Abstract
Human T-cell leukemia virus type 1 (HTLV-1) was the first human retrovirus identified and causes both adult T-cell leukemia/lymphoma and tropical spastic paraparesis/HTLV-1-associated myelopathy, among other disorders. In vitro, HTLV-1 has an extremely broad host cell tropism in that it is capable of infecting most mammalian cell types, although at the same time viral titers remain relatively low. Despite years of study, only recently has a bona fide candidate cellular receptor, glucose transporter 1 (glut-1), been identified. Although glut-1 was shown to bind specifically to the ectodomain of HTLV-1 and HTLV-2 envelope glycoproteins, which was reversible with small interfering RNA directed against glut-1, cellular susceptibility to HTLV upon expression of glut-1 was not established. Here we show that expression of glut-1 in relatively resistant MDBK cells conferred increased susceptibility to both HTLV-1- and HTLV-2-pseudotyped particles. glut-1 also markedly increased syncytium formation in MDBK cells after exposure to HTLV-1. Another assay also demonstrated HTLV-1 envelope-cell fusion in the presence of glut-1. Taken together, these results provide additional evidence that glut-1 is a receptor for HTLV.
Human T-cell leukemia virus type 1 (HTLV-1) was the first retrovirus identified in humans (21, 30, 43, 44). It causes at least two distinct diseases: adult T-cell leukemia/lymphoma (ATLL) (20, 45, 60, 63) and tropical spastic paraparesis/HTLV-1-associated myelopathy (10, 38, 39). HTLV-1 is endemic to certain geographic regions (8, 28), including equatorial Africa (47), southern Japan (1, 14), the southeastern United States (3), and parts of the Caribbean (2) and Latin America (12). Seropositivity increases with age and is higher in women (6, 56, 57). Although up to 35% of the southern Japanese population may be exposed (25, 53), in the United States, among intravenous drug users and persons in sexually transmitted disease clinics, the seroprevalence for HTLV-1 and HTLV-2 (a highly related virus) varies from 0.4 to 17.6 and 0.1 to 2.0%, respectively (22). Both viruses may be transmitted parenterally via blood transfusion or intravenous drug use (19, 37), vertically via breast feeding (35), and sexually (33).
The envelope glycoprotein of HTLV-1 is composed of gp46 (the surface glycoprotein component) noncovalently attached to gp21 (the transmembrane domain). Accumulated evidence suggests that one polypeptide region responsible in part for specific viral absorption and cellular binding resides around amino acid residues 190 to 200 in gp46. Polyclonal antiserum against this portion of gp46 inhibit HTLV-1-induced syncytium formation (24). A monoclonal antibody directed toward a single ectodomain epitope neutralized HTLV-1-induced T-cell proliferation (9). A second monoclonal antibody recognizing an overlapping epitope inhibited both HTLV-1-mediated cell fusion and T-cell transformation (58). Antipeptide antiserum raised against a decapeptide in the same region neutralized virus infection (58). Confirmatory evidence for the importance of this region has been obtained from multiple mutagenesis studies of gp46 (42). However, more direct evidence mapped a portion of the receptor recognition to an amino-terminal fragment of the surface glycoprotein (23). In addition, residue Y114 was critical for cellular binding (26), so the actual receptor binding domain (RBD) may be multipartite or discontinuous.
Although ATL is a disease of CD4+ T cells (43, 44, 64), HTLV-1 has a very broad host cell range in vitro. A wide variety of human cell lines and primary cell types are infectible with HTLV-1 (5, 15, 17, 46, 65). Nonhuman cell lines of many different species are also infectible (34, 62), suggesting that the receptor is both conserved and widely distributed. In fact, one stumbling block has been the identification of a nonsusceptible or resistant cell at the level of viral binding and entry. On the basis of superinfection interference studies, HTLV-1, HTLV-2, and related simian viruses all use the same receptor (50). Mouse-human somatic cell hybrids in conjunction with vesicular stomatitis virus (VSV) pseudotyping localized the receptor to distal 17q in humans (51), although our own work with human immunodeficiency virus (HIV) cores pseudotyped with HTLV envelopes suggested that chromosome 17-containing cell lines were no more susceptible than the parental murine cells (54).
More recently, the RBD of HTLV envelopes has been used as a tool to study the receptor and virus binding, which demonstrated that the receptor is poorly expressed on the cell surface of resting T cells but is up-regulated after T-cell activation (27, 36). Expression of full-length HTLV envelope and RBDs resulted in pronounced cellular metabolic alterations consistent with reduced glucose uptake and consumption (26). Because of this, candidate glucose transporters were tested for their role in HTLV entry and glucose transporter 1 (glut-1) was shown to bind specifically to both HTLV-1 and HTLV-2 ectodomains by both flow cytometric analysis and immunoprecipitation studies. Expression of the RBD also interfered with HTLV pseudotyping, which was reversed by overexpression of glut-1. In addition, small interfering RNA directed against glut-1 specifically reduced HTLV pseudotyping and RBD binding, again reversed with glut-1 (26).
Despite these impressive results, doubts remain about whether glut-1 is the HTLV receptor, especially since nonsusceptible or poorly susceptible cells were not tested because of lack of availability. Here we use the relatively resistant MDBK cell line to show that expression of glut-1 confers increased susceptibility to HTLV, by using both cell-free and cocultivation assays with HTLV pseudotypes. In addition, expression of glut-1 enhances the ability of MDBK cells to form syncytia after exposure to HTLV, and results from a cell fusion assay are also consistent with glut-1 being a component of the HTLV receptor complex. Taken together, these data support the role of glut-1 in HTLV cellular binding and entry.
