Abstract
Modern contraception ushered in an era of improved family planning, but more than 60 years after approval of “the pill,” product gaps and unmet needs still exist. Nearly 250 million women worldwide who want to delay or avoid pregnancy do so ineffectively or not at all, and the principal mechanism of male contraception, condoms, has not changed in 100 years. As a result, about half of the pregnancies that occur globally each year are unintended. Increasing contraceptive options and uptake will curtail abortions, empower women and men, promote healthy families, and moderate population growth that overtaxes the environment. This Review addresses the history of contraception, shortcomings in contraceptive methods, promising approaches for male and female contraception, and simultaneous protection against unintended pregnancy and sexually transmitted infections.
Whereas fewer than 1 billion people inhabited Earth 200 years ago, the human population recently surpassed 8 billion and is on track to exceed 10 billion by 2100 (1). Population growth was fueled by the Industrial Revolution, which introduced more efficient agricultural practices and the refinement of fossil fuels and minerals, which led to global economic growth. Other major influences were advancements in sanitation practices, and new drugs and vaccines that reduced human disease. In 1800, the global child mortality rate was 43%, and the average human life span was 49 years, whereas in 2022, these figures were 3.8% and 73 years, respectively (2, 3). The global fertility rate (the average number of children born per woman) decreased from 5.0 in 1960 to 2.5 today in part because of new contraception practices and burgeoning education and empowerment of women. Even so, the population continues to grow because the global fertility rate (2.5) exceeds the level of sustainable replacement (2.1), and because of “population momentum” (1, 4).
The era of modern contraception arose amid the exponential population growth of the 20th century (Fig. 1). Contraception is defined as the act of intentionally preventing pregnancy by means of devices, practices, medications, or surgery. Modern contraceptives have a sound basis in reproductive biology and evidence of efficacy by using a precise protocol for correct use (1). Examples of modern options now available for women include tubal ligation, barrier methods, hormonal methods [“the pill”, implants and injectables, progestin intrauterine devices (IUDs), or emergency contraception], and nonhormonal IUDs. Only two choices are available for men: vasectomy and condoms (Fig. 2).
Fig. 1.
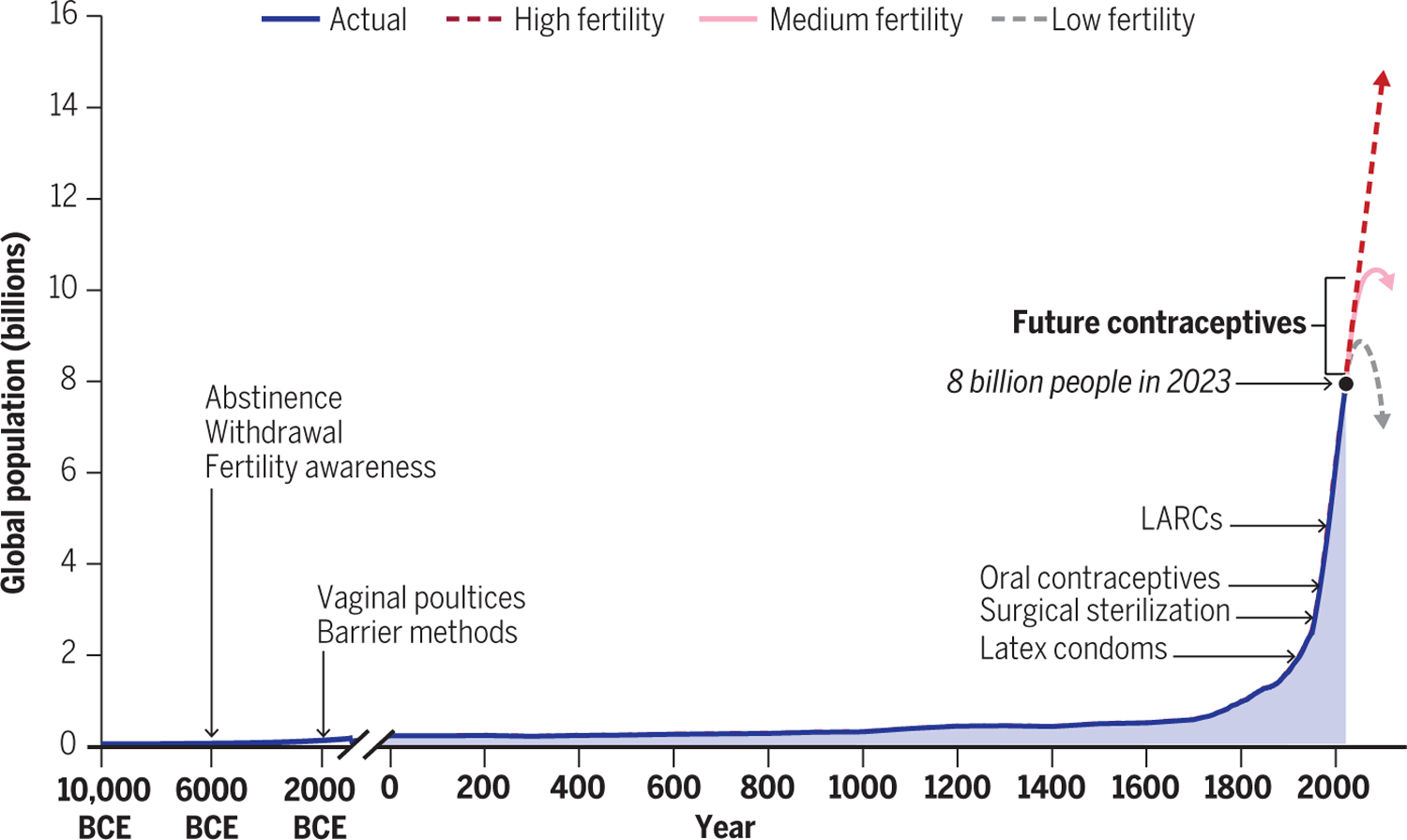
Contraception and human population growth.
