ASCO-Friends study: sponsors say COVID-19–era trial flexibilities had minimal impact on treatment trial data integrity.
Abstract
PURPOSE
The onset of the COVID-19 pandemic created major disruptions in the conduct of cancer clinical trials. In response, regulators and sponsors allowed modifications to traditional trial processes to enable clinical research and care to continue. We systematically evaluated how these mitigation strategies affected data quality and overall trial conduct.
METHODS
This study used surveys and live interviews. Forty-one major industry and National Cancer Institute Network groups (sponsors) overseeing anticancer treatment trials open in the United States from January 2015 to May 2022 were invited to participate. Descriptive statistics were used for survey data summaries. Key themes from interviews were identified.
RESULTS
Twenty sponsors (48.8%; 15 industry and five Network groups) completed the survey; 11/20 (55.0%) participated in interviews. Sponsors predominantly (n = 12; 60.0%) reported large (≥11 trials) portfolios of phase II and/or phase III trials. The proportion of sponsors reporting a moderate (9) or substantial (8) increase in protocol deviations in the initial pandemic wave versus the pre-pandemic period was 89.5% (17/19); the proportion reporting a substantial increased dropped from 42.1% (n = 8/19) in the initial wave to 15.8% (n = 3/19) thereafter. The most commonly adopted mitigation strategies were remote distribution of oral anticancer therapies (70.0%), remote adverse event monitoring (65.0%), and remote consenting (65.0%). Most respondents (15/18; 83.3%) reported that the pandemic had minimal (n = 14) or no impact (n = 1) on overall data integrity.
CONCLUSION
Despite nearly all sponsors observing a temporary increase in protocol deviations, most reported the pandemic had minimal/no impact on overall data integrity. The COVID-19 pandemic accelerated an emerging trend toward greater flexibility in trial conduct, with potential benefits of reduced burden on trial participants and sites and improved patient access to research.
INTRODUCTION
Clinical trials are key to advancing new treatments for patients with cancer. At the outset of the COVID-19 pandemic, patient enrollment and treatment in cancer clinical trials dropped dramatically.1,2 In response, the National Cancer Institute (NCI) and the US Food and Drug Administration (FDA) issued guidance statements that provided greater flexibility for sponsors overseeing clinical trial processes in oncology.3,4 The goal was to allow modifications to trial protocols to mitigate the impact of pandemic-related disruptions on trial participants and clinical research. There is increasing evidence that widespread adoption of these modifications enabled a rebound of cancer treatment trial enrollment following an initial steep decrease.5-9 However, a knowledge gap remains about the impact of COVID-19–era protocol modifications on the quality of clinical trial data.10
CONTEXT
Key Objective
In response to the onset of the COVID-19 pandemic, major cancer clinical trial sponsors were allowed to modify traditional trial processes to enable clinical research and care to continue. Our aim was to evaluate how these mitigation strategies affected data quality and overall trial conduct.
Knowledge Generated
This study shows that major clinical trial sponsors widely adopted the recommended mitigation strategies to help maintain the conduct of clinical trials during the COVID-19 pandemic. Although a temporary increase in protocol deviations was reported by most sponsors, most also reported that the pandemic had minimal/no impact on overall data integrity.
Relevance
Our findings suggest that the strategies implemented during the pandemic to provide greater flexibility in trial conduct may reduce the burden of trial participation for patients and sites with limited adverse consequences for trial data.
To address this, ASCO and Friends of Cancer Research (Friends) convened a task force to evaluate the impact of the COVID-19 pandemic on the conduct of cancer clinical treatment trials. This task force included representation from physician investigators and clinical trial operations executives from academic- and community-based sites, NCI Network group and pharmaceutical industry sponsors, FDA, the NCI Cancer Therapy Evaluation Program (CTEP), patient advocates, biostatisticians, and a contract research organization. The goal was to assess the extent to which trial sponsors perceived that changes to protocols adopted during the pandemic affected data quality, an important consideration when evaluating whether efforts to modernize trial processes may make trials more accessible to patients and speed their conduct without adverse consequences.10,11
METHODS
This study combined surveys with live interviews (Data Supplement, online only). All pharmaceutical companies and NCI Network groups sponsoring at least one anticancer treatment trial before (January 2015-February 2020) and after (March 2020-May 2022) the COVID-19 pandemic were eligible to participate. ClinicalTrials.gov was queried to develop a list of eligible sponsors. The study protocol was reviewed and classified as exempt research by WCG Institutional Review Board (IRB) in April 2022.
Definitions
Trial Eligibility
All survey and interview questions referred to interventional anticancer treatment trials of any modality (eg, systemic therapy [cytotoxic, immune, hormonal, targeted, etc], surgery, or radiation) sponsored by the organization that were open in the United States. Although many industry trials are operated in multiple countries, sponsors were asked to restrict their observations to trial activities located in the United States.