MATERIALS AND METHODS
Plasmids and viral vectors.
pBABE-bleo.cycT1 was constructed by inserting a hemagglutinin epitope-tagged, truncated version of human cyclin T1 (residues 1 to 303; kind gift of Kathy Jones of the Salk Institute) into the SnaBI site of the murine leukemia virus (MLV) vector pBABE-bleo (32). The entire coding sequence of human glut-1 was amplified by PCR with primers 5′-CCATGGAGCCCAGCAGCAAGAA-3′ and 5′-ACTCACACTTGGGAATCAGCCCC-3′ from a HeLa cDNA library, the sequence was confirmed, and it was blunt end ligated into the MLV vector pBABE-MN-IRES-Blasti just upstream of the internal ribosome entry site (IRES). pHIT60 encodes MLV Gag-Pol, driven by the cytomegalovirus immediate-early enhancer-promoter (52), and pME-VSV G encodes VSV glycoprotein G, driven by the SRα promoter. Plasmids encoding HTLV-1 (pSV-HTLV-1 env rre) and HTLV-2 (pSV-HTLV-2 env rre) envelope glycoproteins were as previously described (54), as were pBIV-eGFP and pBH2 (29) (the latter were generously supplied by Tianci Luo of GTI, Gaithersburg, Md.). pcDNA3-Ebola Zaire was constructed by inserting a 2.4-kb cDNA for the Ebola virus glycoprotein (Zaire strain of glycoprotein, gift of Anthony Sanchez of the Centers for Disease Control and Prevention) downstream of the CMV promoter of pCDNA3 (Invitrogen), with glycoprotein expression confirmed by both immunoblotting and pseudotyping. pBIV-puro was constructed by replacing the enhanced green fluorescent protein (eGFP) of pBIV-eGFP with a 1.1-kb simian virus 40 (SV40) promoter-puromycin acetyltransferase cassette. pHIV-neo was constructed by replacing the alkaline phosphatase-encoding gene of pHIV-APΔenv (55) with a 1.7-kb SV40 promoter-neomycin resistance cassette, and pHIV-puro was constructed similarly with a 1.1-kb SV40 promoter-puromycin acetyltransferase cassette. pHIV-hygro was a gift of Dan Littman (Skirball Institute, New York University) and is similar to pHIV-gpt (40) except that the gene for hygromycin B resistance replaces gpt. pHIV-cycT1-IRES-bsd is based upon pHIV-APΔenvΔvifΔvpr (55) and contains a 1.9-kb cassette of truncated cycT1 coupled to blasticidin deaminase (bsd) by an IRES.
pBABE-puro LacZα was constructed by inserting a 400-bp HincII fragment encompassing LacZα from pSCTZα (31) into the SnaBI site of pBABE-puro (32). Plasmid pBABE-puro.LESTReGFP (gift of Dan Littman) encodes a fusion protein of LESTR (also known as fusin or CXCR4) with eGFP at the COOH terminus within MLV vector pBABE-puro. Similarly, plasmid pBABE-puro.GLUT1eYFP encodes a fusion protein of GLUT1 and eYFP at the COOH terminus; details of plasmid construction are available upon request.
Cells and viruses.
All cells were grown in 5% CO2, 37°C water-jacketed incubators and passaged every 3 to 5 days. MDBK cells were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle's high-glucose medium supplemented with 10% fetal calf serum (typically Invitrogen), penicillin, streptomycin, and ciprofloxacin at 20 μg/ml (termed DMEM complete). HOS TK− cells were also obtained from the American Type Culture Collection and similarly maintained, as were 293T cells. C91/PL cells, which chronically release HTLV-1 virions, were grown in RPMI medium with the same supplements as above. 293T-Ω 12 cells were a kind gift of Ned Landau (Salk Institute) and were maintained in DMEM complete supplemented with hygromycin B (Calbiochem) at 0.4 mg/ml.
Pseudotyped retroviral and lentiviral particles were produced by standard calcium phosphate cotransfection of 293T cells with the appropriate vector and envelope. For MLV or bovine immunodeficiency virus (BIV) vector production, the third plasmid encoded Gag-Pol (pHIT60 or pBH2, respectively). After 3 days, vector supernatant was harvested as previously described. For transductions or infections, vector supernatant was added to cells, which were incubated for 12 to 16 h and then refed and 48 to 72 h later either passaged into selective medium or further analyzed. If passaged into antibiotic-containing medium, cells were refed every 3 to 5 days and 9 to 12 days later cell colonies were either pooled and maintained as a stable cell line or fixed and stained with crystal violet in methanol-acetic acid and then enumerated.
Detection of glut-1 protein.
To confirm human glut-1 expression, exponentially growing cells were lysed in radioimmunoprecipitation assay buffer, cell lysates were centrifuged at 100,000 × g for 30 min, and solubilized proteins were separated by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoretic transfer to nitrocellulose, filters were first probed with rabbit anti-glut-1 antiserum diluted 1:1,000 (recognizes an epitope in the carboxy-terminal domain; gift of Mamoun Younes, Baylor College of Medicine), followed by anti-rabbit immunoglobulin G conjugated to horseradish peroxidase at 1:5,000 and developed by enhanced chemiluminescence. For immunofluorescence studies, cells were cytospun onto glass slides; fixed and permeabilized with Ortho Permeafix (Ortho Diagnostics); probed with the same primary antiserum at 1:400, followed by 1:100 anti-rabbit immunoglobulin G conjugated to biotin (Vector Labs) and then 1:500 streptavidin-tetramethyl rhodamine isothiocyanate (Jackson Immunoresearch); and photographed with a DFRT filter after staining nuclei with 4′,6′-diamidino-2-phenylindole (DAPI).
Syncytium and cell fusion assays.
For quantitation of syncytium formation, various amounts of exponentially growing C91/PL cells were cocultured with target adherent cells, with Jurkat cells as a control T-cell line. After 48 h, cells were extensively washed and then fixed and stained with crystal violet. Syncytia were enumerated by counting 5 to 10 randomly chosen microscopic fields.
For the cell fusion assay, 293T-Ω12 cells that stably express LacZΩ polypeptide (16) were transiently transfected with the indicated viral envelope (along with an eYFP expression plasmid as a transfection control) and 48 h later cocultured with target adherent cells. After another 2 days, cell lysates were prepared in accordance with the Galactostar kit manufacturer's (Tropix) instructions. Substrate was added to lysate, the mixture was incubated in the dark for 60 to 90 min, and relative light units were quantified by luminometry.
RESULTS
Relative resistance of MDBK cells to HTLV at the level of viral entry.
