Abstract
Background:
Female pattern hair loss (FPHL) is known to present with characteristic pathological conditions, including reduced overall hair density. Female hormones affect hair condition; however, the detailed mechanism is unknown. Furthermore, research on the topic is complicated by the fact that senescent alopecia often occurs concurrently with FPHL. Therefore, we investigated the effect of estradiol, a female hormone, on hair growth by eliminating aging factors and objectively evaluating hair changes caused by female hormone replacement therapy (HRT).
Objective:
This study was conducted to elucidate the mechanism through which female hormones exert their effects on hair.
Methods:
The study included 11 female patients undergoing HRT who were evaluated before initiating HRT, 3 months after initiating HRT, and 6 months after initiating HRT. The thinning hair score, hair density, telogen hair rate, telogen plucking strength, hair growth rate, and hair thickness were measured and evaluated. Furthermore, hematological tests were performed to assess the general physical condition of the participants.
Results:
HRT increased the telogen hair rate (P = .010, paired t test) at 3 months, improved frontal hairline thinning score (P = .008, Wilcoxon test), and increased the plucking strength (P = .013, paired t test) at 6 months.
Limitations:
The limitation of this study included the relatively small sample size, inability to conduct further long-term tests because of participant burden, and lack of a control group.
Conclusion:
The results suggested that HRT improved the appearance of the frontal hairline. As few studies have analyzed the effects of female hormones on human hair, a novel finding of this study was the effects of estradiol on the plucking strength after excluding age as a factor. We believe that these findings will contribute to understanding FPHL and developing female hormone-related treatments.
Keywords: estradiol, female hormone, female pattern hair loss, hormone replacement therapy
What is known about this subject in regard to women and their families?
Patients with female pattern hair loss have a lower quality of life than those without symptoms.
Furthermore, hair loss is one of the major concerns after menopause.
Little is known about the effects on their families.
What is new from this article as messages for women and their families?
Understanding some of the causes of female pattern hair loss through this study may lead to the development of appropriate treatments.
As a result, patients may be able to reduce their anxiety and achieve a better life.
Introduction
Women with thinning hair have desired the development of hair growth reagents that are sufficiently effective.1 In women with thinning hair, the total hair density decreases; however, thick and long hair is also present in bald lesions,2 and such symptoms tend to begin at the age of >40 years. Therefore, men and women differ in symptoms, onset age, and cause of thinning hair, the cause for which is unknown. A study reported that finasteride, an antiandrogenic agent used to treat androgenetic alopecia, also mentioned an etiology different from it.3
Therefore, we focused on the decrease in the serum levels of the female hormone estradiol (E2), which is associated with menopause, and examined the characteristics of thinning hair in women. Estrogen receptors are present in hair follicles,4 and a decreased hair growth rate and diameter has been observed in pre- and postmenopausal women5; however, the mechanism of E2 effects on hair is unclear. In ovariectomized (OVX) mice with lowered female hormones, hair growth is delayed, new follicle count is reduced, and hair density is decreased.6 Furthermore, in dermal papilla cells responsible for hair growth in OVX mice, angiopoietin-2 acts downstream of female hormones and contributes to hair density control.7
Based on these studies, we hypothesized that thinning hair in women is affected by a decrease in female hormone levels, causing telogen prolongation and abnormalities, an increase in hair follicles without hair (kenogen), and decreasing density.
Regarding changes in hair attributed to E2 administration, hair loss reportedly decreases during hormone replacement therapy (HRT),8 and the external application of E2 valerate (synthetic E2) reduces the telogen hair rate.9
However, a study on female androgenic alopecia has indicated that the evidence to support the efficacy of the antiestrogen agent ICI-182780 is insufficient.10 Based on these findings, it cannot be concluded that the effect mechanism of E2 is fully understood. Moreover, technical evidence regarding the effects of topical estrogen is insufficient because only a few case studies in the literature focused on hormone replacement and the objectivity regarding the method of determining telogen hair is poor.11
Thus, this study was conducted to elucidate the mechanism through which female hormones exert their effects on hair. Additionally, to show the effect on hair, this pilot study involved female patients receiving HRT, evaluated changes over time in hair condition during HRT, and directly investigated the effects of female hormones to obtain highly objective data on the correlation between female sex hormones and hair.
