D). Keyword burst detection of the top 25 keywords with the strongest emergent strength.
Discussion
The immune system is responsible for identifying self and foreign antigens and eliminating harmful foreign antigens from the body. The immune-inflammatory response comprises two primary components: innate and adaptive immune responses. Innate immunity serves as the initial line of defense against foreign pathogens and functions primarily through the non-specific, rapid recognition, binding, and elimination of foreign pathogens by immune cells, including natural killer (NK) cells, monocyte-macrophages, and neutrophils.29 Simultaneously, monocytes, macrophages, and dendritic cells function as proficient antigen presenting cells. Upon pathogenic invasion, these cells present foreign antigens’ MHC-class II molecules to helper T cells, thereby stimulating their activation and eliciting immune responses.30 Through our bibliometric analysis, we found that China exhibited the highest publication output in the field of anesthetics and immune function research, with a total of 109 articles (34.28%), followed by the United States (71, 22.33%) and Japan (22, 6.92%). Among the institutions analyzed, Harvard University was found to be the most productive, with 12 publications (3.77%), followed by Boston Children’s Hospital with 10 publications (3.14%). The individuals with the most prolific publication records were Buggy, Donal J (5, 1.57%) and Yuki, Koichi (5, 1.57%), with Cata, Juan P (4, 1.26%) following closely behind. In terms of citation frequency, Anesthesiology was the most frequently cited journal, with a total of 206 citations (n=206), trailed by Anesth Analg (202) and Brit J Anaesth (179). The study revealed that the term “surgery” was the most commonly utilized keyword, appearing 48 times, followed by “general anesthesia” (41 times) and “breast cancer” (37 times). The investigation identified various contemporary topics of interest, with a primary focus on “metastasis”, “inflammation”, “recurrence”, “anesthesia technique”, and “induction”. Subsequent research endeavors are expected to concentrate on the impact of isoflurane, sevoflurane, and ketamine on immune function.
The precise mechanism by which anesthetic drugs modulate immune function remains elusive. Various anesthetics have been shown to exert immunosuppressive effects via distinct pathways. The administration of local anesthetics, such as lidocaine, through epidural blockade may result in a partial inhibition of sympathetic activity, which could potentially impact the distribution of lymphocyte subsets and the activity of NK cells.31 The disruption of signal transduction pathways may occur through various means, including the interaction between IL-6 secreting monocytes and opioid receptors present on their surface. Binding of opioids to these receptors results in a reduction in intracellular cAMP levels, which may contribute to the secretion of IL-6. Additionally, some scholars propose that morphine can exert both direct and indirect effects on immunocompetent cells by activating μ3 opioid receptor subtypes, leading to the release of nitric oxide.32 The immunomodulatory effects of Tramadol are primarily attributed to the activation of central monoamine neurotransmitters through the regulated inhibition of serotonin and norepinephrine reuptake by central neurons.33 Lipid-soluble inhalation anesthetics, namely halothane, isoflurane, and sevoflurane, have been observed to impact the membrane structure of polymorphonuclear leukocytes (PMNs), resulting in a reduction of their functionality. This effect is attributed to the weakening of the expression of the adhesion molecule CD11b and the inhibition of PMN adhesion to endothelial cells.34 The regulation of amino acid metabolism by Etomicol and Thiopental has been observed to modify amino acid turnover in PMN cells, thereby exerting inhibitory effects.35 Furthermore, the modulation of intracellular calcium concentration through distinct pathways by Ketamine, Thiopental Sodium, and Etomidate, achieved by adjusting calcium concentration, may also impact immune function.36