HTLV is capable of infecting most mammalian cell types, making it difficult to identify a nonsusceptible cell for gene transfer experiments. Previously, normal rat kidney cells and several mouse cell lines, which were thought to be resistant to VSV (HTLV)-pseudotyped particles, were in fact susceptible to HIV (HTLV) pseudotypes (54). Testing of MDBK cells was more problematic since HIV cores pseudotyped with the MLV amphotropic 4070a envelope failed to yield detectable titers (54), even in the presence of pit-2, the receptor for amphotropic virus. This was also true for HIV (VSV G) pseudotypes (data not shown). We decided instead to test BIV pseudotypes since MDBK cells are of bovine origin. Both MDBK and 293T cells were transduced with both BIV-eGFP (HTLV-1) and BIV-eGFP (VSV G) and analyzed by flow cytometry 72 h later. As shown in Fig. 1, MDBK cells were susceptible to BIV (VSV G) but were quite (although not completely) resistant to BIV (HTLV-1)-pseudotyped particles, confirming the original observation with VSV pseudotypes (51). Because of this, we reasoned that a postintegration (transcriptional) block for HIV may be present in MDBK cells. We obtained a truncated human cyclin T1 cDNA construct and introduced it into MDBK cells via an MLV-based vector. After this was done, HIV-eGFP (VSV G) titers were approximately 10,000 IU/ml as determined by epifluorescence microscopy, compared to ∼5 × 106 IU/ml on human cells (a reduction of ∼500-fold). This suggests that although there are other, uncharacterized blocks to HIV transduction in bovine cells, the dynamic range of the VSV G titer in the presence of cyclin T1 is likely sufficient so that HTLV susceptibility can be reliably investigated in MDBK cells.
FIG. 1.
MDBK cells are relatively resistant to BIV (HTLV) particles. MDBK and 293T cells were separately transduced with BIV-eGFP (HTLV-1) (gray bars) or BIV-eGFP (VSV G) (black bars) and subjected to flow cytometry 3 days later. Shown are the transduction efficiencies and mean fluorescence intensities (base of each bar) of eGFP-positive cells.
Confirmation of human glut-1 expression in MDBK cells.
The entire coding sequence of glut-1 was PCR amplified from a HeLa cDNA library and ligated into an MLV vector as part of a bicistronic cassette that included the blasticidin deaminase (bsd) gene. VSV G-pseudotyped MLV particles were produced and used to transduce MDBK cells. After transduction, blasticidin-resistant cells were pooled and maintained in blasticidin at 10 μg/ml. To check for glut-1 expression, cells were lysed and after SDS-PAGE, lysates were immunoblotted with anti-glut-1 antiserum. As shown in Fig. 2, glut-1 was expressed as a heterogeneous set of products with a molecular mass of approximately 55 kDa. This heterogeneity presumably represents different posttranslational modifications, mainly glycosylation, and has been observed previously (26). Note the presence of multiple background bands, one or more of which may represent the endogenous bovine glut-1 protein product.
FIG. 2.
Immunoblot assay of glut-1 in MDBK cells. MDBK cells were lysed, and proteins were separated by SDS-PAGE, blotted onto nitrocellulose, and probed with rabbit anti-glut-1 antiserum, followed by goat anti-rabbit antiserum conjugated to horseradish peroxidase. Lanes: 1, MDBK.cycT1 cells; 2, MDBK.empty cells; 3, MDBK.glut-1 cells; 4, MDBK.cycT1.empty cells; 5, MDBK.cycT1.glut-1 cells. Note the heterogeneous reactivity at 55 kDa; other bands are presumably background or perhaps reflect endogenous glut-1 reactivity.
To confirm glut-1 expression, cells were also subjected to indirect immunofluorescence assay. Blasticidin-resistant cells were fixed, permeabilized, and probed with the same primary anti-glut-1 antiserum, followed by two secondary reagents. As shown in Fig. 3, glut-1 was easily detectable in transduced cells carrying the MLV vector encoding glut-1, whereas only background staining was observed in cells transduced with the empty MLV vector. Note that glut-1 staining appears diffuse, which probably represents both cytoplasmic and plasma membrane localization of glut-1. The cytoplasmic pattern may be an artifact of overexpression from the MLV vector. Taken together, however, these results suggest that glut-1 was expressed in the transduced MDBK cells.
FIG. 3.
Immunofluorescence assay of glut-1 in MDBK cells. Cells were fixed and permeabilized and stained for glut-1 as described in the text. Panels: A, HOS cells (positive control); B, MDBK.empty cells; C, MDBK.glut-1 cells; D, MDBK.cycT1.empty cells; E, MDBK.cycT.glut-1 cells. Note both cytoplasmic and plasma membrane staining in panels C and E.
Conferment of HTLV susceptibility by glut-1.
In order to test the ability of glut-1 to mediate infection by HIV (HTLV)-pseudotyped particles, a truncated version of human cyclin T1 was first introduced into MDBK cells on a separate MLV vector. Note that this version of cyclin T1 lacks the PEST domain, thus making it more stable while retaining full activity as a Tat cofactor (61). Expression of cyclin T1 was confirmed by immunoblotting with anti-hemagglutinin epitope antiserum, and functionality was verified by demonstrating HIV-eYFP (VSV G) pseudotyping in both murine and bovine cells (data not shown). Both empty MLV vector and vector encoding glut-1 were introduced by transduction into MDBK.cycT1 cells, and those cells were maintained in selective medium containing both Zeocin and blasticidin.
In order to test susceptibility to HTLV, a variety of cell-free HIV (HTLV)-pseudotyped particles were prepared by transient transfection of 293T cells and used to infect both MDBK.cycT1.empty and MDBK.cycT1.glut-1 cells, with HOS TK− cells included as a positive control. Infected cells were passaged into selective medium, and colonies were counted after 9 to 12 days. HIV (VSV G) also served as a positive transduction control. As shown in Table 1., for all HIV (HTLV)-pseudotyped particles, titers on MDBK.cycT1 cells were much greater in the presence of glut-1. This was true for both HTLV-1- and HTLV-2-pseudotyped particles.
TABLE 1.
Susceptibility of MDBK cells to HIV(HTLV)-pseudotyped particles
| Vector supernatant | Titer on:
|
||
|---|---|---|---|
| MDBK.cycT1.emptya | MDBK.cycT1.glut1 | HOS TK− | |
| HIV-neo (HTLV-1)d | 1b | 63 | 5,000 |
| HIV-neo (VSV G) | 2,000 | 1,300 | >120,000c |
| HIV-hygro (HTLV-1)e | 8 | 240 | 3,000 |
| HIV-hygro (VSV G) | 7,000 | 6,000 | >120,000 |
| HIV-puro (HTLV-2)f | <1 | 65 | 900 |
| HIV-puro (VSV G) | 50,000 | 40,000 | >120,000 |
Cell-free vector titers are per milliliter. Results are representative of two to four independent experiments.
