Abstract
Skeletal muscle index (SMI) remains a strong predictor of mortality in cirrhosis patients. However, the extent to which SMI varies by race/ethnicity has not been fully evaluated. Among 317 patients, 55% identified themselves as non‐Hispanic White (NHW), 26% Hispanic White (HW), 13% Asian, and 6% Black. There was significant variation in SMI by race/ethnicity; median SMI was lowest in Asian and highest in Black patients. There were significant differences of sarcopenia by race/ethnicity using established SMI cutpoints: 48% NHW, 33% HW, 67% Asian, and 37% Black (P = 0.003). Using these cutpoints, SMI was significantly associated with waitlist mortality only in NHW patients but not in other racial/ethnic groups.
Keywords: body composition, computed tomography, sarcopenia, skeletal muscle index
Introduction
Sarcopenia, or loss of muscle mass, is a prevalent complication of cirrhosis associated with increased pre‐ and post‐transplant morbidity and mortality. 1 , 2 Substantial evidence, to date, has demonstrated the prognostic value of computed tomography (CT)‐based measures of skeletal muscle loss in ambulatory patients with cirrhosis. 1 , 2 However, only sex‐specific cutpoints for sarcopenia, as measured by skeletal muscle index (SMI), exist as prognostic markers for mortality in this population 3 , 4 despite potential differences in skeletal muscle mass across racial/ethnic groups. 5 , 6 Clearly, more information is required to evaluate the applicability of these cutoffs among different racial/ethnic groups. The aims of this brief report were to characterize sarcopenia and the extent to which SMI varies by race/ethnicity and whether these cutpoints accurately predict mortality risk in various racial/ethnic groups.
Methods
We prospectively enrolled adult patients with cirrhosis awaiting liver transplantation in the ambulatory setting at a single transplant center between 2015 and 2018 as part of the Functional Assessment in Liver Transplantation (FrAILT) study, who had available abdominal CT scan within 6 months from enrollment. Baseline was defined as the date of study enrollment. Patient outcomes were obtained prospectively. The primary outcome was waitlist mortality, defined as the combined outcome of death or delisting for being too sick for liver transplantation. Total skeletal muscle area at the L3 vertebrae level (psoas, erector spinae, multifidus, quadratus lumborum, rectus abdominis, transverse abdominis, and internal/external oblique) was quantified using an image analysis software (General Electric Advanced Workstation 4.6, Volume Viewer software, GE Healthcare, Waukesha, WI, USA) and normalized by height to calculate SMI (cm2/m2). Sarcopenia was defined by previously established cutpoints of SMI <50 cm2/m2 for men and <39 cm2/m2 for women, which have been shown to be associated with pre‐transplant mortality independent of age and MELD (Model for End‐Stage Liver Disease) score. 3 Variables were compared between groups using Wilcoxon rank‐sum and Pearson's chi‐square tests, as appropriate. A kernel smoother was used to visually display the distribution of SMI by race/ethnicity. Waitlist survival time was defined as the time from study enrollment to death due to any causes or delisting due to being too sick for transplant. Patients were censored at the date of transplant if they underwent a living or deceased donor liver transplant, date of waitlist removal for reasons other than being too sick (e.g., psychosocial reasons), or last known date of clinical follow‐up. The association between sarcopenia and waitlist mortality was explored using univariable and multivariable Cox regression, with the latter adjusted for a limited number of variables because of the small number of primary outcomes among different racial/ethnic groups. All research was conducted in accordance with both the Declarations of Helsinki and Istanbul. Written consent was provided by all subjects. This study was approved by the Institutional Review Board at the University of California, San Francisco.
Results
Among 317 patients included, 174 (55%) identified themselves as non‐Hispanic White (NHW), 82 (26%) as Hispanic White (HW), 42 (13%) as Asian, and 19 (6%) as Black. Baseline characteristics are presented in Table SS1. The majority were male (69%) with a median age of 61 years and median MELDNa of 13. The primary etiologies differed by race/ethnicity, with the predominant etiology being chronic hepatitis C in NHW (55%), HW (38%), and Black patients (79%) and chronic hepatitis B in Asian patients (45%). Alcohol‐associated and nonalcoholic fatty liver disease was predominately seen in NHW (21% and 11%, respectively) and HW (26% and 24%, respectively), with minimal (≤5%) seen in Asian and Black patients. Prevalence of ascites was higher in NHW (57%) and HW (61%) compared to Asian (34%) and Black (32%) patients. Hepatic encephalopathy was present in 48% of patients, with the highest prevalence in HW (63%).
