Abstract
Vesicular stomatitis virus (VSV) induces apoptosis by at least two mechanisms. The viral matrix (M) protein induces apoptosis via the mitochondrial pathway due to the inhibition of host gene expression. However, in some cell types, the inhibition of host gene expression by VSV expressing wild-type (wt) M protein delays VSV-induced apoptosis, indicating that another mechanism is involved. In support of this, the recombinant M51R-M (rM51R-M) virus, expressing a mutant M protein that is defective in its ability to inhibit host gene expression, induces apoptosis much more rapidly in L929 cells than do viruses expressing wt M protein. Here, we determine the caspase pathways by which the rM51R-M virus induces apoptosis. An analysis of caspase activity, using fluorometric caspase assays and Western blots, indicated that each of the main initiator caspases, caspase-8, caspase-9, and caspase-12, were activated during infection with the rM51R-M virus. The overexpression of Bcl-2, an inhibitor of the mitochondrial pathway, or MAGE-3, an inhibitor of caspase-12 activation, did not delay apoptosis induction in rM51R-M virus-infected L929 cells. However, an inhibitor of caspase-8 activity significantly delayed apoptosis induction. Furthermore, the inhibition of caspase-8 activity prevented the activation of caspase-9, suggesting that caspase-9 is activated by cross talk with caspase-8. These data indicate that VSV expressing the mutant M protein induces apoptosis via the death receptor apoptotic pathway, a mechanism distinct from that induced by VSV expressing the wt M protein.
Cell death via apoptosis is a common outcome of viral infection (32). Vesicular stomatitis virus (VSV), the prototype rhabdovirus, rapidly induces apoptosis in a variety of host cell types (23). Recent results from our laboratory have shown that there are at least two distinct mechanisms by which VSV can induce apoptosis. One mechanism is the result of the activity of the viral matrix (M) protein, and the second mechanism is due to effects of other viral components that have yet to be identified (20, 21). Our hypothesis is that apoptosis induced by M protein occurs via pathways distinct from that induced by other VSV components. Three major caspase-dependent apoptotic pathways have been described; these pathways are the mitochondrion-associated (intrinsic) pathway, the death receptor-mediated (extrinsic) pathway, and the endoplasmic reticulum (ER) stress-associated pathway, which are initiated by caspase-9, caspase-8, and caspase-12, respectively (15, 30, 37). Previous results from our laboratory showed that M protein induces apoptosis via the intrinsic pathway (20, 21). The goal of the experiments presented here was to identify the pathway by which the other viral components induce apoptosis.
The VSV M protein is a structural component of the VSV virion and plays an important role in virus assembly. Independent of its role in virus assembly, M protein is also responsible for many cytopathic effects in VSV-infected cells, including the inhibition of host gene expression (27), as well as the induction of apoptosis (16, 19, 20, 21). M protein inhibits host gene expression at three different levels. M protein inhibits host transcription by interfering with all three host RNA polymerases (1, 41, 42). M protein also inhibits the nucleocytoplasmic transport of host RNA by blocking nuclear pore components, such as the nucleoporin Nup98 (31, 36), and it inhibits host translation by altering the eIF4F complex (13).
The ability of M protein to induce apoptosis is genetically correlated with its ability to inhibit host gene expression and is genetically separable from its role in virus assembly (21). This fact was shown by using a mutant M protein known as M51R-M protein that has an arginine substituted for the methionine at position 51 of the 229-amino-acid sequence. The M51R-M protein is defective in its ability to inhibit host gene expression but is fully functional in virus assembly (9). While wild-type (wt) M protein is able to induce apoptosis in the absence of other viral components, M51R-M protein is unable to do so, further supporting the conclusion that the inhibition of host gene expression by wt M protein is responsible for its apoptosis-inducing function (21). However, in the context of a virus infection, the effect of the M protein mutation on apoptosis induction by VSV is cell type dependent. This was shown by the use of isogenic recombinant viruses that express either wt or mutant M proteins (rwt or rM51R-M viruses, respectively) (20, 21). In HeLa cells, rwt virus induces apoptosis more rapidly than the rM51R-M virus, consistent with the role of wt M protein in apoptosis induction. However, in BHK cells, the rM51R-M virus induces apoptosis more rapidly than rwt virus (20, 21). Since the M51R-M protein alone is unable to induce apoptosis, this led to the conclusion that other viral components besides M protein can also induce apoptosis in infected cells.
Further experiments have led to the development of a model to explain why the inhibition of host gene expression by wt M protein has different effects in different cell types. Some types of cells, such as HeLa cells, do not require new host proapoptotic gene expression in order to enter apoptosis. In these cells, the inhibition of host gene expression by wt M protein activates the intrinsic apoptotic pathway (20) and results in cell death. However, other types of cells, such as BHK cells, do require new host gene expression in order to undergo rapid apoptosis in response to viral infection (20). In these cells, the inhibition of host gene expression by wt M protein serves to delay the onset of apoptosis. Thus, BHK cells infected with the rM51R-M virus, expressing the mutant M protein, are able to express the proapoptotic gene products necessary to allow rapid apoptosis (20). The experiments presented here further test this model by defining the caspase pathways by which the M protein mutant virus induces apoptosis.
We show that the infection of L929 mouse fibroblasts with the rM51R-M virus results in the activation of all three initiator caspases. Furthermore, the inhibition of the intrinsic apoptotic pathway by the overexpression of Bcl-2 or the inhibition of caspase-12 activity by the overexpression of MAGE-3 does not delay rM51R-M virus-induced apoptosis in L929 cells, whereas the inhibition of caspase-8 activity significantly delays apoptosis. These data suggest that, unlike wt virus, the M protein mutant virus induces apoptosis via the extrinsic pathway, requiring the activation of caspase-8. Therefore, VSV demonstrates multiple apoptosis-inducing functions, which induce apoptosis via distinct mechanisms.
MATERIALS AND METHODS
Cells and viruses.
L929 mouse fibroblasts were cultured in Dulbecco's modified Eagle medium supplemented with 7% fetal bovine serum (FBS). The recombinant viruses, rwt virus and the rM51R-M virus, were obtained from cDNA clones and grown as previously described (21). Infections were carried out at a multiplicity of infection of 20 PFU per cell. Stable L929-Bcl-2 cells were generated by transfecting L929 cells with the h-Bcl-2 pcDNA3 plasmid (a gift from Zheng Cui, Wake Forest University School of Medicine), and L929-Bcl-2-empty-vector (EV) cells were generated by transfecting L929 cells with the pcDNA3 plasmid, as previously described (20). L929-MAGE-3 cells were generated by transfecting L929 cells with the pcDNA3.1(+)-MAGE-3 (pNB401) plasmid (a gift from Nobuhiro Morishima, RIKEN) and L929-MAGE-3-EV cells were generated by transfecting cells with the pcDNA3.1(+) plasmid (Invitrogen) as previously described (30). Stably transfected cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% FBS and 200 μg of G418/ml.
Time-lapse microscopy.
