Abstract
Background and Aims
Fontan‐associated liver disease (FALD) is a long‐term complication of the Fontan procedure. Guidelines recommend elastography, but the utility of transient elastography (TE) and two‐dimensional shear wave elastography (2D SWE) is unknown. We aimed to evaluate the relationship between TE and 2D SWE in FALD.
Methods
This prospective cohort study included 25 patients managed in a specialist clinic between January 2018 and August 2021. Trained clinicians performed 2D SWE (GE Logiq‐E9) and TE (FibroScan 503 Touch) on the same day under the same conditions. Laboratory, echocardiography, and imaging data were collected. The atrioventricular systolic‐to‐diastolic duration (AVV S/D ratio) was calculated as a measure of cardiac diastolic function.
Results
We analyzed 40 paired measurements. Median age was 22 years. Median liver stiffness measurement (LSM) was 15.4 kPa (12.1–19.6) by TE and 8.0 kPa (7.0–10.3) (P = 0.001) by 2D SWE. There was weak correlation between the modalities (r = 0.41, P = 0.004). There was no correlation between time since Fontan and LSM by TE (r = 0.15, P = 0.19) or 2D SWE (r = 0.19, P = 0.13). There was no difference in LSM irrespective of whether sonographic cirrhosis was present or absent by TE (17.4 kPa [15.9–23.6] vs. 14.9 kPa [12.0–19.4], respectively, P = 0.6) or 2D SWE (9.0 kPa [2.8–10.5] vs. 8.0 kPa [6.7–10.1], P = 0.46). There was no correlation between AVV S/D ratio and LSM by TE (r = 0.16, P = 0.18) or 2D SWE (r = 0.02, P = 0.45).
Conclusions
In FALD, TE and 2D SWE are poorly correlated. LSM by either modality was not associated with known risk factors for liver fibrosis or Fontan function. Based on these data, the role of elastography in FALD is uncertain.
Keywords: cirrhosis, echocardiography, elastography, fibrosis, Fontan, liver stiffness
We aimed to compare two modalities of liver elastography in Fontan associated liver disease. and found both were poorly correlated. Liver stiffness measurement using either modality was not associated with known risk factors for liver fibrosis or Fontan function. Based on these data, the role of elastography in FALD is uncertain.
Introduction
Fontan surgery is performed on children born with single ventricle anatomy which results in the passive flow of systemic venous blood through the lungs without passing through a ventricle. 1 Although still considered a palliative procedure, refinement of the surgical technique and multidisciplinary management have improved outcomes significantly, with most Fontan patients now surviving well into adulthood. 2 , 3 Long‐term multiorgan complications of Fontan surgery, predominantly resulting from chronic systemic venous hypertension, are now increasingly recognized.
Fontan‐associated liver disease (FALD) is a congestive hepatopathy in which patients universally develop some degree of liver fibrosis by 10 years after the procedure. 4 , 5 , 6 Time since Fontan surgery is the strongest predictor of fibrosis. 4 The reported prevalence of cirrhosis in this population varies 4 , 7 , 8 but its diagnosis has important implications for hepatocellular carcinoma surveillance and endoscopic variceal screening. Moreover, advanced liver disease may complicate candidacy for heart transplantation, sometimes necessitating dual organ transplantation. Early and accurate diagnosis of FALD has proved challenging. 9 , 10 , 11 FALD is often clinically silent, and routine laboratory markers can be normal. 12 , 13 Liver biopsy at 10 years post Fontan has been recommended in some guidelines, 11 although it is not routinely done in most centers. 14
Elastography is widely used in chronic liver disease for noninvasive screening and diagnosis. Modalities include magnetic resonance elastography (MRE), vibration‐controlled transient elastography (TE), and different modalities of ultrasound shear wave elastography (US SWE), with TE and US SWE more readily available than MRE. Available US SWE modalities include point shear wave elastography (also called acoustic radiation force impulse imaging) and 2D shear wave elastography (2D SWE). Position statements based on expert consensus recommend elastography as a noninvasive screening tool for FALD. 11 , 15 , 16 However, hepatic congestion can confound liver stiffness measurements (LSM), and LSM is independently associated with elevated central venous pressure. 17 , 18 Some elastography studies have shown a correlation between LSM and fibrosis score on biopsy, 19 , 20 while others have found no agreement. 5 , 21 The complex interaction between LSM, liver fibrosis, and Fontan hemodynamics may impact the utility of elastography for noninvasive diagnosis of fibrosis or cirrhosis in this context. Moreover, there is heterogeneity in the modalities used to assess LSM in the literature, and their equivalence has not been established.
We aimed to investigate the relationship between paired LSM by TE and 2D SWE in adult Fontan patients, and to examine factors affecting LSM and the correlation between these measures and other noninvasive tests of liver fibrosis.
Methods
Study design and patients
Data were prospectively collected for all patients ≥18 years of age with a Fontan circulation seen at a dedicated multidisciplinary Fontan clinic at a tertiary referral center between January 2018 and August 2021. Patients were excluded if they did not have matched TE and 2D SWE measurements done on the same day or if they were pregnant. Abdominal ultrasound, US SWE, TE, and laboratory tests were performed on the same day. The study was registered and approved by the local hospital ethics committee, including consent waiver.