Fig. 2. Modern contraception methods: Types, usage, and efficacy.
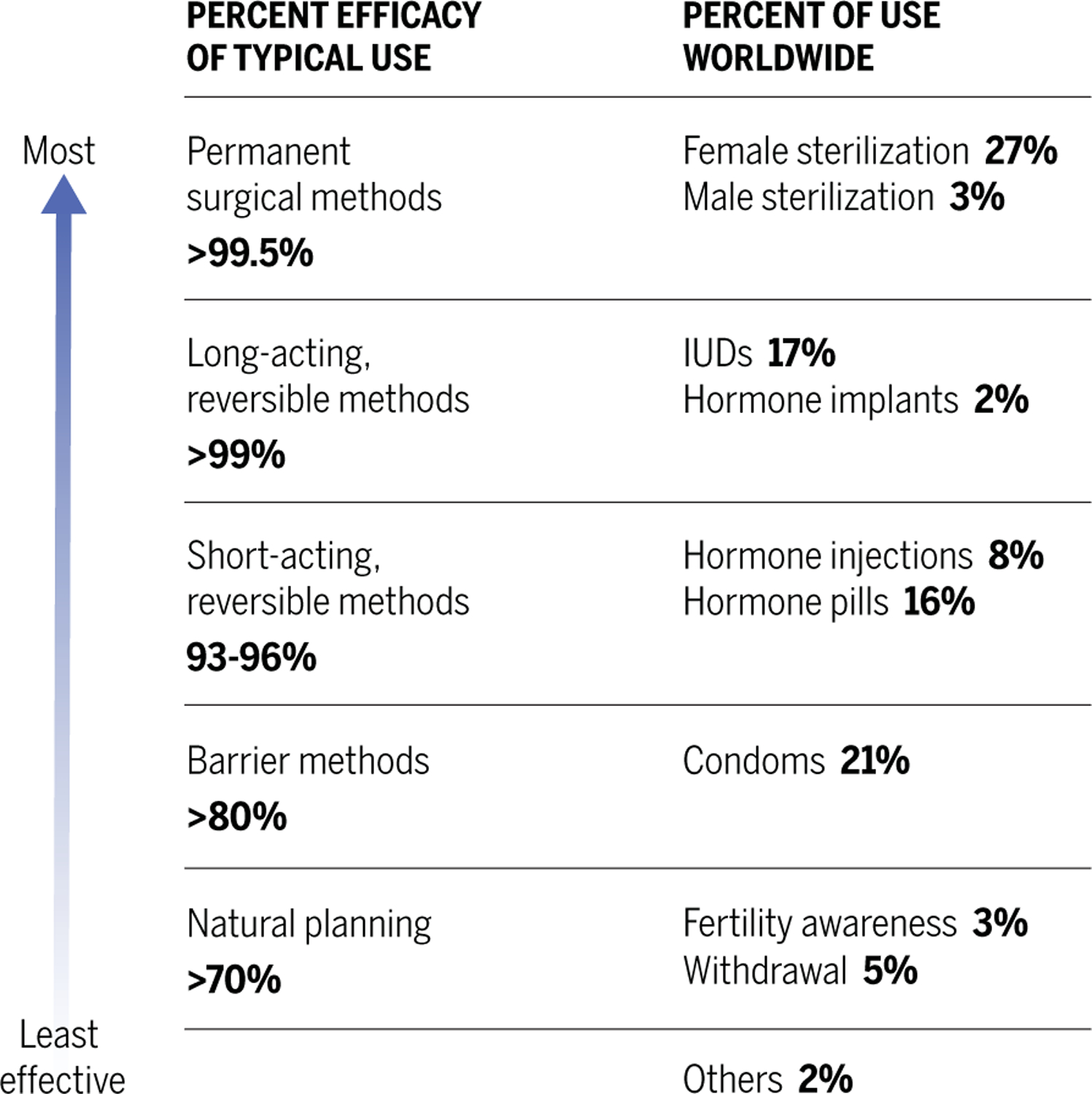
Women now have the potential for reproductive autonomy, and most will use at least one contraception method during their lifetime. However, an estimated 170 million women worldwide who want to avoid pregnancy are not using any contraceptive method at all, and another 80 million women are not using safe and effective modern contraceptives (5). Consequently, of the 210 million pregnancies that occur globally each year, about half are unintended (6). Unintended pregnancies occur disproportionately in young and poor women and are associated with increased incidences of maternal and child mortality and cycles of intergenerational poverty. Worldwide, more than 60% of unintended pregnancies end in abortion (7).
