Abstract
The biological activity of CpG oligodeoxynucleotide 2216 (ODN2216), a Toll-like receptor 9 agonist, was investigated with monocytes from human immunodeficiency virus (HIV)-negative and HIV-positive (HIV+) donors. Exposure of peripheral blood mononuclear cells to CpG ODN2216 led to decreased expression of the monocyte marker CD14 and increased expression of the dendritic cell marker CD83, as well as increased expression of HLA-DR, CD40, CD80, and CD86 among the monocytes. Several features of the CpG ODN-induced maturation were diminished in monocytes from HIV+ donors, and these deficiencies were related to increased viremia but not to CD4 cell counts. Alpha interferon (IFN-α) was implicated as at least a partial mediator of the CpG ODN-induced monocyte maturation. Reduced production of IFN-α in response to CpG ODN and reduced frequencies of plasmacytoid dendritic cells, the principal IFN-α-producing cell type in peripheral blood, were observed in peripheral blood mononuclear cells from HIV+ donors. These deficiencies also were related to levels of plasma HIV RNA. Responses of monocytes from HIV+ donors to direct stimulation with IFN-α also were partially impaired. Thus, reduced production of IFN-α and reduced IFN-α responsiveness may contribute to diminished functional responses to CpG ODN in HIV disease. Application of CpG ODNs in HIV disease for adjuvant or immunoregulatory purposes may be particularly useful for HIV+ donors without high-level viremia.
The immune system requires both innate and adaptive immune mechanisms to detect and control invading pathogenic microorganisms. Professional antigen-presenting cells (APCs), such as dendritic cells (DCs), play an important role in bridging the responses of innate and acquired immunity (15). For example, plasmacytoid dendritic cells (pDC) produce alpha/beta interferon (IFN-α/β) when exposed to viral pathogens or bacterial DNA (15, 25), and the production of interferon by pDC may mediate innate immune functions to help limit the spread of invading pathogens and may also recruit adaptive immune responses by enhancing the function and maturation of APCs (16), including monocytes and myeloid dendritic cells (20, 24). These matured APCs possess greater potential to mediate antigen-dependent activation of T lymphocytes and consequently more effective adaptive immune responses (24).
Knowledge of the pathways that connect innate and adaptive immune responses has led to the design of candidate immunotherapeutics and vaccine adjuvants, including unmethylated CpG oligodeoxynucleotides (ODNs) (17). Bacterial CpG DNA or synthetic CpG oligodeoxynucleotides activate pDC through the Toll-like receptor 9 (TLR9), leading to IFN-α/β production by these cells (17). The resulting cytokine milieu may optimize innate and adaptive immune responses, as has been suggested by mouse studies that examined CpG ODN for adjuvant activity (1). In mice, CpG ODNs increase Th1-type immune responses (6), enhance the processing and delivery of soluble antigens into the class I pathway for presentation to CD8 T cells, and induce CD8 T-cell responses to antigens independently of CD4 help (4, 5). Thus, CpG ODNs appear to have substantial potential as vaccine adjuvants, particularly for the induction of cell-mediated immunity.
Application of these reagents for use in humans may take some refinement, since CpG ODNs are made in various forms that mediate different responses and have different levels of activity in mouse and human cells (17). Class A CpG ODNs are good IFN-α inducers (especially CpG ODN2216), while class B CpG ODNs induce primarily B lymphocyte proliferation responses with interleukin-6-immunoglobulin M secretion (17, 31). Class C CpG ODNs activate B cells and induce IFN-α in pDC, but are less effective than class A CpG ODNs for IFN-α production and may have a greater ability than class B CpG ODNs to activate B cells (12). Thus, the choice of a CpG ODN will require consideration of the desired immune response and testing for that desired outcome in the appropriate clinical setting.
One potential application for CpG ODNs may be in the setting of human immunodeficiency virus (HIV) infection. HIV disease presents a unique challenge for vaccine design since patients may experience profound CD4 cell loss with generalized immunodeficiency and impaired responsiveness to immunization (7, 27, 28). The potential for CpG ODNs to enhance CD8 T-cell responses independently of CD4 cell help (4, 5) may therefore be particularly relevant to HIV disease. CpG responsiveness may, however, be impaired in HIV disease due to the depletion of pDC and possibly also due to qualitative impairments in pDC or other APCs (2, 8, 9, 22, 23). In the present study, we found impairments in maturation of peripheral blood monocytes in response to CpG ODN (CpG ODN2216) among cells from HIV-positive (HIV+) subjects. The defects were related to plasma HIV RNA levels and partially explained by impaired IFN-α production and interferon responsiveness. These impairments may represent an important limitation for CpG ODN applications in uncontrolled HIV infection.
MATERIALS AND METHODS
Antibodies and reagents.
Endotoxin-free CpG ODN2216 (ggGGGACGATCGTCgggggG) and ODN2243 (GC control for CpG ODN2216; ggGGGAGCATGCTCgggggG) were provided by Coley Pharmaceutical Group, Inc. (Wellesley, Mass.) and used at a concentration of 3 μg/ml. The culture medium comprised RPMI 1640 (BioWhittaker, Walkersville, Md.) supplemented with 50 U of penicillin/ml, 50 μg of streptomycin/ml, 2 mM l-glutamine (BioWhittaker), and 10% fetal bovine serum (Sigma-Aldrich, St. Louis, Mo.). Monocyte isolation buffer contains phosphate-buffered saline with 0.5% bovine serum albumin and 2 mM EDTA. Recombinant IFN-α2a (PBL, Piscataway, N.J.) was used at concentrations of 1,000 and 10,000 U/ml. A concentration of 1,000 U of IFN-α/ml, while high, is occasionally detected in peripheral blood mononuclear cell supernatants after overnight incubations with CpG ODN2216. This concentration and a 10-fold-higher concentration were chosen to maximize the potential immunoregulatory activity of IFN-α. Some studies included dose responses based on concentrations ranging from 10 to 10,000 U/ml. Neutralizing anti-IFN-α and -β receptor antibody (PBL) and control mouse immunoglobulin G2a (BD PharMingen, San Diego, Calif.) were used at a concentration of 10 μg/ml. The IFN-α enzyme-linked immunosorbent assay (ELISA) kit was obtained from PBL.
Cell preparation and culture.