Time Windows
We defined the following time periods to organize our evaluation:
Pre–COVID-19: January 2015-December 2019
Immediately pre–COVID-19: January-February 2020
Initial wave: March-April of 2020
Post-initial wave: May 2020-May 2022
Outcomes
The primary data quality metric was protocol deviations, interpreted to represent nonadherence to stated treatment and data collection processes defined prospectively within trial protocols.12 To ensure consistency, the following definition of a protocol deviation was provided: any noncompliance with IRB-approved protocol, including prospectively approved deviations and waivers. Furthermore, a significant or serious protocol deviation was defined as a protocol deviation that increases the potential risk to participants or affects the integrity of study data.
Our terminology is premised on published and anecdotal evidence that the COVID-19 outbreak had both direct (ie, reduced patient willingness to participate in trials) and indirect (mediated through the declaration of a public health emergency [PHE]) effects on the conduct of cancer clinical trials.13 Thus, we generally refer to impact of the COVID-19 pandemic itself—the underlying causal mechanism—even if, in some instances, the PHE was the more proximal cause of a consequence.
Survey
The task force developed a 35-item REDCap questionnaire. The electronic survey collected pre–COVID-19 and COVID-19–era data related to number and types of active treatment trials; trial openings, holds, and closures; organizational protocol deviation definitions; volume and types of protocol deviations collected; mitigation strategies implemented; impact on adverse events (AEs) collected (where AEs were categorized as physician-reported grades 1 [mild] or 2 [moderate] v grades 3 [serious] or 4 [life-threatening] using standard NCI definitions)14; and impact on overall data integrity. Sponsors were not asked to perform any analyses before participating in the survey.
Representatives from eligible sponsors were invited to participate in the survey. Sponsors were encouraged to engage multiple staff within their organization to inform responses. The survey was open from May 10 to August 22, 2022. Sponsors were offered 30 days to complete the survey, with 7- to 45-day extensions allowed by request.
Interviews
All sponsors that completed the survey were invited to be interviewed. Sponsor organizations were interviewed between August 11 and October 3, 2022. Two ASCO and Friends staff members (interviewers) alternated serving as primary interviewer and note-taker. Interviews were conducted via video conference and recorded for analysis purposes.
An interview guide was developed concurrently with the survey. Sponsors received the guide before the interview and were encouraged to select representatives with relevant knowledge of oncology clinical trial conduct and data to participate (one interview per organization). A semistructured interview approach was employed using the interview guide questions and appropriate follow-up questions on the basis of survey responses.
Statistical and Evaluation Methods
Survey data were summarized using descriptive statistics. To compare aggregate trends in the adoption of mitigation strategies between industry and NCI Network groups, each mitigation strategy was treated as an independent opportunity; the total was summed and compared using a Fisher's exact test. To describe how the volume of protocol deviations in the initial wave compared with the pre- and post-pandemic periods, we assessed the difference in paired Likert scale (1 = substantial increase, 2 = moderate increase, 3 = no change, 4 = moderate decrease, 5 = substantial decrease) scores, adjusted for organization type (industry v Network groups) using linear regression.
Interview data were evaluated using a three-step thematic analysis. First, interviewers classified the sponsor representatives' comments into three overarching categories that corresponded to the research objectives: (1) major protocol deviations collected during the pandemic, (2) key takeaways and impacts, and (3) future directions. Second, interviewers reconciled any points of discordance. Finally, interviewers agreed upon commonly occurring themes within the categories.
RESULTS
Forty-one eligible sponsor organizations were invited to participate; 21 (51.2%; all pharmaceutical company sponsors) did not participate, including 11 (26.8%) that did not respond, 8 (19.5%) that declined, and 2 (4.9%) that dropped out before completing the survey. Twenty sponsors (48.8%) completed the survey, including 15 pharmaceutical companies and all five NCI-sponsored Network groups. Representatives from 11/20 participating sponsors (55.0%) were interviewed. The median number of sponsor representatives per call was 3 (range, 1-8). Representatives who provided data for the survey and interviews were in data management, clinical development, regulatory science/affairs, statistics/biostatistics, and medical writing roles. Most (27/34) representatives were in director- or vice president–level roles (including associate, senior, and executive).
Where we did not receive a survey response from all sponsors, the denominator used for analysis is specified; otherwise, it is 20.
Survey Findings
Sponsor Characteristics
In January-February 2020, four sponsors (20.0%) had 0-5 open phase II trials (small portfolios), 4 (20.0%) had 6-10 trials (medium), and 12 (60.0%) had ≥11 (large; Table 1). The majority of all sponsors (60.0%) also had large portfolios of open phase III trials. Among industry sponsors, about half (46.7%) reported large phase II portfolios and about half (46.7) reported large phase III portfolios. NCI Network groups were more likely (P = .05) to have reported large portfolios of both phase II (100%) and phase III (100%) trials.
TABLE 1.
