Abstract
Infectious intracellular mature vaccinia virus particles are wrapped by cisternae, which may arise from trans-Golgi or early endosomal membranes, and are transported along microtubules to the plasma membrane where exocytosis occurs. We used EH21, a dominant-negative form of Eps15 that is an essential component of clathrin-coated pits, to investigate the extent and importance of endocytosis of viral envelope proteins from the cell surface. Several recombinant vaccinia viruses that inducibly or constitutively express an enhanced green fluorescent protein (GFP)-EH21 fusion protein were constructed. Expression of GFP-EH21 blocked uptake of transferrin, a marker for clathrin-mediated endocytosis, as well as association of adaptor protein-2 with clathrin-coated pits. When GFP-EH21 was expressed, there were increased amounts of viral envelope proteins, including A33, A36, B5, and F13, in the plasma membrane, and their internalization was inhibited. Wrapping of virions appeared to be qualitatively unaffected as judged by electron microscopy, a finding consistent with a primary trans-Golgi origin of the cisternae. However, GFP-EH21 expression caused a 50% reduction in released enveloped virions, decreased formation of satellite plaques, and delayed virus spread, indicating an important role for receptor-mediated endocytosis. Due to dynamic interconnection between endocytic and exocytic pathways, viral proteins recovered from the plasma membrane could be used by trans-Golgi or endosomal cisternae to form new viral envelopes. Adherence of enveloped virions to unrecycled viral proteins on the cell surface may also contribute to decreased virus release in the presence of GFP-EH21. In addition to a salvage function, the retrieval of viral proteins from the cell surface may reduce immune recognition.
Poxviruses are large, enveloped DNA viruses that replicate in the cytoplasm of their host cells (23). The assembly of vaccinia virus, the most intensively studied member of the poxvirus family, can be divided into two phases. The first begins with the formation of crescent-shaped membranes in virus factory areas of the cytoplasm and culminates in the production of infectious intracellular mature virions (IMV) (8). The second phase begins with the wrapping of IMV particles by cisternal membranes to form intracellular enveloped virions (IEV) (13). IEV are transported along microtubules to the periphery, where their outer membrane fuses with the plasma membrane (14, 38, 39). The majority of the extracellular virus particles, called cell-associated enveloped virions (CEV), adheres to the plasma membrane and mediates direct cell-to-cell spread (4). The released extracellular enveloped virions (EEV) may contribute to the long-range spread of virus (24).
Seven proteins are known to be associated with IEV- or EEV-specific membranes. Of these, A36 (35), A56 (28), and B5 (11, 19) are type I integral membrane proteins, A33 (25) and A34 (9) are type II integral membrane proteins, and F13 (17) and probably F12 (34) are peripheral membrane proteins. The F13 and B5 proteins are required for wrapping of the IMV, and the other proteins are involved in subsequent steps, including intracellular movement, actin tail formation, and virus spread (29). Most of the IEV and CEV/EEV membrane proteins traffic through the secretory pathway and are detected in the endoplasmic reticulum, Golgi apparatus, and plasma membrane to various extents (21). F13 is exceptional in that it is targeted directly to Golgi membranes (16). When COPII-mediated transport was inhibited, the EEV membrane proteins examined except for F13 were retained in the endoplasmic reticulum and IEV were not formed (15). The latter data indicate that the membranes which wrap IMV must form downstream of the endoplasmic reticulum. The lectin labeling pattern of the IEV outer membranes (27) and phospholipid analysis of purified EEV (30) are consistent with an origin from a late Golgi or post-Golgi compartment. Trans-Golgi cisternae (27) and early endosomes (32) each have been proposed as the progenitors of the wrapping membrane for reasons to be discussed later. The retrieval of viral proteins from the plasma membrane to the endocytic compartment, a potentially important step if wrapping of IMV occurs by early endosomes, has thus far been demonstrated only for B5 (37) and F13 (16), and the significance of this is unknown.
The purpose of the present study was to investigate the receptor-mediated endocytosis of viral membrane proteins and its impact on the formation of extracellular virions. Proteins associated with the plasma membrane that cluster in clathrin-coated pits are internalized through the action of adaptor and accessory proteins (22, 41), including Eps15 (epidermal growth factor receptor pathway substrate clone 15) (2, 31). A dominant-negative form of Eps15 called EH21 and a green fluorescent protein (GFP)-EH21 fusion protein inhibit receptor-mediated endocytosis (1, 3). In a previous study (16), we showed that cotransfection of an expression plasmid encoding GFP-EH21 and a plasmid expressing the vaccinia virus F13 protein resulted in the accumulation of the latter in the plasma membrane. This result provided evidence for receptor-mediated endocytosis of F13, which was inhibited by the dominant-negative form of Eps15. To determine the effect of inhibiting receptor-mediated endocytosis in the context of a virus infection, we have now integrated the GFP-EH21open reading frame (ORF) into the vaccinia virus genome. When endocytosis was inhibited, there were increased amounts of viral envelope proteins in the plasma membrane, and their retrieval was blocked. Concomitantly, there was a 50% reduction in the formation of extracellular virus. We concluded that endocytosis is not essential for wrapping of vaccinia virions but most likely provides a salvage pathway for the recovery of viral proteins. The removal of viral proteins from the plasma membrane may have a second benefit in reducing immune recognition of infected cells.
MATERIALS AND METHODS
Cells and viruses.
BS-C-1 and HeLa cells were propagated in minimum essential medium with Earle’s balanced salts (Quality Biological, Inc.) and Dulbecco’s modified Eagle’s medium (Quality Biological), respectively, supplemented with 10% fetal bovine serum (FBS) at 37°C with 5% CO2 atmosphere. Virus plaque assays were performed on BS-C-1 cells with or without the addition of a methylcellulose overlay. HeLa cells were used to prepare virus stocks (10).
Plasmids.
To construct plasmids pVGFP-EH21, pRGFP-EH21, and pRGFP, coding sequences for EH21 and GFP were amplified by PCR from pGFP-EH21 (1) and inserted into plasmids pVOTE.1 (40) or pRB21 (5) by two- or three-way ligation.
Transfection and infection.
