Abstract
Background:
Since the introduction of the combination treatment of anti-programmed death-ligand 1 antibody atezolizumab and anti-VEGF antibody bevacizumab (AB), median overall survival in HCC has drastically improved. However, evidence on the efficacy and safety of the novel treatment standard in patients with prior exposure to systemic treatment is scarce. The aim of this global, multicenter, observational study was to evaluate the efficacy and safety of AB in patients after previous systemic therapy.
Methods:
We screened our global, multicenter, prospectively maintained registry database for patients who received any systemic therapy before AB. The primary end point was overall survival; secondary end points were time-to-progression, progression-free survival, objective response rate, and safety (rate and severity of adverse events).
Results:
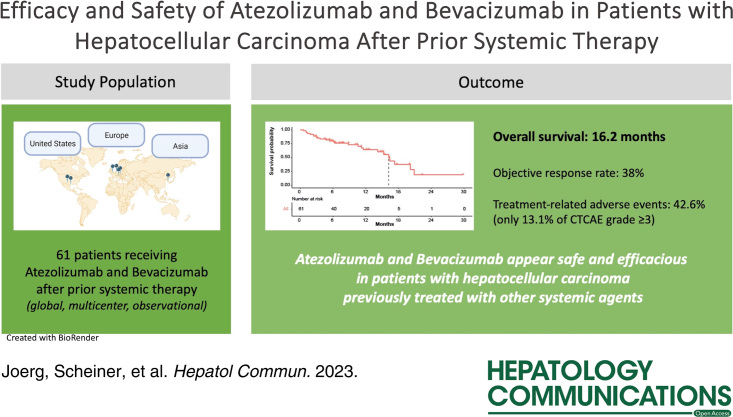
Among 493 patients who received AB for unresectable HCC, 61 patients received prior systemic therapy and were included in this analysis. The median age of the study population was 66 years, with 91.8% males. Predominant risk factors for HCC were viral hepatitis (59%) and alcohol (23%). Overall survival for AB was 16.2 (95% CI, 14.5–17.9) months, time-to-progression and progression-free survival were 4.1 (95% CI, 1.5–6.6) and 3.1 (95% CI, 1.1–5.1) months, respectively. The objective response rate was 38.2% (7.3% with complete and 30.9% with partial response). Overall survival was not influenced by treatment line (2nd vs. >2nd) or previous systemic treatment modality (tyrosine kinase inhibitors vs. immune checkpoint inhibitors). Treatment-related adverse events of all grades according to Common Terminology Criteria for Adverse Events were documented in 42.6% of patients, with only 13.1% of grade ≥3, including one death.
Conclusion:
In this observational study, AB emerges as a safe and efficacious treatment option in patients with HCC previously treated with other systemic therapy.
INTRODUCTION
Despite promising achievements in the treatment landscape of liver cancer,1 incidence and mortality are drastically increasing. Predictions estimate 1.4 million new cases and 1.3 million deaths in 2040, which represents an increase in mortality by 56% compared with 2020.2
The combination of anti-programmed death-ligand 1 antibody atezolizumab and anti-VEGF antibody bevacizumab has revolutionized systemic therapy for unresectable HCC, which represents the majority of primary liver cancer. Based on the IMbrave150 phase 3 clinical trial, this regimen has become the novel standard of care for first-line systemic therapy,3,4 having been proven superior against the multi-tyrosine kinase inhibitor (TKI) sorafenib with an unprecedented median overall survival (OS) of 19.2 months and an objective response rate around 30%.
However, eligibility for the clinical trial was limited to patients without prior exposure to systemic therapy, and therefore, data regarding efficacy and safety in patients who previously received any systemic therapy remain limited. To date, only regorafenib,5 cabozantinib,6 and ramucirumab (for patients with AFP ≥ 400 ng/mL)7 demonstrated a survival advantage against placebo in phase 3 clinical trials enrolling patients with previous systemic therapy (mainly sorafenib).
Overall survival of these regimens ranged between 8.5 and 10.6 months, with a high prevalence of treatment-related adverse events for TKI therapy.5–8 Experience of immunotherapy in the second line mostly includes non-randomized, noncontrolled, or early-phase clinical studies9–13 There are only 2 phase 3 clinical trials evaluating monotherapy with pembrolizumab against programmed cell death protein 1. However, they reported conflicting results. Early evidence from the KEYNOTE-394 study limited to an Asian population reported a survival benefit for pembrolizumab against placebo, while the global KEYNOTE-240 study failed to demonstrate the superiority of pembrolizumab against placebo.14,15
The aim of this global, multicenter, real-world observational study was to assess the efficacy and safety of the combination therapy with atezolizumab and bevacizumab in patients with HCC who previously received other systemic therapies.