Sponsor Responses to Selected Survey Questions
Protocol Deviations
Sponsors' definitions of significant or serious protocol deviations referenced the potential impact on participant safety and data and scientific integrity, similar to the definition provided in study materials. Before the COVID-19 pandemic, nearly all (≥90%) sponsors classified eligibility- or consent-related issues, treatment-related issues, and assessment-, lab-, or imaging-related issues (including missed and out-of-window visits) as protocol deviations, with minor exceptions. Sponsors were evenly divided (yes, 50.0%; no, 50.0%) in considering device-related issues as protocol deviations. A significant minority reported that lab/imaging/test/procedure after withdrawal of consent (20.0%) or imaging performed by a nonqualified site (25.0%) was not considered protocol deviations.
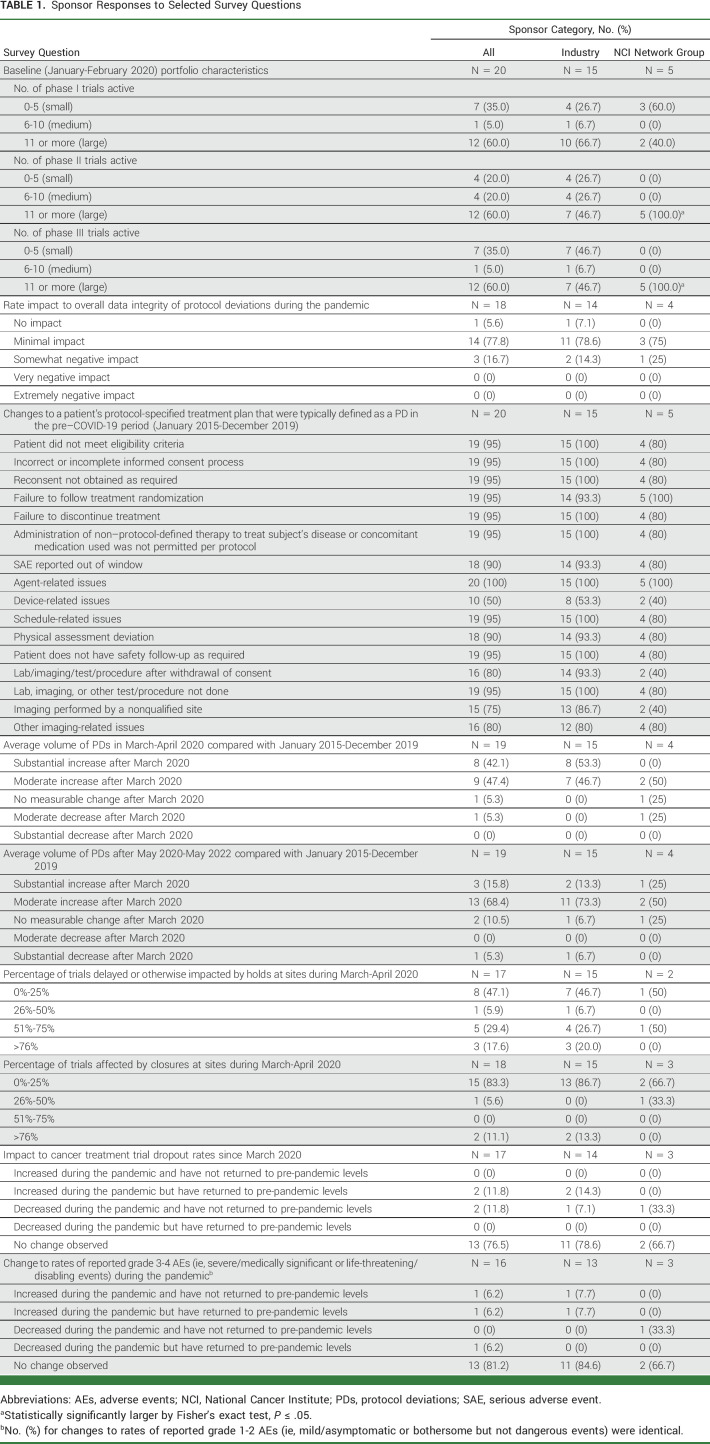
Nearly all sponsors (17/19; 89.5%) reported a moderate (9/19; 47.4%) or substantial (8/19; 42.1%) increase in volume of protocol deviations in the first wave of the COVID-19 pandemic (March-April 2020; Fig 1). After the initial wave (beginning May 2020), the increase in volume compared with the pre-pandemic period was lower (P = .03 in linear regression), with only 3/19 (15.8%) describing the increase as substantial. However, an additional 13/19 (68.4%) reported a moderate increase in protocol deviations after the initial wave, indicating that the level of deviations had not returned to pre-pandemic levels.
FIG 1.
Change in volume of protocol deviations compared with the pre-pandemic period (N = 19; industry = 15, NCI Network groups = 4). The horizontal bars indicate the number of sponsors indicating the level of change in volume of protocol deviations between the initial wave versus the pre–COVID-19 pandemic period (in blue) and between the post-initial wave versus the pre–COVID-19 pandemic period (in red). NCI, National Cancer Institute.