HeLa cells grown on glass coverslips were transfected with DNA by using Lipofectamine 2000 (Invitrogen) in Opti-MEM I medium (Invitrogen) according to the manufacturer's recommendations. After 4 to 5 h at 37°C, fresh medium containing 10% FBS was added to the cells. For infection of HeLa or BS-C-1 cells, virus stocks were diluted in Dulbecco’s modified Eagle’s medium or minimum essential medium, respectively, supplemented with 2.5% FBS to achieve a multiplicity of infection of 2 to 5 PFU per cell. After 1 h at 37°C, the virus inoculum was replaced with fresh medium containing 2.5% FBS. IPTG (isopropyl-β-d-thiogalactopyranoside) at 100 μM was included in the medium where indicated.
Generation of recombinant vaccinia viruses.
For selection of vEH21i, BS-C-1 cells that had been pretreated with medium containing mycophenolic acid, xanthine, and hypoxanthine (12) were infected with a diluted lysate of cells that had been infected with 0.1 PFU of vT7LacOI (40) and transfected with pVGFP-EH21. For isolation of vWEH21, vIEH21, vWGFP, or vIGFP, BS-C-1 cells were infected with a diluted lysate of cells that had been infected with vF13Δ (5) at a multiplicity of 0.1 PFU and transfected with plasmids pRGFP-EH21 or pRGFP; large green plaques were picked after 2 days. After three rounds of plaque purification, the purity of the recombinant viruses was determined by PCR.
Antibodies and chemicals.
Antibody to a peptide sequence at the C terminus of the A36 protein was made in rabbits (42). Mouse anti-A33 monoclonal antibody (MAb) was a gift from A. Schmaljohn; rat MAb 192C to the B5 protein and polyclonal antiserum to the F13 protein were from G. Hiller. Mouse anti-GFP MAb was purchased from Covance. Mouse anti-p230 and anti-α-adaptin MAbs were from Transduction Laboratories and Affinity Bioreagents, respectively. Rhodamine red-conjugated anti-rabbit immunoglobulin G (IgG) and Cy5-conjugated anti-rat IgG were purchased from Jackson Immunoresearch Laboratories. Alexa 568-conjugated anti-rat IgG, Alexa 594-conjugated anti-mouse IgG, and Texas Red-conjugated transferrin (TR-Tfn) protein and FM4-64 were procured from Molecular Probes. Horseradish peroxidase-conjugated anti-mouse IgG was from ICN Biomedicals.
Western blotting.
Infected cells were lysed in ice-cold radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate) and incubated on ice for 10 min. The cell lysate was centrifuged at 20,000 × g for 10 min at 4°C, and the supernatant was analyzed by electrophoresis in a 12% polyacrylamide bis(2-hydroxyethyl)imino]-tris(hydroxymethyl)methane gel with 2-morpholineethanesulfonic acid buffer or a 4 to 12% bis(2-hydroxyethyl)imino]-tris(hydroxymethyl)methane gel with 3-(N-morpholino)propanesulfonic acid buffer. Proteins were transferred to a nitrocellulose membrane, blocked with 5% milk, and probed with mouse anti-GFP antibody diluted in 5% milk-phosphate-buffered saline (PBS) containing 5% milk, followed by horseradish peroxidase-conjugated anti-mouse antibody. The membrane was washed, and proteins were visualized by Super signal chemiluminescence substrate (Pierce).
Confocal microscopy.
Infected cells were washed with PBS and fixed by adding cold 4% paraformaldehyde in PBS, followed by incubation at room temperature for 20 min. Cells were washed twice with PBS and permeabilized for 5 min with 0.2% Triton X-100 in PBS at room temperature. Cells were again washed three times with PBS and incubated with primary antibodies diluted in 10% FBS in PBS for 1 h, followed by secondary antibody diluted in 10% FBS in PBS for 30 min at room temperature. Cells were washed at least three times with PBS after incubation with each antibody. Coverslips were mounted in 20% glycerol and fluorescence was examined under Leica TCS SP2 inverted confocal microscope. Images were analyzed and overlaid by using Adobe Photoshop version 7.0.
Flow cytometry.
Infected HeLa cell monolayers were washed twice with PBS and incubated in EDTA (Gibco) at room temperature. Cell suspensions were washed with ice-cold PBS by centrifugation at 200 × g for 3 min at 4°C. Cells were then stained with rat anti-B5 MAb diluted in 10% FBS in PBS at 4°C for 30 min, washed twice with ice-cold PBS, and incubated with Cy5-conjugated anti-rat IgG diluted in 10% FBS in PBS for 30 min at 4°C. Cells were washed again twice with PBS and analyzed directly. In some experiments the cells were fixed in 4% paraformaldehyde in PBS for 20 min at 4°C before staining. Flow cytometric data were acquired and analyzed in Becton Dickinson FACSCalibur and Flowjo 4.5.9 software, respectively.
Statistical analysis.
Data were analyzed with StatView version 5.0.1 (StatView Software) by using an analysis of variance unpaired t test.
RESULTS
Construction of recombinant vaccinia viruses inducibly and constitutively expressing a GFP-EH21 fusion protein.
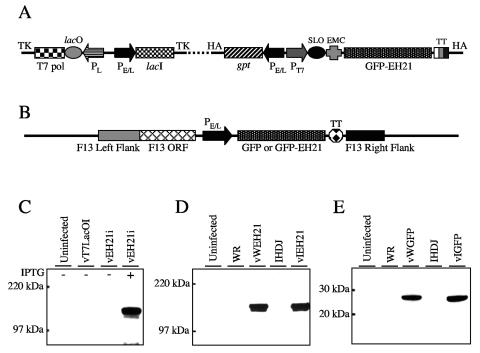
Because of the possibility that constitutive expression of GFP-EH21 might have severe effects on virus replication, our initial recombinant vaccinia virus contained an inducible GFP-EH21 gene. A general strategy for making inducible vaccinia viruses has been described (40) and utilizes a vaccinia virus (strain WR) containing the bacteriophage T7 RNA polymerase gene regulated by an Escherichia coli lac operator and a constitutively expressed E. coli lac repressor. The GFP-EH21-fused ORF, regulated by a T7 promoter and a lac operator, was inserted into a second site in the vaccinia virus genome. All isolation and propagation steps were carried out in the absence of IPTG to prevent induction of EH21, and the recombinant virus was called vEH21i (Fig. 1A).
FIG. 1.