Abdominal ultrasound and liver elastography
Patients underwent standard abdominal ultrasound, 2D SWE (GE Logiq‐E9 platform, GE Healthcare, Chicago, USA) and TE (FibroScan 503 Touch, Probe M/XL, Echosens, Paris, France) on the same day under the same conditions. Patients were fasted for 4 h and measurements were made by trained clinicians according manufacturer's instructions. For TE (Fibroscan), LSM was considered valid when there was a minimum of 10 consecutive valid measurements with a success rate over 60% and an interquartile range to median ratio of <0.30. Results were expressed as a median value in kilopascals (kPa). For 2D SWE, a minimum of five successful measurements of liver stiffness were performed in the same region of interest and were expressed as a median value in kPa as per manufacturer's instruction. In patients with serial paired elastography measurements, each set of paired measurements was separated by a minimum of 6 months. Sonographic cirrhosis was defined as nodularity or the presence of an irregular contour. Sonographic features of portal hypertension were defined as one or more of an enlarged portal vein (>13 mm), reversal of portal blood flow, or splenomegaly, in the presence of sonographic evidence of cirrhosis.
Baseline characteristics and laboratory tests
Demographic and clinical data collected were age, sex, type of congenital heart defect, surgical technique (atriopulmonary connection, lateral tunnel, extracardiac conduit), time since Fontan surgery, medication use, and oxygen saturations by pulse oximetry. Laboratory parameters collected were serum creatinine, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), international normalized ratio (INR), and platelet count. The following formulae were used: FIB‐4 = age (year) × AST [U/L]/(platelets [109/L] × (ALT [U/L])1/2 22 ; APRI = AST (/ULN)/platelet (109/L) × 100. 23 The prognostic score Model for End‐stage Liver Disease eXcluding INR (MELD‐XI) was calculated using MELD‐XI = 5.11 ln(bilirubin [mg/dl] + 11.76 ln(creatinine [mg/dl] + 9.44). 24
Echocardiographic measurements
Two‐dimensional and Doppler echocardiographic assessments were performed using Vivid 7 (General Electric Healthcare, Milwaukee, WI) and IE‐33 ultrasound systems (Philips Medical Systems, Andover, MA) according to the recommendations of the American Society of Echocardiography. 25 , 26 , 27 , 28 A single cardiologist with expertise in echocardiography and congenital heart disease interpreted the echocardiographic data. Dominant ventricular morphology was classed as left ventricular, right ventricular, or biventricular. Durations of systole and diastole were measured from the clearest continuous wave Doppler signal of dominant AV valve regurgitation. Effective systolic duration was measured from the onset to the end of AV valve regurgitation. Effective diastolic duration was measured from the end of AV valve regurgitation to the onset of the subsequent AV valve regurgitation signal. The systolic‐to‐diastolic duration ratio (AVV S/D) was then calculated as a previously validated measure of diastolic function. 29 A cutoff of 1.1 was used as a marker of poor prognosis, as previously demonstrated. 30
Statistical analysis
Statistical analyses were performed using SPSS version 28 (IBM, New York, USA). Data are reported as number (percentage) for categorical variables and mean ± SD or median (25th, 75th percentile) for continuous variables, as appropriate. Pearson coefficient was used for the correlation between TE and 2D SWE and other variables. Wilcoxon signed‐rank test was used for paired nonparametric variables. Mann–Whitney U test and Kruskall–Wallis test were used for independent nonparametric variables. Significance values were adjusted by the Boneforri correction for multiple comparisons. Logistic regression was not performed because of the sample size. A P‐value of <0.05 was considered significant. Areas under the receiver operating characteristic curves (AUCs) were constructed to assess the diagnostic accuracy of noninvasive tests in predicting sonographic cirrhosis.
Results
Study population
Twenty‐nine patients were screened, with 25 having at least one pair of 2D SWE and TE measurements. Four patients were excluded because only one test had been successfully performed. In one patient, 2D SWE failed on three occasions as a result of body habitus. In another, a valid TE score was not obtained because of body habitus. Two patients had valid TE, but 2D SWE was not attempted. In total, 40 paired measurements from 25 patient were included in the analysis, with 12 patients having either two or three paired measurements over multiple annual clinic reviews. The median patient age was 22 years 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ; 14 of 25 (52%) were female. The most common cardiac malformation was double outlet right ventricle in 6 of 25 (24%), with dominant right ventricle anatomy in 14 (66%). Extracardiac was the most common Fontan type in 19 of 25 (76%) and the majority were non‐fenestrated (73%) (Table 1).
Table 1.