L929 cell lines were grown to about 50% confluence in 35-mm-diameter tissue culture dishes and infected with recombinant viruses. Infected cells were incubated for 30 min at room temperature with rocking to allow the attachment of virus to cells. Cells were then transferred to a Zeiss inverted Axiovert phase-contrast time-lapse microscopy system equipped with an incubator and were maintained at 37°C in 5% CO2. Cells were monitored for 24 or 48 h postinfection by a Dage MTI-100 video camera affixed to the microscope at a time-lapse ratio of 600:1. Cells entering apoptosis were quantitated by using the time-date record on the videotape. Membrane blebbing was used as the criterion to distinguish cells entering apoptosis. All cells that underwent membrane blebbing subsequently underwent other morphological changes indicative of apoptosis, including cell shrinkage, membrane blistering, and membrane rupture. Time-lapse microscopy was also used to quantitate the rate of apoptosis induced by actinomycin D (5 μg/ml; Sigma), staurosporine (SSP) (1 μg/ml; Cayman Chemical Co.), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (50 ng/ml; R & D Systems). Cells treated with these drugs were immediately transferred to the time-lapse microscope, and cells entering apoptosis were quantitated as described above.
Analysis of mitochondrial transmembrane potential.
L929 cells were grown to about 50% confluence in 60-mm-diameter tissue culture dishes and were infected with recombinant viruses. The mitochondrial transmembrane potential (ΔΨm) was assessed according to the protocol of Grayson et al. (17). Briefly, cells were incubated in 40 nM 3,3′-dihexyloxacarbocyanide iodide (DiOC6; Molecular Probes) diluted in RPMI medium containing 10% FBS for 30 min at 37°C. The cells were then washed once in ice-cold phosphate-buffered saline containing 2% FBS (pH 7.4) and analyzed by flow cytometry on a FACSCaliber instrument (BD Biosciences), and data were analyzed using CellQuest Pro software (BD Immunocytometry Systems).
Caspase activity assays.
L929 cells were grown to about 50% confluence in 24-well dishes. Cells were infected with the recombinant viruses or treated with SSP or TRAIL as indicated in the figure legends. Each experiment was performed in duplicate as described previously (20). Caspase activity was determined with fluorogenic substrates for caspase-3 (DEVD-AFC; R & D Systems), caspase-8 (IETD-AFC; R & D Systems), and caspase-9 (LEHD-AFC; R & D Systems). Each sample was incubated for 2 h with the peptide substrate, and fluorescence intensities were measured at excitation and emission wavelengths of 400 and 490 nm, respectively. Duplicate samples were lysed in phosphate-buffered saline containing 0.1% sodium dodecyl sulfate (SDS), and total protein concentration was determined by Lowry assay (20).
Western blot analysis.
L929 cell lines were grown to about 75% confluence in six-well dishes and were infected with recombinant viruses. At the indicated times postinfection, cells were solubilized in RIPA buffer containing 1 mM phenylmethylsulfonyl fluoride, 1 mM aprotinin, and 1 mM pepstatin. Proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) on 12% polyacrylamide gels. Following electrophoresis, gels were electroblotted onto polyvinylidene difluoride and blocked in Tris-buffered saline (pH 7.5) containing 5% dry milk. Immunoblots were then probed with antibodies against procaspase-8 (Cell Signaling), procaspase-9 (Cell Signaling), procaspase-12 (Cell Signaling), Bcl-2 (BD Transduction Laboratories), the MAGE-A protein family (Zymed), and actin (Santa Cruz Biotechnology), as previously described (13). Protein band intensities were quantitated by scanning and analysis with Quantity One software (Bio-Rad). Film exposures were used with optical densities that fell within the linear range as determined by serial dilutions of antigen.
Caspase-8 inhibitor.
For time-lapse microscopy experiments, L929 cells were grown to about 50% confluence in 35-mm-diameter tissue culture dishes. For Western blot analysis, L929 cells were grown to about 75% confluence in six-well dishes. Cells were pretreated for 1 h with a synthetic inhibitor of caspase-8 activity (Z-IETD-CHO; BioSource) at a final concentration of 10 μM. Cells were infected with recombinant viruses or were treated with TRAIL. Time-lapse microscopy and Western blot analysis were carried out as described above.
Statistical analysis.
A paired Student's t test was used to compare the significances of individual time points. A P of <0.05 was considered statistically significant.
RESULTS
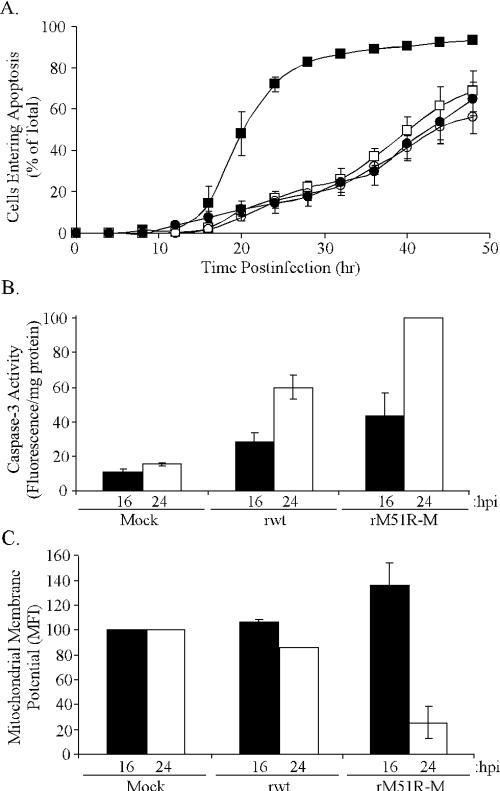
The rM51R-M virus induces apoptosis rapidly in L929 cells. Recombinant VSV expressing the mutant M51R-M protein induces apoptosis more rapidly than wt VSV in BHK cells (20, 21). The goal of the experiments presented here was to define the mechanism by which the M protein mutant virus induces apoptosis. Mouse L929 fibroblasts were used for these experiments to take advantage of murine reagents. Data in Fig. 1 show that, like BHK cells (20, 21), L929 cells infected with rM51R-M virus enter apoptosis more rapidly than those infected with rwt virus. For Fig. 1A, cells entering apoptosis were recorded and quantitated using time-lapse video microscopy. The data are expressed as cumulative percentages of cells entering apoptosis as a function of time postinfection. The rM51R-M virus rapidly induced apoptosis in L929 cells, such that more than 70% of the cells had entered apoptosis by 24 h postinfection, while rwt virus induced apoptosis more slowly, with only about 10% of the cells entering apoptosis by 24 h postinfection.
FIG. 1.