Patients and methods
Study design
Using a quasi-experimental intervention design, we conducted a single-center study to evaluate the effect of female hormones on hair by comparing conditions before and after the start of HRT. The study population included female patients who visited the hospital for improvement of menopausal symptoms. In addition, 3 selection criteria were established: blood E2 concentration <10 pg/mL, follicle-stimulating hormone >25 mIU/mL,12 and absence of hair loss complaints.
Sample size
Before this study, a noninterventional pilot study of hair condition by age was conducted on 30 women with preliminary results (data not shown). From this result, the minimum sample size for the trial estimated using R (The R Foundation) software was 10 participants.
Ethical considerations
Before the study, the principal investigator presented to the potential participants an explanatory document, fully explained the study, and obtained voluntary consent to participate in the study after confirming that they understood the explanation. In the participant selection, the investigators carefully considered the appropriateness of asking them to participate in the study, taking into account human rights protection and the selection and exclusion criteria. The samples collected were used only for the prescribed tests and measurements and were not used for any other purpose. Furthermore, the specimens were properly disposed of after the analysis was completed. Participant information was anonymized, and photographs were taken without revealing the identity of the participants. All trials were conducted at the Haginaka Clinic (Ota-ku, Tokyo, Japan). The study design was approved by a relevant institutional review board. The study was also conducted in accordance with guidelines established by the Helsinki Declaration.
Patients
Patients suffering from menopausal problems were included in the study, without age limitations. However, patients having eczema on the scalp at the start of the study, those using hair growth or flocking agents, and those taking regular supplements for hair or menopause were excluded. All participants were visitors to the Haginaka Clinic and came to the clinic a total of 4 times (at the initial visit, before the start of HRT, and at evaluations 3 and 6 months after the start of HRT), so they were in the study for a maximum of approximately 8 months.
Study intervention
Subjects received 0.625 mg of conjugated estrogens or 1.0 mg of 17-beta-E2 orally on a continuous daily basis. In addition, 10 mg of didrogesterone was orally administrated cyclically for 14 days each month. These interventions methods were determined based on Japanese guidelines for HRT,12 and continued throughout the study.
Baseline evaluation
Hair was parted down the center on the parietal area to the left and right sides and was photographed using a digital camera (D5500; Nikon, Tokyo, Japan) placed perpendicularly to the scalp. The frontal hairline was photographed from the front with the hair raised so that the hairline could be seen. Using the photographs captured, 3 skilled individuals rated the degree of thinning hair using a 4-point Likert-type scale of 1–4 points (Supplementary Fig. S1, http://links.lww.com/IJWD/A29).
Evaluated contents
Blood tests were performed to confirm the effect of HRT, and biochemical, hematological, hair related, and hormone-related factors were evaluated to ascertain changes in the general serum composition as a secondary outcome (Supplementary Table, http://links.lww.com/IJWD/A35). Additionally, a photographic evaluation, phototrichogram, and hair plucking strength test were performed at the beginning and at 3 and 6 months after HRT to verify the condition of the hair. Photographic evaluations were performed in the same manner as the baseline. For the phototrichogram, after confirming the absence of skin abnormalities and ensuring that the skin condition is the same as the surrounding condition within a radius of 5 cm from the center of the parietal scalp, we cut the hair in the aforementioned area to form a 1.5-cm square. The hair in that area was held down with a transparent sheet, and a photograph was taken perpendicular to the scalp using a digital camera (D5500; Nikon) with a 120-mm-f/4.0 lens (Nikon). The same region was photographed after 2–3 days, and the hair density, telogen hair ratio, hair growth rate, and hair thickness were measured based on the photographs. For the plucking strength test, targeting hair within a radius of 5 cm from the center of the parietal area, we selected telogen hair, plucked them using a load measurement device (DPRSX-0.5TR; IMADA Co. Ltd., Toyohashi City, Japan), and measured the maximum load value needed to pull out the hair (plucking strength). The plucking strength with respect to 10 hairs per person was measured.
Evaluation of side effects
During this study, side effects were investigated using the participant’s logbook records, and no events directly related to the trial were identified.