Sponsors were also asked to assess whether more serious/significant protocol deviations were being reported at the time of the survey, compared with pre–COVID-19. Among 16 sponsors providing data, 10 (62.5%) stated that the average number of serious protocol deviations was stable relative to the number of minor protocol deviations. Five (31.3%) reported that the average number of serious protocol deviations had increased compared with the pre-pandemic period, and one sponsor reported a decrease.
Nearly all sponsors (19; 95.0%) collected protocol deviations attributable to the COVID-19 pandemic. Most respondents (17; 85.0%) did not collect data regarding whether protocol deviations were attributable to study staff or participant decision making.
Mitigation Strategies
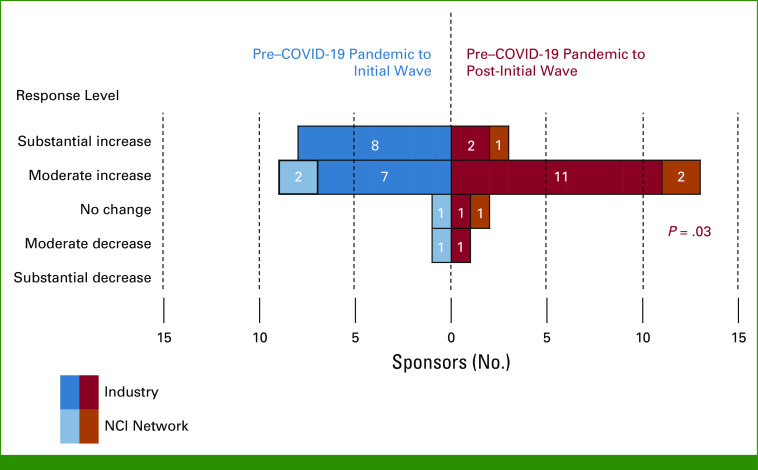
The most common mitigation strategies adopted between January and May 2020 were remote distribution of oral anticancer therapies (70.0%); remote symptom monitoring of AEs (65.0%); and e-consenting or remote informed consent (65.0%; Fig 2). Other commonly (ie, ≥50% overall) adopted strategies included remote collection of patient-reported outcomes (55.0%), remote routine laboratory testing (50.0%), remote imaging (50.0%), and remote study-specific laboratory tests (50.0%). Few sponsors adopted the strategy of remote study-required biopsies (10%). All sponsors reported either yes or no for all 12 specified mitigation strategies; thus, across the 20 sponsors, there were a total of 240 opportunities to adopt the 12 mitigation strategies, and nearly half (110/240; 45.8%) were adopted. This proportion did not differ between NCI Network groups (32/60 opportunities, 53.3%) compared with industry (78/180 opportunities; 43.3%; P = .18).
FIG 2.
Mitigation strategies adopted by sponsors between January and May 2020 (%; N = 20; industry = 15, NCI Network groups = 5). E, electronic; IV, intravenous; NCI, National Cancer Institute.
Impact of Trial Holds and Closures at Study Sites
Among 17 respondents, during the initial wave, trial holds were reported for none/few (0%-25%) sites by eight (47.1%) sponsors, some (26%-50%) sites by one (5.9%) sponsor, most (51%-75%) sites by five (29.4%) sponsors, and nearly all/all (>76%) sites by three (17.6%) sponsors. Among nine sponsors who encountered holds, average hold time at sites in March-April 2020 ranged from 2 to 12 weeks (mean, 7.3; standard deviation, 3.6).
Most sponsors (15/18; 83.3%) reported that closures affected none/few of their sites during the pandemic's first wave.
Trial holds and closures at sites occurred less frequently after the initial wave. Among 17 respondents, trials delayed or affected by holds were reported as much or somewhat lower from May 2020 to May 2022 compared with March-April 2020 by 10 (58.8%) sponsors and the same by 6 (35.3%) sponsors. Similarly, among 16 respondents, trials affected by closures were reported as much or somewhat lower by 9 (56.3%) sponsors and the same by 7 (43.8%) sponsors.
Dropouts and Trial Closures
Among 17 respondents, most (n = 13; 76.5%) reported no change in patient dropout rates during the pandemic. Two industry sponsors observed an increase in dropout rates that had since returned to the pre-pandemic level, and one industry sponsor and one NCI Network group sponsor observed a decrease in dropout rates that had not increased back to the pre-pandemic level.
Among 19 respondents, most (n = 17; 89.5%) observed no change in the number of trials closed because of low accrual during the pandemic, while two respondents (both NCI Network groups) reported a decrease.
AEs
Among 16 respondents, 13 (81.3%) reported no change in rates of both grades 1-2 and 3-4 AEs during the pandemic. One industry sponsor each indicated that rates of reported grades 1-2 and 3-4 AEs increased and have not returned or increased and have returned, respectively, while one NCI Network group reported that levels decreased and have returned.
