Abstract
Of the toxins produced by Bacillus cereus, the emetic toxin is likely the most dangerous but, due to the lack of a suitable assay, the least well known. In this paper, a new, sensitive, inexpensive, and rapid bioassay for detection of the emetic toxin of B. cereus is described. The assay is based on the loss of motility of boar spermatozoa upon 24 h of exposure to extracts of emetic B. cereus strains or contaminated food. The paralyzed spermatozoa exhibited swollen mitochondria, but no depletion of cellular ATP or damage to plasma membrane integrity was observed. Analysis of the purified toxin by electrospray tandem mass spectrometry showed that it was a dodecadepsipeptide with a mass fragmentation pattern similar to that described for cereulide. The 50% effective concentration of the purified toxin to boar spermatozoa was 0.5 ng of purified toxin ml of extended boar semen−1. This amount corresponds to 104 to 105 CFU of B. cereus cells. No toxicity was detected for 27 other B. cereus strains up to 108 CFU ml−1. The detection limit for food was 3 g of rice containing 106 to 107 CFU of emetic B. cereus per gram. Effects similar to those provoked by emetic B. cereus toxin were also induced in boar spermatozoa by valinomycin and gramicidin at 2 and 3 ng ml of extended boar semen−1, respectively. The symptoms provoked by the toxin in spermatozoa indicated that B. cereus emetic toxin was acting as a membrane channel-forming ionophore, damaging mitochondria and blocking the oxidative phosphorylation required for the motility of boar spermatozoa.
Bacillus cereus is known to cause clinical infections, food poisoning, and toxin-induced diarrheagenic and emetic syndromes (8). Even fatal cases of infections (3, 17, 19) and emetic-type food poisoning (15) have been reported recently. B. cereus is ubiquitous in nature but has also been isolated from environments where it may represent a serious health hazard, e.g., infant feed (20), hospital linen (6), and gunpowder (14). B. cereus strains have been shown to produce seven different toxins (11). Of these toxins, the emetic toxin is very likely the most dangerous but, due to the lack of a suitable assay, the least well known.
The documented biological activities of the emetic toxin, cereulide, described by Agata et al. (1) and Isobe et al. (12) are emesis in primates (22, 24) and swelling of mitochondria in HEp-2 cells (21) and in hepatocytes of a fatally food-poisoned patient (15). Even though the structure and mitochondrial toxicity of cereulide were recently elucidated, there has been no rapid detection method for the toxin from bacteria, from food poisoning outbreaks, or from contaminated food. In this paper, we describe an inexpensive, sensitive, rapid, and reproducible bioassay for detection of the emetic mitochondrial toxin, by all criteria identical to cereulide. The assay is based on the loss of motility of boar spermatozoa.
MATERIALS AND METHODS
B. cereus strains used in this study.
Strains F-47, F-528, F-3453, F-4426, and F-5881 were from the Public Health Laboratory Service, London, United Kingdom; strain 4810/72 was from Meijerierna Service AB, Lund, Sweden; strain NC 7401 was from the Nagoya City Public Health Institute, Nagoya, Japan; strains IH41385 and IH41064 were from the National Public Health Laboratory, Helsinki, Finland; and all other strains used were from our own collection.
Media and reagents.
Boar semen lots were obtained from the Artificial Insemination Center (AI Cooperative, Kaarina, Finland) and from the Department of Animal Reproduction (University of Helsinki). The microbial media were from Difco, and gramicidin (a mixture of A, B, C, and D), polymyxin B sulfate, surfactin, ionomycin, N,N-dihexylcarbodiimide, 2,4-dinitrophenol, and proteinase K were from Sigma (St. Louis, Mo.). Valinomycin was from Fluka (Buchs, Switzerland). Anatoxin A and nodularin were gifts of Kaarina Sivonen (University of Helsinki). The microconcentrator membranes were from Amicon Inc., Beverly, Mass. Other chemicals were from local suppliers and were of analytical quality.
Extraction of emetic toxin from pure cultures and directly from food. (i) Cell extracts.
Bacteria were grown on tryptic soy agar plates at 28°C for 10 days to stabilize the growth phase and obtain mainly sporulated and lysed cells, as observed by phase-contrast microscopy. Colonies were scraped from the agar and suspended in water at 100 mg ml−1. Cell extracts were prepared by sonication and freeze-thaw cycles of this suspension. The extracts were filtered (0.2-μm pores), and the permeate, diluted in dimethyl sulfoxide (DMSO), was tested for toxicity.
(ii) Methanol extraction of the toxin from pure cultures.
Five hundred milligrams of cells was collected from tryptic soy agar plates, grown at 28°C for 10 days, and extracted with 100 ml of 100% methanol, as described by Andersson et al. (5). The evaporated residue, diluted in DMSO, was tested for toxicity.
(iii) Methanol extraction of the toxin directly from food.
Boiled rice (60 g, 150 ml) was experimentally infected with 105 to 106 CFU of B. cereus or Bacillus mycoides strains g−1. The rice was kept at room temperature for 2 days. The rice was examined by phase-contrast microscopy and found to contain many spores and lysed cells. The rice was reheated to ca. 80°C (30 min) on day 2 and incubated for two more days at room temperature. On day 4, the rice was extracted with 1 volume of methanol, the extract was evaporated, and the residue, diluted in DSMO, was tested for sperm toxicity.
Biological analyses.
