Abstract
Background
To comprehensively investigate risk factors for proliferative vitreoretinopathy (PVR) after retinal detachment (RD) surgery.
Methods
PubMed, Embase, Cochrane Library, and Web of Science were systematically searched until May 22, 2023. Risk factors included demographic and disease-related risk factors. Odds ratios (ORs) and weighted mean differences (WMDs) were used as the effect sizes, and shown with 95% confidence intervals (CIs). Sensitivity analysis was conducted. The protocol was registered with PROSPERO (CRD42022378652).
Results
Twenty-two studies of 13,875 subjects were included in this systematic review and meta-analysis. Increased age was associated with a higher risk of postoperative PVR (pooled WMD = 3.98, 95%CI: 0.21, 7.75, P = 0.038). Smokers had a higher risk of postoperative PVR than non-smokers (pooled OR = 5.07, 95%CI: 2.21–11.61, P<0.001). Presence of preoperative PVR was associated with a greater risk of postoperative PVR (pooled OR = 22.28, 95%CI: 2.54, 195.31, P = 0.005). Presence of vitreous hemorrhage was associated with a greater risk of postoperative PVR (pooled OR = 4.12, 95%CI: 1.62, 10.50, P = 0.003). Individuals with aphakia or pseudophakia had an increased risk of postoperative PVR in contrast to those without (pooled OR = 1.41, 95%CI: 1.02, 1.95, P = 0.040). The risk of postoperative PVR was higher among patients with macula off versus those with macula on (pooled OR = 1.85, 95%CI: 1.24, 2.74, P = 0.002). Extent of RD in patients with postoperative PVR was larger than that in patients without (pooled WMD = 0.31, 95%CI: 0.02, 0.59, P = 0.036). Patients with postoperative PVR had longer duration of RD symptoms than those without (pooled WMD = 10.36, 95%CI: 2.29, 18.43, P = 0.012).
Conclusion
Age, smoking, preoperative PVR, vitreous hemorrhage, aphakia or pseudophakia, macula off, extent of RD, and duration of RD symptoms were risk factors for postoperative PVR in patients undergoing RD surgery, which may help better identify high-risk patients, and provide timely interventions.
Background
The success of retinal detachment (RD) surgery is dependent on the absence or control of proliferative vitreoretinopathy (PVR) [1]. PVR, one of the most important complications following vitreoretinal surgery, is a fibrotic eye disease with an incidence of 5–11%, and characterized by migration and proliferation of cells following a break in the retina or trauma, leading to formation of membranes in the periretinal area, followed by contraction of the cellular membranes and traction on the retina that causes RD [2–4]. For improvements in the surgical success rate and prevention of this complication, it is necessary to understand the risk factors for PVR in patients after RD surgery.
Several studies have been performed to explore the risk factors for PVR after RD surgery, but there are still inconsistent results. In the study of Xu et al. [5], macular involvement and cigarette smoking are factors predictive of PVR formation after uncomplicated primary RD repair. Bonnet et al. [6] illustrated that preoperative grade-B PVR and preoperative vitreous hemorrhage were associated with a significantly higher incidence of postoperative PVR. A history of uveitis related to an increased risk of PVR following RD surgery, according to another study [7]. In cases of RD with pre-existing PVR, there is a significant risk of progression to further advanced PVR [8–10]. Smoking was reported by Eliott et al. [11] to be a risk factor for PVR after the repair of RD associated with open-globe trauma. Another study showed that patients >70 years and with intraocular pressure lower than 14 mmHg had a higher risk of PVR after RD surgery [12]. At present, only a systematic review protocol of Chaudhary et al. [10] is available, and no study comprehensively evaluates the risk factors for PVR after RD surgery.
This study intended to comprehensively probe into the risk factors for PVR after RD surgery via a systematic review and meta-analysis, and focused on demographic risk factors and disease-related risk factors.
Methods
Search strategy
PubMed, Embase, Cochrane Library, and Web of Science were systematically searched until May 22, 2023 by two independent reviews (JJ Fan, JH Wang). Disagreements were resolved through discussion. English search terms included “Retinal Detachment” OR “Detachment, Retinal” OR “Detachments, Retinal” OR “Retinal Detachments” OR “Retinal Pigment Epithelial Detachment” AND “Vitreoretinopathy, Proliferative” OR “Proliferative Vitreoretinopathies” OR “Vitreoretinopathies, Proliferative” OR “Vitreoretinopathy Neovascular Inflammatory” OR “Inflammatories, Vitreoretinopathy Neovascular” OR “Inflammatory” OR “Vitreoretinopathy Neovascular” OR “Neovascular Inflammatories, Vitreoretinopathy” OR “Neovascular Inflammatory, Vitreoretinopathy” OR “Vitreoretinopathy Neovascular Inflammatories” OR “Proliferative Vitreoretinopathy”. Review studies were hand-searched to identify additional articles. This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The protocol was registered with PROSPERO (CRD42022378652).
Eligibility criteria
Inclusion criteria were: (1) studies on patients undergoing RD surgery; (2) studies reporting whether patients had PVR after RD surgery; (3) studies reporting at least one of the following: demographic risk factors and disease-related risk factors; (4) cohort studies and case-control studies; (5) studies in English; (6) the latest studies when the study population was repeated.
Exclusion criteria were: (1) studies with animal experiments; (2) studies unrelated to the research topic, such as studies on drug testing, etc.; (3) studies for which data are incomplete or data cannot be extracted; (4) conference reports, dissertations, case reports, meta-analyses, reviews.
Data extraction and quality assessment
Extracted data included first author, year of publication, country, study period, study design, sample size, preoperative PVR, sex ratio (male/female), age (years), follow-up time (months), disease type, type of surgery, quality assessment, and risk factor. The quality of the included case-control and cohort studies was evaluated using the modified Newcastle-Ottawa scale (NOS). The scale had a total score of 9, with 0–3 as low quality, 4–6 as medium quality, and 7–9 as high quality [13].