There was significant variation in SMI by race/ethnicity (Fig. 1). Median SMI was highest in Black men (56 cm2/m2) and women (45 cm2/m2) and lowest in Asian men (46 cm2/m2) and women (36 cm2/m2), with similar SMI observed in NHW and HW men (50–52 cm2/m2) and women (41–42 cm2/m2). When using established SMI cutoffs to define sarcopenia, 3 there was a significant difference in the proportion with sarcopenia by race/ethnicity: 48% in NHW, 33% in HW, 67% in Asian, and 37% in Black patients (P = 0.003). After a median (first to third quartile, Q1–Q3) follow‐up of 10 (5–17) months, 78 (25%) patients died or were delisted for being too sick for transplantation. In univariable Cox regression, sarcopenia was significantly associated with waitlist mortality only in NHW patients (HR 2.30, 95% CI: 1.23–4.68, P = 0.02) but not in HW, Asian, or Black patients (P > 0.05 for each). After adjustments for age and MELDNa, sarcopenia remained a strong predictor of waitlist mortality (HR 2.58, 95% CI: 1.23–5.40) in NHW but did not reach statistical significance in HW, Black, and Asian subgroups (Table S2). As expected, older age (per year, HR 1.07, 95% CI: 1.01–1.14) and MELDNa (per 1 point, HR 1.14, 95% CI: 1.07–1.12) were also associated with increased mortality. Interaction terms for sarcopenia and age (P = 0.85) and sarcopenia and MELDNa (P = 0.22) were not statistically significant in NHW patients.
Figure 1.
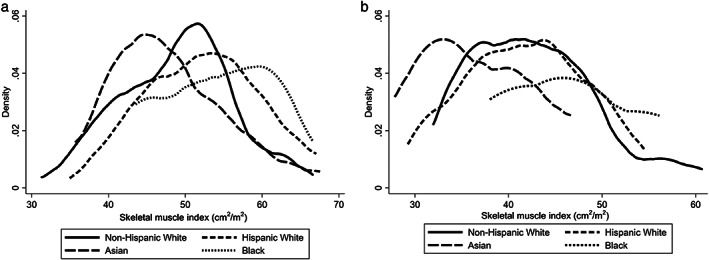
Kernel density plots of computed tomography‐quantified skeletal muscle index (cm2/m2) by self‐identified race/ethnicity among (a) men and (b) women.
Discussion
In this diverse cohort of cirrhosis patients awaiting liver transplantation, we observed significant variation in SMI by self‐identified race/ethnicity, leading to a wide range in the proportion of patients meeting the previously established sex‐specific SMI cutoffs for sarcopenia (range 33–67%). Although data on the prevalence of sarcopenia in different racial/ethnic groups is limited, our results were similar to those reported in prior U.S. studies involving non‐cirrhosis cohorts in which a higher proportion of Asian patients and a lower proportion of HW and Black patients were categorized as sarcopenic, compared to NHW. 6 , 7 Our findings expand on prior data on the variation in SMI among a more limited cohort of Asian and Native Hawaiian/Pacific Islander (NHOPI) patients in comparison to NHW, which demonstrates lower rates of sarcopenia in NHOPI compared to Asian patients (17% vs 60%); however, this cohort did not include HW and Black patients. 8
Observed variation in SMI among different racial/ethnic groups likely extends beyond the level of activity/energy expenditure and/or nutrition/diet, as Black patients still had higher measured skeletal muscle mass when these factors were controlled. 9 As such, potential biological explanations include differences in muscle quality, genetic variability, and/or hormone environment between racial/ethnic groups. Additionally, socioeconomic factors, such as limited access to healthcare resources or lower rates of leisure time physical activity, can further contribute to poorly controlled chronic diseases among minority racial/ethnic groups resulting in late‐stage diagnosis, increased hospitalizations, and disability due to loss of muscle mass. 10 , 11 This was observed among HW and Black patients in our study with higher prevalence of diabetes and hypertension, and in HW patients with a higher proportion of severe/refractory ascites, hepatic encephalopathy, and higher median MELDNa. Lastly, when using the previously established sex‐specific cutpoints, SMI was significantly associated with waitlist mortality only in NHW but not in the other racial/ethnic groups, highlighting the heterogeneity of skeletal muscle measurements and its clinical impact across racial/ethnic groups. Sex‐specific SMI cutoffs for sarcopenia in Asian patients were proposed by the Japanese Society of Hepatology as <42 cm2/m2 in men and <38 cm2/m2 in women, in comparison to the cutoffs of <50 cm2/m2 in men and <39 cm2/m2 in women in North America. When we applied these cutoffs to our present cohort, there was still a significant variation of sarcopenia by race/ethnicity: 24% in NHW, 0% in HW, 33% in Asian, and 16% in Black patients (P = 0.01). Moreover, SMI remained associated with waitlist mortality, though only in NHW patients. 12 Further research to identify different SMI cutoffs is needed to define sarcopenia based on continental norms.
