Abstract
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of ovine pulmonary adenocarcinoma (OPA), a transmissible lung cancer of sheep. The virus can induce tumors rapidly, and we previously found that the JSRV envelope protein (Env) functions as an oncogene, because it can transform mammalian and avian fibroblast cell lines. (N. Maeda, Proc. Natl. Acad. Sci. USA 98:4449-4454, 2001). The molecular mechanisms of JSRV Env transformation are of considerable interest. Several reports suggested that the phosphatidylinositol 3-kinase/Akt pathway is important for transformation of mammalian fibroblasts but not for chicken fibroblasts. In this study, we found that Akt/mTOR is involved in JSRV transformation of mouse NIH 3T3 fibroblasts, because treatment with the mTOR inhibitor rapamycin reduced transformation. We also found that H/N-Ras inhibitor FTI-277 and MEK1/2 inhibitors PD98059 and U0126 strongly inhibited JSRV transformation of NIH 3T3 fibroblasts, suggesting that the H/N-Ras-MEK-mitogen-activated protein kinase (MAPK) p44/42 pathway is necessary for the transformation. In RK3E epithelial cells, the MEK1/2 inhibitors also eliminated transformation, but FTI-277 only partially inhibited transformation. It was noteworthy that p38 MAPK inhibitors enhanced JSRV transformation in both fibroblasts and epithelial cells. Treatment of transformed cells with p38 inhibitors both increased levels of phospho-MEK1/2 and phospho-p44/42 and induced rapid enhancement of the transformed phenotype. Immunohistochemical staining of tumor tissues from naturally and experimentally induced OPA and naturally occurring enzootic nasal adenocarcinoma revealed strong activation of MAPK p44/42 in all cases examined. However, p38 activation was not generally observed. These results indicate that signaling through two pathways (in particular, H/N-Ras-MEK-MAPK and, to a lesser extent, Akt-mTOR) is important for JSRV-induced transformation and that p38 MAPK has a negative regulatory effect on transformation, perhaps via MEK1/2 and p44/42.
Jaagsiekte sheep retrovirus (JSRV) is the causative agent of ovine pulmonary adenocarcinoma (OPA), a transmissible lung cancer in sheep (14). JSRV induces tumors rapidly under experimental conditions (28), and we previously showed that the JSRV envelope protein functions as an oncogene, in that it can morphologically transform mouse NIH 3T3 and rat Rat 208F fibroblasts in vitro (24, 34). The mechanism(s) by which JSRV Env can cause cell transformation is of great interest. We also previously showed that the cytoplasmic tail of the envelope transmembrane (TM) protein is necessary for fibroblast transformation (29). In particular, a putative docking site for phosphatidylinositol 3-kinase (PI3K) in the cytoplasmic tail was implicated, because mutations in this motif (YXXM) eliminated transformation. On the other hand, we subsequently found that disabling PI3K signaling in cells does not prevent JSRV transformation, although in such cells the downstream effector molecule Akt/protein kinase B (PKB) was still phosphorylated (25). Also, Akt is not absolutely required for JSRV transformation of chicken embryo fibroblasts (40). Thus, the role of the PI3K-Akt pathway in JSRV transformation is unclear, although downstream parts of the pathway in particular might be involved.
Akt is known to phosphorylate several downstream substrates that are involved in several signaling pathways associated with human cancers (5). Phosphorylation substrates of Akt include glycogen synthase kinase 3 (GSK-3), the Forkhead transcription factor, the proapoptotic Bad protein, and the mammalian target of rapamycin (mTOR). Akt phosphorylation of GSK-3β, Forkhead, and Bad inactivates these proteins, while phosphorylation of mTOR activates it. In particular, the mammalian target of rapamycin (mTOR) is an important downstream effector of Akt. mTOR (also known as FK506-binding protein [FKBP12], rapamycin-associated protein [FRAP], rapamycin and FKBP12 target [RAFT1], rapamycin target [RAPT1], and sirolimus effector protein [SEP]) is a 289-kDa serine/threonine kinase that is an ortholog of the Saccharomyces cerevisiae target of rapamycin 1 (TOR1) and TOR2 (8, 35). mTOR is a key regulator of cell growth and division at the level of translation (17) by activating (phosphorylating) p70 S6 kinase and phosphorylating (inactivating) 4EBP1, a negative regulatory binding protein of translation initiation factor eIF4G (32). The mTOR inhibitor rapamycin was first identified as an antifungal agent (36, 37). Its specificity for mTOR makes it a good probe for the role of Akt and downstream effectors in various biological processes. Rapamycin has antitumor activity, and clinical trials of rapamycin analogs for treatment of hematologic malignancies are in progress (30). Additionally, Akt has been captured by the acute transforming retrovirus AKT-8 murine leukemia virus (4). The v-Akt protein is myristoylated, and thus, it is constitutively anchored in the plasma membrane where it can constitutively signal through the Akt pathway. In chicken embryo fibroblasts, transformation by v-Akt or an oncogenic form of PI3K (v-P3k) is completely inhibited by rapamycin (2), which indicates that signaling through mTOR is essential for transformation of these cells. Rapamycin does not affect transformation by many other viral or cellular oncogenes, and it can enhance transformation by some (2).
Mitogen-activated protein kinases (MAPKs) are another important group of effector serine/threonine protein kinases in signal transduction. MAPKs are generally grouped into four subfamilies: (i) extracellular signal-regulated kinase 1 (ERK1) and ERK2 (referred to as ERK1/2, also called p44/42), (ii) stress-activated protein kinase/c-Jun NH2-terminal kinase 1 (SAPK/JNK1), SAPK/JNK2, and SAPK/JNK3, (iii) p38 MAPKs p38α, p38β, p38γ, and p38δ, and (iv) big mitogen-activated protein kinase 1 (BMK1)/ERK5. MAPKs are regulated and activated via phosphorylation by MAPK kinases (MAPKKs), and the MAPKKs are in turn activated by MAPKK kinases (MAPKKKs) (20). Several MAPKKK-MAPKK-MAPK pathways have been characterized extensively. The Raf/Mos-MEK1/2-ERK1/2 pathway is activated by stimuli for cell growth or differentiation (e.g., epidermal growth factor [EGF]) (3). The MLK3-MKK3/6-p38α/β/γ/δ and MEKK1/4/ASK1-MKK4/7-SAPK/JNK1/2/3 pathways are activated by oxidative stress, UV radiation, or inflammatory cytokines (interleukin-1 or tumor necrosis factor α) (12). The BMK1/ERK5 pathway has only recently been identified (42). These enzymes in these cascades are specifically regulated by phosphorylation of conserved tyrosine, serine, or threonine residues; ultimately, the cascades result in the translocation of MAPKs to the nucleus, where they phosphorylate and activate specific transcription factors. ERK1/2, p38, and SAPK/JNK typically phosphorylate Elk-1, ATF-2, and c-Jun, respectively (41). In addition, MAPKs can also phosphorylate other nuclear and cytoplasmic proteins and change their activities, e.g., ERK1/2 phosphorylation of ribosomal S6 kinase (16). MAPKKs and MAPKs also are inactivated by dephosphorylation by specific protein phosphatases. Thus, the balance between phosphorylation and dephosphorylation regulates signaling through these pathways.
The Ras proteins are small guanine nucleoside binding proteins that regulate multiple signal transduction pathways (6). They cycle between active (GTP-bound) and inactive (GDP-bound) forms by way of intrinsic Ras GTPase activity as well as by association with cellular regulatory proteins (guanine nucleotide exchange factors [GEFs] and GTPase-activating proteins [GAPs]). Approximately 30% of human cancers contain mutated Ras genes. The activated Ras genes constitutively signal for growth and/or cell division. In particular, the Raf/Mos-MEK1/2-ERK1/2 pathway is downstream of Ras (7, 18). In addition to constitutive MEK1/2-ERK1/2 signaling, oncogenic Ras transiently activates the p38 and SAPK/JNK pathways that typically trigger apoptosis or inflammation. Thus, oncogenic Ras may provide both positive and negative stimulatory signals to cells; the transformed phenotype presumably results from integration of these positive and negative signals as well as cross-talk between the pathways.