The contraception gap is multifaceted. Many women have limited access to comprehensive sexual education and modern contraceptives. Of those with access, some express concerns over side effects or social stigma, whereas others experience opposition for social and cultural reasons (1). These hurdles contribute to inconsistent use and discontinuation. At the same time, many men report willingness to help close the gap and assume greater responsibility for family planning if provided with innovative male contraceptive methods (8).
The importance of contraception extends beyond preventing unintended pregnancies. Contraception plays an essential role in family planning and the ability of individuals and couples to influence the timing of initial and successive pregnancies. Child spacing has clear implications on the health of the child and the mother. Family planning also has economic benefits; it allows families to determine how many children they can support and affords women greater opportunity to pursue education and jobs. Arguably, contraceptive choices are as important during and after the child-bearing stages as they are beforehand.
This Review identifies current problems and gaps in existing contraception options and details three focus areas for future generations of contraceptives: (i) more male methods; (ii) nonhormonal on-demand female contraceptives that have fewer side effects; and (iii) contraception that also protects against sexually transmissible infections (STIs), also called multipurpose prevention technology (MPT).
Contraception past and present
Most women are fertile for at least 30 years and are theoretically capable of giving birth annually during this phase of their life span. Men produce 70 million to 150 million sperm per day, can maintain their fertility well into their latter decades of life, and thus are capable of siring hundreds of offspring. Historical contraceptive options have included abstinence, fertility awareness (monitoring physical signs to estimate ovulation), and withdrawal. In present times, fertility awareness and withdrawal methods are practiced by about 8% of couples desiring contraception (9). These methods have annual failure rates of more than 20% (1).
Women have used vaginal poultices for contraception since at least 2000 BCE, using soft materials such as cotton or wool soaked in various mixtures of oils, fruit juice, herbs, and other substances to form a tampon-like plug. Today, user-applied vaginal methods are limited. Nonoxynol-9 (N-9)—a spermicidal surfactant formulated as a gel, cream, or film—has been available since the 1960s. Phexxi, an acidic vaginal gel that impairs sperm motility by buffering the vaginal pH after intercourse, was introduced in 2020. As with all user-controlled methods, efficacy depends on adherence. The annual failure rate of these vaginal contraceptive methods can be high (N-9, 18 to 28%; Phexxi, 14%) (10, 11).
Male barrier methods (penile sheaths) were used in ancient Egypt and Rome, and a female condom was described in the Greek literature from 3000 BCE (12). Early condoms were made from animal bladders and intestines, or from chemically treated linen (13). During the 1700s, condoms were widely used by men in Europe to prevent sexually transmitted infections. In the 1920s, synthetic latex was invented and led to the manufacture of thin male condoms with high flexibility and increased tensile strength. Synthetic latex condoms remain the predominant male barrier method used today. Modern female condoms are made of latex or nitrile. Condoms offer advantages: they are relatively inexpensive, widely available directly to the end user, provide effective contraception, and protect against most STIs. Disadvantages are the time needed to apply a condom (interrupts intimacy) and decreased sexual sensation. Under perfect use, condoms are 98% effective, but in actual use, they are 87% effective. Diaphragms and cervical caps, two female barrier methods, do not protect users from most STIs and have typical failure rates of 18% (1). Globally, nearly one-quarter of contracepting couples use barrier methods as their primary means of contraception: 21% male condoms, 2% diaphragms and cervical caps, and 1% female condoms (9).
Vasectomy is a surgical procedure that severs and seals the vas deferens, the duct that transports sperm during ejaculation. Female tubal ligation is a surgical procedure that prevents fertilization by cutting and cauterizing the fallopian tubes, which connect the ovaries to the uterus. Both vasectomy and tubal ligation procedures are safe and highly (>99%) effective, but there is a large gender disparity in their uptake. Worldwide, 26.6% of contracepting women have had tubal ligation surgery, whereas only 2.8% of men are vasectomized (5). These procedures are referred to as sterilization, although this designation is some-what misleading. Men and women continue to produce gametes, and both procedures can be successfully reversed with surgery (vaso-vasostomy or tubal reanastomosis), with fertility restored in 40 to 70% of cases (14, 15). Alternatively, many vasectomized men and ligated women can achieve pregnancy through in vitro fertilization technologies, with fertility rates ranging from 30 to 70% (16, 17). However, these methods are expensive and inaccessible to many individuals. Nonsurgical methods to block the vas deferens and readily reversible vasectomies are currently being explored with the hope that they will increase the popularity of vasectomies (18).