Overall Impact on Data Integrity
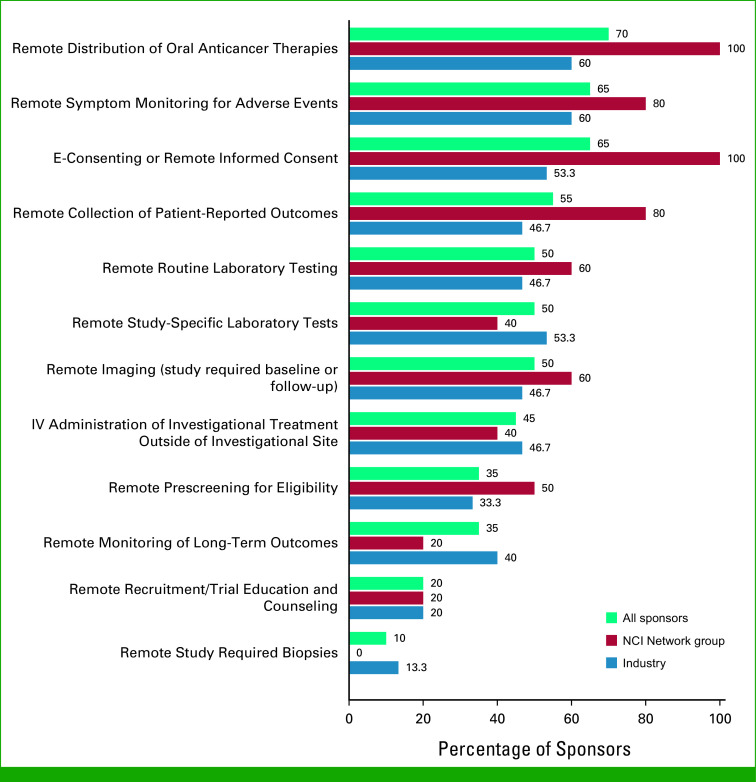
Sponsors were asked to rate the impact of the level of protocol deviations on overall data integrity during the COVID-19 pandemic using a five-point Likert-type scale with undefined response anchors (ie, left up to respondent interpretation). Among 18 respondents, the majority (n = 15; 83.3%) reported a minimal impact (14) or no impact (1) on overall data integrity (Fig 3).
FIG 3.
Overall impact of protocol deviations on data integrity. Overall percentages among those with known data (ie, excluding not reported) are shown at the top of the bars. Counts by sponsor category are shown within the bars in white font. NCI, National Cancer Institute.
Interview Findings
Follow-up interviews were conducted among 11 sponsors (55.0%), including seven industry sponsors and four NCI Network groups. Representatives from NCI CTEP were also interviewed, using a modified version of the sponsor interview guide.
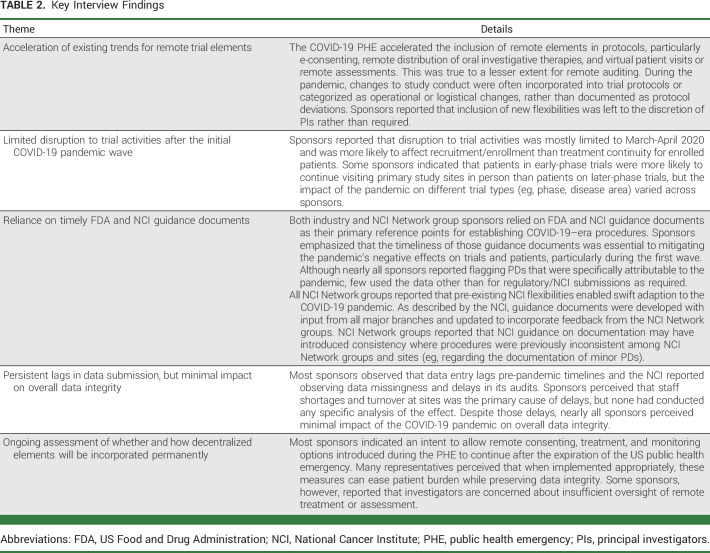
Key findings that emerged from thematic analysis included the perception that the pandemic accelerated the inclusion of remote elements in protocols, especially e-consenting, remote distribution of oral investigative therapies, and virtual patient visits (Table 2). Sponsors reported that disruption to trial activities was mostly limited to March-April 2020 and was more likely to affect recruitment and enrollment rather than treatment continuity.
TABLE 2.
Key Interview Findings
Additionally, sponsors relied upon the FDA and NCI guidance documents for establishing COVID-19–era procedures and indicated that these were essential to mitigating negative effects on trials and patients, particularly during the initial wave. Most sponsors reported that substantial staff shortages and turnover at sites led to persistent data entry lags compared with pre-pandemic timelines, although nearly all sponsors perceived minimal impact of the pandemic on overall data integrity.
Finally, most sponsors reported the intention to allow the mitigation measures to continue after the expiration of the PHE, although some sponsors also reported concern about appropriate clinical oversight of remote treatment or assessment and the regulatory burden of remote auditing.