Statistical analysis
All studies were statistically analyzed using Stata 15.1 (Stata Corporation, College Station, TX, USA). For enumeration data, odds ratios (ORs) and hazard ratios (HRs) were used as the effect sizes, and for measurement data, weighted mean differences (WMDs) acted as the effect size, with 95% confidence intervals (CIs) calculated. The heterogeneity test was carried out for each index. If the heterogeneity statistic I2≥50%, the random-effects model was used for analysis; otherwise, the fixed-effects model was used for analysis. Sensitivity analysis was carried out for all models. P<0.05 was considered to be statistically significant.
Results
Characteristics of the included studies
A total of 6,477 studies were retrieved from the four databases, and 7 additional articles were identified from review studies. After deduplication, 3,495 were left. Subsequently, titles and abstracts and then full texts were subject to screening according to the eligibility criteria. Finally, 22 studies [5,7,11,12,14–30] of 13,875 subjects were included, published between 1984 and 2023. Fig 1 shows a flow chart of study selection. Three studies did not involve patients with preoperative PVR, some patients in 16 studies had preoperative PVR, and 3 studies did not report preoperative PVR. Of the included studies, 7 were cohort studies and 15 were case-control studies. All the studies were evaluated to have medium quality. Basic characteristics of the included studies are illustrated in Table 1.
Fig 1. Flow chart for study screening.
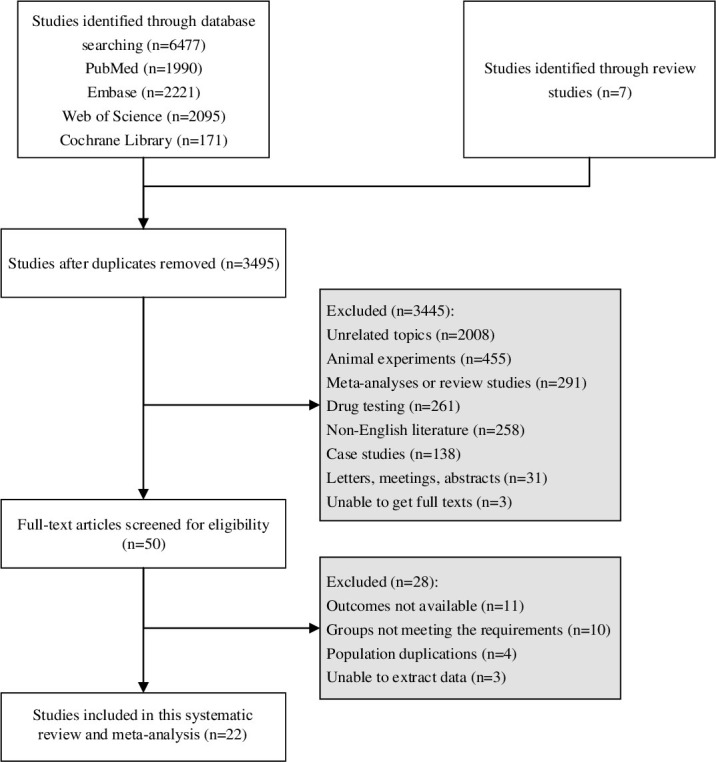
Table 1. Basic characteristics of the included studies.
| Author | Year | Country | Study period | Study design | Sample size | Preoperative PVR | Sex ratio (M/F) | Age (years) | FU time (months) | Disease type | Type of surgery | QA | Risk factor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gao | 2023 | China | 2016–2020 | Case control | 741 | Mixed | 423/318 | ≤40 155, 41–50 126, 51–60 215, 61–70 197, >70 48 | ≥6 | Primary RRD | Vitrectomy | 6 | Sex, age, preoperative PVR |
| Kasetty | 2023 | USA | 2010–2020 | Retrospective cohort | 62 | - | 25/37 | 15–24 10, 25–34 14, 35–45 38 | 33 | RRD | PPV | 4 | Age |
| Loiudice | 2023 | Italy | 2018–2020 | Case control | 85 | Mixed | 58/27 | 61.73±11.24 | 3 | Primary RRD | PPV | 4 | Age, aphakia or pseudophakia, macula off |
| Ferrara | 2022 | UK | 2011–2019 | Retrospective cohort | 8133 | Mixed | 5182/2951 | 59.13 (16–100) | ≥2 | Primary RRD | Vitrectomy 7064, SB 769, vitrectomy+SB 112, pneumatic retinopexy 188 | 4 | Age |
| Kocak | 2022 | Turkey | 2017–2021 | Case control | 150 | No | 97/53 | 57.86±12.40 | 3 | Primary RRD | PPV | 5 | Sex, age, aphakia or pseudophakia, macula off, extent of RD |
| Moussa | 2022 | UK | 2017–2021 | Retrospective cohort | 259 | Mixed | 187/72 | 61 (49–71)* | 6 | Primary RRD | PPV | 4 | Sex, age, preoperative PVR, macula off |
| Patel | 2021 | USA | 2015–2018 | Case control | 69 | Mixed | 51/18 | 42.9±15.5 | ≥3 | Primary RRD | SB | 6 | Sex, age, smoking, preoperative PVR, history of uveitis, surgical history, VH, aphakia or pseudophakia, macula off, IOP, retinal breaks, duration of RD symptoms |
| Zandi | 2021 | Switzerland | - | Case control | 65 | No | 40/25 | 61.3±13.4 | 6 | Primary RRD | PPV | 6 | Sex, age, aphakia or pseudophakia, macula off, retinal breaks |
| Antaki | 2020 | Canada | 2012–2019 | Case control | 506 | Mixed | 341/165 | 18–95 | 52 (3–105) | RRD | PPV | 5 | Sex, age, preoperative PVR, history of uveitis, surgical history, VH, aphakia or pseudophakia, macula off, IOP, retinal breaks, duration of RD symptoms |
| Slingsby | 2020 | USA | 2011–2017 | Retrospective cohort | 523 | - | - | 3–90 | 8.5 | Primary RRD | - | 5 | History of uveitis |
| Xu | 2019 | USA | 2014–2015 | Case control | 74 | No | 45/29 | 61.9±12.0 | 12.4±5.3 | Primary RD | SB 7, PPV 32, SB+PPV 35 | 6 | Sex, age, smoking, VH, aphakia or pseudophakia, IOP, retinal breaks, duration of RD symptoms |