Construction of recombinant vaccinia viruses expressing a GFP-EH21 fusion protein. (A) Diagram of relevant portions of recombinant vaccinia virus vEH21i. Abbreviations: TK, thymidine kinase gene; T7 pol, bacteriophage T7 RNA polymerase gene; lacO, E. coli lac operator; PL, vaccinia virus P11 late promoter; PE/L, vaccinia virus P7.5 early/late promoter; lacI, E. coli lac repressor gene; HA, vaccinia virus hemagglutinin gene; gpt, E. coli guanine phosphotransferase gene; SLO, stem-loop lacO; EMC, encephalomyocarditis virus untranslated leader sequence; GFP-EH21, ORF encoding a fusion of GFP and EH21; TT, transcription termination sequences. (B) Diagram of relevant portions of recombinant vaccinia virus vWEH21, vIEH21, vWGFP, and vIGFP. Additional abbreviations: F13 ORF, ORF encoding vaccinia virus F13 protein; F13 left flank and right flank, DNA sequences on the left and right sides of F13 ORF. Immunoblots showing inducible expression of GFP-EH21 (C), constitutive expression of GFP-EH21 fusion protein (D) or GFP alone (E). HeLa cells were infected for 20 h with the indicated wild-type or recombinant viruses. Proteins from the cell lysates were resolved by polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and detected with mouse anti-GFP MAb.
Because inducible expression of GFP-EH21 had only a small effect on plaque size (to be shown later), it was possible to make additional recombinant vaccinia viruses that constitutively express GFP-EH21 from a strong synthetic early or late promoter. A previously described plaque selection method, which depends on the restoration of the F13L ORF (5), was used to make recombinant viruses vWEH21 and vIEH21 in vaccinia virus strain WR and IHD-J backgrounds, respectively (Fig. 1B). WR, the most widely used laboratory strain of vaccinia virus, makes large amounts of CEV and low amounts of EEV; IHD-J makes more EEV because of a mutation in the A34 ORF (6), a feature useful for some later experiments. The control recombinant viruses vWGFP and vIGFP are similar to vWEH21 and vIEH21 except that they express GFP instead of the fusion protein.
Inducible (Fig. 1C) and constitutive (Fig. 1D) expression of the 140-kDa GFP-EH21 fusion protein or the 25-kDa GFP (Fig. 1E) was demonstrated by Western blotting with an anti-GFP antibody. No band was detected in the cells infected with parental viruses vT7lacOI, WR, and IHD-J or vEH21i in the absence of inducer.
Effect of GFP-EH21 expression on endocytosis of transferrin and localization of adaptor protein 2 (AP-2).
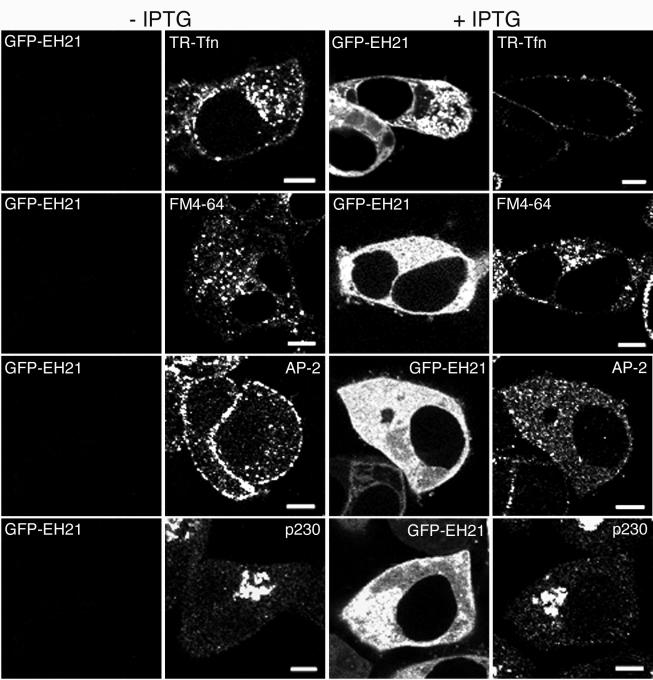
Before investigating the localization of viral proteins in the presence of GFP-EH21, we determined the effects of the inhibitor on transferrin, a serum glycoprotein that binds Fe3+ atoms for delivery to vertebrate cells through clathrin-dependent mechanisms and which is widely used as a marker of receptor-mediated endocytosis (20, 26). In cells infected with vEH21i in the absence of IPTG, expression of GFP-EH21 was not detected by fluorescence microscopy, whereas there was a strong signal in the presence of IPTG (Fig. 2), as expected from the previous Western blots. In the absence of GFP-EH21, a 20-min incubation with TR-Tfn, a fluorescent Texas Red derivative of transferrin, resulted in internalization and cytoplasmic punctate staining, presumably of the perinuclear endocytic compartment (Fig. 2, −IPTG). In cells expressing GFP-EH21, however, there was only surface staining of TR-Tfn (Fig. 2, +IPTG). Similar results were obtained with the recombinant vaccinia viruses that constitutively express GFP-EH21 (not shown). To prove that the effect of GFP-EH21 was specific for clathrin-mediated endocytosis, we carried out further studies with FM4-64, a soluble, nontoxic dye that fluoresces upon nonspecific incorporation into the outer leaflet of the plasma membrane (7). The subsequent internalization of FM4-64 into cytoplasmic membrane vesicles is not dependent on Eps15. Accordingly, we found a similar punctate staining of FM4-64 in cells infected with vEH21i in presence or absence of IPTG (Fig. 2). GFP-EH21 expression also had no adverse effect on the Golgi network, as shown by the similar juxtanuclear staining of p230, a trans-Golgi protein, in the absence or presence of IPTG (Fig. 2).
FIG. 2.
Effect of GFP-EH21 on endocytosis. HeLa cells were infected with vEH21i in the absence or presence of IPTG as indicated. After 8 h, cells were incubated with TR-Tfn (row 1) or FM4-64 (row 2) for 20 min at 37°C, fixed, and examined by confocal microscopy as described previously (1, 16). Alternatively, the cells were fixed, permeabilized, and stained with mouse anti-α adaptin MAb (row 3) and mouse anti-p230 MAb (row 4), followed by Alexa 594-conjugated antibody to mouse IgG. Bars, 10 μm.