Baseline characteristics
| Patient characteristics | |
|---|---|
| Age (years), median (IQR) | 22 (18–29) |
| Female sex, n (%) | 14 (56%) |
| Time since Fontan surgery (years), median (IQR) | 18.6 (±6.2) |
| Main congenital heart defect, n (%) | |
| DORV | 6 (24%) |
| Pulmonary valve atresia | 5 (20%) |
| HLH | 5 (20%) |
| Tricuspid valve atresia | 4 (16%) |
| DILV | 2 (8%) |
| TAPVD | 1 (4%) |
| TGA | 1 (4%) |
| VSD | 1 (4%) |
| Type of Fontan circulation, n (%) | |
| Extra‐cardiac | 19 (76%) |
| Intra‐cardiac | 5 (20%) |
| Atriopulmonary | 1 (4%) |
| Ventricle anatomy, n (%) | |
| Dominant right ventricle | 14 (66%) |
| Dominant left ventricle | 11 (44%) |
| Fenestration, n (%) | |
| No | 18 (72%) |
| Yes | 7 (28%) |
| NYHA class, n (%) | |
| I | 24 (96%) |
| II | 1 (4%) |
| Baseline oxygen saturation (%), mean (±SD) | 93 (±3.7) |
DILV, double inlet left ventricle; DORV, double outlet right ventricle; HLH, hypoplastic left heart; IQR, interquartile range; NYHA, New York Heart Association; SD, standard deviation; TAPVD, total anomalous pulmonary venous return; TGA, transposition of the great arteries; VSD, ventricular septal defect.
Liver stiffness measurement
TE‐derived LSMs were higher than 2D SWE‐derived LSMs in all but one paired measurement. The median difference between TE and SWE was 7.3 kPa (IQR 3.7–12.1). Median LSM by TE was 15.4 kPa (IQR 12.1–19.6) and that by 2D SWE was 8.1 kPa (IQR 7.0–10.3) (P = 0.001) (Fig. 1). There was a weak but significant correlation between LSM using TE versus 2D SWE (r = 0.41, P = 0.004) (Fig. 2).
Figure 1.
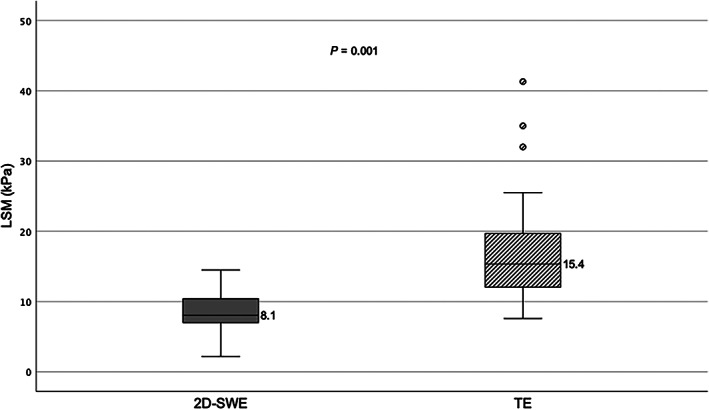
Liver stiffness by transient elastography versus 2D shearwave elastography. 2D SWE, two‐dimensional shear wave elastography; kPa, kilopascal; LSM, liver stiffness measurement; TE, transient elastography.
Figure 2.
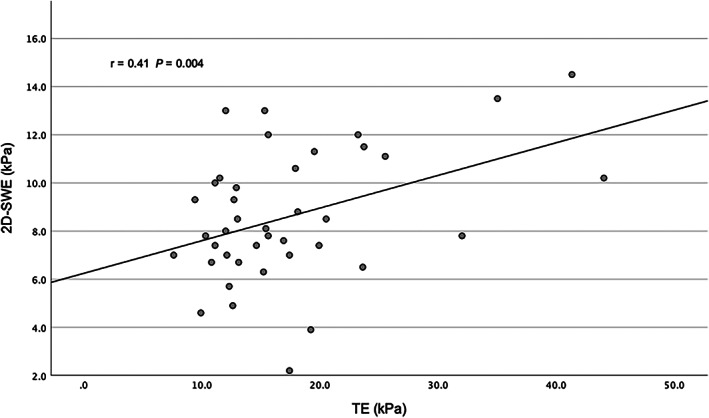
Correlation between siver stiffness using transient elastography and 2D shearwave elastography. 2D SWE, two‐dimensional shear wave elastography; kPa, kilopascal; TE, transient elastography.
Relationship between LSM and time since Fontan surgery
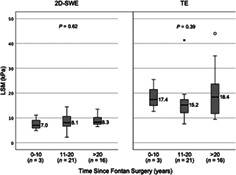
There was no significant correlation between the time since Fontan surgery and LSM by either TE (r = 0.15, P = 0.19) or 2D SWE (r = 0.19, P = 0.13). When patients were grouped per time since Fontan surgery (0–10 years, 11–20 years, and >20 years), there was no difference in LSM, by either modality (Fig. 3).
Figure 3.
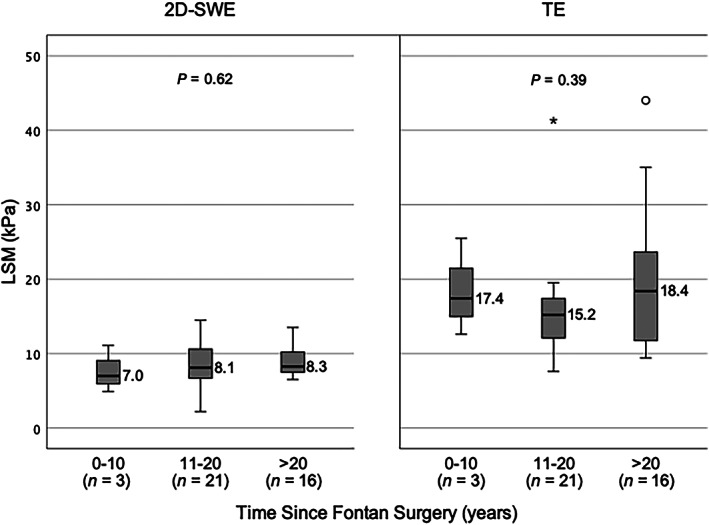
Liver stiffness distribution by time since Fontan. 2D SWE, two‐dimensional shear wave elastography; kPa, kilopascal; LSM, liver stiffness measurement; TE, transient elastography. *denotes extreme outlier.