METHODS
Patient enrollment
We generated a prospectively maintained database, termed atezolizumab bevacizumab (AB real),16 including 493 patients who received atezolizumab and bevacizumab for unresectable HCC in 14 tertiary care centers across Europe, the United States, and Asia. The main inclusion criteria were (i) age ≥18 years, (ii) HCC diagnosis according to clinical guidelines,17 and (iii) systemic therapy with atezolizumab and bevacizumab. Baseline characteristics and outcomes were provided from a medical chart review by each institution. For this project, patients who were treated with atezolizumab and bevacizumab as first-line systemic therapy were excluded (n = 432), and only patients who previously received any type of systemic therapy were kept as the study population (n = 61).
Clinical baseline characteristics, such as demographic data, etiology, and stage of underlying chronic liver disease or cirrhosis [Child-Turcotte-Pugh score, albumin-bilirubin (ALBI) grade], were obtained by medical chart review and provided by each institution. Performance status as indicated by Eastern Cooperative Oncology Group, tumor stage according to the Barcelona Clinic Liver Cancer (BCLC) staging system, and laboratory tests were recorded at the start of therapy. Information about adverse events (AE) was assessed by local investigators and graded according to the Common Terminology Criteria for Adverse Events.
Patients were followed as per clinical guidelines,17,18 which include contrast-enhanced cross-sectional imaging every 2–3 months.
Among our study cohort, 13% (8/61) of patients were previously included in a retrospective analysis of atezolizumab and bevacizumab in HCC with progression after first-line therapy.12 This applies to 3 patients contributed from Hamburg and 5 patients from Vienna. Data on the efficacy of atezolizumab and bevacizumab from the remaining 87% (53/61) patients have not been published yet.
Data analysis
The primary end point of the study was OS from the start of treatment with atezolizumab and bevacizumab. Secondary end points were time-to-progression (TTP), PFS, and investigator-assessed objective response using Response Evaluation Criteria in Solid Tumors 1.1 criteria, as well as safety according to AEs graded by Common Terminology Criteria for Adverse Events v.5.0. For descriptive statistics, continuous variables are reported as median and interquartile ranges and categorical variables as counts and percentages. OS, TTP, and PFS were analyzed using a log-rank test and plotted with Kaplan-Meier curves. The reverse Kaplan-Meier method was used to estimate the median follow-up time. Cox regression modeling was performed for known prognostic clinical variables. A p-value below 0.05 was considered statistically significant. All statistical analyses were conducted on SPSS (IBM, version 26) or R studio (R version 4.2.1).
RESULTS
Clinical characteristics
The overall prospective AB real database currently contains 493 patients from 14 centers globally. For this project, all patients who received previous systemic treatment before atezolizumab and bevacizumab were included in the analysis (n=61) (Figure 1). Baseline characteristics are displayed in Table 1. The median age of the population was 66 (59–71) years, 91.8% of patients were male. Risk factors for HCC were distributed as follows: alcohol in 23%, viral hepatitis in 59%, NASH in 6.6%, and other risk factors in 19.7% of the population. In all, 72.1% of patients had liver cirrhosis. Liver function, according to Child-Pugh Score, was stage A5 in 42.6% (n=26), A6 in 32.8% (n=20), B7 in 14.8% (n=9), B8 in 6.6% (4), B9 in 1.6% (1), and C11 in 1.6% (n=1) of patients. Classification following the ALBI score resulted in 23 patients being classified as grade 1, 34 patients as grade 2, and 4 patients as grade 3. Performance status was mostly adequate, with 98.3% (n=60) of patients being classified as Eastern Cooperative Oncology Group 0 or 1. Tumor stage was assessed according to BCLC staging criteria with 70.5% (n = 43) of patients belonging to BCLC C, 27.9% (n = 17) of patients to BCLC stage B, and 1.6% (n = 1) of patients to BCLC stage A. Extrahepatic tumor extension was present in 44.3% (n = 27) of patients. Median serum AFP levels measured before atezolizumab and bevacizumab exposure were 137 ng/mL IQR 7.1–1.976] and ≥ 400 ng/mL in 39% (n = 23) of patients. With regard to previous systemic treatment, median line of treatment was second line with 63.9% (n=39) of patients having received atezolizumab and bevacizumab as the second line of systemic treatment, 18% (n=11) as third, 6.6% (n=4) as fourth, and 11.4% (n=7) of patients as a further line of treatment, ranging until the seventh line of treatment (n=1, 1.6%). Treatment with atezolizumab and bevacizumab was started after a median of 1 (IQR: 0.4–3.0) month after cessation of the previous regimen. Previous treatment regimens consisted of 1 or multiple TKIs in 47.5% (n = 29), various combinations with immune checkpoint inhibitors (ICI) therapy in 49.2% (n = 30 including n = 5 ICI-monotherapies, n = 3 double ICI-combinations, n = 6 ICI plus anti-VEGF or targeted therapy, n = 13 ICI monotherapy sequentially with TKI treatment, and n = 3 ICI monotherapy with anti-VEGF/targeted therapy or TKI sequentially or combined) as well as 2 patients with prior chemotherapy (3.3%). Overall, 75.4% (n = 46) of patients received at least 1 treatment regimen containing a TKI. The best radiological response to the previous line of systemic treatment was evaluable in 48 (79%) patients. Of these, 1 (2%) patient had a complete response (CR), 6 (10%) had a partial response, 19 (31%) had stable disease, and 22 (36%) had progressive disease as their best radiological response. The most common reason for not starting atezolizumab and bevacizumab as a first-line systemic treatment was lack of approval at the time of systemic treatment initiation (n = 36, 59%), while another 31% of patients (n = 19) decided to participate in clinical trials testing other agents for first line. In 2 patients (3%), atezolizumab and bevacizumab were initially not started at first line due to safety concerns (1 patient with a history of dermatomyositis and fear of autoimmune reactivation, another one with concomitant radiotherapy). In 4 (7%) patients, the reason for not starting atezolizumab and bevacizumab upfront was not well documented.