As the aging population of racial/ethnic groups continues to grow in the United States, it is increasingly important to understand diverse clinical‐, demographic‐ (e.g., age, sex, race/ethnicity, socioeconomic), and disease‐related (e.g., compensated, decompensated) factors impacting body composition, in particular skeletal muscle mass, to promote healthy aging. One limitation of our study pertains to the relatively smaller representation of Asian and Black patients in comparison to NHW and HW participants; however, it still remains one of the more sizeable cohort studies to include a diverse range of racial/ethnic patients for direct comparison of SMI. Our data further supports the need to increase efforts to enhance diversity in cirrhosis cohorts to better understand racial/ethnic variation in the development and presentation of sarcopenia, along with optimal clinical metrics for earlier detection of sarcopenia and intervention(s) to improve functional and health outcomes in older adults with cirrhosis.
Supporting information
Table S1. Baseline demographic and clinical characteristics according to different self‐identified race/ethnicity
Table S2. Multivariable Cox regression for predictors of death or delisted for being too sick for liver transplantation across different self‐identified racial/ethnic groups
Declaration of conflict of interest: The authors report no conflict of interest.
Author contribution: Nghiem B Ha contributed to study concept and design, acquisition of data, interpretation of data, statistical analysis, drafting of the manuscript, and critical revision of the manuscript for important intellectual content. Bo Fan, Srilakshmi Seetharaman, Dorothea S Kent, Frederick Yao, Amy M Shui, Chiung‐Yu Huang, and Sharad I. Wadhwani contributed to interpretation of data and critical revision of the manuscript for important intellectual content. Jennifer C Lai Contributed to study concept and design, acquisition of data, interpretation of data, statistical analysis, drafting of the manuscript, critical revision of the manuscript for important intellectual content, obtaining funding, and study supervision. All authors approved final version of manuscript.
Financial support: This study was funded by NIH R01AG059183 (Jennifer C Lai), NIH P30DK026743 (Chiung‐Yu Huang, Jennifer C Lai, Amy M Shui), NIH R21AG067554 (Jennifer C Lai), KL2TR001870 (Sharad I. Wadhwani), NIH 5T32DK060414–18 (Nghiem B Ha), and NIH K23DK132454 (Sharad I. Wadhwani). These funding agencies played no role in the analysis of the data or the preparation of this manuscript.
References
- 1. Montano‐Loza AJ, Meza‐Junco J, Prado CM et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2012; 10: 166–173, 173 e161, 173.e1. [DOI] [PubMed] [Google Scholar]
- 2. Tandon P, Ney M, Irwin I et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012; 18: 1209–1216. [DOI] [PubMed] [Google Scholar]
- 3. Carey EJ, Lai JC, Wang CW et al. A multicenter study to define sarcopenia in patients with end‐stage liver disease. Liver Transpl. 2017; 23: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lai JC, Tandon P, Bernal W et al. Malnutrition, Frailty, and Sarcopenia in Patients With Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021; 74: 1611–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: A systematic review and meta‐analysis. PloS One. 2017; 12: e0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Silva AM, Shen W, Heo M et al. Ethnicity‐related skeletal muscle differences across the lifespan. Am. J. Hum. Biol. 2010; 22: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wagner DR, Heyward VH. Measures of body composition in blacks and whites: a comparative review. Am. J. Clin. Nutr. 2000; 71: 1392–1402. [DOI] [PubMed] [Google Scholar]
- 8. Sempokuya S, Yokoyama‐Arakakim K, Wong LL, Kalathi S. A pilot study of racial differences in the current definition of sarcopenia among liver transplant candidates. Hawaii J. Health Soc. Welf. 2020; 79: 161–167. [PMC free article] [PubMed] [Google Scholar]
- 9. Hunter GR, Bryan DR, Borges JH, David Diggs M, Carter SJ. Racial differences in relative skeletal muscle mass loss during diet‐induced weight loss in women. Obesity (Silver Spring). 2018; 26: 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al Snih S, Fisher MN, Raji MA, Markides KS, Ostir GV, Goodwin JS. Diabetes mellitus and incidence of lower body disability among older Mexican Americans. J. Gerontol. A Biol. Sci. Med. Sci. 2005; 60: 1152–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mendes de Leon CF, Barnes LL, Bienias JL, Skarupski KA, Evans DA. Racial disparities in disability: recent evidence from self‐reported and performance‐based disability measures in a population‐based study of older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2005; 60: S263–S271. [DOI] [PubMed] [Google Scholar]
- 12. Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol. Res. 2016; 46: 951–963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline demographic and clinical characteristics according to different self‐identified race/ethnicity
Table S2. Multivariable Cox regression for predictors of death or delisted for being too sick for liver transplantation across different self‐identified racial/ethnic groups