Relationship between LSM and Fontan function
The AVV S/D ratio was ≥1.1 in 5 of 40 echocardiographic measurements (12.5%). There was no correlation between AVV S/D ratio and LSM by either TE (r = 0.16, P = 0.18) or 2D SWE (r = 0.02, P = 0.45). There was no significant difference between median LSM by either modality when grouped by AVV S/D ratio. When the magnitude of discordance between LSM by TE and 2D SWE was compared, there was no significant difference when grouped by the AVV S/D ratio. There was also no correlation between the AVV S/D ratio and this discordance (r = 0.18, P = 0.16).
Correlation of LSM with serum noninvasive tests
LSM by TE correlated with APRI (r = 0.34, P = 0.016) and MELD‐XI (r = 0.47, P = 0.001), but 2D SWE did not (APRI r = −0.18, P = 0.14; MELD‐XI r = 0.18, P = 0.14) (Table 2).
Table 2.
Correlations between liver stiffness by 2D shear wave elastography and transient elastography
| 2D SWE (r‐value) | P‐value | TE (r‐value) | P‐value | |
|---|---|---|---|---|
| Time since Fontan | 0.19 | 0.13 | 0.15 | 0.19 |
| AVV S/D | −0.02 | 0.45 | 0.16 | 0.18 |
| MELD‐Xi | 0.18 | 0.14 | 0.47 | 0.001* |
| APRI | −0.18 | 0.14 | 0.34 | 0.016* |
| FIB‐4 score | 0.14 | 0.20 | 0.23 | 0.08 |
| Oxygen saturation | −0.21 | 0.10 | −0.23 | 0.08 |
P ≤ 0.05.
2D SWE, two‐dimensional shear wave elastography; APRI, aspartate aminotransferase to platelet ratio index; AVV S/D, systolic‐to‐diastolic duration ratio; MELD‐Xi, Model for End‐stage Liver Disease eXcluding INR; TE, transient elastography.
Correlation of LSM with sonographic features of cirrhosis
Sonographic features of cirrhosis were found in 6 of 40 (15%) ultrasounds; none had ultrasound evidence of portal hypertension. There was no difference in LSM whether or not these features were present: for TE, 17.4 kPa (IQR 15.9–23.6) vs. 14.9 kPa (IQR 12.0–19.4), respectively (P = 0.24); or 2D SWE: 9.0 kPa (IQR 2.8–10.5) vs. 8.1 kPa (IQR 6.7–10.1), P = 0.43 (Fig. 4). There was low diagnostic accuracy of noninvasive tests (TE, 2D SWE, APRI, FIB‐4, and MELD‐XI) in discriminating sonographic cirrhosis from no sonographic cirrhosis (Table 3). No noninvasive test outperformed the others with wide overlapping confidence intervals.
Figure 4.
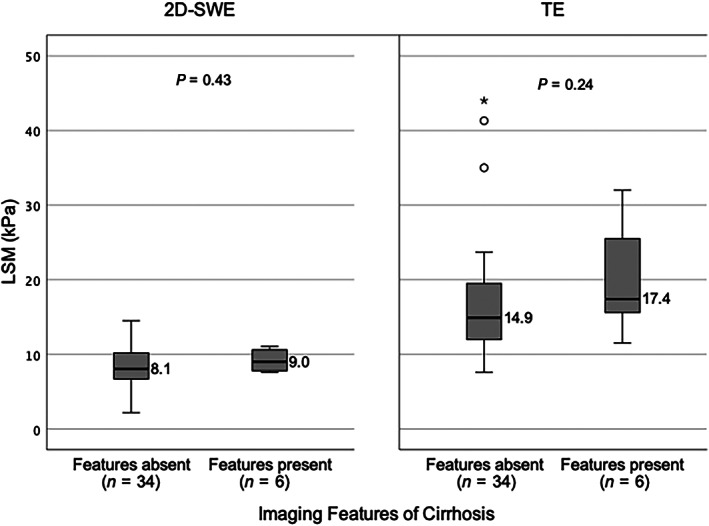
Liver stiffness distribution by sonographic cirrhosis. 2D SWE, two‐dimensional shear wave elastography; kPa, kilopascal; LSM, liver stiffness measurement; TE, transient elastography. *denotes extreme outlier.
Table 3.
Accuracy of noninvasive tests in diagnosing sonographic cirrhosis
| AUC | P‐value | 95% Confidence interval | |
|---|---|---|---|
| TE | 0.67 | 0.24 | 0.41–0.93 |
| 2D SWE | 0.66 | 0.27 | 0.45–0.86 |
| APRI | 0.34 | 0.26 | 0.03–0.65 |
| FIB‐4 | 0.25 | 0.08 | 0.03–0.48 |
| MELD‐Xi | 0.47 | 0.8 | 0.18–0.76 |
2D SWE, two‐dimensional shear wave elastography; APRI, aspartate aminotransferase to platelet ratio index; AUC, area under the curve; MELD‐Xi, Model for End‐stage Liver Disease – excluding INR; TE, transient elastography.