| Mulder | 2018 | the Netherlands | 2014 | Case control | 195 | Mixed | 127/68 | 59.2±11.0 | ≥6 | Primary RRD | SB 70, PPV 125 | 4 | Sex, age, aphakia or pseudophakia |
| Conart | 2017 | France | 2013 | Case control | 100 | Mixed | 55/45 | 59.49±12.93 | ≥6 | Primary RRD | SB 20, PPV 80 | 5 | Age, VH, aphakia or pseudophakia, macula off, extent of RD |
| Eliott | 2017 | USA | 1999–2011 | Case control | 138 | Mixed | 107/31 | 42 ± 22 | ≥6 | RD | SB 3, PPV 70, SB+PPV 65 | 4 | Smoking, preoperative PVR |
| Ricker | 2012 | the Netherlands | 2001–2008 | Case control | 75 | Mixed | 45/20 | 43–79 | 3–80 | Primary RRD | SB | 5 | Sex, VH, aphakia or pseudophakia, macula off |
| Pastor | 2005 | Spain | 1996–2001 | Case control | 335 | Mixed | 214/121 | 54.5 (13–91) | 3 | RD | SB 169, vitrectomy 166 | 5 | Surgical history, aphakia or pseudophakia |
| Kon | 1999 | UK | 1995–1996 | Prospective cohort | 146 | Mixed | 94/42 | 59.0 (16–86) | 8.3 | RRD | PPV | 5 | Preoperative PVR |
| Capeans | 1998 | Spain | - | Case control | 65 | Mixed | 28/37 | 56.8 | 6.6 (3–15) | RRD | SB 63, pneumatic retinopexy 5 | 6 | Sex, aphakia or pseudophakia, macula off |
| Duquesne | 1996 | France | 1984–1993 | Prospective cohort | 390 | Mixed | 244/146 | 50 (4–85) | 12 | Primary RRD | SB 397, vitrectomy 144 | 4 | Preoperative PVR, VH |
| Girard | 1994 | France | 1985–1992 | Case control | 1020 | Mixed | - | - | 17.1±12.8 | RRD | SB 958, vitrectomy 176 | 4 | Preoperative PVR, history of uveitis |
| Cowley | 1989 | USA | 1978–1984 | Case control | 390 | Mixed | - | 58.3 | - | RRD | Vitrectomy 54 | 6 | Preoperative PVR, surgical history, VH, aphakia or pseudophakia, macula off |
| Bonnet | 1984 | France | - | Prospective cohort | 354 | - | - | - | ≥3 | RRD | Release of subretinal fluid 76, intravitreal gas injection 53, SB+ vitrectomy 20 | 6 | Surgical history |
* Median (interquartile range).
PVR, proliferative vitreoretinopathy; M/F, male/female; FU, follow-up; QA, quality assessment; IOP, intraocular pressure; RD, retinal detachment; RRD, rhegmatogenous retinal detachment; SB, scleral buckling; PPV, pars plana vitrectomy; VH, vitreous hemorrhage.
Demographic risk factors
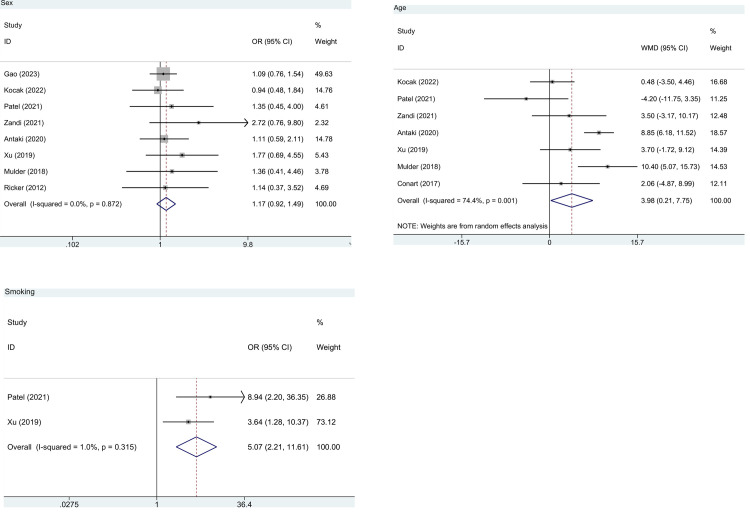
Sex. Eight studies of 1,875 patients reported the association between sex and the risk of postoperative PVR. No significant difference was observed in the risk of postoperative PVR between male and female patients (pooled OR = 1.17, 95%CI: 0.92, 1.49, P = 0.210, I2 = 0.0%) (Fig 2A and Table 2).
Fig 2. Forest plot for demographic risk factors in patients without PVR before RD surgery.
(a) sex; (b) age; (c) smoking. PVR, proliferative vitreoretinopathy; RD, retinal detachment; WMD, weighted mean difference; OR, odds ratio; CI, confidence interval.
Table 2. Risk factors for PVR after RD surgery.
| Risk factor | Pooled OR/WMD (95%CI) | P | I² |
|---|---|---|---|
| Demographic risk factor | |||
| Sex | 1.17 (0.92, 1.49) | 0.210 | 0.0 |
| Age | 3.98 (0.21, 7.75) | 0.038 | 74.4 |
| Smoking | 5.07 (2.21, 11.61) | <0.001 | 1.0 |
| Disease-related risk factor | |||
| Preoperative PVR | 22.28 (2.54, 195.31) | 0.005 | 87.8 |
| History of uveitis | 3.68 (0.81, 16.64) | 0.091 | 55.5 |
| Surgical history | 1.17 (0.54, 2.53) | 0.696 | 0.0 |
| Vitreous hemorrhage | 4.12 (1.62, 10.50) | 0.003 | 52.9 |
| Aphakia or pseudophakia | 1.41 (1.02, 1.94) | 0.040 | 0.0 |
| Macula off | 1.85 (1.24, 2.74) | 0.002 | 47.2 |
| Intraocular pressure | -0.97 (-3.36, 1.42) | 0.427 | 84.0 |
| Extent of RD | 0.31 (0.02, 0.59) | 0.036 | 0.0 |
| Retinal breaks | 0.09 (-0.19, 0.38) | 0.523 | 0.0 |
| Duration of RD symptoms | 10.36 (2.29, 18.43) | 0.012 | 40.7 |
PVR, proliferative vitreoretinopathy; RD, retinal detachment; WMD, weighted mean difference; OR, odds ratio; CI, confidence interval.