IUDs were invented in the early 1900s but were not widely used for contraception in the United States until the late 1950s; by 1974, there were more than 2.5 million users. Unfortunately, one of the IUDs marketed at that time, the Dalkon Shield, was associated with pelvic infections and mortality because of a multifilamentous tail string that allowed bacteria to ascend from the vagina into the uterus. It was removed from the market in 1974, but the damage it caused set the IUD field back several years (19). Two classes of IUDs are currently widely available: the nonhormonal copper IUD (Paragard) and hormonal IUDs (Mirena and others). These newer IUDs are safe, are 99% effective, and produce long-acting reversible contraception (LARC) for 8 to 10 years. IUDs primarily work by preventing fertilization. The copper IUD emits copper ions that are toxic to gametes, and hormonal IUDs block ovulation and thicken cervical mucus, preventing sperm from reaching the fallopian tubes. The effectiveness of IUDs as emergency contraceptives implies that they may also act by preventing implantation of the blastocyst (20). The copper IUD causes heavier menstrual bleeding, which is a serious side effect for some women; hormonal IUDs generally reduce menstrual bleeding, which is often considered an advantage. IUDs earn the highest rate of satisfaction and continuation of all reversible contraceptives: 17% of women using contraception today use IUDs (9).
The first oral hormonal contraceptive pill, Enovid, was approved by the US Food and Drug Administration (FDA) for contraception in 1960. Other oral contraceptives soon followed, and by 1963, 2.3 million women were using some form of oral birth control (21). Serious adverse effects associated with these early pills were soon reported. Epidemiological studies concluded that pill users were more susceptible to thromboembolism than were non-pill users (22). Estrogens, and ethinyl estradiol in particular, were implicated. Over time, both estrogen and progestin components of combination pills were markedly reduced, and safety increased.
In combined hormonal contraceptives, both progestins and estrogens inhibit the hypothalamic-pituitary-ovarian axis, which controls the reproductive cycle. Progestins prevent pregnancy by inhibiting the luteinizing hormone (LH) surge, thus suppressing ovulation, thickening the cervical mucus, and lowering fallopian tube motility. Estrogens prevent pregnancy by suppressing follicle-stimulating hormone (FSH) production, which prevents egg maturation. The addition of estrogen also helps prevent irregular bleeding (23).
Collectively, about 30% of women worldwide currently use contraceptives with hormonal mechanisms of action. The estrogen and/or progestin-only birth control pills, when taken as directed, are >98% effective (24). Furthermore, substantial modifications have allowed for reduced dosages and expanded routes of administration (implants, injectables, intrauterine devices, vaginal rings, and dermal patches), which has increased safety and product choices. However, the estrogens and progestogens in female hormonal methods are still associated with side effects and potential health risks. Estrogens are contraindicated in women at risk for thrombosis (from smoking, hypertension, obesity, or history of thrombosis) and after hormone-dependent cancer. Progestins are associated with abnormal bleeding patterns, headaches, mood swings, and other side effects that can cause end-user dissatisfaction and discontinuation (25). Such risks and drawbacks are reasons for developing additional methods of contraception.
Emerging contraceptives
Male contraceptives
Hormonal
Reversible oligospermia (<15 million sperm/ml of ejaculate) was achieved by the exogenous administration of androgen in 1939 (26), but it was a 1972 publication (27) that ignited interest that continues to the present day (28–30). “Hormonal male contraception” functions by suppressing FSH and LH secretion from the pituitary gland. LH stimulates biosynthesis of testosterone by testicular Leydig cells, which is critical to sustaining spermatogenesis. Suppression of LH results in decreased intratesticular testosterone, spermatogenic failure, and reduced ejaculatory sperm concentrations. Concentrations of <1 million sperm/ml are often achieved, a level deemed compatible with effective contraception. Cessation of male hormonal treatments leads to a return of normal LH and FSH secretion, normal intratesticular testosterone concentrations, and a resumption of spermatogenesis.
Administration of estrogen or progestin along with androgen provides markedly greater suppression of the hypothalamic-pituitary-gonadal (HPG) axis as compared with use of androgen alone (31). Gonadotropin-releasing hormone (GnRH) agonists and antagonists also suppress FSH and LH secretion. To date, many androgenic molecules have been clinically evaluated alone or in combination with estrogens, progestins, GnRH agonists, or GnRH antagonists in a variety of delivery systems, including oral pills, intramuscular injections, subdermal injections, implants, and transdermal gels (28, 32–34).