A spermatozoon toxicity test was performed according to protocols described earlier (5) except that the boar semen was diluted to 12% with a commercial semen extender (MR-A; Kubus, S.A., Madrid, Spain) to a density of 30 × 106 to 50 × 106 sperm cells ml−1. Sperm motility (the amount of motile versus nonmotile sperm cells) was subjectively estimated by phase-contrast microscopy by using a heated stage (37°C), and the exact percentage of highly motile sperm cells was objectively measured with a computerized sperm motility analyzer (4) (HTM-S, version 7.2; Hamilton-Thorn Research, Danvers, Mass.).
Plasma membrane integrity was assayed with a combination of two fluorescent stains, calcein-AM and ethidium homodimer-1 (Molecular Probes, Inc., Eugene, Oreg.), as described by Januskauskas and Rodriguez-Martinez (13). Cellular ATP content was measured by a bioluminescense technique (13) with a Bio-Orbit 1235 luminometer and an ATP Biomass kit 1243-118 (Bio-Orbit, Turku, Finland). Morphology of sperm mitochondria was analyzed with a transmission electron microscope, as described elsewhere (5). Osmolality measurements of extended boar semen were done with an automatic micro-osmometer (Herman Roebeling Messtechnik, Berlin, Germany). Analyses were done in duplicate and the results were averaged.
Purification and analysis of the sperm toxin of B. cereus 4810/72.
An extract of B. cereus 4810/72, prepared by sonication and freeze-thaw cycles of the harvested cells, was precipitated with ammonium sulfate (70%) and centrifuged. The precipitate was dissolved in 90% ethanol, and, after centrifugation, the supernatant was evaporated to dryness under N2. The chloroform-soluble part of the residue was collected, evaporated, and dissolved in 60:40 methanol-water. This solution was injected into a Sep-Pak C18 cartridge (Waters Co., Milford, Mass.) and washed with 80:20 methanol-water. The final elution was done with methanol.
MS analyses.
Electrospray (ESI) mass spectra were collected with an API300 triple quadrupole mass spectrometer (MS) (Perkin-Elmer Sciex Instruments, Thornhill, Ontario, Canada). Samples in 50% methanol-water with 0.5% acetic acid were injected directly into the electrospray chamber with a syringe pump (Harvard Apparatus, South Natick, Mass.) at a flow rate of 3 μl min−1. The instrument was calibrated with a polypropyleneglycol mixture supplied by the instrument manufacturer. MS/MS spectra were acquired by causing collisions between precursor ions and nitrogen collision gas at acceleration voltages of 50 V. Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) MS was performed in the delayed-extraction mode with a BIFLEX MS (Bruker-Franzen Analytik, Bremen, Germany) with a 337-nm nitrogen laser. A thin-layer matrix preparation was used, according to the method of Vorm et al. (26).
RESULTS
Extracts from emetic B. cereus cells inhibited motility and disrupted mitochondria of boar spermatozoa.
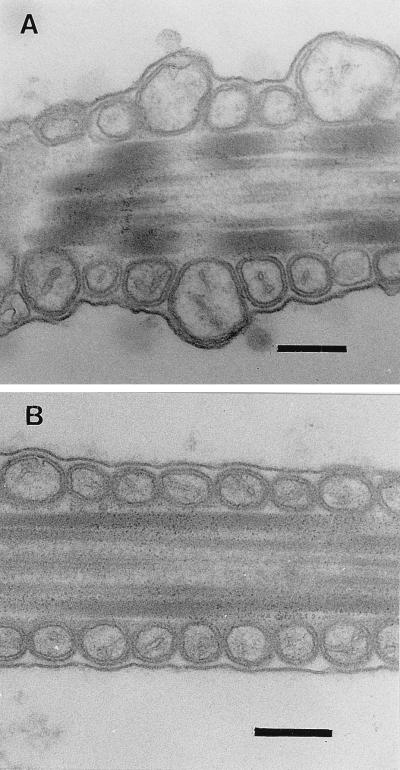
B. cereus 4810/72 and NC 7401, documented producers of emetic toxin (2, 24), were tested for toxicity toward boar spermatozoa. Cell extracts of both strains inhibited sperm motility within 1 day of exposure at a dosage of 2 mg (wet weight) of bacteria ml of extended boar semen−1. Similarly prepared extracts of cells of B. cereus ATCC 14579 (type strain), B. mycoides ATCC 6462 (type strain), and Bacillus licheniformis DSM 13 (type strain) had no effect on sperm motility even after 5 days of exposure (Table 1). Sperm motility subjectively estimated by phase-contrast microscopy was confirmed by the percentage of highly motile sperm cells objectively measured with the Hamilton-Thorn sperm analyzer. The amount of cell extract applied to the extended semen did not affect osmolality or pH. The sperm cells, exposed to extracts of emetic B. cereus strains, exhibited dose-dependent frequencies of swollen mitochondria visible with a transmission electron microscope (Fig. 1A), indicating that the filtrate contained a mitochondrial toxin. No mitochondrial swelling was observed in sperm cells exposed to extracts of B. cereus ATCC 14579T or B. mycoides ATCC 6462T (Fig. 1B).
TABLE 1.