Moussa et al. showed that sex is not a risk factor for postoperative PVR (OR = 0.60, 95%CI: 0.26–1.39, P = 0.234). According to the study of Capeans et al., there is no gender difference between the postoperative PVR group and the postoperative non-PVR group, with a male proportion of 50% vs 42%, respectively.
Age. Age was assessed in 7 studies with 1,155 patients. The combined result exhibited that increased age was associated with a significantly higher risk of postoperative PVR (pooled WMD = 3.98, 95%CI: 0.21, 7.75, P = 0.038, I2 = 74.4%) (Fig 2B and Table 2).
Ferrara et al. found that elderly patients showed more severe PVR (grade C and above), especially after 60 years old. Kasetty et al. illustrated a higher incidence of PVR in young patients. Gao et al., Loiudice et al. and Moussa et al. reported that age is not a risk factor for postoperative PVR.
Smoking
As regards smoking, pooled analysis of 2 studies with 143 patients showed that smokers had a significantly higher risk of postoperative PVR than non-smokers (pooled OR = 5.07, 95%CI: 2.21–11.61, P<0.001, I2 = 1.0%) (Fig 2C and Table 2).
Eliott et al. reported that smoking was a risk factor for redetaching due to postoperative PVR (HR = 1.89, 95%CI: 1.13–3.15, P = 0.01).
Disease-related risk factors
Preoperative PVR
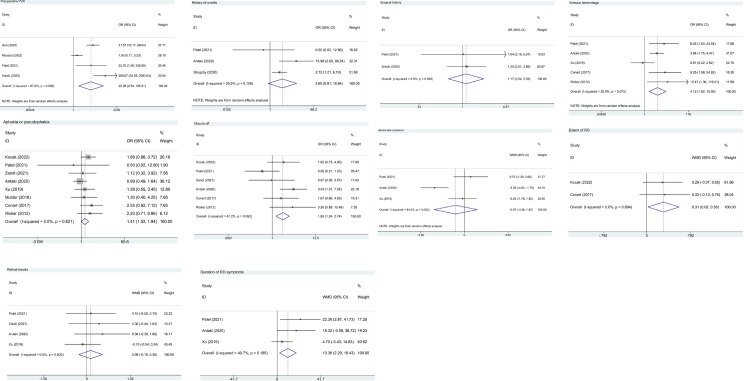
Based on 4 studies of 1,575 patients, presence of preoperative PVR was associated with a significantly greater risk of postoperative PVR (pooled OR = 22.28, 95%CI: 2.54, 195.31, P = 0.005, I2 = 87.8%) (Fig 3A and Table 2).
Fig 3. Forest plot for disease-related risk factors in patients without PVR before RD surgery.
(a) preoperative PVR; (b) history of uveitis; (c) surgical history; (d) vitreous hemorrhage; (e) aphakia or pseudophakia; (f) macula off; (g) intraocular pressure; (h) extent of RD; (i) retinal breaks; (j) duration of RD symptoms. PVR, proliferative vitreoretinopathy; RD, retinal detachment; WMD, weighted mean difference; OR, odds ratio; CI, confidence interval.
The studies of Eliott et al. (HR = 1.75, 95%CI: 1.10, 2.90, P = 0.02), Kon et al. (P<0.05), Duquesne et al. (preoperative PVR grade B: OR = 8.585, P = 0.051; preoperative PVR grade C-D: OR = 50.300, P<0.05), Girard et al. (preoperative PVR grade A: OR = 3.690, P<0.05; preoperative PVR grade B: OR = 1.491, P = 0.047), and Cowley et al. (53.8% vs 11.1%, P<0.01) demonstrated that preoperative PVR was a risk factor for redetaching due to postoperative PVR.
History of uveitis
The data on a history of uveitis were provided by 3 studies with 1,098 patients. The overall result showed no significant difference in the risk of postoperative PVR between patients with and without a history of uveitis (pooled OR = 3.68, 95%CI: 0.81, 16.64, P = 0.091, I2 = 55.5%) (Fig 3B and Table 2).
Girard et al. demonstrated that a history of uveitis was associated with a greater risk of postoperative PVR (OR = 1.656, P = 0.044).
Surgical history
Two studies on 575 patients estimated the risk of postoperative PVR in patients with a history of ophthalmic surgery. A history of ophthalmic surgery was not associated with a greater risk of postoperative PVR (pooled OR = 1.17, 95%CI: 0.54, 2.53, P = 0.696, I2 = 0.0%) (Fig 3C and Table 2).
Pastor et al. (OR = 1.55, 95% CI: 1.14, 9.22), Cowley et al. (P<0.01), and Bonnet et al. (P<0.05) showed that previous ophthalmic surgery was a risk factor for postoperative PVR.
Vitreous hemorrhage
Vitreous hemorrhage was reported in 5 studies with 820 subjects. Presence of vitreous hemorrhage was associated with a significantly greater risk of postoperative PVR (pooled OR = 4.12, 95%CI: 1.62, 10.50, P = 0.003, I2 = 52.9%) (Fig 3D and Table 2).
The study of Duquesne et al. showed that 11.3% of patients with preoperative vitreous hemorrhage and 11.8% of patients without preoperative vitreous hemorrhage developed PVR after surgery, respectively (P = 0.90). Cowley et al. found that severe vitreous hemorrhage was significantly more frequent in the PVR group.
Aphakia or pseudophakia
Eight studies of 1,230 patients demonstrated that individuals with aphakia or pseudophakia had a significantly increased risk of postoperative PVR in contrast to those without (pooled OR = 1.41, 95%CI: 1.02, 1.94, P = 0.040, I2 = 0.0%) (Fig 3E and Table 2).