DISCUSSION
This study represents a systematic evaluation of major clinical trial sponsors about the impact of the COVID-19 pandemic and its associated PHE on the conduct of cancer clinical trials, and thus, provides critical evidence from key collaborators to fill an evidence gap.10 On the basis of survey and interviews, we found that most respondents observed an increase in protocol deviations and many reported persistent lags in data collection >2 years later. However, the majority (83.3%) reported that the pandemic had minimal/no impact on overall data integrity. Sponsors indicated that remote elements were broadly implemented to minimize disruptions to enrollment and care of trial participants.
The COVID-19 pandemic was associated with severe interruptions in routine care for patients with cancer and for patients wishing to receive their care in clinical trials.15-20 In part, this was related to fear of exposure to SARS-CoV-2. In one study, among the one fifth of patients reporting they were less likely to participate in a clinical trial during the pandemic, most reported they were fearful of contracting SARS-CoV-2.13 Patients with cancer are often immunocompromised because of their cancer or its treatment; as such, becoming infected with SARS-CoV-2 while receiving care at clinics is likely to exacerbate their existing clinical risks.21
Given these challenges, enrollment to cancer clinical treatment trials dropped precipitously during the initial COVID-19 pandemic wave.2,8 In response, NCI and FDA provided early guidance to sponsors about mitigation strategies that could help overcome the difficulties of conducting cancer clinical research during a PHE. Many of these mitigation strategies had been previously considered but not widely adopted.22 A focus has been on allowing protocol procedures and processes to be conducted outside of traditional specialized academic centers where the majority of trials are conducted. Proposals to decentralize clinical care outside of trials have included increased use of telemedicine for monitoring and evaluation, with accompanying documented benefits for reducing treatment and participation burdens on patients and their caregivers.23,24 Such proposals can be extended to the conduct of clinical trials as part of a broader effort to modernize clinical research.
Concerns about the potential impacts on data quality of decentralized approaches to clinical trial conduct have previously prevented their widespread adoption.25,26 However, the onset of the COVID-19 pandemic forced their rapid adoption, thus serving as a natural experiment to evaluate their impact. To our knowledge, this study for the first time demonstrates that these mitigation strategies were widely adopted by major sponsors with minimal or no perceived impact on overall data integrity. Many calls to further evaluate whether to permanently incorporate decentralized elements into the conduct of clinical trials on the basis of the experience of the COVID-19 pandemic have been made.11,27-29 Importantly, since the aim of these strategies is also to reduce the burden of trial participation for patients, their adoption may have the salutary effect of improving representation for diverse patient populations. Recently, the FDA highlighted how decentralized trials may reduce barriers in access to trials and improve representation of historically underrepresented patient groups.10
The study is strengthened by high representation of industry organizations and NCI Network groups that sponsor most cancer treatment trials in the United States. The study is limited, however, by its reliance on voluntary survey and interview data alone. Sponsors were not asked to perform analyses before participating in the survey or interviews, although some conducted data aggregation/analysis before reporting findings. The number of days that sponsors spent completing the survey and number of representatives participating in the interviews varied and may have also influenced the results. Thus, the findings rely on variable levels of sponsors' internal analysis and on representatives' perceptions. “Furthermore, sponsors may have been less likely to report negative impacts of the pandemic on their data, leading to a potential bias.” To help mitigate this possibility, a presurvey/interview confidentiality document informed sponsors that their responses would be wholly anonymized in all data presentations. On the basis of hypotheses generated by this work, the ASCO-Friends task force is leading a quantitative evaluation of clinical trial data to provide greater insight into the impact of the pandemic on data quality.
The COVID-19 pandemic affected the conduct of cancer clinical trials and accelerated a trend toward greater flexibility. The strategies implemented during the pandemic to provide greater flexibility in the execution of interventional clinical trial procedures, patient evaluation, and data ascertainment may improve clinical trial access and reduce the burden of participation for sites and patients without compromising trial data. Sponsors continue to include flexibilities in new protocols while still following regulatory requirements and guidance. Future work to quantify the impact of the pandemic on the quality of trial data is vital for informing recommendations about whether more flexible processes may become permanent fixtures in the conduct of oncology clinical trials.
ACKNOWLEDGMENT
The authors thank the participating sponsor organizations and their individual representatives for their essential contributions to this work.