The previous experiments demonstrated that GFP-EH21 expression specifically inhibited clathrin-dependent endocytosis. Next, we analyzed the effect of GFP-EH21 expression on the cellular distribution of AP-2, which is required for assembly of clathrin pits. In cells infected with vEH21i in the absence of IPTG, antibody to AP-2 decorated the plasma membrane (Fig. 2), whereas AP-2 was largely distributed in the cytoplasm when expression of GFP-EH21 was induced. These data indicated that GFP-EH21, expressed by a recombinant vaccinia virus, inhibited the assembly, as well as the endocytic function, of clathrin pits.
Effect of GFP-EH21 expression on the localization of viral envelope proteins.
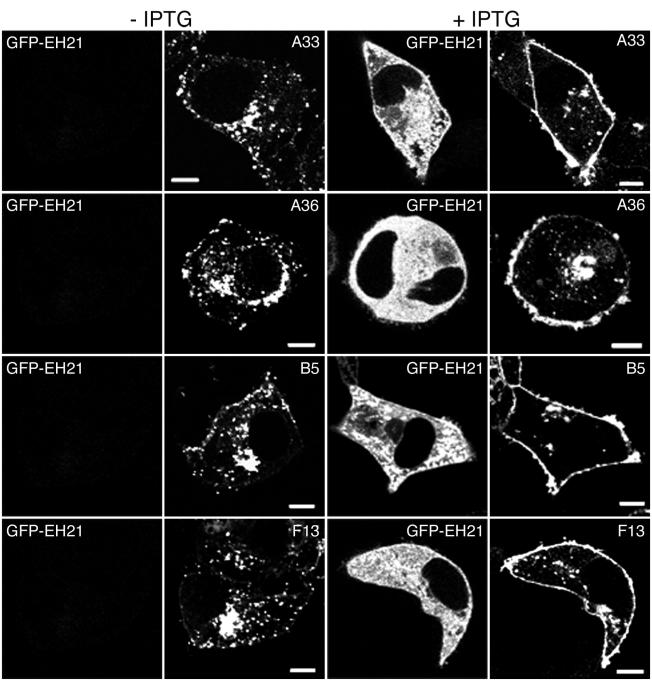
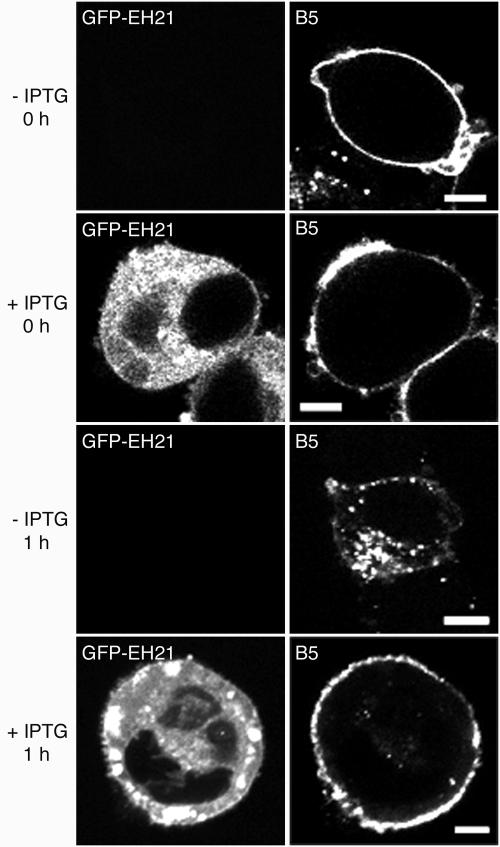
We considered that if viral proteins in the plasma membrane were recycling, blocking receptor-mediated endocytosis should shift the steady state of viral protein on the surface. Specific antibodies were used to localize the viral envelope proteins B5, F13, A33, and A36. Although there was some variation between individual cells, we selected representative ones for Fig. 3. The expected Golgi membrane and peripheral punctate and surface staining of B5, F13, A33, and A36 occurred in the absence of GFP-EH21 expression (Fig. 3, −IPTG). When GFP-EH21 was induced, there was relatively greater staining at the cell surface in each case (Fig. 3, +IPTG). Similar results were also obtained by constitutive expression of GFP-EH21 (not shown).
FIG. 3.
Effect of GFP-EH21on the localization of viral envelope proteins. HeLa cells were infected with vEH21i in the absence or presence of IPTG as indicated. After 8 h the infected cells were fixed, permeabilized, and stained with anti-A33 mouse MAb (row 1), anti-A36 rabbit antibody (row 2), anti-B5 rat MAb (row 3), and anti-F13 rabbit antibody (row 4), followed by the appropriate secondary fluorescent antibodies as follows: Alexa 594-conjugated anti-mouse IgG, rhodamine red-conjugated anti-rabbit, and Alexa 568-conjugated anti-rat IgG. Bars, 10 μm.
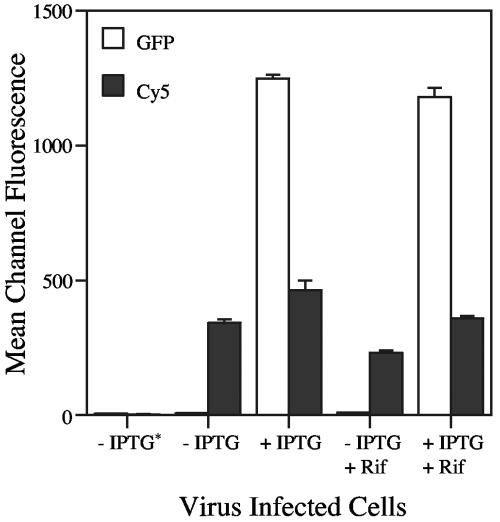
Flow cytometry was used to objectively compare the steady-state amounts of viral protein on the plasma membrane in the presence or absence of GFP-EH21 expression. The results obtained by live staining of unpermeabilized cells infected with vEH21i indicated a statistically significant (P = 0.012) 36% increase in surface expression of B5 when GFP-EH21 was expressed (Fig. 4). When cells were fixed before staining, the overall fluorescence was reduced by nearly half due to poorer antibody binding, but the difference in the presence or absence of IPTG remained 40% (data not shown). The surface fluorescence could represent B5 in the plasma membrane following trafficking through the secretory pathway or fusion of the outer IEV membrane and B5 in enveloped virus particles attached to the outside of the cell. To examine the effect of GFP-EH21 on viral proteins that traffic exclusively through the secretory path, we infected cells with vEH21i in the presence of rifampin, a specific inhibitor of vaccinia virus assembly. Under these conditions, the surface staining of B5 was slightly reduced but the relative increase caused by GFP-EH21 expression (55%) was greater (Fig. 4).