Signal transduction pathways may differ in cells derived from different tissue types. Because JSRV induces tumors of secretory lung epithelial cells, signaling in epithelial cells is of great interest. Recently, Danilkovitch-Miagkova et al. reported JSRV transformation of a human lung epithelial cell line, and they noted activation of both the PI3K-Akt pathway and also the Ras-MEK-MAPK pathway (10).
In this report, we have examined the roles of the Akt-mTOR and H/N-Ras-MEK-MAPK pathways in JSRV-transformed rodent fibroblast and epithelial cell lines. The results indicated the involvement of both pathways in transformation, and that the H/N-Ras-MEK-MAPK pathway appears to play a more important role. The results also indicated that JSRV-induced p38 MAPK negatively regulates transformation.
MATERIALS AND METHODS
Cells and transfection.
Mouse NIH 3T3 cells and rat Rat6 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Rat RK3E cells were grown in DMEM supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin.
Transformation assays were performed as described previously (24). Briefly, cell were seeded at 8 × 105 cells per 10-cm-diameter tissue culture dish or at 5 × 105 cells per 6-cm-diameter dish. After overnight incubation, transfections with 28 μg of plasmid DNA (for 10-cm dishes) or 5 μg of DNA (for 6-cm dishes) were performed as described previously (24) using the CalPhos Mammalian Transfection kit (Clontech) or FuGENE 6 Transfection Reagent (Roche). The cells were cultured for up to 4 weeks posttransfection, with medium changes every 3 days. Dishes were examined under phase-contrast microscopy at various days posttransfection, and the number of transformed foci were recorded. To inhibit focus formation in NIH 3T3 cells, Rat6 cells, and RK3E cells, FTI-277, PD98059, U0126, SB203580, SB202190, SP600125, and rapamycin (Calbiochem) were added at every medium change at the concentrations indicated. Where indicated, inhibitors were added daily.
Plasmid constructs.
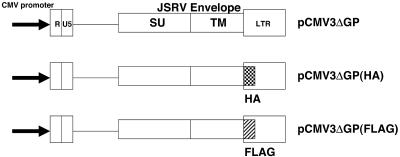
The pCMV3ΔGP expression plasmid for JSRV envelope protein and its hemagglutinin (HA) tag version were described previously (25) (Fig. 1). A pCMV3ΔGP derivative containing a C-terminal FLAG tag was made by using the same procedure as that for the HA-tagged construct. pCMV(neo)MEK-KA(101A) was generously provided by Channing Der, pMKP-1 was provided by Nicholas Tonks, a carboxyl-terminal modulator protein (CTMP) expression plasmid was provided by Brian Hemmings, and a v-akt expression plasmid was provided by Peter Vogt.
FIG. 1.
Plasmids used in this study. The plasmids are described in Materials and Methods. pCMV3ΔGP expresses the JSRV envelope from a cytomegalovirus promoter. The HA tag (checkered box) and FLAG-tag (cross-hatched box) are inserted in wild-type pCMV3ΔGP at the C terminus of the env reading frame.
MAPK assays.
In vitro assays for p44/42 (ERK) MAPK were performed with the p44/42 MAPK Assay kit (Cell Signaling) according to the manufacturer's instructions. Briefly, 5 × 105 parental or transformed cells were seeded and cultured. Twenty-four hours after seeding, cells were serum starved for 20 to 24 h and lysed with cell lysis buffer (20 mM Tris-Cl [pH 7.5], 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 μg of leupeptin/ml) for 1 h. The cell lysate was mixed with immobilized phopsho-p44/42 MAPK (Thr202/Tyr204) monoclonal antibody and incubated at 4°C overnight. The complex was washed with the cell lysis buffer twice and with kinase buffer (25 mM Tris-Cl [pH 7.5], 5 mM β-glycerolphosphate, 2 mM dithiothreitol, 0.1 mM Na3VO4, 10 mM MgCl2) twice and was incubated with 200 μM ATP and 2 μg of Elk-1 fusion protein in kinase buffer at 30°C for 30 min. The reaction was terminated by adding sodium dodecyl sulfate (SDS) sample buffer (0.35 M Tris-HCl [pH 6.8], 10.28% [wt/vol] SDS, 36% [vol/vol] glycerol, 5% β-mercaptoethanol, 0.012% [wt/vol] bromophenol blue) and boiling for 5 min. The supernatant was collected after centrifugation for 1 min in a microcentrifuge and was used for SDS-polyacrylamide gel electrophoresis (PAGE).
EGF treatment.
Recombinant epidermal growth factor (EGF) (R&D Systems) was used to stimulate cells and activate the Ras-MEK-MAPK pathway. Cells were serum starved for 20 to 24 h and stimulated with 100 ng of EGF/ml for 30 min. Cell lysates were subjected to SDS-PAGE and immunoblot analysis along with other samples.
Immunoblotting.
Cells were lysed with Triton lysis buffer (50 mM Tris-Cl [pH 7.4], 150 mM NaCl, 1% Triton X-100, 10% glycerol, and phosphatase inhibitor cocktail 2 [Sigma]) for 30 min. Protein samples (10 to 30 μg per sample) were subjected to SDS-PAGE and immunoblot analysis. To detect an HA or FLAG epitope, anti-HA-tag polyclonal antibody (Sigma) or anti-FLAG-tag polyclonal antibody (Sigma) was used as a first antibody (0.1 μg/ml), respectively. Antibodies against p44/42 MAPK, phospho-p44/42 MAPK (Thr202/Tyr204), MEK1/2, phospho-MEK1/2 (Ser217/221), p38 MAPK, phospho-p38 MAPK (Thr180/Tyr182), SAPK/JNK, and phospho-SAPK/JNK (Thr183/Tyr185) (Cell Signaling) were used as primary antibodies. Secondary antibodies appropriate for the species of the primary antibodies were then used. We used rabbit anti-sheep (DAKO), goat anti-mouse (Caltag Laboratories), or goat anti-rabbit (Pierce) immunoglobulin G (IgG) conjugated with horseradish peroxidase as secondary antibodies. Blots were visualized by the SuperSignal West Pico Chemiluminescent Substrate (Pierce).
IHC staining.
Tumor sections from paraffin-embedded tissues from three naturally occurring cases of OPA, three from experimentally induced OPA with lung fluid, and three tumors from naturally occurring enzootic nasal adenocarcinoma (ENA) were used in the immunohistochemical (IHC) study. Normal lung and nasal mucosa tissues from disease-free sheep were used as negative controls. The IHC procedure was performed with the VECTASTAIN ABC standard peroxidase kit (Vector Laboratories) according to instructions by the manufacturer. Previously, all sections were treated by a routine unmasking treatment with citrate buffer 6.0 and immersed in methanol containing 1% hydrogen peroxide. Antibodies against phospho-p44/42 MAPK (Thr202/Tyr204), phospho-p38 MAPK (Thr180/Tyr182), and phospho-SAPK/JNK (Thr183/Tyr185) (Cell Signaling) were used as primary antibodies, respectively. Primary antibodies were replaced by IHC Tris-buffered saline buffer or normal rabbit serum as a negative controls. Finally, sections were counterstained with Carazzi′s hematoxylin.
RESULTS
Role of Akt and mTOR in JSRV transformation of rodent fibroblasts.
We previously reported that Akt is activated (phosphorylated) in JSRV-transformed NIH 3T3 fibroblasts, even though PI3K is not required for transformation (25). To test if Akt activation is necessary for JSRV transformation, we cotransfected NIH 3T3 cells with the JSRV Env expression plasmid pCMV3ΔGP along with an expression plasmid for the negative regulator of Akt, CTMP. Cotransfection with CTMP showed modest (ca. 22%) inhibition of JSRV-induced transformation, while it had no effect on transformation by other viral or activated cellular oncogenes (v-mos or H-ras) (data not shown). This may have been an underestimate of the inhibitory effect, because some cells may have only taken up pCMV3ΔGP.