Local Anesthetics
Lidocaine is an amide local anaesthetic that was originally used as an antiarrhythmic agent intravenously. It was once proposed that intravenous lidocaine had an analgesic effect that could be useful in perioperative circumstances. Although lidocaine infiltration lowers the discomfort of several emergency department treatments, the impact of topical lidocaine is controversial.37 At the same time, local anesthetics have the ability to impede numerous immunocompetent cell functions, including the suppression of NK cell activity, hindering chemotaxis, adhesion, phagocytosis, and respiratory burst of granulocytes. The incubation of NK cells with whole blood cells containing local anesthetics such as lidocaine, bupivacaine, and ropivacaine results in a significant inhibition of NK cell activity.38 The reduction of cancer cellular migration and proliferation has been attributed to the interference with microtubular extension, cell aggregation, and reattachment by both ester- and amide-linked local anesthetics.39
Nonsteroidal Anti-Inflammatory Drugs
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly utilized during the perioperative phase to provide analgesic effects and reduce opioid consumption. Additionally, they are frequently prescribed for their antipyretic, antithrombotic, anti-inflammatory, and other therapeutic benefits.40 Rezabakhsh et al41 highlighted the well-known effects of aspirin administration (such as inflammatory and immunological responses) in a variety of non-cardiovascular illnesses, particularly in anesthesiology. The inhibition of COX enzymes results in a reduction of both COX-1 and COX-2 expression. The upregulation of PGE2, which is caused by the overexpression of COX-2, leads to an increase in the immunosuppressive IL-10 and a decrease in the antiangiogenic IL-1.42
Opioids
Opioids are acknowledged as immunosuppressive agents, with morphine serving as a prototypical example. Extended exposure to high doses of morphine can impede the proliferation of natural killer cells, T and B lymphocytes, and diminish the production of cytokines, including IL-2, IL-4, and IL-6.43,44
Benzodiazepines
Benzodiazepines exhibit immunomodulatory effects that are characterized by both proinflammatory and anti-inflammatory actions. These effects are observed to be dualistic in nature, with some actions promoting the immune response against infections while others inhibiting it. The activation of peripheral-type receptors is believed to modulate various immune functions such as phagocytosis, chemotaxis, cytokine production, T-cell maturation, and oxidative burst generation.45
Volatile Anesthetic Agents
The innate immunity is known to be affected by the volatile agent, isoflurane, sevoflurane, and halothane, as evidenced by their ability to reduce the cytotoxicity of NK cell.46,47 Isoflurane and sevoflurane have been observed to inhibit the release of IL-1β and tumor necrosis factor α (TNF-α) from human peripheral mononuclear cells, which encompass T and B lymphocytes as well as NK cells, in response to tumor cells in clinical studies. This mechanism may potentially augment tumorigenesis.48,49 Soleimanpour et al50 concluded that despite the unfavorable effects of inhalational halogenated anesthetics (particularly halothane) on hepatic patients, they should be used when necessary. The impact on all systems, especially the immune system, must be evaluated, and appropriate preparations must be made. Yong et al51 found that sevoflurane successfully lowered the expression level of CD4+CD25+FOXP3+ Treg cells in the peripheral blood of T2 and T3 patients with gastric cancer, giving a theoretical basis for the selection of surgical anesthetics for patients with gastric cancer.