No colonies observed after using 5 ml of vector supernatant.
>40,000 colonies using 0.3 ml of vector supernatant.
For HIV-neo, MDBK and HOS cells were selected by using G418 at 2 and 0.5 mg/ml, respectively.
For HIV-hygro, MDBK and HOS cells were selected by using hygromycin B at 0.8 and 0.4 mg/ml, respectively.
For HIV-puro, MDBK and HOS cells were selected by using puromycin at 20 and 5 μg/ml, respectively.
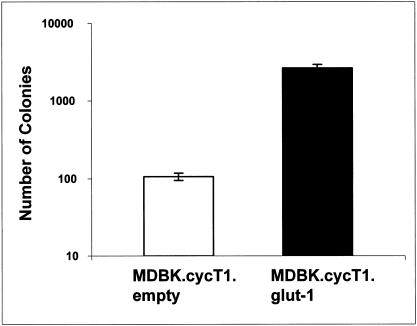
Because cell-free HIV (HTLV) titers were relatively low, we also performed cocultivation experiments in which the viral titers are typically at least 1 order of magnitude greater. Again, HIV (HTLV) pseudotype titers on MDBK.cycT1 cells were much higher in the presence of glut-1 (Fig. 4), whereas there was no appreciable difference in HIV (VSV G) cocultivation titers. These results are thus consistent with glut-1 playing a role in HTLV cellular binding and entry.
FIG. 4.
Cocultivation of HIV (HTLV) with MDBK cells. 293T cells were transfected with both pHIV-neo and pSV-HTLV-1 env rre plasmids, treated with mitomycin C, and then cocultured with the indicated cells. After 48 h, cells were passaged into selective medium containing Geneticin at 2.0 mg/ml. Colonies were enumerated 12 days later. Cocultivation of both cell types with 293T cells transfected with both pHIV-neo and pME VSV G gave approximately equivalent titers of 50,000/2 × 106 293T cells, whereas when envelope glycoprotein was omitted no colonies were observed. Note the logarithmic scale.
To confirm the above results, we also used BIV-pseudotyped particles. Both coculture and cell-free titers of BIV (HTLV-1) and BIV (HTLV-2) particles were markedly higher on MDBK cells in the presence of glut-1 (Table 2.). Note that there was no appreciable difference in BIV (VSV G) titers between the MDBK cell lines in both coculture and cell-free assays. Thus, the results obtained with the BIV-based vectors are consistent with those obtained with the HIV-based vectors.
We also wished to monitor glut-1 protein in real time. To do this, we constructed an MLV vector encoding a glut-1-eYFP fusion protein (eYFP cytosolic at the COOH terminus; Fig. 5A contains a schematic). As a control, the same MLV vector encoding a CXCR4-eGFP fusion was kindly provided by Dan Littman. Transient transfection of both of these constructs into 293T cells showed predominantly (but not exclusively) plasma membrane localization, as determine by epifluorescence microscopy. Similar fluorescence localization results were obtained after MLV (VSV G)-pseudotyped particles were used to transduce HOS TK− cells. In transduced MDBK cells, however, most of the fluorescence was cytosolic in aggregates (not shown). Despite this, MDBK cells expressing the glut-1-eYFP fusion were significantly more sensitive to HIV-cycT1-IRES-bsd (HTLV-2) infection compared to MDBK cells expressing the CXCR4-eGFP fusion, whereas susceptibility to VSV G pseudotypes was approximately the same (Fig. 5B).
FIG. 5.
glut-1-eYFP fusion also allows HIV (HTLV) infection. (A) Cartoon of fusion proteins with the autofluorescent protein portion shown in light grey and the plasma membrane shown as two curved lines. (B) Cell-free pseudotyping results obtained with either 6 ml of HIV-cycT1-IRES-bsd (HTLV-2) (II) or 1 ml of HIV-cycT1-IRES-bsd (VSV G) (G). Closed bars indicate MDBK.cycT1.LESTREGFP cells, and open bars indicate MDBK.cycT1.GLUT1EYFP cells. Average ± standard deviation is shown. *, P = 0.004 compared to HTLV-2 titers on MDBK.cycT1.LESTREGFP cells (Student's t test). Note the logarithmic scale.
Syncytium formation in the presence of glut-1.
Exposure to HTLV-1 and the related retroviruses may cause syncytium formation in susceptible cells. We decided to test whether glut-1 expression in MDBK cells increased syncytium formation after exposure to HTLV-1. C91/PL cells, which release HTLV-1 chronically, were cocultivated with MDBK cells (with or without cyclin T1) that had been transduced with empty or glut-1 MLV vector. Jurkat T cells served as a negative control. As shown in Fig. 6A to F, the presence of glut-1 markedly increased the number of syncytia in MDBK cells after cocultivation with C91/PL cells. Few syncytia were observed in the absence of glut-1 or when the MDBK cells were cocultured with Jurkat cells. These results are quantified in Fig. 6G and are also consistent with glut-1 being part of the HTLV receptor complex.
FIG. 6.
Syncytium formation in the presence of glut-1. Cells were cocultured with either Jurkat T cells (panels A, C, and E) or C91/PL cells (panels B, D, and F) and then fixed and stained with crystal violet. Panels: A and B, HOS cells; C and D, MDBK.empty cells; E and F, MDBK.glut-1 cells; G, quantitation of syncytia. Values are the average number of syncytia per 10× field. Downward arrows indicate that less than one syncytium was present per field. Open boxes, 105 C91/PL cells per 12-well plate; closed boxes, 2 × 105 C91/PL cells per 12-well plate. In the absence of C91/PL cells or in the presence of Jurkat T cells, only rare syncytia were observed for any of the cell lines. For HOS cells in the presence of C91/PL cells, values exceeded 100 (not shown).
Envelope-induced cell fusion in the presence of glut-1.