Pastor et al. illustrated that aphakic or pseudophaki elevated the risk of postoperative PVR (OR = 3.33, 95%CI: 1.54, 7.22). Loiudice et al. found that lens status was not a predictive factor for the development of postoperative PVR (OR = 0.494, 95%CI: 0.035, 7.064). Capeans et al. (10% vs 7%) and Cowley et al. (47.6% vs 42.6%) showed no difference in aphakic or pseudophaki between the postoperative PVR and non-PVR groups.
Macula off
Macula off was assessed in 6 studies with 961 patients. The risk of postoperative PVR was significantly higher among patients with macula off versus those with macula on (pooled OR = 1.85, 95%CI: 1.24, 2.74, P = 0.002, I2 = 47.2%) (Fig 3F and Table 2).
According to Cowley et al., patients with macula off accounted for 84.6% in the postoperative PVR group and 64.6% in the non-PVR group (P = 0.002), indicating that macula off is a risk factor for PVR after RD surgery. Loiudice et al. (OR = 2.13, 95%CI: 0.094, 48.33, P = 0.634), and Moussa et al. (OR = 0.52, 95%CI: 0.15, 1.82, P = 0.309) showed that macula status was not a risk factor for postoperative PVR. Capeans et al. found no difference in macula off between the postoperative PVR and non-PVR groups (30% vs 41%).
Intraocular pressure
Data on intraocular pressure was available in 3 studies of 649 patients. The pooled result indicated no significant association between intraocular pressure and the risk of postoperative PVR (pooled WMD = -0.97, 95%CI: -3.36, 1.42, P = 0.427, I2 = 84.0%) (Fig 3G and Table 2).
Extent of RD
Two studies with 246 patients provided information on extent of RD. Extent of RD in patients with postoperative PVR was significantly larger than that in patients without postoperative PVR (pooled WMD = 0.31, 95%CI: 0.02, 0.59, P = 0.036, I2 = 0.0%) (Fig 3H and Table 2).
Loiudice et al. showed that extent of RD was not a risk factor for postoperative PVR (OR = 0.30, 95%CI: 0.04, 0. 2.26, P = 0.242).
Retinal breaks
Retinal breaks were reported in 4 studies of 714 patients. No significant difference was found in retinal breaks between patients with and without postoperative PVR (pooled WMD = 0.09, 95%CI: -0.19, 0.38, P = 0.523, I2 = 0.0%) (Fig 3I and Table 2).
Loiudice et al. demonstrated that the number of retinal breaks was not associated with PVR after RD surgery (OR = 0.64, 95%CI: 0.52, 2.86, P = 0.643).
Duration of RD symptoms
Three studies with 649 patients evaluated duration of RD symptoms. The overall results showed that patients with postoperative PVR had significantly longer duration of RD symptoms than those without postoperative PVR (pooled WMD = 10.36, 95%CI: 2.29, 18.43, P = 0.012, I2 = 40.7%) (Fig 3J and Table 2).
Sensitivity analysis
Sensitivity analysis was conducted by deleting a study at a time and synthetically assessing the remaining studies. It was found that the pooled results were not affected by one-study deletion, suggesting that the findings of this meta-analysis were stable and robust.
Discussion
This review and meta-analysis first comprehensively evaluated the risk factors for PVR after RD surgery from the perspectives of demographic and disease-related risk factors. It was revealed that age, smoking, preoperative PVR, vitreous hemorrhage, aphakia or pseudophakia, macula off, extent of RD, and duration of RD symptoms were risk factors for postoperative PVR in patients undergoing RD surgery. Combined assessment of demographic and disease-related risk factors may aid in better prediction of the PVR risk following RD surgery. Awareness of these factors in the development of PVR may allow for early lifestyle interventions and careful surgical planning to minimize the risk.
As regards demographic risk factors, older age was associated with a higher risk of PVR after RD surgery. Apart from the included studies that supported the above statement [12,20,26], no other studies are found to support the statement. Future evidence is needed to confirm this finding. A possible explanation for this finding is that increased signaling of pro-inflammatory cytokines in patients with older age can promote the progression of PVR [31,32]. The lack of research on the underlying mechanism necessitates future studies. We also found that smoking was predictive of postoperative PVR formation. Cigarette smoking may influence the integrity of the blood-retinal barrier since the breakdown of the blood-retinal barrier was considered to be related to the formation of PVR [33]. Afterwards, serum components (such as fibronectin and platelet-derived growth factor) invaded the vitreous and accelerated the migration and proliferation of retinal pigment epithelial cells released into the vitreous after RD. Additionally, smoking was correlated with the development of uveitis and retinal neovascularization [34,35]. Wang et al. [36] indicated that the increase of pro-inflammatory cytokine signaling could play an essential role in the development of PVR in smokers. More investigations are needed to clarify the role of smoking in the risk of PVR after RD surgery. Although smoking was reported to be a risk factor for eye diseases [37], a small number of smokers (about 10–13%) realized that smoking can cause visual impairment [38]. In optometric practice, nearly 45% of optometrists did not evaluate patients’ smoking status at the initial visit and 8% of the optometrists never updated patients’ smoking status [39]. Besides, 2.3% of optometrists never explained the adverse effect of smoking on eye health to patients [39]. Thus, it is very important for vitreoretinal subspecialists, comprehensive ophthalmologists, and optometrists who provide primary eye care to highlight the negative impact of smoking. Eye care professionals should provide smoking cessation programs for patients undergoing RD surgery. Quitting smoking after diagnosis of RD should be advocated as a potential timely intervention for this public health concern.
With respect to disease-related risk factors, patients with preoperative PVR, vitreous hemorrhage, aphakia or pseudophakia, macula off, large extent of RD, and long duration of RD symptoms had an elevated risk of postoperative PVR. Scare evidence has been provided on the role of preoperative PVR in such a setting. Bonnet et al. [6] illustrated that preoperative PVR and vitreous hemorrhage were correlated with a significantly higher incidence of postoperative PVR. According to a prior review, individuals with vitreous hemorrhage had high odds of many diseases, including PVR [40]. We assume that the association between vitreous hemorrhage and postoperative PVR is attributed to high levels of cytokines. Preoperative aqueous flare values may increase as extent of RD becomes large [41], and the flare values may predict PVR re-detachment [17], which may contribute to the association between extent of RD and the risk of postoperative PVR. As regards the relationship between duration of RD symptoms and postoperative PVR, we speculated that long duration of RD symptoms provides time for retinal pigment epithelium cells to migrate, proliferate and generate extracellular matrix, resulting in contracted membrane on the anterior or lower surface of the retina, fixed retinal folds and retinal traction [42]. Concerning the associations of aphakia or pseudophakia and macula off with the risk of PVR following RD surgery, more investigations are warranted to confirm and elaborate on these associations.