Another approach to investigating the role of Akt in JSRV transformation would be to study downstream substrates of Akt phosphorylation. Liu et al. previously reported that GSK-3β is phosphorylated in JSRV-transformed rat 208F cells (22), and we have similarly observed GSK-3β phosphorylation in transformed NIH 3T3 cells (Y. Inoshima, N. Maeda, and H. Fan, unpublished data). A potential role for GSK-3β in JSRV transformation remains to be demonstrated. To test if signaling through AKT/mTOR is involved in JSRV transformation, we carried out NIH 3T3 transformation assays in the presence of 10 or 20 ng of rapamycin/ml as shown in Table 1. Rapamycin inhibited JSRV transformation by 42 to 47% while it had no effect on activated H-ras transformation, and it enhanced transformation by v-mos. These results indicated that the Akt/mTOR pathway contributes to JSRV transformation. However, because inhibition of this pathway did not eliminate transformation, other signaling pathways must also be involved.
TABLE 1.
Effect of rapamycin on transformation of NIH 3T3 cellsa
| Inhibitor or DNA | No. of foci in:
|
|||||
|---|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | ||||
| Rapamycin (ng/ml) | 0 | 10 | 0 | 20 | 0 | 20 |
| Transfected DNA | ||||||
| None | 0 | 0 | 0 | 0 | 0 | 0 |
| pcDNA3.1 | 0 | 0 | 0 | 0 | 0 | 0 |
| pCMV3ΔGP | 110 | 59 | 59 | 31 | 48 | 28 |
| H-ras | 88 | 90 | 37 | 48 | 80 | 76 |
| v-mos | 26 | 41 | NTb | NT | 44 | 88 |
NIH 3T3 fibroblasts were transfected with the 28-μg plasmid DNAs as described in Materials and Methods, and the number of transformed foci were scored at 24 to 27 days posttransfection. Rapamycin was added daily at the concentrations indicated. The numbers of foci are shown for each 10-cm-diameter dish. H-ras and v-mos are expression plasmids for activated H-ras and MSV v-mos genes, respectively.
NT, not tested.
Role of the Ras-MEK-MAPK pathway in fibroblast transformation.
Because the results shown in Table 1 suggested the involvement of an additional pathway(s) in JSRV transformation, we considered the possible involvement of the Ras-MEK-MAPK pathway, because this pathway is frequently activated in oncogenically transformed cells. Danilkovitch-Miagkova et al. reported that MAPK p44/42 (ERK1/2) is constitutively phosphorylated (activated) in JSRV-transformed human lung epithelial cells (BEAS-2B) (10). On the other hand, these investigators did not observe p44/42 phosphorylation in JSRV-transformed rat 208F fibroblasts (22). Thus, JSRV-induced MAPK phosphorylation may be cell type and/or species dependent. We also did not detect any differences in phospho-p44/42 levels between parental and JSRV-transformed NIH 3T3 cells (data not shown). Because it was possible that JSRV transformation might involve activation of the Ras-MEK-MAPK pathway at levels below detection by Western blots or enzyme assays, we tested the effects of the MEK-1-specific inhibitor PD98059 on JSRV transformation, as shown in Table 2. At 20 μM the inhibitor strongly inhibited JSRV transformation, even more efficiently than for transformation by activated H-ras. The inhibition of JSRV transformation by PD98059 was dose dependent (Table 2). Thus, MEK-MAPK signaling may be essential for JSRV transformation of NIH 3T3 fibroblasts.
TABLE 2.
Effect of MEK-1 inhibitor PD98059 on transformation of NIH 3T3a
| Inhibitor or DNA | No. of foci in:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | |||||||
| PD98059 (μM) | 0 | 20 | 0 | 20 | 0 | 1 | 5 | 0 | 1 | 5 |
| Transfected DNA | ||||||||||
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| pcDNA3.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-ras | 260 | 100 | NTb | NT | 296 | 152 | 216 | NT | NT | NT |
| pCMV3ΔGP | 188 | 0 | 164 | 6 | 176 | 96 | 56 | 300 | 60 | 56 |
Focus formation assays in NIH 3T3 cells were carried as described in Table 1, except varyious concentrations of the MEK-1 inhibitor PD98059 were used. Foci were scored at 15 to 22 days posttransfection.
NT, not tested.
Because one of the major upstream activators of MEK is Ras protein, we also tested if Ras is involved in transformation. NIH 3T3 cells transfected with pCMV3ΔGP were treated with different concentrations of the H/N-Ras inhibitor FTI-277, as shown in Table 3. At 5 μM FTI-277, transformation by JSRV or activated H-ras was completely eliminated, while transformation by the oncogene v-mos (which can directly activate MAPKK [MEK] and does not involve Ras [26, 31]) was less affected. Inhibition of JSRV transformation by FTI-277 was also dose dependent.
TABLE 3.
Effect of the H/N-Ras inhibitor FTI-277 on transformation of fibroblastsa
| Inhibitor or DNA | No. of foci in:
|
||||
|---|---|---|---|---|---|
| Expt 1 | Expt 2 | ||||
| FTI-277 (μM) | 0 | 5 | 0 | 0.5 | 1 |
| Transfected DNA | |||||
| None | 0 | 0 | 0 | 0 | 0 |
| pcDNA3.1 | 0 | 0 | 0 | 0 | 0 |
| v-mos | 32 | 20 | 23 | 18 | 18 |
| H-ras | 72 | 0 | 61 | 2 | 0 |
| pCMV3ΔGP | 36 | 0 | 47 | 15 | 16 |
NIH 3T3 fibroblasts were tested in transformation assays in the presence of FTI-277 as described for Table 1. Foci were scored at 15 to 17 days posttransfection.
Taken together, these experiments indicated that the H/N-Ras-MEK-MAPK pathway is essential for JSRV transformation of NIH 3T3 fibroblasts. In addition to the pharmacological inhibitors, we also tested cotransfection with expression plasmids for a dominant-negative (kinase dead) MEK1 (MEK-KA[101A]) and for MAPK phosphatase (MKP-1). These plasmids inhibited transformation by JSRV to a degree similar to that for activated H-ras. For MEK-KA[101A], inhibition of H-ras transformation was ca. 22%, versus 34% for JSRV; for MKP-1, inhibition of H-ras transformation was ca. 44%, versus 46% for JSRV.
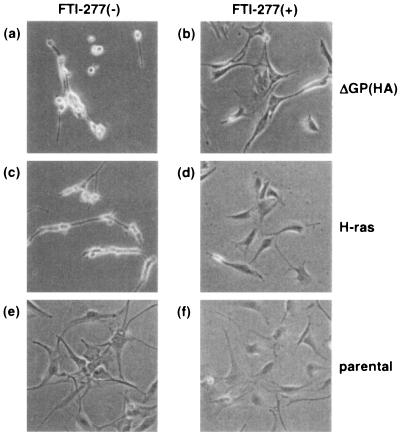
If continued signaling through the H/N-Ras-MEK-MAPK pathway is required for JSRV transformation, then inhibition of this pathway in transformed cells might lead to reversion of the transformed phenotype. Indeed, treatment of transformed NIH 3T3 cells with the H/N-Ras inhibitor FTI-277 led to reversion, as shown in Fig. 2. On the other hand, treatment with rapamycin had relatively little effect on transformation (data not shown), consistent with a secondary role for this pathway in JSRV transformation.
FIG. 2.
Reversion of transformed NIH 3T3 cells treated with the H/N-Ras inhibitor FTI-277. NIH 3T3 cells transformed with pCMV3ΔGP(HA) [3T3(ΔGP(HA))] and with activated H-ras [3T3(H-ras)] were cloned by limiting dilution. Cells were serum starved for 20 to 24 h and treated with dimethyl sulfoxide (vehicle) (a, c, and e) or 5 μM FTI-277 (b, d, and f). Transformed cells were morphologically reversed by treatment with FTI-277 (b and d). Photographs were taken 7 days (a, b, c, and d) or 4 days (e and f) after treatment with FTI-277. Magnification, ×100 in all panels.
Transformation of rat RK3E cells.