Motility of boar spermatozoa after exposure to cell extracts of Bacillus strains
| Cell extract prepared froma: | % Motile spermatozoab after day:
|
|||
|---|---|---|---|---|
| 1 | 2 | 4 | 5 | |
| Emetic strains | ||||
| B. cereus 4810/72 | <5 | <5 | <5 | <5 |
| B. cereus NC 7401 | <5 | <5 | <5 | <5 |
| Other strains | ||||
| B. cereus ATCC 14579T | >60 | >60 | >60 | >50 |
| B. mycoides ATCC 6462T | >60 | >60 | >60 | >50 |
| B. licheniformis DSM 13T | >60 | >60 | >60 | >50 |
| No bacteria | >60 | >60 | >60 | >50 |
Bacillus strains were cultivated for 10 days at 28°C on tryptic soy agar. One hundred mg (wet weight) of cells per ml of distilled water was disrupted by repeated sonication and freezing-thawing and were then filtered (pore size, 0.2 μm).
Motility is given as percent spermatozoa expressing rapid motility, as measured by the Hamilton-Thorn sperm analyzer. The average difference between replicate tests was ≤20% when extracts of bacteria toward the sperm cells of three different boars were compared. The sperm suspension, containing 30 × 106 to 50 × 106 spermatozoa per ml, was exposed to extracts from 2 mg (wet weight) of bacteria per ml of extended boar semen.
FIG. 1.
Thin sections of the middle piece of a boar spermatozoon exposed to cell extracts of B. cereus 4810/72 and ATCC 14579T for 7 days. (A) Mitochondrial damage in the middle piece of a spermatozoon exposed to cell extracts of 2 mg (wet weight) of cells ml−1 of strain 4810/72. The frequency of similarly swollen mitochondria was 75% to over 90% after exposure to extracts from 4 mg (wet weight) of cells ml−1 and <20% after exposure to extracts from 0.5 mg (wet weight) of cells ml−1 (not shown). (B) Middle piece of a spermatozoon exposed to cell extracts of B. cereus ATCC 14579T (2 mg [wet weight] ml−1). Mitochondria of ordinary size with intact membranes are seen. A spermatozoon exposed to the same extract for 25 days exhibited no change of morphology, and those of unexposed spermatozoa were identical to that shown in panel B. Bars, 200 nm.
We tested 32 strains of B. cereus of different origins for sperm toxicity. The results in Table 2 show that cell extracts of five strains, F-5881, F-47, F-4426, 4810/72, and NC 7401, caused the loss of motility of boar spermatozoa at extremely low concentrations, equivalent to 50 ng (dry weight) of B. cereus cells (equivalent to 104 CFU) per ml of diluted boar semen. Extracts of the other 27 strains of B. cereus strains had no effect on the motility of spermatozoa even at 10,000-fold-higher concentrations (≥108 CFU ml−1).
TABLE 2.
Toxicities of cell extracts of B. cereus of different origins
| Source of B. cereus | Toxicity to spermatozoa
|
Strain(s) | |
|---|---|---|---|
| EC50 (mg of bacteria ml−1)a | EC50 (CFU of bacteria ml−1)a | ||
| Food poisoning outbreak | >4 | >108 | IH41385, IH41064, F-3371, F-3453, F-528 |
| Food poisoning outbreak | 0.002–0.0005 | 104–105 | 4810/72, NC 7401, F-47, F4426, F-5881 |
| Paper board machine | >4 | >108 | E1PCA, E60, T3 |
| Liquid packaging board | >4 | >108 | F-A2, EA160, FB12, K5-T9, F-B11, E-21, E-35, K5-T3, Er-25, K5-T4, B922, MC23, B-738, B-667 |
| Live Norway spruce | >4 | >108 | NS61 |
| Dairy products | >4 | >108 | K8, R15, NCFB634, SMR182 |
Expressed as the endpoint dilution of bacteria causing >50% decrease in sperm motility. The extracts were prepared as described in Table 1, footnote a, and diluted to endpoint by twofold dilutions in DMSO. The average difference between duplicate tests was ≤20%.
The spermatozoon-paralyzing agent in the B. cereus extracts was insensitive to heating at 100°C (20 min), treatment with acid (pH 2 [with HCl for 30 min]) or alkali (pH 12 [with NaOH for 30 min]), or the action of proteinase K (100 μg ml−1, pH 7, 3 h at 37°C). The toxic agent was filterable through the microconcentrator membranes, with a nominal cutoff of >100,000 g mol−1, and of >10,000 g mol−1 as a methanol extract, but not as an extract in water or DMSO. The agent paralyzing the sperm cells was thus heat stable and nonpolar, resistant to inactivation by heat, acid, alkali, or protease, and of an apparent molecular size smaller than 10,000 g mol−1.
Isolation, purification, and analysis of the sperm toxin from B. cereus 4810/72.
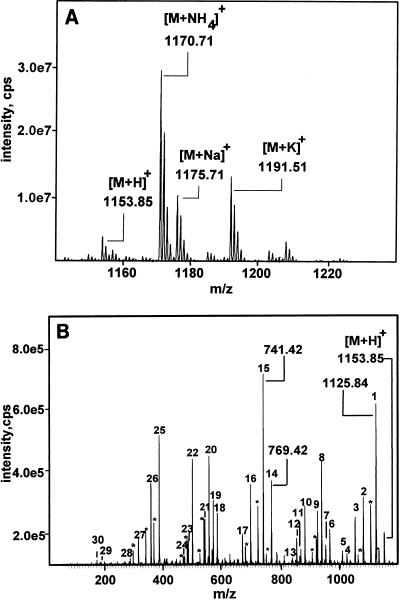
An extract of B. cereus 4810/72 was precipitated with ammonium sulfate, dissolved in chloroform, purified by a single step of Sep-Pak C18 sorption, eluted with methanol, and analyzed by ESI-MS. Four peaks were obtained with m/z of 1,153.85, 1,170.71, 1,175.71, and 1,191.51 (Fig. 2A). These were tentatively identified as the protonated, ammonium, sodium, and potassium adducts, respectively, of a single molecular species.