Hillary Stires
Consulting or Advisory Role: Avalere Health
Emily Dressler
Research Funding: Omada Health
Other Relationship: ICF
Uncompensated Relationships: Society for Clinical Trials
Keith Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Apricity Health, Oncoceutics, FOGPharma, Tvardi Therapeutics, Checkmate Pharmaceuticals, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics, Nextech Invest, Alterome Therapeutics
Consulting or Advisory Role: Novartis, Lilly, Oncoceutics, Tvardi Therapeutics, Takeda, Debiopharm Group, OmRx Oncology, Quanta Therapeutics, Immagene
Peter Fredette
Employment: IQVIA, EQRx
Travel, Accommodations, Expenses: IQVIA, EQRx
Lee Jones
Honoraria: Bayer, Eisai
Peggy McCann
Employment: Merck
Stock and Other Ownership Interests: Merck, Pfizer, CVS Health, GE Healthcare, GlaxoSmithKline, Bristol Meyers Squibb Company
Therica Miller
Consulting or Advisory Role: AOC Oncology
Other Relationship: University of Southern California, AOC Oncology, Alliance Foundation Trials, Children's Hospital Los Angeles, George Washington University, Alphasights
Fran Palmieri
Employment: Sarah Cannon Research Institute
Gary L. Smith
Stock and Other Ownership Interests: Mallinckrodt, Medtronic
Ajjai Alva
Consulting or Advisory Role: AstraZeneca, Merck, Pfizer, BMS
Research Funding: Genentech (Inst), Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Prometheus (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), Roche (Inst), Bayer (Inst), Astellas Pharma (Inst), Arcus Biosciences (Inst), Progenics (Inst), Celgene (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Merck, BMS
No other potential conflicts of interest were reported.
AUTHOR CONTRIBUTIONS
Conception and design: Joseph M. Unger, Hillary Stires, Laura A. Levit, Mark Stewart, Brittany Avin McKelvey, Beverly Canin, Keith Flaherty, Lee Jones, Peggy McCann, Therica Miller, Adedayo A. Onitilo, Timil Patel, Suanna S. Bruinooge, Elizabeth Garrett-Mayer, Ajjai Alva, Caroline Schenkel
Financial support: Joseph M. Unger
Administrative support: Joseph M. Unger, Caroline Schenkel
Provision of study materials or patients: Caroline Schenkel
Collection and assembly of data: Joseph M. Unger, Hillary Stires, Mark Stewart, Brittany Avin McKelvey, Emily Dressler, Peggy McCann, Caroline Schenkel
Data analysis and interpretation: Joseph M. Unger, Hillary Stires, Laura A. Levit, Mark Stewart, Brittany Avin McKelvey, Emily Dressler, Keith Flaherty, Peggy McCann, Therica Miller, Fran Palmieri, Timil Patel, Gary L. Smith, Suanna S. Bruinooge, Xiudong Jennifer Lei, Caroline Schenkel
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Sponsor Perspectives on the Impact of the COVID-19 Pandemic on Interventional Cancer Clinical Trial Protocols and Data Quality
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/op/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Hillary Stires
Consulting or Advisory Role: Avalere Health
Emily Dressler
Research Funding: Omada Health
Other Relationship: ICF
Uncompensated Relationships: Society for Clinical Trials
Keith Flaherty
Stock and Other Ownership Interests: Clovis Oncology, Loxo, X4 Pharma, Strata Oncology, PIC Therapeutics, Apricity Health, Oncoceutics, FOGPharma, Tvardi Therapeutics, Checkmate Pharmaceuticals, Kinnate Biopharma, Scorpion Therapeutics, ALX Oncology, xCures, Monopteros Therapeutics, Vibliome Therapeutics, Transcode Therapeutics, Soley Therapeutics, Nextech Invest, Alterome Therapeutics
Consulting or Advisory Role: Novartis, Lilly, Oncoceutics, Tvardi Therapeutics, Takeda, Debiopharm Group, OmRx Oncology, Quanta Therapeutics, Immagene
Peter Fredette
Employment: IQVIA, EQRx
Travel, Accommodations, Expenses: IQVIA, EQRx
Lee Jones
Honoraria: Bayer, Eisai
Peggy McCann
Employment: Merck
Stock and Other Ownership Interests: Merck, Pfizer, CVS Health, GE Healthcare, GlaxoSmithKline, Bristol Meyers Squibb Company
Therica Miller
Consulting or Advisory Role: AOC Oncology
Other Relationship: University of Southern California, AOC Oncology, Alliance Foundation Trials, Children's Hospital Los Angeles, George Washington University, Alphasights
Fran Palmieri
Employment: Sarah Cannon Research Institute
Gary L. Smith
Stock and Other Ownership Interests: Mallinckrodt, Medtronic
Ajjai Alva
Consulting or Advisory Role: AstraZeneca, Merck, Pfizer, BMS
Research Funding: Genentech (Inst), Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Prometheus (Inst), Mirati Therapeutics (Inst), AstraZeneca (Inst), Roche (Inst), Bayer (Inst), Astellas Pharma (Inst), Arcus Biosciences (Inst), Progenics (Inst), Celgene (Inst), Janssen (Inst)
Travel, Accommodations, Expenses: Merck, BMS
No other potential conflicts of interest were reported.