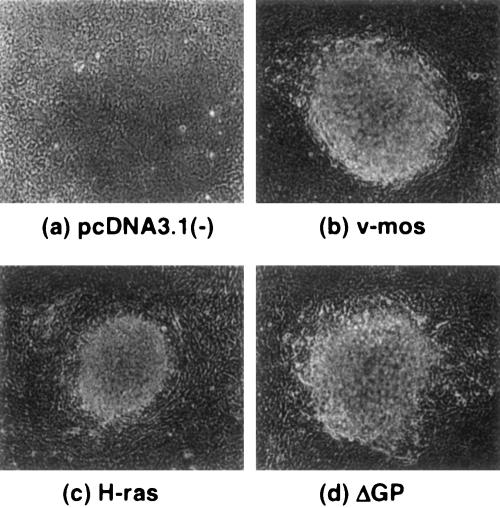
Our previous experiments on JSRV Env transformation utilized rodent fibroblasts, because transformation assays in these cells are well established. However, the natural target cells of JSRV in the sheep are lung epithelial cells, and it is possible that signaling pathways could differ between fibroblasts and epithelial cells. Danilkovitch-Miagkova et al. described the use of the human lung epithelial cell line BEAS-2B for transformation studies by JSRV (10). However, we have not been able to observe transformation of these cells by pCMV3ΔGP DNA transfection or by infection with a retroviral vector expressing JSRV Env (N. Maeda and H. Fan, unpublished). Therefore, we tested another epithelial cell line derived from rat kidney epithelia, RK3E. These cells were immortalized by adenovirus E1A protein, and they can be transformed by activated H-ras as well as by transcription factors such as c-myc or the zinc finger protein gut-enriched Kruppel-like factor (GKLF) (15). As shown in Fig. 3 and Table 4, RK3E cells were readily transformed by JSRV Env and activated H-ras and v-mos. Transformed foci were easily distinguished above the background of nontransformed cells. In these cells, H-ras was somewhat less efficient at transformation than either JSRV Env or v-mos. Epitope-tagged versions of JSRV Env also transformed RK3E cells, with the FLAG-tagged protein being more efficient in transformation than the influenza HA-tagged protein. The epitope-tagged versions of JSRV Env were employed when clones of stably transformed cells were isolated, because the presence of JSRV Env protein could be conveniently monitored by immunofluorescence. We also tested mutant versions of the JSRV Env protein with changes in the YXXM motif in the TM cytoplasmic tail known to affect transformation. The transforming activity of three mutations in the motif were similar to those observed in Rat 208F fibroblasts or NIH 3T3 cells (29), with Y590F eliminating, N592T enhancing, and M593T partially inhibiting transformation.
FIG. 3.
Transformation of immortalized rat kidney epithelial cells, RK3E. RK3E cells were transfected with 5 μg of (a) pcDNA3.1(−) (control), (b) v-mos, (c) H-ras, or (d) pCMV3ΔGP. Foci of transformed cells at day 14 after transfection are shown in panels b, c, and d. Magnification, ×100 in all panels.
TABLE 4.
Transformation of RK3E cellsa
| Transfected DNA | No. of foci in:
|
|||
|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | |
| pcDNA3.1 | 0 | 0 | 0 | 0 |
| v-mos | 1,192 | 1,216 | ||
| H-ras | 488 | 148 | ||
| pCMV3ΔGP | 688 | 664 | 1,316 | 1,372 |
| pCMV3ΔGP(HA) | 288 | |||
| pCMV3ΔGP(FLAG) | 844 | |||
| pCMV3ΔGP(Y590F) | 0 | |||
| pCMV3ΔGP(N592T) | 2,464 | |||
| pCMV3ΔGP(M593T) | 228 | |||
| Day scored | 26 | 17 | 22 | 30 |
Transformation assays in RK3E cells were performed in 6-cm-diameter dishes with 5 μg of plasmid DNA as described in Materials and Methods. The days at which the foci were scored are shown at the bottom of the table.
We also tested the role of the Akt/mTOR pathway in JSRV transformation of RK3E cells. Cotransfection with the expression plasmid for the CTMP protein had a modest (ca. 23%) inhibition of JSRV transformation, while it did not affect H-ras transformation. Rapamycin treatment also partially inhibited JSRV transformation of these cells, while it did not affect H-ras transformation (23) (Table 5); the extent of inhibition (ca. 74%) was greater in these cells than in the NIH 3T3 cells, which suggests that the Akt/mTOR pathway may be more important for JSRV transformation in epithelial cells than in fibroblasts. As expected, transformation of RK3E cells by v-akt was completely inhibited by treatment with rapamycin.
TABLE 5.
Effect of rapamycin on transformation of RK3Ea
| Inhibitor or DNA | No. of foci in:
|
|||
|---|---|---|---|---|
| Expt 1 | Expt 2 | |||
| Rapamycin (ng/ml) | 0 | 5 | 0 | 5 |
| Transfected DNA | ||||
| None | 0 | 0 | 0 | 0 |
| pcDNA3.1 | 0 | 0 | 0 | 0 |
| pCMV3ΔGP | 788 | 108 | 744 | 288 |
| H-ras | 116 | 112 | 68 | 80 |
| v-mos | NTb | NT | 748 | 532 |
| v-akt | NT | NT | 92 | 0 |
The effect of rapamycin on focus formation was investigated in RK3E cells using conditions as described in Table 4. Foci were scored at 17 to 24 days posttransfection.
NT, not tested.
The H/N-Ras-MEK-MAPK pathway is also important for transformation of RK3E cells.
We also tested if the H/N-Ras-MEK-MAPK pathway is necessary for JSRV transformation in the RK3E epithelial cells. In vitro kinase assays on JSRV-transformed RK3E cell extracts showed increased levels of p44/42 activity, as measured by Western blotting for phospho-Elk-1 protein (Fig. 4a). Elk-1 kinase is an established substrate of p44/42. Thus, JSRV transformation leads to activation of this pathway in RK3E cells. We also tested if JSRV transformation of these cells was sensitive to inhibitors of the pathway. As shown in Table 6, JSRV transformation was efficiently inhibited by two MEK1 inhibitors, PD98059 and U0126. On the other hand, the H/N-Ras inhibitor FTI-277 only partially inhibited JSRV transformation, while it completely inhibited activated H-ras transformation (Table 7). Thus, JSRV may activate MEK1/2 and MAPK p44/42 by both H/N-Ras-dependent and -independent mechanisms. Cotransfection of dominant-negative MEK-KA(101A) or MKP-1 along with pCMV3ΔGP also inhibited JSRV transformation; this inhibition was equivalent to the inhibition of transformation observed in cotransfections with the activated H-ras expression plasmid (data not shown).
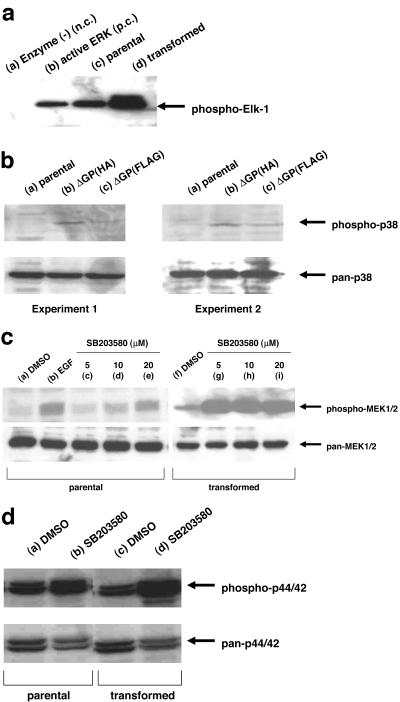
FIG. 4.
Activation of signaling pathways in JSRV-transformed cells. (a) In vitro ERK1/2 kinase assay. Twenty-four hours after seeding, parental (lane c) and JSRV-transformed RK3E (lane d) cells were serum starved for 20 to 24 h, and cell lysates were tested in the in vitro kinase assay for ERK1/2 as described in Materials and Methods. Western blotting for the phosphorylated Elk-1 substrate is shown. Lane a, ATP-substrate mix without any enzyme added; lane b, ATP-substrate mix incubated with purified ERK protein. n.c., negative control; p.c., positive control. (b) Phosphorylation of p38 MAPK in RK3E. Twenty-four hours after seeding, parental (lane a) cells and RK3E cells transformed by pCMV3ΔGP(HA) (lane b) or by pCMV3ΔGP(FLAG) (lane c) were serum starved for 20 to 24 h, and cell lysates were subjected to Western blotting analysis with pan-p38 (lower panel) and phospho-p38 (upper panel) antibodies. (c) Phosphorylation of MEK1/2 in NIH 3T3 cells treated with p38 inhibitor. Cells were serum starved for 20 to 24 h and treated with dimethyl sulfoxide (DMSO) (vehicle) (lanes a and f), 5 μM SB203580 (lanes c and g), 10 μM SB203580 (lanes d and h), 20 μM SB203580 (lanes e and i), or 100 ng of EGF/ml (lane b) for 30 min. Cells lysates were subjected to Western blot analysis with pan-MEK1/2 (lower panel) and phospho-MEK1/2 (upper panel) antibodies. (d) Phosphorylation of ERK1/2 in NIH 3T3 cells treated with p38 inhibitor. Cells were serum starved for 20 to 24 h and treated with dimethyl sulfoxide (vehicle) (lanes a and c) or 10 μM SB203580 (lanes b and d) for 30 min. Cells lysates were subjected to Western blotting analysis with pan-ERK1/2 (lower panel) and phospho-ERK1/2 (upper panel) antibodies.