Nestorone + testosterone (NES+TES) combination gel is a promising product that functions through the HPG axis and has advanced into clinical trials (ClinicalTrials.gov identifier: NCT03452111). NES+TES is a single transdermal gel product that combines nestorone, a progestin, and testosterone and is applied daily by men to the shoulder and upper arm. In a previous trial, NES+TES formulations reduced sperm counts to <1 million sperm/ml in 88 to 89% of subjects, whereas only 23% of men in the testosterone-only group had reductions to that level. No serious adverse effects were reported. The most common side effect in the NES+TES groups was acne (about 25% of subjects). Secondary transfer of NES+TES gel to others is possible but was minimized by washing and covering the site after application (35).
Dimethandrolone undecanoate (DMAU) is a prodrug that is converted into dimethandrolone, which has both androgenic and progestational activity (36). A phase I clinical trial is enrolling participants to evaluate single injections of either intramuscular or subcutaneous DMAU in castor oil–benzyl benzoate over a 5- to 7-month period (ClinicalTrials.gov identifier: NCT02927210).
Nonhormonal molecularly targeted male contraceptives
The past 25 years have been an active period for the development of small-molecule male contraceptive methods that do not function through the HPG axis. This approach identifies molecules that modulate a specific molecular target such as a kinase required for the formation and/or maturation of functional sperm. For the development of a contraceptive that must have minimal side effects, selecting targets exclusively or predominantly expressed in the reproductive system will minimize potential systemic toxicity.
A leading example of a nonhormonal molecularly targeted contraceptive is soluble adenyl cyclase (sAC; ADCY10). Targeted deletions of ADCY10 in mice result in male infertility (37), and two infertile men were identified that have identical frameshift mutations that disrupt sAC’s active site (38). A recent study demonstrated inhibition of sperm motility and short-term infertility in mice after intraperitoneal administration of a sAC inhibitor. Mating behavior was not affected, and fertility was restored within 24 hours, demonstrating reversible on-demand male contraceptive activity (39). Additional research to improve drug-like properties, such as oral bioavailability and compound residence time, may extend the duration of effectiveness and may lead to a clinical trial for this potential on-demand male contraceptive.
Innovative nonhormonal contraceptives for women
Ovaprene is an investigational hormone-free, monthly contraceptive intravaginal ring being developed by Daré Bioscience. Sperm transport through the cervix is prevented by the release of ferrous gluconate (a spermiostatic agent) and a knitted polymer barrier that spans the ring (40). Early clinical trials indicate that the device is safe and highly effective at lowering numbers of progressively motile sperm in the post-coital test. The product is intended to be used continuously for up to one menstrual cycle. A pivotal clinical trial is expected to begin in 2023.
Specific molecular targets from the male reproductive tract (testis, epididymis, and prostate) are being developed as user-controlled vaginal contraceptive products. Inhibitory molecules that target proteins required for sperm function “lie in wait” in the vagina and prevent the movement of sperm into the upper regions of the female reproductive tract, where fertilization occurs.
Immunocontraception
The field of immunocontraception began in the 1900s with discoveries that sperm can elicit immune responses in animals and humans, leading to infertility, and that many infertile men and women have antisperm antibodies (41). Several international agencies started contraceptive vaccine programs directed at inhibiting sperm, oocytes, placenta, and endocrine processes. In the 1980s and 90s, clinical trials were conducted to test the safety and efficacy of a contraceptive vaccine that targets human chorionic gonadotropin (hCG), a hormone secreted by the blastocyst early in pregnancy to ensure uterine receptivity. The vaccine trials demonstrated safety, but antibody levels were highly variable, indicating that some women would not be protected from pregnancy (42).
Today, the immunocontraception approach is undergoing a renaissance owing to breakthroughs in bioengineering and the ability to produce clinical-grade human monoclonal antibodies. The use of defined manufactured monoclonal antibodies for passive immunization bypasses the concerns raised with contraceptive vaccines based on active immunization.
One promising example is an antisperm monoclonal antibody, the “human contraceptive antibody” (HCA). This antibody recognizes a sperm surface carbohydrate epitope, CD52 g, and rapidly agglutinates and immobilizes sperm (43). Formulated into a vaginal film, it was recently tested in women in a phase I clinical trial. The antibody film was safe and effectively eliminated progressively motile sperm in cervical mucus in the post-coital test (44). Second-generation multivalent HCA constructs and alternate antibody delivery systems such as intravaginal rings and topical mRNA application are being explored (45).
Multipurpose prevention technologies
MPTs formally emerged from the microbicide field in 2009 and combine protection against multiple reproductive health risks such as unintended pregnancy, HIV, herpes simplex virus (HSV), and other pathogenic STIs. More than 350 million treatable STIs and 1.5 million new HIV cases are reported each year globally, and 500 million adults are infected with and can potentially transmit HSV (46). For this reason, many sexually active women and men would prefer products that are not only contraceptive but also protective against STIs. Condoms are the only MPT products currently available.