Discussion
To our knowledge, this is the first head‐to‐head comparison of TE and 2D SWE in a Fontan population. We investigated these two modalities using robust methodology, with both tests performed by skilled operators on the same day under the same fasted conditions. Although there was a weak correlation between TE and 2D SWE (r = 0.23), there was significant discordance between paired measurements in individual patients (P = 0.001). Neither test correlated with time since Fontan surgery, a known risk factor for fibrosis, or AVV S/D ratio, a validated surrogate marker of cardiac diastolic filling pressure in the setting of Fontan physiology. 29 LSM measured by TE correlated with APRI and MELD‐XI as serum noninvasive tests for fibrosis, but 2D SWE did not. There was no significant difference in LSM across the modalities whether or not sonographic evidence of cirrhosis was present.
These findings are consistent with the discordant data on elastography for fibrosis assessment in FALD. Studies of elastography in FALD with histological correlation are scant. In 14 patients, Schachter et al. found no agreement between liver biopsy and LSM by SWE, 21 whereas Kutty et al. found a significantly higher LSM (19.8 kPa vs. 13.4 kPa) in10 patients with higher sinusoidal fibrosis scores but no difference when considering the presence of bridging fibrosis or cirrhosis. 19 SWE also overestimated liver fibrosis in 40% of patients. In two studies of TE with liver biopsy, the histologic correlation with LSM was equally underwhelming. In a cohort of 38 patients, Munstermann et al. found that LSM was similar irrespective of whether mild or severe fibrosis was found on biopsy, and did not correlate with the collagen proportionate area or the grade of sinusoidal dilatation. 5 In 10 teenage patients, Wu and colleagues showed that TE overestimated fibrosis by two stages in 50%. LSM was significantly associated with severe centrilobular fibrosis but not portal fibrosis or bridging fibrosis. 30 Although our study did not find any correlation with histology, we had hoped to establish a relationship between these two elastographic modalities and determine an LSM cutoff that could exclude FALD with sonographic cirrhosis. Our data did not support this. With the current available data, it is difficult to have confidence in either TE or SWE for noninvasive assessment of fibrosis or diagnosis of cirrhosis in FALD.
In primary fibrotic liver diseases, a small discordance between TE and 2D SWE is expected, of the order of 1–2 kPa. 31 In FALD studies, the observed LSM by TE has been reported to be higher than by SWE. In three SWE studies in teens or adults, median LSM ranged between 9.1 and 15.6 kPa, 19 , 21 , 32 albeit in nondirect comparison. By contrast, a range of 18.2–22.5 kPa 5 , 30 , 33 , 34 was seen in four TE studies. Our study is the first head‐to‐head comparison of the two modalities. The median discordance of 7.3 kPa was unexpectedly high. It is possible that the unique Fontan hemodynamics and resultant hepatic congestion have a greater effect on TE than SWE; however, the mechanism of this is not clear. The direct relationship between central venous pressure and LSM by TE has been demonstrated by inferior vena cava clamping in pigs, and then in patients with congestive heart failure with a reduction in LSM recorded after cardiac recompensation. 18 This, however, does not explain why this effect would be more pronounced in TE than in 2D SWE. Elastography is best validated in viral hepatitis, where the pattern of liver fibrosis is peri‐portal, rather than the centrilobular and portal fibrosis found in FALD. 4 Fibrogenesis in FALD is thought to be driven by noninflammatory mechanisms, as inflammatory infiltrates are often absent or minimal in biopsy and autopsy. 35 , 36 Elevated post‐sinusoidal systemic venous pressure leads to passive congestion, chronic sinusoidal dilation, peri‐sinusoidal edema, and hypoxia in centrilobular hepatocytes and subsequent arterialized nodule formation. 37 This unique pattern of liver injury and the influence of hepatic congestion may differentially influence mechanical shear wave propagation in TE compared with acoustic shear wave propagation in SWE, a difference not observed in primary fibrotic liver diseases. This requires further exploration. Moreover, the role of tissue viscosity may have significant impacts on shear wave dispersion, which is not measured in most currently available 2D SWE platforms. Viscosity may in part explain the discrepancy between results of the elastography modalities, and therefore viscosity measurement using dispersion imaging with newer 2D SWE platforms should be a focus for future research. In addition, much of the data concerning elastography in FALD are from MRE studies. TE and 2D SWE were chosen for our study because these are point‐of‐care tests and readily available at our center, whereas MRE is not. Our findings highlight the lack of equivalence between elastography modalities in FALD, so caution should be applied when generalizing between techniques.