Study flow diagram
The total time from subject recruitment to study completion was 8 months (Supplementary Fig. S2, http://links.lww.com/IJWD/A30).
Statistical analysis
First, an F test was performed to confirm the normality of each evaluation item. Then, regarding the thinning hair score, the Wilcoxon test was performed. Additionally, Scheffe’s paired comparison was used to evaluate the degree of improvement in thinning hair. Regarding other items, repeated measures analysis of variance (ANOVA) was performed. When a significant difference was confirmed by ANOVA, a comparison was adopted by the paired t test. These analyses were performed using JMP version 14.2.0 (SAS Institute Inc., Cary, NC) or BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan). The level of statistical significance was set at P < .05.
Results
Subject transition and attribute information
A total of 24 Japanese women expressed interest in participating in the study, and 13 were registered according to the criteria. Thereafter, 11 completed the study and 2 withdrew for personal reasons unrelated to the study (Supplementary Fig. S3, http://links.lww.com/IJWD/A31). Data from the individuals who dropped out were not used in the analysis. Among the participants (aged 47–56 years) who completed the trial, hair loss was initially recognized by 2 participants, was noticed only slightly by 3 participants, and was not felt by 6 participants.
Thinning hair score
In the analysis of the thinning hair score, the mean score for the parietal area was 1.61 points before HRT initiation, and it subsequently improved to 1.48 points 3 months after HRT initiation; however, no significant differences were noted (P = .266, Wilcoxon test) (Fig. 1A and B, Supplementary Fig. S4A, http://links.lww.com/IJWD/A32). Furthermore, the mean thinning hair score 6 months after HRT initiation was 1.61 points (P = .844, Wilcoxon test) (Fig. 1A and B, Supplementary Fig. S4A, http://links.lww.com/IJWD/A32). Although the score for frontal hairline was 1.91 points before HRT initiation and improved to 1.61 points 3 months after HRT initiation, no significant difference was observed (P = .109, Wilcoxon test), and 6 months after HRT initiation, the score improved to 1.24 points, and a significant difference was confirmed (P = .008, Wilcoxon) (Fig. 1C and D, Supplementary Fig. S4B, http://links.lww.com/IJWD/A32).
Fig. 1.
Changes in the mean thinning hair score and representative photographs over time: parietal area (A and B) and frontal hairline (C and D). (A and C) The gray lines in the graph show the measured values for each participant, and the bold line indicates the mean value.
Furthermore, we used Scheffe’s paired comparison using blinded photographs as another analytical approach. As a result, 36% of the participants with parietal area images showed improvements from before HRT initiation to 3 months after HRT initiation, and 18% showed improvements 3–6 months after HRT initiation, which were somewhat higher than the percentage of participants who showed deterioration (n = 27, 9% each) (Table 1). With regard to the frontal hairline, amelioration and deterioration cases were the same before initiation and 3 months after HRT initiation (18% each), and only 36% of the participants had improvement from before initiation to 6 months after HRT initiation and from 3 to 6 months after HRT initiation, respectively (Table 2). Moreover, the mean acceptability was based on 7 grades (−3:3 grade, dark; −2:2 grade, thick; −1:1 grade thick; 0: equal; +1:1 grade, thin; +2:2 grade, thin; +3:3 grade, thin).
Table 1.
Scheffe’s paired comparison of the thinning hair scores
| Subjects no. | Average degree of preference | P value | |||||
|---|---|---|---|---|---|---|---|
| Initial | 3 M | 6 M | Main effect | Initial versus 3 M | Initial versus 6 M | 3 M versus 6 M | |
| 1 | 0.28 | 0.00 | −0.28 | 0.0025 | ↑ | ↑ | ↑ |
| 2 | −0.28 | 0.28 | 0.00 | 0.0324 | ↓ | ||
| 3 | −0.06 | 0.06 | 0.00 | 0.4312 | |||
| 4 | 0.22 | −0.44 | 0.22 | 0.0331 | ↑ | ↓ | |
| 5 | 0.28 | −0.17 | −0.11 | 0.0046 | ↑ | ↑ | |
| 6 | −0.33 | 0.11 | 0.22 | 0.0680 | |||
| 7 | −0.11 | −0.11 | 0.22 | 0.0642 | |||
| 8 | −0.06 | 0.39 | −0.33 | 0.0021 | ↓ | ↑ | |
| 9 | −0.50 | 0.17 | 0.33 | 0.0022 | ↓ | ↓ | |
| 10 | 0.11 | 0.00 | −0.11 | 0.0917 | |||
| 11 | 0.39 | −0.11 | −0.28 | 0.0065 | ↑ | ↑ | |
Parietal area; statistical significance: 5%; up arrow: amelioration; down arrow: worsening; M, months.