FIG. 4.
Effect of GFP-EH21 on the cell surface expression of the viral B5 envelope protein. HeLa cells were infected with vEH21i in the absence (−) or presence (+) of IPTG and rifampin (Rif). At 8 h after infection, the cells were detached from the plate and directly stained with anti-B5 rat MAb, followed by the addition of Cy5-conjugated anti-rat IgG, and then analyzed by flow cytometry. The experiment was carried out in triplicate and the data averaged. −IPTG*, a control to which no antibodies were added. GFP, GFP-EH21 fluorescence; Cy5, B5 fluorescence.
The availability of a MAb to the extracellular domain of the B5 protein allowed us to directly investigate the effect of GFP-EH21 expression on viral protein internalization. Cells infected for 8 h with vEH21i in the presence or absence of IPTG were incubated for 1 h on ice with the B5 MAb and then washed and incubated for 1 h at 37°C to allow endocytosis of the MAb-B5 complex. After fixation and permeabilization, the MAb was detected by using a secondary fluorescent antibody. Prior to the 37°C incubation, surface staining was detected in the presence or absence of IPTG (Fig. 5). After incubation at 37°C for 1 h, the fluorescent staining was still mostly at the surface of cells that expressed GFP-EH21 (Fig. 5). In contrast, the staining was largely intracellular in cells that did not express GFP-EH21 (Fig. 5). The punctate staining, which remained at the cell surface under the latter conditions, could represent CEV.
FIG. 5.
Effect of GFP-EH21 on the endocytosis of the viral B5 protein. HeLa cells were infected with vEH21i in the absence or presence of IPTG. After 8 h, the cells were washed twice with ice-cold PBS and incubated with an anti-B5 MAb diluted in PBS for 1 h on ice. Cells were washed twice with PBS and either fixed immediately, permeabilized, and stained with Alexa 568-conjugated anti-rat IgG or incubated with fresh medium (with [+] or without [−] IPTG) for 1 at 37°C before fixing, permeabilizing, and staining. Bar, 10 μm.
Effect of GFP-EH21 expression on IEV formation.
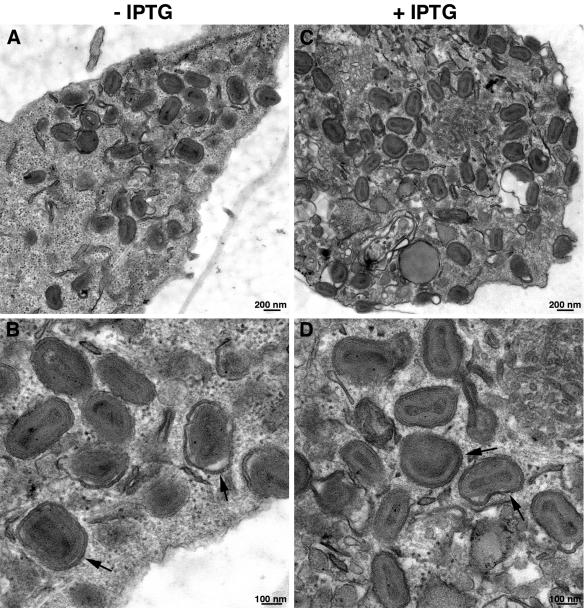
If the membrane that wraps IMV were formed exclusively through the endocytic pathway, there would be a dramatic effect of EH21 on IEV and CEV. To investigate this, we infected BS-C-1 cells with vEH21i in the presence or absence of IPTG and fixed them at 8, 12, and 24 h. At each time point, IEV and CEV were visualized by electron microscopy. Sections of cells infected for 12 h are shown in Fig. 6. The IEV and CEV made in the presence or absence of GFP-EH21 were indistinguishable, indicating that the endocytic recycling pathway is not essential for IMV wrapping. The numbers of such particles in cell sections varied greatly, however, making it difficult to accurately quantify them.
FIG. 6.
Effect of GFP-EH21 on formation of IEV and CEV. BS-C-1 cells were infected with vEH21i for 8 h in the absence (A and B) or presence (C and D) of IPTG. Thin sections were viewed by transmission electron microscopy, and images were obtained at low (A and C) and high (B and D) magnifications. The arrows point to the representative IEV membranes. The magnifications are indicated by the scale bars.
Effect of GFP-EH21 expression on vaccinia virus spread and EEV formation.
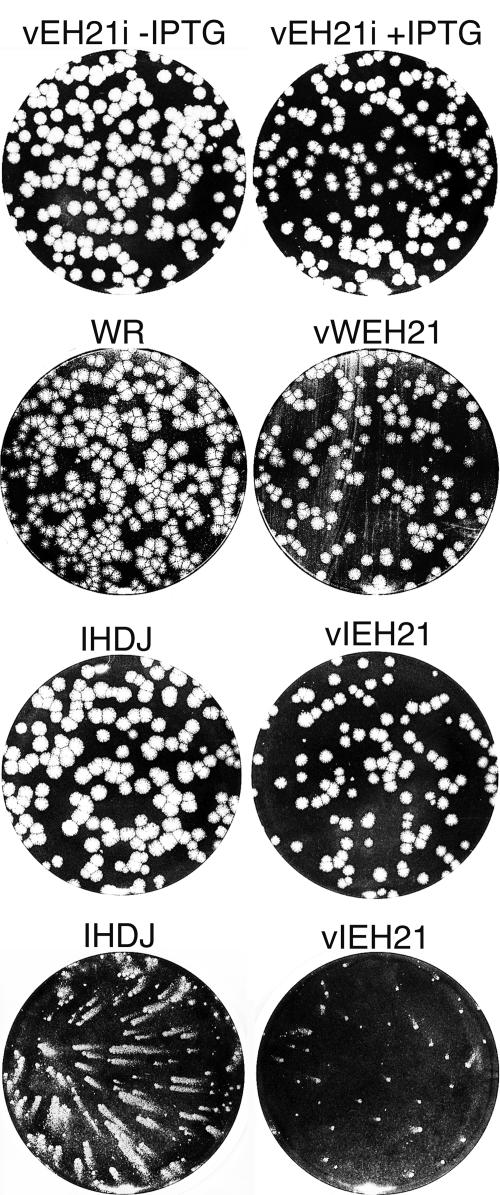
Next, we investigated the effects of inhibiting receptor-mediated endocytosis on virus replication and spread. The majority of plaques formed by vEH21i under a semisolid methylcellulose overlay appeared smaller in the presence of IPTG than in its absence (Fig. 7), suggesting some effect of GFP-EH21 on cell-to-cell spread of virus. However, the reduction in plaque size was relatively minor, and we considered that the effect of GFP-EH21 on envelope formation might be mitigated because induction of recombinant protein expression slightly trails viral late protein synthesis in the inducible expression system. In vWEH21 and vIEH21, however, an early or late promoter regulates GFP-EH21 and consequently expression of the inhibitor starts 3 or 4 h prior to the synthesis of viral membrane proteins. Nevertheless, vWEH21 plaques were still only slightly smaller than those of WR (Fig. 7) or vWGFP (not shown). Both vEH21i and vWEH21 were derived from the WR strain, which forms mostly CEV and very little EEV. The reduction in plaque size appeared more pronounced when vIEH21, which makes more EEV and less CEV, was compared to IHD-J (Fig. 7) or the control virus vIGFP (not shown).