TABLE 6.
Effect of MEK-1 inhibitors on transformation of RK3E cellsa
| Inhibitor or DNA | No. of foci in:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | Expt 5 | Expt 6 | |||||||||||||||
| PD98059 (μM) | 0 | 1 | 10 | 20 | 0 | 1 | 10 | 20 | 0 | 5 | 0 | 5 | ||||||||
| Transfected DNA | ||||||||||||||||||||
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| pcDNA3.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
| H-ras | 152 | 56 | 12 | 24 | NTb | NT | NT | NT | 38 | 8 | NT | NT | ||||||||
| pCMV3ΔGP | 672 | 332 | 12 | 12 | 892 | 256 | 8 | 8 | 200 | 52 | 196 | 20 | ||||||||
| U0126 (μM) | ||||||||||||||||||||
| Transfected DNA | 0 | 1 | 5 | 10 | 0 | 1 | 5 | 10 | ||||||||||||
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||
| pcDNA3.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||
| H-ras | 60 | 16 | 0 | 0 | NT | NT | NT | NT | ||||||||||||
| pCMV3ΔGP | 396 | 8 | 0 | 0 | 392 | 12 | 0 | 0 | ||||||||||||
Effect of MEK-1 inhibitors on RK3E transformation was tested under conditions described in the legend to Table 4. Foci were scored 17 to 19 days posttransfection.
NT, not tested.
TABLE 7.
Effect of the H/N-Ras inhibitor FTI-277 on transformation of RK3Ea
| Transfected DNA | No. of foci with:
|
|
|---|---|---|
| 0 μM FTI-277 | 5 μM FTI-277 | |
| None | 0 | 0 |
| pcDNA3.1 | 0 | 0 |
| v-mos | 848 | 676 |
| H-ras | 56 | 0 |
| pCMV3ΔGP | 908 | 516 |
The effect of FTI-277 on transformation in RK3E cells was assayed under the focus-forming conditions described for Table 4.
Inhibitors of p38 MAPK potentiate JSRV transformation.
The studies described above indicated that the MEK1/2-MAPK p44/42 pathway is essential for transformation by JSRV in both fibroblasts and epithelial cells, and activation of p44/42 MAPK was also found. The activation of p44/42 in JSRV-transformed cells was reasonable, but it also was of interest to investigate if p38 or SAPK/JNK activity was important. The effect of inhibitors of p38 MAPK was therefore tested, as shown in Table 8. When NIH 3T3 cells were transfected with pCMV3ΔGP and incubated in the presence of the p38 inhibitor SB203580 (1 μM), there was a two- to threefold increase in transformation frequency. In contrast, transformation by v-mos was not affected by the inhibitor. The effect was much more dramatic in transfected RK3E cells. There was a 20- to 40-fold enhancement of JSRV transformation; moreover, transformed foci could be easily visualized at earlier times (e.g., 11 days). Another p38 inhibitor SB202190 also showed a similar effect. These results indicated that p38 may negatively regulate JSRV transformation.
TABLE 8.
Effect of p38 inhibitors on JSRV transformationa
| Cell type, inhibitor, or DNA | No. of foci in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Expt 1 | Expt 2 | Expt 3 | Expt 4 | ||||||
| NIH 3T3 | |||||||||
| SB203580 (μM) | 0 | 1 | |||||||
| Transfected DNA | |||||||||
| None | 0 | 0 | |||||||
| pcDNA3.1 | 0 | 0 | |||||||
| v-mos | 100 | 104 | |||||||
| pCMV3ΔGP | 24 | 68 | |||||||
| RK3E | |||||||||
| SB203580 (μM) | 0 | 10 | 20 | 0 | 5 | ||||
| SB202190 (μM) | 0 | 5 | |||||||
| Transfected DNA | |||||||||
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| pcDNA3.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| v-mos | 168 | 180 | 200 | 1676 | 1432 | ||||
| H-ras | 0 | 0 | 28 | ||||||
| pCMV3ΔGP | 56 | 2100 | 2844 | 76 | 1896 | 324 | 4232 | ||
The effects of p38 MAPK inhibitors on transformation of NIH 3T3 (experiment 1) and RK3E cells (experiments 2 to 4) were tested. All assays were conducted in 6-cm dishes with 5 μg of transfected DNA. Foci were scored at 11 to 14 days posttransfection. These foci could be scored earlier due to the enhancement of transformation.
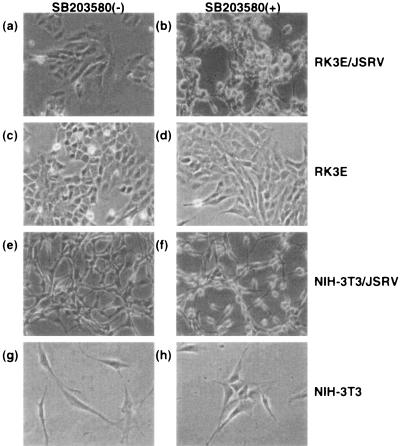
The inhibitory role of p38 in JSRV transformation was also evident in cells stably transformed by JSRV Env. When transformed NIH 3T3 or RK3E cells were treated with SB203580, the cells showed more extreme transformed phenotypes, e.g., more rounded and refractile shapes, within 24 h (Fig. 5). In addition, JSRV-transformed NIH 3T3 and RK3E cells divide under conditions of serum starvation (0.5% serum), while parental cells do not divide, and addition of SB203580 to the growth medium at 5 μM increased the rate of division of serum-starved JSRV-transformed cells (data not shown).
FIG. 5.
Morphological changes of cells treated with SB203580. Cells were serum starved for 20 to 24 h and treated with dimethyl sulfoxide (vehicle) (a, c, e, and g) or 10 μM SB203580 (b, d, f, and h). Morphological changes were observed only in transformed cells treated with SB203580 (b and f). Photographs were taken 24 h after treatment with SB203580. Similar morphological changes were also observed in RK3E cells transformed with FLAG-tagged JSRV Env treated with 10 μM SB203580. Magnification, ×100 in all the panels. Note the increased numbers of rounded and refractile cells in the JSRV-transformed cultures after treatment with SB203580.
We tested the possibility that JSRV transformation (and potentially JSRV Env protein) could lead to induction of p38 activity. Western blotting showed enhanced levels of phospho-p38 in JSRV-transformed cells (Fig. 4B).
One possible mechanism for p38 inhibition could be interference with MEK1/2 and downstream p44/42 MAPK activation, through activation of protein phosphatase 1/2A (PP1/2A; see Discussion). Indeed, when JSRV-transformed NIH 3T3 cells were treated with SB203580, increased levels of phospho-MEK1/2 (Fig. 4C) and phospho-p44/42 (Fig. 4D) were detected. Similarly, treatment of JSRV-transformed RK3E cells with SB203580 or SB202190 led to a marked increase in the levels of phospho-p44/42 (data not shown). When extracts from JSRV-transformed cells were tested for p44/42 activity in an in vitro kinase assay, inhibitor-treated extracts produced enhanced levels of hyperphosphorylated Elk-1 substrate (data not shown). Thus, the p38 inhibitors may enhance JSRV transformation by potentiating positive signaling through the H/N-Ras-MEK-MAPK pathway. Indeed, simultaneous treatment with the MEK1 inhibitor PD98059 and the p38 inhibitor SB203580 resulted in elimination of JSRV-induced foci, as would be expected if the p38 inhibitor enhances transformation through MEK-MAPK (data not shown).