This work may act as a reference for vitreoretinal surgeons, and the identified evidence may help the standardization of clinical practice, with more effective management bringing improved outcomes for patients following RD surgery. Given the risk factors, patients should pay attention to and practice smoking cessation and eye care to prevent and lower the risk of postoperative PVR. Targeted screening strategies and interventions could be developed for individuals at a high risk of PVR after RD surgery. Some limitations of this study should be noted. First, the results of combined analysis may be unstable due to the existence of heterogeneity. Second, confounding factors were not taken into account when evaluating these risk factors. Third, the study only included English literature, which may cause language bias. Besides, of 22 included studies, 9 studies [5,9,11,12,15,17,19,20,22] reported a mixture of vitrectomy and scleral buckling (SB) (and, in 2 studies [15,20], other types of surgery), 1 study [16] reported SB and pneumatic retinopexy, and 1 study [7] did not report the type of surgery. Subgroup analysis could not be performed based on the type of surgery (vitrectomy, SB). The aggressiveness and progression of PVR in post vitrectomy and post SB can be different. Pars plana vitrectomy (PPV) plus SB was associated with a higher risk of PVR re-detachment than PPV alone [11]. Additionally, the surgical method may vary among patients with different demographic characteristics. For example, 73% older patients with rhegmatogenous retinal detachment underwent PPV [43]. Thus, pooled evaluation of PVR in all PPV/SB cases may increase the heterogeneity of this study, which further affect the stability of the results. For another, the results of this pooled evaluation may be applicable to patients undergoing either vitrectomy or SB. Future research is needed for verification regarding heterogeneity and applicability. Furthermore, since most studies reported admixed surgical population (some PPV, some SB in each study) and original data among these studies could not be obtained, separate analysis regarding only cases undergoing micro-incision vitrectomy surgery (MIVS) seems not feasible, which requires more explorations.
Conclusion
Age, smoking, preoperative PVR, vitreous hemorrhage, aphakia or pseudophakia, macula off, extent of RD, and duration of RD symptoms were risk factors for PVR following RD surgery. Future studies are warranted to support our findings.
Supporting information
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was funded by the project “Histone deacetylase HDAC4 promotes retinal pigment epithelium inflammation and mitochondrial dysfunction in high glucose induced diabetes retinopathy (YNKT202207)” received by Jinjin Xiang. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Garweg JG, Tappeiner C, Halberstadt M. Pathophysiology of proliferative vitreoretinopathy in retinal detachment. Survey of ophthalmology. 2013;58(4):321–9. Epub 2013/05/07. doi: 10.1016/j.survophthal.2012.12.004 . [DOI] [PubMed] [Google Scholar]
- 2.Sadaka A, Giuliari GP. Proliferative vitreoretinopathy: current and emerging treatments. Clinical ophthalmology (Auckland, NZ). 2012;6:1325–33. Epub 2012/09/04. doi: 10.2147/OPTH.S27896 ; PubMed Central PMCID: PMC3429288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaub F, Abdullatif AM, Fauser S. [Proliferative vitreoretinopathy prophylaxis: Mission (im)possible]. Der Ophthalmologe: Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2021;118(1):3–9. Epub 2020/07/16. doi: 10.1007/s00347-020-01173-8 . [DOI] [PubMed] [Google Scholar]
- 4.Chiquet C, Rouberol F. [Proliferative vitreoretinopathy: curative treatment]. Journal francais d’ophtalmologie. 2014;37(8):653–9. Epub 2014/07/07. doi: 10.1016/j.jfo.2014.04.002 . [DOI] [PubMed] [Google Scholar]
- 5.Xu K, Chin EK, Bennett SR, Williams DF, Ryan EH, Dev S, et al. PREDICTIVE FACTORS FOR PROLIFERATIVE VITREORETINOPATHY FORMATION AFTER UNCOMPLICATED PRIMARY RETINAL DETACHMENT REPAIR. Retina (Philadelphia, Pa). 2019;39(8):1488–95. Epub 2018/05/23. doi: 10.1097/IAE.0000000000002184 . [DOI] [PubMed] [Google Scholar]
- 6.Bonnet M. The development of severe proliferative vitreoretinopathy after retinal detachment surgery. Grade B: a determining risk factor. Graefe’s archive for clinical and experimental ophthalmology=Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1988;226(3):201–5. Epub 1988/01/01. doi: 10.1007/bf02181181 . [DOI] [PubMed] [Google Scholar]
- 7.Slingsby TJ, Pecen PE, Palestine AG. Outcomes and Complications Associated with Noninfectious Uveitis in Patients Presenting with Rhegmatogenous Retinal Detachment. Ophthalmology Retina. 2020;4(8):823–8. Epub 2020/04/21. doi: 10.1016/j.oret.2020.02.010 . [DOI] [PubMed] [Google Scholar]
- 8.Hooymans JM, De Lavalette VW, Oey AG. Formation of proliferative vitreoretinopathy in primary rhegmatogenous retinal detachment. Doc Ophthalmol. 2000;100(1):39–42. Epub 2000/12/16. doi: 10.1023/a:1002428928803. . [DOI] [PubMed] [Google Scholar]
- 9.Girard P, Mimoun G, Karpouzas I, Montefiore G. Clinical risk factors for proliferative vitreoretinopathy after retinal detachment surgery. Retina (Philadelphia, Pa). 1994;14(5):417–24. Epub 1994/01/01. doi: 10.1097/00006982-199414050-00005 . [DOI] [PubMed] [Google Scholar]