We wished to demonstrate fusion between envelope and glut-1-expressing cells by using a novel alpha-complementation assay recently developed by Landau and colleagues (16). By retroviral vector-mediated gene transfer, LacZα peptide was introduced into MDBK.glut-1 cells and the cells were maintained under puromycin and blasticidin selection. These cells were cocultured with 293T-Ω12 cells that stably express the LacZΩ polypeptide and that had been transiently transfected with HTLV-1 envelope or other viral glycoproteins. After 48 h, cells were lysed and soluble β-galactosidase activity was measured. Negative controls included MDBK.empty cells and cocultivation with parental 293T cells (as well as mock-transduced 293T-Ω12 cells), and positive controls included HOS TK−.lacZα cells and 293T-Ω12 cells that had been transfected with VSV G. Although the dynamic range of the assay was modest, there was a clear increase in LacZ activity in the MDBK.glut-1 cells cocultured with 293T-Ω12 cells that had been transfected with HTLV-1 env, and the magnitude of the effect was similar to that observed when 293T-Ω12 cells were transfected with Ebola Zaire virus glycoprotein (Fig. 7). This result is also consistent with glut-1 mediating HTLV envelope-cell fusion.
FIG. 7.
HTLV-induced cell fusion in the presence of glut-1. 293T Ω12 cells (stably express LacZω protein) were transfected with the indicated glycoproteins and then cocultured with the indicated cells stably expressing LacZα peptide. After 48 h, cells were lysed and soluble β-galactosidase activity was measured. Note the increase in the activity of HTLV-1 env in the presence of glut-1; no increase was observed when 293T cells were transfected or when LacZα peptide was absent.
DISCUSSION
Despite the fact that HTLV-1 was the first human retrovirus discovered, more than 20 years ago (43), identification of its cellular receptor remained elusive until quite recently (26). Major stumbling blocks that have precluded receptor characterization include the fact that most mammalian cell types are susceptible in vitro, viral titers are very modest (even in cocultivation assays), and stable expression of envelope is cytotoxic. Identification of glut-1 as the receptor directly resulted from the perceptive observation that cell glucose metabolism appeared to be altered after expression of either full-length envelope or the RBD (26). glut-1 specifically bound the RBD, and small interfering RNA directed against glut-1 reduced HTLV pseudotyping, which was reversed by overexpression of glut-1. Not demonstrated, however, was the ability of glut-1 to confer susceptibility on HTLV-resistant cells.
Here we confirm that MDBK cells are relatively resistant to HTLV at the level of viral entry, but after expression of glut-1 they became more susceptible to HTLV-pseudotyped particles, in both cell-free and cocultivation assays. glut-1 also increased HTLV-induced syncytium formation and cell fusion mediated by HTLV-1 envelope. These results, taken together with the previous work of Sitbon, Battini, and colleagues, add further support to the concept that glut-1 serves as an HTLV receptor.
We cannot exclude the possibility that other glucose transporters or cell surface proteins play a role in HTLV cellular binding and entry. glut-1 is widely expressed and is very highly conserved among mammalian species, which may explain why it has been nigh impossible to identify an HTLV-resistant cell line. Only recently have glut-1 “knock-down” murine cells become available (13), and it will be of interest to test their susceptibility. W cannot exclude the possibility that other cell surface proteins, in addition to glut-1, are part of the receptor complex. Experiments in which “pseudotyped” particles were produced with glut-1 in order to infect envelope-expressing cells did not yield an informative result. This may be viewed as being consistent with the presence of coreceptor, but it is also conceivable that not enough glut-1 was expressed on the surface of the pseudotyped particles (coupled with poor cell-free infectivity whenever working with HTLV envelope) to achieve an adequate signal over the background noise. Akin to work with HIV, the presence of coreceptor will likely only be inferred if in a specific cell type expression of glut-1 is not sufficient to confer susceptibility to HTLV. Because resistant cell lines are such a rarity, it is hard to envision that a coreceptor for HTLV exists.
One should note that the data presented here do not definitively establish that glut-1 is the HTLV receptor since all of the results are also consistent with its being involved in immediate postentry events, just downstream of receptor binding and entry. Given the fact that it is a multipass transmembrane protein, coupled with existing HTLV RBD binding data (26), the fact that glut-1 does confer susceptibility to HTLV buttresses the argument that it is an integral part of the receptor complex and it would be unprecedented if it were only indirectly or secondarily involved.
One paradox regarding HTLV is that essentially all mammalian cell types are infectible and yet viral titers are uniformly poor or low, even when pseudotyped particles are used for infectivity assays. The question remains whether this is due to limitations in viral envelope expression or stability, immediate postentry restrictions, glut-1 or other receptor-coreceptor expression, or affinity or avidity of virus for receptor. Identification of glut-1 and characterization of its interaction with envelope will help address some of these issues and concerns. Recently it has been suggested that, analogous to HIV infection, HTLV infection is facilitated by the formation of a “virological synapse” between infected and uninfected T cells (18). This synapse accumulates HTLV structural proteins, genome, and T-cell activation markers. Whether glut-1 is present and concentrated within this cell-cell junction is unknown, but if the synapse allows a greater density of glut-1 on the cell surface, that may help explain why cell-cell transmission is much more efficient than cell-free infection. It may be no more than a coincidence that HTLV induces polarization of the cytoskeleton (18) and its putative intracellular partner (GLUT1CBP) is enriched in mammalian midbodies and is required for initiation of cytokinesis (49).
The fact that a fusion protein between glut-1 and eYFP enhances susceptibility to HTLV suggests that a free, cytosolic COOH terminus is not required for HTLV binding and entry. This is true for many other retroviral and lentiviral receptors. This fusion protein may prove to be a useful reagent for examining events that occur at the virological synapse in real time and also for monitoring the effects of HTLV env or RBD on glut-1 intracellular trafficking and down-regulation from the plasma membrane.
Does the identification of glut-1 have therapeutic implications for ATLL or tropical spastic paraparesis/HTLV-1-associated myelopathy? It is doubtful that ongoing viral replication plays a major role in their pathophysiology of either of those illnesses, although the possibility cannot be fully excluded. Certainly for ATLL there is little virus production in malignant clonal CD4 T cells, so it is hard to envision how blockade of the receptor would be of therapeutic benefit. During primary infection, unfortunately, most individuals are asymptomatic and it would be difficult to identify them at the time of seroconversion. In addition, only a minority of seropositive individuals develops any type of clinical illness (and other than HIV coinfection, the risk factors for developing disease are unknown), so it would be hard to justify treating unaffected individuals, even those who are also HIV seropositive.