Blood was drawn into heparin-coated tubes from 24 healthy donors and 55 HIV type 1 (HIV-1)-infected patients. The clinical characteristics of the HIV+ individuals are summarized in Table 1. Peripheral blood mononuclear cells (PBMC) were isolated over a Ficoll-Hypaque cushion. Isolated PBMC (2 × 106 cells/ml) were incubated in 24-well plates for 24 h with CpG ODN, with recombinant IFN-α, or in medium alone. Cell supernatants were stored at −70°C before being analyzed by ELISA (PBL) for IFN-α concentration. To prepare purified monocytes, pDC were depleted by labeling whole blood with anti-human CD123 antibody (StemCell Technologies, Inc., Vancouver, Canada) followed by isolation of CD123-negative cells over a Ficoll-Histopaque cushion. CD123-depleted PBMC were then depleted of T cells, NK cells, B cells, DCs, and basophils by using a MACS monocyte isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Monocytes were examined for purity by flow cytometry (>95% purity) and for viability by trypan blue staining (>98% viability). Monocytes were subsequently cultured in 12-well plates (0.5 × 106 cells/ml) in fresh complete medium with or without CpG ODN or IFN-α for 24 h. Some cells were used in transwell (Costar, Cambridge, Mass.) experiments after purified monocytes were placed in the top wells and whole PBMC were placed in the bottom.
TABLE 1.
Clinical characteristics of 55 HIV + donorsa
| Characteristic | Description or result for donors with indicated no. of copies of virus/ml of plasma
|
|
|---|---|---|
| >400 | ≤400 | |
| No. of donors | 25 | 30 |
| Age | 47 ± 4 | 44 ± 5 |
| Sex (no. of males/no. of females) | 17/8 | 21/9 |
| CD4+ T cells/μl | 152 ± 35 | 403 ± 40 |
| Plasma HIV RNA | ||
| c/ml | 62,778 ± 16,854 | 125 ± 28 |
| Range | 540 to 351,515 | ND to 400 |
Data shown are means ± SEM. ND, not determined.
Allostimulation by monocytes.
PBMC were cultured for 24 h with CpG ODN2216 (3 μg/ml) or in medium alone. Monocytes were subsequently isolated from these cultures by using CD14 microbeads (AutoMACS; Miltenyi Biotec). Monocytes were recovered, washed, and placed in 96-well plates (6 × 104 cells/well). Heterologous PBMC (6 × 105 cells/well) from unrelated healthy individuals were added to each well as responder cells. After 5 days of incubation, cell cultures were pulsed with [3H]thymidine (0.5 μCi per well; MP Biomedicals, Cleveland, Ohio) and then harvested onto glass fiber mats. Radioactivity was measured with a liquid scintillation counter.
Flow cytometry.
Surface molecule expression was monitored by staining cells with the following monoclonal antibodies: anti-CD14-fluorescein isothiocyanate, anti-HLA-DR-peridinin-chlorophyll-protein (PerCP), anti-CD123-phycoerythrin, anti-CD11c-phycoerythrin, anti-CD80-Cychrome, anti-CD86-Cychrome, anti-CD40-Cychrome, anti-CD83-allophycocyanin, and the appropriate isotype control monoclonal antibodies (BD PharMingen). Cells were stained and then washed in wash buffer (phosphate-buffered saline with 1% bovine serum albumin and 0.1% sodium azide), fixed in 1% formaldehyde, and analyzed using a dual-laser flow cytometer (FACSCaliber; Becton Dickinson, San Jose, Calif.) and CellQuest software (BD Bioscience, San Diego, Calif.).
Statistical methods.
Categorical variables were compared by Pearson's chi-square or Fisher's exact test, and continuous variables were analyzed by Student's t test or Mann-Whitney U test as required. Univariate analyses of the correlation between continuous variables were carried out by Spearman's test, and multivariate analyses were conducted by fitting multiple regression models by using a backward stepwise strategy. Exploratory analyses of the independence of association between pairs of predictors of interest (e.g., CD4+-T-cell count and HIV RNA) and marker expression were done by calculating partial correlation coefficients. To test for trends across ordered categories of continuous variables, we used analysis of variance (ANOVA) with polynomial contrasts. All tests are double-sided, and a P value of 0.05 was considered nominally significant.
RESULTS
CpG ODN2216 decreases monocyte expression of CD14 and increases expression of CD83 in PBMC from HIV-negative (HIV−) and HIV+ donors.
To examine the effects of CpG ODN on the phenotypes of human monocytes, PBMC were incubated for 24 h in medium with or without CpG ODN2216 or with control ODN2243. The cells were then processed for flow cytometric analyses.
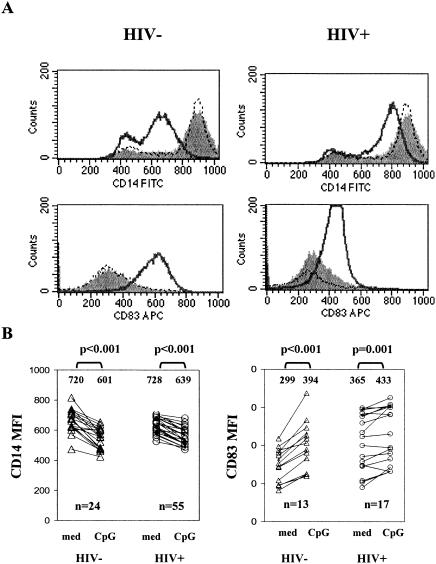
Initially, we assessed the levels of CD14, a characteristic monocyte marker, on cells that displayed forward scatter (cell size) and side scatter (granularity) profiles consistent with monocyte morphology. At baseline, more than 80% of these large granular cells were CD14+. After 24-h culture in medium alone, the intensity of CD14 expression increased above baseline. In contrast, the cell surface density of CD14 molecules diminished dramatically when cells were cultured in the presence of CpG ODN2216, but not when cells were cultured with control ODN2243 (Fig. 1). The down-modulation of CD14 surface expression was observed in cells from HIV− and HIV+ donors. The CpG ODN-induced change (absolute) in mean fluorescence intensity (MFI) of CD14 expression was reduced in cells from HIV+ donors (mean change in MFI, 89 and 119 for HIV+ donors and controls, respectively; P < 0.05).
FIG. 1.
CpG ODN2216 down-regulates monocyte CD14 expression and up-regulates monocyte CD83 expression. PBMC from 24 healthy donors and 55 HIV+ donors were cultured in medium or medium supplemented with either CpG ODN2216 (3 μg/ml) or CpG ODN2243 (3 μg/ml) for 24 h. Cell surface expression of CD14 and CD83 was determined by flow cytometric analysis. For analysis of CD14 expression, monocytes were identified by forward and side scatter characteristics, and for analysis of CD83 expression, monocytes were identified by gating on CD14+ cells. (A) Representative flow histograms. Filled histograms represent CD14 or CD83 expression in cells cultured 24 h without any stimuli, solid lines represent CD14 or CD83 expression in cells cultured with CpG ODN2216, and dashed lines represent CD14 or CD83 expression in cells cultured with control ODN2243. FITC, fluorescein isothiocyanate; APC, allophycocyanin. (B) Summary of results showing the MFI of CD14 or CD83 expression among cells incubated in medium alone (med) or with CpG ODN2216. P values were determined by two-tailed Student's t test. The numbers shown above the data points are the mean values for the respective observations.