Intravenous Anesthetics
Numerous studies have been conducted on the impact of intravenous anesthetic drugs on immunity, with particular emphasis on their effects on PMNs and cytokines.52–54 In emergency procedural sedation, ketamine/propofol combo (ketofol) has less respiratory adverse effects than propofol alone.55 Yu et al56 Found that propofol may suppress T cells less effectively than sevoflurane in children with severe mycoplasmal pneumonia (SMPP). Furthermore, propofol may have a lower impact on Th cell development into Th1 cells and a better Th1/Th2 ratio preservation than sevoflurane. Subsequent investigation utilizing a human cell model revealed that propofol did not impede NK cell-mediated cytotoxicity, in contrast to sevoflurane and isoflurane which hindered LFA-1. This observation implies that although propofol hinders macrophage function, its sparing effect on NK cells may confer advantages in the management of viral and malignant conditions during the perioperative period.46 In vitro studies have demonstrated that Etomidate has the ability to hinder eosinophil chemotaxis, thereby impeding cell-mediated innate immunity.57 Liu et al58 conducted a comprehensive analysis, which revealed that ketamine hinders neutrophil chemotaxis through the suppression of endothelial adhesion molecule expression in both human and rat models. In a systematic review and meta-analysis, Soleimanpour et al59 and they discovered that dexmedetomidine (DEX) injection prior to anesthesia may have a protective effect on liver and intestinal immune function after hepatectomy with vascular occlusion. Wang et al conducted a review and meta-analysis which revealed that DEX administration results in an increase in the quantity of NK cells, B cells, and CD4+ T cells. While the count of CD8+ T cells decreased and the CD4+/CD8+ ratio increased, the Th1/Th2 ratio also significantly increased, indicating the preservation of cell-mediated immunity.60 The regulation of cytokines by anesthetic drugs is a complex topic, with numerous and sometimes contradictory reports. This is due to the intricate nature of the inflammatory response and immune regulation mechanisms within the body. However, it is anticipated that further research will lead to a more cohesive understanding of this phenomenon.
It is evident from the available literature that researchers exhibit significant concern regarding the impact of anesthetic drugs on the immune function. This concern is aimed at facilitating the rational and effective application of diverse anesthetic drugs to different patients, with the ultimate goal of achieving optimal therapeutic outcomes. However, certain experimental findings exhibit dissimilarities or even contradictions, which could be attributed to variances in the design of basic and clinical studies, the selection of distinct tumor types, limitations of observation indicators, imbalanced overall immunity, and disparities between animal models and humans. Hence, further comprehensive investigations are imperative to unveil the correlation between anesthetic agents and immune response, and to provide guidance for perioperative pharmacotherapy in patients with clinical neoplasms and immune deficiencies.
Strengths and Limitations
This research presents the first bibliometric analysis investigating anesthetic’s impact on immune function, which includes an overview, advancements, highlights, and trends. It is recommended that researchers prioritize the most recent and highly cited references and hotspots. Nevertheless, this bibliometric evaluation has certain limitations. Firstly, a time lag may have led to the exclusion of recently published articles. Secondly, the study solely examined papers from the WoS Core Collection, which may have restricted the comprehensiveness of our findings. Finally, the presence of subjective bias in data interpretation was discovered to be inherent. Nonetheless, this investigation can still function as an exemplification of the comprehensive condition and prevailing tendency of this research topic.
Conclusions
Research on the effects of anesthetics on immune functions has progressed and revealed a worldwide pattern. China and Harvard University have been identified as the most productive country and institution, respectively. Anesthesiology has emerged as the most frequently cited journal, while Buggy, Donal J has been recognized as the most productive author. The keyword “surgery” has been the most commonly utilized. Future research endeavors are anticipated to focus on the impact of isoflurane, sevoflurane, and ketamine on immune function.
Acknowledgments
Yufei Wang, Ye Sun and Yunxiang Hu contributed equally to this work and shared the first author.
Funding Statement
There is no funding to report.
Data Sharing Statement
All data analyzed were included in this paper; further requests can be consulted and data can be obtained from the correspondent author.
Disclosure
The authors declare no competing interests.