FIG. 2.
Mass fragmentation of the purified sperm toxin of B. cereus 4810/72. (A) m/z of the molecular ions as determined by ESI-MS. (B) ESI-MS/MS fragmentation of the protonated ion, m/z of 1,153.85, shown in panel A. Peak numbers correspond to the fragment ions assigned in Table 3. Peaks marked with asterisks represent loss of water.
Figure 2B shows the ESI-MS/MS spectrum of the ion with an m/z of 1,153.85 (protonated adduct [Fig. 2A]). The assignments of the fragment ions are shown in Table 3. The mass values of all fragments matched within 0.35 mass units those expected from the published dodecadepsipeptide structure of cereulide (12). In particular, the prominent tetrapeptide cleavages with m/z of 384.43 (peak 14 [Fig. 2B]) and 412.43 (peak 15) from the [M+H]+ match exactly with (Val)1(OLeu)1(Ala)1(OVal)1 and CO(Val)1(OLeu)1(Ala)1(OVal)1, respectively. Cereulide consists of three of these tetrapeptide units forming a cyclic structure. The expected neutral loss of water, 18, was also observed (Fig. 2B). The putative ammonium adduct, m/z of 1,170.71, gave in MS/MS an analogous fragmentation pattern, as well as the [M+H]+ ion through loss of ammonia, thus confirming the assignment.
TABLE 3.
Fragment ions and fragment loss in the MS/MS spectra of the sperm toxin of B. cereus 4810/72
| Fragment ion m/z (no.) | Fragment lossa |
m/zb
|
||
|---|---|---|---|---|
| Obsvd | Calcd | Δ | ||
| 1125.84 (1) | CO | 28.01 | 27.99 | 0.02 |
| 1082.84 (2) | Ala | 71.01 | 71.04 | −0.03 |
| 1054.84 (3) | Val | 99.01 | 99.07 | −0.06 |
| CO-Ala | 99.01 | 99.03 | −0.02 | |
| 1026.84 (4) | CO-Val | 127.01 | 127.06 | −0.05 |
| 1011.63 (5) | CO-OLeu | 142.22 | 142.06 | 0.16 |
| 968.83 (6) | OLeu-Ala | 185.02 | 185.11 | −0.09 |
| 954.63 (7) | OVal-Val | 199.22 | 199.12 | 0.10 |
| CO-Ala-OVal | 199.22 | 199.08 | 0.14 | |
| 940.83 (8) | Val-OLeu | 213.02 | 213.14 | −0.12 |
| CO-OLeu-Ala | 213.02 | 213.10 | −0.08 | |
| 926.63 (9) | CO-OVal-Val | 227.22 | 227.11 | 0.11 |
| 883.62 (10) | Ala-OVal-Val | 270.23 | 270.16 | 0.07 |
| 869.42 (11) | Val-OLeu-Ala | 284.43 | 284.17 | 0.26 |
| 855.62 (12) | CO-Ala-OVal-Val | 298.23 | 298.15 | 0.08 |
| 841.62 (13) | CO-Val-OLeu-Ala | 312.23 | 312.16 | 0.07 |
| 769.42 (14) | Val-OLeu-Ala-OVal, OLeu-Ala-OVal-Val, Ala-OVal-Val-OLeu, and OVal-Val-OLeu-Ala | 384.43 | 384.24 | 0.19 |
| 741.42 (15) | CO-Val-OLeu-Ala-OVal, CO-OLeu-Ala-OVal-Val, CO-Ala-OVal-Val-OLeu, and CO-OVal-Val-OLeu-Ala | 412.43 | 412.23 | 0.20 |
| 698.42 (16) | Ala-OVal-Val-OLeu-Ala | 455.43 | 455.28 | 0.15 |
| 670.42 (17) | Val-OLeu-Ala-OVal-Val | 483.43 | 483.31 | 0.12 |
| 584.22 (18) | OLeu-Ala-OVal-Val-OLeu-Ala | 569.63 | 569.35 | 0.28 |
| 570.42 (19) | OVal-Val-OLeu-Ala-OVal-Val | 583.43 | 583.36 | 0.07 |
| 556.22 (20) | Val-OLeu-Ala-OVal-Val-Oleu | 597.63 | 597.38 | 0.25 |
| 542.22 (21) | CO-OVal-Val-OLeu-Ala-OVal-Val | 611.63 | 611.37 | 0.26 |
| 499.22 (22) | Ala-OVal-Val-Oleu-Ala-OVal-Val | 654.63 | 654.38 | 0.25 |
| 485.42 (23) | Val-OLeu-Ala-OVal-Val-OLeu-Ala | 668.43 | 668.39 | 0.04 |
| 471.42 (24) | CO-Val-OVal-Ala-OLeu-Val-OVal-Ala | 682.43 | 682.37 | 0.06 |
| 385.22 (25) | (-Val-OLeu-Ala-OVal-)2, (-OLeu-Ala-OVal-Val-)2, (-Ala-OVal-Val-OLeu-)2, and (-OVal-Val-OLeu-Ala-)2 | 768.63 | 768.45 | 0.18 |
| 357.22 (26) | CO(-Val-OLeu-Ala-OVal-)2, CO(-OLeu-Ala-OVal-Val-)2, CO(-Ala-OVal-Val-OLeu-)2, and CO(-OVal-Val-OLeu-Ala-)2 | 796.63 | 796.44 | 0.19 |
| 314.03 (27) | Ala(-OVal-Val-OLeu-Ala-)2 and (-Ala-OVal-Val-OLeu)2 Ala | 839.82 | 839.49 | 0.33 |
| 286.03 (28) | (-Val-OLeu-Ala-OVal-)2Val, Val(-OLeu-Ala-OVal-Val-)2, | 867.82 | 867.51 | 0.31 |
| CO-Ala(-OVal-Val-OLeu-Ala-)2, and CO(-Ala-OVal-Val-OLeu-)2 Ala | 867.82 | 867.48 | 0.34 | |
| 186.03 (29) | (-OVal-Val-OLeu-Ala-)2 OVal-Val | 967.82 | 967.56 | 0.26 |
| 172.03 (30) | (-Val-OLeu-Ala-OVal-)2 Val-OLeu | 981.82 | 981.58 | 0.24 |
Val, Ala, OLeu, and OVal are residues of valine, alanine, 2-hydroxyisocaproic acid, and 2-hydroxyisovaleric acid, respectively. The calculated monoisotopic masses (m/z) are 99.07 (Val), 71.04 (Ala), 114.07 (OLeu), 100.05 (OVal), and 27.99 (CO [carbon monoxide]). The fragment ions in the table represent mass values of MS/MS spectra obtained from the precursor ion, m/z of 1153.85. Fragment loss is precursor ion minus fragment ion. The peptide sequence of fragment loss was assigned by using the known structure [(-OLeu-Ala-OVal-Val)3] of cereulide.