FIGURE 1.
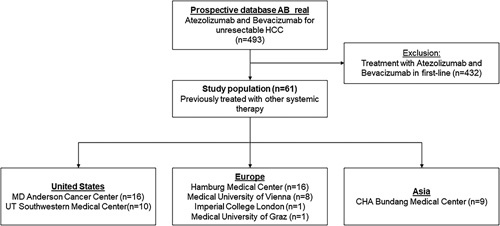
Flow chart of the study with numbers of patients by each contributing center. Abbreviation: AB, antibody bevacizumab.
TABLE 1.
Baseline characteristics
| Study cohort (n = 61) | |
|---|---|
| Age | 66 (59–71) |
| Sex, n (%) | |
| Female | 5 (8.2) |
| Male | 56 (91.8) |
| Cirrhosis present | 44 (72.1) |
| Risk factorb | |
| HBV | 15 (24.6) |
| HCV | 21 (34.4) |
| Alcohol | 14 (23.0) |
| NASH | 4 (6.6) |
| Othera | 12 (19.7) |
| AFP ng/mL | 137 (7–1976) |
| AFP ≥ 400 ng/ml | 23 (39.0) |
| Child-Pugh Score, n (%) | |
| A5 | 26 (42.6) |
| A6 | 20 (32.8) |
| B7 | 9 (14.8) |
| B8 | 4 (6.6) |
| B9 | 1 (1.6) |
| C11 | 1 (1.6) |
| ALBI grade, n (%) | |
| 1 | 23 (37.7) |
| 2 | 34 (55.7) |
| 3 | 4 (6.6) |
| ECOG PS, n (%) | |
| 0 | 31 (50.8) |
| 1 | 29 (47.5) |
| 2 | 1 (1.6) |
| BCLC stage, n (%) | |
| A | 1 (1.6) |
| B | 17 (27.9) |
| C | 43 (70.5) |
| Extrahepatic spread | 27 (44.3) |
| Previous nonsystemic treatmentsc, n (%) | |
| Surgery | 23 (37.7) |
| Ablation | 10 (16.4) |
| TACE | 23 (37.7) |
| TARE | 11 (18.0) |
| EBRT | 7 (11.5) |
| No.of previous systemic treatments, n (%) | |
| 1 | 39 (63.9) |
| 2 | 11 (18.0) |
| 3 | 4 (6.6) |
| 4 | 2 (3.3) |
| 5 | 3 (4.9) |
| 6 | 1 (1.6) |
| 7 | 1 (1.6) |
| Median line of systemic treatment | 2 (2–3) |
| Any previous TKI | 46 (75.4) |
| Any previous IO | 29 (47.5) |
Displayed are medians (IQR) for continuous and frequencies and percentages for categorical variables.
Includes 2 patients with cryptogenic liver disease, 1 patient with Wilson’s disease, 1 patient with alpha-1-antitrypsin-associated liver disease, 1 patient with a history of liver adenoma that progressed to HCC and 6 patients with unknown etiology of liver disease/no underlying liver disease.
Patients can have multiple risk factors, i.e. numbers exceed 100%.
Patients can have multiple previous treatments.
Abbreviations: ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; EBRT, external beam radiotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; IO, immune-oncological therapy; TARE/TACE, transarterial radio/chemoembolisation; TKI, tyrosine kinase inhibitors.