References
- 1.Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S3–23. doi: 10.1016/j.jaci.2009.12.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ackerman RS, Luddy KA, Icard BE, Piñeiro Fernández J, Gatenby RA, Muncey AR. The effects of anesthetics and perioperative medications on immune function: a narrative review. Anesth Analg. 2021;133(3):676–689. doi: 10.1213/ANE.0000000000005607 [DOI] [PubMed] [Google Scholar]
- 3.Yu HP. Role of anesthetic agents on cardiac and immune systems. Shock. 2011;36(6):532–541. doi: 10.1097/SHK.0b013e3182357054 [DOI] [PubMed] [Google Scholar]
- 4.Yokoyama M, Itano Y, Mizobuchi S, et al. The effects of epidural block on the distribution of lymphocyte subsets and natural-killer cell activity in patients with and without pain. Anesth Analg. 2001;92(2):463–469. doi: 10.1213/00000539-200102000-00035 [DOI] [PubMed] [Google Scholar]
- 5.Høgevold HE, Lyberg T, Kähler H, Haug E, Reikerås O. Changes in plasma IL-1beta, TNF-alpha and IL-6 after total Hip replacement surgery in general or regional anaesthesia. Cytokine. 2000;12(7):1156–1159. doi: 10.1006/cyto.2000.0675 [DOI] [PubMed] [Google Scholar]
- 6.Shapiro J, Jersky J, Katzav S, Feldman M, Segal S. Anesthetic drugs accelerate the progression of postoperative metastases of mouse tumors. J Clin Invest. 1981;68(3):678–685. doi: 10.1172/JCI110303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novac MB, Boldeanu L, Rotaru LT, et al. The perioperative effect of anesthetic drugs on the immune response in total intravenous anesthesia in patients undergoing minimally invasive gynecological surgery. Rom J Morphol Embryol. 2021;62(4):961–969. doi: 10.47162/RJME.62.4.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan Z, Chu C, Zhou R, Que B. Effects of oxycodone combined with flurbiprofen axetil on postoperative analgesia and immune function in patients undergoing radical resection of colorectal cancer. Clin Pharmacol Drug Dev. 2021;10(3):251–259. doi: 10.1002/cpdd.818 [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Zhou S. Effect of compound propofol nanoemulsion on immune function in patients with pancreatic cancer. J Nanosci Nanotechnol. 2021;21(2):1390–1396. doi: 10.1166/jnn.2021.18661 [DOI] [PubMed] [Google Scholar]
- 10.Luan T, Li Y, Sun L, et al. Systemic immune effects of anesthetics and their intracellular targets in tumors. Front Med. 2022;9:810189. doi: 10.3389/fmed.2022.810189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Lu J, Qin M, et al. Effects of different anesthesia methods on postoperative immune function in patients undergoing gastrointestinal tumor resection. Sci Rep. 2023;13(1):243. doi: 10.1038/s41598-023-27499-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian X. Influences of etomidate combined with propofol on cognitive function, inflammation and immunity in patients undergoing gastric cancer surgery. Cell Mol Biol. 2023;69(4):81–85. doi: 10.14715/cmb/2023.69.4.12 [DOI] [PubMed] [Google Scholar]
- 13.Liu B, He X, Wang Y, et al. Bibliometric analysis of γδ T cells as immune regulators in cancer prognosis. Front Immunol. 2022;13:874640. doi: 10.3389/fimmu.2022.874640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Liu W, Du Y. Immune response in heart transplantation: a bibliometric analysis. J Thorac Dis. 2022;14(3):635–645. doi: 10.21037/jtd-22-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Z, Sun Y, Shen R, et al. Global scientific trends on the immunomodulation of mesenchymal stem cells in the 21st century: a bibliometric and visualized analysis. Front Immunol. 2022;13:984984. doi: 10.3389/fimmu.2022.984984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Y, Xiao Y, Hu Z, et al. Bibliometric analysis of single-cell sequencing researches on immune cells and their application of DNA damage repair in cancer immunotherapy. Front Oncol. 2023;13:1067305. doi: 10.3389/fonc.2023.1067305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: a retrospective analysis. Anesthesiology. 2008;109(2):180–187. doi: 10.1097/ALN.0b013e31817f5b73 [DOI] [PubMed] [Google Scholar]