Although CD14 expression was reduced among the large granular mononuclear cells incubated with CpG ODN, these cells still expressed detectable levels of CD14, thereby permitting a distinction between CD14+ cells and CD14− cells. Therefore, we were able to assess additional phenotypic changes among these cells by gating specifically on the CD14+ cells.
CD14 expression may be decreased to low levels on monocytes as they mature into APCs that more closely resemble DCs (19). To determine if the reduction in CD14 expression on monocytes was associated with maturation into a DC-like phenotype, we examined the expression of CD83, a typical DC marker (32), on monocytes after overnight incubations of PBMC with CpG ODN. CpG ODN induced the expression of CD83 on CD14+ cells following an overnight incubation (Fig. 1), consistent with the phenotypic change of these cells into a DC-like phenotype. This response was reduced among monocytes from HIV+ donors, as determined by either the percentage of CD83+ cells (mean percentages of CD83+ monocytes ± standard errors of the means [SEM] after CpG ODN treatment were 16 ± 1 and 33 ± 6 for 17 HIV+ donors and 13 controls, respectively; P = 0.045) or the change in staining intensity of CD83 (mean changes in MFI were 40 and 107 for HIV+ donors and controls, respectively; P < 0.05).
CpG ODN2216 induces monocyte expression of HLA-DR, CD40, CD80, and CD86.
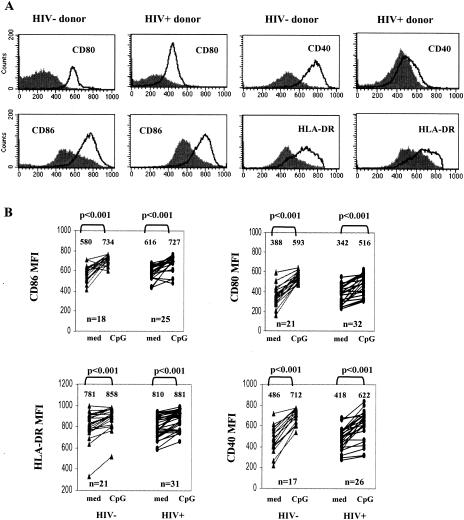
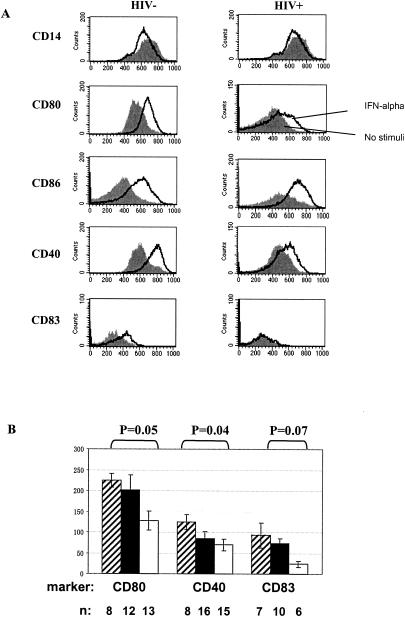
Maturation of professional APCs is characterized by the increased expression of molecules involved in costimulation and antigen presentation. Therefore, we examined the effects of CpG ODN2216 on CD14+ monocyte expression of CD80, CD86, CD40, and HLA-DR. Baseline expression of CD80, CD86, and CD40 was similar in monocytes freshly isolated from healthy donors and from HIV+ donors (P > 0.05); HLA-DR was expressed at higher levels in freshly isolated cells from HIV+ donors than in cells from healthy donors (P = 0.001). A modest increase in the expression of these markers was observed with cells incubated 24 h in medium alone (data not shown). Cells incubated 24 h with CpG ODN2216 strongly up-regulated CD80, CD86, CD40, and HLA-DR surface expression (Fig. 2). Overall, induction (change in MFI) of each of these markers by CpG ODN was similar for patient and control monocytes with the exception of CD40, for which induction was relatively reduced in patient cells (P < 0.05).
FIG. 2.
CpG ODN2216 increases monocyte expression of CD80, CD86, CD40, and HLA-DR. (A) Representative flow histograms from a healthy donor and an HIV+ donor. Filled histograms represent surface molecule expression in cells cultured 24 h without stimuli; solid lines represent surface molecule expression in cells cultured with CpG ODN2216 (3 μg/ml) for 24 h. (B) Summary results of all studies in HIV− and HIV+ donors. Statistically significant differences in the expression of CD80, CD86, CD40, and HLA-DR surface expression among cells incubated in medium alone versus cells incubated with CpG ODN2216 are shown (P values determined by two-tailed nonpaired Student's t test). The numbers shown above the data points are the mean values for the respective observations.
An evaluation of dose response to CpG ODN (0.1 to 27 μg/ml) in cells from seven HIV+ donors and three healthy subjects indicated that monocyte maturation responses (induction of CD40, CD80, and CD83) could be detected between 0.1 and 0.3 μg of CpG ODN/ml but that the responses generally peaked between 1 and 3 μg/ml. Importantly, in cases where patient cells showed defects, these defects could not be corrected by increasing doses of CpG ODN (data not shown).
Decreased monocyte maturation in response to CpG ODN in HIV+ donors is related to plasma HIV RNA levels.
Our initial evaluation of CpG ODN responsiveness in patient monocytes (described above) suggests that there are impairments in the maturation of these cells following incubation with CpG ODN. In particular, monocytes from HIV+ donors demonstrated a reduced induction of CD40 and CD83 as well as an impaired down-modulation of CD14 following incubation with CpG ODN. Multivariate analyses indicated that these defects were correlated with plasma HIV RNA levels independently of CD4 cell counts (P < 0.05) or age and that CD4 cell counts could not independently predict the magnitude of the changes in cell surface markers following activation with CpG ODN (Table 2). Thus, plasma HIV RNA levels are an important predictor of impaired CpG ODN responses.
TABLE 2.
Multiple regression analyses of monocyte maturation responses to CpG ODN2216 and clinical indicesa
| Marker | Regression coefficient |
P value (standard error) for predictor indicated
|
||
|---|---|---|---|---|
| Plasma HIV RNA | Age | CD4 cell count | ||
| CD14 | −10.8 | 0.05 (5.4) | 0.6 | 0.87 |
| CD86 | −24.4 | 0.026 (10.5) | 0.63 | 0.84 |
| CD40 | −22.9 | 0.033 (10.3) | 0.09 | 0.37 |
| CD83 | −18.1 | 0.002 (5.2) | 0.23 | 0.56 |
The relationship between induction of cell surface markers (change in MFI) by CpG ODN and plasma HIV RNA (in log10 copies/ml) after controlling for age and CD4+-T-cell count is shown. The marker was the dependent variable.