VSV expressing mutant M protein induces apoptosis in L929 cells more rapidly than VSV expressing wt M protein. (A) For the time-lapse microscopy analysis, L929 cells were infected with rwt virus (filled circles), rM51R-M virus (filled squares), or rM51R-M virus in the presence of actinomycin D (open squares) or were treated with actinomycin D alone (open circles). The cumulative percentage of cells entering apoptosis was determined as the number of cells that underwent membrane blebbing followed by other morphological changes consistent with apoptosis and is plotted as a function of time postinfection. The data represent the averages ± standard errors of the means (SEM) from three experiments. (B) Caspase-3 activity was assayed in L929 cells that were mock infected or infected with rwt virus or rM51R-M virus for 16 (black bars) or 24 (white bars) h postinfection. Caspase-3 activities in cell lysates were measured with a fluorogenic substrate and are expressed in arbitrary fluorescence units per milligram of total protein, and these data are normalized to the maximum value, expressed by the rM51R-M virus at 24 h postinfection. The data represent the averages ± SEM from three experiments. (C) Mitochondrial transmembrane potential was measured in mock-infected, rwt virus-infected, or rM51R-M virus-infected L929 cells. At 16 (black bars) and 24 (white bars) h postinfection, cells were labeled with DiOC6 and analyzed via flow cytometry. The data are expressed as the mean fluorescence intensity (MFI) of each sample as a percentage of the mock-infected cells. The data represent the averages ± standard deviations for two experiments. (B and C) hpi, hours postinfection.
Our laboratory has shown that BHK cells require new host gene expression in order to undergo rapid apoptosis (21). Since L929 cells rapidly enter apoptosis in response to rM51R-M virus infection, we tested the hypothesis that these cells also require new host gene expression in order to undergo rapid apoptosis. L929 cells were infected with rM51R-M virus in the presence of actinomycin D, a pharmacologic inhibitor of host transcription which has no effect on viral gene expression or viral replication (40). The inhibition of host gene expression by actinomycin D significantly delayed the induction of apoptosis by the rM51R-M virus such that the rate of apoptosis induced by rM51R-M virus was indistinguishable from the rate of apoptosis induced by rwt virus or actinomycin D alone (Fig. 1A). These results support our hypothesis that L929 cells require the expression of new proapoptotic genes to rapidly enter apoptosis.
It was important to confirm the results from time-lapse experiments by using other markers for apoptosis induction. The observation that rM51R-M virus induced apoptosis more rapidly than rwt virus was confirmed by an analysis of caspase-3 activation (Fig. 1B) and the collapse of the mitochondrial transmembrane potential (Fig. 1C). Caspase-3 activation was measured because the activation of this effector caspase is a hallmark of apoptotic cell death. At 16 and 24 h postinfection, cells were lysed with detergent-containing buffer. Cell lysates were assayed for caspase-3 activity by incubation with a fluorogenic caspase-3 substrate (DEVD-AFC). Caspase-3 activity was calculated as arbitrary fluorescence units per milligram of total protein and is expressed as a percentage of the sample with maximum activity (Fig. 1B). Cells infected with either rwt virus or rM51R-M virus had higher levels of active caspase-3 than did mock-infected cells, indicating that both viruses induced apoptosis in these cells. However, the caspase-3 activity at 24 h postinfection with rwt virus was only about 50% of that induced by rM51R-M virus.
The collapse of the mitochondrial transmembrane potential is a common feature of apoptosis. The collapse of ΔΨm may occur directly during activation of the intrinsic apoptotic pathway, preceding the activation of caspases. Alternatively, the collapse of ΔΨm may result from cross talk following activation of the extrinsic pathway (37). A fluorescent probe that partitions into mitochondria (DiOC6) was used to compare ΔΨms following infection with rwt virus and infection with rM51R-M virus. L929 cells were mock infected or infected with rwt virus or rM51R-M virus, and at 16 and 24 h postinfection, cells were labeled with DiOC6 and analyzed via flow cytometry. The data are expressed in Fig. 1C as the mean fluorescent intensity for each sample as a percentage of mock-infected cells. At 16 and 24 h postinfection, cells infected with rwt virus had ΔΨms similar to those of mock-infected cells, indicating that this virus had not induced a collapse of ΔΨm at these time points. Interestingly, rM51R-M virus induced a hyperpolarization of the mitochondrial membrane at 16 h postinfection, as evidenced by a ΔΨm greater than that of mock-infected cells. By 24 h postinfection, the ΔΨm in rM51R-M virus-infected cells had collapsed, consistent with the induction of apoptosis by this time. These results are similar to those of other groups that have observed hyperpolarization prior to the collapse of ΔΨm upon induction of apoptosis in a variety of systems (28, 35). The role of the hyperpolarization in the induction of apoptosis is a matter of debate (reviewed in reference 29). Collectively, the time-lapse microscopy, caspase-3 activity, and collapse of ΔΨm all support the conclusion that rM51R-M virus induces apoptosis more rapidly in L929 cells than does rwt virus.
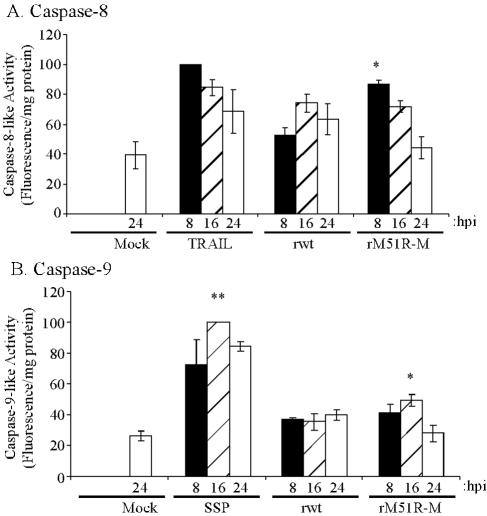
rM51R-M virus induces the activation of all three major initiator caspases.
The induction of apoptosis by wt M protein is dependent on activation of the intrinsic pathway, requiring the activation of the initiator caspase, caspase-9 (21). The rapid induction of apoptosis in L929 cells by the M protein mutant virus raised the possibility that different initiator caspases are involved in rM51R-M virus-infected cells. The activation of caspase-8 and caspase-9 during rwt and rM51R-M virus infection was analyzed using fluorogenic substrates IETD-AFC and LEHD-AFC, respectively. Because these substrates have the potential to be cleaved by other caspases, the activities measured by these substrates are referred to as caspase-8-like or caspase-9-like activities. L929 cells were either mock infected or infected with rwt virus or rM51R-M virus. Cells treated with TRAIL served as a positive control for the activation of caspase-8. Cells treated with SSP served as a positive control for the activation of caspase-9. Cell lysates were prepared at 8, 16, and 24 h postinfection, and caspase activity was measured.
The treatment of L929 cells with TRAIL for 8 h induced caspase-8-like activity that was approximately 2.5-fold higher than that in mock-treated cells (Fig. 2A), but the activity declined following longer periods of treatment. The data in Fig. 2A are expressed as percentages of this positive control at 8 h. At 8 h postinfection, rwt virus did not significantly induce caspase-8-like activity above background levels, and it induced slightly elevated levels at 16 h postinfection. In contrast, at 8 h postinfection, rM51R-M virus induced caspase-8-like activity to a level that was significantly higher than that of mock-infected or rwt virus-infected cells and was almost indistinguishable from the level of activity induced by the positive control, TRAIL. This activity then declined over time, similar to results obtained with TRAIL. These results are consistent with the conclusion that wt M protein delays apoptosis in L929 cells, similar to the results presented in Fig. 1. These results also provide evidence that rM51R-M virus may be activating apoptosis via the caspase-8 pathway.