Aside from treatment efficacy concerns, there is also the potential of serious adverse events. Despite the fact that there are multiple glucose transporters in any given cell, glut-1 is arguably the most critical. Disruption of glucose transport would likely be cytotoxic to most cell types, including those of the central nervous system, especially given the fact that glut-1 is the predominant glucose transporter expressed by endothelial cells at the blood-brain barrier (4, 7, 41). This is further supported by evidence that in humans glut-1 deficiency or haploinsufficiency typically leads to severe learning difficulties, developmental delay, microcephaly, and seizures (11, 48, 59). Whether a small molecule or other compound could be designed to specifically and efficiently interfere with HTLV binding and entry but not glucose transport is unknown. Further molecular characterization of the interaction between HTLV and glut-1 should help resolve some of these questions and illuminate the importance of glut-1 to the replicative cycle of HTLV.
TABLE 2.
Susceptibility of MDBK cells to BIV vector-pseudotyped particles
| Assay and vector | Titer or no. of colonies
|
||
|---|---|---|---|
| MDBK.cycT1.empty | MDBK.cycT1.glut1 | HOS TK− | |
| Cell-free supernatanta | |||
| BIV-puro (no Env) | <1b | <1 | <1 |
| BIV-puro (HTLV-2)e | 1,000 | 20,000 | NDf |
| BIV-puro (HTLV-1) | 100 | 1,500 | 2,000 |
| BIV-puro (VSV G) | 25,000 | 30,000 | 50,000 |
| Cocultivation with cellsc | |||
| BIV-puro (no Env) | <1 | <1 | <1 |
| BIV-puro (HTLV-2) | 750 | 25,000 | ND |
| BIV-puro (HTLV-1) | 500 | 7,500 | 10,000 |
| BIV-puro (VSV G) | >50,000d | >50,000 | >50,000 |
Ten milliliters of culture supernatant was used in all cases except VSV G. Values represent the total number of colonies, except for VSV G, which is per milliliters.
No colonies observed after passaging into selective medium.
Approximately 2 × 106 target cells were cocultured with an equivalent number of 293T virus producers for 48 h prior to passage into selective medium.
An approximate number since cells rapidly reached confluence.
Experiment using HTLV-2 env was performed separately.
ND, not determined.
Acknowledgments
We thank Dan Littman, Tony Sanchez, Kathy Jones, Mamoun Younes, Ned Landau, and Tianci Luo for generous reagent gifts; Andy Rice and Jason Kimata for helpful discussions; and Zeynep Z. Ozen for assistance with the immunofluorescence studies.
This study was supported by the Edward Mallinckrodt, Jr., Foundation. R.E.S. is an Edward Mallinckrodt, Jr., Foundation scholar.
REFERENCES
- 1.Blattner, W. A., D. W. Blayney, G. M. Robert, M. G. Sarngadharan, V. S. Kalyanaraman, P. S. Sarin, E. S. Jaffe, and R. C. Gallo. 1983. Epidemiology of human T-cell leukemia/lymphoma virus. J. Infect. Dis. 147:406-416. [DOI] [PubMed] [Google Scholar]
- 2.Blattner, W. A., V. S. Kalyanaraman, G. M. Robert, T. A. Lister, D. A. Galton, P. S. Sarin, M. H. Crawford, D. Catovsky, M. Greaves, and R. C. Gallo. 1982. The human type-C retrovirus, HTLV, in Blacks from the Caribbean region, and relationship to adult T-cell leukemia/lymphoma. Int. J. Cancer 30:257-264. [DOI] [PubMed] [Google Scholar]
- 3.Blayney, D. W., W. A. Blattner, G. M. Robert, E. S. Jaffe, R. I. Fisher, P. J. Bunn, M. G. Patton, H. R. Rarick, and R. C. Gallo. 1983. The human T-cell leukemia-lymphoma virus in the southeastern United States. JAMA 250:1048-1052. [PubMed] [Google Scholar]
- 4.Bolz, S., C. L. Farrell, K. Dietz, and H. Wolburg. 1996. Subcellular distribution of glucose transporter (GLUT-1) during development of the blood-brain barrier in rats. Cell Tissue Res. 284:355-365. [DOI] [PubMed] [Google Scholar]
- 5.Clapham, P., K. Nagy, P. R. Cheingsong, M. Exley, and R. A. Weiss. 1983. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science 222:1125-1127. [DOI] [PubMed] [Google Scholar]
- 6.Clark, J., C. Saxinger, W. N. Gibbs, W. Lofters, L. Lagranade, K. Deceulaer, A. Ensroth, G. M. Robert, R. C. Gallo, and W. A. Blattner. 1985. Seroepidemiologic studies of human T-cell leukemia/lymphoma virus type I in Jamaica. Int. J. Cancer 36:37-41. [DOI] [PubMed] [Google Scholar]
- 7.Dobrogowska, D. H., and A. W. Vorbrodt. 1999. Quantitative immunocytochemical study of blood-brain barrier glucose transporter (GLUT-1) in four regions of mouse brain. J. Histochem. Cytochem. 47:1021-1030. [DOI] [PubMed] [Google Scholar]
- 8.Edlich, R. F., J. A. Arnette, and F. M. Williams. 2000. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-I). J. Emerg. Med. 18:109-119. [DOI] [PubMed] [Google Scholar]
- 9.Gazzolo, L., and D. M. Duc. 1987. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature 326:714-717. [DOI] [PubMed] [Google Scholar]
- 10.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 11.Gordon, N., and R. W. Newton. 2003. Glucose transporter type1 (GLUT-1) deficiency. Brain Dev. 25:477-480. [DOI] [PubMed] [Google Scholar]
- 12.Gotuzzo, E., C. Arango, A. deq Ueiroz-Campos, and R. E. Isturiz. 2000. Human T-cell lymphotropic virus-I in Latin America. Infect. Dis. Clin. N. Am. 14:211-239, x-xi. [DOI] [PubMed] [Google Scholar]
- 13.Heilig, C. W., T. Saunders, F. C. Brosius III, K. Moley, K. Heilig, R. Baggs, L. Guo, and D. Conner. 2003. Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc. Natl. Acad. Sci. USA 100:15613-15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinuma, Y., H. Komoda, T. Chosa, T. Kondo, M. Kohakura, T. Takenaka, M. Kikuchi, M. Ichimaru, K. Yunoki, I. Sato, R. Matsuo, Y. Takiuchi, H. Uchino, and M. Hanaoka. 1982. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int. J. Cancer 29:631-635. [DOI] [PubMed] [Google Scholar]