Table 2.
Scheffe’s paired comparison of the thinning hair score
| Subjects no. | Average degree of preference | P value | |||||
|---|---|---|---|---|---|---|---|
| Initial | 3 M | 6 M | Main effect | Initial versus 3 M | Initial versus 6 M | 3 M versus 6 M | |
| 1 | 0.17 | −0.06 | −0.11 | 0.2654 | |||
| 2 | 0.22 | −0.06 | −0.17 | 0.0104 | ↑ | ↑ | |
| 3 | −0.17 | 0.22 | −0.06 | 0.0104 | ↓ | ↑ | |
| 4 | 0.00 | −0.22 | 0.22 | 0.1558 | |||
| 5 | −0.22 | 0.11 | 0.11 | 0.3629 | |||
| 6 | 0.17 | −0.06 | −0.11 | 0.0797 | |||
| 7 | 0.17 | 0.11 | −0.28 | 0.0126 | ↑ | ↑ | |
| 8 | 0.28 | 0.61 | −0.89 | 0.0001 | ↑ | ↑ | |
| 9 | −0.28 | 0.44 | −0.17 | 0.0084 | ↓ | ↑ | |
| 10 | 0.39 | −0.11 | −0.28 | 0.0065 | ↑ | ↑ | |
| 11 | −0.17 | −0.11 | 0.28 | 0.0698 | |||
Frontal hairline; statistical significance: 5%; up arrow: amelioration; down arrow: worsening; M, months.
Analysis of the enlarged images of the cut hair site (phototrichogram)
Telogen hair rate
The telogen hair rate indicated a significant change during the test period (P = .012, ANOVA). In the examination of the values according to time point, the telogen hair rate increased significantly to 9.6% 3 months after HRT initiation from 7.3% before HRT initiation (P = .010, paired t test). However, 6 months after HRT initiation, the rate returned to 7.0%, which was the level before HRT initiation (P = .487, paired t test) (Fig. 2A and B, Supplementary Fig. S5, http://links.lww.com/IJWD/A33).
Fig. 2.
(A) Changes in the telogen hair rate over time (the gray lines in the graph show the measured values for each participant, and the bold line indicates the mean value). (B) Phototrichograms of representative participants over time.
Analysis of plucking strength
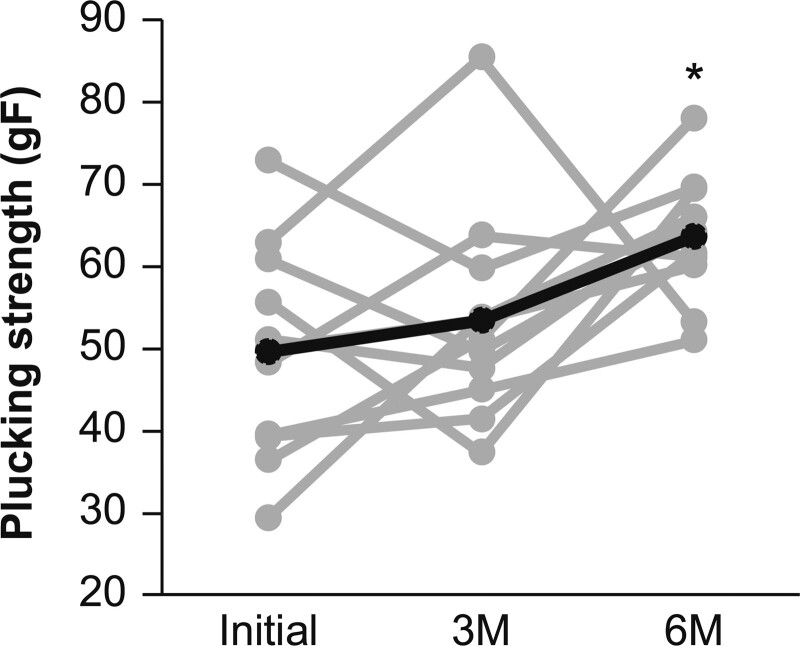
The telogen plucking strength intensity demonstrated a significant change during the period (P = .021, ANOVA). According to time point, the result was 49.7 gF before HRT initiation, but increased to 53.5 and 63.6 gF at 3 and 6 months after HRT initiation, respectively, confirming a significant difference between the telogen plucking strength intensity before and 6 months after HRT initiation (P = .013, paired t test) (Fig. 3).