N-9, a vaginal spermicide introduced in the 1960s for contraception, showed potent efficacy against HIV and other STIs in preclinical research (47) and was tested in a series of clinical trials for protection against HIV-1 transmission. Unfortunately, vaginal administration of N-9 was not only ineffective against HIV (48) but enhanced transmission in high-risk populations because of its inflammatory effects (49). Five other nonspecific microbicides also failed HIV clinical trials (50).
A newer class of MPTs combines potent antiretroviral drugs (ARVs) with previously approved contraceptive hormones. Levonorgestrel was added to a monthly dapivirine intravaginal ring developed to prevent HIV sexual transmission, and this contraceptive-ARV ring demonstrated safety and effective drug-release profiles in phase I clinical trials. However, the dapivirine ring failed to gain FDA approval for HIV prevention because of poor efficacy (29%) in phase III clinical trials (51, 52). This may be revisited because the World Health Organization (WHO) recently approved the dapivirine ring for HIV prevention (53). Three other potent ARVs—tenofovir alafenamide, islatravir, and dolutegravir—have been formulated into long-acting implants that release effective drug concentrations for 6 months to 1 year (54); plans are underway to add a contraceptive hormone such as levonorgestrel to these implants to develop long-acting contraceptive-MPTs. Oral and injectable anti-HIV–contraception combinations are also being explored. Long-acting cabotegravir-rilpivirine injections were recently approved for HIV prevention and could be combined with injectable contraceptives such as DepoProvera or Net-EN.
Several programs are also pursuing combinations of anti-STI and anti-sperm antibodies as MPTs. A vaginal film containing anti-HIV-1 and anti-HSV monoclonal antibodies was shown to be safe and effective (ex vivo) in a phase I clinical trial (55); plans are underway to combine these antibodies with the antisperm antibody HCA to produce an on-demand MPT vaginal film or an intravaginal ring (56). HCA alone may also have a future as a MPT because the sperm antigen recognized by HCA, a glycoprotein produced in the epididymis, inserts into the plasma membrane of sperm and other cells in semen by means of a glycosylphos-phatidylinositol (GPI) anchor. HCA has been shown to bind to sperm, HIV-infected leukocytes, and certain pathogens such as Trichomonas vaginalis and cell-free HIV in semen and may clear infectious cells and organisms by trapping them in sperm agglutinates or mucus (57).
Industry is critical to bring products to market
The primary funders for contraception development are the National Institutes of Health, the US Agency for International Development, and the Bill and Melinda Gates Foundation. These entities are not equipped to bring products to market. Commitment and action are needed from the pharmaceutical industry, which exited from preclinical contraceptive development around 2007 after decades of activity and innovation. However, the past 5 years have seen renewed interest by pharma in emerging contraceptives. Bayer and Organon have entered into agreements with small biotech companies focused on preclinical-stage and early clinical-stage nonhormonal female contraception programs. Hopefully, this is the start of sustained recommitment to the field.
Conclusions and outlook
The long history of contraception is interesting and varied, from futile attempts to magnificent breakthroughs. A notable advance was achieved when hormonally acting female contraceptives were approved more than 60 years ago. Despite this accomplishment, valid concerns persist about the safety and side effects associated with hormonal methods. Although efficacious alternatives exist, these methods do not meet the full range of needs of men and women throughout their reproductive life spans. A contraception “gap” persists, which modern-day research and industry can bridge.
Exciting programs have emerged that demonstrate proof of concept for hormonal and on-demand male contraception. Targets in the male reproductive tract are being pursued as female contraceptives. Moreover, a newer class of contraceptives designated as MPTs are under development to provide both contraception and protection against STIs (Fig. 3).
Fig. 3. Current and future contraceptive methods.