- 15.Ho, D. D., T. R. Rota, and M. S. Hirsch. 1984. Infection of human endothelial cells by human T-lymphotropic virus type I. Proc. Natl. Acad. Sci. USA 81:7588-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland, A. U., C. Munk, G. R. Lucero, L. D. Nguyen, and N. R. Landau. 2004. Alpha-complementation assay for HIV envelope glycoprotein-mediated fusion. Virology 319:343-352. [DOI] [PubMed] [Google Scholar]
- 17.Hoxie, J. A., D. M. Matthews, and D. B. Cines. 1984. Infection of human endothelial cells by human T-cell leukemia virus type I. Proc. Natl. Acad. Sci. USA 81:7591-7595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 19.Jason, J. M., J. S. McDougal, C. Cabradilla, V. S. Kalyanaraman, and B. L. Evatt. 1985. Human T-cell leukemia virus (HTLV-I) p24 antibody in New York City blood product recipients. Am. J. Hematol. 20:129-137. [DOI] [PubMed] [Google Scholar]
- 20.Kalyanaraman, V. S., M. G. Sarngadharan, Y. Nakao, Y. Ito, T. Aoki, and R. C. Gallo. 1982. Natural antibodies to the structural core protein (p24) of the human T-cell leukemia (lymphoma) retrovirus found in sera of leukemia patients in Japan. Proc. Natl. Acad. Sci. USA 79:1653-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalyanaraman, V. S., M. G. Sarngadharan, B. Poiesz, F. W. Ruscetti, and R. C. Gallo. 1981. Immunological properties of a type C retrovirus isolated from cultured human T-lymphoma cells and comparison to other mammalian retroviruses. J. Virol. 38:906-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khabbaz, R. F., I. M. Onorato, R. O. Cannon, T. M. Hartley, B. Roberts, B. Hosein, and J. E. Kaplan. 1992. Seroprevalence of HTLV-1 and HTLV-2 among intravenous drug users and persons in clinics for sexually transmitted diseases. N. Engl. J. Med. 326:375-380. [DOI] [PubMed] [Google Scholar]
- 23.Kim, F. J., I. Seiliez, C. Denesvre, D. Lavillette, F. L. Cosset, and M. Sitbon. 2000. Definition of an amino-terminal domain of the human T-cell leukemia virus type 1 envelope surface unit that extends the fusogenic range of an ecotropic murine leukemia virus. J. Biol. Chem. 275:23417-23420. [DOI] [PubMed] [Google Scholar]
- 24.Kiyokawa, T., H. Yoshikura, S. Hattori, M. Seiki, and M. Yoshida. 1984. Envelope proteins of human T-cell leukemia virus: expression in Escherichia coli and its application to studies of env gene functions. Proc. Natl. Acad. Sci. USA 81:6202-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda, Y., M. Furukawa, Y. Takehara, K. Yoshimura, K. Miyamoto, T. Matsuura, Y. Morishima, K. Tajima, K. Okochi, and Y. Hinuma. 1984. Prevalence of possible adult T-cell leukemia virus-carriers among volunteer blood donors in Japan: a nation-wide study. Int. J. Cancer 33:717-720. [DOI] [PubMed] [Google Scholar]
- 26.Manel, N., F. J. Kim, S. Kinet, N. Taylor, M. Sitbon, and J. L. Battini. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449-459. [DOI] [PubMed] [Google Scholar]
- 27.Manel, N., S. Kinet, J. L. Battini, F. J. Kim, N. Taylor, and M. Sitbon. 2003. The HTLV receptor is an early T-cell activation marker whose expression requires de novo protein synthesis. Blood 101:1913-1918. [DOI] [PubMed] [Google Scholar]
- 28.Manns, A., M. Hisada, and L. La Grenade. 1999. Human T-lymphotropic virus type I infection. Lancet 353:1951-1958. [DOI] [PubMed] [Google Scholar]
- 29.Matukonis, M., M. Li, R. P. Molina, B. Paszkiet, M. Kaleko, and T. Luo. 2002. Development of second- and third-generation bovine immunodeficiency virus-based gene transfer systems. Hum. Gene Ther. 13:1293-1303. [DOI] [PubMed] [Google Scholar]
- 30.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 31.Moosmann, P., and S. Rusconi. 1996. Alpha complementation of LacZ in mammalian cells. Nucleic Acids Res. 24:1171-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy, E. L., J. P. Figueroa, W. N. Gibbs, A. Brathwaite, C. M. Holding, D. Waters, B. Cranston, B. Hanchard, and W. A. Blattner. 1989. Sexual transmission of human T-lymphotropic virus type I (HTLV-I). Ann. Intern. Med. 111:555-560. [DOI] [PubMed] [Google Scholar]
- 34.Nagy, K., P. Clapham, P. R. Cheingsong, and R. A. Weiss. 1983. Human T-cell leukemia virus type I: induction of syncytia and inhibition by patients' sera. Int. J. Cancer 32:321-328. [DOI] [PubMed] [Google Scholar]
- 35.Nakano, S., Y. Ando, K. Saito, I. Moriyama, M. Ichijo, T. Toyama, K. Sugamura, J. Imai, and Y. Hinuma. 1986. Primary infection of Japanese infants with adult T-cell leukaemia-associated retrovirus (ATLV): evidence for viral transmission from mothers to children. J. Infect. 12:205-212. [DOI] [PubMed] [Google Scholar]
- 36.Nath, M. D., F. W. Ruscetti, C. Petrow-Sadowski, and K. S. Jones. 2003. Regulation of the cell-surface expression of an HTLV-I binding protein in human T cells during immune activation. Blood 101:3085-3092. [DOI] [PubMed] [Google Scholar]
- 37.Okochi, K., H. Sato, and Y. Hinuma. 1984. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: seroconversion in recipients. Vox Sang. 46:245-253. [DOI] [PubMed] [Google Scholar]
- 38.Osame, M., M. Matsumoto, K. Usuku, S. Izumo, N. Ijichi, H. Amitani, M. Tara, and A. Igata. 1987. Chronic progressive myelopathy associated with elevated antibodies to human T-lymphotropic virus type I and adult T-cell leukemialike cells. Ann. Neurol. 21:117-122. [DOI] [PubMed] [Google Scholar]
- 39.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. (Letter.) [DOI] [PubMed] [Google Scholar]