Time since Fontan has consistently been shown to correlate with LSM, 30 , 32 , 33 , 38 although Kutty and colleagues did not observe this in their large cohort. 19 We observed no relationship between time since Fontan and LSM. The median time since Fontan in this cohort was 18.6 years, which is considerably longer than in the aforementioned studies. While FALD is considered universal at 10 years post Fontan, it is possible that the association plateaus past this point. The association between LSM and Fontan failure has also been debated, and elevated MRE‐derived LSM has been shown to predict Fontan failure, with an annual increase of >0.3 kPa associated with increasing morbidity. 39 In Brayer et al.'s large cohort, a median LSM of 4.9 kPa was associated with Fontan failure, significantly higher than 4.2 kpa without Fontan failure. 40 We found no association between LSM and the AVV S/D ratio. Furthermore, serial LSM measurements in individual patients showed marked variation, with differences as large as 15 kPa over a 12‐month period. These differences were too large to be explained by fibrosis progression over such short timeframe and are likely related to dynamic hepatic congestion. However, this was not reflected in AVV S/D ratios of these patients. Although based on a small subset of patients, this finding challenges the current recommendations for serial elastography to monitor the progression of FALD. 8 , 11 , 15
Laboratory‐based screening tests and severity scores have also been evaluated in FALD patients. MELD‐XI, designed to predict short‐term survival in patients with cirrhosis on anticoagulants, 24 has been shown to correlate with the extent of fibrosis but an adequate cutoff has not been defined. 41 APRI and FIB‐4 correlate with radiological evidence of fibrosis but not with biopsy evidence of fibrosis in FALD. 42 Our study demonstrated a weak correlation between APRI and MELD‐XI and TE‐derived LSM but not 2D SWE. Combining laboratory‐based scores with elastography may improve diagnostic accuracy and predictive value for FALD, but larger studies with histological correlates are imperative.
In most other liver diseases, LSM values of <10 kpa by TE rule out compensated advanced chronic liver disease. 43 In this cohort, only 3 of 40 (7.5%) TE‐derived LSMs fell below this threshold compared with 28 of 40 (70.0%) of 2D SWE‐derived LSMs. With further validation of 2D SWE against liver biopsies and clarification of the discrepancy between modalities, there is a potential role for 2D SWE‐derived LSM as a negative predictor of FALD. Additionally, peripheral venous pressure measurement can noninvasively estimate central venous pressure and mean circulatory filling pressure 44 and could be investigated as a simple point‐of‐care tool to correct for the influence of volume and congestion on LSM.
The strength of this study is that it is the first to examine paired measures of liver stiffness using the two most widely available modalities, namely TE and 2D SWE, in a robust cohort of adult ambulatory care Fontan patients. Patient inclusion was comprehensive through a dedicated multidisciplinary clinic, minimizing ascertainment bias, and elastography was feasible in the majority of patients. Furthermore, the median time since Fontan of 18.6 years in this study is considerably longer than in most other studies in this area, adding to its generalizability in regard to long‐term screening of the adult Fontan population.
The major limitation of our study is the lack of liver histology and invasive hemodynamics. This was compounded by using sonographic cirrhosis as the reference standard, as nodularity can be a feature of vascular disorders of the liver in the absence of histologic cirrhosis. A large discrepancy between modalities was observed, but conclusions about the accuracy of each cannot be drawn without liver biopsy as a reference standard or the influence of venous pressure on measures of liver stiffness. Indeed, liver biopsy is an imperfect gold standard per se, which is further complicated by the fact there is no agreed histologic grading system in FALD. Although the sample size was robust for our study of Fontan patients, the low prevalence of sonographic cirrhosis in this cohort limited the statistical power of LSM in predicting this finding. Although patients did have repeat measurements, the study's follow‐up period meant that, at most, only three repeat measurements were recorded. Similarly, we were not able to comment on hard clinical endpoints such as survival or hepatic decompensation, as no included patients developed these events during follow‐up. Finally, TE was performed by four hepatologists (NNT, MG, DP, AM) each with experience of over 500 TE measurements, while 2D SWE was performed by multiple trained liver sonographers whose skill level was not objectively assessed.
Conclusion
In the first head‐to‐head comparison of TE and 2D SWE in FALD with low sonographic cirrhosis prevalence, LSM by TE and 2D SWE were found to be poorly correlated. Neither TE nor 2D SWE was associated with known risk factors for biopsy‐proven liver fibrosis or Fontan function. Despite guidelines recommending their routine use, our data add to the ambiguity surrounding the role of elastography in fibrosis detection and disease monitoring in FALD. Given the ubiquity of FALD and the important implications of a diagnosis of cirrhosis, surveillance biopsy, although often unfeasible, remains the only definitive method for diagnosis in this population. This suggests that better noninvasive tests are required in this population.
Acknowledgment
Open access publishing facilitated by The University of Melbourne, as part of the Wiley ‐ The University of Melbourne agreement via the Council of Australian University Librarians.
Declaration of conflict of interest: The authors declare that they have no competing interests.
Author contribution: Madeleine Gill contributed to data collection and analysis and drafted the manuscript. Avik Majumdar and Rachel Cordina conceived the study and reviewed the manuscript. Sanjivan Mudaliar, David Prince, and Nwe Ni Than contributed to data collection and reviewed the manuscript.
Financial support: None.