FIG. 2.
rM51R-M virus induces caspase-8-like and caspase-9-like activities. Fluorometric caspase assays were performed using substrates for caspase-8 (A) and caspase-9 (B). L929 cells were left untreated (Mock), infected with rwt virus or rM51R-M virus, treated with 50 nM TRAIL (A), or treated with 1 μM SSP (B). At 8 (black bars), 16 (hatched bars), and 24 (white bars) h postinfection, cells were lysed and caspase activities were measured with fluorogenic substrates. Caspase activities are expressed in arbitrary fluorescence units per milligram of total protein and are normalized to the maximum values expressed by the positive controls, TRAIL and SSP, respectively. The data represent the averages ± SEM from three experiments. hpi, hours postinfection; *, P is <0.05 relative to mock-infected samples; **, P is <0.001 relative to rM51R-M virus-infected samples at 16 h postinfection.
Analysis of caspase-9-like activity is shown in Fig. 2B. Treatment of L929 cells with SSP induced maximal caspase-9-like activity at 16 h postinfection, which was >3-fold higher than that for mock-treated cells. The data in Fig. 2B are expressed as percentages of this positive control at 16 h. The level of caspase-9-like activity in rwt virus-infected cells did not rise significantly above that of mock-infected cells at any of these times postinfection. The lack of activation of caspase-9 by rwt virus reflects the fact that there is little induction of apoptosis by 24 h postinfection with rwt virus in this cell type (Fig. 1A). An analysis of later times postinfection showed that apoptosis induced by rwt virus involved the activation of the mitochondrial pathway in L929 cells, since it could be inhibited by the overexpression of Bcl-2 (data not shown). Caspase-9-like activity was significantly induced above background levels by 16 h postinfection in rM51R-M virus-infected cells and then decreased by 24 h. However, caspase-9-like activity peaked in rM51R-M virus-infected cells at only half the level activated by SSP. These results indicate that only a low level of caspase-9-like activity was induced in L929 cells by either rwt virus or rM51R-M virus infection.
To confirm the results obtained by fluorometric assays for caspase-8-like and caspase-9-like activities, a Western blot analysis was performed to detect cleavage of procaspase-8 and procaspase-9. In addition, we analyzed the cleavage of procaspase-12, since caspase-12 has been proposed to be an initiator caspase involved in ER stress-induced apoptosis (24). Caspase-12 was not analyzed by the fluorometric assay whose results were graphed in Fig. 2, because fluorogenic substrates with specificity for caspase-12 have yet to be developed. L929 cells were mock infected or infected with rwt virus or rM51R-M virus for 4, 8, or 16 h. At the indicated times postinfection, cell lysates were prepared and analyzed by SDS-PAGE and Western blotting. Figure 3A depicts a representative gel comparing procaspase-8 and actin as a gel loading control, and Fig. 3B shows the quantitation of procaspase-8, expressed as a ratio to actin and normalized to mock-infected controls. There was no significant change in the levels of procaspase-8 in rwt virus-infected cells at any time postinfection when compared to the level in mock-infected cells. In rM51R-M virus-infected cells, procaspase-8 levels were not significantly changed at 4 and 8 h postinfection, but significant cleavage was apparent by 16 h postinfection. In addition, the 43-kDa cleavage product was visible in Western blots of rM51R-M virus-infected cells at 16 h (Fig. 3A). These data support the fluorometric caspase-8 assays, indicating that caspase-8 is activated during rM51R-M virus infection of L929 cells.
FIG. 3.
rM51R-M virus induces cleavage of procaspases. L929 cells were mock infected (M) or infected with rwt virus or rM51R-M virus. At the indicated times postinfection, cell lysates were generated and separated via SDS-PAGE on a 12% polyacrylamide gel, transferred to polyvinylidene difluoride, and analyzed via Western blotting with antibodies for caspase-8 (A and B), caspase-9 (C and D), and caspase-12 (E and F). Representative gels are depicted in panels A, C, and E. Panels B, D, and F show quantitation of procaspase protein expression at 4 (black bars), 8 (hatched bars), and 16 (white bars) h postinfection. These data are normalized to actin protein expression and are shown as percentages of mock-infected samples. The data represent the averages ± SEM from four experiments. hpi, hours postinfection *, P is <0.05 relative to mock-infected samples.
Figure 3C and D show representative blots and their quantitation to analyze procaspase-9 protein expression. As with the assays described above, actin was used to control for protein loading. In rwt virus-infected cells, procaspase-9 levels increased at 4 h postinfection, declining to the levels of procaspase-9 in mock-infected cells by 16 h postinfection. The basis for the increase in procaspase-9 at 4 h is not known, but the subsequent decline of procaspase-9 suggests that some activation of caspase-9 was occurring in these cells. In rM51R-M virus-infected cells, procaspase-9 was cleaved to levels slightly lower than those in mock-infected cells by 16 h postinfection. While the extent of cleavage was small, the difference from that of the mock-infected cells was statistically significant. It was difficult to detect the active, cleaved form of caspase-9 in these Western blots. We attribute this outcome to the rapid turnover of caspase-9 once it has been cleaved. Alternatively, since caspase-9 is activated only in low levels, the quantity of cleavage products present may be below the level of detection. These data support the fluorometric caspase-9 assays, indicating that caspase-9 is activated during rM51R-M virus infection of L929 cells, although the level of activation is low compared to that of the negative controls.
Finally, Fig. 3E and F show an analysis of procaspase-12 protein expression. There was no significant change in the levels of procaspase-12 in rwt virus-infected cells at any time postinfection compared to the levels in mock-infected cells. Interestingly, procaspase-12 expression increased at 8 h postinfection with the rM51R-M virus. While no increases are easily visible in the Western blot in Fig. 3E, the densitometric analysis showed a statistically significant increase in procaspase-12 at 8 h postinfection. This result is consistent with an increase in caspase-12 mRNA expression observed during rM51R-M virus infection (unpublished data). However, a substantial portion of the 55-kDa procaspase-12 was cleaved into the 42-kDa active caspase-12 by 16 h postinfection (Fig. 3E), and the decrease in procaspase-12 was statistically significant (Fig. 3F), indicating that caspase-12 was activated in L929 cells during rM51R-M virus infection. Overall, the data in Fig. 2 and 3 show that there was little if any activation of initiator caspases in cells infected with rwt virus at these times postinfection, while all three initiator caspases were activated in cells infected with rM51R-M virus.
Overexpression of Bcl-2 inhibits procaspase-9 cleavage but does not inhibit apoptosis induced by rM51R-M virus.