Data on upper endoscopy before treatment initiation were available in 48 (79%) patients. Of these, n = 20 (42%) patients had varices at the treatment initiation. While most of these patients (n=19) had esophageal varices, only 4 patients were diagnosed with gastric varices. Overall, 12 patients (60%) had small varices, 7 (35%) patients had medium-sized varices, and one (5%) patient was diagnosed with large varices.
Efficacy
Median OS for the overall cohort was 16.2 (95% CI, 14.5–17.9 Figure 2) months after a median follow-up of 12.2 months (95% CI, 9.5–14.8). 13.1% (n = 8) of patients were still on immunotherapy at the time of data cutoff, and treatment was discontinued in another patient (1.6%) due to ongoing CR. Secondary end points TTP and PFS were 4.1 (95% CI, 1.5–6.6) and 3.1 (95% CI, 1.1–5.1) months, respectively. Median duration of treatment was 3.4 (IQR: 2.0–7.8) months. For evaluable patients (n = 55), the best overall response was assessed as CR in 7.3% (n = 4), partial response in 30.9% (n = 17), stable disease in 27.3% (n=15), and progressive disease in 34.5% (n=19) of patients according to Response Evaluation Criteria in Solid Tumors criteria. For 6 patients (9.8%) no follow-up imaging was available. Disease control rate and objective response rate were 65.5% and 38.2%, respectively.
FIGURE 2.
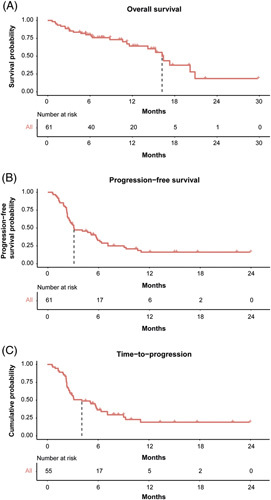
Median overall survival (A), progression-free survival (B), and time-to-progression (C) of the cohort. The latter analysis is limited to patients who had radiologic follow-up data available.
There was no significant difference in median OS between patients receiving atezolizumab and bevacizumab in the second line (n=38) compared to further lines (n = 23) of treatment (16.4 months 95% CI, 13.5–19.3 vs. 15.3 95% CI, 3.6–26.9, p = 0.395). Median OS was 20.2 months (95% CI, 7.1–33.0) in patients without prior exposure to TKI compared to 15.3 months (95% CI, 12.6–18.0) for patients with TKI exposure without reaching significance (p = 0.127). In patients with previous immunotherapy, the median OS was 20.2 months (95% CI, 4.6–35.8), whereas the median OS in patients without prior immunotherapy was 16.2 months (95% CI, 14.0–18.4, p = 0.874). When stratifying patients by liver function according to ALBI grade, superior liver function was significantly associated with improved OS (p < 0.001): ALBI grade 1 17.3 months (95% CI, 12.3–22.3), ALBI grade 2 11.6 months (95% CI, not estimable), and ALBI grade 3 1.3 months (95% CI, 0.027–2.7) (Figure 3). As expected, AFP ≥ 400 ng/ml and ALBI score were independent predictors for worse OS in Cox proportional hazard modeling (HR 3.882, 95% CI, 1.558–9.674, p=0.004, and 4.841, 95% CI, 2.242–10.452, p < 0.001, respectively) (Table 2).
FIGURE 3.
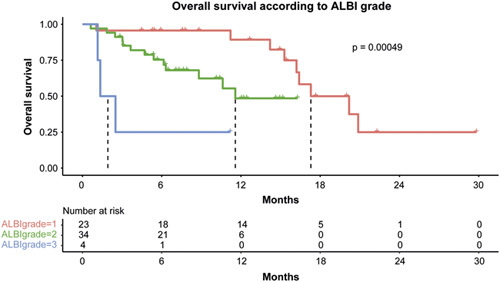
Median overall survival stratified by ALBI grade. Abbreviation: ALBI, albumin-bilirubin.
TABLE 2.
Cox regression model for death
| Univariable analysis | Multivariable analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| HR | Lower | Upper | P | HR | Lower | Upper | P | |
| Age | 0.979 | 0.942 | 1.019 | 0.300 | — | — | — | — |
| Presence of viral hepatitis | 0.956 | 0.432 | 2.119 | 0.913 | — | — | — | — |
| Presence of cirrhosis | 1.100 | 0.455 | 2.656 | 0.833 | — | — | — | — |
| AFP ≥ 400 | 3.103 | 1.318 | 7.306 | 0.010 | 3.882 | 1.558 | 9.674 | 0.004 |
| ALBI score, per point | 3.859 | 1.953 | 7.626 | <0.001 | 4.841 | 2.242 | 10.452 | <0.001 |
| Second vs. later systemic line | 1.417 | 0.633 | 3.171 | 0.397 | — | — | — | — |
| BCLC A/B vs. C | 0.653 | 0.277 | 1.543 | 0.332 | — | — | — | — |
| Previous IO exposure | 1.068 | 0.477 | 2.389 | 0.874 | — | — | — | — |
Abbreviations: ALBI, albumin-bilirubin; BCLC, Barcelona Clinic for Liver Cancer classification; IO, immune-oncological therapy.