- 18.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106–115. doi: 10.1093/bja/aeq164 [DOI] [PubMed] [Google Scholar]
- 19.Myles PS, Peyton P, Silbert B, Hunt J, Rigg JR, Sessler DI. Perioperative epidural analgesia for major abdominal surgery for cancer and recurrence-free survival: randomised trial. BMJ. 2011;342:d1491. doi: 10.1136/bmj.d1491 [DOI] [PubMed] [Google Scholar]
- 20.Sessler DI, Pei L, Huang Y, et al. Recurrence of breast cancer after regional or general anaesthesia: a randomised controlled trial. Lancet. 2019;394(10211):1807–1815. doi: 10.1016/S0140-6736(19)32313-X [DOI] [PubMed] [Google Scholar]
- 21.Kim R. Effects of surgery and anesthetic choice on immunosuppression and cancer recurrence. J Transl Med. 2018;16(1):8. doi: 10.1186/s12967-018-1389-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta A, Björnsson A, Fredriksson M, Hallböök O, Eintrei C. Reduction in mortality after epidural anaesthesia and analgesia in patients undergoing rectal but not colonic cancer surgery: a retrospective analysis of data from 655 patients in central Sweden. Br J Anaesth. 2011;107(2):164–170. doi: 10.1093/bja/aer100 [DOI] [PubMed] [Google Scholar]
- 23.Stollings LM, Jia LJ, Tang P, Dou H, Lu B, Xu Y. Immune Modulation by Volatile Anesthetics. Anesthesiology. 2016;125(2):399–411. doi: 10.1097/ALN.0000000000001195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekandarzad MW, van Zundert AAJ, Lirk PB, Doornebal CW, Hollmann MW. Perioperative Anesthesia Care and Tumor Progression. Anesth Analg. 2017;124(5):1697–1708. doi: 10.1213/ANE.0000000000001652 [DOI] [PubMed] [Google Scholar]
- 25.Cho JS, Lee MH, Kim SI, et al. The effects of perioperative anesthesia and analgesia on immune function in patients undergoing breast cancer resection: a prospective randomized study. Int J Med Sci. 2017;14(10):970–976. doi: 10.7150/ijms.20064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mortezaee K. Enriched cancer stem cells, dense stroma, and cold immunity: interrelated events in pancreatic cancer. J Biochem Mol Toxicol. 2021;35(4):e22708. doi: 10.1002/jbt.22708 [DOI] [PubMed] [Google Scholar]
- 27.Wang YQ, Chen YB, Xu D, Cui YL. Bibliometrics and visualization of the mechanisms of parkinson’s diseases based on animal models. Endocr Metab Immune Disord Drug Targets. 2020;20(10):1560–1568. doi: 10.2174/1871530320666200421103429 [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Zhao S, Tan L, et al. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens Bioelectron. 2022;201:113932. doi: 10.1016/j.bios.2021.113932 [DOI] [PubMed] [Google Scholar]
- 29.Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357(9270):1777–1789. doi: 10.1016/S0140-6736(00)04904-7 [DOI] [PubMed] [Google Scholar]
- 30.Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574(7776):45–56. doi: 10.1038/s41586-019-1593-5 [DOI] [PubMed] [Google Scholar]
- 31.Cata JP, Ramirez MF, Velasquez JF, et al. Lidocaine stimulates the function of natural killer cells in different experimental settings. Anticancer Res. 2017;37(9):4727–4732. doi: 10.21873/anticanres.11879 [DOI] [PubMed] [Google Scholar]