Since all three initiator caspases, caspase-8, caspase-9, and caspase-12, were activated during rM51R-M virus infection, apoptosis inhibitors were used to identify which apoptotic pathways were important for rM51R-M virus-induced apoptosis. To determine if the mitochondrial pathway is important for apoptosis induced by rM51R-M virus, we generated L929 cells that stably overexpress the antiapoptotic protein Bcl-2. Bcl-2 has been shown to exert its antiapoptotic effect by stabilizing the mitochondria, thereby inhibiting the activation of caspase-9 (3). Western blot analysis was used to confirm that these cells overexpress Bcl-2 (Fig. 4A) and that the overexpression of Bcl-2 inhibited cleavage of procaspase-9 following infection with rM51R-M virus (Fig. 4B and C). As with Fig. 3C, it was difficult to visualize a decrease in procaspase-9 in Western blots of infected L929-Bcl-2-EV cells. However, when procaspase-9 was expressed as a ratio to actin, a statistically significant decrease could be observed at 16 h postinfection (Fig. 4C). In contrast, there was no decrease in procaspase-9 in infected cells that overexpress Bcl-2.
FIG. 4.
Inhibition of caspase-9 activity does not delay apoptosis induction by the rM51R-M virus. L929 cells that overexpress Bcl-2- (L929-Bcl-2) or an empty vector control (L929-Bcl-2-EV) were generated. (A) Cell lysates were generated and analyzed via Western blotting as described in the legend to Fig. 3, using an antibody against Bcl-2. (B and C) L929-Bcl-2 and L929-Bcl-2-EV cells were mock infected (M) or infected with rM51R-M virus. At the indicated times postinfection, cell lysates were obtained and analyzed via Western blotting as described above, utilizing an antibody against caspase-9. A representative gel is depicted in panel B. Panel C shows quantitation of procaspase-9 expression at 4 (black bars), 8 (hatched bars), and 16 (white bars) h postinfection. These values are normalized to actin protein expression and are shown as percentages of mock-infected samples. The data represent the averages ± SEM from three experiments. hpi, hours postinfection; *, P is <0.01 relative to mock-infected L929-Bcl-2-EV cells. (D) L929-Bcl-2 cells (open symbols) and L929-Bcl-2-EV cells (filled symbols) were infected with the rM51R-M virus (squares) or treated with 1 μM SSP (circles). Cells entering apoptosis were monitored by time-lapse video microscopy as described in the legend to Fig. 1. The data represent the averages ± SEM from three experiments.
To determine if the inhibition of caspase-9 activity delayed the induction of apoptosis by rM51R-M virus, L929-Bcl-2 and L929-Bcl-2-EV cells were infected with rM51R-M virus or were treated with 1 μM SSP as a positive control. Cells entering apoptosis were quantitated using time-lapse video microscopy, and the numbers are graphed in Fig. 4D as a function of time postinfection. As expected, L929-Bcl-2-EV cells treated with SSP rapidly enter apoptosis, while L929-Bcl-2 cells are resistant to apoptosis induced by SSP. However, L929-Bcl-2 and L929-Bcl-2-EV cells infected with rM51R-M virus both enter apoptosis rapidly, similar to untransfected L929 cells (Fig. 1), indicating that the overexpression of Bcl-2 in L929-Bcl-2 cells did not delay the apoptosis induced by rM51R-M virus. These data suggest that the mitochondrial pathway is not important for the induction of apoptosis by the rM51R-M virus.
Overexpression of MAGE-3 inhibits procaspase-12 cleavage but does not inhibit apoptosis induced by rM51R-M virus.
Morishima et al. recently identified the human cancer antigen MAGE-3 as a specific inhibitor of caspase-12 activation (30). To determine if the caspase-12 pathway is important for apoptosis induced by rM51R-M virus, we generated L929 cells that stably overexpress MAGE-3. A Western blot analysis was used to confirm that these cells overexpress MAGE-3 (Fig. 5A) and that the overexpression of MAGE-3 inhibited cleavage of procaspase-12 in cells infected with rM51R-M virus (Fig. 5B and C).
FIG. 5.
Inhibition of caspase-12 activity does not delay apoptosis induction by rM51R-M virus. L929 cells were generated that overexpress MAGE-3 (L929-MAGE-3) or an empty vector control (L929-MAGE-3-EV). (A) Cell lysates were generated and analyzed via Western blotting as described in the legend to Fig. 3, using an antibody against members of the human MAGE-A family, which includes MAGE-3. (B and C) L929-MAGE-3 and L929-MAGE-3-EV cells were mock infected (M) or infected with the rM51R-M virus. At the indicated times postinfection, cell lysates were obtained and analyzed via Western blotting utilizing an antibody against caspase-12. (B) A representative gel. (C) Quantitation of procaspase-12 expression at 4 (black bars), 8 (hatched bars), and 16 (white bars) h postinfection. These values are normalized to actin protein expression and are presented as percentages of mock-infected samples. The data represent the averages ± SEM from four experiments. hpi, hours postinfection; *, P is <0.05 relative to L929-MAGE-3-EV cells at 4 h postinfection. (D) L929-MAGE-3 (circles) and L929-MAGE-3-EV (squares) cells were infected with rM51R-M virus. Cells entering apoptosis were monitored by time-lapse video microscopy as described in the legend to Fig. 1. The data represent the averages ± SEM from three experiments.
As shown in Fig. 5D, L929-MAGE-3 cells and L929-MAGE-3-EV cells infected with rM51R-M virus enter apoptosis at similar rates. Thus, the inhibition of caspase-12 activity in L929-MAGE-3 cells did not delay the induction of apoptosis induced by rM51R-M virus. These data suggest that the caspase-12 apoptotic pathway is not important for the induction of apoptosis by the rM51R-M virus in L929 cells.
Inhibition of caspase-8 activity delays apoptosis induced by rM51R-M virus.
To determine if the caspase-8 pathway is important for apoptosis induced by rM51R-M virus, L929 cells were left untreated or pretreated for 1 h with a synthetic inhibitor of caspase-8 (Z-IETD-CHO). Pretreatment with Z-IETD-CHO inhibited the cleavage of procaspase-8 following infection with rM51R-M virus, as determined by Western blotting (Fig. 6A and B). In the absence of Z-IETD-CHO, there was a statistically significant decrease in the level of procaspase-8 at 16 h postinfection, similar to results shown in Fig. 3. In the presence of Z-IETD-CHO, there was no reproducible difference in the levels of procaspase-8 at any time postinfection.
FIG. 6.