FIG. 7.
Effect of GFP-EH21 on plaque formation. BS-C-1 cells were infected with vaccinia virus vEH21i with or without IPTG, WR, IHD-J, vWEH21, or vIEH21 as indicated. The cells were covered with a semisolid methylcellulose overlay and incubated for 3 days (rows 1, 2, and 3) or with a liquid overlay and incubated for 1 day (bottom row). Plaques were visualized by staining with crystal violet in 20% ethanol.
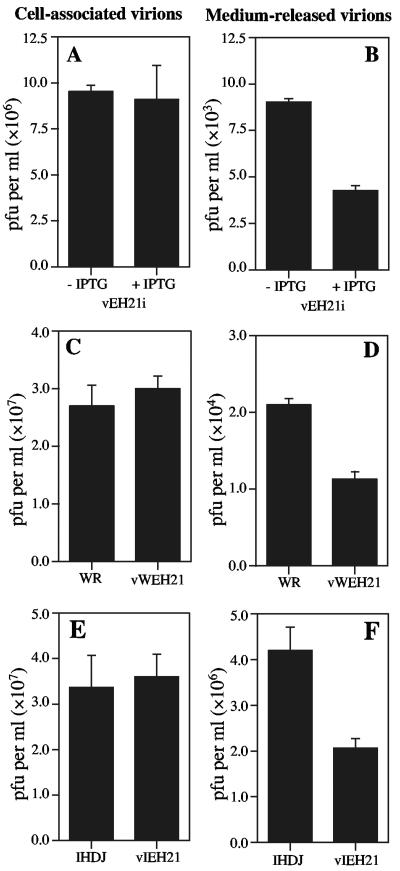
When a liquid overlay is used, instead of a semisolid one, the IHD-J strain forms comet-shaped satellite plaques due to release of large numbers of EEV. Expression of GFP-EH21, greatly reduced the size of the comets (Fig. 7), suggesting an inhibition of EEV formation. One-step growth experiments were carried out to test this explanation. We compared the amounts of cell-associated and released virus in cells infected with vEH21i in the absence of IPTG versus the presence of IPTG, with WR versus vWEH21, and with IHD-J versus vIEH21. In each comparison, we found no difference in the amounts of cell-associated virus but a consistent twofold reduction of virus in the medium (Fig. 8). In each case these differences were statistically significant with P values of 0.005 to 0.0001.
FIG. 8.
Effect of GFP-EH21 on the yields of cell-associated and released virus. BS-C-1 cells were infected with 5 PFU per cell of vEH21i with (+) or without (−) IPTG, WR, IHD-J, vWEH21, or vIEH21 as indicated. After 24 h the medium and cells were harvested separately, and the amount of infectious virus in each was determined by plaque assay. Means with standard deviations from three separate infections are shown.
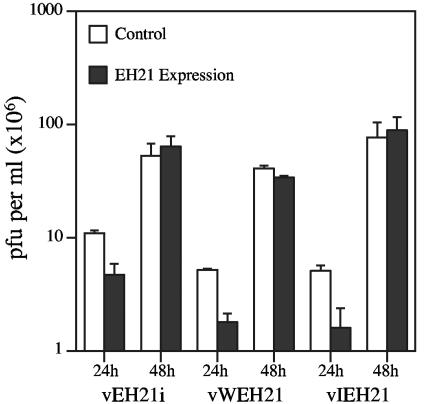
We also measured the rate of virus spread by infecting BS-C-1 cells at a multiplicity of 0.001 PFU per cell. After 24 h, the titers of the recombinant viruses expressing EH21 were about half that of the control viruses, but by 48 h the titers were equivalent (Fig. 9). The differences at 24 h were statistically significant with P values of 0.007 to 0.0002.
FIG. 9.
Effect of GFP-EH21 on virus spread. BS-C-1 cells were infected with 0.001 PFU per cell of vEH21i in the absence (−) or presence (+) of IPTG, of vWEH21 or vWGFP control, or of vIEH21 or vIGFP control. Cell monolayers were harvested at 24-h intervals. Cells were lysed, and virus titers were determined by plaque assay. The data presented are means, with the standard deviations, of three separate infections.
DISCUSSION
Although poxviruses encode about 200 proteins, they depend on cellular processes for many steps in their life cycle. Dominant-negative protein inhibitors can provide a powerful way of blocking a cellular pathway and determining the consequence on virus replication. The inhibitor proteins or plasmids expressing them are usually introduced into cells by injection or transfection. These procedures, however, may result in incomplete and variable expression. Previously, we used a stringently controlled inducible vaccinia virus system (40) for expressing a dominant-negative form of Sar 1 to shut down COPII-mediated transport in infected cells (15). The latter study demonstrated that transport from the endoplasmic reticulum to the Golgi apparatus was unnecessary for synthesizing the primary membrane of IMV particles but was required for wrapping of IMV by cisternal membranes. In the present study, we expressed a dominant-negative inhibitor of receptor-mediated endocytosis to investigate the importance of another cellular pathway for vaccinia virus replication.