- 32.Welters ID, Menzebach A, Goumon Y, et al. Morphine suppresses complement receptor expression, phagocytosis, and respiratory burst in neutrophils by a nitric oxide and mu(3) opiate receptor-dependent mechanism. J Neuroimmunol. 2000;111(1–2):139–145. doi: 10.1016/S0165-5728(00)00401-X [DOI] [PubMed] [Google Scholar]
- 33.Saeed I, La Caze A, Hollmann MW, Shaw PN, Parat MO. New Insights on Tramadol and Immunomodulation. Curr Oncol Rep. 2021;23(11):123. doi: 10.1007/s11912-021-01121-y [DOI] [PubMed] [Google Scholar]
- 34.Heindl B, Reichle FM, Zahler S, Conzen PF, Becker BF. Sevoflurane and isoflurane protect the reperfused Guinea pig heart by reducing postischemic adhesion of polymorphonuclear neutrophils. Anesthesiology. 1999;91(2):521–530. doi: 10.1097/00000542-199908000-00027 [DOI] [PubMed] [Google Scholar]
- 35.Davidson JA, Boom SJ, Pearsall FJ, Zhang P, Ramsay G. Comparison of the effects of four i.v. anaesthetic agents on polymorphonuclear leucocyte function. Br J Anaesth. 1995;74(3):315–318. doi: 10.1093/bja/74.3.315 [DOI] [PubMed] [Google Scholar]
- 36.Eldufani J, Nekoui A, Blaise G. Nonanesthetic effects of ketamine: a review article. Am J Med. 2018;131(12):1418–1424. doi: 10.1016/j.amjmed.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 37.Ghojazadeh M, Sanaie S, Parsian Z, Najafizadeh R, Soleimanpour H. Use of lidocaine for pain management in the emergency medicine: a systematic review and meta-analysis. Pharm Sci. 2019;25(3):177–183. doi: 10.15171/PS.2019.48 [DOI] [Google Scholar]
- 38.Yokoyama M, Nakatsuka H, Itano Y, Hirakawa M. Stellate ganglion block modifies the distribution of lymphocyte subsets and natural-killer cell activity. Anesthesiology. 2000;92(1):109–115. doi: 10.1097/00000542-200001000-00021 [DOI] [PubMed] [Google Scholar]
- 39.Tedore T. Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. Br J Anaesth. 2015;115(Suppl 2):ii34–45. doi: 10.1093/bja/aev375 [DOI] [PubMed] [Google Scholar]
- 40.Day RO, Day RO. Mode of action of non-steroidal anti-inflammatory drugs. Med J Aust. 1988;148(4):195–199. doi: 10.5694/j.1326-5377.1988.tb112818.x [DOI] [PubMed] [Google Scholar]
- 41.Rezabakhsh A, Mahmoodpoor A, Soleimanpour M, Shahsavarinia K, Soleimanpour H. Clinical applications of Aspirin as a multi-potent drug beyond cardiovascular implications: a proof of concept for anesthesiologists- a narrative review. Anesth Pain Med. 2021;11(5):e118909. doi: 10.5812/aapm.118909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandler AB, Dubinett SM. COX-2 inhibition and lung cancer. Semin Oncol. 2004;31(2 Suppl 7):45–52. doi: 10.1053/j.seminoncol.2004.03.045 [DOI] [PubMed] [Google Scholar]
- 43.Sun Q, Li Z, Wang Z, et al. Immunosuppression by opioids: mechanisms of action on innate and adaptive immunity. Biochem Pharmacol. 2023;209:115417. doi: 10.1016/j.bcp.2023.115417 [DOI] [PubMed] [Google Scholar]
- 44.Peng J, Pan J, Wang H, Mo J, Lan L, Peng Y. Morphine-induced microglial immunosuppression via activation of insufficient mitophagy regulated by NLRX1. J Neuroinflammation. 2022;19(1):87. doi: 10.1186/s12974-022-02453-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rochard P, Galiegue S, Tinel N, et al. Expression of the peripheral benzodiazepine receptor triggers thymocyte differentiation. Gene Expr. 2004;12(1):13–27. doi: 10.3727/000000004783992170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tazawa K, Koutsogiannaki S, Chamberlain M, Yuki K. The effect of different anesthetics on tumor cytotoxicity by natural killer cells. Toxicol Lett. 2017;266:23–31. doi: 10.1016/j.toxlet.2016.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyata T, Kodama T, Honma R, et al. Influence of general anesthesia with isoflurane following propofol-induction on natural killer cell cytotoxic activities of peripheral blood lymphocytes in dogs. J Vet Med Sci. 2013;75(7):917–921. doi: 10.1292/jvms.12-0436 [DOI] [PubMed] [Google Scholar]