References
- 1. Cowgill LD. The Fontan procedure: a historical review. Ann. Thorac. Surg. 1991; 51: 1026–1030. [DOI] [PubMed] [Google Scholar]
- 2. Poh CL, d'Udekem Y. Life After Surviving Fontan Surgery: A Meta‐Analysis of the Incidence and Predictors of Late Death. Heart Lung Circ. 2018; 27: 552–559. [DOI] [PubMed] [Google Scholar]
- 3. Schilling C, Dalziel K, Nunn R et al. The Fontan epidemic: Population projections from the Australia and New Zealand Fontan Registry. Int. J. Cardiol. 2016; 219: 14–19. [DOI] [PubMed] [Google Scholar]
- 4. Goldberg DJ, Surrey LF, Glatz AC et al. Hepatic Fibrosis Is Universal Following Fontan Operation, and Severity is Associated With Time From Surgery: A Liver Biopsy and Hemodynamic Study. J. Am. Heart Assoc. 2017; 6: e004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Munsterman ID, Duijnhouwer AL, Kendall TJ et al. The clinical spectrum of Fontan‐associated liver disease: results from a prospective multimodality screening cohort. Eur. Heart J. 2019; 40: 1057–1068. [DOI] [PubMed] [Google Scholar]
- 6. Silva‐Sepulveda JA, Fonseca Y, Vodkin I et al. Evaluation of Fontan liver disease: Correlation of transjugular liver biopsy with magnetic resonance and hemodynamics. Congenit. Heart Dis. 2019; 14: 600–608. [DOI] [PubMed] [Google Scholar]
- 7. Lindsay I, Johnson J, Everitt MD, Hoffman J, Yetman AT. Impact of liver disease after the fontan operation. Am. J. Cardiol. 2015; 115: 249–252. [DOI] [PubMed] [Google Scholar]
- 8. Pundi K, Pundi KN, Kamath PS et al. Liver Disease in Patients After the Fontan Operation. Am. J. Cardiol. 2016; 117: 456–460. [DOI] [PubMed] [Google Scholar]
- 9. Rychik J, Veldtman G, Rand E et al. The precarious state of the liver after a Fontan operation: summary of a multidisciplinary symposium. Pediatr. Cardiol. 2012; 33: 1001–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu FM, Kogon B, Earing MG et al. Liver health in adults with Fontan circulation: A multicenter cross‐sectional study. J. Thorac. Cardiovasc. Surg. 2017; 153: 656–664. [DOI] [PubMed] [Google Scholar]
- 11. Emamaullee J, Zaidi AN, Schiano T et al. Fontan‐Associated Liver Disease: Screening, Management, and Transplant Considerations. Circulation. 2020; 142: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Simpson KE, Esmaeeli A, Khanna G et al. Liver cirrhosis in Fontan patients does not affect 1‐year post‐heart transplant mortality or markers of liver function. J. Heart Lung Transplant. 2014; 33: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bradley E, Hendrickson B, Daniels C. Fontan Liver Disease: Review of an Emerging Epidemic and Management Options. Curr. Treat. Options Cardiovasc. Med. 2015; 17: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Srinivasan A, Guzman AK, Rand EB et al. Percutaneous liver biopsy in Fontan patients. Pediatr. Radiol. 2019; 49: 342–350. [DOI] [PubMed] [Google Scholar]
- 15. Zentner D, Celermajer DS, Gentles T et al. Management of People With a Fontan Circulation: a Cardiac Society of Australia and New Zealand Position statement. Heart Lung Circ. 2020; 29: 5–39. [DOI] [PubMed] [Google Scholar]
- 16. Rychik J, Atz AM, Celermajer DS et al. Evaluation and Management of the Child and Adult With Fontan Circulation: A Scientific Statement From the American Heart Association. Circulation. 2019; 140: CIR0000000000000696. [DOI] [PubMed] [Google Scholar]
- 17. Sugimoto M, Oka H, Kajihama A et al. Non‐invasive assessment of liver fibrosis by magnetic resonance elastography in patients with congenital heart disease undergoing the Fontan procedure and intracardiac repair. J. Cardiol. 2016; 68: 202–208. [DOI] [PubMed] [Google Scholar]
- 18. Millonig G, Friedrich S, Adolf S et al. Liver stiffness is directly influenced by central venous pressure. J. Hepatol. 2010; 52: 206–210. [DOI] [PubMed] [Google Scholar]
- 19. Kutty SS, Peng Q, Danford DA et al. Increased hepatic stiffness as consequence of high hepatic afterload in the Fontan circulation: a vascular Doppler and elastography study. Hepatology. 2014; 59: 251–260. [DOI] [PubMed] [Google Scholar]
- 20. Melero‐Ferrer JL, Osa‐Sáez A, Buendía‐Fuentes F et al. Fontan Circulation in Adult Patients: Acoustic Radiation Force Impulse Elastography as a Useful Tool for Liver Assessment. World J. Pediatr. Congenit. Heart Surg. 2014; 5: 365–371. [DOI] [PubMed] [Google Scholar]
- 21. Schachter JL, Patel M, Horton SR, Mike Devane A, Ewing A, Abrams GA. FibroSURE and elastography poorly predict the severity of liver fibrosis in Fontan‐associated liver disease. Congenit. Heart Dis. 2018; 13: 764–770. [DOI] [PubMed] [Google Scholar]