EH21, a dominant-negative form of Eps15 with a deletion of amino acids 95 to 295, and a related GFP-EH21 fusion protein selectively block clathrin-mediated endocytosis by binding AP-2, which interacts with the cytoplasmic domain of recycling membrane proteins (1, 2, 31). When GFP-EH21 was inducibly or constitutively expressed by recombinant vaccinia viruses, we found increased amounts of several viral proteins (B5, F13, A33, and A36) on the cell surface, suggesting that they are recycled under normal conditions. The viral proteins detected on the cell surface could represent proteins inserted into the plasma membrane via the direct exocytic pathway or when the outer IEV membrane fuses with the plasma membrane, as well as proteins associated with intact CEV particles. This distinction is important because the latter are too large to be retrieved by clathrin-mediated endocytosis. When the drug rifampin was used to prevent virus assembly so that only integral plasma membrane proteins are detected, the cell surface expression of the B5 protein decreased somewhat, but the relative effect of GFP-EH21 inhibition of endocytosis was greater. Flow cytometry indicated that GFP-EH21 expression increased the surface level of B5 by 36% in the absence of rifampin and 55% in the presence of the drug. B5 was previously shown to have functional internalization motifs (37); F13 has a large number of potential internalization motifs and was found to interact with AP-2 (16). We do not know whether endocytosis of A33 and A36 occurs independently or is dependent on their interaction with other proteins. Inspection of their amino acid sequences indicated that A33 has a tyrosine within its short cytoplasmic domain and that A36 has several tyrosines and a dileucine motif in its long cytoplasmic domain, which could represent internalization motifs.
Endocytosis may be important at several different stages of the vaccinia virus life cycle. The low-pH requirement for efficient entry of the EEV form of vaccinia virus suggests that an endocytic pathway is used (18, 36), although vaccinia virus may be too large for internalization through clathrin pits. Endocytosis may also be important for virus assembly. A model, based primarily on the distribution of fluid-phase markers, posits that the cisternae that wrap IMV are derived from early endosomes (32). The evidence for the latter model has been questioned, however, on the grounds that the endocytic and exocytic fluid phases are dynamically interconnected during vaccinia virus infection (27). Schmelz and coworkers (27) favor a trans-Golgi origin based on the localization of viral proteins and the finding of a trans-Golgi marker in the wrapping membrane. The routing of viral membrane proteins from the plasma membrane to early endosomes could be crucial if the latter form the wrapping membranes. However, the salvage of viral proteins from the plasma membrane could be important even if the envelope is formed solely from trans-Golgi cisternae. Despite the inhibition of endocytosis by GFP-EH21, wrapping of IMV appeared qualitatively normal by electron microscopy, indicating that the cisternal membranes form from the exocytic pathway. Nevertheless, EEV formation was reduced by ca. 50%, implying that endocytic membranes are secondarily involved in wrapping or that viral envelope proteins and possibly membrane are limiting in amount and must be retrieved from the plasma membrane for maximal wrapping by trans-Golgi cisternae. The dynamic interconnection between the endocytic and exocytic compartments (22) is consistent with the latter idea. Also, there is evidence that formation of IEV peaks earlier in infection than IMV, a finding consistent with the depletion of membrane precursors (33). The diminished formation of extracellular virus had a greater impact on the number of satellite plaques than on plaque diameters. The lesser effect on plaque size may be due to the ability of CEV to efficiently spread to neighboring cells even if their numbers are moderately reduced. A tighter interaction of CEV with increased amounts of viral proteins on the plasma membrane in the presence of GFP-EH21 is an alternative mechanism to explain the lower amounts of released EEV. Although not investigated in the present study, the retrieval of viral proteins from the plasma membrane may have an additional benefit in reducing immune recognition of infected cells.
Acknowledgments
We thank A. Benmerah for the GFP-EH21 plasmid, A. Weisberg for electron microscopy, and N. Cooper for cell cultures.
REFERENCES
- 1.Benmerah, A., M. Bayrou, N. Cerf-Bensussan, and A. Dautry-Varsat. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303-1311. [DOI] [PubMed] [Google Scholar]
- 2.Benmerah, A., J. Gagnon, B. Begue, B. Megarbane, A. Dautry-Varsat, and N. Cerf-Bensussan. 1995. The tyrosine kinase substrate eps15 is constitutively associated with the plasma membrane adaptor AP-2. J. Cell Biol. 131:1831-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benmerah, A., C. Lamaze, B. Begue, S. L. Schmid, A. Dautry-Varsat, and N. Cerf-Bensussan. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blasco, R., and B. Moss. 1992. Role of cell-associated enveloped vaccinia virus in cell-to-cell spread. J. Virol. 66:4170-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blasco, R., and B. Moss. 1995. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene 158:157-162. [DOI] [PubMed] [Google Scholar]
- 6.Blasco, R., J. R. Sisler, and B. Moss. 1993. Dissociation of progeny vaccinia virus from the cell membrane is regulated by a viral envelope glycoprotein: effect of a point mutation in the lectin homology domain of the A34R gene. J. Virol. 67:3319-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butterworth, M. B., S. I. Helman, and W. J. Els. 2001. cAMP-sensitive endocytic trafficking in A6 epithelia. Am. J. Physiol. Cell Physiol. 280:C752-C762. [DOI] [PubMed] [Google Scholar]
- 8.Dales, S., and L. Siminovitch. 1961. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J. Biophys. Biochem. Cytol. 10:475-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duncan, S. A., and G. L. Smith. 1992. Identification and characterization of an extracellular envelope glycoprotein affecting vaccinia virus egress. J. Virol. 66:1610-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Earl, P. L., B. Moss, L. S. Wyatt, and M. W. Carroll. 1998. Generation of recombinant vaccinia viruses, p. 16.17.1-16.17.19. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 2. Greene Publishing Associates/Wiley Interscience, New York, N.Y. [Google Scholar]
- 11.Engelstad, M., S. T. Howard, and G. L. Smith. 1992. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that forms part of the extracellular virus envelope. Virology 188:801-810. [DOI] [PubMed] [Google Scholar]