Safety
Treatment-related AE of any grade were reported in 26 (42.6%) patients during a median treatment duration of 3.4 (IQR: 2.0–7.8) months. Of these, 4 (6.6%) patients experienced a bleeding event (grade 1 in 3 and grade 3 in 1 patient, respectively). Treatment-related AEs of grade 3 or higher were present in 8 (13.1%) patients and included bleeding (n = 1), proteinuria (n = 1) thrombosis (n = 1 leading to death), infections (n = 2), fever (n = 1), gastric ulcer perforation (n = 1), hyperglycemia (n = 1), and osteomyelitis (n = 1) (one patient suffered two AEs). While treatment could be continued in 1 of these patients, it was ultimately stopped in the remaining 7 patients due to concurrent progressive disease (n = 3) or subsequent death (n = 4, 3 of them unrelated to the respective AEs). Furthermore, 4 (6.6%) patients received corticosteroids, and treatment had to be stopped in additional 2 (3.3%) patients due to toxicity of lower grades.
The proportion of patients experiencing AEs of any grade was not associated with the type of treatment modality (TKI or ICI) or ALBI grade. However, AEs were more common in patients treated in the second line as compared with later lines of treatment (second line: n = 20 (52.6%) vs. later lines: n = 6 (26.1%), p = 0.042) and numerically higher in patients with CPS class B (64.3%, n = 9/14) and C (100%, n = 1/1) as compared with patients with CPS A (34.8%, n = 16/46; p = 0.075).
DISCUSSION
To our knowledge, we report the first global, prospective, multicenter, observational cohort study on the combination of atezolizumab and bevacizumab for unresectable HCC in patients who were previously treated with at least 1 different systemic therapy regimen. Our data suggest strong efficacy of the combination therapy with a median OS of 16.2 months, objective response rates of 65.5%, and PFS and TTP of 4.1 and 3.1 months, respectively. As expected, the ALBI score was an independent predictor of impaired survival in Cox modeling, and patients stratified as grade 1 according to the ALBI score showed a median OS of 17.3 months. This is comparable to the outcome of patients treated with atezolizumab and bevacizumab in the first systemic line within the IMbrave150 trial3,4as well as in real-world studies.16,19 As expected, compromised liver function resulted in a decreased median OS (11.6 mo for ALBI grade 2, 1.3 mo for ALBI grade 3), which is also in line with studies from first-line treatment.20
Importantly, atezolizumab and bevacizumab treatment was not only effective but also safe, with an AE rate of 42.6% overall and an AE grade ≥ 3 rate of 13.1%. This rate is in line with the IMbrave150 trial3,4 and other real-world studies19 on first-line treatment with atezolizumab and bevacizumab. In particular, no grade 3 or higher immune-related events occurred, and only 6.6% of patients required corticosteroid treatment, which could be explained by the high number of patients previously tolerating ICI-based therapies. In conclusion, neither the further line setting nor the fact that we included 25% of patients with advanced liver disease (Child-Turcotte-Pugh B/C) seemed to compromise its safety. Comparable safety data have also been reported in other studies evaluating atezolizumab and bevacizumab in patients with advanced liver disease and impaired liver function.19,21
It is highly likely that patients progressing to multiple lines of treatment while maintaining preserved liver function and adequate performance status will represent a selected HCC subgroup characterized by a favorable tumor biology. However, this is a potential bias inherent to any studies evaluating second-line treatment regimens, and until now, only limited data were available regarding the efficacy of the combination therapy with atezolizumab and bevacizumab in a further-line setting. Currently, only regorafenib, cabozantinib, and ramucirumab (the latter only for patients with AFP ≥ 400 ng/ml) have yielded positive results in phase 3 clinical trials for patients previously treated with TKI systemic therapy (ie, sorafenib).1,5–7 However, the median OS in these studies was only around 10 months (10.6 mo (95% CI, 9.1–12.1), 10.2 months (95% CI, 9.1–12.0), and 8.5 months (95% CI, 7.0–10.6) for regorafenib, cabozantinib, and ramucirumab,5–7 respectively). Noteworthy, objective tumor response rates were remarkably higher for the combination therapy compared to other agents, including our study with 38.2%, which is important as a recent meta-analysis including 34 randomized controlled trials in HCC concluded that achieving an objective response is associated with a significantly favorable prognosis.22 The radiological responses observed in our study included 7.3% of patients with a CR, and in 1 patient, treatment with atezolizumab and bevacizumab could even be stopped due to an ongoing CR, a pattern of response that has hardly ever been observed in patients treated with TKI.