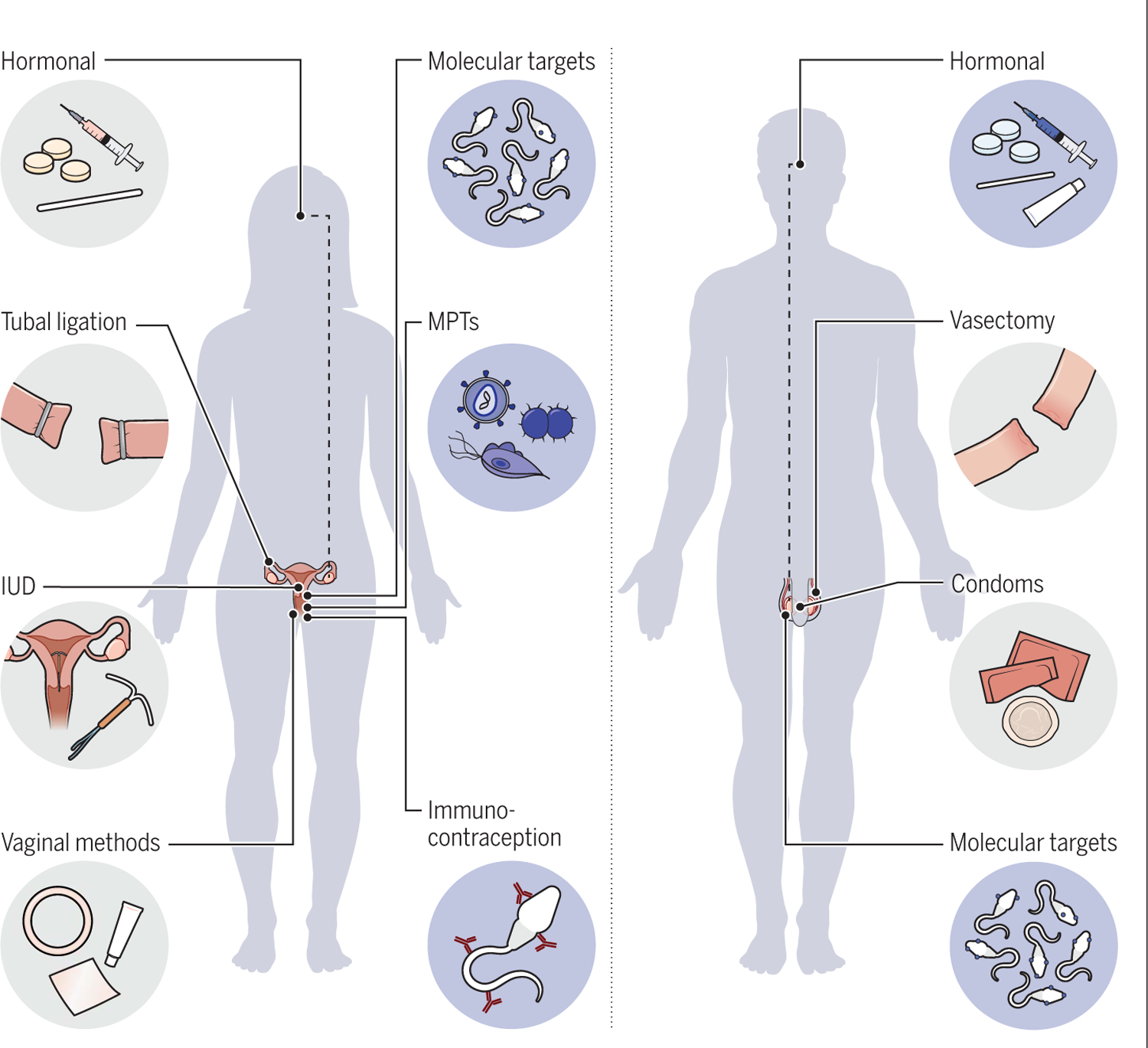
Current contraceptive methods are indicated with gray backgrounds; new contraceptive methods have blue backgrounds.
This renaissance in contraception research has been made possible by renewed commitment from scientists, private foundations, and government institutions. These promising developments await industry involvement to usher them to market and into the hands of people around the world who have long waited for more tools to accomplish their personal goals.
ACKNOWLEDGMENTS
We thank our colleagues—in particular, G. Kopf, W. Wright, K. Whaley, and K. Mayer—for helpful input in early drafts of this manuscript; the anonymous referees for their constructive comments; and J. Politch, E. Mausser, and E. Nador for assistance with the preparation of the manuscript and figures.
Funding:
D.J.A. receives support from P50 HD096957, a contraceptive research center grant from the Eunice Kennedy Shriver National Institutes of Child Health and Human Development (NICHD), National Institutes of Health (NIH). D.S.J. is an employee of NIH. Comments and views of the authors do not necessarily represent the views of the NICHD, NIH, or US federal government.