- 12.Falkner, F. G., and B. Moss. 1988. Escherichia coli gpt gene provides dominant selection for vaccinia virus open reading frame expression vectors. J. Virol. 62:1849-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiller, G., and K. Weber. 1985. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J. Virol. 55:651-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollinshead, M., G. Rodger, H. Van Eijl, M. Law, R. Hollinshead, D. J. Vaux, and G. L. Smith. 2001. Vaccinia virus utilizes microtubules for movement to the cell surface. J. Cell Biol. 154:389-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Husain, M., and B. Moss. 2003. Evidence against an essential role of COPII-mediated cargo transport to the endoplasmic reticulum-Golgi intermediate compartment in the formation of the primary membrane of vaccinia virus. J. Virol. 77:11754-11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Husain, M., and B. Moss. 2003. Intracellular trafficking of a palmitoylated membrane-associated protein component of enveloped vaccinia virions. J. Virol. 77:9008-9019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husain, M., A. Weisberg, and B. Moss. 2003. Topology of epitope-tagged F13L protein, a major membrane component of extracellular vaccinia virions. Virology 308:233-242. [DOI] [PubMed] [Google Scholar]
- 18.Ichihashi, Y. 1996. Extracellular enveloped vaccinia virus escapes neutralization. Virology 217:478-485. [DOI] [PubMed] [Google Scholar]
- 19.Isaacs, S. N., E. J. Wolffe, L. G. Payne, and B. Moss. 1992. Characterization of a vaccinia virus-encoded 42-kilodalton class I membrane glycoprotein component of the extracellular virus envelope. J. Virol. 66:7217-7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Killisch, I., P. Steinlein, K. Romisch, R. Hollinshead, H. Beug, and G. Griffiths. 1992. Characterization of early and late endocytic compartments of the transferrin cycle. Transferrin receptor antibody blocks erythroid differentiation by trapping the receptor in the early endosome. J. Cell Sci. 103:211-232. [DOI] [PubMed] [Google Scholar]
- 21.Lorenzo, M. M., I. Galindo, G. Griffiths, and R. Blasco. 2000. Intracellular localization of vaccinia virus extracellular enveloped virus envelope proteins individually expressed using a Semliki Forest virus replicon. J. Virol. 74:10535-10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxfield, F. R., and T. E. McGraw. 2004. Endocytic recycling. Nat. Rev. Mol. Cell. Biol. 5:121-132. [DOI] [PubMed] [Google Scholar]
- 23.Moss, B. 2001. Poxviridae: the viruses and their replication, p. 2849-2883. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa. [Google Scholar]
- 24.Payne, L. G. 1980. Significance of extracellular virus in the in vitro and in vivo dissemination of vaccinia virus. J. Gen. Virol. 50:89-100. [DOI] [PubMed] [Google Scholar]
- 25.Roper, R. L., L. G. Payne, and B. Moss. 1996. Extracellular vaccinia virus envelope glycoprotein encoded by the A33R gene. J. Virol. 70:3753-3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothenberger, S., B. J. Iacopetta, and L. C. Kuhn. 1987. Endocytosis of the transferrin receptor requires the cytoplasmic domain but not its phosphorylation site. Cell 49:423-431. [DOI] [PubMed] [Google Scholar]
- 27.Schmelz, M., B. Sodeik, M. Ericsson, E. J. Wolffe, H. Shida, G. Hiller, and G. Griffiths. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans-Golgi network. J. Virol. 68:130-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shida, H. 1986. Nucleotide sequence of the vaccinia virus hemagglutinin gene. Virology 150:451-462. [DOI] [PubMed] [Google Scholar]
- 29.Smith, G. L., A. Vanderplasschen, and M. Law. 2002. The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 83:2915-2931. [DOI] [PubMed] [Google Scholar]
- 30.Sodeik, B., R. W. Doms, M. Ericsson, G. Hiller, C. E. Machamer, W. van't Hof, G. van Meer, B. Moss, and G. Griffiths. 1993. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J. Cell Biol. 121:521-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tebar, F., T. Sorkina, A. Sorkin, M. Ericsson, and T. Kirchhausen. 1996. Eps15 is a component of clathrin-coated pits and vesicles and is located at the rim of coated pits. J. Biol. Chem. 271:28727-28730. [DOI] [PubMed] [Google Scholar]
- 32.Tooze, J., M. Hollinshead, B. Reis, K. Radsak, and H. Kern. 1993. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 60:163-178. [PubMed] [Google Scholar]
- 33.Ulaeto, D., D. Grosenbach, and D. E. Hruby. 1996. The vaccinia virus 4c and A-type inclusion proteins are specific markers for the intracellular mature virus particle. J. Virol. 70:3372-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Eijl, H., M. Hollinshead, G. Rodger, W. H. Zhang, and G. L. Smith. 2002. The vaccinia virus F12L protein is associated with intracellular enveloped virus particles and is required for their egress to the cell surface. J. Gen. Virol. 83:195-207. [DOI] [PubMed] [Google Scholar]
- 35.van Eijl, H., M. Hollinshead, and G. L. Smith. 2000. The vaccinia virus A36R protein is a type Ib membrane protein present on intracellular but not extracellular enveloped virus particles. Virology 271:26-36. [DOI] [PubMed] [Google Scholar]
- 36.Vanderplasschen, A., M. Hollinshead, and G. L. Smith. 1998. Intracellular and extracellular vaccinia virions enter cells by different mechanisms. J. Gen. Virol. 79:877-887. [DOI] [PubMed] [Google Scholar]
- 37.Ward, B. M., and B. Moss. 2000. Golgi network targeting and plasma membrane internalization signals in vaccinia virus B5R envelope protein. J. Virol. 74:3771-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward, B. M., and B. Moss. 2001. Vaccinia virus intracellular movement is associated with microtubules and independent of actin tails. J. Virol. 75:11651-11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward, B. M., and B. Moss. 2001. Visualization of intracellular movement of vaccinia virus virions containing a green fluorescent protein-B5R membrane protein chimera. J. Virol. 75:4802-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ward, G. A., C. K. Stover, B. Moss, and T. R. Fuerst. 1995. Stringent chemical and thermal regulation of recombinant gene expression by vaccinia virus vectors in mammalian cells. Proc. Natl. Acad. Sci. USA 92:6773-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wendland, B. 2002. Epsins: adaptors in endocytosis? Nat. Rev. Mol. Cell. Biol. 3:971-977. [DOI] [PubMed] [Google Scholar]
- 42.Wolffe, E. J., A. Weisberg, and B. Moss. 2001. The vaccinia virus A33R protein provides a chaperone function for viral membrane localization and tyrosine phosphorylation of the A36R protein. J. Virol. 75:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]