Inhibition of caspase-8 activity delays apoptosis induction by rM51R-M virus. (A and B) L929 cells were left untreated or pretreated for 1 h with a synthetic caspase-8 inhibitor, Z-IETD-CHO (10 μM), and then mock infected (M) or infected with rM51R-M virus. At the indicated times posttreatment, cell lysates were prepared and analyzed via Western blotting as described in the legend to Fig. 3, using an antibody against caspase-8. A representative gel is depicted in panel A. Panel B shows the quantitation of procaspase-8 expression at 4 (black bars), 8 (hatched bars), and 16 (white bars) h postinfection. These values are normalized to actin protein expression and are presented as percentages of mock-infected samples. The data represent the averages ± SEM from three experiments. *, P is <0.05 relative to untreated, mock-infected L929 cells. (C) L929 cells were left untreated (filled symbols) or pretreated with 10 μM Z-IETD-CHO (open symbols) and then infected with rM51R-M virus (squares) or treated with 50 nM TRAIL (circles). Cells entering apoptosis were monitored by time-lapse video microscopy as described in the legend to Fig. 1. The data represent the averages ± SEM from three experiments.
The effect of the inhibition of caspase-8 activity on the induction of apoptosis in response to rM51R-M virus infection was determined by time-lapse microscopy (Fig. 6C). Cells treated with 50 ng of TRAIL/ml served as a positive control. About 60% of L929 cells treated with TRAIL entered apoptosis by 48 h postinfection, while L929 cells pretreated with Z-IETD-CHO were resistant to apoptosis induced by TRAIL. These results were expected because TRAIL is known to induce apoptosis via the extrinsic pathway. Similarly, pretreatment with Z-IETD-CHO significantly delayed the induction of apoptosis in L929 cells in response to rM51R-M virus infection, compared to the absence of inhibitor (see Fig. 1A). The inhibition of caspase-8 activity did not prevent rwt virus-induced apoptosis (data not shown), confirming previous findings that caspase-8 is not required for apoptosis induction by wt VSV (20). These results show that caspase-8 activity is important for the rapid induction of apoptosis by the rM51R-M virus.
Inhibition of caspase-8 inhibits activation of caspase-9 but does not inhibit activation of caspase-12.
Cross talk between cell death pathways is a widely accepted mechanism for the amplification of apoptotic signal transduction. Caspase-8 can be activated indirectly via the intrinsic pathway following activation of caspase-3 (39). Caspase-9 can be activated via the extrinsic pathway following caspase-8-induced activation of the proapoptotic Bcl-2 family member Bid (25, 26). To determine if cross talk with the extrinsic pathway is responsible for the activation of caspase-9 and caspase-12, Western blot analysis was performed to detect cleavage of procaspase-9 (Fig. 7A and B) and procaspase-12 (Fig. 7C and D) in the presence of the caspase-8 inhibitor (Z-IETD-CHO). In the presence of Z-IETD-CHO, no significant cleavage of procaspase-9 was detected in cells infected with rM51R-M virus (Fig. 7B). However, procaspase-12 was cleaved by 16 h postinfection with the rM51R-M virus in both the presence and the absence of Z-IETD-CHO (Fig. 7D). This fact is shown by the decrease in levels of procaspase-12 to similar extents in the presence and absence of the inhibitor. However, as shown in Fig. 7C, less active caspase-12 appears to be present in cells treated with Z-IETD-CHO, suggesting that turnover may be more rapid in the presence of the inhibitor. These results indicate that caspase-9 was activated by cross talk but caspase-12 was likely activated independently of caspase-8. Furthermore, since pretreatment with Z-IETD-CHO also inhibits the activation of caspase-9 in response to rM51R-M virus infection, it is likely that caspase-12 activation is also independent of caspase-9 activation.
FIG. 7.
Inhibition of caspase-8 activity inhibits activation of caspase-9 but not caspase-12. L929 cells were left untreated or pretreated for 1 h with 10 μM Z-IETD-CHO and then mock infected (M) or infected with the rM51R-M virus. At the indicated times postinfection, cell lysates were prepared and analyzed via Western blotting as described in the legend to Fig. 3, utilizing antibodies against caspase-9 (A and B) and caspase-12 (C and D). Representative gels are depicted in panels A and C. Panels B and D show quantitation of procaspase protein expression at 4 (black bars), 8 (hatched bars), and 16 (white bars) h postinfection. These values are normalized to actin protein expression and are presented as percentages of mock-infected samples. The data represent the averages ± SEM from four experiments. (B and D) hpi, hours postinfection; *, P is <0.05 relative to untreated, mock-infected L929 cells; **, P is <0.05 relative to Z-IETD-CHO- pretreated L929 cells at 8 h postinfection.
DISCUSSION
A particularly interesting aspect of VSV pathogenesis is that this virus is capable of initiating two distinct mechanisms of apoptosis. Previous work from our laboratory illustrated that viruses containing wt M proteins induce apoptosis via the intrinsic pathway due to the ability of wt M protein to inhibit host gene expression (20, 21). However, we also found that viruses containing mutant M proteins are potent inducers of apoptosis in some types of cells, such as BHK cells and, as shown here, L929 cells. The mutant M protein is defective in its ability to inhibit host gene expression and, therefore, unable to induce apoptosis in the absence of other viral components (21). Thus, there must be additional viral components, other than M protein, involved in the induction of apoptosis by VSV.
The two mechanisms of apoptosis induction by VSV are illustrated in Fig. 8 based on results presented here and our previous findings. Infection with rwt virus results in the expression of wt M protein, which inhibits host gene expression (1, 7, 8, 9, 13, 27, 31, 41, 42). This action activates the mitochondrial apoptotic pathway, resulting in the activation of caspase-9 (20, 21). Caspase-9 subsequently activates the executioner caspase, caspase-3. The rwt virus also activates low levels of caspase-8 activity. However, rwt virus-induced apoptosis can be inhibited by the overexpression of Bcl-2 but not by treatment with a caspase-8 inhibitor. Therefore, caspase-9 is the predominant initiator caspase activated by rwt virus infection, and caspase-8 is activated by cross talk with the mitochondrial pathway (20, 21).
FIG. 8.
VSV induces two distinct mechanisms of apoptosis. In rwt virus-infected cells (left), wt M protein inhibits host gene expression. This cellular stress results in the activation of the mitochondrial apoptotic pathway, which involves the activation of caspase-9 and, subsequently, caspase-3. Caspase-8 is activated by cross talk (dashed lines) in this pathway. rM51R-M virus infection (right) induces proapoptotic gene expression. Proapoptotic gene products then activate caspase-8 and, subsequently, caspase-3. It is possible that caspase-8 is activated directly or following the activation of death receptors. Caspase-9 is activated by cross talk in this pathway. Independent of the activation of caspase-8, rM51R-M virus infection induces ER stress, resulting in the activation of caspase-12.