Fig. 3.
Changes in the average plucking strength over time (the gray lines in the graph show the measured values for each participant, and the bold line indicates the mean value).
Other evaluation items
No significant changes were measured in the hair density, hair growth rate, and hair thickness (Supplementary Figs. S6–S8, http://links.lww.com/IJWD/A34).
Correlation analysis of measurement items
Analysis of correlation of parietal area evaluation items
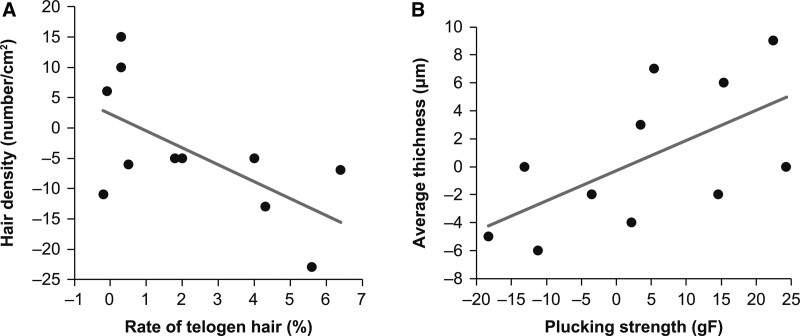
The degree of change from before HRT initiation to 3 months after HRT initiation and from before HRT initiation to 6 months after HRT initiation was calculated, and correlation analysis of all items was performed to evaluate the relationship between the items pertaining to parietal area evaluation. The results showed that regarding changes from before HRT initiation to 3 months after HRT initiation, the telogen hair rate and density showed an inverse correlation (r = −0.626; P = .040; Pearson) (Fig. 4A), whereas the plucking strength and mean hair thickness demonstrated a positive correlation (r = 0.613; P = .045; Pearson) (Fig. 4B). Regarding the changes from before HRT initiation to 6 months after HRT initiation, although some items showed weak correlations, no significant correlation could be confirmed for any item.
Fig. 4.
Scatter plots of the items showing a correlation from before the initiation of hormone replacement therapy (HRT) to 3 months after HRT initiation. (A) Degree of change in the telogen hair rate versus the degree of change in hair density and (B) the mean change in thickness versus the change in plucking strength.
Analysis of the correlation between parietal area evaluation and hematological examination items
Correlation analysis of the rate of change between the parietal area evaluation items (excluding thinning hair score) and hematological examination items revealed that 9 items with P < .05 (Pearson) were extracted 3 months after HRT initiation (Table 3a) and 4 items 6 months after HRT initiation (Table 3b). Many items related to the growth rate 3 months after HRT initiation and the mean hair thickness 6 months after HRT initiation were specifically extracted, and correlations between the mean hair thickness and triglyceride levels and between the mean hair thickness and prolactin were common in both time points.
Table 3.
Results of analysis of the correlation between hair evaluation and hematological examination (comparison between before hormone replacement therapy (HRT) and (a) 3 months (b) 6 months after HRT initiation)
| Hair related items | Blood test | Correlation | P value |
|---|---|---|---|
| (a) | |||
| Average thickness | Triglyceride | 0.801 | .003 |
| Growth rate | Progesterone | −0.747 | .008 |
| Growth rate | Free testosterone | 0.723 | .012 |
| Growth rate | Estradiol | −0.717 | .013 |
| Hair density | AST | −0.692 | .018 |
| Average thickness | Prolactin | −0.689 | .019 |
| Hair density | Leptin | 0.676 | .022 |
| Growth rate | Free T3 | 0.660 | .027 |
| Growth rate | Total cholesterol | 0.649 | .031 |
| (b) | |||
| Average thickness | Serum zinc | −0.755 | .012 |
| Growth rate | Serum iron | −0.741 | .014 |
| Average thickness | Triglyceride | 0.697 | .025 |
| Average thickness | Prolactin | −0.688 | .028 |
AST, aspartate transaminase.