We also tested the effect of the JNK inhibitor SP600125 on JSRV transformation in RK3E cells. The inhibitor showed a modest (ca. twofold) enhancement of transformation, but it was unclear if this was a specific effect, because it also enhanced transformation by v-mos to an equivalent degree (data not shown).
p44/42 MAPK is activated in ovine pulmonary adenocarcinoma (OPA) and enzootic nasal adenocarcinoma (ENA).
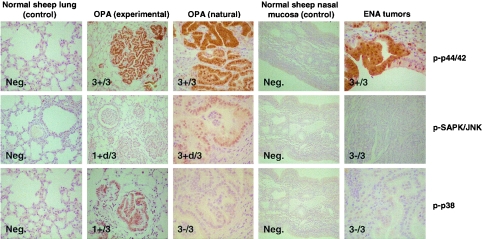
We also examined JSRV-induced lung tumors from experimentally infected sheep as well as from naturally occurring cases of OPA and ENA for expression of activated MAPKs. This was accomplished by immunohistochemistry of tumor samples with antibodies specific for phosphorylated forms of p44/42 (ERK1/2), SAPK/JNK, and p38 as shown in Fig. 6. Tissues from three experimentally induced OPA cases and from three naturally occurring cases were analyzed, along with normal lung tissue from an unaffected animal. All three antibodies were capable of recognizing the ovine proteins, because at least one tumor sample was positive when analyzed by each antibody. Phosphorylated ERK1/2 was consistently expressed in all tumor samples at high levels, consistent with an important role for this kinase in tumorigenesis. On the other hand, not all of the tumors showed expression of phospho-SAPK/JNK or phospho-p38. In particular, phospho-p38 was detected in only one experimentally induced OPA tumor and in none of the naturally occurring ones.
FIG. 6.
Immunohistochemical staining of naturally and experimentally induced OPA and naturally induced ENA. Lung tissues were obtained from one normal lung from a young lamb with no known exposure to JSRV, three experimentally induced OPA cases, and three naturally occurring OPA cases. Also, tissues were obtained from a normal nasal mucosa and from three naturally occurring ENAs. Immunohistochemical staining was performed with antibodies against phospho-ERK1/2 (Thr202/Tyr204), phospho-p38 (Thr180/Tyr182), and phospho-SAPK/JNK (Thr183/Tyr185) as primary antibodies. The numbers in the panels indicate the numbers of lungs showing positive staining (+) out of the total number examined. If available, positively staining tissues are shown. +d indicates weak, diffuse staining. Micrographs are shown at the same magnification (×400), except for those from the experimental OPA (all stains) and the ENA (pSAPK/JNK), which were taken at a lower magnification (×100). Neg., negative.
DISCUSSION
In these studies, we investigated the signaling pathways involved in JSRV Env transformation of rodent fibroblasts and epithelial cells. Our initial focus was on the Akt/mTOR pathway. We previously showed that mutation of a putative PI3K docking site (YXXM) in the cytoplasmic tail of the TM protein eliminated transformation in NIH 3T3 cells (29). Moreover, JSRV-transformed NIH 3T3 cells showed phosphorylation (activation) of Akt, and this phosphorylation could be inhibited by treatment of the cells with the PI3K inhibitor LY294002. On the other hand, disabling PI3K in NIH 3T3 cells does not inhibit JSRV transformation (25). Thus, PI3K is by itself not essential for transformation. However, Akt was nevertheless phosphorylated in JSRV-transformed PI3K-negative cells, which indicated a second PI3K-independent mechanism for Akt activation. Thus, while PI3K is not essential for JSRV transformation, it remained possible that activation of Akt and its downstream substrates are. In particular, mTOR was of interest, because it has been shown that transformation of chicken embryo fibroblasts (CEF) by retroviral oncogene forms of PI3K (v-P3k) or Akt (v-akt) is eliminated by treatment with rapamycin (2). As shown in these experiments, transformation of NIH 3T3 or RK3E cells by JSRV Env protein was partially inhibited by rapamycin (ca. 45% in NIH 3T3 and ca. 74% in RK3E). Similarly, we found that while transformation of CEF by v-P3k and v-akt was eliminated by rapamycin, transformation by JSRV was partially inhibited (data not shown). Thus, the AKT/mTOR pathway is important for JSRV transformation. At the same time, the fact that rapamycin did not completely eliminate transformation indicated that another pathway(s) is also involved in JSRV transformation.
These experiments identified signaling through MEK1/2-MAPK p44/42 as being essential for JSRV transformation in both NIH 3T3 and RK3E cells. Although increased phospho-ERK1/2 was not reliably detected in transformed cells, in vitro assays for kinase activity indicated increased levels. Inhibitors of MEK1 eliminated transformation by JSRV in either cell type, and inhibition was as effective as that for the activated H-ras oncogene. In NIH 3T3 cells, the H/N-Ras inhibitor FTI-277 also eliminated JSRV transformation, indicating that the classical H/N-Ras-MEK-MAPK pathway is essential for transformation. On the other hand, in RK3E cells, FTI-277 only partially inhibited JSRV transformation. Thus, in the epithelial cells there are apparently both H/N-Ras-dependent and -independent mechanisms by which JSRV signals for transformation through MEK1/2. Some of the H/N-Ras-independent signaling can be attributed to K-Ras, because combined treatment with the H/N-Ras inhibitor FTI-277 and the K-Ras inhibitor GGTI-298 increased inhibition of focus formation in RK3E cells from 57% (FTI-277 alone) to 72% (FTI-277 plus GGTI-298) (data not shown).
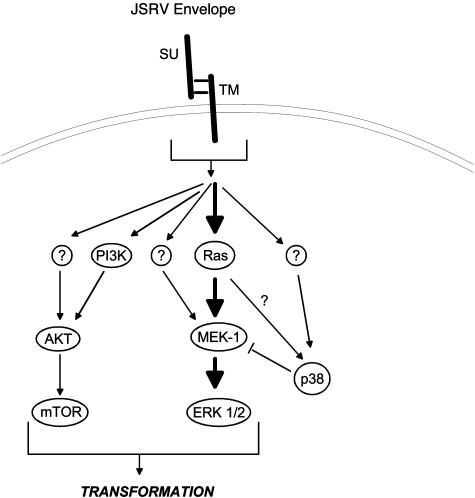
A model for the signaling pathways involved in JSRV transformation is shown in Fig. 7. JSRV Env protein leads to activation of signals through both the Akt/mTOR and H/N-Ras-MEK-MAPK pathways. The transformation phenotype results from integration of signals from these two pathways. Depending upon the particular cell line or type, the relative importance of the two pathways may be different. For instance, in RK3E cells the Akt/mTOR pathway is more important for transformation than it is for NIH 3T3 cells. Also, more than one mechanism may lead to activation of Akt and MEK, depending on cell type. As discussed above, there appears to be both PI3K-dependent and -independent mechanisms for Akt activation in transformed NIH 3T3 cells, and there are H/N-Ras-dependent and -independent mechanisms for activation of MEK-1/2 in RK3E cells. Modulation of H/N-Ras-MEK-MAPK signaling by p38 MAPK also occurs (see below). The signals through these two pathways are presumably phosphorylated proteins, whose activities are changed. Numerous downstream substrates in both of these pathways have been identified (41), and ultimately it will be challenging but important to identify those phosphorylated proteins that are important for transformation. Zavala et al. also reported partial dependence on Akt for JSRV transformation of avian cells (40).
FIG. 7.
Model of JSRV Env-induced transformation. A proposed model for signaling in JSRV Env-induced transformation is shown. JSRV envelope protein leads directly or indirectly to activation of the Ras-MEK-MAPK and PI3K-Akt-mTOR pathways as well as to activation of p38 MAPK, which negatively regulates transformation. Question marks indicate alternate pathways that are inferred from the data, but the identities of the key proteins are not known. Sizes of the arrows reflect the relative importance for transformation. The arrows do not necessarily indicate direct binding and/or activation of the downstream molecules; intermediate proteins or kinases may exist.