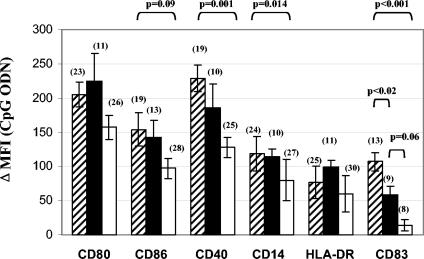
Since increased plasma HIV RNA levels predicted the phenotypic defects, we considered the possibility that viral suppression with highly active antiretroviral therapy (HAART) might reverse the poor responsiveness to CpG ODN. Therefore, we compared, in a cross-sectional analysis, the responses of patients undergoing successful HAART (≤400 copies of virus/ml of plasma) with responses of patients who were either not receiving HAART or receiving HAART with incomplete control of viral replication (>400 copies of virus/ml of plasma). Monocytes from HAART-treated patients who maintained control of viral replication responded to CpG ODN as well as did monocytes from healthy donors for all of the markers examined, with the exception of CD83 induction (Fig. 3) as determined by the change in staining intensity of CD83. This impairment was also observed in an evaluation of the percentage of CD83+ monocytes, although it did not quite reach statistical significance (mean percentage of CD83+ monocytes ± SEM = 26 ± 6 and 33 ± 6 for 9 HIV+ donors and 13 controls, respectively; P = 0.07). In contrast, patients with HIV RNA levels of >400 copies/ml in plasma demonstrated statistically significant defects in the CpG ODN-induced expression of CD40 (P = 0.001) and CD83 (P < 0.001) and down-regulation of CD14 (P = 0.02), as well as nearly statistically significant defects in the induction of CD86 (P = 0.09). For these viremic patients, both the intensity of CD83 staining (Fig. 3) and the percentage of CD83+ monocytes (mean percentage of CD83+ monocytes ± SEM = 4 ± 1 and 33 ± 6 for 8 HIV+ donors and 13 controls, respectively; P = 0.024) were markedly diminished after CpG treatment.
FIG. 3.
HIV viremia is associated with defects in CpG ODN regulation of monocyte surface molecules. Summary data using mean absolute ΔMFIs of CD80, CD86, CD40, CD14, HLA-DR, and CD83 induced by CpG ODN2216 on monocytes of HIV− donors (striped bars), HIV+ donors with undetectable plasma HIV RNA levels (≤400 copies/ml; closed bars) and HIV+ donors with detectable plasma HIV RNA levels (>400 copies/ml; open bars). Group means are compared by ANOVA and Tukey. Sample sizes (n) are shown in parentheses.
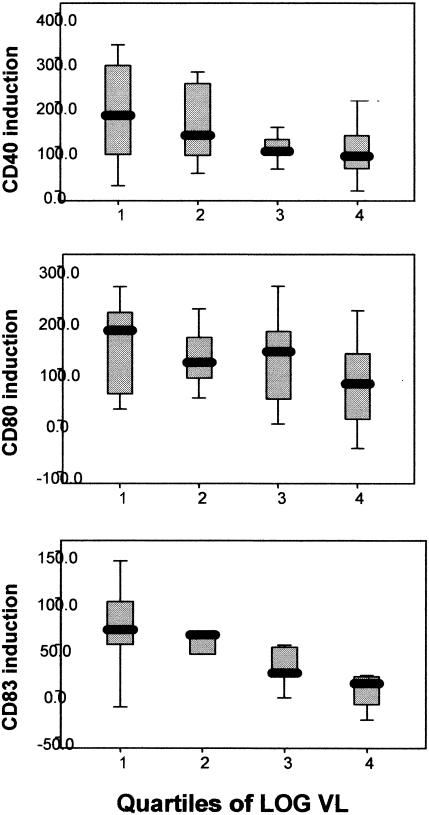
We then divided the HIV+ group into quartiles according to log10 plasma HIV RNA level and to CD4+-T-cell count. When compared across these categories of plasma HIV RNA, the magnitude of induction of markers CD83, CD80, and CD40 demonstrated a consistent direct relationship with plasma HIV RNA (Fig. 4; P values for the trends were 0.004, 0.042, and 0.019 for CD83, CD80, and CD40, respectively). All other markers showed a similar trend in their association with the plasma HIV RNA level. Thus, not only was the detection of virus in the plasma an important predictor of poor responses to CpG ODN, but higher levels of HIV replication were associated with even more pronounced defects.
FIG. 4.
Monocyte maturation responses are related to plasma HIV RNA levels. Monocyte responses to CpG ODN treatment are depicted with HIV+ patient samples subdivided into quartiles based on log10 plasma HIV RNA levels. PBMC were stimulated with CpG ODN2216 (3 μg/ml) for 24 h, and gated monocytes were examined for surface expression of CD40, CD80, and CD83 by flow cytometry. VL, viral load.
CpG ODN fails to enhance accessory cell function of monocytes from HIV+ donors with viremia.
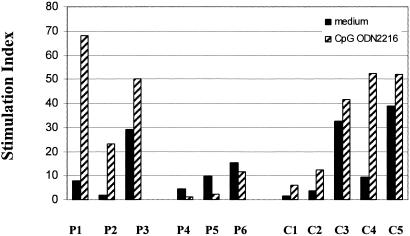
To ascertain if impaired responsiveness to CpG ODN by monocytes from HIV+ persons might result in reduced functional capacity, we compared the capacity of monocytes from HIV+ and HIV− donors to induce allogeneic proliferation responses after exposure to CpG ODN. Monocytes from three viremic HIV+ donors (plasma HIV RNA of >10,000 copies/ml) were compared to cells from three patients with controlled viremia (plasma HIV RNA levels of <400 copies/ml) and cells from four healthy donors for their accessory cell function in allogeneic responses. Monocytes from viremic patients that had been matured with CpG ODN failed to enhance allogeneic responses compared to monocytes that had not been treated with CpG ODN (Fig. 5). Interestingly, CpG ODN exposure seemed to actually impair the allogeneic responses induced by patient monocytes for reasons that are not yet clear. In contrast, monocytes from HIV− donors or HIV+ donors with control of viremia induced a greater allogeneic T-cell proliferation response after CpG ODN exposure. Thus, as is the case for phenotypic maturation of monocytes, functional maturation of monocytes may also be limited in HIV+ individuals with uncontrolled viremia.
FIG. 5.