- 22. Sterling RK, Lissen E, Clumeck N et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006; 43: 1317–1325. [DOI] [PubMed] [Google Scholar]
- 23. Wai CT, Greenson JK, Fontana RJ et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003; 38: 518–526. [DOI] [PubMed] [Google Scholar]
- 24. Heuman DM, Mihas AA, Habib A et al. MELD‐XI: a rational approach to “sickest first” liver transplantation in cirrhotic patients requiring anticoagulant therapy. Liver Transpl. 2007; 13: 30–37. [DOI] [PubMed] [Google Scholar]
- 25. Rudski LG, Lai WW, Afilalo J et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults: A Report from the American Society of Echocardiography: Endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010; 23: 685–713. [DOI] [PubMed] [Google Scholar]
- 26. Lang RM, Badano LP, Mor‐Avi V et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 27. Nagueh SF, Appleton CP, Gillebert TC et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography. J. Am. Soc. Echocardiogr. 2009; 22: 107–133. [DOI] [PubMed] [Google Scholar]
- 28. Zoghbi WA, Enriquez‐Sarano M, Foster E et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and doppler echocardiography. J. Am. Soc. Echocardiogr. 2003; 16: 777–802. [DOI] [PubMed] [Google Scholar]
- 29. Cordina R, Ministeri M, Babu‐Narayan SV et al. Evaluation of the relationship between ventricular end‐diastolic pressure and echocardiographic measures of diastolic function in adults with a Fontan circulation. Int. J. Cardiol. 2018; 259: 71–75. [DOI] [PubMed] [Google Scholar]
- 30. Wu FM, Opotowsky AR, Raza R et al. Transient elastography may identify Fontan patients with unfavorable hemodynamics and advanced hepatic fibrosis. Congenit. Heart Dis. 2014; 9: 438–447. [DOI] [PubMed] [Google Scholar]
- 31. Sharpton SR, Tamaki N, Bettencourt R et al. Diagnostic accuracy of two‐dimensional shear wave elastography and transient elastography in nonalcoholic fatty liver disease. Ther. Adv. Gastroenterol. 2021; 14: 17562848211050436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smaś‐Suska M, Skubera M, Wilkosz T et al. Noninvasive assessment of liver status in adult patients after the Fontan procedure. Pol. Arch. Intern. Med. 2019; 129: 181–188. [DOI] [PubMed] [Google Scholar]
- 33. Fidai A, Dallaire F, Alvarez N et al. Non‐invasive Investigations for the Diagnosis of Fontan‐Associated Liver Disease in Pediatric and Adult Fontan Patients. Front Cardiovasc Med. 2017; 4: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song J, Kim K, Huh J et al. Imaging Assessment of Hepatic Changes after Fontan Surgery. Int. Heart J. 2018; 59: 1008–1014. [DOI] [PubMed] [Google Scholar]
- 35. Fernandez‐Iglesias A, Gracia‐Sancho J. How to Face Chronic Liver Disease: The Sinusoidal Perspective. Front. Med. (Lausanne). 2017; 4: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014; 383: 1749–1761. [DOI] [PubMed] [Google Scholar]
- 37. Bryant T, Ahmad Z, Millward‐Sadler H et al. Arterialised hepatic nodules in the Fontan circulation: hepatico‐cardiac interactions. Int. J. Cardiol. 2011; 151: 268–272. [DOI] [PubMed] [Google Scholar]
- 38. Friedrich‐Rust M, Koch C, Rentzsch A et al. Noninvasive assessment of liver fibrosis in patients with Fontan circulation using transient elastography and biochemical fibrosis markers. J. Thorac. Cardiovasc. Surg. 2008; 135: 560–567. [DOI] [PubMed] [Google Scholar]
- 39. Alsaied T, Possner M, Lubert AM et al. Relation of Magnetic Resonance Elastography to Fontan Failure and Portal Hypertension. Am. J. Cardiol. 2019; 124: 1454–1459. [DOI] [PubMed] [Google Scholar]
- 40. Brayer SW, Zafar F, Lubert AM et al. Relation of Magnetic Resonance Elastography to Fontan Circulatory Failure in a Cohort of Pediatric and Adult Patients. Pediatr. Cardiol. 2021; 42: 1871–1878. [DOI] [PubMed] [Google Scholar]
- 41. Evans WN, Acherman RJ, Ciccolo ML et al. MELD‐XI Scores Correlate with Post‐Fontan Hepatic Biopsy Fibrosis Scores. Pediatr. Cardiol. 2016; 37: 1274–1277. [DOI] [PubMed] [Google Scholar]
- 42. Baek JS, Bae EJ, Ko JS et al. Late hepatic complications after Fontan operation; non‐invasive markers of hepatic fibrosis and risk factors. Heart. 2010; 96: 1750–1755. [DOI] [PubMed] [Google Scholar]
- 43. de Franchis R, Bosch J, Garcia‐Tsao G, Reiberger T, Ripoll C, Baveno VIIF. Baveno VII ‐ Renewing consensus in portal hypertension. J. Hepatol. 2022; 76: 959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Masutani S, Kurishima C, Yana A et al. Assessment of central venous physiology of Fontan circulation using peripheral venous pressure. J. Thorac. Cardiovasc. Surg. 2017; 153: 912–920. [DOI] [PubMed] [Google Scholar]