REFERENCES AND NOTES
- 1.McFarlane I, Ed., State of the World Population 2022 (United Nations Population Fund, 2022). [Google Scholar]
- 2.United Nations, “UN Inter-agency Group for Child Mortality Estimation” (United Nations, 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO, “The Global Health Observatory (Life expectancy at birth)” (WHO, 2023). [Google Scholar]
- 4.Anderson DJ, N. Engl. J. Med 381, 397–399 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haakenstad A et al. , Lancet 400, 295–327 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bearak J et al. , Lancet Glob. Health 8, e1152–e1161 (2020). [DOI] [PubMed] [Google Scholar]
- 7.WHO, “World Health Organization-Adolescent Pregnancy” (WHO, 2022). [Google Scholar]
- 8.MCI, “Potential 17 million men interested in male contraception, consumer survey finds” (MCI, 2019). [Google Scholar]
- 9.Contraceptive Use by Method 2019 (United Nations Population Division, 2019). [Google Scholar]
- 10.Thomas MA et al. , Contracept. X 2, 100031 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trussell J, Contraception 83, 397–404 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finch BE, Green H, in Contraception Through the Ages, Charles C, Ed., (Thomas, 1964). [Google Scholar]
- 13.Khan F, Mukhtar S, Dickinson IK, Sriprasad S, Indian J. Urol 29, 12–15 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deffieux X et al. , Arch. Gynecol. Obstet 283, 1149–1158 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Duijn M, van der Zee JA, Bachour Y, SN Compr. Clin. Med 3, 2193–2203 (2021). [Google Scholar]
- 16.Malacova E, Kemp A, Hart R, Jama-Alol K, Preen DB, Contraception 91, 240–244 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Witherspoon L, Flannigan R, BC Med. J 63, 62–66 (2021). [Google Scholar]
- 18.Colagross-Schouten A, Lemoy MJ, Keesler RI, Lissner E, VandeVoort CA, Basic Clin. Androl 27, 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubacher D, Kavanaugh M, Contraception 98, 467–470 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Turok DK et al. , N. Engl. J. Med 384, 335–344 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asbell B, The Pill: A Biography of the Drug that Changed the World (Random House, 1995). [Google Scholar]
- 22.Risk of thromboembolic disease in women taking oral contraceptives. A preliminary communication to the Medical Research Council by a Subcommittee. BMJ 2, 355–359 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Britton LE, Alspaugh A, Greene MZ, McLemore MR, Am. J. Nurs 120, 22–33 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christin-Maitre S, Best Pract. Res. Clin. Endocrinol. Metab 27, 3–12 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Westhoff CL et al. , Am. J. Obstet. Gynecol 196, 412.e1–412.e6, discussion 412.e6–412.e7 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heckel NJ, Exp. Biol. Med 40, 658–659 (1939). [Google Scholar]
- 27.Reddy PR, Rao JM, Contraception 5, 295–301 (1972). [DOI] [PubMed] [Google Scholar]
- 28.Kogan P, Wald M, Urol. Clin. North Am 41, 145–161 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Thirumalai A, Page ST, Annu. Rev. Med 71, 17–31 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Waites GM, Fertil. Steril 80, 1–15 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Ewing LL, Desjardins C, Irby DC, Robaire B, Nature 269, 409–411 (1977). [DOI] [PubMed] [Google Scholar]
- 32.Chao JH, Page ST, Pharmacol. Ther 163, 109–117 (2016). [DOI] [PubMed] [Google Scholar]
- 33.Piotrowska K, Wang C, Swerdloff RS, Liu PY, Lancet Diabetes Endocrinol. 5, 214–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang C, Swerdloff RS, Curr. Opin. Urol 20, 520–524 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuen F et al. , Andrology 7, 235–243 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Attardi BJ, Hild SA, Reel JR, Endocrinology 147, 3016–3026 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Esposito G et al. , Proc. Natl. Acad. Sci. U.S.A 101, 2993–2998 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akbari A et al. , Hum. Reprod 34, 1155–1164 (2019). [DOI] [PubMed] [Google Scholar]
- 39.Balbach M et al. , Nat. Comm 14, 637 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DareBioscience, “An investigational hormone-free monthly intravaginal contraceptive” (DareBioscience, 2023). [Google Scholar]
- 41.Frayne J, Hall L, J. Reprod. Immunol 43, 1–33 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Jones WR et al. , Lancet 1, 1295–1298 (1988). [DOI] [PubMed] [Google Scholar]
- 43.Baldeon-Vaca G et al. , EBioMedicine 69, 103478 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thurman AR et al. , Am. J. Obstet. Gynecol 10.1016/j.ajog.2023.02.024 (2023). [DOI] [Google Scholar]
- 45.Anderson DJ et al. , Biol. Reprod 103, 275–285 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO, “STIs in 2022: Emerging and re-emerging outbreaks” (WHO, 2022). [Google Scholar]
- 47.Feldblum PJ, Fortney JA, Am. J. Public Health 78, 52–54 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkinson D, Tholandi M, Ramjee G, Rutherford GW, Lancet Infect. Dis 2, 613–617 (2002). [DOI] [PubMed] [Google Scholar]
- 49.Van Damme L et al. , Lancet 360, 971–977 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Notario-Pérez F, Ruiz-Caro R, Veiga-Ochoa MD, Drug Des. Devel. Ther 11, 1767–1787 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baeten JM et al. , N. Engl. J. Med 375, 2121–2132 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nel A et al. , PLOS ONE 11, e0147743 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown ER et al. , J. Int. AIDS Soc 23, e25634 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thoueille P, Choong E, Cavassini M, Buclin T, Decosterd LA, J. Antimicrob. Chemother 77, 290–302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Politch JA et al. , PLOS Med. 18, e1003495 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CAMIHealth, MPT Product Development Database (2023).
- 57.Baldeon-Vaca G et al. , paper presented at the NICHD Contraceptive Research Centers Annual Meeting, Houston, 2019. [Google Scholar]