Failure of CpG ODN to enhance allostimulation by monocytes from HIV+ donors with advanced disease. PBMC from three HIV+ donors (P4, P5, P6) with plasma HIV RNA levels of >10,000 copies/ml and CD4 counts of <200 cells/μl, three HIV+ individuals (P1, P2, P3) with plasma HIV RNA levels of <400 copies per ml and CD4 cell counts of >200 cells/μl, and five healthy individuals (C1, C2, C3, C4, C5) were cultured for 24 h with or without CpG ODN2216. Monocytes were isolated by using anti-CD14 magnetic beads, washed, and plated in 96-well plates with heterologous PBMC from healthy donors at a ratio of 1 monocyte per 10 responder PBMC. After 5 days of incubation, cellular proliferation was measured by [3H]thymidine incorporation. Stimulation indices (y axis) were determined by dividing the response (mean of triplicate wells) of stimulated cells by the response of cells incubated in medium alone. Note that the responder cells used in experiments labeled P4, P5, and P6 were from the same donor as in experiments labeled C3, C4, and C5.
The effects of CpG ODN are mediated indirectly through soluble factors that include IFN-α/β.
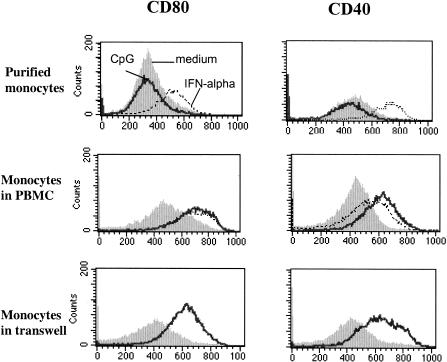
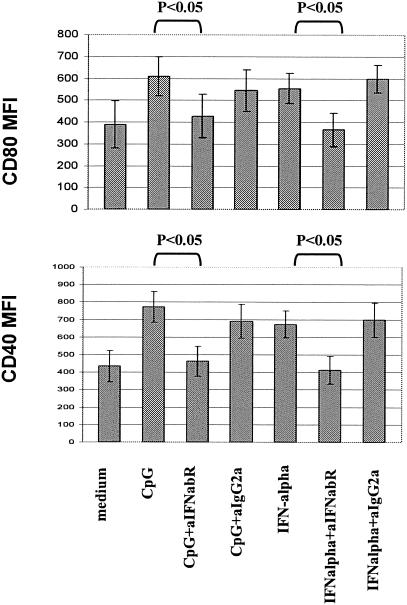
Human monocytes are not known to express TLR9, the receptor for which CpG ODN is a ligand. TLR9-expressing cells in the peripheral blood include pDC and B cells. Activation of pDC by TLR9 agonists induces IFN-α/β production by these cells. Consequently, monocytes are not expected to respond directly to CpG ODN, but may respond indirectly to the inflammatory milieu induced by TLR9 agonist activity (13). Consistent with this idea, we found little change in monocyte phenotype when CpG ODN2216 was added to purified monocyte (>95% CD14+) preparations. In contrast, these purified monocytes did undergo cellular maturation when exposed to IFN-α alone (Fig. 6). Also, when purified monocytes were incubated in transwells with CpG ODN-treated PBMC in communicating wells, cellular maturation of purified monocytes was observed. Thus, soluble factors from cell types other than monocytes appear to initiate the effects of CpG ODN2216 on monocyte maturation, and IFN-α alone is sufficient to mediate these effects. Furthermore, the addition of neutralizing antibody against IFN-α/β receptor but not an isotype control antibody attenuated the induction of CD80 (mean inhibition from four experiments, 48%) and CD40 expression (mean inhibition from four experiments, 68%) among monocytes exposed to CpG ODN in whole PBMC cultures (Fig. 7). These results indicate that IFN-α/β plays at least a partial role in the CpG-induced monocyte maturation.
FIG. 6.
CPG ODN2216 alters monocyte surface phenotype indirectly via soluble factor(s). Monocytes were isolated from PBMC by magnetic bead selection. Purified monocytes were then incubated with CpG ODN or IFN-α. Some purified monocytes were incubated in Transwells with PBMC in communicating wells ± CpG ODN. Induction of CD40 and CD80 expression was examined with purified monocytes (top), among monocytes within PBMC (middle), and with purified monocytes suspended in transwells with PBMC in communicating wells (bottom) following incubation in medium alone (filled histograms) or in medium supplemented with CpG ODN (solid lines) or IFN-α (1,000 U/ml; dashed lines). The experiment shown is representative of the three experiments performed.
FIG. 7.
Blockade of the IFN-α/β receptor interferes with CpG ODN2216 or IFN-α-induced monocyte maturation. PBMC were cultured in medium alone or in medium supplemented with CpG ODN2216 (3 μg/ml) or IFN-α (10,000 U/ml). Murine antibody to the human IFN-α/β receptor (anti-IFNa/bR; 10 μg/ml) or isotype control antibody (10 μg/ml) was added at the beginning of culture as indicated. MFI (y axis) of CD80 and CD40 staining among CD14+ monocytes as determined by flow cytometric analysis is shown. These results are the mean values for four experiments; P values were determined by paired t test. Although not depicted, statistical differences were observed between cells cultured in medium alone and cells cultured with either CpG ODN or IFN-α (P < 0.05). Ig, immunoglobulin.
Monocyte maturation following CpG ODN2216 exposure is related to IFN-α production and is attenuated in the presence of high-level HIV replication.
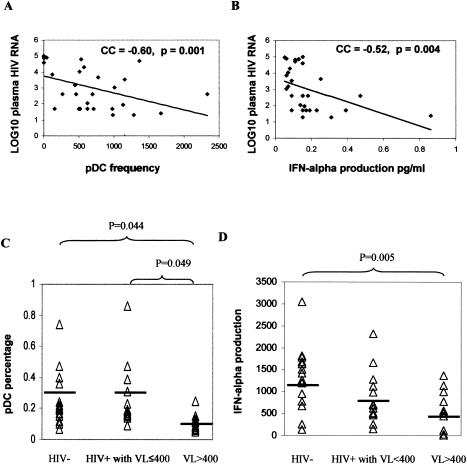
Since monocyte maturation could be induced by IFN-α and since the induction of CD40 and CD80 expression by CpG ODN2216 could be blocked at least partially by anti-IFN-α/β receptor antibodies, we hypothesized that defects in CpG ODN-induced monocyte maturation in HIV disease could be due to reduced IFN-α production in response to the TRL9 agonist. To test this hypothesis, we explored the relationships between IFN-α levels in culture supernatants and monocyte maturation responses. IFN-α levels in culture supernatants of CpG ODN-treated PBMC predicted the magnitude of monocyte maturation for all markers examined (Spearman's correlation, P values < 0.05) except for HLA-DR (data not shown). Because pDC are the primary IFN-α producing cells that respond to TLR9 agonists, we examined the frequencies of pDC in PBMC samples from HIV+ subjects. The percentage of pDC (lineage negative, HLA-DR and CD123+ cells) within freshly isolated PBMC was inversely correlated with plasma HIV RNA levels (Fig. 8A) but not related to CD4 cell counts (Spearman's rank correlation coefficient, 0.145; P = 0.452). Similarly, the amount of IFN-α detected in cell supernatants 1 day after stimulation with CpG ODN was inversely related to plasma HIV RNA levels (Fig. 8B) and also was not related to CD4 cell counts (Spearman's rank correlation coefficient, 0.051; P = 0.794). Cross-sectional analyses indicated that deficiencies in pDC frequencies and IFN-α production in response to CpG ODN are largely confined to patients without control of HIV replication and not observed among HAART-treated HIV+ patients with control of viral replication (Fig. 8C and D). Thus, reduced pDC frequencies and reduced ability to produce IFN-α in response to CpG ODN can at least partially account for poor monocyte maturation responses to CpG ODN in HIV disease.