While these experiments identify two signaling pathways that are important for JSRV transformation, the mechanism(s) by which JSRV Env activates them remains to be determined. One approach is to identify domains and motifs in JSRV Env protein that are important for transformation and/or signaling through these two pathways. We previously showed that the cytoplasmic tail of the TM protein is essential for JSRV transformation (29), which suggests that cytoplasmic cellular proteins bind to the tail, leading to activation of signaling pathways. Indeed, inspection of amino acid sequences in the TM cytoplasmic tail identified a putative PI3K docking site, and mutations of the YXXM motif (Y590F and M593T) eliminated transformation in NIH 3T3 cells (29). However, we and others have not been able to obtain further evidence for binding of PI3K to the TM cytoplasmic tail in pull-down assays, and we have not detected tyrosine phosphorylation of the TM protein. We have performed yeast two-hybrid experiments and coimmunoprecipitation experiments to identify cellular proteins that bind to the cytoplasmic tail (J. W. Kim, R. Pioquinto, N. Maeda, and H. Fan, unpublished data). Candidate interacting proteins have been identified, although so far none are obvious candidates for activating Akt/mTOR or H/N-Ras-MEK-MAPK signaling. We have also carried out alanine scanning mutagenesis of the JSRV TM cytoplasmic tail (S. Hull and H. Fan, unpublished data). Regions of the tail have been identified where alanine mutations eliminate transformation, and other regions are dispensable. These mutants may be valuable for identifying mechanisms of signal transduction.
While our previous experiments highlighted the importance of the cytoplasmic tail of TM in transformation, other regions of JSRV Env are also important, i.e., the ectodomain of TM or the SU protein. We have recently found that SU is also important for JSRV transformation, because mutations in this protein abolish transformation (19). Moreover, the SU and TM domains function independently in transformation, because transformation-negative mutants in SU and TM can complement in cotransfection assays.
Danilkovitch-Miagkova et al. have described studies of JSRV transformation of human BEAS-2B lung epithelial cells (10). They found that in normal BEAS-2B cells, the JSRV receptor protein cell surface hyaluronidase 2 (Hyal-2) is complexed with a membrane-spanning tyrosine kinase-type growth factor receptor, Stk/RON. When Stk/RON is complexed with Hyal-2, the tyrosine kinase activity is not activated, as indicated by the lack of phosphorylation on Stk/RON. However, in JSRV-transformed cells, the JSRV Env protein binds to Hyal-2, blocking formation of the Hyal-2-Stk/RON complex, and this is accompanied by phosphorylation of Stk/RON. In JSRV-transformed BEAS-2B cells, the Ras-MEK-MAPK pathway and the Akt/mTOR pathways are activated, presumably by binding of adaptor proteins to phosphotyrosine residues on Stk/RON. This mechanism of activation is probably not applicable to the NIH 3T3 and RK3E cells studied here. While the human Hyal-2 protein functions as a receptor for JSRV, rodent cells (containing rodent Hyal-2) do not have functional JSRV receptors, and they do not bind JSRV Env protein in vitro (21). On the other hand, it is possible that JSRV SU and/or the ectodomain of TM might bind to another cell surface molecule and trigger signaling (directly or indirectly) through that interaction.
An unexpected and noteworthy finding in these studies was that inhibition of p38 MAPK potentiated JSRV transformation. This was evident in the increase in focus formation efficiency in the presence of p38 inhibitors (particularly for RK3E cells) and also in the enhanced transformation phenotype after treatment of JSRV-transformed cells with these inhibitors. JSRV-transformed cells also showed increased levels of p38 activity. The fact that transformed cells treated with p38 inhibitor showed a rapid enhancement of transformation further indicates that the p38 inhibitors were not simply countering potential proapoptotic effects of p38. These results therefore indicate that JSRV Env leads to positive signals for cell transformation (through H/N-Ras-MEK-MAPK and Akt/mTOR) but also to inhibitory signals via p38. The results also indicate that p38 may be negatively regulating JSRV transformation by reducing signaling through H/N-Ras-MEK-MAPK, because JSRV-transformed NIH 3T3 and RK3E cells treated with p38 inhibitors showed markedly increased levels of phospho-ERK1/2 and ERK1/2 activity. Several recent publications have reported that the balance between ERK1/2 and p38 signaling is important for cell survival (39), although molecular mechanisms for the cross-talk have not been defined. One possibility is that p38 may activate protein phosphatases 1 and 2A (PP1/2A) (38); activation of PP1/2A may dephosphorylate MEK1/2 and thus decrease activation of ERK1/2. In addition, PP2A dephosphorylates phospho-Akt, so p38 could also interfere with signaling through Akt/mTOR. The pathway(s) involved in p38 activation by JSRV Env remains to be determined. Activated H-ras has been shown to constitutively activate ERK1/2 but also to transiently activate p38 and SAPK/JNK. On the other hand, treatment with p38 inhibitors did not enhance transformation by activated H-ras in rat intestinal epithelial (RIE) cells (33) or in the RK3E cells studied here (N. Maeda and H. Fan, unpublished). Thus, activation of p38 by JSRV does not appear to result exclusively from activation of Ras; some other mechanism also must be involved.
Because our ultimate goal is to understand how JSRV causes oncogenic transformation of ovine lung epithelial cells in vivo, we also tested JSRV-induced OPA tumor tissues for activation of the H/N-Ras-MEK-MAPK pathway. Furthermore, we tested tissue from natural ENA, a contagious neoplastic disease induced by enzootic nasal tumor virus (ENTV) (9, 27), which is similar to JSRV. Previous studies reported that ENTV Env also can cause transformation of fibroblasts (1, 13). Therefore, parallel investigations of a mechanism of transformation by ENTV would be very important for studying a mechanism of transformation by JSRV. We had found that OPA tumor tissues do not show phosphorylated Akt, although some tumors induced by the related ovine nasal adenocarcinoma virus (ONAV/ENTV) do (40). As shown here, all OPA tumor tissues (six out of six) showed strong expression of phospho-ERK1/2 by immunohistochemistry. This indicates that signaling through the H/N-Ras-MEK-MAPK pathway indeed is likely important in JSRV-induced ovine tumors. Similar results were obtained with ENA natural tumor sections. It was also interesting that only one out of the six tumors showed the presence of phospho-p38. One possible explanation could be that as OPA tumors progress in the sheep, the transformed cells may lose the capacity to activate p38. This could potentiate signaling through the H/N-Ras-MEK-MAPK pathway, as observed in the in vitro experiments with p38 inhibitors described here. Indeed, we have recently observed that two OPA-derived tumor lines, JS7 and JS8 (11), have constitutively activated ERK1/2, but they are relatively defective for p38 activation (N. Maeda and H. Fan, unpublished).
Acknowledgments
This work was supported by NIH grant CA94188 to H.F. Support of the Cancer Research Institute, grant AGL 2001-1812 (GAN) from Spanish MCYT, and the Chao Family Comprehensive Cancer Center is acknowledged.