- 40.Page, K. A., N. R. Landau, and D. R. Littman. 1990. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J. Virol. 64:5270-5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pardridge, W. M., R. J. Boado, and C. R. Farrell. 1990. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier. Studies with quantitative Western blotting and in situ hybridization. J. Biol. Chem. 265:18035-18040. [PubMed] [Google Scholar]
- 42.Pique, C., T. Tursz, and M. C. Dokhelar. 1990. Mutations introduced along the HTLV-I envelope gene result in a non-functional protein: a basis for envelope conservation? EMBO J. 9:4243-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poiesz, B. J., F. W. Ruscetti, M. S. Reitz, V. S. Kalyanaraman, and R. C. Gallo. 1981. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sezary T-cell leukaemia. Nature 294:268-271. [DOI] [PubMed] [Google Scholar]
- 45.Robert-Guroff, M., Y. Nakao, K. Notake, Y. Ito, A. Sliski, and R. C. Gallo. 1982. Natural antibodies to human retrovirus HTLV in a cluster of Japanese patients with adult T cell leukemia. Science 215:975-978. [DOI] [PubMed] [Google Scholar]
- 46.Saida, T., K. Saida, M. Funauchi, E. Nishiguchi, M. Nakajima, S. Matsuda, M. Ohta, K. Ohta, H. Nishitani, and M. Hatanaka. 1988. HTLV-I myelitis: isolation of virus, genomic analysis, and infection in neural cell cultures. Ann. N. Y. Acad. Sci. 540:636-638. [DOI] [PubMed] [Google Scholar]
- 47.Saxinger, W., W. A. Blattner, P. H. Levine, J. Clark, R. Biggar, M. Hoh, J. Moghissi, P. Jacobs, L. Wilson, R. Jacobson, et al. 1984. Human T-cell leukemia virus (HTLV-I) antibodies in Africa. Science 225:1473-1476. [DOI] [PubMed] [Google Scholar]
- 48.Seidner, G., M. G. Alvarez, J. I. Yeh, K. R. O'Driscoll, J. Klepper, T. S. Stump, D. Wang, N. B. Spinner, M. J. Birnbaum, and D. C. De Vivo. 1998. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat. Genet. 18:188-191. [DOI] [PubMed] [Google Scholar]
- 49.Skop, A. R., H. Liu, J. Yates III, B. J. Meyer, and R. Heald. 2004. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 305:61-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sommerfelt, M. A., and R. A. Weiss. 1990. Receptor interference groups of 20 retroviruses plating on human cells. Virology 176:58-69. [DOI] [PubMed] [Google Scholar]
- 51.Sommerfelt, M. A., B. P. Williams, P. R. Clapham, E. Solomon, P. N. Goodfellow, and R. A. Weiss. 1988. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science 242:1557-1559. [DOI] [PubMed] [Google Scholar]
- 52.Soneoka, Y., P. M. Cannon, E. E. Ramsdale, J. C. Griffiths, G. Romano, S. M. Kingsman, and A. J. Kingsman. 1995. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23:628-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stuver, S. O., N. Tachibana, A. Okayama, F. Romano, T. Yokota, and N. Mueller. 1992. Determinants of HTLV-1 seroprevalence in Miyazaki Prefecture, Japan: a cross-sectional study. J. Acquir. Immune Defic. Syndr. 5:12-18. [PubMed] [Google Scholar]
- 54.Sutton, R. E., and D. R. Littman. 1996. Broad host range of human T-cell leukemia virus type 1 demonstrated with an improved pseudotyping system. J. Virol. 70:7322-7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutton, R. E., H. T. Wu, R. Rigg, E. Bohnlein, and P. O. Brown. 1998. Human immunodeficiency virus type 1 vectors efficiently transduce human hematopoietic stem cells. J. Virol. 72:5781-5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tajima, K., S. Kamura, S. Ito, M. Ito, M. Nagatomo, K. Kinoshita, and S. Ikeda. 1987. Epidemiological features of HTLV-I carriers and incidence of ATL in an ATL-endemic island: a report of the community-based co-operative study in Tsushima, Japan. Int. J. Cancer 40:741-746. [DOI] [PubMed] [Google Scholar]
- 57.Tajima, K., S. Tominaga, T. Suchi, T. Kawagoe, H. Komoda, Y. Hinuma, T. Oda, and K. Fujita. 1982. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: possible horizontal transmission of adult T-cell leukemia virus. Gann 73:893-901. [PubMed] [Google Scholar]
- 58.Tanaka, Y., L. Zeng, H. Shiraki, H. Shida, and H. Tozawa. 1991. Identification of a neutralization epitope on the envelope gp46 antigen of human T cell leukemia virus type I and induction of neutralizing antibody by peptide immunization. J. Immunol. 147:354-360. [PubMed] [Google Scholar]
- 59.Wang, D., P. Kranz-Eble, and D. C. De Vivo. 2000. Mutational analysis of GLUT1 (SLC2A1) in Glut-1 deficiency syndrome. Hum. Mutat. 16:224-231. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe, T., M. Seiki, and M. Yoshida. 1984. HTLV type I (U.S. isolate) and ATLV (Japanese isolate) are the same species of human retrovirus. Virology 133:238-241. [DOI] [PubMed] [Google Scholar]
- 61.Wei, P., M. E. Garber, S. M. Fang, W. H. Fischer, and K. A. Jones. 1998. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell 92:451-462. [DOI] [PubMed] [Google Scholar]
- 62.Weiss, R. A., P. Clapham, K. Nagy, and H. Hoshino. 1985. Envelope properties of human T-cell leukemia viruses. Curr. Top Microbiol. Immunol. 115:235-246. [DOI] [PubMed] [Google Scholar]
- 63.Wong-Staal, F., B. Hahn, V. Manzari, S. Colombini, G. Franchini, E. P. Gelmann, and R. C. Gallo. 1983. A survey of human leukaemias for sequences of a human retrovirus. Nature 302:626-628. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida, M., I. Miyoshi, and Y. Hinuma. 1982. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc. Natl. Acad. Sci. USA 79:2031-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoshikura, H., J. Nishida, M. Yoshida, Y. Kitamura, F. Takaku, and S. Ikeda. 1984. Isolation of HTLV derived from Japanese adult T-cell leukemia patients in human diploid fibroblast strain IMR90 and the biological characters of the infected cells. Int. J. Cancer 33:745-749. [DOI] [PubMed] [Google Scholar]