FIG. 8.
Plasma HIV RNA level is related to pDC frequencies and IFN-α production in response to CpG ODN. (A) PBMC were isolated and examined by flow cytometry to measure the percentage of lineage DR and CD123+ cells (pDC frequencies). This percentage was plotted against the plasma HIV RNA levels (log10 scale). Spearman's correlation coefficients (CC) are shown in the figure. (B) The amount of IFN-α was measured in cell supernatants by ELISA following overnight incubation of PBMC with CpG ODN (3 μg/ml). The relationship between this value and plasma HIV RNA levels is shown. (C) Data were analyzed according to levels of plasma HIV RNA levels of ≤400 or >400 copies/ml, and pDC frequencies were compared to those calculated from healthy donor PBMC samples. (D) A similar comparison is shown for IFN-α production in response to CpG ODN for PBMC samples. Statistical differences in means were determined by ANOVA and Tukey. VL, viral load.
To determine if reduced pDC number and reduced IFN-α production could explain all of the defects in CpG ODN responsiveness, we fitted a multiple regression model of HIV status, pDC number, IFN-α production, and donor age as predictors of the induction of CD83 in monocytes. Induction of CD83 was chosen for the analysis because this marker was the most severely affected in HIV disease. HIV infection was independently predictive of the magnitude of CD83 induction on monocytes (P value, 0.009), while age, pDC number, and IFN-α production were not (P values of 0.22, 0.58, and 0.77, respectively). Based on model estimates, HIV infection was associated with an average decrease in CD83 expression of 98.4 mean fluorescent units. This analysis indicates that the reduced frequency and function of pDC in HIV infection cannot fully explain the defects in CpG ODN responsiveness.
Poor responsiveness to IFN-α may also contribute to impaired induction of CD40 and CD83 expression by CpG ODN in HIV disease.
An alternative hypothesis to explain the defects in CpG ODN responsiveness in HIV disease is that there is a defect in the ability of monocytes from HIV+ donors to respond to IFN-α. To test this possibility, we compared maturation responses between monocytes from HIV− and HIV+ donors as determined by the expression of each of the maturation markers in response to exogenous IFN-α. Treatment of PBMC with IFN-α-2a induced all of the changes that had been observed with CpG ODN, including down-modulation of CD14 and increased expression of CD40, CD80, CD86, and CD83 on the monocyte cell surface (Fig. 9A). Although HLA-DR, CD14, and CD86 were modulated similarly among cells from patients with viremia and cells from HIV− donors (data not shown), the induction of CD80 and CD40 expression in response to IFN-α was impaired in cells from HIV+ donors (Fig. 9B). The induction of CD83 expression was also reduced in monocytes from HIV+ donors with plasma HIV RNA levels of >400 copies/ml, although this result did not quite reach statistical significance. Moreover, the magnitude of the CpG ODN-induced monocyte maturation for each of the markers examined was directly related to the magnitude of IFN-α-induced monocyte maturation (Table 3). Together, these results suggest that IFN-α responsiveness may also play a role in CpG ODN responses.
FIG. 9.
IFN-α induces maturation of monocytes, but this response is impaired for certain markers among cells from HIV+ donors. (A) PBMC from an HIV− and HIV+ donor were incubated with or without IFN-α (1,000 U/ml) for 24 h. After incubation, cell surface expression of CD14, CD80, CD86, and CD40 was determined by flow cytometric analysis. (B) PBMC were stimulated with IFN-α (1,000 U/ml) for 24 h, and gated monocytes were examined for surface expression of CD80, CD40, and CD83 by flow cytometry. Induction (mean ΔMFI) of CD80, CD40, and CD83 by IFN-α on monocytes are shown for HIV− donors (striped bars), HIV+ donors with undetectable plasma HIV RNA levels (≤400 copies/ml; closed bars), and HIV+ donors with detectable plasma HIV RNA levels (>400 copies/ml; open bars). Group means were compared by ANOVA and Tukey.
TABLE 3.
Spearman's correlations between CpG ODN- and IFN-α-induced maturation of monocytesa
| Cell surface marker | Correlation coefficient | P value |
|---|---|---|
| CD80 | 0.49 | 0.003 |
| CD86 | 0.41 | 0.08 |
| CD40 | 0.41 | 0.02 |
| CD14 | 0.43 | 0.006 |
| HLA-DR | 0.53 | 0.03 |
| CD83 | 0.5 | 0.003 |
The absolute change (delta) in MFI was correlated for markers on monocytes after overnight incubation of PBMC with CpG ODN or IFN-α (n1,000 U/ml). Analyses included cells from both HIV+ and HIV− donors.
Importantly, in preliminary studies of dose responses to IFN-α, monocytes from HIV+ donors (n = 6) that responded poorly to IFN-α at low doses (10 to 100 U/ml) sometimes responded to high doses of cytokine (up to 10,000 U/ml), but the responses remained impaired compared to responses of control cells (data not shown). Thus, impaired responses to IFN-α could not be fully corrected with increasing concentrations of cytokine.