Analysis of the correlation between the thinning hair score and hematological examination items
To analyze the correlations between the nonparametric thinning hair score and hematological examination values, the degree of change from before HRT initiation to 3 months after HRT initiation and from before HRT initiation to 6 months after HRT initiation was calculated, and correlation analysis of all items was performed. The results indicated that regarding the changes from before HRT initiation to 3 months after HRT initiation, a significant correlation was found between 2 items: progesterone and parietal thinning hair score (P = .848 and P = .001, respectively, Spearman) and testosterone and parietal thinning hair score (P = .607 and P = .048, respectively, Spearman) (Table 4). However, no significant difference was observed regarding changes from before HRT initiation to 6 months after HRT initiation.
Table 4.
Results of analysis of the correlation between the thinning hair score and hematological examination items (comparison between before HRT initiation and 3 months after HRT initiation)
| Thinning hair score | Blood test | Spearman’s rank correlation coefficient (ρ) | P value (Prob > ρ ) |
|---|---|---|---|
| Parietal | Progesterone | 0.848 | .001 |
| Parietal | Testosterone | 0.607 | .048 |
HRT, hormone replacement therapy.
Discussion
The results of the 2 evaluations of the thinning score suggested that the effects of HRT were stronger on the frontal hairline than on the parietal area. Additionally, items related to thinning hair were not included in the selection criteria of the participants, and no participant had severely thinning hair before HRT initiation (individual and mean evaluation values were ≤3 and ≤2.67, respectively). Therefore, although it was difficult for ameliorating effects to be realized, a significant improvement was observed in the frontal hairline, and clearer effects could be observed in individuals with thinning hair. In female alopecia, diffuse thinning occurs without being limited to a specific area, such as the parietal or frontal area.13 Additionally, both the expression of aromatase and estrogen receptors, which are responsible for the conversion of testosterone to E2, and differences in the site are greater in men, but little difference is noted in women.14 However, clinically, more fine hairs are often present on the frontal hairline than on the parietal area. Therefore, hair thinning treatment is more likely to change at the frontal hairline, and the effects of E2 were exhibited. Further study is needed to clarify the mechanism in detail.
E2 promotes the progression to the telogen phase15; in this study, the progression to the telogen phase possibly occurred early because of the rapid increase in E2 concentration following HRT initiation. However, considering that the telogen hair rate was approximately 10%, the evaluation results of this study were at a relatively low level (7%–10%), and no significant differences were observed 6 months after HRT initiation.
As the telogen plucking strength was significantly ameliorated by HRT in this study, the study was designed for the results to be less affected by age, and female hormones show a certain contribution to the telogen plucking strength. These findings are consistent with the decrease in plucking strength observed in OVX mice, which were created as a model of female alopecia due to decreased female hormone levels.6 Thus, the robustness of the hypothesis based on the animal model was further strengthened.
The inverse correlation between the telogen hair rate and hair density observed from before HRT initiation to 3 months after HRT initiation requires further verification for the same reasons mentioned above. The positive correlation between the plucking strength and mean hair thickness suggested that thicker hair is more difficult to pluck. However, hair thickness is determined by the size of the hair follicle,16 and the anagen phase lasts for 4–7 years.17 Hence, even when HRT was assumed to affect hair thickness, observing large-scale changes that affect the mean value over a 6-month study period may be difficult.