Cells infected with rM51R-M virus (Fig. 8) undergo apoptosis via a separate mechanism. Here, we show that L929 cells require new host gene expression to undergo rapid apoptosis induced by rM51R-M virus (Fig. 1). In these cells, virus infection results in increased proapoptotic gene expression. We propose that this pathway is also active in rwt virus-infected cells but is blocked by the activity of wt M protein. The inability of M51R-M protein to inhibit host gene expression allows increased proapoptotic gene expression in response to an rM51R-M virus infection, resulting in the activation of all three major initiator caspases, caspase-8, caspase-9, and caspase-12 (Fig. 2 and 3), as well as the executioner caspase, caspase-3 (Fig. 1). The overexpression of Bcl-2, an inhibitor of the mitochondrial pathway, and MAGE-3, a specific inhibitor of caspase-12 activation, does not delay apoptosis induced by rM51R-M virus (Fig. 4 and 5). However, treatment with a caspase-8 inhibitor significantly delays apoptosis induced by rM51R-M virus (Fig. 6), indicating that the caspase-8 pathway is the most important apoptotic pathway involved in rM51R-M virus-induced apoptosis. Furthermore, treatment with the caspase-8 inhibitor prevents the activation of caspase-9 but not caspase-12 (Fig. 7), indicating that caspase-8 activates caspase-9 via cross talk. These results are in sharp contrast to our previous data regarding apoptosis induced by rwt virus, and they distinguish two separate mechanisms of apoptosis induced by VSV.
Cross talk between apoptotic pathways is a widely accepted mechanism by which apoptotic cascades are linked and enhanced (33). The intrinsic pathway has been shown to activate caspase-8 via the caspase-9-mediated activation of caspase-3, which targets procaspase-8 for cleavage (39). Our previous data have demonstrated this mechanism of cross talk in apoptosis induced by wt M protein in HeLa cells (20). Here we demonstrated that caspase-9 was activated by cross talk with the caspase-8 pathway in apoptosis induced by rM51R-M virus in L929 cells (Fig. 7). Caspase-8 has been shown to activate the intrinsic pathway following the cleavage of Bid, a proapoptotic member of the Bcl-2 family of proteins (25, 26, 33). The data presented in Fig. 1C are consistent with such a mechanism, showing that the collapse of ΔΨm occurs following infection with rM51R-M virus.
The inhibition of caspase-8 activity did not prevent the cleavage of procaspase-12, suggesting that caspase-12 is activated independently of caspase-8 (Fig. 7C and D). Caspase-12 has previously been implicated as an important initiator caspase in cells infected with other viruses, such as respiratory syncytial virus (6), although other data indicate that caspase-8 is also important for the induction of apoptosis by respiratory syncytial virus (22). Data presented in Fig. 5 show that the inhibition of caspase-12 activity does not significantly delay apoptosis induced by rM51R-M virus, indicating that caspase-12 activity is not required for the induction of apoptosis by rM51R-M virus. However, these data do not rule out the possibility that caspase-12 plays a role in promoting or amplifying apoptosis in response to VSV infection in other types of cells.
Our results have led us to a model in which the two mechanisms of apoptosis induction represent two different types of host responses to virus infection. A primary function of wt M protein is to inhibit host gene expression in order to combat host antiviral responses, such as the synthesis of interferons (2). However, the global inhibition of host gene expression results in cellular stress that activates the mitochondrial apoptotic pathway. In support of this, other intracellular stresses, such as the inhibition of transcription by actinomycin D, induce apoptosis via the mitochondrial apoptotic pathway (20). Therefore, apoptosis induced by wt M protein during rwt virus infection is likely to be a by-product of the virus suppressing the host antiviral response.
On the other hand, we propose that apoptosis induced by rM51R-M virus infection is, in fact, part of the typical antiviral response. This mechanism of apoptosis is dependent on new host gene expression and is, thus, suppressed by the effects of wt M protein. In support of this, the activation of the death receptor pathway is typical of apoptosis induced by interferons, double-stranded RNA, and other factors involved in an antiviral response (4, 5, 10, 14). Furthermore, apoptosis induced by a variety of other viruses, including influenza and reoviruses, is prevented in the presence of a caspase-8 inhibitor, suggesting that the death receptor pathway is the predominant pathway activated by these viruses (5, 11, 12, 34). Even though the induction of apoptosis is a type of antiviral response, data from our laboratory and from others (21, 23) indicate that rapid induction of apoptosis does not inhibit VSV replication in most cell types, suggesting that this aspect of the antiviral response is not very effective against VSV.
Studies are under way to elucidate the mechanism of caspase-8 activation. The activation of caspase-8 may occur via direct cleavage of procaspase-8, as has been observed in the activation of caspase-8 via caspase-3 (39). Also, recent evidence suggests that caspase-8 may be localized to the ER and may play a role in the induction of apoptosis in response to ER stress (18, 38). In this respect, rM51R-M virus-induced caspase-8 activation may be linked to the activation of caspase-12. Alternatively, the proapoptotic gene products may include soluble factors or ligands that are released from the cell and bind cell surface death receptors, thereby facilitating activation of the classical extrinsic pathway.
Acknowledgments
We acknowledge the Microscopy Core Laboratory at Wake Forest University. We thank Maryam Ahmed, John Connor, Zheng Cui, Griffith Parks, and Mark Willingham for helpful advice and comments on the manuscript. We also thank Zheng Cui and Nobuhiro Morishima for sharing plasmid DNAs and Jason Grayson and Sharmila Pejawar for assistance with flow cytometry experiments.
This work was supported by Public Health Service grant AI 32983 from the National Institute of Allergy and Infectious Diseases. D.F.G. was supported by the National Institutes of Health training grant T32 AI 07401. The Microscopy Core Laboratory was supported in part by the core grant for the Comprehensive Cancer Center of Wake Forest University (CA12197) from the National Cancer Institute.