DISCUSSION
CpG ODN2216 is a prototype type A ODN that has biological activity in human cells (17), inducing high levels of IFN-α production by pDC (17-19). In human PBMC, exposure to this CpG ODN promotes monocyte production of IFN-γ-inducible protein 10 mediated at least partially by the induction of IFN-α expression (32). Our results indicate that IFN-α may have an additional role in promoting the maturation of monocytes into dendritic-like cells. A role for IFN-α in the CpG ODN-induced maturation of antigen-presenting cells has been implicated in studies of murine IFN-α/β receptor knock-out mice (3, 18) and in studies of human monocytes exposed to a type A CpG ODN of a different sequence than CpG ODN2216 (4, 29). The latter study indicated that IFN-α production in response to CpG ODN was necessary but not sufficient to induce human monocyte maturation as measured by CD86, CD83, and CD14 expression. Our studies concur, but only in part, confirming that IFN-α/β play at least a contributory role in CpG ODN-induced monocyte maturation. On the other hand, we have shown that IFN-α alone is sufficient to induce these changes in a concentration-dependent manner. Moreover, the capacity of monocytes to mature in response to CpG ODN is directly correlated with IFN-α production. Overall, it would seem that IFN-α plays an important role in the induction of monocyte maturation in response to CpG ODN.
The role of IFN-α in mediating the activity of CpG ODN has clear implications for HIV disease. Induction of IFN-α secretion by a variety of stimuli is reduced in HIV infection (10, 11, 14), and recent studies demonstrated that CpG ODN-induced IFN-α production in PBMC from HIV+ subjects was inversely related to plasma HIV RNA levels and correlated with CD4 cell counts (30). Our work confirms the inverse relationship between IFN-α production and plasma HIV RNA levels but not the correlation with CD4 cell counts. One possible explanation for the different findings is that the previous study did not rule out by multivariate analyses the possible interactions of plasma HIV RNA levels and CD4 cell counts (i.e., patients with high plasma HIV RNA levels tend to have low CD4 cell counts), making it difficult to discern with bivariate analyses the most important variable for predicting the induction of IFN-α by CpG ODN. In contrast, we found by multivariate (data not shown) and even bivariate analyses that plasma HIV RNA levels, but not CD4 cell counts, predicted IFN-α production in response to CpG ODN. Thus, the reduced IFN-α production that accompanies HIV replication may limit the efficacy of CpG ODNs in HIV disease.
Another implication for the importance of IFN-α production in CpG ODN responsiveness rests with the possible quantitative and qualitative defects that may occur in pDC, the primary IFN-α-producing cells in the peripheral blood. Several studies have reported that pDC are often depleted or may be rendered less functional during the course of HIV disease (9, 10, 23, 26). On the other hand, Lor é et al. were unable to demonstrate significant impairments in DC responsiveness to activation through multiple TLRs by using cells obtained from HIV+ persons (21). Our findings also describe reduced frequencies of pDC in PBMC from HIV patients that were correlated with plasma HIV RNA levels but not CD4 cell counts. Nevertheless, our attempts to characterize qualitative defects in pDC in HIV disease have thus far been inconclusive and will require more extensive studies. Finally, it should be recognized that the reduced frequencies of pDC in the peripheral blood may represent a global deficiency in the numbers of these cells or may simply reflect the migration of pDC out of the peripheral circulation. If the latter is the case, then CpG ODNs may yet be effective even in viremic HIV+ individuals as long as the oligonucleotides are delivered to anatomical compartments with resident pDC.
Although the impairments in pDC frequency and IFN-α production that accompany HIV infection represent one potential obstacle for the application of CpG ODNs in HIV infection, our observations indicate that there may also be other important factors involved. This possibility is illustrated by our multivariate analysis of CpG ODN-induced expression of CD83 on monocytes, which indicates that pDC frequencies alone are not sufficient to explain the defects in CD83 induction among HIV+ individuals. In addition, we describe a relative reduction in responsiveness to IFN-α among monocytes from HIV+ persons, particularly for the induction of CD80, CD40, and CD83 expression, indicating that this defect also could contribute to impaired responses to CpG ODN in HIV disease. The mechanisms underlying impairments of IFN-α responsiveness in HIV disease are unknown, but they could be related to subnormal IFN-α/β receptor expression or signaling function in monocytes obtained from HIV+ persons. Our observation that freshly isolated monocytes from HIV+ persons tend to have elevated expression of HLA-DR may represent a phenotypic change reflective of an increased state of activation, and this in turn might influence the potential to further activate these cells. It will be important to explore this possibility as well as other factors that could conceivably influence the ability of these cells to respond to stimuli, such as IFN-α or other cytokines being induced by CpG ODN.
Despite the limitations in CpG ODN responsiveness that we have observed with cells from HIV+ individuals, our observations also indicate that PBMC of patients who achieve control of HIV replication through antiretroviral therapies maintain relatively normal frequencies of pDC and produce normal amounts of IFN-α in response to CpG ODN. Furthermore, these individuals also sustain monocyte maturation responses to CpG ODN that more closely resemble the responses of cells from HIV− individuals. The exception to this “normal” maturation response to CpG ODN includes a reduced induction of CD83 expression among monocytes from patients on successful HAART. It is unclear why this marker is uniquely impaired in this circumstance, and it remains to be determined if the magnitude of these particular impairments will have functional consequences for these cells.
The interplay between pDC and monocytes may have important consequences for immune function. DCs are recognized as the most potent APCs in the body, but these cells are found at relatively low frequencies. Maturation of monocytes into DC-like cells could be a means of amplifying immune responsiveness by recruiting a large pool of phagocytic cells and then enhancing their antigen-presenting capability. We have demonstrated that defects in monocyte maturation are associated with reduced accessory cell function in cells from HIV+ individuals experiencing viremia. Thus, the defects in CpG ODN responsiveness that we describe are likely to have biological relevance. Our results suggest that the interplay between pDC and monocytes may be impaired in HIV+ donors with ongoing viremia and that CpG ODN may be most effective as an immune adjuvant in HIV disease in the setting of HAART-induced control of HIV replication.
Acknowledgments
We thank Arthur M. Krieg for helpful advice and the Coley Pharmaceutical Group for providing the CpG ODN. We also appreciate the assistance of Robert Asaad in the collection of blood samples.
This work was supported in part by the Center for AIDS Research at Case Western Reserve University, University Hospitals of Cleveland (AI-36219), and by NIH grant awards AI-35726, AI-47255, and AI-55793 (Clifford V. Harding) and AI-55793 and AI-38858 (Michael M. Lederman and Scott F. Sieg).