Obsvd, observed; Calcd, calculated; Δ, difference between observed fragment loss and calculated monoisotopic mass.
The data given above, as well as further details to be described elsewhere, indicate that the mitochondrion-toxic, boar sperm-paralyzing agent purified from the emetic strain B. cereus 4810/72 is a cyclic dodecadepsipeptide with a structure identical to that reported for cereulide (2, 12).
Biological properties of the purified sperm-toxic agent from B. cereus 4810/72 compared to other toxins and chemicals.
The sperm-toxic agent isolated as described above from the extract of B. cereus 4810/72 cells is referred to below as the sperm toxin. Yield of the sperm toxin from a 10-day-old culture of B. cereus 4810/72 was 240 ng (wet weight) mg of cells−1 (or 3 fg cell−1), as determined by high-performance liquid chromatography and verified by MALDI-TOF MS.
Toxicity thresholds of the purified sperm toxin toward boar spermatozoa were compared to those of selected bacterial toxins and chemicals (Table 4). The results revealed the sperm toxicities of three materials: the toxin purified from B. cereus 4810/72, valinomycin (a depsipeptide and potassium ionophore [7]), and gramicidin (a membrane channel-forming linear homopeptide, potassium ionophore, and protonophore [7]). Low concentrations (<3 ng ml−1) of these materials caused loss of sperm motility; concentrations of <400 ng ml−1 swelled the mitochondria but did not deplete the cells of ATP even at high concentrations (up to 12,500 ng ml−1). Calcimycin A 23187 (a polyether calcium ionophore [10]) inhibited sperm motility at 32 ng ml−1 and depleted the spermatozoa of ATP at 125 ng ml−1 but caused no morphological damage to mitochondria even at concentrations of 2,000 ng ml−1. Other bioactive peptides tested for sperm toxicity were the polyether calcium ionophore ionomycin (11), the membrane-active lipopeptide polymyxin B sulfate (10), surfactin (a cyclic lipopeptide antibiotic (25), nodularin (a cyanobacterial hepatotoxin [23]), anatoxin A (a cyanobacterial neurotoxin [23]), N,N-dihexylcarbodiimide (an ATPase inhibitor [7]), and 2,4-dinitrophenol (an uncoupler of oxidative phosphorylation [7]). It was found that the sperm cells were relatively insensitive to all of these; the 50% effective concentrations (EC50) for the vitality parameters shown in Table 4 ranged from 100 to >50,000 ng ml−1. In conclusion, sperm cell injuries (loss of motility and swollen mitochondria) caused by B. cereus 4810/72 were similar to those caused by valinomycin and gramicidin but had little resemblance to those caused by the other toxins tested.
TABLE 4.
Toxicity thresholds of selected toxins and chemicals on vitality parameters of boar spermatozoa
| Toxin or chemical | EC50 (ng ml−1)a
|
|||
|---|---|---|---|---|
| Swelling of mitochondriab | Depleted ATP content | Loss of motilityc | Damage to membrane integrityd | |
| Sperm toxin purified from B. cereus 4810/72e | <400 | >1,000 | 0.5 | >1,000 |
| Valinomycin | <400 | >1,000 | 2 | >1,000 |
| Calcimycin A 23187 | >2,000 | 125 | 32 | ND |
| Gramicidin (mixture of A, B, C, and D) | >3,000 | ND | <3 | ND |
| Ionomycin | >1,000 | 10,000 | 1,000 | ND |
| Polymyxin B sulfate | >50,000 | >50,000 | >50,000 | ND |
| Surfactin | >50,000 | >50,000 | >50,000 | ND |
| Nodularin | ND | >40,000 | >40,000 | ND |
| Anatoxin A | ND | >40,000 | >40,000 | ND |
| N,N-Dihexylcarbodiimide | 1,000 | 10,000 | 100–1,000 | ND |
| 2,4-Dinitrophenol | >10,000 | >10,000 | 1,000–10,000 | ND |
Expressed as the endpoint dilution of extended boar semen causing >50% change in vitality parameters compared to spermatozoa exposed to diluent only. The average difference between duplicate samples was ≤20% for all vitality parameters. ND, not done.