REFERENCES
- 1.Ahmed, M., and D. S. Lyles. 1998. Effect of vesicular stomatitis virus matrix protein on transcription directed by host RNA polymerases I, II, and III. J. Virol. 72:8413-8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonsson, B., and J. C. Martinou. 2000. The Bcl-2 protein family. Exp. Cell Res. 256:50-57. [DOI] [PubMed] [Google Scholar]
- 4.Balachandran, S., C. N. Kim, W. C. Yeh, T. W. Mak, K. Bhalla, and G. N. Barber. 1998. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 17:6888-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balachandran, S., P. C. Roberts, T. Kipperman, K. N. Bhalla, R. W. Compans, D. R. Archer, and G. N. Barber. 2000. Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/caspase-8 death signaling pathway. J. Virol. 74:1513-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitko, V., and S. Barik. 2001. An endoplasmic reticulum-specific stress-activated caspase (caspase-12) is implicated in the apoptosis of A549 epithelial cells by respiratory syncytial virus. J. Cell. Biochem. 80:441-454. [DOI] [PubMed] [Google Scholar]
- 7.Black, B. L., G. Brewer, and D. S. Lyles. 1994. Effect of vesicular stomatitis virus matrix protein on host-directed translation in vivo. J. Virol. 68:555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black, B. L., and D. S. Lyles. 1992. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J. Virol. 66:4058-4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black, B. L., R. B. Rhodes, M. McKenzie, and D. S. Lyles. 1993. The role of vesicular stomatitis virus matrix protein in inhibition of host-directed gene expression is genetically separable from its function in virus assembly. J. Virol. 67:4814-4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chawla-Sarkar, M., D. J. Lindner, Y. F. Liu, B. R. Williams, G. C. Sen, R. H. Silverman, and E. C. Borden. 2003. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 8:237-249. [DOI] [PubMed] [Google Scholar]
- 11.Clarke, P., S. M. Meintzer, S. Gibson, C. Widmann, T. P. Garrington, G. L. Johnson, and K. L. Tyler. 2000. Reovirus-induced apoptosis is mediated by TRAIL. J. Virol. 74:8135-8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke, P., and K. L. Tyler. 2003. Reovirus-induced apoptosis: a minireview. Apoptosis 8:141-150. [DOI] [PubMed] [Google Scholar]
- 13.Connor, J. H., and D. S. Lyles. 2002. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 76:10177-10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Der, S. D., Y. L. Yang, C. Weissmann, and B. R. Williams. 1997. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:3279-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desagher, S., and J. C. Martinou. 2000. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10:369-377. [DOI] [PubMed] [Google Scholar]
- 16.Desforges, M., J. Charron, S. Berard, S. Beausoleil, D. F. Stojdl, G. Despars, B. Laverdiere, J. C. Bell, P. J. Talbot, C. P. Stanners, and L. Poliquin. 2001. Different host-cell shutoff strategies related to the matrix protein lead to persistence of vesicular stomatitis virus mutants on fibroblast cells. Virus Res. 76:87-102. [DOI] [PubMed] [Google Scholar]
- 17.Grayson, J. M., N. G. Laniewski, J. G. Lanier, and R. Ahmed. 2003. Mitochondrial potential and reactive oxygen intermediates in antigen-specific CD8+ T cells during viral infection. J. Immunol. 170:4745-4751. [DOI] [PubMed] [Google Scholar]
- 18.Jimbo, A., E. Fujita, Y. Kouroku, J. Ohnishi, N. Inohara, K. Kuida, K. Sakamaki, S. Yonehara, and T. Momoi. 2003. ER stress induces caspase-8 activation, stimulating cytochrome c release and caspase-9 activation. Exp. Cell Res. 283:156-166. [DOI] [PubMed] [Google Scholar]
- 19.Kopecky, S. A., and D. S. Lyles. 2003. The cell-rounding activity of the vesicular stomatitis virus matrix protein is due to the induction of cell death. J. Virol. 77:5524-5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopecky, S. A., and D. S. Lyles. 2003. Contrasting effects of matrix protein on apoptosis in HeLa and BHK cells infected with vesicular stomatitis virus are due to inhibition of host gene expression. J. Virol. 77:4658-4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopecky, S. A., M. C. Willingham, and D. S. Lyles. 2001. Matrix protein and another viral component contribute to induction of apoptosis in cells infected with vesicular stomatitis virus. J. Virol. 75:12169-12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kotelkin, A., E. A. Prikhod'ko, J. I. Cohen, P. L. Collins, and A. Bukreyev. 2003. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand. J. Virol. 77:9156-9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koyama, A. H. 1995. Induction of apoptotic DNA fragmentation by the infection of vesicular stomatitis virus. Virus Res. 37:285-290. [DOI] [PubMed] [Google Scholar]
- 24.Lamkanfi, M., M. Kalai, and P. Vandenabeele. 2004. Caspase-12: an overview. Cell Death Differ. 11:365-368. [DOI] [PubMed] [Google Scholar]
- 25.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 26.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 27.Lyles, D. S. 2000. Cytopathogenesis and inhibition of host gene expression by RNA viruses. Microbiol. Mol. Biol. Rev. 64:709-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuyama, S., J. Llopis, Q. L. Deveraux, R. Y. Tsien, and J. C. Reed. 2000. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat. Cell Biol. 2:318-325. [DOI] [PubMed] [Google Scholar]
- 29.Matsuyama, S., and J. C. Reed. 2000. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 7:1155-1165. [DOI] [PubMed] [Google Scholar]
- 30.Morishima, N., K. Nakanishi, H. Takenouchi, T. Shibata, and Y. Yasuhiko. 2002. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277:34287-34294. [DOI] [PubMed] [Google Scholar]
- 31.Petersen, J. M., L. S. Her, V. Varvel, E. Lund, and J. E. Dahlberg. 2000. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol. 20:8590-8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53:577-628. [DOI] [PubMed] [Google Scholar]
- 33.Roy, S., and D. W. Nicholson. 2000. Cross-talk in cell death signaling. J. Exp. Med. 192:F21-F25. [PMC free article] [PubMed] [Google Scholar]
- 34.Takizawa, T., C. Tatematsu, K. Ohashi, and Y. Nakanishi. 1999. Recruitment of apoptotic cysteine proteases (caspases) in influenza virus-induced cell death. Microbiol. Immunol. 43:245-252. [DOI] [PubMed] [Google Scholar]
- 35.Vander Heiden, M. G., N. S. Chandel, E. K. Williamson, P. T. Schumacker, and C. B. Thompson. 1997. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91:627-637. [DOI] [PubMed] [Google Scholar]
- 36.von Kobbe, C., J. M. van Deursen, J. P. Rodrigues, D. Sitterlin, A. Bachi, X. Wu, M. Wilm, M. Carmo-Fonseca, and E. Izaurralde. 2000. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell 6:1243-1252. [DOI] [PubMed] [Google Scholar]
- 37.Wallach, D., M. Boldin, E. Varfolomeev, R. Beyaert, P. Vandenabeele, and W. Fiers. 1997. Cell death induction by receptors of the TNF family: towards a molecular understanding. FEBS Lett. 410:96-106. [DOI] [PubMed] [Google Scholar]
- 38.Wang, X., Z. Shao, F. S. Zetoune, M. G. Zeidler, K. Gowrishankar, and C. Vincenz. 2003. NRADD, a novel membrane protein with a death domain involved in mediating apoptosis in response to ER stress. Cell Death Differ. 10:580-591. [DOI] [PubMed] [Google Scholar]
- 39.Wieder, T., F. Essmann, A. Prokop, K. Schmelz, K. Schulze-Osthoff, R. Beyaert, B. Dorken, and P. T. Daniel. 2001. Activation of caspase-8 in drug-induced apoptosis of B-lymphoid cells is independent of CD95/Fas receptor-ligand interaction and occurs downstream of caspase-3. Blood 97:1378-1387. [DOI] [PubMed] [Google Scholar]
- 40.Youngner, J. S., E. J. Dubovi, D. O. Quagliana, M. Kelly, and O. T. Preble. 1976. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J. Virol. 19:90-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan, H., S. Puckett, and D. S. Lyles. 2001. Inhibition of host transcription by vesicular stomatitis virus involves a novel mechanism that is independent of phosphorylation of TATA-binding protein (TBP) or association of TBP with TBP-associated factor subunits. J. Virol. 75:4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan, H., B. K. Yoza, and D. S. Lyles. 1998. Inhibition of host RNA polymerase II-dependent transcription by vesicular stomatitis virus results from inactivation of TFIID. Virology 251:383-392. [DOI] [PubMed] [Google Scholar]