- 48.Mitsuhata H, Shimizu R, Yokoyama MM. Suppressive effects of volatile anesthetics on cytokine release in human peripheral blood mononuclear cells. Int J Immunopharmacol. 1995;17(6):529–534. doi: 10.1016/0192-0561(95)00026-X [DOI] [PubMed] [Google Scholar]
- 49.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/S0092-8674(01)00237-9 [DOI] [PubMed] [Google Scholar]
- 50.Soleimanpour H, Safari S, Rahmani F, Ameli H, Alavian SM. The role of inhalational anesthetic drugs in patients with hepatic dysfunction: a review article. Anesth Pain Med. 2015;5(1):e23409. doi: 10.5812/aapm.23409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yong F, Wang H, Li C, Liu W, Wang Z, Jia H. Effect of sevoflurane on CD4+CD25+FOXP3+ regulatory T cells in patients with gastric cancer undergoing radical surgery. Cell Mol Biol. 2023;69(8):214–220. doi: 10.14715/cmb/2023.69.8.33 [DOI] [PubMed] [Google Scholar]
- 52.Väisänen M, Lilius EM, Mustonen L, et al. Effects of ovariohysterectomy on canine blood neutrophil respiratory burst: a chemiluminescence study. Vet Surg. 2004;33(5):551–556. doi: 10.1111/j.1532-950X.2004.04077.x [DOI] [PubMed] [Google Scholar]
- 53.Bredthauer A, Geiger A, Gruber M, et al. Propofol ameliorates exaggerated human neutrophil activation in a LPS sepsis model. J Inflamm Res. 2021;14:3849–3862. doi: 10.2147/JIR.S314192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heller A, Heller S, Blecken S, Urbaschek R, Koch T. Effects of intravenous anesthetics on bacterial elimination in human blood in vitro. Acta Anaesthesiol Scand. 1998;42(5):518–526. doi: 10.1111/j.1399-6576.1998.tb05160.x [DOI] [PubMed] [Google Scholar]
- 55.Ghojazadeh M, Sanaie S, Paknezhad SP, Faghih SS, Soleimanpour H. Using ketamine and propofol for procedural sedation of adults in the emergency department: a systematic review and meta-analysis. Adv Pharm Bull. 2019;9(1):5–11. doi: 10.15171/apb.2019.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu H, Chen L, Yue CJ, et al. Effects of propofol and sevoflurane on T-cell immune function and Th cell differentiation in children with SMPP undergoing fibreoptic bronchoscopy. Ann Med. 2022;54(1):2574–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krumholz W, Abdulle O, Knecht J, Hempelmann G. Effects of i.v. anaesthetic agents on the chemotaxis of eosinophils in vitro. Br J Anaesth. 1999;83(2):333–335. doi: 10.1093/bja/83.2.333 [DOI] [PubMed] [Google Scholar]
- 58.Liu FL, Chen TL, Chen RM. Mechanisms of ketamine-induced immunosuppression. Acta Anaesthesiol Taiwan. 2012;50(4):172–177. doi: 10.1016/j.aat.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 59.Soleimanpour H, Shahsavari Nia K, Sanaie S, Ghojazadeh M, Alavian SM. Use of dexmedetomidine in liver disease: a systematic review and meta-analysis. Hepat Mon. 2019;19(10):e98530. doi: 10.5812/hepatmon.98530 [DOI] [Google Scholar]
- 60.Wang K, Wu M, Xu J, et al. Effects of dexmedetomidine on perioperative stress, inflammation, and immune function: systematic review and meta-analysis. Br J Anaesth. 2019;123(6):777–794. doi: 10.1016/j.bja.2019.07.027 [DOI] [PubMed] [Google Scholar]