- 10.Chaudhary R, Dretzke J, Scott R, Logan A, Blanch R. Clinical and surgical risk factors in the development of proliferative vitreoretinopathy following retinal detachment surgery: a systematic review protocol. Syst Rev. 2016;5(1):107. Epub 2016/07/09. doi: 10.1186/s13643-016-0284-7 ; PubMed Central PMCID: PMC4939038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliott D, Stryjewski TP, Andreoli MT, Andreoli CM. SMOKING IS A RISK FACTOR FOR PROLIFERATIVE VITREORETINOPATHY AFTER TRAUMATIC RETINAL DETACHMENT. Retina (Philadelphia, Pa). 2017;37(7):1229–35. Epub 2016/11/02. doi: 10.1097/IAE.0000000000001361 . [DOI] [PubMed] [Google Scholar]
- 12.Pastor JC, de la Rua ER, Aragon J, Mayo-Iscar A, Martinez V, Garcia-Arumi J, et al. Interaction between surgical procedure for repairing retinal detachment and clinical risk factors for proliferative vitreoretinopathy. Current Eye Research. 2005;30(2):147–53. doi: 10.1080/02713680490904142. PubMed PMID: WOS:000228182000008. [DOI] [PubMed] [Google Scholar]
- 13.Wells GA, Shea BJ, O’Connell D, Peterson J, Tugwell P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. [Google Scholar]
- 14.Antaki F, Kahwati G, Sebag J, Coussa RG, Fanous A, Duval R, et al. Predictive modeling of proliferative vitreoretinopathy using automated machine learning by ophthalmologists without coding experience. Scientific reports. 2020;10(1):19528. Epub 2020/11/13. doi: 10.1038/s41598-020-76665-3 ; PubMed Central PMCID: PMC7658348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonnet M. CLINICAL FACTORS PREDISPOSING TO MASSIVE PROLIFERATIVE VITREORETINOPATHY IN RHEGMATOGENOUS RETINAL-DETACHMENT. Ophthalmologica. 1984;188(3):148–52. doi: 10.1159/000309357. PubMed PMID: WOS:A1984SH26500004. [DOI] [PubMed] [Google Scholar]
- 16.Capeans C, Lorenzo J, Santos L, Suarez A, Copena MJ, Blanco MJ, et al. Comparative study of incomplete posterior vitreous detachment as a risk factor for proliferative vitreoretinopathy. Graefe’s archive for clinical and experimental ophthalmology=Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1998;236(7):481–5. Epub 1998/07/22. doi: 10.1007/s004170050109 . [DOI] [PubMed] [Google Scholar]
- 17.Conart JB, Kurun S, Ameloot F, Tréchot F, Leroy B, Berrod JP. Validity of aqueous flare measurement in predicting proliferative vitreoretinopathy in patients with rhegmatogenous retinal detachment. Acta ophthalmologica. 2017;95(4):e278–e83. Epub 2016/09/30. doi: 10.1111/aos.13254 . [DOI] [PubMed] [Google Scholar]
- 18.Cowley M, Conway BP, Campochiaro PA, Kaiser D, Gaskin H. Clinical risk factors for proliferative vitreoretinopathy. Archives of ophthalmology (Chicago, Ill: 1960). 1989;107(8):1147–51. Epub 1989/08/01. doi: 10.1001/archopht.1989.01070020213027 . [DOI] [PubMed] [Google Scholar]
- 19.Duquesne N, Bonnet M, Adeleine P. Preoperative vitreous hemorrhage associated with rhegmatogenous retinal detachment: a risk factor for postoperative proliferative vitreoretinopathy? Graefe’s archive for clinical and experimental ophthalmology=Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 1996;234(11):677–82. Epub 1996/11/01. doi: 10.1007/BF00292353 . [DOI] [PubMed] [Google Scholar]
- 20.Ferrara M, Al-Zubaidy M, Song A, Avery P, Laidlaw DA, Williamson TH, et al. The effect of age on phenotype of primary rhegmatogenous retinal detachment. Eye (London, England). 2022. Epub 2022/04/28. doi: 10.1038/s41433-022-02061-y . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kon CH, Occleston NL, Aylward GW, Khaw PT. Expression of vitreous cytokines in proliferative vitreoretinopathy: a prospective study. Investigative ophthalmology & visual science. 1999;40(3):705–12. Epub 1999/03/06. . [PubMed] [Google Scholar]
- 22.Mulder VC, van Dijk EHC, van Meurs IA, La Heij EC, van Meurs JC. Postoperative aqueous humour flare as a surrogate marker for proliferative vitreoretinopathy development. Acta ophthalmologica. 2018;96(2):192–6. Epub 2017/10/27. doi: 10.1111/aos.13560 . [DOI] [PubMed] [Google Scholar]
- 23.Patel SN, Salabati M, Mahmoudzadeh R, Obeid A, Kuriyan AE, Yonekawa Y, et al. SURGICAL FAILURES AFTER PRIMARY SCLERAL BUCKLING FOR RHEGMATOGENOUS RETINAL DETACHMENT: Comparison of Eyes With and Without Proliferative Vitreoretinopathy. Retina (Philadelphia, Pa). 2021;41(11):2288–95. Epub 2021/05/19. doi: 10.1097/IAE.0000000000003214 . [DOI] [PubMed] [Google Scholar]
- 24.Ricker L, Kessels AGH, de Jager W, Hendrikse F, Kijlstra A, la Heij EC. Prediction of Proliferative Vitreoretinopathy after Retinal Detachment Surgery: Potential of Biomarker Profiling. American Journal of Ophthalmology. 2012;154(2):347–54. doi: 10.1016/j.ajo.2012.02.004. PubMed PMID: WOS:000307152700019. [DOI] [PubMed] [Google Scholar]