REFERENCES
- 1.Ballas, Z. K., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:1840-1845. [PubMed] [Google Scholar]
- 2.Barron, M. A., N. Blyveis, B. E. Palmer, S. MaWhinney, and C. C. Wilson. 2003. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J. Infect. Dis. 187:26-37. [DOI] [PubMed] [Google Scholar]
- 3.Blackwell, S. E., and A. M. Krieg. 2003. CpG-A-induced monocyte IFN-γ-inducible protein-10 production is regulated by plasmacytoid dendritic cell-derived IFN-α. J. Immunol. 170:4061-4068. [DOI] [PubMed] [Google Scholar]
- 4.Cho, H. J., T. Hayashi, S. K. Datta, K. Takabayashi, J. H. Van Uden, A. Horner, M. Corr, and E. Raz. 2002. IFN-αβ promote priming of antigen-specific CD8+ and CD4+ T lymphocytes by immunostimulatory DNA-based vaccines. J. Immunol. 168:4907-4913. [DOI] [PubMed] [Google Scholar]
- 5.Cho, H. J., K. Takabayashi, P. M. Cheng, M. D. Nguyen, M. Corr, S. Tuck, and E. Raz. 2000. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat. Biotechnol. 18:509-514. [DOI] [PubMed] [Google Scholar]
- 6.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieye, T. N., P. S. Sow, T. Simonart, A. Gueye-Ndiaye, S. J. Popper, M. L. Delforge, A. Dieye, A. D. Sarr, A. Crusiaux, J. P. Van Vooren, M. Devleeschouwer, P. Kanki, S. Mboup, L. Diakhate, and C. M. Farber. 2001. Immunologic and virologic response after tetanus toxoid booster among HIV-1- and HIV-2-infected Senegalese individuals. Vaccine 20:905-913. [DOI] [PubMed] [Google Scholar]
- 8.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101:4505-4511. [DOI] [PubMed] [Google Scholar]
- 9.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574-2576. [DOI] [PubMed] [Google Scholar]
- 10.Feldman, S., D. Stein, S. Amrute, T. Denny, Z. Garcia, P. Kloser, Y. Sun, N. Megjugorac, and P. Fitzgerald-Bocarsly. 2001. Decreased interferon-α production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101:201-210. [DOI] [PubMed] [Google Scholar]
- 11.Ferbas, J., J. Navratil, A. Logar, and C. Rinaldo. 1995. Selective decrease in human immunodeficiency virus type 1 (HIV-1)-induced alpha interferon production by peripheral blood mononuclear cells during HIV-1 infection. Clin. Diagn. Lab. Immunol. 2:138-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann, G., J. Battiany, H. Poeck, M. Wagner, M. Kerkmann, N. Lubenow, S. Rothenfusser, and S. Endres. 2003. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-α induction in plasmacytoid dendritic cells. Eur. J. Immunol. 33:1633-1641. [DOI] [PubMed] [Google Scholar]
- 13.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 14.Howell, D. M., S. B. Feldman, P. Kloser, and P. Fitzgerald-Bocarsly. 1994. Decreased frequency of functional natural interferon-producing cells in peripheral blood of patients with the acquired immune deficiency syndrome. Clin. Immunol. Immunopathol. 71:223-230. [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki, N., and Y. J. Liu. 2002. Natural type I interferon-producing cells as a link between innate and adaptive immunity. Hum. Immunol. 63:1126-1132. [DOI] [PubMed] [Google Scholar]
- 16.Kadowaki, N., S. Antonenko, J. Y. Lau, and Y. J. Liu. 2000. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 18.Krug, A., S. Rothenfusser, V. Hornung, B. Jahrsdorfer, S. Blackwell, Z. K. Ballas, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur. J. Immunol. 31:2154-2163. [DOI] [PubMed] [Google Scholar]
- 19.Krug, A., S. Rothenfusser, S. Selinger, C. Bock, M. Kerkmann, J. Battiany, A. Sarris, T. Giese, D. Speiser, S. Endres, and G. Hartmann. 2003. CpG-A oligonucleotides induce a monocyte-derived dendritic cell-like phenotype that preferentially activates CD8 T cells. J. Immunol. 170:3468-3477. [DOI] [PubMed] [Google Scholar]
- 20.Levy, J. A., I. Scott, and C. Mackewicz. 2003. Protection from HIV/AIDS: the importance of innate immunity. Clin. Immunol. 108:167-174. [DOI] [PubMed] [Google Scholar]
- 21.Loré, K., M. R. Betts, J. M. Brenchley, J. Kuruppu, S. Khojasteh, S. Perfetto, M. Roederer, R. A. Seder, and R. A. Koup. 2003. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 171:4320-4328. [DOI] [PubMed] [Google Scholar]
- 22.Macatonia, S. E., R. Lau, S. Patterson, A. J. Pinching, and S. C. Knight. 1990. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology 71:38-45. [PMC free article] [PubMed] [Google Scholar]
- 23.Pacanowski, J., S. Kahi, M. Baillet, P. Lebon, C. Deveau, C. Goujard, L. Meyer, E. Oksenhendler, M. Sinet, and A. Hosmalin. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98:3016-3021. [DOI] [PubMed] [Google Scholar]
- 24.Shortman, K., and Y. J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151-161. [DOI] [PubMed] [Google Scholar]
- 25.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 26.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98:906-912. [DOI] [PubMed] [Google Scholar]
- 27.Valdez, H., R. Mitsuyasu, A. Landay, A. D. Sevin, E. S. Chan, J. Spritzler, S. A. Kalams, R. B. Pollard, J. Fahey, L. Fox, A. Namkung, S. Estep, R. Moss, D. Sahner, and M. M. Lederman. 2003. Interleukin-2 increases CD4+ lymphocyte numbers but does not enhance responses to immunization: results of A5046s. J. Infect. Dis. 187:320-325. [DOI] [PubMed] [Google Scholar]
- 28.Valdez, H., K. Y. Smith, A. Landay, E. Connick, D. R. Kuritzkes, H. Kessler, L. Fox, J. Spritzler, J. Roe, M. B. Lederman, H. M. Lederman, T. G. Evans, M. Heath-Chiozzi, and M. M. Lederman, and the ACTG 375 team. 2000. Response to immunization with recall and neoantigens after prolonged administration of an HIV-1 protease inhibitor-containing regimen. AIDS 14:11-21. [DOI] [PubMed] [Google Scholar]
- 29.Van Uden, J. H., C. H. Tran, D. A. Carson, and E. Raz. 2001. Type I interferon is required to mount an adaptive response to immunostimulatory DNA. Eur. J. Immunol. 31:3281-3290. [DOI] [PubMed] [Google Scholar]
- 30.Verthelyi, D., M. Gursel, R. T. Kenney, J. D. Lifson, S. Liu, J. Mican, and D. M. Klinman. 2003. CpG oligodeoxynucleotides protect normal and SIV-infected macaques from Leishmania infection. J. Immunol. 170:4717-4723. [DOI] [PubMed] [Google Scholar]
- 31.Verthelyi, D., K. J. Ishii, M. Gursel, F. Takeshita, and D. M. Klinman. 2001. Human peripheral blood cells differentially recognize and respond to two distinct CPG motifs. J. Immunol. 166:2372-2377. [DOI] [PubMed] [Google Scholar]
- 32.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 93:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]