Systemic treatment sequencing remains challenging in patients with HCC.23 Some earlier phase clinical trials have shown promising results for pembrolizumab or nivolumab monotherapy, and nivolumab in combination with ipilimumab in a second-line setting.11,24,25 However, while an Asian phase 3 clinical trial evaluating pembrolizumab against placebo in a second-line setting after sorafenib was positive,14 the global phase 3 clinical trial did not meet its primary end point.15
To our knowledge, there are only 2 studies specifically reporting real-world experience with atezolizumab and bevacizumab after previous systemic therapy, each with a smaller sample size and mostly national cohorts compared to our study. Most recently, a retrospective multicenter study included 12 German and 1 Austrian center and analyzed 50 patients who received atezolizumab and bevacizumab after at least 1 previous line of systemic therapy.12 The authors reported a median OS of 16.0 months (95% CI 5.6–26.4), an objective response rate of 32%, and a disease control rate of 68%, almost identical to our results. Only the median PFS was higher compared to our cohort (7.1 mo, 95% CI 4.4–9.8). Notably, data from our study were collected prospectively and included a global cohort of patients from Europe, the United States, and Asia, which further strengthens the generalizability and reproducibility of our results. Another study from Japan limited their analysis to patients who were treated with molecular targeted therapy before receiving atezolizumab and bevacizumab and focused on tumor growth patterns. This study included 31 patients, of whom 20 patients were previously treated with lenvatinib while the remaining 11 patients received other molecular therapies, including sorafenib, regorafenib, and ramucirumab. Patients with prior lenvatinib treatment showed initial tumor growth followed by shrinkage under atezolizumab and bevacizumab, ultimately resulting in higher objective response rates (21% vs. 9%). However, this did not reach statistical significance and median OS was similar between groups as well (11.6 vs. 11.4 mo).13
Despite our promising findings regarding the efficacy and safety of atezolizumab and bevacizumab in subsequent treatment lines, our study has several limitations. First, the sample size, although multicenter and global, is rather small, and especially subgroup analyses must be considered exploratory and need to be confirmed in larger studies. Due to the variety of different prior systemic therapies and the limited sample size, subgroup analysis of specific prior treatment regimens was not possible. Secondly, we only included patients who actually received the combination treatment, which could render a selection bias toward patients with favorable tumor biology and other known (eg, age, performance status, and liver function) and unknown prognostic factors. Among our study cohort, 13% (8/61) of patients were previously included in a retrospective analysis of atezolizumab and bevacizumab in HCC with progression after first-line therapy.12
In conclusion, our data suggest atezolizumab and bevacizumab as efficacious and safe alternative treatment regimens for patients after previous systemic therapy, including later treatment lines. Nevertheless, larger confirmatory studies are warranted, and the most efficacious sequence of systemic therapy after initial progression is yet to be determined in prospective studies.
Acknowledgments
DATA AVAILABILITY
Data will be made available on reasonable request from the corresponding authors.
AUTHOR CONTRIBUTIONS
Concept, design, and writing of the draft: Vincent Joerg, Bernhard Scheiner, David J. Pinato, Kornelius Schulze, Johann von Felden. All authors: Acquisition and analysis of data. All authors have approved the final version.
FUNDING INFORMATION
Antonio D´Alessio is supported by the National Institute for Health Research (NIHR) Imperial BRC, by grant funding from the European Association for the Study of the Liver (Andrew Burroughs Fellowship) and from Cancer Research UK (RCCPDB- Nov21/100008). ACG research is supported by NIH R01 MD012565, and R01 CA256977. Johann von Felden is supported by the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF grant 01EO2106), the German Cancer Aid (Deutsche Krebshilfe), and the Wilhelm Sander Foundation.