Triglyceride levels are increased by HRT,12 and in this study, while no statistically significant differences were noted, an increasing trend was observed. However, while no significant difference in the average hair thickness was observed, a trend showing a slight increase was noted. It is unlikely that triglycerides have direct effects on hair, and a spurious correlation due to the increase in both factors following HRT is possible. Furthermore, prolactin promotes hair loss by controlling the hair cycle, such as in the case of postpartum hair loss.18 In this study, the mean hair thickness was possibly decreased by the atrophy of the hair root following the promotion of the telogen phase. An inverse correlation between growth rate and E2 was observed 3 months after HRT initiation, which was consistent with previous findings demonstrating that E2 inhibits hair growth.19 Furthermore, no correlation was observed for ferritin, which is connected with hair, suggesting that HRT has an effect independent of ferritin.
Similar to estrogen, progesterone contributes to the physiological control of the hair cycle, and the quantity varies after menopause. Therefore, it might be related to thinning hair in women, which was the focus of this study, an extremely interesting finding. Moreover, testosterone is the cause of androgenetic alopecia in women.14 Therefore, correlating it with the thinning hair score is appropriate.
The limitations of this study included the relatively small sample size, the inability to conduct further long-term tests because of participant burden, and the lack of a control group. Furthermore, considering that the length of 1 hair cycle is approximately 4–6 years, only the effect on the hair follicles just before entering the anagen phase has been confirmed within the 6 months of this study. If the observation is continued for a longer period, the effect on more hair physiology may be manifested; thus, a clearer difference in phenomenon may be confirmed.
Moreover, as extremely complex factors influence hair physiology, the effect of female hormones alone only partially explains the female hair control mechanism. In the future, we would like to establish a control group, consider factors other than female hormones, and conduct a longer follow-up.
Conclusion
The results of this study suggest that female hormone supplementation is effective in ameliorating thinning hair. Significant amelioration of the frontal hairline thinning score and telogen plucking strength due to HRT could be confirmed. Because the thinning hair score was calculated based on photographs, the index of thinning hair might be close to actual evaluations. In addition, selecting participants with a higher telogen hair rate and conducting an experiment or investigating the results for a period longer than 6 months are necessary to determine whether HRT affects telogen hair. As studies on the effects of female hormones on hair in humans are limited, the novel findings of this study might be useful for understanding the causes of thinning hair in women and further development of treatment methods.
Conflicts of interest
Y.E., Y.O., and M.M. are employees of Lion Corporation. J.S. is a collaborator of this study and received rewards from the Lion Corporation. R.U. is compensated by Lion Corporation as a scientific advisor for research. The drugs, reagents, and other items used in this study are not related to Lion Corporation.
Funding
This research was funded by Lion Corporation, Japan.
Study approval
The study design was approved by the institutional review board of the Lion Corporation (no. 238) and the Haginaka Clinic (June 12, 2015). The study was also conducted in accordance with the guidelines established by the Helsinki Declaration.
Author contributions
All authors contributed to the design and performance of the research. YE and RU were responsible for writing paper. YE and YO contributed to the data analysis.
Data availability
The data-sharing plan was developed before this study was conducted. In addition, the internal audit department confirmed that the analysis of the survey was appropriately utilized in accordance with the plans developed (audit no. Q-1150-20150420-CR).
Supplementary data
Supplementary material associated with this article can be found at http://links.lww.com/IJWD/A29, http://links.lww.com/IJWD/A35, http://links.lww.com/IJWD/A30, http://links.lww.com/IJWD/A31, http://links.lww.com/IJWD/A32, http://links.lww.com/IJWD/A33, and http://links.lww.com/IJWD/A34.