As observed by transmission electron microscopy.
Motility, percentage of spermatozoa expressing high motility according to the Hamilton-Thorn sperm motility analyzer.
As observed by vitality staining with fluorescence microscopy.
The concentration of sperm toxin was determined by reverse-phase high-performance liquid chromatography with valinomycin as the standard compound. The column was a Nova-Pak C18, 3.9 by 300 mm; inner diameter, 4 μm. Eluent A, water with 0.1% trifluoroacetic acid (TFA); eluent B, acetonitrile with 0.075% TFA; gradient, from 50% A and 50% B to 100% B in 10 min at a flow rate of 1 ml min−1; detection, 214 nm; injections, 50% methanol with 0.1% TFA.
Direct detection of sperm-paralyzing toxin from methanol extracts of contaminated food.
Extracts of rice contaminated with B. cereus 4810/72 or B. cereus F-5881 inhibited sperm motility at quantities corresponding to 3 g (7.5 ml) of rice (Table 5). No paralysis of motility was observed for extracts prepared from rice contaminated with B. mycoides ATCC 6462T or B. cereus ATCC 14579T, tested up to quantities corresponding to >60 g (150 ml) of boiled rice. Table 5 also shows the sperm toxicities of the inoculant strains of B. cereus and B. mycoides extracted from the rice. The yields of methanol-soluble substances of B. cereus F-5881, 4810/72, and ATCC 14579T and B. mycoides ATCC 6462T were 24, 16, 9, and 13 mg (dry weight), respectively, per gram (wet weight) of cells. The respective EC50 of the methanol-soluble substances toward boar spermatozoa were 0.072 μg (equivalent to 2 × 105 CFU of strain F-5881) ml−1, 0.03 μg (equivalent to 105 CFU of strain 4810/72) ml−1, 17 μg (equivalent to 108 CFU of strain ATCC 14579T) ml−1, and >68 μg (equivalent to >1010 CFU of strain ATCC 6462T) ml−1 of extended boar semen.
TABLE 5.
Toxicity to boar spermatozoa of methanol extracts prepared from boiled rice inoculated with different Bacillus strains 4 days earlier
| Inoculum | EC50 of methanol extracts of inoculum culture (μg ml−1)a | Concn of various parameters equivalent to EC50b
|
||
|---|---|---|---|---|
| B. cereus contamination (CFU ml of rice−1)c | Methanol extracts from inoculated rice (μg of dry solids ml−1)c | Contaminated riced | ||
| B. cereus F-5881 | 0.072 | 106–107 | 38 | 7.5 (3) |
| B. cereus 4810/72 | 0.026 | 106–107 | 30 | 7.5 (3) |
| B. mycoides ATCC 6462T | >68 | >108–109 | >650 | >150 (60) |
| B. cereus ATCC 14579T | 17 | >108–109 | >523 | >150 (60) |
Bacteria were cultivated on tryptic soy agar for 10 days at 28°C, and 500 mg (wet weight) of cells was extracted with methanol. The evaporation residue of the methanol extract was dissolved in DMSO. Extended boar semen was exposed to serial dilutions (in DMSO) of the extract, and sperm motility was measured after 1 and 2 days of exposure.
EC50, endpoint dilution of methanol-extracted solids (dry weight) per milliliter of extended boar semen causing >50% decrease in sperm motility. The average difference between assays of duplicate samples was 20% with semen of different boars.
Aliquots of boiled rice (60 g; two parallel experiments) were inoculated with the Bacillus strains (105 to 106 CFU g−1). After 4 days at room temperature, the rice was extracted with 1 volume of methanol. The evaporated residue of the methanol extract was serially diluted in DMSO, and the motility of boar spermatozoa was measured after 1 and 2 days of exposure. A blank assay was performed with diluent (DMSO) only.
Values are in milliliters; values in parentheses are equivalent amounts, in grams.
Conclusions.
Methanol extracts prepared from inoculated boiled rice, as well as from cultures of B. cereus F-5881 and 4810/72, caused the same injuries on exposed spermatozoa as did the toxin purified from the extract of strain 4810/72. Sperm toxicity of the extract prepared from boiled rice was thus caused by cereulide toxin produced by strains F-5881 and 4810/72 in rice. The results show that the cereulide toxin in food was detectable based on the responses of spermatozoa to a methanol extract prepared directly from the food. Inhibited motility of the boar spermatozoa was observable within 24 h of exposure of the semen dilution to extracts prepared directly from food.
DISCUSSION
In this paper, we describe a rapid bioassay for detection of a sperm toxin produced by B. cereus strains documented as producers of the emetic toxin. The test was readable after 24 h by ordinary phase-contrast light microscopy, required no costly reagents, was easy to perform (5), and was highly reproducible. The average difference between replicate testings was 20% when toxicities towards spermatozoa of three different boars were compared. The EC50 was as low as 0.5 ng of purified toxin per ml of extended boar semen. This amount of toxin is equivalent to 104 CFU of toxin-producing B. cereus organisms per ml of extended boar semen. The test thus is >2 orders of magnitude more sensitive than methods described to date: the monkey (Maccacis muratta) feeding test (22) and vacuolization assay of trypsinized HEp-2 cells (human carcinoma of the larynx) (18).