CONFLICTS OF INTEREST
Bernhard Scheiner is employed by AbbVie, Ipsen, and Gilead. Antonio D´Alessio consults for Roche. Henning Wege consults for Roche, AstraZeneca, and Eisai. Anwaar Saeed advises and received grants from AstraZeneca, Bristol Myers Squibb, Exelixis, and Daiichi Sankyo. He advises Pfizer. He received grants from Clovis, Actuate Therapeutics, Amgen, Biontech, Dragonfly, Incyte, Innovent, KAHR, and Merck. Bertram Bengsch consults for MSD and Roivant. Lorenza Rimassa consults, is on the speakers’ bureau, and received grants from Eisai, Eli Lilly, Incyte, Ipsen, and Roche. He consults, received grants, and is employed by AstraZeneca. He consults and is on the speakers’ bureau for Bayer and Sanofi. He consults and received grants from Exelixis, MSD, Nerviano Medical Sciences, and Zymeworks. He consults for Basilea, Genenta, Hengrui, IQVIA, and Taiho Oncology. He is on the speakers’ bureau for Gilead, Merck Serono, and Servier. He received grants from Agios, BeiGene, and Fibrogen. Arndt Weinmann advises Bristol Myers Squibb, Sanofi, and Wako. He is on the speakers’ bureau for Leo Pharma, Eisai, Ipsen, and Roche. He is employed by Merck and Servier. Rudolf Stauber is employed by Bayer, BMS, and Roche; he is a consultant for Astra Zeneca, Bayer, BMS, Eisai, Ipsen, Lilly, and Roche; he received travel support from Bayer and Roche. Matthias Pinter consults, is on the speakers’ bureau, and is employed by Bayer, Bristol Myers Squibb, Eisai, Eli Lilly, and Roche. He consults and is on the speakers’ bureau for MSD. He consults and is employed by Ipsen. He consults for AstraZeneca. Amit G. Singal consults and advises Genentech, AstraZeneca, Eisai, Bayer, Exelixis, TARGET, FujiFilm Medical Sciences, Glycotest, Exact, GRAIL, and Freenome. David J. Pinato is on the speakers’ bureau, received grants, and is employed by Bristol Myers Squibb. He consults and is on the speakers’ bureau for Eisai. He is on the speakers’ bureau and is employed by Bayer Healthcare. He consults for Astra Zeneca, DaVolterra, Exact, Mina Therapeutics, Mursla, and Roche. He is on the speakers’ bureau for Falk Foundation, Roche, and ViiV Healthcare. He received grants from MSD. Kornelius Schulze consults and advises Bayer. He consults for Ipsen. He advises Bristol Myers Squibb, Eli Lilly, MSD, and Roche. Johann von Felden advises Roche. The remaining authors have no conflicts to report.
Footnotes
Abbreviations: AB, antibody bevacizumab; AE, adverse event; ALBI, albumin-bilirubin; BCLC, Barcelona Clinic Liver Cancer; CR, complete response; EBRT, external beam radiotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; ICI, immune checkpoint inhibitors; IO, immune-oncological therapy; NE, not estimable; OS, overall survival; TARE/TACE, transarterial radio/chemoembolisation; TKI, tyrosine kinase inhibitor; TTP, time-to-progression.
Vincent Joerg and Bernhard Scheiner contributed equally as first authors.
Kornelius Schulze and Johann von Felden contributed equally as senior authors.
Contributor Information
Vincent Joerg, Email: v.joerg@uke.de.
Bernhard Scheiner, Email: bernhard.scheiner@meduniwien.ac.at.
Antonio D´Alessio, Email: a.dalessio@imperial.ac.uk.
Claudia A.M. Fulgenzi, Email: c.fulgenzi@imperial.ac.uk.
Martin Schönlein, Email: m.schoenlein@uke.de.
Lorenz Kocheise, Email: l.kocheise@uke.de.
Ansgar W. Lohse, Email: a.lohse@uke.de.
Samuel Huber, Email: s.huber@uke.de.
Henning Wege, Email: h.wege@uke.de.
Ahmed Kaseb, Email: akaseb@mdanderson.org.
Yinghong Wang, Email: ywang59@mdanderson.org.
Antony Mathew, Email: Antony.J.Mathew@uth.tmc.edu.
Andrew Kuang, Email: Andrew.Kuang@bcm.edu.
Mahvish Muzaffar, Email: muzaffarm@ecu.edu.
Yehia I. Abugabal, Email: YIMohamed@mdanderson.org.
Shadi Chamseddine, Email: schamseddine@mdanderson.org.
Samuel Phen, Email: Samuel.Phen@UTSouthwestern.edu.
Jaekyung Cheon, Email: cheonjk526@gmail.com.
Pei-Chang Lee, Email: tympanum3688@gmail.com.
Lorenz Balcar, Email: lorenz.balcar@meduniwien.ac.at.
Anja Krall, Email: anja.krall@medunigraz.at.
Celina Ang, Email: celina.ang@mssm.edu.
Linda Wu, Email: Linda.Wu2@mountsinai.org.
Anwaar Saeed, Email: dranwaarsaeed1@gmail.com.
Yi-Hsiang Huang, Email: yhhuang@vghtpe.gov.tw.
Bertram Bengsch, Email: bertram.bengsch@uniklinik-freiburg.de.
Lorenza Rimassa, Email: lorenza.rimassa@cancercenter.humanitas.it.
Arndt Weinmann, Email: arndt.weinmann@unimedizin-mainz.de.
Rudolf Stauber, Email: rudolf.stauber@medunigraz.at.
James Korolewicz, Email: j.korolewicz@imperial.ac.uk.
Matthias Pinter, Email: matthias.pinter@meduniwien.ac.at.