Supplementary Material
Footnotes
Published online 1 November 2023
References
- 1.Tsuboi R, Niiyama S, Irisawa R, Harada K, Nakazawa Y, Kishimoto J. Autologous cell-based therapy for male and female pattern hair loss using dermal sheath cup cells: a randomized placebo-controlled double-blinded dose-finding clinical study. J Am Acad Dermatol 2020;83:109–16. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrocini G, Cantelli M, Masarà A, Annunziata MC, Marasca C, Cacciapuoti S. Female pattern hair loss: a clinical, pathophysiologic, and therapeutic review. Int J Womens Dermatol 2018;4:203–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim KH, Kwon SH, Lee YJ, Sim WY, Lew BL. Efficacy of finasteride in female pattern hair loss: a meta-analysis. Ann Dermatol 2021;33:304–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thornton MJ, Taylor AH, Mulligan K, et al. The distribution of estrogen receptor beta is distinct to that of estrogen receptor alpha and the androgen receptor in human skin and the pilosebaceous unit. J Investig Dermatol Symp Proc 2003;8:100–3. [DOI] [PubMed] [Google Scholar]
- 5.Mirmirani P. Hormonal changes in menopause: do they contribute to a “midlife hair crisis” in women? Br J Dermatol 2011;165:7–11. [DOI] [PubMed] [Google Scholar]
- 6.Endo Y, Takahashi M, Obayashi Y, Serizawa T, Murakoshi M, Ohyama M. The ovariectomized mouse simulates the pathophysiology of postmenopausal female pattern hair loss. J Dermatol Sci 2017;87:79–82. [DOI] [PubMed] [Google Scholar]
- 7.Endo Y, Obayashi Y, Ono T, Serizawa T, Murakoshi M, Ohyama M. Reversal of the hair loss phenotype by modulating the estradiol-ANGPT2 axis in the mouse model of female pattern hair loss. J Dermatol Sci 2018;91:43–51. [DOI] [PubMed] [Google Scholar]
- 8.Husmann F. Klinische Erfahrungen mit Climen bei peri- und postmenopausalen Frauen [Clinical experiences with Climen in peri- and postmenopausal women]. Zentralbl Gynakol 1997;119:123–7. [PubMed] [Google Scholar]
- 9.Georgala S, Katoulis AC, Georgala C, Moussatou V, Bozi E, Stavrianeas NG. Topical estrogen therapy for androgenetic alopecia in menopausal females. Dermatology 2004;208:178–9. [DOI] [PubMed] [Google Scholar]
- 10.Gassmueller J, Hoffmann R, Webster A. Topical fulvestrant solution has no effect on male and postmenopausal female androgenetic alopecia: results from two randomized, proof-of-concept studies. Br J Dermatol 2008;158:109–15. [DOI] [PubMed] [Google Scholar]
- 11.Ohnemus U, Uenalan M, Inzunza J, Gustafsson JA, Paus R. The hair follicle as an estrogen target and source. Endocr Rev 2006;27:677–706. [DOI] [PubMed] [Google Scholar]
- 12.Okano H, Higuchi T, Kurabayashi T, et al. ; Subcommittee for Revising the Japanese Guidelines for Hormone Replacement Therapy in the Women's Health Care Committee, Japan Society of Obstetrics and Gynecology. Japan Society of Obstetrics and Gynecology and Japan Society for Menopause and Women’s Health 2017 guidelines for hormone replacement therapy. J Obstet Gynaecol Res 2018;44:1355–68. [DOI] [PubMed] [Google Scholar]
- 13.Dinh QQ, Sinclair R. Female pattern hair loss: current treatment concepts. Clin Interv Aging 2007;2:189–99. [PMC free article] [PubMed] [Google Scholar]
- 14.Sawaya ME, Price VH. Different levels of 5 alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J Invest Dermatol 1997;109:296–300. [DOI] [PubMed] [Google Scholar]
- 15.Ohnemus U, Uenalan M, Conrad F, et al. Hair cycle control by estrogens: catagen induction via estrogen receptor (ER)-alpha is checked by ER beta signaling. Endocrinology 2005;146:1214–25. [DOI] [PubMed] [Google Scholar]
- 16.Chi W, Wu E, Morgan BA. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 2013;140:1676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zubair Z, Kantamaneni K, Jalla K, et al. Prevalence of low serum vitamin D levels in patients presenting with androgenetic alopecia: a review. Cureus 2021;13:e20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gizlenti S, Ekmekci TR. The changes in the hair cycle during gestation and the post-partum period. J Eur Acad Dermatol Venereol 2014;28:878–81. [DOI] [PubMed] [Google Scholar]
- 19.Oh HS, Smart RC. An estrogen receptor pathway regulates the telogen-anagen hair follicle transition and influences epidermal cell proliferation. Proc Natl Acad Sci U S A 1996;93:12525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data-sharing plan was developed before this study was conducted. In addition, the internal audit department confirmed that the analysis of the survey was appropriately utilized in accordance with the plans developed (audit no. Q-1150-20150420-CR).