The cereulide toxin was first isolated from B. cereus NC 7401. We isolated and purified the sperm-toxic agent from a cell extract of B. cereus 4810/72 and found it to be identical to cereulide described by Agata et al. (1, 2) and Isobe et al. (12) in heat stability, resistance to protease, inactivation by acid or alkali, and molecular mass (1,153.85 g mol−1 [protonated form]). Fragmentation analyses by ESI-MS/MS showed that the sperm toxin isolated in the present study was a dodecadepsipeptide with a cleavage pattern identical to that of cereulide.
The sperm toxin was structurally and functionally related to the commercially available potassium ionophore valinomycin. Valinomycin also caused the loss of motility and swelled the sperm mitochondria, but it did not deplete spermatozoal ATP or damage plasma membrane integrity. Similar toxic effects were observed with sperm cells exposed to methanol extracts prepared directly from food contaminated with B. cereus F-5881 and 4810/72. The cereulide toxin was thus extractable directly from food and measurable on the basis of decreased motility of boar spermatozoa within 24 h, allowing toxin detection without the time-consuming process of cultivating and isolating suspected B. cereus strains. The assay utilizes commercially available boar semen and requires no experimental animals. This is the first rapid and sensitive bioassay to be described for detection of the emetic toxin of B. cereus.
Spermatozoa are transcriptionally inactive, which makes them insensitive to toxins affecting synthesis of proteins or nucleic acids or their regulation (9). This excludes interference by other microbial toxins, such as the cyanobacterial toxins (23) and most mycotoxins of importance for foods. However, spermatozoa brilliantly couple external signals to cellular responses such as motility, which is mainly controlled by ion fluxes (27). This makes spermatozoa sensitive indicators for ion channel-forming toxins like potassium ionophores and protonophores and explains the decreased motility of boar spermatozoa exposed to valinomycin and gramicidin as well as their insensitivity to membrane-active agents without ion channel-forming capacity, such as polymyxin and surfactin (25).
Motility in boar spermatozoa (meaning progressive and high motility) is exclusively dependent on oxidative phosphorylation in mitochondria. ATP production by substrate-level phosphorylation is too slow in boar spermatozoa to maintain motility (16). The spermatozoa exposed to cereulide exhibited loss of motility and swollen, disrupted mitochondria. However, vitality staining and measurements of cellular ATP content revealed intact plasma membranes and a supply of ATP explainable by ongoing substrate-level phosphorylation in the cytoplasm. The sperm cells thus were paralyzed but not lethally injured. We conclude that the cereulide intoxicates boar spermatozoa by acting as an ionophore, which leads to loss of motility caused by blockage of oxidative phosphorylation in the mitochondria. Thus, the spermatozoa are paralyzed due to mitochondrial damage. This type of sublethal injury is not detectable with comparable sensitivity in other eukaryotic cells.
ACKNOWLEDGMENTS
This work was financially supported by grants from the Ministry of Agriculture and Forestry (Finland), the Centre of Excellence Fund of the University of Helsinki, the Technology Development Center of Finland, and the Academy of Finland.
We express special gratitude to N. Agata (Nagoya City Public Health Institute, Nagoya, Japan), R. J. Gilbert and A. Scoging (Public Health Laboratory Service, London, United Kingdom), and A. Christiansson (Meijerierna Service AB, Lund, Sweden) for B. cereus strains. We thank Tuire Koro and Mervi Lindman for preparing thin sections. Equipment at the laboratory for electron microscopy of Helsinki University, Biocenter, was at our disposal.
REFERENCES
- 1.Agata N, Mori M, Ohta M, Suwan S, Ohtani I, Isobe M. A novel dodecadepsipeptide, cereulide, isolated from Bacillus cereus causes vacuole formation in HEp-2 cells. FEMS Microbiol Lett. 1994;121:31–34. doi: 10.1111/j.1574-6968.1994.tb07071.x. [DOI] [PubMed] [Google Scholar]
- 2.Agata N, Ohta M, Masashi M, Isobe M. A novel dodecadepsipeptide, cereulide, is an emetic toxin of Bacillus cereus. FEMS Microbiol Lett. 1995;129:17–20. doi: 10.1016/0378-1097(95)00119-P. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama N, Mitani K, Tanaka Y, Hanazono Y, Motoi N, Zarcovic M, Tange T, Hirai H, Yazaki Y. Fulminant septicemic syndrome of Bacillus cereus in a leukemic patient. Intern Med. 1997;36:221–227. doi: 10.2169/internalmedicine.36.221. [DOI] [PubMed] [Google Scholar]
- 4.Andersson M, Katila T. The Hague Congress proceedings 4. The 12th International Congress in Animal Reproduction, The Hague, The Netherlands, 23 to 27 August 1992. 1992. Evaluation of frozen-thawed stallion semen with a motility analyzer; pp. 1837–1839. [Google Scholar]