Amit G. Singal, Email: amit.singal@utsouthwestern.edu.
Hong Jae Chon, Email: hongjaechon@gmail.com.
David J. Pinato, Email: david.pinato@imperial.ac.uk.
Kornelius Schulze, Email: k.schulze@uke.de.
Johann von Felden, Email: j.von-felden@uke.de.
REFERENCES
- 1.Llovet JM, Pinyol R, Kelley RK, El-Khoueiry A, Reeves HL, Wang XW, et al. Molecular pathogenesis and systemic therapies for hepatocellular carcinoma. Nat Cancer. 2022;3:386–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, et al. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng A-L, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862–873. [DOI] [PubMed] [Google Scholar]
- 4.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New England J Med. 2020;382:1894–1905. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Qin S, Merle P, Granito A, Huang Y-H, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Alfa GK, Meyer T, Cheng A-L, El-Khoueiry AB, Rimassa L, Ryoo B-Y, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. New Eng J Med. 2018;379:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu AX, Kang Y-K, Yen C-J, Finn RS, Galle PR, Llovet JM, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:282–296. [DOI] [PubMed] [Google Scholar]
- 8.von Felden J. New systemic agents for hepatocellular carcinoma. Curr Opin Gastroenterol. 2020;36:177–183. [DOI] [PubMed] [Google Scholar]
- 9.Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. JAMA Oncol. 2020;6:e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in combination with apatinib in patients with advanced hepatocellular carcinoma (RESCUE): A Nonrandomized, Open-label, Phase II Trial. Clin Cancer Res. 2021;27:1003–1011. [DOI] [PubMed] [Google Scholar]
- 11.Yau T, Zagonel V, Santoro A, Acosta-Rivera M, Choo SP, Matilla A, et al. Nivolumab plus cabozantinib with or without ipilimumab for advanced hepatocellular carcinoma: results from cohort 6 of the CheckMate 040 Trial. J Clin Oncol. 2023;41:1747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinner F, Pinter M, Scheiner B, Ettrich TJ, Sturm N, Gonzalez-Carmona MA, et al. Atezolizumab plus bevacizumab in patients with advanced and progressing hepatocellular carcinoma: retrospective multicenter experience. Cancers (Basel). 2022;14:5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugimoto R, Satoh T, Ueda A, Senju T, Tanaka Y, Yamashita S, et al. Atezolizumab plus bevacizumab treatment for unresectable hepatocellular carcinoma progressing after molecular targeted therapy: A multicenter prospective observational study. Medicine. 2022;101:e30871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin S, Chen Z, Fang W, Ren Z, Xu R, Ryoo B-Y, et al. Pembrolizumab plus best supportive care versus placebo plus best supportive care as second-line therapy in patients in Asia with advanced hepatocellular carcinoma (HCC): Phase 3 KEYNOTE-394 study. J Clin Oncol. 2022;40:383. [Google Scholar]
- 15.Finn RS, Ryoo B-Y, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193–202. [DOI] [PubMed] [Google Scholar]
- 16.Fulgenzi CAM, Cheon J, D’Alessio A, Nishida N, Ang C, Marron TU, et al. Reproducible safety and efficacy of atezolizumab plus bevacizumab for HCC in clinical practice: Results of the AB-real study. Eur J Cancer. 2022;175:204–213. [DOI] [PubMed] [Google Scholar]
- 17.Galle PR, Forner A, Llovet JM, Mazzaferro V, Piscaglia F, Raoul J-L, et al. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 18.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. [DOI] [PubMed] [Google Scholar]
- 19.D’Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, Schulze K, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child‐Pugh A and B cirrhosis: A real‐world study. Hepatology. 2022;76:1000–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Campani C, Bamba‐Funck J, Campion B, Sidali S, Blaise L, Ganne‐Carrié N, et al. Baseline ALBI score and early variation of serum AFP predicts outcomes in patients with HCC treated by atezolizumab–bevacizumab. Liver Int. 2023;43:708–717. [DOI] [PubMed] [Google Scholar]
- 21.Scheiner B, Roessler D, Phen S, Lim M, Pomej K, Pressiani T, et al. Efficacy and safety of immune checkpoint inhibitor rechallenge in individuals with hepatocellular carcinoma. JHEP Reports. 2023;5:100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kudo M, Montal R, Finn RS, Castet F, Ueshima K, Nishida N, et al. Objective response predicts survival in advanced hepatocellular carcinoma treated with systemic therapies. Clinical Cancer Research. 2022;28:3443–3451. [DOI] [PubMed] [Google Scholar]
- 23.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19:940–952. [DOI] [PubMed] [Google Scholar]
- 25.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. The Lancet. 2017;389:2492–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on reasonable request from the corresponding authors.