REFERENCES
- 1.Unger JM, Blanke CD, LeBlanc M, et al. : Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open 3:e2010651, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakouny Z, Labaki C, Bhalla S, et al. : Oncology clinical trial disruption during the COVID-19 pandemic: A COVID-19 and cancer outcomes study. Ann Oncol 33:836-844, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Institute : Coronavirus guidance, 2022. https://ctep.cancer.gov/investigatorResources/corona_virus_guidance.htm
- 4.US Food and Drug Administration : FDA guidance on conduct of clinical trials of medical products during COVID-19 pandemic, 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-pandemic
- 5.American Association for Cancer Research : AACR report on the impact of COVID-19 on cancer research and patient care, 2022. https://www.AACR.org/COVIDReport
- 6.Boughey JC, Snyder RA, Kantor O, et al. : Impact of the COVID-19 pandemic on cancer clinical trials. Ann Surg Oncol 28:7311-7316, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prindiville SA, Sarosy GA, Loose D, et al. : Patterns of enrollment in cancer treatment trials during the COVID-19 pandemic at National Cancer Institute-designated cancer centers. Cancer J 28:111-117, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unger JM, Xiao H, LeBlanc M, et al. : Cancer clinical trial participation at the 1-year anniversary of the outbreak of the COVID-19 pandemic. JAMA Netw Open 4:e2118433, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waterhouse DM, Harvey RD, Hurley P, et al. : Early impact of COVID-19 on the conduct of oncology clinical trials and long-term opportunities for transformation: Findings from an American Society of Clinical Oncology survey. JCO Oncol Pract 16:417-421, 2020 [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration : Advancing oncology decentralized trials: Modernizing evidence generation, 2022. https://www.fda.gov/about-fda/oncology-center-excellence/advancing-oncology-decentralized-trials
- 11.Pennell NA, Dillmon M, Levit LA, et al. : American Society of Clinical Oncology road to recovery report: Learning from the COVID-19 experience to improve clinical research and cancer care. J Clin Oncol 39:155-169, 2021 [DOI] [PubMed] [Google Scholar]
- 12.Galuchie L, Stewart C, Meloni F: Protocol deviations: A holistic approach from defining to reporting. Ther Innov Regul Sci 55:733-742, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleury ME, Farner AM, Unger JM: Association of the COVID-19 outbreak with patient willingness to enroll in cancer clinical trials. JAMA Oncol 7:131-132, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute : Cancer Therapy Evaluation Program. Common terminology criteria for adverse events (CTCAE), 2023. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm
- 15.Walker MJ, Wang J, Mazuryk J, et al. : Delivery of cancer care in Ontario, Canada, during the first year of the COVID-19 pandemic. JAMA Netw Open 5:e228855, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patt D, Gordan L, Diaz M, et al. : Impact of COVID-19 on cancer care: How the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform 4:1059-1071, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caston NE, Lawhon VM, Smith KL, et al. : Examining the association among fear of COVID-19, psychological distress, and delays in cancer care. Cancer Med 10:8854-8865, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhada S, Stewart D, Cheema E, et al. : Cancer services during the COVID-19 pandemic: Systematic review of patient's and caregiver's experiences. Cancer Manag Res 13:5875-5887, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutcuoglu O, Yazici O, Ozet A, et al. : Harmful consequences of COVID-19 fear in patients with cancer. BMJ Support Palliat Care 10.1136/bmjspcare-2020-002628 [epub ahead of print on December 21, 2020] [DOI] [PubMed]
- 20.Vanni G, Materazzo M, Pellicciaro M, et al. : Breast cancer and COVID-19: The effect of fear on patients' decision-making process. In Vivo 34:1651-1659, 2020. (suppl 3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Zhu F, Xie L, et al. : Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann Oncol 31:894-901, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khozin S, Coravos A: Decentralized trials in the age of real-world evidence and inclusivity in clinical investigations. Clin Pharmacol Ther 106:25-27, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Cox A, Lucas G, Marcu A, et al. : Cancer survivors' experience with telehealth: A systematic review and thematic synthesis. J Med Internet Res 19:e11, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennell NA, Dicker AP, Tran C, et al. : mHealth: Mobile technologies to virtually bring the patient into an oncology practice. Am Soc Clin Oncol Educ Book 37:144-154, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Coert RMH, Timmis JK, Boorsma A, et al. : Stakeholder perspectives on barriers and facilitators for the adoption of virtual clinical trials: Qualitative study. J Med Internet Res 23:e26813, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polhemus AM, Kadhim H, Barnes S, et al. : Accelerating adoption of patient-facing technologies in clinical trials: A pharmaceutical industry perspective on opportunities and challenges. Ther Innov Regul Sci 53:8-24, 2019 [DOI] [PubMed] [Google Scholar]
- 27.Adams DV, Long S, Fleury ME: Association of remote technology use and other decentralization tools with patient likelihood to enroll in cancer clinical trials. JAMA Netw Open 5:e2220053, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu S, Gerber DE, Beg MS: Decentralized clinical trials in oncology: Are we ready for a virtual-first paradigm? J Clin Oncol 41:181-185, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodson N, Wicks P, Morgan J, et al. : Opportunities and counterintuitive challenges for decentralized clinical trials to broaden participant inclusion. NPJ Digit Med 5:58, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]