- 5.Andersson M A, Nikulin M, Köljalg U, Andersson M C, Rainey F A, Reijula K, Hintikka E-L, Salkinoja-Salonen M. Bacteria, molds, and toxins in water-damaged building materials. Appl Environ Microbiol. 1997;63:387–393. doi: 10.1128/aem.63.2.387-393.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrie D, Hoffman P N, Wilson J A, Kramer J M. Contamination of hospital linen by Bacillus cereus. Epidemiol Infect. 1994;113:297–306. doi: 10.1017/s0950268800051724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Booth I. Bacterial transport energetics and mechanisms. In: Anthony C, editor. Bacterial energy transduction. London, England: Academic Press; 1988. pp. 377–428. [Google Scholar]
- 8.Drobniewski F A. Bacillus cereus and related species. Clin Microbiol Rev. 1993;6:324–338. doi: 10.1128/cmr.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadella B M. Lipid changes in the plasma membrane of capacitating boar spermatozoa. In: Rath D, Johnson L, Weitze K, editors. Reproduction in domestic animals, vol. 31. The Third International Conference on Boar Semen Preservation, Mariensee, Germany, August 1995. Berlin, Germany: Paul Parey Scientific Publishers; 1996. pp. 63–70. [Google Scholar]
- 10.Gräfe U. Biochemie der Antibiotika: Struktur-Biosynthese-Wirkmechanismus. Heidelberg, Germany: Spectrum Akademischer Verlag GmbH; 1992. pp. 219–318. [Google Scholar]
- 11.Granum, P. 1994. Bacillus cereus and its toxins. J. Appl. Bacteriol. Symp. 76(Suppl.):61S–66S. [PubMed]
- 12.Isobe M, Ishikawa T, Suwan S, Agata N, Ohta M. Synthesis and activity of cereulide, a cyclic dodecadepsipeptide ionophore as an emetic toxin from Bacillus cereus. Bioorg Med Chem Lett. 1995;5:2855–2858. [Google Scholar]
- 13.Januskauskas A, Rodriguez-Martinez H. Assessment of sperm viability by measurement of ATP, membrane integrity and motility in frozen/thawed bull semen. Acta Vet Scand. 1995;36:571–574. doi: 10.1186/BF03547671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krause A, Freeman R, Sisson P R, Murphy O M. Infection with Bacillus cereus after close-range gunshot injuries. J Trauma. 1996;41:546–548. doi: 10.1097/00005373-199609000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Mahler H, Pasi A, Kramer J M, Schulte P, Scoging A C, Baer W, Kraehenbuehl S. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N Engl J Med. 1997;336:1143–1148. doi: 10.1056/NEJM199704173361604. [DOI] [PubMed] [Google Scholar]
- 16.Mann T, Lutwak-Mann C. Male reproductive function and semen. Heidelberg, Germany: Springer-Verlag; 1982. pp. 196–197. [Google Scholar]
- 17.Marx C, Reimer P, Gross R, Woertler K, Steinmetz M, Peters P E. Meningoenzephalitis durch Bacillus cereus Fortschr. Geb Röentgenstr Nuklearmed. 1997;166:361–363. doi: 10.1055/s-2007-1015441. [DOI] [PubMed] [Google Scholar]
- 18.Mikami T, Horikawa T, Murakami T, Matsumoto T, Yamakawa A, Murayama S, Katagiri S, Shinagawa K, Suzuki M. An improved method for detecting cytostatic toxin (emetic toxin) of Bacillus cereus and its application in food samples. FEMS Microbiol Lett. 1994;119:53–58. doi: 10.1111/j.1574-6968.1994.tb06866.x. [DOI] [PubMed] [Google Scholar]
- 19.Miller J M, Hair J G, Hebert M, Hebert L, Robert S F J. Fulminant bacteremia and pneumonia due to Bacillus cereus. J Clin Microbiol. 1997;35:504–507. doi: 10.1128/jcm.35.2.504-507.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rowan N J, Anderson J G, Anderton A. The bacteriological quality of hospital-prepared infant feeds. J Hosp Infect. 1996;36:259–267. doi: 10.1016/s0195-6701(97)90219-x. [DOI] [PubMed] [Google Scholar]
- 21.Sakurai N, Koike K, Irie Y, Hayashi The rice culture filtrate of Bacillus cereus isolated from emetic type food poisoning causes mitochondrial swelling in a HEp-2 cell. Microbiol Immunol. 1994;38:337–343. doi: 10.1111/j.1348-0421.1994.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 22.Shinagawa K, Konuma H, Sekita H, Sugii S. Emesis of rhesus monkeys induced by intragastric administration with the HEp-2 vacuolation factor (cereulide) produced by Bacillus cereus. FEMS Microbiol Lett. 1995;130:87–90. doi: 10.1016/0378-1097(95)00188-B. [DOI] [PubMed] [Google Scholar]
- 23.Sivonen K. Cyanobacterial toxins and toxin production. Phycologia. 1996;35:12–21. [Google Scholar]
- 24.Turnbull P C B, Kramer J M, Joergensen K, Gilbert R J, Melling J. Properties and production characteristics of vomiting, diarrheal, and necrotizing toxins of Bacillus cereus. Am J Clin Nutr. 1979;32:219–228. doi: 10.1093/ajcn/32.1.219. [DOI] [PubMed] [Google Scholar]
- 25.Vollenbroich D, Pauli G, Özel M, Vater J. Antimycoplasma properties and application in cell culture of surfactin, a lipopeptide antibiotic from Bacillus subtilis. Appl Environ Microbiol. 1997;63:44–49. doi: 10.1128/aem.63.1.44-49.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vorm O, Roepstorff P, Mann M. Improved resolution and very high sensitivity in MALDI-TOF of matrix surfaces made by fast evaporation. Anal Chem. 1994;66:3281–3287. [Google Scholar]
- 27.Zeng Y, Clarke E N, Florman H M. Sperm membrane potential: hyperpolarisation during capacitation regulates zona pellucida-dependent acrosomal secretion. Dev Biol. 1995;171:554–563. doi: 10.1006/dbio.1995.1304. [DOI] [PubMed] [Google Scholar]