- 25.Zandi S, Pfister IB, Garweg JG. Postoperative proliferative vitreoretinopathy development is linked to vitreal CXCL5 concentrations. Scientific reports. 2021;11(1):23989. Epub 2021/12/16. doi: 10.1038/s41598-021-03294-9 ; PubMed Central PMCID: PMC8671512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasetty VM, Aye J, Patel N, Tripathi N, Hessburg T, Kumar N, et al. Outcomes and complications of primary rhegmatogenous retinal detachment repair with pars plana vitrectomy in young adults. International Journal of Retina and Vitreous. 2023;9(1):11. doi: 10.1186/s40942-023-00448-x ; PubMed Central PMCID: PMC9948360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao ZQ, Wu PY, Zhang J, Ke ZS, Hu XT, Zhang ZL, et al. Nomogram for predicting non-proliferative vitreoretinopathy probability after vitrectomy in eyes with rhegmatogenous retinal detachment. International Journal of Ophthalmology. 2023;16(2):215–23. doi: 10.18240/ijo.2023.02.07 ; PubMed Central PMCID: PMC9922634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koçak N, Erduran B, Yeter V. Predictive values of systemic inflammation biomarkers in proliferative vitreoretinopathy associated with primary rhegmatogenous retinal detachment. Clinical & Experimental Optometry. 2022;1–7. Epub 2022/11/14. doi: 10.1080/08164622.2022.2133596 . [DOI] [PubMed] [Google Scholar]
- 29.Loiudice P, Pintor ES, Tronci C, Tatti F, Casini G, Figus M, et al. Safety and efficacy of cryopexy during pars Plana vitrectomy in rhegmatogenous retinal detachment. European Journal of Ophthalmology. 2023;11206721231166976. Epub 2023/03/28. doi: 10.1177/11206721231166976 . [DOI] [PubMed] [Google Scholar]
- 30.Moussa G, Tadros M, Ch’ng SW, Sharma A, Lett KS, Mitra A, et al. Outcomes of Heavy Silicone Oil (Densiron) compared to Silicone Oil in primary rhegmatogenous retinal detachment: a multivariable regression model. International Journal of Retina and Vitreous. 2022;8(1):61. doi: 10.1186/s40942-022-00413-0 ; PubMed Central PMCID: PMC9440647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Frontiers in immunology. 2018;9:586. Epub 2018/04/25. doi: 10.3389/fimmu.2018.00586 ; PubMed Central PMCID: PMC5900450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruunsgaard H, Pedersen M, Pedersen BK. Aging and proinflammatory cytokines. Current opinion in hematology. 2001;8(3):131–6. Epub 2001/04/17. doi: 10.1097/00062752-200105000-00001 . [DOI] [PubMed] [Google Scholar]
- 33.Nagasaki H, Shinagawa K, Mochizuki M. Risk factors for proliferative vitreoretinopathy. Progress in retinal and eye research. 1998;17(1):77–98. Epub 1998/04/16. doi: 10.1016/s1350-9462(97)00007-4 . [DOI] [PubMed] [Google Scholar]
- 34.Yuen BG, Tham VM, Browne EN, Weinrib R, Borkar DS, Parker JV, et al. Association between Smoking and Uveitis: Results from the Pacific Ocular Inflammation Study. Ophthalmology. 2015;122(6):1257–61. Epub 2015/04/04. doi: 10.1016/j.ophtha.2015.02.034 ; PubMed Central PMCID: PMC4446169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel AK, Newcomb CW, Liesegang TL, Pujari SS, Suhler EB, Thorne JE, et al. Risk of Retinal Neovascularization in Cases of Uveitis. Ophthalmology. 2016;123(3):646–54. Epub 2015/12/22. doi: 10.1016/j.ophtha.2015.10.056 ; PubMed Central PMCID: PMC4766036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang V, Heffer A, Roztocil E, Feldon SE, Libby RT, Woeller CF, et al. TNF-α and NF-κB signaling play a critical role in cigarette smoke-induced epithelial-mesenchymal transition of retinal pigment epithelial cells in proliferative vitreoretinopathy. PloS one. 2022;17(9):e0271950. Epub 2022/09/02. doi: 10.1371/journal.pone.0271950 ; PubMed Central PMCID: PMC9436090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galor A, Lee DJ. Effects of smoking on ocular health. Current opinion in ophthalmology. 2011;22(6):477–82. Epub 2011/09/08. doi: 10.1097/ICU.0b013e32834bbe7a . [DOI] [PubMed] [Google Scholar]
- 38.Kennedy RD, Spafford MM, Parkinson CM, Fong GT. Knowledge about the relationship between smoking and blindness in Canada, the United States, the United Kingdom, and Australia: results from the International Tobacco Control Four-Country Project. Optometry (St Louis, Mo). 2011;82(5):310–7. Epub 2011/04/29. doi: 10.1016/j.optm.2010.10.014 ; PubMed Central PMCID: PMC4528643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy RD, Spafford MM, Douglas O, Brûlé J, Hammond D, Fong GT, et al. Patient tobacco use in optometric practice: a Canada-wide study. Optometry and vision science: official publication of the American Academy of Optometry. 2014;91(7):769–77. Epub 2014/06/14. doi: 10.1097/opx.0000000000000303 . [DOI] [PubMed] [Google Scholar]
- 40.Shaikh N, Srishti R, Khanum A, Thirumalesh MB, Dave V, Arora A, et al. Vitreous hemorrhage ‐ Causes, diagnosis, and management. Indian journal of ophthalmology. 2023;71(1):28–38. Epub 2023/01/03. doi: 10.4103/ijo.IJO_928_22 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshi S, Okamoto F, Hasegawa Y, Sugiura Y, Okamoto Y, Hiraoka T, et al. Time course of changes in aqueous flare intensity after vitrectomy for rhegmatogenous retinal detachment. Retina. 2012;32(9):1862–7. doi: 10.1097/IAE.0b013e3182456f38 . [DOI] [PubMed] [Google Scholar]
- 42.Tseng W, Cortez RT, Ramirez G, Stinnett S, Jaffe GJ. Prevalence and risk factors for proliferative vitreoretinopathy in eyes with rhegmatogenous retinal detachment but no previous vitreoretinal surgery. American Journal of Ophthalmology. 2004;137(6):1105–15. doi: 10.1016/j.ajo.2004.02.008 . [DOI] [PubMed] [Google Scholar]
- 43.Patel SN, Starr MR, Obeid A, Ryan EH, Ryan C, Forbes NJ, et al. CHARACTERISTICS AND SURGICAL OUTCOMES OF RHEGMATOGENOUS RETINAL DETACHMENT IN OLDER ADULTS: A Multicenter Comparative Cohort Study. Retina. 2021;41(5):947–56. doi: 10.1097/IAE.0000000000002969 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.