REFERENCES
- 1.Alberti, A., C. Murgia, S. L. Liu, M. Mura, C. Cousens, M. Sharp, A. D. Miller, and M. Palmarini. 2002. Envelope-induced cell transformation by ovine betaretroviruses. J. Virol. 76:5387-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki, M., E. Blazek, and P. K. Vogt. 2001. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc. Natl. Acad. Sci. USA 98:136-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballif, B. A., and J. Blenis. 2001. Molecular mechanisms mediating mammalian mitogen-activated kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 12:397-408. [PubMed] [Google Scholar]
- 4.Bellacosa, A., J. R. Testa, S. P. Staal, and P. N. Tsichlis. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254:274-277. [DOI] [PubMed] [Google Scholar]
- 5.Bellacosa, A., J. R. Testa, R. Moore, and L. Larue. 2004. A portrait of AKT kinases: human cancer and animal models depict a family with strong individualities. Cancer Biol. Ther. 3:268-275. [DOI] [PubMed] [Google Scholar]
- 6.Campbell, S. L., R. Khosravi-Far, K. L. Rossman, G. J. Clark, and C. J. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 7.Chang, F., L. S. Steelman, J. T. Lee, J. G. Shelton, P. M. Navolanic, W. L. Blalock, R. A. Franklin, and J. A. McCubrey. 2003. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 17:1263-1293. [DOI] [PubMed] [Google Scholar]
- 8.Chiu, M. I., H. Katz, and V. Berlin. 1994. RAPT1, a mammalian homolog of yeast Tor, interacts with the FKBP12/rapamycin complex. Proc. Natl. Acad. Sci. USA 91:12574-12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cousens, C., E. Minguijon, M. Garcia, L. M. Ferrer, R. G. Dalziel, M. Palmarini, M. de las Heras, and J. M. Sharp. 1996. PCR-based detection and partial characterization of a retrovirus associated with contagious intranasal tumors of sheep and goats. J. Virol. 70:7580-7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danilkovitch-Miagkova, A., F. M. Duh, I. Kuzmin, D. Angeloni, S. L. Liu, A. D. Miller, and M. I. Lerman. 2003. Hyaluronidase 2 negatively regulates RON receptor tyrosine kinase and mediates transformation of epithelial cells by jaagsiekte sheep retrovirus. Proc. Natl. Acad. Sci. USA 100:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMartini, J. C., J. V. Bishop, T. E. Allen, F. A. Jassim, J. M. Sharp, M. de las Heras, D. R. Voelker, and J. O. Carlson. 2001. Jaagsiekte sheep retrovirus proviral clone JSRV(JS7), derived from the JS7 lung tumor cell line, induces ovine pulmonary carcinoma and is integrated into the surfactant protein A gene. J. Virol. 75:4239-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dent, P., A. Yacoub, P. B. Fisher, M. P. Hagan, and S. Grant. 2003. MAPK pathways in radiation responses. Oncogene 22:5885-5896. [DOI] [PubMed] [Google Scholar]
- 13.Dirks, C., F. M. Duh, S. K. Rai, M. I. Lerman, and A. D. Miller. 2002. Mechanism of cell entry and transformation by enzootic nasal tumor virus. J. Virol. 76:2141-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan, H., M. Palmorini, and J. C. DeMartini. 2003. Transformation and oncogenesis by Jaagsiekte sheep retrovirus. Curr. Top. Microbiol. Immunol. 275:139-177. [DOI] [PubMed]
- 15.Foster, K. W., S. Ren, I. D. Louro, S. M. Lobo-Ruppert, P. McKie-Bell, W. Grizzle, M. R. Hayes, T. R. Broker, L. T. Chow, and J. M. Ruppert. 1999. Oncogene expression cloning by retroviral transduction of adenovirus E1A-immortalized rat kidney RK3E cells: transformation of a host with epithelial features by c-MYC and the zink finger protein GKLF. Cell Growth Differ. 10:423-434. [PubMed] [Google Scholar]
- 16.Frodin, M., and S. Gammeltoft. 1999. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell Endocrinol. 151:65-77. [DOI] [PubMed] [Google Scholar]
- 17.Gingras, A. C., B. Raught, and N. Sonenberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807-826. [DOI] [PubMed] [Google Scholar]
- 18.Gupta, S., R. Plattner, C. J. Der, and E. Stanbridge. 2000. Dissection of Ras-dependent signaling pathways controlling aggressive tumor growth of human fibrosarcoma cells: evidence for a potential novel pathway. Mol. Cell. Biol. 20:9294-9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofacre, A., and H. Fan. 2004.. Multiple domains of the Jaagsiekte sheep retrovirus envelope protein are required for transformation of rodent fibroblasts. J. Virol. 78:10479-10489. [DOI] [PMC free article] [PubMed]
- 20.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 21.Liu, S. L., F. M. Duh, M. I. Lerman, and A. D. Miller. 2003. Role of virus receptor Hyal2 in oncogenic transformation of rodent fibroblasts by sheep betaretrovirus Env proteins. J. Virol. 77:2850-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, S. L., M. I. Lerman, and A. D. Miller. 2003. Putative phosphatidylinositol 3-kinase (PI3K) binding motifs in ovine betaretrovirus Env proteins are not essential for rodent fibroblast transformation and PI3K/Akt activation. J. Virol. 77:7924-7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Louro, I. D., P. McKie-Bell, H. Gosnell, B. C. Brindley, R. P. Bucy, and J. M. Ruppert. 1999. The zinc finger protein GLI induces cellular sensitivity to the mTOR inhibitor rapamycin. Cell Growth Differ. 10:503-516. [PubMed] [Google Scholar]
- 24.Maeda, N., M. Palmarini, C. Murgia, and H. Fan. 2001. Direct transformation of rodent fibroblasts by jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. USA 98:4449-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda, N., Y. Inoshima, D. Fruman, S. M. Brachmann, and H. Fan. 2003. Transformation of mouse fibroblasts by Jaagsiekte sheep retrovirus envelope does not require phosphatidylinositol 3-kinase. J. Virol. 77:9951-9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nebreda, A. R., C. Hill, N. Gomez, P. Cohen, and T. Hunt. 1993. The protein kinase mos activates MAP kinase kinase in vitro and stimulates the MAP kinase pathway in mammalian somatic cells in vivo. FEBS Lett. 333:183-187. [DOI] [PubMed] [Google Scholar]
- 27.Ortin, A., C. Cousens, E. Minguijon, Z. Pascual, M. P. Villarreal, J. M. Sharp, and M. de las Heras. 2003. Characterization of enzootic nasal tumour virus of goats: complete sequence and tissue distribution. J. Gen. Virol. 84:2245-2252. [DOI] [PubMed] [Google Scholar]
- 28.Palmarini, M., J. M. Sharp, M. de las Heras, and H. Fan. 1999. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 73:6964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmarini, M., N. Maeda, C. Murgia, C. De-Fraja, A. Hofacre, and H. Fan. 2001. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J. Virol. 75:11002-11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panwalkar, A., S. Verstovsek, and F. J. Giles. 2003. Mammalian target of rapamycin inhibition as therapy for hematologic malignancies. Cancer 100:657-666. [DOI] [PubMed] [Google Scholar]
- 31.Posada, J., N. Yew, N. G. Ahn, G. F. Vande Woude, and J. A. Cooper. 1993. mos stimulates MAP kinase in Xenopus oocytes and activates a MAP kinase kinase in vitro. Mol. Cell. Biol. 13:2546-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevot, D., J. L. Darlix, and T. Ohlmann. 2003. Conducting the initiation of protein synthesis: the role of elF4G. Biol. Cell. 95:141-156. [DOI] [PubMed] [Google Scholar]
- 33.Pruitt, K., W. M. Pruitt, G. K. Bilter, J. K. Westwick, and C. J. Der. 2002. Raf-independent deregulation of p38 and JNK mitogen-activated protein kinases are critical for Ras transformation. J. Biol. Chem. 277:31808-31817. [DOI] [PubMed] [Google Scholar]
- 34.Rai, S. K., F. M. Duh, V. Vigdorovich, A. Danilkovitch-Miagkova, M. I. Lerman, and A. D. Miller. 2001. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 98:4443-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sabatini, D. M., H. Erdjument-Bromage, M. Lui, P. Tempst, and S. H. Snyder. 1994. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78:35-43. [DOI] [PubMed] [Google Scholar]
- 36.Sehgal, S. N., H. Baker, and C. Vezina. 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. II. Fermentation, isolation and characterization. J. Antibiotics (Tokyo) 28:727-732. [DOI] [PubMed] [Google Scholar]
- 37.Vezina, C., A. Kudelski, and S. N. Sehgal. 1975. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. J. Antibiotics (Tokyo) 28:721-726. [DOI] [PubMed] [Google Scholar]
- 38.Westermarck, J., S.-P. Li, T. Kallunki, J. Han, and V.-M. Kahari. 2001. p38 mitogen-activated protein kinase-dependent activation of protein phosphatases 1 and 2A inhibits MEK1 and MEK2 activity and collagenase 1 (MMP-1) gene expression. Mol. Cell. Biol. 21:2373-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 40.Zavala, G., C. Pretto, Y. H. Chow, L. Jones, A. Alberti, E. Grego, M. de las Heras, and M. Palmarini. 2003. Relevance of Akt phosphorylation in cell transformation induced by jaagsiekte sheep retrovirus. Virology 312:95-105. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, W., and H. T. Liu. 2002. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 12:9-18. [DOI] [PubMed] [Google Scholar]
- 42.Zhou, G., Z. Q. Bao, and J. E. Dixon. 1995. Components of a new human protein kinase signal transduction pathway. J. Biol. Chem. 270:12665-12669. [DOI] [PubMed] [Google Scholar]